- 1Department of Plague and Brucellosis, Shaanxi Center for Disease Control and Prevention, Xi’an, China
- 2National Key Laboratory of Intelligent Tracking and Forecasting for Infectious Diseases, National Institute for Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, China
Introduction: Human brucellosis is a severe public concern in Shaanxi Province, China, and investigating the epidemiological relationship and transmission pattern of B. melitensis is necessary to devise control strategies.
Methods: In this study, a conventional bio-typing approach and whole genome sequencing of single-nucleotide polymorphisms (SNPs) were employed to identify 189 strains.
Results: Based on the conventional bio-typing, 189 Brucella strains were identified as B. melitensis, of which 14 were in bv. 1, 145 were in bv. 3, and 30 were variant, and the Brucella strains were distributed in all ten cities in Shaanxi Province. SNP analysis was used to identify genetic variation in 189 B. melitensis genomes, and maximum-likelihood was used to generate a phylogeny that identified two clades (A and B) and 19 sequence types (STs). The two clades were highly diverse and exclusively of Eastern Mediterranean origin. Clade B contained 18 STs (2-19), with most isolates originating from a broad swath, implying that multiple B. melitensis lineages circulated in Shaanxi. The 19 STs were composed of 3 to 46 strains isolated from different counties and years, suggesting that multiple cross-county brucellosis outbreak events are driven by multiple B. melitensis lineages. Global phylogenetic analysis revealed that clade A was close to GTIIb, and clade B was placed in the GTIIh lineage, expanding the known diversity of B. melitensis from China.
Conclusion: The human brucellosis epidemic in Shaanxi is driven by multiple indigenous circulating B. melitensis lineages, the knowledge of which will contribute to devising a control strategy and providing the foundation for a comprehensive regional phylogeny of this important zoonotic pathogen.
Introduction
Brucellosis is a zoonosis worldwide that has a serious effect on domestic productivity and human health (Rubach et al., 2013). Brucella spp. causes acute febrile illness and potentially debilitating chronic infection in humans, which has a serious negative effect on human health and quality of life. At present, there are 12 Brucella species found globally that cause human brucellosis cases, including six classical species (B. melitensis, B. abortus, B. suis, B. canis, B. ovis, B. neotomae) and six novel Brucella species (B. ceti, B. pinnipedialis, B. inopinata, B. microti, B. papionis, and B. vulpis) originating from mammals and other hosts (Occhialini et al., 2022), and there is one recent potential novel species (B. amazoniensis) isolated from smugglers of gold prospectors from the Amazon region of Brazil (About et al., 2023). However, three species including B. melitensis, B. abortus, and B. suis are responsible for most human brucellosis (Darbandi et al., 2022). In particular, two biovars (bv. 1 and bv. 3) from B. melitensis are the dominant species globally and trigger human brucellosis cases, which frequently occur and re-emerge (Refai, 2002; Zhu et al., 2020). The highest burden of human brucellosis has been observed in Asia and Africa (Wareth et al., 2022). In China, the brucellosis epidemic pattern has changed, with an expansive geographic trend from north to south (Lai et al., 2017). The epidemic situation exhibited a graduate worsening trend when a total of 69,767 cases were reported from 2,083 counties in mainland China in 2021, a 47.7% increase from 2020 (47,425) (Yang et al., 2023). Shaanxi is located in the hinterland of China, adjacent to eight provinces/areas: Shanxi and Henan in the east; Ningxia and Gansu in the west; Sichuan, Chongqing, and Hubei in the south; and Inner Mongolia in the north, the majority of which are historic brucellosis-endemic regions. Animal trade and cross-border movements are not strictly controlled and are frequent between these areas (An et al., 2022).
Currently, there is an ongoing increasing trend in the seroprevalence of human brucellosis from 2008 to 2020 in Shaanxi Province (An et al., 2022), and the number of affected counties increased from 36 in 2008 to 84 in 2020, with geographic expansion gradually toward southern areas (An et al., 2021). Genome epidemiology based on the whole genome sequencing (WGS) of circulating strains is a critical step in understanding the epidemiological relationship and transmission pattern of B. melitensis for further implementation of control measures (Xue et al., 2023). Single-nucleotide polymorphisms (SNPs) are considered the gold standard typing scheme for Brucella and are used to delineate pathogen transmission routes for disease surveillance (Janowicz et al., 2018). Therefore, the purpose of this study was to use conventional bio-typing assays and WGS-SNPs to illustrate the epidemiological relationship and transmission pattern of B. melitensis strains from Shaanxi Province, China, for the better implementation of evidence-based targeted surveillance and control measures.
Methods
Source of strains and the isolation, identification, and preparation of genome DNA
A total of 189 B. meliteneis strains were isolated and identified from human blood samples (n=188) and a goat sample (n=1) from 2013 to 2022, including two in 2013, seven in 2014, six in 2015, eight in 2017, 13 in 2018, 27 in 2019, 30 in 2020, 57 in 2021, and 39 in 2022 (Supplementary Table S1). All of which were isolated from surveillance samples during this period. The strain from the goat sample was involved in an outbreak event (OE2, S2021) and was related to two (S2019 and S2017) of the human cases. Sampling, isolation, and identification were performed according to the standard bacteriological approach (Araj, 2010). Briefly, 5-10 ml of fresh blood was injected into a biphasic culture flask and incubated at 37°C for at least 30 days continuously and the flask was observed and shaken the flask gently every other day. Suspected colonies were further subcultured and identified using conventional bio-typing (Liu et al., 2017) and AMOS-PCR (Bricker and Halling, 1994). Subsequently, single colonies from each culture of these strains were picked and subcultured for 48h and the strains were collected for genome preparation. Then, with reference to a previous study, pure colonies were harvested in 500 µL nuclease-free water and inactivated at 80°C for 30 minutes (Xue et al., 2023). DNA extraction from these strains was performed using the QIAamp DNA Mini Kit (Qiagen, Germany) following the manufacturer’s protocol for gram-negative bacteria. Furthermore, DNA quality was assessed by Qubit (Thermo Fisher Scientific, USA), and it was demonstrated that the resulting product was of good yield and purity.
Genome sequencing, analysis, and visualization of the SNPs
The genome sequences of 189 strains were obtained as previously described (Li et al., 2020). Briefly, the DNA sample was fragmented by sonication to a size of 350bp, and the DNA fragments were then end-polished, A-tailed, and ligated with the full-length adaptor for Illumina sequencing with further PCR amplification. Basic quality control metrics for the raw sequence data were generated using FastQC (https://github.com/s-andrews/FastQC) and the reads were trimmed using FASTQ (Chen et al., 2018) (https://github.com/OpenGene/fastp) to remove low-quality reads and adapter sequences. The high-quality clean data were aligned to contigs and further complete assembly was conducted using the SOAPdenovo method (Li et al., 2010). For SNP analysis of the 189 B. melitensis genomes, as previously reported (Janowicz et al., 2018), SNP quality was calculated using SAMTools (Li et al., 2009), and SNPs were filtered out if the quality was below 30 or if they were within the vicinity of 10 bp of another SNP. The maximum-likelihood trees of the 189 B. melitensis strains were calculated using TreeBeST based on the Maximum-Likelihood Phylogenies (PHYML) algorithm with 1,000 bootstrap replicates (Vilella et al., 2009). Furthermore, a global genetic relationship comparison with 264 strains was conducted with the aforementioned algorithm methods, of which there were 75 strains (Supplementary Table S2) from a previous study (Pisarenko et al., 2018). The tree was visualized and edited using iTOL (Interactive Tree Of Life) v6.5.7 (Letunic and Bork, 2021).
Results
Identification and differentiation of Brucella strains in this study
In this study, 189 strains were identified and differentiated by conventional bio-typing (Table 1) and further confirmed as B. menliensis by the AMOS-PCR assay. In total, 188 strains were isolated from human blood, and only one strain was isolated from a goat. The ages of the patients ranged from 4 to 80 years. The majority of the strains (85.10%, 160/188) were isolated from patients aged 30 to 69 years. Of the samples, 1.6%, 4.2%, 3.2%, and 4.7% were isolated from patients aged < 10, 10–20, 20–30, and > 70 years, respectively. The majority of the patients were male (n=140) and 48 were female. Most patients (94.7%, 178/188) had close contact with sheep/goats, with the most common clinical manifestations being fever (59.72%, 86/144), fatigue (46.53%, 67/144), sweat (40.97%, 59/144), and arthralgia (44.44%, 64/144), and 8.3% (12/144) of the patient were asymptomatic. In this study, 87.77% (165/188) of the strains were isolated from farmers, eight strains were from students, and the remaining strains were obtained from workers, farm technicians, and veterinary personnel.
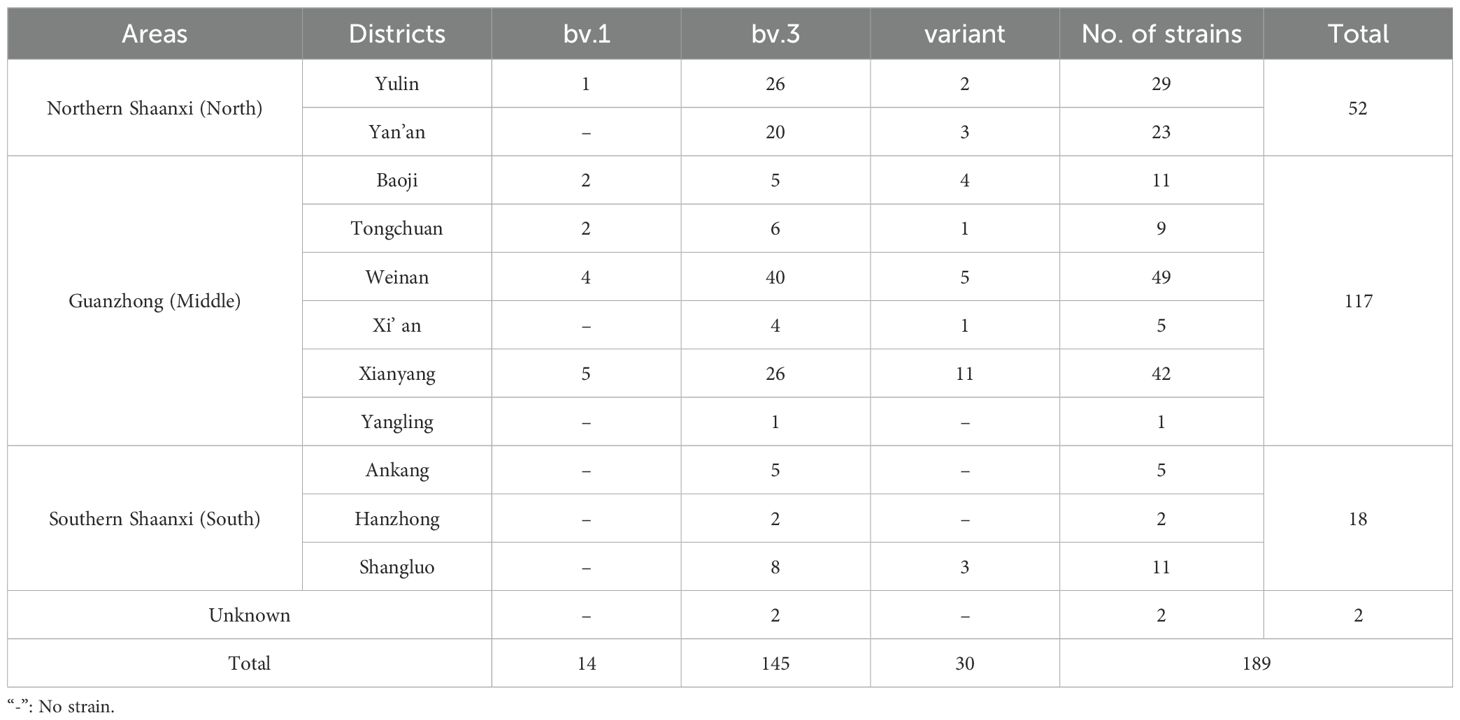
Geographical distribution profile of the 189 B. melitensis strains in this study
From 2003 to 2021,189 Brucella isolates were obtained from all regions in Shaanxi Province (Figure 1, Table 1) including 11 cities and 45 counties. Among the isolates, 52 were in Northern Shaanxi (North), 117 in Guanzhong (Middle), and 18 in Southern Shaanxi (South); the location of the remaining two is unknown (Table 1). The highest number of strains was found in Weinan (n=49), followed by Xianyang (n=42), Yulin (n=29), and Yan’ an (n=23), and the strain range in other regions was 1 to 11 (Table 1). The data show that the source of infection was persistent in this province. Three biovars were found, namely bv. 1 (n=14), bv.3 (n=145), and mutant (n=30), and B. melitensis bv. 3 was the dominant circulating biovar (Table 1, Figure 1).
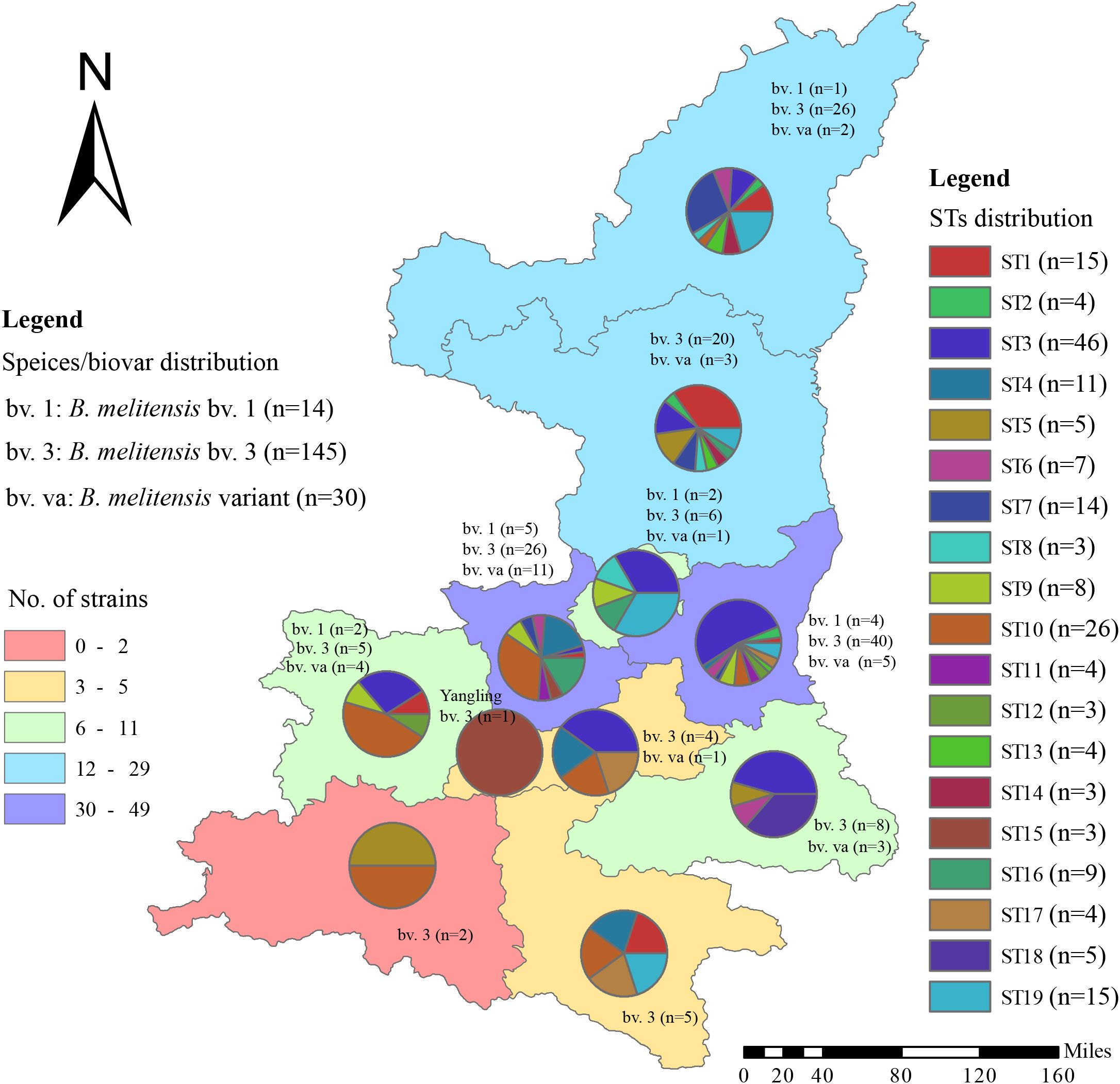
Figure 1. The distribution pattern of the number of strains, species/biovars, and STs of 189 B. melitensis strains from Shaanxi Province. The red dot indicates that the location of one strain was isolated from a goat.
Seasonal distribution of B. melitensis strains and sequence types
A heat map of the isolation times of B. melitensis strains by month showed that most isolates were collected in April (n=35), followed by May (n=33), June, and July (n=32), and the lowest number of strains were collected in December (n=1). Furthermore, 81% (153/189) of the strains were collected from March to July 2013 to 2022 (Figure 2). During the same period, the distribution pattern of the sequence types (STs) was similar to that of the strains examined. Some dominant STs had a unique distribution profile; for example, ST1 was dominant in March, May, and June; ST3 from April to July; ST4 in April and May; ST10 in April and January; and ST19 was dominant in July and June. Most STs were distributed during the epidemic period from March to July, with sporadic cases from January to February and from August to December (Figure 2). The highest ST diversity of STs was found in Weinan (n=13), followed by Yan’an (n=10), Xianyang (n=10), and Yulin (n=10) (Figure 1).
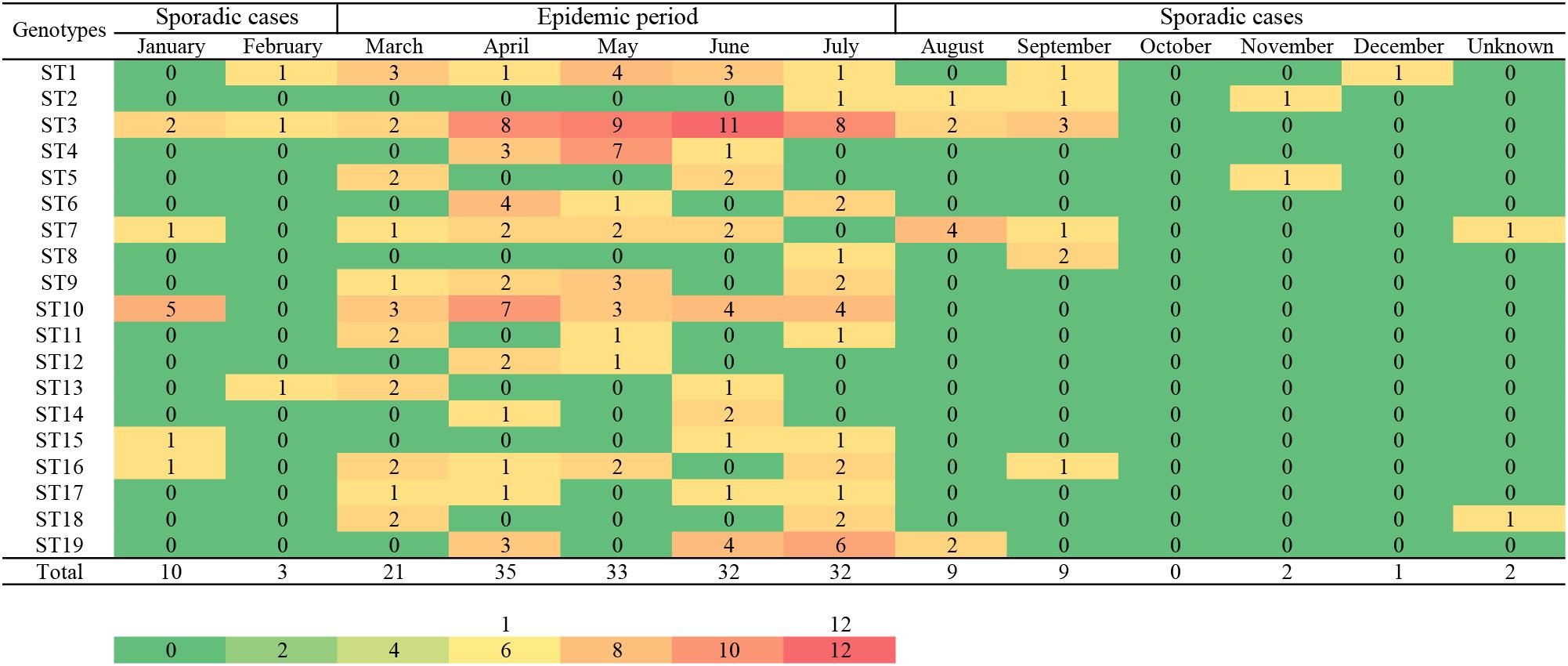
Figure 2. The seasonal distribution profile of the number of strains and STs of the 189 B. melitensis from Shaanxi Province. A heat map for isolation times of Shaanxi B. melitensis strains isolates from 2013 to 2022. The strain isolates were assigned to single nucleotide polymorphism (SNP) sequence types (ST). Color code indicates the number of strains and STs in respective months.
Genome epidemiology of B. melitensis strains based on SNPs
According to the SNP analysis, the 189 strains from the present study were sorted into two clades (A and B) (Figure 3) with 15 strains in clade A and 174 strains in clade B, suggesting that the strains in the present study have two different origins. Furthermore, the 189 strains were divided into 19 STs; ST1 was located in clade A, and the remaining 18 STs belonged to Clade B (Figure 3). The strains from each clade consisted of strains from different counties and years (Figure 1), and each ST contained between 3 and 46 strains. The most strains were observed in ST3, which contained 46 strains and were collected from eight different counties from 2013 to 2022, followed by ST10, which contained 26 strains from six regions from 2017 to 2022. In addition, there were 15 strains from six regions from 2013 to 2021 in ST1 and 15 strains from five regions from 2014 to 2022 in ST19 (Figure 3). These data suggest that multiple cross-county outbreaks of brucellosis were driven by multiple B. melitensis lineages. Importantly, most strains from Northern Shaanxi have identical STs compared to strains from Southern Shaanxi, implying that the strains circulating in the south descended from the northern region, demonstrating that B. meliteneis strains continuously expanded from Northern Shaanxi towards Southern Shaanxi areas (Supplementary Figure S1).
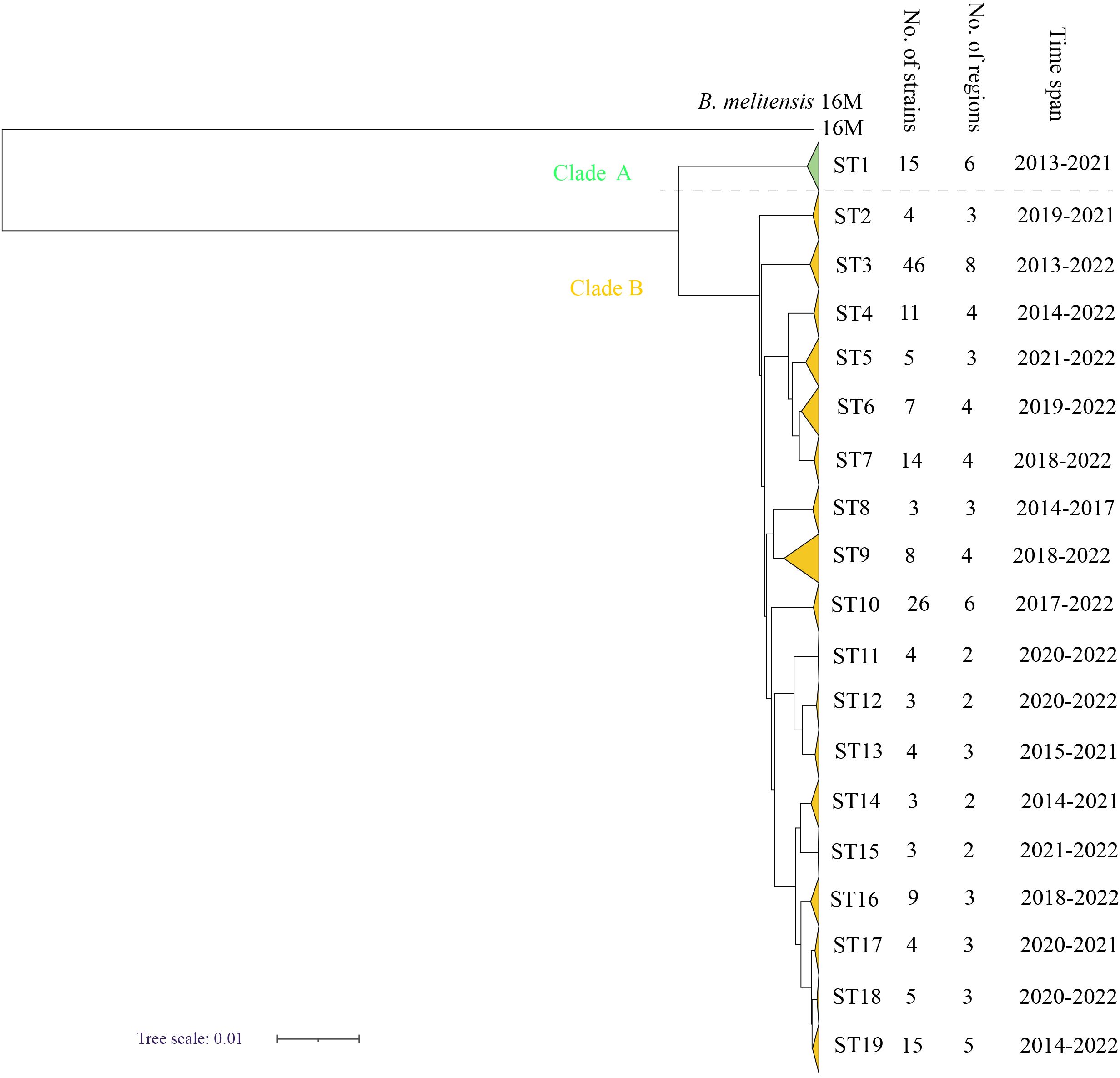
Figure 3. The maximum-likelihood tree generated by the wgSNP matrix of the 189 B. melitensis on the county scale. The phylogeny trees of 189 B. melitensis strains were calculated using TreeBeST based on the Maximum-Likelihood Phylogenies (PHYML) algorithm with 1,000 bootstrap replicates.
Trace-back investigation of human brucellosis outbreak events
From 2020 to 2022, 11 human brucellosis outbreak events (OE1-11) were reported in the surveillance system, and a total of 20 strains were isolated and identified (Supplementary Figure S1). Among 10 EOs (1-9, and 11), all the strains were grouped in the same STs, which was consistent with the field epidemiology survey, and the strains from each outbreak event were isolated from a common family or patient with close epidemiological links. These data indicated that each outbreak event had a common source of infection (Supplementary Figure S1). In contrast, three strains from OE10 were sorted into two different STs; the strains were collected from one family, including the father, wife, and son, and they were all in contact with goats. These data demonstrate that there were two different lineages of B. melitensis circulating in this family (Supplementary Figure S1).
Genetic relationship of B. melitensis strains on a global scale based on SNP analysis
Global SNP phylogenetic analysis showed that the 189 strains were placed in genotype II and were still sorted into two clades (A and B) (Figure 4, Supplementary Figure S2). Clade A contained only ST1, which consisted of 15 strains that were close to strains from GTIIb (2010724553, Syria), and consisted of strains from West Asia and southern Europe, including Syria, Turkey, Iraq, Albania, and Bulgaria, which expands the known diversity of B. melitensis in China (Figure 4, Supplementary Figure S2). Clade B contained 18 STs and was placed in the GTIIh lineage. The strains in these STs had high genetic homogeneity with strains from Gansu, Inner Mongolia, Henan, Shandong, Qinghai, and Xinjiang (Figure 4). These are regions with a high brucellosis burden. The ladder-like phylogram of clade B belonging to genotype II suggests a possible single introduction of East Mediterranean origin. The present analysis demonstrated that multiple indigenous imported B. melitensis lineages from single ancestors co-drove the continuous human brucellosis epidemic in Shaanxi Province.
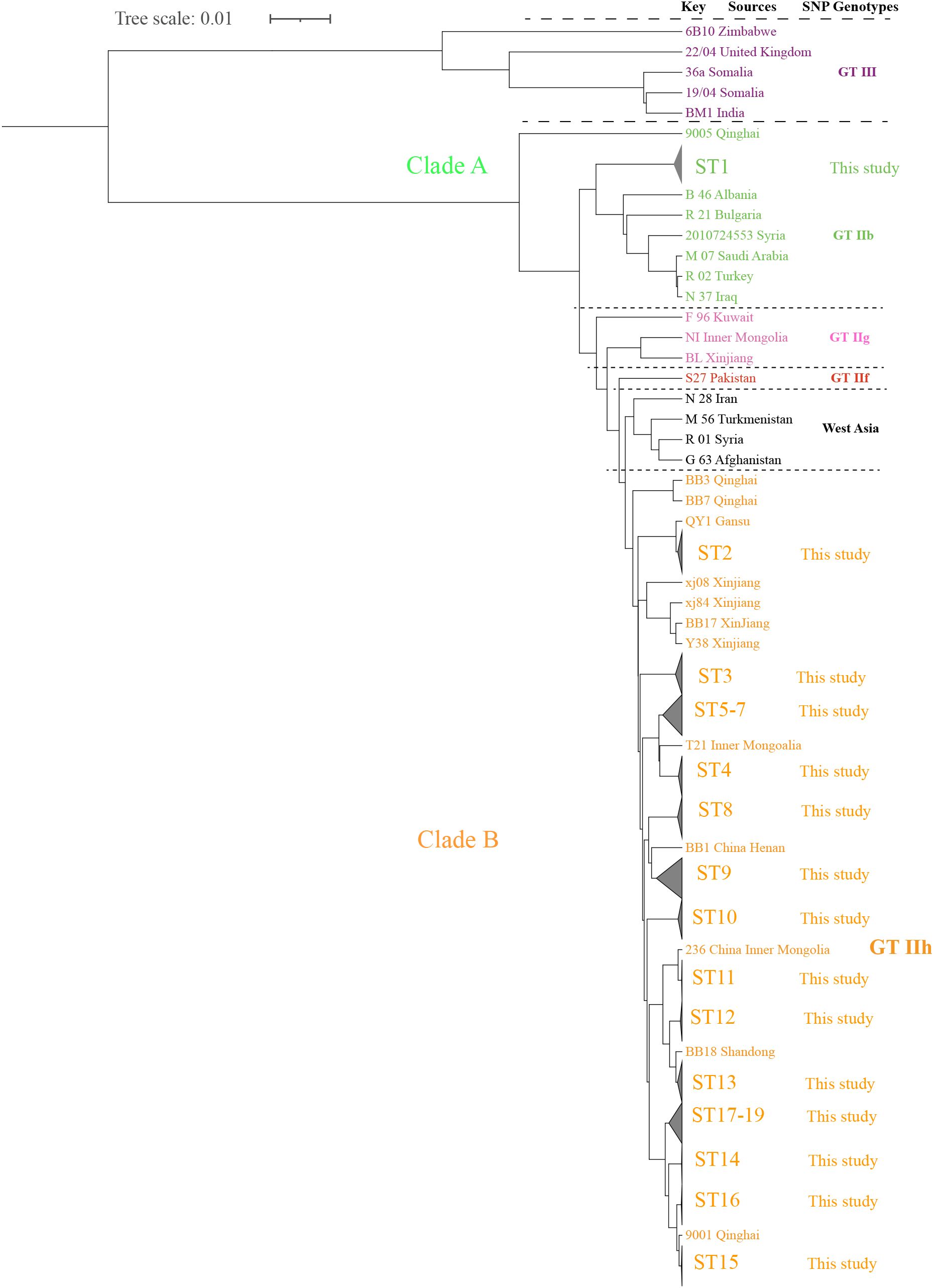
Figure 4. The maximum-likelihood tree of 264 B. melitensis strains on a global scale. The strains from GT II–III are referred to as previously described (Pisarenko et al., 2018), and GT III are marked with purple, GT IIb with green, GT IIg with pink, GT IIf with red, and the strains from the present study (GT IIh) are marked with orange. The phylogeny trees of the 264 B. melitensis strains were calculated using TreeBeST based on the Maximum-Likelihood Phylogenies (PHYML) algorithm with 1,000 bootstrap replicates.
Discussion
In this study, a conventional bio-typing approach and a robust genome epidemiology tool, wgSNP, were applied to identify 189 circulating B. melitensis strains from Shaanxi Province, located in the hinterland of mainland China. Our study showed that there have been multiple-point outbreaks of human brucellosis caused by multiple imported and indigenous epidemic B. melitensis lineages. This study provides genome-based-evidence showed that animal brucellosis is an epidemic in this period (Chang et al., 2020). Furthermore, the present analysis showed that the epidemic period was concentrated from March to July, which may be because these months represent the time of lambing, shearing, and taking care of the lambs in the agricultural and pastoral areas, leading to frequent contact with animals and increasing the risk of infection (Yu et al., 2023). Human brucellosis occurs throughout the year, with an obvious seasonal increase between March and July (Shi et al., 2017). Thus, appropriate strategies for brucellosis prevention and control should be developed during this high-incidence season. The epidemic of animal and human brucellosis in Shaanxi Province may be caused by a lack of controlled movement and the introduction of infected animals and livestock.
The epidemic trend of human brucellosis in Shaanxi Province is consistent with the majority border northern provinces; the number of cases in 2021 increased by 47.7% compared to that in 2020, and approximately 95.5% of the total disease burden was centralized in the northern provinces, especially Inner Mongolia and neighboring provinces (Yang et al., 2023). Human brucellosis is mainly transmitted by infected livestock, such as in those engaged in professions directly linked to agricultural and livestock activities for goats or sheep (Bernardi et al., 2022). B. melitensis is a great challenge for brucellosis control and is distributed worldwide in countries with a high disease burden (Pisarenko et al., 2018). Therefore, enhancing surveillance and controlling the import of small ruminants are important strategies in this region.
In this study, two distinct lineages were identified: clade A was distributed in a limited geographic range, and clade B was widespread throughout all the regions of Shaanxi Province. The lack of geographic expansion of the strains from clade A may be explained by limited host movement (Kamath et al., 2016). To fill this gap in our knowledge, it is necessary to study isolates from animals and conduct a comparative analysis. Interestingly, strains from clade A were similar to strains from GTIIb (2010724553, Syria) (Pisarenko et al., 2018), which consists of strains from West Asia and South Europe, thus expanding the known diversity of B. melitensis in China. Whole genome sequencing of Ethiopian B. abortus isolates expanded the known diversity of an early branching sub-Saharan African lineage (Edao et al., 2023). Further investigation is needed to provide informative evidence to better understand the genetic diversity of strains in this region. Notably, strains from clade B contained the remaining 18 STs that had high genetic homogeneity with strains from northern areas. The ladder-like phylogram of clade B belonging to genotype II suggests a possible single introduction of Mediterranean origin (Pisarenko et al., 2018). The absence of a clear differentiation according to geographic affiliation between these subgroups confirmed the frequent penetration of B. melitensis strains from one province/region to another (Pisarenko et al., 2018). Frequent and active trade and exchange of small ruminants between Shaanxi and other regions of China could promote this process. For example, some areas of Inner Mongolia and Gansu Province are bordered by Shaanxi, which implies that pathogen dispersal in adjacent areas is likely frequent; therefore, further investigation is necessary. The widespread lineage covered a long period and was dominant in Guanzhong, implying that this lineage originated from the development of large-scale goat farming in this area. However, epidemiology surveillance data from animals in this region is lacking. Data from the official veterinary bulletin showed that, in total, 23,399 animal cases were reported in Shaanxi from 2006 to 2021 and the reported number of animal cases per year increased from 248 in 2006 to 4,204 in 2021. In addition, studies reporting isolates from goats and sheep in Shaanxi are rare. One study showed that a total of 110 B. melitensis strains obtained from tissue and milk samples from cattle and small ruminants were serologically positive to Brucella infection from seven northern provinces (such as Gansu and Shaanxi provinces) and 50 counties (Sun et al., 2017). We have previously demonstrated that 66 isolates of Brucella recovered from sheep and yaks in the Inner Mongolia, Xinjiang, Qinghai and Gansu provinces of northwest China in 2015 and 2016, including B. melitensis biovar 3 (n = 58), B. melitensis biovar 1 (n = 1), B. abortus (n = 5), B. suis biovar 3 (n = 2), and B. melitensis biovar 3, were found to be mainly responsible for sheep brucellosis in northwest China (Cao et al., 2018). These data indicate that B. melitensis from small ruminants and cattle is the main driving factor of the human brucellosis epidemics in these regions. Therefore, the increasing trend in the goat population was highly consistent with the incidence of human brucellosis in Shaanxi. The number of goats (in ten thousand) continually increased from 681.32 in 2003 to 723.9 in 2021; however, the number of sheep declined from 195.86 in 2003 to 157.2 in 2021. These data demonstrate that introduced goats are the main reason for the outbreak of human brucellosis, and the transfer and illegal movement of infected goats triggered many outbreaks spanning years with geographic cross-over. Multiple brucellosis outbreak cases among students were caused by consuming raw goat’s milk in this region, which may be the best evidence (An et al., 2021). A meta-analysis showed that the prevalence of brucellosis in ovine and caprine flocks in China increased from 2010 to 2018 (3.2%) compared with 2000-2009 (1.0%), and the pooled prevalence of brucellosis was higher in goat flocks than in sheep flocks in China (Ran et al., 2018). Weak and relaxed surveillance activity in infected animals, the suspension of vaccination or insufficient vaccination of animals, devolvement of the farming economy, livestock products that demand high populations, and fatalities, combined with the inadequate implementation of quarantine and countermeasures for transregional livestock transport and introduction, play a role in driving the widespread expansion (Guan et al., 2018). For example, Xinjiang is one of the largest livestock husbandry sectors in China and has recently experienced an increasing incidence of brucellosis in cattle and small ruminants (Sun et al., 2016), leading to a high incidence of human brucellosis and brucellosis patients who are mainly pastoralists and veterinarians (Zheng et al., 2021). In Qinghai, WGS-SNP analysis demonstrated that an increase in the incidence of human brucellosis may be caused by multiple local circulating lineages (Xue et al., 2023). In Kyrgyzstan, there were different B. melitensis lineages, and strains with high similarity to strains from Turkmenistan, Iran, and Turkey (Kydyshov et al., 2022), which is consistent with our findings that human brucellosis outbreaks in Shaanxi are driven by multiple lineages from single introduced ancestors of B. melitensis strains. In addition, Iranian B. melitensis strains were closely related to those recovered from sheep and humans in Iraq, Afghanistan, Syria, Turkmenistan, and Pakistan. These findings reveal the frequent and uncontrolled livestock exchange that have been introduced between different areas from the past until today. In Egypt, 136 Egyptian B. melitensis strains isolated from animals and humans revealed 99 different cgSNP genotypes, indicating several different incidents and sources of infections, probably from animals imported from other countries to Egypt (Holzer et al., 2022). Brucellosis is an emerging and re-emerging neglected regional communicable disease in some regions of Asia driven by movement and trade (Liu et al., 2020). Therefore, there needs to be effective collaboration locally and nationally, and both animal and human health services authorities need to improve their surveillance capacity of the disease and implement strict measures for animal transboundary movement and create one health approach to curb the spread of the disease.
Conclusion
Our study identified B. melitensis as a causative agent of human brucellosis in Shaanxi Province, and multiple different lineages of B. melitensis were found to drive the epidemic of human brucellosis in this area. The present conclusion provides vital clues for understanding the geographic distribution and transmission pattern of the strains. As this study focused on isolates of human origin, the identity of Brucella species and lineages circulating among animals remains elusive. Implementing animal causative agent surveillance programs and discrimination based on WGS-SNPs are needed to better understand the transmission pattern to facilitate the implementation of targeted control measures to combat brucellosis in Shaanxi and border areas.
Data availability statement
Genome sequences were deposited in the Genome Warehouse in National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation, under accession number that is publicly accessible at https://ngdc.cncb.ac.cn/gwh and accession numbers: GWHFGHQ00000000.1, GWHFGMR00000000.1, GWHFGMS00000000.1, GWHFGMT00000000.1, GWHFGMU00000000.1, GWHFGMV00000000.1, GWHFGMW00000000.1, GWHFGMX00000000.1, GWHFGMY00000000.1, GWHFGMZ00000000.1, GWHFGNA00000000.1, GWHFGNB00000000.1, GWHFGNC00000000.1, GWHFGND00000000.1, GWHFGNE00000000.1, GWHFGNF00000000.1, GWHFGNG00000000.1, GWHFGNH00000000.1, GWHFGNI00000000.1.
Ethics statement
This study was performed in accordance with the principles of the Declaration of Helsinki. The research protocol was reviewed and approved by the Ethics Committee of the Institute for Communicable Disease Control and Prevention, Shaanxi (No.2017-001-01). Informed consent for the study was obtained from patients, isolated Brucella spp. strains were performed for the purpose of diagnosing human brucellosis, and all analyses was based on anonymous status.
Author contributions
CA: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Resources. SN: Methodology, Writing – original draft, Data curation, Formal analysis. BL: Writing – review & editing, Data curation, Formal analysis, Investigation. DZ: Formal analysis, Methodology, Validation, Writing – review & editing. WW: Formal analysis, Writing – review & editing, Resources, Validation. YS: Formal analysis, Methodology, Resources, Software, Writing – review & editing. SF: Formal analysis, Investigation, Validation, Writing – review & editing. DL: Data curation, Formal analysis, Software, Writing – review & editing. ZJL: Conceptualization, Formal analysis, Validation, Writing – review & editing. ZGL: Conceptualization, Investigation, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. WC: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key Research and Development Program of Shaanxi Province (No. 2024SF-YBXM-274) and National Key Research and Development Program of China (No. 2021YF2301105). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1452143/full#supplementary-material
Supplementary Figure 1 | Based on the wgSNP matrix, we generated a maximum-likelihood tree phylogeny tree of the 189 B. melitensis strains on the county scale. Nine outbreak events are marked with red, EO10 is marked with purple, and EO5 and 11 are marked with green.
Supplementary Figure 2 | Phylogenetic analysis of 264 strains on the global scale. Strains from clade (A) are marked with green and strains from Clade (B) are marked with red.
References
About, F., Pastre, T., Boutrou, M., Martinez, A. Y., Melzani, A., Peugny, S., et al. (2023). Novel species of brucella causing human brucellosis, French Guiana. Emerg. Infect. Dis. 29, 333–340. doi: 10.3201/eid2902.220725
An, C. H., Liu, Z. G., Nie, S. M., Sun, Y. X., Fan, S. P., Luo, B. Y., et al. (2021). Changes in the epidemiological characteristics of human brucellosis in Shaanxi Province from 2008 to 2020. Sci. Rep. 11, 17367. doi: 10.1038/s41598-021-96774-x
An, C. H., Nie, S. M., Sun, Y. X., Fan, S. P., Luo, B. Y., Li, Z., et al. (2022). Seroprevalence trend of human brucellosis and MLVA genotyping characteristics of Brucella melitensis in Shaanxi Province, China, during 2008-2020. Transbound Emerg. Dis. 69, e423–e434. doi: 10.1111/tbed.14320
Araj, G. F. (2010). Update on laboratory diagnosis of human brucellosis. Int. J. Antimicrob. Agents 36 Suppl 1, S12–S17. doi: 10.1016/j.ijantimicag.2010.06.014
Bernardi, F., Possa, M. G., Rossi, C. E., Benevenuto, L. G. D., Nascif Junior, I. A., Jesus, J., et al. (2022). Epidemiological characterization of notified human brucellosis cases in Southern Brazil. Rev. Inst Med. Trop. Sao Paulo 64, e38. doi: 10.1590/s1678-9946202264038
Bricker, B. J., Halling, S. M. (1994). Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J. Clin. Microbiol. 32, 2660–2666. doi: 10.1128/jcm.32.11.2660-2666.1994
Cao, X., Li, Z., Liu, Z., Fu, B., Liu, Y., Shang, Y., et al. (2018). Molecular epidemiological characterization of Brucella isolates from sheep and yaks in northwest China. Transbound Emerg. Dis. 65, e425–e433. doi: 10.1111/tbed.2018.65.issue-2
Chang, W., Sun, Y., Fan, S., An, C., Nie, S., Tian, G., et al. (2020). Brucellosis outbreak caused by brucella melitensis - jingyang county, shaanxi province, China, march-May, 2020. China CDC Wkly 2, 753–756. doi: 10.46234/ccdcw2020.204
Chen, S., Zhou, Y., Chen, Y., Gu, J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Darbandi, A., Koupaei, M., Navidifar, T., Shahroodian, S., Heidary, M., Talebi, M. (2022). Brucellosis control methods with an emphasis on vaccination: a systematic review. Expert Rev. Anti Infect. Ther. 20, 1025–1035. doi: 10.1080/14787210.2022.2066521
Edao, B. M., Ameni, G., Berg, S., Tekle, M., Whatmore, A. M., Wood, J. L. N., et al. (2023). Whole genome sequencing of Ethiopian Brucella abortus isolates expands the known diversity of an early branching sub-Saharan African lineage. Front. Microbiol. 14, 1128966. doi: 10.3389/fmicb.2023.1128966
Guan, P., Wu, W., Huang, D. (2018). Trends of reported human brucellosis cases in mainland China from 2007 to 2017: an exponential smoothing time series analysis. Environ. Health Prev. Med. 23, 23. doi: 10.1186/s12199-018-0712-5
Holzer, K., Wareth, G., El-Diasty, M., Abdel-Hamid, N. H., Hamdy, M. E. R., Moustafa, S. A., et al. (2022). Tracking the distribution, genetic diversity and lineage of Brucella melitensis recovered from humans and animals in Egypt based on core-genome SNP analysis and in silico MLVA-16. Transbound Emerg. Dis. 69, 3952–3963. doi: 10.1111/tbed.14768
Janowicz, A., De Massis, F., Ancora, M., Cammà, C., Patavino, C., Battisti, A., et al. (2018). Core genome multilocus sequence typing and single nucleotide polymorphism analysis in the epidemiology of brucella melitensis infections. J. Clin. Microbiol. 56(9):e00517-18. doi: 10.1128/JCM.00517-18
Kamath, P. L., Foster, J. T., Drees, K. P., Luikart, G., Quance, C., Anderson, N. J., et al. (2016). Genomics reveals historic and contemporary transmission dynamics of a bacterial disease among wildlife and livestock. Nat. Commun. 7, 11448. doi: 10.1038/ncomms11448
Kydyshov, K., Usenbaev, N., Berdiev, S., Dzhaparova, A., Abidova, A., Kebekbaeva, N., et al. (2022). First record of the human infection of Brucella melitensis in Kyrgyzstan: evidence from whole-genome sequencing-based analysis. Infect. Dis. Poverty 11, 120. doi: 10.1186/s40249-022-01044-1
Lai, S., Zhou, H., Xiong, W., Gilbert, M., Huang, Z., Yu, J., et al. (2017). Changing epidemiology of human brucellosis, China, 1955-2014. Emerg. Infect. Dis. 23, 184–194. doi: 10.3201/eid2302.151710
Letunic, I., Bork, P. (2021). Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–w296. doi: 10.1093/nar/gkab301
Li, H., Handsaker, B., Wysoker, A., Fennell, T., Ruan, J., Homer, N., et al. (2009). The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078–2079. doi: 10.1093/bioinformatics/btp352
Li, Z., Wang, X. M., Zhu, X., Wang, M., Cheng, H., Li, D., et al. (2020). Molecular characteristics of brucella isolates collected from humans in Hainan Province, China. Front. Microbiol. 11, 452. doi: 10.3389/fmicb.2020.00452
Li, R., Zhu, H., Ruan, J., Qian, W., Fang, X., Shi, Z., et al. (2010). De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 20, 265–272. doi: 10.1101/gr.097261.109
Liu, Z. G., Di, D. D., Wang, M., Liu, R. H., Zhao, H. Y., Piao, D. R., et al. (2017). MLVA genotyping characteristics of human brucella melitensis isolated from ulanqab of inner Mongolia, China. Front. Microbiol. 8, 6. doi: 10.3389/fmicb.2017.00006
Liu, Z., Wang, C., Wei, K., Zhao, Z., Wang, M., Li, D., et al. (2020). Investigation of genetic relatedness of brucella strains in countries along the silk road. Front. Vet. Sci. 7, 539444. doi: 10.3389/fvets.2020.539444
Occhialini, A., Hofreuter, D., Ufermann, C. M., Al Dahouk, S., Köhler, S. (2022). The retrospective on atypical brucella species leads to novel definitions. Microorganisms 10(4):813. doi: 10.3390/microorganisms10040813
Pisarenko, S. V., Kovalev, D. A., Volynkina, A. S., Ponomarenko, D. G., Rusanova, D. V., Zharinova, N. V., et al. (2018). Global evolution and phylogeography of Brucella melitensis strains. BMC Genomics 19, 353. doi: 10.1186/s12864-018-4762-2
Ran, X., Chen, X., Wang, M., Cheng, J., Ni, H., Zhang, X. X., et al. (2018). Brucellosis seroprevalence in ovine and caprine flocks in China during 2000-2018: a systematic review and meta-analysis. BMC Vet. Res. 14, 393. doi: 10.1186/s12917-018-1715-6
Refai, M. (2002). Incidence and control of brucellosis in the Near East region. Vet. Microbiol. 90, 81–110. doi: 10.1016/S0378-1135(02)00248-1
Rubach, M. P., Halliday, J. E., Cleaveland, S., Crump, J. A. (2013). Brucellosis in low-income and middle-income countries. Curr. Opin. Infect. Dis. 26, 404–412. doi: 10.1097/QCO.0b013e3283638104
Shi, Y. J., Lai, S. J., Chen, Q. L., Mu, D., Li, Y., Li, X. X., et al. (2017). Analysis on the epidemiological features of human brucellosis in northern and southern areas of China, 2015-2016. Zhonghua Liu Xing Bing Xue Za Zhi 38, 435–440. doi: 10.3760/cma.j.issn.0254-6450.2017.04.005
Sun, M. J., Di, D. D., Li, Y., Zhang, Z. C., Yan, H., Tian, L. L., et al. (2016). Genotyping of Brucella melitensis and Brucella abortus strains currently circulating in Xinjiang, China. Infect. Genet. Evol. 44, 522–529. doi: 10.1016/j.meegid.2016.07.025
Sun, M., Jing, Z., Di, D., Yan, H., Zhang, Z., Xu, Q., et al. (2017). Multiple locus variable-Number tandem-Repeat and single-Nucleotide polymorphism-Based brucella typing reveals multiple lineages in brucella melitensis currently endemic in China. Front. Vet. Sci. 4, 215. doi: 10.3389/fvets.2017.00215
Vilella, A. J., Severin, J., Ureta-Vidal, A., Heng, L., Durbin, R., Birney, E. (2009). EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 19, 327–335. doi: 10.1101/gr.073585.107
Wareth, G., Dadar, M., Ali, H., Hamdy, M. E. R., Al-Talhy, A. M., Elkharsawi, A. R., et al. (2022). The perspective of antibiotic therapeutic challenges of brucellosis in the Middle East and North African countries: Current situation and therapeutic management. Transbound Emerg. Dis. 69, e1253–e1268. doi: 10.1111/tbed.14502
Xue, H., Zhao, Z., Wang, J., Ma, L., Li, J., Yang, X., et al. (2023). Native circulating Brucella melitensis lineages causing a brucellosis epidemic in Qinghai, China. Front. Microbiol. 14, 1233686. doi: 10.3389/fmicb.2023.1233686
Yang, H., Chen, Q., Li, Y., Mu, D., Zhang, Y., Yin, W. (2023). Epidemic characteristics, high-risk areas and space-time clusters of human brucellosis - China, 2020-2021. China CDC Wkly 5, 17–22. doi: 10.46234/ccdcw2023.004
Yu, X., Fang, M., Li, Y., Yu, J., Cheng, L., Ding, S., et al. (2023). Epidemiological characteristics and spatio-temporal analysis of brucellosis in Shandong province, 2015-2021. BMC Infect. Dis. 23, 669. doi: 10.1186/s12879-023-08503-6
Zheng, Y., Zhang, L., Wang, C., Wang, K., Guo, G., Zhang, X., et al. (2021). Predictive analysis of the number of human brucellosis cases in Xinjiang, China. Sci. Rep. 11, 11513. doi: 10.1038/s41598-021-91176-5
Keywords: brucellosis, Brucella melitensis, species/biovars, WGS-SNPs, genomic
Citation: An C, Nie S, Luo B, Zhou D, Wang W, Sun Y, Fan S, Liu D, Li Z, Liu Z and Chang W (2024) Multiple Brucella melitensis lineages are driving the human brucellosis epidemic in Shaanxi Province, China: evidence from whole genome sequencing-based analysis. Front. Cell. Infect. Microbiol. 14:1452143. doi: 10.3389/fcimb.2024.1452143
Received: 20 June 2024; Accepted: 02 September 2024;
Published: 30 October 2024.
Edited by:
Michael Marceau, Université Lille Nord de France, FranceReviewed by:
Ivo Sirakov, Medical University Sofia, BulgariaAna Pelerito, National Health Institute Doutor Ricardo Jorge (INSA), Portugal
Copyright © 2024 An, Nie, Luo, Zhou, Wang, Sun, Fan, Liu, Li, Liu and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiguo Liu, bGl1emhpZ3VvQGljZGMuY24=; Wenhui Chang, Y2hhbmd3ZW5odWkzQGFsaXl1bi5jb20=
 Cuihong An1
Cuihong An1 Zhenjun Li
Zhenjun Li Zhiguo Liu
Zhiguo Liu