- 1Center for Reproductive Medicine, Jiangxi Maternal and Child Health Hospital, National Clinical Research Center for Obstetrics and Gynecology, Nanchang Medical College, Nanchang, China
- 2Department of Clinical Medicine, School of Queen Mary, Nanchang University, Nanchang, China
- 3Department of Obstetrics, Jiangxi Maternal and Child Health Hospital, National Clinical Research Center for Obstetrics and Gynecology, Nanchang Medical College, Nanchang, China
- 4Key Laboratory of Women’s Reproductive Health of Jiangxi Province, Jiangxi Maternal and Child Health Hospital, National Clinical Research Center for Obstetrics and Gynecology, Nanchang Medical College, Nanchang, China
- 5Reproductive and Genetic Hospital, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China
Background: Observational studies have reported that Helicobacter pylori (H. pylori) infection is associated with a series of pregnancy and neonatal outcomes. However, the results have been inconsistent, and the causal effect is unknown.
Methods: A two-sample Mendelian randomization (MR) study was performed using summary-level statistics for anti-H. pylori IgG levels from the Avon Longitudinal Study of Parents and Children Cohort. Outcome data for pregnancy (miscarriage, preeclampsia-eclampsia, gestational diabetes mellitus, placental abruption, premature rupture of membranes, postpartum hemorrhage) and neonates (birthweight, gestational age, and preterm birth) were sourced from genome-wide association meta-analysis as well as the FinnGen and Early Growth Genetics Consortium. Causal estimates were calculated by five methods including inverse variance weighted (IVW). The heterogeneity of instrumental variables was quantified by Cochran’s Q test, while sensitivity analyses were performed via MR-Egger, MR-PRESSO, and leave-one-out tests.
Results: IVW estimates suggested that genetically predicted anti-H. pylori IgG levels were significantly associated with increased risks of preeclampsia-eclampsia (odds ratio [OR] = 1.12, 95% confidence interval [CI] 1.01–1.24, P = 0.026) and premature rupture of membranes (OR = 1.17, 95% CI 1.05–1.30, P = 0.004). Similar results were obtained for preeclampsia-eclampsia from the MR-Egger method (OR = 1.32, 95% CI 1.06–1.64, P = 0.027) and for premature rupture of membranes from the weighted median method (OR = 1.22, 95% CI 1.06–1.41, P = 0.006). No significant causal effects were found for other outcomes. There was no obvious heterogeneity and horizontal pleiotropy across the MR analysis.
Conclusion: Our two-sample MR study demonstrated a causal relationship of H. pylori infection with preeclampsia-eclampsia and premature rupture of membranes. The findings confirm the epidemiological evidence on the adverse impact of H. pylori in pregnancy. Further studies are needed to elucidate the pathophysiological mechanisms and assess the effectiveness of pre-pregnancy screening and preventive eradication.
Introduction
Helicobacter pylori (H. pylori) is a gram-negative bacterium with urease, catalase, and oxidase activity that colonizes the human stomach (Zamani et al., 2018). It is one of the most common pathogens in the world, infecting more than half of the whole population (Kamboj et al., 2017). Therefore, the health impact of H. pylori infection, such as peptic ulcer disease, chronic gastritis, gastric adenocarcinoma, and gastric cancer, is crucial for public health (Kusters et al., 2006; McColl, 2010). Among pregnant women, the prevalence of H. pylori infection remains high in many countries (Baingana et al., 2014; Mubarak et al., 2014) and it has been suggested that increased susceptibility to H. pylori infection may be due to pregnancy itself (Lanciers et al., 1999).
A systematic review of studies published up to November 17th, 2018, exploring associations of H. pylori infection with pregnancy and neonatal complications, showed significantly increased risks of preeclampsia, gestational diabetes mellitus, spontaneous miscarriage, and low birthweight (Zhan et al., 2019). Consistently, some other studies also reported an adverse effect in certain outcomes (Wanyama et al., 2016; den Hollander et al., 2017; Li et al., 2020; Tang et al., 2021). In particular, for adverse pregnancy outcomes, H. pylori infection is associated with gestational diabetes mellitus (Li et al., 2020; Tang et al., 2021) and preeclampsia (Tang et al., 2021), while for adverse neonatal outcomes, H. pylori infection is associated with low birthweight (den Hollander et al., 2017; Wanyama et al., 2016) and small for gestational age (den Hollander et al., 2017). However, most single studies examined only one or few outcomes without a comprehensive evaluation. In addition, these observational studies were vulnerable to residual confounding, and variations in confounder control could cause heterogeneity between studies, thereby leading to controversial results. Thus, reexamining the impact of infections on a range of pregnancy and neonatal outcomes is essential to safeguard the health of both pregnant women and fetus.
Mendelian randomization (MR) is an epidemiological approach that reveals causality in an unbiased manner, relying on genetic variation as the instrumental variable (IV) to assess whether an exposure leads to a corresponding outcome (Lawlor et al., 2008). Since alleles segregate according to Mendel’s second law of inheritance and genotypes are randomly assigned from parent to offspring without the influence of confounding, the causal sequence is reasonable (Burgess and Thompson, 2021). Given the infeasible conduction of randomized controlled trials, a number of MR studies have assessed the correlation between gut microbiota and pregnancy outcomes (Li C. et al., 2022; Li P. et al., 2022), while H. pylori was underexplored as the most common gastrointestinal pathogen.
In this study, we performed a two-sample MR analysis to evaluate the causal associations between H. pylori infection and nine pregnancy and neonatal outcomes, namely, miscarriage, preeclampsia or eclampsia, gestational diabetes mellitus, placental abruption, premature rupture of membranes, postpartum haemorrhage, birthweight, gestational age, and preterm birth.
Methods and materials
Study design
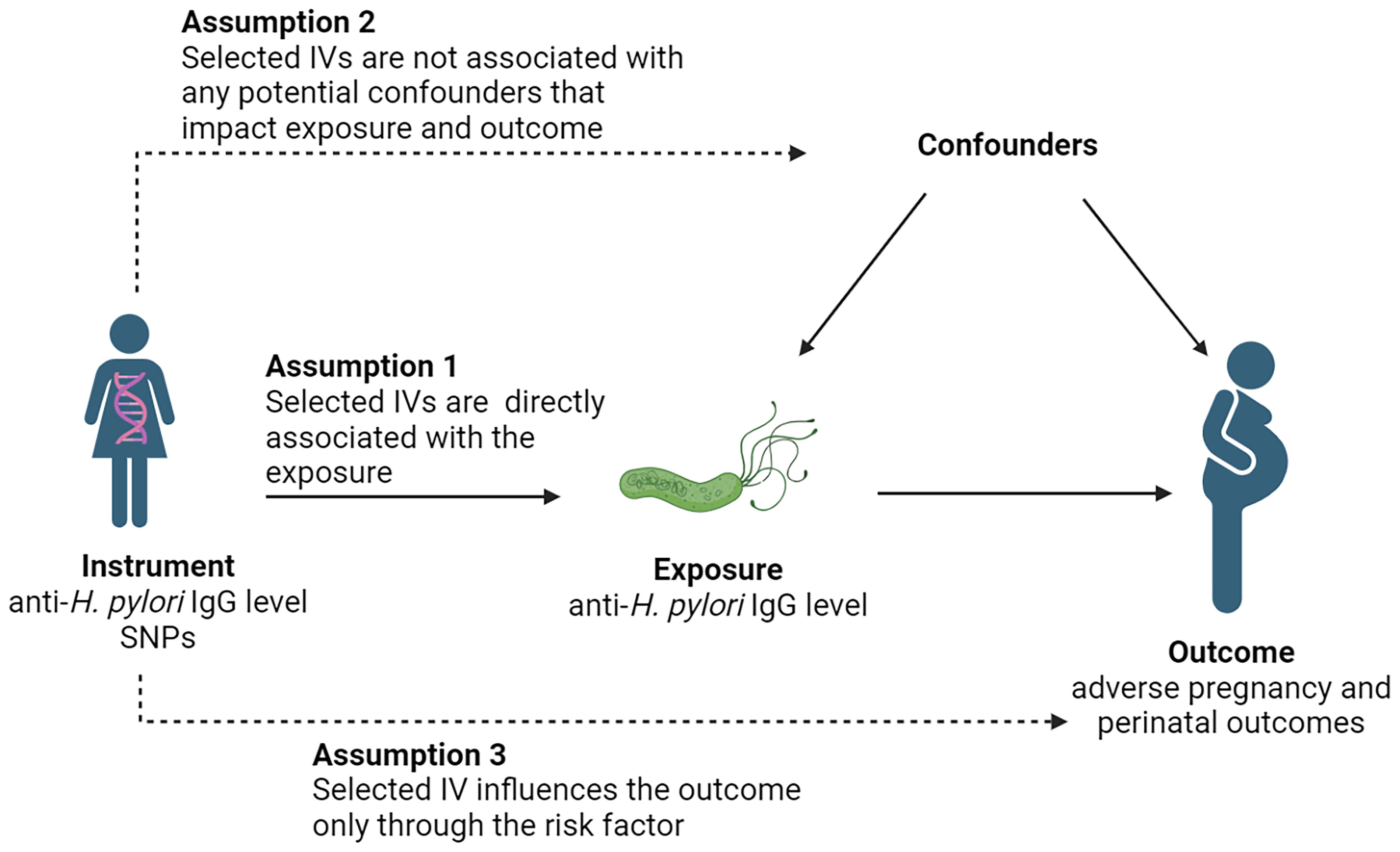
A two-sample MR study was conducted using single nucleotide polymorphisms (SNPs) as IVs (Lawlor et al., 2008). H. pylori infection was defined on the basis of serum-specific IgG antibodies to H. pylori. Three core assumptions were used to ensure the accuracy of results (Davies et al., 2018): 1) each selected IV must be directly associated with the exposure; 2) each selected IV is not associated with any potential confounders that impact exposure and outcome; and 3) each selected IV influences the outcome only through the risk factor (Figure 1).
Data sources
In this MR study, reliable genetic instruments were identified in large genome-wide association studies (GWAS). Exposure data were from the Avon Longitudinal Study of Parents and Children Cohort (ALSPAC), containing 4,735 individuals with anti-H. pylori IgG levels (Chong et al., 2021). Outcome data for miscarriage were obtained from GWAS meta-analysis by Laisk et al. (Laisk et al., 2020), including 49,996 sporadic cases and 174,109 controls. Other summary statistics for preeclampsia or eclampsia (Ncase = 3,903, Ncontrol = 114,735), gestational diabetes mellitus (Ncase = 5,687, Ncontrol = 117,892), placental abruption (Ncase = 294, Ncontrol = 104,247), premature rupture of membranes (Ncase = 3,011, Ncontrol = 104,247), postpartum haemorrhage (Ncase = 44,559, Ncontrol = 202,621), birthweight (N = 210,267), gestational age (N = 84,689), and preterm birth (Ncase = 4,775, Ncontrol = 60,148) were from FinnGen (https://www.finngen.fi/en) and Early Growth Genetics Consortium (www.egg-consortium.org) (Liu et al., 2019). All summary-level data used were harmonized and archived in the Medical Research Council Integrative Epidemiology Unit (MRC-IEU) OpenGWAS (https://gwas.mrcieu.ac.uk/). To reduce potential bias from population stratification, all SNPs and associated data were obtained from studies analyzed separately for those of European ancestry only. The characteristics of each GWAS dataset are detailed in Supplementary Table 1.
IV selection
To ensure adequate screening for IVs, SNPs with genome-wide range significance levels less than the P-value (1×10-5) were selected (Sanna et al., 2019). Then, to check the independence of these variables and the effect of linkage disequilibrium, the SNP for the r2 was set to 0.001 and the clumping window size to 10,000 kb. In addition, IVs with F-statistics <10 were excluded to ensure the strength of association between IVs and exposure. The formula was F = r2×(N−1−K)/[(1−r2) ×K], where r2 represents the exposure variance explained by each IV, N denotes the sample size of the GWAS, and K refers to the number of instruments. Finally, we removed SNPs with minor allele frequency (MAF) less than the threshold of 0.01. Palindromic SNPs were also removed to ensure the effects of SNPs on exposure correspond to the same allele as the effects of SNPs on the outcome.
MR analysis
A total of five methods, including inverse variance weighted (IVW), MR-Egger regression, weighted median, simple mode, and weighted mode, were used to evaluate whether there was a causal association between H. pylori infection and pregnancy and neonatal outcomes. In terms of algorithmic principles, the IVW method could integrate the Wald ratio for each SNP causal effect through meta-analysis. Without the horizontal pleiotropy, the IVW results would be unbiased (Bowden et al., 2017). The MR-Egger method can detect associations when the IV hypothesis does not apply but the weaker hypothesis does. It can also be used to assess horizontal pleiotropy and the results are consistent with IVW when an intercept term equals to zero, indicating the absence of horizontal pleiotropy (Bowden et al., 2015). The weighted median method can provide robust effect estimates when at least fifty percent of the instrumental information is valid, while the weighted mode is reliable if the largest subset of instruments with similar causal effects is valid (Hartwig et al., 2017).
Heterogeneity and sensitivity analysis
Heterogeneity between IVs was analyzed using Cochran’s Q-test. Horizontal pleiotropy was assessed by the intercept of the MR-Egger method (Bowden et al., 2015) as well as the MR-pleiotropy residual sum and outlier (MR-PRESSO) global test (Verbanck et al., 2018). We also performed leave-one-out analyses to monitor whether causal associations were dominated by single SNPs, in which MR was performed iteratively to remove different SNPs using the “mr_leaveoneout_plot” program. All of the above analyses were performed in R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria) and R packages TwoSampleMR (Hemani et al., 2017) and MR-PRESSO (Verbanck et al., 2018) were used.
Results
According to the selection criteria of IVs, we identified a total of 20 SNPs for anti-H. pylori IgG levels. All F-statistics were above 10. The detailed information is shown in Supplementary Table 2.
Under the IVW method, genetically predicted IgG levels were significantly associated with increased risks of preeclampsia or eclampsia (odds ratio [OR] = 1.12, 95% confidence interval [CI] 1.01–1.24, P = 0.026) and premature rupture of membranes (OR = 1.17, 95% CI 1.05–1.30, P = 0.004). For preeclampsia or eclampsia, similar results were obtained via MR-Egger (OR = 1.32, 95% CI 1.06–1.64, P = 0.027). For premature rupture of membranes, the OR estimates obtained from the weighted median (OR = 1.22, 95% CI 1.06–1.41, P = 0.006) were also consistent with those from IVW. No significant associations were observed in sporadic miscarriage, gestational diabetes mellitus, placental abruption, postpartum haemorrhage, birthweight, gestational age, and preterm birth. The results of the five MR analysis methods are displayed in Table 1.
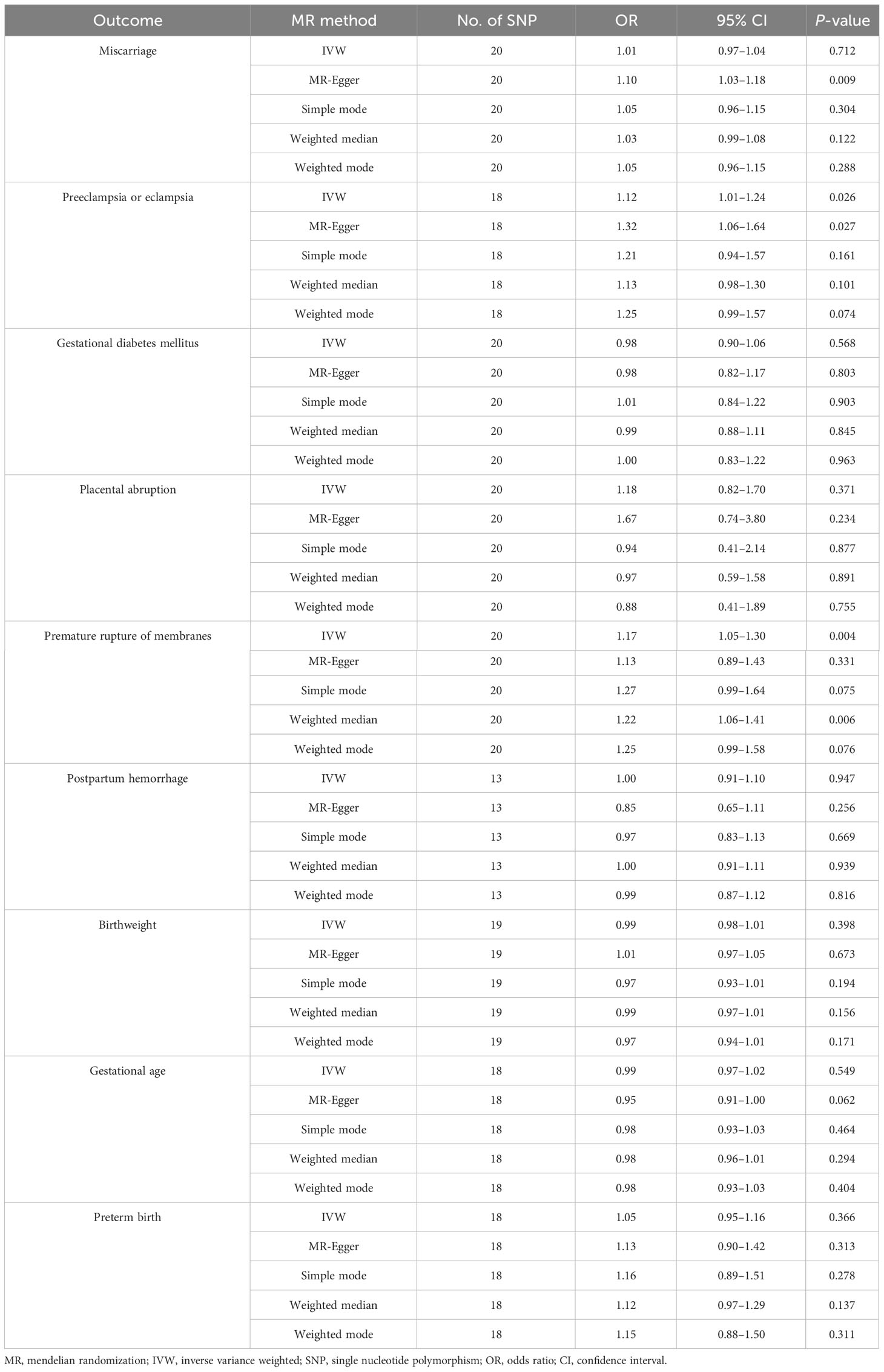
Table 1 MR estimates for the association between H. pylori infection and nine pregnancy and neonatal outcomes.
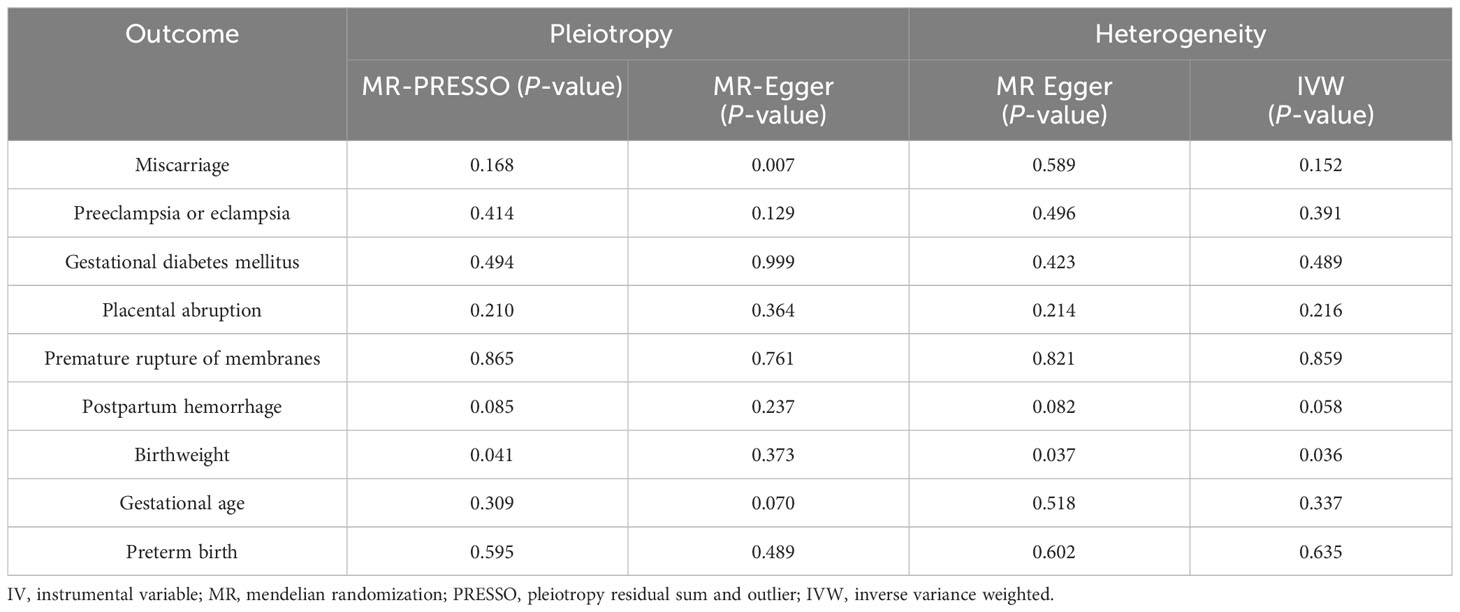
Across the MR study, no evidence of directional pleiotropy and heterogeneity was found in the MR-PRESSO global test, MR-Egger intercept test, and Cochran’s IVW and MR-Egger Q tests, except for analyzing the causal relationship between H. pylori infection and birthweight (Table 2). The results of the leave-one-out permutation analysis showed that the overall risk estimate was not driven by certain SNPs (Figure 2). In addition, potential outliers and the effects of SNPs, individually and jointly, from each MR method were shown in scatter plots (Figure 3), which displayed a similar trend toward a positive MR association of anti-H. pylori IgG levels with preeclampsia or eclampsia and premature rupture of membranes.
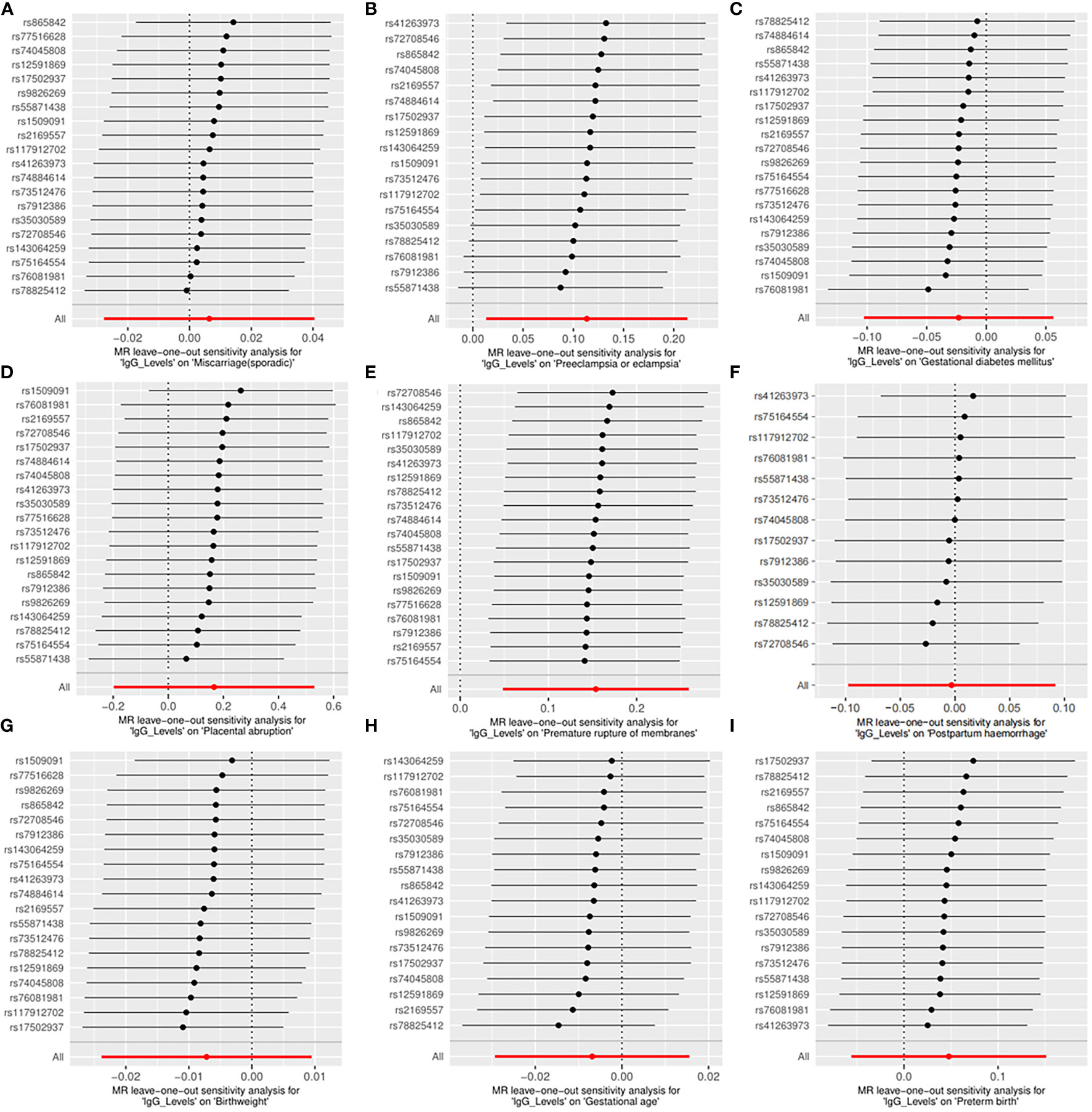
Figure 2 Leave-one-out plots for the causal associations between anti-H. pylori IgG levels and nine pregnancy and neonatal outcomes. (A) Sporadic miscarriage. (B) Preeclampsia or eclampsia. (C) Gestational diabetes mellitus. (D) Placental abruption. (E) Premature rupture of membranes. (F) Postpartum haemorrhage. (G) Birthweight. (H) Gestational age. (I) Preterm birth. MR, mendelian randomization.
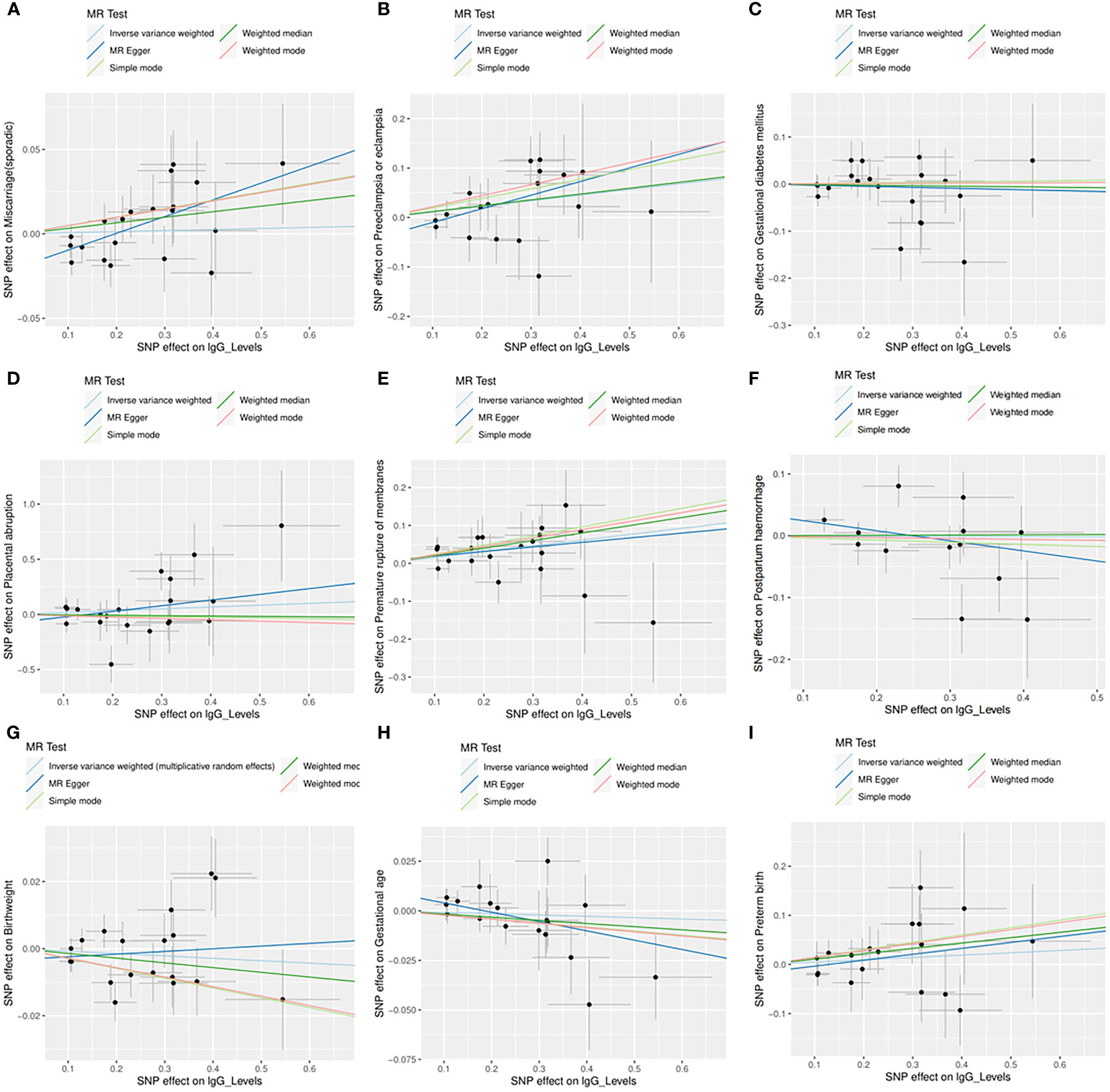
Figure 3 Scatter plots for the causal associations between anti-H. pylori IgG levels and nine pregnancy and neonatal outcomes. (A) Sporadic miscarriage. (B) Preeclampsia or eclampsia. (C) Gestational diabetes mellitus. (D) Placental abruption. (E) Premature rupture of membranes. (F) Postpartum haemorrhage. (G) Birthweight. (H) Gestational age. (I) Preterm birth. Five MR methods are indicated by different colors, including inverse variance weighted (light blue), MR Egger (dark blue), weighed median (dark green), weighted mode (red), and simple mode (light green). SNP, single nucleotide polymorphism; MR, mendelian randomization.
Discussion
In this MR study, we found that anti-H. pylori IgG level was causally associated with preeclampsia or eclampsia and premature rupture of membranes, but not associated with other pregnancy (miscarriage, gestational diabetes mellitus, placental abruption, and postpartum haemorrhage) and neonatal (birthweight, gestational age, and preterm birth) outcomes.
Emerging evidence has shown the role of H. pylori infection in preeclampsia-eclampsia. In 2006, Ponzetto et al. (Ponzetto et al., 2006) first reported that pregnant women with preeclampsia had a 19.2% higher rate of H. pylori seropositivity compared with uncomplicated women. Following that, other groups also demonstrated an epidemiological association between H. pylori infection and preeclampsia, especially for women who were infected with cytotoxin-associated gene A (CagA) positive strains (Mosbah and Nabiel, 2016; Bellos et al., 2018; Nourollahpour Shiadeh et al., 2019). In vitro studies further showed that anti-CagA antibodies could cross-react with cytotrophoblast cells through β-actin, thus reducing their invasiveness by decreasing ERK 1/2 activation, NF-kB translocation and MMP-2 expression (Franceschi et al., 2012). Since trophoblast invasion of maternal decidua is vital for embryo implantation and placental development, the infection-induced autoimmunity may lead to inadequate placentation and preeclampsia onset (Tersigni et al., 2014). In addition, Di Simone et al. (Di Simone et al., 2017) found that H. pylori infection was associated with abnormality of uterine arteries Doppler velocimetry in preeclamptic women, and anti-H. pylori IgG fractions from these women could inhibit endothelial cells’ proliferation, migration and differentiation both in vitro and in vivo. Therefore, H. pylori infection may also have an impact on the angiogenesis and vascular resistance, which constitutes an important mechanism of preeclampsia pathogenesis (Chaiworapongsa et al., 2014).
For the correlation with premature rupture of membranes, the mechanisms are still unclear but may be associated with systematic and local effects of H. pylori infection. On the one hand, H. pylori could stimulate the release of pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor-α, and macrophage migration inhibitory factor (Xia et al., 2005; UstUn et al., 2010; Chen et al., 2022). Among infected patients, systemic indices of inflammation were also observed to be elevated, including white blood cell count and C-reactive protein (Graham et al., 1998; Oshima et al., 2005; UstUn et al., 2010). Given the crucial role of the inflammation-oxidative stress axis in fetal membrane weakening (Menon and Richardson, 2017), the infection may thus lead to premature rupture of membranes. On the other hand, research found that extracellular vesicles (EVs) could be derived from H. pylori-infected gastric epithelial cells and entered the blood circulation (Xia et al., 2020). Additionally, outer membrane vesicles (OMVs) released by H. pylori also existed in the serum samples (Park and Tsunoda, 2022). Both EVs and OMVs could serve as transport vehicles to deliver pathogenic virulence factors (e.g., CagA) to extragastric organs including brain (Qiang et al., 2022; Xie et al., 2023). In this regard, the fetal membrane may be directly affected as well, while further studies are warranted for investigation.
Consistent with previous pooled results (Tang et al., 2021), our study did not support the link between H. pylori infection and preterm birth. However, several cohorts have shown an increased risk of miscarriage (Hajishafiha et al., 2011), gestational diabetes mellitus (Cardaropoli et al., 2015; Li et al., 2020; Tang et al., 2021), and low birth weight (Wanyama et al., 2016; Grooten et al., 2017; Zhan et al., 2019) among infected pregnant women, which were not detected by the current MR analysis. This may be due to the insufficiency of cases as well as residual confounding of observational design. As for placental abruption and postpartum haemorrhage, no relevant research is available thus far and their associations with H. pylori infection remain to be explored.
To our knowledge, this is the first MR study on the association of H. pylori infection with a series of pregnancy and neonatal outcomes, thus eliminating the interference of confounding factors and reverse causation. To avoid sample overlap and its associated bias, we used the exposure and outcome datasets from multiple independent GWAS. An iterative MR analysis with five different approaches was conducted to acquire conservative results, and the absence of pleiotropy and heterogeneity in most sensitivity analyses further rule out the false-positive likelihood.
There are some limitations of the current study that should be acknowledged. Firstly, in order to minimize demographic bias, we selected only data from people of European descent, making the generalizability of our finding to other ethnic populations compromised. Secondly, the GWAS sample size of H. pylori infection was relatively small. Based on the traditional GWAS significance threshold (P <5×10−8), the SNPs obtained were too few for further study. Therefore, we used the locus-wide significance level (P <1×10-5) for SNP selection, which may introduce weak instrument bias to the overall estimates. Thirdly, our analysis was based on summary statistics instead of raw data, and it was not possible to conduct further subgroup analyses on the specific strain of H. pylori (e.g., CagA-positive and CagA-negative) and severe degree or subtype of disease (e.g., early-onset and late-onset preeclampsia). Lastly, serological tests measuring the overall antibody immune response towards H. pylori are incapable of distinguishing ongoing from past infection. For a more specific differentiation, additional information is needed such as 13C-urea breath test, histological examination, or eradication record (Sabbagh et al., 2019). Therefore, the present study using genetically-predicted H. pylori seropositivity only represent the susceptibility to infection, and further studies are needed to compare the outcomes of different infection status.
Conclusion
In summary, this two-sample MR study showed that genetically predicted H. pylori infection was causally associated with increased risks of preeclampsia-eclampsia and premature rupture of membranes. Our findings confirm the epidemiological evidence on the adverse impact of H. pylori in pregnancy. Further studies are needed to thoroughly elucidate the pathophysiological mechanisms and assess the effectiveness of pre-pregnancy screening and preventive eradication.
Data availability statement
All GWAS summary data used are publicly available. The download links are detailed in Supplementary Table 1.
Ethics statement
Ethical approval was not required because this study was based solely on publicly available GWAS summary-level statistics and no individual-level data were used.
Author contributions
JH: Formal analysis, Funding acquisition, Writing – original draft. YL: Formal analysis, Writing – original draft. DX: Investigation, Writing – review & editing. MC: Data curation, Writing – review & editing. QX: Data curation, Writing – review & editing. JC: Data curation, Writing – review & editing. LX: Data curation, Writing – review & editing. LY: Data curation, Writing – review & editing. QW: Data curation, Writing – review & editing. ZL: Conceptualization, Project administration, Writing – review & editing. JW: Conceptualization, Supervision, Writing – review & editing. LT: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82260315), Natural Science Foundation of Jiangxi Province (20224BAB216025), and Central Funds Guiding the Local Science and Technology Development (20221ZDG020071).
Acknowledgments
The authors express their gratitude to all participants and investigators of GWASs for their contributions and data sharing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1343499/full#supplementary-material
Abbreviations
ALSPAC, Avon Longitudinal Study of Parents and Children Cohort; CagA, cytotoxin-associated gene A; CI, confidence interval; EVs, extracellular vesicles; GWAS, genome-wide association studies; H. pylori, Helicobacter pylori; IL, interleukin; IV, instrumental variable; IVW, inverse variance weighted; MAF, minor allele frequency; MR, Mendelian randomization; MRC-IEU, Medical Research Council Integrative Epidemiology Unit; MR-PRESSO, MR-pleiotropy residual sum and outlier; OMVs, outer membrane vesicles; OR, odds ratio; SNPs, single nucleotide polymorphisms.
References
Baingana, R. K., Kiboko Enyaru, J., Davidsson, L. (2014). Helicobacter pylori infection in pregnant women in four districts of Uganda: role of geographic location, education and water sources. BMC Public Health 14, 915. doi: 10.1186/1471-2458-14-915
Bellos, I., Daskalakis, G., Pergialiotis, V. (2018). Helicobacter pylori infection increases the risk of developing preeclampsia: A meta-analysis of observational studies. Int. J. Clin. Pract. 72. doi: 10.1111/ijcp.13064
Bowden, J., Davey Smith, G., Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Bowden, J., Del Greco, M. F., Minelli, C., Davey Smith, G., Sheehan, N., Thompson, J. (2017). A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat. Med. 36, 1783–1802. doi: 10.1002/sim.7221
Burgess, S., Thompson, S. G. (2021). Mendelian randomization: methods for causal inference using genetic variants (Boca Raton, Florida, USA: CRC Press).
Cardaropoli, S., Giuffrida, D., Piazzese, A., Todros, T. (2015). Helicobacter pylori seropositivity and pregnancy-related diseases: a prospective cohort study. J. Reprod. Immunol. 109, 41–47. doi: 10.1016/j.jri.2015.02.004
Chaiworapongsa, T., Chaemsaithong, P., Yeo, L., Romero, R. (2014). Pre-eclampsia part 1: current understanding of its pathophysiology. Nat. Rev. Nephrol. 10, 466–480. doi: 10.1038/nrneph.2014.102
Chen, M., Huang, X., Gao, M., Yang, Z., Fang, Z., Wei, J., et al. (2022). Helicobacter pylori promotes inflammatory factor secretion and lung injury through VacA exotoxin-mediated activation of NF-kappaB signaling. Bioengineered 13, 12760–12771. doi: 10.1080/21655979.2022.2071011
Chong, A. H. W., Mitchell, R. E., Hemani, G., Davey Smith, G., Yolken, R. H., Richmond, R. C., et al. (2021). Genetic analyses of common infections in the avon longitudinal study of parents and children cohort. Front. Immunol. 12, 727457. doi: 10.3389/fimmu.2021.727457
Davies, N. M., Holmes, M. V., Davey Smith, G. (2018). Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362, k601. doi: 10.1136/bmj.k601
den Hollander, W. J., Schalekamp-Timmermans, S., Holster, I. L., Jaddoe, V. W., Hofman, A., Moll, H. A., et al. (2017). Helicobacter pylori colonization and pregnancies complicated by preeclampsia, spontaneous prematurity, and small for gestational age birth. Helicobacter 22. doi: 10.1111/hel.12364
Di Simone, N., Tersigni, C., Cardaropoli, S., Franceschi, F., Di Nicuolo, F., Castellani, R., et al. (2017). Helicobacter pylori infection contributes to placental impairment in preeclampsia: basic and clinical evidences. Helicobacter 22. doi: 10.1111/hel.12347
Franceschi, F., Di Simone, N., D’Ippolito, S., Castellani, R., Di Nicuolo, F., Gasbarrini, G., et al. (2012). Antibodies anti-CagA cross-react with trophoblast cells: a risk factor for pre-eclampsia? Helicobacter 17, 426–434. doi: 10.1111/j.1523-5378.2012.00966.x
Graham, D. Y., Osato, M. S., Olson, C. A., Zhang, J., Figura, N. (1998). Effect of H. pylori infection and CagA status on leukocyte counts and liver function tests: extra-gastric manifestations of H. pylori infection. Helicobacter 3, 174–178. doi: 10.1046/j.1523-5378.1998.08018.x
Grooten, I. J., Den Hollander, W. J., Roseboom, T. J., Kuipers, E. J., Jaddoe, V. W., Gaillard, R., et al. (2017). Helicobacter pylori infection: a predictor of vomiting severity in pregnancy and adverse birth outcome. Am. J. Obstet Gynecol. 216, 512 e1–512 e9. doi: 10.1016/j.ajog.2017.01.042
Hajishafiha, M., Ghasemi-Rad, M., Memari, A., Naji, S., Mladkova, N., Saeedi, V. (2011). Effect of Helicobacter pylori infection on pregnancy rates and early pregnancy loss after intracytoplasmic sperm injection. Int. J. Womens Health 3, 329–335. doi: 10.2147/IJWH.S24424
Hartwig, F. P., Davey Smith, G., Bowden, J. (2017). Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int. J. Epidemiol. 46, 1985–1998. doi: 10.1093/ije/dyx102
Hemani, G., Tilling, K., Davey Smith, G. (2017). Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PloS Genet. 13, e1007081. doi: 10.1371/journal.pgen.1007081
Kamboj, A. K., Cotter, T. G., Oxentenko, A. S. (2017). Helicobacter pylori: the past, present, and future in management. Mayo Clin. Proc. 92, 599–604. doi: 10.1016/j.mayocp.2016.11.017
Kusters, J. G., van Vliet, A. H., Kuipers, E. J. (2006). Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 19, 449–490. doi: 10.1128/CMR.00054-05
Laisk, T., Soares, A. L. G., Ferreira, T., Painter, J. N., Censin, J. C., Laber, S., et al. (2020). The genetic architecture of sporadic and multiple consecutive miscarriage. Nat. Commun. 11, 5980. doi: 10.1038/s41467-020-19742-5
Lanciers, S., Despinasse, B., Mehta, D. I., Blecker, U. (1999). Increased susceptibility to Helicobacter pylori infection in pregnancy. Infect. Dis. Obstet Gynecol. 7, 195–198. doi: 10.1155/S1064744999000332
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N., Davey Smith, G. (2008). Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27, 1133–1163. doi: 10.1002/sim.3034
Li, C., Liu, C., Li, N. (2022). Causal associations between gut microbiota and adverse pregnancy outcomes: A two-sample Mendelian randomization study. Front. Microbiol. 13, 1059281. doi: 10.3389/fmicb.2022.1059281
Li, L., Tan, J., Liu, L., Li, J., Chen, G., Chen, M., et al. (2020). Association between H. pylori infection and health Outcomes: an umbrella review of systematic reviews and meta-analyses. BMJ Open 10, e031951. doi: 10.1136/bmjopen-2019-031951
Li, P., Wang, H., Guo, L., Gou, X., Chen, G., Lin, D., et al. (2022). Association between gut microbiota and preeclampsia-eclampsia: a two-sample Mendelian randomization study. BMC Med. 20, 443. doi: 10.1186/s12916-022-02657-x
Liu, X., Helenius, D., Skotte, L., Beaumont, R. N., Wielscher, M., Geller, F., et al. (2019). Variants in the fetal genome near pro-inflammatory cytokine genes on 2q13 associate with gestational duration. Nat. Commun. 10, 3927. doi: 10.1038/s41467-019-11881-8
McColl, K. E. (2010). Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 362, 1597–1604. doi: 10.1056/NEJMcp1001110
Menon, R., Richardson, L. S. (2017). Preterm prelabor rupture of the membranes: A disease of the fetal membranes. Semin. Perinatol. 41, 409–419. doi: 10.1053/j.semperi.2017.07.012
Mosbah, A., Nabiel, Y. (2016). Helicobacter pylori, Chlamydiae pneumoniae and trachomatis as probable etiological agents of preeclampsia. J. Matern Fetal Neonatal Med. 29, 1607–1612. doi: 10.3109/14767058.2015.1056146
Mubarak, N., Gasim, G. I., Khalafalla, K. E., Ali, N. I., Adam, I. (2014). Helicobacter pylori, anemia, iron deficiency and thrombocytopenia among pregnant women at Khartoum, Sudan. Trans. R. Soc. Trop. Med. Hyg. 108, 380–384. doi: 10.1093/trstmh/tru044
Nourollahpour Shiadeh, M., Riahi, S. M., Adam, I., Saber, V., Behboodi Moghadam, Z., Armon, B., et al. (2019). Helicobacter pylori infection and risk of preeclampsia: a systematic review and meta-analysis. J. Matern Fetal Neonatal Med. 32, 324–331. doi: 10.1080/14767058.2017.1378331
Oshima, T., Ozono, R., Yano, Y., Oishi, Y., Teragawa, H., Higashi, Y., et al. (2005). Association of Helicobacter pylori infection with systemic inflammation and endothelial dysfunction in healthy male subjects. J. Am. Coll. Cardiol. 45, 1219–1222. doi: 10.1016/j.jacc.2005.01.019
Park, A. M., Tsunoda, I. (2022). Helicobacter pylori infection in the stomach induces neuroinflammation: the potential roles of bacterial outer membrane vesicles in an animal model of Alzheimer’s disease. Inflammation Regen. 42, 39. doi: 10.1186/s41232-022-00224-8
Ponzetto, A., Cardaropoli, S., Piccoli, E., Rolfo, A., Gennero, L., Kanduc, D., et al. (2006). Pre-eclampsia is associated with Helicobacter pylori seropositivity in Italy. J. Hypertens. 24, 2445–2449. doi: 10.1097/HJH.0b013e3280109e8c
Qiang, L., Hu, J., Tian, M., Li, Y., Ren, C., Deng, Y., et al. (2022). Extracellular vesicles from helicobacter pylori-infected cells and helicobacter pylori outer membrane vesicles in atherosclerosis. Helicobacter 27, e12877. doi: 10.1111/hel.12877
Sabbagh, P., Mohammadnia-Afrouzi, M., Javanian, M., Babazadeh, A., Koppolu, V., Vasigala, V. R., et al. (2019). Diagnostic methods for Helicobacter pylori infection: ideals, options, and limitations. Eur. J. Clin. Microbiol. Infect. Dis. 38, 55–66. doi: 10.1007/s10096-018-3414-4
Sanna, S., van Zuydam, N. R., Mahajan, A., Kurilshikov, A., Vich Vila, A., Vosa, U., et al. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605. doi: 10.1038/s41588-019-0350-x
Tang, Y., Yang, Y., Lv, Z. (2021). Adverse pregnancy outcomes and Helicobacter pylori infection: A meta-analysis. Int. J. Clin. Pract. 75, e14588. doi: 10.1111/ijcp.14588
Tersigni, C., Franceschi, F., Todros, T., Cardaropoli, S., Scambia, G., Di Simone, N. (2014). Insights into the Role of Helicobacter pylori Infection in Preeclampsia: From the Bench to the Bedside. Front. Immunol. 5, 484. doi: 10.3389/fimmu.2014.00484
UstUn, Y., Engin-UstUn, Y., Ozkaplan, E., Otlu, B., Sait TekerekoGlu, M. (2010). Association of Helicobacter pylori infection with systemic inflammation in preeclampsia. J. Matern Fetal Neonatal Med. 23, 311–314. doi: 10.3109/14767050903121456
Verbanck, M., Chen, C. Y., Neale, B., Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50, 693–698. doi: 10.1038/s41588-018-0099-7
Wanyama, R., Kagawa, M. N., Opio, K. C., Baingana, R. K. (2016). Effect of maternal Helicobacter Pylori infection on birth weight in an urban community in Uganda. BMC Pregnancy Childbirth. 16, 158. doi: 10.1186/s12884-016-0950-8
Xia, H. H., Lam, S. K., Chan, A. O., Lin, M. C., Kung, H. F., Ogura, K., et al. (2005). Macrophage migration inhibitory factor stimulated by Helicobacter pylori increases proliferation of gastric epithelial cells. World J. Gastroenterol. 11, 1946–1950. doi: 10.3748/wjg.v11.i13.1946
Xia, X., Zhang, L., Chi, J., Li, H., Liu, X., Hu, T., et al. (2020). Helicobacter pylori infection impairs endothelial function through an exosome-mediated mechanism. J. Am. Heart Assoc. 9, e014120. doi: 10.1161/JAHA.119.014120
Xie, J., Cools, L., Van Imschoot, G., Van Wonterghem, E., Pauwels, M. J., Vlaeminck, I., et al. (2023). Helicobacter pylori-derived outer membrane vesicles contribute to Alzheimer’s disease pathogenesis via C3-C3aR signalling. J. Extracell Vesicles. 12, e12306. doi: 10.1002/jev2.12306
Zamani, M., Ebrahimtabar, F., Zamani, V., Miller, W. H., Alizadeh-Navaei, R., Shokri-Shirvani, J., et al. (2018). Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol. Ther. 47, 868–876. doi: 10.1111/apt.14561
Keywords: Helicobacter pylori, pregnancy, Mendelian randomization, preeclampsia, premature rupture of membranes
Citation: Huang J, Liu Y, Xu D, Chen M, Xie Q, Chen J, Xia L, Yu L, Wu Q, Li Z, Wang J and Tian L (2024) Causal associations between Helicobacter pylori infection and pregnancy and neonatal outcomes: a two-sample Mendelian randomization study. Front. Cell. Infect. Microbiol. 14:1343499. doi: 10.3389/fcimb.2024.1343499
Received: 05 December 2023; Accepted: 13 February 2024;
Published: 14 March 2024.
Edited by:
Maria Oana Sasaran, “George Emil Palade” University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaReviewed by:
Michał Jacek Sobkowiak, Karolinska Institutet (KI), SwedenVasile Valeriu Lupu, Grigore T. Popa University of Medicine and Pharmacy, Romania
Carolina Romo-Gonzalez, National Institute of Pediatrics, Mexico
Copyright © 2024 Huang, Liu, Xu, Chen, Xie, Chen, Xia, Yu, Wu, Li, Wang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lifeng Tian, dGlhbmxmX2ptY2hoQDEyNi5jb20=; Jiawei Wang, NjE3MjkwNTM1QHFxLmNvbQ==; Zengming Li, bGl6aGVuZ21pbmdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jialyu Huang
Jialyu Huang Yuxin Liu
Yuxin Liu Dingfei Xu1†
Dingfei Xu1† Jia Chen
Jia Chen Leizhen Xia
Leizhen Xia Qiongfang Wu
Qiongfang Wu Zengming Li
Zengming Li Jiawei Wang
Jiawei Wang Lifeng Tian
Lifeng Tian