
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
GENERAL COMMENTARY article
Front. Cell. Infect. Microbiol. , 09 January 2024
Sec. Antibiotic Resistance and New Antimicrobial drugs
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1343608
This article is a commentary on:
Non-pathogenic Neisseria species of the oropharynx as a reservoir of antimicrobial resistance: a cross-sectional study
 Chris Kenyon1,2*
Chris Kenyon1,2*A Commentary on:
Non-pathogenic Neisseria species of the oropharynx as a reservoir of antimicrobial resistance: a cross-sectional study.
by Gaspari V, Djusse ME, Morselli S, Rapparini L, Foschi C, Ambretti S, Lazzarotto T, Piraccini BM and Marangoni A (2023) Front. Cell. Infect. Microbiol. 13:1308550. doi: 10.3389/fcimb.2023.1308550
In this issue, Gaspari et al. report results of the first survey of antimicrobial resistance (AMR) in commensal Neisseria spp. in Italy (Gaspari et al., 2023). They found a high prevalence of ceftriaxone resistance in oral commensal Neisseria spp. (11.7%: breakpoint >0.125mg/L), which did not differ between the general population and men who have sex with men (MSM). This prevalence is very similar to that found in a similar study design in Belgium by Laumen et al. (2022), from 2022, where the prevalence of resistance was 6.9% (same breakpoint), and again did not differ between the MSM and the general population. To the best of our knowledge only one other country (Vietnam) has performed an analogous survey. This was a study in 2029 by Dong et al., who found that the prevalence of ceftriaxone resistance (same breakpoint) in commensal Neisseria was considerably higher (28%) than that found in Belgium or Italy (Dong et al., 2019).
As Gaspari et al. note, horizontal gene transfer of the penA and other genes from commensal Neisseria spp. to N. gonorrhoeae has been an important determinant of gonococcal reduced susceptibility to cephalosporins and other antimicrobials (Manoharan-Basil et al., 2021; Goytia and Wadsworth, 2022). This has led to the proposal that surveillance of AMR in commensal Neisseria could be used as an early warning system of excessive antimicrobial consumption in a population that puts them at risk for AMR (Kenyon et al., 2021; Goytia and Wadsworth, 2022). This proposal depends on the existence of a positive association between antimicrobial consumption and AMR in commensal Neisseria spp. As yet, no study has assessed if such an association exists.
The data from these three studies in Belgium, Italy and Vietnam offer us the opportunity to test this hypothesis. To do this we used Spearman’s correlation to test the association between the prevalence of ceftriaxone resistance in commensal Neisseria spp. and the consumption of cephalosporins in the population. The data for the cephalosporin consumption was taken from IQVIA (IQVIA, Danbury, CT). IQVIA uses national sample surveys that are performed by members of pharmaceutical sales distribution channels to estimate antimicrobial consumption from the volume of antibiotics sold in retail and hospital pharmacies (Klein et al., 2018). Antimicrobial consumption estimates are reported as the number of standard doses per 1000 population per year (DDD). We used data from 2015 which is the most recent year with data available (Klein et al., 2018).
The consumption of cephalosporins varied between 981 DDD in Belgium, 1049 DDD in Italy and 3867 DDD in Vietnam. Spearman’s correlation revealed a positive correlation between cephalosporin consumption and the prevalence of ceftriaxone resistance in each country (Rho 1; P<0.001; Figure 1).
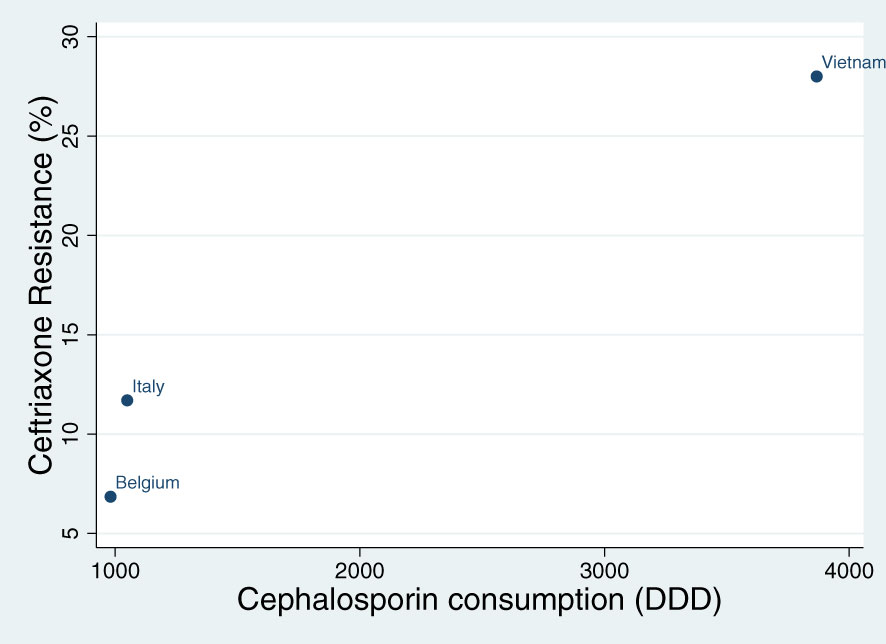
Figure 1 Spearman’s correlation of the association between cephalosporin consumption (defined daily doses per 1000 persons – DDD) and the prevalence of ceftriaxone resistance in commensal Neisseria species in Belgium, Italy and Vietnam (Rho 1; P<0.001).
An important limitation of this analysis is that there were small differences between the studies in terms of setting, selection of participants, sample collection and culturing of commensal Neisseria. All three studies did however obtain oropharyngeal samples with a swab, identified the isolates via oxidase testing and MALDI-TOFF and used Etests to determine the MIC. There was a slightly higher proportion of isolates that were N. perflava in Vietnam than the other two countries. Ceftriaxone susceptibility was not, however, found to vary between the commensal Neisseria species in any of the studies (Gaspari et al., 2023; Dong et al., 2019; Laumen et al., 2022). In addition, there may have been a difference in the prevalence of Neisseria spp. carriage between the three studies. Furthermore, the use of Spearman’s correlation is a crude measure of cross-sectional association that does not take into account variations in antibiotic consumption and resistance over time.
These limitations notwithstanding, this positive association provides evidence that the prevalence of resistance in commensal Neisseria spp. may be a useful early warning system of excessive antimicrobial consumption in a population. It would be very useful for future studies to expand these results to include populations with lower antimicrobial consumption, a broader range of antimicrobials and compare commensal Neisseria with other genera such as oral streptococci as optimal sentinel genus. Finally, it will be important to establish if reducing antimicrobial consumption is followed by a reduced prevalence of AMR.
CK: Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Dong, H. V., Pham, L. Q., Nguyen, H. T., Nguyen, M. X. B., Nguyen, T. V., May, F, et al. (2019). Decreased cephalosporin susceptibility of oropharyngeal neisseria species in antibiotic-using men-who-have-sex-with-men of hanoi, Vietnam. Clin. Infect. Dis. 70 (6), 1169–1175. doi: 10.1093/cid/ciz365
Gaspari, V., Djusse, M. E., Morselli, S., Rapparini, L., Foschi, C., Ambretti, S., et al. (2023). Non-pathogenic Neisseria species of the oropharynx as a reservoir of antimicrobial resistance: a cross-sectional study. Front. Cell. Infection Microbiol. 13, 1308550. doi: 10.3389/fcimb.2023.1308550
Goytia, M., Wadsworth, C. B. (2022). Canary in the coal mine: how resistance surveillance in commensals could help curb the spread of AMR in pathogenic neisseria. Mbio. 13 (5), e01991–e01922. doi: 10.1128/mbio.01991-22
Kenyon, C., Laumen, J., Manoharan-Basil, S. (2021). Choosing new therapies for gonorrhoea: we need to consider the impact on the pan-neisseria genome. A Viewpoint Antibiotics 10 (5), 515. doi: 10.3390/antibiotics10050515
Klein, E. Y., Van Boeckel, T. P., Martinez, E. M., Pant, S., Gandra, S., Levin, S. A., et al. (2018). Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 115 (15), E3463–E3E70. doi: 10.1073/pnas.1717295115
Laumen, J. G. E., Van Dijck, C., Abdellati, S., Pant, S., Gandra, S., Levin, S. A., et al. (2022). Antimicrobial susceptibility of commensal Neisseria in a general population and men who have sex with men in Belgium. Sci. Rep. 12 (1), 1–10. doi: 10.1038/s41598-021-03995-1
Keywords: commensal Neisseria, AMR (antimicrobial resistance), antimicrobial consumption monitoring, Neisseria gonorhoeae, Italy
Citation: Kenyon C (2024) Commentary: Non-pathogenic Neisseria species of the oropharynx as a reservoir of antimicrobial resistance: a cross-sectional study. Front. Cell. Infect. Microbiol. 13:1343608. doi: 10.3389/fcimb.2023.1343608
Received: 23 November 2023; Accepted: 20 December 2023;
Published: 09 January 2024.
Edited by:
Alin Laurentiu Tatu, Dunarea de Jos University, RomaniaReviewed by:
Jordy Evan Sulaiman, University of Wisconsin-Madison, United StatesCopyright © 2024 Kenyon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chris Kenyon, Y2hyaXNrZW55b24wQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.