- 1Department of Laboratory Medicine, The People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, China
- 2Department of endocrinology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, China
- 3Department of Microbiology, Queen Mary Hospital, Hong Kong, Hong Kong SAR, China
- 4Department of Microbiology, Guangxi Jinyu Medical Laboratory Co., Ltd., Nanning, China
- 5Department of Ophthalmology, The People’s Hospital of Guangxi Zhuang Autonomous Region, Guangxi Academy of Medical Sciences, Nanning, China
Background: Pythium insidiosum (P. insidiosum) is the causative agent of pythiosis, an infectious disease with a high morbidity and fatality rate. Pythiosis cases have increased dramatically during the past ten years, particularly in tropical and subtropical areas. Sadly, microbiologists and medical professionals know very little about pythiosis, and the disease is frequently challenging to identify. It is frequently misdiagnosed as a fungal infection.
Methods: We report two cases of pythiosis, one was Pythium keratitis, the other was cutaneous pythiosis. The patient with corneal infection had no underlying disease, while the patient with cutaneous pythiosis had a history of liver cirrhosis, diabetes, and psoriasis. The corneal sample and subcutaneous pus were sent for metagenomic Next-Generation Sequencing (mNGS). To further diagnose the isolated strain, P. insidiosum zoospores were induced to produce by co-incubation with sterile grass leaves in sterile pond water. Their zoospores were used as an inoculum for drug susceptibility testing by disk diffusion and broth microdilution method.
Results: The mNGS of two cases were reported as P. insidiosum. Zoospores were produced after incubation 48h. The zoospores were collected for drug susceptibility assay. All antifungal drugs, antibacterial drugs of β-Lactams, vancomycin, levofloxacin, ciprofloxacin, gentamicin, trimethoprim-sulfamethoxazole, clindamycin have no inhibitory activity against P. insidiosum in vitro. Minocycline, tigecycline, linezolid, erythromycin and azithromycin have significant in vitro activity against P. insidiosum. Based on the susceptibility results, the drug was changed from itraconazole to linezolid and minocycline, along with multiple debridements and drainage for cutaneous pythiosis. The patient was discharged after 24 days of treatment.
Conclusions: Early and accurate identification, combined with aggressive surgical debridement and appropriate drug therapy, can greatly improve patient managements. Conventional culture and zoospore induction remain gold standard for diagnosis; however, DNA-based method should be performed simultaneously. The drug susceptibility testing provides profound effects on proper drug selection against P. insidiosum.
Introduction
Pythiosis caused primarily by the fungus-like aquatic oomycete P. insidiosum, is an emerging but uncommon infection in China. It is the most common species in the Genus Pythium that can cause disease in humans and animals. Pythiosis mainly occurs in tropical and subtropical regions, especially in horses, dogs, and humans (Kaufman, 1998). Infection occurs when the host comes into contact with water contaminated with zoospores after skin damage. The zoospores attach and germinate as hyphae into injured tissue. Agricultural-related activities or water-related recreational activities are considered triggering factors for human pythiosis. P. insidiosum grows by forming filamentous structures similar to fungal hyphae, with broad, irregularly branched, ribbon-like hyphae that can fold easily. Morphologically, it resembles hyphae of Mucorales, although P. insidiosum is not a fungus but a protist. It shows similarities to fungal infections clinically and microbiologically, leading to the widespread use of antifungal drugs for treatment, resulting in high treatment failure rates. Despite some successful reports of antifungal drug treatment (Shenep et al., 1998; Heath et al., 2002), many studies have shown their ineffectiveness. P. insidiosum does not synthesize ergosterol (Sathapatayavongs et al., 1989; Krajaejun et al., 2006b), which is the target of most antifungal drugs. Recent in vitro and in vivo studies suggested that certain antibiotics may be more suitable for treating P. insidiosum infections compared to antifungal drugs. Pythiosis can cause four clinical manifestations: cutaneous and subcutaneous infections, vascular pythiosis, corneal ulcer, and systemic infections (Gaastra et al., 2010). Pythiosis is most commonly reported in South and Southeast Asia, although cases of pythiosis have been reported worldwide (Chitasombat et al., 2020). Recently, an outbreak of pythium keratitis during the rainy season (Thanathanee et al., 2013), as well as severe skin and subcutaneous pythiosis have been reported in China and North America (Perkins et al., 2022; Zhang et al., 2022). Initially, they were misdiagnosed as fungal infections. Conventional culture and zoospores induction remain the gold standard for diagnosis; however, DNA-based methods should also be performed concurrently. Curative surgery, amputation, and surgical debridement of the lesions are the most commonly used and effective treatment methods, but recurrent rates are high (45%) (Gaastra et al., 2010).
We report two cases of pythiosis which were initially misdiagnosed as fungal infections. We propose new diagnostic, drug susceptibility test, and treatment methods to minimize misdiagnosis and ineffective treatment.
Materials and methods
Case presentation
Patient 1
A 67-year-old man presented to our hospital due to leg skin ulcers for 20 days. He had contacted with swamp water after being bitten by ants. Also, the patient had diabetes, liver cirrhosis, and psoriasis. Physical examination showed severe swelling, multiple ulcers and purulent secretions of the left lower limb. Scattered darkening indurations were observed and skin temperature of the indurated area was elevated (Figure 1A). Laboratory findings included leukocyte 7.20×10^9/L, anemia (Hb: 73g/L), thrombocytopenia (85×10^9/L), hyperglycemia (7.05mmol/L), hypoproteinemia (TP: 48.9g/L; Alb: 22.9g/L) and EBV-DNA significantly increased (8.76×10^6 IU/mL). Elevated inflammatory markers included C-reactive protein 67.89mg/L, pocalcitonin 0.76 ng/mL, ESR 57.00mm/h. Magnetic resonance imaging showed abnormal signals in various muscle groups and subcutaneous soft tissues of the left leg, indicating inflammatory lesions and abscesses. Increased signals were observed in the ligaments and tendons of the left foot, indicating inflammatory lesions and surrounding soft tissue swelling (Figure 1B). Local debridement and abscess incision drainage were performed after admission (Figure 1C), and a significant amount of yellow purulent secretion was drained and sent for culture (Figure 1D).
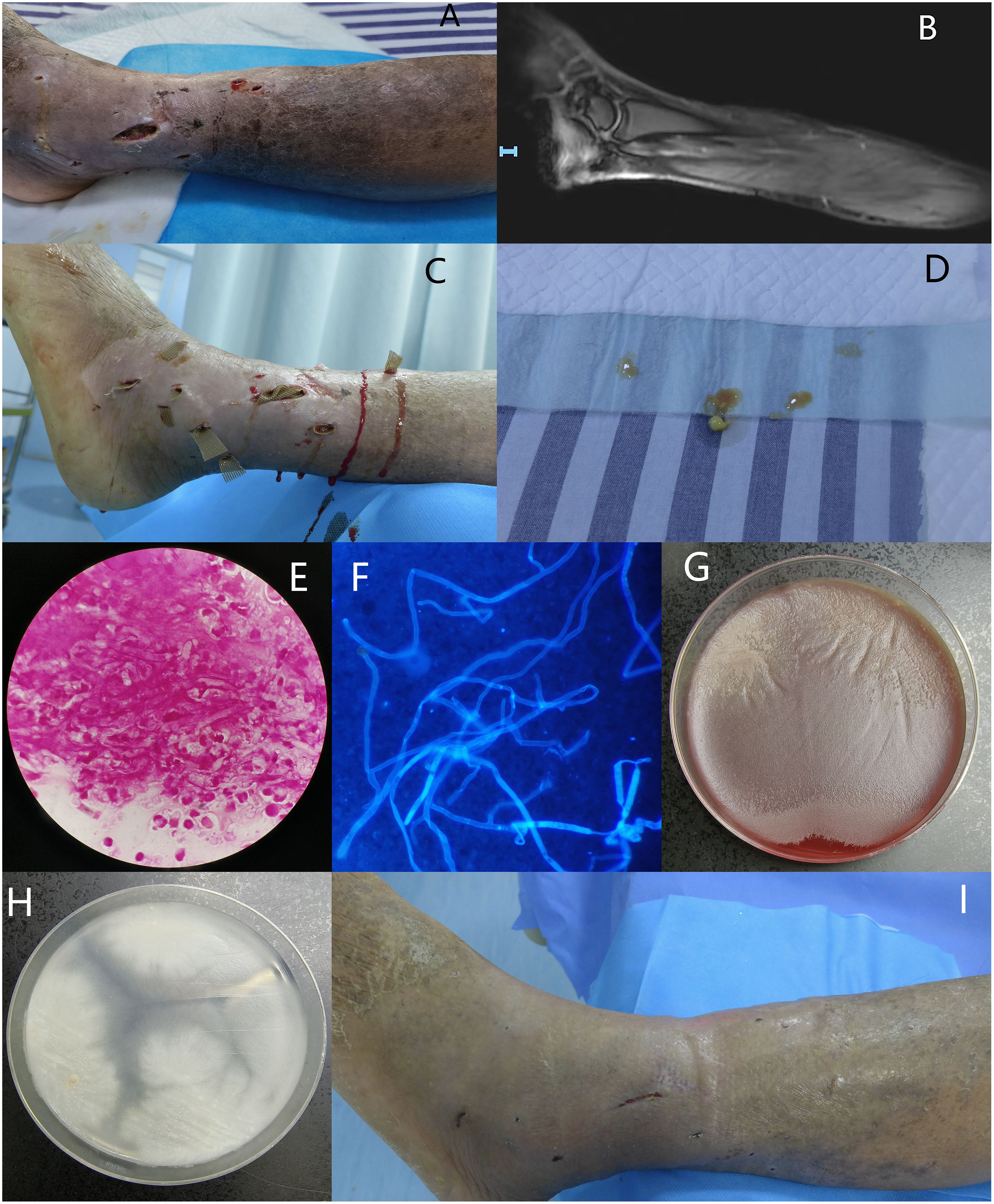
Figure 1. (A) Severe swelling, multiple ulcers and purulent secretions of the left lower limb, with scattered darkening indurations were observed and skin temperature of the indurated area was elevated; (B) Magnetic resonance imaging showed abnormal signals in various muscle groups and subcutaneous soft tissues of the left leg, indicating inflammatory lesions and abscesses. Increased signals were observed in the ligaments and tendons of the left foot, indicating inflammatory lesions and surrounding soft tissue swelling; (C) Subcutaneous abscesses debridement and drainage; (D) A significant amount of yellow purulent secretion was drained; (E, F) Gram staining and calcofluor white fluorescent staining revealed broad, irregularly branched, ribbon-like hyphae, ×1000; (G) Flat greyish-white colony on blood agar with filiform margins incubated at 35°C for 72 hours; (H) White to yellow-white, radiating colonies on PDA with few aerial hyphae incubated at 35°C for 72 hours; (I) After 24 days of treatment, most skin ulcers have successfully healed.
On the second day of admission, Gram staining and calcofluor white-fluorescent staining of the wound secretion revealed broad, irregularly branched, ribbon-like hyphae, highly suggestive of fungal infection (Figures 1E, F). Itraconazole (200mg, once a day) for antifungal treatment and moxifloxacin (0.4g, once a day) for antibacterial treatment were initiated empirically. Flat greyish-white colony on blood agar with filiform margins and white to yellow-white, radiating colonies with few aerial hyphae on potato dextrose agar (PDA) were observed after 72 hours culture at 35°C (Figures 1G, H). Lactophenol cotton blue staining of the colonies showed broad, irregularly branched hyphae. Day 5 after admission, multiple isolated and unconnected subcutaneous abscesses appeared and subcutaneous pus were sent for metagenomic Next-Generation Sequencing, and the result was reported P. insidiosum. Thus, itraconazole was switched to linaconazole (600mg, once a day). After two weeks of treatment with linaconazole, the patient’s platelet count decreased to 26×10^9/L, which was considered as an adverse reaction to linaconazole, and it was discontinued. Minocycline (100mg, twice a day) was then used in combination with moxifloxacin. After 24 days of treatment, most skin ulcers have successfully healed (Figure 1I). The patient was discharged with prescriptions of continue oral minocycline 100mg, twice daily.
Patient 2
A 50-year-old man presented with pain in her left eye after being exposed to contaminated water in a rice field. The left eye showed dense central stromal opacity surrounded by a reticular pattern of subepithelial and superficial stromal infiltration (Figures 2A1, A2). Confocal microscopy displayed a large amount of filamentous structures at the lesion of the left cornea (Figure 2B). Corneal scraping with Gram staining and calcofluor white-fluorescent staining revealed broad, irregularly branched, ribbon-like hyphae (Figures 2C, D). Corneal scraping culture showed flat greyish-white colony on blood agar with filiform margins incubated at 35°C for 48 hours (Figure 2E). The organism was identified as P. insidiosum by zoospores production and metagenomic Next-Generation Sequencing. Unfortunately, due to the patient’s refusal of hospitalization and treatment, we were unable to track the prognosis of this patient.

Figure 2. (A1, A2) The left eye showed dense central stromal opacity surrounded by a reticular pattern of subepithelial and superficial stromal infiltration; (B) Confocal microscopy displayed a large amount of filamentous structures at the lesion of the left cornea; (C, D) Corneal scraping with Gram staining and calcofluor white fluorescent staining revealed broad, irregularly branched, ribbon-like hyphae, ×1000; (E) Corneal scraping culture showed flat greyish-white colony on blood agar with filiform margins incubated at 35°C for 48 hours.
Strain identification
The isolates obtained were identified by examination of colonies morphology and microscopic feature. The first case’s subcutaneous pus and the second case’s isolate were sent for metagenomic Next-Generation Sequencing.
Culture of P. insidiosum for zoospore detection
The colonies from the blood agar plates were used to induce zoospores for the purpose of microbiologically confirming P. insidiosum. A sterile petri dish was filled with sterile pond water, and a few sterile blades of echinochloa crus-galli (approximately 10mm long) were added. 2-3 pieces of agar blocks with P. insidiosum were placed in the petri dish, and the dish was incubated at 35°C for 48 hours. After changing fresh sterile pond water 1 hour, zoosporangiums begin to form on the mycelium. Once fully formed, the biflagellate zoospores break the zoosporangium wall, swim and then encyst about 30 min later. zoosporangiums and zoospores were observed on direct microscopic examination. The flagella of motile zoospores were looked for using Giemsa staining and scanning electron microscopy (SEM).
Sample processing for mNGS
The subcutaneous pus was surgically removed from the skin ulcer and stored in sterile Phosphate Buffered Saline. Then subcutaneous pus was homogenized by vortex for 30 s and centrifuged at 15,000 rpm for 3 min and the supernatant was used for mNGS. The colony of corneal sample grew well on blood agar plate and the resulting colonies were collected and used for mNGS.
Metagenomic sequencing
DNA extraction was performed following JIANSHI BIOTECH Universal DNA/RNA extraction protocol. Library prepared following KingCreate Biotechnology Pathogenic Microorganism Metagenomic DNA Detection Protocol (Miao et al., 2018). Both nucleic acid extraction and library preparation were conducted in parallel with quality control samples. Single-end 75bp sequencing was carried out using Illumina Nextseq 550 System with 75 cycles Reagent Kit. After filtering the low-quality sequencing data by fastp v0.20.0 (Chen et al., 2018) and removing the sequences mapped to the human reference genome using bwa v0.7.10-r789 (Li and Durbin, 2009), the remaining data were aligned to the National Center for Biotechnology Information (NCBI) GenBank database.
Nucleotide identity analysis
The sequences obtained were analyzed using the National Center for Biotechnology Information GenBank database to identify microbial species.
Physiological and morphology studies
Two strains were subcultured on blood agar (BA), Sabouraud medium (SDA) supplemented with chloramphenicol, potato dextrose agar (PDA), and CHROMagar Candida and incubated at 35°C, ambient air to observe growth on different media. Growth rates at different temperatures (25°C and 37°C) on BA, PDA incubated 24 hours, and SDA (without chloramphenicol) incubated for 4d.
Antimicrobial susceptibility testing
By Clinical and Laboratory Standards Institute (CLSI) M38M51S-Ed3 and M100Ed32 protocol, the broth microdilution method and disk diffusion were used for susceptibility testing meanwhile. Zoospores inoculum was obtained by aforementioned zoosporogenesis technique. Upon encystment, the zoospores lose their flagella and become spherical. Zoospores do not produce turbidity as the conidia of filamentous fungi do. Thus, they were counted using a Neubauer chamber. Diluted in RPMI 1640 broth containing L-glutamine and buffered to pH 7.0, yielding a final concentration of 0.4×104~5×104 zoospores/mL for the broth microdilution method and 0.4×106~5×106 zoospores/mL for the disc diffusion method. All experiments were performed in duplicate.
For disk diffusion method, 200 μL of zoospores suspension was spread on the entire surface of a non-supplemented Mueller Hinton agar plate (for antifungal drugs) and Blood Mueller-Hinton with 5% sheep blood agar plate (for antibacterial drugs) by evenly streaking the swab over the entire agar surface. For the broth microdilution method, antimicrobial drugs concentrations ranged from 0.25μg/mL to 32μg/mL. All the plates and microdilution trays were incubated at 35°C, ambient air. The inhibition zone and the MIC were read after 48 hours. MICs were determined by visual observation of 100% growth inhibition compared to the growth in the control well containing no antimicrobial agent, after 48 h of incubation at 35°C. Candida parapsilosis (ATCC 22019) was used as controls for the antifungal drugs susceptibility testing. Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 25923) were used as controls for the for antibacterial drugs susceptibility testing.
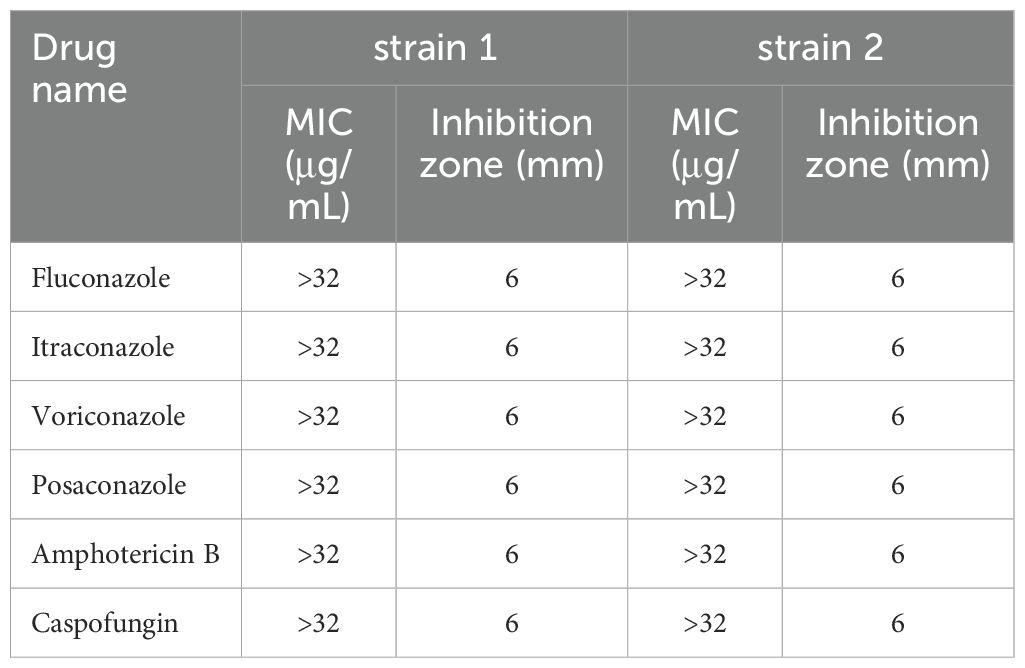
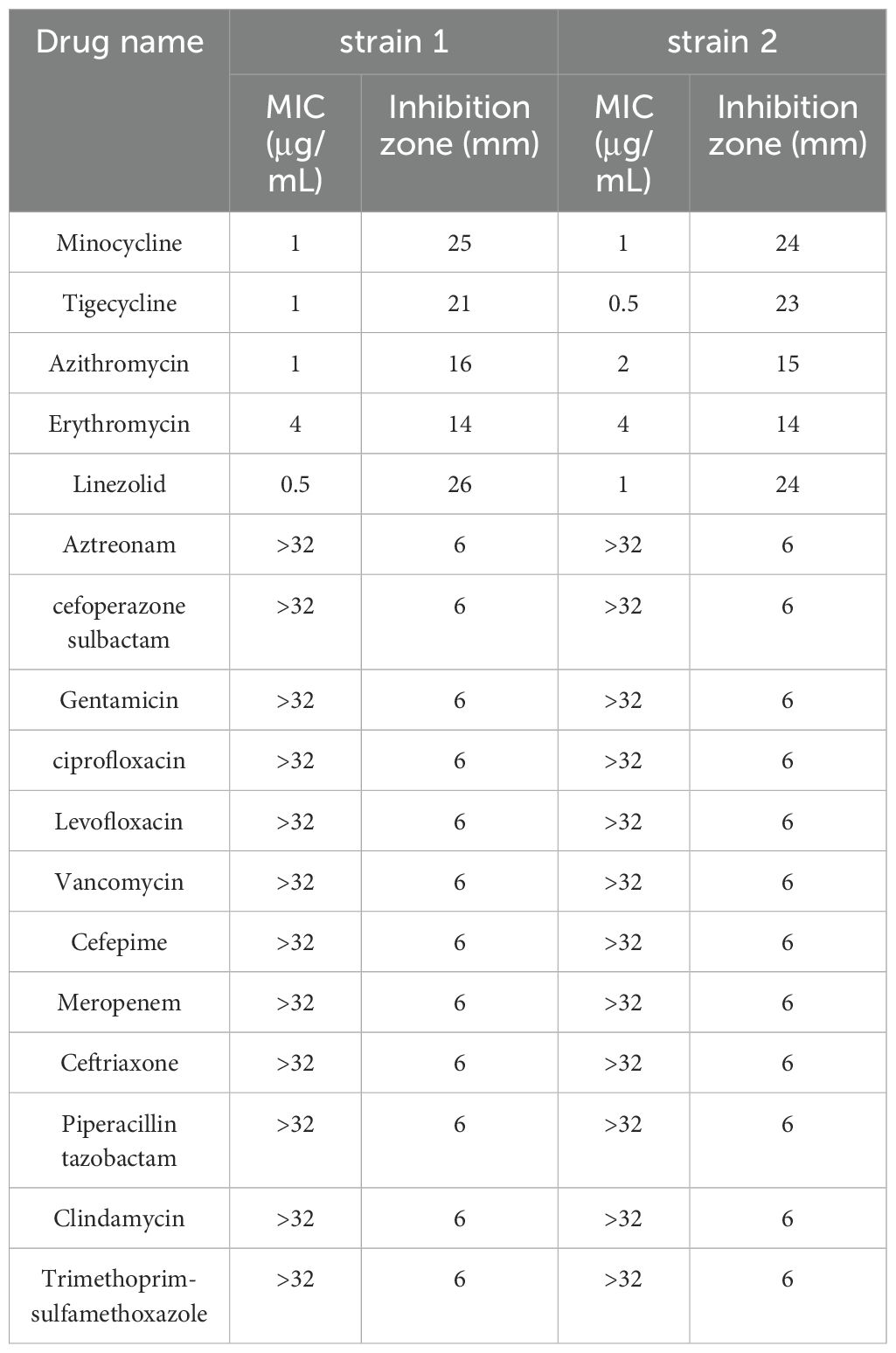
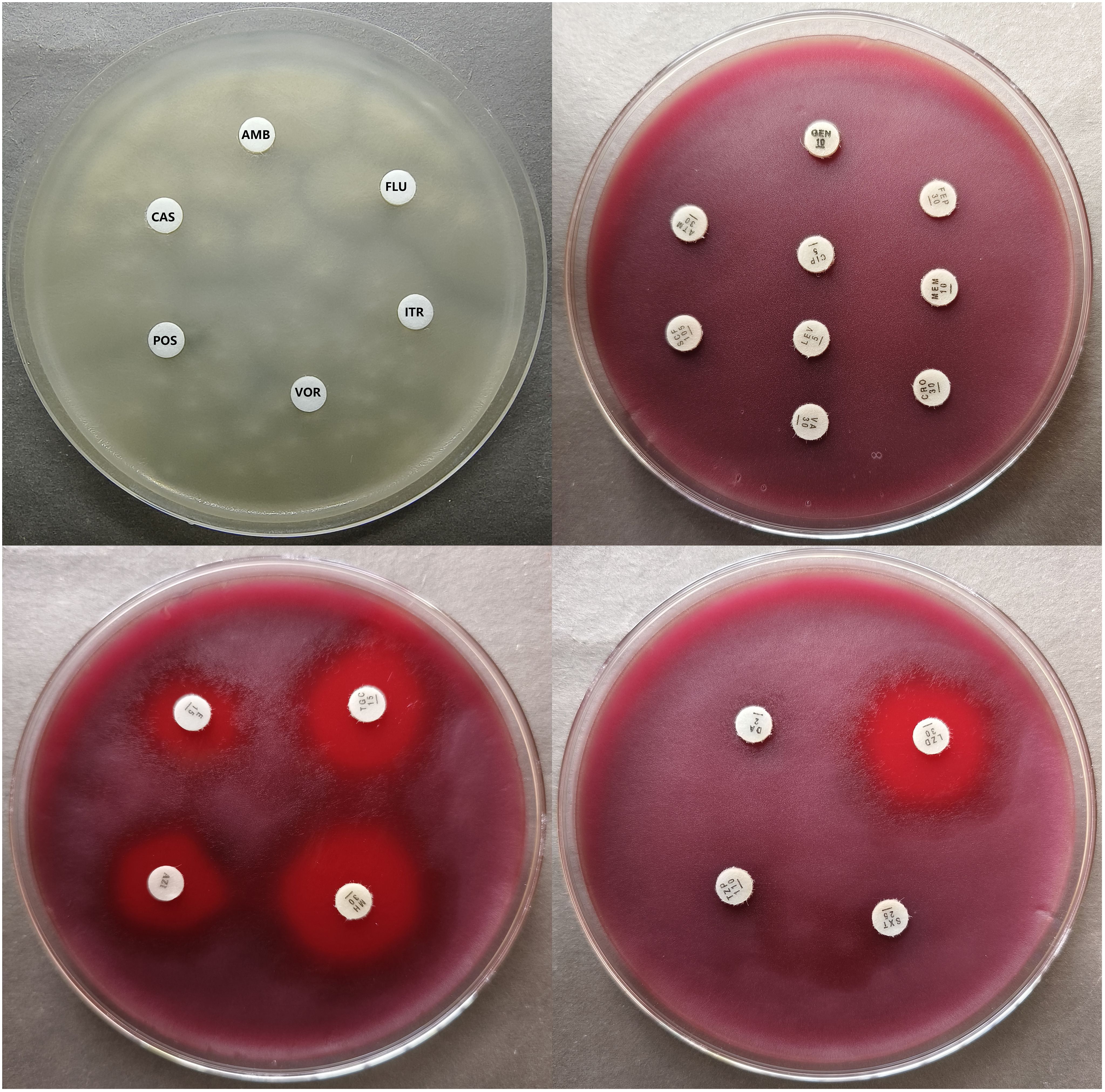
23 antimicrobial agents were tested, including antifungal drugs of amphotericin B (AMB), flucytosine (FLU), itraconazole (ITR), voriconazole (VOR), posaconazole (POS), caspofungin (CAS), and antibacterial drugs of ceftriaxone (CRO), meropenem (MEM), cefepime (FEP), aztreonam (ATM), cefoperazone sulbactam (SCF), piperacillin tazobactam (TZP), vancomycin (VA), levofloxacin (LEV), ciprofloxacin (CIP), gentamicin (GEN), trimethoprim-sulfamethoxazole (SXT), clindamycin (DA), linezolid (LZD), erythromycin (E), azithromycin (AZM), minocycline (MH) and tigecycline (TGC). For disk diffusion method, antifungal drugs were obtained from Sigma Chemical Co. and antibacterial drugs from Oxoid, UK. For the broth microdilution method, all antimicrobial agents susceptibility kits were purchased from Zhuhai DL Biotech. Co., Ltd, China.
Results
Lactophenol cotton blue staining of the colonies showed broad, irregularly branched hyphae. In water culture, sporangium (Figure 3A) and large zoosporangium (20 to 60 µm in diameter, Figure 3B) were observed after induction, zoospores are released after rupture of the zoosporangium membrane. Giemsa staining of zoospores showed two unequal flagella (Figure 3C). Scanning electron microscopy of zoospore showed about 2.5μm in diameter, villose, kidney-like in shape, and two flagella arise from inside a lateral groove (Figures 3D–F). The obtained mNGS nucleotide sequences of two cases were compared with the nearest sequence at the National Center for Biotechnology Information GenBank database. The first homology sequence presenting the highest identity of patient 1 and patient 2 were 100% nucleotide identity with Pythium insidiosum isolate GZ2020 (GenBank No. JAKVDI010000034.1).
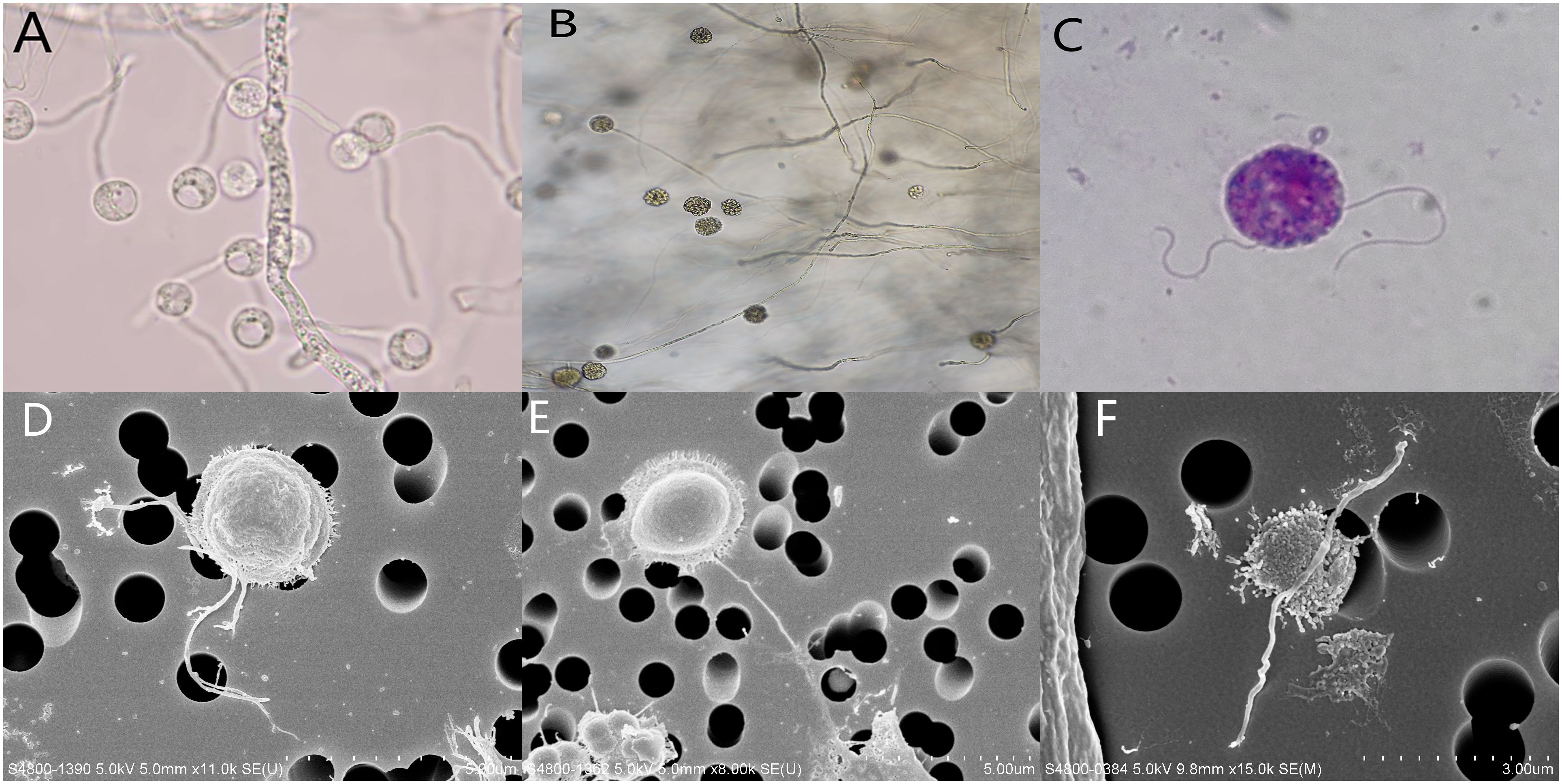
Figure 3. (A) sporangium was observed on direct microscopic examination, ×400; (B) Zoosporangium as observed on direct microscopic examination ×400; (C) Giemsa staining of zoospore showed two unequal flagella, ×1000; (D–F) Scanning electron microscopy of zoospore showed about 2.5μm in diameter, villose, kidney-like in shape, and two flagella arise from inside a lateral groove.
Colonies on blood agar are white to pale yellow, flat or slightly concave, radial, and typically lack aerial hyphae. The mycelium has thin walls, irregular thickness, is readily folded, and is sparsely septate. The growth rate on blood agar was faster than PDA plates, and no colony growth was observed on SDA and CHROMagar Candida (supplemented with chloramphenicol) incubated at 35°C for 24 hours. The results showed that the strain could grow at 25°C and 37°C temperatures, with the 37°C growth rates faster than 25°C on BA. The growth rate of colonies on SDA (not supplemented with chloramphenicol) and PDA does not differ significantly at 25°C and 37°C.
All antifungal drugs showed no inhibition zones for P. insidiosum and the MICs were >32μg/mL, which indicated antifungal drugs have no inhibitory activity against P. insidiosum in vitro. (Table 1). Antibacterial drugs of β-Lactams (ceftriaxone, meropenem, cefepime, aztreonam, cefoperazone-sulbactam, Piperacillin-tazobactam), vancomycin, levofloxacin, ciprofloxacin, gentamicin, trimethoprim-sulfamethoxazole, clindamycin also showed no inhibition zones and the MICs were >32μg/mL (Table 2), which proved that the above-mentioned drugs have no inhibitory activity against P. insidiosum in vitro. MICs of strain 1 and strain 2 for erythromycin were 4μg/mL, for azithromycin was 1μg/mL and 2μg/mL, respectively, for minocycline were 1μg/mL, for tigecycline was 1μg/mL and 0.5μg/mL, respectively, and for linezolid was 0.5μg/mL and 1μg/mL, respectively (Table 2). Minocycline, tigecycline, and linezolid showed significantly inhibition zones (Table 2). The inhibition zones of erythromycin and azithromycin were smaller than minocycline, tigecycline, and linezolid (Table 2). Inhibition zones of the strain 1 after incubating at 35°C for 48 hours were showed in Figure 4. The results showed that minocycline, tigecycline, linezolid, erythromycin and azithromycin have significant in vitro activity against P. insidiosum.
Discussion
Treatment success for pythiosis depends on an early and precise diagnosis. However, medical practitioners’ inexperience of pythiosis hinders prompt and accurate diagnosis, which may lead to incorrect or delayed strategies for treatment. Currently, direct microscopic examination, microbial culture, and induced zoospores assays are the main detection methods. P. insidiosum presented wide, sparsely septate mycelial structure under direct microscopic examination, which is easily misdiagnosed as fungi. The organism is easily mistaken for contaminating fungi and discarded, or is treated incorrectly as fungi resulting in delayed therapy. Zoosporangium and zoospores are characteristic structures of P. insidiosum, which distinguish it from fungi. P. insidiosum grows well on blood agar and potato dextrose agar, but does not grow or grows poorly on SDA plate supplemented with chloramphenicol. The colonies are white or yellow-white, with concave growth, wavy or radiating appearance, and rarely have aerial mycelium. Failure to isolate P. insidiosum may be due to the low-temperature storage of specimens, as the optimal growth temperature for P. insidiosum is 28°C to 32°C, and isolate growth was completely inhibited at 8°C (Krajaejun et al., 2010). At 42°C, most isolates failed to grow, whereas a few isolates grew minimally (Krajaejun et al., 2010). The serological diagnosis of pythiosis usually relies on immunodiffusion tests. Although immunodiffusion tests have high specificity, their sensitivity is poor (Pracharktam et al., 1991). Subsequently, other diagnostic methods have been developed, such as enzyme-linked immunosorbent assay (ELISA) (Krajaejun et al., 2002), immuno-chromatographic tests (Krajaejun et al., 2009), western blotting (Krajaejun et al., 2006a), and PCR assays (Gaastra et al., 2010), which have good specificity and sensitivity. Nevertheless, because pythiosis is rare in China, serological diagnostic testing is not performed commonly because most Chinese hospitals lack the necessary diagnostic supplies and technology. Additionally, such immunological tests cannot diagnose patients with localized infections (e.g., ocular infections). Nucleic acid-based detection (NAT) can effectively and directly detect low levels of P. insidiosum DNA in either trace amounts or culture-negative samples (Sridapan and Krajaejun, 2022). Metagenomic Next-Generation Sequencing is a novel, fast, and robust microbiological detection method, especially in emergency clinical situations where the pathogens are unknown (Sridapan and Krajaejun, 2022). However, this technique is costly and many laboratories are unable to use it, currently limiting its use as a routine microbiological detection analysis (Sridapan and Krajaejun, 2022).
Human pythiosis cases have increased dramatically during the past ten years, infection occurs when an individual has a skin wound or abrasion that comes into contact with the zoospores within the contaminated water (Gaastra et al., 2010). Agricultural related activities or water associated leisure activities are considered to be predisposing factors for human pythiosis (Gaastra et al., 2010). So far reported, there are three Pythium species affecting mammalian hosts: P. aphanidermatum, P. insidiosum and Pythium periculosum (Miraglia et al., 2022). Among them, P. insidiosum is the most common species. The diverse range of Pythium species can greatly affect the epidemiology, diagnosis and management of their infections, especially in the performance of serological diagnosis and effective implementation of immunotherapy of pythiosis (Miraglia et al., 2022). Cutaneous/subcutaneous abscesses caused by P. insidiosum may present with various skin manifestations, such as vesicles/bullae, skin ulcers, cellulitis, chronic swelling, subcutaneous lesions, infiltrative masses and ulcers (Gurnani et al., 2022b). If left untreated, cutaneous/subcutaneous infections may progress to vascular abscesses, leading to limb amputation (Gurnani et al., 2022b). Several studies in Thailand showed that almost all patients with Cutaneous/subcutaneous, vascular, and disseminated pythiosis have underlying hemoglobinopathy-Mediterranean anemia syndrome or blood system diseases including paroxysmal nocturnal hemoglobinuria, aplastic anemia, myeloproliferative disorders, idiopathic thrombocytopenic purpura, and leukemia (Wanachiwanawin et al., 1993; Gurnani et al., 2022b; Chitasombat et al., 2020). Most ocular cases were associated with no underlying diseases (Gurnani et al., 2022b). In our first case, the patient’s left lower limb was infected during contact with swamp water after being bitten by ants. The patient presented with rapidly worsening cutaneous abscesses and ulcers. Decompensated cirrhosis, diabetes, and psoriasis may be potential predisposing factors for pythiosis in the patient.
Most cases of Pythium keratitis have a history of trauma that occurred before (Gurnani et al., 2021). It is difficult to distinguish from fungal keratitis during a slit-lamp examination without a high degree of clinical suspicion. The presence of patchy reticular dot-like subepithelial and stromal infiltrates, multiple infiltrates dispersed throughout the cornea and a stromal infiltrate with hyphated edges that resembles cotton wool are among the distinctive clinical features that differentiate it from fungal keratitis (Anitha and Vanathi, 2021; Gurnani et al., 2022a). Recent in vitro research has led to the recommendation that antibiotics, such as 1% azithromycin and 0.2% linezolid, be used as first-line medications for Pythium keratitis (Gurnani et al., 2022a). However, many cases of Pythium keratitis may not respond well to pharmaceutical treatment because of its high virulence, recurrence rate, and capacity for rapid proliferation. In nonresolving cases, early therapeutic keratoplasty is necessary (Gurnani et al., 2022a). In any case, a proper diagnosis without delay and prompt treatment with the right medications are essential for successful patient management of Pythium keratitis. For our Pythium keratitis case, the patient discontinued the follow-up at the eye clinic. So, its clinical outcome could not be followed although early and correct diagnosis was given.
The treatment for abscesses caused by P. insidiosum involves surgical intervention, medication, and immunotherapy. The key to controlling the disease is a combination of immediate surgical intervention and aggressive medication treatment. Anti-fungal drugs are usually ineffective against P. insidiosum because it does not synthesize ergosterol (Lerksuthirat et al., 2017). A Study showed that itraconazole and terbinafine have synergistic inhibitory effects on the in vitro growth of P. insidiosum and long-term combination therapy with itraconazole and terbinafine has successfully cured P. insidiosum infection (Shenep et al., 1998). Heath, JA, et al. have successfully cured leukemia patients with P. insidiosum pleuropericarditis using liposomal amphotericin B and itraconazole (Heath et al., 2002). However, several studies have shown that antifungal drugs are ineffective against P. insidiosum (Sathapatayavongs et al., 1989; Gurnani et al., 2022b), and Bhupesh Bagga, et al. have compared the rate of therapeutic penetrating keratoplasty (TPK) and proportion of healed ulcers in the group on antifungal therapy (TPK—11/13, 84.6%; Healed—2/13, 15.3%) with the group on antibacterial therapy (TPK—11/17, 64.7%; Healed—6/17, 35.2%). The former has higher rate of TPK and lower proportion of healed ulcers (p=0.21, Fisher’s exact test) (Bagga et al., 2018). Our case suggested that antifungal drugs have no activity in vitro against P. insidiosum.
In a study on in vitro drug sensitivity of pythium, antibiotics such as miltefosine, azithromycin, clarithromycin, josamycin, linezolid, and sutezolid showed good activity and are promising candidate drugs for humans pythiosis (Loreto et al., 2018). Macrolides and tetracycline antibiotics also show good activity to P. insidiosum, with minocycline being considered the most effective drug in testing (Loreto et al., 2011). In our case, we have determined the activity of 17 antibacterial and 6 antifungal drugs against P. insidiosum using broth microdilution and disk diffusion methods in vitro. The lowest MICs and the largest zones of inhibition (disk diffusion) were observed for minocycline, tigecycline and linezolid. All of the zones of inhibition and the MICs were close to the previous studies (Yolanda and Krajaejun, 2020). Up to now, no standard CLSI guideline protocol for in vitro drug susceptibility of P. insidiosum is available. For better interpretation and application of susceptibility test data, it is very necessary to standardize the susceptibility method and correlating drug susceptibility data with clinical outcome of human pythiosis. Maeno et al. successfully treated a 20-year-old Japanese male with P. insidiosum keratitis using a combination of minocycline, linezolid, and chloramphenicol (Maeno et al., 2019). Two cases of keratitis were also successfully cured using a combination of linezolid and azithromycin (Gurnani et al., 2022b, 2023).
In our first case of cutaneous abscess, liver cirrhosis decompensation, diabetes, and psoriasis were considered as potential triggering factors for P. insidiosum infection. Clinicians should have a high suspicion of cutaneous/subcutaneous pythiosis in immunocompromised patients with a history of sewage exposure. It is crucial for patient prognosis. Once diagnosed, patients should receive appropriate treatment, including the use of linezolid, macrolides, minocycline, and tigecycline, which have been proven effective in current research, along with aggressive surgical debridement. Our cases provide a reference for future studies to determine the optimal treatment for cutaneous and subcutaneous pythiosis in immunocompromised patients.
Conclusions
Early and accurate identification, combined with aggressive surgical debridement and appropriate drug therapy, can greatly improve patient managements. Conventional culture and zoospore induction remain gold standard for diagnosis; however, DNA-based method should be performed simultaneously. The drug susceptibility testing provides profound effects on proper drug selection against P. insidiosum.
Data availability statement
The data has been deposited into the National Center for Biotechnology Information GenBank database. GenBank accession number were PQ182808-PQ182826.
Ethics statement
The study was approved by the medical ethics committee of the People’s Hospital of Guangxi Zhuang Autonomous Region. Written informed consent were obtained from the patients for the publication of the cases details.
Author contributions
LH: Writing – original draft. XH: Writing – original draft. NY: Writing – review & editing. HM: Writing – review & editing. LJ: Writing – review & editing. LL: Writing – review & editing. XC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guangxi Natural Science Foundation under Grant No.2024GXNSFBA010122.
Conflict of interest
Author HM was employed by Guangxi Jinyu Medical Laboratory Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anitha, V., Vanathi, M. (2021). Commentary: A retrospective multifactorial analysis of Pythium keratitis and review of the literature. Indian J. Ophthalmol. 69, 1101–1102. doi: 10.4103/ijo.IJO_3660_20
Bagga, B., Sharma, S., Madhuri Guda, S. J., Nagpal, R., Joseph, J., Manjulatha, K., et al. (2018). Leap forward in the treatment of Pythium insidiosum keratitis. Br. J. Ophthalmol. 102, 1629–1633. doi: 10.1136/bjophthalmol-2017-311360
Chen, S., Zhou, Y., Chen, Y., Gu, J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Chitasombat, M. N., Jongkhajornpong, P., Lekhanont, K., Krajaejun, T. (2020). Recent update in diagnosis and treatment of human pythiosis. PeerJ. 8, e8555. doi: 10.7717/peerj.8555
Gaastra, W., Lipman, L. J., De Cock, A. W., Exel, T. K., Pegge, R. B., Scheurwater, J., et al. (2010). Pythium insidiosum: an overview. Vet. Microbiol. 146, 1–16. doi: 10.1016/j.vetmic.2010.07.019
Gurnani, B., Christy, J., Kaur, K., Moutappa, F., Gubert, J. (2023). Successful management of pythium insidiosum keratitis masquerading as dematiaceous fungal keratitis in an immunosuppressed asian male. Ocular Immunol. Inflamm. 2023, 583–586. doi: 10.1080/09273948.2023.2179495
Gurnani, B., Christy, J., Narayana, S., Rajkumar, P., Kaur, K., Gubert, J. (2021). Retrospective multifactorial analysis of Pythium keratitis and review of literature. Indian J. Ophthalmol. 69, 1095–1101. doi: 10.4103/ijo.IJO_1808_20
Gurnani, B., Kaur, K., Venugopal, A., Srinivasan, B., Bagga, B., Iyer, G., et al. (2022a). Pythium insidiosum keratitis - A review. Indian J. Ophthalmol. 70, 1107–1120. doi: 10.4103/ijo.IJO_1534_21
Gurnani, B., Narayana, S., Christy, J., Rajkumar, P., Kaur, K., Gubert, J. (2022b). Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. Eur. J. Ophthalmol. 32, Np87–np91. doi: 10.1177/11206721211006564
Heath, J. A., Kiehn, T. E., Brown, A. E., LaQuaglia, M. P., Steinherz, L. J., Bearman, G., et al. (2002). Pythium insidiosum pleuropericarditis complicating pneumonia in a child with leukemia. Clin. Infect. Dis. 35, E60–E64. doi: 10.1086/342303
Kaufman, L. (1998). Penicilliosis marneffei and pythiosis: emerging tropical diseases. Mycopathologia. 143, 3–7. doi: 10.1023/A:1006958027581
Krajaejun, T., Chongtrakool, P., Angkananukul, K., Brandhorst, T. T. (2010). Effect of temperature on growth of the pathogenic oomycete Pythium insidiosum. Southeast Asian J. Trop. Med. Public Health. 41, 1462–1466.
Krajaejun, T., Imkhieo, S., Intaramat, A., Ratanabanangkoon, K. (2009). Development of an immunochromatographic test for rapid serodiagnosis of human pythiosis. Clin. Vaccine Immunol.: CVI 16, 506–509. doi: 10.1128/CVI.00276-08
Krajaejun, T., Kunakorn, M., Niemhom, S., Chongtrakool, P., Pracharktam, R. (2002). Development and evaluation of an in-house enzyme-linked immunosorbent assay for early diagnosis and monitoring of human pythiosis. Clin. Diagn. Lab. Immunol. 9, 378–382. doi: 10.1128/CDLI.9.2.378-382.2002
Krajaejun, T., Kunakorn, M., Pracharktam, R., Chongtrakool, P., Sathapatayavongs, B., Chaiprasert, A., et al. (2006a). Identification of a novel 74-kiloDalton immunodominant antigen of Pythium insidiosum recognized by sera from human patients with pythiosis. J. Clin. Microbiol. 44, 1674–1680. doi: 10.1128/JCM.44.5.1674-1680.2006
Krajaejun, T., Sathapatayavongs, B., Pracharktam, R., Nitiyanant, P., Leelachaikul, P., Wanachiwanawin, W., et al. (2006b). Clinical and epidemiological analyses of human pythiosis in Thailand. Clin. Infect. Dis. 43, 569–576. doi: 10.1086/506353
Lerksuthirat, T., Sangcakul, A., Lohnoo, T., Yingyong, W., Rujirawat, T., Krajaejun, T. (2017). Evolution of the sterol biosynthetic pathway of pythium insidiosum and related oomycetes contributes to antifungal drug resistance. Antimicrob. Agents Chemother. 61. doi: 10.1128/AAC.02352-16
Li, H., Durbin, R. (2009). Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 25, 1754–1760. doi: 10.1093/bioinformatics/btp324
Loreto, E. S., Mario, D. A., Denardi, L. B., Alves, S. H., Santurio, J. M. (2011). In vitro susceptibility of Pythium insidiosum to macrolides and tetracycline antibiotics. Antimicrob. Agents Chemother. 55, 3588–3590. doi: 10.1128/AAC.01586-10
Loreto, E. S., Tondolo, J. S. M., Oliveira, D. C., Santurio, J. M., Alves, S. H. (2018). In Vitro Activities of Miltefosine and Antibacterial Agents from the Macrolide, Oxazolidinone, and Pleuromutilin Classes against Pythium insidiosum and Pythium aphanidermatum. Antimicrob. Agents Chemother. 62. doi: 10.1128/AAC.01678-17
Maeno, S., Oie, Y., Sunada, A., Tanibuchi, H., Hagiwara, S., Makimura, K., et al. (2019). Successful medical management of Pythium insidiosum keratitis using a combination of minocycline, linezolid, and chloramphenicol. Am. J. Ophthalmol. Case Rep. 15, 100498. doi: 10.1016/j.ajoc.2019.100498
Miao, Q., Ma, Y., Wang, Q., Pan, J., Zhang, Y., Jin, W., et al. (2018). Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin. Infect. Dis. 67, S231–s240. doi: 10.1093/cid/ciy693
Miraglia, B. M., Mendoza, L., Rammohan, R., Vilela, L., Vilela, C., Vilela, G., et al. (2022). Pythium insidiosum complex hides a cryptic novel species: Pythium periculosum. Fungal Biol. 126, 366–374. doi: 10.1016/j.funbio.2022.03.002
Perkins, M. J., Rosario, D. J., Wickes, B. L., Krajaejun, T., Sherwood, J. E., Mody, R. M. (2022). Severe skin and soft tissue pythiosis acquired in a hot spring in the southwestern United States, a case report and review of North American cases. Travel Med. Infect. Dis. 48, 102349. doi: 10.1016/j.tmaid.2022.102349
Pracharktam, R., Changtrakool, P., Sathapatayavongs, B., Jayanetra, P., Ajello, L. (1991). Immunodiffusion test for diagnosis and monitoring of human pythiosis insidiosi. J. Clin. Microbiol. 29, 2661–2662. doi: 10.1128/jcm.29.11.2661-2662.1991
Sathapatayavongs, B., Leelachaikul, P., Prachaktam, R., Atichartakarn, V., Sriphojanart, S., Trairatvorakul, P., et al. (1989). Human pythiosis associated with thalassemia hemoglobinopathy syndrome. J. Infect. Dis. 159, 274–280. doi: 10.1093/infdis/159.2.274
Shenep, J. L., English, B. K., Kaufman, L., Pearson, T. A., Thompson, J. W., Kaufman, R. A., et al. (1998). Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin. Infect. Dis. 27, 1388–1393. doi: 10.1086/515042
Sridapan, T., Krajaejun, T. (2022). Nucleic acid-based detection of pythium insidiosum: A systematic review. J. fungi (Basel Switzerland) 9. doi: 10.3390/jof9010027
Thanathanee, O., Enkvetchakul, O., Rangsin, R., Waraasawapati, S., Samerpitak, K., Suwan-apichon, O. (2013). Outbreak of Pythium keratitis during rainy season: a case series. Cornea 32, 199–204. doi: 10.1097/ICO.0b013e3182535841
Wanachiwanawin, W., Thianprasit, M., Fucharoen, S., Chaiprasert, A., Sudasna, N., Ayudhya, N., et al. (1993). Fatal arteritis due to Pythium insidiosum infection in patients with thalassaemia. Trans. R. Soc. Trop. Med. Hygiene 87, 296–298. doi: 10.1016/0035-9203(93)90135-D
Yolanda, H., Krajaejun, T. (2020). Review of methods and antimicrobial agents for susceptibility testing against Pythium insidiosum. Heliyon 6, e03737. doi: 10.1016/j.heliyon.2020.e03737
Keywords: pythiosis, Pythium insidiosum, pythium keratitis, cutaneous and subcutaneous pythiosis, antimicrobial agent susceptibility
Citation: Hu L, Huang X, Yee NH, Meng H, Jiang L, Liang L and Chen X (2024) Pythium insidiosum: an emerging pathogen that is easily misdiagnosed and given treatment as a fungus. Front. Cell. Infect. Microbiol. 14:1430032. doi: 10.3389/fcimb.2024.1430032
Received: 09 May 2024; Accepted: 05 August 2024;
Published: 29 August 2024.
Edited by:
Maryam Roudbary, The University of Sydney, AustraliaReviewed by:
Sylwia Andrzejczuk, Medical University of Lublin, PolandFatemeh Nikoomanesh, Birjand University of Medical Sciences, Iran
Copyright © 2024 Hu, Huang, Yee, Meng, Jiang, Liang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingchun Chen, MzY5NjA5MDIwQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Liuyang Hu
Liuyang Hu Xiulu Huang
Xiulu Huang Ngan Hung Yee3
Ngan Hung Yee3