- 1Clinical Research Center, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Clinical Laboratory, Zhoupu Hospital, Shanghai University of Medicine and Health Sciences, Shanghai, China
- 3Department of Pharmacy, The State Administration of Traditional Chinese Medicine (SATCM) Third Grade Laboratory of Traditional Chinese Medicine Preparations, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
Objectives: Many studies have confirmed that antibacterial agents can disrupt the human gut microbiota. In China, Tanreqing injection (TRQ) is a drug with antibacterial activity that is widely used in the treatment of respiratory infections. However, its specific influence on gut microbiota remains unclear. This study aimed to investigate the effect of TRQ on the gut microbiota of healthy volunteers.
Methods: Twelve healthy adults received 20 ml of TRQ intravenously daily for 7 consecutive days. At six timepoints (Pre, on D1, D3, D5, D7 and follow-up visit) fecal samples were collected and analyzed using 16S rRNA gene sequencing.
Results: Eleven people were included in the analysis finally. TRQ did not significantly alter gut microbiota diversity or richness (Shannon and Simpson and Chao1 index) in healthy people during the intervention. Gut microbial structure was stable (weighted and unweighted Unifrac). Using a machine learning method based on PLS-DA analysis, the separation trend on D7 at the genus level was found, returning to baseline two days after discontinuation. The abundance of major genus fluctuated on D7 compared with that prior to treatment, including an increase of unclassified_f_Enterobacteriaceae (13.0611%), a decrease of Bifidobacterium and Escherichia-Shigella (6.887%, 10.487%). Functional prediction analysis did not reveal any significant difference.
Conclusions: Our study showed short-term use of TRQ at conventional doses may not cause perturbations to the gut microbiota in healthy adults. This finding provides some useful information for the safe use of TRQ in the treatment of respiratory infections.
Clinical trial registration: https://www.medicalresearch.org.cn/, identifier MR-31-24-014367.
1 Introduction
The gut microbiota is a complex microbial network that is closely related to human health. Gut microbiota significantly influences intestinal barrier function and the maturation of intestinal immunity, thus protecting against pathogen invasion and colonization (Caballero-Flores et al., 2023). In addition, the gut microbiota performs a major role in the absorption and metabolism of nutrients, hormone secretion, and toxin degradation (Li et al., 2022b). Altered gut microbial balance can lead to diseases like intestinal inflammation, kidney disease, autoimmune disorders, and a higher risk of Clostridium difficile infection (Carding et al., 2015; Becattini et al., 2016; Aliberti et al., 2019; Ling et al., 2022). Antibiotics are the main strategies for fighting against infectious diseases, but they can affect the commensal microbiota (Ramirez et al., 2020). The intestine is the most concentrated place for bacteria in the body and is often affected by antibiotics (Dinan and Dinan, 2022). Many studies have confirmed that antibacterial agents can disrupt the human gut microbiota. A broad-spectrum 4-day antibiotics course with vancomycin, gentamycin and meropenem induced shifts in normal human gut microbiota composition (Mikkelsen et al., 2015). In healthy volunteers, a 5–10day course of ciprofloxacin or clindamycin resulted in a drop in microbial diversity and shifts in community composition (Haak et al., 2019). Ceftazidime/avibactam had a significant ecological impact on the normal human gut microbiota (Rashid et al., 2015). In critically ill patients, anti-anaerobic antibiotics are particularly disruptive to gut microbiota, leading to an increased risk of hospital-acquired pneumonia and nosocomial infectious diseases (Chanderraj et al., 2023). Frequent use of antibiotics in patients with chronic kidney disease (CKD) can modify the relative count of bacterial community members and reduce their diversity (Rysz et al., 2021). Gut microbiota dysbiosis and decreased renal clearance cause increased levels of uremic toxins (e.g., Trimethylamine-N-Oxide, p-cresyl and indoxyl sulfates) in CKD (Guo et al., 2024a; Xie et al., 2024a; Zhou et al., 2024a). Gut-microbiota-derived uremic toxins induce gastrointestinal inflammation, and ultimately diarrhea (Li et al., 2021; Mosterd et al., 2021; Zhou et al., 2024b). Antibiotics dramatically reduce gut microbe abundance and diversity, leading to an increased risk of adverse clinical outcomes and the development of bacterial antibiotic resistance (Palleja et al., 2018; Rysz et al., 2021; Duan et al., 2022). Therefore, studying the effects of interventions on the gut microbiota will facilitate the adoption of appropriate therapeutic regimens and reduce the aforementioned risks (Dethlefsen and Relman, 2011).
Several studies have shown that Chinese medicine intervention induced structural changes in gut microbiota (Wu and Tan, 2019; Li et al., 2024b; Xie et al., 2024b). Tanreqing injection (National Drug Permit No. Z20030054) is a commonly used drug for treating respiratory infections in China (Fan et al., 2023; Ma et al., 2024). It has several activities such as antibacterial, antiviral and anti-inflammatory (Li et al., 2024a; Guo et al., 2024b). Since Tanreqing injection (TRQ) first became commercially available in 2003, it has been recommended by many clinical guidelines and treatment protocols, such as Guideline on treating community-acquired pneumonia and the COVID-19 Diagnosis and Treatment Guidelines of China (Chun et al., 2021; Wang et al., 2023). TRQ is a standardized formulation consisting of Radix scutellariae, Flos lonicerae, Fructus forsythiae, Fel ursi, and Cornu gorais, with baicalin, chlorogenic acid, ursodeoxycholic acid, and chenodeoxycholic acid as the major bioactive constituents (Wang et al., 2021; Xie et al., 2021). TRQ has good antibacterial activity, it not only affects the expression of virulence genes in Staphylococcus aureus but also targets cell division to inhibit cell growth, ultimately leading to cell death (Yang et al., 2022). Meanwhile, TRQ can also inhibit the quorum sensing system of Pseudomonas aeruginosa, the formation of Klebsiella pneumoniae biofilm, and the drug resistance of Pseudomonas aeruginosa (Li et al., 2024a; Yang et al., 2020; Zhang et al., 2024). TRQ is an effective drug for combating bacterial infections, its standard treatment involves a 7-day course of 20 ml intravenous doses for adults. Recently, some studies have found that compared to antibiotics alone, TRQ combined with antibiotics has higher antibacterial activity against respiratory infections, particularly against drug-resistant strains such as Streptococcus pneumoniae and Staphylococcus aureus (Pan et al., 2016; Zhang et al., 2019; Li et al., 2022a). The combination treatment has become a popular option due to improved efficacy and reduced drug resistance (Tong et al., 2021; Zhou et al., 2021; Peng et al., 2022; Han et al., 2023). Although TRQ is a commonly used drug for the treatment of respiratory infections, its effect on gut microbiota is unknown.
The study aimed to assess the effect of TRQ on the gut microbiota of healthy adult volunteers at the recommended dose and regimen, which will facilitate the safe use of TRQ clinically.
2 Methods
2.1 Study design
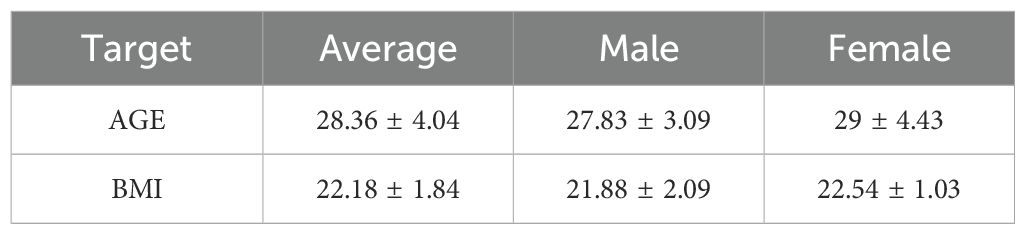
Inclusion criteria for men and women were age 18 to 45 years and non-clinically significant findings in the medical history, physical examination, vital signs, electrocardiogram and blood laboratory results. Each volunteer had a body mass index (BMI) between 19 and 26kg/m2 (Table 1). The volunteers were willing to take adequate contraceptive measures during the entire study period and for 90 days after completion of the study. Volunteers understood and signed an informed consent form at the screening visit prior to any investigational procedure. Exclusion criteria were as follows: underlying known or previous clinically significant oncologic, pulmonary, hepatic, gastrointestinal, cardiovascular, hematologic, metabolic, neurological, immunologic, nephrological, endocrine, or psychiatric disease or current infection; drug or food allergy, drug or alcohol abuse; persons who had used drugs, vitamins, dietary supplements or herbal supplements within 14 days before the first dose of study drug; consumption of Seville oranges or products containing Seville orange components, grapefruit, grapefruit juice, or juices containing grapefruit, within 14 days before the first dose of the study drug; pregnant, risk of becoming pregnant, or breastfeeding during the study period; persons with a positive test for human immunodeficiency virus, hepatitis B virus surface antigen, or anti-hepatitis C virus antibodies. They were secondary excluded if they did not provide any fecal sample before the first dose of the study drug, or if more than 1 fecal sample was missing between day 1 and day 7 after treatment initiation.
2.2 Ethics and treatments
We recruited 12 adult healthy volunteers (6 males, 6 females) for an open, single-center clinical trial at the Clinical Research Center of Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China from August to September 2023 (Figure 1). All volunteers signed an informed consent form before screening. The trial was approved by the Ethics Committee of Shuguang Hospital of Shanghai University of Traditional Chinese Medicine on June 7, 2023 (2023-1332-99-01). This trial has been registered in the national health Security information platform and medical research registration information system (no.: MR-31-24-014367). Subjects did not consume any supplements or foods during the trial that might affect gut function or microbiota. Drinking and other conditions such as uniform meals were therefore restricted for all subjects.
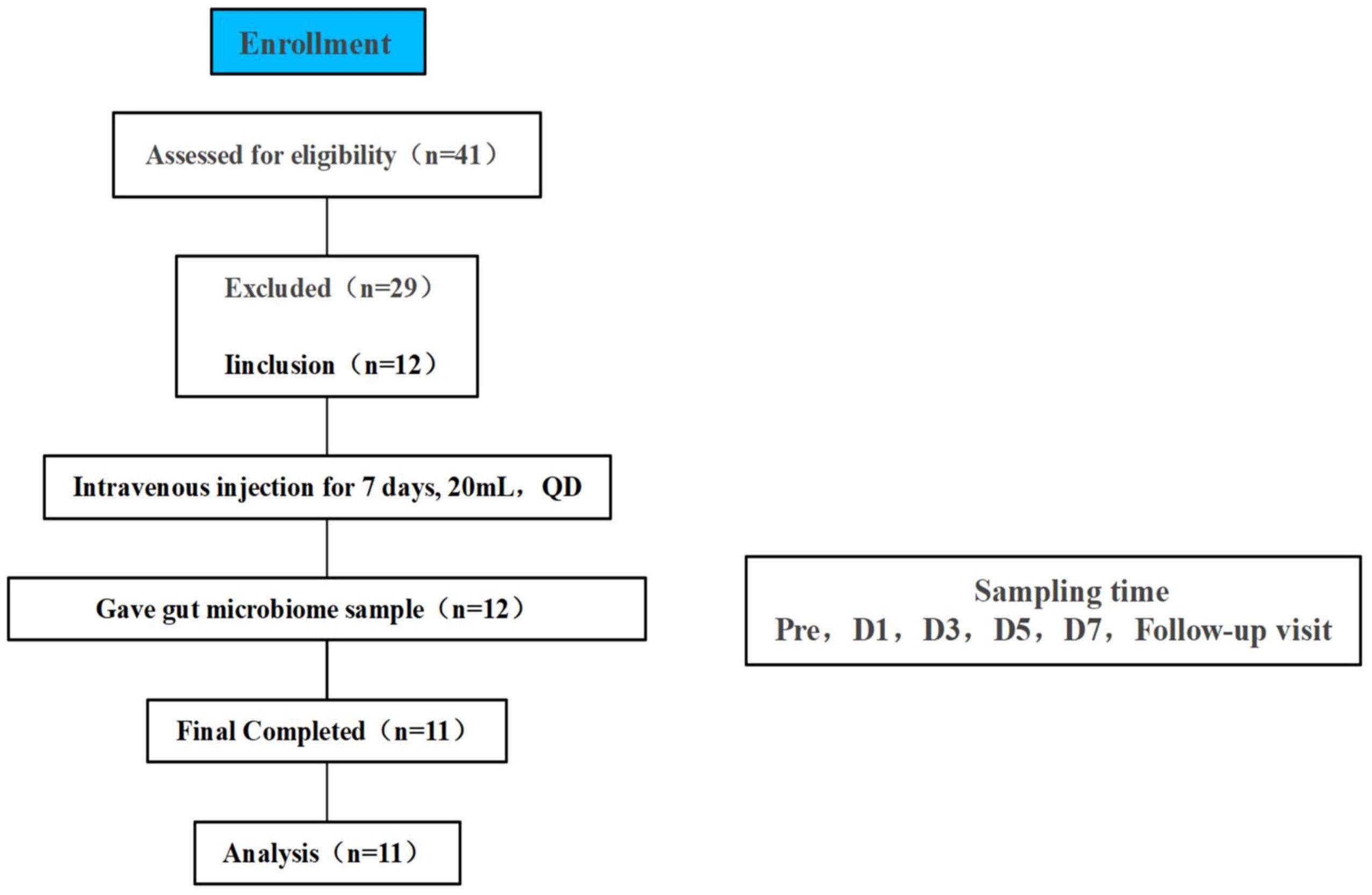
Figure 1. Flowchart of study subjects, a total of 41 volunteers were recruited for initial screening, 12 healthy volunteers participated in this study through the screening conditions, 1 volunteer did not collect a stool sample at the time of Pre therefore 11 people were finally included in the final analysis, TRQ was administered intravenously for 7 consecutive days at 20 mL once daily.
Volunteers were intervened by TRQ (20mL/24hours) from day1 to day7. Briefly, 20mL TRQ was mixed with 230mL 0.9% sodium chloride solution as 120 minutes intravenous infusions using an automatic high precision infusion pump. Day 1 was defined as the first day of antibiotic treatment.
TRQ (no.: 2304105) was obtained by the Shanghai Kai Bao Pharmaceutical Co. Ltd. (Shanghai, China). 0.9% Sodium chloride injection (no.: WA2306027) came from Huaren Pharmaceutical Co. Ltd(Qingdao, China)was used for dilutions.
2.3 Sample collection
Fecal samples were collected from healthy volunteers at six time points: before dosing (Pre), the first day of dosing (D1), third day of dosing (D3), fifth day of dosing (D5), seventh day of dosing (D7), and two days of discontinuation (Follow-up visit). Stool samples were placed in sterile containers and transferred to a -80°C refrigerator within 30 minutes of defecation for storage until processing.
2.4 Sample processing and sequencing
Total microbial genomic DNA was extracted from the above samples using the OMEGA Stool DNA Kit (Omega Bio-tek, Georgia, U.S.) according to the manufacturer’s instructions. The quality and concentration of DNA were determined by 1.0% agarose gel electrophoresis and a NanoDrop® ND-2000 spectrophotometer (Thermo Scientific Inc., USA) and kept at -80 °C prior to further use. The hypervariable region V3-V4 of the bacterial 16S rRNA gene sequencing were amplified with primer pairs 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R(5’-GGACTACHVGGGTWTCTAAT-3’) by an ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, USA) (Liu et al., 2016). The PCR mixture consisted of 4 μL 5× Fast Pfu buffer, 2 μL 2.5 mM dNTPs, 0.8 μL each primer, 0.4 μL FastPfu polymerase, 0.2 μL BSA, 10 ng template DNA, and ddH2O up to 20 µL. PCR cycling conditions: initial denaturation at 95°C for 3 min; 27 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s; final extension at 72°C for 10 min, and hold at 10°C. All samples were amplified in triplicate. The PCR product was extracted from 2% agarose gel and purified. Then quantified using Quantus™ Fluorometer (Promega, USA).
Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina PE300 platform (Illumina, San Diego, USA) according to the standard protocols by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China).
2.5 Data processing and statistical analysis
Bioinformatic analysis of the gut microbiota was carried out using the Majorbio Cloud platform (https://cloud.majorbio.com). Based on the OTUs information, rarefaction curves and alpha diversity indices including observed OTUs, Shannon index and Simpson index and Chao1 index were calculated with Mothur v1.30.2 (Schloss et al., 2009). Sample microbial community similarities were analyzed with PCoA using weighted and unweighted Unifrac distances, via the Vegan v2.4.3 package. Treatment variation and significance were assessed with the PERMANOVA test using the Vegan v2.4.3 package. Partial least square discriminant analysis (PLS-DA) was used to analyze small between-group differences and large within-group variations, effectively identifying the affecting variables. PcoA and PLS-DA were utilized to quantify compositional differences, thus indicating the beta diversity within the bacterial community samples.
The linear discriminant analysis (LDA) effect size (LEfSe) was used to analyze the dominant flora to detect intergroup differences in the sample microbiota. LEfSe analysis (http://huttenhower.sph.harvard.edu/LEfSe) identified significantly abundant bacterial taxa from phylum to genera among groups (Segata et al., 2011); PICRUSt2 analysis predicted the functional composition of microbial genomes by calculating Clusters of Orthologous Groups (COG) and Kyoto Encyclopedia of Genes and Genomes (KEGG) abundances, normalizing OTU tables, and comparing green gene ID. Bugbase phenotype prediction utilized pre-calculated files to predict microbial phenotypes post-OTU normalization. To control for the risk of false positives due to multiple testing, False Discovery Rate (FDR) correction was applied in this study. All p-values from statistical tests were adjusted using FDR. In this paper, only those P < 0.05 were considered statistically significant.
3 Result
Eleven subjects were included in the analysis, and one of them was not sampled at Pre due to the lack of pre-and post-test mechanical controls. No adverse reactions were observed at any time point during the study.
3.1 α- and β- Diversities
To know the effect of TRQ on gut microbial community structure, we performed 16S rRNA gene sequencing and analysis. Using the α-diversity index to assess species richness and evenness (Shannon and Simpson and Chao1 diversity indices). Species diversity during administration and two days after drug withdrawal with median Shannon index values of 2.90, 3.20, 3.10, 3.11, 3.20 and 3.12. These changes were not statistically significant (P> 0.05) (Figures 2A–C). Furthermore, TRQ had no significant effect on β-diversity of microbial communities (Figures 2D, E). Sample dispersion across time points was not significant in both weighted and unweighted Unifrac distances analyses. Besides, although the indexes of richness and diversity failed to show a notable change, partial least squares-discriminant analysis (PLS-DA) plots showed that a distinct dissociation trend emerged on D7 at the genus level (Figure 3A), returning to baseline two days after discontinuation. However, no significant sample dispersion at the phylum level (Figure 3B). For the genus level, subjects L05, L08 and L09 exhibited pronounced dissociation on D7. But the PERMANOVA test analysis did not reveal any significant differences between the groups. Similarly, the LEfSe tool did not identify any distinctive microbial biomarkers (Supplementary Figure S1, LDA > 2).
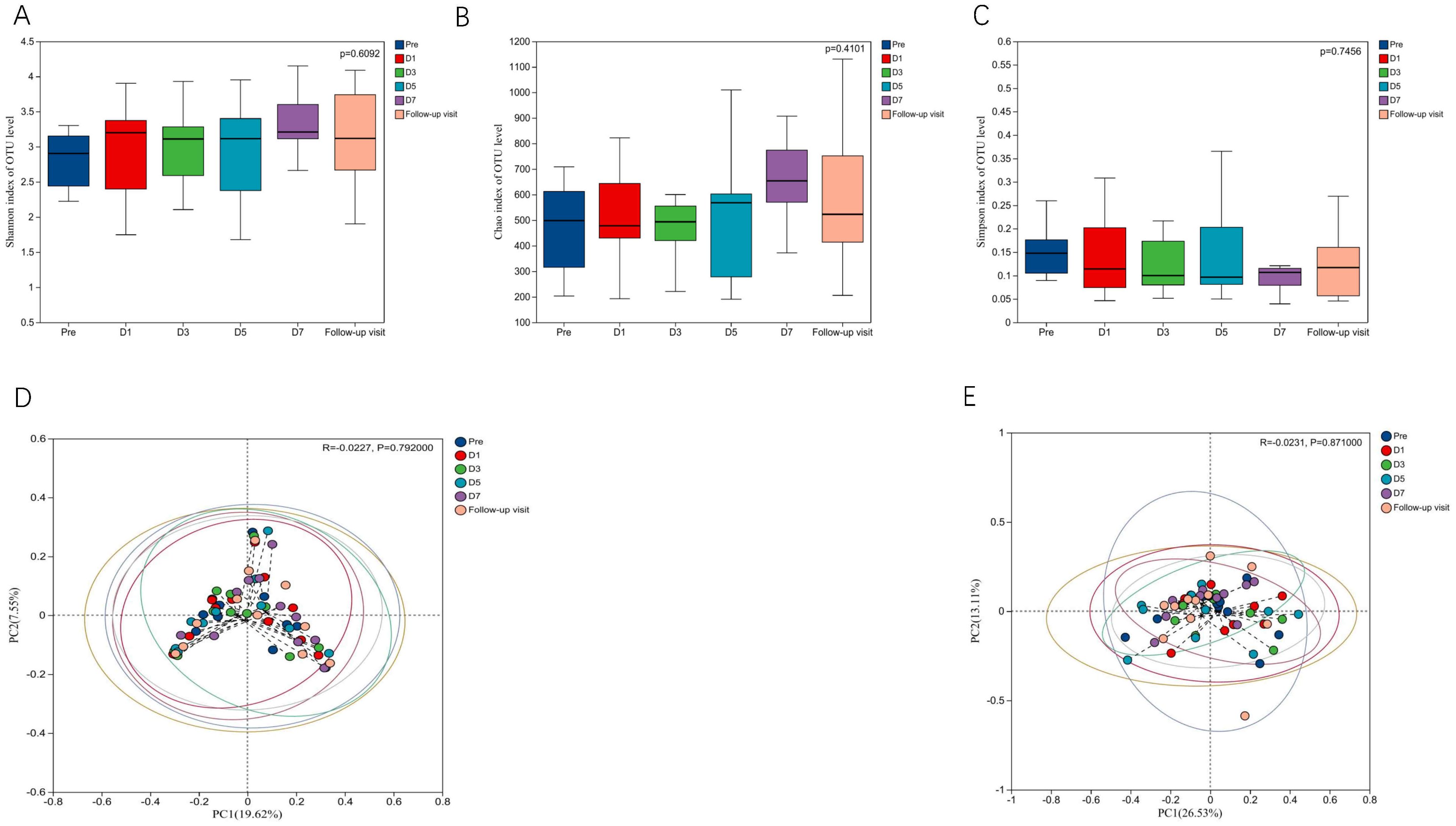
Figure 2. Effects of TRQ on α and β diversity of gut microbiota in healthy volunteers. (A) Shannon index and (B) Simpson index and (C) Chao1 index were used to assess species richness and evenness to indicate changes in α diversity levels, and data are shown in a box plot with the middle horizontal line representing the median. (D) Unweighted and (E) weighted PCoA were used to show the similarities and differences between samples at each time point, and to evaluate the changes in gut microbial community structure by looking at the clustering and dispersion of sample points. Changes in gut microbial community structure were evaluated by testing the clustering and dispersion of sample points, and the samples at different time points were represented by different colors.
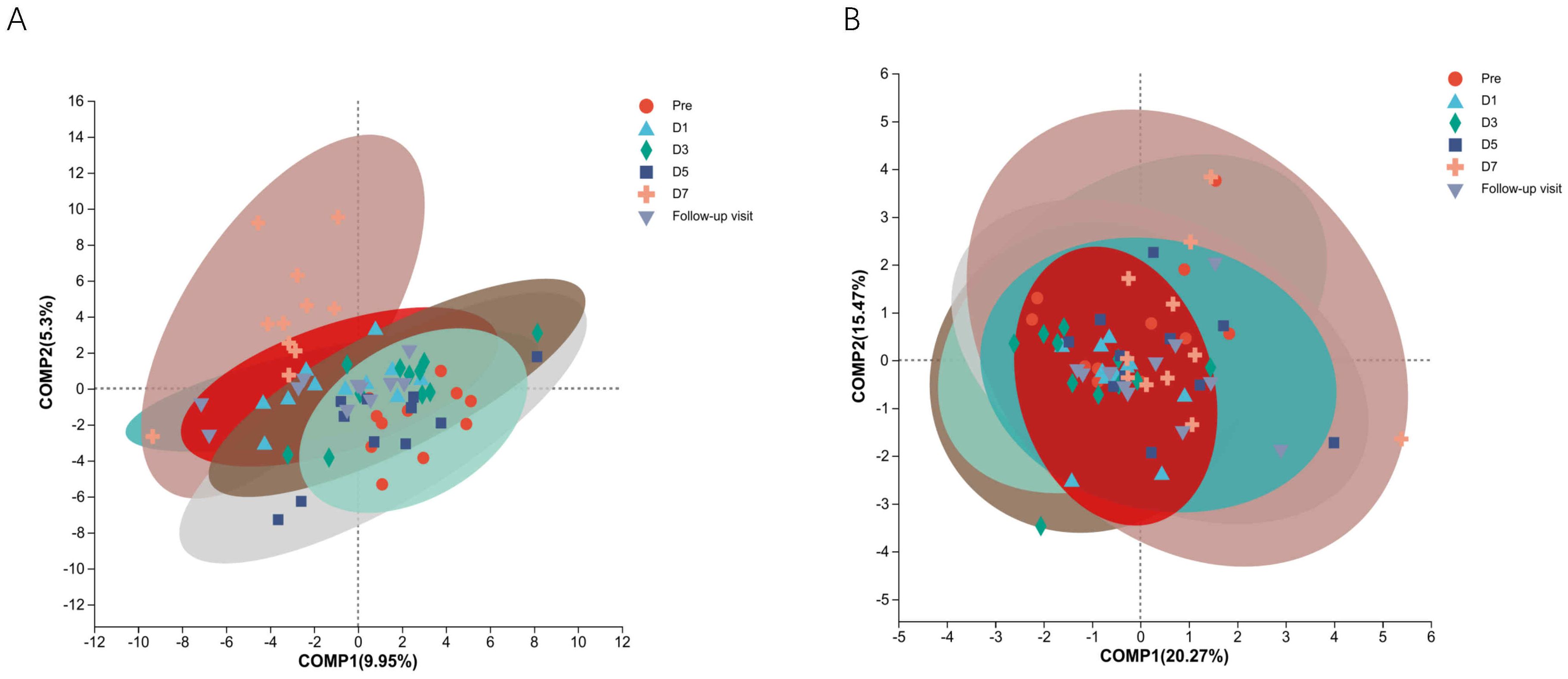
Figure 3. The study utilized Partial Least Squares Discriminant Analysis (PLS-DA) to identify the structural differences in microbial communities between groups, specifically samples from different time points. (A) Shows information on structural differences between time point samples at the genus level; (B) Shows information on structural differences between time point samples at the phylum level. Different colors and shapes represent different time points and samples.
3.2 Effects on microbiota community composition
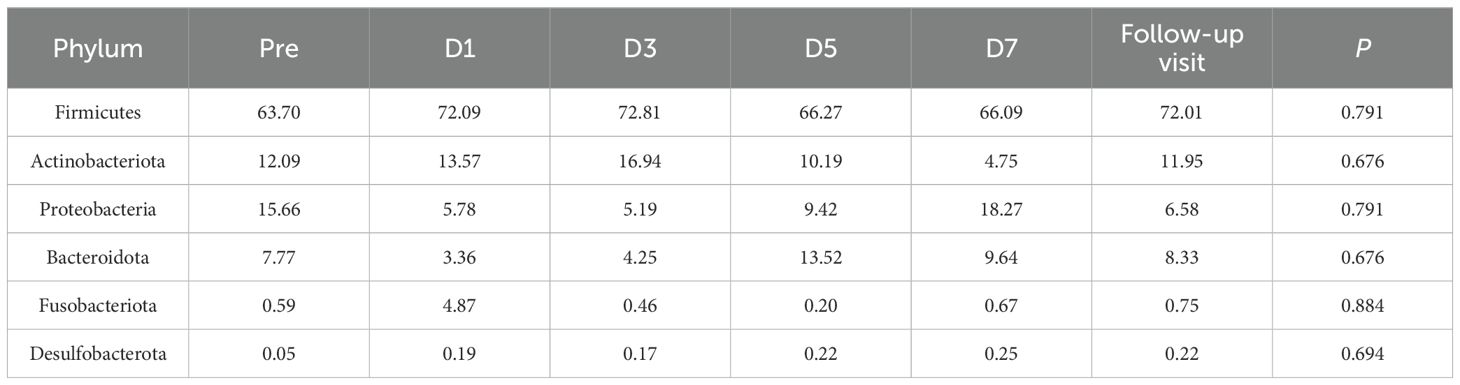
β-diversity analysis indicates changes in gut microbial community structure on D7. To explore the potential reasons for these changes, we examined the major gut microbial flora across all taxonomic levels. The dominant flora has an important effect on the stability of microbial communities. Our study found that the gut microbiome mainly consists of four phyla: Firmicutes, Actinobacteriota, Proteobacteria and Bacteroidota (Figure 4A). In exploratory analysis of mean abundance at the phylum level, it was observed that Firmicutes increased on D1, D3, and the follow-up visit, while Actinobacteriota decreased on D7. Additionally, there was a decrease in Proteobacteria during dosing (Table 2). Class, order, and family level analyses showed increases in Clostridia, Lachnospirales, and Lachnospiraceae on D3. Gammaproteobacteria, Enterobacteriales and Enterobacteriaceae decreased during administration; Actinobacteria, Bifidobacteriales and Bifidobacteriaceae were reduced on D7. Two days after drug withdrawal, there was an increase in Negativicutes, Veillonellales-Selenomonadales and Selenomonadaceae (Supplementary Figures S2-S4, Supplementary Tables S1-S3). At the genus level, Bifidobacterium decreased by 6.887% on D7, specifically in subjects L05 and L08. Escherichia-Shigella decreased by 10.487%, especially in subjects L03, L06 and L09. Unclassified_f_Enterobacteriacea increased by 13.0611%, particularly in subjects L05, L08 and L09 (Figure 4B, Supplementary Table S4). No differences in the aforementioned species were observed after multiple tests analysis.
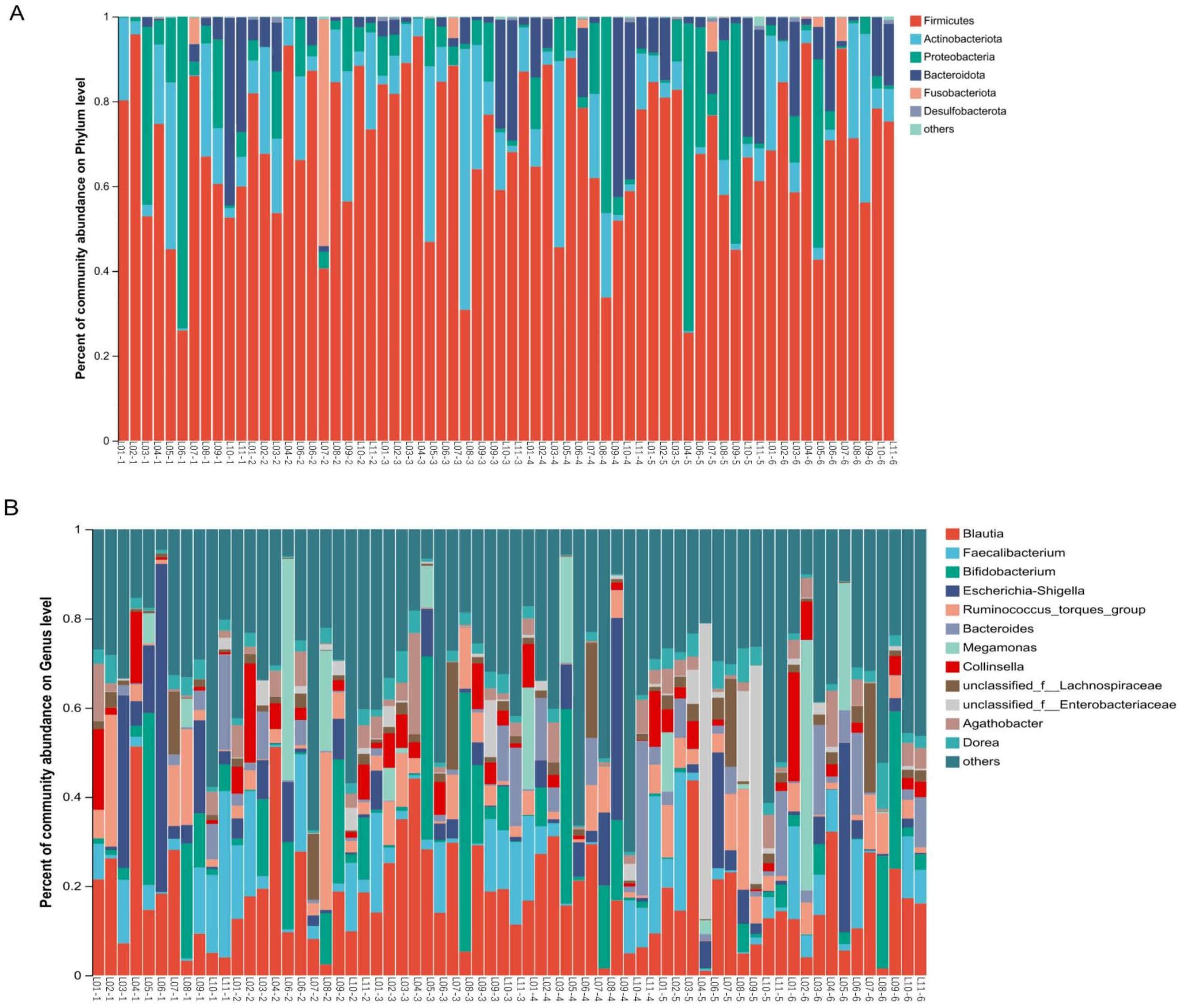
Figure 4. Effects of TRQ use for one week on gut microbial composition (phylum and genus level) in healthy subjects. Fecal samples were collected at various time points: before the trial began (Pre), on the first day of administration (D1), on the third day of administration (D3), on the fifth day of administration (D5), on the seventh day of administration (D7), and two days after discontinuation of the drug (Follow-up visit). Each bar represents a single sample, and the names of the upper and lower samples correspond to each other. The colors used to distinguish Phylum (A) and Genus (B) represents the occupancy. The top 6 bacterial phyla in abundance were at the Phylum level, while the top 12 bacterial genera in abundance were at the Genus level (the others were combined with a relative abundance of less than 1%). The L represents the subject and the number represents the sampling time (1-Pre, 2-D1, 3-D3, 4-D5, 5-D7, 6-Follow up visit).
Significant variations in bacteria at the phylum to genus levels were observed, including Leuconostocaceae, Bacillaceae, Weissella, and Cyanobacteria, accounting for 0-0.3% of the total abundance (P < 0.05, Supplementary Table S5). To minimize mutual interference among samples at different time points, two-group difference tests were conducted separately on species composition for Pre, D7 and Follow-up visit. The results from multiple tests analysis showed no differential species detected.
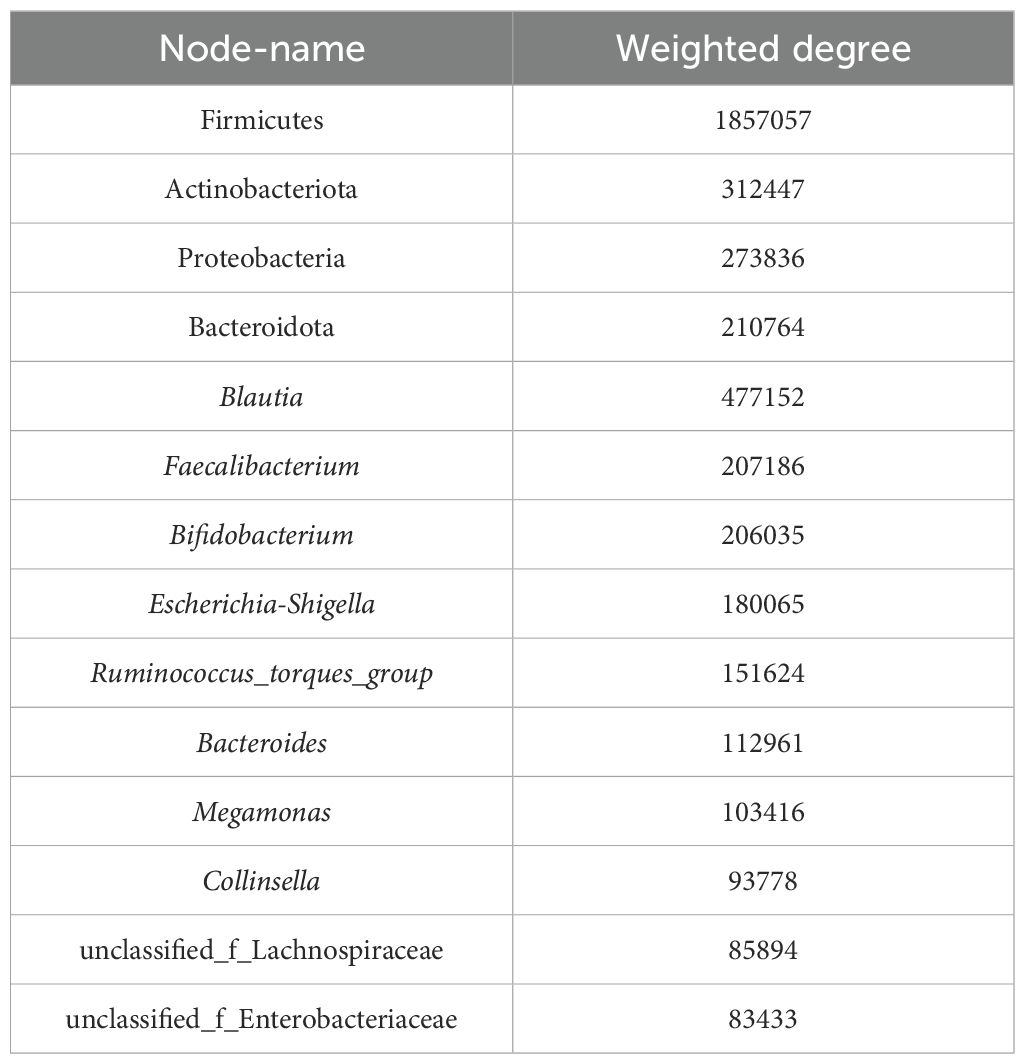
Co-occurrence network analysis was conducted at both the phylum and genus levels to assess the significance of dominant gut microflora (Figure 5). At the phylum level, species with high weighted degrees were identified, including Firmicutes, Actinobacteriota, Proteobacteria, and Bacteroidota. Genus-level species Blautia, Faecalibacterium, Bifidobacterium, Escherichia-Shigella, Ruminococcus_torques_group, Bacteroides, Megamonas, Collinsella, unclassified_f_Lachnospiraceae, and unclassified_f_Enterobacteriaceae exhibited high weighted-degree (Table 3). As illustrated in Figure 4, these species have a vital role in the community and have a significant impact on its structure and function.
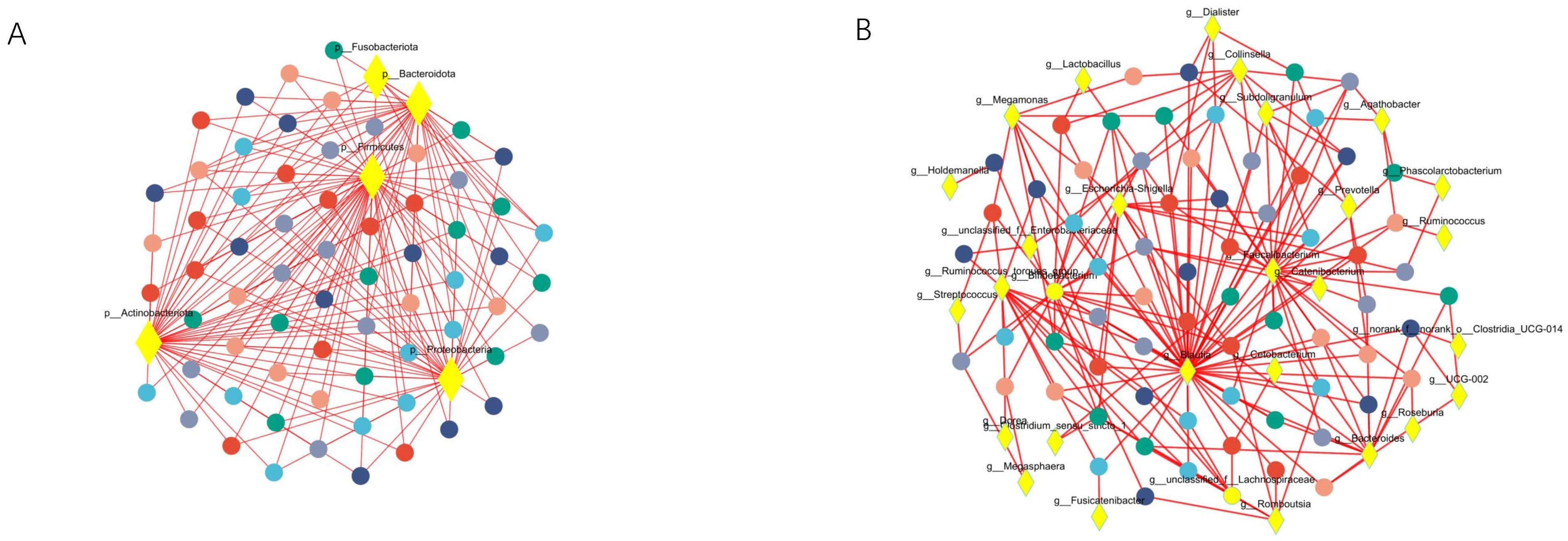
Figure 5. Co-occurrence network analysis was performed to observe the relationship between species and samples, so as to identify the central species in the community. (A) Represents the results of the analysis at the phylum level. (B) Represents the results at the genus level. The diamond-shaped nodes in yellow represent species at the taxonomic level. Circular nodes of different colors represent samples at different time points, and edges represent correlations.
3.3 Prediction of changes in the function of the intestinal microbiota
This study used the PICRUSt2 tool to predict changes in gut microbiota function before and after TRQ administration. KEGG pathway analysis results showed the abundance of predicted functions related to metabolism, global and overview maps, carbohydrate metabolism, ko01100, and ko01110 were high (Supplementary Figures S5-S7). COG analysis revealed high abundance in amino acid transport and metabolism, carbohydrate transport and metabolism, translation, ribosomal structure and biogenesis, as well as transcription demonstrated (Supplementary Figure S8). No statistically significant differences in functional categories were detected before and after TRQ treatment, according to the Kruskal-Wallis H test. BugBase phenotype prediction indicated a higher predominance of Anaerobic and Gram-Positive, with no statistical significance found for all assessed phenotypic categories (Supplementary Table S6).
4 Discussion and conclusion
Gut microbiota performs several vital functions by aiding digestion, stimulating and regulating the immune system and preventing the growth of pathogens (Scott et al., 2024). Therapeutic and prophylactic drugs with antibacterial properties can disrupt the normal gut microbiota structure that have both short- and long-term health consequences (Rashid et al., 2016; Ramirez et al., 2020). One study documented healthy volunteers treated for 1 week or less with antibiotics reported effects on their bacterial flora that persisted 6 months to 2 years after treatment, including a dramatic loss in diversity as well as in representation of specific taxa, and insurgence of antibiotic-resistant strains (Becattini et al., 2016). Gut microbiota dysbiosis also increases the generation of uremic toxins, reduces the production of short-chain fatty acids, and affects metabolism such as adenine, which may increase an individual’s risk of metabolic syndrome, renal injury, and inflammatory bowel diseases, as well as susceptibility to infectious diseases (Chen, 2020; Ramirez et al., 2020; Rysz et al., 2021; Li et al., 2023; Zhou et al., 2024a). Knowledge of how these drugs interact with the normal gut microbiota can help clinicians select appropriate drugs (Feng et al., 2020). TRQ is a drug with antibacterial activity that is widely used in the treatment of respiratory infections, and understanding its potential effects on the gut microbiota is necessary.
In this study, we observed that the use of TRQ did not result in significant changes in the gut microbiota of healthy individuals. Antibiotic treatment reduces the diversity of gut microbiota species, and a loss of species diversity is a common feature of a disturbed gut microbiota (Lange et al., 2016; Ramirez et al., 2020). Alpha-diversity is used to evaluate the richness of each sample, and its decrease is associated with disease (Li et al., 2022b). It has been reported that within a few days after treatment with antibiotics such as Cefprozil, ciprofloxacin, vancomycin, and metronidazole, the Shannon and Chao1 indices of healthy individuals significantly decrease, indicating that antibiotics can markedly affect the species diversity of the gut microbial communities. Additionally, significant differences were observed between samples before and after antibiotic treatment in both weighted and unweighted PCoA, revealing that the beta diversity of the microbial communities was affected by the antibiotics (Burdet et al., 2019; Haak et al., 2019). Our study showed that in alpha-diversity analysis, Shannon, Chao1, and Simpson indices did not find any significant differences, and no significant dispersion occurred in the weighted and unweighted PCoA (P>0.05). These results indicates that no bacterial species were extinguished by TRQ.
PLS-DA can offer a discerning visual analysis, more intuitively find differences between groups (Zhuang et al., 2016). Genus-level analysis revealed a separation trend in the PLS-DA model, returning to baseline after discontinuation. This implies potential alterations in the gut microbial community structure at a more detailed classification level. Significantly discrete sample points in the model corresponded to subjects L05, L08, and L09. The fluctuations in the abundance of unclassified_f_Enterobacteriaceae, Bifidobacterium, and Escherichia-Shigella were observed in these three subjects only. These changes indicate individual differences. The segregation trend in the PLS-DA model likely stems from the discrete sample points of subjects L05, L08, and L09. However, no statistically significant were observed. Bacteria with significant changes at phylum and genus levels accounted for less than 1% of total abundances (Supplementary Table S5), suggesting a minimal impact on community structure. These findings indicate that TRQ does not significantly affect the gut microbial community’s overall structure.
In the context of the KEGG database, Pathway1, Pathway2, and Pathway3 together depict a multilayered network ranging from broad biological functions to specific metabolic mechanisms (Antonov et al., 2008). Analysis of KEGG Pathway1 of gut microbial function, which are generally divided into six major categories: metabolism, genetic information processing, environmental information processing, cellular processes, human diseases, and organismal systems (Xie et al., 2024b). At this macroscopic level, no significant difference in results was found. Consistent with Pathway1, analyses of Pathway2 and Pathway3 also indicated no significant functional alterations. Furthermore, to substantiate our findings, we utilized the COG database for functional predictions and the Bugbase platform for phenotypic assessments of gut microbes. These findings revealed no significant differences, thereby indicating the microbial community’s functional and phenotypic stability under the study’s conditions. However, due to the limitations of PICRUSt2, the analysis of predicted gut microbiota function only provided some preliminary results. Functional changes in the gut microbiota should be confirmed by combining metabolomics and macro genomics in the future.
TRQ is widely used to treat respiratory infections in China (Guo et al., 2024b). Recently, TRQ combined with antibiotics is popular in the treatment of respiratory infection due to its improved efficacy and reduced drug resistance (Peng et al., 2022; Han et al., 2023). Understanding the effects of TRQ on the gut microbiota is important for its safe application. This study first reported the effect of TRQ on the gut microbiota. Family genetic background, dietary habits and lifestyle can influence the gut microbiota and lead to inter-individual differences (Anwar et al., 2021), so we adopted a self-controlled clinical trial design. Self-controlled clinical trial design can not only effectively control individual differences and reduce sample size, but also improve the internal validity of the study to draw more reliable conclusions (Iwagami and Takeuchi, 2021). In this study we employed the most commonly used dose of TRQ, which is 20ml. The composition of gut microbiota varies dynamically, thus we selected 6 time points for fecal sample collection. The healthy, adult gut microbiome is relatively stable in an individual and resilient to perturbation (Anwar et al., 2021). The effect of TRQ on gut microbiota can be evaluated more objectively in healthy volunteers. However, our study had a relatively small sample size, and the age distribution of the participants was uneven. When applying the research results, these limitations need to be considered. Further studies will involve more participants of different age groups to better understand the effects of TRQ on the gut microbiota.
Despite these limitations, the findings of this pilot study provide valuable information for the appropriate use of TRQ. Gut microbiota dysbiosis and the rise in antibiotic-resistant strains are the major problems faced by antibiotic therapy. With the increased use of antibiotics, these problems are likely to become more acute or more prevalent in the future. Indeed, new therapeutical agents will be necessary to substitute or complement the use of antibiotic treatments. TRQ, as a natural medicine, has unique advantages in preventing and treating respiratory infections (Ma et al., 2019; Ye et al., 2020). TRQ provides an option for patients with limited treatment options due to antibiotic resistance or Gut microbiota dysbiosis.In conclusion, the study indicates that short-term use of TRQ at conventional doses may not cause perturbations to the gut microbiota in healthy adults. This finding provides some useful information for the safe use of TRQ in the treatment of respiratory infections.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The Ethics Committee of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine reviewed and approved the present study in written form [2023-1332-99-01]. This trial has been registered in the national health Security information platform and medical research registration information system [no.: MR-31-24-014367].
Author contributions
SYL: Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing. WZ: Investigation, Formal analysis, Writing – review & editing. SJL: Investigation, Writing – review & editing. YZ: Investigation, Writing – review & editing. WL: Conceptualization, Writing – review & editing. WY: Conceptualization, Project administration, Writing – review & editing. MH: Conceptualization, Investigation, Formal analysis, Writing – original draft, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by shanghai Kai Bao Pharmaceutical Co. Ltd, the National Natural Science Foundation of China Program (82104635,81874362), Shanghai Education Commission Collaborative Innovation Center: Clinical Evaluation Platform for Traditional Chinese and Western Medicine (A1-U21-205-0103), and Construction of Demonstration Research Unit of Shanghai Shenkang Centre (SHDC2022CRW010). The funding sources were not involved in the design, analysis, and interpretation of data or decision to present these results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1428476/full#supplementary-material
References
Aliberti, S., Cook, G. S., Babu, B. L., Reyes, L. F., H. Rodriguez, A., Sanz, F., et al. (2019). International prevalence and risk factors evaluation for drug-resistant Streptococcus pneumoniae pneumonia. J. Infect. 79, 300–311. doi: 10.1016/j.jinf.2019.07.004
Antonov, A. V., Dietmann, S., Mewes, H. W. (2008). KEGG spider: interpretation of genomics data in the context of the global gene metabolic network. Genome Biol. 9, R179. doi: 10.1186/gb-2008-9-12-r179
Anwar, H., Iftikhar, A., Muzaffar, H., Almatroudi, A., Allemailem, K. S., Navaid, S., et al. (2021). Biodiversity of gut microbiota: impact of various host and environmental factors. BioMed. Res. Int. 2021, 1–9. doi: 10.1155/2021/5575245
Becattini, S., Taur, Y., Pamer, E. G. (2016). Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 22, 458–478. doi: 10.1016/j.molmed.2016.04.003
Burdet, C., Nguyen, T. T., Duval, X., Ferreira, S., Andremont, A., Guedj, J., et al. (2019). Impact of antibiotic gut exposure on the temporal changes in microbiome diversity. Antimicrob. Agents chemother. 63, e00820–e00819. doi: 10.1128/AAC.00820-19
Caballero-Flores, G., Pickard, J. M., Nunez, G. (2023). Microbiota-mediated colonization resistance: mechanisms and regulation. Nat. Rev. Microbiol. 21, 347–360. doi: 10.1038/s41579-022-00833-7
Carding, S., Verbeke, K., Vipond, D. T., Corfe, B. M., Owen, L. J. (2015). Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 26, 26191. doi: 10.3402/mehd.v26.26191
Chanderraj, R., Baker, J. M., Kay, S. G., Brown, C. A., Hinkle, K. J., Fergle, D. J., et al. (2023). In critically ill patients, anti-anaerobic antibiotics increase risk of adverse clinical outcomes. Eur. Resp. J. 61. doi: 10.1183/13993003.00910-2022
Chen, P. (Ed.) (2020). Gut Microbiota and Pathogenesis of Organ Injury (Singapore: Springer). doi: 10.1007/978-981-15-2385-4
Chun, H. S., Choi, S. H., Song, H. S. (2021). A meta-analysis of treatment effects on viral pneumonia using TCM injections specified in the clinical guideline for COVID-19 in China. J. Pharmacopunct. 24, 107–121. doi: 10.3831/KPI.2021.24.3.107
Dethlefsen, L., Relman, D. A. (2011). Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc. Natl. Acad. Sci. 108, 4554–4561. doi: 10.1073/pnas.1000087107
Dinan, K., Dinan, T. (2022). Antibiotics and mental health: The good, the bad and the ugly. J. Intern. Med. 292, 858–869. doi: 10.1111/joim.13543
Duan, H., Yu, L., Tian, F., Zhai, Q., Fan, L., Chen, W. (2022). Antibiotic-induced gut dysbiosis and barrier disruption and the potential protective strategies. Crit. Rev. Food Sci. Nutr. 62, 1427–1452. doi: 10.1080/10408398.2020.1843396
Fan, G., Wang, D., Wu, S., Li, D., Ren, X., Dong, F., et al. (2023). Better response to Tanreqing injection in frequent acute exacerbation of chronic obstructive pulmonary disease (AECOPD) patients-Real-world evidence from a nationwide registry (ACURE) study. Front. Pharmacol. 14. doi: 10.3389/fphar.2023.1118143
Feng, W., Liu, J., Ao, H., Yue, S., Peng, C. (2020). Targeting gut microbiota for precision medicine: Focusing on the efficacy and toxicity of drugs. Theranostics 10, 11278–11301. doi: 10.7150/thno.47289
Guo, M., Fang, L., Chen, M., Shen, J., Tan, Z., He, W. (2024a). Dysfunction of cecal microbiota and CutC activity in mice mediating diarrhea with kidney-yang deficiency syndrome. Front. Microbiol. 15. doi: 10.3389/fmicb.2024.1354823
Guo, R., Liu, H., Su, R., Mao, Q., Zhao, M., Zhang, H., et al. (2024b). Tanreqing injection inhibits influenza virus replication by promoting the fusion of autophagosomes with lysosomes: An integrated pharmacological study. J. Ethnopharmacol. 331, 118159. doi: 10.1016/j.jep.2024.118159
Haak, B. W., Lankelma, J. M., Hugenholtz, F., Belzer, C., de Vos, W. M., Wiersinga, W. J. (2019). Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 74, 782–786. doi: 10.1093/jac/dky471
Han, J., Cao, L., Liang, Y., Wang, Y. (2023). Clinical observation of Tanreqing combined with azithromycin in sequential treatment of mycoplasma pneumonia in children. Minerva Pediatr. 75, 161. doi: 10.23736/S2724-5276.22.07117-8
Iwagami, M., Takeuchi, Y. (2021). Introduction to self-controlled study design. Ann. Clin. Epidemiol. 3, 67–73. doi: 10.37737/ace.3.3_67
Lange, K., Buerger, M., Stallmach, A., Bruns, T. (2016). Effects of antibiotics on gut microbiota. Digest. Dis. (Basel Switzerland) 34, 260–268. doi: 10.1159/000443360
Li, Y., Li, H., Tong, H., Maegelev, M., Gu, Z. (2022b). Outer membrane vesicles derived from heatstroke-associated intestinal microbiota promote multiple organ injury in mice. Microb. Pathog. 170, 105653. doi: 10.1016/j.micpath.2022.105653
Li, D., Li, Y., Wang, J., Yang, W., Cui, K., Su, R., et al. (2024a). In-depth analysis of the treatment effect and synergistic mechanism of TanReQing injection on clinical multi-drug resistant Pseudomonas aeruginosa. Microbiol. Spectr. 12, e0272623. doi: 10.1128/spectrum.02726-23
Li, X., Qiao, B., Wu, Y., Deng, N., Yuan, J., Tan, Z. (2024b). Sishen Pill inhibits intestinal inflammation in diarrhea mice via regulating kidney-intestinal bacteria-metabolic pathway. Front. Pharmacol. 15. doi: 10.3389/fphar.2024.1360589
Li, X., Wei, S., Ma, X., Li, H., Jing, M., Liu, H., et al. (2022a). Efficacy and safety of Tanreqing injection combined with antibiotics against Streptococcus pneumoniae pneumonia: A systematic review and meta-analysis. J. Clin. Pharm. Ther. 47, 1159–1172. doi: 10.1111/jcpt.13706
Li, Y., Xia, S., Jiang, X., Feng, C., Gong, S., Ma, J., et al. (2021). Gut microbiota and diarrhea: an updated review. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.625210
Li, X., Zhu, J., Wu, Y., Tan, Z. (2023). Correlation between kidney function and intestinal biological characteristics of adenine and folium sennaeInduced diarrhea model in mice. Turk J. Gastroenterol. 34, 4–12. doi: 10.5152/tjg.2022.211010
Ling, Z., Liu, X., Cheng, Y., Yan, X., Wu, S. (2022). Gut microbiota and aging. Crit. Rev. Food Sci. Nutr. 62, 3509–3534. doi: 10.1080/10408398.2020.1867054
Liu, C., Zhao, D., Ma, W., Guo, Y., Wang, A., Wang, Q., et al. (2016). Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 100, 1421–1426. doi: 10.1007/s00253-015-7039-6
Ma, Y., Chen, M., Guo, Y., Liu, J., Chen, W., Guan, M., et al. (2019). Prevention and treatment of infectious diseases by traditional Chinese medicine: a commentary. APMIS 127, 372–384. doi: 10.1111/apm.12928
Ma, N., Pan, B., Ge, L., Li, M., Zhu, H., Deng, X., et al. (2024). Efficacy and safety of Tanreqing injection for cough caused by acute trachea-bronchitis disease: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 321, 117429. doi: 10.1016/j.jep.2023.117429
Mikkelsen, K. H., Frost, M., Bahl, M. I., Licht, T. R., Jensen, U. S., Rosenberg, J., et al. (2015). Effect of antibiotics on gut microbiota, gut hormones and glucose metabolism. PloS One 10, e0142352. doi: 10.1371/journal.pone.0142352
Mosterd, C. M., Kanbay, M., van den Born, B. J. H., van Raalte, D. H., Rampanelli, E. (2021). Intestinal microbiota and diabetic kidney diseases: the Role of microbiota and derived metabolites inmodulation of renal inflammation and disease progression. Best Pract. Res. Clin. Endoc. Metab. 35, 101484. doi: 10.1016/j.beem.2021.101484
Palleja, A., Mikkelsen, K. H., Forslund, S. K., Kashani, A., Allin, K. H., Nielsen, T., et al. (2018). Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 3, 1255–1265. doi: 10.1038/s41564-018-0257-9
Pan, T., Liu, X., Xiang, S., Ji, W. (2016). Treatment for patients with multidrug resistant Acinetobacter baumannii pulmonary infection. Exp. Ther. Med. 11, 1345–1347. doi: 10.3892/etm.2016.3051
Peng, Y., Chen, Z., Li, H., Han, Y., Sun, D., Li, Y., et al. (2022). Traditional Chinese medicine injections combined with oseltamivir for influenza: systematic review and network meta-analysis. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.848770
Ramirez, J., Guarner, F., Bustos Fernandez, L., Maruy, A., Sdepanian, V. L., Cohen, H. (2020). Antibiotics as major disruptors of gut microbiota. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.572912
Rashid, M. U., Rosenborg, S., Panagiotidis, G., Holm, J., Soderberg, L. K., Weintraub, A., et al. (2016). Ecological effect of solithromycin on normal human oropharyngeal and intestinal microbiota. Antimicrob. Agents Chemother. 60, 4244–4251. doi: 10.1128/AAC.00461-16
Rashid, M., Rosenborg, S., Panagiotidis, G., Löfdal, K. S., Weintraub, A., Nord, C. E. (2015). Ecological effect of ceftazidime/avibactam on the normal human intestinal microbiota. Int. J. Antimicrob. Agents. 46, 60–65. doi: 10.1016/j.ijantimicag.2015.02.027
Rysz, J., Franczyk, B., Ławiński, J., Olszewski, R., Ciałkowska-Rysz, A., Gluba-Brzózka, A. (2021). The impact of CKD on uremic toxins and gut microbiota. Toxins 13, 252. doi: 10.3390/toxins13040252
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Scott, S. A., Fu, J., Chang, P. V. (2024). Dopamine receptor D2 confers colonization resistance via microbial metabolites. Nat. (London). 628, 180–185. doi: 10.1038/s41586-024-07179-5
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12, R60. doi: 10.1186/gb-2011-12-6-r60
Tong, Y., Wen, J., Yang, T., Li, H., Wei, S., Jing, M., et al. (2021). Clinical efficacy and safety of Tanreqing injection combined with antibiotics versus antibiotics alone in the treatment of pulmonary infection patients after chemotherapy with lung cancer: A systematic review and meta-analysis. Phytother. Res. 35, 122–137. doi: 10.1002/ptr.6790
Wang, M., Liu, H., Chen, Y., Yu, J., Lin, J., Sun, Z., et al. (2023). Guideline on treating community-acquired pneumonia with Chinese patent medicines. Pharmacol. Res. 196, 106919. doi: 10.1016/j.phrs.2023.106919
Wang, L., Ma, Y., He, Y., Deng, J., Huang, D., Zhang, X., et al. (2021). Systematic investigation of the pharmacological mechanism of Tanreqing injection in treating respiratory diseases by UHPLC/Q-TOF-MS/MS based on multiple in-house chemical libraries coupled with network pharmacology. J. Pharm. Biomed. Anal. 202, 114141. doi: 10.1016/j.jpba.2021.114141
Wu, X. M., Tan, R. X. (2019). Interaction between gut microbiota and ethnomedicine constituents. Nat. Prod. Rep. 36, 788–809. doi: 10.1039/c8np00041g
Xie, S., Deng, N., Fang, L., Shen, J., Tan, Z., Cai, Y. (2024a). TMAO is involved in kidney-yang deficiency syndrome diarrhea by mediating the “gut-kidney axis. Heliyon 10, e35461. doi: 10.1016/j.heliyon.2024.e35461
Xie, S., Fang, L., Deng, N., Shen, J., Tan, Z., Peng, X. (2024b). Targeting the gut-kidney axis in diarrhea with kidney-yang deficiency syndrome: the role of sishen pills in regulating TMAO-mediated inflammatory response. Med. Sci. Monitor. 30, e944185. doi: 10.12659/MSM.944185
Xie, L., Wang, Y., Yin, H., Li, J., Xu, Z., Sun, Z., et al. (2021). Identification of the absorbed ingredients and metabolites in rats after an intravenous administration of Tanreqing injection using high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 44, 2097–2112. doi: 10.1002/jssc.202000898
Yang, W., Cui, K., Tong, Q., Ma, S., Sun, Y., He, G., et al. (2022). Traditional Chinese medicine tanreqing targets both cell division and virulence in staphylococcus aureus. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.884045
Yang, W., Wei, Q., Tong, Q., Cui, K., He, G., Lin, L., et al. (2020). Traditional Chinese medicine tanreqing inhibits quorum sensing systems in pseudomonas aeruginosa. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.517462
Ye, L., Zhang, J., Xiao, W., Liu, S. (2020). Efficacy and mechanism of actions of natural antimicrobial drugs. Pharmacol. Ther. 216, 107671. doi: 10.1016/j.pharmthera.2020.107671
Zhang, W., He, M., Kong, N., Niu, Y., Li, A., Yan, Y. (2024). Study on the inhibition activity and mechanism of Tanreqing against Klebsiella pneumoniae biofilm formation in vitro and in vivo. Front. Cell. Infect. Microbiol. 14. doi: 10.3389/fcimb.2024.1368450
Zhang, F., Sun, L., Zhai, J., Xia, T., Jiang, W., Li, M., et al. (2019). The effect of tanreqing injection on the pharmacokinetics of sirolimus in rats. BioMed. Res. Int. 2019, 1854323. doi: 10.1155/2019/1854323
Zhou, M., Li, X., Liu, J., Wu, Y., Tan, Z., Deng, N. (2024a). Adenine’s impact on mice’s gut and kidney varies with the dosage administered and relates to intestinal microorganisms and enzyme activities. 3 Biotech. 14, 88. doi: 10.1007/s13205-024-03959-y
Zhou, M., Li, X., Wang, X., Deng, N., Cai, Y., Tan, Z. (2024b). The dysfunction in intestinal microorganisms and enzyme activity as significant contributors to diarrhea with kidney-yang deficiency syndrome. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1324938
Zhou, D., Xie, L., Shi, X., Liu, F., Wu, S., Zhang, S., et al. (2021). A meta-analysis of the clinical efficacy of Tanreqing injection combined with antibiotics vs antibiotics alone for treating pulmonary infection secondary to intracerebral hemorrhage. Med. (Baltimore). 100, e24905. doi: 10.1097/MD.0000000000024905
Keywords: Tanreqing injection, gut microbiota, microbial diversity, healthy volunteers, TCM
Citation: Li S, Zhang W, Liu S, Zhou Y, Liu W, Yuan W and He M (2024) Effects of Tanreqing injection on the gut microbiota in healthy volunteers. Front. Cell. Infect. Microbiol. 14:1428476. doi: 10.3389/fcimb.2024.1428476
Received: 06 May 2024; Accepted: 13 September 2024;
Published: 04 October 2024.
Edited by:
Nenqun Xiao, Hunan University of Chinese Medicine, ChinaReviewed by:
Chengxing Long, Hunan University of Humanities, Science and Technology, ChinaHaoqing Shao, Hunan University of Medicine, China
Yang Ping, Jiamusi University, China
Copyright © 2024 Li, Zhang, Liu, Zhou, Liu, Yuan and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min He, aGVtaW5taWFvQDE2My5jb20=; Weian Yuan, d2VpYW5fMTk4MEAxNjMuY29t; Wei Liu, bHdoemF5bEAxNjMuY29t
 Shiyu Li
Shiyu Li Wenxia Zhang
Wenxia Zhang Sijie Liu
Sijie Liu Yichen Zhou
Yichen Zhou Wei Liu
Wei Liu Weian Yuan
Weian Yuan Min He
Min He