- 1Stomatological Hospital of Chongqing Medical University, Chongqing, China
- 2Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences, Chongqing, China
- 3Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing, China
Oral lichen planus (OLP), a T-lymphocyte-mediated disease of the oral mucosa, has a complex pathogenesis that involves a number of factors. The disease is characterized by recurrent episodes and requires continuous follow up, and there is no curative treatment available. Erosive lichen planus, among others, has a risk of malignant transformation and requires standardized treatment to control its progression. Different clinical subtypes of oral lichen planus require appropriate treatment. Pharmacological treatments are the most widely available and have the greatest variety of options and a number of novel pharmacological treatments are presented as highlights, including JAK enzyme inhibitors. The second is photodynamic therapy, which is the leading physiological treatment. In addition, periodontal treatment and psychological treatment should not be neglected. In this review, we briefly discuss the most recent developments in therapies for oral lichen planus after summarizing the most widely used clinical treatments, aiming to provide different proposals for future clinical treatment.
1 Introduction
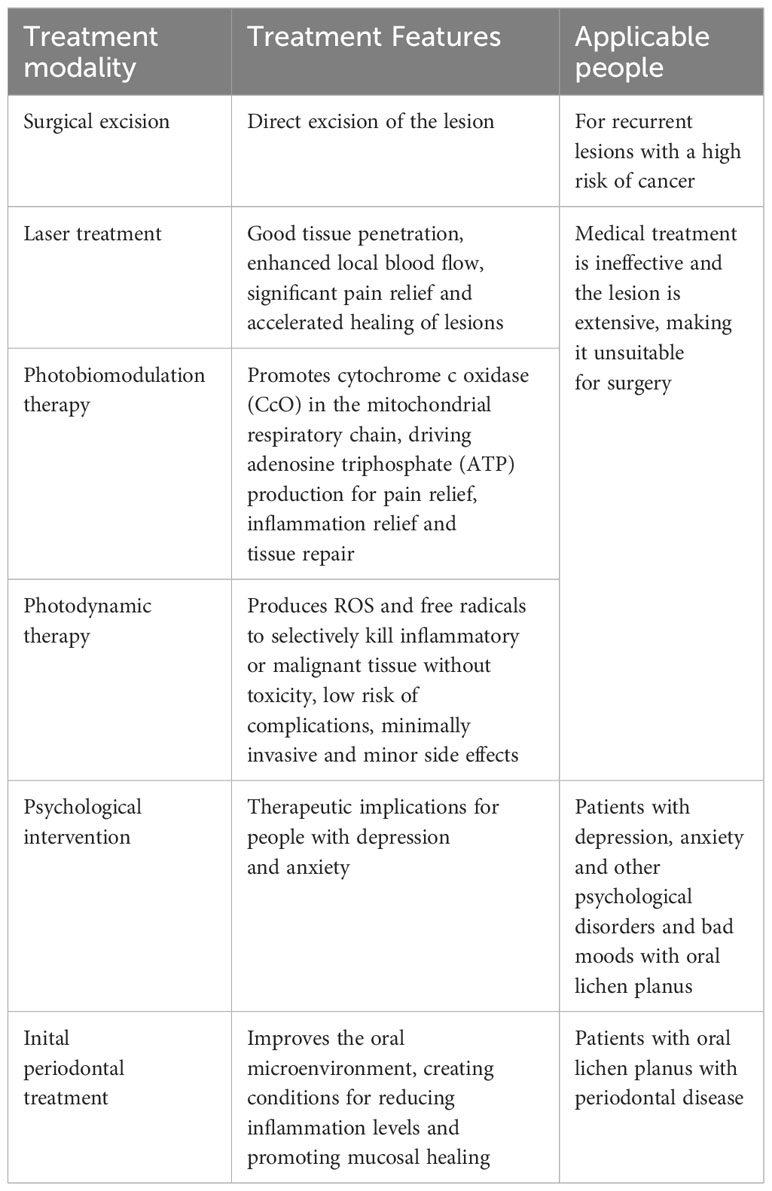
Erasmus Wilson first identified lichen planus (LP) in 1869 (Saeed et al., 2022), and oral lichen planus (OLP) is the name for the lesions that develop in the oral mucosa, a chronic inflammatory condition with an autoimmune component and an unknown cause (Carrozzo et al., 2019). OLP is approximately 0.98 percent prevalent worldwide, and the majority of patients are middle-aged women (Li et al., 2020). OLP lesions tend to occur on the buccal mucosa, ventral, and dorsal parts of the tongue’s mucous membranes (Figure 1). The typical clinical feature is a bilateral white or reticulated pattern, known as the Wickham striations. Clinically, OLP can be categorized as erosive or non-erosive (Didona and Hertl, 2022). The disorder is presently categorized by the World Health Organization (WHO) as an oral potentially malignant disease due to the possibility of its malignant transformation, with an erosive or ulcerative form considered a high-risk factor (Giuliani et al., 2019). To date, the potential for OLP to become malignant remains a highly controversial issue, with incidence rates fluctuating between 0 and 3.5% (Fitzpatrick et al., 2014), and most authors emphasize that clinical treatment should focus on symptom-based forms of atrophic and erosive OLP (EOLP) (Eisen et al., 2005). As a result, EOLP has become challenging to manage the lesions, prevent recurrence, and minimize side effects complications. There is currently no cure for OLP, and the standard first-line treatment consists of topical corticosteroids (Society of Oral Medicine and Chinese Stomatological Association, 2022). Klieb et al. proposed that the management of OLP can be effectively addressed through a multidisciplinary approach. For instance, patients with OLP who complain of dysphagia need the assistance of a gastroenterologist to rule out esophageal lichen planus (Shavit et al., 2020). The correlation between oral lichen planus (OLP), diabetes mellitus (DM), and hypertension was initially documented in 1990, indicating that OLP should not be regarded solely as a condition confined to the oral mucosa. Hence, it is imperative to conduct a meticulous examination of the patient’s medical history to identify any concurrent comorbidities (Lamey et al., 1990). Secondly, anxiety and psychological depression are risk factors for OLP that should not be ignored, and the onset of OLP itself leads to sleep disturbances and mood disorders, which should be closely monitored and psychologically counseled accordingly (Adamo et al., 2015). This review summarize the existing and latest advancements in OLP treatments to provide ideas for future clinical treatment.
Figure 1 Clinical manifestations of different types of OLP (A) Right buccal mucosa: reticular pattern affecting the mucosa, a large erosive area and atrophy seen in the center. (B) The white patches and a reticular pattern called Wickham’s striae. (C) Right tongue margin: erosive type. (D) Left tongue margin: non-erosive type. OLP, oral lichen planus.
2 Pharmacological treatment
The major therapeutic agents for EOLP include corticosteroids, calcineurin inhibitors (cyclosporin-A, tacrolimus), hydroxychloroquine, and total glucosides of paeony, etc. Each type of drug will be discussed in turn below.
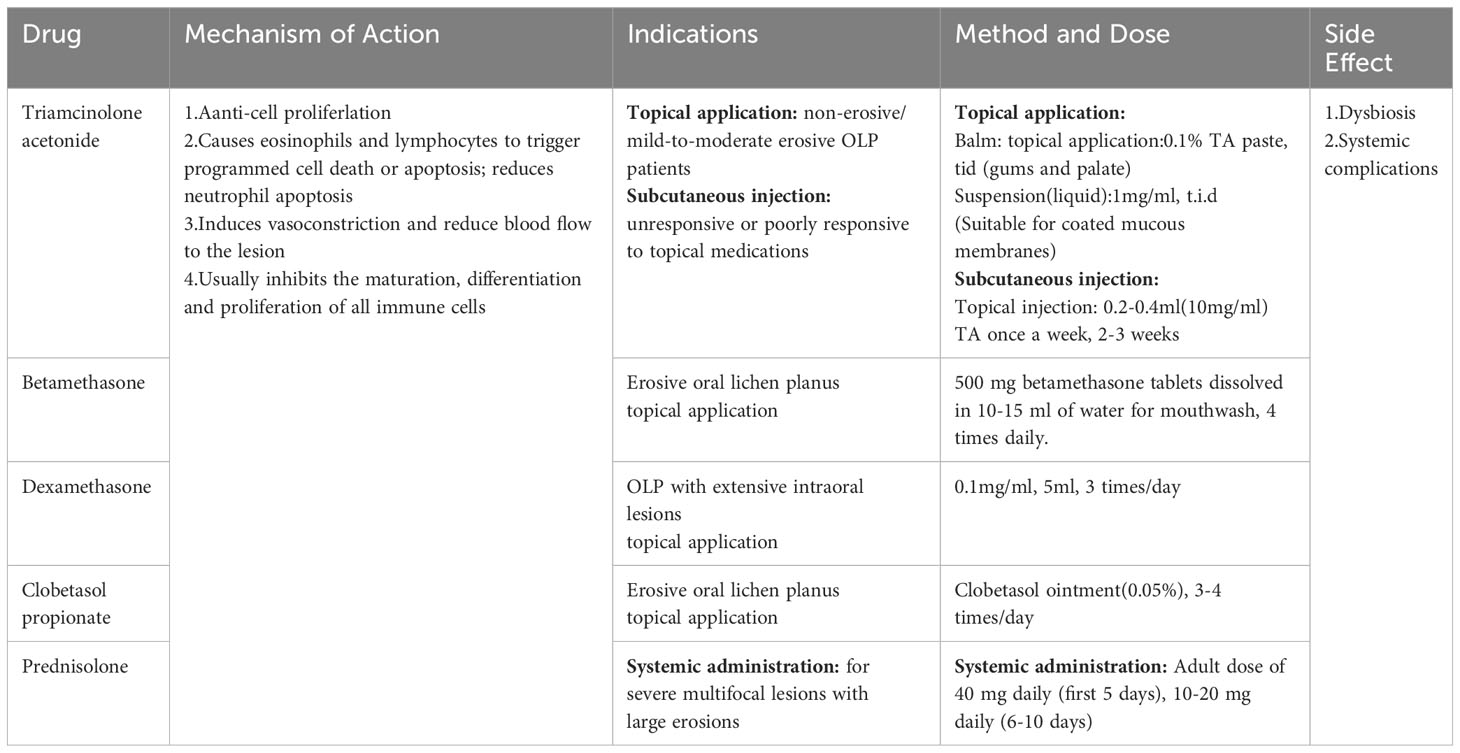
2.1 Corticosteroids
In development, metabolism, and immunity, adrenal steroid hormones (known as glucocorticoids) can have an important impact, and corticosteroids (CS), which are synthetic analogs of glucocorticoids, have anti-inflammatory and immunosuppressive effects (Ayyar and Jusko, 2020). In their comparison of the costs and effectiveness of several OLP treatment interventions, Sandhu et al. concluded that topical steroids continue to be the most economical and effective choice at the moment (Sandhu et al., 2022). In clinical practice, the most frequently employed agents are triamcinolone acetonide, betamethasone, clobetasol, and dexamethasone, and for systemic application, prednisone (Alrashdan et al., 2016b). In vivo, CS inhibits the maturation, differentiation, and proliferation of virtually all immune cells and reduces the inflammatory response by inducing vasoconstriction and reducing blood flow to the lesion (Claman, 1975). Topical application and intra- and submucosal injections of steroids are appropriate for mild to moderate lesions (Serafini et al., 2023). Systemic medications are indicated for recalcitrant and severe multifocal lesions, such as large erosive lesions, in which conventional medications have failed. However, CS have limitations, with the most prevalent side effect being opportunistic fungal infections. The reason for this is because corticosteroids’ immunosuppressive effects, can disturb the oral microbiome rendering the mouth susceptible to a variety of bacterial, parasitic, and viral infections (Hodgens and Sharman, 2023). As a result, CS are frequently used clinically in combination with antifungal drugs such as miconazole or chlorhexidine. On the other hand, CS topical therapy appears to affect the microbiota in OLP in some way. For example, following four weeks of using mouthwash containing 0.05% dexamethasone, Jeong et al. observed alterations in the composition of the microbial population in the majority of OLP patients (Ku et al., 2021), and this could be among the risk factors that cause fungal infections.
EOLP has been successfully treated with clobetasol patches (Rivelin-CLO) in clinical trials recently. This is a patch named Rivelin-CLO that adheres to mucosal surfaces and offers targeted delivery, dose control, and ease of continuous administration compared to traditional forms (Brennan et al., 2022). The innovative regimen was more effective, comfortable, and easy to apply, improving patient compliance. In addition, no adverse effects such as Candida infection were observed during the observation period, suggesting a high safety profile of the drug. However, topical clobetasol propionate gel has been linked to hypoadrenocorticism (Einarsdottir et al., 2023), and the adhesion ability of the patch is affected by the site of the lesion and oral activity, which may cause lingual border and alveolar mucosa patch dislodgement. In another lab study, CS was delivered to a human keratinocyte-T cell co-culture model using a new electrostatic spun mucosal adhesion patch, reducing activated Jurkat T cell IL-2 release. This medication delivery method also enhances CS action in confined lesions (Said et al., 2021). This mode of administration has fewer side effects and adverse reactions, making it a safe first-line therapy for the treatment of EOLP (Table 1).
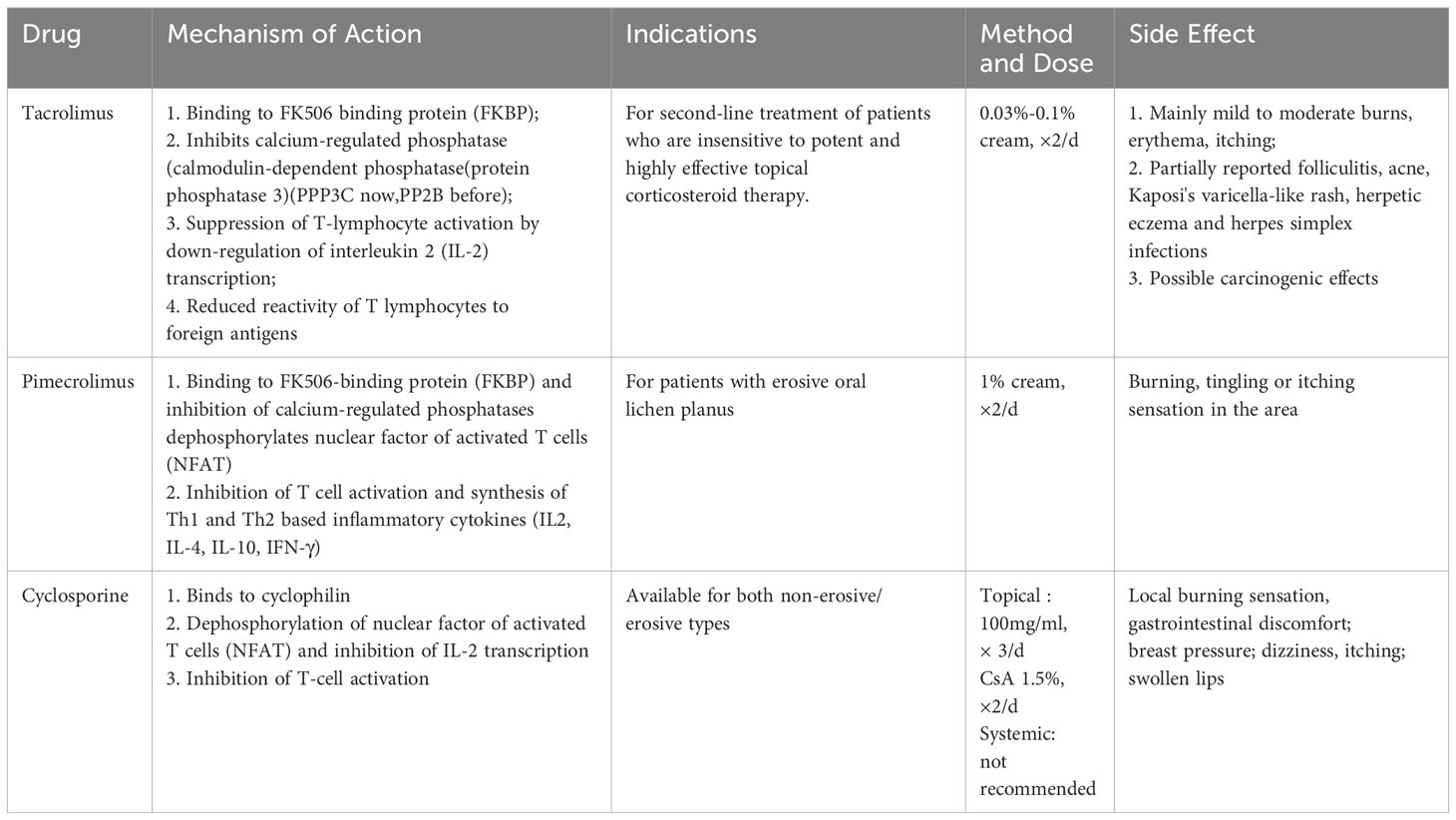
2.2 Calcineurin inhibitors
Calcineurin inhibitors are immunomodulators that combine with lecithin in T lymphocytes (cyclosporine binds to cyclophilin; tacrolimus and pimecrolimus bind to the FK506 binding protein), and these medications are used in the management of immune-mediated lesions (Tobón-Arroyave et al., 2004). Calcium phosphatase inhibitors inhibit the expression of pro-inflammatory factors by binding to macrophillin-12 and dephosphorylating NFAT (Yang et al., 2016) (Table 2).
2.2.1 Tacrolimus
A 5-year trial found that Tacrolimus inhibited lesion progression and improved subjective complaints (Utz et al., 2022). Another study found that the tacrolimus group showed increased mesenchyme expression of the apoptosis marker caspase-3, which may indicate impaired T-cell viability and reduce local inflammation, explaining its role in OLP immunology (Ibrahim et al., 2023). However, one of the side effects of tacrolimus is that topical application of tacrolimus can release neuropeptides such as substance P to stimulate sensory neurons and produce a transient burning or painful sensation (Riano Arguelles et al., 2006). Topical tacrolimus is a crucial second-line treatment for refractory EOLP, and Calcium phosphate inhibitors may help with refractory erosive OLP if steroids fail. Todd et al. reviewed 13 patients with OLP treated with topical tacrolimus, 11 of whom had a definite treatment effect. Seven of them had no subsequent recurrence (Rozycki et al., 2002). Notably, oral tacrolimus caused intestinal microecological dysregulation by decreasing levels of regenerating islet-derived protein 3 in the ileum and increasing intestinal permeability, which resulted in a significant increase in Bacteroides anomalies and Lactobacillus lactis (Toral et al., 2018). On the other hand, Guo et al. demonstrated that tacrolimus can be metabolized by a variety of intestinal commensal bacteria to a novel metabolite, 9-hydroxytacrolimus, a finding that explains the reduced efficacy of oral tacrolimus (Guo et al., 2019). Furthermore, no microbe-specific investigations on the topical administration of tacrolimus to the oral mucosa have been described.
2.2.2 Cyclosporine A
Cyclosporine A (CsA), an immunosuppressive peptide, is another calcium phosphatase inhibitor that selectively and reversibly suppresses CD4+ T cell activation (Guada et al., 2016). CsA also used for the oral treatment of refractory LP (Jungell and Malmstrom, 1996; Wang H. et al., 2016). Farahnaz et al. discovered that cyclosporine and methotrexate were successful in treating 33 cases of resistant EOLP (Fatemi Naeini et al., 2020). However, hypertension, altered renal function, gingival hyperplasia, and skin carcinogenicity are all adverse effects connected to oral administration of CsA (Lanese and Conger, 1993; Rouimi et al., 2018; Didona et al., 2022). Cyclosporine mouthwash is useless because mucosal cytochrome P450 deactivates it (Itin et al., 1992), and its mechanism of action relies on its interaction with systemic T-lymphocytes (Sieg et al., 1995). In terms of microorganisms, CsA has been shown in a rat model to reduce the amount of Enterobacteriaceae and Clostridium clusters I and XIV (Olek et al., 2023).
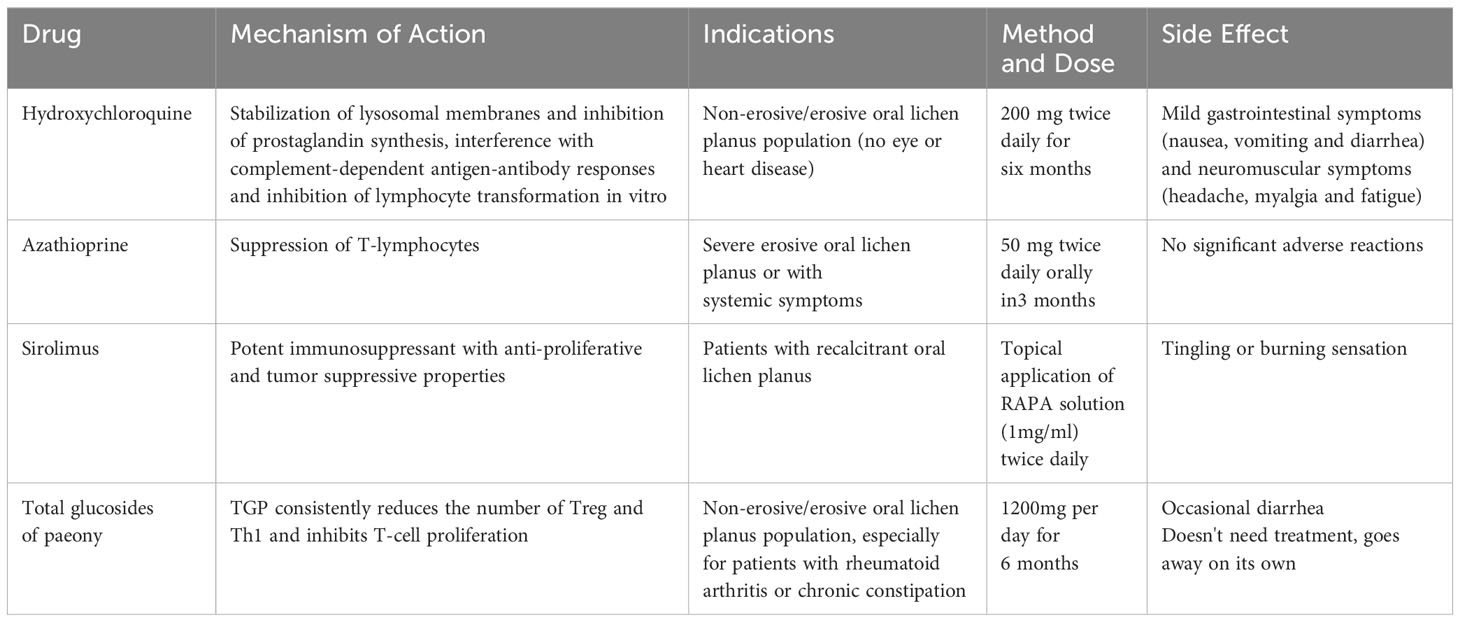
2.3 Hydroxychloroquine
The antimalarial drug hydroxychloroquine (HCQ) has been used to treat autoimmune conditions such as lupus erythematosus and rheumatoid arthritis. It modulates the immune system by stabilizing lysosomal enzymes and reducing the production of inflammatory cytokines (Bhanja et al., 2021). Oral HCQ may be used to treat EOLP 90% of OLP patients who received a 6-month pharmacological treatment for HCQ improved (Eisen, 1993). Yeshurun et al. discovered that 81 percent of 21 EOLP patients improved after three months of HCQ treatment. However, it is important to note that long-term use of hydroxychloroquine can result in irreversible retinal damage, hyperpigmentation, and elevated serum creatinine levels. It has been suggested that patients who have not responded to HCQ treatment for 2-4 months should not continue treatment with hydroxychloroquine to avoid accumulation of doses leading to side effects (Yeshurun et al., 2019) (Table 3). HCQ diminished regulatory T cells (Tregs) in the peripheral blood of OLP patients, indicating a potential pharmacological mechanism (Zhu et al., 2014). Unfortunately, this study has not been followed up further, while a number of scholars have reached opposite results regarding the mechanism of action of HCQ on Tregs (An et al., 2017; Sadeghpour et al., 2020; Hu et al., 2022). Therefore, a great number of fundamental studies still need to be conducted to thoroughly investigate the efficacy of HCQ in the therapy of OLP.
HCQ can exert antibacterial and antiviral effects by alkalinizing the intracellular pH of infected cells and inhibiting the glycosylation of viral proteins (Rolain et al., 2007). Keshavarzi found that HCQ had a direct antifungal effect on both A. fumigatus and A. nidulans (Keshavarzi, 2016). However, for the oral cavity, there are no reports on the inhibition of the growth of Candida albicans in the oral cavity.
2.4 Platelet-rich fibrin
By centrifuging venous blood at various speeds, with or without thrombin and anticoagulants, to create a fibrin clot including platelets and white blood cells, autologous platelet concentrate (APC) is created (Mijiritsky et al., 2021). Biocompatible products containing cytokines, platelets, leukocytes and fibrin, which can be considered slow-release systems ensuring continuous delivery of active ingredients and growth factors for about 2 weeks (Ding et al., 2021), have now been used to treat scalp LP and genital sclerosing LP satisfactorily (Casabona et al., 2010; Bolanča et al., 2016). The first study on the application of first-generation APC platelet-rich plasma (PRP) in oral and maxillofacial surgery was published in 1997 by Whitman et al., but its extraction steps were cumbersome and could cause immune rejection or infection, so second-generation APC was born (Whitman et al., 1997; Choukroun and Miron, 2017). Second-generation APC platelet-rich fibrin (PRF), first identified in 2001, promotes vascular regeneration, regulates local immunity, and hastens wound healing (Bennardo et al., 2020). The PRF serves as a biodegradable scaffold for microangiogenesis and can direct epithelial cells to migrate to its surface (Dohan et al., 2006; Li et al., 2013). The benefits of PRF over PRP include, but are not limited to, the fact that it does not require anticoagulants, promotes faster wound healing, and boosts the immune system (Gunasekaran et al., 2021). In recent years, scholars have found PRF local injections to be comparable to tretinoin (TA) in relieving OLP pain, suggesting a role in EOLP pain control (Bennardo et al., 2021). However, one study found that TA showed better treatment results on the visual pain analog scale (VAS) and semi-quantitative REU (reticulo-erosion-ulcer) scores when both were injected at the same lesion site (Al-Hallak et al., 2023). Platelet-rich fibrin (i-PRF) and CS for EOLP both reduce pain and lesion size, but i-PRF is less expensive (Saglam et al., 2021). Patients resistant to topical corticosteroids may therefore profit from i-PRF.
2.5 Hyaluronic acid
Hyaluronic acid (HA) stimulates angiogenesis, moistens wounds, reduces exudation, possesses vasoprotective properties, and functions as fibrin in inflammatory and injured tissues to promote tissue regeneration. It is used in oral surgery and periodontal treatment. It was found that HA was comparable to 0.1% triamcinolone in reducing the VAS score and size of the lesions in OLP (Hashem et al., 2019). In a clinical study, effective control of OLP was observed in patients using an anti-inflammatory mouthwash containing 0.3% HA as well as topical tacrolimus, but the tacrolimus group showed better clinical performance at 3-month follow-up (Polizzi et al., 2023). The above studies suggest that HA may be effective in short-term localized applications, but its ability to play a role in long-term disease control is debatable.
2.6 Anti-metabolic drugs
Anti-metabolic drugs such as azathioprine act to inhibit cell proliferation by inhibiting the purine pathway and interfering with DNA synthesis (Broen and van Laar, 2020). Adverse effects of such drugs may lead to bone marrow toxicity and gastrointestinal and hepatic mucosal damage (Verma et al., 2001). In contrast, antimetabolites are rarely used clinically for the treatment of OLP (Table 3).
2.7 mTOR inhibitors
The mTOR signaling pathway regulates cell survival, proliferation, translation, autophagy, and cytoskeletal reorganization. It regulates cellular responses to dietary and oxygen status changes (Paghdal and Schwartz, 2007). Sirolimus binds to cell membrane proteins similarly to tacrolimus and was the first mTOR inhibitors (mTORIs) to receive approval from the US Food and Drug Administration (Yang et al., 2016). The IL-2 signaling pathway is blocked by sirolimus, which inhibits the mammalian target of rapamycin by directly interacting with the mammalian rapamycin complex 1 (mTORC1). This prevents the activation of T and B cells (Treister et al., 2021). Soria et al. treated seven patients with EOLP using topical Sirolimus; after three months, four patients were in complete remission and two were in partial remission. Although myelosuppression and hyperlipidemia are the most common adverse effects of oral sirolimus for the treatment of chronic refractory EOLP, sirolimus is not well absorbed into the bloodstream and side effects are rare (Soria et al., 2009) (Table 3).
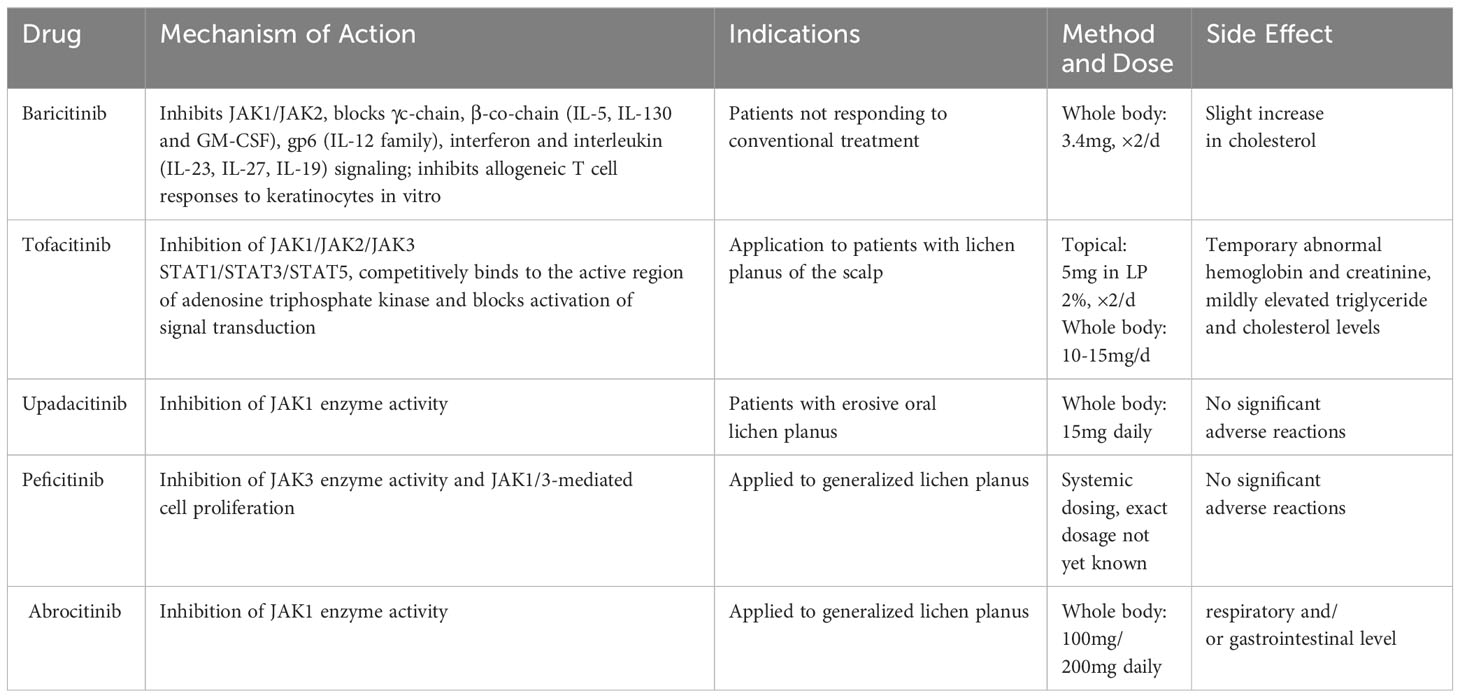
2.8 JAK/STAT signaling pathway inhibitors
Accidentally, Janus kinases and Signal Transducers and Activators of Transcription (JAKs and STATs) were discovered while studying the effects of cytokines such as interferon and interleukin (Stark and Darnell, 2012). JAKs are intracellular second messengers that are necessary for the cell to receive signals from extracellular cytokines (Schroder et al., 2004). JAK kinase has four isoforms (JAK1, JAK2, JAK3 and Tyk2), and STAT protein contains seven isoforms (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6) (Nishio et al., 2001). JAKs and STATs are phosphorylated by type I and type II cytokine receptors, and then STATs enter the nucleus and regulate transcription as well as immune and blood processes (Leonard and O'Shea, 1998; Arbouzova and Zeidler, 2006; Yazdi et al., 2016; Sabat et al., 2019) (Figure 2).
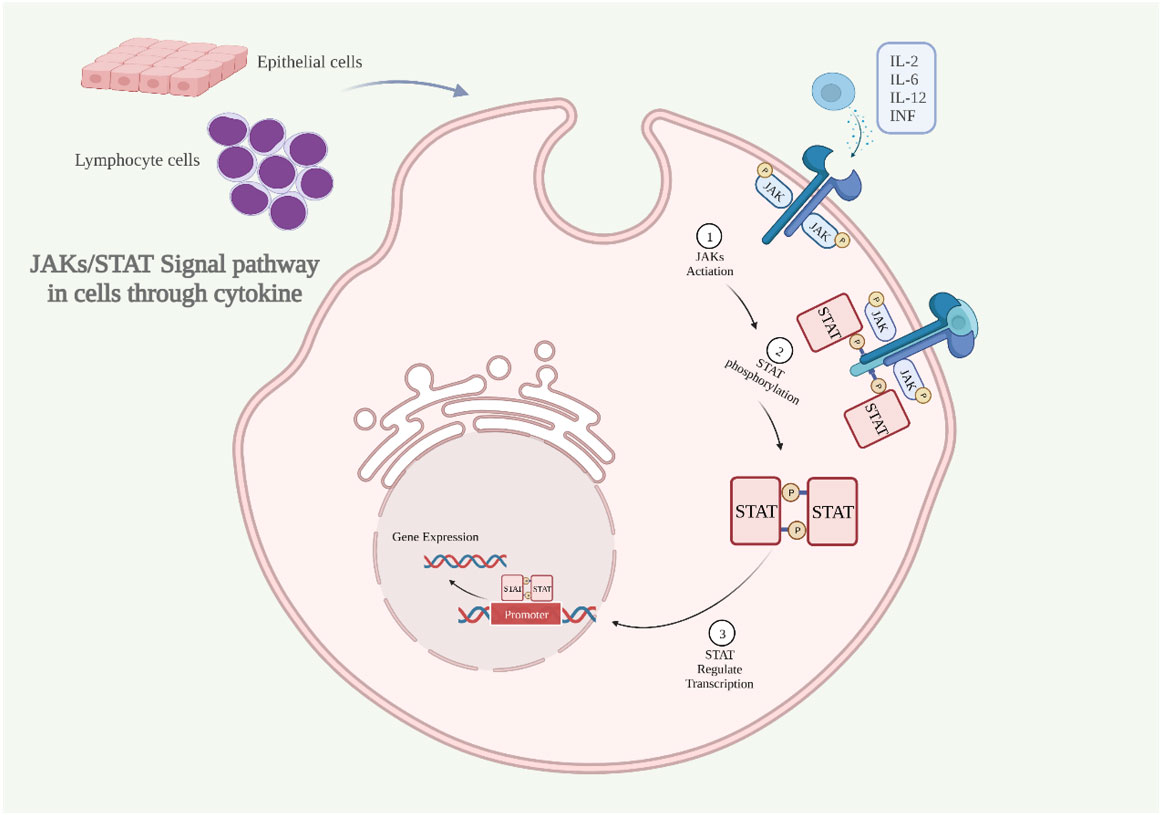
Figure 2 The JAKs/STAT signaling pathway is activated through cytokine (A) Inflammatory cytokines bind to the receptor, dimerizing it, activating JAKs, and phosphorylating the intracellular portion of the receptor. (B) STAT proteins bind at sites containing phosphorylated tyrosine. (C) STAT dimerizes and translocates into the nucleus to regulate gene expression. IL, interleukin; INF, interferon; JAKs and STAT, Janus kinases and Signal Transducers and Activators of Transcription (Created with BioRender.com).
It was discovered that JAK1 plays a crucial role in immunological and inflammatory responses and is engaged in IL-2 and IL-6 signaling (Migone et al., 1998; Haan et al., 2002). Abduelmula et al. found that MHCI expression was found to increase in keratinocytes, mainly through the JAK2/STAT1 pathway and inhibition of this pathway protects keratinocytes from cytotoxic responses (Abduelmula et al., 2023). JAK3, on the other hand, was found to be upregulated in peripheral blood Th1 and Th17 cells from OLP patients, accompanied by upregulation of STAT2 and STAT6 expression (Ociepa et al., 2022). Furthermore, IFN-γand CXCL10 were strongly expressed in lesions as well as in the serum of patients with LP, which was regulated by JAK1/2 (Flier et al., 2001). The aforementioned findings imply that the JAK/STAT pathway might be crucial in OLP.
The therapeutic effect of JAK inhibitors on OLP has been clinically proven. Tofacitinib inhibits JAK1/2/3 and, to a lesser extent, TYK2 (Hodge et al., 2016). Abrahim et al. found an overall effectiveness rate of 70% for tofacitinib in the treatment of LP by meta-analysis (Abduelmula et al., 2023). Baricitinib inhibited JAK1/2, and Moussa et al. discovered that a 63-year-old female patient with left buccal erosion improved after one month of oral baricitinib treatment, and that her oral irritation and distress were nearly eliminated after four months (Moussa et al., 2022). The adverse effects of tofacitinib and baricitinib seem to contain herpes zoster, occurrence of malignancy, and gastrointestinal perforation (Genovese et al., 2016; Strangfeld et al., 2017; Winthrop et al., 2017; Burmester et al., 2018). So far, they have not been found in the treatment of OLP. Upadacitinib is a JAK1 inhibitor but can also inhibit JAK2. Oral lesions in an EOLP patient receiving 15 mg of upadacitinib daily improved over time and experienced no side effects from the therapy. This outcome agrees with Neda’s observations (Kooybaran et al., 2021; Balestri et al., 2022). According to a recent case report from 2022, abrocitinib is a selective inhibitor of the JAK1 enzyme, patients with OLP who received a daily dose of 200 mg alone for 8 weeks and then 100mg for 4 weeks demonstrated full recovery of the right cheek lesion. The patient responded well to the medication, and there were no untoward occurrences (Gooderham et al., 2019; Solimani et al., 2023). However, adverse reactions have occurred in the context of treatment for atopic dermatitis (AD), generally at the respiratory and/or gastrointestinal level (Niculet et al., 2022). Peficitinib, a novel JAK3 inhibitor, was found to reduce enzyme activity and cell proliferation in an efficient manner (Qiu et al., 2019). One study reported that topical use of JAK3 enzyme inhibitors in cutaneous LP may have therapeutic promise (Samadi et al., 2017). Although tofacitinib, baricitinib, and upadacitinib all inhibit JAK2, there is no FDA-approval for the use of selective JAK2 inhibitor in OLP at the moment (Spinelli et al., 2021). It should be emphasized that it is not clear which isoform of the JAK enzyme is prominent in the pathogenesis of OLP, and more research is needed to develop more targeted drugs in the future (Table 4).
2.9 Antioxidant therapy
Some evidence suggests that the pathogenesis of OLP can be mediated by the oxidative stress (OS) state, which is also believed to facilitate the process of OLP’s malignant transformation (Amirchaghmaghi et al., 2016). The presence of reactive oxygen species (ROS) at the lesion increases the T lymphocyte-mediated inflammatory response, upregulates the expression of intercellular adhesion molecule (ICAM)-1 in keratin-forming cells, and disrupts their lipid membrane, resulting in increased T cell infiltration and ROS production (Gupta et al., 2017; Bao et al., 2022). Patients with OLP had higher levels of oxidative stress and lower levels of antioxidants, suggesting that oxidative stress contributes to the development of OLP (Wang et al., 2021). Many antioxidants have been used as adjuvant therapy for OLP, such as glutathione, coenzyme Q, lipoic acid, carotenoids, vitamins A, C, and E, and resveratrol (Thongprasom et al., 2011; Pisoschi and Pop, 2015). After 12 weeks of treatment, OLP patients who received topical selenium (Se) had substantially lower pain scores (NRS) than those who received corticosteroids, and the topical Se group experienced longer-lasting pain relief (Qataya et al., 2020). This suggests that Se alone may be helpful in the management of OLP with longer-lasting effects, improved pain alleviation, and no adverse effects.
2.10 Chinese herbal medicine treatment
Chinese medicine employs yin and yang, as well as the five elements, to restore internal organ function. Certain botanicals are immunomodulatory, anti-inflammatory, metabolism-promoting, and microcirculation-improving, according to modern pharmacology (Duan, 2015; Pang et al., 2018; You et al., 2020). Herbal medications with minimal adverse effects, such as Liu Wei Di Huang Wan and Tripterygium glycosides, are used to treat chronic oral diseases in China and other Asian countries (Ghahremanlo et al., 2019). Curcumin, a plant extract from turmeric with anti-inflammatory, antioxidant, and antitumor properties, has recently been studied in relation to OLP. Early OLP therapy with 6,000 mg per day of curcumin was effective (Chainani-Wu et al., 2012b; Chainani-Wu et al., 2012a). The use of curcumin and turmeric extracts to treat OLP, however, is disputed by certain researchers (Zeng et al., 2022).
2.11 Total glucosides of paeony
Total glucosides of paeony (TGP) is an active compound extracted from the root of Paeonia lactiflora that has immunomodulatory effects, and its components include paeoniflorin, hydroxypaeoniflorin, paeoniflorin, leuconidin, benzoylpaeoniflorin, etc. It is often used as an analgesic and anti-inflammatory drug for the treatment of diseases such as rheumatoid arthritis (RA) and skin ailment psoriasis (Jiang et al., 2020; Zhang and Wei, 2020). A double-blind, randomized, placebo-controlled study showed that TGP and Acitretin together improved the effectiveness of antipsoriasis treatment and decreased liver damage (Yu et al., 2017; Zheng et al., 2019).
Although TGP is used in the treatment of autoimmune diseases, its specific modulation of OLP is unknown (Jiang et al., 2020). TGP consistently reduces the number of Treg and Th1 and inhibits T-cell proliferation (Wang Y. et al., 2016). Wang et al. found that the NF-κB signaling pathway was significantly activated in OLP tissues, and TGP seems to inhibit the production of IL-6 and TNF-α in LPS-induced HaCaT cells in a dose-dependent manner, thus inhibiting the phosphorylation of IκBα and NF-κB p65 proteins (Yoke et al., 2006; Wang Y. et al., 2016). On the other hand, YAN et al. observed in a clinical study that peripheral blood IFN-γ and IL-10 levels were significantly elevated in patients with OLP after treatment with TGP, suggesting that TGP may play a role by increasing the expression of IFN-γ and IL-10 in peripheral blood (Yan and Z, 2016). These seem to reveal the tip of the iceberg of the mechanism of action of TGP for OLP treatment. The combination of TGP with triamcinolone acetonide and tacrolimus had a satisfactory therapeutic effect, and the percentage of fungal infections was not significantly different from the normal population (Marable et al., 2016). During TGP treatment, no liver or kidney damage or other side effects were observed, and a small number of patients experience diarrhea, which dissipates quickly (Li et al., 2014; Feng et al., 2019; Luo et al., 2019). The above findings suggest that TGP may be a strong candidate for the treatment of OLP (Table 3).
In terms of microbiology, it has been found that the combination of TGP and hydroxychloroquine can increase the growth of a variety of beneficial bacteria, inhibit the growth of dominant pathogenic bacteria, increase the diversity and abundance of intestinal microorganisms. Among them, the abundance of Lactobacillus, Bacteroides undulatus and Vibrio desulfuricans was significantly increased and the abundance of Bacteroides and alloprevotella was significantly decreased in the TGP+hydroxychloroquine group (Lu et al., 2020). However, the current study could not construct a complete intestinal ecosystem, and further studies are needed in the future to investigate the specific mechanisms of the microbial effects of TGP and hydroxychloroquine.
2.12 Micronutrients and EOLP
Micronutrients, such as vitamins and trace minerals, are crucial in determining one’s susceptibility to a number of systemic and oral disorders (Bhattacharya et al., 2016). A recent study reveals that OLP patients had lower levels of essential micronutrients for normal metabolism, such as iron, zinc, calcium, vitamin D, and vitamin B12, and higher levels of oxidants and homocysteine (Gholizadeh and Sheykhbahaei, 2021). Vitamin D inhibits B-lymphocyte differentiation and immunoglobulin secretion, and a growing number of studies have confirmed the link between vitamin D deficiency and OLP (Kujundzic et al., 2016; Zhao et al., 2019). The oral mucosa is exposed to environmental factors that compromise the immune system and increase tissue permeability due to epithelial thinning, mucosal inflammation, and atrophy from iron deficiency. Also, cell differentiation and proliferation require iron, which explains why supplemental iron decreases OLP lesions (Maggini et al., 2007). In particular, CD8+ and NK cells benefit from vitamin B12’s regulatory action as an immunomodulator of cellular immunity (Permoda-Osip et al., 2014). The combination of vitamin B12 and immunomodulatory drugs was effective in reducing autoantibody levels, pain, and lesion severity in patients with OLP (Chang et al., 2009; Lin et al., 2011).
2.13 Microbial agents
It has been reported that the oral cavity is home to more than 700 types of bacteria and 100 species of fungi (Escapa et al., 2018). Microorganisms are linked to the inflammatory cytokine production in OLP patients, including IL-1, IL-10, IL-17, and IFN-γ, the activation of T cells, and the elevation of protein expression linked to the response to oxidative stress (Jung and Jang, 2022). A variety of bacteria and fungi, including Lactobacillus, Bifidobacterium, and Saccharomyces species, also have distinct effects on the immune response. They control and modify the T regulatory/T helper 17 (Treg/Th17) axis, which shields the host from infections and reduces the overabundance of effector T cell responses (Ashraf and Shah, 2014; Yu et al., 2019). Accordingly, microbial therapy might make a great difference in the treatment of OLP.
Notably, the current microbiological therapies for OLP seem to be ineffective (Keller and Kragelund, 2018; Kragelund and Keller, 2019; Cosgarea et al., 2020; Marlina et al., 2022). Maukonen et al. were unable to detect any probiotics included in oral capsules in saliva samples (Maukonen et al., 2008). Svante et al. discovered that chewing gum containing L. reuteri lowered the levels of pro-inflammatory cytokines in gingival sulcus fluid (Twetman et al., 2009). Therefore, direct contact may be required for probiotics to colonize the oral mucosa. Nevertheless, other research indicates that probiotic colonization of oral biofilms seems to be a somewhat transient process (Yli-Knuuttila et al., 2006). Horz et al. discovered, for example, that Streptococcus salivarius K12 only colonized the mouth momentarily (Horz et al., 2007). After giving probiotic-containing dairy items to their patients for three days, Ravn et al. discovered that the probiotics had vanished from the surface of the teeth and were only slightly present in the saliva and oral mucosa (Ravn et al., 2012). The above results may be due to the inability of the current mode of delivery to act directly on the lesion site, and a probiotic delivery mechanism that operates directly on the lesion site might be created in the future, which would be a daring and interesting attempt.
3 Non-pharmacological treatment
3.1 Photodynamic therapy
Photodynamic therapy (PDT), a non-invasive, safe, and non-toxic treatment for oral precancerous lesions, has been shown to be beneficial (Ch, 2012). PDT consists of photosensitizers, light, and reactive oxygen species. Through biochemical interactions, singlet oxygen and free radicals from PDT induce cell death, membrane disruption, and protein inactivation at the lesion site (Hesse et al., 2020). According to Cosgarea et al., methylene blue-mediated photodynamic therapy (MB-PDT) decreased the number of CD4+ and CD8+ T cells in OLP-lesions and their capacity to produce IL-17 (Cosgarea et al., 2020). Mostafa et al. included 20 patients with EOLP and compared the effects of MB-PDT with topical CS and found that MB-PDT was effective in reducing pain and minimizing lesions (Mostafa et al., 2017). Jajarm et al. compared the efficacy of toluidine blue-mediated photodynamic therapy (TB-PDT) with topical corticosteroids in EOLP. During a one-month follow-up, no significant difference was found between the two, while the CS group seemed to be more effective in terms of pain relief (Jajarm et al., 2015). Chen et al. noted that saliva and soft tissue movements in the oral cavity result in incomplete photosensitizers absorption, and the more of the photosensitizer retained in the local lesion, the better the efficacy of PDT (He et al., 2020). Clinicians need to take these factors fully into account when selecting photosensitizers. Notably, Saleh et al. concluded that PDT is a more effective and safer treatment option for some patients with diabetes and hypertension (Saleh and Khashaba, 2022), and Sulewska et al. included 12 elderly patients with EOLP who showed significant healing or even complete remission of the lesions during a follow-up period of up to 12 months after treatment (Sulewska et al., 2017). The above results suggest that PDT offers a non-invasive treatment for oral mucosal lesions and may become an alternative and complementary method to those currently in use (Table 5).
The effect of PDT on the oral microbiome has been poorly studied, and the available evidence suggests that PDT reduces clinical infections caused by drug-resistant Gram-positive and Gram-negative bacteria (Carpenter et al., 2015). For example, antimicrobial photodynamic therapy was applied to the treatment of peri-implantitis in a study by Dörtbudak et al (Magnet et al., 2001). This was also reported to be beneficial for the recovery of OLP patients treated with PDT to avoid complications due to postoperative infections (Cieplik et al., 2014; Kang et al., 2019). However, antimicrobial efficacy in the deeper layers of the oral biofilm remains controversial because bacteria embedded in the biofilm have a higher tolerance to antimicrobial agents (Mah and O'Toole, 2001; Marsh, 2004). Cosgarea et al. studied and analyzed OLP patients 28 days after PDT treatment, an analysis that included 18 oral microorganisms and found that the bacteria were not statistically different despite varying reductions in numbers (Cosgarea et al., 2020). More rigorous evidence for the effect of PDT on the oral microbiome is required, and that its antibacterial capacity is unlikely to be achieved by modifying the microbial makeup.
3.2 Photobiomodulation therapy
In 2014, low-level laser therapy evolved into photobiomodulation (PBM). PBM stimulates cytochrome c oxidase (CcO) in the mitochondrial respiratory chain, which generates adenosine triphosphate (ATP) and reduces tissue injury by momentarily elevating reactive oxygen species (ROS) in cellular organization (Kemper, 2018; Flores Luna et al., 2020). PBM is considered a novel approach to OLP treatment because of its ability to reduce pain, alleviate inflammation, and promote tissue repair in different pathological conditions (Nammour et al., 2021). Photobiomodulation is helpful at reducing pain and lesions in atrophic or erosive OLP, according to randomized, controlled, double-blind research, with no significant difference from topical steroid hormone use and showing good results at late follow-up (Ruiz Roca et al., 2022) (Table 5).
It is important to note that, like PDT, PBM is capable of causing some effects on microorganisms. Fukui et al. suggested that PBM irradiation may affect the metabolism and growth of Porphyromonas gingivalis, by the mechanism that visible and near-infrared light affects the bacterial cell cycle and growth mechanisms through major interactions with photosensitive molecules (Dadras et al., 2006). In addition, PBM may modulate the oral microbiome by improving salivary gland function, levels of interleukin-1 receptor antagonist, interleukin-10, and stimulate the immune system (Ailioaie and Litscher, 2021).
3.3 Laser treatment
Laser irradiation has been postulated to have an anti-inflammatory effect. It decreases the chemotaxis of polymorphic nuclei. and has a thermal effect, leading to microbial cell wall degradation, protein denaturation, and ultimately fungal cell death (Agha-Hosseini et al., 2012; Momeni et al., 2022). Potentially cancerous conditions are treated with CO2 laser technology (Flynn et al., 1988; Humphreys et al., 1998; Dong et al., 2019). The CO2 laser’s thermal impact carbonizes and vaporizes tissue, closing blood arteries and lymphatic vessels, destroying nerve endings, and sterilizing incisions.
The main laser treatments are neodymium-doped yttrium aluminum garnet (Nd : YAG) and Erbium-doped yttrium aluminum garnet (Er : YAG) laser (ERL) irradiation (de Pauli Paglioni et al., 2020). The Nd : YAG laser, a 1064 nm infrared laser, penetrates deeply into tissues, increases local blood flow, reduces pain quickly, and speeds up recovery (Kolli et al., 2021) (Table 5). Khater et al. treated EOLP patients with Nd : YAG laser three times a week for one month and found that it greatly reduced clinical symptoms without major side effects (Khater and Khattab, 2020). After irradiating Candida albicans and Streptococcus pyogenes strains in vitro using a Nd : YAG laser, Grezch-Lesniak discovered that Candida albicans and Streptococcus pyogenes counts declined and a substantial fall in bacterial metabolic levels was also observed (Grzech-Leśniak et al., 2019). As a result, in addition to its therapeutic purpose, the Nd : YAG laser also has potential for fungal control following topical hormone treatment in patients with OLP. Er : YAG-based high-intensity laser therapy (HILT) is a safe and effective method for removing OLP tissue (Fornaini et al., 2012; Broccoletti et al., 2015; Tarasenko et al., 2021). Er : YAG therapy in conjunction with 0.5% H2O2, 0.5% NaOCl, or 0.03% chlorhexidine effectively decreased the oral flora of Streptococcus gordonii, Clostridium nucleatum, and Porphyromonas gingivalis (Golob Deeb et al., 2019). The observation that the oral detection rate of P. gingivalis in OLP patients was substantially greater than in the healthy population (Ertugrul et al., 2013), this may imply that the mechanism of action of Er : YAG laser in the therapy of OLP involves a reduction in the microbial load.
3.4 Surgical excision
In general, EOLP lesions are difficult to treat and frequently recur. Surgical treatment may be an option for long-term, untreated lesions due to the increased risk of malignancy (Tarasenko et al., 2021). In a case report, the decision to excise the right buccal mucosal lesion was made after the use of local steroids, systemic steroids, hydroxychloroquine, and even intralesional steroids. Surprisingly, after 2 years of follow-up, recurrence was not detected (Hadiuzzaman et al., 2013). In addition, a subset of physicians have attempted to treat refractory EOLP with free palatal mucosa grafts (Kim et al., 2018). However, the efficacy and treatment criteria for this treatment modality have not been clearly established (Yan et al., 2017). Surgical excision looks to be more complete than conservative treatments and reduce lesion recurrence, although recurrence rate comparisons require larger samples and longer-term follow-up. Second, surgery is more costly, has limited therapeutic value, and is only suitable for those with limited and persistent erosions and lesions in risk areas with some cancer risk (Table 5).
3.5 Psychological intervention
Modern culture and medicine have made psychological factors more critical in many conditions including OLP (Hampf et al., 1987). There is growing evidence that mood disturbances is an important factor in the onset and amplification of OLP, especially in patients suffering from depression, anxiety, and acute stress (Song et al., 2021). Paranoia, anxiety, and depression were more pronounced in the reticular and erosive OLP group compared to the control group (Ivanovski et al., 2005). Depressive symptoms were seen in 41.66% of OLP patients and 22.91% of control patients in a clinical observation involving 48 people (Alves et al., 2015). In addition, patients with more severe lesions tend to have more pronounced anxiety (Zucoloto et al., 2019). Gabriella et al. found a significant effect of different personality types on the outcome of OLP patients, suggesting that psychological factors of personality play an integral role in OLP pathogenesis (Gabriella et al., 2021). Another study used the GAD-7 and PHQ-9 to compare moods in OLP patients and healthy people. The GAD-7 and PHQ-9 scores of OLP patients and controls differed significantly, suggesting that mental illnesses may be linked to OLP (Wiriyakijja et al., 2020). The aforementioned evidence suggests that doctors should pay attention to psychological issues in OLP patients to increase effectiveness and tailor pharmacological and psychological treatment approaches (Table 5).
3.6 Initial periodontal treatment
Chronic periodontitis (CP), the most common reason for adult tooth loss, is a chronic inflammatory disease that is primarily caused by bacteria and driven by the host immune system (Rai et al., 2016). Significant risk factors for OLP progression include dietary habits, tension, and poor oral hygiene; therefore, it is necessary to investigate the relationship between periodontal health and OLP (Xue et al., 2005). In previous studies, there was a complex interaction between OLP and CP. Hu et al. used the RAE scoring system to evaluate intraoral lesions in OLP patients with CP and the average gingival index (GI-Avg) to evaluate primary gingival inflammation and found a mutually reinforcing effect between OLP and CP (Wu et al., 2022). ZHAO et al. discovered that patients with EOLP exhibited significant improvement in OLP lesions and symptoms following one month of periodontal treatment, indicating that periodontal treatment is clinically significant for EOLP (Zhao, 2018). A meta-analysis revealed that the clinical indicators of bleeding on probing and probing depth were substantially linked with OLP, which may be owing to the patient’s incapacity to perform appropriate oral hygiene maintenance due to the uncomfortable sensation at the site of the lesion (Sanadi et al., 2023). The initial periodontal treatment in treatment of EOLP is promising. When treating OLP, particularly EOLP, clinicians should also take the patient’s periodontal health into account (Table 5).
Besides, smoking, diabetes, and obesity are among the many frequent systemic risk factors for periodontal disease and smoking appears to be able to interrelate with OLP alone, in addition to affecting OLP through periodontitis (Genco and Borgnakke, 2013). Smoking enhances the expression of TLR-34 and CD34 in OLP lesions, which can promote an inflammatory response. It may also lead to increased microvascularization in OLP lesions, which exacerbates cancerous tendencies (Kłosek et al., 2011; Amin et al., 2020). Furthermore, Alrashdan et al. discovered that smoking lowers macrophage expression in OLL, which may change immune surveillance and malignant transformation pathways (Alrashdan et al., 2016a). A meta-analysis of OLP patient data revealed that smokers had a significantly greater rate of malignant transformation than nonsmokers (Aghbari et al., 2017). It is suggested that we should not only focus on the patient’s disease state but also monitor and manage the series of behaviors of clinical patients that are not conducive to disease recovery.
4 Summary and outlook
OLP is a chronic inflammatory disease characterized by relapses and delayed healing, and thus has a prolonged detrimental effect on patients` quality of life. For asymptomatic reticular OLP, routine observation and testing are usually adequate. Low, brief doses of systemic steroids taken orally may be used to treat acute exacerbations or persistent, considerably deteriorating OLP lesions, but prolonged use is to be avoided. Topical calcium phosphatase inhibitors are the second most affordable and efficient form of therapy after topical corticosteroids, according to a comparison of the financial benefits of various therapeutic approaches. PDT sessions are the most expensive, despite evidence that they are more effective and have fewer side effects than other treatment methods (Sandhu et al., 2022). Although JAK inhibitors are less commonly used in OLP, there is a potential that they may be effective therapeutic agents for OLP, especially when conventional drugs are ineffective. If conservative treatments are not effective, laser and surgical treatments are also available, depending on the lesions and the patient’s wishes.
Most of the evidence regarding the involvement of microorganisms in the pathogenesis of OLP has not been conclusively demonstrated. More studies are required to unveil the interactions between the oral microbiome (bacteria, host metabolites, and microecological environment) and OLP (Berg et al., 2020). In addition to this, there is a need to explore the efficacy of oral probiotics as well as the mode of administration, which usually requires more and longer-term research observations.
The main goal of clinical OLP treatment is to lessen the severity of local inflammation while reducing disease recurrence and preventing malignant transformation. Owing to the risk of malignancy in EOLP, timely treatment plan adjustments, frequent medical follow-up, and a biopsy if needed are required. In addition to conventional medications and physical therapy, treatment strategies should also take into account the patient’s psychological characteristics, oral hygiene, systemic underlying diseases, smoking and alcohol abuse, and other adverse habits to obtain a more favorable therapeutic outcome (Table 5). Overall, a multi-faceted, multi-dimensional approach to the treatment of EOLP may be the only solution that can truly reduce disease recurrence and even restore immune homeostasis and cure the disease.
Author contributions
TW: Investigation, Project administration, Writing – original draft, Writing – review & editing. YB: Investigation, Writing – original draft, Writing – review & editing. YJ: Investigation, Writing – review & editing. FC: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Joint project of Chongqing Health Commission and Science and Technology Bureau (2020MSXM005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abduelmula, A., Bagit, A., Mufti, A., Yeung, K. C. Y., Yeung, J. (2023). The use of janus kinase inhibitors for lichen planus: an evidence-based review. J. Cutan Med. Surg. 27 (3), 271–276. doi: 10.1177/12034754231156100
Adamo, D., Ruoppo, E., Leuci, S., Aria, M., Amato, M., Mignogna, M. D. (2015). Sleep disturbances, anxiety and depression in patients with oral lichen planus: a case-control study. J. Eur. Acad. Dermatol. Venereol. 29 (2), 291–297. doi: 10.1111/jdv.12525
Agha-Hosseini, F., Moslemi, E., Mirzaii-Dizgah, I. (2012). Comparative evaluation of low-level laser and CO2 laser in treatment of patients with oral lichen planus. Int. J. Oral. Maxillofac. Surg. 41 (10), 1265–1269. doi: 10.1016/j.ijom.2012.06.001
Aghbari, S. M. H., Abushouk, A. I., Attia, A., Elmaraezy, A., Menshawy, A., Ahmed, M. S., et al. (2017). Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20095 patient data. Oral. Oncol. 68, 92–102. doi: 10.1016/j.oraloncology.2017.03.012
Ailioaie, L. M., Litscher, G. (2021). Probiotics, photobiomodulation, and disease management: controversies and challenges. Int. J. Mol. Sci. 22 (9), 4942. doi: 10.3390/ijms22094942
Al-Hallak, N., Hamadah, O., Mouhamad, M., Kujan, O. (2023). Efficacy of injectable platelet-rich fibrin in the treatment of symptomatic oral lichen planus. Oral. Dis. 29 (5), 2256–2264. doi: 10.1111/odi.14261
Alrashdan, M. S., Angel, C., Cirillo, N., McCullough, M. (2016a). Smoking habits and clinical patterns can alter the inflammatory infiltrate in oral lichenoid lesions. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 121 (1), 49–57. doi: 10.1016/j.oooo.2015.08.020
Alrashdan, M. S., Cirillo, N., McCullough, M. (2016b). Oral lichen planus: a literature review and update. Arch. Dermatol. Res. 308 (8), 539–551. doi: 10.1007/s00403-016-1667-2
Alves, M. G., do Carmo Carvalho, B. F., Balducci, I., Cabral, L. A., Nicodemo, D., Almeida, J. D. (2015). Emotional assessment of patients with oral lichen planus. Int. J. Dermatol. 54 (1), 29–32. doi: 10.1111/ijd.12052
Amin, N. R., Yussif, N., Ahmed, E. (2020). The effect of smoking on clinical presentation and expression of TLR-2 and CD34 in Oral lichen Planus patients: clinical and immunohistochemical study. BMC Oral. Health 20 (1), 129. doi: 10.1186/s12903-020-01118-2
Amirchaghmaghi, M., Hashemy, S. I., Alirezaei, B., Jahed Keyhani, F., Kargozar, S., Vasigh, S., et al. (2016). Evaluation of plasma isoprostane in patients with oral lichen planus. J. Dent. (Shiraz). 17 (1), 21–25.
An, N., Chen, Y., Wang, C., Yang, C., Wu, Z. H., Xue, J., et al. (2017). Chloroquine autophagic inhibition rebalances Th17/Treg-mediated immunity and ameliorates systemic lupus erythematosus. Cell Physiol. Biochem. 44 (1), 412–422. doi: 10.1159/000484955
Arbouzova, N. I., Zeidler, M. P. (2006). JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development. 133 (14), 2605–2616. doi: 10.1242/dev.02411
Ashraf, R., Shah, N. P. (2014). Immune system stimulation by probiotic microorganisms. Crit. Rev. Food Sci. Nutr. 54 (7), 938–956. doi: 10.1080/10408398.2011.619671
Ayyar, V. S., Jusko, W. J. (2020). Transitioning from basic toward systems pharmacodynamic models: lessons from corticosteroids. Pharmacol. Rev. 72 (2), 414–438. doi: 10.1124/pr.119.018101
Balestri, R., Bortolotti, R., Rech, G., Girardelli, C. R., Zorzi, M. G., Magnano, M. (2022). Treatment of oral erosive lichen planus with upadacitinib. JAMA Dermatol. 158 (4), 457–458. doi: 10.1001/jamadermatol.2022.0147
Bao, J., Chen, C., Yan, J., Wen, Y., Bian, J., Xu, M., et al. (2022). Antioxidant therapy for patients with oral lichen planus: A systematic review and meta-analysis. Front. Pharmacol. 13, 1030893. doi: 10.3389/fphar.2022.1030893
Bennardo, F., Bennardo, L., Del Duca, E., Patruno, C., Fortunato, L., Giudice, A., et al. (2020). Autologous platelet-rich fibrin injections in the management of facial cutaneous sinus tracts secondary to medication-related osteonecrosis of the jaw. Dermatol. Ther. 33 (3), e13334. doi: 10.1111/dth.13334
Bennardo, F., Liborio, F., Barone, S., Antonelli, A., Buffone, C., Fortunato, L., et al. (2021). Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: a pilot study. Clin. Oral. Investig. 25 (6), 3747–3755. doi: 10.1007/s00784-020-03702-w
Berg, G., Rybakova, D., Fischer, D., Cernava, T., Vergès, M. C., Charles, T., et al. (2020). Microbiome definition re-visited: old concepts and new challenges. Microbiome. 8 (1), 103.
Bhanja, D. B., Sil, A., Chandra, A., Biswas, S. K. (2021). Addisonian-like acrofacial hyperpigmentation following long-term hydroxychloroquine therapy in oral lichen planus. BMJ Case Rep. 14 (1), e240727. doi: 10.1136/bcr-2020-240727
Bhattacharya, P. T., Misra, S. R., Hussain, M. (2016). Nutritional aspects of essential trace elements in oral health and disease: an extensive review. Scientifica (Cairo). 2016, 5464373. doi: 10.1155/2016/5464373
Bolanča, Ž, Goren, A., Getaldić-Švarc, B., Vučić, M., Šitum, M. (2016). Platelet-rich plasma as a novel treatment for lichen planopillaris. Dermatol. Ther. 29 (4), 233–235. doi: 10.1111/dth.12343
Brennan, M. T., Madsen, L. S., Saunders, D. P., Napenas, J. J., McCreary, C., Ni Riordain, R., et al. (2022). Efficacy and safety of a novel mucoadhesive clobetasol patch for treatment of erosive oral lichen planus: A phase 2 randomized clinical trial. J. Oral. Pathol. Med. 51 (1), 86–97. doi: 10.1111/jop.13270
Broccoletti, R., Cafaro, A., Gambino, A., Romagnoli, E., Arduino, P. G. (2015). Er:YAG laser versus cold knife excision in the treatment of nondysplastic oral lesions: A randomized comparative study for the postoperative period. Photomed Laser Surg. 33 (12), 604–609. doi: 10.1089/pho.2015.3967
Broen, J. C. A., van Laar, J. M. (2020). Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat. Rev. Rheumatol. 16 (3), 167–178. doi: 10.1038/s41584-020-0374-8
Burmester, G. R., Kremer, J. M., Van den Bosch, F., Kivitz, A., Bessette, L., Li, Y., et al. (2018). Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 391 (10139), 2503–2512. doi: 10.1016/S0140-6736(18)31115-2
Carpenter, B. L., Situ, X., Scholle, F., Bartelmess, J., Weare, W. W., Ghiladi, R. A. (2015). Antiviral, antifungal and antibacterial activities of a BODIPY-based photosensitizer. Molecules. 20 (6), 10604–10621. doi: 10.3390/molecules200610604
Carrozzo, M., Porter, S., Mercadante, V., Fedele, S. (2019). Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontol 2000. 80 (1), 105–125. doi: 10.1111/prd.12260
Casabona, F., Priano, V., Vallerino, V., Cogliandro, A., Lavagnino, G. (2010). New surgical approach to lichen sclerosus of the vulva: the role of adipose-derived mesenchymal cells and platelet-rich plasma in tissue regeneration. Plast. Reconstr Surg. 126 (4), 210e–211e. doi: 10.1097/PRS.0b013e3181ea9386
Chainani-Wu, N., Collins, K., Silverman, S., Jr. (2012a). Use of curcuminoids in a cohort of patients with oral lichen planus, an autoimmune disease. Phytomedicine. 19 (5), 418–423. doi: 10.1016/j.phymed.2011.11.005
Chainani-Wu, N., Madden, E., Lozada-Nur, F., Silverman, S., Jr. (2012b). High-dose curcuminoids are efficacious in the reduction in symptoms and signs of oral lichen planus. J. Am. Acad. Dermatol. 66 (5), 752–760. doi: 10.1016/j.jaad.2011.04.022
Chang, J. Y., Chiang, C. P., Hsiao, C. K., Sun, A. (2009). Significantly higher frequencies of presence of serum autoantibodies in Chinese patients with oral lichen planus. J. Oral. Pathol. Med. 38 (1), 48–54. doi: 10.1111/j.1600-0714.2008.00686.x
Choukroun, J., Miron, R. J. (2017). “Platelet rich fibrin: A second-generation platelet concentrate” in Platelet Rich Fibrin in Regenerative Dentistry: Biological Background and Clinical Indications. Eds. Miron, R. J., Choukroun, J.. doi: 10.1002/9781119406792.ch1
Cieplik, F., Tabenski, L., Buchalla, W., Maisch, T. (2014). Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 5, 405. doi: 10.3389/fmicb.2014.00405
Claman, H. N. (1975). How corticosteroids work. J. Allergy Clin. Immunol. 55 (3), 145–151. doi: 10.1016/0091-6749(75)90010-X
Cosgarea, R., Pollmann, R., Sharif, J., Schmidt, T., Stein, R., Bodea, A., et al. (2020). Photodynamic therapy in oral lichen planus: A prospective case-controlled pilot study. Sci. Rep. 10 (1), 1667.
Dadras, S., Mohajerani, E., Eftekhar, F., Hosseini, M. (2006). Different photoresponses of Staphylococcus aureus and Pseudomonas aeruginosa to 514, 532, and 633 nm low level lasers in vitro. Curr. Microbiol. 53 (4), 282–286. doi: 10.1007/s00284-005-0490-3
de Pauli Paglioni, M., Migliorati, C. A., Schausltz Pereira Faustino, I., Linhares Almeida Mariz, B. A., Oliveira Corrêa Roza, A. L., Agustin Vargas, P., et al. (2020). Laser excision of oral leukoplakia: Does it affect recurrence and Malignant transformation? A systematic review and meta-analysis. Oral. Oncol. 109, 104850. doi: 10.1016/j.oraloncology.2020.104850
Didona, D., Caposiena Caro, R. D., Sequeira Santos, A. M., Solimani, F., Hertl, M. (2022). Therapeutic strategies for oral lichen planus: State of the art and new insights. Front. Med. (Lausanne). 9, 997190. doi: 10.3389/fmed.2022.997190
Didona, D., Hertl, M. (2022). Detection of anti-desmoglein antibodies in oral lichen planus: What do we know so far. Front. Immunol. 13, 1001970. doi: 10.3389/fimmu.2022.1001970
Ding, Z. Y., Tan, Y., Peng, Q., Zuo, J., Li, N. (2021). Novel applications of platelet concentrates in tissue regeneration (Review). Exp. Ther. Med. 21 (3), 226. doi: 10.3892/etm.2021.9657
Dohan, D. M., Choukroun, J., Diss, A., Dohan, S. L., Dohan, A. J., Mouhyi, J., et al. (2006). Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 101 (3), e37–e44.
Dong, Y., Chen, Y., Tao, Y., Hao, Y., Jiang, L., Dan, H., et al. (2019). Malignant transformation of oral leukoplakia treated with carbon dioxide laser: a meta-analysis. Lasers Med. Sci. 34 (1), 209–221. doi: 10.1007/s10103-018-2674-7
Duan, C. (2015). Observation of Buzhong Yiqi Decoction combined with western medicine in erosive oral lichen planus. Modern J. Integrated Traditional Chin. Western Med. 24, 4020–4022.
Einarsdottir, M. J., Bankvall, M., Robledo-Sierra, J., Rödström, P. O., Bergthorsdottir, R., Trimpou, P., et al. (2023). Topical clobetasol treatment for oral lichen planus can cause adrenal insufficiency. Oral. Dis. 1–9.
Eisen, D. (1993). Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: An open trial. J. Am. Acad. Dermatol. 28 (4), 609–612. doi: 10.1016/0190-9622(93)70082-5
Eisen, D., Carrozzo, M., Bagan Sebastian, J. V., Thongprasom, K. (2005). Number V Oral lichen planus: clinical features and management. Oral. Dis. 11 (6), 338–349. doi: 10.1111/j.1601-0825.2005.01142.x
Ertugrul, A. S., Arslan, U., Dursun, R., Hakki, S. S. (2013). Periodontopathogen profile of healthy and oral lichen planus patients with gingivitis or periodontitis. Int. J. Oral. Sci. 5 (2), 92–97. doi: 10.1038/ijos.2013.30
Escapa, I. F., Chen, T., Huang, Y., Gajare, P., Dewhirst, F. E., Lemon, K. P. (2018). New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): a resource for the microbiome of the human aerodigestive tract. mSystems. 3 (6), e00187–e00118. doi: 10.1128/mSystems.00187-18
Fatemi Naeini, F., Mohaghegh, F., Jelvan, M., Asilian, A., Saber, M. (2020). Cyclosporine or methotrexate, which one is more promising in the treatment of lichen planopilaris?; A comparative clinical trial. Int. Immunopharmacol. 86, 106765. doi: 10.1016/j.intimp.2020.106765
Feng, Z., Zhang, B. Q., Zhu, Y. M., Yu, B. B., Fu, L., Zhou, L. L., et al. (2019). The effectiveness and safety of total glucosides of paeony in primary Sjögren's syndrome: A systematic review and meta-analysis. Front. Pharmacol. 10, 550. doi: 10.3389/fphar.2019.00550
Fitzpatrick, S. G., Hirsch, S. A., Gordon, S. C. (2014). The Malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J. Am. Dent. Assoc. 145 (1), 45–56. doi: 10.14219/jada.2013.10
Flier, J., Boorsma, D. M., van Beek, P. J., Nieboer, C., Stoof, T. J., Willemze, R., et al. (2001). Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J. Pathol. 194 (4), 398–405. doi: 10.1002/1096-9896(200108)194:4<397::AID-PATH899>3.0.CO;2-S
Flores Luna, G. L., de Andrade, A. L. M., Brassolatti, P., Bossini, P. S., Anibal, F. F., Parizotto, N. A., et al. (2020). Biphasic dose/response of photobiomodulation therapy on culture of human fibroblasts. Photobiomodul Photomed Laser Surg. 38 (7), 413–418.
Flynn, M. B., White, M., Tabah, R. J. (1988). Use of carbon dioxide laser for the treatment of premalignant lesions of the oral mucosa. J. Surg. Oncol. 37 (4), 232–234. doi: 10.1002/jso.2930370404
Fornaini, C., Raybaud, H., Augros, C., Rocca, J. P. (2012). New clinical approach for use of Er:YAG laser in the surgical treatment of oral lichen planus: a report of two cases. Photomed Laser Surg. 30 (4), 234–238. doi: 10.1089/pho.2011.3116
Gabriella, D., Klemens, R., Xiao-Hui, R. F., Corinna, B., Eva, H. (2021). Effect of personality traits on the oral health-related quality of life in patients with oral lichen planus undergoing treatment. Clin. Oral. Investig. 25 (4), 2381–2389. doi: 10.1007/s00784-020-03561-5
Genco, R. J., Borgnakke, W. S. (2013). Risk factors for periodontal disease. Periodontol 2000. 62 (1), 59–94. doi: 10.1111/j.1600-0757.2012.00457.x
Genovese, M. C., van Vollenhoven, R. F., Pacheco-Tena, C., Zhang, Y., Kinnman, N. (2016). VX-509 (Decernotinib), an oral selective JAK-3 inhibitor, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheumatol. 68 (1), 46–55. doi: 10.1002/art.39473
Ghahremanlo, A., Boroumand, N., Ghazvini, K., Hashemy, S. I. (2019). Herbal medicine in oral lichen planus. Phytother. Res. 33 (2), 288–293. doi: 10.1002/ptr.6236
Gholizadeh, N., Sheykhbahaei, N. (2021). Micronutrients profile in oral lichen planus: a review literature. Biol. Trace Elem Res. 199 (3), 912–924. doi: 10.1007/s12011-020-02221-9
Giuliani, M., Troiano, G., Cordaro, M., Corsalini, M., Gioco, G., Lo Muzio, L., et al. (2019). Rate of Malignant transformation of oral lichen planus: A systematic review. Oral. Dis. 25 (3), 693–709. doi: 10.1111/odi.12885
Golob Deeb, J., Smith, J., Belvin, B. R., Lewis, J., Grzech-Leśniak, K. (2019). Er:YAG laser irradiation reduces microbial viability when used in combination with irrigation with sodium hypochlorite, chlorhexidine, and hydrogen peroxide. Microorganisms. 7 (12), 612.
Gooderham, M. J., Forman, S. B., Bissonnette, R., Beebe, J. S., Zhang, W., Banfield, C., et al. (2019). Efficacy and safety of oral janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: A phase 2 randomized clinical trial. JAMA Dermatol. 155 (12), 1371–1379. doi: 10.1001/jamadermatol.2019.2855
Grzech-Leśniak, K., Nowicka, J., Pajączkowska, M., Matys, J., Szymonowicz, M., Kuropka, P., et al. (2019). Effects of Nd:YAG laser irradiation on the growth of Candida albicans and Streptococcus mutans: in vitro study. Lasers Med. Sci. 34 (1), 129–137. doi: 10.1007/s10103-018-2622-6
Guada, M., Beloqui, A., Kumar, M. R., Préat, V., del Carmen Dios-Viéitez, M., Blanco-Prieto, M. J. (2016). Reformulating cyclosporine A (CsA): More than just a life cycle management strategy. J. Controlled Release. 225, 269–282. doi: 10.1016/j.jconrel.2016.01.056
Gunasekaran, S., Sakthivel, S., Babu, G., Vijayan, V. (2021). Clinical application of platelet-rich fibrin in pediatric dentistry. J. Health Allied Sci. NU. 12 (02), 186–190.
Guo, Y., Crnkovic, C. M., Won, K. J., Yang, X., Lee, J. R., Orjala, J., et al. (2019). Commensal gut bacteria convert the immunosuppressant tacrolimus to less potent metabolites. Drug Metab. Dispos. 47 (3), 194–202. doi: 10.1124/dmd.118.084772
Gupta, A., Mohan, R. P., Gupta, S., Malik, S. S., Goel, S., Kamarthi, N. (2017). Roles of serum uric acid, prolactin levels, and psychosocial factors in oral lichen planus. J. Oral. Sci. 59 (1), 139–146. doi: 10.2334/josnusd.16-0219
Haan, C., Heinrich, P. C., Behrmann, I. (2002). Structural requirements of the interleukin-6 signal transducer gp130 for its interaction with Janus kinase 1: the receptor is crucial for kinase activation. Biochem. J. 361 (Pt 1), 105–111. doi: 10.1042/bj3610105
Hadiuzzaman, M., Rahman, M. H., Ansari, N. P. (2013). A case of recalcitrant oral lichen planus. J. Dentistry Oral. Hygiene. 5 (1), 1–3.
Hampf, B. G., Malmström, M. J., Aalberg, V. A., Hannula, J. A., Vikkula, J. (1987). Psychiatric disturbance in patients with oral lichen planus. Oral. Surg. Oral. Med. Oral. Pathol. 63 (4), 429–432. doi: 10.1016/0030-4220(87)90254-4
Hashem, A. S., Issrani, R., Elsayed, T. E. E., Prabhu, N. (2019). Topical hyaluronic acid in the management of oral lichen planus: A comparative study. J. Investig. Clin. Dent. 10 (2), e12385. doi: 10.1111/jicd.12385
He, Y., Deng, J., Zhao, Y., Tao, H., Dan, H., Xu, H., et al. (2020). Efficacy evaluation of photodynamic therapy for oral lichen planus: a systematic review and meta-analysis. BMC Oral. Health 20 (1), 302. doi: 10.1186/s12903-020-01260-x
Hesse, J., Schmalfuss, A., Kvaal, S. I. (2020). Photodynamic therapy of oral lichen planus. Photochem. Photobiol. Sci. 19 (10), 1271–1279. doi: 10.1039/d0pp00249f
Hodge, J. A., Kawabata, T. T., Krishnaswami, S., Clark, J. D., Telliez, J. B., Dowty, M. E., et al. (2016). The mechanism of action of tofacitinib - an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. Clin. Exp. Rheumatol. 34 (2), 318–328.
Hodgens, A., Sharman, T. (2023). “Corticosteroids,” in StatPearls [Internet]. (Treasure Island (FL): StatPearls Publishing).
Horz, H. P., Meinelt, A., Houben, B., Conrads, G. (2007). Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral. Microbiol. Immunol. 22 (2), 126–130. doi: 10.1111/j.1399-302X.2007.00334.x
Hu, Y., Li, Z., Chen, G., Li, Z., Huang, J., Huang, H., et al. (2022). Hydroxychloroquine alleviates EAU by inhibiting uveitogenic T cells and ameliorating retinal vascular endothelial cells dysfunction. Front. Immunol. 13, 859260. doi: 10.3389/fimmu.2022.859260
Humphreys, T. R., Malhotra, R., Scharf, M. J., Marcus, S. M., Starkus, L., Calegari, K. (1998). Treatment of superficial basal cell carcinoma and squamous cell carcinoma in situ with a high-energy pulsed carbon dioxide laser. Arch. Dermatol. 134 (10), 1247–1252. doi: 10.1001/archderm.134.10.1247
Ibrahim, S. S., Ragy, N. I., Nagy, N. A., El-Kammar, H., Elbakry, A. M., Ezzatt, O. M. (2023). Evaluation of muco-adhesive tacrolimus patch on caspase-3 induced apoptosis in oral lichen planus: a randomized clinical trial. BMC Oral. Health 23 (1), 99. doi: 10.1186/s12903-023-02803-8
Itin, P., Surber, C., Büchner, S. (1992). Lack of effect after local treatment with a new ciclosporin formulation in recalcitrant erosive oral lichen planus. Dermatology. 185 (4), 262–265. doi: 10.1159/000247464
Ivanovski, K., Nakova, M., Warburton, G., Pesevska, S., Filipovska, A., Nares, S., et al. (2005). Psychological profile in oral lichen planus. J. Clin. Periodontol. 32 (10), 1034–1040. doi: 10.1111/j.1600-051X.2005.00829.x
Jajarm, H. H., Falaki, F., Sanatkhani, M., Ahmadzadeh, M., Ahrari, F., Shafaee, H. (2015). A comparative study of toluidine blue-mediated photodynamic therapy versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus: a randomized clinical controlled trial. Lasers Med. Sci. 30 (5), 1475–1480. doi: 10.1007/s10103-014-1694-1
Jiang, H., Li, J., Wang, L., Wang, S., Nie, X., Chen, Y., et al. (2020). Total glucosides of paeony: A review of its phytochemistry, role in autoimmune diseases, and mechanisms of action. J. Ethnopharmacol. 258, 112913. doi: 10.1016/j.jep.2020.112913
Jung, W., Jang, S. (2022). Oral microbiome research on oral lichen planus: current findings and perspectives. Biol. (Basel). 11 (5), 723. doi: 10.3390/biology11050723
Jungell, P., Malmstrom, M. (1996). Cyclosporin A mouthwash in the treatment of oral lichen planus. Int. J. Oral. Maxillofac. Surg. 25 (1), 60–62. doi: 10.1016/S0901-5027(96)80014-2
Kang, S. M., Jung, H. I., Kim, B. I. (2019). Susceptibility of oral bacteria to antibacterial photodynamic therapy. J. Oral. Microbiol. 11 (1), 1644111. doi: 10.1117/12.2507675
Keller, M. K., Kragelund, C. (2018). Randomized pilot study on probiotic effects on recurrent candidiasis in oral lichen planus patients. Oral. Dis. 24 (6), 1107–1114. doi: 10.1111/odi.12858
Kemper, K. J. (2018). "Let there be light." Research on phototherapy, light therapy, and photobiomodulation for healing - Alternative therapy becomes mainstream. Complement Ther. Med. 41, A1–a6.
Keshavarzi, F. (2016). Fungistatic effect of hydroxychloroquine, lessons from a case. Med. Mycol Case Rep. 13, 17–18. doi: 10.1016/j.mmcr.2016.09.003
Khater, M. M., Khattab, F. M. (2020). Efficacy of 1064 Q switched Nd:YAG laser in the treatment of oral lichen planus. J. Dermatolog Treat. 31 (6), 655–659. doi: 10.1080/09546634.2019.1638881
Kim, D. H., Lim, B. R., Na, G. H., Kim, E. J., Park, K. (2018). Surgical excision and mucosal advancement flap: Treatment for refractory lichen planus of the lip. Dermatol. Ther. 31 (5), e12695. doi: 10.1111/dth.12695
Kłosek, S. K., Sporny, S., Stasikowska-Kanicka, O., Kurnatowska, A. J. (2011). Cigarette smoking induces overexpression of c-Met receptor in microvessels of oral lichen planus. Arch. Med. Sci. 7 (4), 706–712.
Kolli, H., Evers, C., Murray, P. I. (2021). Nd:YAG laser posterior capsulotomy in adult patients with uveitis. Ocul Immunol. Inflamm. 29 (7-8), 1537–1539.
Kooybaran, N. R., Petzold, G., Ströbel, P., Schön, M. P., Mössner, R. (2021). Alleviation of erosive oral and esophageal lichen planus by the JAK1 inhibitor upadacitinib. J. Dtsch Dermatol. Ges. 19 (12), 1778–1780.
Kragelund, C., Keller, M. K. (2019). The oral microbiome in oral lichen planus during a 1-year randomized clinical trial. Oral. Dis. 25 (1), 327–338.
Ku, J.-K., Park, S.-Y., Hwang, K.-G., Yun, P.-Y. (2021). The effect of mouthrinse with 0.05% dexamethasone solution on the oral bacterial community of oral lichen planus patients: Prospective pilot study. Appl. Sci. 11 (14), 6286.
Kujundzic, B., Zeljic, K., Supic, G., Magic, M., Stanimirovic, D., Ilic, V., et al. (2016). Association of vdr, cyp27b1, cyp24a1 and mthfr gene polymorphisms with oral lichen planus risk. Clin. Oral. Investig. 20 (4), 781–789.
Lamey, P. J., Gibson, J., Barclay, S. C., Miller, S. (1990). Grinspan's syndrome: a drug-induced phenomenon? Oral. Surg. Oral. Med. Oral. Pathol. 70 (2), 184–185. doi: 10.1016/0030-4220(90)90116-A
Lanese, D. M., Conger, J. D. (1993). Effects of endothelin receptor antagonist on cyclosporine-induced vasoconstriction in isolated rat renal arterioles. J. Clin. Invest. 91 (5), 2144–2149. doi: 10.1172/JCI116440
Leonard, W. J., O'Shea, J. J. (1998). Jaks and STATs: biological implications. Annu. Rev. Immunol. 16 (1), 293–322. doi: 10.1146/annurev.immunol.16.1.293
Li, S., Chen, A. J., Fang, S., Li, H. (2014). Successful treatment of necrobiotic xanthogranuloma with total glucosides of paeony. Dermatol. Ther. 27 (5), 304–306. doi: 10.1111/dth.12133
Li, Q., Pan, S., Dangaria, S. J., Gopinathan, G., Kolokythas, A., Chu, S., et al. (2013). Platelet-rich fibrin promotes periodontal regeneration and enhances alveolar bone augmentation. BioMed. Res. Int. 2013, 638043. doi: 10.1155/2013/638043
Li, C., Tang, X., Zheng, X., Ge, S., Wen, H., Lin, X., et al. (2020). Global prevalence and incidence estimates of oral lichen planus: A systematic review and meta-analysis. JAMA Dermatol. 156 (2), 172–181. doi: 10.1001/jamadermatol.2019.3797
Lin, H. P., Wang, Y. P., Chia, J. S., Chiang, C. P., Sun, A. (2011). Modulation of serum gastric parietal cell antibody level by levamisole and vitamin B12 in oral lichen planus. Oral. Dis. 17 (1), 95–101. doi: 10.1111/j.1601-0825.2010.01711.x
Lu, W. W., Fu, T. X., Wang, Q., Chen, Y. L., Li, T. Y., Wu, G. L. (2020). The effect of total glucoside of paeony on gut microbiota in NOD mice with Sjögren's syndrome based on high-throughput sequencing of 16SrRNA gene. Chin. Med. 15, 61. doi: 10.1186/s13020-020-00342-w
Luo, J., Song, W. J., Xu, Y., Chen, G. Y., Hu, Q., Tao, Q. W. (2019). Benefits and safety of tripterygium glycosides and total glucosides of paeony for rheumatoid arthritis: an overview of systematic reviews. Chin. J. Integr. Med. 25 (9), 696–703. doi: 10.1007/s11655-019-3221-5
Maggini, S., Wintergerst, E. S., Beveridge, S., Hornig, D. H. (2007). Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 98 Suppl 1, S29–S35. doi: 10.1017/S0007114507832971
Magnet, S., Courvalin, P., Lambert, T. (2001). Resistance-nodulation-cell division-type efflux pump involved in aminoglycoside resistance in Acinetobacter baumannii strain BM4454. Antimicrob. Agents Chemother. 45 (12), 3375–3380. doi: 10.1128/AAC.45.12.3375-3380.2001
Mah, T. F., O'Toole, G. A. (2001). Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9 (1), 34–39. doi: 10.1016/S0966-842X(00)01913-2
Marable, D. R., Bowers, L. M., Stout, T. L., Stewart, C. M., Berg, K. M., Sankar, V., et al. (2016). Oral candidiasis following steroid therapy for oral lichen planus. Oral. Dis. 22 (2), 140–147. doi: 10.1111/odi.12399
Marlina, E., Goodman, R. N., Mercadante, V., Shephard, M., McMillan, R., Hodgson, T., et al. (2022). A proof of concept pilot trial of probiotics in symptomatic oral lichen planus (CABRIO). Oral. Dis. 28 (8), 2155–2167. doi: 10.1111/odi.14014
Marsh, P. D. (2004). Dental plaque as a microbial biofilm. Caries Res. 38 (3), 204–211. doi: 10.1159/000077756
Maukonen, J., Mättö, J., Suihko, M. L., Saarela, M. (2008). Intra-individual diversity and similarity of salivary and faecal microbiota. J. Med. Microbiol. 57 (Pt 12), 1560–1568. doi: 10.1099/jmm.0.47352-0
Migone, T. S., Rodig, S., Cacalano, N. A., Berg, M., Schreiber, R. D., Leonard, W. J. (1998). Functional cooperation of the interleukin-2 receptor beta chain and Jak1 in phosphatidylinositol 3-kinase recruitment and phosphorylation. Mol. Cell Biol. 18 (11), 6416–6422. doi: 10.1128/MCB.18.11.6416
Mijiritsky, E., Assaf, H. D., Peleg, O., Shacham, M., Cerroni, L., Mangani, L. (2021). Use of PRP, PRF and CGF in periodontal regeneration and facial rejuvenation-A narrative review. Biology (Basel) 10 (4), 317. doi: 10.3390/biology10040317
Momeni, E., Didehdar, M., Sarlak, E., Safari, M. (2022). In vitro effect of a high-intensity laser on candida albicans colony count. J. Lasers Med. Sci. 13, e59. doi: 10.34172/jlms.2022.59
Mostafa, D., Moussa, E., Alnouaem, M. (2017). Evaluation of photodynamic therapy in treatment of oral erosive lichen planus in comparison with topically applied corticosteroids. Photodiagnosis Photodyn. Ther. 19, 56–66. doi: 10.1016/j.pdpdt.2017.04.014
Moussa, A., Colla, T., Morrison, B., Sinclair, R. (2022). Effective treatment of oral lichen planus with the JAK inhibitor baricitinib. Australas. J. Dermatol. 63 (2), 276–277. doi: 10.1111/ajd.13811
Nammour, S., El Mobadder, M., Brugnera, A. J., Namour, M., Houeis, S., Heysselaer, D., et al. (2021). Photobiomodulation Therapy vs. Corticosteroid for the Management of Erosive/Ulcerative and Painful Oral Lichen Planus. Assessment of Success Rate during One-Year Follow-Up: A Retrospective Study. Healthcare (Basel). 9 (9), 1137.
Niculet, E., Bobeica, C., Stefanopol, I. A., Pelin, A. M., Nechifor, A., Onisor, C., et al. (2022). Once-daily abrocitinib for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents aged 12 years and over: A short review of current clinical perspectives. Ther. Clin. Risk Manage. 18, 399–407. doi: 10.2147/TCRM.S338661
Nishio, H., Matsui, K., Tsuji, H., Tamura, A., Suzuki, K. (2001). Immunolocalisation of the janus kinases (JAK)—signal transducers and activators of transcription (STAT) pathway in human epidermis. J. Anat. 198 (Pt 5), 581–589. doi: 10.1046/j.1469-7580.2001.19850581.x
Ociepa, K., Danilewicz, M., Wągrowska-Danilewicz, M., Peterson-Jęckowska, R., Wójcicka-Rubin, A., Lewkowicz, N., et al. (2022). Expression of the selected proteins of JAK/STAT signaling pathway in diseases with oral mucosa involvement. Int. J. Mol. Sci. 24 (1), 323. doi: 10.3390/ijms24010323
Olek, K., Kuczaj, A. A., Warwas, S., Hrapkowicz, T., Przybyłowski, P., Tanasiewicz, M. (2023). Gut microbiome in patients after heart transplantation-current state of knowledge. Biomedicines. 11 (6), 1588. doi: 10.3390/biomedicines11061588
Paghdal, K. V., Schwartz, R. A. (2007). Sirolimus (rapamycin): from the soil of Easter Island to a bright future. J. Am. Acad. Dermatol. 57 (6), 1046–1050. doi: 10.1016/j.jaad.2007.05.021
Pang, Y., Wei, J., Mu, H. (2018). Clinical observation and influence on immune function and inflammatory factors of Jiangxian decoction with erosive oral lichen planus with Yin deficiency and internal heat syndrome. Hebei J. TCM. 40, 1487–1490.
Permoda-Osip, A., Skibinska, M., Bartkowska-Sniatkowska, A., Kliwicki, S., Chlopocka-Wozniak, M., Rybakowski, J. K. (2014). Factors connected with efficacy of single ketamine infusion in bipolar depression. Psychiatr. Pol. 48 (1), 35–47. doi: 10.12740/PP/21175
Pisoschi, A. M., Pop, A. (2015). The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 97, 55–74. doi: 10.1016/j.ejmech.2015.04.040
Polizzi, A., Santonocito, S., Lo Giudice, A., Alibrandi, A., De Pasquale, R., Isola, G. (2023). Analysis of the response to two pharmacological protocols in patients with oral lichen planus: A randomized clinical trial. Oral. Dis. 29 (2), 755–763. doi: 10.1111/odi.13960
Qataya, P. O., Elsayed, N. M., Elguindy, N. M., Ahmed Hafiz, M., Samy, W. M. (2020). Selenium: A sole treatment for erosive oral lichen planus (Randomized controlled clinical trial). Oral. Dis. 26 (4), 789–804. doi: 10.1111/odi.13285
Qiu, Q., Feng, Q., Tan, X., Guo, M. (2019). JAK3-selective inhibitor peficitinib for the treatment of rheumatoid arthritis. Expert Rev. Clin. Pharmacol. 12 (6), 547–554. doi: 10.1080/17512433.2019.1615443
Rai, N. P., Kumar, P., Mustafa, S. M., Divakar, D. D., Kheraif, A. A., Ramakrishnaiah, R., et al. (2016). Relation between periodontal status and pre-cancerous condition (Oral lichen planus): A pilot study. Adv. Clin. Exp. Med. 25 (4), 763–766.
Ravn, I., Dige, I., Meyer, R. L., Nyvad, B. (2012). Colonization of the oral cavity by probiotic bacteria. Caries Res. 46 (2), 107–112. doi: 10.1159/000336960
Riano Arguelles, A., Martino Gorbea, R., Iglesias Zamora, M. E., Garatea Crelgo, J. (2006). Topic tacrolimus, alternative treatment for oral erosive lichen planus resistant to steroids: a case report. Med. Oral. Patol Oral. Cir Bucal. 11 (6), E462–E466.
Rolain, J. M., Colson, P., Raoult, D. (2007). Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents. 30 (4), 297–308. doi: 10.1016/j.ijantimicag.2007.05.015
Rouimi, F., Bouillot, A., Baudouin, C., Labbé, A. (2018). [Topical cyclosporine A and risk of ocular surface neoplasia]. J. Fr Ophtalmol. 41 (2), 122–128. doi: 10.1016/j.jfo.2017.09.005
Rozycki, T. W., Rogers, R. S., 3rd, Pittelkow, M. R., McEvoy, M. T., el-Azhary, R. A., Bruce, A. J., et al. (2002). Topical tacrolimus in the treatment of symptomatic oral lichen planus: a series of 13 patients. J. Am. Acad. Dermatol. 46 (1), 27–34. doi: 10.1067/mjd.2002.119648
Ruiz Roca, J. A., López Jornet, P., Gómez García, F. J., Marcos Aroca, P. (2022). Effect of photobiomodulation on atrophic-erosive clinical forms of oral lichen planus: A systematic review. Dent. J. (Basel). 10 (12), 221.
Sabat, R., Wolk, K., Loyal, L., Döcke, W. D., Ghoreschi, K. (2019). T cell pathology in skin inflammation. Semin. Immunopathol. 41 (3), 359–377. doi: 10.1007/s00281-019-00742-7
Sadeghpour, S., Ghasemnejad Berenji, M., Nazarian, H., Ghasemnejad, T., Nematollahi, M. H., Abroon, S., et al. (2020). Effects of treatment with hydroxychloroquine on the modulation of Th17/Treg ratio and pregnancy outcomes in women with recurrent implantation failure: clinical trial. Immunopharmacol Immunotoxicol. 42 (6), 632–642. doi: 10.1080/08923973.2020.1835951
Saeed, S., Choudhury, P., Ahmad, S. A., Alam, T., Panigrahi, R., Aziz, S., et al. (2022). Vitamin D in the treatment of oral lichen planus: A systematic review. Biomedicines 10 (11), 2964. doi: 10.3390/biomedicines10112964
Saglam, E., Ozsagir, Z. B., Unver, T., Alinca, S. B., Toprak, A., Tunali, M. (2021). Efficacy of injectable platelet-rich fibrin in the erosive oral lichen planus: a split-mouth, randomized, controlled clinical trial. J. Appl. Oral. Sci. 29, e20210180. doi: 10.1590/1678-7757-2021-0180
Said, Z., Murdoch, C., Hansen, J., Siim Madsen, L., Colley, H. E. (2021). Corticosteroid delivery using oral mucosa equivalents for the treatment of inflammatory mucosal diseases. Eur. J. Oral. Sci. 129 (2), e12761. doi: 10.1111/eos.12761
Saleh, W., Khashaba, O. (2022). Clinical responses of patients with systemic diseases to the photodynamic therapy of oral lichen planus. Photodiagnosis Photodyn. Ther. 39, 102972. doi: 10.1016/j.pdpdt.2022.102972
Samadi, A., Ahmad Nasrollahi, S., Hashemi, A., Nassiri Kashani, M., Firooz, A. (2017). Janus kinase (JAK) inhibitors for the treatment of skin and hair disorders: a review of literature. J. Dermatolog Treat. 28 (6), 476–483. doi: 10.1080/09546634.2016.1277179
Sanadi, R. M., Khandekar, P. D., Chaudhari, S. R., Javali, M. A., Gurav, N. U. (2023). Association of periodontal disease with oral lichen planus: A systematic review and meta analysis. J. Oral. Maxillofac. Pathol. 27 (1), 173–180. doi: 10.4103/jomfp.jomfp_178_22
Sandhu, S., Klein, B. A., Al-Hadlaq, M., Chirravur, P., Bajonaid, A., Xu, Y., et al. (2022). Oral lichen planus: comparative efficacy and treatment costs-a systematic review. BMC Oral. Health 22 (1), 161. doi: 10.1186/s12903-022-02168-4
Schroder, K., Hertzog, P. J., Ravasi, T., Hume, D. A. (2004). Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75 (2), 163–189. doi: 10.1189/jlb.0603252
Serafini, G., De Biase, A., Lamazza, L., Mazzucchi, G., Lollobrigida, M. (2023). Efficacy of topical treatments for the management of symptomatic oral lichen planus: A systematic review. Int. J. Environ. Res. Public Health 20 (2), 1202. doi: 10.3390/ijerph20021202
Shavit, E., Hagen, K., Shear, N. (2020). Oral lichen planus: a novel staging and algorithmic approach and all that is essential to know. F1000Res. 9, F1000. doi: 10.12688/f1000research.18713.1
Sieg, P., Von Domarus, H., Von Zitzewitz, V., Iven, H., Färber, L. (1995). Topical cyclosporin in oral lichen planus: a controlled, randomized, prospective trial. Br. J. Dermatol. 132 (5), 790–794. doi: 10.1111/j.1365-2133.1995.tb00728.x
Society of Oral Medicine and Chinese Stomatological Association. (2022). Guideline for the diagnosis and treatment of oral lichen planus (revision). Zhonghua Kou Qiang Yi Xue Za Zhi 57 (2), 115–121. doi: 10.3760/cma.j.cn112144-20211115-00505
Solimani, F., Mesas-Fernández, A., Dilling, A., Nast, A., Hilke, F. J., Ghoreschi, F. C., et al. (2023). The Janus kinase 1 inhibitor abrocitinib for the treatment of oral lichen planus. J. Eur. Acad. Dermatol. Venereol. 39, e996–e998. doi: 10.1111/jdv.19069
Song, X., Wu, X., Wang, C., Sun, S., Zhang, X. (2021). Case report: treatment of oral lichen planus with a focus on psychological methods. Front. Psychiatry 12, 731093. doi: 10.3389/fpsyt.2021.731093
Soria, A., Agbo-Godeau, S., Taïeb, A., Francès, C. (2009). Treatment of refractory oral erosive lichen planus with topical rapamycin: 7 cases. Dermatology. 218 (1), 22–25. doi: 10.1159/000172830
Spinelli, F. R., Colbert, R. A., Gadina, M. (2021). JAK1: Number one in the family; number one in inflammation? Rheumatol. (Oxford). 60 (Suppl 2), ii3–ii10. doi: 10.1093/rheumatology/keab024
Stark, G. R., Darnell, J. E. (2012). The JAK-STAT pathway at twenty. Immunity. 36 (4), 503–514. doi: 10.1016/j.immuni.2012.03.013
Strangfeld, A., Richter, A., Siegmund, B., Herzer, P., Rockwitz, K., Demary, W., et al. (2017). Risk for lower intestinal perforations in patients with rheumatoid arthritis treated with tocilizumab in comparison to treatment with other biologic or conventional synthetic DMARDs. Ann. Rheum Dis. 76 (3), 504–510. doi: 10.1136/annrheumdis-2016-209773
Sulewska, M., Duraj, E., Sobaniec, S., Graczyk, A., Milewski, R., Wróblewska, M., et al. (2017). A clinical evaluation of the efficacy of photodynamic therapy in the treatment of erosive oral lichen planus: A case series. Photodiagnosis Photodyn. Ther. 18, 12–19. doi: 10.1016/j.pdpdt.2017.01.178
Tarasenko, S., Stepanov, M., Morozova, E., Unkovskiy, A. (2021). High-level laser therapy versus scalpel surgery in the treatment of oral lichen planus: a randomized control trial. Clin. Oral. Investig. 25 (10), 5649–5660. doi: 10.1007/s00784-021-03867-y
Thongprasom, K., Carrozzo, M., Furness, S., Lodi, G. (2011). Interventions for treating oral lichen planus. Cochrane Database Syst. Rev. 7), Cd001168. doi: 10.1002/14651858.CD001168.pub2
Tobón-Arroyave, S. I., Villegas-Acosta, F. A., Ruiz-Restrepo, S. M., Vieco-Durán, B., Restrepo-Misas, M., Londoño-López, M. L. (2004). Expression of caspase-3 and structural changes associated with apoptotic cell death of keratinocytes in oral lichen planus. Oral. Dis. 10 (3), 173–178. doi: 10.1046/j.1601-0825.2003.00998.x
Toral, M., Romero, M., Rodríguez-Nogales, A., Jiménez, R., Robles-Vera, I., Algieri, F., et al. (2018). Lactobacillus fermentum Improves Tacrolimus-Induced Hypertension by Restoring Vascular Redox State and Improving eNOS Coupling. Mol. Nutr. Food Res. 62 (14), e1800033. doi: 10.1002/mnfr.201800033
Treister, N., Li, S., Soiffer, R., Cutler, C. (2021). Topical sirolimus for management of refractory oral chronic graft-versus-host disease. Oral. Dis. 27 (6), 1451–1454. doi: 10.1111/odi.13676
Twetman, S., Derawi, B., Keller, M., Ekstrand, K., Yucel-Lindberg, T., Stecksen-Blicks, C. (2009). Short-term effect of chewing gums containing probiotic Lactobacillus reuteri on the levels of inflammatory mediators in gingival crevicular fluid. Acta Odontol Scand. 67 (1), 19–24. doi: 10.1080/00016350802516170
Utz, S., Suter, V. G. A., Cazzaniga, S., Borradori, L., Feldmeyer, L. (2022). Outcome and long-term treatment protocol for topical tacrolimus in oral lichen planus. J. Eur. Acad. Dermatol. Venereol. 36 (12), 2459–2465. doi: 10.1111/jdv.18457
Verma, K. K., Mittal, R., Manchanda, Y. (2001). Azathioprine for the treatment of severe erosive oral and generalized lichen planus. Acta Derm Venereol. 81 (5), 378–379.
Wang, J., Yang, J., Wang, C., Zhao, Z., Fan, Y. (2021). Systematic review and meta-analysis of oxidative stress and antioxidant markers in oral lichen planus. Oxid. Med. Cell Longev. 2021, 9914652. doi: 10.1155/2021/9914652
Wang, Y., Zhang, H., Du, G., Wang, Y., Cao, T., Luo, Q., et al. (2016). Total glucosides of paeony (TGP) inhibits the production of inflammatory cytokines in oral lichen planus by suppressing the NF-κB signaling pathway. Int. Immunopharmacol. 36, 67–72. doi: 10.1016/j.intimp.2016.04.010
Wang, H., Zhang, D., Han, Q., Zhao, X., Zeng, X., Xu, Y., et al. (2016). Role of distinct CD4(+) T helper subset in pathogenesis of oral lichen planus. J. Oral. Pathol. Med. 45 (6), 385–393. doi: 10.1111/jop.12405
Whitman, D. H., Berry, R. L., Green, D. M. (1997). Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J. Oral. Maxillofac. Surg. 55 (11), 1294–1299. doi: 10.1016/S0278-2391(97)90187-7
Winthrop, K. L., Curtis, J. R., Lindsey, S., Tanaka, Y., Yamaoka, K., Valdez, H., et al. (2017). Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol. 69 (10), 1960–1968. doi: 10.1002/art.40189
Wiriyakijja, P., Porter, S., Fedele, S., Hodgson, T., McMillan, R., Shephard, M., et al. (2020). Validation of the HADS and PSS-10 and psychological status in patients with oral lichen planus. Oral. Dis. 26 (1), 96–110. doi: 10.1111/odi.13220
Wu, Y., Xu, H., Wang, Y., Li, C., Tang, G., Hua, H., et al. (2022). An improved scoring system for monitoring oral lichen planus: A preliminary clinical study. Oral. Dis. 29, 3337–3345. doi: 10.1111/odi.14273
Xue, J. L., Fan, M. W., Wang, S. Z., Chen, X. M., Li, Y., Wang, L. (2005). A clinical study of 674 patients with oral lichen planus in China. J. Oral. Pathol. Med. 34 (8), 467–472. doi: 10.1111/j.1600-0714.2005.00341.x
Yan, J. J., Kuo, M. Y., Chiang, C. P. (2017). Free palatal mucosal graft for treatment of erosive oral lichen planus. J. Formos Med. Assoc. 116 (7), 560–562. doi: 10.1016/j.jfma.2017.02.008
Yan, X.-H., Z, B. (2016). Influence of total glucosides paeony on the expression of IFN-γand IL-10 in peripheral blood of patients with oral lichen planus. J. Oral. Sci. Res. 32 (5), 534.
Yang, H., Wu, Y., Ma, H., Jiang, L., Zeng, X., Dan, H., et al. (2016). Possible alternative therapies for oral lichen planus cases refractory to steroid therapies. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 121 (5), 496–509. doi: 10.1016/j.oooo.2016.02.002
Yazdi, A. S., Röcken, M., Ghoreschi, K. (2016). Cutaneous immunology: basics and new concepts. Semin. Immunopathol. 38 (1), 3–10. doi: 10.1007/s00281-015-0545-x
Yeshurun, A., Bergman, R., Bathish, N., Khamaysi, Z. (2019). Hydroxychloroquine sulphate therapy of erosive oral lichen planus. Australas. J. Dermatol. 60 (2), e109–ee12. doi: 10.1111/ajd.12948
Yli-Knuuttila, H., Snäll, J., Kari, K., Meurman, J. H. (2006). Colonization of Lactobacillus rhamnosus GG in the oral cavity. Oral. Microbiol. Immunol. 21 (2), 129–131. doi: 10.1111/j.1399-302X.2006.00258.x
Yoke, P. C., Tin, G. B., Kim, M. J., Rajaseharan, A., Ahmed, S., Thongprasom, K., et al. (2006). A randomized controlled trial to compare steroid with cyclosporine for the topical treatment of oral lichen planus. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 102 (1), 47–55. doi: 10.1016/j.tripleo.2005.09.006
You, Y., Huang, X., Chen, Y., You, Y. (2020). Efficacy and safety of traditional Chinese medicine for erosive oral lichen planus: A protocol for systematic review and meta analysis. Med. (Baltimore). 99 (48), e23375. doi: 10.1097/MD.0000000000023375
Yu, C., Fan, X., Li, Z., Liu, X., Wang, G. (2017). Efficacy and safety of total glucosides of paeony combined with acitretin in the treatment of moderate-to-severe plaque psoriasis: a double-blind, randomised, placebo-controlled trial. Eur. J. Dermatol. 27 (2), 150–154. doi: 10.1684/ejd.2016.2946
Yu, R., Zuo, F., Ma, H., Chen, S. (2019). Exopolysaccharide-producing bifidobacterium adolescentis strains with similar adhesion property induce differential regulation of inflammatory immune response in Treg/Th17 axis of DSS-colitis mice. Nutrients. 11 (4), 782. doi: 10.3390/nu11040782
Zeng, L., Yang, T., Yang, K., Yu, G., Li, J., Xiang, W., et al. (2022). Curcumin and curcuma longa extract in the treatment of 10 types of autoimmune diseases: A systematic review and meta-analysis of 31 randomized controlled trials. Front. Immunol. 13, 896476. doi: 10.3389/fimmu.2022.896476
Zhang, L., Wei, W. (2020). Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 207, 107452. doi: 10.1016/j.pharmthera.2019.107452
Zhao, L. (2018). Clinical effect of initial periodontal therapy combined with topical medication therapy on erosive oral lichen planus. Int. J. Clin. Exp. Med. 11 (7), 7346–7351.
Zhao, B., Xu, N., Li, R., Yu, F., Zhang, F., Yang, F., et al. (2019). Vitamin D/VDR signaling suppresses microRNA-802-induced apoptosis of keratinocytes in oral lichen planus. FASEB J. 33 (1), 1042–1050. doi: 10.1096/fj.201801020RRR
Zheng, Q., Jiang, W., Sun, X., Ma, T., Xu, W., Shen, F., et al. (2019). Total glucosides of paeony for the treatment of psoriasis: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine. 62, 152940. doi: 10.1016/j.phymed.2019.152940
Zhu, Y., Li, J., Bai, Y., Wang, X., Duan, N., Jiang, H., et al. (2014). Hydroxychloroquine decreases the upregulated frequencies of Tregs in patients with oral lichen planus. Clin. Oral. Investig. 18 (8), 1903–1911. doi: 10.1007/s00784-013-1176-z
Keywords: oral lichen planus, erosive type, pharmacological treatment, nonpharmacological treatment, microbes
Citation: Wu T, Bai Y, Jing Y and Chen F (2024) What can we learn from treatments of oral lichen planus? Front. Cell. Infect. Microbiol. 14:1279220. doi: 10.3389/fcimb.2024.1279220
Received: 19 August 2023; Accepted: 22 January 2024;
Published: 15 February 2024.
Edited by:
Lionel Breton, IDEC Therapeutic/CILIA Consult, FranceReviewed by:
Xiang Wang, Nanjing University, ChinaMohammad Alrashdan, University of Sharjah, United Arab Emirates
Ioanina Parlatescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2024 Wu, Bai, Jing and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fangchun Chen, NTAwMTE4QGhvc3BpdGFsLmNxbXUuZWR1LmNu
 Tingting Wu
Tingting Wu Yang Bai
Yang Bai Yin Jing1,2,3
Yin Jing1,2,3 Fangchun Chen
Fangchun Chen