
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 23 April 2024
Sec. Veterinary and Zoonotic Infection
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1371916
 Zhaoyang Xu†
Zhaoyang Xu† Qian Zhang†
Qian Zhang† Mengjun Wu
Mengjun Wu Yanyan Zhang
Yanyan Zhang Zhonghua Li
Zhonghua Li Hanxiao Li
Hanxiao Li Chenmin Yu
Chenmin Yu Xiaohan Zhang
Xiaohan Zhang Di Zhao
Di Zhao Lei Wang
Lei Wang Yongqing Hou
Yongqing Hou Tao Wu*
Tao Wu*Porcine epidemic diarrhea virus (PEDV) has become a challenging problem in pig industry worldwide, causing significant profit losses. Lactobacillus rhamnosus GG (LGG) has been regarded as a safe probiotic strain and has been shown to exert protective effects on the intestinal dysfunction caused by PEDV. This study evaluated the effect of LGG on the gut health of lactating piglets challenged with PEDV. Fifteen piglets at 7 days of age were equally assigned into 3 groups (5 piglets per group): 1) control group (basal diet); 2) PEDV group: (basal diet + PEDV challenged); 3) LGG + PEDV group (basal diet + 3×109 CFU/pig/day LGG + PEDV). The trial lasted 11 days including 3 days of adaptation. The treatment with LGG was from D4 to D10. PEDV challenge was carried out on D8. PEDV infection disrupted the cell structure, undermined the integrity of the intestinal tract, and induced oxidative stress, and intestinal damage of piglets. Supplementation of LGG improved intestinal morphology, enhanced intestinal antioxidant capacity, and alleviated jejunal mucosal inflammation and lipid metabolism disorders in PEDV-infected piglets, which may be regulated by LGG by altering the expression of TNF signaling pathway, PPAR signaling pathway, and fat digestion and absorption pathway.
Porcine epidemic diarrhea (PED) is a highly contagious intestinal disease caused by the porcine epidemic diarrhea virus (PEDV) and is considered one of the most significant threats to the pig farming industry. In recent years, the global spreading of PED has caused enormous economic losses to the pig farming industry. PEDV, as well as transmissible gastroenteritis virus and porcine enteric A coronavirus, belongs to the genus α-coronavirus in the family Coronaviridae (Gong et al., 2017). The main PEDV transmission route is fecal-oral, but airborne transmission via the fecal-nasal route may play a role in pig-to-pig and farm-to-farm spread (Gerber et al., 2014; Yuan et al., 2022). Piglets up to 7 days of age are particularly susceptible to the virus and thus cause acute diarrhea, vomiting, dehydration and high mortality in newborn piglets (Sun et al., 2016). In recent years, various mutations and recombinations have occurred between different strains and even between PEDV and other coronaviruses. The lack of updated effective vaccination in controlling PEDV infections has caused continuous problems in the pig industry (Du et al., 2019).
Lactobacillus rhamnosus GG (LGG), a Gram-positive parthenogenetic anaerobic bacterium first extracted from the gastrointestinal tract of healthy human beings in 1983 (Shi et al., 2019), is one of the most widely studied probiotic organisms for atopic diseases. The main characteristics of LGG are the strong ability to survive and reproduce under low pH conditions of gastric acid and bile, easy to colonize and function in the intestinal tract, with a high level of immunological activity (Hou et al., 2023). Some studies have shown that colonization of LGG in neonatal mice enhances intestinal functional maturation and IgA production and confers lifelong health consequences on protection from intestinal injury and inflammation (Yan et al., 2016). Oral administration of Lactobacillus rhamnosus powder to mice alleviated antibiotic-induced intestinal injury (Cresci et al., 2013). Moreover, LGG may be effective in ameliorating E. coli K88-induced diarrhea in weaned piglets by modulating the intestinal flora, enhancing the intestinal antibody defenses, and modulating the production of systemic inflammatory factors (Zhang et al., 2010). In addition, dietary Lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by Porcine Rotavirus (Mao et al., 2016). Moreover, studies have shown that treatment with L. rhamnosus can significantly increase the villus height of ileum, promote the proliferation of T Cell proliferation, and therefore improve the absorption function and immune capacity of intestines in piglets (Shonyela et al., 2020).
Lactobacillus rhamnosus has been applied in medical research and animal husbandry production, but there are few reports on the resistance of Lactobacillus rhamnosus powder to PEDV infection. Our research group has previously established the intestinal injury model of PEDV-infected piglets. Therefore, the present study aimed to explore the protective effect of Lactobacillus rhamnosus GG powder on the intestinal tract of PEDV-infected piglets.
All animal procedures used in the present study were approved by the Institutional Animal Care and Use Committee of Wuhan Polytechnic University (Index number: 202205003). A total of 15 healthy 7-day-old piglets (Duroc × Landrace × Yorkshire) were used for the experiment. Piglets were housed separately in two environment-controlled nursery rooms (28-30°C) to avoid cross-infection and given ad libitum access to water throughout the study. The freeze-dried Lactobacillus rhamnosus GG powder containing 3 ×109 colony forming units (CFU)/g was purchased from Hubei Haohua biotechnology Co., Ltd (Wuhan, China), and stored in the sealed packet at 4°C until used. A commercial milk replacer from Shanghai Nouriz Dairy Co., Ltd (Shanghai, China) was served as a basal diet for piglets, formulated to satisfy the requirements of suckling piglets. Before feeding, the milk replacer was dissolved in warm water (45–55°C) to form a liquid feed (dry matter content of 20%). Pigs were fed with the liquid feed every 3 h between 8:00 am and 8:00 pm.
The healthy piglets (half male and half female) at 7 day of age (Duroc × Landrace × Yorkshire, BW = 3.51 ± 0.33 kg) were used in this experiment. Piglets were housed in clean pens with strict control of cross-infection.The trial lasted 11 days (D0-D10), including three days of adaptation (D1-D3). The treatment with LGG was from D4 to D10. The piglets were orally administered with PEDV at a dose of 106 TCID50 (50% tissue culture infectious dose) per pig on D8, We used the dose of infection in our previous study and pretest (Zhang et al., 2022; Song et al., 2024), while the control group was orally administered with an equal volume of PBS solution. Fifteen healthy crossbred piglets were chosen and the commercially available milk replacer (Nouriz shanghai, China) was used as a basic diet. After 3-day adaptation, the piglets were assigned randomly, based on body weight (BW) and litter origin, to 3 groups: (5 piglets/group): 1) control group - piglets fed the basal diet; 2) PEDV group: piglets fed the basal diet, and were orally administered with 3 ml 1×106 TCID50/pig PEDV; 3) LGG+PEDV group-piglets fed the basal diet supplemented with 50 mg/kg BW LGG supplementation in diet, and were orally administered with 3 ml 1×106 TCID50/pig PEDV.
Intestine sample was collected from the jugular vein of piglets on D11 and then all animals were slaughtered under sodium pentobarbital anesthesia (50 mg/kg BW, iv). The abdomen of piglets was opened immediately and the small intestine was separated from the mesentery onto a chilled stainless-steel tray, then the 5-cm segment samples were cut respectively at distal duodenum, mid-jejunum and mid-ileum. The samples were gently flushed with ice-cold PBS and placed in chilled formalin solution (10%), mucosa was collected by scraping using a sterile glass microscope slide at 4°C, rapidly frozen in liquid nitrogen, then processed by embedding and staining for the observation of intestinal morphology. All the samples were collected within 10 min. Intestinal tissues were stored at −80°C until analysis. Piglet challenge PEDV, administration of LGG powder and sample collection process are shown in Figure 1.
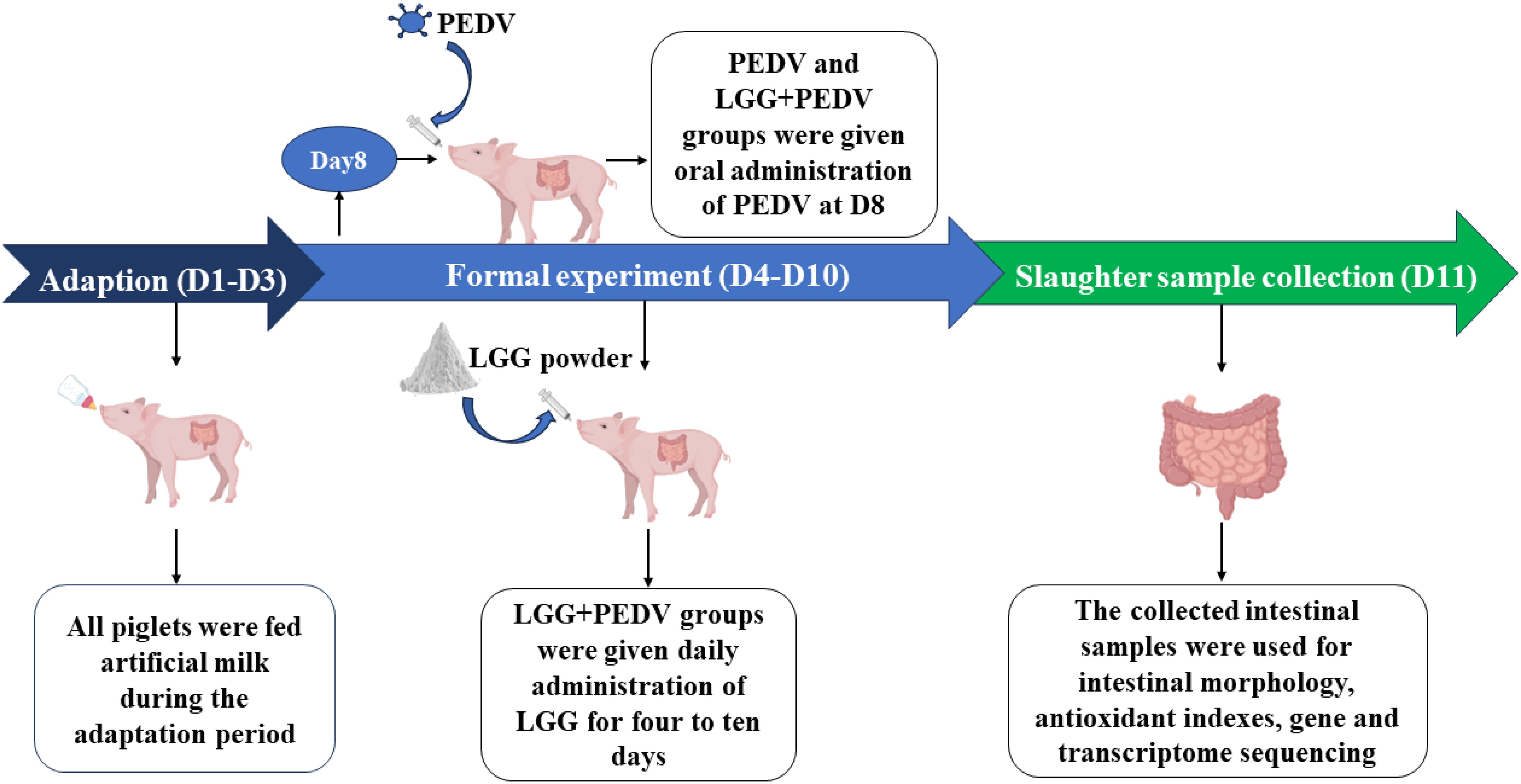
Figure 1 Workflow for PEDV challenge, administration of LGG powder, and sample collection in piglets.
The procedure of preparing samples for measuring intestinal morphology was performed as described previously (Wu et al., 2018). Briefly, the samples were dehydrated and embedded in paraffin, sectioned at a thickness of 4 mm, and stained with haematoxylin and eosin. Morphological examination was conducted with a light microscope (Leica microsystems, Wetzlar, Germany) with the Leica Application Suite image analysis software (Leica microsystems, Wetzlar, Germany). Eight areas per slide were inspected randomly in a double-blind manner. Intestinal villus height and crypt depth were measured to calculate the ratio of villus height to crypt depth.
Mucosa of duodenum, jejunum, ileum, and colon were used for analysis of antioxidative enzymes and oxidation-related products. The activities of catalase (CAT), total superoxide dismutase (T-SOD), myeloperoxidase (MPO), and the concentration of hydrogen peroxide (H2O2) and malondialdehyde (MDA) were determined by using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), according to the protocols of manufacturer.
The gene expression levels were quantitated using the method of qRT-PCR assays and the primers used in the present study was shown in Table 1, which were either verified experimentally using the conditions in previous publications including the housekeeping gene encoding ribosomal protein L19 (RPL19) or were designed using Primer Express software version 3.0 (Applied Biosystems). The qRT-PCR was performed using the SYBR ® Premix Ex Taq TM (Takara, Dalian, China) on an Applied Biosystems 7500 Fast qRT-PCR System (Foster City, CA). The total volume of PCR reaction system was 50 μL, containing 0.2 µM of each primer, 25 µL of SYBR ® Premix Ex Taq TM (2×), and 4 µL of cDNA. All PCR assays were performed in triplicate on a 96-well real-time PCR plate (Applied Biosystems) under the following conditions (two-step amplification): 95°C for 30 sec, followed by 40 cycles of 95°C for 5 sec and 55-65°C for 31 sec depending on the melting temperature of each pair of primers. A subsequent melting curve (95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec) with continuous fluorescence measurement was constructed. Data were analyzed using the 2-ΔΔCt method as described (Yi et al., 2016).
Total RNA was isolated from the Jejunum tissue using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The integrity and purity of the total RNA was checked before construction of the sequencing library. The NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) was used to generate the sequencing libraries, which were sequenced on an Illumina Hiseq platform (Illumina, San Diego, CA, USA) to generate paired-end reads. Thereafter, we used DESeq2 R package (1.18.1) to profile gene expression in the PEDV vs. Contral and LGG+PEDV vs. PEDV. Differential expression genes (DEGs) were filtered based on a P-value < 0.05 and a fold-change ≥1.5 or ≤ 0.67. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was carried out by Python Scipy based on differentially expressed genes, and the first 20 pathways with the smallest P- adjust, the most significant enrichment, were selected for display. A P-value < 0.05 was considered as a significance threshold.
All data were analyzed by one-way ANOVA using the GLM procedure of SPSS 20.0 software appropriate for a 2×2 factorial design (SPSS Inc. Chicago, IL, USA). The factors of models included the main effects of LGG treatment (supplemented or unsupplemented with LGG in the diet) and the PEDV challenge. P<0.05 was considered to indicate statistical significance, and P<0.10 was considered to indicate statistical tendency. All data were expressed as mean ± standard deviation.
Small intestine is the main place for animals to digest and absorb nutrients. The structure and morphology of intestinal mucosa are closely related to the intestinal health of animals. In order to investigate the effect of LGG powder on intestinal morphology of PEDV-infected piglets, we observed and analyzed the villus growth in the small intestine of piglets. The microscopic pictures of intestinal mucosal are shown in Figure 2. The effects of LGG on the intestinal morphology of piglets is shown in Table 2. Compared with none-challenged piglets, PEDV infection decreased villus height/crypt depth ratio in all small intestinal segments (P< 0.05), decreased the villus height, and increased the crypt depth in the jejunum and ileum (P< 0.05). LGG supplementation decreased crypt depth and increased villus height/crypt depth ratio in the jejunum and ileum (P< 0.05), and increased the villus height in the ileum, as compared to the PEDV group.
Several representative antioxidant enzymes and oxidative damage products were detected in this experiment to reveal the effect of PEDV and LGG on intestinal antioxidant capacity in the intestinal tract of piglets, as shown in Figure 3. Compared with none-challenged piglets, the PEDV infection significantly increased MPO activity in the duodenum (P<0.05). The activity of CAT in the jejunum was significantly decreased in PEDV group (P<0.05). PEDV infection significantly decreased CAT activity in the colon (P< 0.05). Compared with the PEDV group, the LGG supplementation decreased MPO activity, in the duodenum. Compared with the PEDV group, the LGG supplementation significantly decreased the H2O2 concentration in the duodenum, increased CAT activity in the jejunum (P< 0.05). PEDV infection and LGG supplementation has no significantly effect in the ileum. LGG supplementation could significantly increased CAT and T-SOD activity in the colon (P<0.05).
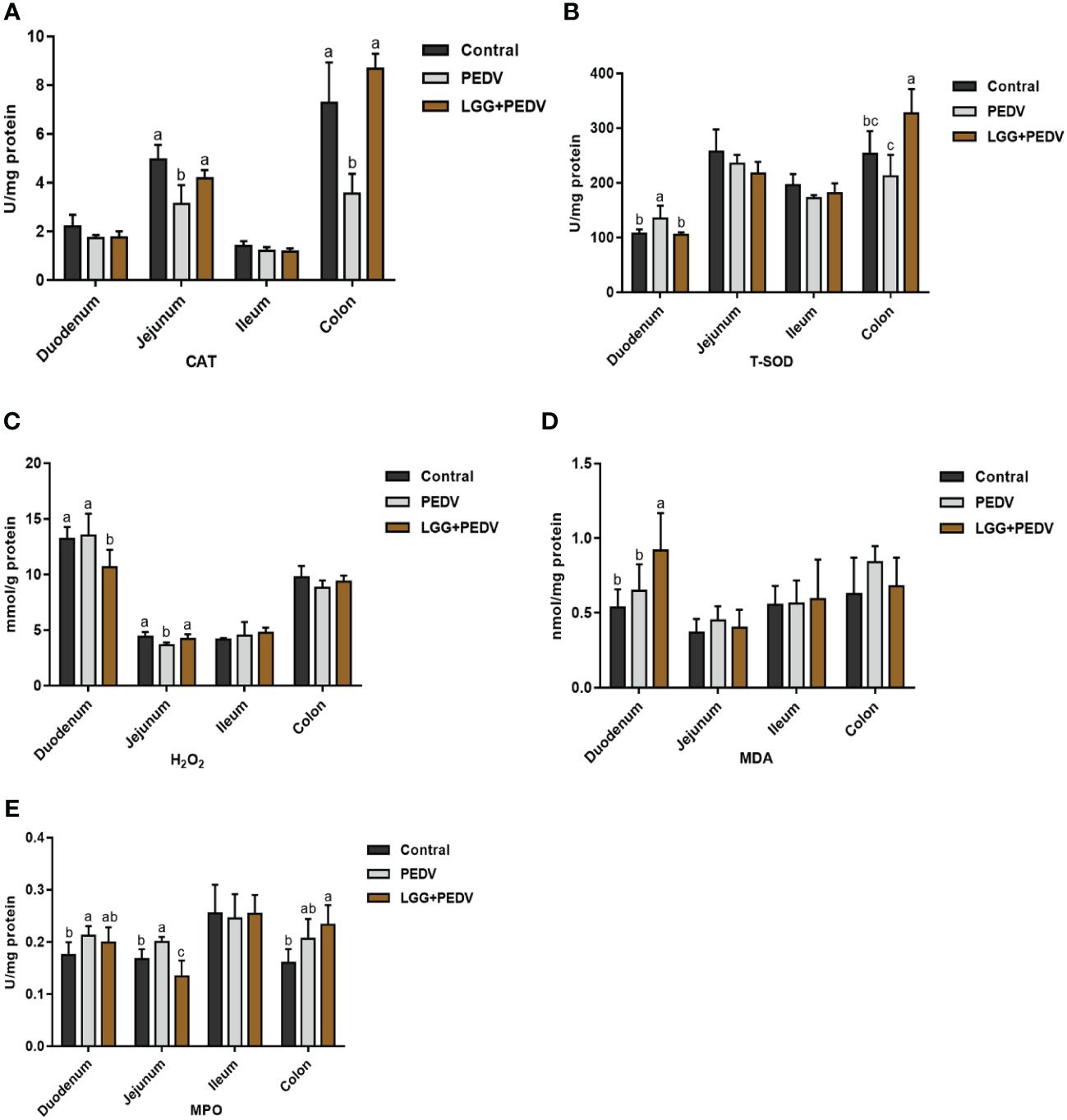
Figure 3 Effects of Lactobacillus rhamnosus GG on the intestinal antioxidant capacity of piglets (A) CAT, catalase; (B) T-SOD, total superoxide, and dismutase; (C) H2O2, hydrogen peroxide; (D) MDA, malondialdehyde. (E) MPO, Myeloperoxidase. a,b,c, Values within a column not sharing a common superscript letter indicate significant difference at P < 0.05.
PEDV infection in piglets usually induces the expression of inflammatory factors and the disturbance of lipid metabolism. To further investigate the effects of LGG powder supplementation on the immune and lipid metabolism status of PEDV-infected piglets, we performed qRT-PCR analysis for some anti-inflammatory and lipid metabolism-related genes. The regulation of jejunal intestinal gene expression is shown in Figure 4. Compared with none-challenged piglets, the PEDV infection significantly increased expression levels of PEDV M, PEDV N, OASL, CXCL2, IL-8 and IL-1β (P<0.05), and decreased expression levels of ISG15, IRF3, APOA1, APOC3, FABP1, FABP2, ACSL3 of SLC27A2 in the jejunum (P<0.05). LGG Powder supplementation decreased expression levels of PEDV M, PEDV N, OASL, CXCL2, IL-8 and IL-1β (P<0.05), and increased expression levels of ISG15, APOA1, FABP1, FABP2 (P<0.05).
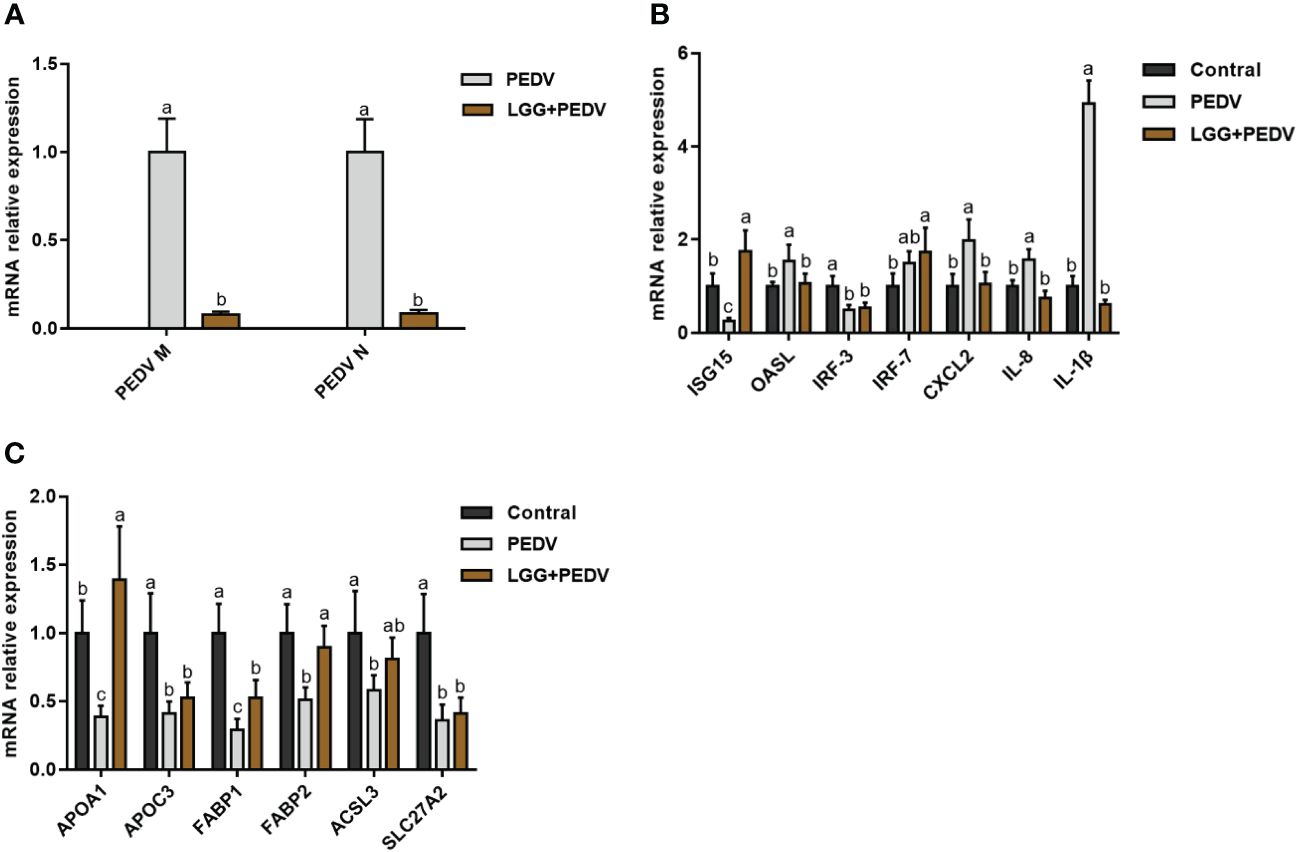
Figure 4 (A) Effects of Lactobacillus rhamnosus GG on the viral gene expression in the jejunum of piglets. (B) Effects of Lactobacillus rhamnosus GG on the Anti-Inflammatory gene expression in the jejunum of piglets. (C) Effects of Lactobacillus rhamnosus GG on the lipid metabolism gene expression in the jejunum of piglets. ISG15, interferon-stimulated gene 15; OASL, 2’-5’-oligoadenylate synthetase; IRF3, Interferon regulatory factor 3; IRF7, Interferon regulatory factor 7; CXCL2, CXC motif chemokine ligand 2; APOA1, Apolipoprotein A1; APOC3, Apolipoprotein C3; FABP1, Fatty Acid Binding Protein-1; FABP2, Fatty acid binding protein 2; ACSL3, acyl-CoA synthetase long-chain family member 3; SLC27A2, Solute carrier family 27 member 2; a,b,c, Values within a column not sharing a common superscript letter indicate significant difference at P < 0.05.
Transcriptome sequencing technology (RNA-seq) is an effective tool for mining differentially expressed genes and regulatory networks in specific environments, thus we used transcriptome sequencing technology to explore the key pathways of LGG powder action on PEDV-infected piglets. Compared with none-challenged piglets, 1806 DEGs (936 up-regulated and 870 down-regulated) were identified in the PEDV group (Figure 5A). KEGG enrichment analysis showed the most significant pathway including Chemical carcinogenesis-DNA adducts, Hematopoietic cell lineage, Retinol metabolism, Drug metabolism-other enzymes, Cytokine-cytokine receptor interaction, TNF signaling pathway, Lipid and atherosclerosis, Drug metabolism-cytochrome P450, Osteoclast differentiation, Rheumatoid arthritis, Fat digestion and absorption, Bile secretion, PPAR signaling pathway, et al. (Figure 5C).Compared with the PEDV group, 1785 DEGs (1059 up-regulated and 726 down-regulated) were identified in the LGG+PEDV group (Figure 5B). KEGG enrichment analysis showed the most significant pathway including Peroxisome, Retinol metabolism, Hematopoietic cell lineage, Fructose and mannose metabolism, Chemical carcinogenesis-DNA adducts, PPAR signaling pathway, Cytokine-cytokine receptor interaction, Drug metabolism-other enzymes, Fat digestion and absorption, Glutathione metabolism, steroid hormone biosynthesis, Drug metabolism-cytochrome P450, Arginine and proline metabolism, Galactose metabolism, Fatty acid degradation, Protein digestion and absorption, Glycerolipid metabolism, et al. (Figure 5D). Clustering heatmap indicated that DEG samples selected from each group presented a similar expression pattern (Figure 6).
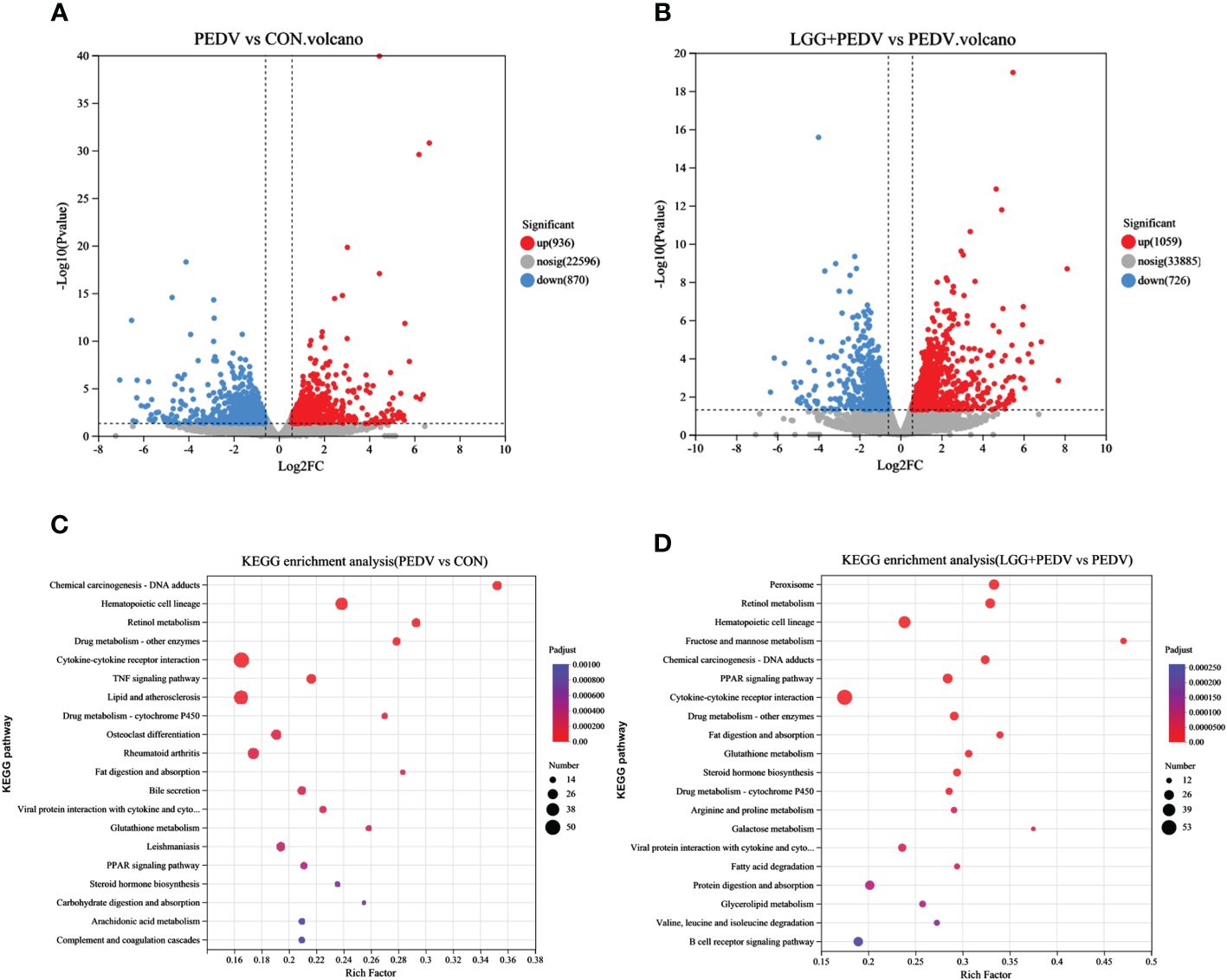
Figure 5 Jejunum mucosal transcriptome. (A) Volcano plot of DEG in PEDV and CON group. Up-regulation, none difference, and down-regulation are represented by red, grey, and blue dots, respectively. (B) Volcano plot of DEG in LGG+PEDV and PEDV group. (C) KEGG pathway enrichment analysis of DEG in PEDV and CON groups. The abscissa is the name of the pathway, and the ordinate is the P-adjust showing the enrichment of each pathway; the number of genes in the circles shows the number of differentially expressed genes enriched in the pathway. (D) KEGG pathway enrichment analysis of DEG in LGG+PEDV and PEDV group The abscissa is the name of the pathway, and the ordinate is the P-adjust showing the enrichment of each pathway; the number of genes in the circles shows the number of differentially expressed genes enriched in the pathway.
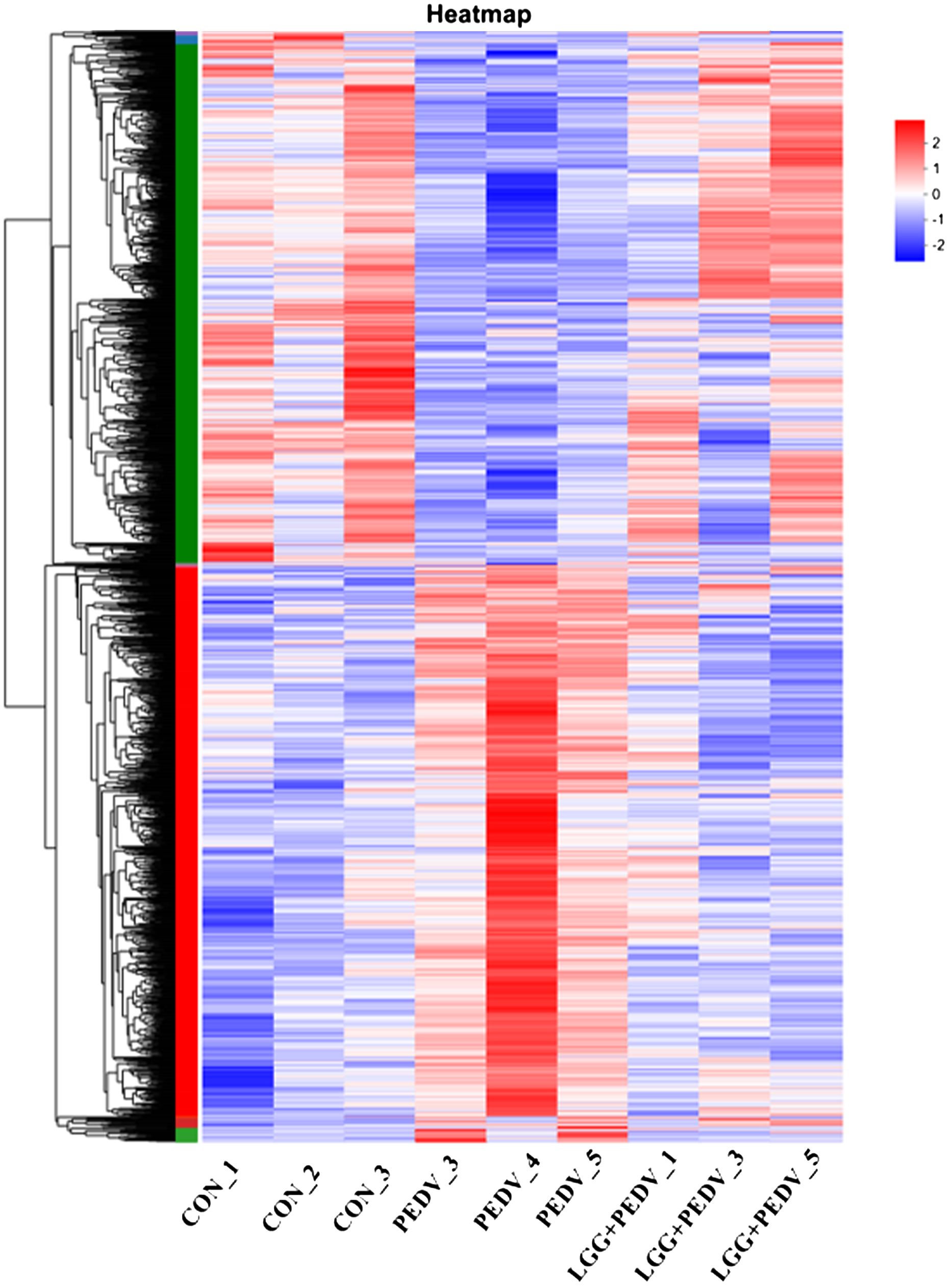
Figure 6 The red color indicates up-regulated genes, and the green color indicates genes showing down-regulated. Each row represents a gene, and each column is a sample.
The transcriptome data and qRT-PCR results are shown in Figure 7. Ten genes identified by qRT-PCR were consistent with the transcriptome data, and the fold changes were basically the same, which indicated the consistency between the qRT-PCR and transcriptome data.
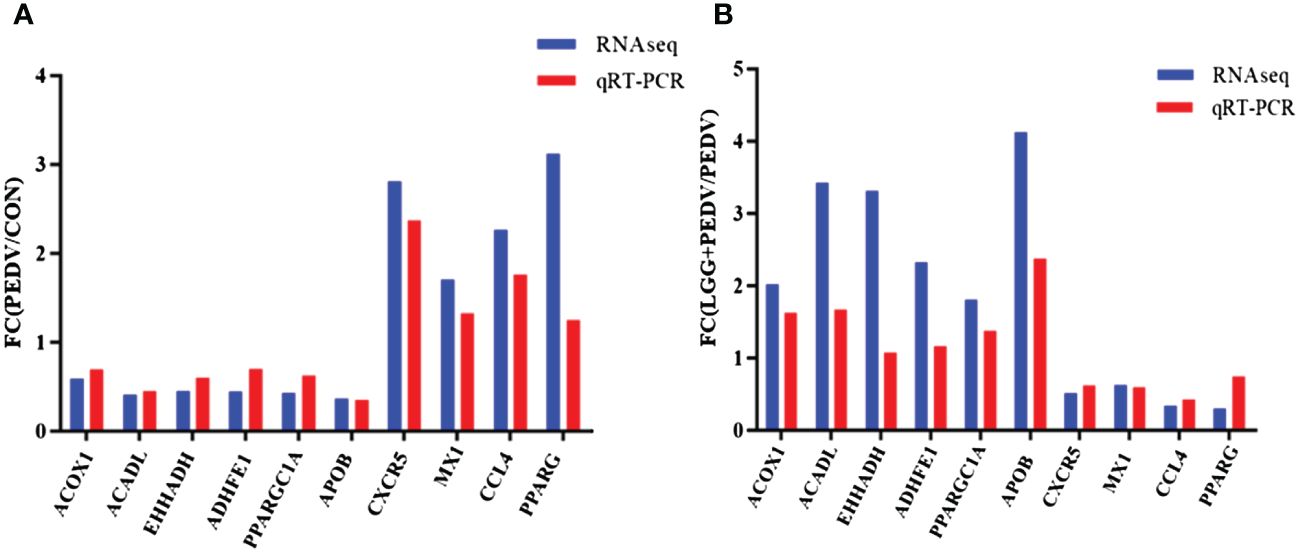
Figure 7 (A) Fold change indicates the ratio of average expression of PEDV-affected mRNA relative to controls. (B) Fold change indicates the ratio of average expression of LGG-affected mRNA relative to PEDV.
The digestive and absorptive functions of piglets are closely related to the morphology integrity of the intestinal mucosal, and the villus height, crypt depth and villus height/crypt depth ratio are important indicators of intestinal morphology structure and integrity. The shortening of small intestinal mucosal epithelial villi and deepening of crypts represent shrinkage of intestinal mucosal epithelial villi and decreased absorption capacity; the increase in VH/CD represents an increase in the specific area of the mucosal and an increase in the digestive and absorptive capacity (Puppel et al., 2015; Choi et al., 2020). In addition, It has been shown that the addition of Lactobacillus delbrueckii to the diet significantly increased the ratio of villus height to crypt depth in the jejunum and ileum, significantly decreased crypt depth in the jejunum, and increased villus height in the ileum in weaned piglets (Wang et al., 2021). Dietary administration of 5 × 1010 CFU/kg Lactobacillus Royale LR1 (LR1) to weaned piglet diets significantly increased villus height of the ileum as well as the ratio of ileal and jejunal villus height to crypt depth (Yi et al., 2018). A study by Wang et al. showed that the mRNA expression of intestinal occludin and ZO-1, and the protein expression level of occludin were increased by L. plantarum 299v treatment (Wang et al., 2019). Lactobacillus possibly improves intestinal barrier function by up-regulating the expression of Tight junction proteins, which are critical structural components of intestinal epithelial cells. The beneficial effect of Lactobacillus rhamnosus GG on improving the mucosal barrier function in the intestine of weaned piglets has been proved (Mao et al., 2016). Interestingly, In this experiment, supplementation of Lactobacillus rhamnosus GG powder significantly alleviated the intestinal morphology of PEDV-infected 7-day-old piglets, indicating that the Lactobacillus rhamnosus GG could play a function in the early life of piglets.
T-SOD and CAT belong to the main radical scavenging capacity of the organism, and their activity directly reflects the antioxidant capacity of the organism. MDA is a lipid peroxidation product, and its content directly indicates the degree of lipid peroxidation of the organism, which has cytotoxicity (Shi et al., 2014). MDA is an important index for the evaluation of lipid oxidation damage in the body, and can also indirectly reflect the damage of intestinal mucosal cells. MPO is a protein enriched in neutrophils, and higher activity indicates a more intense inflammatory response (Kolářová et al., 2021). In the present study, PEDV infection resulted in a significant decrease in CAT and T-SOD activities, a significant increase in MPO activity, and a significant increase in MDA content in the intestine, suggesting that PEDV successfully induced oxidative damage in the intestinal mucosa of piglets. Supplemented with Lactobacillus rhamnosus powder significantly increased CAT and T-SOD activity and reduced MDA content in the intestine. Consistently, previous studies have found that erythrocyte CAT and T-SOD activities were elevated in piglets fed Lactobacillus FT28, which effectively increased the antioxidant level of piglets (Dowarah et al., 2018). Dietary administration of Lactobacillus rhamnosus GG to the diet can reduce the damage of deoxynivalenol to the renal antioxidant system of piglets and effectively alleviate the oxidative damage of the kidneys (Ma et al., 2022). Lactobacillus rhamnosus GG can inhibit oxidative stress and apoptosis, thereby counteracting rotavirus-induced intestinal epithelial cell damage (Buccigrossi et al., 2022). In addition, supplementation of the diet with Lactobacillus rhamnosus GG (LGG) has been reported to significantly increase T-SOD activity and significantly decrease MDA levels in foal plasma (Shi et al., 2023). The results of this experiment were similar to those of previous studies, indicating that the addition of Lactobacillus rhamnosus GG powder to the diets could improve the antioxidant enzyme activities, enhance the body’s ability to scavenge free radicals, and alleviate the oxidative damage of the body in PEDV-infected piglets.
Transcriptomic techniques provide us with a powerful tool to discover potential biochemical pathways and reveal biological insights. The present study focused on the TNF signaling pathway, PPAR signaling pathway, fat digestion and absorption, and fatty acid degradation. The innate immune response plays an important role in the defense of mammalian cells against viral infections. After viral infection, an intrinsic antiviral immune response is initiated, and the body synthesizes interferon to induce the expression of antiviral molecules and to interfere with viral replication (Yang et al., 2022; Jung et al., 2023). In this study, PEDV infection significantly increased the content of Mx1 and OASL, whereas L. rhamnosus LGG supplementation powder decreased the levels of Mx1 and OSAL, similar to the control group. The recent study has shown that inflammatory cytokines, and small amounts of pro-inflammatory cytokines may have a protective effect against viral invasion (Huan et al., 2017), however, overproduction of cytokines disrupt the host immune response and affects the integrity of the small intestinal barrier, further exacerbating intestinal immune barrier damage (Liu et al., 2012), therefore, effective control of inflammatory cytokines expression is important for piglet gut health. The TNF signaling pathway may have been activated due to PEDV infection in this study, and this was also corroborated by the results of qRT-PCR. In the jejunal mucosa, we detected a significant increase in the mRNA expression of the inflammatory factors downstream of the TNF signaling pathway, IL-β, CXCL2, and IL-8, which suggests that a severe inflammatory response was induced in the cells of the jejunal mucosa (Qian et al., 2022). Whereas, L. rhamnosus GG supplementation decreased the levels of IL-β, CXCL2, and IL-8, suggesting its effects on alleviation of the inflammation. It has been reported that supplementation of piglets with lactic acid bacteria-fermented formula milk significantly decreased the levels of pro-inflammatory cytokines and inhibited the expression of inflammatory cytokines CXCL9 and CXCL10 in the colonic mucosa (Lin et al., 2023), and supplementation of ETEC-infected piglets with L. rhamnosus LB1 decreased the levels of TNF-α and IL-1β (Wu et al., 2021). Lactobacillus rhamnosus inhibits E. coli-induced expression of pro-inflammatory cytokines TNF-α and IL-1β in piglets (Li et al., 2012). Similar results were obtained in the present study, suggesting that L. rhamnosus GG powder may alleviate the inflammatory response of PEDV-infected piglets by down-regulating the expression of TNF signaling pathway.
Peroxisome proliferator-activated receptors (PPARα, PPARβ/δ, and PPARγ) belong to the transcription factor family, is a nuclear hormone receptor activated by fatty acids and their derivatives (Peng et al., 2021). PPARα plays a role in removing or recycling cellular lipids by regulating the expression of genes related to lipid metabolism in liver and skeletal muscle, PPARβ/δ are involved in cell proliferation and lipid oxidation, and PPARγ can promote adipocyte differentiation. In this study, PEDV infection significantly decreased the levels of mRNAs of key differentially expressed genes in the PPAR signaling pathway and the fat digestion and absorption pathway, such as ACADL, ACOX1, APOA1, APOC3, EHHADH, FABP1, FABP2, ACSL3, SLC27A2, APOB, etc, which was in line with the recent studies, that PEDV infection significantly suppressed the expression of genes related to lipid metabolism (Mao et al., 2022). Long-chain acyl-CoA dehydrogenase (ACADL) is the first step in fatty acid oxidation and plays a vital role in long-chain fatty acid β-oxidation, and its deficiency or deletion also causes mitochondrial metabolic disorder and affects lipid metabolism (Ji and Friedman, 2021). ACOX1 gene is strongly associated with fatty acid metabolism and triglyceride deposition in piglets (Zuo et al., 2007). Apolipoprotein A1 (APOA1) is the main apolipoprotein of plasma HDL and is involved in the regulation of intracellular lipid levels (Cochran et al., 2021), and it has also been reported that APOA1 is associated with the development of inflammatory and oxidative processes in the body (Madahian et al., 2014). Apolipoprotein A-IMilano (apoAIM), a naturally occurring cysteine mutant of apoAI with dimers as its effective form, and recombinant HDL-APOA1M (rHDL- APOA1M) formed from APOA1M and HDL has significant anti-inflammatory and anti-oxidant functions (Zhang et al., 2015). In this study, the mRNA expression of the APOA1 gene and other genes related to lipid metabolism was significantly down-regulated in the jejunal mucosal tissues of piglets infected with PEDV, suggesting that PEDV infection caused disorders of intestinal lipid metabolism and inflammatory responses in piglets. Supplementation of PEDV piglets with Lactobacillus rhamnosus GG powder significantly up-regulated the expression of mRNAs of key differentially expressed genes in the PPAR signaling pathway and the fat digestion and absorption pathway, such as ACADL, ACOX1, APOA1, APOC3, EHHADH, FABP1, FABP2, ACSL3, SLC27A2, APOB, etc., and then alleviated the disorders of lipid metabolism in the intestinal tract due to PEDV infection. The results were similar to a previous study conducted on Intestinal porcine cells (IPEC‐1) that L. plantarum inhibited the reduction in peroxisome proliferator‐activated receptor‐γ (PPAR‐γ) expression caused by ETEC K88 (Yang et al., 2021). Differently, Oral administration of Lactobacillus plantarum 299v down-regulated PPAR signaling pathway gene expression in the ileum of weaned male 4-week-old pigs (Hulst et al., 2015). Such variable results might be attributed to the variations in the types of probiotics, animals, cells, and experiment times.
Supplementation of Lactobacillus rhamnosus GG powder improved intestinal morphology, enhanced intestinal antioxidant capacity, and alleviated jejunal mucosal inflammation and lipid metabolism disorders in PEDV-infected piglets, which may be regulated by Lactobacillus rhamnosus GG powder by altering the expression of TNF signaling pathway, PPAR signaling pathway and fat digestion and absorption pathway.
The datasets presented in this study can be found in online repositories. The names of the repository and accession number(s) can be found below: NCBI; PRJNA1099660.
The animal studies were approved by The Institutional Animal Care and Use Committee of Wuhan Polytechnic University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
ZX: Writing – original draft. QZ: Formal analysis, Writing – review & editing. MW: Data curation, Writing – review & editing. YZ: Investigation, Writing – review & editing. ZL: Investigation, Writing – review & editing. HL: Project administration, Writing – review & editing. CY: Project administration, Writing – review & editing. XZ: Project administration, Writing – review & editing. DZ: Data curation, Writing – review & editing. LW: Data curation, Writing – review & editing. YH: Funding acquisition, Writing – review & editing. TW: Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (U22A20514), National Key R&D Program of China (2022YFD1300404-2) and the Wuhan Knowledge Innovation Project (2022020801010392).
We thank our students and technicians for their contributions to this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Buccigrossi, V, Poeta, M, Cioffi, V, Terranova, S, Nunziata, F, Lo Vecchio, A, et al. (2022). Lacticaseibacillus rhamnosus GG Counteracts Rotavirus-Induced Ion Secretion and Enterocyte Damage by Inhibiting Oxidative Stress and Apoptosis Through Specific Effects of Living and Postbiotic Preparations. Front Cell Infect Microbiol. 12, 854989. doi: 10.3389/fcimb.2022.854989
Choi, J., Wang, L., Liu, S., Lu, P., Zhao, X., Liu, H., et al. (2020). Effects of a microencapsulated formula of organic acids and essential oils on nutrient absorption, immunity, gut barrier function, and abundance of enterotoxigenic Escherichia coli F4 in weaned piglets challenged with E. coli F4. J. Anim. Sci. 98, skaa259. doi: 10.1093/jas/skaa259
Cochran, B. J., Ong, K. L., Manandhar, B., Rye, K. A. (2021). APOA1: a protein with multiple therapeutic functions. Curr. Atheroscler Rep. 23, 11. doi: 10.1007/s11883-021-00906-7
Cresci, G., Nagy, L. E., Ganapathy, V. (2013). Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN J. Parenter Enteral Nutr. 37, 763–774. doi: 10.1177/0148607113486809
Dowarah, R., Verma, A. K., Agarwal, N., Singh, P., Singh, B. R. (2018). Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PloS One 13, e0192978. doi: 10.1371/journal.pone.0192978
Du, J., Luo, J., Yu, J., Mao, X., Luo, Y., Zheng, P., et al. (2019). Manipulation of intestinal antiviral innate immunity and immune evasion strategies of porcine epidemic diarrhea virus. BioMed. Res. Int. 2019, 1862531. doi: 10.1155/2019/1862531
Gerber, P. F., Gong, Q., Huang, Y. W., Wang, C., Holtkamp, D., Opriessnig, T., et al. (2014). Opriessnig, Detection of antibodies against porcine epidemic diarrhea virus in serum and colostrum by indirect ELISA. Vet. J. 202, 33–36. doi: 10.1016/j.tvjl.2014.07.018
Gong, L., Li, J., Zhou, Q., Xu, Z., Chen, L., Zhang, Y., et al. (2017). A new bat-HKU2-like coronavirus in swine, China, 2017. Emerg. Infect. Dis. 23, 1607–1609. doi: 10.3201/eid2309.170915
Hou, Y., Zheng, S., Zou, F., Wang, D., Da, H., Zhou, Y., et al. (2023). Lactobacillus rhamnosus 76 alleviates airway inflammation in ovalbumin-allergic mice and improves mucus secretion by down-regulating STAT6/SPDEF pathway. Immunobiology 228, 152712. doi: 10.1016/j.imbio.2023.152712
Huan, C. C., Wang, H. X., Sheng, X. X., Wang, R., Wang, X., Mao, X. (2017). Glycyrrhizin inhibits porcine epidemic diarrhea virus infection and attenuates the proinflammatory responses by inhibition of high mobility group box-1 protein. Arch. Virol. 162, 1467–1476. doi: 10.1007/s00705-017-3259-7
Hulst, M., Gross, G., Liu, Y., Hoekman, A., Niewold, T., van der Meulen, J., et al. (2015). Oral administration of Lactobacillus plantarum 299v modulates gene expression in the ileum of pigs: prediction of crosstalk between intestinal immune cells and sub-mucosal adipocytes. Genes Nutr. 10, 10. doi: 10.1007/s12263-015-0461-7
Ji, H., Friedman, M. I. (2021). Reduced hepatocyte fatty acid oxidation in outbred rats prescreened for susceptibility to diet-induced obesity. Int. J. Obes. (Lond). 32, 1331–1334. doi: 10.1038/ijo.2008.71
Jung, K. I., McKenna, S., Vijayamahantesh, V., He, Y., Hahm, B. (2023). Protective versus Pathogenic Type I Interferon Responses during Virus Infections. Viruses. 15, 1916. doi: 10.3390/v15091916
Kolářová, H., Víteček, J., Černá, A., Černík, M., Přibyl, J., Skládal, P., et al. (2021). Myeloperoxidase mediated alteration of endothelial function is dependent on its cationic charge. Free Radic. Biol. Med. 162, 14–26. doi: 10.1016/j.freeradbiomed.2020.11.008
Li, H., Zhang, Y., Xie, J., Wang, C., Yi, D., Wu, T., et al. (2023). Dietary supplementation with mono-lactate glyceride enhances intestinal function of weaned piglets. Anim. (Basel) 13, 1303. doi: 10.3390/ani13081303
Li, X. Q., Zhu, Y. H., Zhang, H. F., Yue, Y., Cai, Z. X., Lu, Q. P., et al. (2012). Risks associated with high-dose Lactobacillus rhamnosus in an Escherichia coli model of piglet diarrhea: intestinal microbiota and immune imbalances. PloS One 7, e40666. doi: 10.1371/journal.pone.0040666
Lin, A., Yan, X., Xu, R., Wang, H., Su, Y., Zhu, W. (2023). Effects of lactic acid bacteria-fermented formula milk supplementation on colonic microbiota and mucosal transcriptome profile of weaned piglets. Animal. 17, 100959. doi: 10.1016/j.animal.2023.100959
Liu, Y., Chen, F., Odle, J., Lin, X., Jacobi, S. K., Zhu, H. (2012). Fish oil enhances intestinal integrity and inhibits TLR4 and NOD2 signaling pathways in weaned pigs after LPS challenge. J. Nutr. 142, 2017–2024. doi: 10.3945/jn.112.164947
Ma, K., Bai, Y., Li, J., Ren, Z., Li, J., Zhang, J., et al. (2022). Lactobacillus rhamnosus GG ameliorates deoxynivalenol-induced kidney oxidative damage and mitochondrial injury in weaned piglets. Food Funct. 13, 3905–3916. doi: 10.1039/D2FO00185C
Madahian, S., Navab, K. D., Pourtabatabaei, N., Seyedali, S., Safar, S., Vazirian, S. (2014). Inflammation, high density lipoprotein and endothelium. Curr. Med. Chem. 21, 2902–2909. doi: 10.2174/0929867321666140414105530
Mao, X., Gu, C., Hu, H., Tang, J., Chen, D., Yu, B., et al. (2016). Dietary lactobacillus rhamnosus GG supplementation improves the mucosal barrier function in the intestine of weaned piglets challenged by porcine rotavirus. PloS One 11, e0146312. doi: 10.1371/journal.pone.0146312
Mao, J., Huang, X., Shan, Y., Xu, J., Gao, Q., Xu, X., et al. (2022). Transcriptome analysis revealed inhibition of lipid metabolism in 2-D porcine enteroids by infection with porcine epidemic diarrhea virus. Vet. Microbiol. 273, 109525. doi: 10.1016/j.vetmic.2022.109525
Peng, L., Yang, H., Ye, Y., Ma, Z., Kuhn, C., Rahmeh, M., et al. (2021). Role of peroxisome proliferator-activated receptors (PPARs) in trophoblast functions. Int. J. Mol. Sci. 22, 433. doi: 10.3390/ijms22010433
Puppel, K., Kapusta, A., Kuczyńska, B. (2015). The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 95, 2179–2184. doi: 10.1002/jsfa.7015
Qian, K., Shan, L., Shang, S., Li, T., Wang, S., Wei, M., et al. (2022). Manganese enhances macrophage defense against Mycobacterium tuberculosis via the STING-TNF signaling pathway. Int. Immunopharmacol. 113, 109471. doi: 10.1016/j.intimp.2022.109471
Shi, Y. H., Wang, J., Guo, R., Wang, C. Z., Yan, X. B., Xu, B., et al. (2014). Effects of alfalfa saponin extract on growth performance and some antioxidant indices of weaned piglets. Livest Sci. 167, 257–262. doi: 10.1016/j.livsci.2014.05.032
Shi, J., Zhao, G., Huang, X., Li, X., Ma, Y., Yang, K. (2023). Effects of lactobacillus rhamnosus supplementation on growth performance, immune function, and antioxidant capacity of newborn foals. J. Equine Vet. Sci. 129, 104501. doi: 10.1016/j.jevs.2023.104501
Shi, J., Zhao, G., Huang, X., Li, X., Ma, Y., Yang, K., et al. (2019). Effects of lactobacillus rhamnosus supplementation on growth performance, immune function, and antioxidant capacity of newborn foals. J. Equine Vet. Sci. 129, 104501. doi: 10.1016/j.jevs.2023.104501
Shonyela, S. M., Feng, B., Yang, W., Yang, G., Wang, C. (2020). The regulatory effect of Lactobacillus rhamnosus GG on T lymphocyte and the development of intestinal villi in piglets of different periods. AMB Express. 10, 76. doi: 10.1186/s13568-020-00980-1
Song, Z., Deng, C., Chen, Q., Zhao, S., Li, P., Wu, T., et al. (2024). Protective effects and mechanisms of ellagic acid on intestinal injury in piglets infected with porcine epidemic diarrhea virus. Front. Immunol. 1. doi: 10.3389/fimmu.2024.1323866
Sun, D., Wang, X., Wei, S., Chen, J., Feng, L. (2016). Epidemiology and vaccine of porcine epidemic diarrhea virus in China: a mini-review. J. Vet. Med. Sci. 78, 355–363. doi: 10.1292/jvms.15-0446
Wang, X. L., Liu, Z. Y., Li, Y. H., Yang, L. Y., Yin, J., He, J. H., et al. (2021). Effects of dietary supplementation of lactobacillus delbrueckii on gut microbiome and intestinal morphology in weaned piglets. Front. Vet. Sci. 8. doi: 10.3389/fvets.2021.692389
Wang, Q., Sun, Q., Qi, R., Wang, J., Qiu, X., Liu, Z., et al. (2019). Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 103, 1908–1918. doi: 10.1111/jpn.13198
Wu, T., Li, K., Yi, D., Wang, L., Zhao, D., Lv, Y., et al. (2018). Dietary supplementation with trihexanoin enhances intestinal function of weaned piglets. Int. J. Mol. Sci. 19, 3277. doi: 10.3390/ijms19103277
Wu, T., Shi, Y., Zhang, Y., Zhang, M., Zhang, L., Ma, Z. (2021). Lactobacillus rhamnosus LB1 Alleviates Enterotoxigenic Escherichia coli-Induced Adverse Effects in Piglets by Improving Host Immune Response and Anti-Oxidation Stress and Restoring Intestinal Integrity. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.724401
Yan, F., Liu, L., Cao, H., Moore, D. J., Washington, M. K., Wang, B., et al. (2016). Neonatal colonization of mice with LGG promotes intestinal development and decreases susceptibility to colitis in adulthood. Mucosal Immunol. 10, 117–127. doi: 10.1038/mi.2016.43
Yang, J., Qiu, Y., Hu, S., Zhu, C., Wang, L., Wen, X., et al. (2021). Lactobacillus plantarum inhibited the inflammatory response induced by enterotoxigenic Escherichia coli K88 via modulating MAPK and NF-κB signalling in intestinal porcine epithelial cells. J. Appl. Microbiol. 130, 1684–1694. doi: 10.1111/jam.14835
Yang, J., Tian, G., Chen, D., Zheng, P., Yu, J., Mao, X., et al. (2022). Dietary 25-hydroxyvitamin D3 supplementation alleviates porcine epidemic diarrhea virus infection by improving intestinal structure and immune response in weaned pigs. Anim. (Basel). 9, 627. doi: 10.3390/ani9090627
Yi, D., Hou, Y., Wang, L., Zhao, D., Ding, B., Wu, T., et al. (2016). Gene expression profiles in the intestine of lipopolysaccharide-challenged piglets. Front. Biosci. (Landmark Ed). 21, 487–501. doi: 10.2741/4404
Yi, H., Wang, L., Xiong, Y., Wen, X., Wang, Z., Yang, X., et al. (2018). Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 96, 2342–2351. doi: 10.1093/jas/sky129
Yuan, C., Zhang, P., Liu, P., Li, Y., Li, J., Zhang, E., et al. (2022). A novel pathway for porcine epidemic diarrhea virus transmission from sows to neonatal piglets mediated by colostrum. J. Virol. 96, e0047722. doi: 10.1128/jvi.00477-22
Zhang, Y., Li, Q., Wang, Z., Dong, Y., Yi, D., Wu, T., et al. (2023). Dietary supplementation with a complex of cinnamaldehyde, carvacrol, and thymol negatively affects the intestinal function in LPS-challenged piglets. Front. Vet. Sci. 10. doi: 10.3389/fvets.2023.1098579
Zhang, X., Wang, L., Chen, B. (2015). Recombinant HDL (Milano) protects endotoxin-challenged rats from multiple organ injury and dysfunction. Biol. Chem. 396, 53–60. doi: 10.1515/hsz-2014-0188
Zhang, Z., Wang, S., Zheng, L., Hou, Y., Guo, S., Wang, L., et al. (2022). Tannic acid-chelated zinc supplementation alleviates intestinal injury in piglets challenged by porcine epidemic diarrhea virus. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.1033022
Zhang, L., Xu, Y. Q., Liu, H. Y., Lai, T., Ma, J. L., Wang, J. F. (2010). Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhea: Effects on diarrhea incidence, fecal microflora and immune responses. Vet. Microbiol. 2010, 141, 142–148. doi: 10.1016/j.vetmic.2009.09.003
Zhang, J., Zhao, D., Yi, D., Wu, M., Chen, H., Wu, T., et al. (2019). Microarray analysis reveals the inhibition of intestinal expression of nutrient transporters in piglets infected with porcine epidemic diarrhea virus. Sci. Rep. 9, 19798. doi: 10.1038/s41598-019-56391-1
Keywords: Lactobacillus rhamnosus GG, PEDV, gut health, gut functions, piglets
Citation: Xu Z, Zhang Q, Wu M, Zhang Y, Li Z, Li H, Yu C, Zhang X, Zhao D, Wang L, Hou Y and Wu T (2024) Lactobacillus rhamnosus GG powder supplementation alleviates intestinal injury in piglets challenged by porcine epidemic diarrhea virus. Front. Cell. Infect. Microbiol. 14:1371916. doi: 10.3389/fcimb.2024.1371916
Received: 17 January 2024; Accepted: 28 March 2024;
Published: 23 April 2024.
Edited by:
Sara Louise Cosby, Belfast, United KingdomReviewed by:
Zhanbo Zhu, Heilongjiang Bayi Agricultural University, ChinaCopyright © 2024 Xu, Zhang, Wu, Zhang, Li, Li, Yu, Zhang, Zhao, Wang, Hou and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Wu, d3RhbzA1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.