- 1Biomedical Research Center, Qatar University, Doha, Qatar
- 2Department of Biomedical Sciences, College of Health Sciences, Qatar University (QU) Health, Qatar University, Doha, Qatar
- 3Vice President for Medical and Health Sciences Office, QU Health, Qatar University, Doha, Qatar
- 4Qatar Biobank for Medical Research, Qatar Foundation, Doha, Qatar
Pregnancy is a dynamic physiological process involving significant hormonal, immune, and metabolic changes to support fetal growth and development. This study investigates the changes in salivary microbiome and biochemical markers from the second to the third trimester of pregnancy. Saliva samples were collected from 45 pregnant women enrolled in the Qatar Birth Cohort study at two time points (second and third trimesters). DNA was extracted and subjected to 16S rRNA gene sequencing using Oxford Nanopore Technology. Microbial diversity and taxonomic analyses were performed, along with correlation analyses between microbial abundance and clinical parameters. Biochemically, significant increases in BMI, pulse rate, HbA1c, LDL, total cholesterol, and triglycerides were observed in the third trimester compared to the second. Microbial diversity analysis revealed significant changes in microbial richness and composition. Taxonomy analysis showed a significant 3-fold increase in Bacteroidota. Also, a significant decline in Selenomonas and a significant increase in Veillonella, specifically Veillonella dispar and Veillonella atypica, as well as an increase in Granulicatella were observed in the third trimester, along with a significant decrease in Streptococcus sanguinis. Correlation analysis during the second trimester revealed positive associations between BMI, cholesterol, LDL, and Selenomonas, and negative correlations with Streptococcus and Gemella. In the third trimester, BMI was negatively correlated with Campylobacter, glucose levels were negatively correlated with Neisseria, and triglyceride levels were negatively correlated with Prevotella. These findings highlight significant biochemical and microbial shifts during pregnancy, underscoring the importance of monitoring oral health and metabolic changes in pregnant women.
Introduction
Pregnancy represents a remarkable transformation for a woman’s body. A cascade of hormonal, immune, and metabolic changes orchestrate the nurturing and development of a healthy fetus (Murray and Hendley, 2020). In recent years, scientific curiosity has turned towards the fascinating role of the human microbiome during this critical period (Fasano and Flaherty, 2021).
The gut microbiome, long recognized for its influence on digestion and overall health, exhibits dramatic shifts throughout pregnancy (Yao et al., 2021). Research suggests a rise in specific bacterial groups like Proteobacteria and Actinobacteria, while others, particularly butyrate-producing bacteria, see a decline (Fu et al., 2019). These alterations are believed to be adaptations that support the body’s heightened metabolic demands during pregnancy, ultimately contributing to fetal growth (De Siena et al., 2021). Additionally, the gut microbiome might play a role in regulating weight gain through mechanisms like nutrient absorption and immune system stimulation (Yoo et al., 2020).
The oral cavity harbors another complex and diverse microbial ecosystem – the oral microbiome – which is also thought to be susceptible to the hormonal and immunological fluctuations that occur during pregnancy (Sedghi et al., 2021). Studies have shown an increase in total bacterial counts in pregnant women, including some bacteria associated with gum disease (Jang et al., 2021; Saadaoui et al., 2021). Interestingly, correlations have been observed between oral infections and pregnancy complications, suggesting a potential link between the health of the oral microbiome and the course of pregnancy (Wen et al., 2023). Furthermore, a mother’s oral microbiome may influence the development of her infant’s oral microbiome during the perinatal period, potentially impacting their future oral and systemic health (Nardi et al., 2021).
This complex community of microorganisms residing in various body sites, including the gut and oral cavity, undergoes distinct alterations throughout gestation (Sedghi et al., 2021; Mohammed et al., 2024). Investigating these microbiome fluctuations across trimesters holds promise for elucidating potential associations with maternal health and pregnancy outcomes (Baud et al., 2023). Therefore, this study aims to examine changes in the salivary microbiome composition during pregnancy across the second and third trimesters. By leveraging the Qatari Birth Cohort (QbiC) we aim to explore the dynamic shifts in the oral microbiomes and illuminate the intricate interplay between the oral microbiome and the course of pregnancy.
Methodology
Sample collection and participant criteria
The proposed study was designed to gather saliva samples from pregnant women enrolled in the Qatar Birth Cohort study at Qatar Biobank (QBB). These samples were obtained from 45 pregnant women taken at two different time points (second and third trimesters), making a total of 90 samples selected from the QBB repository. Pregnant women who have lived in Doha for at least 15 years and were anticipated to deliver their babies in Qatar were eligible to participate in this study. Participants had to be residents in the study area, aged over 18 years, without any communication handicaps. Women with clinically diagnosed metabolic, metastatic, or chronic infectious diseases were excluded from the study. Additionally, relevant information on physical activity, feeding habits, socio-economic status, smoking habits, disease history, medication, and family history were obtained from the Qatar Biobank.
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Approval for the study was obtained from the Institutional Review Board (IRB) of QBB, with the ethical approval reference number [QF-QBB-QBIC-RES-ACC-0225-0129]. All participants provided written informed consent before their inclusion in the study. Confidentiality of the participants’ data was strictly maintained throughout the research process, and the samples were anonymized to ensure privacy. The study adhered to all relevant national and international regulations and guidelines for research involving human subjects.
DNA extraction
Saliva samples collected from pregnant women were retrieved from the QBB repository and transported on ice from to Qatar University. Genomic DNA extraction of the samples was achieved using the DNeasy Blood & Tissue Kits (Qiagen®, Germany) according to the manufacturer guidelines.
Library preparation and sequencing
The extracted DNA from the different saliva specimens were subjected to purification and quantification assessment using the Nanodrop ratio A260/A280 and the Qubit dsDNA High Sensitivity Assay kit, respectively. A total of 10ng of pure DNA was utilized for the library preparation using ONT’s 16S Barcoding Kit (SQK-16S024) according to the manufacturer’s guidelines. Briefly, specimen genomic DNA was subjected to barcoding and amplification of the full length of 16S rRNA gene using LongAmp Hot Start Taq 2X Master Mix (New England Biolabs), and the primer set 27F/1492R containing 5’ tags to facilitate the ulterior ligase-free attachment of the Sequencing Adapters. The PCR end products were then purified using CleanMag® Magnetic Beads (Paragon Genomics) at a 0.6X ratio. All barcoded libraries were pooled in Elution Buffer, pH8, at equal ratios of 60 fmoles. The Rapid Sequencing Adapter (RAP) was then incorporated into the pooled libraries and incubated for 5 min at room temperature before adding the Sequencing Buffer (SQB) and the Loading Beads (LB) followed by loading a primed Flongle Flow Cell R9.4.1 (FLO-FLG001). The sequencing was launched under high accuracy base calling parameter and with a QScore >7. Base calling was performed using MinKNOW software (3.6.5).
Bioinformatics & statistical analysis
Fastq_pass files were analyzed using EPI2ME software (V5.1.3) using the 16S workflow (wf-16S, V0.0.3) and minimap2 aligner (2.26-r1175) under the default conditions: reads size ranging from 1200bp to 1700bp using a Blast E-value filter of [e=0.01] and showing the minimum coverage was 80% and 80% identity. The resolution was set to the species level. Phylum, genus and species read csv files were downloaded from EPI2ME and the relative abundance was calculated. The relative abundance was filtered to be ≥ 5%. R programming language version 4.3.1 (2023) was utilized for filtration and statistical analysis. The alpha diversity indices (sobs and Shannon) and beta diversity (Bray-Curtis dissimilarity) were computed using vegan package 2.6.4 (Oksanen et al., 2015). Significance tests for clinical were conducted using either a paired t-test or paired Wilxcon test, depending on the data distribution. Microbial significance was assessed using a paired t-test. A p-value less than 0.05 was considered statistically significant. The Spearman correlation test was performed to indicate correlation between the microbial taxa at the genus level and clinical tests. Plots were generated using ggplot2 package version 3.4.3 (Wickham, 2016) and indicating the significance ggsignif package was used version 3.4.3 (Ahlmann-Eltze and Patil, 2021).
Results
Characteristics of the study population
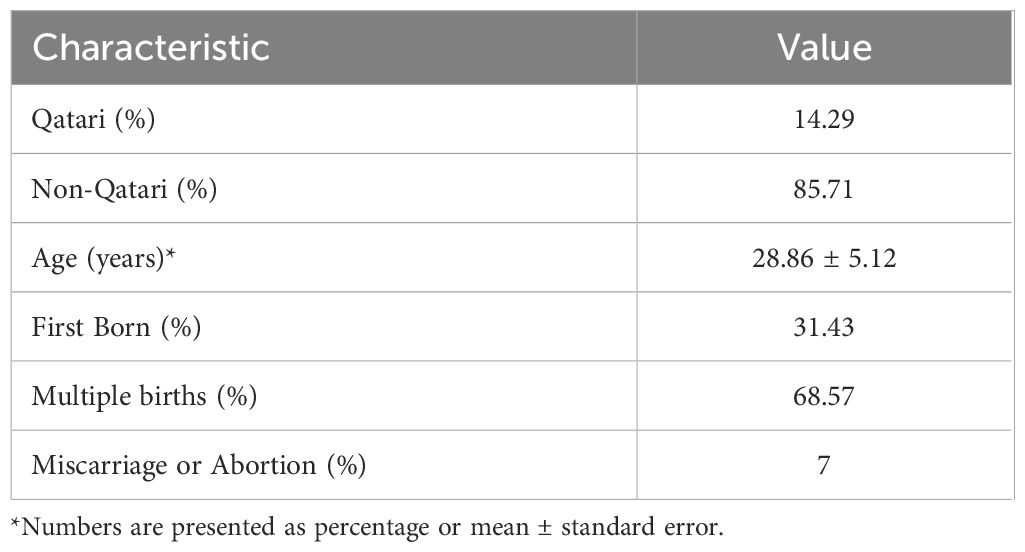
Demographic data of the 45 pregnant women was obtained from Qatar Biobank in their second and third trimesters (Table 1). After excluding individuals with missing information, the study included 35 females, among whom 14% were Qatari. The mean age of the participants was 29 ± 5.12 years. Approximately 31% of the participants were experiencing their first pregnancy, while about 68% had multiple births, and 7% had experienced a previous miscarriage. Participants who took painkillers, antipyretics, or antibiotics accounted for 51.43% of the total, and all were included in the analysis.
Clinical changes during second and third pregnancy trimesters
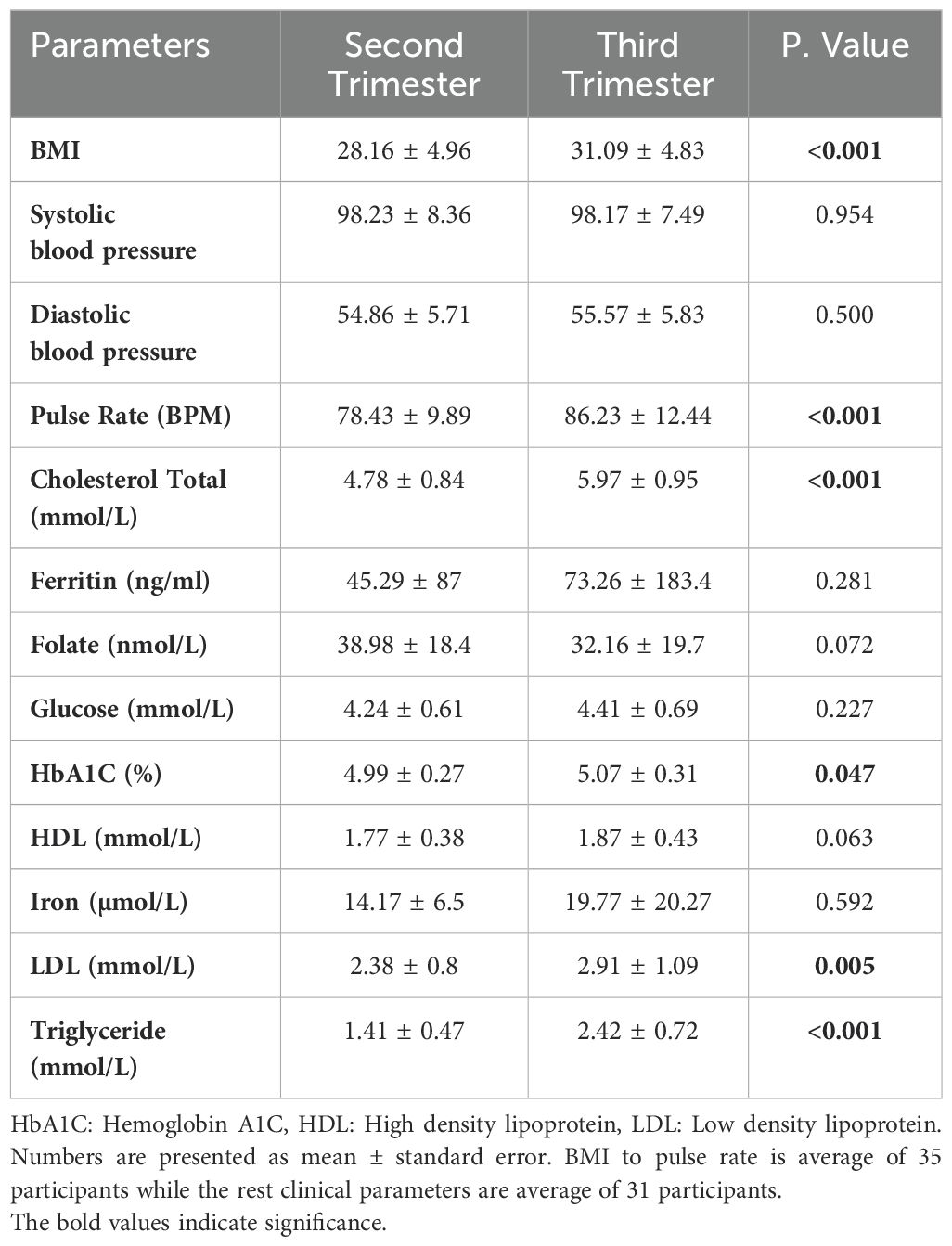
The body mass index (BMI) and pulse rate showed a significant increase in the third trimester compared to the second trimester (p< 0.001), Table 2. Due to missing clinical data from either the second or third trimester in four individuals, these participants were excluded from the study. Consequently, a total of 31 participants were included in the analysis of clinical characteristics. There was a significant increase in HbA1c and LDL levels, p value of 0.047 and 0.005, respectively. Additionally, there was a more profound significant increase in total cholesterol (1.25 fold) and triglycerides (1.75 fold), Table 2.
Microbial diversity
Alpha diversity, which measures the richness and evenness of microbial species within a sample, showed significant changes from the second to the third trimester of pregnancy. Specifically, the observed species (Sobs, Figure 1B) decreased from 499.6 ± 343.3 in the second trimester to 388.0 ± 222.7 in the third trimester, with a highly significant p-value of less than 0.0001. Similarly, the Shannon diversity index (Figure 1A), which accounts for both abundance and evenness of the species present, also showed a significant decrease from 3.15 ± 0.34 in the second trimester to 3.05 ± 0.31 in the third trimester (p < 0.0001). This reduction in the Shannon diversity index indicates a decrease in both the number of species and their evenness during later stages of pregnancy, possibly indicating a shift towards a less complex microbial community as pregnancy progresses.
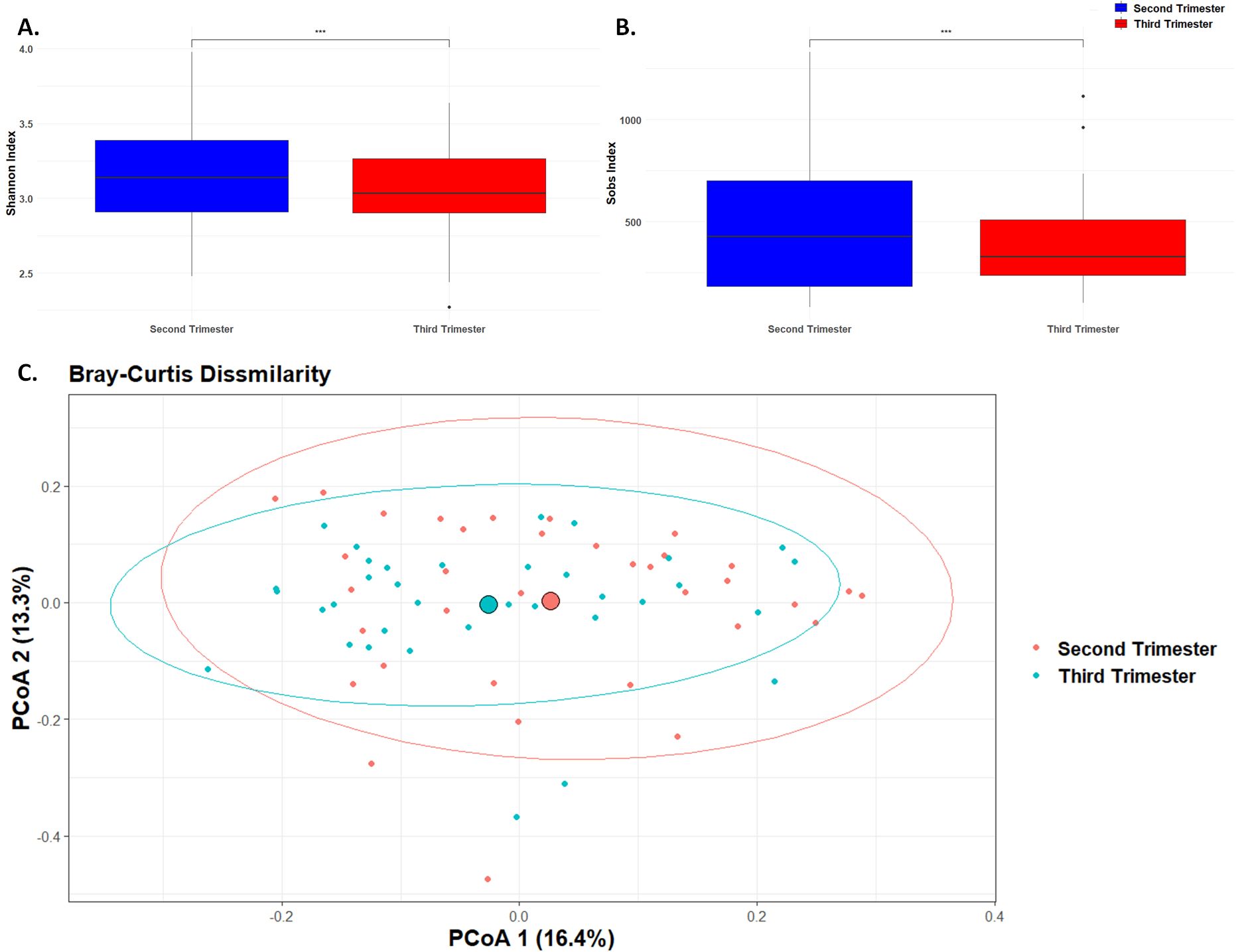
Figure 1. Microbial Diversity. Box plot of differences in observed species richness and evenness using (A). Shannon diversity index and (B). Sobs value. (*** p< 0.001). (C). Principal component analysis plot representing beta diversity distance matrices of the Bray–Curtis distance comparing the sample distribution between the second and third trimesters. The red dots represent samples from the second trimester, and the blue dots represent samples from the third trimester.
Beta diversity, which measures the differences in microbial community composition between samples, was assessed using the Bray-Curtis dissimilarity index (Figure 1C). The Bray-Curtis dissimilarity index indicated a significant shift in the overall microbial community composition between the second and third trimesters (p < 0.01). This indicates significant changes in the overall composition and structure of the microbial community as pregnancy progresses, likely reflecting shifts in species abundance or presence.
Taxonomy analysis
The 16S rRNA gene sequencing analysis at the phylum level detected a total of four phyla: Firmicutes, Proteobacteria, Bacteroidota, and Fusobacteria (Figure 2A). Among these, Firmicutes was the most abundant, with a relative abundance of 84.69% in the second trimester and 82.98% in the third trimester (Figure 2A.1). There were minimal changes in the relative abundance of most bacterial phyla between the second and third trimesters. Firmicutes showed a slight, non-significant decrease with a fold change of -1.02 (p = 0.54), while Proteobacteria exhibited a minor, non-significant increase from 9.27% to 10.28%, corresponding to a fold change of 1.11 (p = 0.71). Fusobacteria remained relatively stable, with a minimal change from 0.26% to 0.25% and a fold change of -1.01 (p = 0.99, Figure 2A.2). In contrast, Bacteroidota displayed a significant increase in relative abundance, rising from 1.15% in the second trimester to 3.34% in the third trimester, with a fold change of 2.92 (p = 0.03, Figures 2A.2, A.3). These findings suggest that while most bacterial phyla remained stable, Bacteroidota underwent a significant shift during the course of pregnancy.
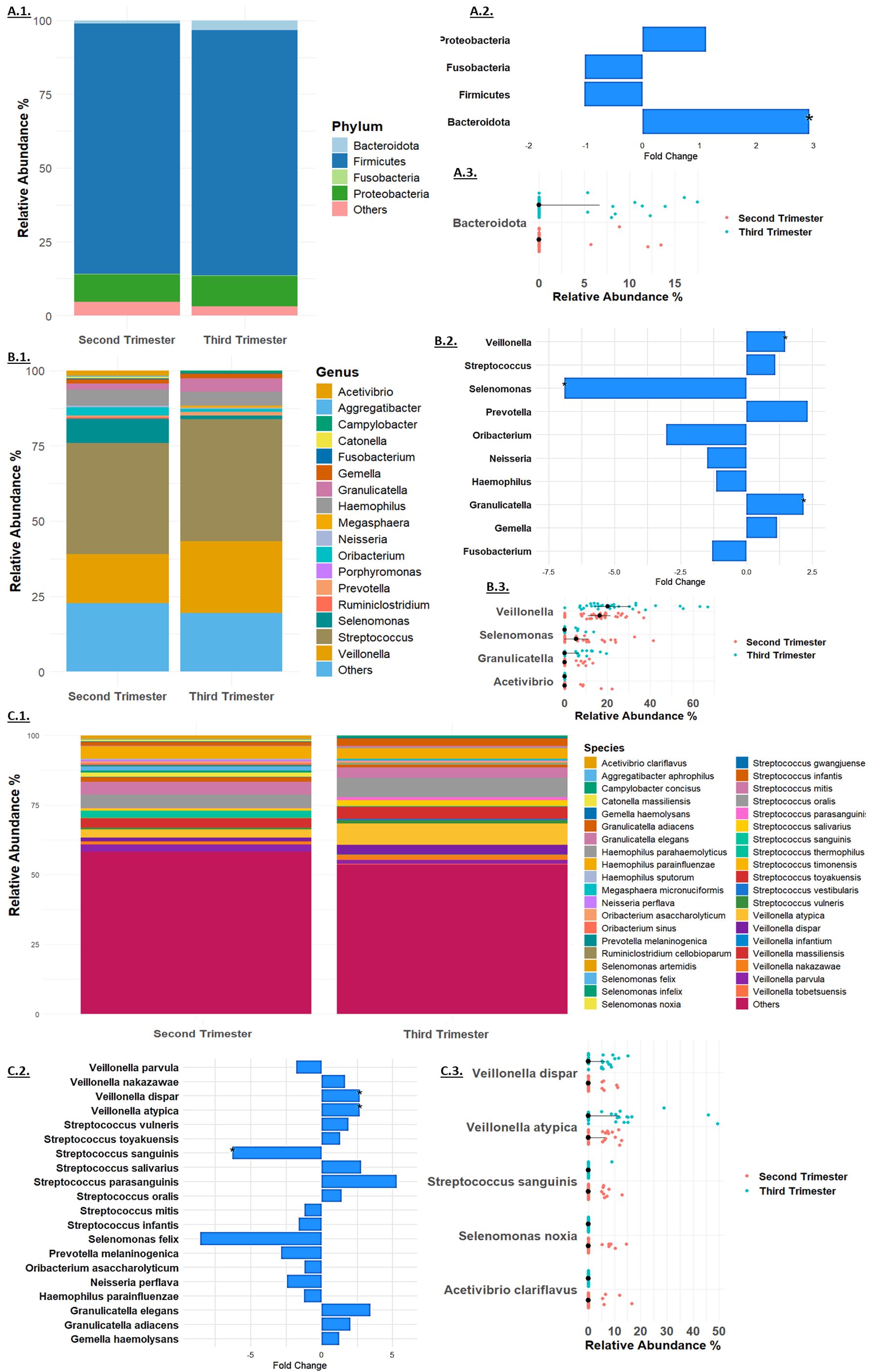
Figure 2. Taxonomy Analysis at the (A). Phylum, (B). Genus and (C). Species levels. Microbial relative mean abundance at the (A.1). Phylum, (B.1). Genus level and (C.1) Species levels. Relative fold change of taxa (A.2). Phylum, (B.2). Genus and (C.2). Species levels. Taxa that exhibited significant change at the (A.3). Phylum, (B.3). Genus and (C.3). Species levels in each sample in the second trimester (red dots) and third trimester (blue dots).
At the genus level (Figure 2B), Streptococcus was the most abundant, increasing slightly from 37.01% to 40.46% (fold change 1.09, p = 0.35, Figure 2B.1). Significant changes were observed in other genera: Granulicatella increased significantly from 2.05% to 4.47% (fold change 2.18, p = 0.03), and Veillonella rose from 16.19% to 23.87% (fold change 1.47, p = 0.013). Selenomonas showed a significant decrease from 8.03% to 1.16% (fold change -6.90, p = 0.0014), while Acetivibrio exhibited a notable reduction to non-detectable levels in the third trimester (p = 0.04, Figures 2B.2, B.3). Other genera, such as Gemella, Haemophilus, Prevotella, and Fusobacterium, showed minor changes, and several genera including Ruminiclostridium, Oribacterium, Catonella, Aggregatibacter, Porphyromonas, Megasphaera, and Campylobacter were non-detectable in the third trimester.
At the species level (Figure 2C), Streptococcus mitis was the most abundant, with a relative abundance of 4.52% in the second trimester and 3.80% in the third trimester (fold change -1.19, p = 0.51, Figure 2C.1). Significant changes were observed in several other species: Granulicatella adiacens increased from 1.36% to 2.79% (fold change 2.05, p = 0.07), and Veillonella dispar rose from 1.26% to 3.38% (fold change 2.68, p = 0.04). Veillonella atypica also increased significantly from 2.88% to 7.69% (fold change 2.67, p = 0.03). Streptococcus sanguinis showed a significant decrease from 1.61% to 0.26% (fold change -6.28, p = 0.04, Figures 2C.2, C.3). Several species exhibited reductions to non-detectable levels in the third trimester, including Selenomonas noxia (p = 0.02), Selenomonas timonensis, Acetivibrio clariflavus (p = 0.04), and others. These findings underscore the dynamic shifts in the microbiome composition during pregnancy, highlighting the significant changes at multiple taxonomic levels.
Correlation analysis
During the second trimester, significant correlations were observed between various physiological markers and microbial taxa. Specifically, BMI, cholesterol levels, and LDL were positively associated with Selenomonas abundance, while inversely correlated with Streptococcus. Additionally, BMI exhibited a negative correlation with Gemella. Diastolic blood pressure showed a positive correlation with Selenomonas abundance and a negative correlation with Ruminiclostridium (Figure 3A).
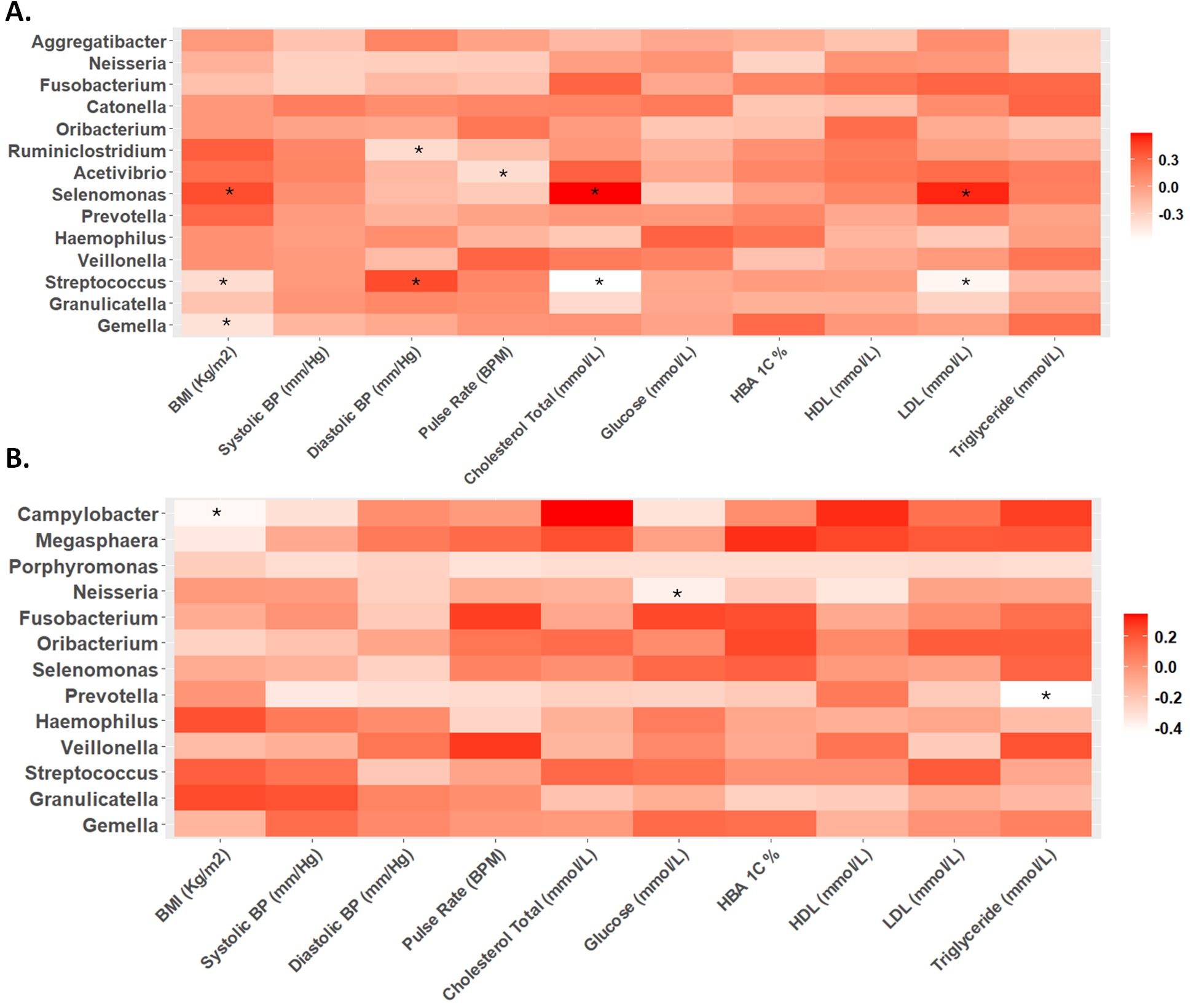
Figure 3. Heat maps of Spearman correlation analysis between relative abundance of microbes at genus level and biochemical parameters in the (A) second and (B) third trimester. The color intensity (white to red) indicates the r-value, with asterisks denoting significance (p-value < 0.05).
In contrast, during the third trimester, different correlation trends were observed. While the positive correlation between diastolic blood pressure and Selenomonas, as well as the negative correlation between diastolic blood pressure and Ruminiclostridium, remained, other significant correlations emerged. BMI showed a negative correlation with Campylobacter, and glucose levels were inversely correlated with Neisseria. Triglyceride levels displayed a negative correlation with Prevotella abundance (Figure 3B).
Discussion
Pregnancy is a transformative period marked by numerous physiological changes, including fluctuations in hormone levels and immune responses (Nuriel-Ohayon et al., 2016). Emerging evidence has highlighted alterations in the oral microbiome during this time. The hormonal and immune modulations associated with pregnancy may significantly influence oral microbiota composition, with potential consequences for both maternal and fetal health (Mockridge and Maclennan, 2019; Saadaoui et al., 2021). Understanding these dynamic changes is crucial for enhancing maternal oral health and, by extension, broader systemic health during this critical period.
Our study demonstrated several significant shifts in both clinical and microbial parameters throughout pregnancy. The observed increase in pulse rate, a typical physiological response to pregnancy, aligns with known cardiovascular changes driven by elevated estrogen, progesterone, and prostaglandin levels (La et al., 2022). These hormones contribute to increased diastolic and stroke volumes, along with a higher heart rate, resulting in a progressive rise in cardiac output during the latter stages of pregnancy (Morton, 2021; La et al., 2022). This heightened cardiac output primarily serves the growing fetus and prepares the mother’s body for postpartum lactation (Zakaria et al., 2022).
Regarding lipid metabolism, pregnancy induces dynamic alterations that transition from an anabolic state in the first two trimesters to a catabolic phase in the third trimester, as insulin sensitivity decreases (Elkus and Popovich, 1992; Herrera, 2002; Buggio et al., 2019). Our study found significant increases in cholesterol, LDL, and triglycerides during pregnancy, consistent with the typical metabolic shifts of pregnancy (Herrera, 2002). These lipid changes are influenced by hormonal fluctuations and metabolic adaptations, which also have an impact on gut microbiota diversity and composition (Napso et al., 2018). Oral diseases such as periodontal disease and gingivitis are more likely during pregnancy due to increased sex steroid hormones and potential microbiome dysbiosis, emphasizing the importance of periodontal care (Boggess and Society for Maternal-Fetal Medicine Publications Committee, 2008; Silk et al., 2008; Balan et al., 2018; Figuero et al., 2020; Rapone et al., 2020; Giannella et al., 2023; Wild and Feingold, 2023).
The notable shifts in both alpha and beta diversity in our cohort suggest significant changes in microbial composition as pregnancy progresses. Specifically, the decline in alpha diversity in the third trimester likely reflects physiological adaptations to pregnancy, such as increased insulin resistance and weight gain (Nuriel-Ohayon et al., 2016; Zakaria et al., 2022). This reduction in diversity could be beneficial for fetal growth, illustrating how the maternal microbiome adapts to meet the needs of pregnancy.
Further, our findings on weight gain align with previous studies showing that changes in microbial diversity are associated with normal weight gain during pregnancy, especially in relation to the increased abundance of Bacteroides (Santacruz et al., 2010; Li et al., 2023; Sinha et al., 2023; Strobel et al., 2023). The increased presence of Bacteroides, a member of the Bacteroidetes phylum, has been linked to improved lipid profiles and folic acid levels, supporting the maternal metabolic adaptations required for a healthy pregnancy (Santacruz et al., 2010). This is consistent with previous research reporting a similar trend in the relative abundance of Bacteroidetes and Firmicutes, with Bacteroides showing a notable increase from the second to the third trimester (Gao et al., 2024). However, it is important to note that we did not observe any significant changes at the species level within the Bacteroidota phylum; nonetheless, the increase in the overall Bacteroidota abundance during pregnancy may still suggest broader functional roles within the maternal microbiome.
At the genus level, our observation of a decrease in the relative abundance of Selenomonas during the third trimester may be linked to hormonal changes and immune modulation, which are known to alter the oral microbiome (Balan et al., 2018; Xu et al., 2020; Giannella et al., 2023). Although literature on Selenomonas during pregnancy is sparse, previous studies suggest its abundance is associated with gestational diabetes mellitus (GDM) and other pregnancy complications (DiGiulio et al., 2015; Tuominen et al., 2018). The reduced abundance of Selenomonas in our study cohort could reflect positive oral health behaviors among participants, a factor previously linked to improved pregnancy outcomes (Silk et al., 2008).
Conversely, the increase in the mean relative abundance of Veillonella and Granulicatella during the third trimester is consistent with their roles as opportunistic pathogens, often associated with oral diseases such as gingivitis (Eribe and Olsen, 2017; Knapp et al., 2017; Yang et al., 2019). Veillonella, in particular, has been implicated in the translocation of bacteria from the oral cavity to the placenta in murine models, suggesting its potential involvement in maternal-fetal microbial transmission (Fardini et al., 2010; Gomez-Arango et al., 2017). Its increase in our study further supports findings that pregnancy promotes the growth of certain gram-negative anaerobic bacteria in the oral cavity due to hormonal changes (Beighton et al., 2008; Yokoyama et al., 2008; Gürsoy et al., 2009; MaChado et al., 2012; Bäckhed et al., 2015; Fujiwara et al., 2017; Massoni et al., 2019).
Granulicatella, another gram-positive bacterium, has been linked to various infections and metabolic disturbances, including dyslipidemia and obesity (Gardenier et al., 2011; Crusell et al., 2018; Wu et al., 2018; Sparvoli et al., 2020; Zhao et al., 2020; Aranaz et al., 2021; Dong et al., 2021; Li et al., 2022; Gao et al., 2024). The significant increase in its abundance during pregnancy, as observed in our study, may have implications for lipid metabolism, maternal inflammation, and potential complications such as gestational diabetes (Crusell et al., 2018). Our findings contribute to the growing body of research indicating that Granulicatella plays a role in the maternal microbiome and metabolic health during pregnancy.
Streptococcus sanguinis, a component of the core microbiome, exhibited higher abundance in the second trimester, suggesting that hormonal changes and dietary habits during pregnancy influence its prevalence (Santacroce et al., 2023). Additionally, correlations between specific bacterial genera and metabolic parameters indicate complex interplays during pregnancy. For instance, the negative correlation between Streptococcus levels and metabolic markers supports previous findings regarding probiotics and their role in improving lipid profiles and inflammatory markers during pregnancy (Asemi et al., 2012; Jafarnejad et al., 2016).
Gemella, linked to maternal health, showed a tendency to decrease as BMI increased, suggesting that maternal weight may influence microbial composition (Yao et al., 2021). The correlation between diastolic blood pressure and Selenomonas in the second trimester further illustrates the relationship between cardiovascular alterations and microbial dynamics (Fu et al., 2019). Studies indicate that elevated DBP is associated with adverse pregnancy outcomes, highlighting the need for further exploration of this correlation (Yoo et al., 2020; De Siena et al., 2021).
In the third trimester, negative correlations between BMI, glucose, and Campylobacter, Neisseria, and Prevotella suggest that metabolic changes influence oral microbiota composition (Zhang et al., 2010). Specifically, Campylobacter levels were associated with oral hygiene practices, indicating a link between oral health and microbial ecology (La et al., 2022). The negative correlation between Neisseria and glucose levels during the third trimester implies its potential role in glucose metabolism, which warrants further investigation (Morse Stephen et al., 1974; Exley Rachel et al., 2005; Exley Rachel et al., 2007; Crusell et al., 2020; Ren et al., 2023).
Conclusion
In conclusion, our study reveals significant changes in both clinical and microbial parameters during pregnancy, underscoring the intricate relationship between maternal health and the microbiome. These findings enhance our understanding of how these changes may influence maternal and fetal health outcomes, emphasizing the need for vigilant clinical oversight throughout pregnancy. Recognizing these alterations is crucial for establishing appropriate benchmarks for maternal health. Additionally, while medication use, including antibiotics, was recorded, the lack of specific exclusion criteria for antibiotic use should be considered a limitation, as it may impact microbiome composition. Further research is warranted to explore the mechanisms underlying these changes and their potential long-term effects on both maternal and offspring health.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: NCBI - PRJNA1195396.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Qatar BioBank. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FB: Formal analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. LIM: Formal analysis, Writing – review & editing. HA-H: Writing – original draft. SS: Data curation, Writing – review & editing. SB: Data curation, Writing – review & editing. ZZ: Writing – review & editing. EF: Resources, Writing – review & editing. MA-A: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by a grant from Qatar National Research Fund (UREP26-104-3-044) and Qatar University’s collaborative grant (QUCG-BRC-23/24-183). FB is the recipient of the L’Oréal-UNESCO for Women in Science Middle East Regional Young Talents award 2022.
Acknowledgments
We would like to acknowledge the contributions of undergraduate students involved in the UREP project: Shahd Nasr, Salma Bouabidi, Rahaf Nader, Huda Farah, Muram Elamin, and Sara Suleiman.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahlmann-Eltze, C., Patil, I. (2021). ggsignif: R package for displaying significance brackets for ‘ggplot2. PsyArxiv. doi: 10.31234/osf.io/7awm6
Aranaz, P., Ramos-Lopez, O., Cuevas-Sierra, A., Martinez, J. A., Milagro, F.I., Riezu-Boj, J. I. (2021). A predictive regression model of the obesity-related inflammatory status based on gut microbiota composition. Int. J. Obes. 45, 2261–2268. doi: 10.1038/s41366-021-00904-4
Asemi, Z., Samimi, M., Tabasi, Z., Talebian, P., Azarbad, Z., Hydarzadeh, Z., et al. (2012). Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. J. Matern Fetal Neonatal Med. 25, 1552–1556. doi: 10.3109/14767058.2011.640372
Bäckhed, F., Roswall, J., Peng, Y., Feng, Q., Jia, H., Kovatcheva-Datchary, P., et al. (2015). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703. doi: 10.1016/j.chom.2015.04.004
Balan, P., Chong, Y. S., Umashankar, S., Swarup, S., Loke, W. M., Lopez, V., et al. (2018). Keystone species in pregnancy gingivitis: a snapshot of oral microbiome during pregnancy and postpartum period. Front. Microbiol. 9, 2360. doi: 10.3389/fmicb.2018.02360
Baud, A., Hillion, K.-H., Plainvert, C., Tessier, V., Tazi, A., Mandelbrot, L., et al. (2023). Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci. Rep. 13, 9061. doi: 10.1038/s41598-023-36126-z
Beighton, D., Clark, D., Hanakuka, B., Gilbert, S., Do, T. (2008). The predominant cultivable Veillonella spp. of the tongue of healthy adults identified using rpoB sequencing. Oral. Microbiol. Immunol. 23, 344–347. doi: 10.1111/j.1399-302X.2007.00424.x
Boggess, K. A., Society for Maternal-Fetal Medicine Publications Committee (2008). Maternal oral health in pregnancy. Obstetrics gynecology 111, 976–986. doi: 10.1097/AOG.0b013e31816a49d3
Buggio, L., Somigliana, E., Borghi, A., Vercellini, P. (2019). Probiotics and vaginal microecology: fact or fancy? BMC women's Health 19, 1–6. doi: 10.1186/s12905-019-0723-4
Crusell, M. K. W., Brink, L. R., Nielsen, T., Allin, K. H., Hansen, T., Damm, P., et al. (2018). Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6, 1–19. doi: 10.1186/s40168-018-0472-x
Crusell, M. K. W., Hansen, T. H., Nielsen, T., Allin, K. H., Rühlemann, M. C., Damm, P., et al. (2020). Gestational diabetes and the human salivary microbiota: a longitudinal study during pregnancy and postpartum. BMC Pregnancy Childbirth 20, 69. doi: 10.1186/s12884-020-2764-y
De Siena, M., Laterza, L., Matteo, M. V., Mignini, I., Schepis, T., Rizzatti, G., et al. (2021). Gut and reproductive tract microbiota adaptation during pregnancy: new insights for pregnancy-related complications and therapy. Microorganisms 9, 473. doi: 10.3390/microorganisms9030473
DiGiulio, D. B., Callahan, B. J., McMurdie, P. J., Costello, E. K., Lyell, D. J., Robaczewska, A., et al. (2015). Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. 112, 11060–11065. doi: 10.1073/pnas.1502875112
Dong, T., Zhao, F., Yuan, K., Zhu, X., Wang, N., Xia, F., et al. (2021). Association between serum thyroid-stimulating hormone levels and salivary microbiome shifts. Front. Cell. Infection Microbiol. 11, 603291. doi: 10.3389/fcimb.2021.603291
Elkus, R., Popovich, J., Jr (1992). Respiratory physiology in pregnancy. Clinics chest Med. 13, 555–565. doi: 10.1016/S0272-5231(21)01125-4
Eribe, E. R., Olsen, I. (2017). Leptotrichia species in human infections II. J. Oral. Microbiol. 9, 1368848. doi: 10.1080/20002297.2017.1368848
Exley Rachel, M., Goodwin, L., Mowe, E., Shaw, J., Smith, H., Read, R. C., et al. (2005). Neisseria meningitidis lactate permease is required for nasopharyngeal colonization. Infection Immun. 73, 5762–5766. doi: 10.1128/IAI.73.9.5762-5766.2005
Exley Rachel, M., Wu, H., Shaw, J., Schneider, M. C., Smith, H., Jerse, A. E., et al. (2007). Lactate acquisition promotes successful colonization of the murine genital tract by neisseria gonorrhoeae. Infection Immun. 75, 1318–1324. doi: 10.1128/IAI.01530-06
Fardini, Y., Chung, P., Dumm, R., Joshi, N., Han, Y. W. (2010). Transmission of diverse oral bacteria to murine placenta: evidence for the oral microbiome as a potential source of intrauterine infection. Infection Immun. 78, 1789–1796. doi: 10.1128/IAI.01395-09
Fasano, A., Flaherty, S. (2021). Gut feelings: The microbiome and our health (MIT Press). doi: 10.7551/mitpress/11291.001.0001
Figuero, E., Han, Y. W., Furuichi, Y. (2020). Periodontal diseases and adverse pregnancy outcomes: Mechanisms. Periodontology 2000 83, 175–188. doi: 10.1111/prd.12295
Fu, X., Liu, Z., Zhu, C., Mou, H., Kong, Q. (2019). Nondigestible carbohydrates, butyrate, and butyrate-producing bacteria. Crit. Rev. Food Sci. Nutr. 59, S130–S152. doi: 10.1080/10408398.2018.1542587
Fujiwara, N., Tsuruda, K., Iwamoto, Y., Kato, F., Odaki, T., Yamane, N., et al. (2017). Significant increase of oral bacteria in the early pregnancy period in Japanese women. J. Invest. Clin. dentistry 8, e12189. doi: 10.1111/jicd.2017.8.issue-1
Gao, Y., Zhang, J., Chen, H., Jin, X., Lin, Z., Fan, C., et al. (2024). Dynamic changes in the gut microbiota during three consecutive trimesters of pregnancy and their correlation with abnormal glucose and lipid metabolism. Eur. J. Med. Res. 29, 117. doi: 10.1186/s40001-024-01702-0
Gardenier, J. C., Hranjec, T., Sawyer, R. G., Bonatti, H. (2011). Granulicatella adiacens bacteremia in an elderly trauma patient. Surg. Infect. (Larchmt) 12, 251–253. doi: 10.1089/sur.2010.059
Giannella, L., Grelloni, C., Quintili, D., Fiorelli, A., Montironi, R., Alia, S., et al. (2023). Microbiome changes in pregnancy disorders. Antioxidants 12, 463. doi: 10.3390/antiox12020463
Gomez-Arango, L. F., Barrett, H. L., McIntyre, H. D., Callaway, L. K., Morrison, M., Nitert, M. D. (2017). Contributions of the maternal oral and gut microbiome to placental microbial colonization in overweight and obese pregnant women. Sci. Rep. 7, 2860. doi: 10.1038/s41598-017-03066-4
Gürsoy, M., Haraldsson, G., Hyvönen, M., Sorsa, T., Pajukanta, R., Könönen, E. (2009). Does the frequency of Prevotella intermedia increase during pregnancy? Oral. Microbiol. Immunol. 24, 299–303. doi: 10.3748/wjg.v21.i31.9239
Herrera, E. (2002). Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 19, 43–55. doi: 10.1385/ENDO:19:1:43
Jafarnejad, S., Saremi, S., Jafarnejad, F., Arab, A. (2016). Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Nutr. Metab 2016. p, 5190846. doi: 10.1155/2016/5190846
Jang, H., Patoine, A., Wu, T. T., Castillo, D. A., Xiao, J. (2021). Oral microflora and pregnancy: a systematic review and meta-analysis. Sci. Rep. 11, 16870. doi: 10.1038/s41598-021-96495-1
Knapp, S., Brodal, C., Peterson, J., Qi, F., Kreth, J., Merritt, J. (2017). Natural competence is common among clinical isolates of Veillonella parvula and is useful for genetic manipulation of this key member of the oral microbiome. Front. Cell. Infection Microbiol. 7, 139. doi: 10.3389/fcimb.2017.00139
La, X., Zhang, G., Cui, L., Zhang, L., Zhou, Q., Mu, C., et al. (2022). Profile of the oral microbiota from preconception to the third trimester of pregnancy and its association with oral hygiene practices. J. Oral. Microbiol. 14, 2053389. doi: 10.1080/20002297.2022.2053389
Li, M., Li, K., Tang, S., Lv, Y., Wang, Q., Wang, Z., et al. (2022). Restoration of the gut microbiota is associated with a decreased risk of hepatic encephalopathy after TIPS. JHEP Rep. 4, 100448. doi: 10.1016/j.jhepr.2022.100448
Li, M., Jiang, H., Chen, A., Zheng, H., Shen, L., Chen, W., et al. (2023). Dynamic changes in gut microbiota during pregnancy among Chinese women and influencing factors: A prospective cohort study. Front. Microbiol. 14, 1114228. doi: 10.3389/fmicb.2023.1114228
MaChado, F. C., Cesar, D. E., Assis, A. V. D. A., Diniz, C. G., Ribeiro, R. A. (2012). Detection and enumeration of periodontopathogenic bacteria in subgingival biofilm of pregnant women. Braz. Oral. Res. 26, 443–449. doi: 10.1590/S1806-83242012000500011
Massoni, R. S. D. S., Aranha, A. M. F., Matos, F. Z., Guedes, O. A., Borges, Á. H., Miotto, M., et al. (2019). Correlation of periodontal and microbiological evaluations, with serum levels of estradiol and progesterone, during different trimesters of gestation. Sci. Rep. 9, 11762. doi: 10.1038/s41598-019-48288-w
Mockridge, A., Maclennan, K. (2019). Physiology of pregnancy. Anaesthesia Intensive Care Med. 20, 397–401. doi: 10.1016/j.mpaic.2019.05.001
Mohammed, L. I., Razali, R., Zakaria, Z. Z., Benslimane, F. M., Cyprian, F., Al-Asmakh, M. (2024), “Smoking induced salivary microbiome dysbiosis and is correlated with lipid biomarkers”, BMC Oral Health, 24, 608. doi: 10.1186/s12903-024-04340-4
Morse Stephen, A., Stein, S., Hines, J. (1974). Glucose metabolism in neisseria gonorrhoeae. J. Bacteriology 120, 702–714. doi: 10.1128/jb.120.2.702-714.1974
Morton, A. (2021). Physiological changes and cardiovascular investigations in pregnancy. Heart Lung Circ. 30, e6–e15. doi: 10.1016/j.hlc.2020.10.001
Murray, I., Hendley, J. (2020). “Change and adaptation in pregnancy,” in Myles' Textbook for Midwives E-Book: Myles' Textbook for Midwives E-Book (London: Elsevier), 197.
Napso, T., Yong, H. E. J., Lopez-Tello, J., Sferruzzi-Perri, A. N. (2018). The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front. Physiol. 9, 1091. doi: 10.3389/fphys.2018.01091
Nardi, G. M., Grassi, R., Ndokaj, A., Antonioni, M., Jedlinski, M., Rumi, G., et al. (2021). Maternal and neonatal oral Microbiome Developmental patterns and correlated factors: a systematic review—does the Apple fall close to the Tree? Int. J. Environ. Res. Public Health 18, 5569. doi: 10.3390/ijerph18115569
Nuriel-Ohayon, M., Neuman, H., Koren, O. (2016). Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. p, 1031. doi: 10.3389/fmicb.2016.01031
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P., O’Hara, B., et al. (2015). Vegan: Community Ecology Package Vol. 2 (R Package Version 2.2-1), 1–2.
Rapone, B., Ferrara, E., Montemurro, N., Converti, I., Loverro, M., Loverro, M. T., et al. (2020). Oral microbiome and preterm birth: correlation or coincidence? A narrative review. Open Access Macedonian J. Med. Sci. 8, 123–132. doi: 10.3889/oamjms.2020.4444
Ren, Y., Hao, L., Liu, J., Wang, P., Ding, Q., Chen, C., et al. (2023). Alterations in the gut microbiota in pregnant women with pregestational type 2 diabetes mellitus. mSystems 8, e0114622. doi: 10.1128/msystems.01146-22
Saadaoui, M., Singh, P., Al Khodor, S. (2021). Oral microbiome and pregnancy: A bidirectional relationship. J. Reprod. Immunol. 145, 103293. doi: 10.1016/j.jri.2021.103293
Santacroce, L., Passarelli, P. C., Azzolino, D., Bottalico, L., Charitos, I. A., Cazzolla, A. P., et al. (2023). Oral microbiota in human health and disease: A perspective. Exp. Biol. Med. (Maywood) 248, 1288–1301. doi: 10.1177/15353702231187645
Santacruz, A., Collado, M. C., García-Valdés, L., Segura, M. T., Martín-Lagos, J. A., Anjos, T., et al. (2010). Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 104, 83–92. doi: 10.1017/S0007114510000176
Sedghi, L., DiMassa, V., Harrington, A., Lynch, S. V., Kapila, Y. L. (2021). The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2000 87, 107–131. doi: 10.1111/prd.12393
Silk, H., Douglass, A. B., Douglass, J. M., Silk, L. (2008). Oral health during pregnancy. Am. Fam Physician 77, 1139–1144.
Sinha, T., Brushett, S., Prins, J., Zhernakova, A. (2023). The maternal gut microbiome during pregnancy and its role in maternal and infant health. Curr. Opin. Microbiol. 74, 102309. doi: 10.1016/j.mib.2023.102309
Sparvoli, L. G., Cortez, R. V., Daher, S., Padilha, M., Sun, S. Y., Nakamura, M. U., et al. (2020). Women's multisite microbial modulation during pregnancy. Microbial Pathogenesis 147, 104230. doi: 10.1016/j.micpath.2020.104230
Strobel, K. M., Juul, S. E., Hendrixson, D. T. (2023). Maternal nutritional status and the microbiome across the pregnancy and the post-partum period. Microorganisms 11, 1569. doi: 10.3390/microorganisms11061569
Tuominen, H., Rautava, S., Syrjänen, S., Collado, M. C., Rautava, J. (2018). HPV infection and bacterial microbiota in the placenta, uterine cervix and oral mucosa. Sci. Rep. 8, 9787. doi: 10.1038/s41598-018-27980-3
Wen, X., Fu, X., Zhao, C., Yang, L., Huang, R. (2023). The bidirectional relationship between periodontal disease and pregnancy via the interaction of oral microorganisms, hormone and immune response. Front. Microbiol. 14, 1070917. doi: 10.3389/fmicb.2023.1070917
Wild, R., Feingold, K. R. (2023). Effect of pregnancy on lipid metabolism and lipoprotein levels. In: Feingold, K. R., Anawalt, B., Blackman, M. R., et al eds. Endotext [Internet]. (South Dartmouth (MA): MDText.com, Inc.). Available at: https://www.ncbi.nlm.nih.gov/books/NBK498654/. (Accessed 11 November 2024).
Wu, Y., Chi, X., Zhang, Q., Chen, F., Deng, X. (2018). Characterization of the salivary microbiome in people with obesity. PeerJ 6, e4458. doi: 10.7717/peerj.4458
Xu, Y., Zhang, M., Zhang, J., Sun, Z., Ran, L., Ban, Y., et al. (2020). Differential intestinal and oral microbiota features associated with gestational diabetes and maternal inflammation. Am. J. Physiol. Endocrinol. Metab. 319, E247–e253. doi: 10.1152/ajpendo.00266.2019
Yang, I., Knight, A. K., Dunlop, A. L., Corwin, E. J. (2019). Characterizing the subgingival microbiome of pregnant african american women. J. Obstetric Gynecologic Neonatal Nurs. 48, 140–152. doi: 10.1016/j.jogn.2018.12.003
Yao, Y., Cai, X., Ye, Y., Wang, F., Chen, F., Zheng, C. (2021). The role of microbiota in infant health: from early life to adulthood. Front. Immunol. 12, 708472. doi: 10.3389/fimmu.2021.708472
Yokoyama, M., Hinode, D., Yoshioka, M., Fukui, M., Tanabe, S., Grenier, D., et al. (2008). Relationship between Campylobacter rectus and periodontal status during pregnancy. Oral. Microbiol. Immunol. 23, 55–59. doi: 10.1111/j.1399-302X.2007.00391.x
Yoo, J. Y., Groer, M., Dutra, S., Sarkar, A., McSkimming, D. (2020). Gut microbiota and immune system interactions. Microorganisms 8, 1587. doi: 10.3390/microorganisms8101587
Zakaria, Z. Z., Al-Rumaihi, S., Al-Absi, R. S., Farah, H., Elamin, M., Nader, R., et al. (2022). Physiological changes and interactions between microbiome and the host during pregnancy. Front. Cell Infect. Microbiol. 12, 824925. doi: 10.3389/fcimb.2022.824925
Zhang, L., Budiman, V., Day, A. S., Mitchell, H., Lemberg, D. A., Riordan, S. M., et al. (2010). Isolation and detection of Campylobacter concisus from saliva of healthy individuals and patients with inflammatory bowel disease. J. Clin. Microbiol. 48, 2965–2967. doi: 10.1128/JCM.02391-09
Keywords: pregnancy, oral microbiome, gut microbiota, salivary microbiome, Qatari birth cohort (QbiC), oxford nanopore sequencing
Citation: Benslimane FM, Mohammed LI, Abu-Hijleh H, Suleiman S, Boughattas S, Zakaria ZZ, Fthenou E and Al-Asmakh M (2024) Metabarcoding analysis of oral microbiome during pregnancy. Front. Cell. Infect. Microbiol. 14:1477703. doi: 10.3389/fcimb.2024.1477703
Received: 08 August 2024; Accepted: 31 October 2024;
Published: 17 December 2024.
Edited by:
Soumyadev Sarkar, Arizona State University, United StatesReviewed by:
Allison E. Mann, University of Wyoming, United StatesMara Roxana Rubinstein, CONICET Institute for Biomedical Research (BIOMED), Argentina
Copyright © 2024 Benslimane, Mohammed, Abu-Hijleh, Suleiman, Boughattas, Zakaria, Fthenou and Al-Asmakh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maha Al-Asmakh, bWFoYS5hbGFzbWFraEBxdS5lZHUucWE=
 Fatiha M. Benslimane
Fatiha M. Benslimane Layla I. Mohammed
Layla I. Mohammed Haya Abu-Hijleh
Haya Abu-Hijleh Sara Suleiman
Sara Suleiman Sonia Boughattas
Sonia Boughattas Zain Zaki Zakaria
Zain Zaki Zakaria Eleni Fthenou
Eleni Fthenou Maha Al-Asmakh
Maha Al-Asmakh