- 1Department of Laboratory Medicine, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 3Department of Clinical Laboratory, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
Introduction: Intraamniotic infection is crucial in preterm prelabor rupture of membranes(PPROM), a clinical condition resulting from the invasion of vaginal opportunistic microbes into the amniotic cavity. Although previous studies have suggested potential associations between infection and PPROM, the role of vaginalopportunistic bacteria in PPROM has received limited attention.
Methods: This study aimed to confirm the vaginal bacterial etiology of PPROM. We investigated vaginal microbiotas using automatic analysis of vaginal discharge, microbiological tests, and 16s rRNA genehigh-throughput sequencing.
Results: The research findings revealed that the proportion of parabasal epitheliocytes, leukocytes, toxic leukocytes, and bacteria with diameters smaller than 1.5 um was significantly higher in the PPROM group than that in the normal full-term labor (TL) group. The top three vaginal opportunistic bacterial isolates in all participants were 9.47% Escherichia coli, 5.99% Streptococcus agalactiae, and 3.57% Enterococcus faecalis. The bacterial resistance differed, but all the isolates were sensitive to nitrofurantoin. Compared with the vaginal microbiota dysbiosis (VMD) TL (C) group, the VMD PPROM (P) group demonstrated more operational taxonomic units, a high richness of bacterial taxa, and a different beta-diversity index. Indicator species analysis revealed that Lactobacillus jensenii, Lactobacillus crispatus, and Veillonellaceae bacterium DNF00626 were strongly associated with the C group. Unlike the C group, the indicator bacteria in the P group were Enterococcus faecalis, Escherichia coli, and Streptococcus agalactiae.
Discussion: These findings provide solidevidence that an abnormal vaginal microbiome is a very crucial risk factorclosely related to PPROM. There were no unique bacteria in the vaginalmicrobiota of the PPROM group; however, the relative abundance of bacteria inthe abnormal vaginal flora of PPROM pregnancies differed. Antibiotics should bereasonably selected based on drug sensitivity testing. The findings presented in this paper enhance our understanding of Streptococcus agalactiae, Enterococcus faecalis, and Escherichia coli vaginal bacterial etiology of PPROM in Western China.
Introduction
Preterm prelabor rupture of membranes (PPROM) is the rupture of membranes before the onset of labor, precisely 37 weeks of gestation, occurring in approximately 2%–4% of pregnancies and representing 40%~50% of preterm births (Siegler et al., 2020; Ambalpady et al., 2022). It is associated with severe adverse pregnancy outcomes, including maternal factors such as chorioamnionitis, endometritis, puerperium infection, dystocia, placental abruption, placental retention, and postpartum hemorrhage. Meanwhile, neonatal risks include complications of prematurity, such as respiratory distress, neonatal infection, neonatal premature septicemia, pulmonary hyaline membrane disease, and bronchopulmonary dysplasia (Bouchghoul et al., 2019).
PPROM arises from various causes and mechanisms, such as genetic predisposition, behavioral factors, environmental factors, obstetric complications, and intraamniotic infection (Bonasoni et al., 2022). Among these, intraamniotic infection has been demonstrated to be commonly associated with PPROM (Yang and Wang, 2023). However, the effect of abnormal vaginal bacteria on PPROM remains unclear.
The vaginal flora of pregnant women is dominated by Lactobacillus species (Pramanick et al., 2021). When Lactobacillus species decrease opportunistic pathogens increase, vaginal microflora dysbiosis occurs (Ma et al., 2022), which can lead to several adverse pregnancy outcomes, including PROM, preterm birth, intrauterine growth restriction, intrauterine infections, chorioamnionitis, Low birth weight infants, miscarriages, and stillbirths (Kumar et al., 2022). Bacteria commonly found in intrauterine tissues in association with preterm labor (PTB) are of vaginal origin, and one of the most common routes is via the vaginal canal (Tian et al., 2023). It has been revealed that treatment for some vaginal infections can reduce the incidence of PTB (Lacroix et al., 2022). However, only two studies in a meta-analysis of 12 studies found an association between PPROM and vaginal microflora dysbiosis (Juliana et al., 2020). Further studies have revealed that treating bacterial vaginosis (BV) with oral or vaginal metronidazole or vaginal clindamycin before 28 weeks of gestation does not reduce the incidence of PTB (Rebouças et al., 2019). Accordingly, additional research is needed.
This study aimed to investigate the vaginal bacterial etiology of PPROM by exploring the association between the vaginal microbiome in PPROM and normal full-term labor (TL), antibiotic susceptibility testing of the major isolates, and vaginal microecological status.
Materials and methods
The study was reviewed and approved by the ethics committee of West China Second University Hospital of Sichuan University (Medical Research 2020 NO. 050 and 2023 NO. 131).
Study design and patients
Singleton pregnant women were eligible if their gestational age was >14 weeks based on the last menstrual period or obstetrical estimation and were planning to receive ongoing prenatal and delivery in the Obstetric and Gynecological Department of West China Second University Hospital from January 2020 to December 2023. Written informed consent forms were obtained from all participants.
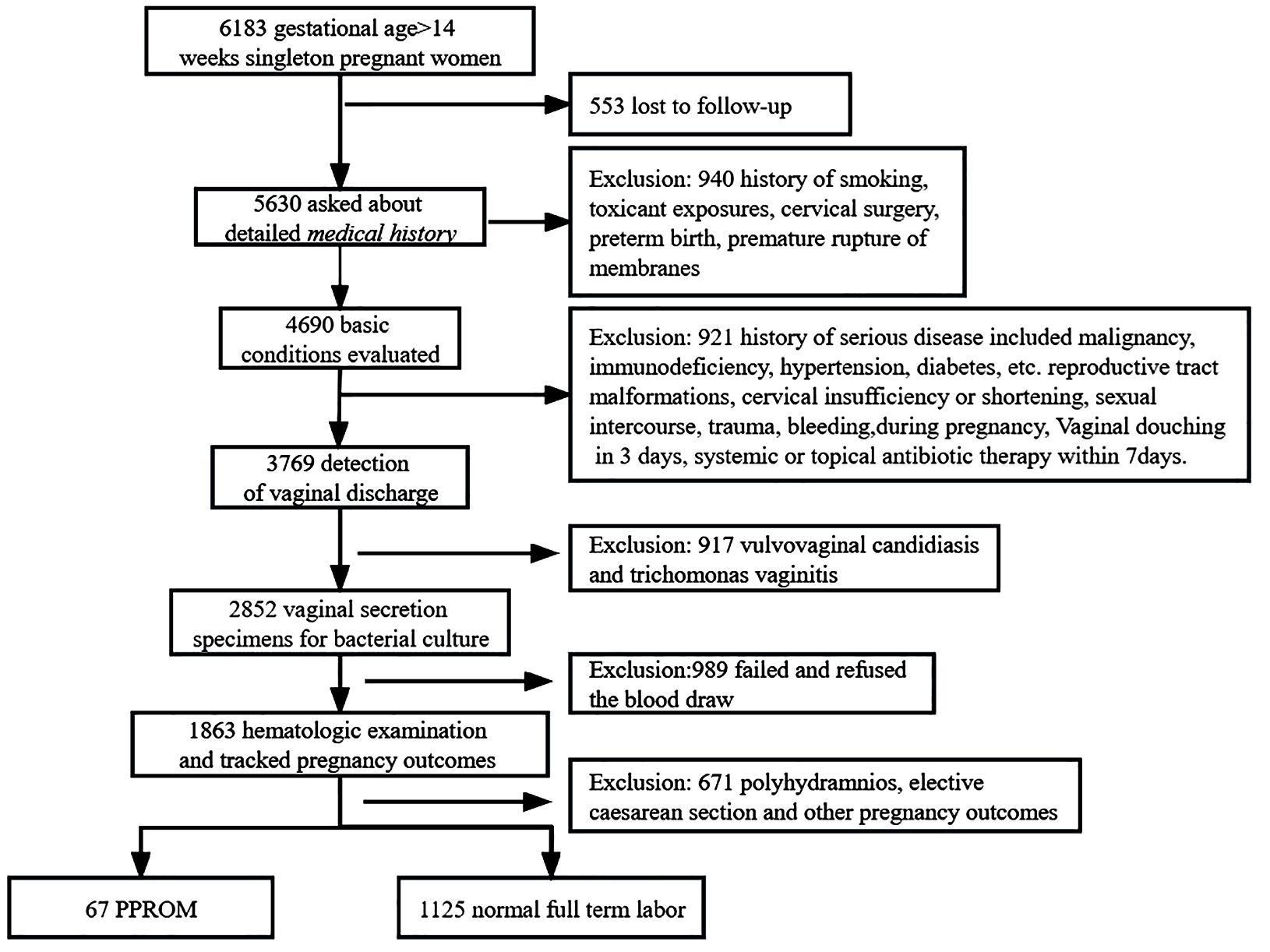
The exclusion criteria were as follows: (a) history of substance abuse, smoking, or toxic chemical exposure; contractions; preterm birth; cervical dilatation; or premature rupture of membranes; (b) severe medical diseases, such as hypertension, diabetes mellitus, malignant tumors, and abnormal immune function; (c) presence of specific pathogens, including fungal, trichomonad, viral, mycoplasma, and chlamydia, in the female reproductive system; (d) use of antibiotics within 7 days or inability to follow up, such as due to psychiatric illness; (e) vaginal bleeding, abdomen injury, and sexual intercourse during pregnancy. The inclusion and exclusion criteria for the study subject are detailed in the flowchart (Figure 1).
Vaginal discharge collection
We collected vaginal secretions during routine prenatal check-ups for pregnant women beyond 14 gestational weeks. The time interval between the collection of vaginal secretions and the gestational week of delivery ranges from 2 to 20 days. We collected vaginal discharge that was taken from the posterior fornix or vaginal wall, via three sterile nylon-flocked swabs (Mairuikelin Technology Co. Science and Technology Ltd., Shenzhen, China). All sterile nylon-flocked swabs were put into sterile tubes (Di RuiI Industrial Company, Changchun, China) immediately and sent to the Department of Laboratory Medicine, West China Second University Hospital, Sichuan University. One swab was used for automatic analysis, another was analyzed for microbiological tests, and the last one was stored at −80°C immediately for subsequent deoxyribonucleic (DNA) extraction.
Automatic analysis of vaginal discharge images
Automatic analysis of vaginal discharge images was performed using an automatic vaginal infections analyzer GMD-S600 (Di Rui Industrial Company, Changchun, China) with plane laminar liquid flow technology, high-speed camera technology, and artificial intelligent recognition technology. We upgraded a novel software analyzer of bacterial morphotype grading; calculated the average gray value, geometrical shape, and bacterial color (Ye et al., 2024); and input the characteristic value into an efficient support vector machine and back-propagation classifier to finish the recognition and classification. The new parameters and settings used in this study provided data on the long diameter of each bacterium, toxic leukocytes, and parabasal epitheliocytes (PBCs).
Microbiological tests
Vaginal discharge samples were inoculated within 2 h of sampling. The swabs were streaked aerobically on Columbia sheep blood agar (Mérieux Shanghai Science and Technology Development Limited Company, Shanghai, China) and MacConkey plates (Antu Bioengineering Institute Co., Ltd., Zhengzhou, China) for 24–48 h at 35°C. Anaerobic culture was performed on fastidious anaerobic agar plates (Kailin Trading Co., Ltd., Jiangmen, China) for 7 d days at 35°C.
One colony was picked from the total bacterial population after confirmation through Gram staining; then, the strain identification was performed using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Assays were repeated twice per strain; Escherichia coli ATCC 25922 was used as the quality control strain. Following the manufacturer instructions, the antimicrobial susceptibility testing (AST) of Enterobacteriaceae, Pseudomonas, and Acinetobacter isolates was determined using the Gram-negative susceptibility card AST-GN13/16; Staphylococcaceae and Enterococcus isolates were tested using the AST-GP67 (all bioMérieux); Streptococcus isolates were tested using the TDR STR-AST (Tian Diren Streptococcus antimicrobial susceptibility testing) detection kit (Tian Diren, China).
16s rRNA gene high-throughput sequencing
Pregnant women with VMD underwent 16S rRNA gene amplification and sequencing. The DNA was extracted from clinical vaginal swabs using the FastDNA® SPIN Kit for Soil (MP Biomedicals, USA). The 16S polymerase chain reaction amplification targeted the V3–V4 hypervariable region with Forward Primer (5′–3′): 341CCTACGGGNGGCWGCAG and Reverse Primer 805 (5′–3′): GACTACHVGGGTATCTAATCC (Chengdu Qingke Technology, Co., China). Libraries were multiplexed and sequenced on an Illumina MiSeq platform (MiSeq Reagent Kit v3, USA) at 2 × 250 bp. Finally, the sequencing data were bioinformatically analyzed. Microbial diversity was analyzed using usearch, Mothur, QIIME1, and QIIME2 and displayed with Mothur and R version 3.5.3 software (Aberhe et al., 2020; Trifi et al., 2020; Kizilirmak et al., 2021). The Linear discriminant analysis effect size analysis (LEfSe) is performed to determine the features that most likely to explain differences between groups. A |LDA|>2 and a P<0.05 are used for screening.
Data analysis
Statistical analyses and construction of statistical charts were performed using GraphPad Software version 8.0 (GraphPad Software Inc., CA, USA). The normal distribution of data is expressed as mean ± standard deviation, assessed using the t-test. However, non-normally distributed data are expressed as median (interquartile range) applied using the Mann–Whitney U test. Constituent ratios were assessed using the chi-square or Fisher’s exact probability tests.
Results
Basic information and clinical features
A total of 3,769 vaginal discharge samples from 4,690 pregnant women were included in this study. A total of 1,863 cases were tracked for pregnancy outcomes; 3.6% (67/1863) of cases displayed PPROM, and 60.39% (1,125/1,863) controls demonstrated normal full-term labor (TL). A total of 671 participants were excluded due to vaginal bleeding, trauma, sexual intercourse during pregnancy, polyhydramnios, abortion, preterm delivery, placental abruption, placenta praevia, term premature rupture of membranes, and elective cesarean.
The PPROM group aged 19–44, and the TL group aged 17–49. The two groups demonstrated non-significant differences in age (P > 0.05). Analysis of the prepregnancy body mass index (BMI) revealed that 56.72% (38/67) were overweight (BMI of 25–29.9) in the PPROM group, whereas 30.04% (338/1,125) were overweight in the TL group. The two groups did not differ statistically, in the rate of cesarean sections. The high incidence of clinical chorioamnionitis and neonatal asphyxia in the PPROM group weighed less than in the TL group (Table 1).
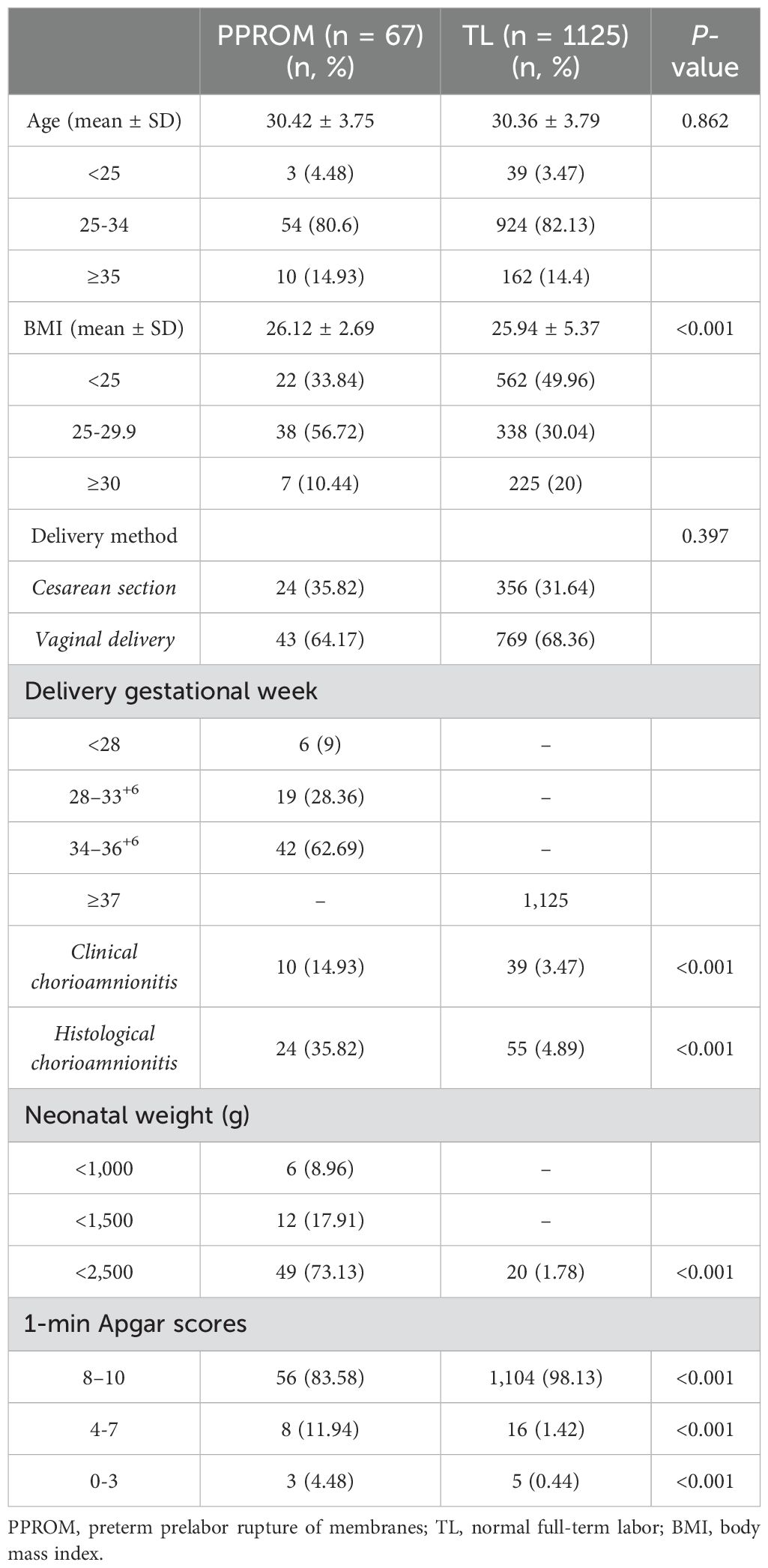
Table 1. Baseline demographics and clinical characteristics were compared between the PPROM group and the TL group.
Vaginal discharge examination
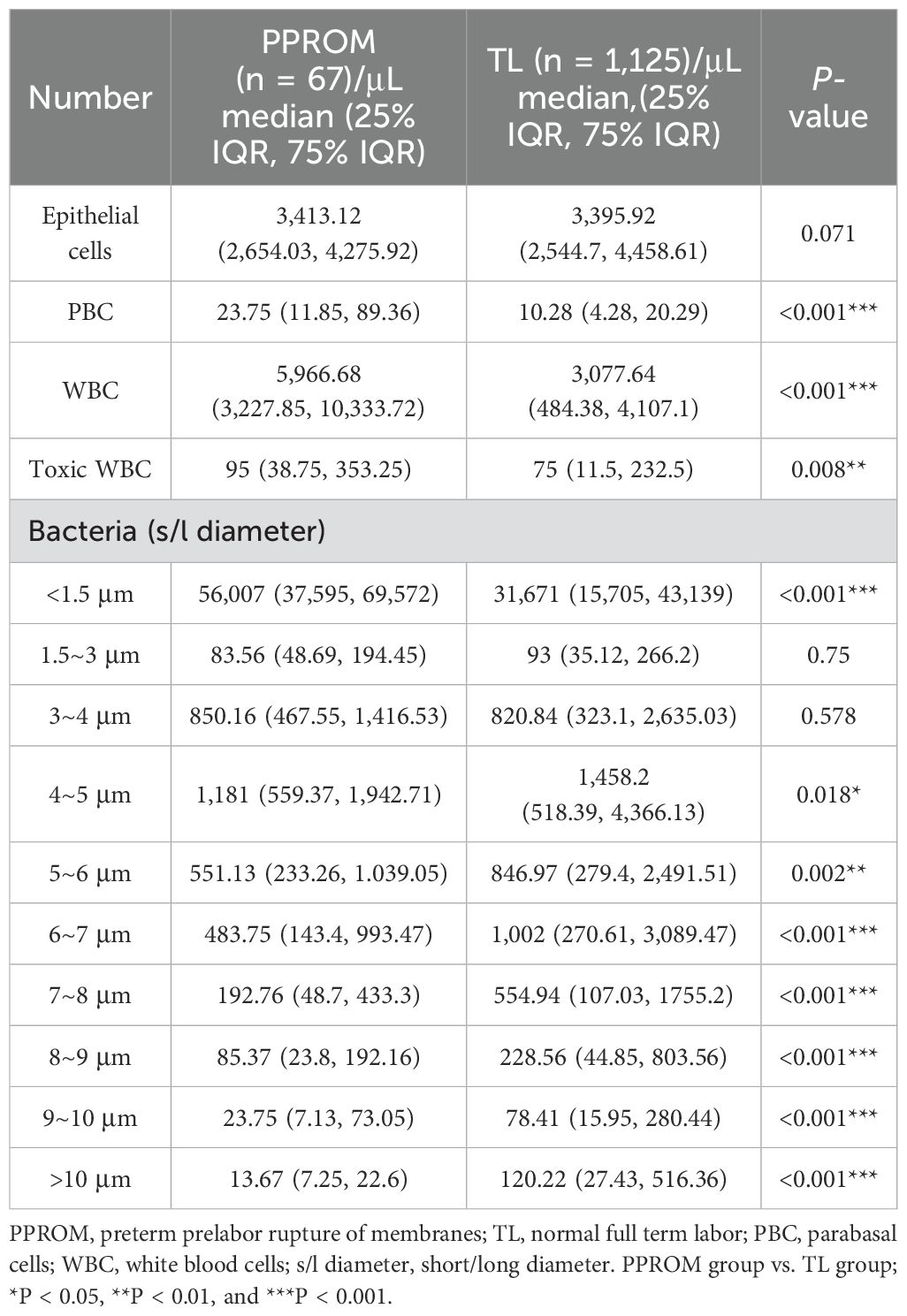
Vaginal discharge examination and delivery had a time interval between 2 and 20 days before PPROM in the PPROM group. In the TL group, one pregnant woman presented with vaginal flora disorders, whereas the prevalence was higher in the PPROM group, with five cases (χ2 = 6, P < 0.001). We found that PPROM has a significant positive correlation with vaginal flora disorders (Spearman correlation coefficient: 0.42, P < 0.001). The proportion of PBC, leukocytes, toxic leukocytes, and bacteria with diameters smaller than 1.5 μm was also significantly higher in the PPROM group than that in the TL group (Table 2).
Vaginal opportunistic bacterial isolates
A total of 102 vaginal opportunistic bacterial isolates were collected from 67 pregnant women with PPROM. Among them, Enterococcus faecalis was the most common bacteria, comprising 21.57% (22/102) of total isolates. This was followed in order, and the proportion of Escherichia coli and Lactobacillus iners was 16.67% (17/102). In contrast, A total of 2,000 vaginal bacterial isolates were collected from 1,125 pregnant women in the TL group. The most common vaginal bacterial isolates identified were Lactobacillus species., which included Lactobacillus crispatus (32.6%, 652/2000), Lactobacillus jensenii (20.8%, 199/2,000), and Lactobacillus iners (7.2%, 144/2,000). The proportion of Escherichia coli was 9.1% (182/2,000) (Figure 2).
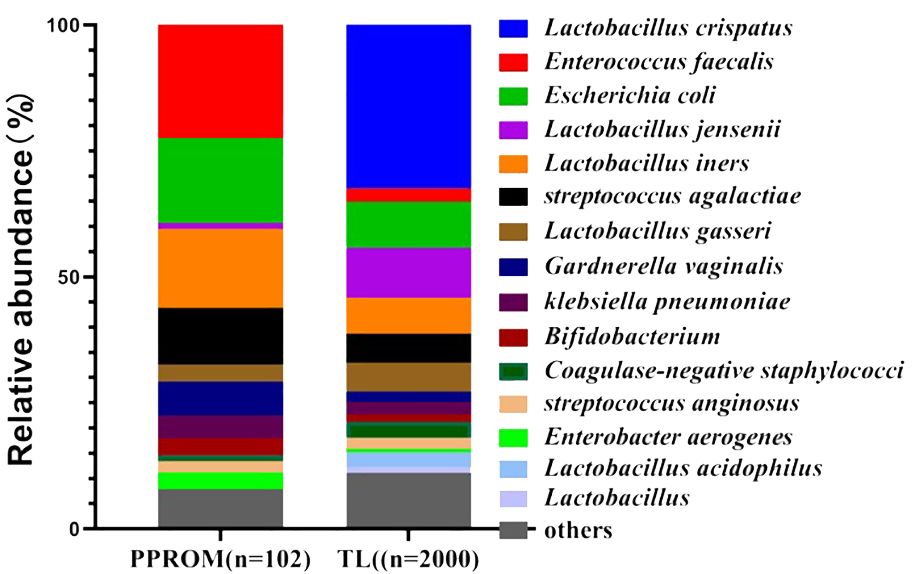
Figure 2. Vaginal microbiota based on the relative abundance of the PPROM group and TL group. PPROM, preterm prelabor rupture of membranes, TL, normal full term labor.
Drug sensitivity tests
The top three vaginal opportunistic bacterial isolates in all participants were Escherichia coli (9.47%, 199/2,102), Streptococcus agalactiae (5.99%, 126/2,102), and Enterococcus faecalis (3.57%, 75/2,102). Moreover, 16.08% (32/199) of Escherichia coli were extended-spectrum beta-lactamase producers, 36.51% (46/126) of Streptococcus agalactiae were multidrug-resistant organisms (MDROs), 20% (15/75) of Enterococcus faecalis were high-level streptomycin-resistant organisms, and 18.67% (14/75) of Enterococcus faecalis were high-level gentamicin-resistant organisms.
All isolates were sensitive to nitrofurantoin. In addition, Escherichia coli was highly susceptible to amikacin (100%), ertapenem (100%), imipenem (100%), cefepime (98.49%), piperacillin/tazobactam (97.99%), aztreonam (97.49%), cefotetan (96.98%), and ceftazidime (93.97%). However, Streptococcus agalactiae and Enterococcus faecalis display uniform high susceptibility to vancomycin (100%) and linezolid (100%). For Streptococcus agalactiae, no resistance to ampicillin, cefuroxime, ceftriaxone, cefotaxime, cefepime, meropenem, daptomycin, teicoplanin, cotrimoxazole, rifampin, penicillin, or amoxicillin-clavulanate was observed. Enterococcus faecalis isolates were susceptible to tigecycline (100%), ampicillin (100%), and penicillin (100%).
However, Escherichia coli was resistant to ampicillin (52.76%), ampicillin/sulbactam (62.31%), and levofloxacin (70.35%). Notably, 36.51% of the Streptococcus agalactiae were classified as MDROs, with the most common MDR pattern being co–non-susceptibility to erythromycin (29.37%), tetracycline (33.33%), and clindamycin (38.89%). Enterococcus faecalis was resistant to clindamycin (0%), quinupristin-dalfopristin (0%), erythromycin (4%), and tetracycline (13.33%). The antimicrobial susceptibilities of Escherichia coli were performed using the AST-GN card. These isolates were 100% susceptible to nitrofurantoin, whereas the lowest sensitivity rate was 52.76% for ampicillin. The antimicrobial susceptibilities of Streptococcus agalactiae were determined using the TDR STR-AST detection reagent. These isolates were 100% susceptible to vancomycin, whereas the lowest sensitivity rate was 52.76% for erythromycin. The antimicrobial susceptibilities of the Enterococcus faecalis were performed using the AST-GP card. These isolates were 100% susceptible to vancomycin, and the lowest sensitivity rate was 52.76% for ampicillin. However, all isolates were resistant to clindamycin (Figure 3; Table 3).
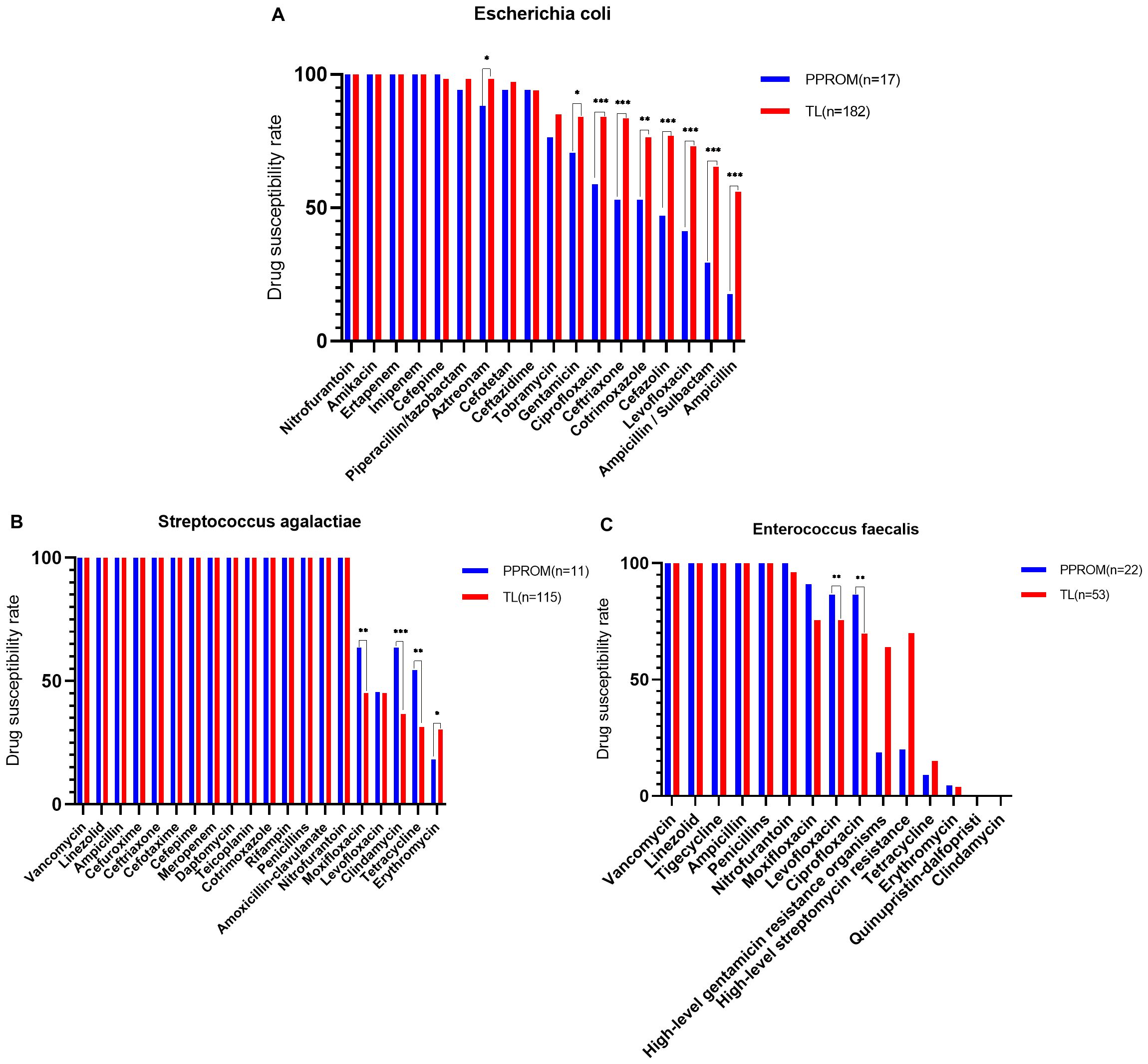
Figure 3. The top 3 vaginal opportunistic bacterial isolates tested for antimicrobial susceptibility of pregnant women. The rate of antimicrobial susceptibilities decreased from left to right: (A) Escherichia coli was susceptible to nitrofurantoin (100%) and the lowest sensitivity rate of 52.76% to ampicillin, (B) Streptococcus agalactiae was 100% sensitivity to vancomycin and the lowest sensitivity rate of 52.76% to Erythromycin, (C) Enterococcus faecalis is 100% sensitive to vancomycin and completely resistant to clindamycin.
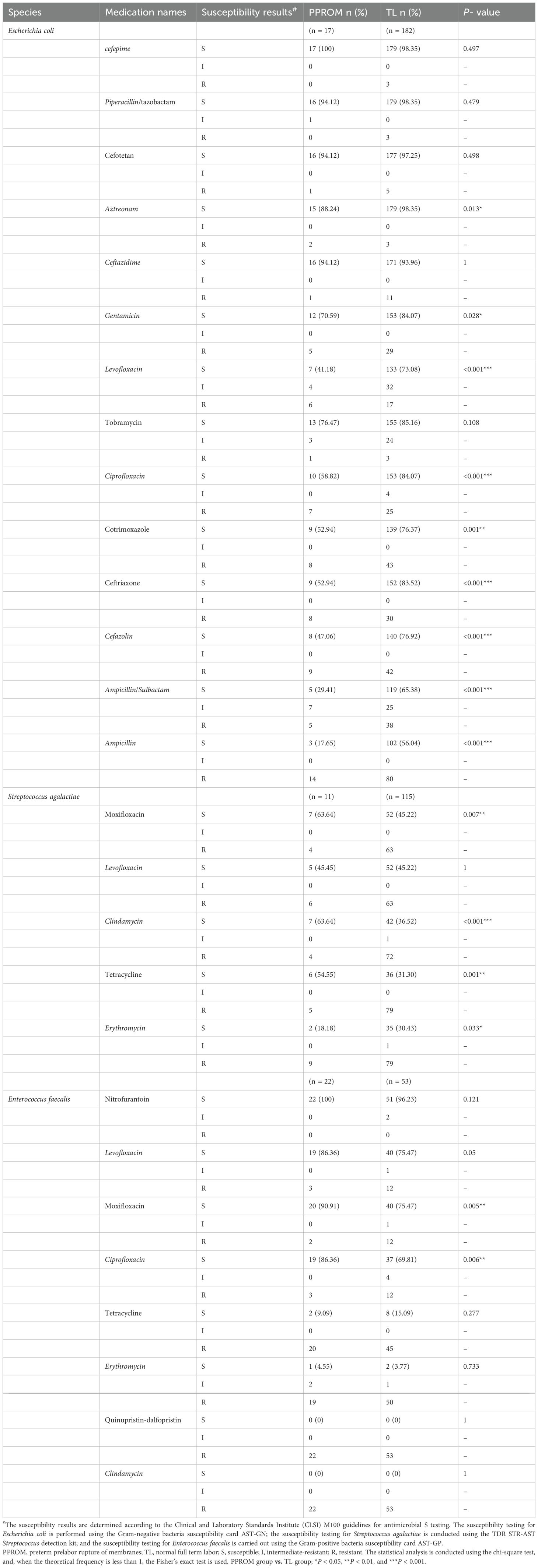
Table 3. The drug sensitivity tests of the top three vaginal opportunistic bacterial isolates in vaginal secretion samples from the PPROM and TL groups.
Bacterial profiles of vaginal specimens
A total of 80 VMD swab specimens underwent 16S rRNA gene amplification and sequencing. The 66 swab specimens of the pregnant women with VMD produced 4,898,416 reads, averaging 74,218 reads per sample. Unfortunately, 14 samples that did not satisfy the quality control criteria were excluded. A total of 4,436,059 high-quality sequences belonged to two groups, with a length distribution concentrated at 426–431, 408–413, and 420–425 bp. The flat Shannon–Wiener curve indicated that the sequencing data were sufficiently large to reflect most microbial information in these samples. A total of 2,884 operational taxonomic units (OTUs) were clustered into two groups. However, 1,986 (1,965~2,109) OTUs were detected in the VMD-PPROM (P) group, compared with 853 (799~909) OTUs in the VMD-TL (C) group. Venn analysis identified 241 common OTUs in the two groups, compared with 1,724 and 558 unique OTUs in the P and C groups, respectively.
The difference in vaginal flora alpha- and beta- diversity between the two groups was noticeable. Alpha- diversity from the directly observed OTUs revealed that the flora variety in the P group was greater than that in the C group. Alpha- diversity indices based on OTUs using Chao1 and abundance-based coverage estimates present a high richness of bacterial taxa in the P group, with the lowest values recorded for the C group. Beta-diversity index analysis revealed differences in vaginal microbiota across the groups through partial least squares discriminant analysis (Figure 4).
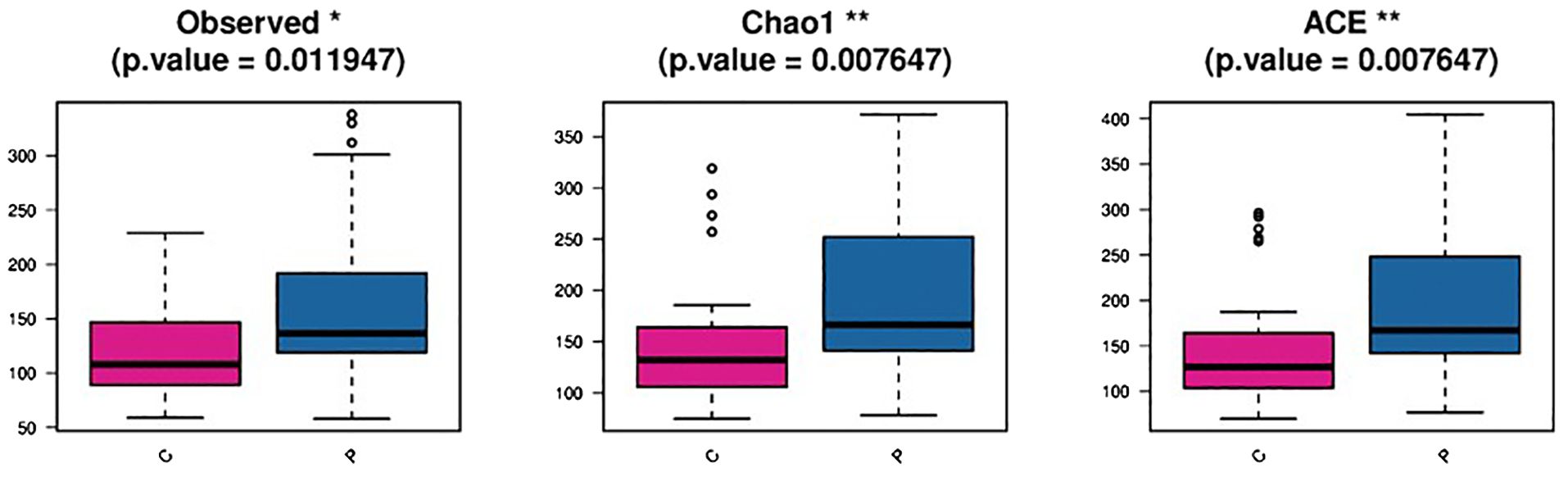
Figure 4. Diversity analysis among groups in the P and C group C: the vaginal microbiota dysbiosis of normal full term labor group, P: the vaginal microbiota dysbiosis of preterm prelabor rupture of membranes group.
Indicator species analysis revealed that Lactobacillus jensenii, Lactobacillus crispatus, and Veillonellaceae bacterium DNF00626 were strongly associated with the C group. In contrast, the indicator bacteria in the P group were Enterococcus faecalis, Escherichia coli, and Streptococcus agalactiae (Figure 5).
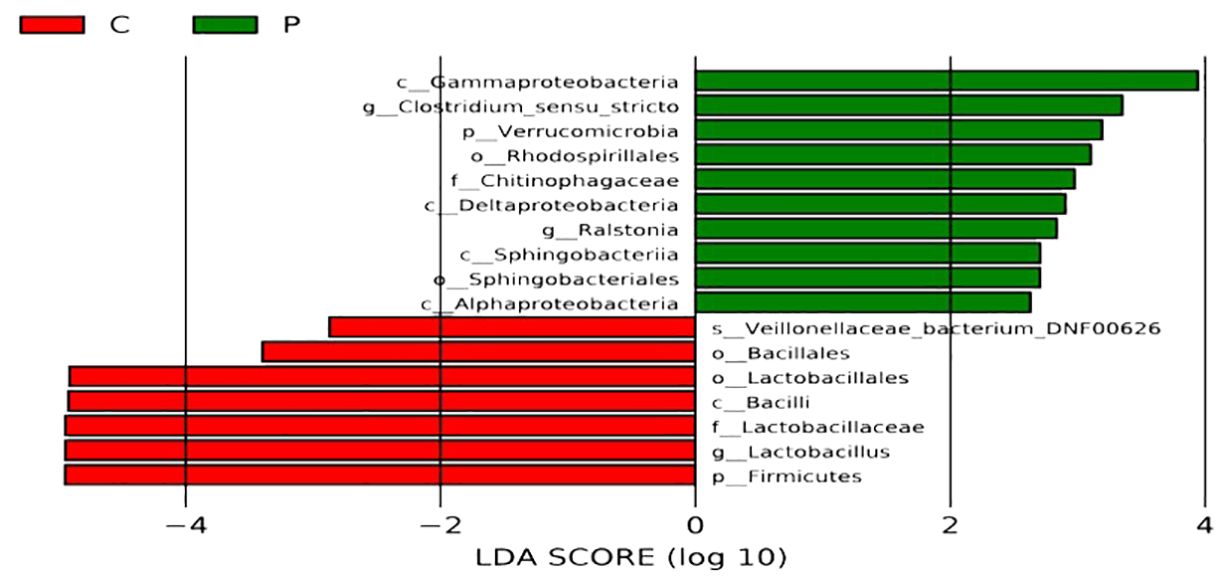
Figure 5. Analyses of differences for bacterial communities within groups. C: the vaginal microbiota dysbiosis of normal full term labor group, P: the vaginal microbiota dysbiosis of preterm prelabor rupture of membranes group. The Linear Discriminant Analysis Effect Size (LEfSe) method was used to select species that are most likely to explain the differences between groups. The non-parametric Kruskal-Wallis rank sum test was employed to screen for species with significantly different abundances between different groups; pairwise Wilcoxon rank sum tests were conducted for sub-group difference analysis; Linear Discriminant Analysis was used to assess the effect size of each significantly different species, that is, the LDA score log (10) with a larger absolute value indicating that the species is more likely to distinguish between groups. The bars of different colors represent the differential species in different groups, and the length of the bars is proportional to the size of the LDA score value.
Conclusion
Our study indicated that 3.6% of the women experience PPROM, which is the upper end of the range reported in China. This can be attributed to two reasons: (1) as our hospital is a tertiary medical center for severe illness, referral bias cannot be entirely excluded; and (2) the prevalence of PPROM is increasing annually owing to environmental pollution, social pressure, and nutritional problems. Notably, BMI has been implicated as a factor contributing to PPROM. Specifically, both underweight and overweight and overweight conditions increase the risk of PPROM (Lynch et al., 2020). Our results regarding prepregnancy BMI demonstrated that more overweight pregnant women but fewer obese participants experienced PPROM. In the next step, a multicenter prospective longitudinal cohort will be designed. Comparable rates of cesarean deliveries were found among women with PPROM and those in the TL group. In contrast to the TL group, the PPROM group displayed a higher rate of chorioamnionitis. In addition, the PPROM group demonstrated a significantly lower proportion of newborns who weighed over 2,500 grams, and lower 1-min Apgar scores. These results paralleled previous literature reports (Stranik et al., 2021; Mengistu et al., 2022).
An abnormal vaginal microbiome, particularly a reduction of Lactobacillus species, is a significant risk factor closely related to PPROM (Pan et al., 2020; Yang et al., 2023). This study results also paralleled findings reported in the literature (Klerk et al., 2022), although in different ways. In this study, PPROM pregnant women were not predominantly colonized with Group B Streptococcus, but with various bacteria in the genital tract. However, despite no biomarkers being identified in the study, differences in bacterial abundance were observed in the abnormal vaginal flora of PPROM pregnancies. The main isolates identified in the bacterial cultures included Enterococcus faecalis, Escherichia coli, and Streptococcus agalactiae. However, high-throughput sequencing of the 16sRNA gene revealed Enterococcus faecalis, Atopobium sp.S3PFAA1-4, Streptococcus anginosus, bifidobacterium longus, Streptococcus agalactiae, and Escherichia coli as the primary isolates. In the literature, considerable differences exist, and four factors may have influenced these results. The first reason is the varying factors, such as ethnicity and geography (Ma et al., 2018; Pan et al., 2020; Tarca et al., 2021). The second factor is the inconsistent timing of urine sample collection during pregnancy (Pan et al., 2020; Bonasoni et al., 2022; Spiliopoulos et al., 2022). The third reason is the differences in the control group. The literature reports differ from this result (Wu et al., 2022); our control group was the VMD of the TL group, and mixed and abnormal vaginal microbes, such as Gardnerella and Prevotella species, were observed in both groups. The fourth reason is that routine prophylactic antibiotics used during pregnancy may have affected the diagnosis of early-onset neonatal sepsis in mothers with PPROM. Although PPROM has been linked to VMD, the use of antibiotics remains controversial. Studies have demonstrated that more aggressive antibiotic treatment for BV during pregnancy, including the administration of antibiotics such as clindamycin before 22 weeks, should result in a lower incidence of PPROM (Mohr et al., 2019; Ambalpady et al., 2022). However, other studies have highlighted that the abnormal vaginal bacteria can persist from PPROM to early delivery despite antibiotic treatment, and meta-analyses suggest that antibiotics may not prevent PPROM (Sullivan and Soper, 2015; Lorthe et al., 2020). Moreover, studies have indicated that prophylactic use of erythromycin can lead to an aggravated imbalance of vaginal microbiota in PPROM (Kovo et al., 2021).
The American College of Obstetricians and Gynecologists guidelines recommend the use of ampicillin/amoxicillin combined with erythromycin in PPROM pregnant women to prevent verticality spread (Faksh et al., 2011). However, some research in China has revealed that Escherichia coli in isolated bacteria from PPROM pregnant women’s membranes demonstrated resistance to tetracycline, ampicillin, piperacillin, trimethoprim-sulfamethoxazole, and cefazolin. In addition, Enterococcus faecalis was resistant to erythromycin, tetracycline, rifampicin, and ciprofloxacin, whereas Streptococcus agalactiae exhibited a high resistance rate to clindamycin, erythromycin, and tetracycline. Cefotaxime or the combination of ceftazidime and azithromycin improves maternal and infant outcomes in pregnant women with premature rupture of membranes at term (Hume-Nixon et al., 2021). The drug sensitivity test of vaginal secretions isolated from pregnant women in our study indicated that Streptococcus agalactiae is highly sensitive to ampicillin. However, Escherichia coli has low sensitivity to ampicillin, whereas Streptococcus agalactiae and Enterococcus faecalis are generally resistant to erythromycin, paralleling domestic research results (Bekele et al., 2022; Carey et al., 2022; Haindongo et al., 2022; Jiao et al., 2022). Considering that studies have indicated erythromycin to be effective in preventing PPROM, this study found that most bacterial strains isolated from the vaginal secretions of pregnant women were highly sensitive to antibiotics such as furantoin. As furantoin is classified as Class B in the FDA’s drug pregnancy safety classification, it may be a reasonable choice for managing in PPROM.
Data availability statement
The data presented in the study are deposited in the the Sequence Read Archive repository, accession number PRJNA1136794. This data has been published.
Ethics statement
The studies involving humans were approved by the ethics committee of West China Second University Hospital of Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Software, Validation, Writing – original draft. XW: Formal analysis, Investigation, Supervision, Writing – review & editing. JH: Software, Supervision, Validation, Visualization, Writing – review & editing. YJ: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review & editing. FY: Conceptualization, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Supported by Sichuan Science and Technology Program 2023NSFSC1602, 2023YFS0223. This work was supported by Sichuan Science and Technology Program (2023NSFSC1602 and 2023YFS0223).
Acknowledgments
We would like to express our gratitude to the Science and Technology Department of Sichuan Province and the West China Second Hospital of Sichuan University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1471027/full#supplementary-material
References
Aberhe, W., Hailay, A., Zereabruk, K., Mebrahtom, G., Haile, T. (2020). Non-adherence to inhaled medications among adult asthmatic patients in Ethiopia: a systematic review and meta-analysis. Asthma Res. Pract. 6, 12. doi: 10.1186/s40733-020-00065-7
Ambalpady, P. A., Samantroy, S., Mishra, A., Panda, J., Pattnaik, D., Jena, P. (2022). Microbiome diversity in vaginal fluid and sensitivity patterns in preterm premature rupture of membrane cases. Cureus 14, e20999. doi: 10.7759/cureus.20999
Bekele, H., Debella, A., Getachew, T., Balis, B., Tamiru, D., Eyeberu, A., et al. (2022). Prevalence of group B streptococcus recto-vaginal colonization, vertical transmission, and antibiotic susceptibility among pregnant women in Ethiopia: A systematic review and meta-analysis. Front. Public Health 10, 851434. doi: 10.3389/fpubh.2022.851434
Bonasoni, M. P., Comitini, G., Pati, M., Russello, G., Vizzini, L., Bardaro, M., et al. (2022). Second trimester fetal loss due to citrobacter koseri infection: A rare cause of preterm premature rupture of membranes (PPROM). Diagnostics (Basel) 12, 159. doi: 10.3390/diagnostics12010159
Bouchghoul, H., Kayem, G., Schmitz, T., Benachi, A., Sentilhes, L., Dussaux, C., et al. (2019). Outpatient versus inpatient care for preterm premature rupture of membranes before 34 weeks of gestation. Sci. Rep. 9, 4280. doi: 10.1038/s41598-019-40585-8
Carey, A. M., Capik, S. F., Giebel, S., Nickodem, C., Piñeiro, J. M., Scott, H. M., et al. (2022). Prevalence and profiles of antibiotic resistance genes mph(a) and qnrb in extended-spectrum beta-lactamase (esbl)-producing escherichia coli isolated from dairy calf feces. Microorganisms 10, 411. doi: 10.3390/microorganisms10020411
Faksh, A., Wax, J. R., Lucas, F. L., Cartin, A., Pinette, M. G. (2011). Preterm premature rupture of membranes ≥ 32 weeks' gestation: impact of revised practice guidelines. Am. J. Obstet. Gynecol. 205, 340.e341–345. doi: 10.1016/j.ajog.2011.05.036
Haindongo, E. H., Funtua, B., Singu, B., Hedimbi, M., Kalemeera, F., Hamman, J., et al. (2022). Antimicrobial resistance among bacteria isolated from urinary tract infections in females in Namibia 2016-2017. Antimicrob. Resist. Infect. Control 11, 33. doi: 10.1186/s13756-022-01066-2
Hume-Nixon, M., Quach, A., Reyburn, R., Nguyen, C., Steer, A., Russell, F. (2021). A Systematic Review and meta-analysis of the effect of administration of azithromycin during pregnancy on perinatal and neonatal outcomes. EClinicalMedicine 40, 101123. doi: 10.1016/j.eclinm.2021.101123
Jiao, J., Wu, W., Shen, F., Liu, Z., Zhou, H., Fan, G., et al. (2022). Clinical profile and risk factors of group B streptococcal colonization in mothers from the eastern district of China. J. Trop. Med. 2022, 5236430. doi: 10.1155/2022/5236430
Juliana, N. C.A., S., M. J. M., Al-Nasiry, S., Morré, S. A., Petersn, R. P.H., Ambrosino, E. (2020). The association between vaginal microbiota dysbiosis, bacterial vaginosis, and aerobic vaginitis, and adverse pregnancy outcomes of women living in Sub-Saharan Africa: A systematic review. Front. Public Health 8, 567885. doi: 10.3389/fpubh.2020.567885
Kizilirmak, J. M., Fischer, L., Krause, J., Soch, J., Richter, A., Schott, B. H. (2021). Learning by insight-like sudden comprehension as a potential strategy to improve memory encoding in older adults. Front. Aging Neurosci. 13, 661346. doi: 10.3389/fnagi.2021.661346
Klerk, D. H., van Avezaath, L. K., Loeffen, E. A. H., Hulscher, J. B. F., Kooi, E. M. W. (2022). Fetal-neonatal exposure to antibiotics and NEC development: A systematic review and meta-analysis. Front. Pediatr.10, 1102884. doi: 10.3389/fped.2022.1102884
Kovo, M., Roitman, D., Mizrachi, Y., Gonen, N., Bar, J., Oron, A., et al. (2021). Effects of 'rescue' dose of antenatal corticosteroids on placental histopathology in preterm births. Placenta 107, 41–45. doi: 10.1016/j.placenta.2021.03.002
Kumar, M., Saadaoui, M., Al Khodor, S. (2022). Infections and pregnancy: effects on maternal and child health. Front. Cell Infect. Microbiol. 12, 873253. doi: 10.3389/fcimb.2022.873253
Lacroix, G., Gouyer, V., Rocher, M., Gottrand, F., Desseyn, J. L. (2022). A porous cervical mucus plug leads to preterm birth induced by experimental vaginal infection in mice. iScience 25, 104526. doi: 10.1016/j.isci.2022.104526
Lorthe, E., Moreira, C., Weber, T., Huusom, L. D., Schmidt, S., Maier, R. F., et al. (2020). Unit policies regarding tocolysis after preterm premature rupture of membranes: association with latency, neonatal and 2-year outcomes (EPICE cohort). Sci. Rep. 10, 9535. doi: 10.1038/s41598-020-65201-y
Lynch, T. A., Malshe, A., Colihan, S., Meyers, J., Li, D., Holloman, C., et al. (2020). Impact of maternal obesity on perinatal outcomes in preterm prelabor rupture of membranes ≥34 weeks. Am. J. Perinatol. 37, 467–474. doi: 10.1055/s-0039-1698833
Ma, B., McComb, E., Gajer, P., Yang, H., Humphrys, M., Okogbule-Wonodi, A. C., et al. (2018). Microbial biomarkers of intestinal barrier maturation in preterm infants. Front. Microbiol. 9, 2755. doi: 10.3389/fmicb.2018.02755
Ma, L., Zhang, Z., Li, L., Zhang, L., Lin, Z., Qin, H. (2022). Vitamin D deficiency increases the risk of bacterial vaginosis during pregnancy: Evidence from a meta-analysis based on observational studies. Front. Nutr. 9, 1016592. doi: 10.3389/fnut.2022.1016592
Mengistu, S., Debella, A., Mulatu, T., Mesfin, F., Danusa, K. T., Dheresa, M. (2022). Stillbirth and associated factors among women who gave birth at Hiwot Fana specialized university hospital, Harar, Eastern Ethiopia. Front. Pediatr. 10, 820308. doi: 10.3389/fped.2022.820308
Mohr, S., Amylidi-Mohr, S. K., Stadelmann, P., Sculean, A., Persson, R., Eick, S., et al. (2019). Systemic inflammation in pregnant women with periodontitis and preterm prelabor rupture of membranes: A prospective case-control study. Front. Immunol. 10, 2624. doi: 10.3389/fimmu.2019.02624
Pan, J., Tian, X., Huang, H., Zhong, N. (2020). Proteomic study of fetal membrane: inflammation-triggered proteolysis of extracellular matrix may present a pathogenic pathway for spontaneous preterm birth. Front. Physiol. 11, 800. doi: 10.3389/fphys.2020.00800
Pramanick, R., Nathani, N., Warke, H., Mayadeo, N., Aranha, C. (2021). Vaginal dysbiotic microbiome in women with no symptoms of genital infections. Front. Cell Infect. Microbiol. 11, 760459. doi: 10.3389/fcimb.2021.760459
Rebouças, K. F., E., J. J., Peixoto, R. C., Costa, A. P. F., Cobucci, R. N., Gonçalves, A. K. (2019). Treatment of bacterial vaginosis before 28 weeks of pregnancy to reduce the incidence of preterm labor. Int. J. Gynaecol. Obstet. 146, 271–276. doi: 10.1002/ijgo.12829
Siegler, Y., Weiner, Z., Solt, I. (2020). ACOG practice bulletin no. 217: prelabor rupture of membranes. Obstet. Gynecol. 136, 1061. doi: 10.1097/AOG.0000000000003700
Spiliopoulos, M., Haddad, A., Al-Kouatly, H. B., Haleema, S., Paidas, M. J., Iqbal, S. N., et al. (2022). MicroRNA analysis in maternal blood of pregnancies with preterm premature rupture of membranes reveals a distinct expression profile. PloS One 17, e0277098. doi: 10.1371/journal.pone.0277098
Stranik, J., Kacerovsky, M., Soucek, O., Kolackova, M., Musilova, I., Pliskova, L., et al. (2021). IgGFc-binding protein in pregnancies complicated by spontaneous preterm delivery: a retrospective cohort study. Sci. Rep. 11, 6107. doi: 10.1038/s41598-021-85473-2
Sullivan, S. A., Soper, D. (2015). Antibiotic prophylaxis in obstetrics. Am. J. Obstet. Gynecol. 212, 559–560. doi: 10.1016/j.ajog.2015.01.039
Tarca, A. L., Pataki, B., Romero, R., Sirota, M., Guan, Y., Kutum, R., et al. (2021). Crowdsourcing assessment of maternal blood multi-omics for predicting gestational age and preterm birth. Cell Rep. Med. 2, 100323. doi: 10.1016/j.xcrm.2021.100323
Tian, M., Li, Q., Zheng, T., Yang, S., Chen, F., Guan, W., et al. (2023). Maternal microbe-specific modulation of the offspring microbiome and development during pregnancy and lactation. Gut Microbes 15, 2206505. doi: 10.1080/19490976.2023.2206505
Trifi, H., Najjari, A., Achouak, W., Barakat, M., Ghedira, K., Mrad, F., et al. (2020). Metataxonomics of Tunisian phosphogypsum based on five bioinformatics pipelines: Insights for bioremediation. Genomics 112, 981–989. doi: 10.1016/j.ygeno.2019.06.014
Wu, L., Liu, Y., Liu, Z., Chen, H., Shen, S., Wei, Y., et al. (2022). Serum urea acid and urea nitrogen levels are risk factors for maternal and fetal outcomes of pregnancy: a retrospective cohort study. Reprod. Health 19, 192. doi: 10.1186/s12978-022-01496-6
Yang, Y., Liu, Z., Chen, X., Qin, F., Zhou, W. (2023). Association between vaginal microbiomes and neonatal septicemia in pregnant women with preterm premature rupture of membranes based on metagenome sequencing. Am. J. Transl. Res. 15, 4544–4557.
Yang, S. T., Wang, P. H. (2023). Preterm premature rupture of membranes (PPROM) and secondary intra-amniotic infection/inflammation. Taiwan J. Obstet. Gynecol. 62, 187–188. doi: 10.1016/j.tjog.2022.09.007
Keywords: vaginal opportunistic pathogens, PPROM, automatic analysis, microbiological tests, 16S rDNA sequencing technique
Citation: Tang Y, Wang X, Huang J, Jiang Y and Yu F (2024) The microbiome biomarkers of pregnant women’s vaginal area predict preterm prelabor rupture in Western China. Front. Cell. Infect. Microbiol. 14:1471027. doi: 10.3389/fcimb.2024.1471027
Received: 26 July 2024; Accepted: 07 October 2024;
Published: 31 October 2024.
Edited by:
Lifeng Zhu, Nanjing University of Chinese Medicine, ChinaReviewed by:
Megan L. Falsetta, University of Rochester, United StatesNoble K. Kurian, B. S. Abdur Rahman Crescent Institute Of Science And Technology, India
Copyright © 2024 Tang, Wang, Huang, Jiang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongmei Jiang, Smlhbmd5b25nbWVpNjhAeWVhaC5uZXQ=; Fan Yu, eXVmYW4xOTc4QHllYWgubmV0
†These authors have contributed equally to this work
 Yuanting Tang
Yuanting Tang Xia Wang
Xia Wang Jialing Huang
Jialing Huang Yongmei Jiang
Yongmei Jiang Fan Yu
Fan Yu