- CBR Division, Defence Science and Technology Laboratory (Dstl), Salisbury, United Kingdom
Animal models of infectious disease often serve a crucial purpose in obtaining licensure of therapeutics and medical countermeasures, particularly in situations where human trials are not feasible, i.e., for those diseases that occur infrequently in the human population. The common marmoset (Callithrix jacchus), a Neotropical new-world (platyrrhines) non-human primate, has gained increasing attention as an animal model for a number of diseases given its small size, availability and evolutionary proximity to humans. This review aims to (i) discuss the pros and cons of the common marmoset as an animal model by providing a brief snapshot of how marmosets are currently utilized in biomedical research, (ii) summarize and evaluate relevant aspects of the marmoset immune system to the study of infectious diseases, (iii) provide a historical backdrop, outlining the significance of infectious diseases and the importance of developing reliable animal models to test novel therapeutics, and (iv) provide a summary of infectious diseases for which a marmoset model exists, followed by an in-depth discussion of the marmoset models of two studied bacterial infectious diseases (tularemia and melioidosis) and one viral infectious disease (viral hepatitis C).
1 Introduction
1.1 The common marmoset (Callithrix jacchus)
The common marmoset (Callithrix jacchus), henceforth referred to as the marmoset, is a Neotropical new-world (now increasingly referred to as platyrrhines) non-human primate (NHP) native to the north-eastern regions of Brazil. Having diverged from humans some 33 million years ago, the common marmoset is phylogenetically and anatomically more similar to humans than rats or mice which diverged approximately 96 million years ago. As such, a significant degree of cross-reactivity of reagents designed for human targets with those in the marmoset is observed (Barton et al., 1984; Neubert et al., 1996; Kireta et al., 2005; Jagessar et al., 2013; Neumann et al., 2016). However, a greater evolutionary distance exists between the divergence of new-world NHPs from humans compared with old-world (now increasingly referred to as catarrhines) NHPs (e.g. rhesus macaques and cynomolgus macaques) from humans, with the latter occurring some 23 million years ago (Mansfield, 2003). Consequently, there exists more physiological and immunological differences between humans and marmosets than humans and old-world primates, which have traditionally been used as NHP models of various human diseases. Nevertheless, the marmoset represents an attractive alternative to the old-world primates and this is reflected by their increasing use in the field of biomedical science.
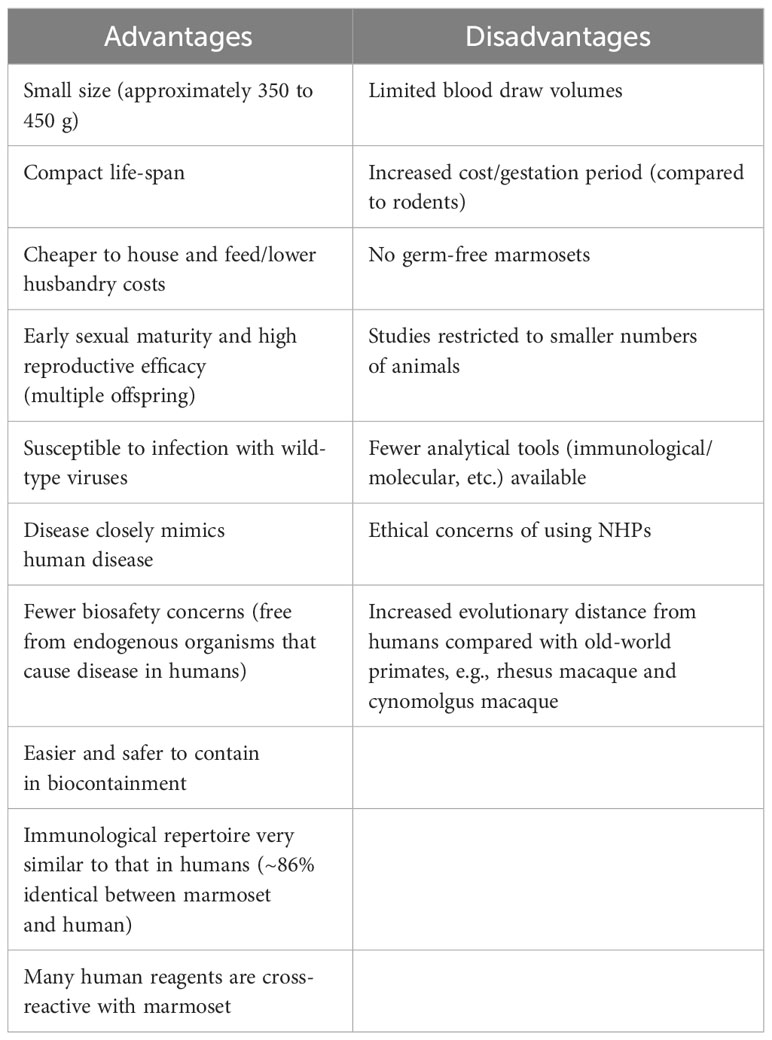
Whilst a comprehensive review of the basic biology and physiology of the marmoset is beyond the scope of this manuscript, the reader is directed to a number of excellent reviews published on these topics (Abbott et al., 2003; Orsi et al., 2011; ‘T Hart et al., 2012; Preuss, 2019). Here, a brief overview of the marmoset is presented to provide the reader with sufficient background to appreciate the pros and cons of using this new-world primate as a model of infectious diseases. This information is also summarized in Table 1. The marmoset is considerably smaller than the old-world primates, weighing around 350 to 450 g with a body size comparable to that of a rat (Orsi et al., 2011). As such, animals are more easily handled and the associated costs (e.g., husbandry, housing, feeding, etc.) are reduced considerably. Additionally, their smaller size makes biocontainment both safer and cheaper. The small size of the marmoset means smaller amounts of a given test substance/therapeutic can be administered, again reducing costs and aiding where manufacture is difficult. Aside from their small size, marmosets have a compact life-span and reach sexual maturity in approximately 1.5 years. Marmosets are easily bred in captivity and frequently give birth to multiple offspring; these offspring are born as bone marrow chimeric twins that are the result of fusion of the placental bloodstreams (Benirschke et al., 1962; Sweeney et al., 2012). Consequently, marmoset twins are immunologically highly comparable. In this regard, the marmoset is biologically unique; researchers can exploit this aspect of their biology to perform paired experimental analyses, i.e., where one sibling receives treatment with a given therapeutic and the other receives a placebo. Such paired analyses are highly beneficial, particularly in pre-clinical studies. Further, marmoset twins have been used in adoptive transfer experiments in the study of the pathogenesis of multiple sclerosis (MS) (Massacesi et al., 1995; Genain and Hauser, 1997). Importantly, marmosets are a naturally outbred species and are exposed to environmental factors (e.g., bacteria) that shape their developing immune systems. As the links between the environmental microbiome and host immune system continue to emerge, this feature of the marmoset is particularly advantageous as it better reflects the human condition. Marmosets are susceptible to infection with many wild-type viruses that, in their native forms, either do not cause disease or cause a different disease in the mouse (Mansfield, 2003; Carrion and Patterson, 2012). Indeed, to render mice vulnerable to infection, an adapted rodent virus is frequently used. These viruses, although based on the wild-type virus, are genetically modified and thus may fail to recapitulate human disease (Sarkar and Heise, 2019). Finally, and of particular importance to infection models, marmosets are not known to carry endogenous viruses that cause disease in humans (Abbott et al., 2003). Thus, with fewer biosafety considerations the marmoset represents an animal model that is safer, cheaper and less labor intensive.
Whilst the marmoset presents a number of practical advantages, it is vital that the potential disadvantages of the species are not overlooked. For example, though the marmoset is comparatively cheaper and easier to handle than the larger old-world primates, mice are both considerably smaller and cheaper than the marmoset. Whilst the small size of the marmoset may be advantageous, this may also limit what procedures/techniques can be performed. For example, the amount of blood that can be obtained from a live marmoset is typically 1% of its body weight (Jagessar et al., 2013; ‘T Hart, 2019). A study wishing to perform comprehensive immunophenotyping of marmoset immune cells may not be feasible given the limited amount of blood available at each blood draw – particularly those studies incorporating large panels that require multiple controls. While outbred animals are more representative to humans, this heterogeneity may produce more variability in experimental outcome, necessitating greater numbers. Studies involving NHPs are also limited to a smaller number of animals, which can negatively influence statistical power. Finally, and most importantly, any study involving NHPs is subject to ethical concerns, concerns for the wellbeing of the animals and ever-growing societal and political pressures. Any study using NHPs will require specialist facilities and trained staff, including veterinary staff.
1.2 Marmosets in biomedical research
Marmosets have been used in biomedical research for many decades. Over the past twenty or so years, marmoset research has increased in pace with biomedical research in general, driven in part by a growing inventory of reagents and analytical tools. Notable advances include the sequencing of the marmoset genome (Worley et al., 2014), the generation of transgenic animals by germline transmission (Sasaki et al., 2009; Tomioka et al., 2017a; Tomioka et al., 2017b), the creation of gene knockout marmoset models (Kumita et al., 2019; Yoshimatsu et al., 2019) and an ever-growing array of marmoset-specific reagents, including microarrays (Datson et al., 2007), ELISA and ELISPOT assays (Zhu et al., 2016), and monoclonal antibodies (Kametani et al., 2009). A number of marmoset-specific monoclonal antibodies are available commercially; however, these are specific to a few targets and conjugated to only few commonly used fluorophores. In spite of the challenges presented by reagent availability and technical issues, the marmoset has been utilized as an appropriate animal model in a number of contexts, including infectious disease, autoimmunity, neurobiology and, more traditionally, in developmental biology, reproductive biology, toxicology/drug development, and behavioral research. Since the focus of this review is infectious disease, a comprehensive discussion of each of these areas of research is simply not feasible. The reader is directed to a number of excellent review articles, which outline the value of the marmoset in these contexts (Mansfield, 2003; ‘T Hart et al., 2012; Okano et al., 2012; Han et al., 2022; Inoue et al., 2022).
Marmoset models utilized in neuroscience, behavioral science and reproductive biology are very well characterized, and there is a wealth of published literature in these areas. In contrast, one area that remains relatively unexplored is the marmoset immune system and the mechanisms of immune regulation. As noted, this is partly due to the limited availability of analytical tools and reagents that cross-react with the marmoset. Given their phylogenetic similarity to humans, the marmoset immune system is likely more similar to our own than that of a mouse. Nevertheless, much of our understanding of the molecular basis of the human immune system has been elucidated or predicted using murine experimental models. Thus, to understand the value of the marmoset in immunology research, a more in-depth characterization of the marmoset immune system is required. Such an endeavor would lead to the development of a wider array of analytical tools and reagents specific for the marmoset. A greater characterization of the marmoset immune system would benefit a number of existent marmoset disease models. In the proceeding section, important immunological features that are relevant to the study of infectious disease are outlined, with an emphasis on the reagents and techniques developed for the marmoset.
2 Marmoset immunology: like mice and man?
To best utilize the marmoset in immunological research, we need to understand the marmoset immune system. To use the marmoset as a surrogate of human diseases and conditions, we need to be confident that what we see in the marmoset actually recapitulates what we see in humans. Though many aspects of marmoset immunology remain elusive, several important findings that highlight the similarities and differences between marmoset and man have been reported over the years.
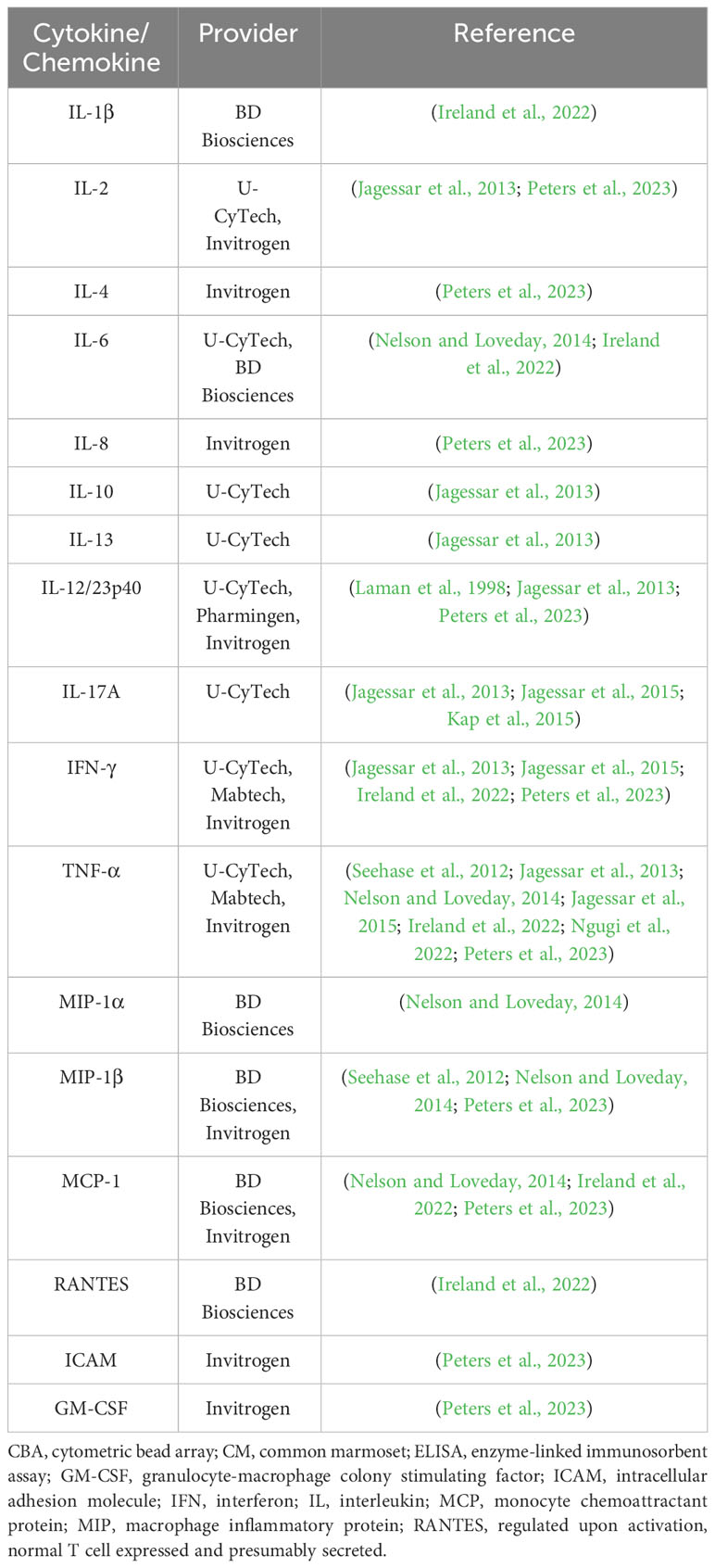
The ability of the immune system to recognize foreign (non-self) antigens is central to the adaptive immune response. One indicator of an immune systems breadth is the variability of the molecules involved in antigen recognition, [i.e., major histocompatibility complex (MHC) molecules, T-cell receptors (TCRs) and immunoglobulins (Igs)] (Kametani et al., 2018). The structure of the MHC in the marmoset has been elucidated. In the marmoset, class I MHC molecules are encoded by Caja genes (Caja-B, Caja-G, Caja-F loci), which are orthologs of the human leukocyte antigen (HLA) genes (classical: HLA-A, HLA-B, HLA-C; non-classical: HLA-G, HLA-E) in humans (Shiina et al., 2011). Caja genes exhibit a high degree of homology with human HLA genes, particularly Caja-G and HLA-G, which are evolutionarily closely related (Kametani et al., 2018). Importantly, HLA orthologs have not been identified in rodents (Kametani et al., 2018), reflecting the increased evolutionary distance between mouse and man. In spite of these similarities, marmoset Caja genes are associated with multiple alleles at each locus, but the diversity is nevertheless limited in the marmoset compared to that in man (Shiina et al., 2011; Kono et al., 2014). Furthermore, the human homolog of Caja-G (i.e., HLA-G) is a non-classical MHC molecule, represented by a single gene locus with a low number of alleles. The expression of Caja-G is restricted to cells of the placenta and on certain regulatory T-cells (Ferreira et al., 2017; Kametani et al., 2018; Zhuang et al., 2021). HLA-G has been suggested to possess immunosuppressive functions (Lin and Yan, 2016). Conversely, in the marmoset, Caja-G is ubiquitously expressed and polymorphic, more akin to human classical class I HLA molecules (Van Der Wiel et al., 2013; Kono et al., 2014; Li et al., 2014a; Neehus et al., 2016). The specific function of Caja-G in the marmoset in unclear, but it may possess immune activating functions (Münz et al., 1999; Neehus et al., 2016; Kametani et al., 2018). Uncovering the role of Caja-G in the marmoset may provide valuable insight into the immunological mechanisms in the species. Orthologs of the genes encoding HLA-G ligands in man (LILRB1 and LILRB2) have also been predicted (Kametani et al., 2018). Aside from the class I HLA molecules, functional homologs of human HLA-DR and HLA-DQ (both encoding class II MHC molecules) are present in the marmoset but, relative to humans, the diversity of these molecules is restricted (Antunes et al., 1998). Nevertheless, the function of these class II orthologs appears to be similar to their human counterpart (Kametani et al., 2018). Evidence to support the divergence of Caja-DRB and the DRB*W16 allele in the marmoset has been reported (Prasad et al., 2006; Prasad et al., 2007). Aside from HLA molecules, the homology of the TCR repertoire between humans and marmosets is high, displaying a greater than 90% homology between man and marmoset in the CDR3-FR4 region (Matsutani et al., 2011; Kitaura et al., 2012). Homology of human and marmoset immunoglobulins are yet to be fully-characterized. Yet, in a recent study of primate genomes and transcriptomes by Olivieri and colleagues, immunoglobulin genes were identified (Olivieri and Gambón Deza, 2018). In the marmoset, an isotype of each class of immunoglobulin was identified. Notably, the CH2 exon of the IgD gene is absent in the marmoset, whilst the CH1 and CH3 exons are evolutionarily conserved (Olivieri and Gambón Deza, 2018). The diversity of the B-cell response in the marmoset is, however, predicted to be more restricted (Griffiths et al., 2006; Kametani et al., 2018). For those molecules involved in immune effector responses (e.g., cytokines), complementary DNA (cDNA) sequences and amino acid sequences between marmosets and humans were 86% identical, compared with 61% between mouse and humans (Kohu et al., 2008). Numerous approaches have been adopted for the analysis of marmoset cytokines and chemokines, measuring the level of expression at the protein (i.e., by enzyme-linked immunosorbent assays (ELISAs) and cytometric bead arrays (CBAs)) and mRNA (i.e., by quantitative polymerase chain reaction (qPCR)) level (Fujii et al., 2013; Jagessar et al., 2013; Ngugi et al., 2022). The assessment of intracellular cytokines has also been performed using flow cytometric techniques (Mietsch et al., 2020). A list of assays designed for analysis of serum cytokines and chemokines that are reported to work in the marmoset are presented in Table 2.
To understand the process of immune cell differentiation in the marmoset, there is a need to understand the primate hematopoietic system, and how this compares to humans. The markers CD34 and CD117 are used to identify hematopoietic stem cells (HSCs) in mice and humans (Okada et al., 1992; Galy et al., 1995). Human HSCs are CD34+ CD117lo, whereas mice HSCs are CD34- CD117+ (Papayannopoulou et al., 1991; Okada et al., 1992; Gunji et al., 1993; Galy et al., 1995; Mestas and Hughes, 2004). Identification and characterization of marmoset HSCs was made possible by the development of anti-marmoset CD34 and CD117 monoclonal antibodies (Izawa et al., 2004; Kametani et al., 2009; Shimada et al., 2015). Marmosets are reported to express both CD34 and CD117; however, the differentiation of CD117+ cells into cells of the erythroid and myeloid (but not lymphoid) lineages was not dependent on CD34 expression (Ito et al., 2002; Matsumura et al., 2003; Kametani et al., 2006; Ito et al., 2008a). Whilst the specific biological function of CD34 is unclear in humans, in the marmoset it may enhance engraftment following HSC transplantation, like the situation in humans (Kametani et al., 2018). When human HSCs were transplanted into NOG immunodeficient mice, B-cell development preceded T-cell development and CD4 and CD8 T-cells developed simultaneously (Ito et al., 2002; Yahata et al., 2002; Matsumura et al., 2003; Kametani et al., 2006). In contrast, following transplantation of marmoset HSCs into NOG mice, CD8 T-cell development occurred predominantly, with no B-cell or CD4 T-cell development (Kametani et al., 2018). These findings illustrate a key species difference in the hematopoietic system between human and marmoset. Efforts should be taken to understand how this difference might influence the function of the immune system.
A significant hurdle in the study of marmoset immunology is the lack of specific reagents and analytical tools. The limited availability of marmoset-specific monoclonal antibodies is particularly problematic and limits our ability to survey the immunological landscape of the marmoset. Unsurprisingly, increased interest in the marmoset in biomedicine has led to a number of groups developing and evaluating reagents (including monoclonal antibodies) designed specifically for the marmoset, leading to the commercial availability of marmoset reagents. Nevertheless, whilst progress has been made, there remains a pressing (and as of yet unmet) need for the wider availability of validated anti-marmoset antibodies. A comprehensive discussion of these reagents is beyond the scope of this review. However, a number of marmoset-specific antibodies against common surface antigens are reported in the literature, including anti-marmoset CD45, CD3, CD4, CD8 and CD25 (Brok et al., 2001; Ito et al., 2008b; Kametani et al., 2009; Jagessar et al., 2013; Neumann et al., 2016; Gordeychuk et al., 2018). Marmoset-specific anti-CD34 and anti-CD117 antibodies were also developed as described earlier (Izawa et al., 2004; Kametani et al., 2009; Shimada et al., 2015). Whilst this list is by no means exhaustive, it is worth pointing out that, to the best of our knowledge, the only marmoset-specific monoclonal antibodies currently available commercially recognize and bind CD45 and CD8. Unfortunately, the availability of fluorochromes for conjugation is limited. Numerous studies have evaluated anti-human monoclonal antibodies for cross-reactivity with marmoset antigens. Indeed, one report showed that 126 out of 331 monoclonal antibodies tested cross-reacted with peripheral blood mononuclear cells (PBMCs) from the marmoset (Brok et al., 2001). More recently, Neumann and colleagues evaluated a panel of 120 monoclonal antibodies for cross-reactivity against the marmoset, including testing of 97 different antibody clones (49 of which were not tested previously) against cell-surface markers, intracellular markers, chemokine receptors and cytokines (Neumann et al., 2016). Finally, it should be noted here that, despite the similarities between the human and the marmoset in terms of immune molecules, not all anti-human antibodies will cross-react with the marmoset; likewise, anti-marmoset CD4 and CD8 antibodies failed to cross-react with the corresponding antigen in humans (Gordeychuk et al., 2018). It is pivotal that care is taken to properly design, test and validate immunophenotyping panels, giving researchers the assurance and confidence in the data they generate.
An in-depth, comprehensive picture of the marmoset immune system is still lacking, though a snapshot of fundamental cellular immune components in healthy animals has begun to emerge (Nelson and Loveday, 2014; Neumann et al., 2016; Gordeychuk et al., 2018; Mietsch et al., 2020; Ngugi et al., 2022). All data discussed here relate to marmoset whole blood since data from other tissues is limited. Briefly, the constitution of the marmoset immune system is remarkably similar to our own: in blood, the majority (over 80%) of cells express CD45, the common leukocyte antigen; monocytes represent a minor proportion of CD45+ cells (<5%), whilst over 40% of cells were lymphocytes (Ross et al., 2012; Nelson and Loveday, 2014; Neumann et al., 2016; Gordeychuk et al., 2018; Mietsch et al., 2020; Ngugi et al., 2022). In terms of the distribution of immune cell subsets, reports from numerous research groups, including two from our own, are largely agreeable: total T-cells (CD3+) represent between 50 and 70% of lymphocytes, with between 20 and 30% of cells being B-cells (CD20+); the frequency of natural killer (NK) cells and γδ T-cells is low (<5%); within the CD3 T-cell compartment, 50 to 60% and 30 to 40% of cells express either the CD4 or CD8 co-receptors, respectively; and a small proportion of cells (<3%) express both CD4 and CD8 (Nelson and Loveday, 2014; Neumann et al., 2016; Gordeychuk et al., 2018; Mietsch et al., 2020; Ngugi et al., 2022). In one report, the frequency of cytotoxic T-cells (CD8+) was reported to be significantly higher in the marmoset than that seen in humans (Fujii et al., 2013), possibly due to the small number of animals and/or the CD8 antibody clone. Finally, neutrophils comprised approximately 35% of circulating cells (Nelson and Loveday, 2014; Neumann et al., 2016; Gordeychuk et al., 2018; Mietsch et al., 2020; Ngugi et al., 2022). Taken together, the frequency of immune cells in the marmoset mirrors humans better than how mice mirror humans.
3 Modelling infectious diseases in the marmoset: tularemia, melioidosis and hepatitis C virus
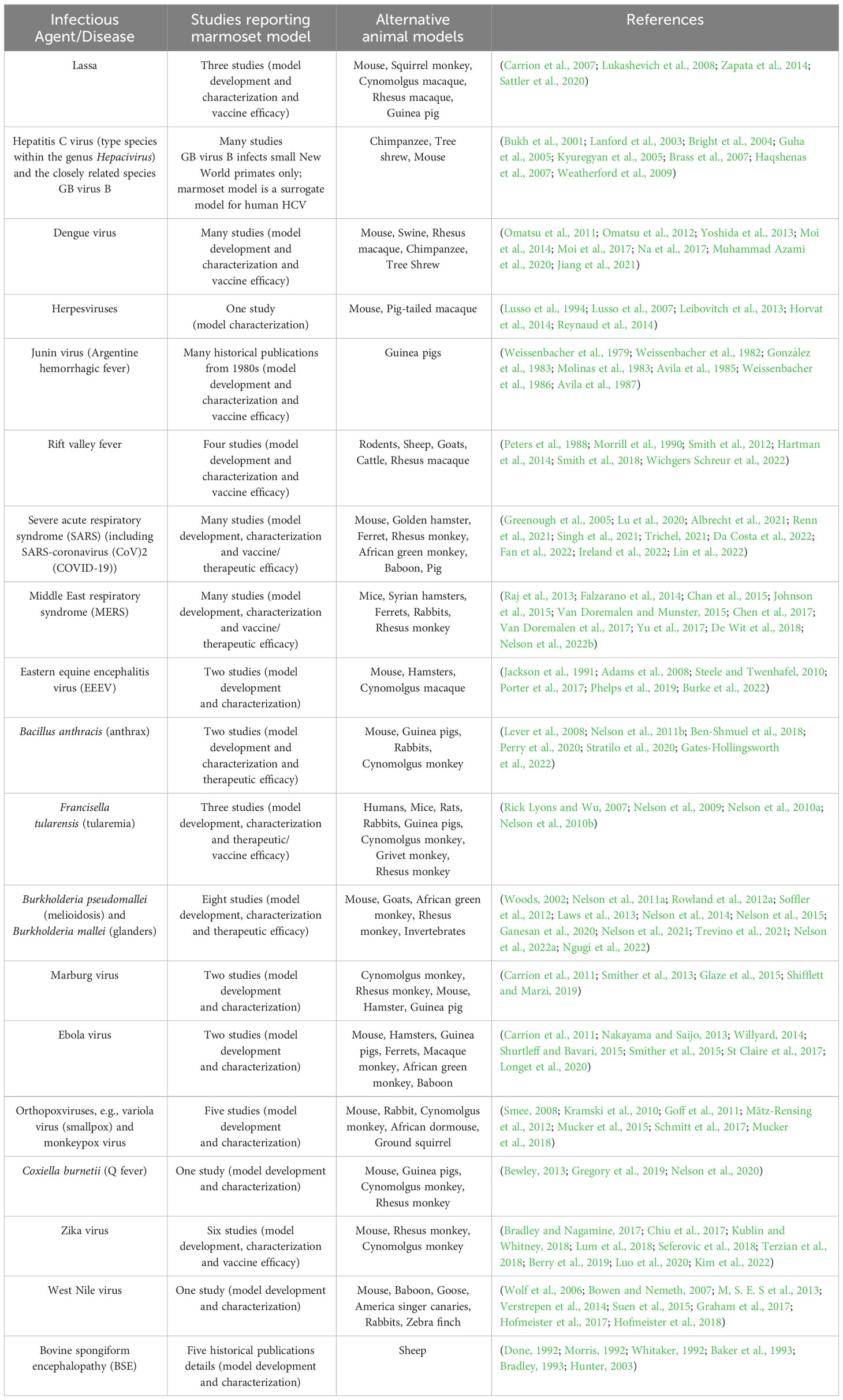
The marmoset has been used as an experimental model of several infectious diseases; this information, along with a summary of both the number of studies utilizing a marmoset disease model and alternative animal models, is provided in Table 3. A comprehensive discussion of each of these models is beyond the scope of this manuscript, thus the final section of this review will examine two experimental models of bacterial infection and one of viral infection that have been successfully developed in the marmoset: Francisella tularensis and Burkholderia pseudomallei, the etiological agents of tularemia and melioidosis, respectively, and hepatitis C virus (HCV) and the related GB virus B (GBV-B). Tularemia and melioidosis (and their respective causative agents) were selected for discussion given their potential for use as biological warfare agents; hepatitis C was selected since the marmoset has been shown to be susceptible to infection and therefore represents an important surrogate model. Whilst a discussion of the marmoset models of Ebola, Zika and influenza viruses would have been extremely interesting, these agents were not selected for further discussion in this review.
3.1 Animal models of infectious disease: introducing the 3 R’s and the animal efficacy rule
For many infectious diseases, disease incidence is too low to model in human populations. Studies involving humans are obviously subject to significant ethical concerns and, where diseases are fatal, human challenge studies are impossible. Nevertheless, modelling the efficacy of a potential medical countermeasure is a crucial step towards drug/therapy licensure (Gronvall et al., 2007; Dicarlo et al., 2011; Aebersold, 2012). Animal models are frequently used in an attempt to better understand disease pathogenesis in humans and to support both the identification of diagnostic correlates and effective treatment regimens (Gronvall et al., 2007). The use of animals in scientific research is tightly regulated and animals are used for research within an ethical framework. In the United Kingdom (UK), the Animals (Scientific Procedures) Act 1986 extends this ethical framework by imposing a set of comprehensive legal requirements for any institution wishing to undertake research involving animals (Hollands, 1986). In essence, research proposals involving animals are carefully reviewed to assess factors such as any harm animals might incur, the protocols and procedures involved, the number and types of animal used and the value of the study in terms of the potential benefits. Additionally, UK government introduced additional controls in 1998, namely the Ethical Review Process, with the aims of providing independent ethical advice for projects (Pietrzykowski, 2021). This move to promote an ethical analysis of a project and to enhance awareness of animal welfare issues is a fundamental part of engaging with the concept of the 3R’s (replacement, reduction and refinement) (Russell and Burch, 1959; Fenwick et al., 2009; Hubrecht and Carter, 2019). In a recent monography, ‘t Hart proposed a fourth R: relevance and particularly the clinical relevance of an animal model (‘T Hart, 2019). It is perhaps the relevance where the marmoset excels over murine models of infection. The FDA established the animal efficacy rule (or simply the animal rule) in 2002; this was later authorized by the United States Congress (Allio, 2018). The animal efficacy rule applies to all studies that aim to develop and/or test the efficacy of a given therapy against a life-threatening or life-changing biological, chemical, radiological or nuclear agent and where human efficacy trials are either unethical or not feasible.
3.2 Francisella tularensis
Francisella tularensis is a small, gram-negative, facultative intracellular coccobacillus and the causative agent of tularemia in humans (Wayne Conlan and Oyston, 2007). The bacterium was first isolated in 1911 from ground squirrels in Tulare County, California, and later in 1914 from a human in Ohio (Mccoy and Chapin, 1912; Wherry and Lamb, 1914). Three subspecies have been described: i) subsp. tularensis (type A strains), ii) subsp. holarctica (type B strains), and iii) subsp. mediasiatica; a fourth strain, generally considered a separate species given its aquatic reservoir and low virulence in humans, is F. novicida (Caspar and Maurin, 2017). Type A and B strains are responsible for the vast majority of tularemia cases in humans, with the type A strain being most virulent (Maurin, 2015). F. tularensis is a highly pathogenic organism that can cause severe and sometimes fatal disease in humans. An important aspect to F. tularensis virulence is its ability to replicate within eukaryotic cells, such as in the cytosol of macrophages (Steiner et al., 2014). Tularemia is a zoonotic disease; cases of the disease are typically sporadic or occur in small familial groups (Tärnvik and Berglund, 2003; Janse et al., 2018). Infection occurs via direct contact with infected animals, consumption of contaminated food or water, exposure to contaminated environments or via arthropod bites (e.g., mosquitoes and tics) (Keim et al., 2007; Carvalho et al., 2014). Lagomorphs and small rodents are the primary hosts of the pathogen (Maurin and Gyuranecz, 2016).
Tularemia symptoms vary depending on the route of exposure; six clinical forms of the disease have been described, namely: i) ulceroglandular, ii) glandular, iii) oropharyngeal, iv) oculoglandular, v) pneumonic, and vi) typhoidal (Yeni et al., 2021). Ulceroglandular and glandular forms (with or without skin ulcers at the inoculation site, respectively) result from skin exposure (e.g., via arthropods) and patients present with regional lymphadenopathy (Caspar and Maurin, 2017; Balestra et al., 2018). Oculoglandular tularemia results from exposure via the ocular conjunctiva and patients typically present with painful conjunctivitis and regional lymphadenopathy (Kantardjiev et al., 2007). Oropharyngeal tularemia usually results from ingestion of contaminated meat or water, leading to pharyngitis and regional lymphadenopathy (Steinrücken and Graber, 2014). Patients presenting with the pneumonic form of disease, caused by inhalation of airborne particles, experience cough, fever and dyspnea; mediastinal or hilar lymphadenopathy is sometimes observed (Gill and Cunha, 1997; Williams et al., 2019). Finally, typhoidal disease is characterized by systemic disease with neurological manifestations that mimic the symptoms of typhoid. Frequently, no symptoms of localized infection are observed, nor is the site of bacterial entry (Faucher et al., 2012). Complications of infection with F. tularensis include skin eruptions, abscess formation, suppuration of lymph nodes and the emergence of secondary infectious locations.
The potential of airborne transmission of F. tularensis infection, its ability to cause severe human disease and low infectious dose has led to the bacterium’s classification as a potential bioterrorism agent (Dennis et al., 2001). Diagnosis is challenging and is based on clinical and epidemiological features, serological tests and detection of microbial DNA by PCR. Since the isolation of the bacterium from blood and tissues of infected individuals occurs in less than 20% of cases, antibiotic susceptibility testing is difficult (Maurin et al., 2011). Treatment of tularemia is with antibiotics; the aminoglycosides, fluoroquinolones or tetracycline classes of antibiotic are recommended (Dennis et al., 2001; Ellis et al., 2002). No licensed tularemia vaccine is currently available, although a live attenuated vaccine is still in use in certain parts of the world where it is reserved to treat the most at-risk persons.
3.2.1 Common marmoset model of tularemia
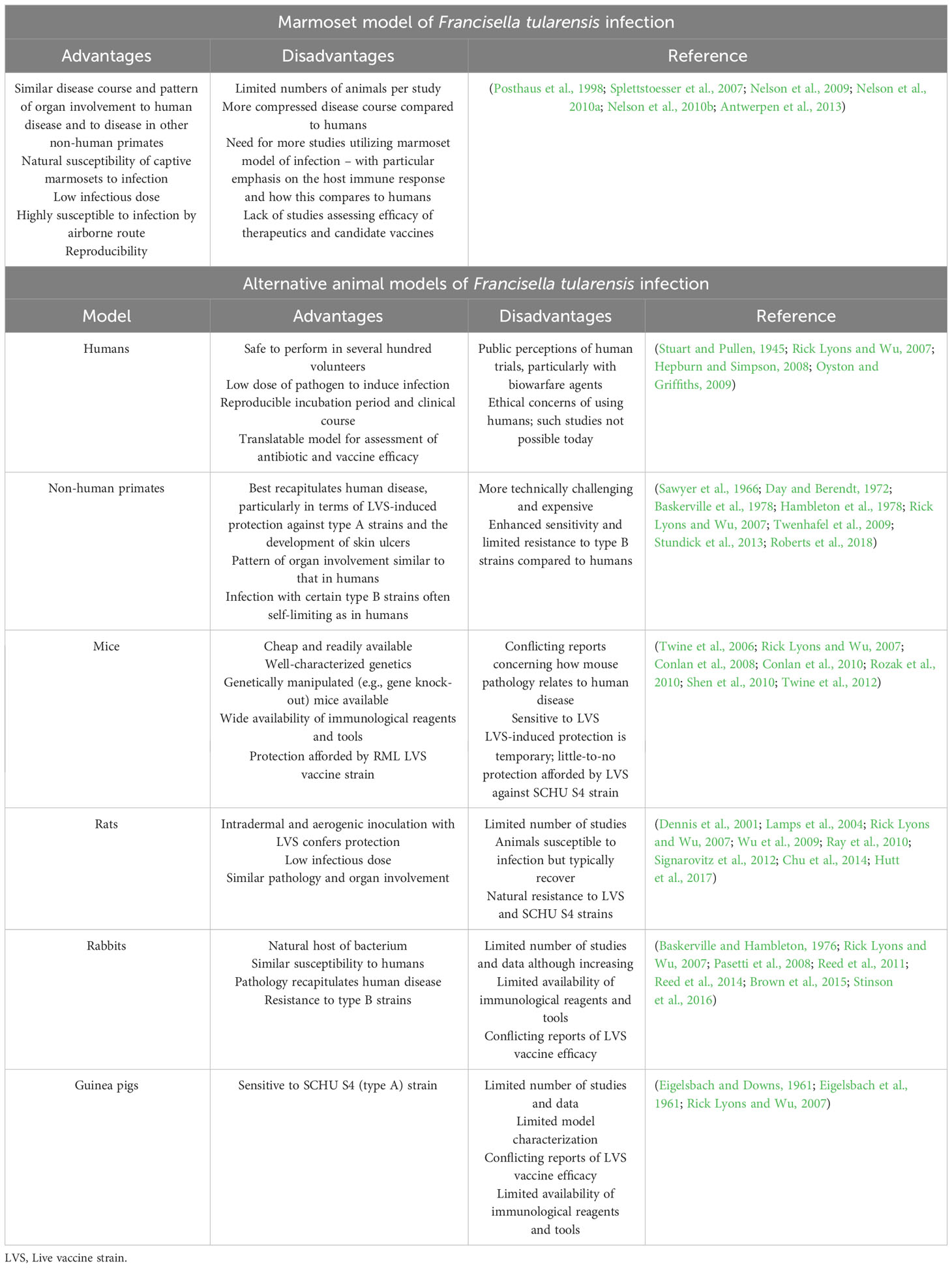
A number of animal models of F. tularensis infection have been developed, including mice, rats, rabbits, guinea pigs and non-human primates (e.g., cynomolgus and rhesus monkeys). The advantages and disadvantages of these various animal models (and how they compare with the marmoset model) are presented in Table 4. To the best of our knowledge, we are the only group to report on a marmoset model of F. tularensis infection to-date (Nelson et al., 2009; Nelson et al., 2010a; Nelson et al., 2010b). In this section, the marmoset model of inhalational tularemia will be discussed with a particular emphasis on the immunological features. The reader is directed to the above publications for full details of the model.
The marmoset as an NHP model of tularemia has a number of advantages (see Table 4); importantly, the course and progression of disease accurately recapitulated human disease – including the development of ulcers, a feature not observed in any other animal model (Nelson et al., 2010b; Roberts et al., 2018). Evidence of an immune response was demonstrated by the production of pro-inflammatory cytokines with disease progression. For example, at 72 hrs post-challenge, monocyte chemoattractant protein (MCP)-1 (CCL2) was detectable in the spleen, lungs and blood and the level increased until death (Nelson et al., 2010b). Additional cytokines, including macrophage inflammatory protein (MIP-1α; CCL3), MIP-1β (CCL4), interleukin (IL-6), IL-1β and regulated on activation, normal T-cell expressed and secreted (RANTES; CCL5), were upregulated in all organs at 96 hrs post-challenge (Nelson et al., 2010b). Interestingly, MIP-1α and IL-6 were first observed shortly prior to death, akin to the murine model of inhalational tularemia (Conlan et al., 2008; Nelson et al., 2010b). Neutrophils and natural killer (NK) cells were the first cells to arrive at the site of infection (24 hrs post-challenge), followed by macrophages, T-cells and additional influx of NK cells (48 hrs post-challenge) (Nelson et al., 2010b). A decline in the percentage of neutrophils in the lung and blood at 72 hrs post-challenge was observed, raising important questions concerning the role of neutrophils in response to F. tularensis infection. Indeed, studies assessing the importance of neutrophils in the response to F. tularensis infection are conflicting. Using the neutrophil-depleting antibody RB6-8C5, Sjöstedt and colleagues found that mice depleted of neutrophils were vulnerable to otherwise sublethal doses of F. tularensis, delivered either intraveneously or intradermally, suggesting a key role for neutrophils in controlling bacterial replication (Sjöstedt et al., 1994). Meanwhile, KuoLee and colleagues demonstrated that depleting the number of neutrophils had no effect on the bacterial burden or time to death (Kuolee et al., 2011). It has been suggested that the role of neutrophils in response to infection with F. tularensis may be dependent on the site of infection and that, in some cases, excessive neutrophil recruitment may contribute to the over-production of pro-inflammatory cytokines that ultimately lead to sepsis (Malik et al., 2007; Metzger et al., 2013; Steiner et al., 2014). Notably, whilst infection with the type A strain rapidly induced neutrophil recruitment in the marmoset, the type B (but not type A) strain led to neutrophil influx in the mouse, highlighting an important difference between the two species (Hall et al., 2008; Nelson et al., 2010b). By 72 hrs post-challenge, the number of B-cells and T-cells in the spleen and blood increased (Nelson et al., 2010b). By 96 hrs post-challenge, the number of neutrophils in the blood and organs returned to normal levels; a concomitant decline in the number of NK cells, both B- and (CD4+) T-lymphocytes and macrophages in the lungs was also observed (Nelson et al., 2010b). The proportion of CD8+ T-cells and γδ T-cells in the spleen and lung were increased 96 hrs post-challenge (Sumida et al., 1992; Poquet et al., 1998; Kroca et al., 2000; Nelson et al., 2010b). γδ T-cells are thought to play a role in the innate immune response and thought to be important in human infections with F. tularensis (Rowland et al., 2012a; Rowland et al., 2012b). An increase of γδ T-cells in the blood was not observed, consistent with reports in humans, where cells were discerned approximately one week post-infection (Kroca et al., 2000).
Having shown the marmoset model of tularemia effectively recapitulates human disease, a follow-up study by our research group evaluated the efficacy of levofloxacin, a fluoroquinolone shown to be effective against F. tularensis (Hepburn and Simpson, 2008). Fluoroquinolones have a number of advantages over current treatment protocols, including their broad-spectrum activity (important when diagnosis is difficult), bactericidal effects, tolerability and oral administration (Fish, 2003; Hepburn and Simpson, 2008). Further, levofloxacin is effective as a single daily dose which will likely increase compliance (Nelson et al., 2010a). Indeed, levofloxacin is approved for the treatment of inhalational anthrax in both children and adults (Deziel et al., 2005; Li et al., 2010). To achieve licensure of any therapeutic agent for a given disease under the animal rule (discussed earlier), the efficacy and safety profile must first be assessed in a NHP. In our study, all animals that received levofloxacin for ten days post-exposure survived and showed no clinical signs of disease, indicating the efficacy of oral levofloxacin against inhalational tularemia (Nelson et al., 2010a).
In summary, the common marmoset model of tularemia effectively and accurately recapitulates human disease and has numerous advantages over alternative animal models. It will be useful for the evaluation and licensure of medical countermeasures by the FDA.
3.3 Burkholderia pseudomallei
Burkholderia pseudomallei is a gram-negative, intracellular pathogens and the agent responsible for melioidosis (Whitlock et al., 2007; Wiersinga et al., 2018). B. pseudomallei is classified as Tier 1 Select Agents by the Centers for Disease Control and Prevention (CDC) given its potential use in bioterrorism (Peacock et al., 2008). Melioidosis was first described as a ‘glanders-like disease’ in 1913 by Alfred Whitmore (Whitmore, 1913). As an environmental saprophyte, B. pseudomallei is found in wet soils and contaminated water in endemic areas; B. pseudomallei is endemic in northern Australia and north east Thailand, and an emerging disease in India, China and potentially the United States (Ashdown and Clarke, 1992; Dance, 2000; Cheng and Currie, 2005; Limmathurotsakul et al., 2016). Most cases of infection occur through contact of broken skin with contaminated soil and water, although numerous other routes of exposure have been documented including ingestion and inhalation of bacteria (Webling, 1980; White et al., 1989; Abbink et al., 2001; Holland et al., 2002; Ralph et al., 2004; Baker et al., 2011; Limmathurotsakul and Peacock, 2011; Bzdyl et al., 2022). Melioidosis presents as a systemic disease; symptoms are frequently non-specific, vary from person-to-person, and can mimic several other clinical scenarios making diagnosis challenging (Yee et al., 1988; White, 2003; Cheng and Currie, 2005). The immunocompromised are particularly vulnerable to infection; risk factors for more severe disease include diabetes and lung and kidney disease (Ip et al., 1995; Northfield et al., 2002; Kronsteiner et al., 2019; Bzdyl et al., 2022). Treatment paradigms are complex and slow: an initial intensive phase requiring intravenous antibiotics (ceftazidime or meropenem) for 14 days is followed by an eradication phase, where antimicrobials (co-trimoxazole and doxycycline as combination therapy or equally efficacious co-trimoxazole monotherapy) are taken orally for a prolonged period to kill residual bacteria (Cheng et al., 2004; Chusri et al., 2012; Lipsitz et al., 2012; Chetchotisakd et al., 2014; Dance, 2014; Fisher and Harris, 2014). Disease relapse is common given the nature of the microorganism (i.e., it is intracellular and can evade the host immune response) despite prolonged antimicrobial therapy (Limmathurotsakul et al., 2008; Dance, 2014; Mariappan et al., 2021). No licensed vaccine is currently available.
3.3.1 Common marmoset model of melioidosis
Despite early studies of experimental melioidosis in rhesus macaques, much of our understanding of the pathogenesis and the effectiveness of therapies against melioidosis and glanders has emerged from small animal models, specifically mice and hamsters (Warawa, 2010; Amemiya et al., 2017). Reports from the 1990s described experimental infections of baboons with B. pseudomallei and B. mallei (Manzeniuk et al., 1999). More recently, our group established and characterized a common marmoset model of B. pseudomallei infection following inhalational challenge (Nelson et al., 2011a). An African green monkey and rhesus macaque model of experimental infection has also been described (Miller et al., 1948; Yeager et al., 2012). The advantages and disadvantages of these various animal models, and how they compare with the marmoset model, are presented in Table 5. In this section, the marmoset model of melioidosis is discussed with particular emphasis on the immunological features. The reader is directed to the above publications for full details of the model.
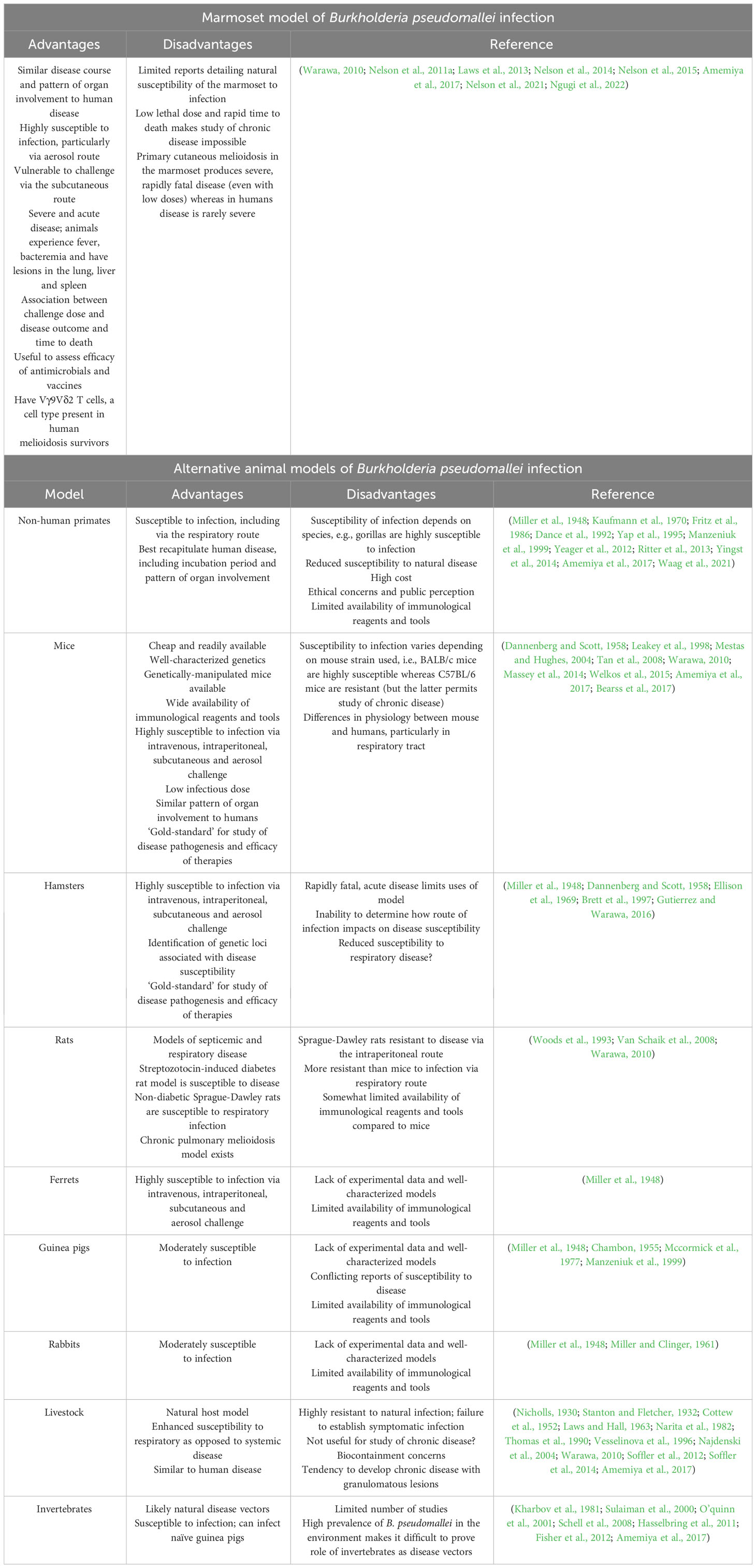
Table 5 Marmoset and alternative animal models of Burkholderia pseudomallei infection (melioidosis).
Work from our research group has led to the development of a marmoset model of experimental melioidosis caused by three natural routes of exposure to B. pseudomallei, i.e., through broken skin, inhalation and ingestion (Nelson et al., 2011a; Nelson et al., 2014; Nelson et al., 2015; Nelson et al., 2021; Nelson et al., 2022a; Ngugi et al., 2022). Clinically, this is important as the route of exposure, whilst often difficult to determine at disease presentation, is likely to impact on the efficacy of medical countermeasures. Whilst early studies of experimental melioidosis in the marmoset reported limited immunological findings, a recent study by Ngugi and colleagues provided the most complete and comprehensive analysis of the immunological features of acute pneumonic disease resulting from B. pseudomallei exposure to-date (Ngugi et al., 2022). Significantly, features of the marmoset immune response to infection (e.g., neutrophil and macrophage migration and activation, T-cell activation and the production of pro-inflammatory mediators) mimicked acute disease in humans and was associated with disease prognosis, providing additional evidence as to the validity of the model. The proceeding section will focus predominantly on neutrophils, though other immunological components will be noted.
Notably, naïve marmoset neutrophils exhibited a rather different phenotype compared to the human counterpart. Specifically, HLA-DR (MHC II) was constitutively expressed on naïve marmoset neutrophils whereas in humans HLA-DR expression is not typically observed on resting neutrophils (Meinderts et al., 2019; Ngugi et al., 2022). Additionally, expression of the classical marker used to identify human neutrophils, CD16 (the Fc receptor gamma III), was lower on marmoset neutrophils (Silvestre-Roig et al., 2019; Ngugi et al., 2022). Considering that the proportion of circulating cells (and particularly neutrophils) in the marmoset more closely resembles that in humans, the significance of these phenotypic variations is unclear and the marmoset remains a viable model of human disease. Most importantly, both the proportions and cellular phenotypes changed during the course of the disease providing an objective, quantitative metric of disease progress and thus the opportunity to assess the efficacy of therapeutic interventions. In this study, the proportion of circulating neutrophils increased during the first 48 hrs post-challenge, after which the number declined significantly (and below baseline levels) in terminal animals. Meanwhile, the proportion of neutrophils in the lung declined 12 hrs post-challenge which is contrary to the scenario in the mouse, whereby neutrophil influx into the lung is observed post-challenge (Laws et al., 2011). At 36 hrs post-challenge, neutrophil proportion began to recover, returning to near-baseline levels by 48 hrs post-challenge. The authors noted, however, that since cell typing was proportional, it was not clear whether the apparent decline in the number of neutrophils in the lung was the result of neutrophil death [as a result of bactericidal processes (Kaplan and Radic, 2012)] or merely indicative of enhanced lymphocyte infiltration. Concomitantly, the proportion of circulating T (but not B) lymphocytes declined as the disease progressed. As noted, lymphocyte proportions were increased in the lung at 12 hrs post-challenge and continued to increase until 36 hrs post-challenge, after which levels declined. Changes to the proportions of cells in the spleen were similar to those observed in blood. In addition to changes to the proportion of cells in the various tissues, phenotypic changes were observed in neutrophils immediately following challenge. Significantly, expression of HLA-DR (which is constitutively expressed on marmoset neutrophils) dropped as disease progressed in the blood, lung and spleen. In blood, significantly reduced expression of HLA-DR was observed at all-time points post-challenge; in the lung and spleen, a significant decline in the proportion of neutrophil HLA-DR expression was observed by 12 hrs post-challenge and before the onset of clinical signs of disease, e.g., fever. Taken together, these findings provide additional evidence to support the use of the marmoset model of melioidosis for assessing medical countermeasures. Encouragingly, these findings regarding HLA-DR, CD54 and CD16 were also observed in a more recent, related study with B. pseudomallei (Nelson et al., 2022a).
Considering the role of neutrophils as first-responders to injury and insult, and their documented significance in early melioidosis (Easton et al., 2007; Laws et al., 2011),the fact that neutrophils showed the most significant variation of all cellular parameters assessed is not surprising. In the mouse, neutrophils play a central role in the acute response to aerosol infection (Easton et al., 2007). Though susceptibility to infection is largely pre-determined depending on the specific mouse strain (Warawa, 2010), marmosets are considered to demonstrate enhanced sensitivity to (particularly) aerosol challenge and this may be due to the tendency for a decline in the proportion of neutrophils in the lung during the early stages of infection (Nelson et al., 2022a; Ngugi et al., 2022). Alternative explanations should not be disregarded. These include the possibility that early neutrophil influx into the lung does occur, yet neutrophils are not detectable by flow cytometry because they are infected and degraded. In this scenario, subsequent neutrophil recruitment and activation occurs too late to counteract an already rapidly escalating bacterial burden. Encouragingly, the pattern of neutrophil recruitment in the marmoset mirrors that observed in other NHP models in the rhesus macaque and African green monkey (Yeager et al., 2012). Additional evidence implicating neutrophils as key players in early melioidosis include the association between excessively high or low neutrophil counts and poorer outcomes in humans, and the increased susceptibility of individuals with certain conditions (e.g., diabetes) associated with suboptimal neutrophil function (Chanchamroen et al., 2009; Saengmuang et al., 2014; Jenjaroen et al., 2015).
With a marmoset-specific candidate biomarker indicative of infection (a reduction in neutrophil HLA-DR expression), our research group recently evaluated the efficacy of co-trimoxazole using the marmoset model of experimental melioidosis (Nelson et al., 2022a). In this study, animals were challenged by one of three exposure routes: inhalational, ingestion or subcutaneous. Once fever had developed, a proportion of the animals were administered oral co-trimoxazole; all remaining animals received a placebo. A second-dose was administered 12 hrs after the first, followed by one dose every 12 hrs up until a total of 28 doses was delivered. With respect to the immunological perturbations, the proportion of neutrophils increased at the onset of fever, yet there was a drop in the level of HLA-DR expression that continued until animals succumbed to disease. HLA-DR expression was at a normal level by day 15 post-challenge in those animals that received oral co-trimoxazole. In addition to validating the observation of decreased HLA-DR expression with the onset of fever in an independent study, the immunophenotyping panel was also expanded and incorporated markers for CD16 (Fc gamma receptor III, expressed on NK cells, macrophages and neutrophils, plays a role in the internalization of exogenous antigens by binding the Fc portion of IgG immune complexes),CD66b (an activation marker on granulocytes), CD80 (a co-stimulation marker used by professional phagocytes to aid in MHC to T-cell receptor interactions) and CD54 (intracellular adhesion molecule-1 (ICAM-1), an adhesion molecule involved in lymphocyte homing and activation). Expression of all these markers decreased in the placebo group; meanwhile, neutrophil CD16 expression returned to normal levels in the co-trimoxazole treatment group. Upon treatment cessation, animals either survived, relapsed and succumbed to disease or exhibited abnormal immunological perturbations indicative of subclinical disease. Importantly, those animals that survived without relapse maintained normal levels of HLA-DR expression on neutrophils. A decline in neutrophil HLA-DR expression was observed in those animals that would later relapse and succumb to disease; likewise, elevated circulating IFN-γ was detectable and indicative of relapse up to three days prior to death. At post-mortem, a reduced proportion of neutrophils in the blood was the only indicator of fatal disease. Minor immunological changes were observed between those animals that succumbed, recovered and later relapsed and those that survived. For example, there was a somewhat increased proportion of CD69+ CD8+ T-cells and decreased expression of CD40, CD16 and CD64 on macrophages. Interestingly, whereas neutrophil influx into the lung was a feature of those animals that received the placebo, there was no evidence for this in animals that received treatment and later relapsed. Akin to the situation in humans (Jenjaroen et al., 2015; Nithichanon et al., 2018), there was evidence of T-cell activation (indicated by expansion of the cytotoxic T-cell proportion and expression of CD16 and CD69) in animals that survived until the study end. The population of γδ T-cells was also expanded in survivors, providing additional evidence to support an important role for this cell type in the response to infection (Haque et al., 2006; Andreu-Ballester et al., 2013; Laws et al., 2013; Kronsteiner et al., 2019). Notably, a re-stimulation assay of splenic T-cells taken from those animals that survived revealed enhanced IFN-γ production compared with the negative control (Nelson et al., 2022a). In those animals that survived to the study end, high antibody titers were observed. Yet the relative protective value of the humoral response in humans is limited, despite the importance of vaccine-induced humoral immunity having been demonstrated in animal studies (Burtnick et al., 2018; Khakhum et al., 2019; Chaichana et al., 2020; Chaichana et al., 2021).
In summary, the common marmoset model of melioidosis has been well characterized and shown to recapitulate human disease and exhibit a higher degree of similarity to human disease compared with other animal models. It will no doubt have value in the evaluation and licensure of medical countermeasures.
3.4 Hepatitis C virus
Viral hepatitis, broadly defined as inflammation of the liver caused by a virus, represents a major health care burden worldwide (Estes et al., 2018; Jefferies et al., 2018). The hepatotropic viruses (types A to E) are the most important and common cause of hepatitis, with types B and C being most prevalent globally (Lim et al., 2020; Castaneda et al., 2021). Infection occurs either via ingestion of contaminated food or water (types A and E) or by contact with infected bodily fluids, i.e., blood (types B, C and D) (Loader et al., 2019). Hepatitis B can be transmitted from mother to baby at birth (Loader et al., 2019). Hepatitis A and D is typically acute and self-limiting, whereas types B, C and E can establish chronic disease (Loader et al., 2019; Castaneda et al., 2021). Chronic viral hepatitis is the leading cause of liver cirrhosis and hepatocellular carcinoma (Lin et al., 2014).
Tissue tropism of the phylogenetically unrelated hepatitis viruses for differentiated hepatocytes may explain the narrow range of susceptible hosts, namely humans and NHPs (Pfaender et al., 2014). Consequently, much of our knowledge of human viral hepatitis has stemmed from NHP models of infection. The proceeding discussion will focus on animal models of hepatitis C virus (and the closely related species GB virus B; the advantages and disadvantages of which are presented in Table 6) specifically. For reviews of animal models of the other hepatitis viruses, see (Purcell and Emerson, 2001; Manickam and Reeves, 2014; Protzer, 2017; Guo et al., 2018; Burwitz et al., 2020; Liu et al., 2021; Zhang et al., 2021).
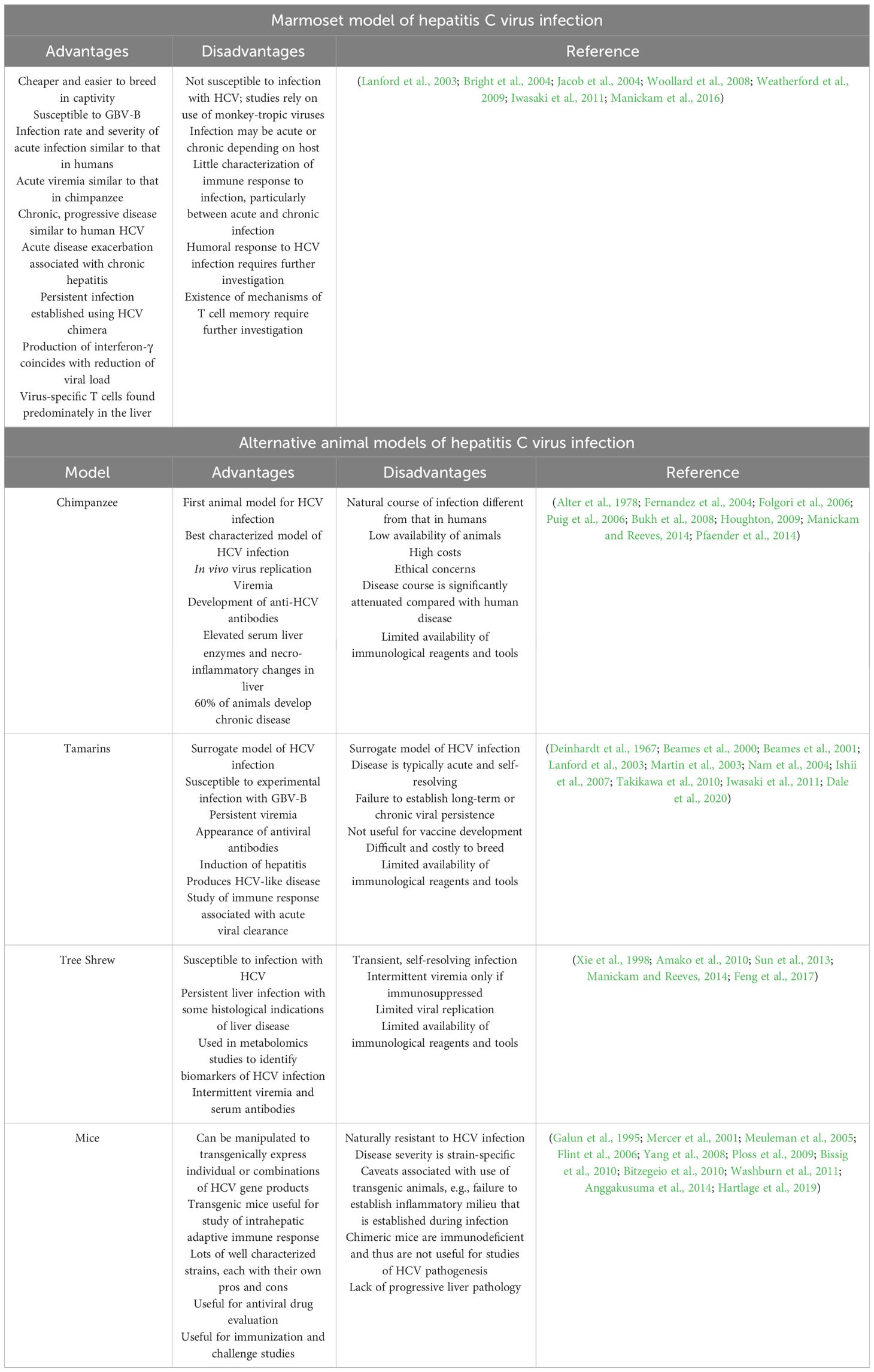
Of all hepatitis viruses, hepatitis C virus (HCV) has the most restricted host range, capable of producing infection in humans and chimpanzees only (Folgori et al., 2006; Puig et al., 2006). As such, the majority of early studies of hepatitis C relied almost exclusively on chimpanzees, giving rise to first generation vaccines and a number of novel therapeutics. However, the search for alternative animal models of hepatitis C was fueled by increasing costs and ethical concerns surrounding the use of chimpanzees in biomedical research. Studies of the closely related GB virus B (Deinhardt et al., 1967), which infects new-world primates and produces disease similar to that caused by HCV in humans, were fundamental in expanding both the number and availability of alternative animal models.
3.4.1 Common marmoset model of viral hepatitis C
The search for a more robust animal model of human HCV infection, particularly one permitting testing of vaccine efficacy, is important and remains a pressing unmet need in hepatitis C research. Whilst highly effective treatments for HCV infection exist, these are often prohibitively expensive and, consequently, are unavailable to those most at-risk individuals (Etzion and Ghany, 2015; Chahal et al., 2016). The development of preventative measures (like vaccines) is therefore key.
Development of a surrogate common marmoset model (Parks et al., 1969; Lanford et al., 2003; Bright et al., 2004; Kyuregyan et al., 2005; Haqshenas et al., 2007) of human HCV infection (with the NHP-specific GBV-B and, later, HCV chimera) followed earlier studies performed in tamarins (Deinhardt et al., 1967; Beames et al., 2000; Beames et al., 2001) which, compared to marmosets, are difficult and costly to breed in captivity. Though tamarins are susceptible to GBV-B infection, the utility of the tamarin model (and indeed monkey models more generally) of HCV infection was highly debated given the inability to establish chronic infection, a hallmark of human HCV infection (Lanford et al., 2003; Weatherford et al., 2009). The usefulness of the tamarin model was also limited by the availability of animals (Weatherford et al., 2009). Early studies in the marmoset revealed the susceptibility of the species to GBV-B infection, with animals developing acute viraemia (albeit to a lower level compared with that seen in tamarins) (Parks et al., 1969; Lanford et al., 2003; Bright et al., 2004). Interestingly, the level of viraemia in the marmoset was similar to that seen in chimpanzees (107 copies/mL or less) which have been shown to develop persistent infections (Fernandez et al., 2004; Bukh et al., 2008). Thus, it has been suggested that lower viral loads in the acute phase of the infection may actually support viral persistence and the development of chronic inflammation (Iwasaki et al., 2011). Indeed, Iwasaki and colleagues were the first to show that infection of the marmoset with GBV-B produced a chronic and progressive disease similar to human hepatitis C, as indicated by fibrosis and recurrent increases of the liver enzyme alanine transaminase (ALT) (Iwasaki et al., 2011). Further, one marmoset experienced piecemeal necrosis and elevated ALT levels four years post-infection, indicative of an acute exacerbation associated with chronic hepatitis (Iwasaki et al., 2011), itself a feature of human viral hepatitis (Perrillo, 1997). Notably, marmosets infected with GBV-B were shown to exhibit two distinct phenotypes: susceptible and partially resistant (Weatherford et al., 2009). In contrast, HCV chimera (carrying core, E1, E2 and p7 structural proteins of HCV) causes persistent infection in marmosets (Li et al., 2014b). Since long-term viral persistence was established in animals with lower viral loads during the acute phase on infection (i.e., within the first 2 weeks post-infection), it seems reasonable to conclude that animals with the partially-resistant phenotype (where viral growth is restricted) will support the development of chronic infection. Viral persistence in those animals with lower viral loads may be the result of diminished early antiviral immune responses (Iwasaki et al., 2011). Data concerning the innate and adaptive immune response to infection in animals exhibiting acute disease compared with those that progress to develop chronic disease are still lacking and will prove critical in deciphering the mechanisms responsible for the establishment of chronic infection.
The induction of type I interferons represents one of the first responses to infection with HCV. HCV utilizes a NS3/4A protease to inactivate these early antiviral responses, possibly leading to viral persistence (Kaukinen et al., 2006). An interferon-inactivating NS3/4A protease is also present in GBV-B (Li et al., 2014b). In humans and chimpanzees, both CD4+ and CD8+ T cells play an important role in the response to HCV infection (Cooper et al., 1999; Lechner et al., 2000; Day et al., 2002; Woollard et al., 2003). The generation of virus-specific T cells that recognize multiple viral epitopes is crucial for viral clearance. Indeed, the accumulation of HCV-specific CD4+ and CD8+ T cells (recognizing multiple viral epitopes) in the liver is associated with acute resolving infection (He et al., 1999; Grabowska et al., 2001; Woollard et al., 2008). Conversely, a weaker T cell response against a limited number of viral epitopes is associated with viral persistence and chronic disease (Woollard et al., 2008). In the marmoset, IFN-γ production was first detectable five weeks post-infection, coinciding with a 1000-fold reduction in viral load (Woollard et al., 2008). A T cell response against NS3/N54A epitope (but no other viral epitope) was observed predominantly in the liver at week seven post-infection, coinciding with the clearance of viraemia (Woollard et al., 2008). At this point, virus-specific T cells appear in peripheral blood (Woollard et al., 2008). Akin to the situation in humans and chimpanzees, virus-specific T cells are present in higher frequencies in the liver than in the blood, suggesting the accumulation of T cells in the liver at the site of viral replication (He et al., 1999; Grabowska et al., 2001; Woollard et al., 2008). It is currently unclear whether the anti-HCV adaptive immune response is mediated by CD4+ or CD8+ T cells. Recently, the role of regulatory T cells (Tregs) in the response to HCV infection has gained increasing attention. Tregs, a unique type of CD4+ T cell with suppressor functions, are important in maintaining immune tolerance (Sakaguchi et al., 2008). In the context of an infection, Tregs can modulate effector T cell responses and, by inhibiting the anti-viral functions of specific T cells, may permit viral persistence (Boer et al., 2015; Liu et al., 2023). In chronically infected individuals, Treg populations are maintained, whereas the suppressor function of Tregs was diminished in individuals with acute resolving infection (Liu et al., 2023). The phenotype and role of Tregs in the marmoset is yet to be determined.
Another important aspect of the immune response against HCV is memory. In chimpanzees, virus-specific memory cells are essential for protection against reinfection (Grakoui et al., 2003; Shoukry et al., 2003). Marmosets were also protected from reinfection for several months after clearance of primary infection, pointing to the existence of virus-specific memory cells (Woollard et al., 2008). Consistently, T cell responses were both greater in magnitude and occurred faster following secondary infection, indicating recall of memory T cells (Bright et al., 2004; Woollard et al., 2008). In comparison to cell-mediated mechanisms of immunity, the humoral response to HCV infection is less well defined and requires further investigation.
In summary, the marmoset is susceptible to infection with both GBV-B and HCV chimeras and develops a hepatitis C-like disease, the pathology of which mirrors that of human HCV infection. Varying susceptibility phenotypes are likely genetically-determined, with some animals more likely to exhibit viral persistence and therefore chronic infection. In this sense, the marmoset may represent a valuable surrogate model of human hepatitis C.
4 Discussion
The common marmoset, a new-world primate, offers a number of advantages over the more traditional old-world primates; their small size, compact life-span and reduced husbandry costs are particularly notable, especially in the context of high containment research where their small size makes them both easier and safer to house. Their evolutionary proximity to humans makes them a more accurate and representative model of human disease compared to the more frequently used murine models. Critically, demonstration of the efficacy of medical countermeasures in a representative animal model is central to obtaining licensure under the FDA animal rule. Taken together, the marmoset represents an attractive alternative animal model. Further research in this area with increased focus on the development of marmoset-specific immunological reagents and tools will undoubtedly increase the utility of the marmoset in all areas of biomedical research.
Author contributions
IH: Investigation, Writing – original draft, Writing – review & editing. TL: Conceptualization, Writing – review & editing. MN: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the UK Ministry of Defence Chief Scientific Advisor.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
‘T Hart, B. A. (2019). Experimental autoimmune encephalomyelitis in the common marmoset: a translationally relevant model for the cause and course of multiple sclerosis. Primate. Biol. 6, 17–58. doi: 10.5194/pb-6-17-2019
‘T Hart, B. A., Abbott, D. H., Nakamura, K., Fuchs, E. (2012). The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discovery Today 17, 1160–1165. doi: 10.1016/j.drudis.2012.06.009
Abbink, F. C., Orendi, J. M., De Beaufort, A. J. (2001). Mother-to-child transmission of Burkholderia pseudomallei. N. Engl. J. Med. 344, 1171–1172. doi: 10.1056/NEJM200104123441516
Abbott, D. H., Barnett, D. K., Colman, R. J., Yamamoto, M. E., Schultz-Darken, N. J. (2003). Aspects of common marmoset basic biology and life history important for biomedical research. Comp. Med. 53, 339–350.
Adams, A. P., Aronson, J. F., Tardif, S. D., Patterson, J. L., Brasky, K. M., Geiger, R., et al. (2008). Common marmosets (Callithrix jacchus) as a nonhuman primate model to assess the virulence of eastern equine encephalitis virus strains. J. Virol. 82, 9035–9042. doi: 10.1128/JVI.00674-08
Aebersold, P. (2012). FDA experience with medical countermeasures under the animal rule. Adv. Prev. Med. 2012, 507571. doi: 10.1155/2012/507571
Albrecht, L., Bishop, E., Jay, B., Lafoux, B., Minoves, M., Passaes, C. (2021). COVID-19 research: lessons from non-human primate models. Vaccines (Basel). 9, 886. doi: 10.3390/vaccines9080886
Allio, T. (2018). The FDA Animal Rule and its role in protecting human safety. Expert Opin. Drug Saf. 17, 971–973. doi: 10.1080/14740338.2018.1518429
Alter, H. J., Purcell, R. H., Holland, P. V., Popper, H. (1978). Transmissible agent in non-A, non-B hepatitis. Lancet 1, 459–463. doi: 10.1016/S0140-6736(78)90131-9
Amako, Y., Tsukiyama-Kohara, K., Katsume, A., Hirata, Y., Sekiguchi, S., Tobita, Y., et al. (2010). Pathogenesis of hepatitis C virus infection in Tupaia belangeri. J. Virol. 84, 303–311. doi: 10.1128/JVI.01448-09
Amemiya, K., Bozue, J. A., Cote, C. K., Deshazer, D., Soffler, C., Welkos, S. L., et al. (2017). Animal models for melioidosis. Curr. Trop. Med. Rep. 4, 208–222. doi: 10.1007/s40475-017-0131-5
Andreu-Ballester, J. C., Tormo-Calandín, C., Garcia-Ballesteros, C., Pérez-Griera, J., Amigó, V., Almela-Quilis, A., et al. (2013). Association of γδ T cells with disease severity and mortality in septic patients. Clin. Vaccine Immunol. 20, 738–746. doi: 10.1128/CVI.00752-12
Anggakusuma, Colpitts, C. C., Schang, L. M., Rachmawati, H., Frentzen, A., Pfaender, S., et al. (2014). Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 63, 1137–1149. doi: 10.1136/gutjnl-2012-304299
Antunes, S. G., De Groot, N. G., Brok, H., Doxiadis, G., Menezes, A. A., Otting, N., et al. (1998). The common marmoset: a new world primate species with limited Mhc class II variability. Proc. Natl. Acad. Sci. U.S.A. 95, 11745–11750. doi: 10.1073/pnas.95.20.11745
Antwerpen, M. H., Schacht, E., Kaysser, P., Splettstoesser, W. D. (2013). Complete Genome Sequence of a Francisella tularensis subsp. holarctica Strain from Germany Causing Lethal Infection in Common Marmosets. Genome Announc. 1. e00135-12. doi: 10.1128/genomeA.00135-12
Ashdown, L. R., Clarke, S. G. (1992). Evaluation of culture techniques for isolation of pseudomonas pseudomallei from soil. Appl. Environ. Microbiol. 58, 4011–4015. doi: 10.1128/aem.58.12.4011-4015.1992
Avila, M. M., Frigerio, M. J., Weber, E. L., Rondinone, S., Samoilovich, S. R., Laguens, R. P., et al. (1985). Attenuated Junin virus infection in Callithrix jacchus. J. Med. Virol. 15, 93–100. doi: 10.1002/jmv.1890150112
Avila, M. M., Samoilovich, S. R., Laguens, R. P., Merani, M. S., Weissenbacher, M. C. (1987). Protection of Junín virus-infected marmosets by passive administration of immune serum: association with late neurologic signs. J. Med. Virol. 21, 67–74. doi: 10.1002/jmv.1890210109
Baker, H. F., Ridley, R. M., Wells, G. A. (1993). Experimental transmission of BSE and scrapie to the common marmoset. Vet. Rec. 132, 403–406. doi: 10.1136/vr.132.16.403
Baker, A., Tahani, D., Gardiner, C., Bristow, K. L., Greenhill, A. R., Warner, J. (2011). Groundwater seeps facilitate exposure to Burkholderia pseudomallei. Appl. Environ. Microbiol. 77, 7243–7246. doi: 10.1128/AEM.05048-11
Balestra, A., Bytyci, H., Guillod, C., Braghetti, A., Elzi, L. (2018). A case of ulceroglandular tularemia presenting with lymphadenopathy and an ulcer on a linear morphoea lesion surrounded by erysipelas. Int. Med. Case Rep. J. 11, 313–318. doi: 10.2147/IMCRJ.S178561
Barton, R. W., Thrall, R. S., Neubauer, R. H. (1984). Binding of human lymphocyte-specific monoclonal antibodies to common marmoset lymphoid cells. Cell Immunol. 84, 446–452. doi: 10.1016/0008-8749(84)90119-9
Baskerville, A., Hambleton, P. (1976). Pathogenesis and pathology of respiratory tularaemia in the rabbit. Br. J. Exp. Pathol. 57, 339–347.
Baskerville, A., Hambleton, P., Dowsett, A. B. (1978). The pathology of untreated and antibiotic-treated experimental tularaemia in monkeys. Br. J. Exp. Pathol. 59, 615–623.
Beames, B., Chavez, D., Guerra, B., Notvall, L., Brasky, K. M., Lanford, R. E. (2000). Development of a primary tamarin hepatocyte culture system for GB virus-B: a surrogate model for hepatitis C virus. J. Virol. 74, 11764–11772. doi: 10.1128/JVI.74.24.11764-11772.2000
Beames, B., Chavez, D., Lanford, R. E. (2001). GB virus B as a model for hepatitis C virus. Ilar. J. 42, 152–160. doi: 10.1093/ilar.42.2.152
Bearss, J. J., Hunter, M., Dankmeyer, J. L., Fritts, K. A., Klimko, C. P., Weaver, C. H., et al. (2017). Characterization of pathogenesis of and immune response to Burkholderia pseudomallei K96243 using both inhalational and intraperitoneal infection models in BALB/c and C57BL/6 mice. PloS One 12, e0172627. doi: 10.1371/journal.pone.0172627
Benirschke, K., Anderson, J. M., Brownhill, L. E. (1962). Marrow chimerism in marmosets. Science 138, 513–515. doi: 10.1126/science.138.3539.513
Ben-Shmuel, A., Glinert, I., Sittner, A., Bar-David, E., Schlomovitz, J., Brosh, T., et al. (2018). Treating anthrax-induced meningitis in rabbits. Antimicrob. Agents Chemother. 62, e00298-18. doi: 10.1128/AAC.00298-18
Berry, N., Ferguson, D., Ham, C., Hall, J., Jenkins, A., Giles, E., et al. (2019). High susceptibility, viral dynamics and persistence of South American Zika virus in New World monkey species. Sci. Rep. 9, 14495. doi: 10.1038/s41598-019-50918-2
Bissig, K. D., Wieland, S. F., Tran, P., Isogawa, M., Le, T. T., Chisari, F. V., et al. (2010). Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J. Clin. Invest. 120, 924–930. doi: 10.1172/JCI40094
Bitzegeio, J., Bankwitz, D., Hueging, K., Haid, S., Brohm, C., Zeisel, M. B., et al. (2010). Adaptation of hepatitis C virus to mouse CD81 permits infection of mouse cells in the absence of human entry factors. PloS Pathog. 6, e1000978. doi: 10.1371/journal.ppat.1000978
Boer, M. C., Joosten, S. A., Ottenhoff, T. H. (2015). Regulatory T-cells at the interface between human host and pathogens in infectious diseases and vaccination. Front. Immunol. 6, 217. doi: 10.3389/fimmu.2015.00217
Bowen, R. A., Nemeth, N. M. (2007). Experimental infections with West Nile virus. Curr. Opin. Infect. Dis. 20, 293–297. doi: 10.1097/QCO.0b013e32816b5cad
Bradley, R. (1993). The research programme on transmissible spongiform encephalopathies in Britain with special reference to bovine spongiform encephalopathy. Dev. Biol. Stand. 80, 157–170.
Brass, V., Moradpour, D., Blum, H. E. (2007). Hepatitis C virus infection: in vivo and in vitro models. J. Viral Hepat. 14 Suppl 1, 64–67. doi: 10.1111/j.1365-2893.2007.00918.x
Brett, P. J., Deshazer, D., Woods, D. E. (1997). Characterization of Burkholderia pseudomallei and Burkholderia pseudomallei-like strains. Epidemiol. Infect. 118, 137–148. doi: 10.1017/S095026889600739X
Bright, H., Carroll, A. R., Watts, P. A., Fenton, R. J. (2004). Development of a GB virus B marmoset model and its validation with a novel series of hepatitis C virus NS3 protease inhibitors. J. Virol. 78, 2062–2071. doi: 10.1128/JVI.78.4.2062-2071.2004
Brok, H. P., Hornby, R. J., Griffiths, G. D., Scott, L. A., Hart, B. A. (2001). An extensive monoclonal antibody panel for the phenotyping of leukocyte subsets in the common marmoset and the cotton-top tamarin. Cytometry 45, 294–303. doi: 10.1002/1097-0320(20011201)45:4<294::AID-CYTO10002>3.0.CO;2-C
Brown, V. R., Adney, D. R., Bielefeldt-Ohmann, H., Gordy, P. W., Felix, T. A., Olea-Popelka, F. J., et al. (2015). Pathogenesis and immune responses of francisella tularensis strains in wild-caught cottontail rabbits (sylvilagus spp.). J. Wildl. Dis. 51, 564–575. doi: 10.7589/2015-02-030
Bukh, J., Apgar, C. L., Govindarajan, S., Purcell, R. H. (2001). Host range studies of GB virus-B hepatitis agent, the closest relative of hepatitis C virus, in New World monkeys and chimpanzees. J. Med. Virol. 65, 694–697. doi: 10.1002/jmv.2092
Bukh, J., Thimme, R., Meunier, J. C., Faulk, K., Spangenberg, H. C., Chang, K. M., et al. (2008). Previously infected chimpanzees are not consistently protected against reinfection or persistent infection after reexposure to the identical hepatitis C virus strain. J. Virol. 82, 8183–8195. doi: 10.1128/JVI.00142-08
Burke, C. W., Erwin-Cohen, R. A., Goodson, A. I., Wilhelmsen, C., Edmundson, J. A., White, C. E., et al. (2022). Efficacy of western, eastern, and Venezuelan equine encephalitis (WEVEE) virus-replicon particle (VRP) vaccine against WEEV in a non-human primate animal model. Viruses 14, 1502. doi: 10.3390/v14071502
Burtnick, M. N., Shaffer, T. L., Ross, B. N., Muruato, L. A., Sbrana, E., Deshazer, D., et al. (2018). Development of subunit vaccines that provide high-level protection and sterilizing immunity against acute inhalational melioidosis. Infect. Immun. 86, e00724-17. doi: 10.1128/IAI.00724-17
Burwitz, B. J., Zhou, Z., Li, W. (2020). Animal models for the study of human hepatitis B and D virus infection: New insights and progress. Antiviral Res. 182, 104898. doi: 10.1016/j.antiviral.2020.104898
Bzdyl, N. M., Moran, C. L., Bendo, J., Sarkar-Tyson, M. (2022). Pathogenicity and virulence of Burkholderia pseudomallei. Virulence 13, 1945–1965. doi: 10.1080/21505594.2022.2139063
Carrion, R., Jr., Brasky, K., Mansfield, K., Johnson, C., Gonzales, M., Ticer, A., et al. (2007). Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J. Virol. 81, 6482–6490. doi: 10.1128/JVI.02876-06
Carrion, R., Jr., Patterson, J. L. (2012). An animal model that reflects human disease: the common marmoset (Callithrix jacchus). Curr. Opin. Virol. 2, 357–362. doi: 10.1016/j.coviro.2012.02.007
Carrion, R., Jr., Ro, Y., Hoosien, K., Ticer, A., Brasky, K., de la Garza, M., et al. (2011). A small nonhuman primate model for filovirus-induced disease. Virology 420, 117–124. doi: 10.1016/j.virol.2011.08.022
Carvalho, C. L., Lopes De Carvalho, I., Zé-Zé, L., Núncio, M. S., Duarte, E. L. (2014). Tularaemia: a challenging zoonosis. Comp. Immunol. Microbiol. Infect. Dis. 37, 85–96. doi: 10.1016/j.cimid.2014.01.002
Caspar, Y., Maurin, M. (2017). Francisella tularensis Susceptibility to Antibiotics: A Comprehensive Review of the Data Obtained In vitro and in Animal Models. Front. Cell Infect. Microbiol. 7, 122. doi: 10.3389/fcimb.2017.00122
Castaneda, D., Gonzalez, A. J., Alomari, M., Tandon, K., Zervos, X. B. (2021). From hepatitis A to E: A critical review of viral hepatitis. World J. Gastroenterol. 27, 1691–1715. doi: 10.3748/wjg.v27.i16.1691
Chahal, H. S., Marseille, E. A., Tice, J. A., Pearson, S. D., Ollendorf, D. A., Fox, R. K., et al. (2016). Cost-effectiveness of early treatment of hepatitis C virus genotype 1 by stage of liver fibrosis in a US treatment-naive population. JAMA Intern. Med. 176, 65–73. doi: 10.1001/jamainternmed.2015.6011
Chaichana, P., Jenjaroen, K., Chumseng, S., Sumonwiriya, M., Rongkard, P., Kronsteiner, B., et al. (2021). Role of burkholderia pseudomallei-specific igG2 in adults with acute melioidosis, Thailand. Emerg. Infect. Dis. 27, 463–470. doi: 10.3201/eid2702.200213
Chaichana, P., Kronsteiner, B., Rongkard, P., Teparrukkul, P., Limmathurotsakul, D., Chantratita, N., et al. (2020). Serum from melioidosis survivors diminished intracellular burkholderia pseudomallei growth in macrophages: A brief research report. Front. Cell Infect. Microbiol. 10, 442. doi: 10.3389/fcimb.2020.00442
Chambon, L. (1955). Isolation of Whitmore’s bacillus from external environment. Annales. l’Institut. Pasteur. 89, 229–235.
Chan, J. F., Yao, Y., Yeung, M. L., Deng, W., Bao, L., Jia, L., et al. (2015). Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-coV infection in a nonhuman primate model of common marmoset. J. Infect. Dis. 212, 1904–1913. doi: 10.1093/infdis/jiv392
Chanchamroen, S., Kewcharoenwong, C., Susaengrat, W., Ato, M., Lertmemongkolchai, G. (2009). Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect. Immun. 77, 456–463. doi: 10.1128/IAI.00503-08
Chen, Z., Bao, L., Chen, C., Zou, T., Xue, Y., Li, F., et al. (2017). Human neutralizing monoclonal antibody inhibition of middle east respiratory syndrome coronavirus replication in the common marmoset. J. Infect. Dis. 215, 1807–1815. doi: 10.1093/infdis/jix209
Cheng, A. C., Currie, B. J. (2005). Melioidosis: epidemiology, pathophysiology, and management. Clin. Microbiol. Rev. 18, 383–416. doi: 10.1128/CMR.18.2.383-416.2005
Cheng, A. C., Fisher, D. A., Anstey, N. M., Stephens, D. P., Jacups, S. P., Currie, B. J. (2004). Outcomes of patients with melioidosis treated with meropenem. Antimicrob. Agents Chemother. 48, 1763–1765. doi: 10.1128/AAC.48.5.1763-1765.2004
Chetchotisakd, P., Chierakul, W., Chaowagul, W., Anunnatsiri, S., Phimda, K., Mootsikapun, P., et al. (2014). Trimethoprim-sulfamethoxazole versus trimethoprim-sulfamethoxazole plus doxycycline as oral eradicative treatment for melioidosis (MERTH): a multicentre, double-blind, non-inferiority, randomised controlled trial. Lancet 383, 807–814. doi: 10.1016/S0140-6736(13)61951-0
Chiu, C. Y., Sánchez-San Martín, C., Bouquet, J., Li, T., Yagi, S., Tamhankar, M., et al. (2017). Experimental zika virus inoculation in a new world monkey model reproduces key features of the human infection. Sci. Rep. 7, 17126. doi: 10.1038/s41598-017-17067-w
Chu, P., Cunningham, A. L., Yu, J. J., Nguyen, J. Q., Barker, J. R., Lyons, C. R., et al. (2014). Live attenuated Francisella novicida vaccine protects against Francisella tularensis pulmonary challenge in rats and non-human primates. PloS Pathog. 10, e1004439. doi: 10.1371/journal.ppat.1004439
Chusri, S., Hortiwakul, T., Charoenmak, B., Silpapojakul, K. (2012). Outcomes of patients with melioidosis treated with cotrimoxazole alone for eradication therapy. Am. J. Trop. Med. Hyg. 87, 927–932. doi: 10.4269/ajtmh.2012.12-0136
Conlan, J. W., Shen, H., Golovliov, I., Zingmark, C., Oyston, P. C., Chen, W., et al. (2010). Differential ability of novel attenuated targeted deletion mutants of Francisella tularensis subspecies tularensis strain SCHU S4 to protect mice against aerosol challenge with virulent bacteria: effects of host background and route of immunization. Vaccine 28, 1824–1831. doi: 10.1016/j.vaccine.2009.12.001
Conlan, J. W., Zhao, X., Harris, G., Shen, H., Bolanowski, M., Rietz, C., et al. (2008). Molecular immunology of experimental primary tularemia in mice infected by respiratory or intradermal routes with type A Francisella tularensis. Mol. Immunol. 45, 2962–2969. doi: 10.1016/j.molimm.2008.01.022
Cooper, S., Erickson, A. L., Adams, E. J., Kansopon, J., Weiner, A. J., Chien, D. Y., et al. (1999). Analysis of a successful immune response against hepatitis C virus. Immunity 10, 439–449. doi: 10.1016/S1074-7613(00)80044-8
Cottew, G., Sutherland, A., Meehan, J. (1952). Melioidosis in sheep in Queensland: description of an outbreak. Aust. Vet. J. 28, 113–123. doi: 10.1111/j.1751-0813.1952.tb05138.x
Da Costa, C. B. P., Cruz, A. C. M., Penha, J. C. Q., Castro, H. C., Da Cunha, L. E. R., Ratcliffe, N. A., et al. (2022). Using in vivo animal models for studying SARS-CoV-2. Expert Opin. Drug Discovery 17, 121–137. doi: 10.1080/17460441.2022.1995352
Dale, J. M., Hood, S. P., Bowen, O., Bright, H., Cutler, K. L., Berry, N., et al. (2020). Development of hepatic pathology in GBV-B-infected red-bellied tamarins (Saguinus labiatus). J. Med. Virol. 92, 3584–3595. doi: 10.1002/jmv.25769
Dance, D. A. (2000). Ecology of Burkholderia pseudomallei and the interactions between environmental Burkholderia spp. and human-animal hosts. Acta Trop. 74, 159–168. doi: 10.1016/S0001-706X(99)00066-2
Dance, D. (2014). Treatment and prophylaxis of melioidosis. Int. J. Antimicrob. Agents 43, 310–318. doi: 10.1016/j.ijantimicag.2014.01.005
Dance, D. A., King, C., Aucken, H., Knott, C. D., West, P. G., Pitt, T. L. (1992). An outbreak of melioidosis in imported primates in Britain. Vet. Rec. 130, 525–529. doi: 10.1136/vr.130.24.525
Dannenberg, A. M., Jr., Scott, E. M. (1958). Melioidosis: pathogenesis and immunity in mice and hamsters. I. Studies with virulent strains of Malleomyces pseudomallei. J. Exp. Med. 107, 153–166. doi: 10.1084/jem.107.1.153
Datson, N. A., Morsink, M. C., Atanasova, S., Armstrong, V. W., Zischler, H., Schlumbohm, C., et al. (2007). Development of the first marmoset-specific DNA microarray (EUMAMA): a new genetic tool for large-scale expression profiling in a non-human primate. BMC Genomics 8, 190. doi: 10.1186/1471-2164-8-190
Day, W. C., Berendt, R. F. (1972). Experimental tularemia in Macaca mulatta: relationship of aerosol particle size to the infectivity of airborne Pasteurella tularensis. Infect. Immun. 5, 77–82. doi: 10.1128/iai.5.1.77-82.1972
Day, C. L., Lauer, G. M., Robbins, G. K., Mcgovern, B., Wurcel, A. G., Gandhi, R. T., et al. (2002). Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J. Virol. 76, 12584–12595. doi: 10.1128/JVI.76.24.12584-12595.2002
Deinhardt, F., Holmes, A. W., Capps, R. B., Popper, H. (1967). Studies on the transmission of human viral hepatitis to marmoset monkeys. I. Transmission of disease, serial passages, and description of liver lesions. J. Exp. Med. 125, 673–688. doi: 10.1084/jem.125.4.673
Dennis, D. T., Inglesby, T. V., Henderson, D. A., Bartlett, J. G., Ascher, M. S., Eitzen, E., et al. (2001). Tularemia as a biological weapon: medical and public health management. Jama 285, 2763–2773. doi: 10.1001/jama.285.21.2763
De Wit, E., Feldmann, F., Okumura, A., Horne, E., Haddock, E., Saturday, G., et al. (2018). Prophylactic and therapeutic efficacy of mAb treatment against MERS-CoV in common marmosets. Antiviral Res. 156, 64–71. doi: 10.1016/j.antiviral.2018.06.006
Deziel, M. R., Heine, H., Louie, A., Kao, M., Byrne, W. R., Basset, J., et al. (2005). Effective antimicrobial regimens for use in humans for therapy of Bacillus anthracis infections and postexposure prophylaxis. Antimicrob. Agents Chemother. 49, 5099–5106. doi: 10.1128/AAC.49.12.5099-5106.2005
Dicarlo, A. L., Poncz, M., Cassatt, D. R., Shah, J. R., Czarniecki, C. W., Maidment, B. W. (2011). Development and licensure of medical countermeasures for platelet regeneration after radiation exposure. Radiat. Res. 176, 134–137. doi: 10.1667/RR2610.1
Done, J. T. (1992). Infection of a marmoset with the BSE agent. Vet. Rec. 130, 279. doi: 10.1136/vr.130.13.279
Easton, A., Haque, A., Chu, K., Lukaszewski, R., Bancroft, G. J. (2007). A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J. Infect. Dis. 195, 99–107. doi: 10.1086/509810
Eigelsbach, H. T., Downs, C. M. (1961). Prophylactic effectiveness of live and killed tularemia vaccines. I. Production of vaccine and evaluation in the white mouse and Guinea pig. J. Immunol. 87, 415–425. doi: 10.4049/jimmunol.87.4.415
Eigelsbach, H. T., Tulis, J. J., Overholt, E. L., Griffith, W. R. (1961). Aerogenic immunization of the monkey and Guinea pig with live tularemia vaccine. Proc. Soc. Exp. Biol. Med. 108, 732–734. doi: 10.3181/00379727-108-27049
Ellis, J., Oyston, P. C., Green, M., Titball, R. W. (2002). Tularemia. Clin. Microbiol. Rev. 15, 631–646. doi: 10.1128/CMR.15.4.631-646.2002
Ellison, D. W., Baker, H. J., Mariappan, M. (1969). Melioidosis in Malaysia. I. A method for isolation of Pseudomonas pseudomallei from soil and surface water. Am. J. Trop. Med. Hyg. 18, 694–697. doi: 10.4269/ajtmh.1969.18.694
Estes, J. D., Wong, S. W., Brenchley, J. M. (2018). Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 18, 390–404. doi: 10.1038/s41577-018-0005-7
Etzion, O., Ghany, M. G. (2015). A cure for the high cost of hepatitis C virus treatment. Ann. Intern. Med. 162, 660–661. doi: 10.7326/M15-0674
Falzarano, D., De Wit, E., Feldmann, F., Rasmussen, A. L., Okumura, A., Peng, X., et al. (2014). Infection with MERS-CoV causes lethal pneumonia in the common marmoset. PloS Pathog. 10, e1004250. doi: 10.1371/journal.ppat.1004250
Fan, C., Wu, Y., Rui, X., Yang, Y., Ling, C., Liu, S., et al. (2022). Animal models for COVID-19: advances, gaps and perspectives. Signal Transduct. Target. Ther. 7, 220. doi: 10.1038/s41392-022-01087-8
Faucher, J. F., Chirouze, C., Coutris, C., Fery-Blanco, C., Maurin, M., Hoen, B. (2012). Typhoidal tularemia: 2 familial cases. Case Rep. Infect. Dis. 2012, 214215. doi: 10.1155/2012/214215
Feng, Y., Feng, Y. M., Lu, C., Han, Y., Liu, L., Sun, X., et al. (2017). Tree shrew, a potential animal model for hepatitis C, supports the infection and replication of HCV in vitro and in vivo. J. Gen. Virol. 98, 2069–2078. doi: 10.1099/jgv.0.000869
Fenwick, N., Griffin, G., Gauthier, C. (2009). The welfare of animals used in science: how the “Three Rs” ethic guides improvements. Can. Vet. J. 50, 523–530.
Fernandez, J., Taylor, D., Morhardt, D. R., Mihalik, K., Puig, M., Rice, C. M., et al. (2004). Long-term persistence of infection in chimpanzees inoculated with an infectious hepatitis C virus clone is associated with a decrease in the viral amino acid substitution rate and low levels of heterogeneity. J. Virol. 78, 9782–9789. doi: 10.1128/JVI.78.18.9782-9789.2004
Ferreira, L. M. R., Meissner, T. B., Tilburgs, T., Strominger, J. L. (2017). HLA-G: at the interface of maternal-fetal tolerance. Trends Immunol. 38, 272–286. doi: 10.1016/j.it.2017.01.009
Fish, D. N. (2003). Levofloxacin: update and perspectives on one of the original ‘respiratory quinolones’. Expert Rev. Anti Infect. Ther. 1, 371–387. doi: 10.1586/14787210.1.3.371
Fisher, D. A., Harris, P. N. (2014). Melioidosis: refining management of a tropical time bomb. Lancet 383, 762–764. doi: 10.1016/S0140-6736(13)62143-1
Fisher, N. A., Ribot, W. J., Applefeld, W., Deshazer, D. (2012). The Madagascar hissing cockroach as a novel surrogate host for Burkholderia pseudomallei, B. mallei and B. Thailandensis. BMC Microbiol. 12, 117. doi: 10.1186/1471-2180-12-117
Flint, M., Von Hahn, T., Zhang, J., Farquhar, M., Jones, C. T., Balfe, P., et al. (2006). Diverse CD81 proteins support hepatitis C virus infection. J. Virol. 80, 11331–11342. doi: 10.1128/JVI.00104-06
Folgori, A., Capone, S., Ruggeri, L., Meola, A., Sporeno, E., Ercole, B. B., et al. (2006). A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat. Med. 12, 190–197. doi: 10.1038/nm1353
Fritz, P. E., Miller, J. G., Slayter, M., Smith, T. J. (1986). Naturally occurring melioidosis in a colonized rhesus monkey (Macaca mulatta). Lab. Anim. 20, 281–285. doi: 10.1258/002367786780808749
Fujii, Y., Kitaura, K., Matsutani, T., Shirai, K., Suzuki, S., Takasaki, T., et al. (2013). Immune-related gene expression profile in laboratory common marmosets assessed by an accurate quantitative real-time PCR using selected reference genes. PloS One 8, e56296. doi: 10.1371/journal.pone.0056296
Galun, E., Burakova, T., Ketzinel, M., Lubin, I., Shezen, E., Kahana, Y., et al. (1995). Hepatitis C virus viremia in SCID–>BNX mouse chimera. J. Infect. Dis. 172, 25–30. doi: 10.1093/infdis/172.1.25
Galy, A., Travis, M., Cen, D., Chen, B. (1995). Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity 3, 459–473. doi: 10.1016/1074-7613(95)90175-2
Ganesan, N., Embi, N., Hasidah, M. S. (2020). Potential of repurposing chloroquine as an adjunct therapy for melioidosis based on a murine model of Burkholderia pseudomallei infection. Trop. BioMed. 37, 303–317.
Gates-Hollingsworth, M. A., Kolton, C. B., Hoffmaster, A. R., Meister, G. T., Moore, A. E., Green, H. R., et al. (2022). Rapid capsular antigen immunoassay for diagnosis of inhalational anthrax: preclinical studies and evaluation in a nonhuman primate model. mBio 13, e0093122. doi: 10.1128/mbio.00931-22
Genain, C. P., Hauser, S. L. (1997). Creation of a model for multiple sclerosis in Callithrix jacchus marmosets. J. Mol. Med. (Berl). 75, 187–197. doi: 10.1007/s001090050103
Glaze, E. R., Roy, M. J., Dalrymple, L. W., Lanning, L. L. (2015). A comparison of the pathogenesis of marburg virus disease in humans and nonhuman primates and evaluation of the suitability of these animal models for predicting clinical efficacy under the ‘Animal rule’. Comp. Med. 65, 241–259.
Goff, A. J., Chapman, J., Foster, C., Wlazlowski, C., Shamblin, J., Lin, K., et al. (2011). A novel respiratory model of infection with monkeypox virus in cynomolgus macaques. J. Virol. 85, 4898–4909. doi: 10.1128/JVI.02525-10
González, P. H., Laguens, R. P., Frigerio, M. J., Calello, M. A., Weissenbacher, M. C. (1983). Junin virus infection of Callithrix jacchus: pathologic features. Am. J. Trop. Med. Hyg. 32, 417–423. doi: 10.4269/ajtmh.1983.32.417
Gordeychuk, I. V., Tukhvatulin, A. I., Petkov, S. P., Abakumov, M. A., Gulyaev, S. A., Tukhvatulina, N. M., et al. (2018). Assessment of the parameters of adaptive cell-mediated immunity in naïve common marmosets (Callithrix jacchus). Acta Naturae. 10, 63–69.
Grabowska, A. M., Lechner, F., Klenerman, P., Tighe, P. J., Ryder, S., Ball, J. K., et al. (2001). Direct ex vivo comparison of the breadth and specificity of the T cells in the liver and peripheral blood of patients with chronic HCV infection. Eur. J. Immunol. 31, 2388–2394. doi: 10.1002/1521-4141(200108)31:8<2388::aid-immu2388>3.0.co;2-l
Graham, J. B., Swarts, J. L., Lund, J. M. (2017). A mouse model of west nile virus infection. Curr. Protoc. Mouse Biol. 7, 221–235. doi: 10.1002/cpmo.33
Grakoui, A., Shoukry, N. H., Woollard, D. J., Han, J. H., Hanson, H. L., Ghrayeb, J., et al. (2003). HCV persistence and immune evasion in the absence of memory T cell help. Science 302, 659–662. doi: 10.1126/science.1088774
Greenough, T. C., Carville, A., Coderre, J., Somasundaran, M., Sullivan, J. L., Luzuriaga, K., et al. (2005). Pneumonitis and multi-organ system disease in common marmosets (Callithrix jacchus) infected with the severe acute respiratory syndrome-associated coronavirus. Am. J. Pathol. 167, 455–463. doi: 10.1016/S0002-9440(10)62989-6
Gregory, A. E., Van Schaik, E. J., Russell-Lodrigue, K. E., Fratzke, A. P., Samuel, J. E. (2019). Coxiella burnetii intratracheal aerosol infection model in mice, Guinea pigs, and nonhuman primates. Infect. Immun. 87, e00178-19. doi: 10.1128/IAI.00178-19
Griffiths, G. D., Hornby, R. J., Jagger, C. P., Brown, A. P., Stoten, A., Pearce, P. C., et al. (2006). Development of methods to measure humoral immune responses against selected antigens in the common marmoset (Callithrix jacchus) and the effect of pyridostigmine bromide administration. Int. Immunopharmacol. 6, 1755–1764. doi: 10.1016/j.intimp.2006.08.005
Gronvall, G. K., Trent, D., Borio, L., Brey, R., Nagao, L., On Behalf Of The Alliance For, B (2007). The FDA animal efficacy rule and biodefense. Nat. Biotechnol. 25, 1084–1087. doi: 10.1038/nbt1007-1084
Guha, C., Lee, S.-W., Chowdhury, N. R., Chowdhury, J. R. (2005). Cell culture models and animal models of viral hepatitis. Part II: hepatitis C. Lab. Anim. 34, 39–47. doi: 10.1038/laban0205-39
Gunji, Y., Nakamura, M., Osawa, H., Nagayoshi, K., Nakauchi, H., Miura, Y., et al. (1993). Human primitive hematopoietic progenitor cells are more enriched in KITlow cells than in KIThigh cells. Blood 82, 3283–3289. doi: 10.1182/blood.V82.11.3283.3283
Guo, W. N., Zhu, B., Ai, L., Yang, D. L., Wang, B. J. (2018). Animal models for the study of hepatitis B virus infection. Zool. Res. 39, 25–31. doi: 10.24272/j.issn.2095-8137.2018.013
Gutierrez, M. G., Warawa, J. M. (2016). Attenuation of a select agent-excluded Burkholderia pseudomallei capsule mutant in hamsters. Acta Trop. 157, 68–72. doi: 10.1016/j.actatropica.2015.12.006
Hall, J. D., Woolard, M. D., Gunn, B. M., Craven, R. R., Taft-Benz, S., Frelinger, J. A., et al. (2008). Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect. Immun. 76, 5843–5852. doi: 10.1128/IAI.01176-08
Hambleton, P., Baskerville, A., Harris-Smith, P. W., Bailey, N. E. (1978). Changes in whole blood and serum components of grivet monkeys with experimental respiratory Francisella tularensis infection. Br. J. Exp. Pathol. 59, 630–639.
Han, H. J., Powers, S. J., Gabrielson, K. L. (2022). The common marmoset-biomedical research animal model applications and common spontaneous diseases. Toxicol. Pathol. 50, 628–637. doi: 10.1177/01926233221095449
Haqshenas, G., Dong, X., Netter, H., Torresi, J., Gowans, E. J. (2007). A chimeric GB virus B encoding the hepatitis C virus hypervariable region 1 is infectious in vivo. J. Gen. Virol. 88, 895–902. doi: 10.1099/vir.0.82467-0
Haque, A., Easton, A., Smith, D., O’garra, A., Van Rooijen, N., Lertmemongkolchai, G., et al. (2006). Role of T Cells in Innate and Adaptive Immunity against Murine Burkholderia pseudomallei Infection. J. Infect. Dis. 193, 370–379. doi: 10.1086/498983
Hartlage, A. S., Murthy, S., Kumar, A., Trivedi, S., Dravid, P., Sharma, H., et al. (2019). Vaccination to prevent T cell subversion can protect against persistent hepacivirus infection. Nat. Commun. 10, 113. doi: 10.1038/s41467-019-09105-0
Hartman, A. L., Powell, D. S., Bethel, L. M., Caroline, A. L., Schmid, R. J., Oury, T., et al. (2014). Aerosolized rift valley fever virus causes fatal encephalitis in african green monkeys and common marmosets. J. Virol. 88, 2235–2245. doi: 10.1128/JVI.02341-13
Hasselbring, B. M., Patel, M. K., Schell, M. A. (2011). Dictyostelium discoideum as a model system for identification of Burkholderia pseudomallei virulence factors. Infect. Immun. 79, 2079–2088. doi: 10.1128/IAI.01233-10
He, X. S., Rehermann, B., López-Labrador, F. X., Boisvert, J., Cheung, R., Mumm, J., et al. (1999). Quantitative analysis of hepatitis C virus-specific CD8(+) T cells in peripheral blood and liver using peptide-MHC tetramers. Proc. Natl. Acad. Sci. U.S.A. 96, 5692–5697. doi: 10.1073/pnas.96.10.5692
Hepburn, M. J., Simpson, A. J. (2008). Tularemia: current diagnosis and treatment options. Expert Rev. Anti Infect. Ther. 6, 231–240. doi: 10.1586/14787210.6.2.231
Hofmeister, E. K., Lund, M., Shearn Bochsler, V. (2018). West nile virus infection in american singer canaries: an experimental model in a highly susceptible avian species. Vet. Pathol. 55, 531–538. doi: 10.1177/0300985818760377
Hofmeister, E. K., Lund, M., Shearn-Bochsler, V., Balakrishnan, C. N. (2017). Susceptibility and antibody response of the laboratory model zebra finch (Taeniopygia guttata) to west nile virus. PloS One 12, e0167876. doi: 10.1371/journal.pone.0167876
Holland, D. J., Wesley, A., Drinkovic, D., Currie, B. J. (2002). Cystic fibrosis and burkholderia pseudomallei infection: an emerging problem? Clin. Infect. Dis. 35, e138–e140. doi: 10.1086/344447
Hollands, C. (1986). The animals (scientific procedures) act 1986. Lancet 2, 32–33. doi: 10.1016/S0140-6736(86)92571-7
Horvat, B., Berges, B. K., Lusso, P. (2014). Recent developments in animal models for human herpesvirus 6A and 6B. Curr. Opin. Virol. 9, 97–103. doi: 10.1016/j.coviro.2014.09.012
Houghton, M. (2009). Discovery of the hepatitis C virus. Liver. Int. 29 Suppl 1, 82–88. doi: 10.1111/j.1478-3231.2008.01925.x
Hubrecht, R. C., Carter, E. (2019). The 3Rs and humane experimental technique: implementing change. Anim. (Basel). 9, 754. doi: 10.3390/ani9100754
Hunter, N. (2003). Scrapie and experimental BSE in sheep. Br. Med. Bull. 66, 171–183. doi: 10.1093/bmb/66.1.171
Hutt, J. A., Lovchik, J. A., Dekonenko, A., Hahn, A. C., Wu, T. H. (2017). The Natural History of Pneumonic Tularemia in Female Fischer 344 Rats after Inhalational Exposure to Aerosolized Francisella tularensis Subspecies tularensis Strain SCHU S4. Am. J. Pathol. 187, 252–267. doi: 10.1016/j.ajpath.2016.09.021
Inoue, T., Yurimoto, T., Seki, F., Sato, K., Sasaki, E. (2022). The common marmoset in biomedical research: experimental disease models and veterinary management. Exp. Anim. 72, 140–150. doi: 10.1538/expanim.22-0107
Ip, M., Osterberg, L. G., Chau, P. Y., Raffin, T. A. (1995). Pulmonary melioidosis. Chest 108, 1420–1424. doi: 10.1378/chest.108.5.1420
Ireland, R. E., Davies, C. D., Keyser, E., Findlay, J. S. F., Eastaugh, L., Laws, T. R., et al. (2022). Histopathological and immunological findings in the common marmoset following exposure to aerosolized SARS-coV-2. Viruses 14, 1580. doi: 10.3390/v14071580
Ishii, K., Iijima, S., Kimura, N., Lee, Y. J., Ageyama, N., Yagi, S., et al. (2007). GBV-B as a pleiotropic virus: distribution of GBV-B in extrahepatic tissues in vivo. Microbes Infect. 9, 515–521. doi: 10.1016/j.micinf.2007.01.010
Ito, M., Hiramatsu, H., Kobayashi, K., Suzue, K., Kawahata, M., Hioki, K., et al. (2002). NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100, 3175–3182. doi: 10.1182/blood-2001-12-0207
Ito, M., Kobayashi, K., Nakahata, T. (2008a). NOD/Shi-scid IL2rgamma(null) (NOG) mice more appropriate for humanized mouse models. Curr. Top. Microbiol. Immunol. 324, 53–76. doi: 10.1007/978-3-540-75647-7_3
Ito, R., Maekawa, S., Kawai, K., Suemizu, H., Suzuki, S., Ishii, H., et al. (2008b). Novel monoclonal antibodies recognizing different subsets of lymphocytes from the common marmoset (Callithrix jacchus). Immunol. Lett. 121, 116–122. doi: 10.1016/j.imlet.2008.09.007
Iwasaki, Y., Mori, K.-I., Ishii, K., Maki, N., Iijima, S., Yoshida, T., et al. (2011). Long-term persistent GBV-B infection and development of a chronic and progressive hepatitis C-like disease in marmosets. Front. Microbiol. 2. doi: 10.3389/fmicb.2011.00240
Izawa, K., Tani, K., Nakazaki, Y., Hibino, H., Sugiyama, H., Kawasaki, A., et al. (2004). Hematopoietic activity of common marmoset CD34 cells isolated by a novel monoclonal antibody MA24. Exp. Hematol. 32, 843–851. doi: 10.1016/j.exphem.2004.06.007
Jackson, A. C., Sengupta, S. K., Smith, J. F. (1991). Pathogenesis of Venezuelan equine encephalitis virus infection in mice and hamsters. Vet. Pathol. 28, 410–418. doi: 10.1177/030098589102800509
Jacob, J. R., Lin, K. C., Tennant, B. C., Mansfield, K. G. (2004). GB virus B infection of the common marmoset (Callithrix jacchus) and associated liver pathology. J. Gen. Virol. 85, 2525–2533. doi: 10.1099/vir.0.80036-0
Jagessar, S. A., Heijmans, N., Blezer, E. L., Bauer, J., Weissert, R., T Hart, B. A. (2015). Immune profile of an atypical EAE model in marmoset monkeys immunized with recombinant human myelin oligodendrocyte glycoprotein in incomplete Freund’s adjuvant. J. Neuroinflamm. 12, 169. doi: 10.1186/s12974-015-0378-5
Jagessar, S. A., Vierboom, M., Blezer, E. L., Bauer, J., Hart, B. A., Kap, Y. S. (2013). Overview of models, methods, and reagents developed for translational autoimmunity research in the common marmoset (Callithrix jacchus). Exp. Anim. 62, 159–171. doi: 10.1538/expanim.62.159
Janse, I., van der Plaats, R. Q. J., De Roda Husman, A. M., Van Passel, M. W. J. (2018). Environmental surveillance of zoonotic francisella tularensis in the Netherlands. Front. Cell Infect. Microbiol. 8, 140. doi: 10.3389/fcimb.2018.00140
Jefferies, M., Rauff, B., Rashid, H., Lam, T., Rafiq, S. (2018). Update on global epidemiology of viral hepatitis and preventive strategies. World J. Clin. cases 6, 589–599. doi: 10.12998/wjcc.v6.i13.589
Jenjaroen, K., Chumseng, S., Sumonwiriya, M., Ariyaprasert, P., Chantratita, N., Sunyakumthorn, P., et al. (2015). T-cell responses are associated with survival in acute melioidosis patients. PloS Negl. Trop. Dis. 9, e0004152. doi: 10.1371/journal.pntd.0004152
Jiang, L., Lu, C., Sun, Q. (2021). Tree shrew as a new animal model for the study of dengue virus. Front. Immunol. 12, 621164. doi: 10.3389/fimmu.2021.621164
Johnson, R. F., Via, L. E., Kumar, M. R., Cornish, J. P., Yellayi, S., Huzella, L., et al. (2015). Intratracheal exposure of common marmosets to MERS-CoV Jordan-n3/2012 or MERS-CoV EMC/2012 isolates does not result in lethal disease. Virology 485, 422–430. doi: 10.1016/j.virol.2015.07.013
Kametani, Y., Shiina, M., Katano, I., Ito, R., Ando, K., Toyama, K., et al. (2006). Development of human-human hybridoma from anti-Her-2 peptide-producing B cells in immunized NOG mouse. Exp. Hematol. 34, 1240–1248. doi: 10.1016/j.exphem.2006.05.006
Kametani, Y., Shiina, T., Suzuki, R., Sasaki, E., Habu, S. (2018). Comparative immunity of antigen recognition, differentiation, and other functional molecules: similarities and differences among common marmosets, humans, and mice. Exp. Anim. 67, 301–312. doi: 10.1538/expanim.17-0150
Kametani, Y., Suzuki, D., Kohu, K., Satake, M., Suemizu, H., Sasaki, E., et al. (2009). Development of monoclonal antibodies for analyzing immune and hematopoietic systems of common marmoset. Exp. Hematol. 37, 1318–1329. doi: 10.1016/j.exphem.2009.08.003
Kantardjiev, T., Padeshki, P., Ivanov, I. N. (2007). Diagnostic approaches for oculoglandular tularemia: advantages of PCR. Br. J. Ophthalmol. 91, 1206–1208. doi: 10.1136/bjo.2007.117523
Kap, Y. S., Van Driel, N., Arends, R., Rouwendal, G., Verolin, M., Blezer, E., et al. (2015). Immune modulation by a tolerogenic myelin oligodendrocyte glycoprotein (MOG)10-60 containing fusion protein in the marmoset experimental autoimmune encephalomyelitis model. Clin. Exp. Immunol. 180, 28–39. doi: 10.1111/cei.12487
Kaplan, M. J., Radic, M. (2012). Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 189, 2689–2695. doi: 10.4049/jimmunol.1201719
Kaufmann, A. F., Alexander, A. D., Allen, M. A., Cronin, R. J., Dillingham, L. A., Douglas, J. D., et al. (1970). Melioidosis in imported non-human primates. J. Wildl. Dis. 6, 211–219. doi: 10.7589/0090-3558-6.4.211
Kaukinen, P., Sillanpää, M., Kotenko, S., Lin, R., Hiscott, J., Melén, K., et al. (2006). Hepatitis C virus NS2 and NS3/4A proteins are potent inhibitors of host cell cytokine/chemokine gene expression. Virol. J. 3, 66. doi: 10.1186/1743-422X-3-66
Keim, P., Johansson, A., Wagner, D. M. (2007). Molecular epidemiology, evolution, and ecology of Francisella. Ann. N. Y. Acad. Sci. 1105, 30–66. doi: 10.1196/annals.1409.011
Khakhum, N., Bharaj, P., Myers, J. N., Tapia, D., Kilgore, P. B., Ross, B. N., et al. (2019). Burkholderia pseudomallei ΔtonB Δhcp1 Live Attenuated Vaccine Strain Elicits Full Protective Immunity against Aerosolized Melioidosis Infection. mSphere 4, e00570-18. doi: 10.1128/mSphere.00570-18
Kharbov, D., Velianov, D., Raucheva, T. (1981). [Ecological aspects of Pseudomonas pseudomallei interactions with ixodid ticks]. Acta Microbiol. Bulg. 8, 17–24.
Kim, I. J., Lanthier, P. A., Clark, M. J., de la Barrera, R. A., Tighe, M. P., Szaba, F. M., et al. (2022). Author Correction: Efficacy of an inactivated Zika vaccine against virus infection during pregnancy in mice and marmosets. NPJ Vaccines 7, 99. doi: 10.1038/s41541-022-00520-x
Kireta, S., Zola, H., Gilchrist, R. B., Coates, P. T. (2005). Cross-reactivity of anti-human chemokine receptor and anti-TNF family antibodies with common marmoset (Callithrix jacchus) leukocytes. Cell Immunol. 236, 115–122. doi: 10.1016/j.cellimm.2005.08.017
Kitaura, K., Fujii, Y., Matsutani, T., Shirai, K., Suzuki, S., Takasaki, T., et al. (2012). A new method for quantitative analysis of the T cell receptor V region repertoires in healthy common marmosets by microplate hybridization assay. J. Immunol. Methods 384, 81–91. doi: 10.1016/j.jim.2012.07.012
Kohu, K., Yamabe, E., Matsuzawa, A., Onda, D., Suemizu, H., Sasaki, E., et al. (2008). Comparison of 30 immunity-related genes from the common marmoset with orthologues from human and mouse. Tohoku. J. Exp. Med. 215, 167–180. doi: 10.1620/tjem.215.167
Kono, A., Brameier, M., Roos, C., Suzuki, S., Shigenari, A., Kametani, Y., et al. (2014). Genomic sequence analysis of the MHC class I G/F segment in common marmoset (Callithrix jacchus). J. Immunol. 192, 3239–3246. doi: 10.4049/jimmunol.1302745
Kramski, M., Mätz-Rensing, K., Stahl-Hennig, C., Kaup, F. J., Nitsche, A., Pauli, G., et al. (2010). A novel highly reproducible and lethal nonhuman primate model for orthopox virus infection. PloS One 5, e10412. doi: 10.1371/journal.pone.0010412
Kroca, M., Tärnvik, A., Sjöstedt, A. (2000). The proportion of circulating gammadelta T cells increases after the first week of onset of tularaemia and remains elevated for more than a year. Clin. Exp. Immunol. 120, 280–284. doi: 10.1046/j.1365-2249.2000.01215.x
Kronsteiner, B., Chaichana, P., Sumonwiriya, M., Jenjaroen, K., Chowdhury, F. R., Chumseng, S., et al. (2019). Diabetes alters immune response patterns to acute melioidosis in humans. Eur. J. Immunol. 49, 1092–1106. doi: 10.1002/eji.201848037
Kublin, J. L., Whitney, J. B. (2018). Zika virus research models. Virus Res. 254, 15–20. doi: 10.1016/j.virusres.2017.07.025
Kumita, W., Sato, K., Suzuki, Y., Kurotaki, Y., Harada, T., Zhou, Y., et al. (2019). Efficient generation of Knock-in/Knock-out marmoset embryo via CRISPR/Cas9 gene editing. Sci. Rep. 9, 12719. doi: 10.1038/s41598-019-49110-3
Kuolee, R., Harris, G., Conlan, J. W., Chen, W. (2011). Role of neutrophils and NADPH phagocyte oxidase in host defense against respiratory infection with virulent Francisella tularensis in mice. Microbes Infect. 13, 447–456. doi: 10.1016/j.micinf.2011.01.010
Kyuregyan, K. K., Poleschuk, V. F., Zamyatina, N. A., Isaeva, O. V., Michailov, M. I., Ross, S., et al. (2005). Acute GB virus B infection of marmosets is accompanied by mutations in the NS5A protein. Virus Res. 114, 154–157. doi: 10.1016/j.virusres.2005.06.009
Laman, J. D., Van Meurs, M., Schellekens, M. M., De Boer, M., Melchers, B., Massacesi, L., et al. (1998). Expression of accessory molecules and cytokines in acute EAE in marmoset monkeys (Callithrix jacchus). J. Neuroimmunol. 86, 30–45. doi: 10.1016/S0165-5728(98)00024-1
Lamps, L. W., Havens, J. M., Sjostedt, A., Page, D. L., Scott, M. A. (2004). Histologic and molecular diagnosis of tularemia: a potential bioterrorism agent endemic to North America. Mod. Pathol. 17, 489–495. doi: 10.1038/modpathol.3800087
Lanford, R. E., Chavez, D., Notvall, L., Brasky, K. M. (2003). Comparison of tamarins and marmosets as hosts for GBV-B infections and the effect of immunosuppression on duration of viremia. Virology 311, 72–80. doi: 10.1016/S0042-6822(03)00193-4
Laws, L., Hall, W. (1963). Melioidosis in Animals in North Queensland. 1. Incidence and Pathology, with special reference to Central Nervous System Lesions. Queensland. J. Agric. Sci. 20, 499–513.
Laws, T. R., Nelson, M., Bonnafous, C., Sicard, H., Taylor, C., Salguero, F. J., et al. (2013). In vivo manipulation of γ9(+) T cells in the common marmoset (Callithrix Jacchus) with phosphoantigen and effect on the progression of respiratory melioidosis. PloS One 8, e74789. doi: 10.1371/journal.pone.0074789
Laws, T. R., Smither, S. J., Lukaszewski, R. A., Atkins, H. S. (2011). Neutrophils are the predominant cell-type to associate with Burkholderia pseudomallei in a BALB/c mouse model of respiratory melioidosis. Microb. Pathog. 51, 471–475. doi: 10.1016/j.micpath.2011.07.002
Leakey, A. K., Ulett, G. C., Hirst, R. G. (1998). BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb. Pathog. 24, 269–275. doi: 10.1006/mpat.1997.0179
Lechner, F., Wong, D. K., Dunbar, P. R., Chapman, R., Chung, R. T., Dohrenwend, P., et al. (2000). Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191, 1499–1512. doi: 10.1084/jem.191.9.1499
Leibovitch, E., Wohler, J. E., Cummings Macri, S. M., Motanic, K., Harberts, E., Gaitán, M. I., et al. (2013). Novel marmoset (Callithrix jacchus) model of human Herpesvirus 6A and 6B infections: immunologic, virologic and radiologic characterization. PloS Pathog. 9, e1003138. doi: 10.1371/journal.ppat.1003138
Lever, M. S., Stagg, A. J., Nelson, M., Pearce, P., Stevens, D. J., Scott, E. A., et al. (2008). Experimental respiratory anthrax infection in the common marmoset (Callithrix jacchus). Int. J. Exp. Pathol. 89, 171–179. doi: 10.1111/j.1365-2613.2008.00581.x
Li, F., Nandy, P., Chien, S., Noel, G. J., Tornoe, C. W. (2010). Pharmacometrics-based dose selection of levofloxacin as a treatment for postexposure inhalational anthrax in children. Antimicrob. Agents Chemother. 54, 375–379. doi: 10.1128/AAC.00667-09
Li, T., Xu, Y., Yin, S., Liu, B., Zhu, S., Wang, W., et al. (2014a). Characterization of major histocompatibility complex class I allele polymorphisms in common marmosets. Tissue Antigens 84, 568–573. doi: 10.1111/tan.12453
Li, T., Zhu, S., Shuai, L., Xu, Y., Yin, S., Bian, Y., et al. (2014b). Infection of common marmosets with hepatitis C virus/GB virus-B chimeras. Hepatology 59, 789–802. doi: 10.1002/hep.26750
Lim, H. K., Jeffrey, G. P., Ramm, G. A., Soekmadji, C. (2020). Pathogenesis of viral hepatitis-induced chronic liver disease: role of extracellular vesicles. Front. Cell Infect. Microbiol. 10, 587628. doi: 10.3389/fcimb.2020.587628
Limmathurotsakul, D., Chaowagul, W., Chantratita, N., Wuthiekanun, V., Biaklang, M., Tumapa, S., et al. (2008). A simple scoring system to differentiate between relapse and re-infection in patients with recurrent melioidosis. PloS Negl. Trop. Dis. 2, e327. doi: 10.1371/journal.pntd.0000327
Limmathurotsakul, D., Golding, N., Dance, D. A., Messina, J. P., Pigott, D. M., Moyes, C. L., et al. (2016). Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat. Microbiol. 1, 15008. doi: 10.1038/nmicrobiol.2015.8
Limmathurotsakul, D., Peacock, S. J. (2011). Melioidosis: a clinical overview. Br. Med. Bull. 99, 125–139. doi: 10.7861/clinmed.2022-0014
Lin, Q., Lu, C., Hong, Y., Li, R., Chen, J., Chen, W., et al. (2022). Animal models for studying coronavirus infections and developing antiviral agents and vaccines. Antiviral Res. 203, 105345. doi: 10.1016/j.antiviral.2022.105345
Lin, J., Wu, J. F., Zhang, Q., Zhang, H. W., Cao, G. W. (2014). Virus-related liver cirrhosis: molecular basis and therapeutic options. World J. Gastroenterol. 20, 6457–6469. doi: 10.3748/wjg.v20.i21.6457
Lin, A., Yan, W. H. (2016). HLA-G as an inhibitor of immune responses. Methods Mol. Biol. 1371, 3–9. doi: 10.1007/978-1-4939-3139-2_1
Lipsitz, R., Garges, S., Aurigemma, R., Baccam, P., Blaney, D. D., Cheng, A. C., et al. (2012). Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei Infectio. Emerg. Infect. Dis. 18, e2. doi: 10.3201/eid1812.120638
Liu, Y., Maya, S., Ploss, A. (2021). Animal models of hepatitis B virus infection-success, challenges, and future directions. Viruses 13, 777. doi: 10.3390/v13050777
Liu, B., Zhang, E., Ma, X., Luo, S., Wang, C., Zhang, L., et al. (2023). Early phase of specific cellular immune status associates with HCV infection outcomes in marmosets. Viruses 15, 1082. doi: 10.3390/v15051082
Loader, M., Moravek, R., Witowski, S. E., Driscoll, L. M. (2019). A clinical review of viral hepatitis. Jaapa 32, 15–20. doi: 10.1097/01.JAA.0000586300.88300.84
Longet, S., Mellors, J., Carroll, M. W., Tipton, T. (2020). Ebolavirus: comparison of survivor immunology and animal models in the search for a correlate of protection. Front. Immunol. 11, 599568. doi: 10.3389/fimmu.2020.599568
Lu, S., Zhao, Y., Yu, W., Yang, Y., Gao, J., Wang, J., et al. (2020). Comparison of nonhuman primates identified the suitable model for COVID-19. Signal Transduction. Targeted. Ther. 5, 157. doi: 10.1038/s41392-020-00269-6
Lukashevich, I. S., Carrion, R., Jr., Salvato, M. S., Mansfield, K., Brasky, K., Zapata, J., et al. (2008). Safety, immunogenicity, and efficacy of the ML29 reassortant vaccine for Lassa fever in small non-human primates. Vaccine 26, 5246–5254. doi: 10.1016/j.vaccine.2008.07.057
Lum, F. M., Zhang, W., Lim, K. C., Malleret, B., Teo, T. H., Koh, J. J., et al. (2018). Multimodal assessments of Zika virus immune pathophysiological responses in marmosets. Sci. Rep. 8, 17125. doi: 10.1038/s41598-018-35481-6
Luo, S., Zhao, W., Ma, X., Zhang, P., Liu, B., Zhang, L., et al. (2020). A high infectious simian adenovirus type 23 vector based vaccine efficiently protects common marmosets against Zika virus infection. PloS Negl. Trop. Dis. 14, e0008027. doi: 10.1371/journal.pntd.0008027
Lusso, P., Crowley, R. W., Malnati, M. S., Di Serio, C., Ponzoni, M., Biancotto, A., et al. (2007). Human herpesvirus 6A accelerates AIDS progression in macaques. Proc. Natl. Acad. Sci. U.S.A. 104, 5067–5072. doi: 10.1073/pnas.0700929104
Lusso, P., Secchiero, P., Crowley, R. W. (1994). In vitro susceptibility of Macaca nemestrina to human herpesvirus 6: a potential animal model of coinfection with primate immunodeficiency viruses. AIDS Res. Hum. Retroviruses 10, 181–187. doi: 10.1089/aid.1994.10.181
Malik, M., Bakshi, C. S., Mccabe, K., Catlett, S. V., Shah, A., Singh, R., et al. (2007). Matrix metalloproteinase 9 activity enhances host susceptibility to pulmonary infection with type A and B strains of Francisella tularensis. J. Immunol. 178, 1013–1020. doi: 10.4049/jimmunol.178.2.1013
Manickam, C., Rajakumar, P., Wachtman, L., Kramer, J. A., Martinot, A. J., Varner, V., et al. (2016). Acute liver damage associated with innate immune activation in a small nonhuman primate model of hepacivirus infection. J. Virol. 90, 9153–9162. doi: 10.1128/JVI.01051-16
Manickam, C., Reeves, R. K. (2014). Modeling HCV disease in animals: virology, immunology and pathogenesis of HCV and GBV-B infections. Front. Microbiol. 5, 690. doi: 10.3389/fmicb.2014.00690
Manzeniuk, I. N., Galina, E. A., Dorokhin, V. V., Kalachev, I., Borzenkov, V. N., Svetoch, E. A. (1999). [Burkholderia mallei and Burkholderia pseudomallei. Study of immuno- and pathogenesis of glanders and melioidosis. Heterologous vaccines]. Antibiot. Khimioter. 44, 21–26.
Mariappan, V., Vellasamy, K. M., Barathan, M., Girija, A. S. S., Shankar, E. M., Vadivelu, J. (2021). Hijacking of the host’s immune surveillance radars by burkholderia pseudomallei. Front. Immunol. 12, 718719. doi: 10.3389/fimmu.2021.718719
Martin, A., Bodola, F., Sangar, D. V., Goettge, K., Popov, V., Rijnbrand, R., et al. (2003). Chronic hepatitis associated with GB virus B persistence in a tamarin after intrahepatic inoculation of synthetic. Proc. Natl. Acad. Sci. U.S.A. 100, 9962–9967. doi: 10.1073/pnas.1731505100
Massacesi, L., Genain, C. P., Lee-Parritz, D., Letvin, N. L., Canfield, D., Hauser, S. L. (1995). Active and passively induced experimental autoimmune encephalomyelitis in common marmosets: a new model for multiple sclerosis. Ann. Neurol. 37, 519–530. doi: 10.1002/ana.410370415
Massey, S., Yeager, L. A., Blumentritt, C. A., Vijayakumar, S., Sbrana, E., Peterson, J. W., et al. (2014). Comparative Burkholderia pseudomallei natural history virulence studies using an aerosol murine model of infection. Sci. Rep. 4, 4305. doi: 10.1038/srep04305
Matsumura, T., Kametani, Y., Ando, K., Hirano, Y., Katano, I., Ito, R., et al. (2003). Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/gammac(null) (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp. Hematol. 31, 789–797. doi: 10.1016/S0301-472X(03)00193-0
Matsutani, T., Fujii, Y., Kitaura, K., Suzuki, S., Tsuruta, Y., Takasaki, T., et al. (2011). Increased positive selection pressure within the complementarity determining regions of the T-cell receptor β gene in New World monkeys. Am. J. Primatol. 73, 1082–1092. doi: 10.1002/ajp.20976
Mätz-Rensing, K., Stahl-Hennig, C., Kramski, M., Pauli, G., Ellerbrok, H., Kaup, F. J. (2012). The pathology of experimental poxvirus infection in common marmosets (Callithrix jacchus): further characterization of a new primate model for orthopoxvirus infections. J. Comp. Pathol. 146, 230–242. doi: 10.1016/j.jcpa.2011.06.003
Maurin, M. (2015). Francisella tularensis as a potential agent of bioterrorism? Expert Rev. Anti Infect. Ther. 13, 141–144. doi: 10.1586/14787210.2015.986463
Maurin, M., Gyuranecz, M. (2016). Tularaemia: clinical aspects in Europe. Lancet Infect. Dis. 16, 113–124. doi: 10.1016/S1473-3099(15)00355-2
Maurin, M., Pelloux, I., Brion, J. P., Del Banõ, J. N., Picard, A. (2011). Human tularemia in France 2006-2010. Clin. Infect. Dis. 53, e133–e141. doi: 10.1093/cid/cir612
Mccormick, J. B., Weaver, R. E., Hayes, P. S., Boyce, J. M., Feldman, R. A. (1977). Wound infection by an indigenous Pseudomonas pseudomallei-like organism isolated from the soil: case report and epidemiologic study. J. Infect. Dis. 135, 103–107. doi: 10.1093/infdis/135.1.103
Mccoy, G. W., Chapin, C. W. (1912). Further observations on a plague-like disease of rodents with a preliminary note on the causative agent, bacterium tularense. J. Infect. Dis. 10, 61–72. doi: 10.1093/infdis/10.1.61
Meinderts, S. M., Baker, G., Van Wijk, S., Beuger, B. M., Geissler, J., Jansen, M. H., et al. (2019). Neutrophils acquire antigen-presenting cell features after phagocytosis of IgG-opsonized erythrocytes. Blood Adv. 3, 1761–1773. doi: 10.1182/bloodadvances.2018028753
Mercer, D. F., Schiller, D. E., Elliott, J. F., Douglas, D. N., Hao, C., Rinfret, A., et al. (2001). Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 7, 927–933. doi: 10.1038/90968
Mestas, J., Hughes, C. C. (2004). Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738. doi: 10.4049/jimmunol.172.5.2731
Metzger, D. W., Salmon, S. L., Kirimanjeswara, G. (2013). Differing effects of interleukin-10 on cutaneous and pulmonary Francisella tularensis live vaccine strain infection. Infect. Immun. 81, 2022–2027. doi: 10.1128/IAI.00024-13
Meuleman, P., Libbrecht, L., De Vos, R., De Hemptinne, B., Gevaert, K., Vandekerckhove, J., et al. (2005). Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology 41, 847–856. doi: 10.1002/hep.20657
Mietsch, M., Paqué, K., Drummer, C., Stahl-Hennig, C., Roshani, B. (2020). The aging common marmoset’s immune system: From junior to senior. Am. J. Primatol. 82, e23128. doi: 10.1002/ajp.23128
Miller, R., Jr., Clinger, D. I. (1961). Melioidosis pathogenesis in rabbits. II. A simplified surgical technique for in vivo observations of pathologic changes in abdominal viscera. Arch. Pathol. 71, 635–640.
Miller, W. R., Pannell, L., Cravitz, L., Tanner, W. A., Rosebury, T. (1948). Studies on Certain Biological Characteristics of Malleomyces mallei and Malleomyces pseudomallei: II. Virulence and Infectivity for Animals. J. Bacteriol. 55, 127–135. doi: 10.1128/jb.55.1.127-135.1948
Moi, M. L., Ami, Y., Muhammad Azami, N. A., Shirai, K., Yoksan, S., Suzaki, Y., et al. (2017). Marmosets (Callithrix jacchus) as a non-human primate model for evaluation of candidate dengue vaccines: induction and maintenance of specific protective immunity against challenges with clinical isolates. J. Gen. Virol. 98, 2955–2967. doi: 10.1099/jgv.0.000913
Moi, M. L., Takasaki, T., Omatsu, T., Nakamura, S., Katakai, Y., Ami, Y., et al. (2014). Demonstration of marmosets (Callithrix jacchus) as a non-human primate model for secondary dengue virus infection: high levels of viraemia and serotype cross-reactive antibody responses consistent with secondary infection of humans. J. Gen. Virol. 95, 591–600. doi: 10.1099/vir.0.060384-0
Molinas, F. C., Giavedoni, E., Frigerio, M. J., Calello, M. A., Barcat, J. A., Weissenbacher, M. C. (1983). Alteration of blood coagulation and complement system in neotropical primates infected with Junin virus. J. Med. Virol. 12, 281–292. doi: 10.1002/jmv.1890120408
Morrill, J. C., Jennings, G. B., Johnson, A. J., Cosgriff, T. M., Gibbs, P. H., Peters, C. J. (1990). Pathogenesis of Rift Valley fever in rhesus monkeys: role of interferon response. Arch. Virol. 110, 195–212. doi: 10.1007/BF01311288
Morris, T. H. (1992). Infection of a marmoset with the BSE agent. Vet. Rec. 130, 359–360. doi: 10.1136/vr.130.16.359
M, S. E. S, Ellis, A., Karaca, K., Minke, J., Nordgren, R., Wu, S., et al. (2013). Domestic goose as a model for West Nile virus vaccine efficacy. Vaccine 31, 1045–1050. doi: 10.1016/j.vaccine.2012.12.044
Mucker, E. M., Chapman, J., Huzella, L. M., Huggins, J. W., Shamblin, J., Robinson, C. G., et al. (2015). Susceptibility of marmosets (Callithrix jacchus) to monkeypox virus: A low dose prospective model for monkeypox and smallpox disease. PloS One 10, e0131742. doi: 10.1371/journal.pone.0131742
Mucker, E. M., Wollen-Roberts, S. E., Kimmel, A., Shamblin, J., Sampey, D., Hooper, J. W. (2018). Intranasal monkeypox marmoset model: Prophylactic antibody treatment provides benefit against severe monkeypox virus disease. PloS Negl. Trop. Dis. 12, e0006581. doi: 10.1371/journal.pntd.0006581
Muhammad Azami, N. A., Takasaki, T., Kurane, I., Moi, M. L. (2020). Non-Human Primate Models of Dengue Virus Infection: A Comparison of Viremia Levels and Antibody Responses during Primary and Secondary Infection among Old World and New World Monkeys. Pathogens 9, 247. doi: 10.3390/pathogens9040247
Münz, C., Stevanović, S., Rammensee, H. G. (1999). Peptide presentation and NK inhibition by HLA-G. J. Reprod. Immunol. 43, 139–155. doi: 10.1016/S0165-0378(99)00029-7
Na, W., Yeom, M., Choi, I. K., Yook, H., Song, D. (2017). Animal models for dengue vaccine development and testing. Clin. Exp. Vaccine Res. 6, 104–110. doi: 10.7774/cevr.2017.6.2.104
Najdenski, H., Kussovski, V., Vesselinova, A. (2004). Experimental Burkholderia pseudomallei infection of pigs. J. Vet. Med. B. Infect. Dis. Vet. Public Health 51, 225–230. doi: 10.1111/j.1439-0450.2004.00754.x
Nakayama, E., Saijo, M. (2013). Animal models for Ebola and Marburg virus infections. Front. Microbiol. 4, 267. doi: 10.3389/fmicb.2013.00267
Nam, J. H., Faulk, K., Engle, R. E., Govindarajan, S., St Claire, M., Bukh, J. (2004). In vivo analysis of the 3’ untranslated region of GB virus B after in vitro mutagenesis of an infectious cDNA clone: persistent infection in a transfected tamarin. J. Virol. 78, 9389–9399. doi: 10.1128/JVI.78.17.9389-9399.2004
Narita, M., Loganathan, P., Hussein, A., Jamaluddin, A., Joseph, P. (1982). Pathological changes in goats experimentally inoculated with Pseudomonas pseudomallei. Natl. Inst. Anim. Health Q. 22, 170–179.
Neehus, A. L., Wistuba, J., Ladas, N., Eiz-Vesper, B., Schlatt, S., Müller, T. (2016). Gene conversion of the major histocompatibility complex class I Caja-G in common marmosets (Callithrix jacchus). Immunology 149, 343–352. doi: 10.1111/imm.12652
Nelson, M., Burton, N., Nunez, A., Butcher, W., Ngugi, S., Atkins, T. P. (2022a). Efficacy of co-trimoxazole against experimental melioidosis acquired by different routes of infection. Antimicrob. Agents Chemother., e0070822. doi: 10.1128/aac.00708-22
Nelson, M., Dean, R. E., Salguero, F. J., Taylor, C., Pearce, P. C., Simpson, A. J., et al. (2011a). Development of an acute model of inhalational melioidosis in the common marmoset (Callithrix jacchus). Int. J. Exp. Pathol. 92, 428–435. doi: 10.1111/j.1365-2613.2011.00791.x
Nelson, M., Lever, M. S., Dean, R. E., Pearce, P. C., Stevens, D. J., Simpson, A. J. (2010a). Bioavailability and efficacy of levofloxacin against Francisella tularensis in the common marmoset (Callithrix jacchus). Antimicrob. Agents Chemother. 54, 3922–3926. doi: 10.1128/AAC.00390-10
Nelson, M., Lever, M. S., Dean, R. E., Savage, V. L., Salguero, F. J., Pearce, P. C., et al. (2010b). Characterization of lethal inhalational infection with Francisella tularensis in the common marmoset (Callithrix jacchus). J. Med. Microbiol. 59, 1107–1113. doi: 10.1099/jmm.0.020669-0
Nelson, M., Lever, M. S., Savage, V. L., Salguero, F. J., Pearce, P. C., Stevens, D. J., et al. (2009). Establishment of lethal inhalational infection with Francisella tularensis (tularaemia) in the common marmoset (Callithrix jacchus). Int. J. Exp. Pathol. 90, 109–118. doi: 10.1111/j.1365-2613.2008.00631.x
Nelson, M., Loveday, M. (2014). Exploring the innate immunological response of an alternative nonhuman primate model of infectious disease; the common marmoset. J. Immunol. Res. 2014, 913632. doi: 10.1155/2014/913632
Nelson, M., Nunez, A., Ngugi, S. A., Atkins, T. P. (2021). The lymphatic system as a potential mechanism of spread of melioidosis following ingestion of Burkholderia pseudomallei. PloS Negl. Trop. Dis. 15, e0009016. doi: 10.1371/journal.pntd.0009016
Nelson, M., Nunez, A., Ngugi, S. A., Sinclair, A., Atkins, T. P. (2015). Characterization of lesion formation in marmosets following inhalational challenge with different strains of Burkholderia pseudomallei. Int. J. Exp. Pathol. 96, 414–426. doi: 10.1111/iep.12161
Nelson, M., O’brien, L. M., Davies, C., Keyser, E., Butcher, W., Smither, S. J., et al. (2022b). Comparison of experimental middle east respiratory syndrome coronavirus infection acquired by three individual routes of infection in the common marmoset. J. Virol. 96, e0173921. doi: 10.1128/jvi.01739-21
Nelson, M., Salguero, F. J., Dean, R. E., Ngugi, S. A., Smither, S. J., Atkins, T. P., et al. (2014). Comparative experimental subcutaneous glanders and melioidosis in the common marmoset (Callithrix jacchus). Int. J. Exp. Pathol. 95, 378–391. doi: 10.1111/iep.12105
Nelson, M., Salguero, F. J., Hunter, L., Atkins, T. P. (2020). A novel marmoset (Callithrix jacchus) model of human inhalational Q fever. Front. Cell Infect. Microbiol. 10, 621635. doi: 10.3389/fcimb.2020.621635
Nelson, M., Stagg, A. J., Stevens, D. J., Brown, M. A., Pearce, P. C., Simpson, A. J., et al. (2011b). Post-exposure therapy of inhalational anthrax in the common marmoset. Int. J. Antimicrob. Agents 38, 60–64. doi: 10.1016/j.ijantimicag.2011.03.003
Neubert, R., Foerster, M., Nogueira, A. C., Helge, H. (1996). Cross-reactivity of antihuman monoclonal antibodies with cell surface receptors in the common marmoset. Life Sci. 58, 317–324. doi: 10.1016/0024-3205(95)02291-0
Neumann, B., Shi, T., Gan, L. L., Klippert, A., Daskalaki, M., Stolte-Leeb, N., et al. (2016). Comprehensive panel of cross-reacting monoclonal antibodies for analysis of different immune cells and their distribution in the common marmoset (Callithrix jacchus). J. Med. Primatol. 45, 139–146. doi: 10.1111/jmp.12216
Ngugi, S., Laws, T., Simpson, A. J., Nelson, M. (2022). The Innate Immune Response in the Marmoset during the Acute Pneumonic Disease Caused by Burkholderia pseudomallei. Infect. Immun. 90, e0055021. doi: 10.1128/iai.00550-21
Nicholls, L. (1930). Melioidosis, with special reference to the dissociation of Bacillus whitmori. Br. J. Exp. Pathol. 11, 393.
Nithichanon, A., Rinchai, D., Buddhisa, S., Saenmuang, P., Kewcharoenwong, C., Kessler, B., et al. (2018). Immune control of burkholderia pseudomallei–common, high-frequency T-cell responses to a broad repertoire of immunoprevalent epitopes. Front. Immunol. 9, 484. doi: 10.3389/fimmu.2018.00484
Northfield, J., Whitty, C. J. M., Macphee, I. A. M. (2002). Burkholderia pseudomallei infection, or melioidosis, and nephrotic syndrome. Nephrol. Dialysis Transplant. 17, 137–139. doi: 10.1093/ndt/17.1.137
Okada, S., Nakauchi, H., Nagayoshi, K., Nishikawa, S., Miura, Y., Suda, T. (1992). In vivo and in vitro stem cell function of c-kit- and Sca-1-positive murine hematopoietic cells. Blood 80, 3044–3050.
Okano, H., Hikishima, K., Iriki, A., Sasaki, E. (2012). The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin. Fetal. Neonatal. Med. 17, 336–340. doi: 10.1016/j.siny.2012.07.002
Olivieri, D. N., Gambón Deza, F. (2018). Immunoglobulin genes in primates. Mol. Immunol. 101, 353–363. doi: 10.1016/j.molimm.2018.07.020
Omatsu, T., Moi, M. L., Hirayama, T., Takasaki, T., Nakamura, S., Tajima, S., et al. (2011). Common marmoset (Callithrix jacchus) as a primate model of dengue virus infection: development of high levels of viraemia and demonstration of protective immunity. J. Gen. Virol. 92, 2272–2280. doi: 10.1099/vir.0.031229-0
Omatsu, T., Moi, M. L., Takasaki, T., Nakamura, S., Katakai, Y., Tajima, S., et al. (2012). Changes in hematological and serum biochemical parameters in common marmosets (Callithrix jacchus) after inoculation with dengue virus. J. Med. Primatol. 41, 289–296. doi: 10.1111/j.1600-0684.2012.00552.x
O’quinn, A. L., Wiegand, E. M., Jeddeloh, J. A. (2001). Burkholderia pseudomallei kills the nematode Caenorhabditis elegans using an endotoxin-mediated paralysis. Cell Microbiol. 3, 381–393. doi: 10.1046/j.1462-5822.2001.00118.x
Orsi, A., Rees, D., Andreini, I., Venturella, S., Cinelli, S., Oberto, G. (2011). Overview of the marmoset as a model in nonclinical development of pharmaceutical products. Regul. Toxicol. Pharmacol. 59, 19–27. doi: 10.1016/j.yrtph.2010.12.003
Oyston, P. C., Griffiths, R. (2009). Francisella virulence: significant advances, ongoing challenges and unmet needs. Expert Rev. Vaccines 8, 1575–1585. doi: 10.1586/erv.09.114
Papayannopoulou, T., Brice, M., Broudy, V. C., Zsebo, K. M. (1991). Isolation of c-kit receptor-expressing cells from bone marrow, peripheral blood, and fetal liver: functional properties and composite antigenic profile. Blood 78, 1403–1412. doi: 10.1182/blood.V78.6.1403.1403
Parks, W. P., Melnick, J. L., Voss, W. R., Singer, D. B., Rosenberg, H. S., Alcott, J., et al. (1969). Characterization of marmoset hepatitis virus. J. Infect. Dis. 120, 548–559. doi: 10.1093/infdis/120.5.548
Pasetti, M. F., Cuberos, L., Horn, T. L., Shearer, J. D., Matthews, S. J., House, R. V., et al. (2008). An improved Francisella tularensis live vaccine strain (LVS) is well tolerated and highly immunogenic when administered to rabbits in escalating doses using various immunization routes. Vaccine 26, 1773–1785. doi: 10.1016/j.vaccine.2008.01.005
Peacock, S. J., Schweizer, H. P., Dance, D. A., Smith, T. L., Gee, J. E., Wuthiekanun, V., et al. (2008). Management of accidental laboratory exposure to Burkholderia pseudomallei and B. mallei. Emerg. Infect. Dis. 14, e2. doi: 10.3201/eid1407.071501
Perrillo, R. P. (1997). The role of liver biopsy in hepatitis C. Hepatology 26, 57s–61s. doi: 10.1002/hep.510260710
Perry, M. R., Ionin, B., Barnewall, R. E., Vassar, M. L., Reece, J. J., Park, S., et al. (2020). Development of a Guinea pig inhalational anthrax model for evaluation of post-exposure prophylaxis efficacy of anthrax vaccines. Vaccine 38, 2307–2314. doi: 10.1016/j.vaccine.2020.01.068
Peters, C. J., Jones, D., Trotter, R., Donaldson, J., White, J., Stephen, E., et al. (1988). Experimental Rift Valley fever in rhesus macaques. Arch. Virol. 99, 31–44. doi: 10.1007/BF01311021
Peters, J., Maselli, D. J., Mangat, M., Coalson, J. J., Hinojosa, C., Giavedoni, L., et al. (2023). A marmoset model for Mycobacterium avium complex pulmonary disease. PloS One 18, e0260563. doi: 10.1371/journal.pone.0260563
Pfaender, S., Brown, R. J., Pietschmann, T., Steinmann, E. (2014). Natural reservoirs for homologs of hepatitis C virus. Emerg. Microbes Infect. 3, e21. doi: 10.1038/emi.2014.19
Phelps, A. L., O’brien, L. M., Eastaugh, L. S., Davies, C., Lever, M. S., Ennis, J., et al. (2019). Aerosol infection of Balb/c mice with eastern equine encephalitis virus; susceptibility and lethality. Virol. J. 16, 2. doi: 10.1186/s12985-018-1103-7
Pietrzykowski, T. (2021). Ethical review of animal research and the standards of procedural justice: A european perspective. J. Bioethical. Inq. 18, 525–534. doi: 10.1007/s11673-021-10111-5
Ploss, A., Evans, M. J., Gaysinskaya, V. A., Panis, M., You, H., De Jong, Y. P., et al. (2009). Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature 457, 882–886. doi: 10.1038/nature07684
Poquet, Y., Kroca, M., Halary, F., Stenmark, S., Peyrat, M. A., Bonneville, M., et al. (1998). Expansion of Vgamma9 Vdelta2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect. Immun. 66, 2107–2114. doi: 10.1128/IAI.66.5.2107-2114.1998
Porter, A. I., Erwin-Cohen, R. A., Twenhafel, N., Chance, T., Yee, S. B., Kern, S. J., et al. (2017). Characterization and pathogenesis of aerosolized eastern equine encephalitis in the common marmoset (Callithrix jacchus). Virol. J. 14, 25. doi: 10.1186/s12985-017-0687-7
Posthaus, H., Welle, M., Mörner, T., Nicolet, J., Kuhnert, P. (1998). Tularemia in a common marmoset (Callithrix jacchus) diagnosed by 16S rRNA sequencing. Vet. Microbiol. 61, 145–150. doi: 10.1016/S0378-1135(98)00180-1
Prasad, S., Humphreys, I., Kireta, S., Gilchrist, R. B., Bardy, P., Russ, G. R., et al. (2006). MHC Class II DRB genotyping is highly predictive of in-vitro alloreactivity in the common marmoset. J. Immunol. Methods 314, 153–163. doi: 10.1016/j.jim.2006.06.009
Prasad, S., Humphreys, I., Kireta, S., Gilchrist, R. B., Bardy, P., Russ, G. R., et al. (2007). The common marmoset as a novel preclinical transplant model: identification of new MHC class II DRB alleles and prediction of in vitro alloreactivity. Tissue Antigens 69 Suppl 1, 72–75. doi: 10.1111/j.1399-0039.2006.760_7.x
Preuss, T. M. (2019). Critique of pure marmoset. Brain Behav. Evol. 93, 92–107. doi: 10.1159/000500500
Protzer, U. (2017). The bumpy road to animal models for HBV infection. Nat. Rev. Gastroenterol. Hepatol. 14, 327–328. doi: 10.1038/nrgastro.2017.44
Puig, M., Mihalik, K., Tilton, J. C., Williams, O., Merchlinsky, M., Connors, M., et al. (2006). CD4+ immune escape and subsequent T-cell failure following chimpanzee immunization against hepatitis C virus. Hepatology 44, 736–745. doi: 10.1002/hep.21319
Purcell, R. H., Emerson, S. U. (2001). Animal models of hepatitis A and E. Ilar. J. 42, 161–177. doi: 10.1093/ilar.42.2.161
Raj, V. S., Mou, H., Smits, S. L., Dekkers, D. H., Müller, M. A., Dijkman, R., et al. (2013). Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature 495, 251–254. doi: 10.1038/nature12005
Ralph, A., Mcbride, J., Currie, B. J. (2004). Transmission of Burkholderia pseudomallei via breast milk in northern Australia. Pediatr. Infect. Dis. J. 23, 1169–1171. doi: 10.1097/01.inf.0000145548.79395.da
Ray, H. J., Chu, P., Wu, T. H., Lyons, C. R., Murthy, A. K., Guentzel, M. N., et al. (2010). The Fischer 344 rat reflects human susceptibility to francisella pulmonary challenge and provides a new platform for virulence and protection studies. PloS One 5, e9952. doi: 10.1371/journal.pone.0009952
Reed, D. S., Smith, L. P., Cole, K. S., Santiago, A. E., Mann, B. J., Barry, E. M. (2014). Live attenuated mutants of Francisella tularensis protect rabbits against aerosol challenge with a virulent type A strain. Infect. Immun. 82, 2098–2105. doi: 10.1128/IAI.01498-14
Reed, D. S., Smith, L., Dunsmore, T., Trichel, A., Ortiz, L. A., Cole, K. S., et al. (2011). Pneumonic tularemia in rabbits resembles the human disease as illustrated by radiographic and hematological changes after infection. PloS One 6, e24654. doi: 10.1371/journal.pone.0024654
Renn, M., Bartok, E., Zillinger, T., Hartmann, G., Behrendt, R. (2021). Animal models of SARS-CoV-2 and COVID-19 for the development of prophylactic and therapeutic interventions. Pharmacol. Ther. 228, 107931. doi: 10.1016/j.pharmthera.2021.107931
Reynaud, J. M., Jégou, J. F., Welsch, J. C., Horvat, B. (2014). Human herpesvirus 6A infection in CD46 transgenic mice: viral persistence in the brain and increased production of proinflammatory chemokines via Toll-like receptor 9. J. Virol. 88, 5421–5436. doi: 10.1128/JVI.03763-13
Rick Lyons, C., Wu, T. H. (2007). Animal models of Francisella tularensis infection. Ann. N. Y. Acad. Sci. 1105, 238–265. doi: 10.1196/annals.1409.003
Ritter, J. M., Sanchez, S., Jones, T. L., Zaki, S. R., Drew, C. P. (2013). Neurologic melioidosis in an imported pigtail macaque (Macaca nemestrina). Vet. Pathol. 50, 1139–1144. doi: 10.1177/0300985813485249
Roberts, L. M., Powell, D. A., Frelinger, J. A. (2018). Adaptive immunity to francisella tularensis and considerations for vaccine development. Front. Cell Infect. Microbiol. 8, 115. doi: 10.3389/fcimb.2018.00115
Ross, C. N., Davis, K., Dobek, G., Tardif, S. D. (2012). Aging phenotypes of common marmosets (Callithrix jacchus). J. Aging Res. 2012, 567143. doi: 10.1155/2012/567143
Rowland, C. A., Hartley, M. G., Flick-Smith, H., Laws, T. R., Eyles, J. E., Oyston, P. C. (2012a). Peripheral human γδ T cells control growth of both avirulent and highly virulent strains of Francisella tularensis in vitro. Microbes Infect. 14, 584–589. doi: 10.1016/j.micinf.2012.02.001
Rowland, C. A., Laws, T. R., Oyston, P. C. (2012b). An assessment of common marmoset (Callithrix jacchus) γ9(+) T cells and their response to phosphoantigen in vitro. Cell Immunol. 280, 132–137. doi: 10.1016/j.cellimm.2012.12.002
Rozak, D. A., Gelhaus, H. C., Smith, M., Zadeh, M., Huzella, L., Waag, D., et al. (2010). CpG oligodeoxyribonucleotides protect mice from Burkholderia pseudomallei but not Francisella tularensis Schu S4 aerosols. J. Immune Based. Ther. Vaccines 8, 2. doi: 10.1186/1476-8518-8-2
Russell, W. M. S., Burch, R. L. (1959). The principles of humane experimental technique (Springfield, Illinois: Methuen).
Saengmuang, P., Kewcharoenwong, C., Tippayawat, P., Nithichanon, A., Buddhisa, S., Lertmemongkolchai, G. (2014). Effect of host factors on neutrophil functions in response to Burkholderia pseudomallei in healthy Thai subjects. Jpn. J. Infect. Dis. 67, 436–440. doi: 10.7883/yoken.67.436
Sakaguchi, S., Yamaguchi, T., Nomura, T., Ono, M. (2008). Regulatory T cells and immune tolerance. Cell 133, 775–787. doi: 10.1016/j.cell.2008.05.009
Sarkar, S., Heise, M. T. (2019). Mouse models as resources for studying infectious diseases. Clin. Ther. 41, 1912–1922. doi: 10.1016/j.clinthera.2019.08.010
Sasaki, E., Suemizu, H., Shimada, A., Hanazawa, K., Oiwa, R., Kamioka, M., et al. (2009). Generation of transgenic non-human primates with germline transmission. Nature 459, 523–527. doi: 10.1038/nature08090
Sattler, R. A., Paessler, S., Ly, H., Huang, C. (2020). Animal models of lassa fever. Pathogens 9, 197. doi: 10.3390/pathogens9030197
Sawyer, W. D., Dangerfield, H. G., Hogge, A. L., Crozier, D. (1966). Antibiotic prophylaxis and therapy of airborne tularemia. Bacteriol. Rev. 30, 542–550. doi: 10.1128/br.30.3.542-550.1966
Schell, M. A., Lipscomb, L., Deshazer, D. (2008). Comparative genomics and an insect model rapidly identify novel virulence genes of Burkholderia mallei. J. Bacteriol. 190, 2306–2313. doi: 10.1128/JB.01735-07
Schmitt, A., Gan, L. L., Abd El Wahed, A., Shi, T., Ellerbrok, H., Kaup, F. J., et al. (2017). Dynamics of pathological and virological findings during experimental calpox virus infection of common marmosets (Callithrix jacchus). Viruses 9, 363. doi: 10.3390/v9120363
Seehase, S., Lauenstein, H. D., Schlumbohm, C., Switalla, S., Neuhaus, V., Förster, C., et al. (2012). LPS-induced lung inflammation in marmoset monkeys - an acute model for anti-inflammatory drug testing. PloS One 7, e43709. doi: 10.1371/journal.pone.0043709
Seferovic, M., Sánchez-San Martín, C., Tardif, S. D., Rutherford, J., Castro, E. C. C., Li, T., et al. (2018). Publisher correction: experimental zika virus infection in the pregnant common marmoset induces spontaneous fetal loss and neurodevelopmental abnormalities. Sci. Rep. 8, 16131. doi: 10.1038/s41598-018-34068-5
Shen, H., Harris, G., Chen, W., Sjostedt, A., Ryden, P., Conlan, W. (2010). Molecular immune responses to aerosol challenge with Francisella tularensis in mice inoculated with live vaccine candidates of varying efficacy. PloS One 5, e13349. doi: 10.1371/journal.pone.0013349
Shifflett, K., Marzi, A. (2019). Marburg virus pathogenesis - differences and similarities in humans and animal models. Virol. J. 16, 165. doi: 10.1186/s12985-019-1272-z
Shiina, T., Kono, A., Westphal, N., Suzuki, S., Hosomichi, K., Kita, Y. F., et al. (2011). Comparative genome analysis of the major histocompatibility complex (MHC) class I B/C segments in primates elucidated by genomic sequencing in common marmoset (Callithrix jacchus). Immunogenetics 63, 485–499. doi: 10.1007/s00251-011-0526-8
Shimada, S., Nunomura, S., Mori, S., Suemizu, H., Itoh, T., Takabayashi, S., et al. (2015). Common marmoset CD117+ hematopoietic cells possess multipotency. Int. Immunol. 27, 567–577. doi: 10.1093/intimm/dxv031
Shoukry, N. H., Grakoui, A., Houghton, M., Chien, D. Y., Ghrayeb, J., Reimann, K. A., et al. (2003). Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 197, 1645–1655. doi: 10.1084/jem.20030239
Shurtleff, A. C., Bavari, S. (2015). Animal models for ebolavirus countermeasures discovery: what defines a useful model? Expert Opin. Drug Discovery 10, 685–702. doi: 10.1517/17460441.2015.1035252
Signarovitz, A. L., Ray, H. J., Yu, J. J., Guentzel, M. N., Chambers, J. P., Klose, K. E., et al. (2012). Mucosal immunization with live attenuated Francisella novicida U112ΔiglB protects against pulmonary F. tularensis SCHU S4 in the Fischer 344 rat model. PloS One 7, e47639. doi: 10.1371/journal.pone.0047639
Silvestre-Roig, C., Fridlender, Z. G., Glogauer, M., Scapini, P. (2019). Neutrophil diversity in health and disease. Trends Immunol. 40, 565–583. doi: 10.1016/j.it.2019.04.012
Singh, D. K., Singh, B., Ganatra, S. R., Gazi, M., Cole, J., Thippeshappa, R., et al. (2021). Responses to acute infection with SARS-CoV-2 in the lungs of rhesus macaques, baboons and marmosets. Nat. Microbiol. 6, 73–86. doi: 10.1038/s41564-020-00841-4
Sjöstedt, A., Conlan, J. W., North, R. J. (1994). Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect. Immun. 62, 2779–2783. doi: 10.1128/iai.62.7.2779-2783.1994
Smee, D. F. (2008). Progress in the discovery of compounds inhibiting orthopoxviruses in animal models. Antiviral Chem. Chemother. 19, 115–124. doi: 10.1177/095632020801900302
Smith, D. R., Bird, B. H., Lewis, B., Johnston, S. C., Mccarthy, S., Keeney, A., et al. (2012). Development of a novel nonhuman primate model for Rift Valley fever. J. Virol. 86, 2109–2120. doi: 10.1128/JVI.06190-11
Smith, D. R., Johnston, S. C., Piper, A., Botto, M., Donnelly, G., Shamblin, J., et al. (2018). Attenuation and efficacy of live-attenuated Rift Valley fever virus vaccine candidates in non-human primates. PloS Negl. Trop. Dis. 12, e0006474. doi: 10.1371/journal.pntd.0006474
Smither, S. J., Nelson, M., Eastaugh, L., Laws, T. R., Taylor, C., Smith, S. A., et al. (2013). Experimental respiratory Marburg virus haemorrhagic fever infection in the common marmoset (Callithrix jacchus). Int. J. Exp. Pathol. 94, 156–168. doi: 10.1111/iep.12018
Smither, S. J., Nelson, M., Eastaugh, L., Nunez, A., Salguero, F. J., Lever, M. S. (2015). Experimental respiratory infection of marmosets (Callithrix jacchus) with ebola virus kikwit. J. Infect. Dis. 212 Suppl 2, S336–S345. doi: 10.1093/infdis/jiv371
Soffler, C., Bosco-Lauth, A. M., Aboellail, T. A., Marolf, A. J., Bowen, R. A. (2012). Development and characterization of a caprine aerosol infection model of melioidosis. PloS One 7, e43207. doi: 10.1371/journal.pone.0043207
Soffler, C., Bosco-Lauth, A. M., Aboellail, T. A., Marolf, A. J., Bowen, R. A. (2014). Pathogenesis of percutaneous infection of goats with Burkholderia pseudomallei: clinical, pathologic, and immunological responses in chronic melioidosis. Int. J. Exp. Pathol. 95, 101–119. doi: 10.1111/iep.12068
Splettstoesser, W. D., Mätz-Rensing, K., Seibold, E., Tomaso, H., Al Dahouk, S., Grunow, R., et al. (2007). Re-emergence of Francisella tularensis in Germany: fatal tularaemia in a colony of semi-free-living marmosets (Callithrix jacchus). Epidemiol. Infect. 135, 1256–1265. doi: 10.1017/S0950268807008035
St Claire, M. C., Ragland, D. R., Bollinger, L., Jahrling, P. B. (2017). Animal models of ebolavirus infection. Comp. Med. 67, 253–262.
Steele, K. E., Twenhafel, N. A. (2010). REVIEW PAPER: pathology of animal models of alphavirus encephalitis. Vet. Pathol. 47, 790–805. doi: 10.1177/0300985810372508
Steiner, D. J., Furuya, Y., Metzger, D. W. (2014). Host-pathogen interactions and immune evasion strategies in Francisella tularensis pathogenicity. Infect. Drug Resist. 7, 239–251. doi: 10.2147/IDR.S53700
Steinrücken, J., Graber, P. (2014). Oropharyngeal tularemia. Cmaj 186, E62. doi: 10.1503/cmaj.122097
Stinson, E., Smith, L. P., Cole, K. S., Barry, E. M., Reed, D. S. (2016). Respiratory and oral vaccination improves protection conferred by the live vaccine strain against pneumonic tularemia in the rabbit model. Pathog. Dis. 74, ftw079. doi: 10.1093/femspd/ftw079
Stratilo, C. W., Jager, S., Crichton, M., Blanchard, J. D. (2020). Evaluation of liposomal ciprofloxacin formulations in a murine model of anthrax. PloS One 15, e0228162. doi: 10.1371/journal.pone.0228162
Stuart, B. M., Pullen, R. L. (1945). Tularemia pneumonia: review of American literature and a report of 15 additional cases. Am. J. Med. Sci. 210, 223–236. doi: 10.1097/00000441-194508000-00013
Stundick, M. V., Albrecht, M. T., Houchens, C. R., Smith, A. P., Dreier, T. M., Larsen, J. C. (2013). Animal models for Francisella tularensis and Burkholderia species: scientific and regulatory gaps toward approval of antibiotics under the FDA Animal Rule. Vet. Pathol. 50, 877–892. doi: 10.1177/0300985813486812
Suen, W. W., Uddin, M. J., Wang, W., Brown, V., Adney, D. R., Broad, N., et al. (2015). Experimental west nile virus infection in rabbits: an alternative model for studying induction of disease and virus control. Pathogens 4, 529–558. doi: 10.3390/pathogens4030529
Sulaiman, S., Othman, M. Z., Aziz, A. H. (2000). Isolations of enteric pathogens from synanthropic flies trapped in downtown Kuala Lumpur. J. Vector. Ecol. 25, 90–93.
Sumida, T., Maeda, T., Takahashi, H., Yoshida, S., Yonaha, F., Sakamoto, A., et al. (1992). Predominant expansion of V gamma 9/V delta 2 T cells in a tularemia patient. Infect. Immun. 60, 2554–2558. doi: 10.1128/iai.60.6.2554-2558.1992
Sun, H., Zhang, A., Yan, G., Piao, C., Li, W., Sun, C., et al. (2013). Metabolomic analysis of key regulatory metabolites in hepatitis C virus-infected tree shrews. Mol. Cell Proteomics 12, 710–719. doi: 10.1074/mcp.M112.019141
Sweeney, C., Ward, J., Vallender, E. J. (2012). Naturally occurring, physiologically normal, primate chimeras. Chimerism 3, 43–44. doi: 10.4161/chim.20729
Takikawa, S., Engle, R. E., Faulk, K. N., Emerson, S. U., Purcell, R. H., Bukh, J. (2010). Molecular evolution of GB virus B hepatitis virus during acute resolving and persistent infections in experimentally infected tamarins. J. Gen. Virol. 91, 727–733. doi: 10.1099/vir.0.015750-0
Tan, G. G., Liu, Y., Sivalingam, S. P., Sim, S. H., Wang, D., Paucod, J. C., et al. (2008). Burkholderia pseudomallei aerosol infection results in differential inflammatory responses in BALB/c and C57Bl/6 mice. J. Med. Microbiol. 57, 508–515. doi: 10.1099/jmm.0.47596-0
Tärnvik, A., Berglund, L. (2003). Tularaemia. Eur. Respir. J. 21, 361–373. doi: 10.1183/09031936.03.00088903
Terzian, A. C. B., Zini, N., Sacchetto, L., Rocha, R. F., Parra, M. C. P., Del Sarto, J. L., et al. (2018). Evidence of natural Zika virus infection in neotropical non-human primates in Brazil. Sci. Rep. 8, 16034. doi: 10.1038/s41598-018-34423-6
Thomas, A. D., Forbes-Faulkner, J. C., D’arcy, T. L., Norton, J. H., Hoffmann, D. (1990). Experimental infection of normal and immunosuppressed pigs with Pseudomonas pseudomallei. Aust. Vet. J. 67, 43–46. doi: 10.1111/j.1751-0813.1990.tb07692.x
Tomioka, I., Ishibashi, H., Minakawa, E. N., Motohashi, H. H., Takayama, O., Saito, Y., et al. (2017a). Transgenic monkey model of the polyglutamine diseases recapitulating progressive neurological symptoms. eNeuro 4, 0250-16. doi: 10.1523/ENEURO.0250-16.2017
Tomioka, I., Nogami, N., Nakatani, T., Owari, K., Fujita, N., Motohashi, H., et al. (2017b). Generation of transgenic marmosets using a tetracyclin-inducible transgene expression system as a neurodegenerative disease model. Biol. Reprod. 97, 772–780. doi: 10.1093/biolre/iox129
Trevino, S. R., Dankmeyer, J. L., Fetterer, D. P., Klimko, C. P., Raymond, J. L. W., Moreau, A. M., et al. (2021). Comparative virulence of three different strains of Burkholderia pseudomallei in an aerosol non-human primate model. PloS Negl. Trop. Dis. 15, e0009125. doi: 10.1371/journal.pntd.0009125
Trichel, A. M. (2021). Overview of nonhuman primate models of SARS-coV-2 infection. Comp. Med. 71, 411–432. doi: 10.30802/AALAS-CM-20-000119
Twenhafel, N. A., Alves, D. A., Purcell, B. K. (2009). Pathology of inhalational Francisella tularensis spp. tularensis SCHU S4 infection in African green monkeys (Chlorocebus aethiops). Vet. Pathol. 46, 698–706. doi: 10.1354/vp.08-VP-0302-T-AM
Twine, S., Shen, H., Harris, G., Chen, W., Sjostedt, A., Ryden, P., et al. (2012). BALB/c mice, but not C57BL/6 mice immunized with a ΔclpB mutant of Francisella tularensis subspecies tularensis are protected against respiratory challenge with wild-type bacteria: association of protection with post-vaccination and post-challenge immune responses. Vaccine 30, 3634–3645. doi: 10.1016/j.vaccine.2012.03.036
Twine, S. M., Shen, H., Kelly, J. F., Chen, W., Sjöstedt, A., Conlan, J. W. (2006). Virulence comparison in mice of distinct isolates of type A Francisella tularensis. Microb. Pathog. 40, 133–138. doi: 10.1016/j.micpath.2005.12.004
Van Der Wiel, M. K., Otting, N., De Groot, N. G., Doxiadis, G. G., Bontrop, R. E. (2013). The repertoire of MHC class I genes in the common marmoset: evidence for functional plasticity. Immunogenetics 65, 841–849. doi: 10.1007/s00251-013-0732-7
Van Doremalen, N., Falzarano, D., Ying, T., De Wit, E., Bushmaker, T., Feldmann, F., et al. (2017). Efficacy of antibody-based therapies against Middle East respiratory syndrome coronavirus (MERS-CoV) in common marmosets. Antiviral Res. 143, 30–37. doi: 10.1016/j.antiviral.2017.03.025
Van Doremalen, N., Munster, V. J. (2015). Animal models of Middle East respiratory syndrome coronavirus infection. Antiviral Res. 122, 28–38. doi: 10.1016/j.antiviral.2015.07.005
Van Schaik, E., Tom, M., Devinney, R., Woods, D. E. (2008). Development of novel animal infection models for the study of acute and chronic Burkholderia pseudomallei pulmonary infections. Microbes Infect. 10, 1291–1299. doi: 10.1016/j.micinf.2008.07.028
Verstrepen, B. E., Fagrouch, Z., Van Heteren, M., Buitendijk, H., Haaksma, T., Beenhakker, N., et al. (2014). Experimental infection of rhesus macaques and common marmosets with a European strain of West Nile virus. PloS Negl. Trop. Dis. 8, e2797. doi: 10.1371/journal.pntd.0002797
Vesselinova, A., Najdenski, H., Nikolova, S., Kussovski, V. (1996). Experimental melioidosis in hens. Zentralbl. Veterinarmed. B. 43, 371–378. doi: 10.1111/j.1439-0450.1996.tb00328.x
Waag, D. M., Chance, T. B., Trevino, S. R., Rossi, F. D., Fetterer, D. P., Amemiya, K., et al. (2021). Comparison of three non-human primate aerosol models for glanders, caused by Burkholderia mallei. Microb. Pathog. 155, 104919. doi: 10.1016/j.micpath.2021.104919
Warawa, J. M. (2010). Evaluation of surrogate animal models of melioidosis. Front. Microbiol. 1, 141. doi: 10.3389/fmicb.2010.00141
Washburn, M. L., Bility, M. T., Zhang, L., Kovalev, G. I., Buntzman, A., Frelinger, J. A., et al. (2011). A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology 140, 1334–1344. doi: 10.1053/j.gastro.2011.01.001
Wayne Conlan, J., Oyston, P. C. (2007). Vaccines against Francisella tularensis. Ann. N. Y. Acad. Sci. 1105, 325–350. doi: 10.1196/annals.1409.012
Weatherford, T., Chavez, D., Brasky, K. M., Lanford, R. E. (2009). The marmoset model of GB virus B infections: adaptation to host phenotypic variation. J. Virol. 83, 5806–5814. doi: 10.1128/JVI.00033-09
Webling, D. D. (1980). Genito-urinary infections with Pseudomonas pseudomallei in Australian Aboriginals. Trans. R. Soc. Trop. Med. Hyg. 74, 138–139. doi: 10.1016/0035-9203(80)90036-X
Weissenbacher, M. C., Calello, M. A., Colillas, O. J., Rondinone, S. N., Frigerio, M. J. (1979). Argentine hemorrhagic fever: a primate model. Intervirology 11, 363–365. doi: 10.1159/000149059
Weissenbacher, M. C., Calello, M. A., Merani, M. S., Mccormick, J. B., Rodriguez, M. (1986). Therapeutic effect of the antiviral agent ribavirin in Junín virus infection of primates. J. Med. Virol. 20, 261–267. doi: 10.1002/jmv.1890200308
Weissenbacher, M. C., Coto, C. E., Calello, M. A., Rondinone, S. N., Damonte, E. B., Frigerio, M. J. (1982). Cross-protection in nonhuman primates against Argentine hemorrhagic fever. Infect. Immun. 35, 425–430. doi: 10.1128/iai.35.2.425-430.1982
Welkos, S. L., Klimko, C. P., Kern, S. J., Bearss, J. J., Bozue, J. A., Bernhards, R. C., et al. (2015). Characterization of burkholderia pseudomallei strains using a murine intraperitoneal infection model and in vitro macrophage assays. PloS One 10, e0124667. doi: 10.1371/journal.pone.0124667
Wherry, W. B., Lamb, B. H. (1914). Infection of man with bacterium tularense. J. Infect. Dis. 15, 331–340. doi: 10.1093/infdis/15.2.331
Whitaker, D. A. (1992). Infection of a marmoset with the BSE agent. Vet. Rec. 130, 251. doi: 10.1136/vr.130.12.251-b
White, N. J., Dance, D. A., Chaowagul, W., Wattanagoon, Y., Wuthiekanun, V., Pitakwatchara, N. (1989). Halving of mortality of severe melioidosis by ceftazidime. Lancet 2, 697–701. doi: 10.1016/S0140-6736(89)90768-X
Whitlock, G. C., Mark Estes, D., Torres, A. G. (2007). Glanders: off to the races with Burkholderia mallei. FEMS Microbiol. Lett. 277, 115–122. doi: 10.1111/j.1574-6968.2007.00949.x
Whitmore, A. (1913). An account of a glanders-like disease occurring in rangoon. J. Hyg. (Lond). 13, 1–34.1. doi: 10.1017/S0022172400005234
Wichgers Schreur, P. J., Mooij, P., Koopman, G., Verstrepen, B. E., Fagrouch, Z., Mortier, D., et al. (2022). Safety and immunogenicity of four-segmented Rift Valley fever virus in the common marmoset. NPJ Vaccines 7, 54. doi: 10.1038/s41541-022-00476-y
Wiersinga, W. J., Virk, H. S., Torres, A. G., Currie, B. J., Peacock, S. J., Dance, D. A. B., et al. (2018). Melioidosis. Nat. Rev. Dis. Primers 4, 17107. doi: 10.1038/nrdp.2017.107
Williams, M. S., Baker, M. R., Guina, T., Hewitt, J. A., Lanning, L., Hill, H., et al. (2019). Retrospective analysis of pneumonic tularemia in operation whitecoat human subjects: disease progression and tetracycline efficacy. Front. Med. 6. doi: 10.3389/fmed.2019.00229
Willyard, C. (2014). Advances in marmoset and mouse models buoy Ebola research. Nat. Med. 20, 1356–1357. doi: 10.1038/nm1214-1356
Wolf, R. F., Papin, J. F., Hines-Boykin, R., Chavez-Suarez, M., White, G. L., Sakalian, M., et al. (2006). Baboon model for West Nile virus infection and vaccine evaluation. Virology 355, 44–51. doi: 10.1016/j.virol.2006.06.033
Woods, D. E. (2002). The use of animal infection models to study the pathogenesis of melioidosis and glanders. Trends Microbiol. 10, 483–4; discussion 484-5. doi: 10.1016/S0966-842X(02)02464-2
Woods, D. E., Jones, A. L., Hill, P. J. (1993). Interaction of insulin with Pseudomonas pseudomallei. Infect. Immun. 61, 4045–4050. doi: 10.1128/iai.61.10.4045-4050.1993
Woollard, D. J., Grakoui, A., Shoukry, N. H., Murthy, K. K., Campbell, K. J., Walker, C. M. (2003). Characterization of HCV-specific Patr class II restricted CD4+ T cell responses in an acutely infected chimpanzee. Hepatology 38, 1297–1306. doi: 10.1053/jhep.2003.50478
Woollard, D. J., Haqshenas, G., Dong, X., Pratt, B. F., Kent, S. J., Gowans, E. J. (2008). Virus-specific T-cell immunity correlates with control of GB virus B infection in marmosets. J. Virol. 82, 3054–3060. doi: 10.1128/JVI.01153-07
Worley, K. C., Warren, W. C., Rogers, J., Locke, D., Muzny, D. M., Mardis, E. R., et al. (2014). The common marmoset genome provides insight into primate biology and evolution. Nat. Genet. 46, 850–857. doi: 10.1038/ng.3042
Wu, T. H., Zsemlye, J. L., Statom, G. L., Hutt, J. A., Schrader, R. M., Scrymgeour, A. A., et al. (2009). Vaccination of Fischer 344 rats against pulmonary infections by Francisella tularensis type A strains. Vaccine 27, 4684–4693. doi: 10.1016/j.vaccine.2009.05.060
Xie, Z. C., Riezu-Boj, J. I., Lasarte, J. J., Guillen, J., Su, J. H., Civeira, M. P., et al. (1998). Transmission of hepatitis C virus infection to tree shrews. Virology 244, 513–520. doi: 10.1006/viro.1998.9127
Yahata, T., Ando, K., Nakamura, Y., Ueyama, Y., Shimamura, K., Tamaoki, N., et al. (2002). Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice. J. Immunol. 169, 204–209. doi: 10.4049/jimmunol.169.1.204
Yang, F., Robotham, J. M., Nelson, H. B., Irsigler, A., Kenworthy, R., Tang, H. (2008). Cyclophilin A is an essential cofactor for hepatitis C virus infection and the principal mediator of cyclosporine resistance in vitro. J. Virol. 82, 5269–5278. doi: 10.1128/JVI.02614-07
Yap, E. H., Thong, T. W., Tan, A. L., Yeo, M., Tan, H. C., Loh, H., et al. (1995). Comparison of Pseudomonas pseudomallei from humans, animals, soil and water by restriction endonuclease analysis. Singapore. Med. J. 36, 60–62.
Yeager, J. J., Facemire, P., Dabisch, P. A., Robinson, C. G., Nyakiti, D., Beck, K., et al. (2012). Natural history of inhalation melioidosis in rhesus macaques (Macaca mulatta) and African green monkeys (Chlorocebus aethiops). Infect. Immun. 80, 3332–3340. doi: 10.1128/IAI.00675-12
Yee, K. C., Lee, M. K., Chua, C. T., Puthucheary, S. D. (1988). Melioidosis, the great mimicker: a report of 10 cases from Malaysia. J. Trop. Med. Hyg. 91, 249–254.
Yeni, D. K., Büyük, F., Ashraf, A., Shah, M. (2021). Tularemia: a re-emerging tick-borne infectious disease. Folia Microbiol. (Praha). 66, 1–14. doi: 10.1007/s12223-020-00827-z
Yingst, S. L., Facemire, P., Chuvala, L., Norwood, D., Wolcott, M., Alves, D. A. (2014). Pathological findings and diagnostic implications of a rhesus macaque (Macaca mulatta) model of aerosol-exposure melioidosis (Burkholderia pseudomallei). J. Med. Microbiol. 63, 118–128. doi: 10.1099/jmm.0.059063-0
Yoshida, T., Omatsu, T., Saito, A., Katakai, Y., Iwasaki, Y., Kurosawa, T., et al. (2013). Dynamics of cellular immune responses in the acute phase of dengue virus infection. Arch. Virol. 158, 1209–1220. doi: 10.1007/s00705-013-1618-6
Yoshimatsu, S., Okahara, J., Sone, T., Takeda, Y., Nakamura, M., Sasaki, E., et al. (2019). Robust and efficient knock-in in embryonic stem cells and early-stage embryos of the common marmoset using the CRISPR-Cas9 system. Sci. Rep. 9, 1528. doi: 10.1038/s41598-018-37990-w
Yu, P., Xu, Y., Deng, W., Bao, L., Huang, L., Xu, Y., et al. (2017). Comparative pathology of rhesus macaque and common marmoset animal models with Middle East respiratory syndrome coronavirus. PloS One 12, e0172093. doi: 10.1371/journal.pone.0172093
Zapata, J. C., Goicochea, M., Nadai, Y., Eyzaguirre, L. M., Carr, J. K., Tallon, L. J., et al. (2014). Genetic variation in vitro and in vivo of an attenuated Lassa vaccine candidate. J. Virol. 88, 3058–3066. doi: 10.1128/JVI.03035-13
Zhang, X., Wang, X., Wu, M., Ghildyal, R., Yuan, Z. (2021). Animal models for the study of hepatitis B virus pathobiology and immunity: past, present, and future. Front. Microbiol. 12, 715450. doi: 10.3389/fmicb.2021.715450
Zhu, S., Li, T., Liu, B., Xu, Y., Sun, Y., Wang, Y., et al. (2016). Infection of common marmosets with GB virus B chimeric virus encoding the major nonstructural proteins NS2 to NS4A of hepatitis C virus. J. Virol. 90, 8198–8211. doi: 10.1128/JVI.02653-15
Keywords: common marmoset, immunology, inflammation, animal models, Francisella tularensis, Burkholderia pseudomallei, hepatitis C virus
Citation: Herron ICT, Laws TR and Nelson M (2024) Marmosets as models of infectious diseases. Front. Cell. Infect. Microbiol. 14:1340017. doi: 10.3389/fcimb.2024.1340017
Received: 17 November 2023; Accepted: 29 January 2024;
Published: 23 February 2024.
Edited by:
Namita Rout, Tulane University, United StatesReviewed by:
Deepa Machiah Kodandera, Emory Primate Research Center, United StatesCorinna Ross, Texas Biomedical Research Institute, United States
Crown copyright © 2024 Dstl. Authors: Herron, Laws and Nelson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian C. T. Herron, aWhlcnJvbkBkc3RsLmdvdi51aw==
 Ian C. T. Herron
Ian C. T. Herron Thomas R. Laws
Thomas R. Laws Michelle Nelson
Michelle Nelson