- 1Department of Microbiology & Microbial Biotechnology, Faculty of Life Sciences and Biotechnology, Shahid Beheshti University, Tehran, Iran
- 2Department of Virology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 3Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh, Assam, India
- 4Vista Aria Rena Gene Inc., Gorgan, Golestan, Iran
Flavonoids, a diverse group of polyphenolic compounds found in various plant-based foods, have garnered attention for their potential in combating Hepatitis B Virus (HBV) infection. Flavonoids have demonstrated promising anti-HBV activities by interfering with multiple stages of the HBV life cycle, making them promising candidates for novel antiviral agents. Certain plant families, such as Theaceae, Asteraceae, Lamiaceae, and Gentianaceae, are of particular interest for their flavonoid-rich members with anti-HBV activities. Evidences, both in vitro and in vivo, supports the anti-HBV potential of flavonoids. These subsets of compound exert their anti-HBV effects through various mechanisms, including inhibiting viral entry, disrupting viral replication, modulating transcription factors, enhancing the immune response, and inducing autophagy. The antioxidant properties of flavonoids play a crucial role in modulating oxidative stress associated with HBV infection. Several flavonoids like epigallocatechin gallate (EGCG), proanthocyanidin (PAC), hexamethoxyflavone, wogonin, and baicalin have shown significant anti-HBV potential, holding promise as therapeutic agents. Synergistic effects between flavonoids and existing antiviral therapies offer a promising approach to enhance antiviral efficacy and reduce drug resistance. Challenges, including limited bioavailability, translation from preclinical studies to clinical practice, and understanding precise targets, need to be addressed. Future research should focus on clinical trials, combination therapies, and the development of flavonoid derivatives with improved bioavailability, and optimizing their effectiveness in managing chronic HBV infections.
Introduction
Infection with the Hepatitis B Virus (HBV) is a major global health concern with far-reaching consequences. It is a huge public health concern because it causes acute and chronic liver damage and is the root cause of hepatocellular carcinoma (HCC), one of the deadliest cancers in the world (Mohebbi et al., 2016, 2018). HBV is a partly double-stranded DNA virus that is spread through contact with contaminated blood and other bodily fluids, making it a highly contagious infection. More than 2 billion individuals globally are anticipated to have been exposed to HBV, with 300 million people living with chronic HBV (CHB) infections by 2023 (Jeng et al., 2023). Chronic HBV infection has a global impact, contributing to a significant morbidity and mortality burden. It is a leading cause of liver cirrhosis and death from liver disease (Martyn et al., 2023; Wang et al., 2023). Notably, HBV is responsible for roughly 887,000 fatalities per year, primarily owing to cirrhosis and HCC, making it a serious concern for world health. The Western Pacific and African areas experience the largest proportion of HBV-related mortality (Hyun Kim and Ray Kim, 2018; Amponsah-Dacosta, 2021; Hsu et al., 2023). This scenario is further complicated by the prevalence of perinatal and vertical HBV transmission from infected mothers to their children, which maintains the infection throughout generations (di Filippo Villa and Navas, 2023; Naderi et al., 2023a, 2023b, 2023c). This is especially troublesome in areas with high incidence and low immunization coverage (Al-Amleh, 2020).
The limitations of conventional antiviral therapy in dealing with HBV is becoming increasingly clear. While therapeutics are available for managing HBV, such as nucleos(t)ide analogues like lamivudine, adefovir, telbivudine, tenofovir, and entecavir, they may require long-term usage and may give rise to the development of drug-resistant viral strains (Rezanezhadi et al., 2019; Mohebbi et al., 2021). In this scenario, search for new potential antiviral agents has focused on natural chemicals (Mohebbi et al., 2022, 2023). Plant-derived compounds have a long history of medicinal and therapeutic use, and up-to-date scientific studies have begun to uncover their antiviral properties. These compounds offer several benefits, including a wide range of structural diversity, well-established safety profiles, and the capacity to target different phases of the viral lifecycle, limiting the possibility of developing resistance (Behl et al., 2021; Abookleesh et al., 2022).
Flavonoids represent a diverse group of polyphenolic compounds found in various plant-based foods, including fruits, grains, vegetables, and beverages. These compounds have been of particular interest due to their well-documented antioxidant, anti-inflammatory, and immunomodulatory properties (Panche et al., 2016; Dias et al., 2021; Bié et al., 2023). Their diverse chemical structures provide them exceptional versatility and the ability to interact with different targets in the viral lifecycle. Flavonoids have been found to interfere with several phases of the viral lifecycle, including viral entrance (Tsukuda et al., 2017), replication (Xu et al., 2020), and assembly (Ninfali et al., 2020). Their mechanisms of action are often multifaceted, making them intriguing candidates for antiviral therapy. Given the global impact of HBV infection and the need for alternative antiviral strategies, this comprehensive review explores the potential of flavonoids as agents to interfere HBV replication and, potentially, alleviate the associated complications. Furthermore, this review study describes the types and sources of flavonoids, the mechanism(s) by which they exert their antiviral activities, and the experimental and clinical data that supports their prospective use as anti-HBV candidate(s).
Main text
Search strategy
The search methodology used in this study included a thorough evaluation of the existing literature on flavonoids that are effective agents against HBV infection. A systematic search approach was executed across several databases, including Google Scholar, PubMed, Scopus, and Web of Science, using appropriate terms, including “flavonoids,” “HBV” or “Hepatitis B virus” and “antiviral.” Articles, reviews, and research published prior to the date of the search were evaluated for inclusion. The inclusion criteria were studies that investigated the effect(s) of flavonoids on HBV at the molecular, cellular, animal model, and clinical trial levels. Exclusion criteria included non-English research, studies unrelated to flavonoids or HBV, and studies with insufficient relevance to the subject of this study. No precise publication date was used, and studies with related information were retrieved. Following the selection of relevant literature, data on biological activities of flavonoids, modes of action, chemical origin, and any related experimental or clinical evidence were extracted. The chemical structures of anti-HBV flavonoids were obtained from original research publications and databases.
Flavonoids: types and sources
Flavonoids are a diverse group of polyphenolic compounds found in a wide range of plant-based foods. They are characterized by a common structure composed of two aromatic rings (A and B) connected by a three-carbon chain that forms an oxygenated heterocycle (C ring) (Dias et al., 2021). This structural diversity has led to the classification of flavonoids into several distinct subclasses, including flavones, flavonols, flavanones, isoflavonoids, anthocyanins, and Chalcones (Panche et al., 2016; Chen et al., 2023).
Citrus fruits, particularly oranges and grapefruits, are rich sources of flavanones. Naringenin and hesperetin are well-known flavanones, and they have been associated with antioxidant and anti-inflammatory properties (Alam et al., 2014; Khan et al., 2020). Flavan-3-ols, also known as flavanols, feature a double bond between C2 and C3. These compounds are prevalent in various plant-derived foods, particularly in fruits like apples, apricots, and cherries, and in beverages such as tea (Hollman et al., 2000; Chen et al., 2023). Catechin and epicatechin are two common flavan-3-ols known for their antioxidant and cardiovascular health-promoting effects (Bernatova, 2018). Furthermore, Flavonols are characterized by a double bond between C2 and C3 in the C ring, similar to flavan-3-ols, but with an added 3-hydroxyl group. Onions, apples, and grapes are examples of dietary sources rich in flavonols. Quercetin, a well-studied flavonol, is recognized for its antioxidant and anti-inflammatory properties (Zhang et al., 2020). In addition, Anthocyanidins are water-soluble flavonoids responsible for the vibrant red, blue, and purple colors in many fruits and vegetables. Berries, red grapes, and red cabbage are examples of foods rich in anthocyanidins (Khoo et al., 2017). Cyanidin, delphinidin, and malvidin are some prominent anthocyanidins that have demonstrated antioxidant and anti-inflammatory effects (Merecz-Sadowska et al., 2023). Flavones are another flavonoid subclass featuring a double bond between C2 and C3 in the C ring. These compounds are often found in leafy green vegetables like spinach, and in spices such as parsley and celery. Apigenin and luteolin are common flavones known for their potential anti-inflammatory and anticancer properties (Salehi et al., 2019; Do Socorro Chagas et al., 2022). Also, isoflavones, notably genistein and daidzein, are predominantly found in soy-based products such as tofu, soy milk, and tempeh. These compounds have a structure resembling 17β-estradiol, the primary female sex hormone, and are classified as phytoestrogens (Alshehri et al., 2021; Kim, 2021). Understanding the diverse classes of flavonoids and their sources is vital in harnessing the potential health benefits of these natural compounds. The presence of flavonoids in various plant-based foods underscores the importance of a balanced and colorful diet in promoting overall health and well-being. In terms of antiviral properties, these chemicals provide interesting options for explore and therapeutic development, as evidenced by their well-documented anti-HBV activities.
Plants and their flavonoid constituents with potential anti-HBV activities
The potential herbal medicine and plant-derived constituents on HBV life cycle have been studied (Mohebbi et al., 2018; Indrasetiawan et al., 2019; Roy et al., 2022). In the ongoing search of effective antiviral therapies against HBV, flavonoids have emerged as promising candidates due to their versatile antiviral properties. The understanding of the significance of plants and their families, particularly those rich in flavonoids is very important. These flavonoids have demonstrated promising anti-HBV activities in various in vitro and in vivo models. These natural substances provide a potential opportunity for the development of new antiviral treatments. This study reviews the potential of several flavonoids that have been found to interfere with the HBV life cycle at different stages. This enhances the prospect of producing innovative antivirals.
Figure 1 represent the plants and their described flavonoid constituents with active anti-HBV activities. The most frequently reported plant family was Lamiaceae, and its species contains promising flavonoids (wogonin and apigenin) and triterpenoids (ursolic acid and betulinic acid). Further reported plant families were included Theaceae, home to Camellia sinensis, which yields (-)-Epigallocatechin-3-gallate (EGCG) with potent anti-HBV activity (Huang et al., 2014a). Further exploration of this family might reveal additional members with flavonoids sharing similar properties. The Asteraceae family, represented by Dandelion (Taraxacum officinale), also contains flavonoids with anti-HBV activities (Yang et al., 2020). The Lamiaceae family, represented by Scutellaria baicalensis, has provided flavonoids like baicalin and wogonin, both having anti-HBV activities (Guo et al., 2007; Huang et al., 2000). The Gentianaceae family, hosting Swertia macrosperma and its anti-HBV swermacrolactones, holds potential for revealing novel flavonoid compounds with antiviral potential (Wang et al., 2013). The scientific examination of these plant families includes phytochemical analysis, biological tests, computational approaches, and clinical validation, all with the goal of advancing antiviral research and providing hope to individuals suffering by HBV infections. Table 1 provides details on the plants and their flavonoid compounds.
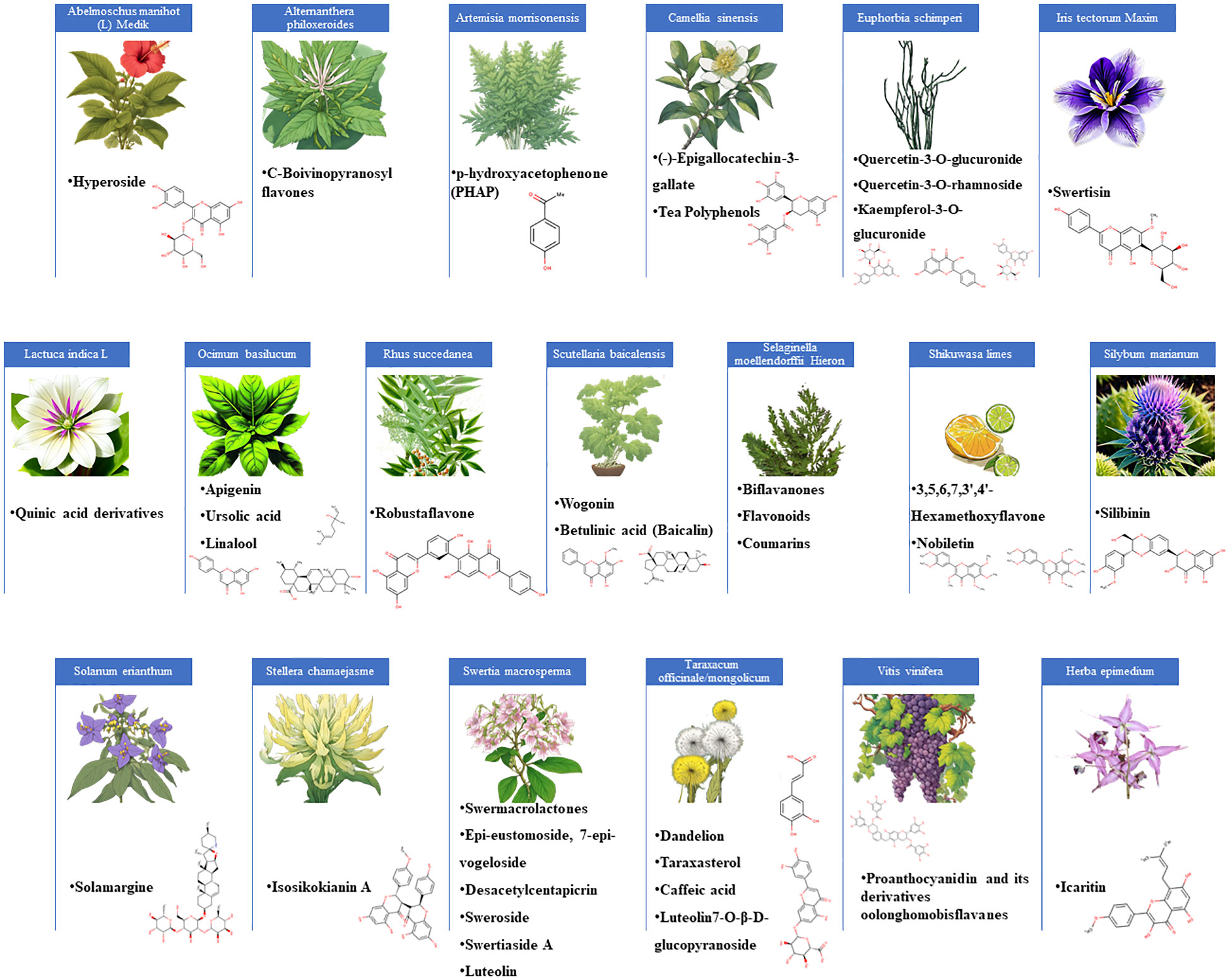
Figure 1 An illustration of various plants known for their flavonoid constituents with potent anti-HBV activities.
Mechanisms of antiviral effects of flavonoids
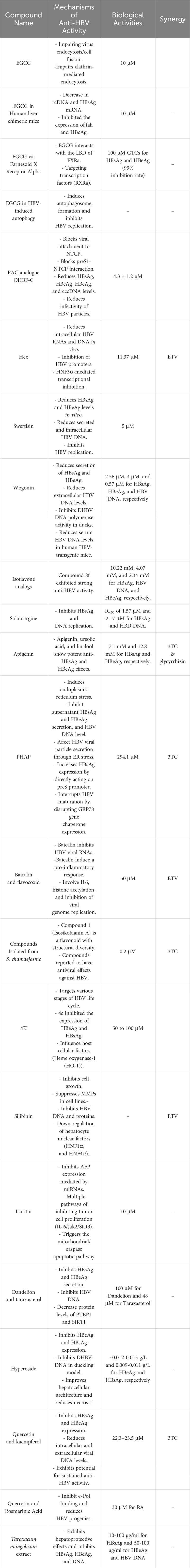
Several flavonoids with significant anti-HBV activity have been reviewed in the present study (Table 2). Compounds showed different mechanisms of action that make them potential candidates for further research and drug development. Here, the mechanisms by which flavonoids exert their anti-HBV actions are addressed. Accordingly, the biological activities of flavonoids, in vitro and in vivo data, clinical insights, synergistic effects with existing antiviral medicines, challenges, and future approaches are all reviewed.
Flavonoids exhibit a variety of antiviral mechanisms (Figure 2), which contribute to their potential against HBV. One of the key mechanisms include inhibition of viral entry. Accordingly, EGCG and proanthocyanidin (PAC) suppress HBV infection by interfering with the virus’s endocytosis and cell fusion, respectively (He et al., 2011; Tsukuda et al., 2017). They block viral attachment to specific cell surface receptors, Na+ taurocholate co-transporting polypeptide (NTCP), thereby preventing viral entry, resulting in reduction of HBV antigens and DNA. EGCG is one of the most studied flavonoids, and its anti-HBV mechanisms of action are explored thoroughly. In this context, EGCG, a green tea ingredient, suppresses HBV infection at different viral state of replication, including entry (Huang et al., 2014a), DNA synthesis (He et al., 2011), gene expression (Wang et al., 2020), and replication (He et al., 2011). It primarily hinders the entry of HBV into hepatocytes by impairing the virus’s interaction with NTCP, clathrin-mediated endocytosis, and cell fusion steps at 10 μM concentration in a dose- and time-dependent manner (Huang et al., 2014a). EGCG is more effective in inhibiting HBV infection compared to other green tea catechins. It can also inhibit HBV infection when added during the inoculation process. Furthermore, EGCG reduces HBV infection by decreasing HBV cccDNA and mRNA levels, affecting core and HBsAg protein levels (Huang et al., 2014a). It also reduces HBV DNA synthesis, but does not inhibit HBV replication, assembly, or release, suggesting its anti-HBV mechanism primarily targets the synthesis of HBV DNA through interfering viral entry. EGCG has also been found to suppress the expression of HBeAg and HBsAg, downregulate preCore mRNA levels, and inhibit HBV core promoter activity at 10 μM concentration (He et al., 2011) EGCG has been demonstrated to reduce HBsAg and HBeAg (99% inhibition rate) in a dose-dependent manner by targeting the transcription factor Farnesoid X Receptor Alpha (FXRa), indicating its potential as an anti-HBV medication (Xu et al., 2016). EGCG’s therapeutic effect on HBV infection is supported by in vivo studies (Lai et al., 2018), indicating its promise as an antiviral agent. Additionally, EGCG can induce autophagosome formation and opposes HBV-induced incomplete autophagy, further contributing to its antiviral effects.
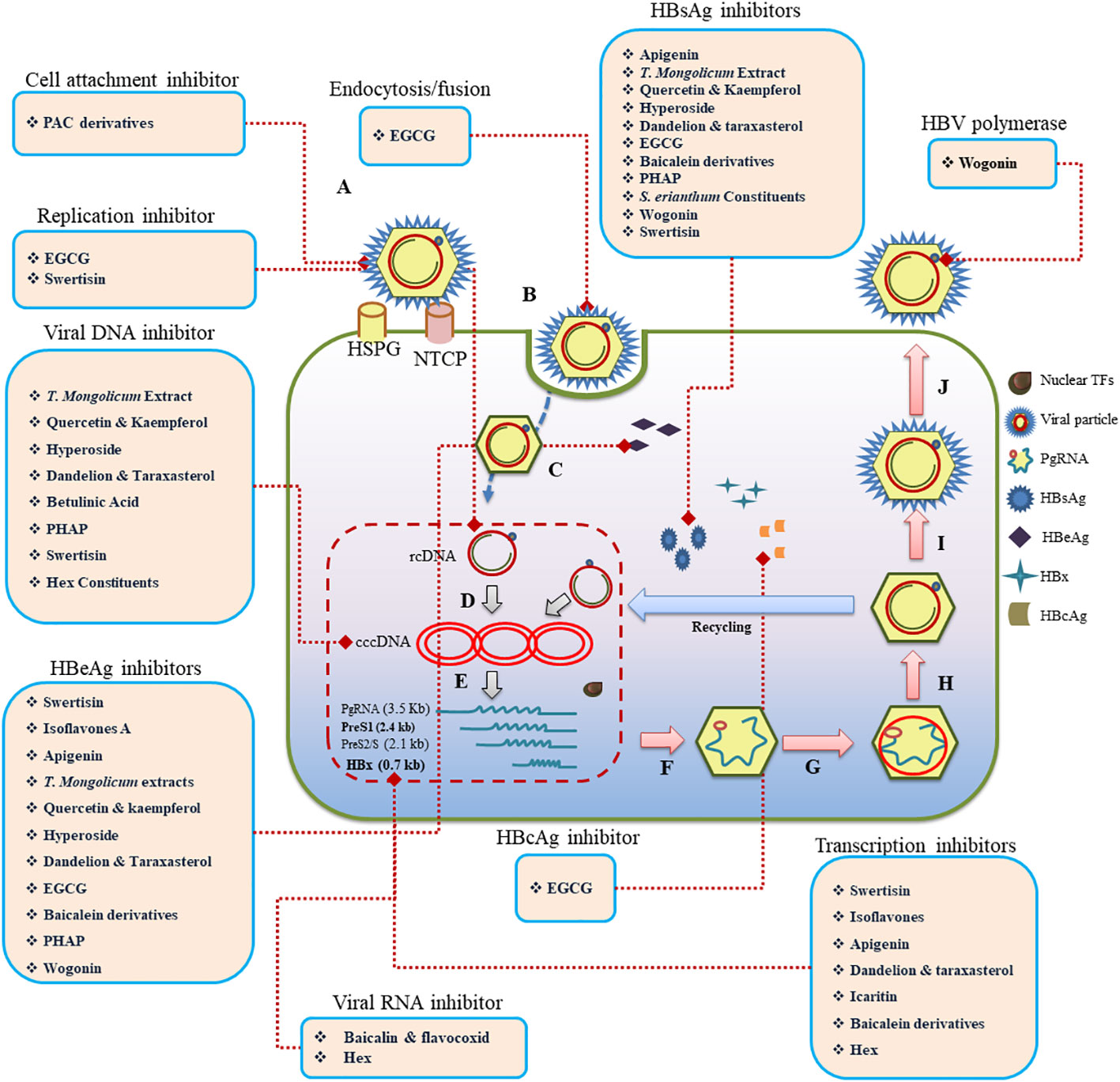
Figure 2 Schematic representation of the life cycle, highlighting key stages from viral attachment to budding (A–J). Flavonoids acting on various stages of the process of HBV infection are provided within rectangles. Nuclear TFs, Nuclear transcription factors; Pg RNA, pregenomic RNA; HBsAg, HBV surface antigen; HBeAg, HBV accessory “e” antigen; HBx, HBV transactivating protein X; HBcAg, HBV core antigen; rcDNA, relaxed circular DNA; cccDNA, covalently closed circular DNA; HSPG, heparan sulphate proteoglycan; NTC, N taurocholate co-transporting polypeptide.
Flavonoids further exerts their antiviral effects on viral replication. Compounds, including baicalin and betulinic acid (BA), inhibit the replication of HBV viral RNAs, disrupting viral replication and reducing the production of new virions. Modulation of transcription factors is another mechanism by which compounds like wogonin (Huang et al., 2000) and rosmarinic acid (Tsukamoto et al., 2018) influence host cellular factors related to HBV replication. They target hepatocytes’ protein, ϵ-Pol (Tsukamoto et al., 2018), affecting viral transcription. Flavonoids are well-known for their antioxidant properties, which play a crucial role in modulating oxidative stress associated with HBV infection. By reducing the levels of reactive oxygen species (ROS) and inhibiting lipid peroxidation, flavonoids help protect hepatocytes and liver tissues from oxidative damage. These antioxidant effects can contribute to the overall reduction in liver inflammation and damage associated with CHB infection. Enhancement of immune response by flavonoids, like baicalin, induce an endogenous pro-inflammatory response to HBV, producing an autocrine IFN-γ reaction and promoting the expression of IFN α/β and IFN-γ (Chirumbolo, 2018), contributing to the host cell’s antiviral response. Further details are provided in Table 2.
Experimental evidences of anti-HBV effects of flavonoids
Several studies have emphasized flavonoids’ anti-HBV potential. These studies mainly employed both in vitro and in vivo methods to assess the efficiency of flavonoids against HBV. In vitro research has been conducted to investigate the mechanisms by which flavonoids suppress HBV. Flavonoids have been to decrease HBsAg and HBeAg secretion shown in studies using hepatoma cell lines (HepG2.2.15 and HuH-7), as well as to decrease viral RNA and intracellular/extracellular HBV DNA levels in a dose- and time-dependent manner (Guo et al., 2007; Huang et al., 2017; Xu et al., 2020; Tan et al., 2021). Furthermore, molecular docking analysis have revealed that flavonoids can interact with viral proteins, transcription factors, and nucleotides, potentially interfering with viral replication (Parvez et al., 2021).
Animal models have offered critical insights into the flavonoids’ anti-HBV properties. In vivo tests using human liver chimeric mice (Lai et al., 2018), ducks (Guo et al., 2007), and infected ducklings (Wu et al., 2007) have shown that flavonoids such as EGCG, hyperoside, and quercetin significantly inhibit HBV replication, resulting in decreased viral DNA and antigen levels. These studies also indicated flavonoids’ hepatoprotective characteristics, including better liver histology and reduced liver damage (Wu et al., 2007). Furthermore, the data from these studies reveals a probable link between flavonoid consumption and a decreased risk of HBV infection and its associated repercussions. However, translating promising findings of in vitro and in vivo studies into clinical practice remains a significant gap.
Synergistic effects and combination therapies
Synergies between flavonoids and existing antiviral therapies, such as nucleos(t)ide analogs, have been explored. The combination of flavonoids with standard drugs may enhance antiviral efficacy, reduce drug resistance, and offer potential for more effective HBV management.
EGCG has demonstrated promising results in the combination therapy. EGCG inhibits HBV entry into hepatocytes and lowers cccDNA levels, which are required for the virus’s persistence. By limiting the establishment of cccDNA, EGCG may complement traditional anti-HBV drugs that primarily target viral replication (He et al., 2011). This synergy tackles the key difficulty in HBV treatment, the removal of viral cccDNA within the hepatocyte repositories. PAC is another promising compound, which exhibits potential for combination therapy with tenofovir, a widely used antiretroviral drug for HBV treatment (Tsukuda et al., 2017). Combining PAC with tenofovir could potentially enhance the inhibition of viral entry, making it a valuable addition to current treatment strategies.
3,5,6,7,3’,4’-Hexamethoxyflavone (Hex) is another flavonoid demonstrating potential for combination therapy. Hex reduced HBsAg levels and intracellular HBV RNA and DNA, targeting the critical steps in viral lifecycle, including antigen expression and replication (Tan et al., 2021). Therefore, combining Hex with current anti-HBV drugs may offer a comprehensive approach to reduce viral load and enhance therapeutic efficacy. Baicalin, found in Flavocoxid, presents a multifaceted approach to combat HBV infection. It inhibits HBV viral RNA, modulates NF-κB, induces proinflammatory responses to HBV, and down-regulate HNF1α and HNF4α (Chirumbolo, 2018). Flavocoxid, a baicalin-containing formula, demonstrated antiviral activity against HBV (Pollicino et al., 2018). However, further research is needed to ascertain the full potential of baicalin and flavocoxid in combination therapies.
In addition, baicalin in lipid-based nanoemulsions and hyperoside nanocrystals improve AUC and Cmax values. Higher Cmax in lymph nodes has been found to be a viable drug delivery method for CHB therapy (Shen et al., 2016; Xu et al., 2019). While this points to potential synergy with other anti-HBV drugs, especially those targeting lymphatic absorption, further research is needed to delineate the specific combinations and their extent of synergistic effects. These compounds, along with their respective biological activities, indicate their potential as valuable candidates for combination therapy in the context of HBV treatment. Such combinations could help enhance the overall effectiveness of anti-HBV drugs and address various stages of the HBV life cycle. However, it’s important to further investigate the specific drug combinations, dosage, and treatment regimens to optimize their antiviral effects. The synergy of compounds with other anti-HBV drugs offers a promising approach to enhance the effectiveness of CHB treatment, and present a fertile ground for further research and clinical trials.
Challenges and future perspectives
While flavonoids have promise anti-HBV properties, different challenges must be addressed. Flavonoid molecules may have low absorption, limiting their therapeutic use. Strategies for improving their absorption and delivery are critical. Furthermore, converting in vitro and animal study results to human trials is a difficult undertaking that necessitates extensive clinical research. Flavonoids exert their effects through a variety of methods, making it critical to understand their specific targets and interactions with viral components. Future research is needed on well-designed clinical studies to determine the effectiveness of flavonoids as supplementary therapy for persistent HBV infections. Additionally, combination therapies with flavonoid compounds could hold the key to more effective HBV treatment strategies. Moreover, the comprehension of the structure and biological activities of anti-HBV flavonoid compounds (depicted in Figure 3) enables the construction of models with significant precision using computational tools and artificial intelligence, facilitating drug development research.
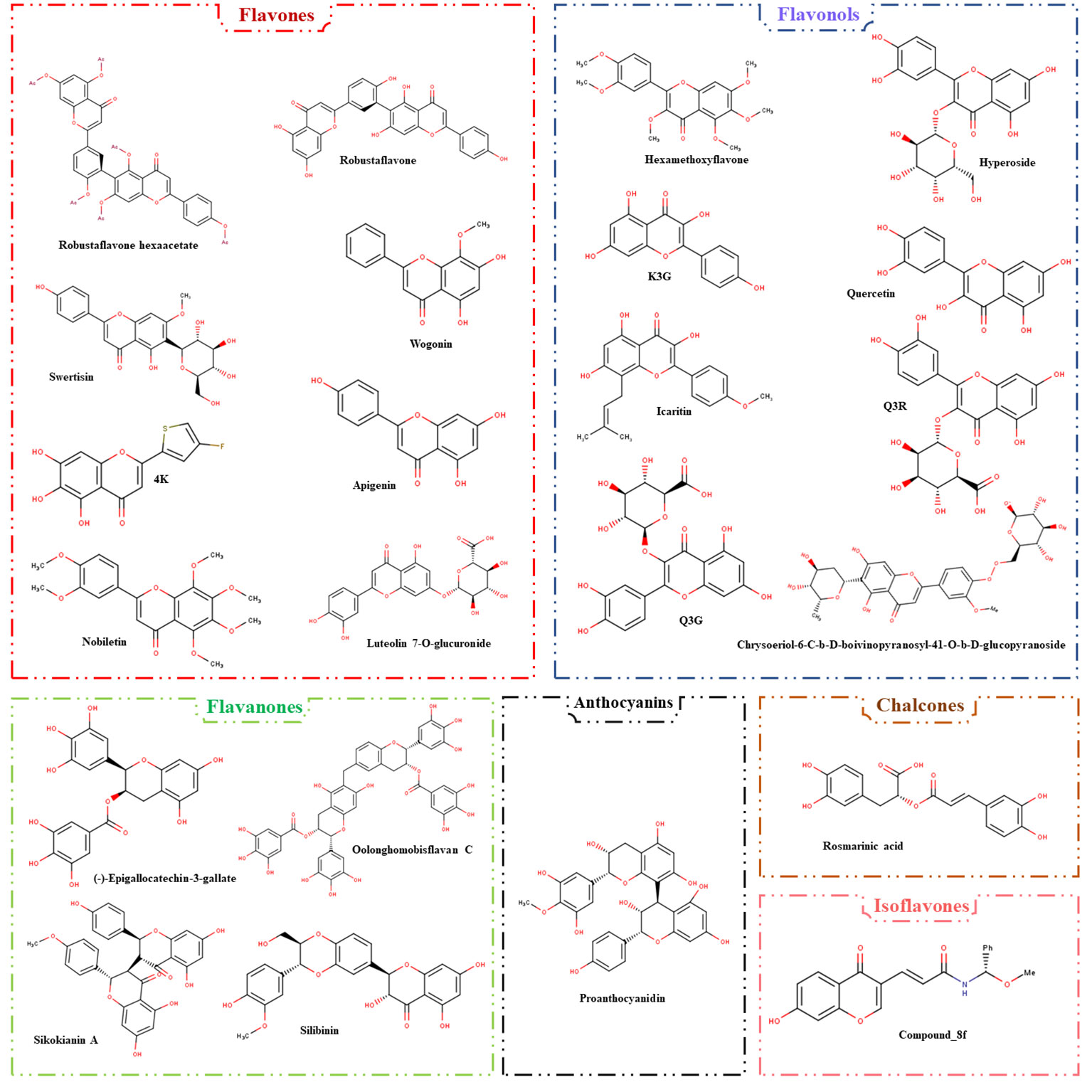
Figure 3 The classification of flavonoids with anti-HBV activities. The compounds are designated into six flavonoid subclasses, including flavones, flavonols, flavanones, anthocyanins, chalcones, and isoflavones.
Conclusion
Flavonoids, a diverse group of polyphenolic compounds found in various plant-based foods, exhibit great potential in the context of antiviral activities and particularly against HBV infection. This group of natural compounds includes different subclasses, each with unique variations and potential health benefits. Understanding the diverse classes of flavonoids and their dietary sources is crucial for harnessing their potential health benefits. In the pursuit of effective antiviral therapies against HBV, flavonoids have emerged as promising candidates. Different studies revealed the potential of flavonoids to interfere with multiple stages of the HBV life cycle, opening up avenues for novel antiviral agents. Key plant families, including Theaceae, Asteraceae, Lamiaceae, and Gentianaceae, are of particular interest, as they house plants rich in flavonoids with anti-HBV activities. The scientific exploration of these plant families involves various methods, including phytochemical analysis, biological assays, computational approaches, and clinical validation. This multidisciplinary approach is striving to advance antiviral research and provide hope for those affected by HBV infections.
Flavonoids have demonstrated diverse mechanisms of action, making them promising for HBV therapy. These mechanisms include inhibiting viral entry, disrupting viral replication, modulating transcription factors, enhancing the immune response, and inducing autophagy. Important flavonoids such as EGCG, PAC, hexamethoxyflavone, wogonin, and baicalin exhibit significant anti-HBV activity, highlighting their potential as therapeutic agents. However, it is essential to conduct further research to optimize their clinical application. Additionally, Flavonoids’ antioxidant properties play a vital role in mitigating the oxidative stress associated with HBV infection, reducing reactive ROS, and inhibiting lipid peroxidation. These effects help protect hepatocytes and liver tissues from oxidative damage, contributing to the overall reduction in liver inflammation and damage.
Experimental evidence, both in vitro and in vivo, underscores the anti-HBV potential of flavonoids. These compounds have been shown to inhibit viral replication, reduce viral DNA and antigen levels, and exhibit hepatoprotective effects in animal models. Translating these promising results into clinical practice remains a significant challenge. Also, synergistic effects between flavonoids and existing antiviral therapies have been explored, offering a potential avenue to enhance antiviral efficacy and reduce drug resistance. Flavonoids like EGCG, PAC, hexamethoxyflavone, and baicalin have shown promise in combination therapies with standard HBV drugs. These combinations could provide a more comprehensive approach to reducing viral load and enhancing therapeutic efficacy.
Above all, challenges remain, including the limited bioavailability of flavonoid and almost all other plant-derived compounds, which hinders their clinical application. Additionally, the bioactive dosage of these compounds is not adequate to exerts their anti-viral activities when consumed from the plants or fruits. Improving strategies for absorption, delivery, and dosage are essential. Additionally, translating in vitro and animal study results to human trials is complex and requires rigorous clinical research. Understanding the precise targets and interactions of flavonoids with viral components is crucial. Future research should focus on well-designed clinical trials, further exploration of combination therapies, and the development of flavonoid derivatives with enhanced bioavailability to optimize their effectiveness in managing chronic HBV infections. Flavonoids offer a promising avenue for the development of novel antiviral therapies against HBV, underscoring their significance in the scientific quest for effective HBV treatment. Also, integration of artificial intelligence and computer-aided drug discovery approaches for establishing reliable models based-on active flavonoids against HBV will be promising in the future studies.
Author contributions
MN: Data curation, Formal analysis, Investigation, Writing – original draft. ZS: Visualization, Writing – original draft. UG: Data curation, Writing – review & editing. AM: Conceptualization, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
Author AM was employed by Vista Aria Rena Gene Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abookleesh, F. L., Al-Anzi, B. S., Ullah, A. (2022). Potential antiviral action of alkaloids. Molecules 27, 903. doi: 10.3390/molecules27030903
Alam, M. A., Subhan, N., Rahman, M. M., Uddin, S. J., Reza, H. M., Sarker, S. D. (2014). Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 5, 404. doi: 10.3945/an.113.005603
Al-Amleh, S. (2020). Prevalence of hepatitis B virus among children of HBsAg-positive mothers in Hebron district, Palestine. Transl. Gastroenterol. Hepatol. 5. doi: 10.21037/tgh
Alshehri, M. M., Sharifi-Rad, J., Herrera-Bravo, J., Jara, E. L., Salazar, L. A., Kregiel, D., et al. (2021). Therapeutic potential of isoflavones with an emphasis on daidzein. Oxid. Med. Cell Longev 2021. doi: 10.1155/2021/6331630
Amponsah-Dacosta, E. (2021). Hepatitis B virus infection and hepatocellular carcinoma in sub-Saharan Africa: Implications for elimination of viral hepatitis by 2030? World J. Gastroenterol. 27, 6025. doi: 10.3748/wjg.v27.i36.6025
Behl, T., Rocchetti, G., Chadha, S., Zengin, G., Bungau, S., Kumar, A., et al. (2021). Phytochemicals from plant foods as potential source of antiviral agents: an overview. Pharmaceuticals 14, 381. doi: 10.3390/ph14040381
Bernatova, I. (2018) Biological activities of (–)-epicatechin and (–)-epicatechin-containing foods: Focus on cardiovascular and neuropsychological health. Biotechnol. Adv. 36, 666–681. doi: 10.1016/J.BIOTECHADV.2018.01.009
Bié, J., Sepodes, B., Fernandes, P. C. B., Ribeiro, M. H. L. (2023). Polyphenols in health and disease: gut microbiota, bioaccessibility, and bioavailability. Compounds 3, 40–72. doi: 10.3390/compounds3010005
Cao, Y., Tan, N. H., Chen, J. J., Zeng, G. Z., Ma, Y. B., Fitoterapia, Y. P. W., et al. (2010). Bioactive flavones and biflavones from Selaginella moellendorffii Hieron. Fitoterapia 81, 253–258. doi: 10.1016/j.fitote.2009.09.007
Chen, S., Wang, X., Cheng, Y., Gao, H., Chen, X. (2023). A review of classification, biosynthesis, biological activities and potential applications of flavonoids. Molecules 28, 4982. doi: 10.3390/molecules28134982
Cheng, Z., Sun, G., Guo, W., Huang, Y., Sun, W., Zhao, F., et al. (2015). Inhibition of hepatitis B virus replication by quercetin in human hepatoma cell lines. Virol. Sin. 30, 261–268. doi: 10.1007/s12250-015-3584-5
Chiang, L. C., Ng, L. T., Cheng, P. W., Chiang, W., Lin, C. C. (2005). Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 32, 811–816. doi: 10.1111/j.1440-1681.2005.04270.x
Chirumbolo, S. (2018). Baicalin in flavocoxid may act against hepatitis B virus via a pro-inflammatory pathway. Inflammation Res. 67, 203–205. doi: 10.1007/s00011-017-1111-x
Chou, S. C., Huang, T. J., Lin, E. H., Huang, C. H., Chou, C. H. (2012). Antihepatitis B virus constituents of Solanum erianthum. Nat. Prod. Commun. 7, 153–156. doi: 10.1177/1934578X1200700205
Dias, M. C., Pinto, D. C. G. A., Silva, A. M. S. (2021). Plant flavonoids: chemical characteristics and biological activity. Molecules 26, 5377. doi: 10.3390/molecules26175377
di Filippo Villa, D., Navas, M. C. (2023). Vertical transmission of hepatitis B virus—An update. Microorganisms 11, 1140. doi: 10.3390/microorganisms11051140
Do Socorro Chagas, M. S., Behrens, M. D., Moragas-Tellis, C. J., Penedo, G. X. M., Silva, A. R., Gonçalves-De-Albuquerque, C. F. (2022). Flavonols and flavones as potential anti-inflammatory, antioxidant, and antibacterial compounds. Oxid. Med. Cell Longev. 2022. doi: 10.1155/2022/9966750
Ghasemi, R., Ghaffari, S. H., Momeny, M., Pirouzpanah, S., Yousefi, M., Malehmir, M., et al. (2013). Multitargeting and antimetastatic potentials of silibinin in human HepG-2 and PLC/PRF/5 hepatoma cells. Nutr. Cancer 65, 590–599. doi: 10.1080/01635581.2013.770043
Guo, Q., Zhao, L., You, Q., Yang, Y., Gu, H., Song, G., et al. (2007). Anti-hepatitis B virus activity of wogonin in vitro and in vivo. Antiviral Res 74, 16–24. doi: 10.1016/j.antiviral.2007.01.002
He, W., Li, L., Liao, Q., Liu, C. L. (2011). Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication-inducible cell line. World J. Gastroenterol. 17, 1507–1514. doi: 10.3748/wjg.v17.i11.1507
Hollman, P. C. H., Aarts, I. C. W. (2000). Flavonols, flavones and flavanols–nature, occurrence and dietary burden. J. Sci. Food. Agric. 80, 1081–1093. doi: 10.1002/(SICI)1097-0010(20000515)80:7%3C1081::AID-JSFA566%3E3.0.CO;2-G
Hsu, Y. C., Huang, D. Q., Nguyen, M. H. (2023). Global burden of hepatitis B virus: current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. 20, 524–537. doi: 10.1038/s41575-023-00760-9
Hu, Z., Hu, J., Ren, F., Xu, H., Tan, M., Wang, Q., et al. (2020). Nobiletin, a novel inhibitor, inhibits HBsAg production and hepatitis B virus replication. Biochem. Biophys. Res. Commun. 523, 802–808. doi: 10.1016/j.bbrc.2019.12.099
Huang, R. L., Chen, C. C., Huang, H. L., Chang, C. G., Chen, C. F., Chang, C., et al. (2000). Anti-hepatitis B virus effects of wogonin isolated from Scutellaria baicalensis. Planta Med. 66, 694–698. doi: 10.1055/s-2000-9775
Huang, T., Liu, S., Kuo, Y., Chen, C. (2014b). Antiviral activity of chemical compound isolated from Artemisia morrisonensis against hepatitis B virus in vitro. Antiviral Res. 101, 97–104. doi: 10.1016/j.antiviral.2013.11.007
Huang, H., Tao, M., Hung, T., Chen, J. (2014a). (−)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antiviral Res. 111, 100–111. doi: 10.1016/j.antiviral.2014.09.009
Huang, H., Zhou, W., Zhu, H., Zhou, P., Shi, X. (2017). Baicalin benefits the anti-HBV therapy via inhibiting HBV viral RNAs. Toxicol. Appl. Pharmacol. 323, 36–43. doi: 10.1016/j.taap.2017.03.016
Hyun Kim, B., Ray Kim, W. (2018). Epidemiology of hepatitis B virus infection in the United States. Clin. Liver Dis. (Hoboken) 12, 1. doi: 10.1002/CLD.732
Indrasetiawan, P., Aoki-Utsubo, C., Hanafi, M., Hartati, S. R. I., Wahyuni, T. S., Kameoka, M., et al. (2019). Antiviral activity of cananga odorata against hepatitis B virus. Kobe J. Med. Sci. 65, E71.
Jeng, W. J., Papatheodoridis, G. V., Lok, A. S. F. (2023). Hepatitis B. Lancet 401, 1039–1052. doi: 10.1016/S0140-6736(22)01468-4
Jia, Y. Y., Guan, R. F., Wu, Y. H., Yu, X. P., Lin, W. Y., Zhang, Y. Y., et al (2014). Taraxacum mongolicum extract exhibits a protective effect on hepatocytes and an antiviral effect against hepatitis B virus in animal and human cells. Mol. Med. Rep. 9, 1381–1387. doi: 10.3892/mmr.2014.1925
Khan, A., Ikram, M., Hahm, J. R., Kim, M. O. (2020). Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: special focus on neurological disorders. Antioxidants 9, 1–15. doi: 10.3390/antiox9070609
Khoo, H. E., Azlan, A., Tang, S. T., Lim, S. M. (2017). Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 61. doi: 10.1080/16546628.2017.1361779
Kim, I. S. (2021). Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 10, 1064. doi: 10.3390/antiox10071064
Kim, K., Kim, Y., letters, K. L.-B. (2007). Isolation of quinic acid derivatives and flavonoids from the aerial parts of Lactuca indica L. and their hepatoprotective activity in vitro. Bioorg. Med. Chem. Lett. 17, 6739–6743. doi: 10.1016/j.bmcl.2007.10.046
Lai, Y. H., Sun, C. P., Huang, H. C., Chen, J. C., Liu, H. K., Huang, C. (2018). Epigallocatechin gallate inhibits hepatitis B virus infection in human liver chimeric mice. BMC Complement Altern. Med. 18, 1–7. doi: 10.1186/s12906-018-2316-4
Li, B., Guo, Q., Tian, Y., Liu, S., Wang, Q., Chen, L., et al. (2016). New anti-HBV C-boivinopyranosyl flavones from alternanthera philoxeroides. Molecules 21, 336. doi: 10.3390/molecules21030336
Lv, D. D., Wang, Y. J., Wang, M. L., Chen, E. Q., Tao, Y. C., Zhang, D. M., et al. (2021). Effect of silibinin capsules combined with lifestyle modification on hepatic steatosis in patients with chronic hepatitis B. Sci. Rep. 11, 1–9. doi: 10.1038/s41598-020-80709-z
Ma, J., Li, T., Han, X., Yuan, H., Liang, H., Wang, Y., et al. (2017). Discovery and mechanism of action of Novel Baicalein modified derivatives as potent antihepatitis agent. Virology 507, 199–205. Available at: https://www.sciencedirect.com/science/article/pii/S0042682217301046.
Martyn, E., Eisen, S., Longley, N., Harris, P., Surey, J., Norman, J., et al. (2023). The forgotten people: Hepatitis B virus (HBV) infection as a priority for the inclusion health agenda. Elife 12. doi: 10.7554/eLife.81070
Merecz-Sadowska, A., Sitarek, P., Kowalczyk, T., Zajdel, K., Jęcek, M., Nowak, P., et al. (2023). Food anthocyanins: malvidin and its glycosides as promising antioxidant and anti-inflammatory agents with potential health benefits. Nutrients 15, 3016. doi: 10.3390/nu15133016
Mohebbi, A., Azadi, F., Hashemi, M. M., Askari, F. S., Razzaghi, N. (2021). Havachoobe (Onosma dichroanthum boiss) root extract decreases the hepatitis B virus surface antigen secretion in the PLC/PRF/5 cell line. Intervirology 64, 22–26. doi: 10.1159/000512140
Mohebbi, A., Ebrahimi, M., Askari, F. S., Shaddel, R., Mirarab, A., Oladnabi, M. (2022). QSAR Modeling of a Ligand-Based Pharmacophore Derived from Hepatitis B Virus Surface Antigen Inhibitors. Acta Microbiol Bulg 38. Available at: https://actamicrobio.bg/archive/issue-3-2022/amb-3-2022-article-5.pdf (Accessed February 22, 2024)
Mohebbi, A., Ghorbanzadeh, T., Naderifar, S., Khalaj, F., Askari, F. S., Salehnia Sammak, A. (2023). A fragment-based drug discovery developed on ciclopirox for inhibition of Hepatitis B virus core protein: An in silico study. PloS One 18, e0285941. doi: 10.1371/journal.pone.0285941
Mohebbi, A., Lorestani, N., Tahamtan, A., Kargar, N. L., Tabarraei, A. (2018). An overview of hepatitis B virus surface antigen secretion inhibitors. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00662
Mohebbi, A., Mohammadi, S., Memarian, A. (2016). Prediction of HBF-0259 interactions with hepatitis B Virus receptors and surface antigen secretory factors. Virusdisease 27, 234–241. doi: 10.1007/s13337-016-0333-9
Naderi, M., Hosseini, S. M., Behnampour, N., Shahramian, I., Moradi, A. (2023a). Association of HLADQ-B1 polymorphisms in three generations of chronic hepatitis B patients. Virus Res. 325. doi: 10.1016/j.virusres.2022.199036
Naderi, M., Hosseini, S. M., Behnampour, N., Shahramian, I., Moradi, A. (2023b). Mutations in the S gene of hepatitis B virus in three generations of patients with chronic hepatitis B. Virus Genes 59, 662–669. doi: 10.1007/s11262-023-02012-z
Naderi, M., Hosseini, S. M., Besharat, S., Behnampour, N., Shahramian, I., Moradi, A. (2023c). Clinical and virological aspects of core and pre-core mutations in three generations of chronic hepatitis B virus patients. Future Virol. 18, 349–358. doi: 10.2217/FVL-2022-0216
Ninfali, P., Antonelli, A., Magnani, M., Scarpa, E. S. (2020). Antiviral properties of flavonoids and delivery strategies. Nutrients 12, 2534. doi: 10.3390/nu12092534
Panche, A. N., Diwan, A. D., Chandra, S. R (2016). Flavonoids: an overview. J. Nutr. Sci. 5. doi: 10.1017/JNS.2016.41
Parvez, M. K., Ahmed, S., Al-Dosari, M. S., Abdelwahid, M. A. S., Arbab, A. H., Al-Rehaily, A. J., et al. (2021). Novel anti-hepatitis B virus activity of euphorbia schimperi and its quercetin and kaempferol derivatives. ACS Omega 6, 29100–29110. doi: 10.1021/acsomega.1c04320
Pollicino, T., Musolino, C., Irrera, N., Bitto, A., Lombardo, D., Timmoneri, M., et al. (2018). Flavocoxid exerts a potent antiviral effect against hepatitis B virus. Inflammation Res. 67, 89–103. doi: 10.1007/s00011-017-1099-2
Rezanezhadi, M., Mohebbi, A., Askari, F. S., Hosseini, S. D., Tabarraei, A. (2019). Hepatitis B virus reverse transcriptase polymorphisms between treated and treatment-naïve chronically infected patients. Virusdisease 30, 219–226. doi: 10.1007/s13337-018-00510-5
Roy, A., Roy, M., Gacem, A., Datta, S., Zeyaullah, M., Muzammil, K., et al. (2022). Role of bioactive compounds in the treatment of hepatitis: A review. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.1051751
Salehi, B., Venditti, A., Sharifi-Rad, M., Kręgiel, D., Sharifi-Rad, J., Durazzo, A., et al. (2019). The therapeutic potential of apigenin. Int. J. Mol. Sci. 20, 1305. doi: 10.3390/ijms20061305
Shen, B., Wu, N., Shen, C., Zhang, F., Wu, Y., Xu, P., et al. (2016). Hyperoside nanocrystals for HBV treatment: process optimization, in vitro and in vivo evaluation. Drug Dev. Ind. Pharm. 42, 1772–1781. doi: 10.3109/03639045.2016.1173051
Tan, M., Ren, F., Yang, X. (2021). Anti-HBV therapeutic potential of small molecule 3, 5, 6, 7, 3′, 4′-Hexamethoxyflavone in vitro and in vivo. Virology 560, 66–75. Available at: https://www.sciencedirect.com/science/article/pii/S0042682221001112.
Tsukamoto, Y., Ikeda, S., Uwai, K., Taguchi, R., Chayama, K., Sakaguchi, T., et al. (2018). Rosmarinic acid is a novel inhibitor for hepatitis b virus replication targeting viral epsilon RNA-polymerase interaction. PloS One 13. doi: 10.1371/journal.pone.0197664
Tsukuda, S., Watashi, K., Hojima, T., Isogawa, M., Iwamoto, M., Omagari, K., et al. (2017). A new class of hepatitis B and D virus entry inhibitors, proanthocyanidin and its analogs, that directly act on the viral large surface proteins. Hepatology 65, 1104–1116. doi: 10.1002/hep.28952
Wang, H. L., Geng, C. A., Ma, Y. B., Zhang, X. M., Chen, J. J. (2013). Three new secoiridoids, swermacrolactones A–C and anti-hepatitis B virus activity from Swertia macrosperma. Fitoterapia 89, 183–187. doi: 10.1016/j.fitote.2013.06.002
Wang, Z., Li, Y., Guo, Z., Zhou, X., Lu, M., et al. (2020). ERK1/2-HNF4α axis is involved in epigallocatechin-3-gallate inhibition of HBV replication. Acta Pharmacol. Sin 41, 278–285. doi: 10.1038/s41401-019-0302-0
Wang, M., Yan, L., Wang, J., Jin, Y., Zheng, Z. J. (2023). Global burden of hepatitis B attributable to modifiable risk factors from 1990 to 2019: a growing contribution and its association with socioeconomic status. Global Health 19. doi: 10.1186/s12992-023-00922-z
Wu, L., Yang, X., Huang, Z., Liu, H., Sinica, G. (2007). In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol Sin 28, 404–409. doi: 10.1111/j.1745-7254.2007.00510.x
Xia, C., Tang, W., Geng, P., Zhu, H., Zhou, W. (2020). Baicalin down-regulating hepatitis B virus transcription depends on the liver-specific HNF4α-HNF1α axis. Toxicol Appl Pharmacol 403, 115131. doi: 10.1016/j.taap.2020.115131
Xu, H. Y., Ren, J. H., Su, Y., Ren, F., Zhou, Y., et al. (2020). Anti-hepatitis B virus activity of swertisin isolated from Iris tectorum Maxim. J. Ethnopharmacol. 257, 112787. doi: 10.1016/j.jep.2020.112787
Xu, J., Gu, W., Li, C., Li, X., Xing, G., Li, Y., et al. (2016). Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J. Nat. Med. 70, 584–591. doi: 10.1007/s11418-016-0980-6
Xu, Q., Zhou, A., Wu, H., Bi, Y. (2019). Development and in vivo evaluation of baicalin-loaded W/O nanoemulsion for lymphatic absorption. Pharm. Dev. Technol. 24, 1155–1163. doi: 10.1080/10837450.2019.1646757
Yang, G., Chen, D. (2008). Biflavanones, flavonoids, and coumarins from the roots of Stellera chamaejasme and their antiviral effect on hepatits B virus. Chem. Biodivers 5, 1419–1424. doi: 10.1002/cbdv.200890130
Yang, Y., Ying, G., Wu, S., Wu, F., Chen, Z. (2020). In vitro inhibition effects of hepatitis B virus by dandelion and taraxasterol. Infect. Agent Cancer 15, 1–10. doi: 10.1186/s13027-020-00309-4
Zembower, D. E., Lin, Y. M., Flavin, M. T., Chen, F. C., Korba, B. E. (1998). Robustaflavone, a potential non-nucleoside anti-hepatitis B agent. Antiviral Res. 39, 81–88. doi: 10.1016/S0166-3542(98)00033-3
Zhang, C., Li, H., Jiang, W., Zhang, X., Li, G. (2016). Icaritin inhibits the expression of alpha-fetoprotein in hepatitis B virus-infected hepatoma cell lines through post-transcriptional regulation. Oncotarget 7, 83755. doi: 10.18632%2Foncotarget.13194
Zhang, Y. M., Zhang, Z. Y., Wang, R. X. (2020). Protective mechanisms of quercetin against myocardial ischemia reperfusion injury. Front. Physiol. 11. doi: 10.3389/fphys.2020.00956
Keywords: flavonoids, Hepatitis B virus, antiviral therapy, herbal medicine, natural compounds
Citation: Naderi M, Salavatiha Z, Gogoi U and Mohebbi A (2024) An overview of anti-Hepatitis B virus flavonoids and their mechanisms of action. Front. Cell. Infect. Microbiol. 14:1356003. doi: 10.3389/fcimb.2024.1356003
Received: 14 December 2023; Accepted: 12 February 2024;
Published: 29 February 2024.
Edited by:
Haitao Wen, The Ohio State University, United StatesReviewed by:
Arup Banerjee, Regional Centre for Biotechnology (RCB), IndiaTooba Mahboob, UCSI University, Malaysia
Copyright © 2024 Naderi, Salavatiha, Gogoi and Mohebbi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alireza Mohebbi, QWxpcmV6YWEyc0BnbWFpbC5jb20=
†ORCID: Alireza Mohebbi, orcid.org/0000-0003-2489-585X
 Malihe Naderi
Malihe Naderi Zahra Salavatiha2
Zahra Salavatiha2 Urvashee Gogoi
Urvashee Gogoi Alireza Mohebbi
Alireza Mohebbi