- 1Department of Biochemistry and Pharmacology, Uzhhorod National University, Uzhhorod, Ukraine
- 2Department of Microbiology, Virology, and Immunology, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
Antimicrobial resistance (AMR) is one of the global health challenges of the 21st century. Data regarding AMR mechanisms in Leptospira interrogans, the causative agents of leptospirosis, have been relatively limited. Therefore, our study aimed to identify resistance genes and explore potential resistance mechanisms specific to particular serovars. We conducted a comprehensive analysis of 98 Leptospira strains, representing 10 common serovars, using whole-genome sequencing (WGS) FASTA files. Employing the PATRIC tool from the Bacterial and Viral Bioinformatics Resource Center (BV-BRC), we scrutinized the genomes for AMR genes. Our investigation revealed 32 genes associated with AMR, with 20 key genes consistently prevalent across most strains. Notably, we identified unique efflux pump systems in serovar Pomona, indicating distinctive resistance mechanisms in this serovar. In summary, our findings shed light on the genetic landscape of AMR in Leptospira, uncovering both common and serovar-specific resistance elements. The presence of unique efflux pump systems in serovar Pomona introduces a novel dimension to our understanding of resistance mechanisms. These insights underscore the importance of tailored intervention strategies and collaborative efforts between human and veterinary healthcare professionals, as well as environmental scientists, to address the complex dynamics of leptospirosis and its implications for antibiotic resistance.
1 Introduction
Antimicrobial resistance (AMR) presents significant challenges in addressing bacterial infections across both animal and human populations (Arnold et al., 2016; Furness et al., 2017). The emergence and dissemination of antimicrobial-resistant microbes over the past few decades pose a severe global health threat (Pulingam et al., 2022). AMR not only increase morbidity and mortality but also substantially impacts treatment duration and costs (Murray et al., 2022).
Although leptospirosis is endemic in tropical geographic areas, it is known as one of the most considerable global zoonotic bacterial infections, which may lead to establishing epidemics on large scales as a result of flooding and strong rainfall (Haake and Levett, 2015; Rajapakse, 2022). The zoonotic infection of leptospirosis annually may cause one million cases with a mortality rate of 6.86% (approximately 60,000 deaths) around the world (Adler and de la Peña Moctezuma, 2010; Petakh and Nykyforuk, 2022; Petakh et al., 2023; Petakh et al., 2022a; Petakh et al., 2022b; Petakh et al., 2022c). The genus Leptospira comprises over 20 species based on DNA relatedness, with more than 350 serovars identified based on surface agglutinating lipopolysaccharide antigens (Adler and de la Peña Moctezuma, 2010; Fouts et al., 2016). These species are broadly categorized into three groups. Saprophytic species like Leptospira biflexa are not associated with disease. Pathogenic species such as Leptospira interrogans and Leptospira borgpetersenii cause leptospirosis globally, ranging from mild or asymptomatic infection to severe forms resulting in multiple organ failure and death. An intermediate group, including Leptospira fainei and Leptospira licerasiae, may be associated with infection and mild disease (Fouts et al., 2016).
Leptospira spp. exhibit intrinsic resistance to various antimicrobial agents, though the specific mechanisms responsible remain unidentified (Adler et al., 1986; Vinod Kumar et al., 2016; Petakh et al., 2024a; Petakh et al., 2024b). Nevertheless, resistance to sulfonamides, neomycin, actidione, polymyxin, nalidixic acid, vancomycin, and rifampicin has facilitated the development of selective media for isolating leptospires (Schönberg, 1981).
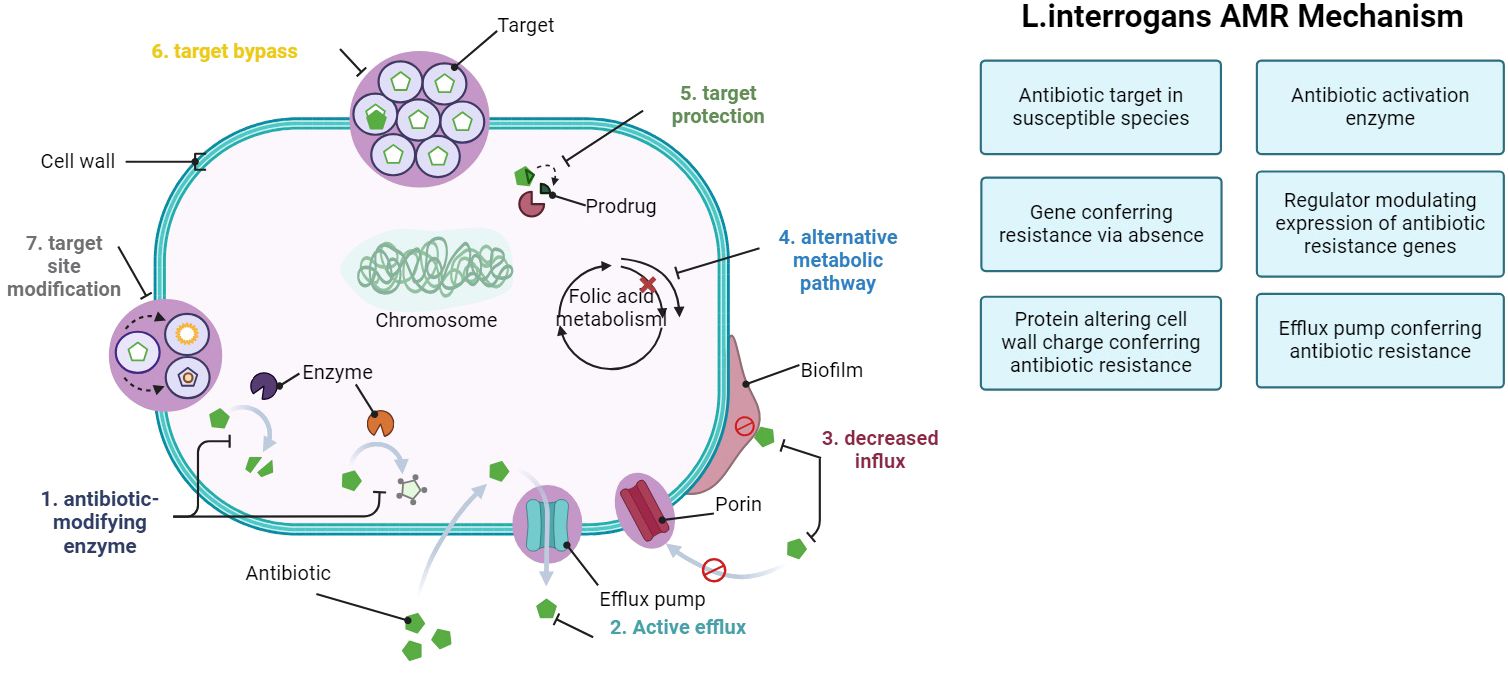
In recent research, genes linked to AMR have been explored using the Comprehensive Antibiotic Resistance Database (CARD) definition (McArthur et al., 2013; MaChado et al., 2022). The CARD definition outlines different modes of AMR action:
1. Antibiotic target in susceptible species: genes associated with antibiotic-sensitive wild-type bacterial components may undergo mutations, rendering them not susceptible.
2. Protein altering cell wall charge conferring antibiotic resistance: Genes involved in cell wall alteration, leading to changes in charge that confer resistance to antibiotics.
3. Gene conferring resistance via absence: deletion of specific genes or gene products results in resistance. For instance, deletion of a porin gene can block the entry of drugs into the cell.
4. Antibiotic inactivation enzyme: genes encoding enzymes that catalyze the inactivation of antibiotics, resulting in resistance: inactivation includes chemical modification, destruction, etc.
5. Efflux pump conferring antibiotic resistance: subunits of efflux proteins that pump antibiotics out of a cell to confer resistance.
6. Antibiotic target protection protein: these proteins confer antibiotic resistance by binding the antibiotic target to prevent antibiotic binding.
7. Antibiotic target modifying enzyme: enzymes that confer resistance by modifying (mutational alteration or enzymatic modification) antibiotic targets.
8. Regulator modulating expression of antibiotic resistance genes: mechanism activated by the presence of a specific antibiotic.
Current recommendations for treating human leptospirosis involve penicillin, ampicillin, ceftriaxone, or cefotaxime (Adler and de la Peña Moctezuma, 2010; Haake and Levett, 2015). Alternatives, particularly for those with allergies or in non-hospital settings, include oral doxycycline or azithromycin. In veterinary settings, a penicillin-streptomycin combination is the preferred therapy for acute leptospirosis, although ampicillin, amoxicillin, tetracyclines, tulathromycin, and third-generation cephalosporins have also been utilized (Ellis, 2015). Tilmicosin presents an additional alternative (Alt et al., 2001).
The apparent absence of significant antimicrobial resistance emergence in Leptospira prompts the question of why this has not occurred (Liegeon et al., 2018). Leptospiral infections are typically monomicrobial, limiting opportunities for horizontal resistance gene acquisition. Moreover, there is no experimental evidence of foreign DNA uptake by Leptospira spp., although genomic analyses support this notion. Finally, human leptospirosis is a dead-end infection, with human-to-human transmission being extremely rare (Trott et al., 2018).
The study of antimicrobial resistance (AMR) mechanisms in Leptospira interrogans serovars is crucial due to the increasing global burden of leptospirosis, a zoonotic disease caused by this bacterium (Boss et al., 2019). Understanding the mechanisms that contribute to AMR in different serovars is essential for developing effective treatment strategies and controlling the spread of resistant strains. In this comprehensive study, we aim to investigate the genetic and phenotypic characteristics of AMR in L. interrogans serovars, shedding light on the factors that drive resistance and providing valuable insights for future therapeutic interventions.
2 Materials and methods
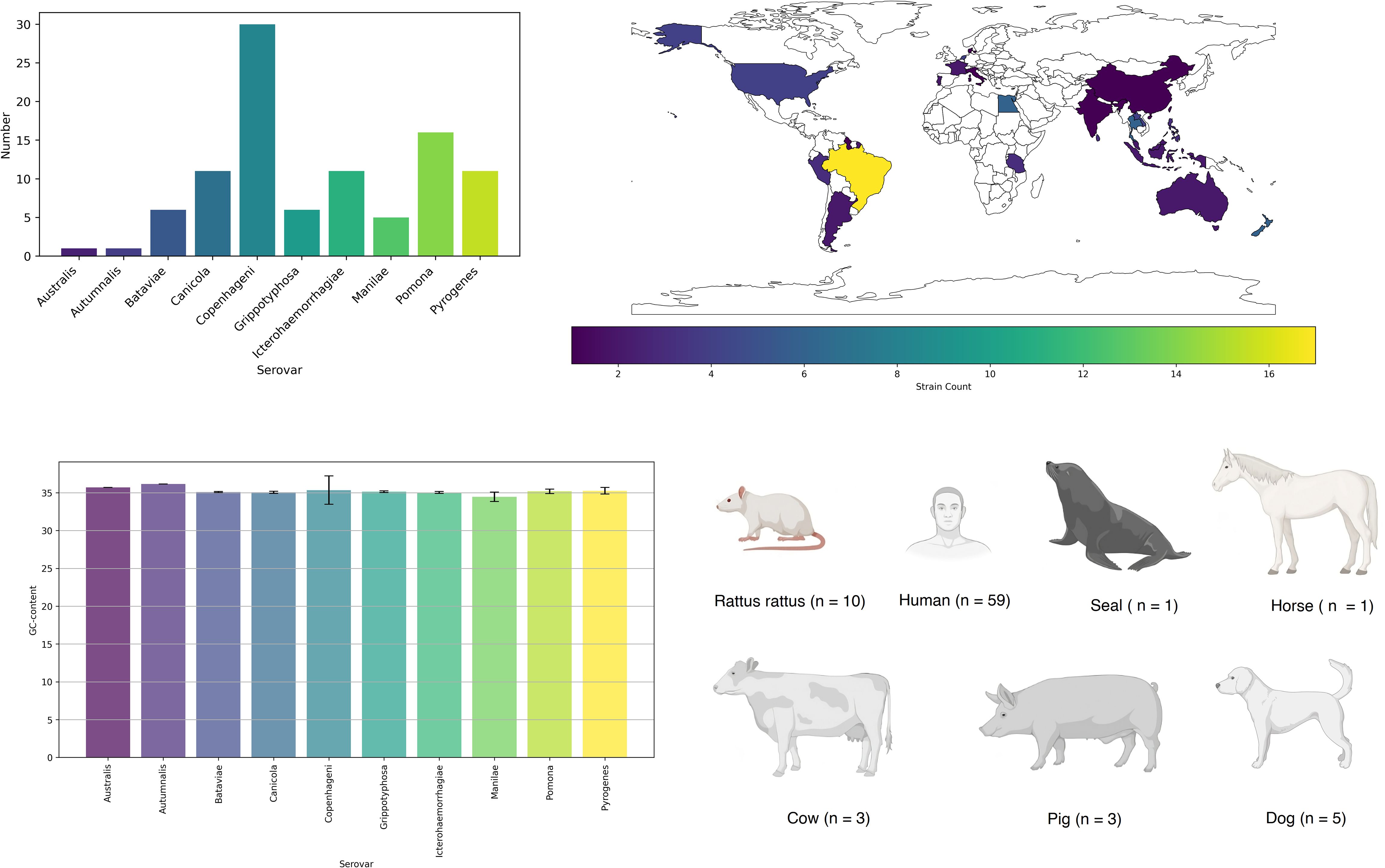
We conducted an analysis of FASTA files obtained from the whole-genome sequencing (WGS) of 98 strains, representing 10 common serovars in humans and animals (Oliveira et al., 2017). The distribution of strains among the serovars is as follows: Australis (n = 1); Autumnalis (n = 1); Bataviae (n = 6); Canicola (n = 11); Copenhageni (n = 30); Grippotyphosa (n = 6); Icterohaemorrhagiae (n = 11); Manilae (n = 5); Pomona (n = 16); Pyrogenes (n = 11) (Supplementary Materials). Strains were collected during 1958 – 2020 years.
We employed the PATRIC tool from the Bacterial and Viral Bioinformatics Resource Center (BV-BRC) to analyze the genomes for antimicrobial resistance (AMR) genes. The Genome Annotation Service in PATRIC uses the k-mer-based AMR gene detection method, which utilizes PATRIC’s curated collection of representative AMR gene sequence variants and assigns to each AMR gene a functional annotation and a broad mechanism of antibiotic resistance (Wattam et al., 2017).
3 Results
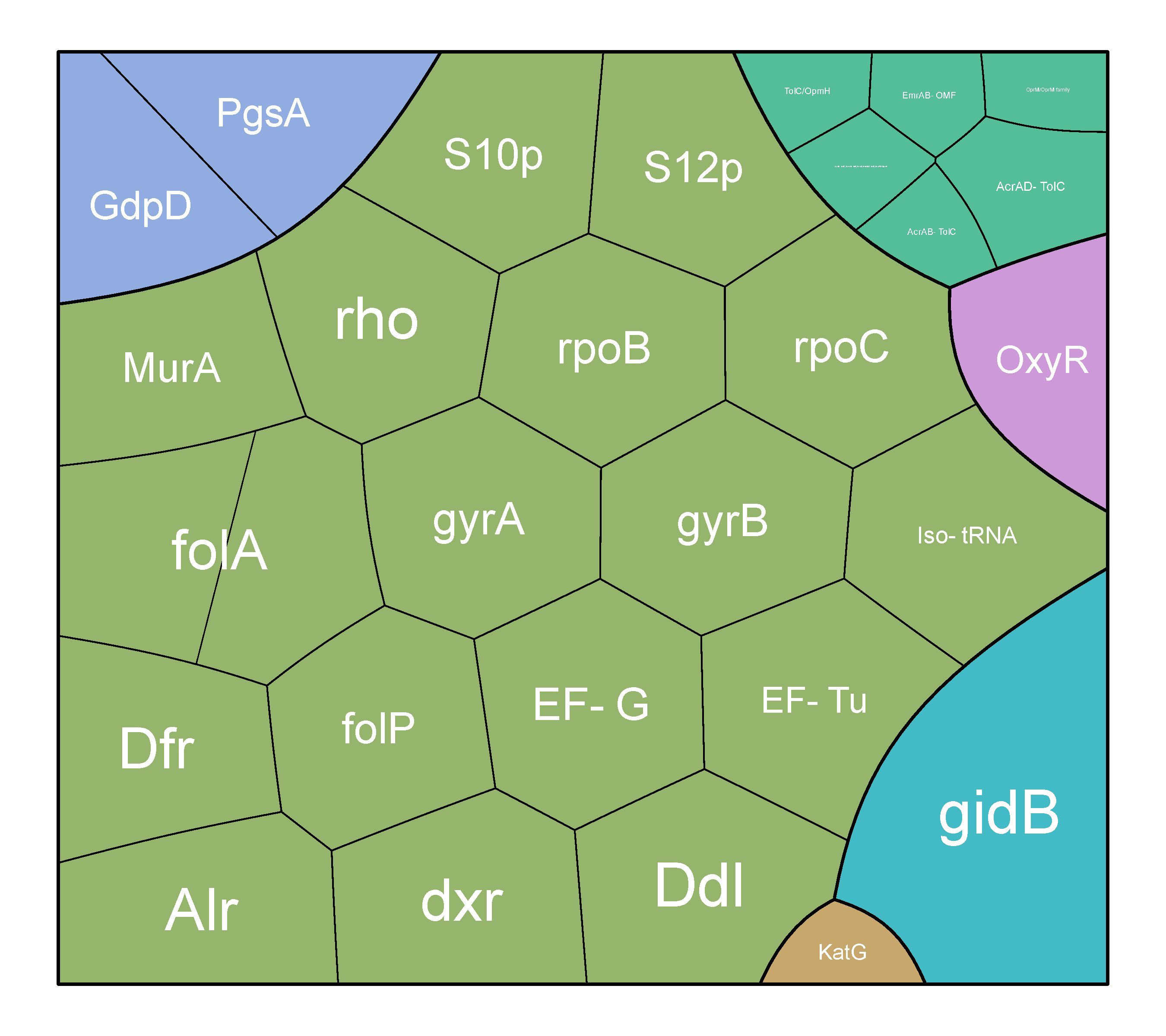
The majority of strains were isolated in Brazil (n = 17) and the United Kingdom (n = 16) (Figure 1). Most of the strains were obtained from humans (n = 59). In general, five main mechanisms of resistance to antibiotics were identified: gene conferring resistance via absence; protein altering cell wall charge conferring antibiotic resistance; antibiotic activation enzyme; regulator modulating expression of antibiotic resistance genes; efflux pump conferring antibiotic resistance (Figure 2).
The three predominant and widespread mechanisms were antibiotic target in susceptible species, gene conferring resistance via absence, and protein altering cell wall charge conferring antibiotic resistance, all of which were detected in all studied strains.
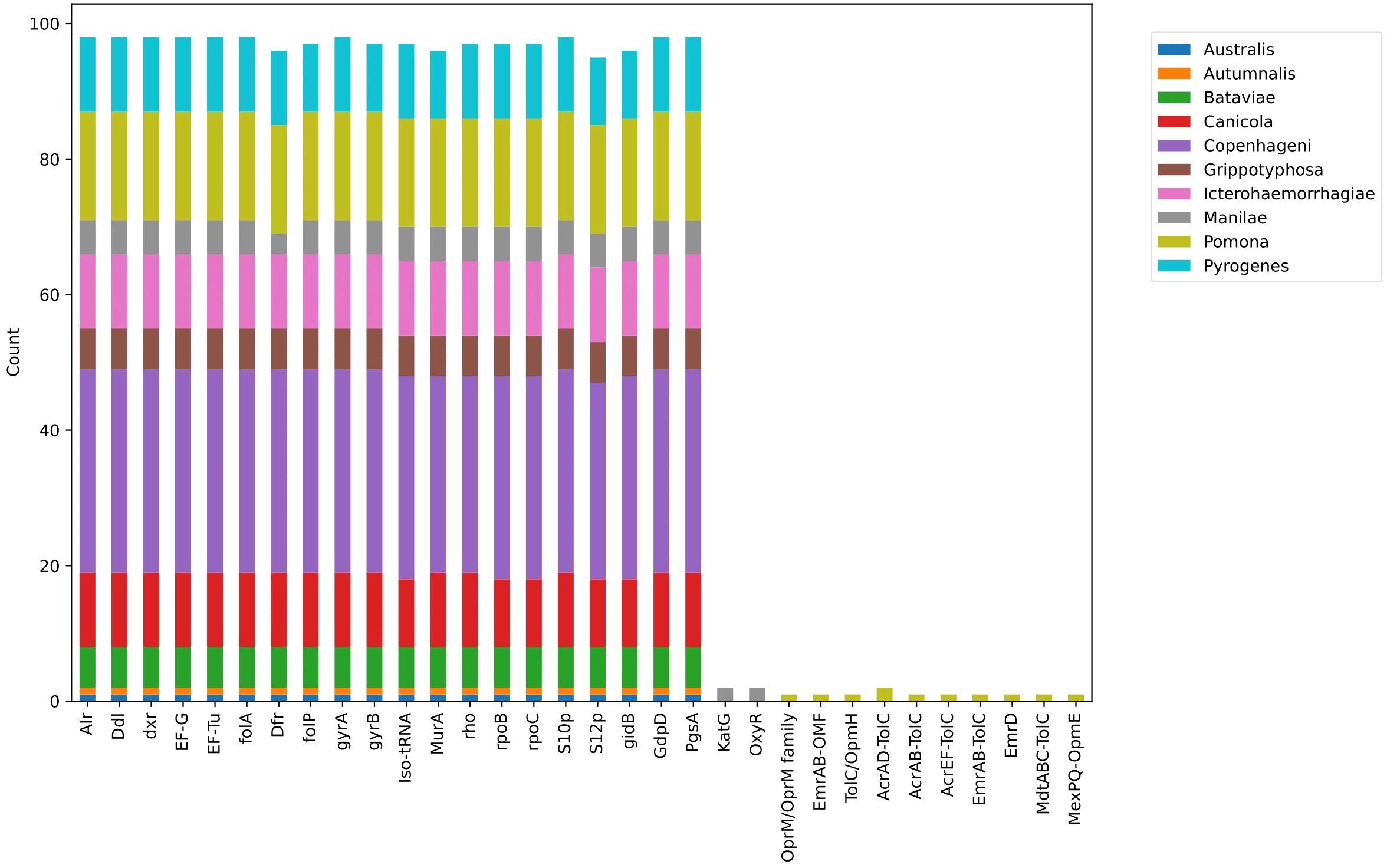
Seven genes (alr, ddl, dxr, EF-G, EF-Tu, folA, dfr) were observed in all studied strains. These genes are part of the antibiotic target in susceptible species. Antibiotic-sensitive wild-type bacterial components might undergo mutations that render them non-susceptible. All serovars had the folP gene, which encodes dihydropteroate synthase, the target enzyme of sulfonamides, except for one strain of serovar Pyrogenes (Leptospira interrogans serovar Pyrogenes str. R168). Additionally, Leptospira interrogans serovar Pyrogenes str. 200701872 did not have the gyrB gene, which encodes the B subunit of the DNA gyrase. Leptospira interrogans serovar Copenhageni str. M20 and Leptospira interrogans serovar Pyrogenes str. R168 did not have the MurA gene, a key enzyme involved in bacterial cell wall peptidoglycan synthesis and a target for the antimicrobial agent fosfomycin. Leptospira interrogans serovar Canicola strain Tande did not have the rpoB gene encoding the beta subunit of RNA polymerase (rpoB), the rpoC gene encoding DNA-directed RNA polymerase subunit beta, and the S12p gene encoding the small ribosomal protein.
Two mechanisms, antibiotic activation enzyme and regulator modulating expression of antibiotic resistance genes, were found among two strains of serovar Manilae. Specifically, the catalase-peroxidase enzyme KatG, encoded by the katG gene, and oxyR, the canonical orchestrator of the H2O2 detoxification response, were identified. Additionally, four strains of serovar Pomona exhibited efflux pump conferring antibiotic resistance (Figure 3). Specifically, in Leptospira interrogans serovar Pomona strain EMY7780, OprM/OprM family and TolC/OpmH were identified. In Leptospira interrogans serovar Pomona strain ESR8, AcrAB-TolC, AcrAD-TolC, and TolC/OpmH were observed. Strain P5661 of Leptospira interrogans serovar Pomona showed AcrAD-TolC, AcrEF-TolC, EmrAB-TolC, EmrD, MdtABC-TolC, and MexPQ-OpmE. Lastly, in Leptospira interrogans serovar Pomona strain Pom_str68, EmrAB-OMF was found.
4 Discussion
In our study of 98 types of Leptospira bacteria, we discovered 32 genes linked to antimicrobial resistance (AMR). Out of these, 20 key genes consistently stood out in most strains. The highest number of genes was associated with a mechanism that makes antibiotics less effective in susceptible species, involving 17 different genes (Figure 4).
Some crucial genes, such as alr and ddl, encode D-alanine racemase (Alr) and D-alanine:D-alanine ligase (Ddl), which are peptidoglycan biosynthetic enzymes. Feng reported that the overproduction of Alr in Mycobacterium smegmatis determines a d-Cycloserine resistance phenotype (Feng and Barletta, 2003).
Another important gene is murA, which is a key enzyme involved in bacterial cell wall peptidoglycan synthesis and is a target for the antimicrobial agent fosfomycin, a structural analog of the MurA substrate phosphoenol pyruvate (Kumar et al., 2009).
The dxr gene plays a significant role in building the cell membranes of bacteria. Fosmidomycin is a phosphonic antibiotic that inhibits 1-deoxy-D-xylulose 5-phosphate reductoisomerase (Dxr), the first committed step of the non-mevalonate pathway of isoprenoid biosynthesis. In Mycobacterium tuberculosis, Dxr is encoded by Rv2870c, and although the antibiotic has been shown to inhibit the recombinant enzyme, mycobacteria are intrinsically resistant to fosmidomycin at the whole-cell level (Brown and Parish, 2008).
For DNA replication and repair, DNA gyrase subunits (gyrA and gyrB) are like the repair crew for the bacterial DNA (Chien et al., 2016). Efflux pumps and mutations in gyrA, associated with fluoroquinolone resistance, have been induced in vitro in Brucella spp. strains. Think of these as defense mechanisms that bacteria develop to resist certain antibiotics.
An interesting discovery was made regarding certain strains of serovar Pomona. These strains possess different efflux pump systems that expel drugs, impacting bacterial resistance. This emphasizes the need to understand these variations for effective treatment. In study L. interrogans serovar Pomona isolates from swine in Brazil were found to be resistant to fluoroquinolones (Miraglia et al., 2008). We think that overexpression of efflux pumps might be the reason.
In a separate study by Antara Chakraborty and colleagues, 46 Leptospira isolates from rats were tested for their susceptibility to 14 antimicrobial agents (Chakraborty et al., 2010). All of the strains were found to be sensitive to ampicillin, cefotaxime, ciprofloxacin, norfloxacin, doxycycline, erythromycin, and streptomycin. In contrast, the tested isolates showed resistance to amphotericin B, 5-fluorouracil, fosfomycin, trimethoprim, sulfamethoxazole, neomycin, and vancomycin.
5 Conclusions
In the end, our research on AMR mechanisms in Leptospira interrogans serovars found 32 genes linked to resistance, with 20 key genes present in all strains. We believe these genes form the basis that ensures leptospira’s resistance to certain types of antibiotics. The diverse resistance profiles observed among different Leptospira interrogans serovars underscore the complexity of antimicrobial resistance mechanisms in this bacterium.
The identified serovar-specific resistance mechanisms raise the prospect of tailoring therapeutic approaches based on the prevalent serovar in a specific geographical area. A nuanced understanding of the resistance patterns associated with each serovar could lead to more effective and targeted treatment strategies.
Efflux pump systems identified in serovar Pomona, introduce a dimension of complexity to the management of leptospirosis. The potential contribution of these systems to AMR highlights the need for future research.
Given its status as a zoonotic disease, understanding the interplay between human, animal, and environmental factors is crucial for comprehensive intervention strategies. The identified resistance mechanisms should prompt collaborative efforts between human and veterinary healthcare professionals, as well as environmental scientists, to address the multifaceted nature of leptospirosis and its potential impact on antibiotic resistance dynamics.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
PP: Conceptualization, Investigation, Visualization, Writing – original draft. OK: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1384427/full#supplementary-material
References
Adler, B., de la Peña Moctezuma, A. (2010). Leptospira and leptospirosis. Vet. Microbiol. 140, 287–296. doi: 10.1016/j.vetmic.2009.03.012
Adler, B., Faine, S., Christopher, W. L., Chappel, R. J. (1986). Development of an improved selective medium for isolation of leptospires from clinical material. Vet. Microbiol. 12, 377–381. doi: 10.1016/0378-1135(86)90087-8
Alt, D. P., Zuerner, R. L., Bolin, C. A. (2001). Evaluation of antibiotics for treatment of cattle infected with Leptospira borgpetersenii serovar hardjo. J. Am. Vet. Med. Assoc. 219, 636–639. doi: 10.2460/javma.2001.219.636
Arnold, K. E., Williams, N. J., Bennett, M. (2016). 'Disperse abroad in the land': the role of wildlife in the dissemination of antimicrobial resistance. Biol. letters. 12, 20160137–20160145. doi: 10.1098/rsbl.2016.0137
Boss, J., Dance, D. A. B., Chanthongthip, A., Newton, P. N., Wuthiekanun, V., Robinson, M. T. (2019). Antimicrobial susceptibility testing of leptospira spp. in the lao people's democratic republic using disk diffusion. Am. J. Trop. Med. Hyg. 100, 1073–1078. doi: 10.4269/ajtmh.18-0955
Brown, A. C., Parish, T. (2008). Dxr is essential in Mycobacterium tuberculosis and fosmidomycin resistance is due to a lack of uptake. BMC Microbiol. 8, 78. doi: 10.1186/1471-2180-8-78
Chakraborty, A., Miyahara, S., Villanueva, S. Y., Gloriani, N. G., Yoshida, S. (2010). In vitro sensitivity and resistance of 46 Leptospira strains isolated from rats in the Philippines to 14 antimicrobial agents. Antimicrobial. Agents chemother. 54, 5403–5405. doi: 10.1128/AAC.00973-10
Chien, J. Y., Chiu, W. Y., Chien, S. T., Chiang, C. J., Yu, C. J., Hsueh, P. R. (2016). Mutations in gyrA and gyrB among Fluoroquinolone- and Multidrug-Resistant Mycobacterium tuberculosis Isolates. Antimicrobial. Agents chemother. 60, 2090–2096. doi: 10.1128/AAC.01049-15
Ellis, W. A. (2015). Animal leptospirosis. Curr. topics Microbiol. Immunol. 387, 99–137. doi: 10.1007/978-3-662-45059-8_6
Feng, Z., Barletta, R. G. (2003). Roles of Mycobacterium smegmatis D-alanine:D-alanine ligase and D-alanine racemase in the mechanisms of action of and resistance to the peptidoglycan inhibitor D-cycloserine. Antimicrobial. Agents chemother. 47, 283–291. doi: 10.1128/AAC.47.1.283-291.2003
Fouts, D. E., Matthias, M. A., Adhikarla, H., Adler, B., Amorim-Santos, L., Berg, D. E., et al. (2016). What makes a bacterial species pathogenic?:Comparative genomic analysis of the genus leptospira. PloS Negl. Trop. Dis. 10, e0004403. doi: 10.1371/journal.pntd.0004403
Furness, L. E., Campbell, A., Zhang, L., Gaze, W. H., McDonald, R. A. (2017). Wild small mammals as sentinels for the environmental transmission of antimicrobial resistance. Environ. Res. 154, 28–34. doi: 10.1016/j.envres.2016.12.014
Haake, D. A., Levett, P. N. (2015). Leptospirosis in humans. Leptospira leptospirosis. 387, 65–97. doi: 10.1007/978-3-662-45059-8_5
Kumar, S., Parvathi, A., Hernandez, R. L., Cadle, K. M., Varela, M. F. (2009). Identification of a novel UDP-N-acetylglucosamine enolpyruvyl transferase (MurA) from Vibrio fischeri that confers high fosfomycin resistance in Escherichia coli. Arch. Microbiol. 191, 425–429. doi: 10.1007/s00203-009-0468-9
Liegeon, G., Delory, T., Picardeau, M. (2018). Antibiotic susceptibilities of livestock isolates of leptospira. Int. J. antimicrobial. agents. 51, 693–699. doi: 10.1016/j.ijantimicag.2017.12.024
MaChado, D., Barbosa, J. C., Almeida, D., Andrade, J. C., Freitas, A. C., Gomes, A. M. (2022). Insights into the Antimicrobial Resistance Profile of a Next Generation Probiotic Akkermansia muciniphila DSM 22959. Int. J. Environ. Res. Public Health 19, 9152–9167. doi: 10.3390/ijerph19159152
McArthur, A. G., Waglechner, N., Nizam, F., Yan, A., Azad, M. A., Baylay, A. J., et al. (2013). The comprehensive antibiotic resistance database. Antimicrobial. Agents chemother. 57, 3348–3357. doi: 10.1128/AAC.00419-13
Miraglia, F., Moreno, A. M., Gomes, C. R., Paixão, R., Liuson, E., Morais, Z. M., et al. (2008). Isolation and characterization of Leptospira interrogans from pigs slaughtered in São Paulo State, Brazil. Braz. J. Microbiol. 39, 501–507. doi: 10.1590/S1517-83822008000300017
Murray, C. J. L., Ikuta, K. S., Sharara, F., Swetschinski, L., Robles Aguilar, G., Gray, A. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet (London England). 399, 629–655. doi: 10.1016/S0140-6736(21)02724-0
Oliveira, M. A. A., Leal, É., Correia, M. A., Serufo Filho, J. C., Dias, R. S., Serufo, J. C. (2017). Human leptospirosis: occurrence of serovars of Leptospira spp. in the state of Minas Gerais, Brazil, from 2008 to 2012. Braz. J. Microbiol. 48, 483–488. doi: 10.1016/j.bjm.2016.12.010
Petakh, P., Isevych, V., Griga, V., Kamyshnyi, A. (2022a). The risk factors of severe leptospirosis in the Transcarpathian region of Ukraine–search for “red flags”. Arch. Balk Med. Union. 57, 231–237. doi: 10.31688/ABMU
Petakh, P., Isevych, V., Kamyshnyi, A., Oksenych, V. (2022b). Weil's disease-immunopathogenesis, multiple organ failure, and potential role of gut microbiota. Biomolecules 12, 1830–1847. doi: 10.3390/biom12121830
Petakh, P., Isevych, V., Mohammed, I. B., Nykyforuk, A., Rostoka, L. (2022c). Leptospirosis: prognostic model for patient mortality in the transcarpathian region, Ukraine. Vector borne zoon. Dis. (Larchmont NY). 22, 584–588. doi: 10.1089/vbz.2022.0063
Petakh, P., Nykyforuk, A. (2022). Predictors of lethality in severe leptospirosis in Transcarpathian region of Ukraine. Le infezioni medicina. 30, 272–276. doi: 10.53854/liim-3002-13
Petakh, P., Oksenych, V., Kamyshna, I., Boisak, I., Lyubomirskaya, K., Kamyshnyi, O. (2024a). Exploring the complex interplay: gut microbiome, stress, and leptospirosis. Front. Microbiol. 15, 1345684–1345700. doi: 10.3389/fmicb.2024.1345684
Petakh, P., Oksenych, V., Kamyshnyi, O. (2024b). Exploring Leptospira interrogans FDAARGOS_203: Insights into AMR and Anti-Phage Defense. Microorganisms 12, 3, 546. doi: 10.3390/microorganisms12030546
Petakh, P., Rostoka, L., Isevych, V., Kamyshnyi, A. (2023). Identifying risk factors and disease severity in leptospirosis: A meta-analysis of clinical predictors. Trop. doctor. 53, 464–469. doi: 10.1177/00494755231187673
Pulingam, T., Parumasivam, T., Gazzali, A. M., Sulaiman, A. M., Chee, J. Y., Lakshmanan, M., et al. (2022). Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 170, 106103. doi: 10.1016/j.ejps.2021.106103
Rajapakse, S. (2022). Leptospirosis: clinical aspects. Clin. Med. 22, 14. doi: 10.7861/clinmed.2021-0784
Schönberg, A. (1981). Studies on the effect of antibiotic substances on leptospires and their cultivation from material with a high bacterial count. Zentralblatt fur Bakteriologie 1 Abt Originale A: Medizinische Mikrobiologie Infektionskrankheiten und Parasitologie. 249, 400–406. doi: 10.1016/S0174-3031(81)80096-0
Trott, D. J., Abraham, S., Adler, B. (2018). Antimicrobial resistance in leptospira, brucella, and other rarely investigated veterinary and zoonotic pathogens. Microbiol. spect. 6. doi: 10.1128/microbiolspec.ARBA-0029-2017
Vinod Kumar, K., Lall, C., Raj, R. V., Vedhagiri, K., Sunish, I. P., Vijayachari, P. (2016). In vitro antimicrobial susceptibility of pathogenic leptospira biofilm. Microbial. Drug resist. (Larchmont NY). 22, 511–514. doi: 10.1089/mdr.2015.0284
Keywords: Leptospira interrogans, antibiotic, resistance, leptospiral serovars, leptospirosis
Citation: Petakh P and Kamyshnyi O (2024) AMR mechanisms in L. interrogans serovars: a comprehensive study. Front. Cell. Infect. Microbiol. 14:1384427. doi: 10.3389/fcimb.2024.1384427
Received: 09 February 2024; Accepted: 26 March 2024;
Published: 11 April 2024.
Edited by:
Nayeem Ahmad, Arabian Gulf University, BahrainReviewed by:
M. Oves, King Abdulaziz University, Saudi ArabiaPankaj Dhaka, Guru Angad Dev Veterinary and Animal Sciences University, India
Dalia Hamza, Cairo University, Egypt
Copyright © 2024 Petakh and Kamyshnyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavlo Petakh, cGF2bG8ucGV0YWtoQHV6aG51LmVkdS51YQ==; Oleksandr Kamyshnyi, a2FteXNobnlpX29tQHRkbXUuZWR1LnVh
 Pavlo Petakh
Pavlo Petakh Oleksandr Kamyshnyi
Oleksandr Kamyshnyi