- 1Department of Pulmonary and Critical Care Medicine, Peking University Third Hospital, Beijing, China
- 2Center of Infectious Disease, Peking University Third Hospital, Beijing, China
- 3Department of Infectious Diseases, Peking University Third Hospital, Beijing, China
- 4Department of Laboratory Medicine, Peking University Third Hospital, Beijing, China
- 5Institute of Medical Technology, Peking University Health Science Center, Beijing, China
- 6Beijing Key laboratory of Emerging Infectious Diseases, Institute of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Background: Neutrophil plays a pivotal role in the management of Klebsiella pneumoniae infection. Delineate the clinical characteristics and prognostic utility of neutrophil in severe patients with K. pneumoniae infection are crucial for clinical management and prognostic assessment.
Methods: K. pneumoniae patients with different infection sites were enrolled from Medical Information Mart for Intensive Care IV and eICU Collaborative Research Database. Temporal variations of neutrophil counts within 30 days of clinical onset were examined using locally weighted scatterplot smoothing curves. Logistic regression analysis was performed to assess the relationship between neutrophil counts and hospital mortality.
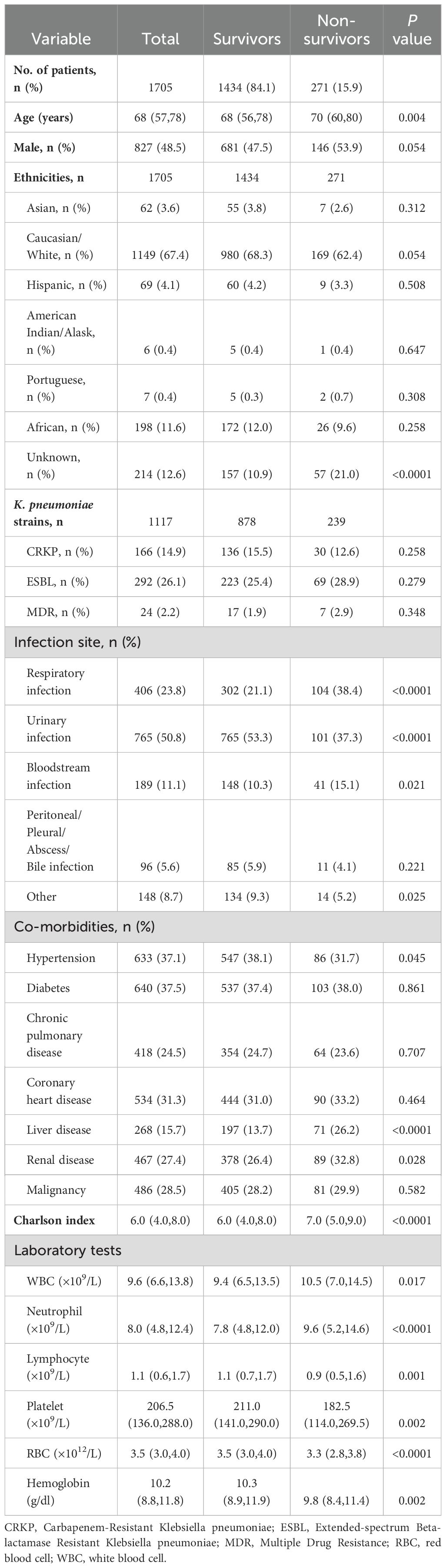
Results: A total of 1,705 patients caused by K. pneumonia were included in the study. The non-survivor group exhibited a comparatively older age and a higher proportion of K. pneumoniae infections originating from respiratory and bloodstream sources compared to the survivor group (38.4% vs 21.1%, p<0.0001, and 15.1% vs 10.3%, p=0.021). Patients combined with multiple drug resistance strains, respiratory infection, liver disease, and above 60 years exhibited a specific dynamic process of neutrophil levels. Neutrophils counts peaked at admission and 1-2 weeks later. There was a ‘U’-shaped relationship between neutrophil counts and hospital mortality.
Conclusions: Neutrophils in K. pneumoniae infected patients have distinctive features and dynamic clinical trajectories. Close monitoring of severe patients infected with K. pneumoniae upon admission and during the first 1-2 weeks after admission is of utmost importance, particularly for patients with a neutrophil count exceeding 8.0×109/L.
1 Introduction
Klebsiella pneumoniae is a prevalent Gram-negative bacterium known for its ability to cause various infectious diseases, including pneumonia, hepatic abscess, bloodstream infections, and urinary tract infections (Holmes et al., 2021). K. pneumoniae, first identified by Carl Friedländer in 1882 as a causative agent of pneumonia, continues to be a prevalent nosocomial pathogen globally (Pendleton et al., 2013). It remains one of the world’s most prevalent nosocomial pathogens and is a leading cause of neonatal sepsis, ranking among the top three causative agents in most settings (Okomo et al., 2019). Notably, Klebsiella species are particularly notorious for their ability to develop resistance to multiple antibiotics, including extended-spectrum β-lactam (ESBL) and carbapenems. The World Health Organization recognizes ESBL-producing and carbapenem-resistant K. pneumoniae (CRKP) as a critical public health threat (Tacconelli, 2017). In Europe alone, K. pneumoniae strains reportedly account for >90,000 infections, >7,000 deaths annually and 25% of the total disability-adjusted life years lost to multidrug-resistant (MDR) bacterial infections, resulting in poorer clinical outcomes (Musicha et al., 2017). The host’s immune response, as a crucial of infectious disease outcomes, is crucial to pathogen clearance and preventing disseminated infection (Metzemaekers et al., 2023; van der Geest et al., 2023). A thorough understanding the clinical characteristics of immune cells, especially during K. pneumoniae infections, is crucial for optimizing clinical management and improving patient outcomes.
Neutrophils, the front-line soldiers of the innate immune system, are known for their pivotal role in pathogen elimination (Burn et al., 2021; Tsai et al., 2021; Fine et al., 2020). They are equipped with various bactericidal mechanisms, including phagocytosis, degranulation, and the release of reactive oxygen species and neutrophil extracellular traps (Liew and Kubes, 2019). Neutrophils recognize K. pneumoniae through pattern recognition receptors (PRRs) that detect pathogen-associated molecular patterns (PAMPs). This recognition triggers an inflammatory response, leading to the activation of neutrophils and the release of cytokines and chemokines that recruit more immune cells to the site of infection (Chalifour et al., 2004). Increased neutrophil levels frequently serve as a critical indicator of bacterial infection, reflecting the body’s immune response to pathogenic invasion. However, the role of neutrophils during infectious diseases is intricate (Koenderman et al., 2022; Metzemaekers et al., 2020). While neutrophils can effectively eliminate pathogens, they can also create a microenvironment conducive to pathogen survival and proliferation (Metzemaekers et al., 2020; Xu et al., 2021). In addition, sustained elevations in neutrophil counts contribute to tissue damage, exacerbate disease’s severity, and worsen clinical prognosis (Pechous, 2017; Hou et al., 2018; Nguyen et al., 2020). Thus, elucidating the multifaceted roles of neutrophils during infectious diseases are of paramount importance, offering insights that can enhance clinical management and improve patient outcomes.
Clinically, the correlation between neutrophil counts and hospital mortality rates has been well-established in patients with infectious diseases (Salciccioli et al., 2015; Liu et al., 2022). In sepsis and bloodstream infections caused by organisms such as Staphylococcus aureus and Escherichia coli, neutrophil counts and their functional status have been correlated with disease severity and patient outcomes (von Köckritz-Blickwede and Winstel, 2022; Bruns et al., 2011). Similarly, in viral infections, particularly influenza and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), neutrophilia and neutrophil activation have been associated with worse outcomes and increased mortality (Jimeno et al., 2021). These studies have highlighted the importance of neutrophils in the pathogenesis of these infections, as well as their potential prognostic value. However, the specific role and prognostic value of neutrophils in infections caused by K. pneumoniae have not been as extensively studied. Moreover, there is a lack of data that specifically delineates the clinical profiles of neutrophils during K. pneumoniae infections, especially among critically ill individuals whose immune response is frequently dysregulated and may exhibit distinct neutrophil dynamics. Giving the importance of understanding neutrophil clinical attributes for effective clinical management, there is a pressing need for focused studies that explore the behavior of neutrophils and their influence on outcomes in severe patients with K. pneumoniae infections.
In the present study, we present a detailed analysis of neutrophil characteristics and clinical trajectory in severe patients with K. pneumoniae infections using two extensive databases: the Medical Information Mart for Intensive Care (MIMIC)-IV and the eICU Collaborative Research Database (eICU-CRD). Moreover, we thoroughly investigated the association between neutrophil levels and mortality among the enrolled patients, which holds significant implications for prognostic assessment and clinical management.
2 Methods
2.1 Data description
In this study, we collected data from two databases of patient cohorts: MIMIC-IV v2.0, and the eICU-CRD v2.0. MIMIC-IV v2.0 (https://physionet.org/content/mimiciv/2.0/) is a publicly available database that consists of 76,540 admissions to the Beth Israel Deaconess Medical Centre (BIDMC; Boston, MA, USA) from 2008 to 2019. The eICU-CRD v2.0 database (https://physionet.org/content/eicu-crd/2.0/) is a multi-center and publicly available dataset that includes over 200,000 discharged patients to ICU across the United States during 2014–2015. Both databases provided demographic details (including age, sex, clinical history, and et al), laboratory measurements, diagnosis, length of stay in the hospital and other comprehensive information. All patient-related information in both databases is anonymous, and no informed consent is required. Data were extracted by author CD (certification number 50491649).
2.2 Study population
All patients in the MIMIC-IV v2.0 and eICU-CRD v2.0 databases were eligible for inclusion for the present investigation. The inclusion criteria were as follows: (i) age 18≥ years; (ii) a positive microbial culture for K. pneumoniae, obtained within the 48h-period preceding admission; and (iii) a definitive diagnosis of infection, corroborated by microbial culture results and corresponding International Classification of Diseases (ICD)-9 and ICD-10 diagnostic codes. The exclusion criteria included: (i) patients with duplicated records of infection; and (ii) multiple microbiological cultures from the same site; and (iii) incomplete data (individuals having more than 20% of the required variables missing) or absence of neutrophil results (shown as Supplementary Figure S1). To manage missing data, we employed the method of multiple imputation. The primary outcome was the in-hospital mortality, while secondary outcomes included ICU mortality, and hospital- and ICU length of stay (LOS).
2.3 Data extraction
Patient demographics, including age, gender, ICU and hospital LOS, ICU and hospital mortality rates were extracted as part of the demographic data. Comorbidities such as hypertension, diabetes, chronic pulmonary disease, coronary heart disease, liver disease, renal disease, and malignancy were also recorded. The Charlson index, a widely accepted measure of comorbidity burden, was calculated for each patient. The diagnosis of comorbidities was determined by the recorded ICD-9 and ICD-10 codes. Microbiology and laboratory played a crucial role in our analysis. Detailed information regarding the bacteria isolated, site of infection, and hematological parameters (including white blood cell counts, neutrophil counts, lymphocyte counts, red blood cell counts, and platelet counts) during their hospital stay were collected. In cases where patients had multiple hospitalizations, only the data related to the first hospitalization admission were collected. All enrolled patients had no record of being readmitted to the hospital within 7 days of their discharge due to infection. The data were extracted using Navicate Premium software (version 15.0) through a running Structured Query Language (SQL).
2.4 Statistical analysis
Continuous variables were presented as median with interquartile range (IQR) or mean with standard deviation (SD) and their statistical significance was assessed using the t-test or Wilcoxon rank sum test. Categorical variables were reported as frequencies and percentages, and their statistical significance was analyzed using the chi-square (χ2) or Fisher’s exact tests. To investigate the relationship between neutrophil counts and hospital mortality rates, as well as the dynamic fluctuations of neutrophils in K. pneumoniae infection within 30 days of clinical onset, locally weighted scatterplot smoothing (LOWESS) curves were constructed. Logistic regression models were employed to analyze the impact of neutrophil levels on hospital mortality, using the neutrophil range of 4.8-8.0×109/L serving as the reference group. The stepwise backward elimination method was employed to establish the final logistic regression model, with a significance level of 0.05. All statistical analyses were performed using the Stata (v 17.0) or R (v 4.2.2) software. Two-sided p-values <0.05 were considered statistically significant.
3 Results
3.1 Population and baseline characteristics
A total of 126,420 positive microbiological culture records of K. pneumoniae documented in both MIMIC-IV v2.0 database (n=125,440) and the eICU-CRD v2.0 database (n=980). Patient inclusion was grounded in the confirmation of these positive culture results, combined with corresponding diagnostic codes, which collectively indicated a definitive K. pneumoniae infection. The final analysis involved 1,705 patients, including 1,434 survivors and 271 non-survivors, with a mortality rate of 15.9% (Supplementary Figure S1). In the combined cohort of the two databases, the non-survivor group exhibited a comparatively older age and a higher proportion of K. pneumoniae infections originating from respiratory and bloodstream sources compared to the survivor group (38.4% vs 21.1%, p<0.0001, and 15.1% vs 10.3%, p=0.021, Table 1). There were no significant differences in different types of K. pneumoniae strains between the survivor group and the non-survivor group (p>0.05). In terms of co-morbidities, the non-survival group had a higher incidence of liver disease and kidney disease, along with Charlson index, compared to the survival group. Conversely, the incidence of hypertension in the non-survival group was lower than that observed in the survival group. Additionally, the non-survivor group demonstrated lower urinary infection rates than the non-survivor group (37.3% vs 53.3%, p<0.0001), as well as decreased counts of lymphocyte, platelet, RBC, and hemoglobin (Table 1). The baseline characteristics of each database are presented in Supplementary Tables S1, S2.
3.2 Differences and dynamic clinical trajectory of neutrophils in patients with K. pneumoniae infection among different groups
Neutrophil counts from the enrolled patients throughout their 30-day hospitalization were analyzed to investigate the characteristics and dynamic profiles among groups in different infection sites and survival conditions. The non-survivor group exhibited significantly higher neutrophil values at admission (p<0.0001), as well as at minimum and maximum levels (p=0.136 and p<0.0001, respectively), compared to the survivor group (Figure 1A). As shown in Figures 2A, B; Supplementary Figures S2A, B, the non-survivor group displayed a relatively high levels of neutrophil levels compared the survivor group no matter after antibiotics use or admission. In addition, the survivor group demonstrated a fluctuating pattern with a peak occurring approximately two weeks after admission. There were no significant differences in neutrophil values among different ethnicities (Figure 1B). In terms of different infection sites, the respiratory infection group had significantly higher neutrophil values compared to the urinary infection group and bloodstream infection group (p<0.0001 and p=0.0055, respectively, Figure 1C). Following antibiotic administration, the disparity in neutrophil levels among the three groups diminished (Figures 2C, D). Furthermore, patients combined with multiple drug resistance (MDR) strains, liver disease, above 60 years, and respiratory infection exhibited a specific dynamic process of neutrophil levels. In detail, their neutrophil counts increased within the first week, gradually declining after reaching a peak at approximately 10 days following admission (Figures 3A–D; Supplementary Figures S2C-D, S3, S4A-C). These dynamic profiles provide important insights into the temporal changes in neutrophil counts and their association with patient outcomes.
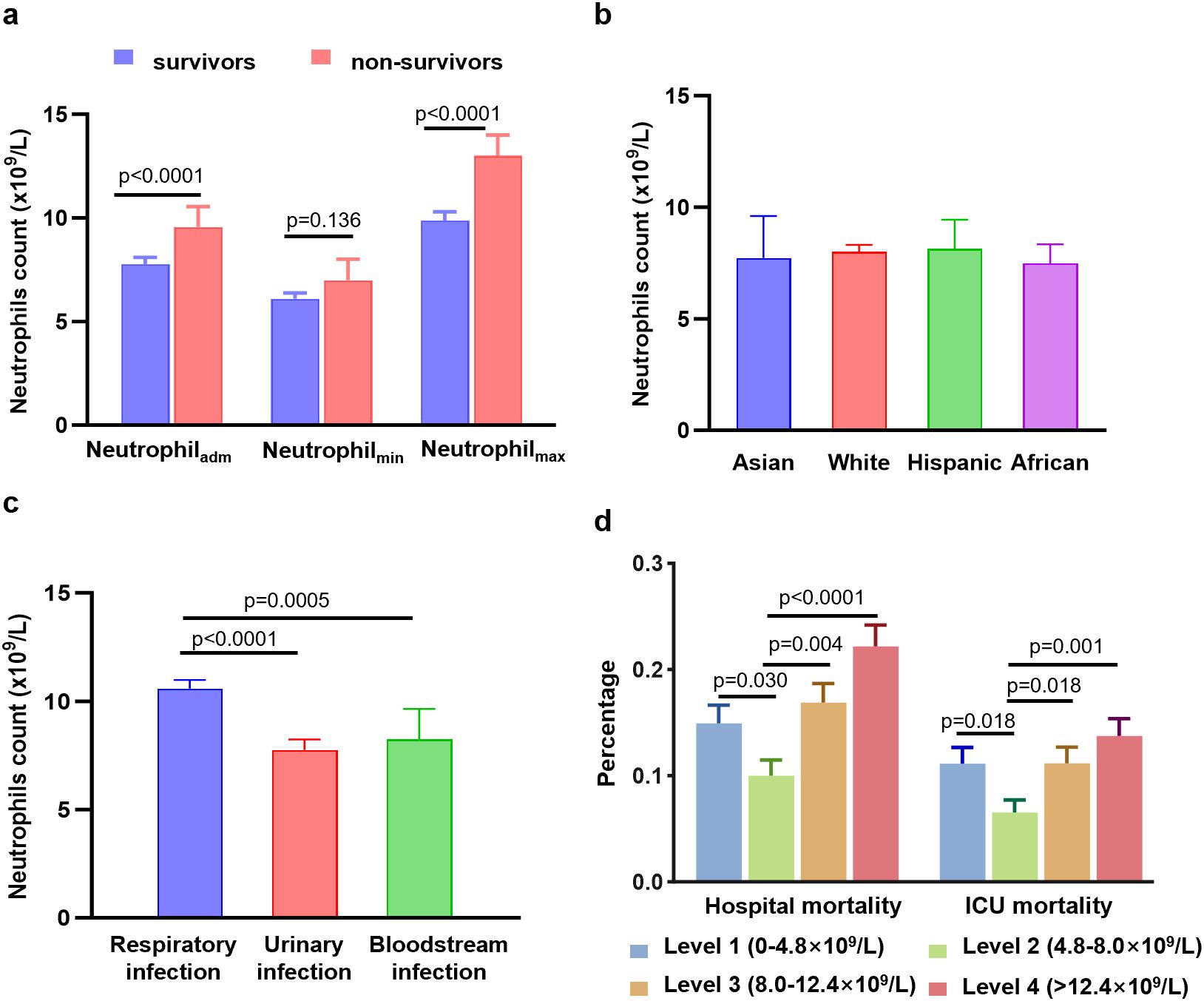
Figure 1. Differences in neutrophil counts, hospital mortality, and ICU mortality among different groups. (A) Differences in neutrophil counts at the admission values, minimum values, and maximum values between the survivor group and non-survivor group. (B) Differences in neutrophil counts among different ethnicities. (C) Differences in neutrophil counts among different infection sites. (D) Differences in hospital mortality and ICU mortality among four level groups of neutrophils. Data are presented as media (95% CI).
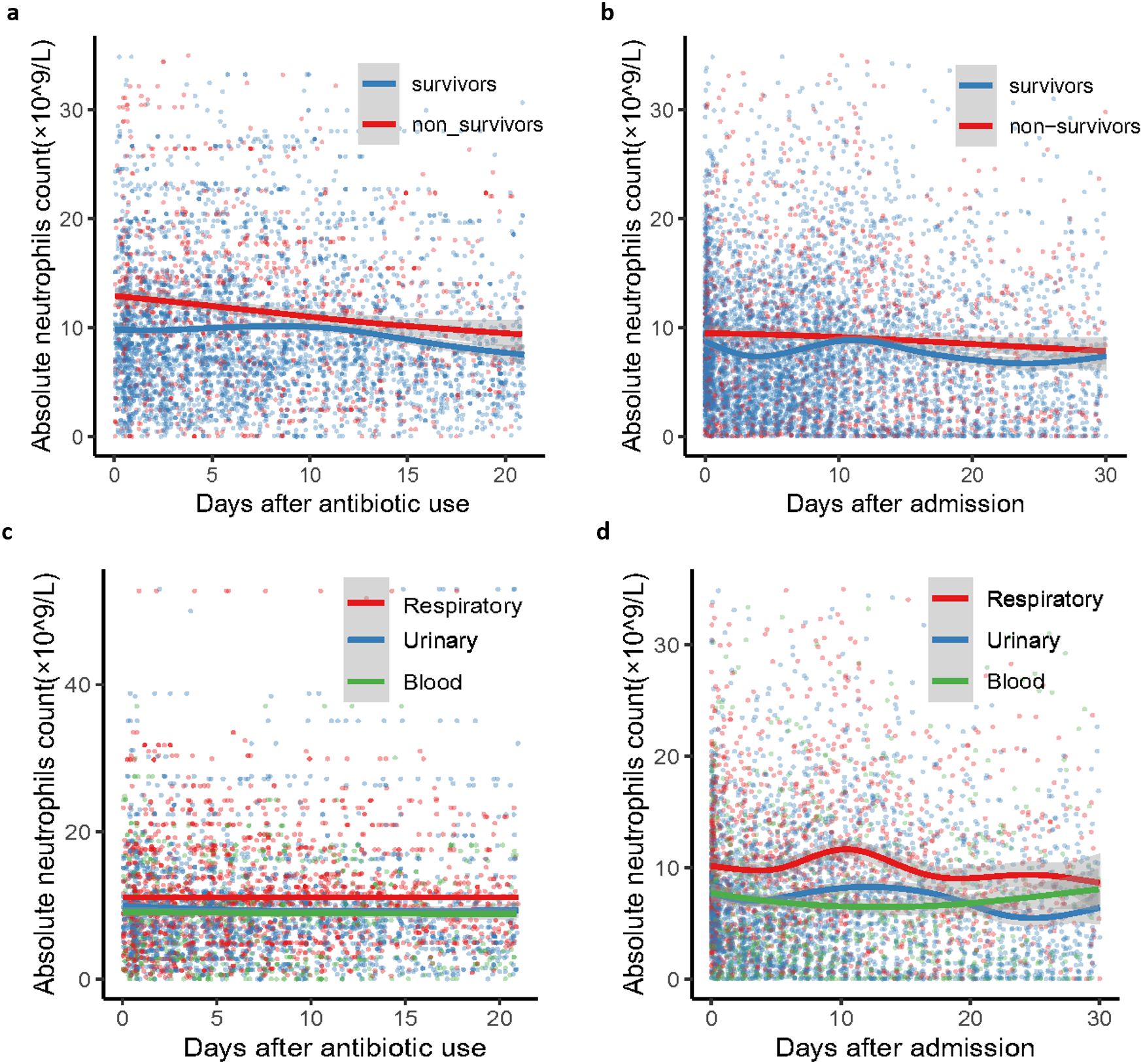
Figure 2. Dynamic clinical trajectory of neutrophil counts among survivor group, non-survivor group and groups of K. pneumoniae infected patients with respiratory, blood and urinary. (A) survivor group and non-survivor group of neutrophil dynamic clinical trajectory after antibiotic use. (B) survivor group and non-survivor group of neutrophil dynamic clinical trajectory after admission. (C) K. pneumoniae infected patients with respiratory, blood and urinary groups of neutrophil dynamic clinical trajectory after antibiotic use. (D) D K. pneumoniae infected patients with respiratory, blood and urinary groups of neutrophil dynamic clinical trajectory after admission. The Confidence interval (CI) at 95% is indicated as a gray shadow.

Figure 3. Dynamic clinical trajectory of neutrophil counts among different K. pneumoniae strains and different combination situations. (A) different K. pneumoniae strains of neutrophil dynamic clinical trajectory after antibiotic use. (B) different K. pneumoniae strains of neutrophil dynamic clinical trajectory after admission. (C) non-liver disease group and liver disease group of neutrophil dynamic clinical trajectory after admission. (D) age <60 years group and age ≥60 years group of neutrophil dynamic clinical trajectory after admission. The Confidence interval (CI) at 95% is indicated as a gray shadow. CRKP, Carbapenem-Resistant Klebsiella pneumoniae; ESBL, extended-spectrum beta-lactamase; MDR, multiple drug resistance.
3.3 Association between neutrophil counts and hospital mortality in patients with K. pneumoniae infection
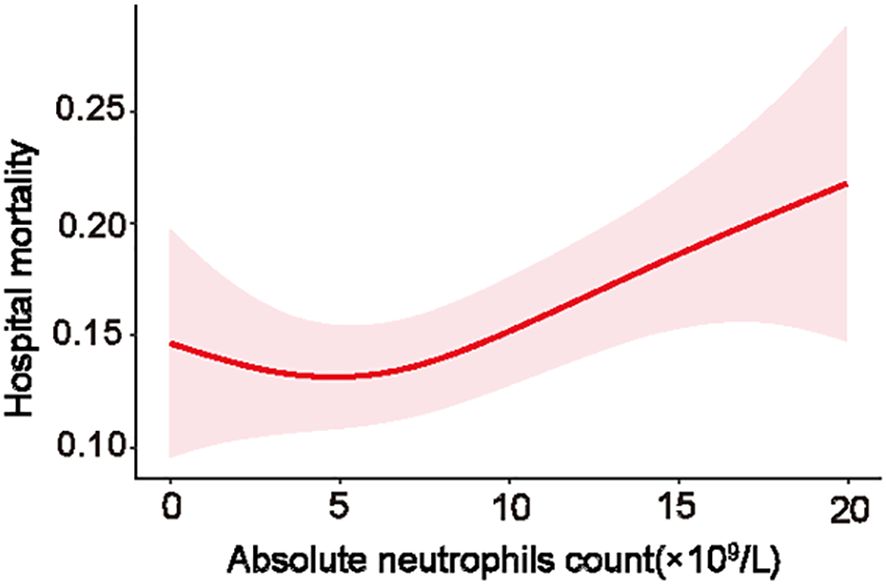
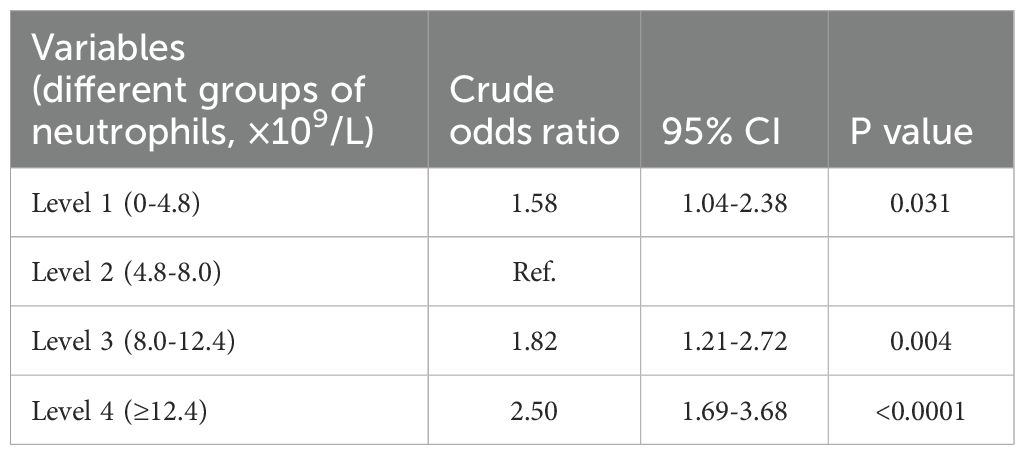
To further explore the relationship between neutrophil count and hospital mortality in patients infected with K. pneumoniae, a LOWESS curve analysis was conducted (Figure 4). Results revealed a ‘U’-shaped relationship, indicating that hospital mortality rates were lowest when the neutrophil count was 5.0×109/L. Subsequently, we divided the neutrophil levels into four groups based on the median and IQR, with the reference group set as 4.8-8.0×109/L for all comparisons and logistic regression models. Despite not statistically significant, the hospital mortality and ICU mortality rates were higher in level 1, level 3, and level 4 groups than the reference group of level 2 (Figure 1D; Supplementary Figure S4D). Univariate analysis revealed that higher neutrophil levels were associated with an increased risk of hospital mortality, with the OR increasing stepwise from level 1 (OR: 1.58; [95% CI 1.04 - 2.38], p=0.031) to level 4 (OR: 2.5; [95% CI 1.69 - 3.68], p<0.0001). These trends were consistently observed in each individual database (Table 2; Supplementary Table S3).
3.4 Multivariate logistic analysis for the risk factors of hospital mortality in patients with K. pneumoniae infection
To determine whether the neutrophil count independently influenced hospital mortality in patients infected with K. pneumoniae, a logistic regression model was employed. Each group of neutrophils levels was analyzed after adjusting for covariates with p values of <0.05 in univariate analyses, including age, Charlson’s comorbidity index, comorbidities, and different routes of infection. Using level 2 used as the reference group, the logistic regression analysis revealed that the neutrophil levels were an increased risk of hospital mortality. The odds ratio (OR) showed a stepwise increment from level 3 (OR: 1.53; 95% CI 1.09-2.16) to level 4 (OR: 2.01; 95% CI 1.45-2.79) (Table 3). These results were consistent with the findings obtained from the MIMIC database (Supplementary Table S3). Notably, a neutrophil count of ≥8.0×109/L was identified as an independent risk factor for hospital mortality in patients with K. pneumoniae infection. This suggests that higher neutrophil levels are associated with an increased likelihood of hospital mortality in this patient population.
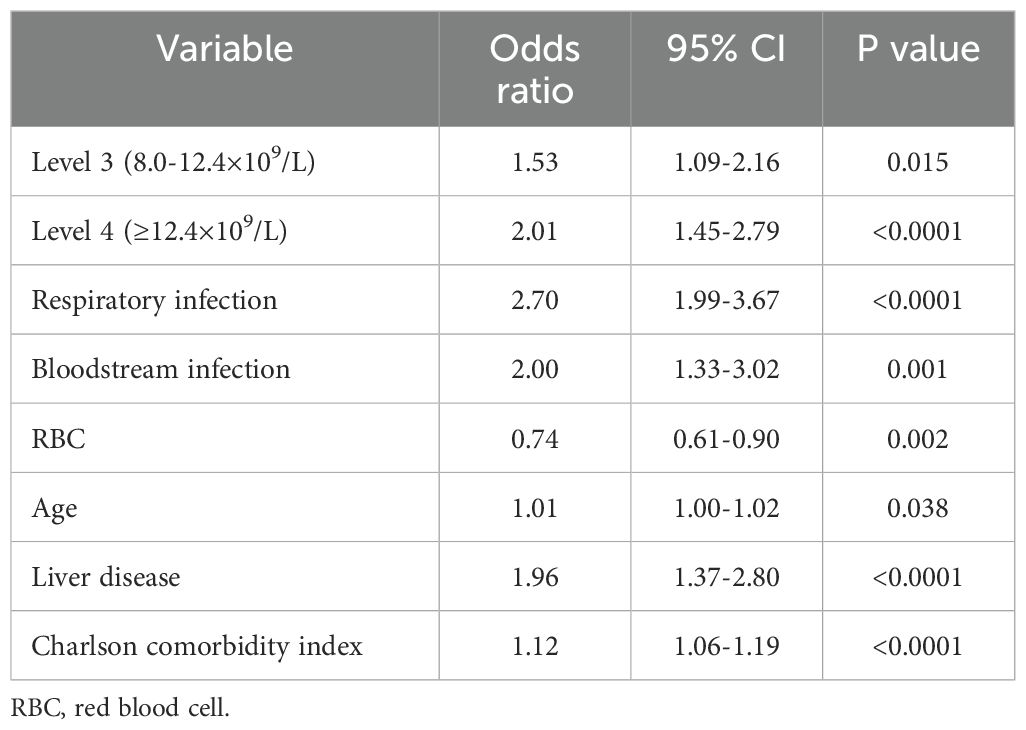
Table 3. Adjusted odds ratio using neutrophil as the design variable in multivariate logistic regression.
4 Discussion
K. pneumoniae infections pose a significant challenge due to its elevated morbidity and mortality rates. Considering the limited clinical understanding of neutrophils’ involvement in such infections, we aimed to address this knowledge gap by comprehensive analyses using two large clinical databases. Our findings revealed that non-survivors had significantly higher neutrophil counts compared to survivors. Patients with respiratory infections, without liver disease, and below 60 years of age exhibited higher neutrophil levels and had different dynamic profiles. The peak levels of neutrophils were observed at the time of admission or approximately 1-2 weeks later. Importantly, neutrophil counts appeared to follow a “U”-shaped relationship between neutrophil counts and hospital mortality. Patients with a neutrophil count ≥ 8.0×109/L emerged as a subgroup of particular concern during hospitalization, as this parameter was identified as an independent risk factor for hospital mortality in patients with K. pneumoniae infection. These findings contribute to our understanding of the pathophysiology of K. pneumoniae infections and assist clinicians in identifying patients who may require closer monitoring and more intensive management strategies.
Our findings revealed a crucial aspect regarding the timing of neutrophil peaks, which occurred at the time of admission or approximately 1-2 weeks later. This observation reflects a dynamic pattern of neutrophil response during the course of infection and holds significant clinical implications in the management of K. pneumoniae infected patients. In the human body, the circulatory lifespan of neutrophils averages around 5.4 days (Pillay et al., 2010). However, during inflammatory processes, the longevity of neutrophils can be magnified manifold, ensuring the recruitment of primed neutrophils to the precise sites of inflammation (Koenderman et al., 2022). Elevated levels of neutrophils remained detectable even at 7 to 14 days post-infection. Of particular interest, the rapid recruitment of neutrophils within the initial 48 hours following infection does not exhibit a direct correlation with a reduction in bacterial burden (Ahn et al., 2016; Peñaloza et al., 2019; Wang et al., 2020). The resolution of inflammation involves a multifaceted interplay between anti-inflammatory and pro-resolving phases, which can influence the generation and recruitment of neutrophils. This intricate balance between pro-inflammatory and anti-inflammatory factors throughout the progression of infection, coupled with the re-emergence of pathogens, likely contributes to the dynamic profiles exhibited by neutrophils (Ji and Fan, 2021; Bruserud et al., 2023).
The observation of a distinctive ‘U’-shaped relationship between neutrophil levels and hospital mortality is an interesting finding, which suggests that there might be an optimal range of neutrophil counts for fighting K. pneumoniae infections. Notably, while higher and lower levels of neutrophils were both correlated with increased mortality rates, this association does not necessarily imply a direct causative relationship. In present study, non-survivors exhibited higher neutrophil counts than survivors. Furthermore, patients with elevated or decreased neutrophil levels (groups of level 1, 3, and 4) exhibited increased hospital and ICU mortalities compared to the reference group (group of level 2). This finding likely reflects the intricate interplay between neutrophils and other factors in the context of severe infections. While an increase in neutrophil counts, or neutrophilia, is a typical response to bacterial infections like K. pneumoniae and is generally perceived as advantageous as it indicates the host’s active immune response, the scenario in severe infections is less clear-cut. In such cases, very severe infections might precipitate either an increase or a decrease in neutrophil counts and functions. This can lead to an immune system malfunction, coupled with multiple organ dysfunction, which may further intensify disease progression (Pechous, 2017; Metzemaekers et al., 2020; Zanza et al., 2022). As neutrophil counts are influenced by a multitude of factors, including the severity of the infection, the host’s immune response, and the overall clinical condition, it is essential to interpret neutrophil levels within the broader clinical context.
Our study identified neutrophil counts were independent risk factors for hospital mortality. This finding aligns with previous research (Lv et al., 2022). Importantly, we validated a critical cut-off value at 8.0×109/L, which serves as significant biomarker for differentiating patients at increased risk of mortality due to elevated neutrophil levels. These findings emphasize the necessity for close surveillance of neutrophil dynamics in the clinical setting, especially in cases where they exceed the established cut-off. Integrating this biomarker with other clinical data empower healthcare professionals to make more informed decisions about patient management. This facilitates the implementation of targeted therapeutic interventions, encompassing early antimicrobial therapy and adjunctive treatments, thereby optimizing patient care and outcomes.
In current study, patients with respiratory infections caused by K. pneumonia exhibited higher levels of neutrophils compare to those with bloodstream infections and other sites of infections. Neutrophils in K. pneumoniae patients with different infection sites have distinctive features and dynamic clinical trajectories. Previous studies have demonstrated that the lungs serve as a natural reservoir of mature neutrophils, which can be rapidly mobilized to sites of inflammation or infection (Ostrand-Rosenberg et al., 2023). Furthermore, it has been suggested that all blood neutrophils undergo a temporary pulmonary sequestration due to the lungs receiving the entire cardiac output (Summers et al., 2010; Fay et al., 2016; Pararajasingam et al., 1999). Considering these factors, the higher neutrophil levels observed in patients with respiratory infections may be attributed to the recruitment and accumulation of neutrophils in the lung tissues. Since neutrophil counts have been identified as an independent risk factor for hospital mortality, it is necessary to promptly identify the site of infection, particularly focusing on patients with respiratory infections.
In present study, patients who had liver disease or were aged ≥ 60 years displayed lower neutrophil levels in comparison to the control group consisting of patients without liver disease or younger than 60 years old. Concurrently, we conducted a comprehensive analysis, comparing and illustrating the fluctuations of neutrophil levels across both groups. This analysis provided a detailed depiction of neutrophil dynamics in relation to liver disease status and age demographics. The liver plays a central role as an immunological organ, housing various myeloid immune cells, including neutrophils, macrophages, and Kupffer cells (Kubes and Jenne, 2018; Ahmed et al., 2021). Therefore, the presence of liver disease may contribute to a decrease in neutrophil levels. It is important to note that while the absolute number of neutrophils does not vary significantly with age, there is evidence to suggest that aging is associated with impaired phagocytosis, chemotaxis, and superoxide generation functions of neutrophils (Hornigold et al., 2022; Lewis et al., 2022). Additionally, elderly patients may experience decreased anti-pathogen activity due to increased apoptosis of neutrophils, resulting in a higher susceptibility to more frequent and severe infections (Hornigold et al., 2022; Wen et al., 2022).
Despite significant findings regarding the dynamic profiles of neutrophils and their association with hospital mortality, several potential limitations in this study should be acknowledged. Firstly, retrospective analyses inherently possess limitations in establishing causal associations definitively. A more comprehensive understanding of the immunological mechanisms encompassing both the quantitative dynamics and functional integrity of neutrophils is warranted. Secondly, the database lacked comprehensive data on inflammatory biomarkers such as C-reactive protein and procalcitonin, which precluded any in-depth analysis or comparison of these markers with neutrophil counts in this study. Thirdly, while our findings are focused on primary K. pneumoniae infections, the potential influence of coinfections and reinfections on neutrophil counts was not addressed. Further studies should take these factors into account to refine the understanding of neutrophil dynamics in the context of infectious diseases. Lastly, while the statistical association between hospital mortality and neutrophil levels has been demonstrated, it is essential to include data from other clinical databases that help confirm the robustness of the associations. Meanwhile, its practical application in clinical practice should be evaluated across different clinical scenarios.
In summary, neutrophils in K. pneumoniae infected patients have distinctive features and dynamic clinical trajectories. Closely monitoring severe patients infected with K. pneumoniae upon admission and during the first 1-2 weeks after admission is of utmost importance. This is particularly significant for patients with a neutrophil count exceeding 8.0×109/L, as elevated neutrophils levels were correlated with a poor prognosis in individuals with K. pneumonia infection. To effectively manage severe cases of K. pneumoniae infections, it is essential to further investigate the intricate mechanisms of neutrophils during K. pneumoniae infections.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://physionet.org/content/mimiciv/2.0/ https://physionet.org/content/eicu-crd/2.0/.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
CD: Conceptualization, Data curation, Funding acquisition, Methodology, Writing – original draft, Writing – review & editing. ML: Data curation, Methodology, Writing – original draft. JZ: Data curation, Formal analysis, Supervision, Writing – original draft. CL: Data curation, Formal analysis, Writing – original draft. PY: Methodology, Software, Writing – original draft. JY: Methodology, Software, Writing – original draft. LZ: Conceptualization, Writing – review & editing. NS: Conceptualization, Funding acquisition, Writing – review & editing, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Beijing Key Clinical Specialty Funding (No. 010071) and National Natural Science Foundation of China (No.82202415).
Acknowledgments
The authors gratefully acknowledge Lu Xu Ph.D. and Hongling Chu Ph.D. for their assistance with data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1406168/full#supplementary-material
Abbreviations
eICU-CRD, eICU Collaborative Research Database; ICD International Classification of Diseases; IQR, interquartile ranges; Klebsiella pneumoniae, K. pneumoniae; LOESS, locally weighted scatterplot smoothing; LOS, length of stays; MIMIC IV, Medical Information Mart for Intensive Care IV; RBC, red blood cell.
References
Ahmed, O., Robinson, M. W., O’Farrelly, C. (2021). Inflammatory processes in the liver: divergent roles in homeostasis and pathology. Cell Mol. Immunol. 18, 1375–1386. doi: 10.1038/s41423-021-00639-2
Ahn, D., Peñaloza, H., Wang, Z., Wickersham, M., Parker, D., Patel, P., et al. (2016). Acquired resistance to innate immune clearance promotes Klebsiella pneumoniae ST258 pulmonary infection. JCI Insight 1, e89704. doi: 10.1172/jci.insight.89704
Bruns, T., Peter, J., Hagel, S., Herrmann, A., Stallmach, A. (2011). The augmented neutrophil respiratory burst in response to Escherichia coli is reduced in liver cirrhosis during infection. Clin. Exp. Immunol. 164, 346–356. doi: 10.1111/j.1365-2249.2011.04373.x
Bruserud, Ø., Mosevoll, K. A., Bruserud, Ø., Reikvam, H., Wendelbo, Ø. (2023). The regulation of neutrophil migration in patients with sepsis: the complexity of the molecular mechanisms and their modulation in sepsis and the heterogeneity of sepsis patients. Cells 12, 1003. doi: 10.3390/cells12071003
Burn, G. L., Foti, A., Marsman, G., Patel, D. F., Zychlinsky, A. (2021). The neutrophil. Immunity 54, 1377–1391. doi: 10.1016/j.immuni.2021.06.006
Chalifour, A., Jeannin, P., Gauchat, J.-F., Blaecke, A., Malissard, M., N’Guyen, T., et al. (2004). Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 104, 1778–1783. doi: 10.1182/blood-2003-08-2820
Fay, M. E., Myers, D. R., Kumar, A., Turbyfield, C. T., Byler, R., Crawford, K., et al. (2016). Cellular softening mediates leukocyte demargination and trafficking, thereby increasing clinical blood counts. Proc. Natl. Acad. Sci. U.S.A. 113, 1987–1992. doi: 10.1073/pnas.1508920113
Fine, N., Tasevski, N., McCulloch, C. A., Tenenbaum, H. C., Glogauer, M. (2020). The neutrophil: constant defender and first responder. Front. Immunol. 11. doi: 10.3389/fimmu.2020.571085
Holmes, C. L., Anderson, M. T., Mobley, H. L. T., Bachman, M. A. (2021). Pathogenesis of gram-negative bacteremia. Clin. Microbiol. Rev. 34, e00234–e00220. doi: 10.1128/CMR.00234-20
Hornigold, K., Chu, J. Y., Chetwynd, S. A., Machin, P. A., Crossland, L., Pantarelli, C., et al. (2022). Age-related decline in the resistance of mice to bacterial infection and in LPS/TLR4 pathway-dependent neutrophil responses. Front. Immunol. 13. doi: 10.3389/fimmu.2022.888415
Hou, Q., Liu, F., Chakraborty, A., Jia, Y., Prasad, A., Yu, H., et al. (2018). Inhibition of IP6K1 suppresses neutrophil-mediated pulmonary damage in bacterial pneumonia. Sci. Transl. Med. 10, eaal4045. doi: 10.1126/scitranslmed.aal4045
Ji, J., Fan, J. (2021). Neutrophil in reverse migration: role in sepsis. Front. Immunol. 12. doi: 10.3389/fimmu.2021.656039
Jimeno, S., Ventura, P. S., Castellano, J. M., García-Adasme, S. I., Miranda, M., Touza, P., et al. (2021). Prognostic implications of neutrophil-lymphocyte ratio in COVID-19. Eur. J. Clin. Invest. 51, e13404. doi: 10.1111/eci.13404
Koenderman, L., Tesselaar, K., Vrisekoop, N. (2022). Human neutrophil kinetics: a call to revisit old evidence. Trends Immunol. 43, 868–876. doi: 10.1016/j.it.2022.09.008
Kubes, P., Jenne, C. (2018). Immune responses in the liver. Annu. Rev. Immunol. 36, 247–277. doi: 10.1146/annurev-immunol-051116-052415
Lewis, E. D., Wu, D., Meydani, S. N. (2022). Age-associated alterations in immune function and inflammation. Prog. Neuropsychopharmacol. Biol. Psychiatry 118, 110576. doi: 10.1016/j.pnpbp.2022.110576
Liew, P. X., Kubes, P. (2019). The neutrophil’s role during health and disease. Physiol. Rev. 99, 1223–1248. doi: 10.1152/physrev.00012.2018
Liu, W., Yang, C., Liao, Y.-G., Wan, F., Lin, L., Huang, X., et al. (2022). Risk factors for COVID-19 progression and mortality in hospitalized patients without pre-existing comorbidities. J. Infect. Public Health 15, 13–20. doi: 10.1016/j.jiph.2021.11.012
Lv, D., Zuo, Y., Wang, Y., Wang, Z., Xu, Y. (2022). Predictors of Occurrence and 30-Day Mortality for Co-Infection of Carbapenem-Resistant Klebsiella pneumoniae and Carbapenem-Resistant Acinetobacter baumannii. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.919414
Metzemaekers, M., Gouwy, M., Proost, P. (2020). Neutrophil chemoattractant receptors in health and disease: double-edged swords. Cell Mol. Immunol. 17, 433–450. doi: 10.1038/s41423-020-0412-0
Metzemaekers, M., Malengier-Devlies, B., Gouwy, M., De Somer, L., Cunha, F., de, Q., et al. (2023). Fast and furious: The neutrophil and its armamentarium in health and disease. Med. Res. Rev. 43, 1537–1606. doi: 10.1002/med.21958
Musicha, P., Cornick, J. E., Bar-Zeev, N., French, N., Masesa, C., Denis, B., et al. (2017). Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi, (1998-2016): a surveillance study. Lancet Infect. Dis. 17, 1042–1052. doi: 10.1016/S1473-3099(17)30394-8
Nguyen, G. T., Shaban, L., Mack, M., Swanson, K. D., Bunnell, S. C., Sykes, D. B., et al. (2020). SKAP2 is required for defense against K. pneumoniae infection and neutrophil respiratory burst. Elife 9, e56656. doi: 10.7554/eLife.56656
Okomo, U., Akpalu, E. N. K., Le Doare, K., Roca, A., Cousens, S., Jarde, A., et al. (2019). Aetiology of invasive bacterial infection and antimicrobial resistance in neonates in sub-Saharan Africa: a systematic review and meta-analysis in line with the STROBE-NI reporting guidelines. Lancet Infect. Dis. 19, 1219–1234. doi: 10.1016/S1473-3099(19)30414-1
Ostrand-Rosenberg, S., Lamb, T. J., Pawelec, G. (2023). Here, there, and everywhere: myeloid-derived suppressor cells in immunology. J. Immunol. 210, 1183–1197. doi: 10.4049/jimmunol.2200914
Pararajasingam, R., Nicholson, M. L., Bell, P. R., Sayers, R. D. (1999). Non-cardiogenic pulmonary oedema in vascular surgery. Eur. J. Vasc. Endovasc Surg. 17, 93–105. doi: 10.1053/ejvs.1998.0750
Pechous, R. D. (2017). With friends like these: the complex role of neutrophils in the progression of severe pneumonia. Front. Cell Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00160
Peñaloza, H. F., Noguera, L. P., Ahn, D., Vallejos, O. P., Castellanos, R. M., Vazquez, Y., et al. (2019). Interleukin-10 produced by myeloid-derived suppressor cells provides protection to carbapenem-resistant klebsiella pneumoniae sequence type 258 by enhancing its clearance in the airways. Infect. Immun. 87, e00665–e00618. doi: 10.1128/IAI.00665-18
Pendleton, J. N., Gorman, S. P., Gilmore, B. F. (2013). Clinical relevance of the ESKAPE pathogens. Expert Rev. Anti Infect. Ther. 11, 297–308. doi: 10.1586/eri.13.12
Pillay, J., den Braber, I., Vrisekoop, N., Kwast, L. M., de Boer, R. J., Borghans, J. A. M., et al. (2010). In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116, 625–627. doi: 10.1182/blood-2010-01-259028
Salciccioli, J. D., Marshall, D. C., Pimentel, M. A. F., Santos, M. D., Pollard, T., Celi, L. A., et al. (2015). The association between the neutrophil-to-lymphocyte ratio and mortality in critical illness: an observational cohort study. Crit. Care 19, 13. doi: 10.1186/s13054-014-0731-6
Summers, C., Rankin, S. M., Condliffe, A. M., Singh, N., Peters, A. M., Chilvers, E. R. (2010). Neutrophil kinetics in health and disease. Trends Immunol. 31, 318–324. doi: 10.1016/j.it.2010.05.006
Tacconelli, E. (2017). Global priority listof antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization (WHO). Available at: https://www.aidsdatahub.org/sites/default/files/resource/who-global-priority-list-antibiotic-resistant-bacteria.pdf.
Tsai, C.-Y., Hsieh, S.-C., Liu, C.-W., Lu, C.-S., Wu, C.-H., Liao, H.-T., et al. (2021). Cross-Talk among Polymorphonuclear Neutrophils, Immune, and Non-Immune Cells via Released Cytokines, Granule Proteins, Microvesicles, and Neutrophil Extracellular Trap Formation: A Novel Concept of Biology and Pathobiology for Neutrophils. Int. J. Mol. Sci. 22, 3119. doi: 10.3390/ijms22063119
van der Geest, R., Fan, H., Peñaloza, H. F., Bain, W. G., Xiong, Z., Kohli, N., et al. (2023). Phagocytosis is a primary determinant of pulmonary clearance of clinical Klebsiella pneumoniae isolates. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1150658
von Köckritz-Blickwede, M., Winstel, V. (2022). Molecular prerequisites for neutrophil extracellular trap formation and evasion mechanisms of staphylococcus aureus. Front. Immunol. 13. doi: 10.3389/fimmu.2022.836278
Wang, N., Bai, X., Tang, B., Yang, Y., Wang, X., Zhu, H., et al. (2020). Primary characterization of the immune response in pigs infected with Trichinella spiralis. Vet. Res. 51, 17. doi: 10.1186/s13567-020-0741-0
Wen, S. W., Shim, R., Hall, P., Bedo, J., Wilson, J. L., Nicholls, A. J., et al. (2022). Lung imaging reveals stroke-induced impairment in pulmonary intravascular neutrophil function, a response exacerbated with aging. J. Immunol. 208, 2019–2028. doi: 10.4049/jimmunol.2100997
Xu, Q., Yang, X., Chan, E. W. C., Chen, S. (2021). The hypermucoviscosity of hypervirulent K. pneumoniae confers the ability to evade neutrophil-mediated phagocytosis. Virulence 12, 2050–2059. doi: 10.1080/21505594.2021.1960101
Keywords: K. pneumoniae, neutrophil, infection, innate immunity, prognosis, severe patients
Citation: Du C, Lu M, Zheng J, Liu C, Yang P, Yi J, Zhu L and Shen N (2024) Distinctive features and prognostic utility of neutrophil in severe patients with Klebsiella pneumoniae infection. Front. Cell. Infect. Microbiol. 14:1406168. doi: 10.3389/fcimb.2024.1406168
Received: 25 March 2024; Accepted: 14 August 2024;
Published: 03 September 2024.
Edited by:
Shu-Hua Wang, The Ohio State University, United StatesReviewed by:
Pedro Xavier-Elsas, Federal University of Rio de Janeiro, BrazilJuan Xicohtencatl-Cortes, Hospital Infantil de México Federico Gómez, Mexico
Kasturi Banerjee, CSL, United States
Copyright © 2024 Du, Lu, Zheng, Liu, Yang, Yi, Zhu and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Shen, c2hlbm5pbmdwdXRoQDE2My5jb20=; Liuluan Zhu, emh1bGl1bHVhbkBjY211LmVkdS5jbg==
†These authors have contributed equally to this work
 Chunjing Du
Chunjing Du Ming Lu2,3†
Ming Lu2,3† Chao Liu
Chao Liu Ping Yang
Ping Yang Juan Yi
Juan Yi Liuluan Zhu
Liuluan Zhu Ning Shen
Ning Shen