- 1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 2Department of Pulmonary and Critical Care Medicine, The People’s Hospital of Dazu, Chongqing, China
- 3Department of Radiology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 4Department of Clinical Laboratory, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
- 5Department of Pathology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China
Background: Aeromonas dhakensis is associated with soft tissue infection, bacteremia and gastroenteritis. Involvement of respiratory system in adults is extremely rare. We report a case of fulminant pneumonia and bacteremia due to A. dhakensis in a patient without underlying diseases.
Case presentation: A 26-year-old man became ill suddenly with pneumonia after swimming in a river. Despite intensive support measures in the intensive care unit, he died 13 hours after admission and 4 days after his first symptoms. Autopsy showed abundant Gram-negative bacteria, massive inflammatory cell infiltration, edema, necrosis and hemorrhage in lung tissue. A. dhakensis was isolated from blood culture taken at admission and bronchoalveolar lavage fluid (BALF) after intubation. Moreover, A. dhakensis was also detected in lung tissue by metagenomic next-generation sequencing (mNGS) assay. The infection may have come from river water.
Conclusion: In patients who develop a fulminant pneumonia after contacting an aquatic environment, A. dhakensis should be alerted and mNGS may aid in the detection of aquatic pathogens by being more sensitive and specific versus traditional bacterial culture.
Introduction
Aeromonas dhakensis, is a facultative anaerobic Gram-negative bacillus and often isolated from aquatic environments, including rivers and lakes. It is emerging as an important human pathogen that can cause severe soft tissue infections, gastroenteritis, and fatal bloodstream infections (Shin et al., 2013; Chen et al., 2014b, 2016; Chang et al., 2018; Huang et al., 2020a). A. dhakensis (Huys et al., 2002) Beaz-Hidalgo et al., 2015, was first isolated from children with diarrhea and described as A. hydrophila subsp. dhakensis Huys et al., 2002 (Huys et al., 2002), and latter was also classified as A. aquariorum Martinez-Murcia et al., 2008 (Martinez-Murcia et al., 2008). After A. hydrophila subsp. dhakensis and A. aquariorum being confirmed as the same taxon, both of them have been reclassified as A. dhakensis sp. nov. comb nov (Beaz-Hidalgo et al., 2013, 2015). A. dhakensis bacteremia was observed to cause a higher mortality rate than non-A. dhakensis species (Chen et al., 2014b). A fatal case of A. dhakensis severe sepsis in an old man with liver cirrhosis was reported in Korea (Shin et al., 2013), two severe dengue patients with A. dhakensis bacteremia and necrotizing fasciitis passed out due to shock and multiorgan failure in southern Taiwan (Chang et al., 2018), and a young man with A. dhakensis septicemia accompanying chronic hepatitis B virus infection after the ingestion of a meal of raw snakehead fish died from multiple organ failure in Mainland China (Huang et al., 2020a). However, community-acquired bacterial pneumonia due to this organism is extremely rare, especially in a non-immunocompromised host. Here, we describe fulminant fatal pulmonary infection and bacteremia due to A. dhakensis in an immunocompetent man.
Case report
A 26-year-old man was admitted to our hospital complaining of cough, fatigue, fever, hemoptysis and dyspnea. Three days before admission, the patient became ill after swimming in a river, his condition did not improve significantly after treatment with oral cefuroxime (0.25 g, every 12h) prescribed by a local clinic. One day prior to admission, the patient’s symptoms became exacerbated and included fever, sputum expectoration, chest pain, wheezing, shortness of breath and hemoptysis. He had no gastrointestinal complaints in his course of illness. He had a five-year history of light beer consumption. He had smoked approximately 20 cigarettes per day for 4 years. He had been in good health without history of diabetes mellitus, liver cirrhosis, chronic lung disease, immunosuppression, recurrent infection, malignancies, chemotherapy, or steroid use. His vital signs were as follows: respiratory rate, 18 breaths/minute; pulse rate, 82 beats/minute and regular; blood pressure, 129/81 mmHg; and temperature, 37.5°C. His heart sound was normal. Coarse breath sounds and scattered wet rales could be auscultated in the bilateral lower lungs.
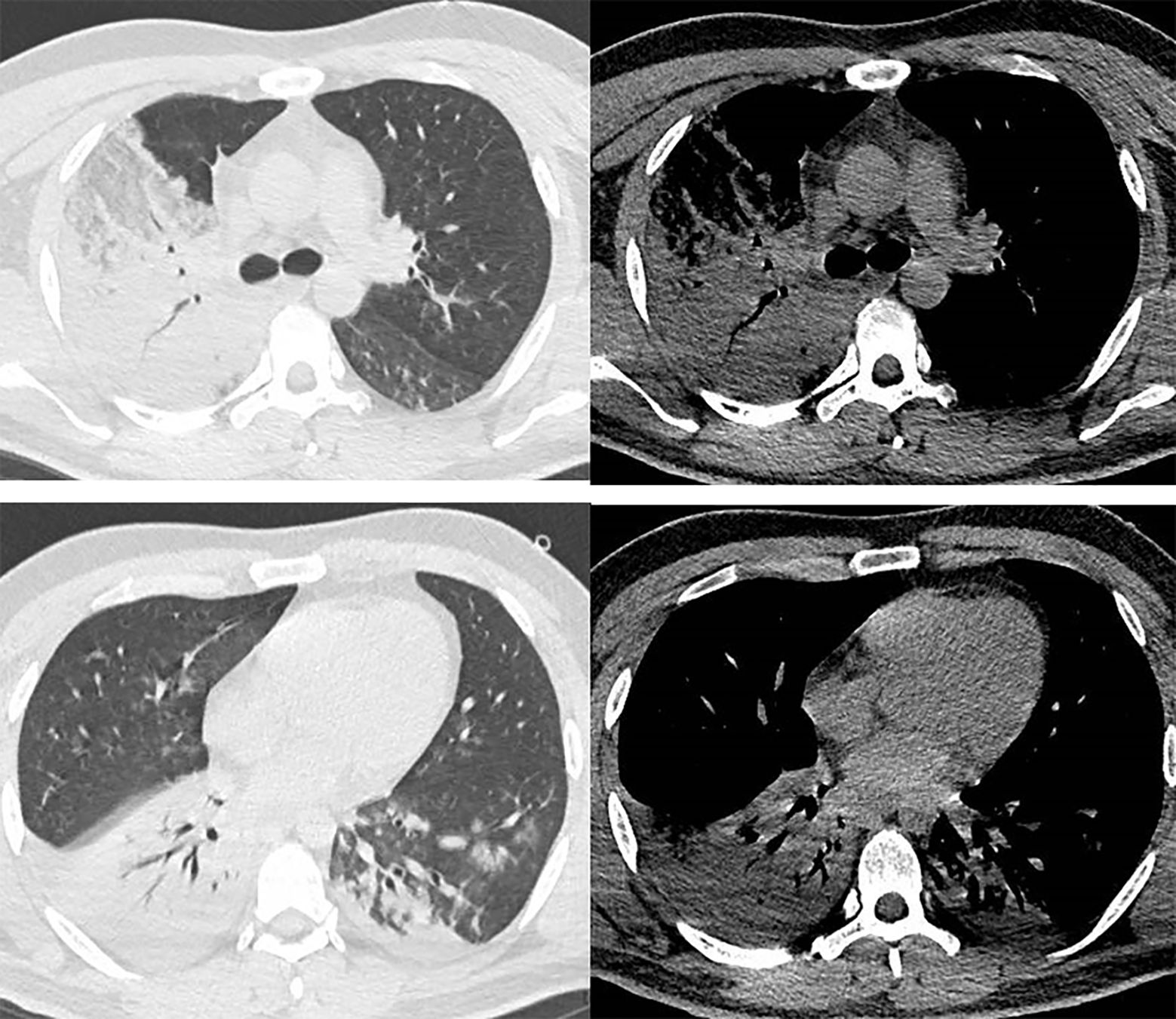
Laboratory findings on admission were as follows: white blood cell (WBC) 7060/mm3 (normal value 3500 - 9500/mm3); lymphocyte 1870/mm3 (normal value 1100 - 3200/mm3); alanine aminotransferase (ALT) 150 IU/L (normal value 13 - 69 IU/L); aspartate transferase (AST) 84 IU/L (normal value 15 - 46 IU/L); serum creatinine (Cre) 2.23 mg/dL (normal range: 0.66 -1.24 mg/dL); serum urea nitrogen (BUN) was 97.29 mg/dL (normal value 36.20 - 80.32 mg/dL); serum uric acid (UA) 6.87 mg/dL (normal value 2.35 - 5.72 mg/dL); C-reactive protein (CRP) was 8.67 mg/dL (normal value 0 - 0.8 mg/dL); Procalcitonin (PCT) 99.57 ng/mL (normal value 0 - 0.05 ng/mL); Prothrombin time international normalized ratio (PT-INR) was 1.87 (normal value 0 80 - 1.2); and D-dimer was 10.48 μg/mL (normal value 0 - 0.55 μg/mL). HIV antibody and rheumatological work-up were negative. Hepatitis B virus, hepatitis C virus infection and live cirrhosis were not detected. Arterial blood gas analysis revealed pH 7.32, PaCO2 43 mmHg, PaO2 37 mmHg, HCO3- 22.21 mmol/L, Lac 4.8 mmol/L, SaO2 65% with the patient breathing room air. Chest computed tomography(CT)scan on arrival at our hospital revealed consolidation in his right lung and an infiltrating shadow in his left lower lobe (Figure 1).
Non-invasive ventilation was begun and empirical antimicrobial treatment with imipenem-cilastatin (0.5 g: 0.5 g, every 8 h) and linezolid(0.6 g, every 12h)was intravenously administered 1 hour after his arrival. Two sets of blood cultures were obtained at the same time. However, his arterial blood gas tensions deteriorated rapidly. 3 hours later, he was intubated for invasive ventilation. Massive bloody secretions were suctioned from the endotracheal tube. Bronchoscopy demonstrated diffuse alveolar hemorrhage (DAH) and bronchoalveolar lavage fluid (BALF) was obtained for pathogen culture. The patient went into severe acute respiratory distress syndrome (ARDS), DAH and shock quickly. An extracorporeal membrane oxygenation (ECMO) evaluation was requested for severe ARDS and fast-deteriorating hypoxemia, and, given the likely reversible nature of his pulmonary disease and hypoxemia. However, his family refused to initiate ECMO support due to huge expense. Despite intensive supportive efforts, he eventually died 13 hours after admission.
We sought to further confirm the diagnosis after death, a percutaneous lung biopsy was performed to obtain lung tissue samples for pathological examination and metagenome next-generation sequencing (mNGS) assay performed on a BGISEQ-500 platform (Beijing Genomics Institute, Wuhan, China). Microscopically lung tissue was infiltrated with leucocytes and the alveoli were flooded with fluid and erythrocytes. Zones of edema, necrosis and hemorrhage were present (Figure 2A). Numerous small Gram-negative bacteria were seen with inflammatory cells (Figure 2B). The BALF and two sets of blood cultures all yielded Aeromonas hydrophila, identified by VITEK® MS MALDI-TOF system (BioMeírieux, Marcy I’ Etoile, France) but re-identified as A. dhakensis (accession number: GCF_905132925.1) by housekeeping gene sequencing (rpoD and gyrB) as described in the previous study (Zhang et al., 2023). mNGS of the lung puncture tissue also suggested A. dhakensis. A total of 100226 unique DNA reads and 103244 RNA reads mapping of the A. dhakensis genome were reported. The raw mNGS sequence results have been uploaded to the NCBI data (accession number: PRJNA1120325). To confirm the strain’s identification as A. dhakensis, the sequences were aligned with the reference genome of A. dhakensis (accession number: GCF_905132925.1), as detailed in Supplementary Material 1. The MUMmer analysis showed a red line in the forward direction and a blue line in the reverse direction, indicating that the genomes were mostly co-linear (Figure 3). A substantial similarity of 80% between the two was noted. The test for A. dhakensis susceptibility to various antimicrobial agents was performed by the microbroth dilution method according to Clinical and Laboratory Standards Institute (CLSI) 2015 guidelines (CLSI, 2015), which revealed that A. dhakensis was resistant to trimethoprim-sulfamethoxazole and cefoxitin, and sensitive to piperacillin tazobactam, imipenem, meropenem, levofloxacin, ciprofloxacin, cefuroxime, cefepime, ceftriaxone, cefoperazone sulbactam, ceftazidime and amikacin.
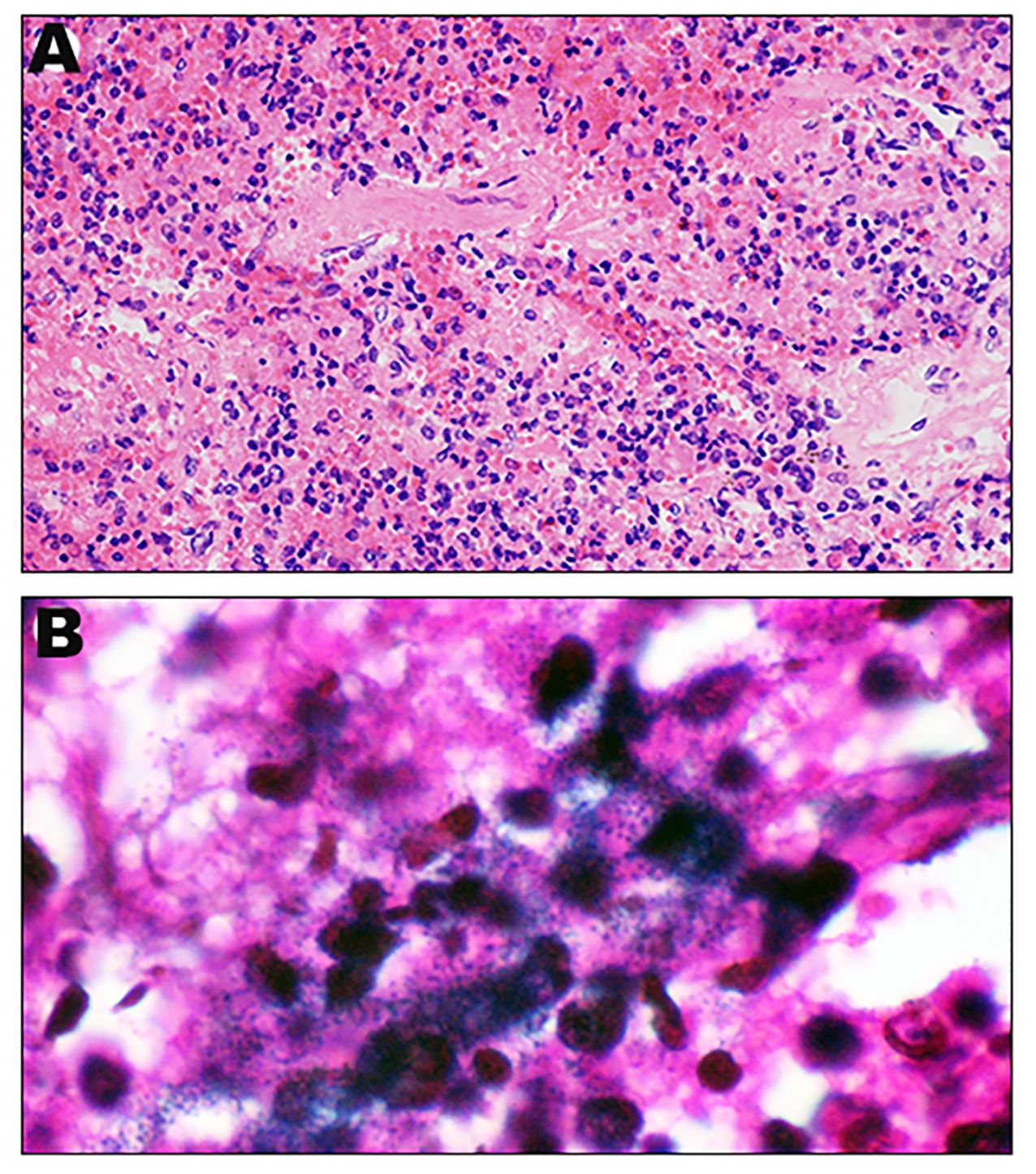
Figure 2 Histopathological appearance of lung: Representative H&E sections of lung ( 400) (A). Representative Gram stain sections of lung (
400) (A). Representative Gram stain sections of lung ( 1000) (B).
1000) (B).
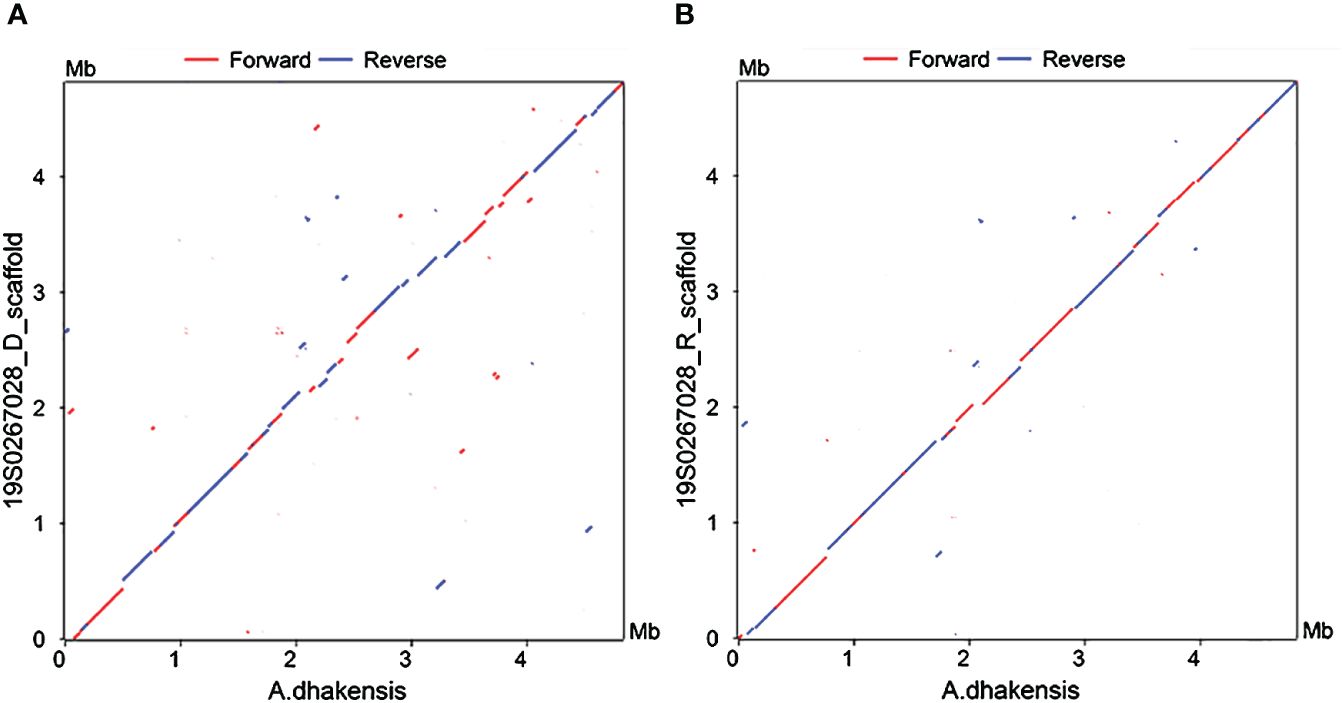
Figure 3 Nucleotide-based alignments with NUCmer. X-axis: reference genome of A dhakensis. Y-axis: (A) 19S0267028_D and (B) 19S0267028_R. Aligned segments are presented as dots or lines in the NUCmer alignment and were generated by the MUMmer plot script.
Discussion and conclusions
This is a rare report of fatal hemorrhagic pneumonia and bacteremia due to A. dhakensis in a fit young man. Spontaneous bacterial peritonitis, soft-tissue infection, biliary tract infection and primary bacteremia are major origins of A. dhakensis bacteremia (Kitagawa et al., 2020). Pneumonia as a source of A. dhakensis bacteremia has been mentioned in an earlier study (Wu et al., 2015). It also has been reported that A. dhakensis can be recovered from the respiratory tract or lung with a low rate (Fernandez-Bravo and Figueras, 2020; Puah et al., 2022). Here, pneumonia may well be the source of A. dhakensis bacteremia, because it was found that massive bacteria were scattered in lung tissue and isolated from both bronchial secretion and blood in this case.
Aeromonas bacteremia usually occurs in patients with weakened immune system and underlying conditions including liver cirrhosis, diabetes mellitus, malignancy etc (Wu et al., 2015). However, no predisposing underlying diseases were identified in this patient. Despite immediate and intensive therapy with antibiotics, the patient’s medical condition acutely deteriorated and the patient died. Fulminant pneumonia due to A. dhakensis after swimming showed a too rapid fatal clinical course for the therapy to be effective. Due to the unknown etiology, infection severity, sepsis and rapid clinical decline, a broad-spectrum antibiotic (imipenem) and an antibiotic with methicillin resistant staph aureus coverage were used in our patient initially. However, volume of distribution (Vd) of imipenem could change according to the severity of illness, inflammation, and shock. The high Vd of imipenem may lead to subtherapeutic concentrations among sepsis or septic shock patients and further influence their prognosis (Huang et al., 2020c). A high loading dose of imipenem should be considered in the treatment of sepsis or septic shock (Huang et al., 2020b). In addition, a high prevalence of carbapenem resistance in A. dhakensis clinical isolates has been reported (Puah et al., 2022). Monotherapy with a routine dose of imipenem may not be an appropriate treatment choice for this case with high A. dhakensis burden in lung tissue and blood. However, the antimicrobial susceptibility tests performed on the A. dhakensis strain after the patient’s death showed susceptibility to both imipenem and meropenem. Aeromonas spp. exhibited best susceptibility to aminoglycosides (Sun et al., 2021), suggesting that aminoglycosides might be recommended for initial therapy of Aeromonas-associated bacteremia. Therefore, a higher dose of imipenem plus aminoglycosides might be required in A. dhakensis pneumonia and bacteremia empirically.
ECMO can provide temporary pulmonary assistance to prolong the time frame for diagnosis and specific treatment in severe ARDS patients (Resch et al., 2009). It has also been reported that ECMO was feasible in ARDS along with DAH (Abrams et al., 2015; Zhang and Yu, 2022). VV-ECMO as a bridging strategy to pulmonary recovery was successfully used in a trauma patient with fulminant A. hydrophila pneumonia and ARDS (Issa and Napolitano, 2011). Thus, ECMO may be effective to provide oxygenation support, ensure sufficient time to initiate treatment measures and allow for further therapy to take effect in this patient. When invasive mechanical ventilation fails to maintain adequate blood oxygen levels, ECMO should be initiated immediately, especially in the patients with reversible and fulminant severe pneumonia. Meanwhile, hemorrhagic complications and aggravated DAH should be avoided by low-level, delayed, or even no systemic anticoagulation.
The majority of Aeromonas pneumonia cases has been reported after freshwater or saltwater near drowning (Reines and Cook, 1981; Goncalves et al., 1992; Ender et al., 1996; Ender and Dolan, 1997; Miyake et al., 2000; Cousin and Pittet, 2024). A. hydrophila was the most common aeromonad reported to cause pneumonia in drowning patients previously (Ender and Dolan, 1997). Aeromonas pneumonia is possibly due to the aspiration of a large amount of water containing a high concentration of bacteria that can quickly lead to hemorrhagic pneumonia. Therefore, the most important relevant factor in this case may be his recent swim in the river. In the environment, A. dhakensis has been recovered from river water, cooling-system water pond, fish tank water and fish (Chen et al., 2016; Huang et al., 2020a). Oral ingestion or abraded open wounds can serve as the portals of entry of A. dhakensis infection (Janda and Abbott, 2010). In immunocompetent hosts, respiratory infection due to this pathogen may occur by ingestion or even aspiration of contaminated water as documented in previous studies (Miyake et al., 2000; Mukhopadhyay et al., 2003). In addition, predisposing factors for Aeromonas pneumonia involve alcohol and cigarette consumption (Baddour and Baselski, 1988) and our patient consumed alcohol regularly and smoked 20 cigarettes per day. His bad habits may leave him vulnerable to A. dhakensis infection.
According to the literature, A. dhakensis bacteremia is more lethal than bacteremia due to other Aeromonas species (Wu et al., 2015; Chen et al., 2016; Zhang et al., 2023). Moreover, A. dhakensis is more virulent than A. hydrophila and an independent mortality risk factor in monomicrobial Aeromonas bacteremia (Wu et al., 2015). In the present study, the patient died from respiratory failure and septic shock 13 hours postadmission despite all intensive supportive efforts. The clinical features were hemoptysis, sepsis, progressive respiratory failure and high mortality rate. Histopathological examination showed severe hemorrhagic pneumonia with necrosis and numerous small gram-negative bacteria with a remarkable inflammatory cellular reaction, which may suggest the virulence of this pathogen and could explain the rapid clinical course. The pathogenesis of such an extremely rapid clinical course remains to be further clarified.
Pneumonia with A. dhakensis might be underreported, underdiagnosed, or misdiagnosed. Many similarities in the clinical features, predisposing factors and pathological manifestations exist between A. dhakensis pneumonia and A. hydrophila pneumonia. A. dhakensis is often misidentified as A. hydrophila, A. veronii, or A. caviae by commercial phenotypic tests in the clinical settings (Wu et al., 2015), which may bring about misdiagnosis and non-effective treatment. Corrective identification of the pathogen may be of great importance for the successful treatment of the patients with A. dhakensis infection, which relies on molecular identification with the sequences of housekeeping genes (Soler et al., 2004). In this patient, A. dhakensis in the blood was misidentified as A. hydrophila, or A. caviae by VITEK MALDI-TOF system, while it was identified by mNGS assay or rpoD sequencing correctly. Therefore, laboratories should be alerted to the possibility that A. dhakensis could be a potentially pathogenic bacterium with important antimicrobial resistances (Chen et al., 2014a, 2016; Puah et al., 2022). It has been reported that A. dhakensis could be accurately and efficiently identified by MALDI-TOF MS (Chen et al., 2014a; Fernandez-Bravo and Figueras, 2020). However, A. dhakensis is not included in the commercial database of MALDI-TOF system. Once A. dhakensis is added to the commercial MALDI-TOF database, it may be not difficult to accurately identify it (Fernandez-Bravo and Figueras, 2020; Kitagawa et al., 2020). Meanwhile, mNGS is also an effective tool for the detection of A. dhakensis.
In summary, although A. dhakensis rarely causes pneumonia, it does occur as a fulminant type of pneumonia occasionally in patients with or without underlying conditions. Clinicians should be rapidly aware of and accurately identify this rare organism, and select proper antibiotics with adequate doses in the treatment of A. dhakensis pneumonia and bacteremia. ECMO may be required in the rescue treatment of fulminant fatal pneumonia with DAH.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJNA1120325.
Ethics statement
The studies involving humans were approved by the Ethical Committee of the First Affiliated Hospital of Chongqing Medical Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
LJ: Conceptualization, Data curation, Funding acquisition, Writing – original draft. QZ: Data curation, Writing – review & editing. DL: Data curation, Methodology, Writing – review & editing. JG: Data curation, Writing – review & editing. XZ: Data curation, Methodology, Writing – review & editing. QS: Methodology, Writing – review & editing. XH: Conceptualization, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the In-hospital Cultivation Fund of the First Affiliated Hospital of Chongqing Medical University (Grant No. PYJJ2019-207, YEZX_27). The funding bodies had no role in the study design, data collection and analysis, interpretation of data, and writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1359422/full#supplementary-material
Abbreviations
A. dhakensis, Aeromonas dhakensis; BALF, bronchoalveolar lavage fluid; mNGS, metagenomic next-generation sequencing; CT, chest computed tomography; ARDS, acute respiratory distress syndrome; ECMO, extracorporeal membrane oxygenation; DAH, diffuse alveolar hemorrhage.
References
Abrams, D., Agerstrand, C. L., Biscotti, M., Burkart, K. M., Bacchetta, M., Brodie, D. (2015). Extracorporeal membrane oxygenation in the management of diffuse alveolar hemorrhage. ASAIO J. 61, 216–218. doi: 10.1097/MAT.0000000000000183
Baddour, L. M., Baselski, V. S. (1988). Pneumonia due to Aeromonas hydrophila-complex: epidemiologic, clinical, and microbiologic features. South Med. J. 81, 461–463. doi: 10.1097/00007611-198804000-00013
Beaz-Hidalgo, R., Latif-Eugenin, F., Hossain, M. J., Berg, K., Niemi, R. M., Rapala, J., et al. (2015). Aeromonas aquatica sp. nov., Aeromonas Finlandiensis sp. nov. and Aeromonas lacus sp. nov. isolated from Finnish waters associated with cyanobacterial blooms. Syst. Appl. Microbiol. 38, 161–168, . doi: 10.1016/j.syapm.2015.02.005
Beaz-Hidalgo, R., Martinez-Murcia, A., Figueras, M. J. (2013). Reclassification of Aeromonas hydrophila subsp. dhakensis Huys et al. 2002 and Aeromonas aquariorum Martinez-Murcia et al. 2008 as Aeromonas dhakensis sp. nov. comb nov. and emendation of the species Aeromonas hydrophila. Syst. Appl. Microbiol. 36, 171–176. doi: 10.1016/j.syapm.2012.12.007
Chang, H. L., Chen, P. L., Lin, S. Y., Chen, T. C., Chang, K., Ko, W. C., et al. (2018). Two fatal cases of Aeromonas dhakensis bacteremia and necrotizing fasciitis in severe dengue patients. J. Microbiol. Immunol. Infect. 51, 692–694. doi: 10.1016/j.jmii.2018.03.003
Chen, P. L., Lamy, B., Ko, W. C. (2016). Aeromonas dhakensis, an increasingly recognized human pathogen. Front. Microbiol. 7, 793. doi: 10.3389/fmicb.2016.00793
Chen, P. L., Lee, T. F., Wu, C. J., Teng, S. H., Teng, L. J., Ko, W. C., et al. (2014a). Matrix-assisted laser desorption ionization-time of flight mass spectrometry can accurately differentiate Aeromonas dhakensis from A. hydrophila, A. caviae, and A. veronii. J. Clin. Microbiol. 52, 2625–2628. doi: 10.1128/JCM.01025-14
Chen, P. L., Wu, C. J., Chen, C. S., Tsai, P. J., Tang, H. J., Ko, W. C. (2014b). A comparative study of clinical Aeromonas dhakensis and Aeromonas hydrophila isolates in southern Taiwan: A. dhakensis is more predominant and virulent. Clin. Microbiol. Infect. 20, O428–O434. doi: 10.1111/1469-0691.12456
CLSI (2015). Methods for antimicrobial dilution. and disk susceptibility testing of infrequent isolated or fastidious bacteria. 3rd Edition (Wayne, PA: CLSI).
Cousin, V. L., Pittet, L. F. (2024). Microbiological features of drowning-associated pneumonia: a systematic review and meta-analysis. Ann. Intensive Care 14, 61. doi: 10.1186/s13613-024-01287-1
Ender, P. T., Dolan, M. J. (1997). Pneumonia associated with near-drowning. Clin. Infect. Dis. 25, 896–907. doi: 10.1086/515532
Ender, P. T., Dolan, M. J., Dolan, D., Farmer, J. C., Melcher, G. P. (1996). Near-drowning-associated Aeromonas pneumonia. J. Emerg. Med. 14, 737–741. doi: 10.1016/S0736-4679(96)00183-7
Fernandez-Bravo, A., Figueras, M. J. (2020). An update on the genus Aeromonas: taxonomy, epidemiology, and pathogenicity. Microorganisms 8, 129. doi: 10.3390/microorganisms8010129
Goncalves, J. R., Brum, G., Fernandes, A., Biscaia, I., Correia, M. J., Bastardo, J. (1992). Aeromonas hydrophila fulminant pneumonia in a fit young man. Thorax 47, 482–483. doi: 10.1136/thx.47.6.482
Huang, M., Chen, H., Li, C., Liu, Y., Gan, C., El-Sayed Ahmed, M. A. E., et al. (2020a). Rapid fulminant progression and mortality secondary to Aeromonas dhakensis septicemia with hepatitis B virus infection following the ingestion of snakehead fish in Mainland China: A Case Report. Foodborne Pathog. Dis. 17, 743–749. doi: 10.1089/fpd.2019.2780
Huang, Y., Xu, K., Zhan, Y., Zha, X., Liu, S., Xie, J., et al. (2020b). Comparable effect of two-step versus extended infusions on the pharmacokinetics of imipenem in patients with sepsis and septic shock. Adv. Ther. 37, 2246–2255. doi: 10.1007/s12325-020-01339-5
Huang, Y., Yang, J., Xie, J., Liu, L., Liu, S., Guo, F., et al. (2020c). Association between pathophysiology and volume of distribution among patients with sepsis or septic shock treated with imipenem: A prospective cohort study. J. Infect. Dis. 221, S272–S278. doi: 10.1093/infdis/jiz651
Huys, G., Kampfer, P., Albert, M. J., Kuhn, I., Denys, R., Swings, J. (2002). Aeromonas hydrophila subsp. dhakensis subsp. nov., isolated from children with diarrhoea in Bangladesh, and extended description of Aeromonas hydrophila subsp. hydrophila (Chester 1901) Stanier 1943 (approved lists 1980). Int. J. Syst. Evol. Microbiol. 52, 705–712. doi: 10.1099/00207713–52-3–705
Issa, N., Napolitano, L. M. (2011). Aeromonas pneumonia in a trauma patient requiring extracorporeal membrane oxygenation for severe acute respiratory distress syndrome: case report and literature review. Surg. Infect. (Larchmt). 12, 241–245. doi: 10.1089/sur.2010.037
Janda, J. M., Abbott, S. L. (2010). The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 23, 35–73. doi: 10.1128/CMR.00039-09
Kitagawa, H., Ohge, H., Yu, L., Kayama, S., Hara, T., Kashiyama, S., et al. (2020). Aeromonas dhakensis is not a rare cause of Aeromonas bacteremia in Hiroshima, Japan. J. Infect. Chemother. 26, 316–320.doi:10.1016/j.jiac.2019.08.020. doi: 10.1016/j.jiac.2019.08.020
Martinez-Murcia, A. J., Saavedra, M. J., Mota, V. R., Maier, T., Stackebrandt, E., Cousin, S. (2008). Aeromonas aquariorum sp. nov., isolated from aquaria of ornamental fish. Int. J. Syst. Evol. Microbiol. 58, 1169–1175. doi: 10.1099/ijs.0.65352-0
Miyake, M., Iga, K., Izumi, C., Miyagawa, A., Kobashi, Y., Konishi, T. (2000). Rapidly progressive pneumonia due to Aeromonas hydrophila shortly after near-drowning. Intern. Med. 39, 1128–1130. doi: 10.2169/internalmedicine.39.1128
Mukhopadhyay, C., Bhargava, A., Ayyagari, A. (2003). Aeromonas hydrophila and aspiration pneumonia: a diverse presentation. Yonsei Med. J. 44, 1087–1090. doi: 10.3349/ymj.2003.44.6.1087
Puah, S. M., Khor, W. C., Aung, K. T., Lau, T. T. V., Puthucheary, S. D., Chua, K. H. (2022). Aeromonas dhakensis: clinical isolates with high carbapenem resistance. Pathogens 11, 833. doi: 10.3390/pathogens11080833
Reines, H. D., Cook, F. V. (1981). Pneumonia and bacteremia due to Aeromonas hydrophila. Chest 80, 264–267. doi: 10.1378/chest.80.3.264
Resch, M., Kurz, K., Schneider-Brachert, W., Tintelnot, K., Birner, C., Schichtl, T., et al. (2009). Extracorporeal membrane oxygenation (ECMO) for severe acute respiratory distress syndrome (ARDS) in fulminant blastomycosis in Germany. BMJ Case Rep. 2009, bcr07.2008.0392. doi: 10.1136/bcr.07.2008.0392
Shin, G. W., You, M. J., Cho, H. S., Yi, S. W., Lee, C. S. (2013). Severe sepsis due to Aeromonas aquariorum in a patient with liver cirrhosis. Jpn J. Infect. Dis. 66, 519–522. doi: 10.7883/yoken.66.519
Soler, L., Yanez, M. A., Chacon, M. R., Aguilera-Arreola, M. G., Catalan, V., Figueras, M. J., et al. (2004). Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int. J. Syst. Evol. Microbiol. 54, 1511–1519. doi: 10.1099/ijs.0.03048-0
Sun, Y., Zhao, Y., Xu, W., Fang, R., Wu, Q., He, H., et al. (2021). Taxonomy, virulence determinants and antimicrobial susceptibility of Aeromonas spp. isolated from bacteremia in southeastern China. Antimicrob. Resist. Infect. Control. 10, 43. doi: 10.1186/s13756–021-00911–0
Wu, C. J., Chen, P. L., Hsueh, P. R., Chang, M. C., Tsai, P. J., Shih, H. I., et al. (2015). Clinical implications of species identification in monomicrobial Aeromonas bacteremia. PloS One 10, e0117821. doi: 10.1371/journal.pone.0117821
Zhang, D., Li, W., Hu, X., Huang, H., Zhang, X. (2023). Accurate identification and virulence detection of Aeromonas: a single-center retrospective study on the clinical features and outcomes associated with Aeromonas bacteremia in Southwestern China. Jpn J. Infect. Dis. 76, 7–13. doi: 10.7883/yoken.JJID.2022.101
Keywords: Aeromonas dhakensis, pneumonia, bacteremia, metagenomic next-generation sequencing, housekeeping gene sequencing
Citation: Jiang L, Zhao Q, Li D, Gao J, Zhang X, Shu Q and Han X (2024) Fulminant fatal pneumonia and bacteremia due to Aeromonas dhakensis in an immunocompetent man: a case report and literature review. Front. Cell. Infect. Microbiol. 14:1359422. doi: 10.3389/fcimb.2024.1359422
Received: 21 December 2023; Accepted: 13 June 2024;
Published: 15 July 2024.
Edited by:
Maria Jose Figueras, University of Rovira i Virgili, SpainReviewed by:
Apichai Tuanyok, University of Florida, United StatesJ. Michael Janda, Kern County Public Health Laboratory, United States
Copyright © 2024 Jiang, Zhao, Li, Gao, Zhang, Shu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Han, aHhsMDkzQDE2My5jb20=
 Lei Jiang1
Lei Jiang1 Xiaoli Han
Xiaoli Han