- 1Department of Infectious Disease, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
- 2Department of Radiology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, China
Background and aims: Limited data have been reported on achieving functional cure using pegylated interferon (Peg-IFN) alpha-2b treatment for postpartum hepatitis B e antigen (HBeAg)-negative women with chronic hepatitis B virus (HBV) infection. This study was to assess the effectiveness and safety of Peg-IFN alpha-2b in HBV postpartum women without HBeAg and identify factors linked to the functional cure.
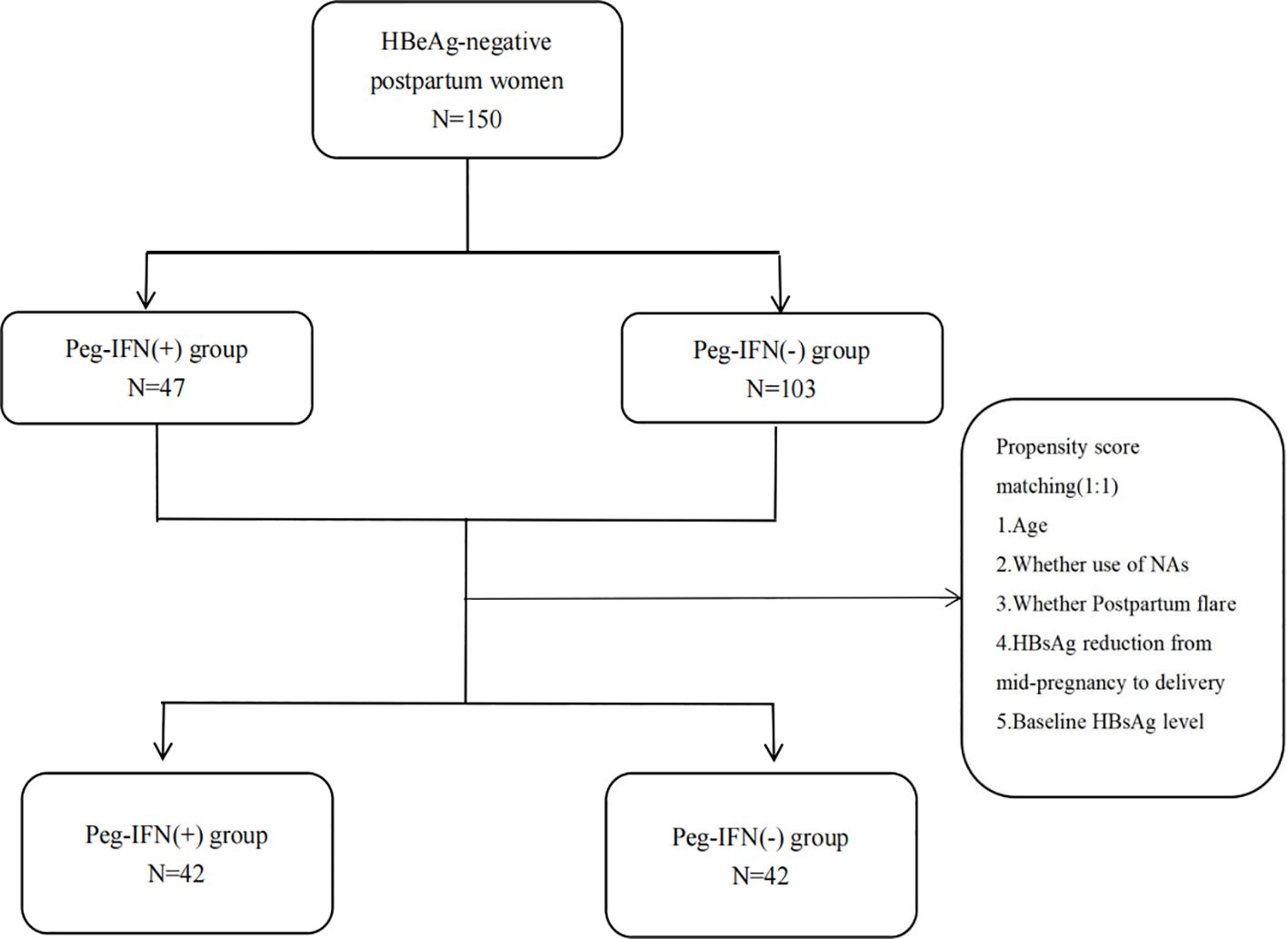
Methods: A total of 150 HBeAg-negative postpartum women were retrospectively recruited.47 patients received Peg-IFN alpha-2b [Peg-IFN(+) group] and 103 patients did not [Peg-IFN(-) group]. Propensity score matching (PSM) was used to adjust the baseline imbalance between the two groups. The patients were followed for at least 48 weeks. The primary endpoints were hepatitis B surface antigen(HBsAg) loss and HBsAg seroconversion at 48 weeks. Logistic regression analysis was used to assess factors associated with HBsAg loss at 48 weeks.
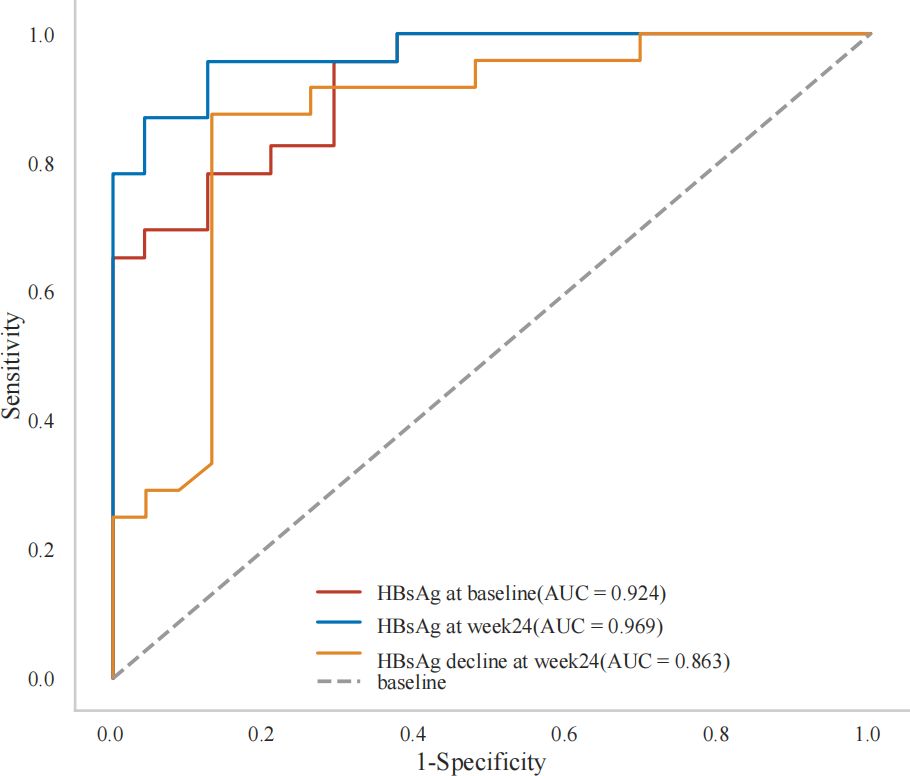
Results: At week 48,the HBsAg loss and seroconversion rate in Peg-IFN(+) group were 51.06%(24/47) and 40.43%(19/47), respectively. Even after PSM, Peg-IFN(+) group still showed higher HBsAg loss rate (50.00% vs 7.14%,p<0.001) and higher HBsAg seroconversion rate (38.10% vs 2.38%,p<0.001). Baseline HBsAg levels (Odds Ratio [OR]: 0.051, 95% Confidence Interval [CI]: 0.003-0.273, P=0.010), HBsAg at week 24 (OR:0.214, 95%CI:0.033-0.616, P=0.022), HBsAg decline at week 24 (OR:4.682, 95%CI: 1.624-30.198, P=0.022) and postpartum flare (OR:21.181, 95%CI:1.872-633.801, P=0.030) were significantly associated with HBsAg loss at week 48 after Peg-IFN alpha-2b therapy. Furthermore, the receiver operating characteristic curve (ROC) showed that the use of baseline HBsAg<182 IU/mL, HBsAg at week24 < 4 IU/mL and HBsAg decline at week24>12IU/mL were good predictors of HBsAg loss. No serious adverse events were reported.
Conclusion: Peg-IFN alpha-2b treatment could achieve a high rate of HBsAg loss and seroconversion in HBeAg-negative postpartum women with reliable safety, particularly for patients experience postpartum flare and have low baseline HBsAg levels.
1 Introduction
In 2022, about 3.2% of the global population had chronic hepatitis B virus (HBV) infection (Polaris Observatory Collaborators, 2023). HBV is the primary cause of cirrhosis and hepatocellular carcinoma (HCC) worldwide (Hsu et al., 2023).
“Functional cure” has become the paramount therapeutic objective for chronic HBV infection, aided by medical advancements (Hou et al., 2017),which denotes hepatitis B (HBsAg) loss in chronic hepatitis B (CHB) patients, with or without hepatitis B surface antibodies (HBsAb).While covalently closed circular DNA(cccDNA) may persist in the liver, there is an amelioration of liver inflammation and histopathology, markedly reducing end-stage liver disease risk. However, spontaneous HBsAg reversal is exceedingly rare, occurring at a rate of roughly 1% annually (Zhou et al., 2019). Even with long term use of nucleos(t)ide analogues (NAs), annual HBsAg clearance rate is still below 2% (Levrero et al., 2018; Tang et al., 2018; Terrault et al., 2018; Wu et al., 2019).
Peg-IFN alpha possesses immunomodulatory and antiviral properties, making a functional cure more achievable. However, responses differ among individuals, and its effectiveness is notable in only a specific group of patients, often referred to as the advantaged population (Wieland et al., 2005; Sadler and Williams, 2008; Belloni et al., 2012). Several studies (Chinese Society of Infectious Disease Chinese Society of Hepatology, and Chinese Medical Association, 2019; De Ridder et al., 2021; Ren et al., 2021; Huang et al., 2022; Wang et al., 2022a) have suggested that individuals who are HBeAg-negative with HBsAg levels below 3000 IU/ml, and particularly below 1500 IU/ml, are more likely to attain a functional cure with Peg-IFN alpha.
Concerning HBeAg-negative mothers with a low HBsAg level and low HBV DNA level, the postpartum period’s significant hormonal changes and immune system restructuring influence HBV infection’s natural course (Carr et al., 1981; Lin et al., 1993; Wegmann et al., 1993; Marzi et al., 1996; Elenkov, 2004; Groer et al., 2015). However, current guidelines for pregnant women with HBV concentrate on mother-to-child transmission (MTCT), and there is debate about postpartum treatment approaches.
Prior studies have observed that replacing telbivudine with Peg-IFN alpha-2a alongside adefovir treatment postnatally, when postnatal alanine aminotransferase (ALT) levels exceeded twice the upper limit of normal(ULN), leads to HBeAg titer decreasing by over 20% from baseline, resulting in a 56.7% HBeAg seroconversion rate and a 26.7% HBsAg loss rate (Lu et al., 2015).This rate surpasses or matches that of the general chronic HBV population during immune activation (Lau et al., 2005; Buster et al., 2008; Marcellin et al., 2016). Two small studies (Guo et al., 2023; Han et al., 2021) from the American Association for the Study of Liver Diseases (ASALD), which are still ongoing, have found that Peg-IFN can achieve HBsAg loss rates of over 40% in postpartum women with low HBsAg levels. Additionally, other studies (Liu et al., 2017; Feng et al., 2023) have demonstrated that regardless of pregnancy ALT levels, continuing oral NAs monotherapy for antiviral therapy in the postpartum period aids in reducing HBsAg and HBeAg levels. This treatment yields an HBeAg seroconversion rate of approximately 10-15%, making it markedly more effective than it for the general chronic HBV population during the immune-tolerant phase.
Thus, the postpartum phase presents an opportune time for chronic HBV treatment and disease regression. HBeAg-negative postpartum mothers may have a heightened likelihood of achieving a functional cure with Peg-IFN alpha compared to other HBeAg-negative patients. However, most prior studies on the functional cure with Peg-IFN have excluded postpartum women due to the special physiological state of the postpartum period and the difficulty of postpartum follow-up and management.
This study aimed to assess the therapeutic efficacy and safety of Peg-IFN alpha-2b in achieving a functional cure rate for chronic HBV infection in postpartum HBeAg-negative women.
2 Method
2.1 Study design
This retrospective cohort study enrolled 150 HBV postpartum women between January 2018 and December 2022 at the First Affiliated Hospital of Xi’an Jiaotong University, China. The patients were categorized into two groups based on Peg-IFN alpha-2b treatment: 47 received Peg-IFN alpha-2b (the Peg-IFN (+) group) and 103 did not (the Peg-IFN (-) group). Postpartum women who did not receive Peg-IFN alpha-2b were monitored at least every 24 weeks like other HBV-infected patients, while those who received Peg-IFN alpha-2b were monitored at least every 12 weeks. The inclusion criteria were: 1) postpartum women (12-72 weeks post-delivery) with chronic HBV infection, HBsAg positive for ≥6 months, and HBsAg ≤ 3000IU/mL at baseline; 2) HBeAg negative at baseline; 3) HBV DNA ≤ 2000IU/ml at baseline; 4) followed up for ≥48 weeks. The exclusion criteria were: 1) co-infection with hepatitis C, hepatitis D, or HIV; 2) alcoholic liver disease or autoimmune liver disease; 3) decompensated cirrhosis or HCC; 4) undergoing radiotherapy, chemotherapy, or immunosuppressive therapy; 5) serum creatinine levels <50 ml/min/1.73 m2; 6) mental disorders; 7) other contraindications for Peg-IFN alpha-2b use.
This study received approval from the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University (XJTU1AF2024LSK-2021-464) and adhered to the 1975 Declaration of Helsinki, clinical practice guidelines (Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese GRADE Center, 2019; Kumar et al., 2022), and local regulatory requirements. Written informed consent was obtained from all patients regarding their participation in the study.
2.2 Primary outcome and secondary outcomes
The primary outcome was the rate of HBsAg loss and seroconversion at 48 weeks. HBsAg loss was defined as HBsAg levels dropping below 0.05 IU/mL, with or without the emergence of HBsAb. HBsAg seroconversion was defined as HBsAg levels dropping below 0.05 IU/mL, accompanied by HBsAb levels exceeding 10 mIU/mL.
Secondary outcomes included: (1) the rate of HBsAg loss and seroconversion at 24 weeks; (2) reduction in HBsAg levels from baseline to 24 weeks and 48 weeks; (3) rate of HBV DNA undetectability at 24 weeks and 48 weeks. HBV DNA undetectability was defined as HBV DNA levels below 20 IU/mL.
2.3 Other measurements
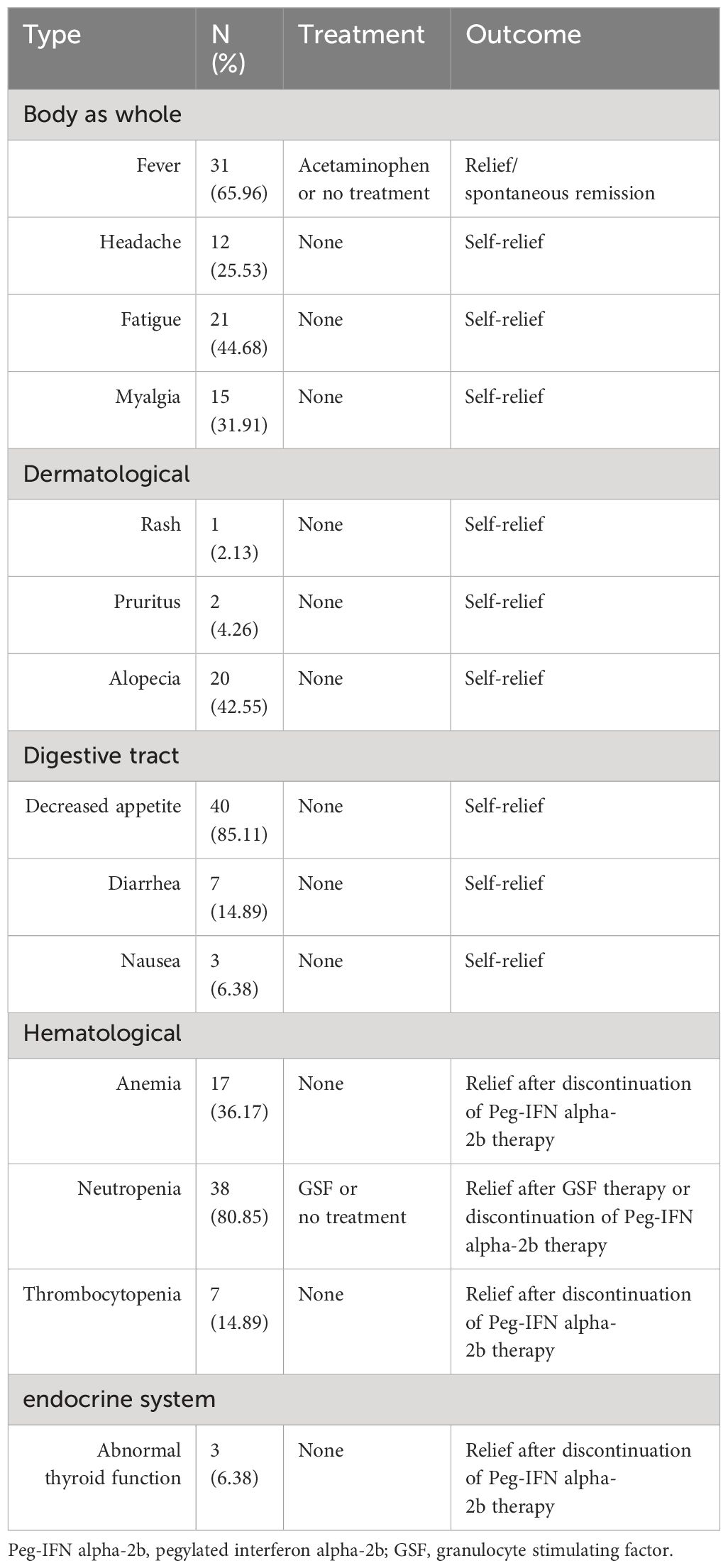
Clinical data and laboratory test results were retrieved from the Electronic Medical Records (EMR) system. The clinical data included age, NAs usage, occurrence of postpartum flare (ALT>2 ULN within 12 weeks post-delivery, ULN=40 U/L), time from delivery to study enrollment, and HBsAg reduction from mid-pregnancy to delivery. The laboratory test results comprised a routine blood test, liver function and hepatitis B-related markers. The safety assessment included systemic symptoms, skin symptoms, gastrointestinal symptoms, blood tests and endocrine tests.
2.4 Antiviral regimens
Patients previously treated with NAs during pregnancy continued NAs post-delivery, with the option to add Peg-IFN alpha-2b therapy (Xiamen Amoytop Biotech Co, LTD, Xiamen, China, 135μg, subcutaneous injection, once weekly) for a minimum of 48 weeks based on patient preference. The NAs treatment used was tenofovir disoproxil fumarate. Those not treated with NAs during pregnancy either underwent postpartum monitoring alone or chose to add Peg-IFN alpha-2b therapy for a minimum of 48 weeks at their discretion. Patients could stop the treatment before 48 weeks after they achieved HBsAg seroconversion. The median course of the Peg-IFN alpha-2b therapy was 48 (range: 36 – 80) weeks.
2.5 Propensity score matching method
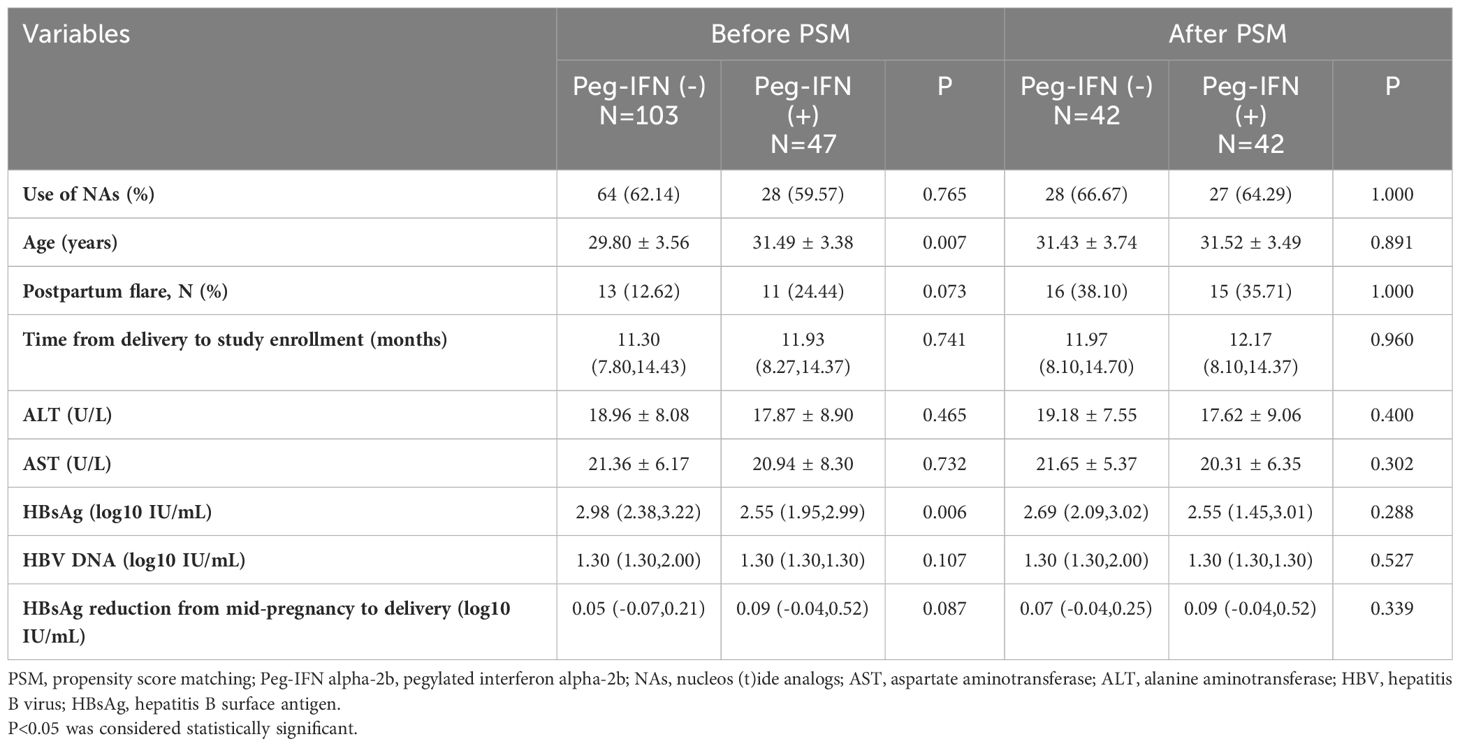
The PSM method was employed to match the Peg-IFN (-) group to the Peg-IFN (+) group at a 1:1 ratio based on the following covariates: age, occurrence of postpartum flare, NAs usage, HBsAg reduction from mid-pregnancy to delivery, and baseline HBsAg level, aiming to address imbalances in confounding factors in the retrospective cohort study (Figure 1). The R package “matchit” facilitated the one-to-one matching process.
2.6 Statistical analysis
Before PSM, continuous variables with a normal distribution were expressed as means ± standard deviations (SDs) and compared between groups using t-tests. For continuous variables not following a normal distribution, median (interquartile range [IQR]) values were presented., and group differences were assessed using the Mann-Whitney U-test. Categorical variables were presented as the number of patients (with corresponding proportions) and differences between groups were evaluated using the Chi-squared test or Fisher’s exact test.
After PSM, paired t-tests or Signed Wilcoxon tests were utilized to compare continuous variables between the pair-matched groups. The McNemar test was employed to compare categorical variables between the matched groups. Univariable and multivariable logistic regressions were used to identify the factors independently associated with HBsAg loss within the Peg-IFN (+) group. Odds ratios (OR) and the 95% confidence interval (95% CI) were reported for the factors. For continuous predictors, the receiver operating characteristic (ROC) curve analyses were used to determine the optimal cut-off values by utilizing the Youden index. A p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 29.0 software (IBM, Armonk, NY, USA) and R software (version 3.6.3).
3 Results
3.1 Baseline characteristics of patients enrolled in the study
The baseline characteristics of patients before and after PSM are presented by group in Table 1. Prior to PSM, age and baseline HBsAg (log10 IU/mL) showed significant differences between the two groups (P<0.05). The median onset time of Peg-IFN alpha-2b therapy for the Peg-IFN (+) group was 11.93 (8.27 to 14.37) months. Following PSM, 42 patients in the Peg-IFN(-) group were matched one-to-one with patients in the Peg-IFN(+) group based on age, postpartum flare, NAs usage, HBsAg reduction from mid-pregnancy to delivery, and the baseline HBsAg level. Subsequently, no statistical differences were observed in baseline characteristics between the two groups after PSM.
3.2 Outcomes before and after PSM
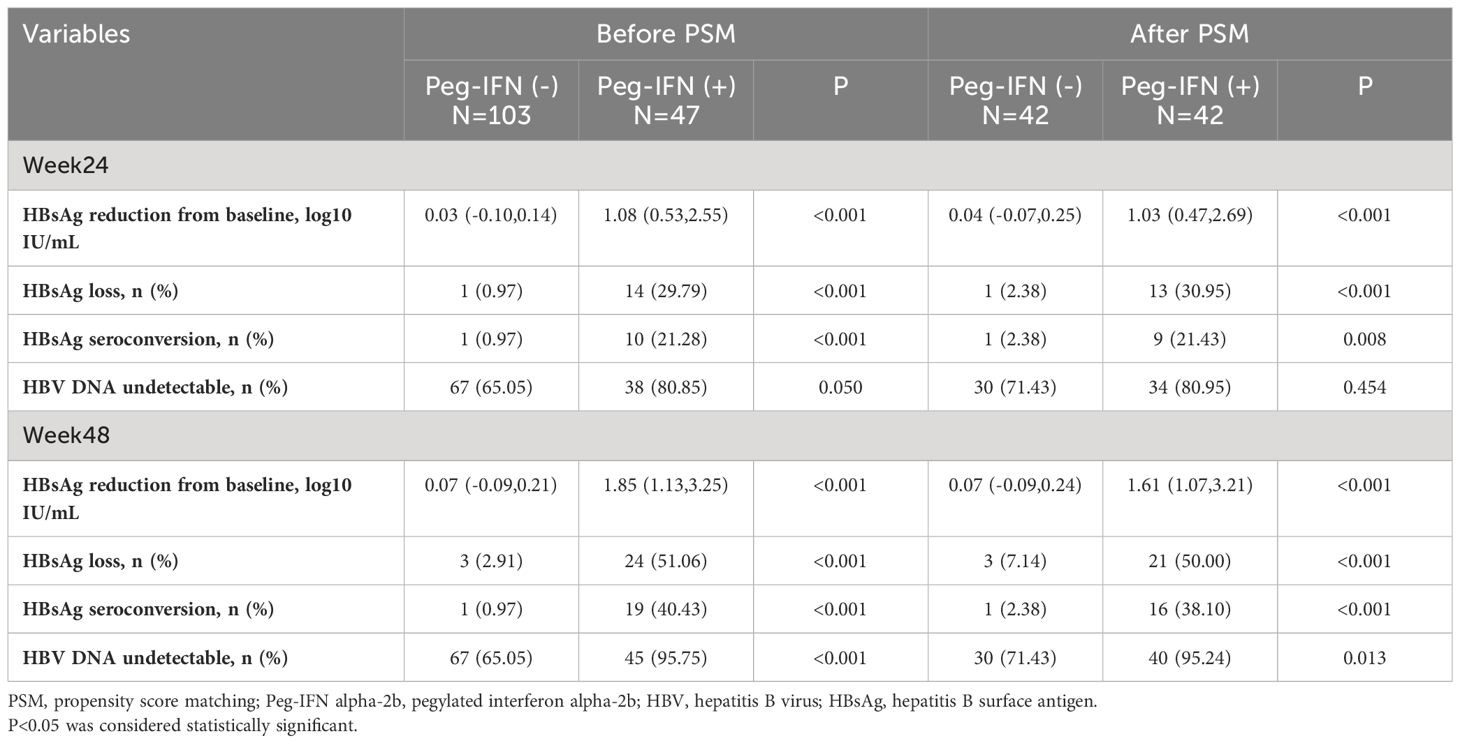
Before PSM, the Peg-IFN (+) group achieved both higher HBsAg loss rate (week 24: 29.79% vs. 0.97%, P<0.001; week 48: 51.06% vs. 2.91%, P<0.001) and HBsAg seroconversion rate (week 24: 21.28% vs. 0.97%, P<0.001, week 48: 40.43% vs. 0.97%, P<0.001). Please refer to Table 2.
After PSM, compared to patients in the Peg-IFN (-) group, those in the Peg-IFN (+) group exhibited greater HBsAg reduction (1.03 [0.47, 2.69] vs. 0.04 [-0.07, 0.25] log10 IU/mL, p < 0.001), a higher rate of HBsAg loss (30.95% vs. 2.38%, p < 0.001), and a higher rate of HBsAg seroconversion (21.43% vs. 2.38%, p = 0.008) at week 24. No significant difference was observed in the rate of HBV DNA undetectability at week 24.
At week 48, the disparities in the HBsAg reduction (1.61 [1.07,3.21] vs. 0.07 [-0.09,0.24] log10 IU/mL, p < 0.001), rate of HBsAg loss (50.00% vs. 7.14%, p < 0.001), and seroconversion (38.10% vs. 2.38%, p < 0.001) were even more pronounced. Additionally, a higher proportion of patients in the Peg-IFN (+) group achieved HBV DNA undetectability at week 48 (95.24% vs. 71.43%, p = 0.013). Please refer to Figure 2.
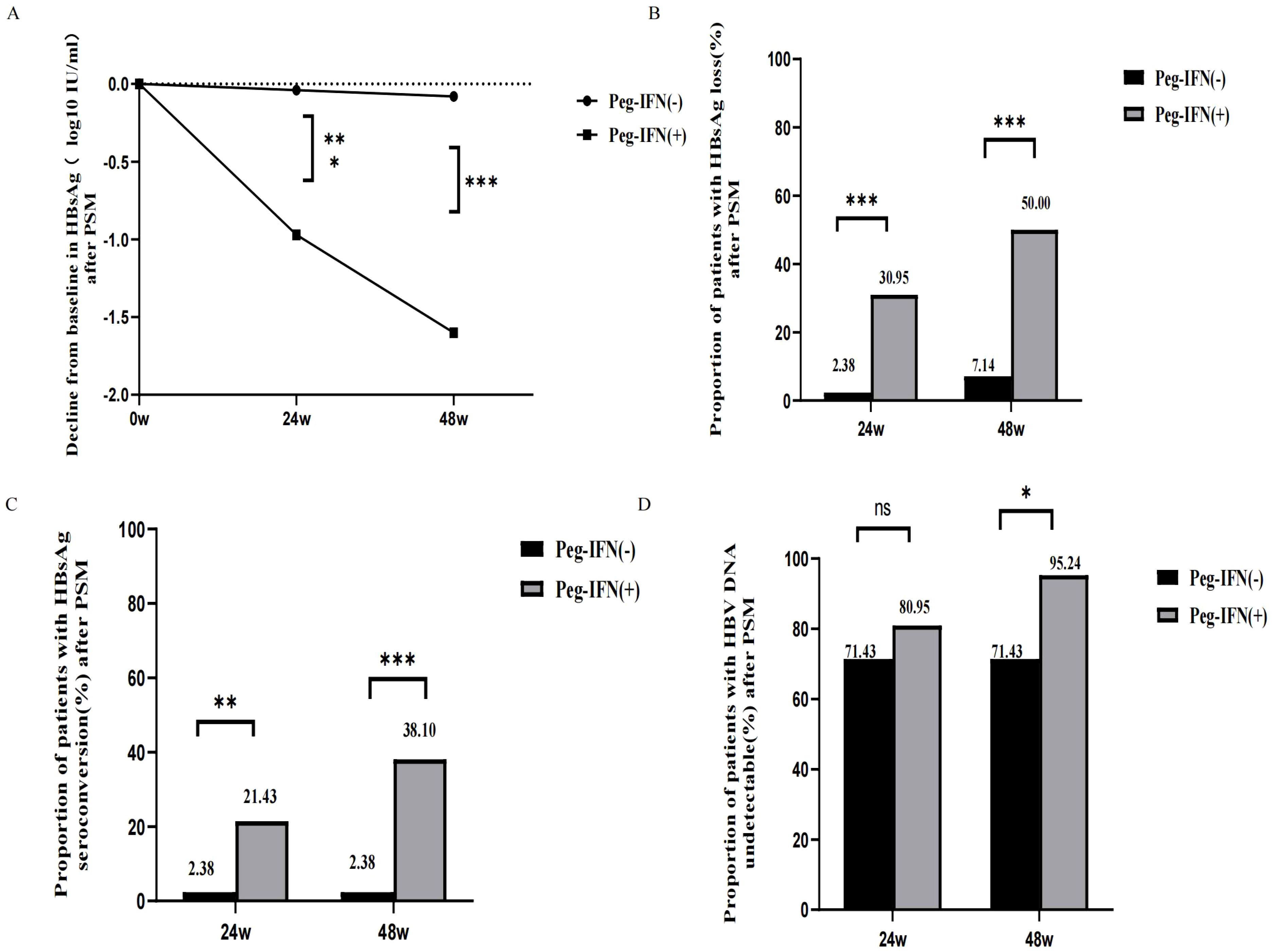
Figure 2 Outcome after PSM. (A) Decline from baseline in HBsAg after PSM. (B) HBsAg loss rates in week 24 and week 48 after PSM. (C) HBsAg seroconversion rates in week 24 and week 48 after PSM. (D) HBV DNA undetectable rates in week 24 and week 48 after PSM. NS, non-significant (p > 0.05), (*P < 0.05, **P <0.01, ***P < 0.001).
3.3 Predictors of functional cure
The results of univariate and multivariate analyses for factors associated with HBsAg loss are presented in Table 3A. In the univariate analyses, a lower HBsAg level at baseline (OR:0.043, 95% CI: 0.007-0.276, P = 0.003), a lower HBsAg level at week 12 (OR:0.188, 95% CI:0.076-0.462, P <0.001), a greater HBsAg decline at week 12(OR:4.283, 95% CI:1.511-12.141, P = 0.006), a lower HBsAg level at week 24 (OR:0.161, 95% CI:0.063-0.413, P <0.001), a greater HBsAg decline at week 24 (OR:4.202, 95% CI:1.885-9.368, P <0.001),postpartum elevated ALT(>ULN)(OR:4.320, 95% CI: 1.179-15.827, P =0.027) and postpartum flare (OR:7.269, 95% CI:1.353-39.045, P =0.021) were associated with a higher likelihood of HBsAg loss after 48- weeks of Peg-IFN alpha-2b therapy. Multivariate logistic regression revealed that the HBsAg level at baseline (OR:0.051, 95% CI:0.003-0.273, P = 0.010), HBsAg level at week 24 (OR:0.214, 95% CI: 0.033-0.616, P = 0.022), HBsAg decline at week 24 (OR:4.682, 95% CI: 1.624-30.198, P = 0.022) and postpartum flare (OR:21.181, 95% CI: 1.872-633.801, P = 0.030) remained associated with a higher likelihood of HBsAg loss after 48 weeks of Peg-IFN alpha-2b therapy.
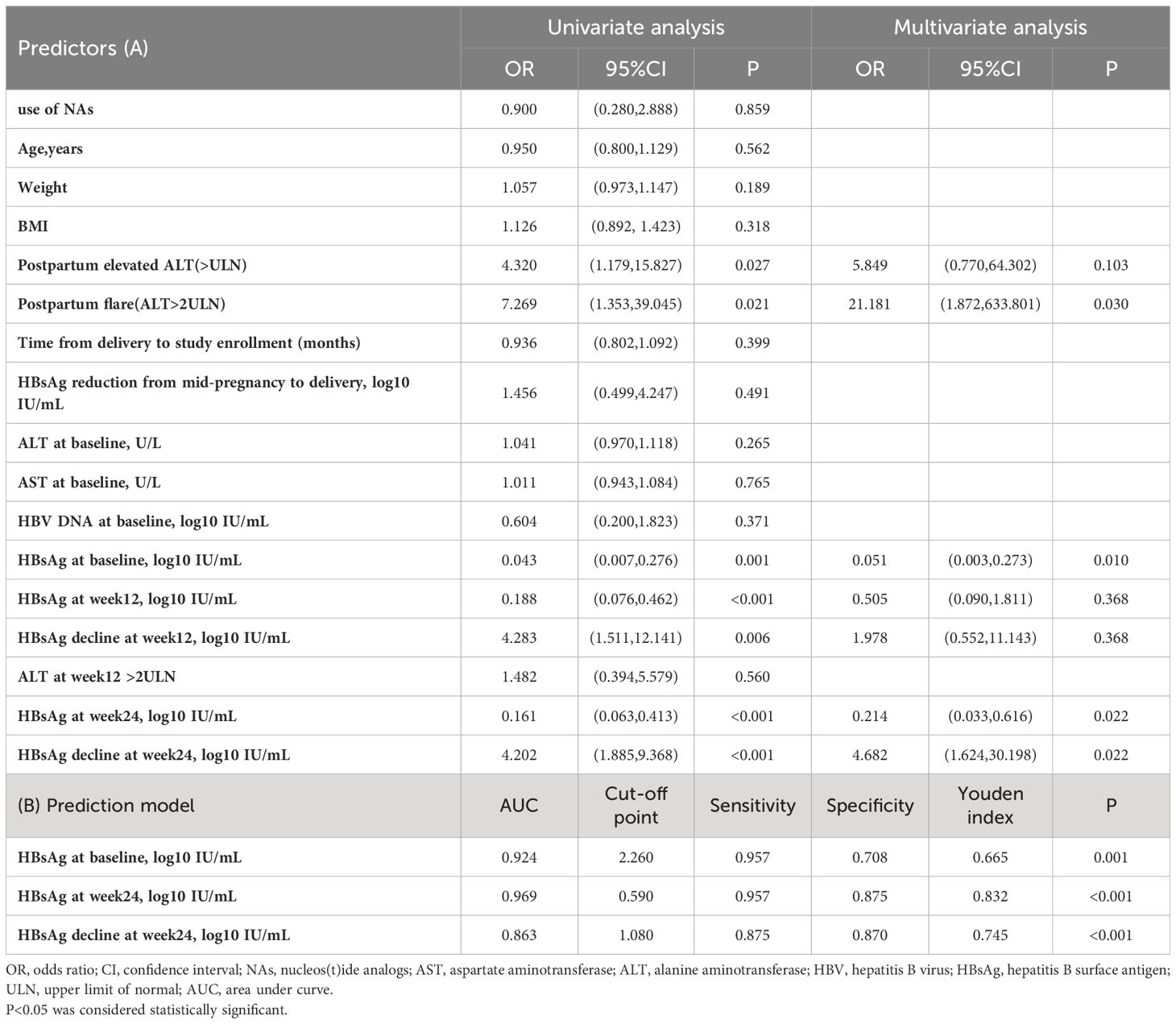
Table 3 (A) The predictive factors for HBsAg loss at week 48 in Peg-IFN-treated patients and (B) prediction of HBsAg loss at week 48 in patients who received Peg-IFN treatment.
Furthermore ROC analysis also showed that baseline HBsAg<182 IU/mL,HBsAg at week24 < 4 IU/mL and HBsAg decline at week24>12IU/mL were all independent predictors of a functional cure (Table 3B, Figure 3).
3.4 Safety
No serious adverse events occurred among the patients in the Peg-IFN (+) group. The most common adverse events included fever (31/47, 65.96%), fatigue (21/47, 44.68%), alopecia (20/47, 42.55%), decreased appetite (40/47, 85.11%) and neutropenia (38/47, 80.85%) (Table 4). A total of 17 out of 47 (36.17%) patients treated with Peg-IFN alpha-2b had anemia, with hemoglobin levels were between 90-110 g/L. Among patients with thrombocytopenia, 5 out of 47 (10.64%) had platelet counts <75×109 but >50×109. The patients above were not given the specific treatment and relieved after the end of the Peg-IFN alpha-2b therapy. Among patients with neutropenia, 18 out of 47 (38.3%) had absolute neutrophil counts <1.0×109/L, with 6 (12.77%) patients having counts <0.75×109/L. The counts were improved to >0.75×109 for all six patients after treatment with granulocyte stimulating factor (GSF).
Among three patients with abnormal thyroid function, one patient (thyroid stimulating hormone[TSH] 0.21 uIU/mL) had already achieved HBsAg seroconversion before thyroid abnormalities were detected. Her abnormal thyroid function resolved after the Peg-IFN alpha-2b therapy stopped. Another patient (TSH 0.11 uIU/mL) was observed that the thyroid function normalize after 5 weeks following of suspending Peg-IFN therapy. The third patient had subclinical hypothyroidism (TSH 5.05 uIU/mL) and the thyroid function returned to normal after 6 weeks of interferon suspension, prompting us to continue the original Peg-IFN dose. None of the three had associated clinical symptoms.
4 Discussion
Peg-IFN alpha therapy is currently the main approach for achieving functional cure, but its effectiveness varies widely in different people. Postpartum mothers can undergo significant changes in the activity of immune cells and immune factors due to hormonal shifts and immune system reconstitution, potentially making this period a good opportunity to achieve functional HBV cures for mothers with chronic HBV infection and low HBV DNA levels and low HBsAg levels (Liu et al., 2017; Feng et al., 2023; Zhang et al., 2023). However, current guidelines (Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese GRADE Center, 2019; Kumar et al., 2022) on whether and how to use antiviral therapy postpartum remain contentious. Most studies on the functional cure induced by interferon exclude this population due to the challenges of managing and following them up during this physiological state. Limited data have been reported on the functional cure of Peg-IFN alpha-2b treatment for postpartum HBeAg-negative women with chronic HBV infection. This study aimed to evaluate the effectiveness and safety of Peg-IFN alpha-2b in this group, providing data to support that postpartum women with HBV could be an advantageous population for a functional cure with Peg-IFN alpha.
A total of 51.06% and 40.43% of the patients in this study achieved HBsAg loss and seroconversion, respectively, at 48 weeks after treatment with Peg-IFN alpha-2b. Even after PSM matching, these rates remained significantly higher than in the group that did not receive Peg-IFN alpha-2b. The functional cure rate among the HBeAg-negative HBV mothers after Peg-IFN application was notably higher, surpassing the 10-40% cure rate seen in similar non-postpartum populations in other studies (Broquetas et al., 2020; Wu et al., 2020; Chu et al., 2022).
At present, the mechanism that induces postpartum HBsAg loss is not fully understood. Zhang et al. (2023) described the potential mechanisms that immune tolerance during the pregnancy could be broken due to the decrease of estrogen, progesterone and HCG after delivery. The immune system was transformed from Th2 and Treg dominant in pregnancy to Th1 and Th17 dominant after delivery. Natural killer (NK) cells, helper T (Th)cells, CD8+ T cells and multiple cytokines such as IFN-r, IL-2 and TNF-α could be involved (Joshi et al., 2017; Huang et al., 2021; Zhang et al., 2023). The change of immune system could last for at least 1 year after delivery (Samadi Kochaksaraei et al., 2020; Zhang et al., 2023). Studies (Huang et al., 2021; Song et al., 2022) also observed the CD4+ T cell and CD8+ T cell activation in patients with postpartum flares. There is an elevated frequency of CD8+ T cells expressing perforin and granzyme B. Among HBV-infected pregnant women, about 10-30% (Giles et al., 2015; Chang et al., 2018; Wang et al., 2022b; Zhang et al., 2023) of pregnant women have postpartum hepatitis, which may be related to reactivation in the immune system. Thus, the postpartum period might be an opportune time to achieve a functional cure for HBV.
Additionally, none of the 47 individuals included in the study experienced serious adverse events, demonstrating both the safety of Peg-IFN alpha-2b in maternity care and the potential effectiveness of treating HBeAg-negative HBV mothers in terms of achieving functional cure.
After further analysis of the factors influencing the maternal functional cure of HBV, we found a robust association between lower HBsAg levels at baseline and a rapid decline in HBsAg levels early in the treatment process with heightened rates of HBsAg loss at 48 weeks. Analogous observations have occurred in other non-postpartum cohorts (Ning et al., 2014; Hu et al., 2018; Chu et al., 2022).
We found that women with abnormal postnatal liver function, especially those with postnatal liver function >2ULN, which is postpartum flare, were more likely to achieve functional cure with Peg-IFN alpha-2b. Indeed, previous studies (Buster et al., 2009; Liaw et al., 2011) have found that patients with higher liver function at baseline have better outcomes with Peg-IFN, but it is important to note that the most HBV-infected mothers were close to or in immune tolerant carrier state or inactive HBV carrier state prior to pregnancy. These populations are characterized by consistently normal ALT or AST levels and infrequent pathological changes in liver tissue. They are in a relatively stable condition, with a low likelihood of liver function abnormalities (Chu and Liaw, 2007; Chang and Liaw, 2014). Morever, the timing of occurrence is uncertain as well. It is difficult to identify the onset of elevated liver function to start interferon therapy. The incidence of postpartum hepatic flare (Giles et al., 2015; Chang et al., 2018; Wang et al., 2022b; Zhang et al., 2023) is much higher than the incidence of hepatic abnormalities in the general population, with at least about 7.3% incidence of postpartum flare even in the HBeAg-negative mothers (Giles et al., 2015). Moreover, the time of occurrence of postpartum flare was more concentrated, mostly within 3 months postpartum (Wang et al., 2022b). This also means that we can identify those who can be clinically cured by interferon through regular monitoring of liver function in the postpartum population during the first 3 months after delivery.
Postpartum flare may represent an opportunity to achieve a functional cure.
However, this study had several limitations. Firstly, as this was a retrospective study, data were missing regarding pregnancy and the number of subjects included was relatively small. Recent studies (Chu et al., 2007; Chu et al., 2013; Tuna et al., 2024) have suggested a potential correlation between lipid metabolism and HBsAg seroclearance in HBV patients. However, the lack of data on changes in lipids and weight hindered our ability to delve deeper into this correlation. Secondly, no clear definition of postpartum in the literature, and after reviewing the literature (Liu et al., 2017; Feng et al., 2023; Zhang et al., 2023), we included women before 18 months postpartum in this study. In addition, we found that the relationship between the time of initiation of treatment in the postpartum period and the rate of HBsAg loss was not significant, which could be due to the limited sample size, this finding remains to be further confirmed by prospective large-data studies. Therefore, we suggest conducting a broader, prospective study involving multiple centers and a larger pool of patients, commencing from early pregnancy, to analyze various potential influences during pregnancy and postpartum.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WZ: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Methodology, Software. LY: Data curation, Investigation, Writing – original draft. YZ: Data curation, Investigation, Writing – review & editing. LS: Project administration, Supervision, Writing – review & editing. YH: Project administration, Resources, Supervision, Writing – review & editing. TC: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing, Writing – original draft. JZ: Conceptualization, Methodology, Supervision, Writing – review & editing, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Shaanxi Key Research and Development Program (2021ZDLSF-04-14) and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University, China (XJTU1AF-CRF-2020-001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary

References
Belloni, L., Allweiss, L., Guerrieri, F., Pediconi, N., Volz, T., Pollicino, T., et al. (2012). IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J. Clin. Invest. 122, 529–537. doi: 10.1172/JCI58847
Broquetas, T., Garcia-Retortillo, M., Micó, M., Canillas, L., Puigvehí, M., Cañete, N., et al. (2020). Hepatitis B surface antigen and hepatitis B core-related antigen kinetics after adding pegylated-interferon to nucleos(t)ids analogues in hepatitis B e antigen-negative patients. World J. Hepatol. 12, 1076–1088. doi: 10.4254/wjh.v12.i11.1076
Buster, E. H., Flink, H. J., Cakaloglu, Y., Simon, K., Trojan, J., Tabak, F., et al. (2008). Sustained HBeAg and HBsAg loss after long-term follow-up of HBeAg-positive patients treated with peginterferon alpha-2b. Gastroenterology 135, 459–467. doi: 10.1053/j.gastro.2008.05.031
Buster, E. H., Hansen, B. E., Lau, G. K., Piratvisuth, T., Zeuzem, S., Steyerberg, E. W., et al. (2009). Factors that predict response of patients with hepatitis B e antigen-positive chronic hepatitis B to peginterferon-alfa. Gastroenterology 137, 2002–2009. doi: 10.1053/j.gastro.2009.08.061
Carr, B. R., Parker, C. R., Jr, Madden, J. D., MacDonald, P. C., Porter, J. C. (1981). Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am. J. Obstet Gynecol 139, 416–422. doi: 10.1016/0002-9378(81)90318-5
Chang, C. Y., Aziz, N., Poongkunran, M., Javaid, A., Trinh, H. N., Lau, D. T., et al. (2018). Serum aminotransferase flares in pregnant and postpartum women with current or prior treatment for chronic hepatitis B. J. Clin. Gastroenterol. 52, 255–261. doi: 10.1097/MCG.0000000000000822
Chang, M. L., Liaw, Y. F. (2014). Hepatitis B flares in chronic hepatitis B: pathogenesis, natural course, and management. J. Hepatol. 61, 1407–1417. doi: 10.1016/j.jhep.2014.08.033
Chinese Society of Infectious Disease Chinese Society of Hepatology, and Chinese Medical Association (2019). The expert consensus on clinical cure (functional cure) of chronic hepatitis B. Zhonghua gan zang bing za zhi 27, 594–603. doi: 10.3760/cma.j.issn.1007-3418.2019.08.003
Chinese Society of Infectious Diseases, Chinese Medical Association; Chinese GRADE Center (2019). 2019 Chinese practice guideline for prevention and treatment of hepatitis B virus motherto-child transmission. Chin. J. Infect. 37, 388–396. doi: 10.3760/cma.j.issn.1000-6680.2019.07.002
Chu, J. H., Huang, Y., Xie, D. Y., Deng, H., Wei, J., Guan, Y. J., et al. (2022). Real-world study on HBsAg loss of combination therapy in HBeAg-negative chronic hepatitis B patients. J. Viral Hepat 29, 765–776. doi: 10.1111/jvh.13722
Chu, C. M., Liaw, Y. F. (2007). Spontaneous relapse of hepatitis in inactive HBsAg carriers. Hepatol. Int. 1, 311–315. doi: 10.1007/s12072-007-9002-9
Chu, C. M., Lin, D. Y., Liaw, Y. F. (2007). Does increased body mass index with hepatic steatosis contribute to seroclearance of hepatitis B virus (HBV) surface antigen in chronic HBV infection? Int. J. Obes. (Lond) 31, 871–875. doi: 10.1038/sj.ijo.0803479
Chu, C. M., Lin, D. Y., Liaw, Y. F. (2013). Clinical and virological characteristics post HBsAg seroclearance in hepatitis B virus carriers with hepatic steatosis versus those without. Dig Dis. Sci. 58, 275–281. doi: 10.1007/s10620-012-2343-9
De Ridder, F., Sonneveld, M. J., Lenz, O., Janssen, H. L. A., Talloen, W., Hansen, B. E. (2021). Mean HBsAg decline at week 24 of PEG-IFN-based treatment predicts subsequent rate of HBsAg clearance - suggesting a valuable endpoint for early development HBV trials. J. Viral Hepat 28, 1563–1569. doi: 10.1111/jvh.13599
Elenkov, I. J. (2004). Glucocorticoids and the th1/th2 balance. Ann. N Y Acad. Sci. 1024, 138–146. doi: 10.1196/annals.1321.010
Feng, Y., Yao, N., Shi, L., Zhu, Y., Liu, J., He, Y., et al. (2023). Efficacy and safety of long-term postpartum antiviral therapy in hepatitis B virus-infected mothers receiving prophylactic tenofovir disoproxil fumarate treatment. Eur. J. Gastroenterol. Hepatol. 35, 212–218. doi: 10.1097/MEG.0000000000002476
Giles, M., Visvanathan, K., Lewin, S., Bowden, S., Locarnini, S., Spelman, T., et al. (2015). Clinical and virological predictors of hepatic flares in pregnant women with chronic hepatitis B. Gut 64, 1810–1815. doi: 10.1136/gutjnl-2014-308211
Groer, M. E., Jevitt, C., Ji, M. (2015). Immune changes and dysphoric moods across the postpartum. Am. J. Reprod. Immunol. 73, 193–198. doi: 10.1111/aji.12322
Guo, Y., Jiang, L., Liu, M., Zhu, Y., Wu, Y., Xia, J., et al. (2023). Efficacy and Safety of Pegylated Interferon alpha-2b in HBV postpartum women with Low HBsAg Levels: an Exploratory Study in Southwest China. Abstract (1413-C). Hepatolgy 78 (S1), pS514–S515. doi: 10.1097/HEP.0000000000000581
Han, G. R., Zhou, G. L., Chen, C., Wang, C. X. (2021). Efficacy of pegylated interferon alpha-2b therapy in nucleos(t)ide analogue-treated HBV postpartum women: an exploratory study. Poster Abstracts. Hepatology 74 (S1), 527A. doi: 10.1002/hep.32188
Hou, J., Wang, G., Wang, F., Cheng, J., Ren, H., Zhuang, H., et al. (2017). Guideline of prevention and treatment for chronic hepatitis B (2015 update). J. Clin. Transl. Hepatol. 5, 297–318. doi: 10.14218/JCTH.2016.00019
Hsu, Y. C., Huang, D. Q., Nguyen, M. H. (2023). Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. 20, 524–537. doi: 10.1038/s41575-023-00760-9
Hu, P., Shang, J., Zhang, W., Gong, G., Li, Y., Chen, X., et al. (2018). HBsAg loss with peg-interferon alfa-2a in hepatitis B patients with partial response to nucleos(t)ide analog: new switch study. J. Clin. Transl. Hepatol. 6, 25–34. doi: 10.14218/JCTH.2017.00072
Huang, M., Gao, Y., Yin, X., Zhang, X., Hao, Y., Hu, J., et al. (2021). Characterization of T cell immunity in chronic hepatitis B virus-infected mothers with postpartum alanine transaminase flare. BMC Infect. Dis. 21, 922. doi: 10.1186/s12879-021-06634-2
Huang, D., Wu, D., Wang, P., Wang, Y., Yuan, W., Hu, D., et al. (2022). End-of-treatment HBcrAg and HBsAb levels identify durable functional cure after Peg-IFN-based therapy in patients with CHB. J. Hepatol. 77, 42–54. doi: 10.1016/j.jhep.2022.01.021
Joshi, S. S., Wong, D., Castillo, E., Swain, M. G., Coffin, C. S. (2017). Peripartum cytokine flares in a multiethnic cohort of chronic hepatitis B carriers does not correlate with hepatitis B virus suppression or increased risk of liver disease. Am. J. Reprod. Immunol. 78, 10. doi: 10.1111/aji.12707
Kumar, M., Abbas, Z., Azami, M., Belopolskaya, M., Dokmeci, A. K., Ghazinyan, H., et al. (2022). Asian Pacific association for the study of liver (APASL) guidelines: hepatitis B virus in pregnancy. Hepatol. Int. 16, 211–253. doi: 10.1007/s12072-021-10285-5
Lau, G. K., Piratvisuth, T., Luo, K. X., Marcellin, P., Thongsawat, S., Cooksley, G., et al. (2005). Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl. J. Med. 352, 2682–2695. doi: 10.1056/NEJMoa043470
Levrero, M., Subic, M., Villeret, F., Zoulim, F. (2018). Perspectives and limitations for nucleo(t)side analogs in future HBV therapies. Curr. Opin. Virol. 30, 80–89. doi: 10.1016/j.coviro.2018.04.006
Liaw, Y. F., Jia, J. D., Chan, H. L., Han, K. H., Tanwandee, T., Chuang, W. L., et al. (2011). Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology 54, 1591–1599. doi: 10.1002/hep.24555
Lin, H., Mosmann, T. R., Guilbert, L., Tuntipopipat, S., Wegmann, T. G. (1993). Synthesis of T helper 2-type cytokines at the maternal-fetal interface. J. Immunol. 151, 4562–4573. doi: 10.4049/jimmunol.151.9.4562
Liu, J., Wang, J., Jin, D., Qi, C., Yan, T., Cao, F., et al. (2017). Hepatic flare after telbivudine withdrawal and efficacy of postpartum antiviral therapy for pregnancies with chronic hepatitis B virus. J. Gastroenterol. Hepatol. 32, 177–183. doi: 10.1111/jgh.13436
Lu, J., Zhang, S., Liu, Y., Du, X., Ren, S., Zhang, H., et al. (2015). Effect of Peg-interferon α-2a combined with Adefovir in HBV postpartum women with normal levels of ALT and high levels of HBV DNA. Liver Int. 35, 1692–1699. doi: 10.1111/liv.12753
Marcellin, P., Ahn, S. H., Ma, X., Caruntu, F. A., Tak, W. Y., Elkashab, M., et al. (2016). Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology 150, 134–144.e10. doi: 10.1053/j.gastro.2015.09.043
Marzi, M., Vigano, A., Trabattoni, D., Villa, M. L., Salvaggio, A., Clerici, E., et al. (1996). Characterization of type 1 and type 2 cytokine production profile in physiologic and pathologic human pregnancy. Clin. Exp. Immunol. 106, 127–133. doi: 10.1046/j.1365-2249.1996.d01-809.x
Ning, Q., Han, M., Sun, Y., Jiang, J., Tan, D., Hou, J., et al. (2014). Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J. Hepatol. 61, 777–784. doi: 10.1016/j.jhep.2014.05.044
Polaris Observatory Collaborators (2023). Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol. Hepatol. 8, 879–907. doi: 10.1016/S2468-1253(23)00197-8
Ren, P., Li, H., Huang, Y., Jiang, J., Guo, S., Cao, Z., et al. (2021). A simple-to-use tool for predicting response to peginterferon in HBV DNA suppressed chronic hepatitis B patients in China. Antiviral Res. 194, 105163. doi: 10.1016/j.antiviral.2021.105163
Sadler, A. J., Williams, B. R. (2008). Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 8, 559–568. doi: 10.1038/nri2314
Samadi Kochaksaraei, G., Castillo, E., Sadler, M. D., Seow, C. H., Barkema, H. W., Martin, S. R., et al. (2020). Real-world clinical and virological outcomes in a retrospective multiethnic cohort study of 341 untreated and tenofovir disoproxil fumarate-treated chronic hepatitis B pregnant patients in North America. Aliment Pharmacol. Ther. 52, 1707–1716. doi: 10.1111/apt.16123
Song, A., Liu, Y., Cao, Z., Lu, J., Ren, S., Zheng, S., et al. (2022). Clinical features and T cell immune characteristics of postpartum hepatitis flare in pregnant women with HBeAg-positive chronic HBV infection. Front. Immunol. 13. doi: 10.3389/fimmu.2022.881321
Tang, L. S. Y., Covert, E., Wilson, E., Kottilil, S. (2018). Chronic hepatitis B infection: A review. JAMA 319, 1802–1813. doi: 10.1001/jama.2018.3795
Terrault, N. A., Lok, A. S. F., McMahon, B. J., Chang, K. M., Hwang, J. P., Jonas, M. M., et al. (2018). Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67, 1560–1599. doi: 10.1002/hep.29800
Tuna, N., Çalıca Utku, A., Vatan, A., Ogutlu, A., Guclu, E., Karabay, O. (2024). The association between weight loss and hepatitis B surface antigen seroclearence in chronic hepatitis B patients. Hepat Mon 24, e142264. doi: 10.5812/hepatmon-142264
Wang, W. X., Jia, R., Gao, Y. Y., Liu, J. Y., Luan, J. Q., Qiao, F., et al. (2022a). Quantitative anti-HBc combined with quantitative HBsAg can predict HBsAg clearance in sequential combination therapy with PEG-IFN-α in NA-suppressed chronic hepatitis B patients. Front. Immunol. 13. doi: 10.3389/fimmu.2022.894410
Wang, X., Song, A., Lin, X., Lu, J., Zheng, S., Ma, L., et al. (2022b). Clinical characteristics of hepatitis flares during pregnancy and postpartum in Chinese chronic hepatitis B virus carriers-a prospective cohort study of 417 cases. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1031291
Wegmann, T. G., Lin, H., Guilbert, L., Mosmann, T. R. (1993). Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol. Today 14, 353–356. doi: 10.1016/0167-5699(93)90235-D
Wieland, S. F., Eustaquio, A., Whitten-Bauer, C., Boyd, B., Chisari, F. V. (2005). Interferon prevents formation of replication-competent hepatitis B virus RNA-containing nucleocapsids. Proc. Natl. Acad. Sci. U.S.A. 102, 9913–9917. doi: 10.1073/pnas.0504273102
Wu, D., Wang, P., Han, M., Chen, Y., Chen, X., Xia, Q., et al. (2019). Sequential combination therapy with interferon, interleukin-2 and therapeutic vaccine in entecavir-suppressed chronic hepatitis B patients: the Endeavor study. Hepatol. Int. 13, 573–586. doi: 10.1007/s12072-019-09956-1
Wu, F. P., Yang, Y., Li, M., Liu, Y. X., Li, Y. P., Wang, W. J., et al. (2020). Add-on pegylated interferon augments hepatitis B surface antigen clearance vs continuous nucleos(t)ide analog monotherapy in Chinese patients with chronic hepatitis B and hepatitis B surface antigen ≤ 1500 IU/mL: An observational study. World J. Gastroenterol. 26, 1525–1539. doi: 10.3748/wjg.v26.i13.1525
Zhang, L., Jiang, T., Yang, Y., Deng, W., Lu, H., Wang, S., et al. (2023). Postpartum hepatitis and host immunity in pregnant women with chronic HBV infection. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1112234
Keywords: postpartum therapy, functional cure, advantageous population, HBsAg loss, Peg-IFN alpha, postpartum women
Citation: Zhong W, Yan L, Zhu Y, Shi L, He Y, Chen T and Zheng J (2024) A high functional cure rate was induced by pegylated interferon alpha-2b treatment in postpartum hepatitis B e antigen-negative women with chronic hepatitis B virus infection: an exploratory study. Front. Cell. Infect. Microbiol. 14:1426960. doi: 10.3389/fcimb.2024.1426960
Received: 02 May 2024; Accepted: 24 July 2024;
Published: 08 August 2024.
Edited by:
Chinyere Onubogu, Nnamdi Azikiwe University, NigeriaReviewed by:
Nazan Tuna, Namik Kemal University, TürkiyeWolfram Gerlich, University of Giessen, Germany
Copyright © 2024 Zhong, Yan, Zhu, Shi, He, Chen and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianyan Chen, Y2hlbnRpYW55YW5AeGp0dWZoLmVkdS5jbg==; Jie Zheng, amllemhlbmdAeGp0dS5lZHUuY24=
 Wenting Zhong
Wenting Zhong Lanzhi Yan1
Lanzhi Yan1 Yingli He
Yingli He Tianyan Chen
Tianyan Chen Jie Zheng
Jie Zheng