
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 03 June 2024
Sec. Molecular Bacterial Pathogenesis
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1337861
 Kimona Rampersadh1
Kimona Rampersadh1 M. Taariq Salie1
M. Taariq Salie1 Kelin C. Engel1
Kelin C. Engel1 Clinton Moodley2,3
Clinton Moodley2,3 Liesl J. Zühlke4,5
Liesl J. Zühlke4,5 Mark E. Engel1,5*
Mark E. Engel1,5*Introduction: It is currently unclear what the role of Group A streptococcus (GAS) virulence factors (VFs) is in contributing to the invasive potential of GAS. This work investigated the evidence for the association of GAS VFs with invasive disease.
Methods: We employed a broad search strategy for studies reporting the presence of GAS VFs in invasive and non-invasive GAS disease. Data were independently extracted by two reviewers, quality assessed, and meta-analyzed using Stata®.
Results: A total of 32 studies reported on 45 putative virulence factors [invasive (n = 3,236); non-invasive (n = 5,218)], characterized by polymerase chain reaction (PCR) (n = 30) and whole-genome sequencing (WGS) (n = 2). The risk of bias was rated as low and moderate, in 23 and 9 studies, respectively. Meta-,analyses of high-quality studies (n = 23) revealed a significant association of speM [OR, 1.64 (95%CI, 1.06; 2.52)] with invasive infection. Meta-analysis of WGS studies demonstrated a significant association of hasA [OR, 1.91 (95%CI, 1.36; 2.67)] and speG [OR, 2.83 (95%CI, 1.63; 4.92)] with invasive GAS (iGAS). Meta-analysis of PCR studies indicated a significant association of speA [OR, 1.59 (95%CI, 1.10; 2.30)] and speK [OR, 2.95 (95%CI, 1.81; 4.80)] with invasive infection. A significant inverse association was observed between prtf1 [OR, 0.42 (95%CI, 0.20; 0.87)] and invasive infection.
Conclusion: This systematic review and genomic meta-analysis provides evidence of a statistically significant association with invasive infection for the hasA gene, while smeZ, ssa, pnga3, sda1, sic, and NaDase show statistically significantly inverse associations with invasive infection. SpeA, speK, and speG are associated with GAS virulence; however, it is unclear if they are markers of invasive infection. This work could possibly aid in developing preventative strategies.
Group A streptococcus (GAS) is responsible for a range of disease, causing both superficial and invasive disease (Tapiainen et al., 2016; Espadas-Maciá et al., 2018; CDC, 2022). GAS invasive disease is characterized by the isolation of strains from normally sterile sites in the body, e.g., blood, cerebrospinal fluid, pleural fluid, joint fluid, pericardial fluid, or peritoneal fluid, or non-sterile sites such as wounds associated with necrotizing fasciitis (NF) and streptococcal toxic shock syndrome (STSS). Where GAS strains are isolated from patients with pharyngitis, impetigo, scarlet fever, and erysipelas, the disease is regarded as non-invasive/superficial. Since 2005, the global burden from invasive GAS diseases is reported to be approximately 517,000 deaths with figures disproportionately higher in developing countries as compared to those in developed countries (Carapetis et al., 2005).
GAS are genetically diverse, with various complements of virulence factors that engage a vast variety of host defenses (Walker et al., 2014). Among virulence factors associated with the pathogenesis of GAS, the M protein and streptococcal pyrogenic exotoxins (Spes) are the major ones (Shannon et al., 2019). In addition, GAS produces surface proteins, known as adhesins, including pilli (Spy0130, Spy0128, Cpa), fibronectin-binding proteins (PrtF1, PrtF2, SfbI, SfbII, SOF, Fbaa, and Fbab), collagen-like proteins (Scl1, Scl2), laminin-binding proteins (Lbp, Shr), and plasminogen-binding proteins (GAPDH, SEN), which have also been reported (Walker et al., 2014). GAS also produces numerous secreted factors, such as streptokinase (Ska), engaged in interactions with complements; streptolysin S (SLS), a promoter involved in fibrinolysis and neutrophil modulation; and hyaluronidase and cysteine proteinases, which are often considered to be virulence factors (Walker et al., 2014; Barnett et al., 2015).
The M protein is a key surface virulence factor encoded by the emm gene, which displays marked variability in the 5' hypervariable region and forms the basis for emm genotyping (DebRoy et al., 2018). To date, in excess of 250 different emm types have been reported (Sanderson-Smith et al., 2014). M protein is associated with several stages in GAS pathogenesis, namely, adhesion, internalization, evasion of the immune system, and tissue invasion. The contribution of the M protein to virulence is attributed to immune modulatory effects, mediated by the binding of host proteins such as immunoglobulins and fibrinogen, as well as providing antiphagocytic functions critical for GAS survival in tissues and bodily fluids (Smeesters et al., 2010). In an effort to predict the basic genetic features of GAS isolates, Sanderson-Smith et al. introduced a cluster-based classification for GAS (Sanderson-Smith et al., 2014). This system classifies emm types into clusters that have the same or similar sequences as well as host binding properties, allowing for previously characterized GAS emm types to be classified into 48 emm clusters, complementing the emm typing scheme, which may assist in improving studies associated with M protein function, epidemiological surveillance, GAS virulence determinants, and therapeutic developments such as vaccines (Sanderson-Smith et al., 2014).
Spes are secreted proteins displaying the traits of superantigens (SAgs), which putatively play a role in the pathogenesis of invasive infections. Superantigens or exotoxins have thus far been described as the most potent proteins involved in stimulating T-cell proliferation and differentiation. Superantigens have the ability to circumvent the usual antigen processing and presentation by cross-linking MHC class II molecules and the Vβ region of the antigen receptor on a subset of T lymphocytes (Fraser and Proft, 2008; Zeppa et al., 2017), leading to T-cell proliferation. This induces a huge secretion of inflammatory cytokines (Herman et al., 1991). Overproduction of these cytokines can lead to shock, tissue damage, and organ failure. There have been more than 40 bacterial superantigens reported in the literature, of which 12 distinct extracellular superantigens have been elucidated in GAS, which include Spes (A, C, G, H, I, J, K, L, M), streptococcal mitogenic exotoxins (smeZ) 1 and 2, and the streptococcal superantigen (ssa) (Proft and Fraser, 2003; Commons et al., 2008; Berman et al., 2014; Reglinski et al., 2019). Superantigens implicated in GAS virulence have been associated with diseases such as scarlet fever, STSS, and rheumatic fever (Barnett et al., 2015). Emm types have been reported to be associated with specific superantigens, and these associations vary in GAS populations collected from various geographical locations (Commons et al., 2008).
GAS cell surface proteins include various adhesins, which allows for bacterial–host interactions, permitting GAS colonization to diverse tissues in the human body (Walker et al., 2014). GAS surface proteins use three known mechanisms to attach to the bacterial surface, namely, covalent binding to the peptidoglycan through a C-terminal LPxTG motif, which is recognized by sortase A (Barnett and Scott, 2002); covalent attachment to the cell membrane via N-terminal modifications with lipoproteins (Nobbs et al., 2009); and non-covalent binding to cell surface components (Nobbs et al., 2009). Secreted GAS virulence factors target numerous components of the immune response. The host immune response is avoided through several mechanisms, such as interference of the chemokine gradient via degradation, hindering of neutrophil migration, damaging of host cells through pore-forming toxins, degradation of neutrophil extracellular traps via DNases, cleavage of circulating host effector proteins, destruction of epithelial barriers and extracellular matrix proteins, degradation of macrophage proliferation and function, and evading of intracellular activities once inside the host (Barnett et al., 2022).
Given that there are currently no syntheses of existing studies, we sought to provide an evidence-based assessment, from published articles, of the key virulence factors associated with invasive GAS infection. We envisaged that the results of this study will serve to inform further research addressing the role of GAS virulence factors in both invasive and non-invasive GAS infections.
This systematic review was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocols (Moher et al., 2009).
This systematic review sought to identify the genomic elements associated with invasive GAS infection. Using the PEO (population, exposure, and outcome) mnemonic, where P refers to children or adults, E to GAS virulence factors, and outcome to invasive disease, the review question was, Are specific GAS virulence genes associated with invasive disease in patients with GAS-associated infection?
To maximize sensitivity, a broad search strategy was designed. The main search included individual searches using Medical Subject Headings (MeSH). A combination of terms relating to “invasive”, “virulence”, and “pathogenic” were used (Supplementary Table S1—available at https://doi.org/10.25375/uct.23708346). The search was carried out, independently, by two reviewers among several databases including Medline (accessed via PubMed), Scopus, and Web of Science from the earliest published data to 19 July 2023. Search results were complemented with snowballing searches in Google Scholar, thesis databases, and conference proceedings and scanning the reference lists of the articles. The search strategy was modified to suit the vocabulary of individual database(s). The search was not restricted by language or date of publication.
We included studies reporting sequencing of the genetic elements associated with invasive and non-invasive GAS infection across all age groups, ethnicities, and socioeconomic and educational backgrounds, globally. Invasive infection was broadly defined as recovery of GAS isolates from normally sterile sites with samples, including cerebrospinal fluid (CSF), blood, and synovial and pleural fluids. We considered published articles; all study designs were considered for inclusion. In addition, articles published in other languages with complete English abstracts were considered. Studies incorporating polymerase chain reaction (PCR)/whole-genome sequencing (WGS) were prioritized, given the superiority of these methods in producing molecular sequence data (Chochua et al., 2017; Plainvert et al., 2018).
We excluded opinion pieces, letters, narrative reviews, and any other publications lacking primary data and/or unambiguous method descriptions. Where publications utilized the same data, the most recent and complete versions were considered.
Search results from all aforementioned databases and reference search results were managed with the EndNote referencing software. A data extraction form was compiled, which included predefined criteria. Data extraction was conducted by KR and verified by a second reviewer (KE) and a third reviewer (TS).
The internal and external validity and generalizability of the included study results were evaluated for risk of bias. An assessment of the risk of bias informed the evaluation of heterogeneity in the pooled analysis. A quality assessment tool for evaluating prevalence studies as suggested by Hoy and colleagues (and adapted by Salie et al.) was adapted for the purpose of this review; the revised version allows for a composite score to assist with a relative comparison between the studies, thereby reducing reviewers’ subjectivity (Salie et al., 2020). Briefly, Salie et al. added a quantitative scoring system to the risk of bias table, allocating four points for external validly score and six points for internal validity. Six domains were considered for this review. The scoring system tool classifies studies into different categories based on their overall scores: high risk if the score is 1–2 points, moderate risk for 3–4 points, and low risk if it falls within the range of 5–6 points.
We conducted statistical analyses using Stata version 14.1 (Stata Corp., College Station, TX, USA) to determine the overall the effect size (odds ratio and 95%CI) of association between virulence factors and invasive GAS disease. Meta-analyses are presented by tables. Where a meta-analysis was not feasible, because data were either too heterogeneous or insufficient to allow for meaningful pooling, we compiled a narrative report of the results.
The literature search identified 1,185 articles for consideration for inclusion from the respective electronic databases (Figure 1). Following deduplication and handsearching, 695 articles were subjected to screening of titles and abstracts, of which 59 articles required full-text review. Finally, 32 articles met the inclusion criteria and were included in the review. A single restriction fragment length polymorphism (RFLP) study was excluded since this review only included sequence-based methods. A detailed list of the excluded studies is documented in Supplementary Table S2 (available at https://doi.org/10.25375/uct.23708346).
The study characteristics of included studies are presented in Table 1. The included articles comprised molecular studies, published between 1992 and 2022, reporting on the association of GAS infection with descriptions of genetic elements (invasive, n = 3,236; non-invasive, n = 5,218). The 32 articles reported in this systematic review comprised 33 datasets from 22 countries, namely, Poland (1 article), USA (2), Italy (3), Belgium (2), China (2), Germany (2), Norway (3), Sweden (2), Denmark (1), Australia (1), Bulgaria (1), Canada (1), France (1), Hong Kong (1), India (1), Ireland (1), Japan (1), Pakistan (1), Romania (1), Spain (1), Taiwan (1), and Tunisia (1); the remaining study comprised samples from 12 countries. Where invasive disease was not defined, we accepted the authors’ classification of invasiveness. Methods for detecting the virulence factors included PCR (n = 30) and WGS (n = 2).
The risk of bias (ROB) was assessed using the Hoy criteria as modified by Salie et al. (2020). The risk of bias was rated as low and moderate in 23 and 9 studies, respectively. Clinical phenotypes were clearly defined in the majority of studies. Considering the six domains relating to our review, most studies were assessed as having a moderate to low risk of bias (Supplementary Table S3—available at https://doi.org/10.25375/uct.23708346), and one study lacked clarity for assessing the risk of bias (Muhtarova et al., 2017). The sampling frame for all, but one study (Golińska et al., 2016), was a true or close representation of the target population. The data collected from all included studies were directly from participants rather than through a proxy, verifying the reliability of the sample collected. The participants of the included studies were clearly described, providing adequate control definition. Both the study instrument used to measure the parameter of interest and the mode of data collection used were well described.
Emm types (n = 119) were provided in 27 studies. For interest, we list the emm types documented according to type of infection. A total of 25 studies provided data on the emm types in invasive and non-invasive isolates. The most prevalent emm types (n = 22) are presented in Supplementary Figure S1—available at https://doi.org/10.25375/uct.23708346; emm1 and emm12 were found in 25 and 22 studies, respectively. A total of 12 emm types (emm1, emm3, emm11, emm27, emm49, emm76, emm81, emm82, emm83, emm89, emm90, and emm92) were significantly associated with invasive GAS infection, while 6 emm types (emm2, emm4, emm6, emm12, emm77, and emm104) were inversely associated with invasive GAS infection (Supplementary Table S4—available at https://doi.org/10.25375/uct.23708346).
A total of 32 studies (n = 8,454 isolates) were amenable to meta-analysis. We found 45 different virulence elements among the studies (Supplementary Table S5—available at https://doi.org/10.25375/uct.23708346). When pooling the data from high-quality studies (any method), the only two genes with a significant associations with invasive disease were speM [8 studies, n = 1,758; OR, 1.64 (95%CI, 1.06; 2.52), I2 = 24.7] and prtf1 [3 studies, n = 424; OR, 0.42 (95%CI, 0.20; 0.87), I2 = 0%], of which only ptrf1 had a significant association when analyzed with data coming from only one method (PCR) (Table 2). The other two genes with significant association with invasive GAS (iGAS) using data obtained with the PCR method, speA [20 studies, n = 3,571; OR, 1.59 (95%CI, 1.10; 2.30), I2 = 64.4%] and speK [4 studies, n = 840; OR, 2.95 (95%CI, 1.81; 4.80), I2 = 0%], were found to be inversely associated with iGAS in the WGS study. Moreover, most of the genes identified by the WGS method came from a single study (Supplementary Table S6—available at https://doi.org/10.25375/uct.23708346).
As a subgroup, WGS-based studies rated as having a low risk of bias demonstrated significant associations of hasA [1 study, n = 653; OR, 1.91 (95%CI, 1.36; 2.67)] and speG [1 study, n = 653; OR, 2.83 (95%CI, 1.63; 4.92)] with iGAS infection. In contrast, significant inverse associations were observed for speA [1 study, n = 653; OR, 0.44 (95%CI, 0.27; 0.73)], speK [2 studies, n = 678; OR, 0.26 (95%CI, 0.15; 0.45)], ssa [1 study, n = 653; OR, 0.15 (95%CI, 0.08; 0.26)], smeZ [1 study, n = 653; OR, 0.42 (95%CI, 0.29; 0.61)], NaDase 330G [1 study, n = 653; OR, 0.34 (95%CI, 0.22; 0.52)], sic [1 study, n = 653; OR, 0.42 (95%CI, 0.26; 0.68)], sda1 [1 study, n = 653; OR, 0.62 (95%CI, 0.41; 0.94)], and pnga3 [1 study, n = 653; OR, 0.26 (95%CI, 0.19; 0.37)] with invasive GAS infections (Table 3). No heterogeneity was observed in the studies comprising the meta-analysis.
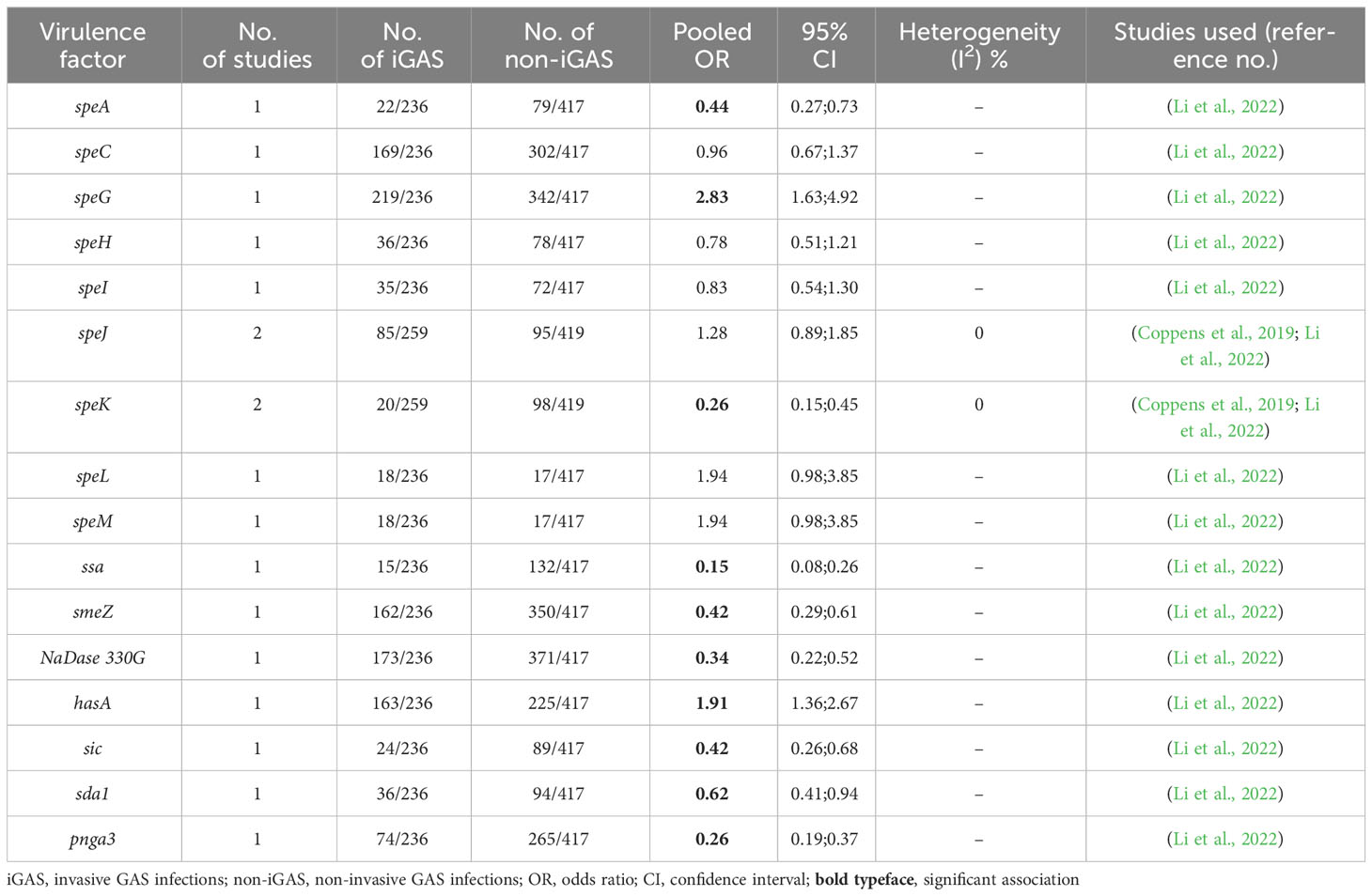
Table 3 Study data used in meta-analyses of virulence factors and invasive infection (lab method: WGS, low ROB).
Where reported, emm types (2,797 isolates) associated with invasive infection represented 27 different emm clusters (out of a possible 48) (Supplementary Table S7—available at https://doi.org/10.25375/uct.23708346) (Sanderson-Smith et al., 2014). Of these, six emm clusters were significantly associated with invasive GAS infection: AC3 [emm1; n = 25 studies; OR, 1.63 (95%CI, 1.44; 1.84], AC5 [emm3; n = 21 studies; OR, 2.15 (95%CI, 1.75; 2.64)], and E3 [emm9, emm25, emm44, emm49, emm58, emm82, emm87, emm103, emm113, emm118, emm180, emm183, and emm219; n = 16 studies; OR, 1.38 (95%CI, 1.13; 1.68)]. Clusters AC4 [emm12, emm39; n = 23 studies; OR, 0.40 (95%CI, 0.35; 0.47)], E1 [emm4, emm60, emm78, emm165, emm176; n = 22 studies; OR, 0.53 (95%CI, 0.45; 0.63)], and M6 [emm6; n = OR, 0.59 (95%CI, 0.45; 0.79)] were inversely associated with invasive GAS infection (Table 4).
More than 97% of the cluster-associated isolates belonged to 11 emm clusters (in decreasing order of frequency: E4, AC3, E6, E3, E1, AC4, AC5, E2, M6, D4, M5) (Supplementary Table S7—available at https://doi.org/10.25375/uct.23708346). A total of 20 clusters (n = 4,099 isolates) were documented among the isolates associated with the significant virulence factors (speA, speG, speK, speM, ssa, smeZ) (Supplementary Table S8—available at https://doi.org/10.25375/uct.23708346). Five clusters (AC4, E2, E3, E4, E6) were frequently seen with speG and smeZ; cluster E1 with ssa and smeZ; cluster AC3 with speA, speG, and smeZ; and cluster AC5 with speA, speG, ssa, and smeZ. Clusters D4 and M6 were frequently seen with smeZ, and cluster M18 with speA (Supplementary Table S9—available at https://doi.org/10.25375/uct.23708346).
This review comprising a global investigation of virulence factors in invasive and non-invasive GAS infection provides reliable evidence for the association of GAS genetic elements with invasive disease. We identified 45 GAS putative virulence factors across 32 studies in our systematic review. Meta-analysis of high-quality studies identified a significant association, correlating positively and inversely, between genes and invasive disease. Below is the synthesis of virulence factors determined to be significantly associated with invasive disease as assembled through our review.
Among the chromosomally encoded superantigens, a lack of association between smeZ and invasive GAS disease was observed in this review, which is in agreement with an earlier study (Rogers et al., 2007). In this review, a significant association of speG and invasive GAS disease was seen. SpeG has been implicated in modulating host inflammatory responses and inhibiting complement activation (Friães et al., 2012). However, when considering WGS studies only, no associations were seen between speG and invasive disease, correlating with reports elsewhere (Proft and Fraser, 2003; Proft et al., 2003). Furthermore, speG has been reported in both invasive and non-invasive GAS, suggesting that virulence may, instead, be mediated by other elements in invasive GAS disease.
SpeA, speK, and speM are phage-encoded superantigens, mainly acquired via horizontal gene transfer; their differences result from the loss or acquisition of prophages. SpeA has been shown to promote bacterial adhesion and may play a role in invasion and dissemination of the bacterium. Most of the strains associated with severe streptococcal infections have been shown to produce the SpeA toxin (Yu and Ferretti, 1989; Hauser et al., 1991). In this review, we found that speA was associated with invasive GAS infection among PCR-based studies, which is commensurate with reports elsewhere (Hauser et al., 1991; Musser et al., 1991). However, when referring to the WGS studies, we also observed a significant inverse association of speA with invasive GAS infection. SpeK is a pseudogene characterized by an incomplete open reading frame (ORF) (Ferretti et al., 2001). Individuals infected with the M3 GAS strain MGAS315, containing phage genes, exhibited antibodies against speK, suggesting that this protein is produced in vivo (Beres et al., 2002). Our findings showed that speK had a significant association with invasive GAS among PCR-based studies. However, in WGS studies only, we observed a significant inverse association of speK with invasive GAS infection, contrasting with the PCR-based results. Our findings revealed that speM was significantly associated with invasive GAS infection.
Streptococcal superantigen (ssa) has been described in M3-related toxic shock syndrome isolates (Mollick et al., 1993), suggesting it to be a potential GAS virulence factor. In this review, we observed a significant inverse association with ssa and invasive GAS infection. Sda1 encodes for an extracellular nuclease that displays a strong sequence non-specific nuclease activity on DNA substrates and is thought to play a role in evasion of the host’s innate immune response by degradation of the DNA component of neutrophil extracellular traps (NETs) and macrophage extracellular traps (Uchiyama et al., 2012). Although earlier studies using a murine model indicated the significance of sda1 in enhancing GAS virulence during necrotizing fasciitis (Buchanan et al., 2006), our findings reveal an inverse correlation between sda1 and invasive disease. This implies that virulence may be influenced by factors beyond sda1, considering the critical role of extracellular DNA-degrading activity in GAS invasive disease virulence (Uchiyama et al., 2012).
Hyaluronan synthase (hasA) increases virulence by aiding in evasion of the host immune system (Dougherty and Van de Rijn, 1994; Wessels, 2019). HasA plays an important role in invasive GAS disease (Ashbaugh et al., 1998). This is highlighted by the enhanced production of capsules by the invasive disease-associated serotype M3 isolates relative to other isolates and by the in vivo selection of isolates during invasive diseases that show enhanced capsule production (Shea et al., 2011). This review found that hasA is significantly associated with invasive infection in WGS-based studies. Unfortunately, the included study in our review looked at isolates from a single location and at a single time; two of the three most highly represented serotypes are M89 and M4, which are known to partially (M89, certain clades) or fully (M4) lack the has operon and are not in the top three M types listed in the study overall (Li et al., 2022) (Supplementary Figure S1).
The enzymatic activity of NAD+–glycohydrolase (NADase) is essential in GAS virulence; NADase works interdependently with streptolysin O (SLO), a pore-forming toxin, to facilitate pore formation during GAS infection (Mozola and Caparon, 2015). Despite several clinical GAS isolates being deficient in NADase activity, they may still exhibit cytotoxicity comparable to that of NADase-proficient strains (Riddle et al., 2010; Chandrasekaran et al., 2013). NADaseG330D, a frequently occurring genetic variation characterized by the presence of aspartate at position 330 of NADase, exhibits a lack of observable NADase activity (Chandrasekaran et al., 2013). Nevertheless, NADaseG330D remains a potent virulence factor and demonstrates the ability to interact with SLO in a manner similar to that of the wild-type NADase (Velarde et al., 2017). In this study, however, NADaseG330D was inversely associated with invasive GAS infection.
This review showed that PrtF1 was inversely associated with invasive GAS infection in PCR-based studies. Protein F1 (PrtF1/sfb1) is a fibronectin-binding protein, reported to promote epithelial cell adhesion and internalization. Hyland et al. demonstrated that PrtF1 expression elicits increased invasion of epithelial cells and resistance to phagocytosis, when expressed in M1 Streptococcus pyogenes strains (Hyland et al., 2007).
Westman et al. illustrated that streptococcal inhibitor of the complement (sic) is associated with invasive infection; sic is a secreted virulence factor that confers protection to GAS and performs multifunctional activities such as interfering with complement function and binding to various ligands essential for host colonization (Fernie-King et al., 2004; Pence et al., 2010; Frick et al., 2011; Westman et al., 2018). The contrast of the findings in this review showing an inverse association of sic with invasive disease may be due to Westman et al. only focusing on specific serotypes in iGAS, thus suggesting that virulence factors other than sic mediate invasive infection.
A single study by Chochua et al. found that pnga3 was present in 55.6% of 1,454 invasive GAS isolates. Pnga3 is a clade 3 upregulated promoter of the nga operon that encodes NADase and streptolysin O (Chochua et al., 2017). In this review, one study documented pnga3 to be inversely associated with invasive GAS infection as compared with non-invasive isolates. However, data on the association of pnga3 and clinical phenotypes are relatively scarce, thus requiring more studies to correlate these findings.
This review found 12 emm types significantly associated with invasive GAS infection. Similar patterns of emm types causing invasive disease were observed in other studies (O’Brien et al., 2002; Sharkawy et al., 2002; Naseer et al., 2016). Utilizing the cluster classification of the numerous emm types, 11 prevalent emm clusters were observed in invasive GAS isolates: clusters AC3, AC5, and E3 were found to be significantly associated with invasive GAS infection. Our findings correlate, albeit in a different order, with previous studies describing these clusters and their corresponding emm types in invasive infection (Smeesters et al., 2017; Friães et al., 2019; Jabang et al., 2021; Zangarini et al., 2023). We observed a close relationship between emm cluster and the significant virulence-associated factors, which correlates with results from China (Lu et al., 2017). More than 70% of isolates from the major cluster A-C3 (emm1) harbored speA, speG, and smeZ, correlating with a study performed by Gergova et al. (2019). The link between emm type/cluster and occurrence of virulence factors may be greatly conserved for the most virulent emm types, rendering them more pathogenic (Vlaminckx et al., 2003).
Collectively, our data contribute to an understanding of the interrelational nature of emm type/clusters and other virulence determinants in streptococcal pathogenesis and clinical outcomes. The vast amount of functional redundancy among superantigens emphasizes the biological significance of these elements and also suggests that host factors have a substantial contribution in the outcome of GAS infection.
One of the strengths of this review is the use of multiple databases and a broad inclusive search strategy so as to prevent overlooking eligible articles. Quality was assured through the inclusion, only, of articles of high quality, thus allowing for comparisons across the studies. Challenges in conducting this review arose from unclear definitions of invasive disease, requiring assumptions on the part of the reviewer (based on the isolation site reported) as well as variation in methods used to identify the virulence factors in this review.
We acknowledge the limitation of the PCR method employed in earlier association studies, which may have been confounded by allelic variants not as yet detected (Commons et al., 2014); thus, the range of primer sequences may not have been optimal to identify several allelic variants of the single superantigen. In comparison, WGS methods offer comprehensive detection capabilities as they can identify sequences rapidly and accurately without the need for prior knowledge of specific targets. Secondly, we acknowledge that low numbers of isolates included in studies may impact meta-analyses; thus, we conducted subgroup analysis according to molecular method. Unfortunately, not having individual data precluded us from studying a potential combined effect of virulence factors.
In conclusion, we acknowledge that the limitation of only focusing on gene data has implications in interpreting these results; it must be borne in mind that the PCR and WGS methods do not confirm function, especially if single‐nucleotide polymorphisms (SNPs) or insertions and deletions (INDELs) are present, given that uncharacterized SNPs may negate function. Nevertheless, this systematic review provides the latest data on the association of virulence factors with iGAS, presenting evidence for a possible relationship between the hasA gene and invasive infection. Also, we document an inverse association of smeZ, sic, sda1, and pnga3 genes with iGAS; this inverse association with invasive infection may be due to the presence of unknown virulence genes in GAS lineages. There is mixed evidence regarding the association of SpeA, speK, and speG with GAS virulence; thus, it is unclear if they are markers of invasive infection. The occurrence of specific genes encoding these virulence factors will serve to inform further research addressing the role of GAS virulence factors in both invasive and non-invasive GAS infections.
KR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. MS: Data curation, Methodology, Validation, Writing – review & editing. KE: Data curation, Validation, Writing – review & editing. CM: Supervision, Validation, Writing – review & editing. LZ: Funding acquisition, Investigation, Resources, Writing – review & editing. ME: Conceptualization, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – review & editing
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research reported in this publication was supported by the South African Medical Research Council (SAMRC) under a Self-Initiated Research Grant. KR, KE, and TS are also funded by a grant from the SAMRC. LZ also receives support from the National Research Foundation of South Africa (NRFSA), as well as the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement, via the African Research Leader Award (MR/S005242/1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The views and opinions expressed are those of the authors and do not necessarily represent the official views of the SAMRC.
Engel, Mark; Rampersadh, Kimona (2023). Supplementary Material - Group A streptococcus (GAS) virulence factors associated with invasive disease: a systematic review. University of Cape Town. Journal contribution. https://doi.org/10.25375/uct.23708346.v4
Supplementary Figure 1 | The most prevalent emm types identified amongst GAS studies.
Supplementary Table 1 | Search strategy for PubMed database.
Supplementary Table 2 | Characteristics of excluded studies.
Supplementary Table 3 | Risk of bias of the included studies.
Supplementary Table 4 | List of emm types significantly associated with invasive GAS infection.
Supplementary Table 5 | Study data of all virulence factors in all the studies (classification-cell surface, secretory, both or other).
Supplementary Table 6 | Meta-analyses of the association of secretory virulence factors and invasive infection (lab method: PCR; Low ROB).
Supplementary Table 7 | Distribution of emm cluster in invasive GAS infections.
Supplementary Table 8 | Extract of the more significant emm clusters associated with the significant virulence factors.
Supplementary Table 9 | Summary of emm clusters (>2 studies) associated with the significant virulence factors.
Ashbaugh, C. D., Warren, H. B., Carey, V. J., Wessels, M. R. (1998). Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J. Clin. Invest. 102 (3), 550–560. doi: 10.1172/JCI3065
Barnett, T. C., Cole, J. N., Rivera-Hernandez, T., Henningham, A., Paton, J. C., Nizet, V., et al. (2015). Streptococcal toxins: role in pathogenesis and disease. Cell. Microbiol. 17 (12), 1721–1741. doi: 10.1111/cmi.12531
Barnett, T., Indraratna, A., Sanderson-Smith, M. (2022). Secreted virulence factors of Streptococcus pyogenes. In: Ferretti, JJ, Stevens, DL, Fischetti, VA editors. Streptococcus pyogenes: Basic Biology to Clinical Manifestations [Internet]. 2nd ed. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2022 Oct 8. Chapter 13. PMID: 36479759.
Barnett, T. C., Scott, J. R. (2002). Differential recognition of surface proteins in Streptococcus pyogenes by two sortase gene homologs. J. Bacteriol. 184 (8), 2181–2191. doi: 10.1128/JB.184.8.2181-2191.2002
Bencardino, D., Di Luca, M. C., Petrelli, D., Prenna, M., Vitali, L. A. (2019). High virulence gene diversity in Streptococcus pyogenes isolated in Central Italy. PeerJ 7, e6613. doi: 10.7717/peerj.6613
Beres, S. B., Sylva, G. L., Barbian, K. D., Lei, B., Hoff, J. S., Mammarella, N. D., et al. (2002). Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. 99 (15), 10078–10083. doi: 10.1073/pnas.152298499
Berman, H. F., Tartof, S. Y., Reis, J. N., Reis, M. G., Riley, L. W. (2014). Distribution of superantigens in group A streptococcal isolates from Salvador, Brazil. BMC Infect. Dis. 14 (1), 1–6. doi: 10.1186/1471-2334-14-294
Bianco, S., Allice, T., Zucca, M., Savoia, D. (2006). Survey of phenotypic and genetic features of Streptococcus pyogenes strains isolated in Northwest Italy. Curr. Microbiol. 52, 33–39. doi: 10.1007/s00284-005-0067-1
Buchanan, J. T., Simpson, A. J., Aziz, R. K., Liu, G. Y., Kristian, S. A., Kotb, M., et al. (2006). DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 16 (4), 396–400. doi: 10.1016/j.cub.2005.12.039
Carapetis, J. R., Steer, A. C., Mulholland, E. K., Weber, M. (2005). The global burden of group A streptococcal diseases. Lancet Infect. Dis. 5 (11), 685–694. doi: 10.1016/S1473-3099(05)70267-X
CDC (2022). Increase in invasive group A strep infections (Atlanta: CDC). Available at: https://www.cdc.gov/groupastrep/igas-infections-investigation.html.
Chan, J. C., Chu, Y.-W., Chu, M.-Y., Cheung, T. K., Lo, J. Y. (2009). Epidemiological analysis of Streptococcus pyogenes infections in Hong Kong. Pathology 41 (7), 681–686. doi: 10.3109/00313020903257723
Chandrasekaran, S., Ghosh, J., Port, G. C., Koh, E.-i., Caparon, M. G. (2013). Analysis of polymorphic residues reveals distinct enzymatic and cytotoxic activities of the Streptococcus pyogenes NAD+ glycohydrolase. J. Biol. Chem. 288 (27), 20064–20075. doi: 10.1074/jbc.M113.481556
Chochua, S., Metcalf, B. J., Li, Z., Rivers, J., Mathis, S., Jackson, D., et al. (2017). Population and whole genome sequence based characterization of invasive group A streptococci recovered in the United States during 2015. MBio 8 (5), e01422–e01417. doi: 10.1128/mBio.01422-17
Commons, R., Rogers, S., Gooding, T., Danchin, M., Carapetis, J., Robins-Browne, R., et al. (2008). Superantigen genes in group A streptococcal isolates and their relationship with emm types. J. Med. Microbiol. 57 (10), 1238–1246. doi: 10.1099/jmm.0.2008/001156-0
Commons, R. J., Smeesters, P. R., Proft, T., Fraser, J. D., Robins-Browne, R., Curtis, N. (2014). Streptococcal superantigens: categorization and clinical associations. Trends Mol. Med. 20 (1), 48–62. doi: 10.1016/j.molmed.2013.10.004
Coppens, J., Xavier, B. B., Loens, K., Lammens, C., Ieven, M., Matheeussen, V., et al. (2019). Remarkable genome stability among emm1 group a Streptococcus in Belgium over 19 Years. Genome Biol. Evol. 11 (5), 1432–1439. doi: 10.1093/gbe/evz093
Creti, R., Gherardi, G., Imperi, M., von Hunolstein, C., Baldassarri, L., Pataracchia, M., et al. (2005). Association of group a streptococcal emm types with virulence traits and macrolide-resistance genes is independent of the source of isolation. J. Med. Microbiol. 54 (10), 913–917. doi: 10.1099/jmm.0.46035-0
DebRoy, S., Li, X., Kalia, A., Galloway-Pena, J., Shah, B. J., Fowler, V. G., Jr., et al. (2018). Identification of a chimeric emm gene and novel emm pattern in currently circulating strains of emm4 Group A Streptococcus. Microbial. Genomics 4 (11). doi: 10.1099/mgen.0.000235
DelVecchio, A., Maley, M., Currie, B. J., Sriprakash, K. S. (2002). NAD-glycohydrolase production and speA and speC distribution in group A streptococcus (GAS) isolates do not correlate with severe GAS diseases in the Australian population. J. Clin. Microbiol. 40 (7), 2642–2644. doi: 10.1128/JCM.40.7.2642-2644.2002
Descheemaeker, P., Van Loock, F., Hauchecorne, M., Vandamme, P., Goossens, H. (2000). Molecular characterisation of group A streptococci from invasive and non-invasive disease episodes in Belgium during 1993–1994. J. Med. Microbiol. 49 (5), 467–471. doi: 10.1099/0022-1317-49-5-467
Dougherty, B. A., Van de Rijn, I. (1994). Molecular characterization of hasA from an operon required for hyaluronic acid synthesis in group A streptococci. J. Biol. Chem. 269 (1), 169–175. doi: 10.1016/S0021-9258(17)42330-1
Ekelund, K., Darenberg, J., Norrby-Teglund, A., Hoffmann, S., Bang, D., Skinhøj, P., et al. (2005). Variations in emm type among group A streptococcal isolates causing invasive or noninvasive infections in a nationwide study. J. Clin. Microbiol. 43 (7), 3101–3109. doi: 10.1128/JCM.43.7.3101-3109.2005
Engel, M., Rampersadh, K. (2023). Supplementary Material - Group A streptococcus (GAS) virulence factors associated with invasive disease: a systematic review. University of Cape Town. J Contrib. doi: 10.25375/uct.23708346.v4
Espadas-Maciá, D., Macián, E. M. F., Borrás, R., Gisbert, S. P., Bonet, J. I. M. (2018). Streptococcus pyogenes infection in paediatrics: from pharyngotonsillitis to invasive infections. Anales Pediatría (English Ed) 88 (2), 75–81. doi: 10.1016/j.anpede.2017.02.013
Fernie-King, B. A., Seilly, D. J., Lachmann, P. J. (2004). The interaction of streptococcal inhibitor of complement (SIC) and its proteolytic fragments with the human beta defensins. Immunology 111 (4), 444–452. doi: 10.1111/j.0019-2805.2004.01837.x
Ferretti, J. J., McShan, W. M., Ajdic, D., Savic, D. J., Savic, G., Lyon, K., et al. (2001). Complete genome sequence of an M1 strain of Streptococcus pyogenes. Proc. Natl. Acad. Sci. 98 (8), 4658–4663. doi: 10.1073/pnas.071559398
Fraser, J. D., Proft, T. (2008). The bacterial superantigen and superantigen-like proteins. Immunol. Rev. 225 (1), 226–243. doi: 10.1111/j.1600-065X.2008.00681.x
Friães, A., Melo-Cristino, J., Ramirez, M. (2019). Changes in emm types and superantigen gene content of Streptococcus pyogenes causing invasive infections in Portugal. Sci. Rep. 9 (1), 18051. doi: 10.1038/s41598-019-54409-2
Friães, A., Pinto, F. R., Silva-Costa, C., Ramirez, M., Melo-Cristino, J., Infections PGftSoS (2012). Group A streptococci clones associated with invasive infections and pharyngitis in Portugal present differences in emm types, superantigen gene content and antimicrobial resistance. BMC Microbiol. 12 (1), 280. doi: 10.1186/1471-2180-12-280
Frick, I.-M., Shannon, O., Åkesson, P., Mörgelin, M., Collin, M., Schmidtchen, A., et al. (2011). Antibacterial activity of the contact and complement systems is blocked by SIC, a protein secreted by Streptococcus pyogenes. J. Biol. Chem. 286 (2), 1331–1340. doi: 10.1074/jbc.M110.178350
Gergova, R., Muhtarova, A., Mitov, I., Setchanova, L., Mihova, K., Kaneva, R., et al. (2019). Relation between emm types and virulence gene profiles among Bulgarian Streptococcus pyogenes clinical isolates. Infect. Dis. 51 (9), 668–675. doi: 10.1080/23744235.2019.1638964
Golińska, E., van der Linden, M., Więcek, G., Mikołajczyk, D., Machul, A., Samet, A., et al. (2016). Virulence factors of Streptococcus pyogenes strains from women in peri-labor with invasive infections. Eur. J. Clin. Microbiol. Infect. Dis. 35 (5), 747–754. doi: 10.1007/s10096-016-2593-0
Haukness, H. A., Tanz, R. R., Thomson, R. B., Jr., Pierry, D. K., Kaplan, E. L., Beall, B., et al. (2002). The heterogeneity of endemic community pediatric group a streptococcal pharyngeal isolates and their relationship to invasive isolates. J. Infect. Dis. 185 (7), 915–920. doi: 10.1086/339407
Hauser, A. R., Stevens, D., Kaplan, E., Schlievert, P. (1991). Molecular analysis of pyrogenic exotoxins from Streptococcus pyogenes isolates associated with toxic shock-like syndrome. J. Clin. Microbiol. 29 (8), 1562–1567. doi: 10.1128/jcm.29.8.1562-1567.1991
Herman, A., Kappler, J. W., Marrack, P., Pullen, A. M. (1991). Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu. Rev. Immunol. 9 (1), 745–772. doi: 10.1146/annurev.iy.09.040191.003525
Hraoui, M., Boubaker, I. B.-B., Doloy, A., Samir, E., Redjeb, S. B., Bouvet, A. (2011). Epidemiological markers of Streptococcus pyogenes strains in Tunisia. Clin. Microbiol. Infect. 17 (1), 63–68. doi: 10.1111/j.1469-0691.2010.03174.x
Hsueh, P.-R., Wu, J.-J., Tsai, P.-J., Liu, J.-W., Chuang, Y.-C., Luh, K.-T. (1998). Invasive group A streptococcal disease in Taiwan is not associated with the presence of streptococcal pyrogenic exotoxin genes. Clin. Infect. Dis. 26 (3), 584–589. doi: 10.1086/514567
Hyland, K. A., Wang, B., Cleary, P. P. (2007). Protein F1 and Streptococcus pyogenes resistance to phagocytosis. Infect. Immun. 75 (6), 3188–3191. doi: 10.1128/IAI.01745-06
Jabang, S., Erhart, A., Darboe, S., Baldeh, A.-K., Delforge, V., Watson, G., et al. (2021). Molecular epidemiology of group A Streptococcus infections in the Gambia. Vaccines 9 (2), 124. doi: 10.3390/vaccines9020124
Jing, H. B., Ning, B. A., Hao, H. J., Zheng, Y. L., Chang, D., Jiang, W., et al. (2006). Epidemiological analysis of group a streptococci recovered from patients in china. J. Med. Microbiol. 55 (8), 1101–1107. doi: 10.1099/jmm.0.46243-0
Khan, R. M. A., Anwar, S., Pirzada, Z. A. (2020). Streptococcus pyogenes strains associated with invasive and non-invasive infections present possible links with emm types and superantigens. Iran. J. Basic. Med. Sci. 23 (1), 133. doi: 10.22038/IJBMS.2019.38635.9164
Kittang, B. R., Skrede, S., Langeland, N., Haanshuus, C. G., Mylvaganam, H. (2011). emm gene diversity, superantigen gene profiles and presence of SlaA among clinical isolates of group A, C and G streptococci from western Norway. Eur. J. Clin. Microbiol. Infect. Dis. 30, 423–433. doi: 10.1007/s10096-010-1105-x
Li, Y., Dominguez, S., Nanduri, S. A., Rivers, J., Mathis, S., Li, Z., et al. (2022). Genomic characterization of group A streptococci causing pharyngitis and invasive disease in colorado, USA, June 2016–April 2017. J. Infect. Dis. 225 (10), 1841–1851. doi: 10.1093/infdis/jiab565
Lintges, M., van der Linden, M., Hilgers, R. D., Arlt, S., Al-Lahham, A., Reinert, R. R., et al. (2010). Superantigen genes are more important than the emm type for the invasiveness of group a streptococcus infection. J. Infect. Dis. 202 (1), 20–28. doi: 10.1086/653082
Lu, B., Fang, Y., Fan, Y., Chen, X., Wang, J., Zeng, J., et al. (2017). High prevalence of macrolide-resistance and molecular characterization of Streptococcus pyogenes isolates circulating in China from 2009 to 2016. Front. Microbiol. 8, 1052. doi: 10.3389/fmicb.2017.01052
Luca-Harari, B., Straut, M., Cretoiu, S., Surdeanu, M., Ungureanu, V., van der Linden, M., et al. (2008). Molecular characterization of invasive and non-invasive Streptococcus pyogenes isolates from Romania. J. Med. Microbiol. 57 (11), 1354–1363. doi: 10.1099/jmm.0.2008/001875-0
Maripuu, L., Eriksson, A., Norgren, M. (2008). Superantigen gene profile diversity among clinical group A streptococcal isolates. FEMS Immunol. Med. Microbiol. 54 (2), 236–244. doi: 10.1111/j.1574-695X.2008.00469.x
Meehan, M., Murchan, S., Gavin, P. J., Drew, R. J., Cunney, R. (2018). Epidemiology of an upsurge of invasive group a streptococcal infections in irelan–2015. J. Infect. 77 (3), 183–190. doi: 10.1016/j.jinf.2018.05.010
Michaelsen, T., Andreasson, I., Langerud, B., Caugant, D. (2011). Similar superantigen gene profiles and superantigen activity in norwegian isolates of invasive and non-invasive group A streptococci. Scandinavian J. Immunol. 74 (5), 423–429. doi: 10.1111/j.1365-3083.2011.02594.x
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Internal Med. 151 (4), 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Mollick, J. A., Miller, G. G., Musser, J. M., Cook, R. G., Grossman, D., Rich, R. R. (1993). A novel superantigen isolated from pathogenic strains of Streptococcus pyogenes with aminoterminal homology to staphylococcal enterotoxins B and C. J. Clin. Invest. 92 (2), 710–719. doi: 10.1172/JCI116641
Mozola, C. C., Caparon, M. G. (2015). Dual modes of membrane binding direct pore formation by S treptolysin O. Mol. Microbiol. 97 (6), 1036–1050. doi: 10.1111/mmi.13085
Muhtarova, A., Gergova, R., Setchanova, L., Mitov, I. (2017). Distribution of super-antigens and toxins in Bulgarian invasive and non-invasive clinical isolates Streptococcus pyogenes. Acta Microb. Bulgar. 33, 151–156.
Murakami, J., Kawabata, S., Terao, Y., Kikuchi, K., Totsuka, K., Tamaru, A., et al. (2002). Distribution of emm genotypes and superantigen genes of Streptococcus pyogenes isolated in Japan, 1994–9. Epidemiol. Infect. 128 (3), 397–404. doi: 10.1017/S0950268802006854
Musser, J. M., Hauser, A. R., Kim, M. H., Schlievert, P. M., Nelson, K., Selander, R. K. (1991). Streptococcus pyogenes causing toxic-shock-like syndrome and other invasive diseases: clonal diversity and pyrogenic exotoxin expression. Proc. Natl. Acad. Sci. 88 (7), 2668–2672. doi: 10.1073/pnas.88.7.2668
Mylvaganam, H., Bjorvatn, B., Osland, A. (2000). Distribution and sequence variations of selected virulence genes among group A streptococcal isolates from western Norway. Apmis 108 (11), 771–778. doi: 10.1034/j.1600-0463.2000.d01-28.x
Nandi, S., Chakraborti, A., Bakshi, D. K., Rani, A., Kumar, R., Ganguly, N. K. (2002). Association of pyrogenic exotoxin genes with pharyngitis and rheumatic fever/rheumatic heart disease among indian isolates of streptococcus pyogenes. Lett. Appl. Microbiol. 35 (3), 237–241. doi: 10.1046/j.1472-765X.2002.01176.x
Naseer, U., Steinbakk, M., Blystad, H., Caugant, D. (2016). Epidemiology of invasive group A streptococcal infections in Norway 2010–2014: a retrospective cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1639–1648. doi: 10.1007/s10096-016-2704-y
Nobbs, A. H., Lamont, R. J., Jenkinson, H. F. (2009). Streptococcus adherence and colonization. Microbiol. Mol. Biol. Rev. 73 (3), 407–450. doi: 10.1128/MMBR.00014-09
O’Brien, K. L., Beall, B., Barrett, N. L., Cieslak, P. R., Reingold, A., Farley, M. M., et al. (2002). Epidemiology of invasive group A streptococcus disease in the United States, 1995–1999. Clin. Infect. Dis. 35 (3), 268–276. doi: 10.1086/341409
Pence, M. A., Rooijakkers, S. H., Cogen, A. L., Cole, J. N., Hollands, A., Gallo, R. L., et al. (2010). Streptococcal inhibitor of complement promotes innate immune resistance phenotypes of invasive M1T1 group A Streptococcus. J. Innate Immun. 2 (6), 587–595. doi: 10.1159/000317672
Plainvert, C., Longo, M., Seringe, E., Saintpierre, B., Sauvage, E., Ma, L., et al. (2018). A clone of the emergent Streptococcus pyogenes emm89 clade responsible for a large outbreak in a post-surgery oncology unit in France. Med. Microbiol. Immunol. 207, 287–296. doi: 10.1007/s00430-018-0546-1
Plainvert, C., Dinis, M., Ravins, M., Hanski, E., Touak, G., Dmytruk, N., et al. (2014). Molecular epidemiology of sil locus in clinical streptococcus pyogenes strains. J. Clin. Microbiol. 52 (6), 2003–2010. doi: 10.1128/jcm.00290-14
Proft, T., Fraser, J. D. (2003). Bacterial superantigens. Clin. Exp. Immunol. 133 (3), 299–306. doi: 10.1046/j.1365-2249.2003.02203.x
Proft, T., Webb, P. D., Handley, V., Fraser, J. D. (2003). Two novel superantigens found in both group A and group C Streptococcus. Infect. Immun. 71 (3), 1361–1369. doi: 10.1128/IAI.71.3.1361-1369.2003
Reglinski, M., Sriskandan, S., Turner, C. E. (2019). Identification of two new core chromosome-encoded superantigens in Streptococcus pyogenes; speQ and speR. J. Infect. 78 (5), 358–363. doi: 10.1016/j.jinf.2019.02.005
Riddle, D. J., Bessen, D. E., Caparon, M. G. (2010). Variation in Streptococcus pyogenes NAD+ glycohydrolase is associated with tissue tropism. J. Bacteriol. 192 (14), 3735–3746. doi: 10.1128/JB.00234-10
Rivera, A., Rebollo, M., Miro, E., Mateo, M., Navarro, F., Gurguí, M., et al. (2006). Superantigen gene profile, emm type and antibiotic resistance genes among group A streptococcal isolates from Barcelona, Spain. J. Med. Microbiol. 55 (8), 1115–1123. doi: 10.1099/jmm.0.46481-0
Rogers, S., Commons, R., Danchin, M. H., Selvaraj, G., Kelpie, L., Curtis, N., et al. (2007). Strain prevalence, rather than innate virulence potential, is the major factor responsible for an increase in serious group A streptococcus infections. J. Infect. Dis. 195 (11), 1625–1633. doi: 10.1086/513875
Salie, T., Engel, K., Moloi, A., Muhamed, B., Dale, J. B., Engel, M. E. (2020). Systematic review and meta-analysis of the prevalence of group A Streptococcal emm clusters in Africa to inform vaccine development. Msphere 5 (4), e00429–e00420. doi: 10.1128/mSphere.00429-20
Sanderson-Smith, M., De Oliveira, D. M., Guglielmini, J., McMillan, D. J., Vu, T., Holien, J. K., et al. (2014). A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J. Infect. Dis. 210 (8), 1325–1338. doi: 10.1093/infdis/jiu260
Schmitz, F.-J., Beyer, A., Charpentier, E., Normark, B. H., SChade, M., Fluit, A. C., et al. (2003). Toxin-gene profile heterogeneity among endemic invasive European group A streptococcal isolates. J. Infect. Dis. 188 (10), 1578–1586. doi: 10.1086/379230
Shannon, B. A., McCormick, J. K., Schlievert, P. M. (2019). Toxins and superantigens of group A streptococci. Microbiol. Spectrum. 7 (1), 12. doi: 10.1128/9781683670131.ch5
Sharkawy, A., Low, D. E., Saginur, R., Gregson, D., Schwartz, B., Jessamine, P., et al. (2002). Severe group A streptococcal soft-tissue infections in Ontario: 1992–1996. Clin. Infect. Dis. 34 (4), 454–460. doi: 10.1086/338466
Shea, P. R., Beres, S. B., Flores, A. R., Ewbank, A. L., Gonzalez-Lugo, J. H., Martagon-Rosado, A. J., et al. (2011). Distinct signatures of diversifying selection revealed by genome analysis of respiratory tract and invasive bacterial populations. Proc. Natl. Acad. Sci. 108 (12), 5039–5044. doi: 10.1073/pnas.1016282108
Smeesters, P. R., Laho, D., Beall, B., Steer, A. C., Van Beneden, C. A. (2017). Seasonal, geographic, and temporal trends of emm clusters associated with invasive group A streptococcal infections in US multistate surveillance. Clin. Infect. Dis. 64 (5), 694–695. doi: 10.1093/cid/ciw807
Smeesters, P. R., McMillan, D. J., Sriprakash, K. S. (2010). The streptococcal M protein: a highly versatile molecule. Trends Microbiol. 18 (6), 275–282. doi: 10.1016/j.tim.2010.02.007
Strus, M., Heczko, P., Golińska, E., Tomusiak, A., Chmielarczyk, A., Dorycka, M., et al. (2017). The virulence factors of group A streptococcus strains isolated from invasive and non-invasive infections in Polish and German centres, 2009–2011. Eur. J. Clin. Microbiol. Infect. Dis. 36 (9), 1643–1649. doi: 10.1007/s10096-017-2978-8
Tapiainen, T., Launonen, S., Renko, M., Saxen, H., Salo, E., Korppi, M., et al. (2016). Invasive group A streptococcal infections in children. Pediatr. Infect. Dis. J. 35 (2), 123–128. doi: 10.1097/INF.0000000000000945
Tyler, S., Johnson, W., Huang, J., Ashton, F., Wang, G., Low, D., et al. (1992). Streptococcal erythrogenic toxin genes: detection by polymerase chain reaction and association with disease in strains isolated in Canada from 1940 to 1991. J. Clin. Microbiol. 30 (12), 3127–3131. doi: 10.1128/jcm.30.12.3127-3131.1992
Uchiyama, S., Andreoni, F., Schuepbach, R. A., Nizet, V., Zinkernagel, A. S. (2012). DNase Sda1 allows invasive M1T1 group A Streptococcus to prevent TLR9-dependent recognition. PloS Pathogens. 8 (6), e1002736. doi: 10.1371/journal.ppat.1002736
Velarde, J. J., O’Seaghdha, M., Baddal, B., Bastiat-Sempe, B., Wessels, M. R. (2017). Binding of NAD+-glycohydrolase to streptolysin O stabilizes both toxins and promotes virulence of group A Streptococcus. MBio 8 (5), 10–1128. doi: 10.1128/mbio.01382-17
Vlaminckx, B. J., Mascini, E. M., Schellekens, J., Schouls, L. M., Paauw, A., Fluit, A. C., et al. (2003). Site-specific manifestations of invasive group A streptococcal disease: type distribution and corresponding patterns of virulence determinants. J. Clin. Microbiol. 41 (11), 4941–4949. doi: 10.1128/JCM.41.11.4941-4949.2003
Walker, M. J., Barnett, T. C., McArthur, J. D., Cole, J. N., Gillen, C. M., Henningham, A., et al. (2014). Disease manifestations and pathogenic mechanisms of group A Streptococcus. Clin. Microbiol. Rev. 27 (2), 264–301. doi: 10.1128/CMR.00101-13
Wessels, M. R. (2019). Capsular polysaccharide of group A Streptococcus. Microbiol. Spectrum. 7 (1). doi: 10.1128/microbiolspec.gpp3-0050-2018
Westman, J., Chakrakodi, B., Snäll, J., Mörgelin, M., Bruun Madsen, M., Hyldegaard, O., et al. (2018). Protein SIC secreted from Streptococcus pyogenes forms complexes with extracellular histones that boost cytokine production. Front. Immunol. 9, 236. doi: 10.3389/fimmu.2018.00236
Yu, C.-E., Ferretti, J. J. (1989). Molecular epidemiologic analysis of the type A streptococcal exotoxin (erythrogenic toxin) gene (speA) in clinical Streptococcus pyogenes strains. Infect. Immun. 57 (12), 3715–3719. doi: 10.1128/iai.57.12.3715-3719.1989
Yu, D., Liang, Y., Lu, Q., Meng, Q., Wang, W., Huang, L., et al. (2021). Molecular characteristics of streptococcus pyogenes isolated from chinese children with different diseases. Front. Microbiol. 12, 722225. doi: 10.3389/fmicb.2021.722225
Zangarini, L., Martiny, D., Miendje Deyi, V. Y., Hites, M., Maillart, E., Hainaut, M., et al. (2023). Incidence and clinical and microbiological features of invasive and probable invasive streptococcal group A infections in children and adults in the Brussels-Capital Region, 2005–2020. Eur. J. Clin. Microbiol. Infect. Dis. 42 (5), 555–567. doi: 10.1007/s10096-023-04568-y
Keywords: Group A streptococcus, Streptococcus pyogenes, invasive disease, virulence factors, superantigens
Citation: Rampersadh K, Salie MT, Engel KC, Moodley C, Zühlke LJ and Engel ME (2024) Presence of Group A streptococcus frequently assayed virulence genes in invasive disease: a systematic review and meta-analysis. Front. Cell. Infect. Microbiol. 14:1337861. doi: 10.3389/fcimb.2024.1337861
Received: 13 November 2023; Accepted: 18 January 2024;
Published: 03 June 2024.
Edited by:
Justin Merritt, Oregon Health and Science University, United StatesReviewed by:
Tarcisio Brignoli, University of Milan, ItalyCopyright © 2024 Rampersadh, Salie, Engel, Moodley, Zühlke and Engel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mark E. Engel, bWFyay5lbmdlbEBtcmMuYWMuemE=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.