- 1Dermatology Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 2Section of Microbiology, Department of Medical and Surgical Sciences, Alma Mater Studiorum - University of Bologna, Bologna, Italy
- 3Section of Dermatology, Department of Medical and Surgical Sciences, Alma Mater Studiorum - University of Bologna, Bologna, Italy
- 4Microbiology Unit, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
Commensal Neisseria species of the oropharynx represent a significant reservoir of antimicrobial resistance determinants that can be transferred to Neisseria gonorrhoeae. This aspect is particularly crucial in ‘men having sex with men’ (MSM), a key population in which pharyngeal co-colonization by N. gonorrhoeae and non-pathogenic Neisseria species is frequent and associated with the emergence of antimicrobial resistance. Here, we explored the antimicrobial susceptibility of a large panel of non-pathogenic Neisseria species isolated from the oropharynx of two populations: a group of MSM attending a ‘sexually transmitted infection’ clinic in Bologna (Italy) (n=108) and a group of males representing a ‘general population’ (n=119). We collected 246 strains, mainly belonging to N. subflava (60%) and N. flavescens (28%) species. Their antimicrobial susceptibility was evaluated assessing the minimum inhibitory concentrations (MICs) for azithromycin, ciprofloxacin, cefotaxime, and ceftriaxone using E-test strips. Overall, commensal Neisseria spp. showed high rates of resistance to azithromycin (90%; median MICs: 4.0 mg/L), and ciprofloxacin (58%; median MICs: 0.12 mg/L), whereas resistance to cephalosporins was far less common (<15%). Neisseria strains from MSM were found to have significantly higher MICs for azithromycin (p=0.0001) and ciprofloxacin (p<0.0001) compared to those from the general population. However, there was no significant difference in cephalosporin MICs between the two groups. The surveillance of the antimicrobial resistance of non-pathogenic Neisseria spp. could be instrumental in predicting the risk of the spread of multi-drug resistant gonorrhea. This information could be an early predictor of an excessive use of antimicrobials, paving the way to innovative screening and prevention policies.
1 Introduction
Neisseria gonorrhoeae is the causative agent of gonorrhea, one of the most common bacterial sexually transmitted infection (STI), worldwide (European Centre for Disease Prevention and Control, 2020).
This microorganism is becoming increasingly resistant to many antimicrobials, including last-resort drugs such as ceftriaxone and azithromycin, holding the status of ‘super-bug’ (Unemo et al., 2021). Antimicrobial resistance (AMR) in N. gonorrhoeae represents a major health concern globally, requiring elevated treatment costs and the establishment of large surveillance programs (Cole et al., 2022).
N. gonorrhoeae infections can be found at extra-genital sites, such as the oropharynx, mainly in ‘men who have sex with men’ (MSM) reporting unprotected oral sex (Gaspari et al., 2019).
The oropharyngeal mucosa is a particularly suitable niche for N. gonorrhoeae replication and persistence, and it represents a pivotal site for the emergence of multi-drug resistance (Marangoni et al., 2020a). Indeed, several studies have demonstrated that for N. gonorrhoeae most of resistance determinants are acquired from the commensal non-pathogenic Neisseria species found in the oropharyngeal environment (Marangoni et al., 2020b; Morselli et al., 2022).
It has been reported that the mosaic penA alleles, associated with decreased susceptibility and resistance to cephalosporins, have emerged by DNA transformation and recombination with partial penA genes, particularly those from commensal oropharyngeal Neisseria species, such as N. perflava, N. cinerea, N. flavescens, N. polysaccharea (Laumen et al., 2022; Manoharan-Basil et al., 2023).
Other genes responsible for the acquisition of macrolide, fluoroquinolone and/or multi-drug resistance (e.g., mtrCDE, rplB, rplD, rplV, parC, and gyrA) can be acquired by N. gonorrhoeae from non-pathogenic Neisseria species (Fiore et al., 2020; Manoharan-Basil et al., 2021; Laumen et al., 2022).
Thus, commensal Neisseria species have gained more and more attention, since they can act as a significant reservoir for genetic material, conferring resistance in N. gonorrhoeae, and, thus, potentially, leading to the emergence of multi-drug resistant gonorrhea.
Until now, only a few studies have assessed the antimicrobial resistance rates of commensal Neisseria of the oropharynx in adult populations. Among them, two surveys, carried out among MSM attending STI clinics, reported a reduced susceptibility to azithromycin and ceftriaxone in non-pathogenic Neisseria strains (Dong et al., 2020; Laumen et al., 2021).
Recently, Laumen et al. showed that azithromycin and ciprofloxacin resistance rates in commensal Neisseria are significantly higher among MSM compared to the general population, but not associated with a recent antimicrobial exposure (Laumen et al., 2022).
To expand the epidemiology of antimicrobial susceptibility in non-pathogenic Neisseria spp., the primary aim of this cross-sectional study was to assess the resistance patterns of oropharyngeal Neisseria species, by comparing two populations: (i) a group of MSM, a key population where the pharyngeal co-colonization by N. gonorrhoeae and non-pathogenic Neisseria species is particularly frequent and associated to AMR emergence (Lewis, 2013; Kenyon and Schwartz, 2018) and (ii) a group of male subjects, matched for age, ethnicity and HIV-status, representative of the general population in the same geographical area (Bologna, Italy).
To this purpose, we obtained by culture 264 pharyngeal non-pathogenic Neisseria strains (127 from MSM and 119 from the general population) and we assessed their susceptibility by E-test strips to four antimicrobials, namely azithromycin, ciprofloxacin, cefotaxime, and ceftriaxone.
2 Materials and methods
2.1 Study population
Two groups of subjects were considered for the study: (i) a group of white HIV-negative MSM attending the STI Outpatient Clinic of S. Orsola University Hospital of Bologna (Italy) for STI screening between January and July 2022, (ii) white males from a ‘general population’, who attended family doctors/local clinics of the Bologna area for sore throat between August and December 2022. For this last group, subjects were enrolled if negative for pharyngeal Group A Streptococcus (GAS; S. pyogenes). Information about the sexual orientation of the male subjects from the general population was not available. For both groups, individuals below the age of 18 were excluded.
(i) MSM were screened for pharyngeal sexually transmitted infections (i.e., Chlamydia trachomatis and N. gonorrhoeae) as a part of the routine clinical/diagnostic workflow. A pharyngeal swab (E-swab; Copan, Brescia, Italy) was collected from each participant and processed by Alinity m STI assay (Abbott Molecular, Illinois, USA), a real-time PCR test detecting the presence of C. trachomatis and/or N. gonorrhoeae nucleic acids. Starting from the same pharyngeal swab, a bacterial culture was performed in order to isolate commensal Neisseria strains (see paragraph below).
(ii) From the male individuals belonging to the ‘general population’ a pharyngeal swab (E-swab) was submitted to the Microbiology Unit of S. Orsola Hospital for the detection of GAS by bacterial culture. The same sample was used for the recovery of Neisseria strains as described below.
To mitigate the effect of potential confounding factors on the pharyngeal microbial communities, the two groups were matched for several variables, in addition to sex and ethnicity, such as age (no difference in the mean age) and HIV-status (all HIV-negative) (details on Supplementary Table S1).
The study protocol was approved by the Ethical Committee of St. Orsola-Malpighi Hospital (78/2017/U/Tess).
2.2 Strain collection
Pharyngeal swabs were plated onto Columbia blood agar and modified Thayer-Martin agar plates (Kima, Piove di Sacco, Italy) using the streak plate technique and incubated at 37°C at 5% CO2 for 48h. Afterwards, suggestive Neisseria colonies (oxidase-positive greyish-yellowish colonies) were isolated on chocolate agar and incubated for 24-48h at the same conditions described above. Bacterial identification at the species level was obtained through MALDI-TOF mass spectrometry (MALDI-TOF MS) (Bruker, Bremen, Germany), following manufacturer’s instructions. All the strains belonging to Neisseria species were collected and frozen at -80°C for further analyses. Strains belonging to N. meningitidis species were excluded.
2.3 Antimicrobial susceptibility testing
For each strain, an antimicrobial susceptibility test (AST) was performed using E-test strips (bioMérieux, Marcy-l’Étoile, France), following the ‘European Committee on Antimicrobial Susceptibility Testing’ (EUCAST) guidelines (available at https://www.eucast.org). The following antimicrobials were tested: ciprofloxacin, azithromycin, cefotaxime, and ceftriaxone. Minimum inhibitory concentration (MIC) values, expressed as mg/L, were interpreted (susceptible/resistant) using the clinical breakpoint established by EUCAST for Neisseria gonorrhoeae (available at https://www.eucast.org/clinical_breakpoints).
2.4 Data analysis and statistics
Statistical analyses were performed employing Prism 5.02 version for Windows (GraphPad Software, San Diego, CA, USA). Significant differences among the study groups (e.g., MSM vs general population; different Neisseria species) were searched by the Chi-square test or Mann-Whitney/Kruskal-Wallis test, based on the variables. A significance level of p < 0.05 was considered.
3 Results
3.1 Study population and strains isolated
During the study period, a total of 246 Neisseria strains were collected and analyzed. In particular, 127 strains were recovered from 108 MSM (89 subjects with a single strain, 19 with two strains belonging to different species) and 119 from 87 males of the general population (58 subjects with single strain, 26 with two strains, and 3 with three strains belonging to different species). In the MSM population, 9 patients suffered from pharyngeal gonorrhea (9/108; 8.3%), as indicated by the detection of N. gonorrhoeae by nucleic acid amplification test (NAAT).
The most frequently isolated Neisseria species was N. subflava (59.7%, 147/246), followed by N. flavescens (28%; 69/246). The other species were far less common: 3.2% N. perflava (8/246), 3.2% N. macacae (8/246), 2.0% N. mucosa (5/246), 1.6% Neisseria spp. (4/246), 1.2% N. sicca (3/246), 0.8% N. lactamica (2/246). The distribution of the different species among the two study groups was quite similar, except for N. mucosa, which was only detected in the general population, and N. lactamica, which was exclusively found in the MSM group (Supplementary Table S2). No strains of N. gonorrhoeae were recovered by culture.
3.2 Overall antimicrobial resistance
As shown in Supplementary Table S3, the overall resistance rate for azithromycin and ciprofloxacin exceeded 90% (91.1%) and 55% (57.7%), respectively. On the other hand, resistance to 3rd generation cephalosporins (i.e., cefotaxime and ceftriaxone) was far less common (<15%). No significant differences were found among the different Neisseria species (Table S2). N. macacae exhibited the lowest resistance rate to all the antimicrobials tested. N. mucosa strains were characterized by the highest median MIC values for azithromycin and ciprofloxacin.
The distribution of MIC values stratified by Neisseria species is displayed in Figure 1; Table S4. For azithromycin, most strains showed MIC values ranging from 1.5 to 6 mg/L (median MIC value: 4.0). In contrast, for both cefotaxime and ceftriaxone the values ranged between 0.023 and 0.064 mg/L (median MIC values: 0.06 and 0.04, respectively). Ciprofloxacin was characterized by a bimodal MIC distribution with a first peak between 0.006 and 0.016 and a second between 0.19 and 0.75 mg/L (median MIC value: 0.12).
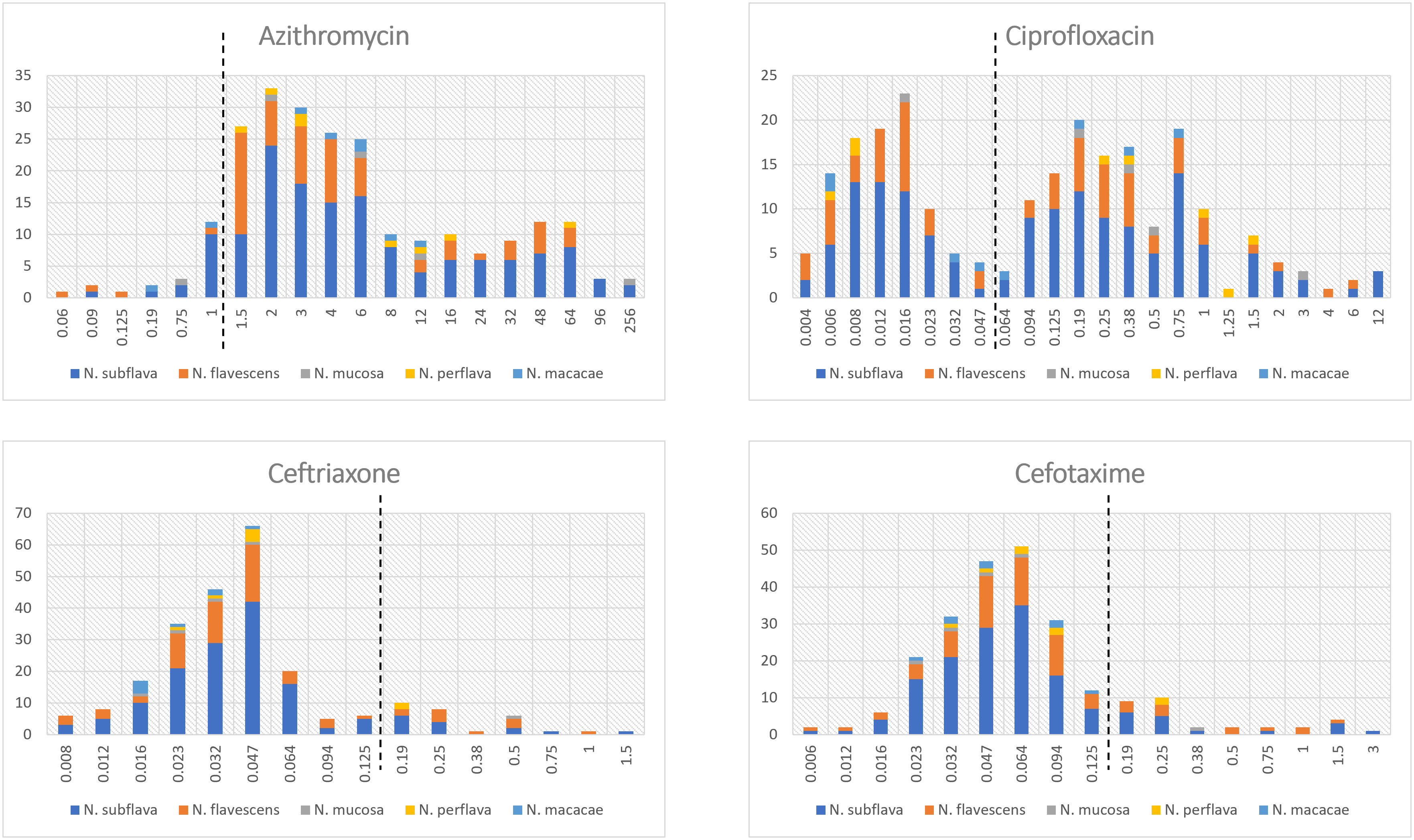
Figure 1 Distribution of MIC values (mg/L) stratified by Neisseria species. The dotted lines represent the clinical breakpoints (categorizing susceptible/resistant strains) for each antimicrobial set by the EUCAST guidelines for Neisseria gonorrhoeae. X-axis: MIC values; Y-axis: number of isolates.
Two strains of Neisseria subflava exhibited azithromycin MIC values ≥256 mg/L.
Multiple strains of different species isolated from the same subject showed similar patterns of resistance, often with identical MIC values or with a deviation of one or two-fold dilutions (data not shown).
Among the strains resistant to ceftriaxone (n=29), 89.6% of cases (26/29) also displayed resistance to azithromycin, 68.9% (20/29) to ciprofloxacin and 75.8% (22/29) to cefotaxime, thus indicating the presence of multi-drug resistant strains.
3.3 Comparison between the two populations
Interesting data emerged when comparing resistance rates/MIC values between the two populations. As shown in Table 1, median MIC values for azithromycin and ciprofloxacin were significantly higher in the MSM group in comparison to the general population (p=0.0001 and p<0.0001, respectively). On the other hand, no differences were observed in the MIC values for both the cephalosporins tested (i.e., cefotaxime, p=0.78; ceftriaxone, p=0.89). Similar results were obtained when MIC values were categorized in sensitive/resistant following EUCAST N. gonorrhoeae clinical breakpoints (Table S5 in the Supplementary Material). The distribution of MIC values stratified by the two populations (MSM vs general population) is displayed in Figure 2 (azithromycin and ciprofloxacin) and in Figure 3 (3rd generation cephalosporins).
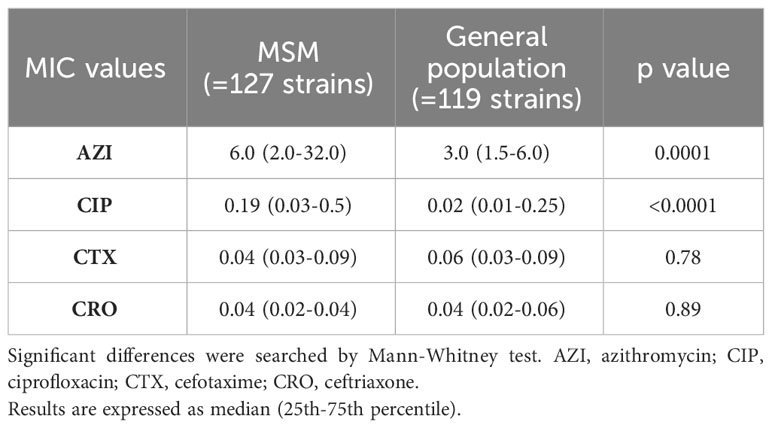
Table 1 Comparison of MIC values (mg/L) of Neisseria strains belonging to the two populations (MSM vs general population).
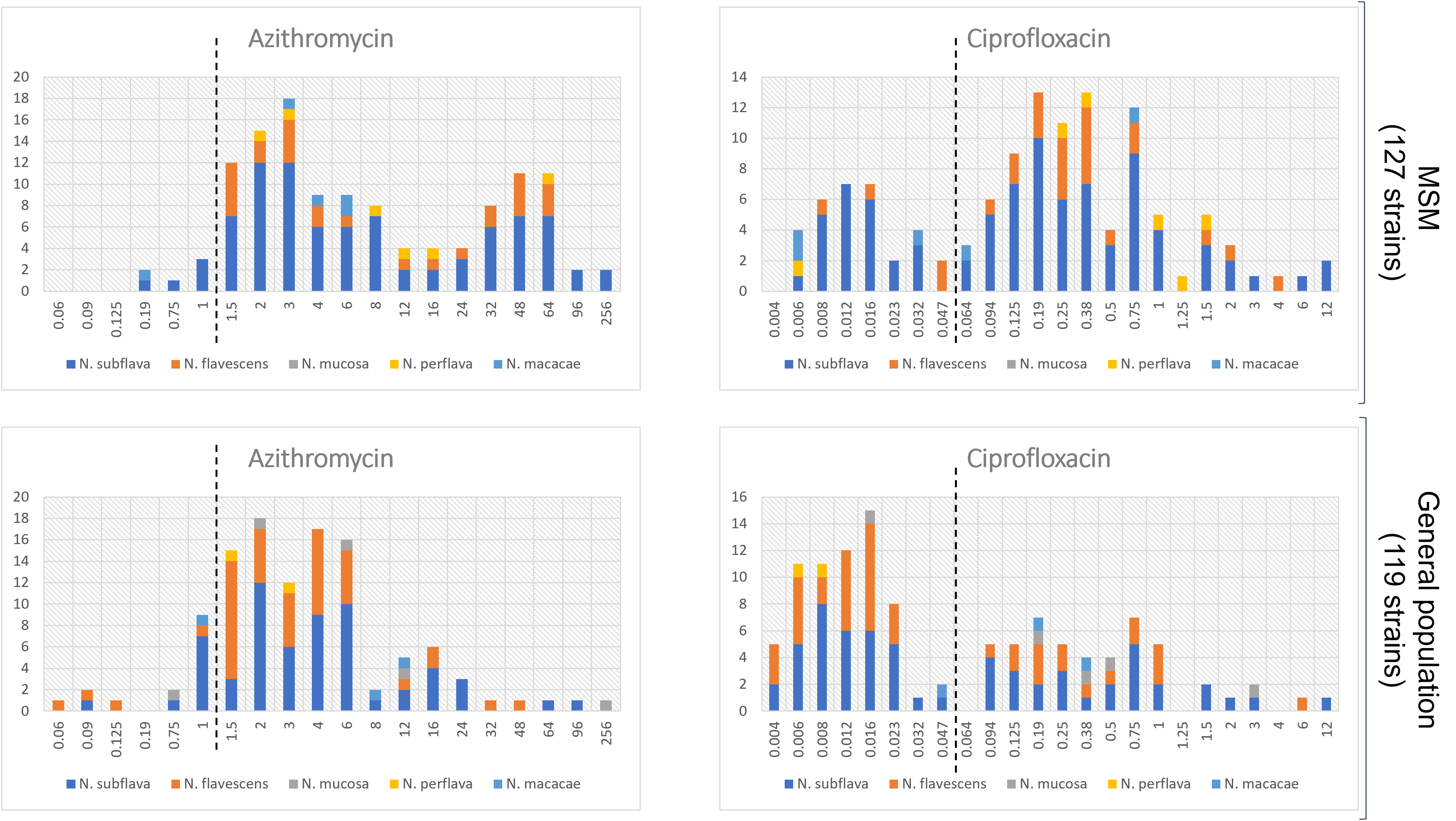
Figure 2 Distribution of MIC values (mg/L) for azithromycin and ciprofloxacin stratified by the two populations: MSM vs general populations. Only Neisseria species with at least 5 strains were considered. The dotted lines represent the clinical breakpoints (categorizing susceptible/resistant strains) for each antimicrobial set by the EUCAST guidelines for Neisseria gonorrhoeae. X-axis, MIC values; Y-axis, number of isolates.
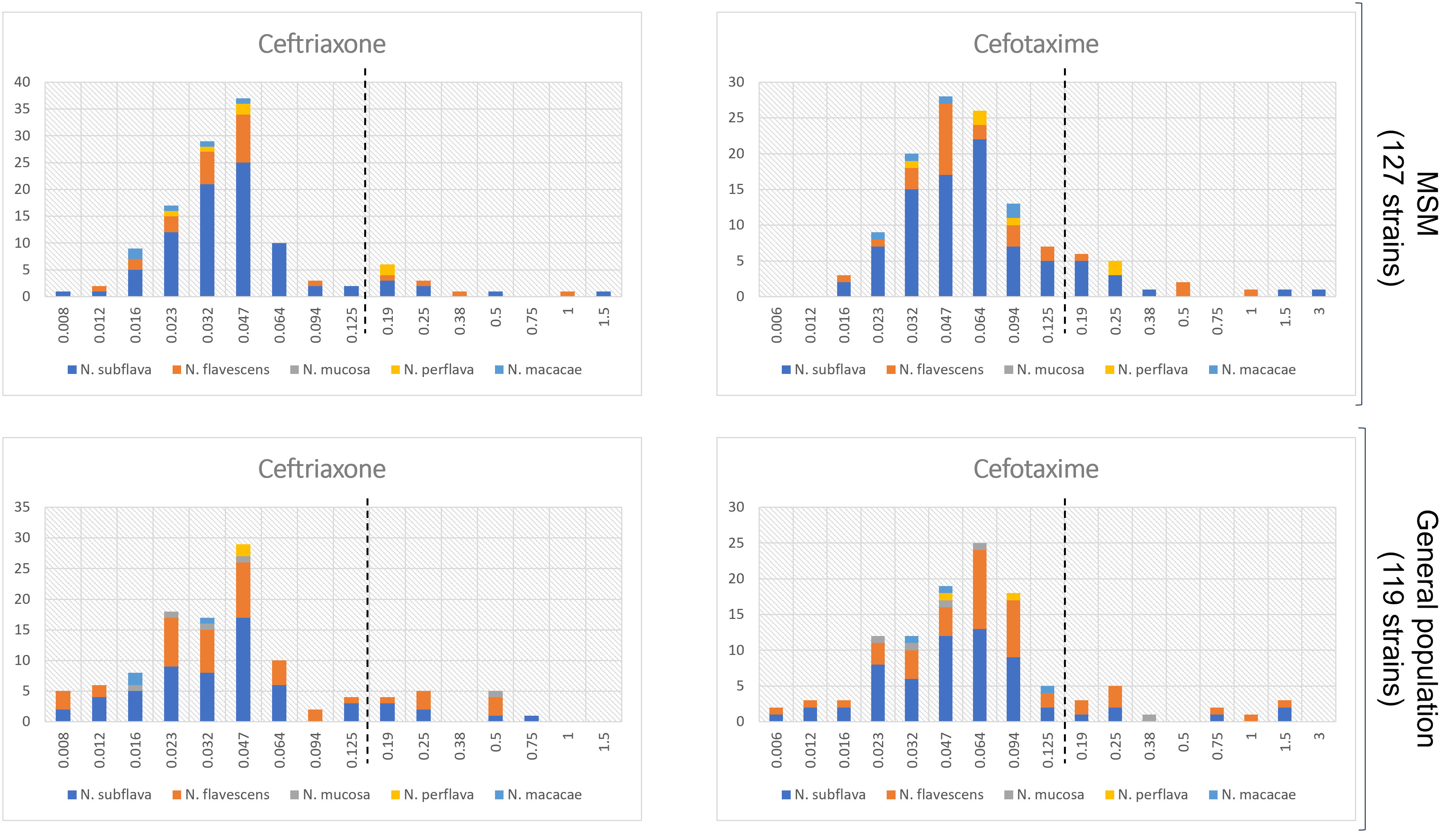
Figure 3 Distribution of MIC values (mg/L) for cefotaxime and ceftriaxone stratified by the two populations: MSM vs general populations. Only Neisseria species with at least 5 strains were considered. The dotted lines represent the clinical breakpoints (categorizing susceptible/resistant strains) for each antimicrobial set by the EUCAST guidelines for Neisseria gonorrhoeae. X-axis, MIC values; Y-axis, number of isolates.
Finally, in the MSM group, pharyngeal N. gonorrhoeae infection did not significantly affect the detection of antimicrobial resistance in commensal Neisseria species for any of the drugs tested (data not shown).
4 Discussion
In this study, we explored the antimicrobial susceptibility of a large collection of non-pathogenic Neisseria species (n=246), isolated from the oropharyngeal mucosa in two distinct groups of individuals: MSM attending an STI clinic and males, representative of the general population. Considering that the pharyngeal bacterial communities could be influenced by several factors, such as sex, hormonal levels, age, and chronic conditions (Proctor and Relman, 2017; Marangoni et al., 2020a; Bach et al., 2023), the two groups of males were matched for age, ethnicity, and HIV-status. Moreover, subjects were enrolled in the same geographical area, within a close time period.
The importance of this study lies in the fact that commensal Neisseria species of the oropharynx play a pivotal role for the emergence of multi-drug resistant gonorrhea. In fact, they represent a significant reservoir for genetic determinants of antimicrobial resistance, which can be transferred to N. gonorrhoeae (de Block et al., 2022).
At first, we observed that N. subflava and N. flavescens were the most common pharyngeal non-pathogenic Neisseria species isolated from our study populations, accounting for 87% of the total of strains. In our setting, other Neisseria species, such as N. mucosa (2%), N. lactamica (0.8%) and N. macacae (3.2%) were far less common.
This distribution is in line with a study of Dong et al., reporting a high prevalence of N. subflava and N. flavescens recovered from the oropharynx of a group of MSM in Vietnam in 2016-2017 (Dong et al., 2020). In a recent survey in Belgium, N. subflava and N. mucosa were the most common isolated strains, along with some minority species (i.e., N. cinerea, N. oralis, N. elongata) that we did not have the chance to observe in the present study (Laumen et al., 2022).
Thus, the different distribution of pharyngeal non-pathogenic Neisseria species could depend on the different geographical areas, different socio-demographic factors (e.g., age, sex), different habits and behaviors (e.g., antimicrobials exposure, unsafe sex, smoking).
Second, regardless of the Neisseria species and population considered, we found a concerning overall rate of resistance for azithromycin (about 90%; median MIC values: 4.0 mg/L) and ciprofloxacin (about 58%; median MIC values: 0.12 mg/L). Notably, for azithromycin, we detected two strains of N. subflava with alarmingly high-level resistance (MIC ≥256 mg/L): this phenomenon has been associated with the acquisition of an msrD gene, likely from oral streptococci (de Block et al., 2021).
Thus, the study of other commensal bacteria of the oropharynx (e.g., streptococci) could expand the knowledge about the dynamics between Neisseria species and other microbes in term of antimicrobial resistance spread.
On the contrary, we observed that most Neisseria strains remained susceptible to both cefotaxime (resistance rate 13%; median MIC values: 0.06 mg/L) and ceftriaxone (resistance rate 11.7%; median MIC values: 0.04 mg/L).
Similar results emerged from a recent survey in Belgium that evaluated MICs of oropharyngeal Neisseria spp. isolated from 96 individuals in 2019. The authors found remarkably high rates of azithromycin resistance (median MIC values: 3.0 mg/L), with higher susceptibility to ceftriaxone (median MIC values: 0.047 mg/L) (Laumen et al., 2022).
In other countries (i.e., Vietnam), the proportion of pharyngeal Neisseria species with reduced susceptibility to ceftriaxone reaches about the 30%, especially in participants who used at least 1 antibiotic in the 6 months prior to the enrolment (Dong et al., 2020).
A recent systematic review on the global epidemiology of antimicrobial resistance in commensal Neisseria species highlights an increasing trend in MICs over time for all the antimicrobials in various geographical areas (Vanbaelen et al., 2022).
The reasons behind the increasing antimicrobial resistance in commensal Neisseria spp. are not fully understood at this time. Presumably, the exposure to broad-spectrum antimicrobials is able to select for antimicrobial resistance in non-pathogenic Neisseria species, that seem to be less susceptible to eradication compared to pathogenic Neisseria (de Block et al., 2021). In fact, populations with high levels of antimicrobial consumption have been shown to be at high risk for the emergence of antimicrobial resistance in commensal Neisseria species (Laumen et al., 2022).
In Italy, both the community consumption of antibiotics and the prevalence of bacterial resistance are higher than in other European countries (Cangini et al., 2021). Consequently, a likely explanation for the high rates of antimicrobial resistance found in commensal Neisseria species could be the excessive and inappropriate use of antimicrobials within the general Italian population (Cangini et al., 2021). Population level antimicrobial consumption may have selected for circulating commensal Neisseria species with increased MIC values.
Noteworthy results emerged when the resistance rates/MIC values were stratified by the two study populations. Neisseria strains from the MSM group were characterized by significantly higher MIC values for azithromycin (p=0.0001) and ciprofloxacin (p<0.0001), but not for cephalosporins (p=0.8), than those from the general population.
These results, in line with the data by Laumen et al. (Laumen et al., 2022), could be explained by several reasons. MSM are often exposed to an excess of antimicrobials, including macrolide, fluoroquinolones, cephalosporins and tetracyclines (Kenyon and Schwartz, 2018). An explanation of the large drug consumption among MSM is the practice of screening for gonorrhea and chlamydia, with high rates of asymptomatic infections detected and treated (Kenyon et al., 2020; Wardley et al., 2023). In this context, it has been observed that the pharyngeal environment of MSM using pre-exposure prophylaxis (PrEP) for HIV prevention is enriched with antibiotic resistance genes (Van Dijck et al., 2023).
In addition, MSM often represent a ‘closed’ community with high frequency of interpersonal contacts - like kissing and attending the same crowded recreational events - during which transmission of commensal microorganisms of the oropharynx may occur (Aral, 2022).
In fact, it has been shown that the oral Neisseria communities of partners are more similar than those of unrelated individuals. Thus, commensal Neisseria species can be transmitted between sexual partners, presumably through intimate kissing (Van Dijck et al., 2020).
In conclusion, we found that, in our geographical area (Bologna, Italy), non-pathogenic pharyngeal Neisseria species showed worrisome rates of resistance to azithromycin and ciprofloxacin. Strains from MSM were significantly less susceptible to azithromycin and ciprofloxacin, but not to cephalosporins, than those from the general population.
To the best of our knowledge this is the first report on the susceptibility of pharyngeal commensal Neisseria species in Italy and one of the few works that compared the resistance rates in two different groups. Although our results largely confirm those of Laumen et al. (Laumen et al., 2022), we included a significantly larger panel of Neisseria strains, testing them for an additional antimicrobial (i.e., cefotaxime). Moreover, we included individuals from a different geographical area (Belgium vs Italy), comparing only male groups (vs males and females) matched for several variables, potentially affecting the composition of pharyngeal microbiome.
Data about the antimicrobial resistance of non-pathogenic Neisseria spp. in populations where pharyngeal gonorrhea is quite common (e.g., MSM) could help predict the risk of the spread of multi-drug resistant N. gonorrhoeae strains. Moreover, this information could be an early predictor of an excessive use or misuse of antimicrobials, paving the way to innovative screening programs and prevention policies. In this scenario, the assessment of antimicrobial susceptibility of commensal Neisseria species in selected populations could be added as part of routine testing in clinical microbiology laboratories.
However, this study has a number of major limitations, including, (i) the lack of information about antibiotic exposure and about behavioral factors (e.g., number of sexual partners, sexual orientation of the general population), (ii) a single center design, (iii) the absence of strains belonging to N. gonorrhoeae species (iv) the culture-based approach starting from a pharyngeal swab, potentially leading to miss specific or minority commensal Neisseria species.
Further perspectives include the use of metagenomic approaches to better profile the pharyngeal Neisseria species and their related resistome. In addition, we are planning to test non-pathogenic Neisseria strains for other antimicrobials (i.e., doxycycline), as well as to include commensal oral microbes different from Neisseria species (e.g., streptococci). Finally, it would be useful to explore the resistance rates of commensal Neisseria in groups with different levels of antibiotic exposure, to better understand the relationship between the type/dose of drug and the risk of acquisition of antimicrobial resistance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by ethical committee of St. Orsola-Malpighi Hospital (78/2017/U/Tess). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
VG: Investigation, Methodology, Writing – original draft. MD: Data curation, Formal analysis, Writing – original draft. SM: Data curation, Formal analysis, Writing – original draft. LR: Investigation, Writing – original draft. CF: Conceptualization, Formal analysis, Supervision, Writing – original draft, Writing – review & editing. SA: Resources, Writing – original draft. TL: Resources, Writing – original draft. BP: Conceptualization, Writing – original draft. AM: Conceptualization, Supervision, Resources, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article. This research received no specific grant from any funding agency in the public, commercial, or notfor-profit sectors.
Acknowledgments
We would like to thank Dr. Laura Dionisi, Dr. Egle Pizzirani and Dr. Anna Chiara Lombardi for providing excellent technical support during the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1308550/full#supplementary-material
References
Aral, S. O. (2022). Determinants of STD epidemics: implications for phase appropriate intervention strategies. Sex Transm Infect. 78, i3–13. doi: 10.1136/sti.78.suppl_1.i3
Bach, L., Ram, A., Ijaz, U. Z., Evans, T. J., Haydon, D. T., Lindström, J. (2023). The effects of smoking on human pharynx microbiota composition and stability. Microbiol. Spectr. 11, e0216621. doi: 10.1128/spectrum.02166-21
Cangini, A., Fortinguerra, F., Di Filippo, A., Pierantozzi, A., Da Cas, R., Villa, F., et al. (2021). Monitoring the community use of antibiotics in Italy within the National Action Plan on antimicrobial resistance. Br. J. Clin. Pharmacol. 87, 1033–1042. doi: 10.1111/bcp.14461
Cole, M. J., Day, M., Jacobsson, S., Amato-Gauci, A. J., Spiteri, G., Unemo, M., et al. (2022). The European response to control and manage multi- and extensively drug-resistant Neisseria gonorrhoeae. Euro Surveill 27, 2100611. doi: 10.2807/1560-7917.ES.2022.27.18.2100611. European Gonorrhoea Response Plan Group.
de Block, T., González, N., Abdellati, S., Laumen, J. G. E., Van Dijck, C., De Baetselier, I., et al. (2022). Successful Intra- but Not Inter-species Recombination of msr(D) in Neisseria subflava. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.855482
de Block, T., Laumen, J. G. E., Van Dijck, C., Abdellati, S., De Baetselier, I., Manoharan-Basil, S. S., et al. (2021). WGS of commensal neisseria reveals acquisition of a new ribosomal protection protein (MsrD) as a possible explanation for high level azithromycin resistance in Belgium. Pathogens 10, 384. doi: 10.3390/pathogens10030384
Dong, H. V., Pham, L. Q., Nguyen, H. T., Nguyen, M. X. B., Nguyen, T. V., May, F., et al. (2020). Decreased cephalosporin susceptibility of oropharyngeal neisseria species in antibiotic-using men who have sex with men in hanoi, Vietnam. Clin. Infect. Dis. 70, 1169–1175. doi: 10.1093/cid/ciz365
European Centre for Disease Prevention and Control (2020). “Gonorrhea,” in ECDC annual epidemiological report for 2018 (Stockholm: ECDC).
Fiore, M. A., Raisman, J. C., Wong, N. H., Hudson, A. O., Wadsworth, C. B. (2020). Exploration of the Neisseria Resistome Reveals Resistance Mechanisms in Commensals That May Be Acquired by N. gonorrhoeae through Horizontal Gene Transfer. Antibiotics (Basel). 9, 656. doi: 10.3390/antibiotics9100656
Gaspari, V., Marangoni, A., D'Antuono, A., Roncarati, G., Salvo, M., Foschi, C., et al. (2019). Pharyngeal Chlamydia and gonorrhea: a hidden problem. Int. J. STD AIDS 30, 732–738. doi: 10.1177/0956462419838922
Kenyon, C., Baetselier, I., Wouters, K. (2020). Screening for STIs in PrEP cohorts results in high levels of antimicrobial consumption. Int. J. STD AIDS 31, 1215–1218. doi: 10.1177/0956462420957519
Kenyon, C. R., Schwartz, I. S. (2018). Effects of sexual network connectivity and antimicrobial drug use on antimicrobial resistance in neisseria gonorrhoeae. Emerg. Infect. Dis. 24, 1195–1203. doi: 10.3201/eid2407.172104
Laumen, J. G. E., Van Dijck, C., Abdellati, S., De Baetselier, I., Serrano, G., Manoharan-Basil, S. S., et al. (2022). Antimicrobial susceptibility of commensal Neisseria in a general population and men who have sex with men in Belgium. Sci. Rep. 12, 9. doi: 10.1038/s41598-021-03995-1
Laumen, J. G. E., Van Dijck, C., Abdellati, S., Manoharan-Basil, S. S., De Baetselier, I., Martiny, D., et al. (2021). Markedly reduced azithromycin and ceftriaxone susceptibility in commensal neisseria species in clinical samples from belgian men who have sex with men. Clin. Infect. Dis. 72, 363–364. doi: 10.1093/cid/ciaa565
Lewis, D. A. (2013). The role of core groups in the emergence and dissemination of antimicrobial-resistant N gonorrhoeae. Sex Transm Infect. 89, iv47–iv51. doi: 10.1136/sextrans-2013-051020
Manoharan-Basil, S. S., Gestels, Z., Abdellati, S., Akomoneh, E. A., Kenyon, C. (2023). Evidence of horizontal gene transfer within porB in 19 018 whole-genome Neisseria spp. isolates: a global phylogenetic analysis. Microb. Genom 9, mgen001041. doi: 10.1099/mgen.0.001041
Manoharan-Basil, S. S., Laumen, J. G. E., Van Dijck, C., De Block, T., De Baetselier, I., Kenyon, C. (2021). Evidence of Horizontal Gene Transfer of 50S Ribosomal Genes rplB, rplD, and rplY in Neisseria gonorrhoeae. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.683901
Marangoni, A., Ceccarani, C., Camboni, T., Consolandi, C., Foschi, C., Salvo, M., et al. (2020a). Pharyngeal microbiome alterations during Neisseria gonorrhoeae infection. PloS One 15, e0227985. doi: 10.1371/journal.pone.0227985
Marangoni, A., Marziali, G., Salvo, M., D'Antuono, A., Gaspari, V., Foschi, C., et al. (2020b). Mosaic structure of the penA gene in the oropharynx of men who have sex with men negative for gonorrhea. Int. J. STD AIDS 31, 230–235. doi: 10.1177/0956462419889265
Morselli, S., Gaspari, V., Cantiani, A., Salvo, M., Foschi, C., Lazzarotto, T., et al. (2022). Meningococcal carriage in 'Men having sex with men' With pharyngeal gonorrhoea. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.798575
Proctor, D. M., Relman, D. A. (2017). The landscape ecology and microbiota of the human nose, mouth, and throat. Cell Host Microbe 21, 421–432. doi: 10.1016/j.chom.2017.03.011
Unemo, M., Lahra, M. M., Escher, M., Eremin, S., Cole, M. J., Galarza, P., et al. (2021). WHO global antimicrobial resistance surveillance for Neisseria gonorrhoeae 2017-18: a retrospective observational study. Lancet Microbe 2 (11), e627–e636. doi: 10.1016/S2666-5247(21)00171-3
Vanbaelen, T., Van Dijck, C., Laumen, J., Gonzalez, N., De Baetselier, I., Manoharan-Basil, S. S., et al. (2022). Global epidemiology of antimicrobial resistance in commensal Neisseria species: A systematic review. Int. J. Med. Microbiol. 312, 151551. doi: 10.1016/j.ijmm.2022.151551
Van Dijck, C., Laumen, J. G. E., de Block, T., Abdellati, S., De Baetselier, I., Tsoumanis, A., et al. (2023). The oropharynx of men using HIV pre-exposure prophylaxis is enriched with antibiotic resistance genes: a cross-sectional observational metagenomic study. J. Infect. 86, 329–337. doi: 10.1016/j.jinf.2023.02.006
Van Dijck, C., Laumen, J. G. E., Manoharan-Basil, S. S., Kenyon, C. (2020). Commensal neisseria are shared between sexual partners: implications for gonococcal and meningococcal antimicrobial resistance. Pathogens 9, 228. doi: 10.3390/pathogens9030228
Keywords: Neisseria, Neisseria gonorrhoeae, oropharynx, antimicrobial resistance, AMR, MSM
Citation: Gaspari V, Djusse ME, Morselli S, Rapparini L, Foschi C, Ambretti S, Lazzarotto T, Piraccini BM and Marangoni A (2023) Non-pathogenic Neisseria species of the oropharynx as a reservoir of antimicrobial resistance: a cross-sectional study. Front. Cell. Infect. Microbiol. 13:1308550. doi: 10.3389/fcimb.2023.1308550
Received: 06 October 2023; Accepted: 02 November 2023;
Published: 22 November 2023.
Edited by:
Marion Skalweit, Case Western Reserve University, United StatesReviewed by:
Lewis S. Musoke, United States Department of Veterans Affairs, United StatesPuja Van Epps, United States Department of Veterans Affairs, United States
Copyright © 2023 Gaspari, Djusse, Morselli, Rapparini, Foschi, Ambretti, Lazzarotto, Piraccini and Marangoni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claudio Foschi, Y2xhdWRpby5mb3NjaGkyQHVuaWJvLml0
†These authors have contributed equally to this work and share first authorship
 Valeria Gaspari1†
Valeria Gaspari1† Marielle Ezekielle Djusse
Marielle Ezekielle Djusse Sara Morselli
Sara Morselli Luca Rapparini
Luca Rapparini Claudio Foschi
Claudio Foschi Simone Ambretti
Simone Ambretti Tiziana Lazzarotto
Tiziana Lazzarotto Bianca Maria Piraccini
Bianca Maria Piraccini Antonella Marangoni
Antonella Marangoni