
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 12 November 2024
Sec. Parasite and Host
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1480347
Babesia ovis, commonly associated with ovine babesiosis, poses a significant threat to sheep health, often resulting in severe clinical manifestations and high mortality rates. However, the impact of B. ovis on goats has remained uncertain, prompting us to investigate its pathogenicity in caprine hosts. Experimental infections using B. ovis-infected blood inoculation and infected tick infestation, were conducted on spleen-intact (n=5) and splenectomized (n=5) goats. The experimental infection was performed using fresh blood obtained from a B. ovis-infected splenectomized sheep. One spleen-intact sheep served as a control for the experimental infection with B. ovis-infected Rhipicephalus bursa ticks. While all experimentally infected sheep (#501, #575) displayed severe clinical symptoms and high parasitemia, goats exhibited resistance, showing no significant clinical manifestations or sustained parasitemia. Notably, B. ovis was detected in two spleen-intact goats via nested PCR, prompting further investigation into their role as reservoirs for tick-borne transmission. These goats were then infested with Babesia spp.-free R. bursa larvae (0.1 gr) and adults (50 females and 50 males) for transstadial and transovarial transmission experiments respectively. Results indicated that chronically B. ovis-infected spleen-intact goats are not significant sources for maintaining the tick-borne transmission cycle of the parasite. These findings highlight the differential susceptibility of goats to B. ovis infection compared to sheep and their limited role as reservoirs for parasite transmission. Understanding the role of goats in B. ovis transmission and their resistance mechanisms can inform effective control measures and reduce economic losses in affected regions. Further research into caprine babesiosis and host immunological responses is essential to fully elucidate their possible role as reservoirs of the parasite, and underlying mechanisms of host susceptibility and parasite pathogenesis.
Goats, often referred to as the "poor man's cow," are renowned for their resilience and adaptability to harsh environments, making them indispensable assets for small-scale farmers worldwide (Kumar et al., 2006; Nair et al., 2021). In some countries, including Turkiye, goat breeding holds not only economic significance but also cultural importance, especially within nomadic communities (Alkan and Ugur, 2015). However, despite their hardiness, goats are susceptible to various infections, including babesiosis caused by Babesia spp., which poses a significant threat to livestock globally.
Babesiosis, the disease caused by Babesia spp., is known for its impact on livestock, particularly affecting sheep, cattle, and goats (Uilenberg, 2006; Schnittger et al., 2012, 2022; Ozubek et al., 2020). The economic consequences of babesiosis are profound, encompassing direct losses from animal morbidity and mortality, as well as indirect losses from reduced productivity and heightened veterinary expenses (Ozubek et al., 2020). Small ruminant babesiosis is commonly caused by Babesia ovis, B. motasi, and B. crassa. Babesia taylori and B. foliata were identified in India years ago, but information on these species remains limited (Schnittger et al., 2022). Over the past two decades, new species or genotypes of Babesia that affect sheep and goats have been identified, including Babesia sp. Xinjiang (Guan et al., 2009), Babesia aktasi (Ozubek et al., 2023a), and B. motasi-like species, such as B. motasi Lintanensis and B. motasi Hebeinensis (Wang et al., 2023).
Babesia ovis poses a significant threat to sheep, often causing severe clinical and hematological issues, with mortality rates reaching up to 50% (Yeruham et al., 1998a, 1998b; De Waal, 2000; Ceylan et al., 2021). The epidemiology of B. ovis is closely tied to the biology and ecology of Rhipicephalus bursa ticks (Yeruham et al., 1998a). Rhipicephalus bursa is a two-host species that can transmit B. ovis to hosts only during its adult stage (Erster et al., 2016). While the impact of B. ovis on sheep is well-documented, its effect on goats remains a subject of debate within the veterinary and scientific communities. Despite assertions in various review articles suggesting that B. ovis does not induce clinical infection in goats (Friedhoff, 1997; De Waal, 2000; Schnittger et al., 2022), recent studies have presented contrasting findings, indicating inflammatory responses and altered hematological parameters in naturally infected goats (Esmaeilnejad et al., 2012, 2020). Furthermore, molecular studies have detected B. ovis in goats across various geographical regions, including Turkiye (Aktaş et al., 2005; Aktas et al., 2007; Inci et al., 2010; Aktas and Özübek, 2017; Ulucesme et al., 2023), Tunisia (Rjeibi et al., 2014), Uganda (Tumwebaze et al., 2020), and Philippines (Galon et al., 2022), fueling discussions on the susceptibility of goats to the pathogen and its clinical implications (Schnittger et al., 2022).
Given the significance of goat farming for economic stability (Nair et al., 2021), clarifying the role of B. ovis in goat health is imperative. This study aimed to address the existing discrepancies in the literature by conducting controlled experimental infections in goats using both infected blood and infected ticks, mimicking natural transmission routes. Through comprehensive assessment, we determined whether B. ovis can indeed cause clinical babesiosis in goats. By elucidating the interactions between B. ovis and caprine hosts, in this study we provided new and valuable insights into the pathogenic potential of the parasite in goats. Such insights are crucial for developing effective management strategies to safeguard goat health and productivity, thereby ensuring the sustainability of small ruminant farming systems.
This study was conducted in compliance with Turkish animal welfare regulations. All animal experiments received approval from the Firat University Animal Experiment Ethics Committee, under protocol number 2018/100.
In this study, we used sheep and goats that were free of Babesia, Anaplasma, and Theileria infections. To select the experimental animals, we collected blood samples from apparently healthy sheep and goats aged 5 to 8 months. The samples were collected in both serum and EDTA tubes. We employed nested PCR (nPCR) with general primers to investigate the presence of these species. For Anaplasma, we used primers Ec9/Ec12A (Kawahara et al., 2006) and 16S8FE/B-GA1B (Bekker et al., 2002). For Babesia and Theileria, we used primers Nbab1F/Nbab1R (Oosthuizen et al., 2008) and RLBF2/RLBR2 (Georges et al., 2001), respectively. Six sheep and twelve goats were determined to be negative for these pathogens by nPCR. These animals were then transported to the Elazıg Veterinary Control Institute Directorate, where the experimental infections took place. They were kept in a tick-free environment and provided with feed and water ad libitum (Firat et al., 2024). Sheep (#501) and goat (#803, #862, #4K, #804, #SAN) underwent splenectomy at Firat University Veterinary Hospital and were subsequently housed in individual compartments to facilitate a two-week recovery period prior to the commencement of the experiment. The surgical procedure adhered to established protocols, ensuring standardized techniques for anesthesia and analgesia administration (Sevinc et al., 2007).
The experimental infection was carried out as previously described by Guan et al. (2009) and Ozubek et al. (2023b). Briefly, the Babesia ovis/Alacakaya stabilate used in this study was originally obtained from a naturally infected sheep (Firat et al., 2024). To ensure the purity of the B. ovis stabilate and eliminate any potential contamination from other hemoparasites, such as Theileria or Anaplasma, the stabilate was subjected to transovarial passage through R. bursa ticks. Following this procedure, the stabilate was cryopreserved in a cryobank. Before experimental infection, the stabilate was reactivated by thawing and intravenously administered (15 mL) to a splenectomized sheep (#501). Once parasitemia reached 5.2%, 15 mL of fresh B. ovis-infected blood was intravenously administered to splenectomized goats (#803, #862), and 30 mL to spleen-intact goats (#008, #796) (Guan et al., 2009; Ozubek et al., 2023b; Firat et al., 2024). Blood samples were collected from the experimental donor animals daily for 60 days post-infection for analysis using microscopy and nested-PCR (nPCR) (Figure 1).
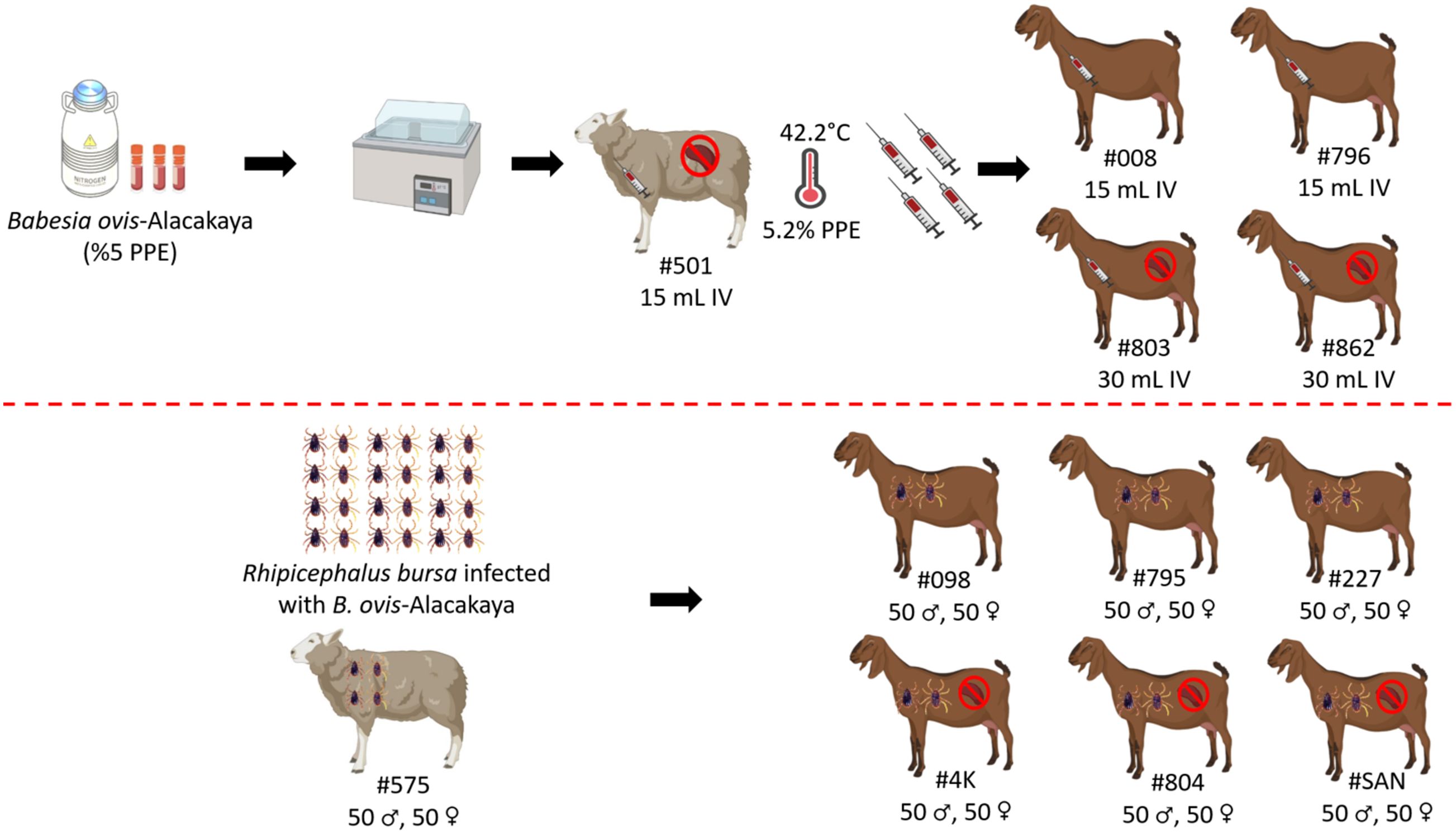
Figure 1. Schematic representation of sheep and goats used in experimental infection with infected blood and ticks with B. ovis-Alacakaya. Figure was created using Biorender.com.
Rhipicephalus bursa ticks used in the experimental infections were obtained from a colony regularly maintained in our laboratory (Firat et al., 2024; Ulucesme et al., 2024). For the experimental infection, adult R. bursa ticks infected with B. ovis Alacakaya were used. The process of obtaining infected ticks is briefly described as follows: a splenectomized lamb experimentally infected with the B. ovis Alacakaya stabilate (Firat et al., 2024) was infested with 15 female and 10 male Babesia spp.-free R. bursa ticks. Engorged females were collected from the experimentally infected lamb and placed in an incubator (25 ± 1°C and 70 ± 10% relative humidity) to obtain infected larvae. Since R. bursa can only transmit B. ovis in the adult stage (Erster et al., 2016), the obtained larvae were used to infest a rabbit. Engorged nymphs were then collected from this rabbit. Finally, the engorged nymphs were placed in an incubator to obtain B. ovis-infected adult R. bursa ticks. As a control, one sheep (#575) was infested with 50 females and 50 males R. bursa adult ticks infected with B. ovis. Additionally, the same number of female and male R. bursa ticks were used to infest both splenectomized (#4K, #804, #SAN) and spleen-intact goats (#098, #795, #227). Following the experimental infection with the infected ticks, all animals (#575, #098, #795, #227, #4K, #804, #SAN) were monitored for clinical symptoms of ovine babesiosis, including increased body temperature, anemia, jaundice, and hemoglobinuria. Additionally, the animals were monitored for 60 days post-infection for B. ovis parasitemia using microscopy and nPCR methods (Figure 1).
Experiments on transstadial and transovarial transmission were conducted to determine if goats infected with B. ovis serve as a source of parasite transmission by R. bursa ticks. For the transstadial transmission experiment, infected goats (#098, #795) were each infested with 0.1 g Babesia spp.-free R. bursa larvae (Firat et al., 2024). Engorged nymphs were collected from these larvae-infested goats (#098, #795) and incubated at 25 ± 1°C and 70 ± 10% relative humidity (RH). After incubation, unfed adult ticks obtained from goats #098 and #795 were used to infest splenectomized sheep #K2 and #K4, respectively (50 females and 50 males per sheep).
For the transovarial transmission experiment, Babesia spp.-free adult R. bursa ticks were used to infest goats #098 and #795 (50 females and 50 males per goat). Engorged females were collected from each goat, placed in separate plastic containers, and incubated under the same conditions to obtain larvae. Larvae obtained from engorged females collected from goats #098 and #795 were used to infest #Rabbit-1 and #Rabbit-2, respectively. Engorged nymphs were collected from each rabbit and incubated under the same conditions to obtain unfed adult R. bursa ticks. Unfed adult ticks obtained from #Rabbit-1 and #Rabbit-2 were used to infest splenectomized sheep #K1 and #K4, respectively (Figure 2). Additionally, all splenectomized sheep (#K1, #K2, #K3, #K4) infested with adult R. bursa were monitored for 60 DPI for B. ovis using microscopy and nPCR methods.
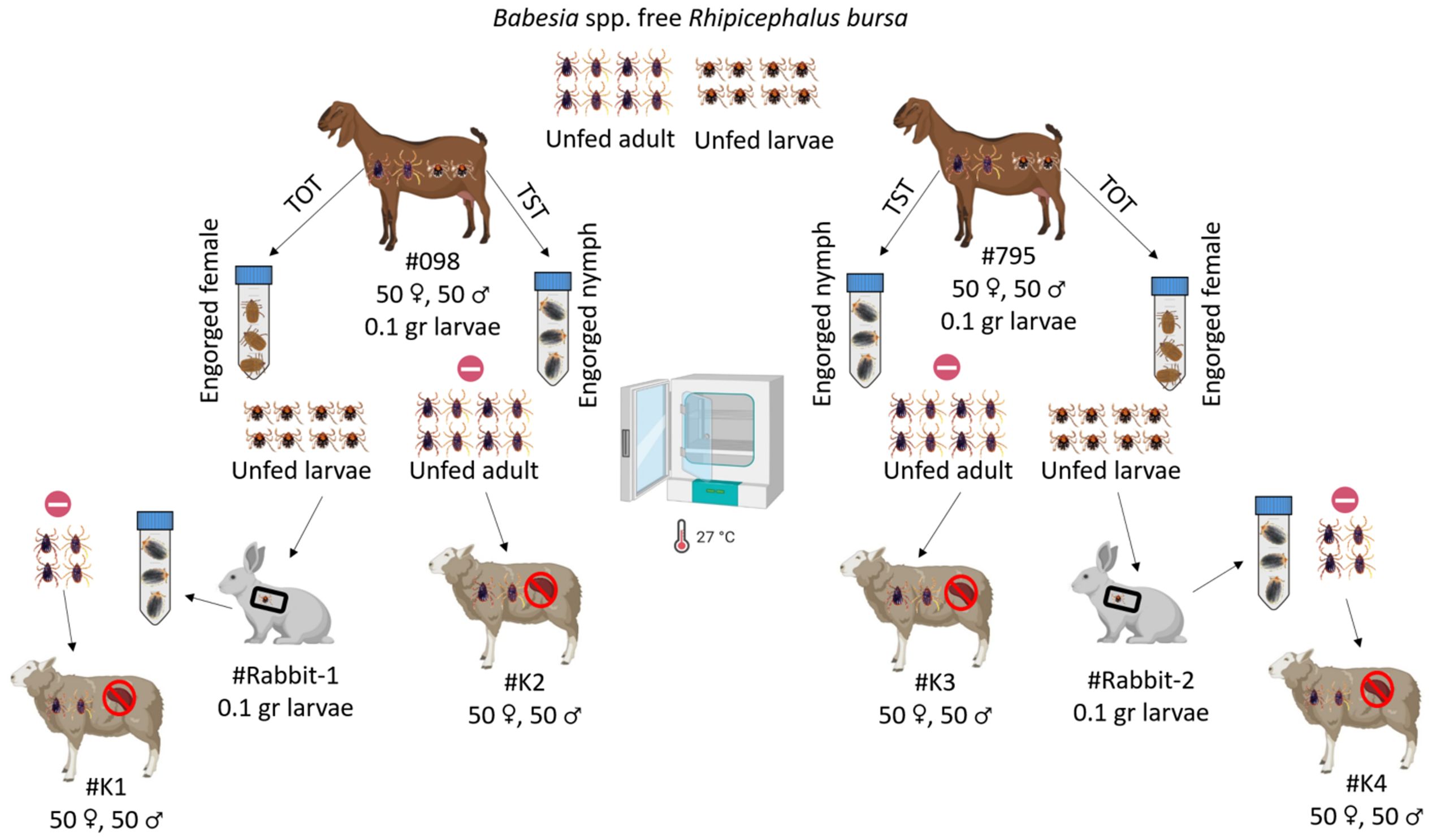
Figure 2. Schematic representation of transstadial (TST) and transovarial (TOT) transmission experiments using Babesia spp.-free R. bursa larvae and adults on goats infected with B. ovis.  : represent nPCR negative. Figure was created using Biorender.com.
: represent nPCR negative. Figure was created using Biorender.com.
Unfed adult ticks obtained from goats #098 and #795 and rabbits #Rabbit-1 and #Rabbit-2 were analyzed for the presence of B. ovis using nPCR. For this analysis, 60 unfed adult ticks (30 females and 30 males) from each goat and rabbit were divided into 12 pools (6 female pools and 6 male pools), with each pool containing 5 ticks (Table 1). These pools were stored in a -20°C for DNA isolation and subsequent nPCR analysis.
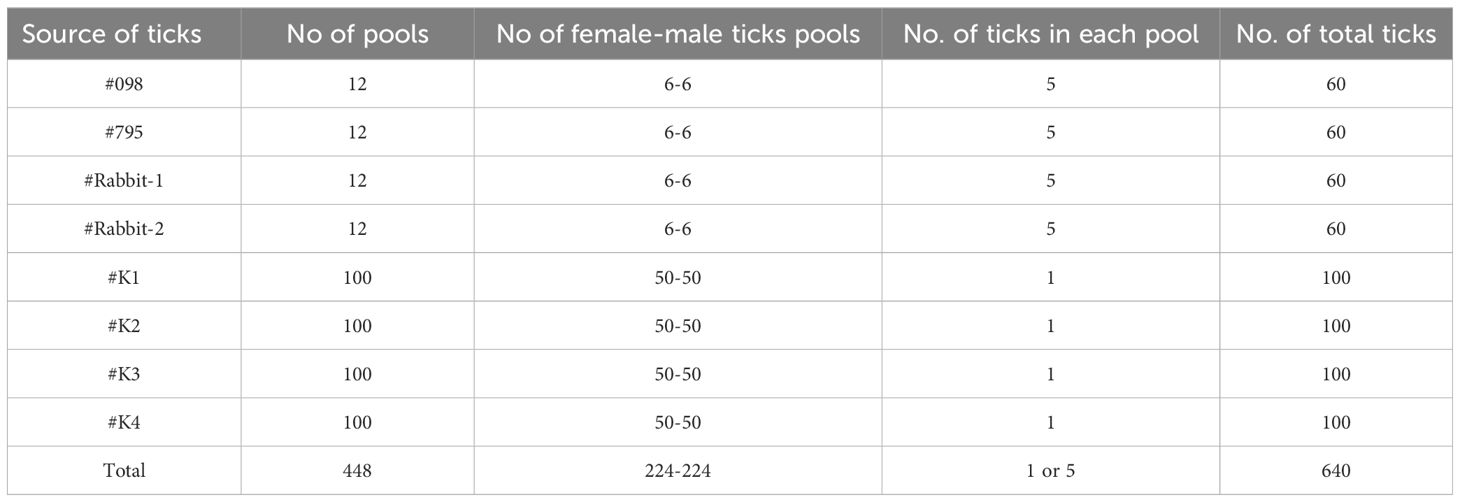
Table 1. Source and pooling of adult R. bursa from transstadial and transovarial transmission experiments.
For microscopic examination, thin blood smears were prepared from the ear tip of sheep, fixed in absolute methanol for 5 minutes, and stained with 10% Giemsa solution for 30 minutes. The slides were examined for intra-erythrocytic piroplasms using oil immersion at 100x magnification. Parasitemia, defined as the percentage of erythrocytes containing parasites, was assessed by counting infected erythrocytes in 20 randomly selected microscopic fields from the edge regions of the blood smear and compared with the total erythrocyte count. Mean PPE was calculated by dividing the number of parasitized erythrocytes by the total erythrocyte count. If no parasites were detected in 20 fields, the smears were recorded as negative for piroplasms (Sevinc et al., 2007; Ozubek and Aktas, 2017).
For the detection of B. ovis using nPCR, genomic DNA was extracted from 200 µL of EDTA-anticoagulated blood samples collected from sheep, goat, and R. bursa ticks used in experimental infections. The extraction was performed using the PureLinkTM Genomic DNA Mini Kit (Invitrogen Corporation, Carlsbad, USA) according to the manufacturer's instructions. The extracted DNA was then stored at −20°C until needed. The nPCR assay for identifying B. ovis DNA utilized two sets of primers: Nbab1F/Nbab1R (Oosthuizen et al., 2008) and BboF/BboR (Aktaş et al., 2005). For the detection of R. bursa DNA, PCR was conducted with the primers 16S + 1 and 16S – 1 (Black and Piesman, 1994), following established protocols.
For investigating experimental infection of goats via direct blood inoculation, a B. ovis Alacakaya stabilate with a ~5% parasitemia percentage (PPE) stored in a cryobank was intravenously administered to a splenectomized donor sheep (#501) (Figure 1). Parasites were identified in the peripheral blood on the third day following infection. As the infection progressed, fever was observed, reaching peak temperatures between 41.0°C and 42.2°C, corresponding to an increase in parasitemia. On day 7, microscopic examination of peripheral blood smears revealed a PPE level of 5.2%. For the experimental infection of goats, 90 ml of the sheep infected blood was collected (Figure 3). At this point, the sheep exhibited severe clinical symptoms of babesiosis, including hemoglobinuria, jaundice, reduced appetite, and lethargy. Following blood collection, the sheep received treatment with imidocarb dipropionate (1.2 mg/kg). After treatment, the fever decreased to 38.7°C, and parasites were not detected in circulation upon microscopical examination by day 9 post-infection (DPI).
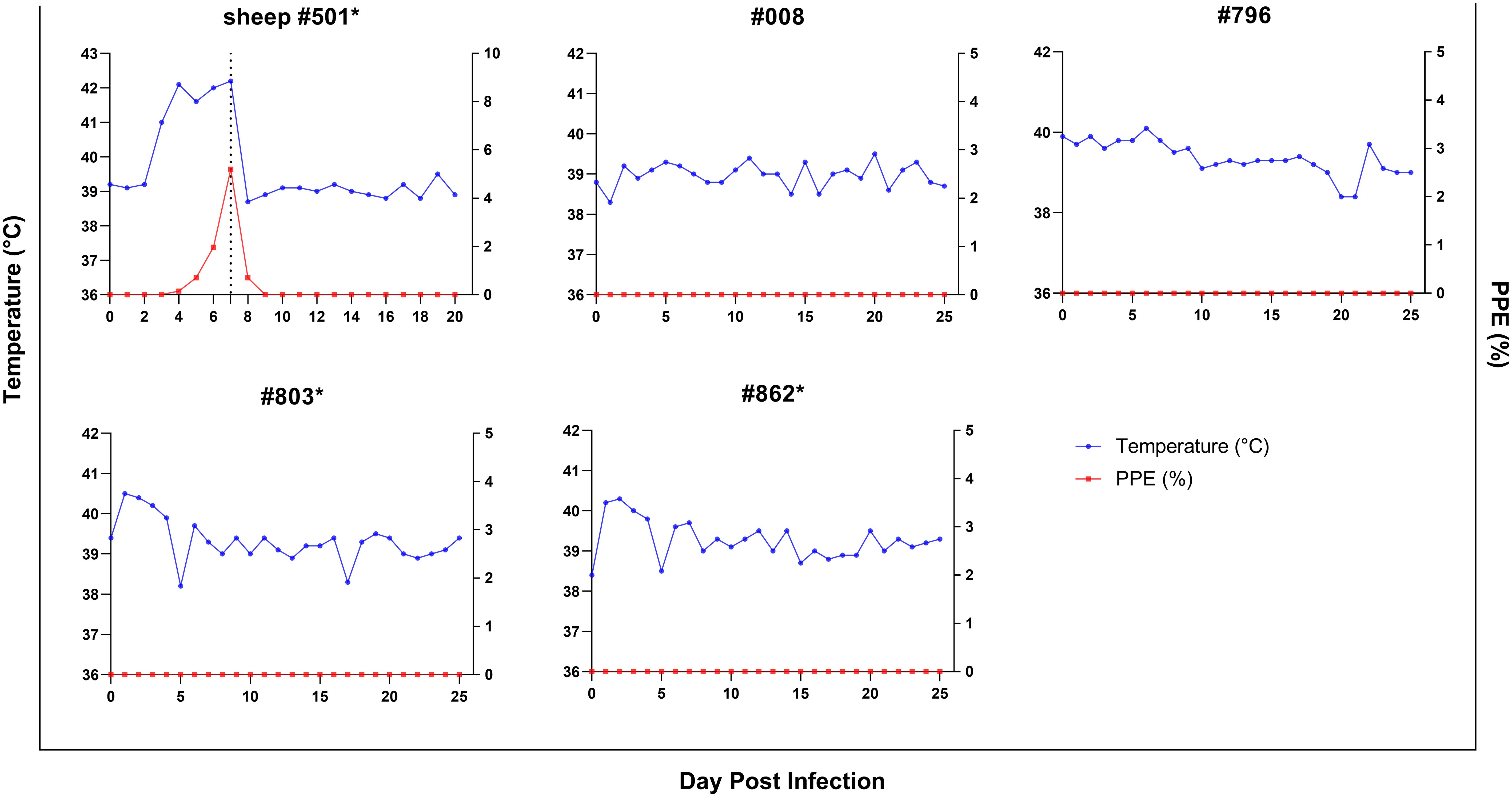
Figure 3. Parasitemia (PPE) (red lines) and body temperature (blue lines)in goats in splenectomized (*) and spleen-intact groups experimentally infected with B. ovis-infected fresh blood obtained from sheep #501. Day 7 in #501 shows the time 90 ml blood was drawn for the experimental infection of goats.
Splenectomized goats (#803, #862) and spleen-intact goats (#008, #796) were infected with fresh-B. ovis infected blood from donor sheep #501 (Figure 1). The presence of B. ovis was monitored using microscopy and nPCR for the first 25 days, and only by nPCR from days 26 to 60. During this period, B. ovis remained undetected by any of these methods. However, B. ovis was identified by nPCR in goats #796 and #803 on day 1 post-infection (Figure 3 and Figure 4). Additionally, no symptoms, such as fever, anemia, or jaundice, were observed in any of the goats throughout the experiment.
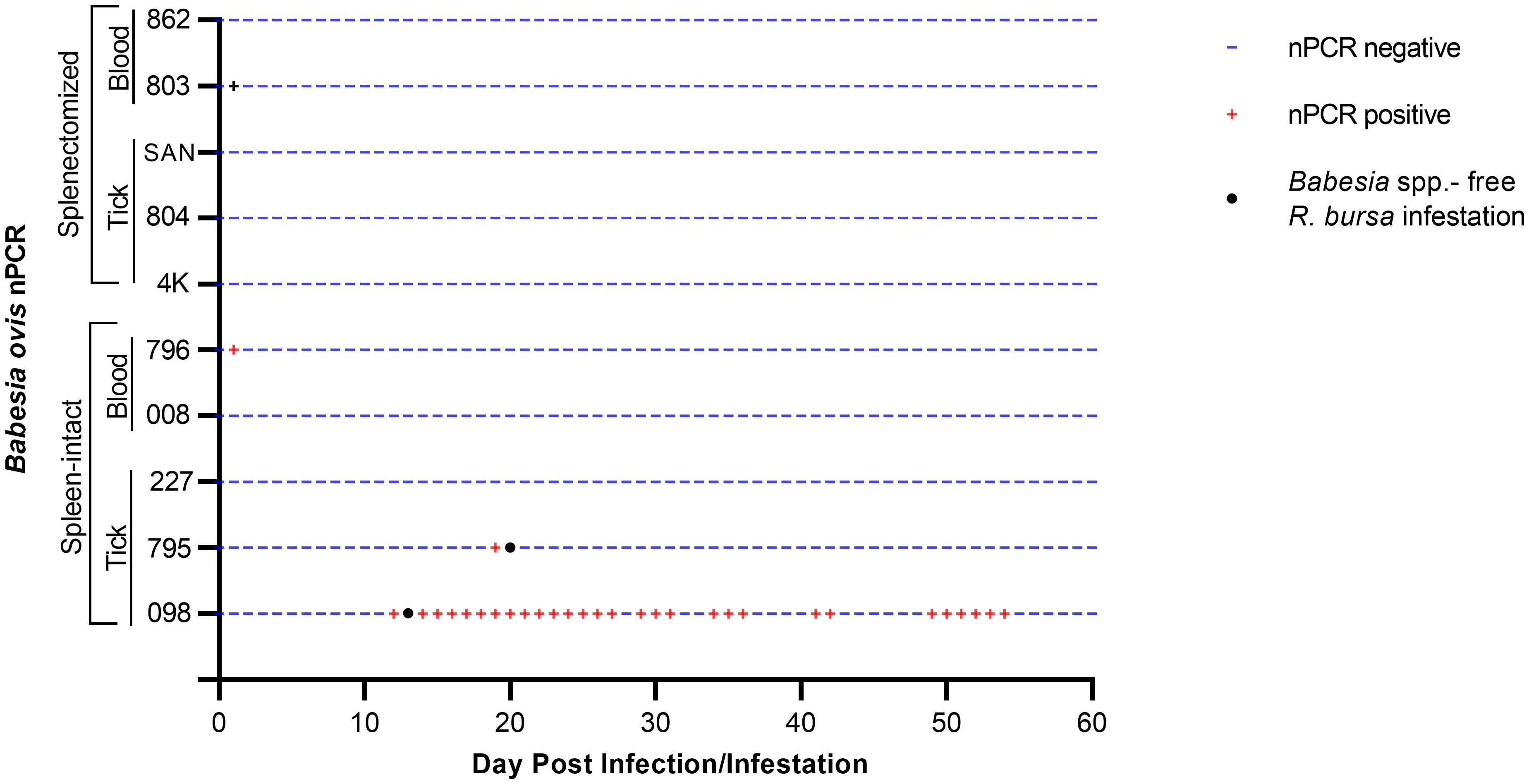
Figure 4. Monitoring of the presence of B. bovis DNA by nPCR in splenectomized and spleen-intact goats following a 60-day period of experimental.
In addition, experimental infection was conducted using B. ovis-infected ticks. For control purposes, sheep #575 was infested with 50 female and 50 male B. ovis-infected R. bursa ticks. On the seventh day following infestation, parasites were detected in the sheep peripheral blood. As the infection progressed, the sheep developed a fever, with temperatures peaking at 42.5°C on the 8th day post-infestation. The maximum percentage of parasitemia (PPE) was recorded as 0.6% on the 12th day, and the sheep succumbed to acute babesiosis on the 20th day after infestation (Figure 5).
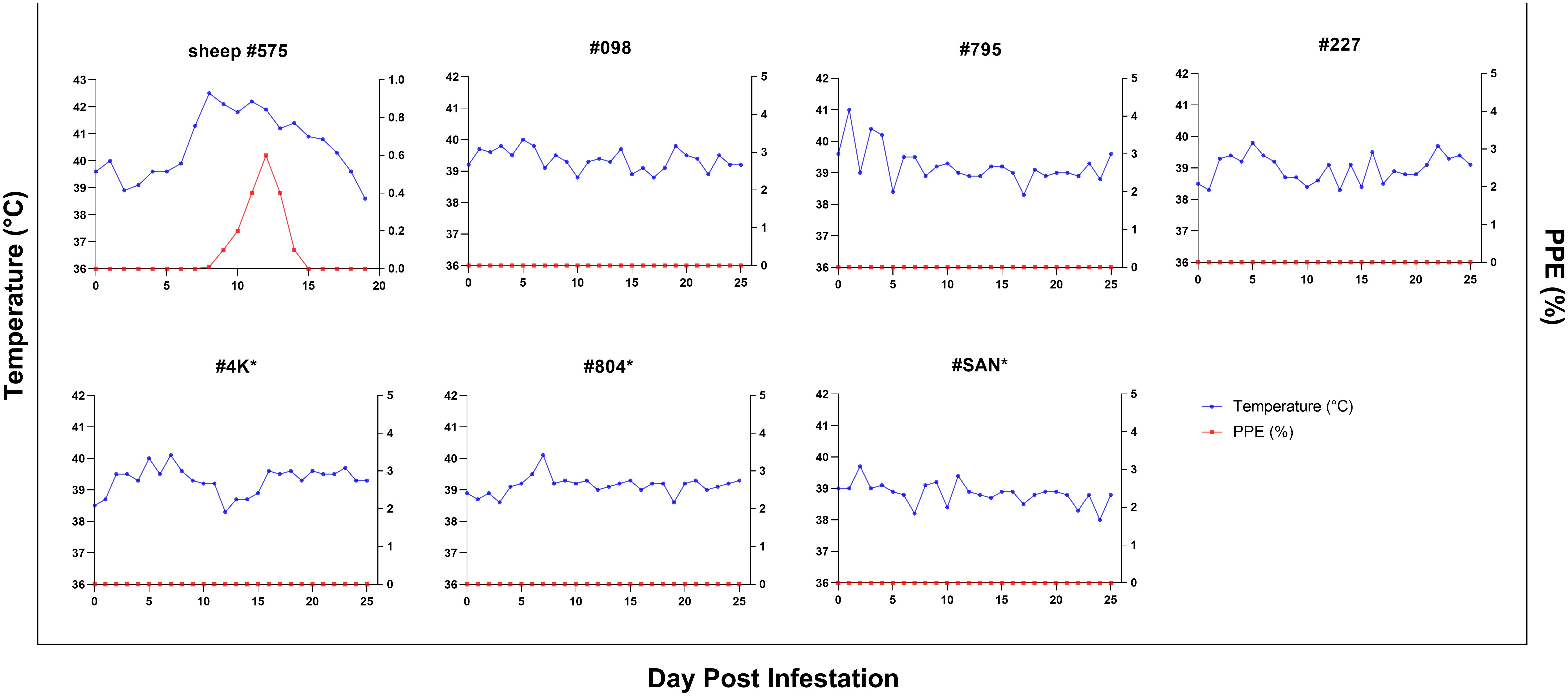
Figure 5. Parasitemia (PPE) and body temperature of goats in splenectomized (*) and spleen-intact groups infested with R. bursa ticks infected with B. ovis. #575 represents a spleen-intact control sheep treated in identical fashion.
The same colony of infected ticks was used to infest splenectomized (#4K, #804, #SAN) and spleen-intact goats (#098, #795, #227), with each goat receiving 50 female and 50 male ticks. Goats were monitored for the presence of B. ovis by microscopy and nPCR, as in the experiment with infected blood described above, for 60 days after infestation. Clinical signs of babesiosis were not observed in any of the goats. However, B. ovis DNA was detected by nPCR in goat #098 starting on the 12th day and continuing until the 54th day post-infestation, in goat #795, B. ovis was detected by nPCR only on the 19th day, while in goats #4K, #804, #SAN, and #227, B. ovis was not detected at all during the course of the experiment (Figure 4).
To evaluate transstadial transmission, Babesia spp.-free R. bursa larvae were used to infest goats (#098, #795) that were found to be positive by nPCR. Engorged nymphs were collected from the goats 12-16 days post-infestation. The collected engorged nymphs were incubated (25 ± 1°C, 70 ± 10% RH) to obtain unfed adult ticks. The unfed adult ticks (50 females, 50 males) were then used to infest splenectomized sheep (#K2, #K3). The infested ticks were collected 8-10 days after feeding. All collected male and female ticks from #K2 and #K3 were individually placed into Eppendorf tubes, and after DNA extraction, examination using nPCR determined that they were negative for B. ovis. The splenectomized sheep were monitored for 60 days using both microscopic examination and nPCR, and both transmissions. Sheep #K2 and #K3 were negative for B. ovis throughout the observation period.
To assess transovarial transmission, engorged female ticks were collected from goats #098 and #795. Unfed offspring larvae were obtained from these ticks and used to infest rabbits (#Rabbit-1, #Rabbit-2). Fourteen to sixteen days after infestation, engorged nymphs were collected from the rabbits. These nymphs developed into unfed adult ticks, which were then used to infest splenectomized sheep (#K1, #K4). The ticks engorged within 8-10 days of feeding and were collected for analysis. As in the transstadial transmission experiment, both male and female ticks were examined by nPCR and found to be negative for B. ovis. The transmission sheep #K1 and #K4 were monitored for 60 days using both microscopic examination and nPCR, and both were determined to be negative for B. ovis.
The pathogenesis of babesiosis in goats remains under-researched, despite its potential to cause fatal infections in sheep. Goats and sheep usually co-exist on farms and there is the need to determine whether goats can act as reservoirs for the parasite in order to establish effective strategies for the control of B. ovis. Taking these factors into consideration, this study aimed to investigate the pathogenicity of B. ovis in splenectomized and spleen-intact goats. The study revealed significant insights into the host specificity and transmission dynamics of B. ovis. Notably, B. ovis did not cause overt clinical infection in goats, even after direct inoculation with infected blood or experimental infestation with B. ovis-infected ticks. Infections were observed in only two spleen-intact goats (#098, #795) infected via ticks, but these infections were subclinical and asymptomatic. This suggests goats may have species-specific immunity or resistance, as they showed no typical babesiosis symptoms like fever, anemia, and jaundice. More importantly, the species-specific resistance against this parasite occurred independently of the spleen and mode of infection. In contrast, intravenous administration of a B. ovis Alacakaya stabilate to sheep resulted in severe clinical symptoms and high parasitemia, showing high virulence. Additionally, experimental infestation with B. ovis-infected R. bursa ticks led to clinical infection in sheep, with detectable parasitemia, high fever, anemia, and eventual mortality. Babesia ovis was detected by nPCR in some goats but did not establish a sustained infection, indicating innate resistance. Our findings align with previous review papers suggesting that B. ovis presents sub- clinically in goats (Friedhoff, 1997; De Waal, 2000; Schnittger et al., 2022). However, a previous study has reported natural infection of B. ovis in goats, with PPE ranging from 0.1% to 1%, and canonical symptoms of babesiosis, such as fever, anemia, and icterus, in the infected animals. These studies, however, did not provide information on the duration of parasitemia, treatment, or recovery outcomes (Esmaeilnejad et al., 2012, 2020).
Research on Babesia bovis, the primary causative agent of bovine babesiosis, has shown high levels of genetic differentiation and diversity worldwide (Ozubek et al., 2020). Although population genetics studies on B. ovis are limited, a study reported high genetic diversity and differentiation in the B. ovis population in Turkiye (Mira et al., 2020). Different genetic strains of B. ovis may cause babesiosis in goats; however, the B. ovis Alacakaya strain used in our study did not induce any clinical infection in goats, despite being highly virulent for sheep. Additionally, different goat breeds may contribute to the development of clinical babesiosis. In this study, local breed hairy goats were used for experimental infection, but natural cases of babesiosis have been reported in Marghoz and Raeini goats in Iran (Esmaeilnejad et al., 2020). Another study found that while Chinese Tan sheep erythrocytes are susceptible to Babesia sp BQ1 (Lintan), French Vendéen sheep erythrocytes are not, indicating breed-specific susceptibility (Guan et al., 2010a). A similar study was conducted using virulent and attenuated strains of B. bovis to experimentally infect water buffaloes (Benitez et al., 2018). This experimental infection did not result in any clinical signs of babesiosis in the water buffaloes. During the experimental infection with the attenuated strain, B. bovis was detectable using nPCR, whereas it was not detectable with the virulent strain. As a result, it has been reported that buffaloes control babesiosis caused by B. bovis much more effectively than cattle (Benitez et al., 2018). Moreover, the experimental challenge of Nilgai antelope with a virulent B. bovis strain resulted in no signs of infection. Animals were nPCR-negative for the parasite and did not develop antibodies to B. bovis, further illustrating the variability in susceptibility among different species (Johnson et al., 2024). Collectively, these findings suggest that genetic and immunological factors intrinsic to each host species or breed play a crucial role in determining the outcome of Babesia infections.
In contrast to this study, experimental infections with B. motasi, Babesia sp. Lintan, and B. aktasi in goats yielded results, indicating varying degrees of clinical manifestations despite low to high parasitemia levels (Lewis et al., 1981; Guan et al., 2010b; Ozubek et al., 2023b). Babesia motasi infections caused mild anemia, moderate parasitemia, and mild fever in spleen-intact goats (Lewis et al., 1981). The varying clinical outcomes observed in goats infected with different species of Babesia highlight the complexity of host-parasite interactions. Understanding the factors governing these differences is essential for targeted interventions, such as vaccines or therapeutics, to combat these infections. Despite being a closely related species, goats exhibit notable resistance to tick-borne pathogens compared to sheep, leading to lower mortality rates. Investigating the innate and adaptive immunological responses and genetic factors conferring this resistance could provide novel avenues for livestock health management. Furthermore, goats' well-known resistance to diseases and their ability to thrive in harsh environments further support the findings of this study (Nair et al., 2021). Their robust immune system, characterized by a higher proportion of lymphocytes in circulation compared to neutrophils (Daramola and Adeloye, 2009), likely contributes to their resistance against tick-borne and other pathogens. Additionally, their resistance to experimental infections with the louping-ill virus (LIV) (Reid et al., 1984) and the toxic effects of oak ingestion further demonstrate their capacity to withstand various challenges (Smith and Sherman, 2009), as consistent with our data.
The data suggest that resistance to B. ovis in naïve goats is independent of spleen function and indicates the possible involvement of innate immune mechanisms in controlling the infection. This observation highlights the importance of considering innate immune responses, rather than adaptive mechanisms, as playing important roles in their resistance to Babesia infections (Torina et al., 2020; Bastos et al., 2022). Consistently, both spleen-intact and splenectomized goats, which had never been exposed to B. ovis, demonstrated resistance to infection in this study. This innate resistance may be the result of evolutionary adaptations that have led to unique immune responses in goats. The rapid activation of innate immune components, such as macrophages and neutrophils, along with the involvement of pattern recognition receptors (PRRs) like Toll-like receptors (TLRs) and nucleotide-binding oligomerization domain (NOD)-like receptors, could significantly contribute to the early and effective defense against Babesia infections (Torina et al., 2020). Understanding these mechanisms could provide valuable insights into developing control strategies for Babesia and other tick-borne diseases in livestock. Furthermore, the absence of infected erythrocytes in goats suggests a poor affinity of the parasite for the target erythrocytes. This raises the possibility of differences in erythrocyte surface characteristics and potential receptors between goats and sheep, which may limit the parasite's ability to invade. Conducting further experiments, such as culturing B. ovis in goat versus sheep erythrocytes, along with comparative analyses of erythrocyte surface molecules or distinct erythrocyte metabolism, could shed light on these observations. A better understanding of these mechanisms could ultimately be exploited to design more effective control measures against ovine babesiosis.
To investigate the epidemiological significance of goats in the transmission cycle of B. ovis, we conducted tick transmission experiments to determine whether goats could serve as reservoirs capable of transmitting B. ovis to R. bursa ticks. The rationale for these experiments is based on findings from other tick-borne protozoan infections, where both acute and chronic infections have been shown to contribute to vector transmission of certain Babesia and Theileria species (Yeruham et al., 2001; Gray et al., 2002; Howell et al., 2007; Ueti et al., 2012; Erster et al., 2016; Peckle et al., 2022). Previous research has shown that ticks feeding on animals with either acute or chronic infections can sometimes acquire and transmit Theileria and Babesia species. For instance, Howell et al. (2007) demonstrated that ticks feeding on persistently infected calves were capable of transmitting infection, though this ability depended on both the infection status and the timing of vector exposure. In some cases, larvae failed to transmit the pathogen from persistently infected animals, while in other instances, successful transmission occurred (Howell et al., 2007). Similarly, Gray et al. (2002) found that Ixodes ricinus ticks could be infected by Babesia microti HK and GI strains when feeding on acutely, but not chronically, infected gerbils (Gray et al., 2002). In some instances, ticks feeding on animals with chronic infections have been able to acquire and transmit the pathogen. For example, Peckle et al. (2022) showed that chronically infected horses successfully transmitted Theileria equi to R. microplus ticks (Peckle et al., 2022). Similarly, Ueti et al. (2012) reported that even horses treated with chemotherapeutic agents like imidocarb, which appeared healthy, still posed a transmission risk because subclinical carriers could continue to sustain the pathogen cycle within tick populations (Ueti et al., 2012). Based on these observations, we hypothesized that goats, which can harbor B. ovis and share environments with sheep, might contribute to the maintenance and transmission of the parasite to tick vectors. To test this, we designed a tick transmission experiment in which goats were infested with both larvae and adult R. bursa ticks, following methodologies similar to those used in transmission studies of other Babesia and Theileria species. Although limited in size and scope, our study involved infesting goats with both life stages of R. bursa. While molecular methods confirmed the presence of B. ovis in goats, we did not observe any transmission of the parasite to the ticks. This suggests that goats may not play a significant epidemiological role in the transmission of B. ovis. This outcome aligns with findings in the literature indicating that sufficient parasitemia is crucial for pathogen transmission. Studies on Babesia species have shown that the level of parasitemia in host animals directly impacts the ability of ticks to acquire the pathogen (Yeruham et al., 2001; Gray et al., 2002; Howell et al., 2007; Firat et al., 2024). In our study, the absence of detectable transmission may be due to low or transient parasitemia in goats, preventing ticks from acquiring B. ovis during feeding. These results suggest that, while acute and chronic infections of Babesia and Theileria species can sometimes lead to successful pathogen transmission, goats may not achieve the parasitemia threshold necessary for effective transmission of B. ovis to ticks. This finding has significant implications for understanding the role of different hosts in the transmission dynamics of tick-borne pathogens. It supports the focus on sheep as the primary reservoir in B. ovis control strategies and suggests that goats may have limited epidemiological importance in the transmission cycle of B. ovis. Further studies are needed to confirm this and clarify the potential role of goats in B. ovis epidemiology.
In conclusion, several lines of evidence in this study suggest that goats are not suitable hosts for B. ovis, as they exhibit resistance to clinical disease and do not support tick-borne transmission. Further research into the immunological responses of goats to Babesia infections could provide valuable insights into the mechanisms underlying host resistance or susceptibility. Additionally, assessing the economic impact of babesiosis in goats would be crucial for implementing effective control measures and mitigating losses in affected regions. Therefore, it is essential to consider ovine and caprine babesiosis separately due to their distinct pathogenicity profiles and host responses. It is likely that the factors contributing to this resistance may be related to innate immunity mechanisms rather than adaptive immunity. Understanding these innate immune responses in goats could pave the way for developing new strategies to control babesiosis in other susceptible species. Further studies in this area are warranted to fully elucidate the underlying mechanisms and to develop targeted interventions. Moreover, future studies should also focus on examining "heterologous" goats, from different breeds or from regions that are not endemic to B. ovis. This could help determine if the observed resistance is consistent across various goat populations and provide a broader understanding of the factors contributing to host resistance. In addition, it is crucial to conduct additional transmission studies to explore the role of goats in the epidemiology of B. ovis, in order to determine whether they effectively contribute to the parasite's life cycle under specific conditions. Such comprehensive research will be instrumental in enhancing our knowledge of babesiosis and improving control strategies in diverse geographic and ecological settings.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by Firat University Animal Experiment Ethics Committee. The study was conducted in accordance with the local legislation and institutional requirements.
SO: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MU: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. CS: Formal analysis, Writing – review & editing. RB: Formal Analysis, Writing – review & editing. MA: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported financially by Performance Research Project (Veterinary Faculty VF.23.28 and VF.23.32) from the Scientific Research Project, Firat University.
We are grateful to Aleyna Karoglu, Arda Eyvaz, Havva Nur Cevik and Halil Unal for the excellent technical and administrative support. We would like to acknowledge the use of OpenAI's ChatGPT-4o for assistance with language editing
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aktas, M., Altay, K., Dumanli, N. (2007). Determination of prevalence and risk factors for infection with Babesia ovis in small ruminants from Turkey by polymerase chain reaction. Parasitol. Res. 100, 797–802. doi: 10.1007/s00436-006-0345-2
Aktas, M., Özübek, S. (2017). Transstadial transmission of Hepatozoon canis by Rhipicephalus sanguineus (Acari: Ixodidae) in field conditions. J. Med. Entomol. 54, 1044–1048. doi: 10.1093/jme/tjx050
Aktaş, M., Altay, K., Dumanlı, N. (2005). Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet. Parasitol. 133, 277–281. doi: 10.1016/j.vetpar.2005.05.057
Alkan, H., Ugur, T. (2015). Views of hair goat breeders concerning the relationship between nomadic livestock and forestry: an example from the Turkey. Int. J. Environ. Res. 9, 969–976. doi: 10.22059/ijer.2015.984
Bastos, R. G., Alzan, H. F., Rathinasamy, V. A., Cooke, B. M., Dellagostin, O. A., Barletta, R. G., et al. (2022). Harnessing mycobacterium bovis BCG trained immunity to control human and bovine babesiosis. Vaccines 10, 123. doi: 10.3390/vaccines10010123
Bekker, C. P., De Vos, S., Taoufik, A., Sparagano, O. A., Jongejan, F. (2002). Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 89, 223–238. doi: 10.1016/S0378-1135(02)00179-7
Benitez, D., Mesplet, M., Echaide, I., de Echaide, S. T., Schnittger, L., Florin-Christensen, M. (2018). Mitigated clinical disease in water buffaloes experimentally infected with Babesia bovis. Ticks Tick-Borne Dis. 9, 1358–1363. doi: 10.1016/j.ttbdis.2018.04.012
Black, W. C., Piesman, J. (1994). Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. 91, 10034–10038. doi: 10.1073/pnas.91.21.10034
Ceylan, O., Xuan, X., Sevinc, F. (2021). Primary tick-borne protozoan and rickettsial infections of animals in Turkey. Pathogens 10, 231. doi: 10.3390/pathogens10020231
Daramola, J. O., Adeloye, A. A. (2009). Physiological adaptation to the humid tropics with special reference to the West African Dwarf (WAD) goat. Trop. Anim. Health Prod. 41, 1005–1016. doi: 10.1007/s11250-008-9267-6
De Waal, D. T. (2000). Global importance of piroplasmosis. J. Protozool. Res. 10, 106–127. doi: 10.32268/jprotozoolres.10.3_106
Erster, O., Roth, A., Wolkomirsky, R., Leibovich, B., Savitzky, I., Shkap, V. (2016). Transmission of Babesia ovis by different Rhipicephalus bursa developmental stages and infected blood injection. Ticks Tick-Borne Dis. 7, 13–19. doi: 10.1016/j.ttbdis.2015.07.017
Esmaeilnejad, B., Rajabi, S., Tavassoli, M., Rashnavadi, M., Seif, F., Aligolzadeh, A., et al. (2020). Evaluation of inflammatory biomarkers in goats naturally infected with Babesia ovis. Parasitol. Res. 119, 4151–4158. doi: 10.1007/s00436-020-06829-7
Esmaeilnejad, B., Tavassoli, M., Asri-Rezaei, S., Dalir-Naghadeh, B., Malekinejad, H. (2012). Status of lipid peroxidation and antioxidant enzymes in goats naturally infected with Babesia ovis. Acta Parasitol. 57 (3), 228–234. doi: 10.2478/s11686-012-0038-1
Firat, R., Ulucesme, M. C., Aktaş, M., Ceylan, O., Sevinc, F., Bastos, R. G., et al. (2024). Role of Rhipicephalus bursa larvae in transstadial transmission and endemicity of Babesia ovis in chronically infected sheep. Front. Cell. Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1428719
Friedhoff, K. T. (1997). Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia39, 99–109.
Galon, E. M., Ybañez, R. H., Macalanda, A. M., Estabillo, G. R., Montano, M. T. R., Veedor, M. D., et al. (2022). First molecular identification of Babesia, Theileria, and Anaplasma in goats from the Philippines. Pathogens 11, 1109. doi: 10.3390/pathogens11101109
Georges, K., Loria, G. R., Riili, S., Greco, A., Caracappa, S., Jongejan, F., et al. (2001). Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 99, 273–286. doi: 10.1016/S0304-4017(01)00488-5
Gray, J., Von Stedingk, L. V., Gürtelschmid, M., Granström, M. (2002). Transmission studies of Babesia microti in Ixodes ricinus ticks and gerbils. J. Clin. Microbiol. 40, 1259–1263. doi: 10.1128/JCM.40.4.1259-1263.2002
Guan, G., Ma, M., Moreau, E., Liu, J., Lu, B., Bai, Q., et al. (2009). A new ovine Babesia species transmitted by Hyalomma anatolicum anatolicum. Exp. Parasitol. 122, 261–267. doi: 10.1016/j.exppara.2009.05.001
Guan, G., Moreau, E., Brisseau, N., Luo, J., Yin, H., Chauvin, A. (2010a). Determination of erythrocyte susceptibility of Chinese sheep (Tan mutton breed) and French sheep (Vendéen breed) to Babesia sp. BQ1 (Lintan) by in vitro culture. Vet. Parasitol. 170, 37–43. doi: 10.1016/j.vetpar.2010.02.006
Guan, G., Moreau, E., Liu, J., Hao, X., Ma, M., Luo, J., et al. (2010b). Babesia sp. BQ1 (Lintan): molecular evidence of experimental transmission to sheep by Haemaphysalis qinghaiensis and Haemaphysalis longicornis. Parasitol. Int. 59, 265–267. doi: 10.1016/j.parint.2009.12.002
Howell, J. M., Ueti, M. W., Palmer, G. H., Scoles, G. A., Knowles, D. P. (2007). Persistently infected calves as reservoirs for acquisition and transovarial transmission of Babesia bovis by Rhipicephalus ( Boophilus ) microplus. J. Clin. Microbiol. 45, 3155–3159. doi: 10.1128/JCM.00766-07
Inci, A., Ica, A., Yildirim, A., Düzlü, Ö. (2010). Identification of Babesia and Theileria species in small ruminants in Central Anatolia (Turkey) via reverse line blotting. Turk. J. Vet. Anim. Sci. 34, 205–210. doi: 10.3906/vet-0902-15
Johnson, T. L., Persinger, K. A., Taus, N. S., Davis, S. K., Poh, K. C., Kappmeyer, L. S., et al. (2024). Nilgai antelope display no signs of infection upon experimental challenge with a virulent Babesia bovis strain. Parasitol. Vectors 17, 245. doi: 10.1186/s13071-024-06316-3
Kawahara, M., Rikihisa, Y., Lin, Q., Isogai, E., Tahara, K., Itagaki, A., et al. (2006). Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl. Environ. Microbiol. 72, 1102–1109. doi: 10.1128/AEM.72.2.1102-1109.2006
Kumar, S., Vaid, R. K., Sagar, R. L. (2006). Contribution of goats to livelihood security of small ruminant farmers in semiarid region. Indian J. Small Rumin. 12, 61–66.
Lewis, D., Holman, M. R., Purnell, R. E., Young, E. R., Herbert, I. V., Bevan, W. J. (1981). Investigations on Babesia motasi isolated from Wales. Res. Vet. Sci. 31, 239–243. doi: 10.1016/S0034-5288(18)32501-3
Mira, A., Unlu, A. H., Bilgic, H. B., Bakirci, S., Hacilarlioglu, S., Karagenc, T., et al. (2020). High genetic diversity and differentiation of the Babesia ovis population in Turkey. Transbound Emerg. Dis. 67, 26–35. doi: 10.1111/tbed.13174
Nair, M. R. R., Sejian, V., Silpa, M. V., Fonsêca, V. F. C., De Melo Costa, C. C., Devaraj, C., et al. (2021). Goat as the ideal climate-resilient animal model in tropical environment: revisiting advantages over other livestock species. Int. J. Biometeorol. 65, 2229–2240. doi: 10.1007/s00484-021-02179-w
Oosthuizen, M. C., Zweygarth, E., Collins, N. E., Troskie, M., Penzhorn, B. L. (2008). Identification of a novel Babesia sp. from a sable antelope ( Hippotragus Niger Harris 1838). J. Clin. Microbiol. 46, 2247–2251. doi: 10.1128/JCM.00167-08
Ozubek, S., Aktas, M. (2017). Molecular and parasitological survey of ovine piroplasmosis, including the first report of Theileria annulata (Apicomplexa: Theileridae) in sheep and goats from Turkey. J. Med. Entomol. 54, 212–220. doi: 10.1093/jme/tjw134
Ozubek, S., Bastos, R. G., Alzan, H. F., Inci, A., Aktas, M., Suarez, C. E. (2020). Bovine babesiosis in Turkey: Impact, current gaps, and opportunities for intervention. Pathogens 9, 1041. doi: 10.3390/pathogens9121041
Ozubek, S., Ulucesme, M. C., Aktas, M. (2023a). Discovery of a novel species infecting goats: morphological and molecular characterization of Babesia aktasi n. sp. Pathogens 12, 113. doi: 10.3390/pathogens12010113
Ozubek, S., Ulucesme, M. C., Bastos, R. G., Alzan, H. F., Laughery, J. M., Suarez, C. E., et al. (2023b). Experimental infection of non-immunosuppressed and immunosuppressed goats reveals differential pathogenesis of Babesia aktasi n. sp. Front. Cell. Infect. Microbiol. 13, 1277956. doi: 10.3389/fcimb.2023.1277956
Peckle, M., Santos, H., Pires, M., Silva, C., Costa, R., Vitari, G., et al. (2022). Dynamics of Theileria equi infection in Rhipicephalus (Boophilus) microplus during the parasitic phase in a chronically infected horse. Pathogens 11, 525. doi: 10.3390/pathogens11050525
Reid, H. W., Buxton, D., Pow, I., Finlayson, J. (1984). Transmission of louping-ill virus in goat milk. Vet. Rec. 114, 163–165. doi: 10.1136/vr.114.7.163
Rjeibi, M. R., Gharbi, M., Mhadhbi, M., Mabrouk, W., Ayari, B., Nasfi, I., et al. (2014). Prevalence of piroplasms in small ruminants in North-West Tunisia and the first genetic characterisation of Babesia ovis in Africa. Parasite 21, 23. doi: 10.1051/parasite/2014025
Schnittger, L., Ganzinelli, S., Bhoora, R., Omondi, D., Nijhof, A. M., Florin-Christensen, M. (2022). The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 121, 1207–1245. doi: 10.1007/s00436-022-07424-8
Schnittger, L., Rodriguez, A. E., Florin-Christensen, M., Morrison, D. A. (2012). Babesia: a world emerging. Infect. Genet. Evol. 12, 1788–1809. doi: 10.1016/j.meegid.2012.07.004
Sevinc, F., Turgut, K., Sevinc, M., Ekici, O. D., Coskun, A., Koc, Y., et al. (2007). Therapeutic and prophylactic efficacy of imidocarb dipropionate on experimental Babesia ovis infection of lambs. Vet. Parasitol. 149, 65–71. doi: 10.1016/j.vetpar.2007.07.014
Torina, A., Villari, S., Blanda, V., Vullo, S., La Manna, M. P., Shekarkar Azgomi, M., et al. (2020). Innate immune response to tick-borne pathogens: Cellular and molecular mechanisms induced in the hosts. Int. J. Mol. Sci. 21, 5437. doi: 10.3390/ijms21155437
Tumwebaze, M. A., Byamukama, B., Tayebwa, D. S., Byaruhanga, J., Angwe, M. K., Galon, E. M., et al. (2020). First molecular detection of Babesia ovis, Theileria spp., Anaplasma spp., and Ehrlichia ruminantium in goats from western Uganda. Pathogens 9, 895. doi: 10.3390/pathogens9110895
Ueti, M. W., Mealey, R. H., Kappmeyer, L. S., White, S. N., Kumpula-McWhirter, N., Pelzel, A. M., et al. (2012). Re-emergence of the apicomplexan Theileria equi in the United States: elimination of persistent infection and transmission risk. PloS One 7, e44713. doi: 10.1371/journal.pone.0044713
Uilenberg, G. (2006). Babesia—a historical overview. Vet. Parasitol. 138, 3–10. doi: 10.1016/j.vetpar.2006.01.035
Ulucesme, M. C., Ozubek, S., Aktas, M. (2024). Incompetence of vector capacity of rhipicephalus bursa to transmit Babesia aktasi following feeding on clinically infected goat with high level of parasitemia. Vet. Sci. 11, 309. doi: 10.3390/vetsci11070309
Ulucesme, M. C., Ozubek, S., Karoglu, A., Turk, Z. I., Olmus, I., Irehan, B., et al. (2023). Small Ruminant Piroplasmosis: High prevalence of Babesia aktasi n. sp. in goats in Türkiye. Pathogens 12, 514. doi: 10.3390/pathogens12040514
Wang, J., Chen, K., Ren, Q., Zhang, S., Yang, J., Wang, Y., et al. (2023). Comparative genomics reveals unique features of two Babesia motasi subspecies: Babesia motasi lintanensis and Babesia motasi hebeiensis. Int. J. Parasitol. 53, 265–283. doi: 10.1016/j.ijpara.2023.02.005
Yeruham, I., Hadani, A., Galker, F. (1998a). Some epizootiological and clinical aspects of ovine babesiosis caused by Babesia ovis—A review. Vet. Parasitol. 74, 153–163. doi: 10.1016/S0304-4017(97)00143-X
Yeruham, I., Hadani, A., Galker, F. (2001). The effect of the ovine host parasitaemia on the development of Babesia ovis (Babes 1892) in the tick Rhipicephalus bursa (Canestrini and Fanzago 1877). Vet. Parasitol. 96, 195–202. doi: 10.1016/S0304-4017(00)00433-7
Keywords: Babesia ovis, experimental infection, goat, pathogenicity, Rhipicephalus bursa
Citation: Ozubek S, Ulucesme MC, Suarez CE, Bastos RG and Aktas M (2024) Assessment of Babesia ovis pathogenicity in goats: implications for transmission dynamics and host resistant. Front. Cell. Infect. Microbiol. 14:1480347. doi: 10.3389/fcimb.2024.1480347
Received: 13 August 2024; Accepted: 15 October 2024;
Published: 12 November 2024.
Edited by:
Ana Gonçalves Domingos, New University of Lisbon, PortugalReviewed by:
Pablo Colunga-Salas, Universidad Veracruzana, MexicoCopyright © 2024 Ozubek, Ulucesme, Suarez, Bastos and Aktas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sezayi Ozubek, c296dWJla0BmaXJhdC5lZHUudHI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.