- 1Department of Biochemistry, Central University of Punjab, Bathinda, Punjab, India
- 2Department of Dermatology, Mahatma Gandhi Medical Services Complex, Shimla, Himachal Pradesh, India
- 3Department of Dermatology, Indira Gandhi Medical College and Hospital, Shimla, Himachal Pradesh, India
- 4Department of Microbiology and Immunology, McGill University, Montreal, QC, Canada
Visceral leishmaniasis (VL) caused by L. donovani in South-East Asian endemic countries including India, Nepal and Bangladesh has been the primary focus of the ongoing VL elimination program. With a major reduction in VL cases resulting from the elimination program during the last two decades, the efforts are now focused on the challenges posed by potential reservoirs within the asymptomatic cases, HIV-co-infection VL cases and Post Kala-azar Dermal Leishmaniasis (PKDL) cases that continue to sustain the parasite transmission cycle in known and newer endemic zones. This article brings attention to a new potential parasite reservoir in the form of atypical cutaneous leishmaniasis (ACL) cases caused by novel L. donovani genetic variants. L. donovani mediated ACL is an emerging phenomenon in recent endemic sites that now justify a need for implementing molecular surveillance tools to identify region-specific L. donovani variants with dermotropic capabilities and potential to revert to visceral disease. A timely detection of novel ACL causing L. donovani genetic lineages in South-East Asian endemic regions is necessary to halt the spread of ACL and is potentially crucial for the sustainability of the advances made by the VL elimination.
Introduction
Leishmaniasis is among the most neglected infectious tropical diseases and is caused by an intracellular protozoan parasite belonging to the genus Leishmania. More than 20 Leishmania species are prevalent in different parts of Africa, Central-South America, Mediterranean regions, Southern Europe and South-East Asia. Leishmania species-specific disease manifestation range from fatal systemic visceral leishmaniasis (VL) also known as Kala-Azar caused by the L. donovani/L. infantum complex, cutaneous leishmaniasis (CL) caused by L. tropica/L. major species in the Old World and mucocutaneous leishmaniasis (MCL) and CL caused by the L. Viannia subgenus in the New World (Alvar et al., 2012; World Health Organization, 2023). Due to the fatal nature of VL caused by L. donovani with a significant global burden, operational efforts are ongoing toward VL disease control and elimination. In 2005, with a share of ~70% in the global VL burden in South-East Asia, a disease elimination initiative was formulated by the WHO for the elimination of VL by 2015 in India, Bangladesh and Nepal with the objective to reduce the number of cases to less than one per 10,000 population in all endemic districts (World Health Organization, 2015). Elimination efforts included improved case detection, case management including rapid diagnosis and treatment, vector control and community education that have resulted in a dramatic reduction in VL cases in South-East Asia to less than 20% of the global burden (World Health Organization, 2022). Notwithstanding this progress, challenges remain including the emergence of novel L. donovani lineages in new areas acting as potential reservoirs for future outbreaks that could threaten the sustainability of the VL elimination program as discussed within.
VL elimination targets and the challenges ahead
The VL elimination target in South-East Asia countries is defined as an annual incidence of less than one case per 10,000 population in endemic regions. On this scale, 99% of implementation units are reported to achieve the elimination threshold (Yajima et al., 2023). Among the VL endemic countries in the region, India reports a dramatic decrease in VL cases from 9241 in 2014 to 26 cases in 2024 (National Center for Vector Borne Diseases Control, 2024). Nepal reached the elimination target in 2013 although the emergence of several new VL cases in previously non-endemic low population districts breached the one case per 10,000 threshold (Epidemiology and Disease Control Division, 2019; Shrestha et al., 2019; Banjara and Joshi, 2020; Pandey et al., 2023). Bangladesh reached and sustained the elimination target for 3 years in all endemic districts in 2023 and has now entered the maintenance phase (World Health Organization, 2024). India is close to reaching the threshold. Bhutan and Thailand document sporadic cases in different districts and continue to accelerate case detection surveillance programs for disease elimination (Yangzom et al., 2012; Leelayoova et al., 2017; World Health Organization, 2021a; Dorji et al., 2024). Reaching the last remaining elimination target in South-East Asia by 2026 is supported by a regional strategic framework in alignment with the new neglected diseases elimination roadmap, 2021-2030 by the WHO (World Health Organization, 2021a, 2022).
Challenges to the VL elimination program remain and include the emergence of new endemic sites, the continued presence of Post Kala-azar Dermal Leishmaniasis (PKDL) and asymptomatic cases as potential reservoirs, the emergence of HIV-co-infection cases and more recently atypical cutaneous leishmaniasis (ACL) cases as depicted in Figure 1 (Manomat et al., 2017; World Health Organization, 2021a, 2022; Singh and Sundar, 2022; Ruang-Areerate et al., 2023; National Center for Vector Borne Diseases Control, 2024). Consequently, VL elimination programs have recognized the need for mandatory reporting of HIV-VL and PKDL cases and a need to develop strategies to identify asymptomatic cases for parasite detection in low disease burden settings (World Health Organization, 2021b, 2022; Singh and Sundar, 2022; Kumar et al., 2023; Ruang-Areerate et al., 2023). The availability of a L. donovani antigen leishmanin skin test (LST) to identify people previously infected will be important to support surveillance of transmission caused by asymptomatic and PKDL cases (Dey et al., 2023).
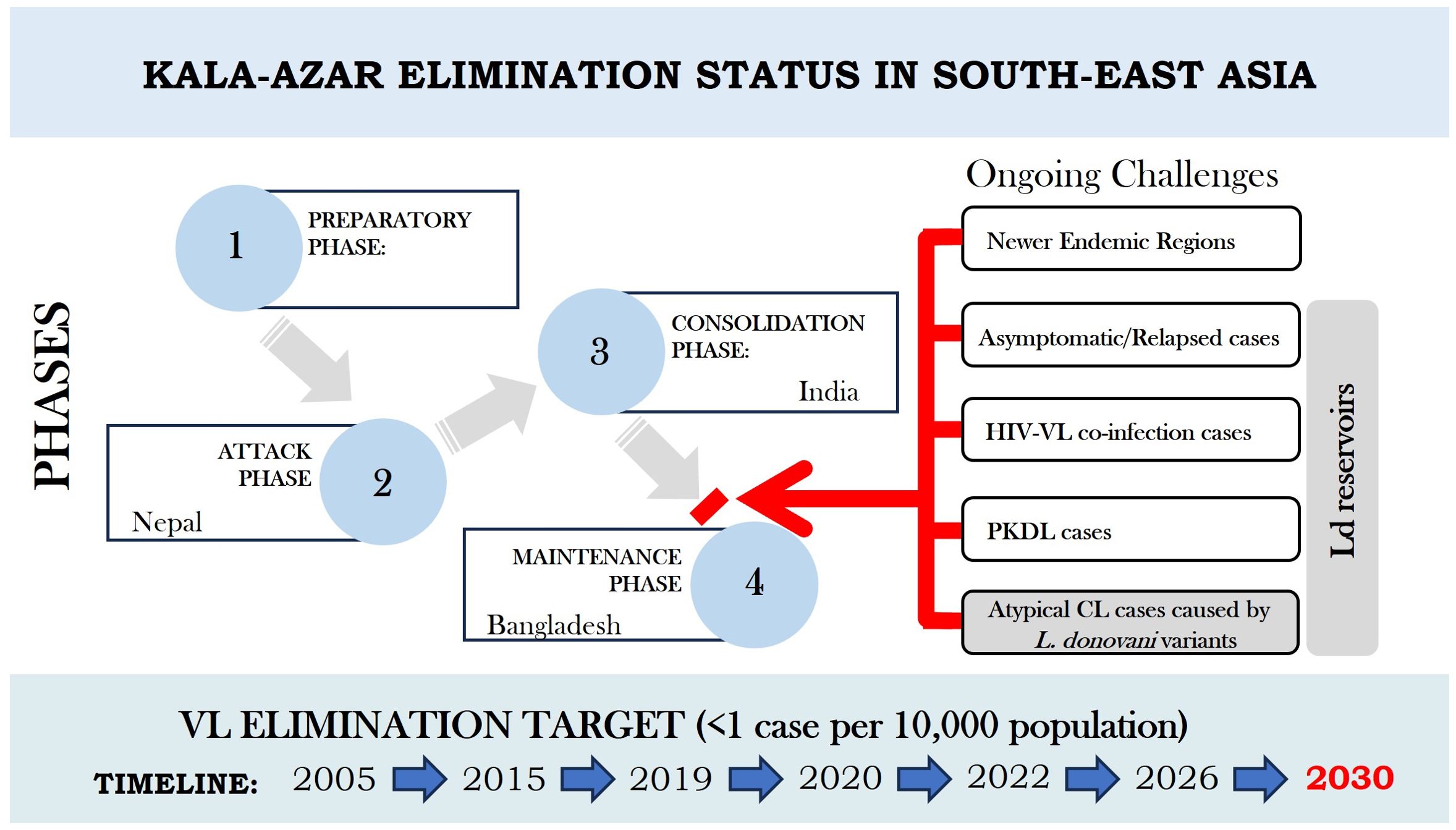
Figure 1. Current Scenario of Kala-azar Elimination programme in the Indian Subcontinent with extended timelines. Country specific phases achieved as per elimination targets are shown in terms of different phases viz preparatory phase, attack phase, consolidation phase and maintenance phase. Ongoing challenges in the way of India to move from consolidation phase to maintenance phase are indicated by red arrow and bar. The challenges also imply to Nepal to move further to achieve elimination target and to Bangladesh to maintain status of VL elimination through an integrated operational approach.
Despite the reduction in VL cases approaching the elimination target, VL cases from newer pockets in previously non-endemic and endemic regions continue to be documented in India, Nepal, and Bhutan (Hirve et al., 2017; Banjara and Joshi, 2020; Tobgay et al., 2021; Singh and Sundar, 2022). It is important to highlight that reaching the elimination target does not mean the disease is gone; it means VL is no longer a major public health problem. Even in districts that have reached the elimination threshold, the L. donovani parasite can still persist and the possibility of an outbreak remains if surveillance is not maintained. Further, the emerging challenge of Atypical Cutaneous Leishmaniasis (ACL) caused by L. donovani is on an increase in Sri Lanka, India and Nepal.
Atypical cutaneous leishmaniasis in South East Asis: An emerging threat
The clinical manifestations of leishmaniasis were historically largely determined by the Leishmania species with L. donovani/L. infantum typically causing VL and L. major/L. tropica causing CL. This association between species and clinical presentation is however undergoing a paradigm shift in some regions where L. donovani is increasingly associated with CL, also known as Atypical Cutaneous Leishmaniasis (ACL). This trend is especially present in newer endemic sites. The characteristic features of ACL caused by L. donovani vs typical cutaneous leishmaniasis (CL) caused by L. tropica complex are largely indistinguishable. The ACL specific lesions mostly appeared as localized cutaneous skin lesions with characteristic CL lesion-specific raised borders, serous crusting and ulceration along with epidermal changes exhibiting acanthosis, papillomatosis and granulomatous inflammation with no one feature that can differentiate them. ACL/CL lesions are distinguished from PKDL that typically exhibit macular and papular rashes over different parts of the body. With this scenario, a diagnostic test that can molecularly differentiate disease specific causative parasite species/strain is required.
Molecular identification of the parasite species using species-specific PCR/PCR-RFLP initially identified genetically divergent and region-specific L. donovani ACL isolates from Sri Lanka, India, Nepal and Bhutan that were distinct from the VL-causing Mon-2 L. donovani zymodeme from Bihar India, Nepal and Bangladesh (Ranasinghe et al., 2013; Zhang et al., 2014; Kumar et al., 2015; Siriwardana et al., 2019; Bastola et al., 2020; Pal et al., 2020; Thakur et al., 2020; Tharakan et al., 2020; Rai et al., 2023). It is important to recognize that ACL represents a new L. donovani reservoir and that this reservoir may have the potential to evolve or revert into VL causing variants fuelling future VL outbreaks and threaten the VL elimination program advances.
Comparative genome-wide analysis of L. donovani isolates from VL and ACL cases from known and newer endemic sites can demonstrate the emergence of region-specific parasite genotypes, their origin and genetic relatedness. In this context, whole genome sequencing (WGS) has helped to decipher the genetic makeup of isolates from the Indian Subcontinent (ISC) as a heterogeneous L. donovani population with discrete genetic lineages circulating in endemic lowlands of Nepal and Bihar India (identified as the core group, CG) and a rare and emerging ISC1 lineage in the highlands of Nepal (Imamura et al., 2016; Cuypers et al., 2018; Seblova et al., 2019). More recently, isolates from VL endemic sites in western Nepal have been genetically identified as L. donovani sub-lineages also within the ISC1 clade (Monsieurs et al., 2024). In a neighbouring state of India, Himachal Pradesh, WGS of three L. donovani ACL isolates exhibit genetic heterogeneity among themselves and originate from the ISC1 clade with evidence for intraspecies hybrids and non-hybrid lineages (Lypaczewski et al., 2022, 2024). In Sri Lanka, L. donovani variants from ACL cases are distinct from the ISC1 clade, but are in some cases remarkably derived from interspecies hybridization between Ethiopian L. donovani strains with the CL causing species L. major and L. tropica (Lypaczewski and Matlashewski, 2021). Considering the emergence of these novel L. donovani variants such as in the ISC1 cluster in India and Nepal and the distinct Sri Lanka clusters, an evolving L. donovani genome is supporting the emergence of ACL endemic zones and expansion of newer mountainous niches for VL. This phenomenon underscores the urgent need to recognize the evolution of L. donovani as a new challenge to the VL elimination program and to public health in the affected areas.
Discussion
The impact of the VL elimination program has been impressive with Bangladesh reaching the elimination target and the number of cases in India and Nepal dramatically reduced. Surveillance through active and passive case detection, improved case management with rapid diagnosis and effective treatments and vector control have all contributed to this success. Concomitant with this success, the occurrence of multiple clinical entities with skin manifestations associated with atypical CL caused by novel L. donovani variants and PKDL caused by yet different L. donovani strains can be misdiagnosed as typical CL caused by L. tropica complex. In lieu of the new outbreak areas with these disease formats, public health authorities should increase surveillance in these regions and should establish molecular diagnostics strategies to differentiate parasite species/strains associated with each type of local pathology. The evolution of novel L. donovani lineages in new locations including North East India, Nepalese highlands, Bhutan and Sri Lanka that cause ACL and VL is now becoming more apparent. These novel L. donovani lineages, such as the ISC1 lineage in newer endemic pockets in Himachal and the highlands of Nepal could eventually undermine the advances made by the VL elimination program.
A molecular surveillance strategy is necessary to follow the movement and expansion of these and other region-specific L. donovani lineages. Complete genome sequencing data could provide the necessary insights into parasite transmission patterns in newer geographical niches in relation to the disease phenotype. Knowledge gained will address important questions such as identifying the principal reservoir(s) for transmission and whether ACL associated L. donovani lineages can potentially visceralize to cause asymptomatic, VL or PKDL cases. The genome sequence surveillance data could be coupled with the re-introduction of the leishmanin skin test (LST) to determine the extend of new and previous transmission (Dey et al., 2023).This information could justify the initiation of targeted ACL elimination programs to stop the migration of this disease into more densely susceptible populations. Much has been learned from the successes of the VL elimination program and this now needs to be leveraged toward ensuring there are no major disease outbreaks of novel L. donovani lineages.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
MJ: Conceptualization, Supervision, Visualization, Writing – original draft. DS: Visualization, Writing – review & editing. LP: Writing – review & editing. RN: Project administration, Writing – review & editing. AN: Project administration, Writing – review & editing. GM: Project administration, Writing – review & editing. PL: Writing – review & editing, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Indian Council of Medical Research, ICMR [6/9-7(272/KA/2021/ECD-II] and Canadian Institutes of Health Research, [CIHR 192246].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alvar, J., Velez, I. D., Bern, C., Herrero, M., Desjeux, P., Cano, J., et al. (2012). Leishmaniasis worldwide and global estimates of its incidence. PloS One 7, e35671. doi: 10.1371/journal.pone.0035671
Banjara, M. R., Joshi, A. B. (2020). Evidence for visceral leishmaniasis elimination in Nepal. Lancet Glob Health 8, e161–e162. doi: 10.1016/S2214-109X(19)30538-8
Bastola, A., Shrestha, M., Lamsal, M., Shrestha, S., Prajapati, S., Adhikari, A., et al. (2020). A case of high altitude cutaneous leishmaniasis in a non-endemic region in Nepal. Parasitol. Int. 74, 101991. doi: 10.1016/j.parint.2019.101991
Cuypers, B., Berg, M., Imamura, H., Dumetz, F., De Muylder, G., Domagalska, M. A., et al. (2018). Integrated genomic and metabolomic profiling of ISC1, an emerging leishmania donovani population in the Indian subcontinent. Infect. Genet. Evol. 62, 170–178. doi: 10.1016/j.meegid.2018.04.021
Dey, R., Alshaweesh, J., Singh, K. P., Lypaczewski, P., Karmakar, S., Klenow, L., et al. (2023). Production of leishmanin skin test antigen from leishmania donovani for future reintroduction in the field. Nat. Commun. 14, 7028. doi: 10.1038/s41467-023-42732-2
Dorji, T., Dorjee, S., Wangdi, T., Tshokey, T., Pradhan, A. R., Penjor, K., et al. (2024). Efforts toward the elimination of visceral leishmaniasis in South Asia: A review of progress in Bhutan. Am. J. Trop. Med. Hyg 110, 633–638. doi: 10.4269/ajtmh.23-0642
Epidemiology and Disease Control Division. (2019). National Guideline on Kala-azar Elimination Program (Nepal: Department of Health Services).
Hirve, S., Kroeger, A., Matlashewski, G., Mondal, D., Banjara, M. R., Das, P., et al. (2017). Towards elimination of visceral leishmaniasis in the Indian subcontinent-translating research to practice to public health. PloS Negl. Trop. Dis. 11, e0005889. doi: 10.1371/journal.pntd.0005889
Imamura, H., Downing, T., Van Den Broeck, F., Sanders, M. J., Rijal, S., Sundar, S., et al. (2016). Evolutionary genomics of epidemic visceral leishmaniasis in the Indian subcontinent. Elife 5, e12613. doi: 10.7554/eLife.12613.031
Kumar, A., Singh, V. K., Tiwari, R., Madhukar, P., Rajneesh, Kumar, S., et al. (2023). Post kala-azar dermal leishmaniasis in the Indian sub-continent: Challenges and strategies for elimination. Front. Immunol. 14, 1236952. doi: 10.3389/fimmu.2023.1236952
Kumar, N. P., Srinivasan, R., Anish, T. S., Nandakumar, G., Jambulingam, P. (2015). Cutaneous leishmaniasis caused by leishmania donovani in the tribal population of the agasthyamala biosphere reserve forest, Western Ghats, Kerala, India. J. Med. Microbiol. 64, 157–163. doi: 10.1099/jmm.0.076695-0
Leelayoova, S., Siripattanapipong, S., Manomat, J., Piyaraj, P., Tan-Ariya, P., Bualert, L., et al. (2017). Leishmaniasis in Thailand: A review of causative agents and situations. Am. J. Trop. Med. Hyg 96, 534–542. doi: 10.4269/ajtmh.16-0604
Lypaczewski, P., Chauhan, Y., Paulini, K., Thakur, L., Chauhan, S., Roy, E. I., et al. (2024). Emerging leishmania donovani lineages associated with cutaneous leishmaniasis, himachal Pradesh, India 2023. Emerg. Infect. Dis. 30, 1957–1959. doi: 10.3201/eid3009.231595
Lypaczewski, P., Matlashewski, G. (2021). Leishmania donovani hybridisation and introgression in nature: A comparative genomic investigation. Lancet Microbe 2, e250–e258. doi: 10.1016/S2666-5247(21)00028-8
Lypaczewski, P., Thakur, L., Jain, A., Kumari, S., Paulini, K., Matlashewski, G., et al. (2022). An intraspecies leishmania donovani hybrid from the Indian subcontinent is associated with an atypical phenotype of cutaneous disease. iScience 25, 103802. doi: 10.1016/j.isci.2022.103802
Manomat, J., Leelayoova, S., Bualert, L., Tan-Ariya, P., Siripattanapipong, S., Mungthin, M., et al. (2017). Prevalence and risk factors associated with leishmania infection in Trang Province, Southern Thailand. PloS Negl. Trop. Dis. 11, e0006095. doi: 10.1371/journal.pntd.0006095
Monsieurs, P., Cloots, K., Uranw, S., Banjara, M. R., Ghimire, P., Burza, S., et al. (2024). Source tracing of leishmania donovani in emerging foci of visceral leishmaniasis, Western Nepal. Emerg. Infect. Dis. 30, 611–613. doi: 10.3201/eid3003.231160
National Center for Vector Borne Diseases Control. (2024). Kala-azar Situation in India. Available online at: https://ncvbdc.mohfw.gov.in/index1.php?lang=1&level=2&sublinkid=5945&lid=3750. (accessed September 8, 2024).
Pal, A., Saha, A., Chatterjee, S., Saha, S. (2020). A case of mutilating localized cutaneous leishmaniasis caused by leishmania donovani from Bhutan. Indian J. Dermatol. Venereol Leprol 86, 536–539. doi: 10.4103/ijdvl.IJDVL_801_19
Pandey, K., Dumre, S. P., Shah, Y., Acharya, B. K., Khanal, L., Pyakurel, U. R., et al. (2023). Forty years, (1980-2019) of visceral leishmaniasis in Nepal: Trends and elimination challenges. Trans. R Soc. Trop. Med. Hyg 117, 460–469. doi: 10.1093/trstmh/trad001
Rai, T., Shrestha, S., Prajapati, S., Bastola, A., Parajuli, N., Ghimire, P. G., et al. (2023). Leishmania donovani persistence and circulation causing cutaneous leishmaniasis in unusual-foci of Nepal. Sci. Rep. 13, 12329. doi: 10.1038/s41598-023-37458-6
Ranasinghe, S., Wickremasinghe, R., Munasinghe, A., Hulangamuwa, S., Sivanantharajah, S., Seneviratne, K., et al. (2013). Cross-sectional study to assess risk factors for leishmaniasis in an endemic region in Sri Lanka. Am. J. Trop. Med. Hyg 89, 742–749. doi: 10.4269/ajtmh.12-0640
Ruang-Areerate, T., Ruang-Areerate, P., Manomat, J., Naaglor, T., Piyaraj, P., Mungthin, M., et al. (2023). Genetic variation and geographic distribution of leishmania orientalis and leishmania Martiniquensis among Leishmania/HIV co-infection in Thailand. Sci. Rep. 13, 23094. doi: 10.1038/s41598-023-50604-4
Seblova, V., Dujardin, J. C., Rijal, S., Domagalska, M. A., Volf, P. (2019). Isc1, a new leishmania donovani population emerging in the Indian sub-continent: Vector competence of phlebotomus argentipes. Infect. Genet. Evol. 76, 104073. doi: 10.1016/j.meegid.2019.104073
Shrestha, M., Khatri-Chhetri, M., Poudel, R. C., Maharjan, J., Dumre, S. P., Manandhar, K. D., et al. (2019). Molecular evidence supports the expansion of visceral leishmaniasis towards non-program districts of Nepal. BMC Infect. Dis. 19, 444. doi: 10.1186/s12879-019-4083-3
Singh, O. P., Sundar, S. (2022). Visceral leishmaniasis elimination in India: Progress and the road ahead. Expert Rev. Anti Infect. Ther. 20, 1381–1388. doi: 10.1080/14787210.2022.2126352
Siriwardana, Y., Zhou, G., Deepachandi, B., Akarawita, J., Wickremarathne, C., Warnasuriya, W., et al. (2019). Trends in recently emerged leishmania donovani induced cutaneous leishmaniasis, Sri Lanka, for the first 13 years. BioMed. Res. Int. 2019, 4093603. doi: 10.1155/2019/4093603
Thakur, L., Singh, K. K., Kushwaha, H. R., Sharma, S. K., Shankar, V., Negi, A., et al. (2020). Leishmania donovani infection with atypical cutaneous manifestations, Himachal Pradesh, India 2014-2018. Emerg. Infect. Dis. 26, 1864–1869. doi: 10.3201/eid2608.191761
Tharakan, S. J., Peter Cv, D., Karthik, R., Rupa, V., Rose, W., Thomas, M., et al. (2020). Case report: A single-center case series on skin manifestations of leishmaniasis from a non-endemic state in Southern India. Am. J. Trop. Med. Hyg 104, 928–933. doi: 10.4269/ajtmh.20-0938
Tobgay, T., Dorjee, S., Pradhan, A., Wangdi, T., Drukpa, T., Namgye, R., et al. (2021). Is leishmaniasis donovani elimination feasible in Bhutan? A review of current prevention and control mechanisms in Bhutan. Bhutan Health J. 6, 27–30. doi: 10.47811/bhj.106
World Health Organization. (2015). Regional Strategic Framework for Elimination of Kala-azar from the South-East Asia Region, (2005-2015) (Regional Office for South-East Asia, New Delhi, India: World Health Organization).
World Health Organization. (2021a). Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030. Geneva.
World Health Organization. (2021b). Weekly epidemiological record 2021, vol. 96, 35 [full issue. Weekly Epidemiological Rec. = Relevé épid miologique hebdomadaire 96, 401–420.
World Health Organization. (2022). Regional Strategic Framework for Accelerating and Sustaining Elimination of kala-azar in the South-east Asia Region 2022-2026 (Regional Office for South-East Asia, New Delhi, India: World Helath Organization).
World Health Organization. (2023). Leishmaniasis. Available online at: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. (accessed September 8, 2024).
World Health Organization. (2024). Global report on neglected tropical diseases 2024 stronger together, towards 2030. (Geneva: World Health Organization).
Yajima, A., Lin, Z., Mohamed, A. J., Dash, A. P., Rijal, S. (2023). Finishing the task of eliminating neglected tropical diseases (NTDs) in who South-East Asia Region: Promises kept, challenges, and the way forward. Lancet Reg. Health Southeast Asia 18, 100302. doi: 10.1016/j.lansea.2023.100302
Yangzom, T., Cruz, I., Bern, C., Argaw, D., Den Boer, M., Velez, I. D., et al. (2012). Endemic transmission of visceral leishmaniasis in Bhutan. Am. J. Trop. Med. Hyg 87, 1028–1037. doi: 10.4269/ajtmh.2012.12-0211
Keywords: Atypical, cutaneous leishmaniasis, Leishmania donovani, VL-elimination, South East Asia
Citation: Jain M, Sangma DA, Parida L, Negi R, Negi A, Matlashewski G and Lypaczewski P (2024) Atypical cutaneous leishmaniasis: a new challenge to VL elimination in South-East Asia. Front. Cell. Infect. Microbiol. 14:1454002. doi: 10.3389/fcimb.2024.1454002
Received: 24 June 2024; Accepted: 17 September 2024;
Published: 04 November 2024.
Edited by:
Rajiv Kumar, Banaras Hindu University, IndiaReviewed by:
Arun Kumar Haldar, Central Drug Research Institute (CSIR), IndiaCopyright © 2024 Jain, Sangma, Parida, Negi, Negi, Matlashewski and Lypaczewski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manju Jain, bWFuanVqYWlubWRhQGdtYWlsLmNvbQ==
 Manju Jain
Manju Jain Diya A’gitok Sangma
Diya A’gitok Sangma Lipsalely Parida
Lipsalely Parida Rohit Negi
Rohit Negi Ajeet Negi
Ajeet Negi Greg Matlashewski
Greg Matlashewski Patrick Lypaczewski
Patrick Lypaczewski