
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 07 June 2024
Sec. Antibiotic Resistance and New Antimicrobial drugs
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1390053
 Han Wu1,2†
Han Wu1,2† Manning Li1,2†
Manning Li1,2† Chunyi Shou2
Chunyi Shou2 Fangfang Shi2
Fangfang Shi2 Xiaolu Song2
Xiaolu Song2 Qingfeng Hu3
Qingfeng Hu3 Ying Wang4
Ying Wang4 Yirui Chen2*
Yirui Chen2* Xiangmin Tong2,5*
Xiangmin Tong2,5*Background: Bloodstream infection (BSI) represent a prevalent complication in haematological malignancies (HMs). Typically, Patients with BSI usually undergo empirical treatment pending pathogen identification. The timely and effective management of BSIs significantly influences patient prognosis. However, pathogen distribution in BSIs exhibits regional variation. In this study, we investigated the clinical characteristics, pathogen spectrum, drug resistance, risk factors of short-term prognosis and long-term prognostic factors of acute myeloid leukemia (AML) patients with BSI at Zhejiang Provincal People’s Hospital.
Methods: From 2019 to 2021, a total of 56 AML patients with BSI were treated in the Department of Haematology at Zhejiang Province People’s Hospital. Data regarding pathogen spectrum and drug resistance were collected for analysis. The patients were stratified into non-survivor cohort and survivor cohort within 30 days after BSI, and the predictors of 30-days mortality were identified through both univariate and multivariate Logistic regression analyses. Furthermore, Kaplan-Meier survival analysis and Cox regression analysis were employed to ascertain the risk factors associated with poor prognosis in AML patients complicated by BSI.
Results: A total of 70 strains of pathogenic bacteria were isolated from 56 AML patients with BSI. Gram-negative bacteria constituted the predominant pathogens (71.4%), with Klebsiella pneumoniae being the most prevalent (22.9%). Gram-positive bacteria and fungi accounted for 22.9% and 5.7%, respectively. Univariate and multivariate analyses revealed significant differences in total protein, albumin levels, and the presence of septic shock between the non-survivor cohort and the survior cohort 30 days post-BSI. COX regression analysis showed that agranulocytosis duration exceeding 20 days (HR:3.854; 95% CI: 1.451–10.242) and septic shock (HR:3.788; 95% CI: 1.729–8.299) were independent risk factors for poor prognosis in AML patients complicated by BSI. Notably, the mortality rate within 30 days after Stenotrophomonas maltophilia infection was up to 71.4%.
Conclusions: In this study, Gram-negative bacteria, predominantly Klebsiella pneumoniae, constituted the primary pathogens among AML patients with BSIs. Serum albumin levels and the presence of septic shock emerged as independent risk factors for mortality within 30 days among AML patients with BSI. In terms of long-term prognosis, extended agranulocytosis duration exceeding 20 days and septic shock were associated with elevated mortality rates in AML patients with BSI. Additionally, in our centre, Stenotrophomonas maltophilia infection was found to be associated with a poor prognosis. Early intervention for Stenotrophomonas maltophilia infection in our centre could potentially improve patient outcomes.
Acute myeloid leukaemia (AML) is a malignant proliferation of haematopoietic cells. The massive proliferation of abnormal blasts and immature cells in the bone marrow prevents normal haematopoiesis. Thus, the bone marrow and peripheral blood are characterised by an increase in immature leukocytes, mainly blasts (Assi et al., 2019; Wrighton, 2019). Immature cells accumulate in the bone marrow and replace normal bone marrow cells, megakaryocytes, and red blood cells, leading to loss of normal bone marrow function, bleeding, anaemia, and infection-related complications (McKenzie, 2005). Several factors related to the prognosis of patients with AML, such as infection, disease relapse, and haemorrhage. In addition, haematopoietic stem cell transplantation, chemotherapy regimens, and duration of agranulocytosis are closely related to patient prognosis. Among these, the incidence of bloodstream infections (BSIs) in haematological malignancies is 4–90% (Tumbarello et al., 2012; Samonis et al., 2013; Kolonen et al., 2017). Furthermore, the widespread utilisation of antibiotics has led to a gradual increase in the incidence of drug-resistant bacteria. Inappropriate empirical antimicrobial therapy or repeated infections contribute to the rise in the incidence of multiple drug resistance and mortality rates in patients (Moghnieh et al., 2015; Tang et al., 2020). Fortunately, by taking prompt preventive measures, we can significantly reduce the incidence of BSIs and lower the occurrence of related complications. This underscores the critical importance of early intervention. Such measures not only contribute to enhancing patient survival rates but also notably improve their overall quality of life, thereby creating a more conducive environment for their treatment. Hence, the significance of early BSIs prevention cannot be underestimated. However, the distribution of pathogenic bacteria in BSIs of patients in different regions varies greatly, which makes it difficult for early intervention of BSIs.
Gram-positive bacteria plays a predominant role as pathogens in certain haematology centres in Europe and America (Mikulska et al., 2014). However, the situation is markedly different in haematological wards in China, where Gram-negative bacteria are more prevalent (Deng et al., 2012). This disparity not only involves the types of pathogens but also extends to the establishment of medical infrastructure and the formulation of prevention and control strategies. Furthermore, regional variations also impact the antimicrobial susceptibility of specific pathogens (Deng et al., 2012; Mikulska et al., 2014). Resistance patterns of the same pathogenic bacteria may differ among different regions underscoring the need to consider the local microbial environment and resistance profiles when devising treatment plans and preventive strategies. This consideration is particularly crucial in the treatment of haematologic patients, where drug selection and administration must be tailored to the patient’s condition, the type of pathogenic bacteria, and the local susceptibility data. This integration ensures the optimal therapeutic outcomes and prognostic results (Deng et al., 2012; Mikulska et al., 2014). Therefore, as the etiological differences in different regions, there are some difficulties in empirical determination of medication for patients with BSIs and of prophylactic medication in patients at high risk of BSIs.
This study conducted a retrospective analysis of pathogen distribution, drug resistance, the risk factors associated with mortality within 30 days of BSI, and long-term prognosis of AML patients with BSI in a subset of 56 AML patients with BSIs, including 18 cases that resulted in death. This study provides a robust basis for clinically determining treatment approaches for AML patients with concurrent BSI and for improving patient prognosis in China.
A retrospective analysis was conducted on 56 AML patients who received treatment at the Hematology Department of Zhejiang People’s Hospital. The deadline for follow-up is January 1, 2024. The age, sex, platelet count, activated partial thromboplastin time, total protein, albumin, glucocorticoid, septic shock, agranulocytosis duration, agranulocytosis to infection, history of infection, prior HSCT history and chemotherapy cycles were collected from 56 patients. Patients who died within 30 days after BSI constituted non-survivor cohort, while those who still survive formed the survivor cohort. Patients in the cohort who died within 30 days after BSI had an average age of 48 years, with 9 males and 9 females. In the other cohort, the average age was 52 years, with 25 males and 18 females. (Table 1).
In addition, the pathogens of AML patients with BSI in the Hematology Department of Zhejiang people’s Hospital from 2013 to 2021 and the mortality within 30 days after bloodstream infection were collected retrospectively.
1. Body temperature > 38.0°C or < 36.0°C, possibly accompanied by chills; aerobic bacteria, anaerobic bacteria, and fungal culture should be completed before the use of antibacterial drugs or during high fever and chills.
2. Poisoning symptoms without obvious infection lesions: Body temperature > 38.0°C or < 36.0°C may be accompanied by chills hypotension, oliguria or high lactic acid blood levels, but no pathogens were isolated from respiratory tract, urinary tract, gastrointestinal tract and skin.
3. Antigen substance of pathogenic bacteria detected in blood culture is consistent with signs and symptoms: Pathogenic microorganisms were isolated from blood culture, accompanied by one of the following symptoms: fever, chills, hypoxia and hypotension (China MoHotPsRo, 2005).
4. Agranulocytosis: absolute neutrophil count (ANC) in peripheral blood < 0.5×109/L or ANC < 0.5×109/L predicted to occur after 48 hours (Chen Xin, 2020).
Peripheral blood or bone marrow blasts ≥ 20% or peripheral blood or bone marrow blasts < 20%, with t (8, 21) (q22;q22), inv (16) (p13q22), t (16; 16) (p13;q22), t (15; 17) (q22;q12) or other recurrent genetic abnormalities (Leonard et al., 2017).
Septic shock exhibits low blood pressure necessitating vasopressor therapy to maintain an average blood pressure of 65 mmHg or higher, or serum lactate levels are greater than 2 mmol/L after full fluid resuscitation (Shankar-Hari et al., 2016).
1. Patient diagnosed with AML.
2. Positive blood culture results.
1. Patient diagnosed with non-AML.
2. Patients who fail to perform blood culture or whose blood culture results are negative.
3. Patients with multiple positive blood cultures with the same pathogen during the same hospitalisation were recorded as once.
All variables were statistically processed using SPSS software (version 25.0). Non-normal distribution was expressed as median and interquartile range, while normal distribution was expressed as mean ± standard deviation P < 0.05 was considered statistically significant. The distribution of pathogen spectrum between groups was analysed by Fisher’s exact test. Independent sample T-tests and logistic regression analysis were applied to assess the risk factors impacting the prognosis within 30 days after infection. Additionally, Kaplan-Meier survival analysis and Cox regression analysis were conducted to identify risk factors for poor prognosis of AML patients with BSI.
66 strains of bacteria and 4 strains of fungi were isolated from 70 strains, of which 50 were Gram-negative bacteria (71.4%). Specifically, there were 16 strains of Klebsiella pneumoniae (22.9%), 10 strains of Escherichia coli (14.2%), 9 strains of Pseudomonas aeruginosa (12.9%), 7 strains of Stenotrophomonas maltophilia (10%), 2 strains of Enterobacter cloacae (2.9%), 1 strain each of Proteus vulgaris, Enterobacter aerogenes, Aeromonas sobria, Oral ciliates, Klebsiella oxytoca and Meningeal Septicemia Elisabia (1.4%, respectively). Additionally, there are 16 strains of Gram-positive bacteria (22.9%), 5 strains were Enterococcus faecium (7.1%), and 2 strains each of Streptococcus mitis, Staphylococcus epidermidis, Staphylococcus hominis subsp hominis, and Bacillus cereus (2.9%), and 1 strain each of Staphylococcus aureus, Hemolytic Staphylococcus, and Corynebacterium Gerschi(1.4%). 4 strains of fungi were Candida tropicalis (5.7%) (Table 2).
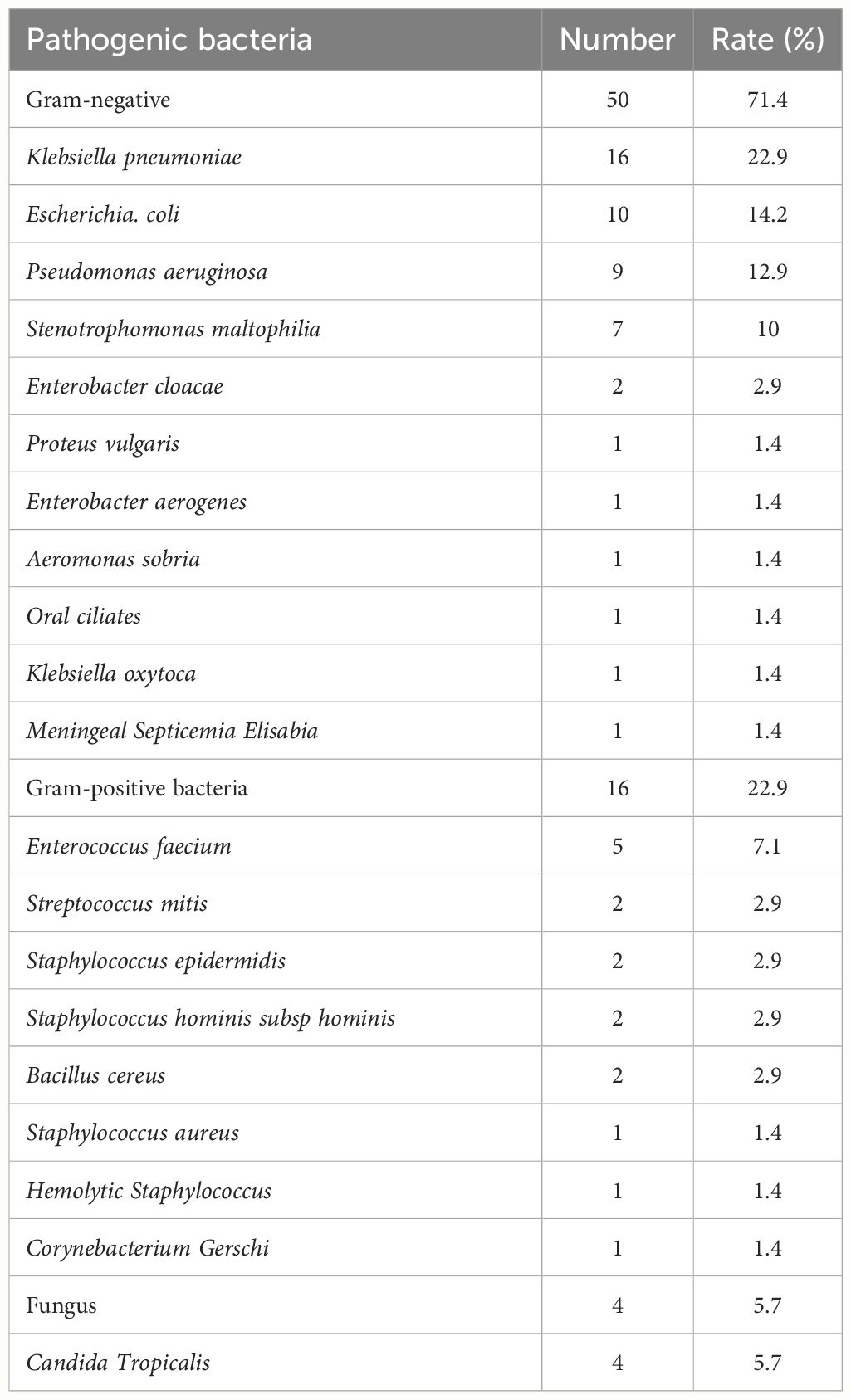
Table 2 Distribution of pathogenic bacteria (70 strains) in 56 patients with acute myeloid leukaemia with BSI.
Compared to the period of 2013–2015, where there were no reported cases, the incidence of Carbapenem-Resistant Enterobacter (CRE) has shown a steady increase, reaching 5% during 2016–2018 and escalating to 11.4% during 2019–2021. Similarly, the incidence of Extended-Spectrum β-Lactamases (ESBL) (22.5%) and Multi-Drug Resistance (MDR) (15%) during 2016–2018 surpassed that of ESBL (14.9%) and MDR (0%) during 2013–2015, as well as exceeding the rates of ESBL (7.1%) and MDR (11.4%) during 2019–2021. However, the mortality rate within 30 days after MDR infection during 2019–2021 (25%) was higher than that observed in 2013–2015 (0%) and 2016–2018 (16.7%) (Supplementary Table 1).
Gram-negative bacteria: There were 16 strains of Klebsiella pneumoniae, with a resistance rate of over 65% to cephalosporins, quinolones, and penicillins. Escherichia coli is sensitive to tigecycline, and the drug resistance rate is 0%. The resistance rate to piperacillin/tazobactam, ceftazidime/sulbactam, ceftolozane/tazobactam, ertapenem, and amikacin was 10%. It exhibits a resistance rate of 60% to ampicillin/Sulbactam, ciprofloxacin, levofloxacin, sulfamethoxazole/trimethoprim (TMP-SMX), and the resistance rate to ampicillin was as high as 80%. The resistance rate of Pseudomonas aeruginosa to piperacillin/tazobactam, ceftazidime, cefepime, cefoperazone/sulbactam, meropenem, ciprofloxacin, and levofloxacin was 55.6% (Table 3). Eight strains of CRE exhibited complete resistance to piperacillin/tazobactam, ceftriaxone, cefepime, cefoperazone, ciprofloxacin, and levofloxacin but showed sensitivity to amikacin. (Supplementary Table 2). Six strains of CRE were detected from sixteen strains of Klebsiella pneumoniae. Ten strains of Escherichia coli produced three strains of ESBL. Five of nine Pseudomonas aeruginosa strains produced carbapenem-resistant Pseudomonas aeruginosa (CRPA). There were eight strains of MDR, including two strains of Klebsiella pneumoniae, three strains of Escherichia coli, two strains of Enterococcus faecium and one strain of Streptococcus mitis (Supplementary Table 3).
Gram-positive bacteria: Enterococcus faecium and Staphylococcus haemolyticus aureus demonstrate a resistance rate of 0% to linezolid, vancomycin, daptomycin, and tigecycline. They exhibit a 100% resistance rate to penicillin. The five strains of Enterococcus faecium were resistant to ampicillin and penicillin and had high resistance to erythromycin and high concentration of gentamicin (Supplementary Table 4).
Fungi: A total of four strains of fungi were isolated, all of which were Candida tropicalis, and drug sensitivity test was not performed.
In 56 patients with AML, with 18 deaths within 30 days after BSI. Among these 18 patients, 5 cases were infected by Pseudomonas aeruginosa, 4 cases by Klebsiella pneumoniae, 3 cases by Escherichia coli, 1 case by Candida tropicalis, 1 case by Enterobacter cloacae, 1 case by Staphylococcus aureus, 1 case by Enterococcus faecalis, and 1 case by Staphylococcus epidermidis.
Univariate analysis showed that, compared to the non-survivor cohort, survior cohort had lower activated partial thromboplastin time and direct bilirubin levels. Additionally, total protein and albumin levels, the number of patients without septic shock, and the number of patients with neutrophil reduction lasting more than 20 days were all higher than those in the non-survivor cohort (P<0.05) (Table 1). Following logistic regression, significant differences were observed between the two cohorts in terms of total protein (P=0.017, HR: 0.861; 95% CI: 0.761–0.974), albumin (P=0.005, HR: 0.740; 95% CI: 0.599–0.914), and septic shock (P=0.046, HR: 4.399; 95% CI:1.025–18.870) (Table 4).
In addition to logistic regression analysis, factors that were clinically considered to be related to the long-term prognosis of patients with infection were added for survival analysis. Kaplan-Meier survival analysis showed that prior HSCT history and history of infection were not related to the poor prognosis of AML patients with BSI (Figures 1A, B). Total protein, albumin, septic shock and agranulocytosis duration>20 days were related to the poor prognosis of AML patients after BSI (Figures 1C–F). COX regression analysis showed that agranulocytosis duration >20 days (HR:3.854; 95% CI: 1.451–10.242) and septic shock (HR:3.788; 95% CI: 3.1.729–8.299) were predictors of poor long-term prognosis in patients with AML after BSI (Table 5).

Figure 1 Survival Analysis of AML Complicated with BSI. Each label has the following meanings:the Impact of Prior Hematopoietic stem cell transplantation (A), History of infection (B), Total protein (C), Albumin (D), Septic shock (E) and Agranulocytosis time (F) on the Prognosis of AML Patients with BSI. W/O: without; HSCT: Hematopoietic stem cell transplantation.
BSI is a serious systemic infectious disease caused by various pathogenic microorganisms that invade the blood circulation and release toxins and metabolites (Jang-yong et al., 2020). It is one of the common complications of AML patients and is associated with poor prognosis (Collin et al., 2001; Nørgaard et al., 2006). Leukopenia, especially the decrease in neutrophils, can be caused by patients with AML themselves and chemotherapy, which leads to immunodeficiency, and the incidence of BSIs is significantly increased. Previous exposure to antibiotics or inappropriate empirical antibiotic treatment contributes to an increase in the incidence of BSI, drug resistance and mortality. This trend was corroborated by Italian studies conducted by Gudiol C et al. and Trecarichi EM et al., wherein the incidence of MDR escalated from 13.7% in 2011 to 31.5% in 2023, accompanied by an increase in mortality rates from 24% to 34.4% (Gudiol et al., 2011; Trecarichi et al., 2023).
The distribution of types and drug resistance rates of pathogenic bacteria of BSIs varies in different regions and times. Studies have shown that Gram-positive bacteria were the most common cause of BSIs in cancer patients before 2000, and the infection rate of Gram-positive bacteria reached 76% in 2000 (Wisplinghoff et al., 2003; Ortega et al., 2005). Trecarichi et al. found that the pathogenic bacteria of BSIs in patients with HMs changed from Gram-positive bacteria to Gram-negative bacteria from 2006 to 2016 (Trecarichi and Tumbarello, 2014; Trecarichi et al., 2015). A study on BSIs in 357 AML patients in Finland from 2003 to 2011 showed that most of the pathogens were Gram-positive bacteria (65.7%), with coagulase-negative staphylococci accounting for the highest proportion at 24.7% among Gram-positive bacteria (Kolonen et al., 2017). An Italian study on adult haematological malignancies from 2009 to 2012 revealed that Gram-negative bacteria constituted 52.8% of infections (Trecarichi et al., 2015).
Gram-negative bacteria have been the main cause of infection in related studies in China, of which Escherichia coli is the most common (Chen et al., 2010; Li et al., 2022). Escherichia coli typically colonizes in the gastrointestinal tract. During the treatment of AML, cytotoxic chemotherapy alters the integrity of the gastrointestinal mucosa and impairs the immune system, increasing the risk of invasive infections caused by the translocation of colonising bacteria (Montassier et al., 2015; Garcia-Vidal et al., 2018). Similarly, gram-negative bacteria are the main pathogens in our centre. However, in our study, Klebsiella pneumoniae took precedence among Gram-negative bacteria. Some patients with potential prior infection, having received β-lactam cephalosporins and quinolones antibiotics for infection treatment, exhibited Klebsiella pneumoniae in the screening. Previous antimicrobial therapy, particularly β-lactams and cephalosporins, can also elevate the risk of multidrug resistance, leading to a high incidence of Klebsiella pneumoniae in the region (Garcia-Vidal et al., 2018). This emphasizes the need for cautious use of broad-spectrum antibiotics in our centre. In the study by Chen et al., the results are similar to those of this study, with Klebsiella pneumoniae being the predominant gram-negative bacterium (Esbenshade et al., 2015; Chen et al., 2019). In our centre, the predominant pathogen spectrum comprises Gram-negative bacteria. Standard empirical treatment typically involves broad-spectrum β-lactam antibiotics, while enterococci exhibit complete resistance to β-lactam antibiotics (Kristich et al., 2014; Gagetti et al., 2019). Consequently, the incidence of enterococci has exhibited a progressive increase from 2013 to 2021 in our centre. Enterococci has become predominant Gram-positive bacteria from 2019 to 2021 in our centre, likely attributed to the inadequacy of empirical antimicrobial therapy to address enterococcal infections. In cases where a Gram-positive bacterial infection is suspected, the addition of vancomycin to empirical treatment regimens may be considered.
In other studies, Gram-positive bacteria are mainly represented by Streptococcus viridans and Staphylococcus epidermidis (Chen et al., 2019; Carvalho et al., 2020), which is totally different from the situation in our centre. The clinical medication principle involves tailoring treatments based on blood culture and susceptibility results to ensure efficacy while minimising the development of drug-resistant pathogens. However, blood pathogen cultures and susceptibility testing can be time-consuming. Clinical treatment for BSI often relies on empirical approaches. As antimicrobial drug usage increases, pathogen resistance grows, making empirical anti-infection treatment more challenging. Thus, understanding the microbial antimicrobial spectra and resistance profiles in different healthcare centres is crucial for guiding clinical empirical drug administration, improving susceptibility testing, and reducing overall risk.
In our centre, the resistance rate of Klebsiella pneumoniae to cephalosporins and quinolones was high, and the resistance rate to aminoglycosides was low. The resistance rates of Escherichia coli to β-lactam/β-lactamase inhibitors, sulfonamides and cephalosporins were high, their resistance rate to carbapenems was low.
Therefore, aminoglycosides and carbapenems can be recommended for AML patients with BSI suspected of being infected with Gram-negative bacteria, which consistent with the findings of Trecarichi et al (Trecarichi et al., 2015; Åttman et al., 2021). Although the predominance of Gram-positive bacteria in our study differs from other studies, consistent with our results, most of them exhibited high sensitivity to vancomycin (Chen et al., 2019; Li et al., 2022). Therefore, in our centre, empirical treatment with vancomycin may be considered for AML patients suspected of Gram-positive bacterial infection prior to obtaining definitive pathogen and drug sensitivity results.
Studies conducted by Gudiol C et al. have highlighted the consequences of the inappropriate use of antibiotics, demonstrating an associated increase in drug resistance (Gudiol et al., 2011; Trecarichi et al., 2023). Comparing drug resistance rates from 2013 to 2021 in our centre revealed a gradual upward trend, attributed to the use of carbapenem in empirical anti-infective therapy. Specifically, the incidence of ESBL and MDR increased during the period of 2016–2018 compared to 2013–2015, potentially linked to the irrational use of antibiotics. Kollef MH et al. found that prolonged antibiotic exposure was a risk factor for MDR (Munoz-Price et al., 2016; Kollef et al., 2021). Interestingly, our study observed a decreasing trend in MDR incidence during 2019–2021 compared to 2016–2018, potentially attributed to reduce antibiotic exposure duration and decreased use of empirical antibacterial drugs in our centre.
Frequent exposure to initially inappropriate empirical antibiotic treatment may play a pivotal role in the risk of MDR-related death, hospital bacterial colonisation or infection and viral reactivation are also closely related to MDR morbidity and mortality (Hotchkiss et al., 2013; Zilberberg et al., 2014; van Vught et al., 2016). Compared with non-MDR infection, patients with MDR bacterial infection have an increased risk of mortality (Cornaglia et al., 2011; Vardakas et al., 2013). Our data also revealed an increased mortality rate within 30 days attributable to MDR infections, despite a declining trend in MDR incidence. However, due to the limited sample size, further data collection is warranted for comprehensive research. Among the 8 patients with MDR infection from 2019 to 2021, only one patient had previous colonisation of MDR strain (Enterobacter asburiae), which differed from the MDR strain (Escherichia coli) causing BSI. This patient received antifungal treatment combined with cephalosporin, with subsequent adjustment of antibiotic therapy based on drug sensitivity results. Prior to the identification of the causative pathogen in bloodstream infections, 6 out of the remaining 7 patients received empirical antifungal therapy, primarily in combination with β-lactam, carbapenem, or quinolone antibiotics, until the pathogens of BSI were identified.
Septic shock, denotes a complex syndrome of septicemia induced by microbial agents and their toxic byproducts, resulting in shock (Shankar-Hari et al., 2016). The use of chemotherapeutic drugs leads to neutropenia, which leads to severe immunodeficiency and increases the probability of septic shock (Na et al., 2022).The incidence of septic shock in patients with HMs ranged from 20.7% to 31% (Royo-Cebrecos et al., 2022; Wang et al., 2023; Zhang et al., 2023). In this study, the incidence of septic shock was 37.3%, with 50% of cases resulting in death within 30 days after BSI. The elevated incidence of septic shock in our centre may be associated with neutropenia occurrence among patients, with over half of them experiencing neutropenia lasting over 20 days (Na et al., 2022; Islas-Muñoz et al., 2024).
In addition, coagulation disorders associated with haematological malignancies can also increase mortality during septic shock (Lemiale et al., 2023). This study found that septic shock or the use of vasoactive drugs is an independent risk factor for the death of patients with AML after BSI. Royo-Cebrecs et al’ s study is similar to our findings (Chen et al., 2020; Royo-Cebrecos et al., 2022).
Albumin plays a crucial role in mitigating inflammation, maintaining vascular endothelial integrity, and regulating acid-base balance. It also acts to inhibit the progression of inflammation and damage to microcirculation and tissues (Ferrer et al., 2018; Hariri et al., 2018). In the context of infection, capillary permeability increases, causing albumin to seep from the plasma into the interstitial space, resulting in hypoalbuminemia (Gradel et al., 2018). In this study, lower levels of albumin were found to be indicative of adverse outcomes within 30 days following BSI. Notably, research conducted by Benjamin M. Greenberg and others has demonstrated a linear association between albumin levels and the prognosis of bacteremic patients (Greenberg et al., 2005; Magnussen et al., 2016). Diminished albumin levels in patients can lead to immunosuppression following BSI, contributing to poorer patient outcomes (McSorley et al., 2017). Furthermore, low albumin levels in patients with potential infectious foci can lead to infection recurrence (Leal et al., 2018; Abdel-Razik et al., 2020). At the same time, reduced albumin levels are associated with a decreased effectiveness of antibiotic treatment (Ibrahim et al., 2000; Holmes et al., 2018). Low albumin levels serve as a risk factor for treatment failure in Gram-negative bacteremia (Hashiguchi et al., 2020).
Agranulocytosis refers to neutropenia, bone marrow suppression caused by the use of chemotherapeutic drugs or by haematological tumours hinders the generation of an effective immune response, and patients with reduced immunity are prone to infection. The longer the agranulocytosis, the higher the incidence of infection, and the mortality rate of blood stream infection in patients with agranulocytosis and fever in patients with HMs can reach 42% (Zuber et al., 2012; Wohlfarth et al., 2014). The longer the duration of agranulocytosis, the worse the prognosis of HMs patients with infection (Darmon et al., 2002; Li et al., 2018). This study also confirmed this result. However, this study suggests that the duration of neutropenia is not an independent risk factor for death within 30 days after BSI. Tang’s study is consistent with our findings (Tang et al., 2018).This may be due to the early application of empirical antibacterial drugs to timely control the infection caused by agranulocytosis, which reduced the incidence of serious complications and mortality (Falcone et al., 2020; Zhang et al., 2020). This correlation requires additional data for further study and verification.
Stenotrophomonas maltophilia is an opportunistic pathogen that often occurs in hospitals and in immunosuppressed patients (Looney et al., 2009; Adegoke et al., 2017). The infection rate of this bacterium increased gradually because of the low immune function and the wide use of broad-spectrum antibiotics (Said et al., 2024). TMP-SMX is the first choice of treatment for Stenotrophomonas maltophilia infection, but TMP-SMX treatment may cause myelosuppression, hyperkalaemia and hypersensitivity syndrome, and Stenotrophomonas maltophilia infection is relatively rare. Hence, TMP-SMX is not generally used for empirical treatment of BSIs (Brooke, 2012; Chang et al., 2015). In this study, seven patients with Stenotrophomonas maltophilia infection were included, and no drug sensitivity test was performed. Five patients died within 30 days after BSI with Stenotrophomonas maltophilia, with a mortality rate of 71.4%. Sumida et al. also reported similar findings that Stenotrophomonas maltophilia infection was associated with adverse outcomes in patients (Cho et al., 2014; Sumida et al., 2015). A history of recurrent infections and prior antibiotic use are risk factors for Stenotrophomonas maltophilia infection (Apisarnthanarak et al., 2003; Bao et al., 2020). In our study, all the patients with Stenotrophomonas maltophilia infection had a history of repeated infection before BSI, but no multiple infection between Stenotrophomonas maltophilia and other bacteria were found during BSI (Apisarnthanarak et al., 2003; Bao et al., 2020). Among seven patients with Stenotrophomonas maltophilia infection, 5 patients who died within 30 days after BSI had a history of empirical use of carbapenem antibiotics before the pathogen was identified. Stenotrophomonas maltophilia exhibits resistance to some empirically used clinical treatments, particularly β-lactam antibiotics (including carbapenems) and aminoglycosides. As a result, routine empiric therapy often fails to achieve timely and effective control of the Stenotrophomonas maltophilia infection before blood culture results become available (Gales et al., 2019; Mojica et al., 2019).
Nonetheless, these results must be interpreted with caution and a number of limitations should be borne in mind. This study is a single-centre study in Zhejiang Province People’s Hospital, with limited sample size and certain regional characteristics. Thus, patients from other places were still need to be included. In addition, some factors that have been associated with the prognosis of patients with post-infection AML in other studies were not relevant in this study and may require confirmation with a larger sample size.
In this study, 56 AML patients complicated by BSIs were primarily infected with Gram-negative bacteria. Aminoglycosides and carbapenems are recommended for empirical treatment based on the pathogenic spectrum and drug resistance status, pending definitive drug sensitivity results. Serum albumin levels and the presence of septic shock emerged as independent risk factors for mortality within 30 days among AML patients with BSI. Furthermore, prolonged agranulocytosis exceeding 20 days and septic shock were associated with heightened mortality rates in terms of long-term prognosis for AML patients with BSI. In addition, Stenotrophomonas maltophilia infection may be associated with poor prognosis, with TMP-SMX serving as a potential treatment option to manage infection and enhance patient outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was approved by the research involving humans were approved by the ethics committee of Zhejiang people's Hospital (QT2023130). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/ next of kin in accordance with the national legislation and institutional requirements.
HW: Software, Validation, Writing – original draft, Writing – review & editing. ML: Writing – original draft, Data curation, Writing – review & editing. CS: Data curation, Writing – review & editing, Formal analysis. FS: Writing – review & editing, Data curation. XS: Writing – review & editing. QH: Data curation, Writing – review & editing. YW: Writing – review & editing. YC: Writing – review & editing. XT: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Zhejiang Natural Science Foundation Exploration General Project (LY24H080004), Youth Talent Project of Zhejiang traditional Chinese Medicine Science and Technology Program (2022ZQ012), Zhejiang Science and Technology Program of traditional Chinese Medicine (2023ZL269, 2024ZL255), Zhejiang Medical and Health Science and Technology Plan Project (2024KY768).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1390053/full#supplementary-material
Abdel-Razik, A., Abdelsalam, M., Gad, D. F., Abdelwahab, A., Tawfik, M., Elzehery, R., et al. (2020). Recurrence of spontaneous bacterial peritonitis in cirrhosis: novel predictors. Eur. J. Gastroenterol. Hepatol. 32, 718–726. doi: 10.1097/MEG.0000000000001578
Adegoke, A. A., Stenström, T. A., Okoh, A. I. (2017). Stenotrophomonas maltophilia as an emerging ubiquitous pathogen: looking beyond contemporary antibiotic therapy. Front. Microbiol. 8, 2276. doi: 10.3389/fmicb.2017.02276
Apisarnthanarak, A., Mayfield, J. L., Garison, T., McLendon, P. M., DiPersio, J. F., Fraser, V. J., et al. (2003). Risk factors for Stenotrophomonas maltophilia bacteremia in oncology patients: a case-control study. Infect. Control Hosp Epidemiol. 24, 269–274. doi: 10.1086/502197
Assi, S. A., Imperato, M. R., Coleman, D. J. L., Pickin, A., Potluri, S., Ptasinska, A., et al. (2019). Subtype-specific regulatory network rewiring in acute myeloid leukemia. Nat. Genet. 51, 151–162. doi: 10.1038/s41588-018-0270-1
Åttman, E., Syrjänen, J., Lyytikäinen, O., Ollgren, J., Sinisalo, M., Vuento, R., et al. (2021). Healthcare-associated blood stream infections in hematological patients in Finland during the years 2006–2016. Eur. J. Haematol. 107, 311–317.
Bao, H., Qiao, Y., Liu, D., Chen, J., Wu, X., Hu, X., et al. (2020). The clinical impact of Stenotrophomonas maltophilia bacteremia on the 30-day mortality rate in patients with hematologic disorders: a single-institution experience. Infection. 48, 205–212. doi: 10.1007/s15010-019-01369-4
Brooke, J. S. (2012). Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin. Microbiol. Rev. 25, 2–41. doi: 10.1128/CMR.00019-11
Carvalho, A. S., Lagana, D., Catford, J., Shaw, D., Bak, N. (2020). Bloodstream infections in neutropenic patients with haematological Malignancies. Infect. Dis. Health 25, 22–29. doi: 10.1016/j.idh.2019.08.006
Chang, Y. T., Lin, C. Y., Chen, Y. H., Hsueh, P. R. (2015). Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front. Microbiol. 6, 893. doi: 10.3389/fmicb.2015.00893
Chen, S., Liu, S., Yuan, X., Mai, H., Lin, J., Wen, F. (2019). Etiology, drug sensitivity profiles and clinical outcome of bloodstream infections: A retrospective study of 784 pediatric patients with hematological and neoplastic diseases. Pediatr. Hematol. Oncol. 36, 482–493. doi: 10.1080/08880018.2019.1667462
Chen, C. Y., Tsay, W., Tang, J. L., Tien, H. F., Chen, Y. C., Chang, S. C., et al. (2010). Epidemiology of bloodstream infections in patients with haematological Malignancies with and without neutropenia. Epidemiol. Infect. 138, 1044–1051. doi: 10.1017/S0950268809991208
Chen, X. C., Xu, J., Wu, D. P. (2020). Clinical characteristics and outcomes of breakthrough candidemia in 71 hematologic Malignancy patients and/or allogeneic hematopoietic stem cell transplant recipients: A single-center retrospective study from China, 2011–2018. Clin. Infect. Dis. 71, S394–S3s9. doi: 10.1093/cid/ciaa1523
Chen Xin, F. S.-Z. (2020). Interpretation of guidelines for clinical use of antibiotics in chinese neutropenia patients with fever(2020 edition). Chin. J. Hematology. 19, 14–17.
Cho, S. Y., Kang, C. I., Kim, J., Ha, Y. E., Chung, D. R., Lee, N. Y., et al. (2014). Can levofloxacin be a useful alternative to trimethoprim-sulfamethoxazole for treating Stenotrophomonas maltophilia bacteremia? Antimicrob. Agents Chemother. 58, 581–583. doi: 10.1128/AAC.01682-13
Collin, B. A., Leather, H. L., Wingard, J. R., Ramphal, R. (2001). Evolution, incidence, and susceptibility of bacterial bloodstream isolates from 519 bone marrow transplant patients. Clin. Infect. Dis. 33, 947–953. doi: 10.1086/322604
Cornaglia, G., Giamarellou, H., Rossolini, G. M. (2011). Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect. Dis. 11, 381–393.
Darmon, M., Azoulay, E., Alberti, C., Fieux, F., Moreau, D., Le Gall, J. R., et al. (2002). Impact of neutropenia duration on short-term mortality in neutropenic critically ill cancer patients. Intensive Care Med. 28, 1775–1780. doi: 10.1007/s00134-002-1528-7
Deng, Q., Li, Q., Lin, X. M., Li, Y. M. (2012). [Epidemiology and antimicrobial resistance of clinical isolates about hospital infection from patients with hematological diseases]. Zhonghua Xue Ye Xue Za Zhi. 33, 994–999.
Esbenshade, A. J., Pentima, M. C., Zhao, Z., Shintani, A., Esbenshade, J. C., Simpson, M. E., et al. (2015). Development and validation of a prediction model for diagnosing blood stream infections in febrile, non-neutropenic children with cancer. Pediatr. Blood Cancer. 62, 262–268. doi: 10.1002/pbc.25275
Falcone, M., Bassetti, M., Tiseo, G., Giordano, C., Nencini, E., Russo, A., et al. (2020). Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit. Care 24, 29. doi: 10.1186/s13054-020-2742-9
Ferrer, R., Mateu, X., Maseda, E., Yébenes, J. C., Aldecoa, C., De Haro, C., et al. (2018). Non-oncotic properties of albumin. A multidisciplinary vision about the implications for critically ill patients. Expert Rev. Clin. Pharmacol. 11, 125–137. doi: 10.1080/17512433.2018.1412827
Gagetti, P., Bonofiglio, L., García Gabarrot, G., Kaufman, S., Mollerach, M., Vigliarolo, L., et al. (2019). Resistance to β-lactams in enterococci. Rev. Argent Microbiol. 51, 179–183. doi: 10.1016/j.ram.2018.01.007
Gales, A. C., Seifert, H., Gur, D., Castanheira, M., Jones, R. N., Sader, H. S. (2019). Antimicrobial Susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii Complex and Stenotrophomonas maltophilia Clinical Isolates: Results From the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect. Dis. 6, S34–s46. doi: 10.1093/ofid/ofy293
Garcia-Vidal, C., Cardozo-Espinola, C., Puerta-Alcalde, P., Marco, F., Tellez, A., Agüero, D., et al. (2018). Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PloS One 13, e0199531. doi: 10.1371/journal.pone.0199531
Gradel, K. O., Vinholt, P. J., Magnussen, B., Pedersen, C., Jensen, T. G., Kolmos, H. J., et al. (2018). Hypoalbuminaemia as a marker of trans-capillary leakage in community-acquired bacteraemia patients. Epidemiol. Infect. 146, 648–655. doi: 10.1017/S0950268818000274
Greenberg, B. M., Atmar, R. L., Stager, C. E., Greenberg, S. B. (2005). Bacteraemia in the elderly: predictors of outcome in an urban teaching hospital. J. Infect. 50, 288–295. doi: 10.1016/j.jinf.2004.06.014
Gudiol, C., Tubau, F., Calatayud, L., Garcia-Vidal, C., Cisnal, M., Sánchez-Ortega, I., et al. (2011). Bacteraemia due to multidrug-resistant Gram-negative bacilli in cancer patients: risk factors, antibiotic therapy and outcomes. J. Antimicrob. Chemother. 66, 657–663. doi: 10.1093/jac/dkq494
Hariri, G., Joffre, J., Deryckere, S., Bigé, N., Dumas, G., Baudel, J. L., et al. (2018). Albumin infusion improves endothelial function in septic shock patients: a pilot study. Intensive Care Med. 44, 669–671. doi: 10.1007/s00134-018-5075-2
Hashiguchi, Y., Oda, K., Katanoda, T., Nosaka, K., Jono, H., Saito, H. (2020). Clinical evaluation of cefotiam in the treatment of bacteremia caused by Escherichia coli, Klebsiella species, and Proteus mirabilis: A retrospective study. J. Infect. Chemother. 26, 1158–1163. doi: 10.1016/j.jiac.2020.06.007
Holmes, N. E., Robinson, J. O., van Hal, S. J., Munckhof, W. J., Athan, E., Korman, T. M., et al. (2018). Morbidity from in-hospital complications is greater than treatment failure in patients with Staphylococcus aureus bacteraemia. BMC Infect. Dis. 18, 107. doi: 10.1186/s12879-018-3011-2
Hotchkiss, R. S., Monneret, G., Payen, D. (2013). Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 13, 260–268. doi: 10.1016/S1473-3099(13)70001-X
Ibrahim, E. H., Sherman, G., Ward, S., Fraser, V. J., Kollef, M. H. (2000). The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 118, 146–155. doi: 10.1378/chest.118.1.146
Islas-Muñoz, B., Volkow-Fernández, P., Silva-Zamora, J., Ramírez-Ibarguen, A., Cornejo-Juárez, P. (2024). Mortality in patients with hematological Malignancies, febrile neutropenia, and septic shock. J. Infect. Dev. Ctries. 18, 235–242. doi: 10.3855/jidc.17451
Jang-yong, Y., Hua, Z., Yiqi, F., Qing, Y., Jinwei, H., Jianying, Z. (2020). Analysis of clinical features and risk factors of mortality in carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Chin. J. Infect. Cheemother. 20, 388–395.
Kollef, M. H., Shorr, A. F., Bassetti, M., Timsit, J. F., Micek, S. T., Michelson, A. P., et al. (2021). Timing of antibiotic therapy in the ICU. Crit. Care 25, 360. doi: 10.1186/s13054-021-03787-z
Kolonen, A., Sinisalo, M., Huttunen, R., Syrjänen, J., Aittoniemi, J., Huhtala, H., et al. (2017). Bloodstream infections in acute myeloid leukemia patients treated according to the Finnish Leukemia Group AML-2003 protocol - a prospective nationwide study. Infect. Dis. (Lond) 49, 799–808. doi: 10.1080/23744235.2017.1347814
Kristich, C. J., Rice, L. B., Arias, C. A. (2014). “Enterococcal infection—Treatment and antibiotic resistance,” in Enterococci: from commensals to leading causes of drug resistant infection. Eds. Gilmore, M. S., Clewell, D. B., Ike, Y., Shankar, N. (Massachusetts Eye and Ear Infirmary, Boston).
Leal, J. A., Fausto, M. A., Carneiro, M., Tubinambás, U. (2018). Prevalence of hypoalbuminemia in outpatients with HIV/AIDS. Rev. Soc. Bras. Med. Trop. 51, 203–206. doi: 10.1590/0037-8682-0093-2017
Lemiale, V., Mabrouki, A., Miry, L., Mokart, D., Pène, F., Kouatchet, A., et al. (2023). Sepsis-associated coagulopathy in onco-hematology patients presenting with thrombocytopenia: a multicentric observational study. Leuk Lymphoma. 64, 197–204. doi: 10.1080/10428194.2022.2136971
Leonard, J. P., Martin, P., Roboz, G. J. (2017). Practical implications of the 2016 revision of the world health organization classification of lymphoid and myeloid neoplasms and acute leukemia. J. Clin. Oncol. 35, 2708–2715. doi: 10.1200/JCO.2017.72.6745
Li, M., Du, M., Li, H., Liu, D., Liu, Y. (2022). Epidemiology, resistant pathogens, and causes of early death in cases of bloodstream infection in patients with hematological Malignancies from 2012–2019. Infect. Med. (Beijing). 1, 23–30. doi: 10.1016/j.imj.2022.02.002
Li, S. T., Tang, L., Chen, X. M., Li, X. M. (2018). [Influencing factors of nosocomial infection after chemotherapy in adult patients with acute myeloid leukemia and its control strategy]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 26, 412–416.
Looney, W. J., Narita, M., Mühlemann, K. (2009). Stenotrophomonas maltophilia: an emerging opportunist human pathogen. Lancet Infect. Dis. 9, 312–323. doi: 10.1016/S1473-3099(09)70083-0
Magnussen, B., Oren Gradel, K., Gorm Jensen, T., Kolmos, H. J., Pedersen, C., Just Vinholt, P., et al. (2016). Association between hypoalbuminaemia and mortality in patients with community-acquired bacteraemia is primarily related to acute disorders. PloS One 11, e0160466. doi: 10.1371/journal.pone.0160466
McKenzie, S. B. (2005). Advances in understanding the biology and genetics of acute myelocytic leukemia. Clin. Lab. Sci. 18, 28–37.
McSorley, S.T. DRD, Roxburgh, C. S. D., McMillan, D. C., Horgan, P. G. (2017). How and why systemic inflammation worsens quality of life in patients with advanced cancer. Expert Rev. Qual Life Cancer Care 2017, 2:167–2:175.
Mikulska, M., Viscoli, C., Orasch, C., Livermore, D. M., Averbuch, D., Cordonnier, C., et al. (2014). Aetiology and resistance in bacteraemias among adult and paediatric haematology and cancer patients. J. Infect. 68, 321–331. doi: 10.1016/j.jinf.2013.12.006
Moghnieh, R., Estaitieh, N., Mugharbil, A., Jisr, T., Abdallah, D. I., Ziade, F., et al. (2015). Third generation cephalosporin resistant Enterobacteriaceae and multidrug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis. Front. Cell Infect. Microbiol. 5, 11. doi: 10.3389/fcimb.2015.00011
Mojica, M. F., Rutter, J. D., Taracila, M., Abriata, L. A., Fouts, D. E., Papp-Wallace, K. M., et al. (2019). Population Structure, Molecular Epidemiology, and β-Lactamase Diversity among Stenotrophomonas maltophilia Isolates in the United States. mBio 10. doi: 10.1128/mBio.00405-19
Montassier, E., Gastinne, T., Vangay, P., Al-Ghalith, G. A., Bruley des Varannes, S., Massart, S., et al. (2015). Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol. Ther. 42, 515–528. doi: 10.1111/apt.13302
Munoz-Price, L. S., Frencken, J. F., Tarima, S., Bonten, M. (2016). Handling time-dependent variables: antibiotics and antibiotic resistance. Clin. Infect. Dis. 62, 1558–1563. doi: 10.1093/cid/ciw191
Na, S. J., Oh, D. K., Park, S., Lee, Y. J., Hong, S. B., Park, M. H., et al. (2022). Clinical characteristics and outcomes of neutropenic sepsis: A multicenter cohort study. Shock. 57, 659–665. doi: 10.1097/SHK.0000000000001907
Nørgaard, M., Larsson, H., Pedersen, G., Schønheyder, H. C., Sørensen, H. T. (2006). Risk of bacteraemia and mortality in patients with haematological Malignancies. Clin. Microbiol. Infect. 12, 217–223. doi: 10.1111/j.1469-0691.2005.01298.x
Ortega, M., Rovira, M., Almela, M., Marco, F., de la Bellacasa, J. P., Martínez, J. A., et al. (2005). Bacterial and fungal bloodstream isolates from 796 hematopoietic stem cell transplant recipients between 1991 and 2000. Ann. Hematol. 84, 40–46. doi: 10.1007/s00277-004-0909-0
Royo-Cebrecos, C., Laporte-Amargós, J., Peña, M., Ruiz-Camps, I., Puerta-Alcalde, P., Abdala, E., et al. (2022). Pseudomonas aeruginosa bloodstream infections in patients with cancer: differences between patients with hematological Malignancies and solid tumors. Pathogens 11. doi: 10.3390/pathogens11101132
Said, M. S., Tirthani, E., Lesho, E. (2024). Stenotrophomonas maltophilia (StatPearls. Treasure Island (FL: StatPearls Publishing LLC). StatPearls Publishing Copyright © 2024.
Samonis, G., Vardakas, K. Z., Maraki, S., Tansarli, G. S., Dimopoulou, D., Kofteridis, D. P., et al. (2013). A prospective study of characteristics and outcomes of bacteremia in patients with solid organ or hematologic Malignancies. Support Care Cancer. 21, 2521–2526. doi: 10.1007/s00520-013-1816-5
Shankar-Hari, M., Phillips, G. S., Levy, M. L., Seymour, C. W., Liu, V. X., Deutschman, C. S., et al. (2016). Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama. 315, 775–787. doi: 10.1001/jama.2016.0289
Sumida, K., Chong, Y., Miyake, N., Akahoshi, T., Yasuda, M., Shimono, N., et al. (2015). Risk factors associated with stenotrophomonas maltophilia bacteremia: A matched case-control study. PloS One 10, e0133731. doi: 10.1371/journal.pone.0133731
Tang, Y., Cheng, Q., Yang, Q., Liu, J., Zhang, D., Cao, W., et al. (2018). Prognostic factors and scoring model of hematological Malignancies patients with bloodstream infections. Infection. 46, 513–521. doi: 10.1007/s15010-018-1151-3
Tang, Y., Wu, X., Cheng, Q., Li, X. (2020). Inappropriate initial antimicrobial therapy for hematological Malignancies patients with Gram-negative bloodstream infections. Infection. 48, 109–116. doi: 10.1007/s15010-019-01370-x
Trecarichi, E. M., Giuliano, G., Cattaneo, C., Ballanti, S., Criscuolo, M., Candoni, A., et al. (2023). Bloodstream infections due to Gram-negative bacteria in patients with hematologic Malignancies: updated epidemiology and risk factors for multidrug-resistant strains in an Italian perspective survey. Int. J. Antimicrob. Agents. 61, 106806. doi: 10.1016/j.ijantimicag.2023.106806
Trecarichi, E. M., Pagano, L., Candoni, A., Pastore, D., Cattaneo, C., Fanci, R., et al. (2015). Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic Malignancies: an Italian multicentre prospective survey. Clin. Microbiol. Infect. 21, 337–343. doi: 10.1016/j.cmi.2014.11.022
Trecarichi, E. M., Tumbarello, M. (2014). Antimicrobial-resistant Gram-negative bacteria in febrile neutropenic patients with cancer: current epidemiology and clinical impact. Curr. Opin. Infect. Dis. 27, 200–210. doi: 10.1097/QCO.0000000000000038
Tumbarello, M., Trecarichi, E. M., Caira, M., Candoni, A., Pastore, D., Cattaneo, C., et al. (2012). Derivation and validation of a scoring system to identify patients with bacteremia and hematological Malignancies at higher risk for mortality. PloS One 7, e51612. doi: 10.1371/journal.pone.0051612
van Vught, L. A., Klein Klouwenberg, P. M., Spitoni, C., Scicluna, B. P., Wiewel, M. A., Horn, J., et al. (2016). Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. Jama. 315, 1469–1479. doi: 10.1001/jama.2016.2691
Vardakas, K. Z., Rafailidis, P. I., Konstantelias, A. A., Falagas, M. E. (2013). Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: the study, the patient, the bug or the drug? J. Infect. 66, 401–414. doi: 10.1016/j.jinf.2012.10.028
Wang, J., Wang, M., Zhao, A., Zhou, H., Mu, M., Liu, X., et al. (2023). Microbiology and prognostic prediction model of bloodstream infection in patients with hematological Malignancies. Front. Cell Infect. Microbiol. 13, 1167638. doi: 10.3389/fcimb.2023.1167638
Wisplinghoff, H., Seifert, H., Wenzel, R. P., Edmond, M. B. (2003). Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological Malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis. 36, 1103–1110. doi: 10.1086/374339
Wohlfarth, P., Staudinger, T., Sperr, W. R., Bojic, A., Robak, O., Hermann, A., et al. (2014). Prognostic factors, long-term survival, and outcome of cancer patients receiving chemotherapy in the intensive care unit. Ann. Hematol. 93, 1629–1636. doi: 10.1007/s00277-014-2141-x
Wrighton, K. H. (2019). Regulatory networks in AML. Nat. Rev. Cancer. 19, 6–7. doi: 10.1038/s41568-018-0092-6
Zhang, L., Zhen, S., Shen, Y., Zhang, T., Wang, J., Li, J., et al. (2023). Bloodstream infections due to Carbapenem-Resistant Enterobacteriaceae in hematological patients: assessment of risk factors for mortality and treatment options. Ann. Clin. Microbiol. Antimicrob. 22, 41. doi: 10.1186/s12941-023-00586-y
Zhang, Y., Zheng, Y., Dong, F., Ma, H., Zhu, L., Shi, D., et al. (2020). Epidemiology of febrile neutropenia episodes with gram-negative bacteria infection in patients who have undergone chemotherapy for hematologic Malignancies: A retrospective study of 10 years’ Data from a single center. Infect. Drug Resist. 13, 903–910. doi: 10.2147/IDR.S241263
Zilberberg, M. D., Shorr, A. F., Micek, S. T., Vazquez-Guillamet, C., Kollef, M. H. (2014). Multi-drug resistance, inappropriate initial antibiotic therapy and mortality in Gram-negative severe sepsis and septic shock: a retrospective cohort study. Crit. Care 18, 596. doi: 10.1186/s13054-014-0596-8
Keywords: acute myeloid leukaemia, bloodstream infections, distribution of pathogenic bacteria, drug resistance, prognosis, risk factors
Citation: Wu H, Li M, Shou C, Shi F, Song X, Hu Q, Wang Y, Chen Y and Tong X (2024) Pathogenic spectrum and drug resistance of bloodstream infection in patients with acute myeloid leukaemia: a single centre retrospective study. Front. Cell. Infect. Microbiol. 14:1390053. doi: 10.3389/fcimb.2024.1390053
Received: 22 February 2024; Accepted: 10 May 2024;
Published: 07 June 2024.
Edited by:
Ghassan Dbaibo, American University of Beirut, LebanonReviewed by:
Wei-Han Huang, Hualien Tzu Chi Hospital, TaiwanCopyright © 2024 Wu, Li, Shou, Shi, Song, Hu, Wang, Chen and Tong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangmin Tong, dG9uZ3hpYW5nbWluQDE2My5jb20=; Yirui Chen, ZGFpc3lfY2hlbjE5ODZAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.