- 1Department of Infectious Diseases, The First Hospital of Shanxi Medical University, Taiyuan, China
- 2Graduate School, Shanxi Medical University, Taiyuan, China
- 3Department of Infectious Disease Prevention and Control, Shanxi Provincial Center for Disease Control and Prevention, Taiyuan, China
Japanese encephalitis (JE) is a naturally occurring localized disease caused by the Japanese encephalitis virus, which is spread by the Culex tritaeniorhynchus. China has a high rate of JE. Shanxi, located in North China, has a high prevalence of adult JE. Adult JE has more severe complications, mortality, and a higher disease burden, making it a public health issue. This retrospective study examined the dynamic epidemic changes, high-risk areas of JE, and clinical characteristics and prognostic factors of adult JE in Shanxi Province. The findings revealed that July to September was the primary epidemic season of JE and that JE cases were mainly in individuals over the age of 40. The incidence of JE from 2005 to 2022 demonstrated a positive spatial correlation with significant clustering characteristics, with high-incidence clusters in the south and southeast. Multivariate logistic regression analysis revealed that higher cerebrospinal fluid pressure, higher white blood cell counts, higher neutrophil percentage, deep coma, and lower albumin were independent factors for poor prognosis of adult JE. The developed risk prediction model holds great promise in early prognosis assessment of patients, providing a basis for clinical decision-making and early clinical intervention.
1 Introduction
Japanese encephalitis (JE) is a natural localized disease caused by the Japanese encephalitis virus (JEV), spread by the Culex tritaeniorhynchus, with pigs as the primary source of infection (Oliveira et al., 2018). Less than 1% of JEV-infected patients develop neurological disease, with encephalitis fatality rates ranging from 20-30%, and 30-50% of survivors continuing to have neurologic, cognitive, or psychiatric symptoms (Symptoms & Treatment | Japanese Encephalitis | CDC, 2022). JE is more prevalent in Southeast Asia and the Western Pacific. JEV transmission is seasonal throughout temperate Asia, peaking in the summer and autumn. Transmission can occur all year in the subtropics and tropics, with a surge during the rainy season. JE primarily affects children, but adult JE cases have increased due to a decrease in pediatric patients through childhood immunization programs in endemic areas, an increase in tourism to JE risk areas, and a gradual decrease in JEV neutralizing antibodies with age (Hills et al., 2023). Statistics from several countries and regions show that the age distribution of JE is steadily becoming more adult, with more severe complications and mortality (Yin et al., 2015) and a higher disease burden (Wang et al., 2021), making adult JE a public health concern.
There is no specific antiviral therapy for JE; supportive care is the primary approach to JE management. Several treatments, including dexamethasone (anti-inflammatory), IFN-α2a (antiviral), immunoglobulin (viral neutralization and anti-inflammatory), and minocycline (anti-inflammatory), have entered randomized clinical trials. However, none of these treatments improved the prognosis of JE patients (Ashraf et al., 2021). Immunoprophylaxis is considered to be the most effective approach to JE prevention. JE vaccine is derived from genotype III (GIII) of JE (Rajaiah and Kumar, 2022), with weak protection against GI and GV strains (Cao et al., 2016; Hegde and Gore, 2017), although GI, GIII, GV co-exist in China (Li et al., 2014). The GV strain is more pathogenic in mice than the GI/III (de Wispelaere et al., 2015). The outbreak of JE in Australia in 2022 suggests that novel genotypes might be introduced by migrating birds or mosquitoes and spread throughout the globe (Pham et al., 2022). Therefore, managing and preventing adult JE in endemic areas must include cost-effectiveness and practicality.
China has a high incidence of JE. Following the incorporation of the JE vaccine into the Expanded Program on Immunization, the incidence of JE decreased in most parts of China (excluding North China) (Shi et al., 2022b), while less developed economy regions have gradually become JE epidemic areas (Shi et al., 2022a). Adult JE was more prevalent in some provinces than the national average, notably among patients over 40 years old. The 6 provinces north of the Yangtze River were hotspots for adult JE, while Shanxi, in North China, had a high incidence of adult JE (Li et al., 2016). A study of adult JE in southern Shanxi Province revealed that having pigsties near dwellings is a potential risk factor for the prevalence of adult JE (Ren et al., 2017). However, there is no current description of JE distribution characteristics in Shanxi Province.
This retrospective study used geographic information system (GIS) spatial analysis and JE surveillance data between 2005 and 2022 in Shanxi Province to describe the dynamic epidemic changes and high-risk areas of JE in Shanxi Province and to manage and prevent the occurrence of JE in high-risk areas at an early stage. Furthermore, it is imperative to understand the clinical characteristics and prognostic factors of adult JE, given that early symptoms of adult JE are nonspecific and the prognosis is poor. The findings can guide the diagnosis and treatment of adult JE and conduct early intervention and prognostic estimation.
2 Methods
2.1 Study region
Shanxi Province is located in the east wing of the Loess Plateau in western North China, between 34°34’ to 40°44’ north latitude and 110°14’ to 114°33’ east longitude. It is a parallelogram oblique from northeast to southwest, having a total area of 156,700 square kilometers. The precipitation from June to August accounts for nearly 60% of the annual precipitation. It has jurisdiction over 117 counties or districts of 11 prefecture-level cities.
2.2 Study population and data collection
Data on JE cases from January 1, 2005 to December 31, 2022 were derived from the China Information System for Disease Control and Prevention, including age, date of onset, and clinical outcomes. Shanxi Center for Disease Control and Prevention provided the geographical information for each case.
This study retrospectively recruited adult patients diagnosed with JE from the First Hospital of Shanxi Medical University between January 2012 and December 2022 and the Yuncheng Second Hospital in 2006. All patients fulfilled the following criteria by the World Health Organization recommend: presence of clinical criteria of acute encephalitis syndrome and (1) detectable JE specific IgM in CSF or serum, or (2) evidence of seroconversion or a 4-fold increase of IgM or IgG in the convalescence phase by the ELISA method, or (3) isolation of virus from blood, CSF fluid or tissue, or (4) detection of JE-virus genome in serum, plasma, blood, CSF or tissue(Solomon et al., 2008). Patients aged <18 years, with other causes of consciousness disruption, encephalitis and meningitis, and with a significant lack of clinical data were excluded. General information (age, sex, underlying diseases), clinical characteristics (fever, headache, vomiting, convulsion, consciousness disturbance, and neurological signs), serological indicators (blood routine, liver and kidney function, ions), cerebrospinal fluid (CSF) (pressure, routine, biochemical, cytology), and brain MRI results were collected. These patients were classified into two groups according to GCS score at discharge: good outcomes (≥8 scores) and poor outcomes (<8 scores). Furthermore, the influencing factors of poor outcomes were investigated.
This retrospective study involved no personal information such as names and required no ethical statement.
2.3 Spatiotemporal analysis
The epidemiological characteristics of JE distribution such as annual cases and death, incidence and mortality, Monthly cumulative JE cases, and age group were plotted using Microsoft Excel 2021. The annual distribution of JE cases by county within Microsoft Excel were linked through the unique value of county level and included in the attribute table of Shanxi Province base map using ArcGIS. JE cases were classified into 6 levels: 0, 1-2, 3-5, 6-10, 11-20, and > 20 cases, with data from ArcGIS software shown on a county-level map of Shanxi Province. The yearly geographical distribution of JE was made according to the JE frequency. Add up the annual distribution of JE cases by county from January 1, 2005 to December 31, 2022 to draw spatiotemporal distribution of total JE cases. The Local Indicators of Spatial Association (LISA) was utilized to examine the spatial autocorrelation of the JE distribution and the Z-score, P-value and local Moran’s I coefficient were calculated. A local Moran’s I coefficient >0 indicates that the data have a positive spatial correlation. In contrast, a local Moran’s I coefficient <0 indicates that the data have a negative spatial correlation, and a local Moran’s I coefficient = 0 indicates that the data are random.
2.4 Statistical analysis
Data were analyzed using the IBM SPSS Statistics 26.0 and R4.3.1 software. Count data were described by the number of cases (percentage) and compared across groups using Chi-square tests or Fisher exact probability. Measurement data with a normal distribution were presented as mean ± standard deviation; an independent sample t-test was employed to compare the two groups. The measurement data with non-normal distribution were presented as median and interquartile distance; the Mann-Whitney U test was used to compare the two groups. The influencing factors of prognosis were examined using Logistic stepwise regression (backward elimination) to develop a prediction model. The p-values > 0.05 used for excluding variables at each step. The results were presented as odds ratio (OR) and 95% confidence interval (CI), and the forest map was generated using the “forestplot” package in R software. The predictive capacity of the prediction model was evaluated using the Receiver operating characteristic (ROC) curve, defined by area under the curve (AUC) and 95%CI. p < 0.05 (double-tailed) denoted statistical significance. The “ResourceSelection” package in R software was used to evaluate the calibration ability of the model using the calibration curve and Hosmer-Lemeshow test. The p > 0.05 indicated a good fit of the model in the Hosmer-Lemeshow test. A nomogram diagram was generated using the “rms” package.
3 Results
3.1 Epidemiological features of JE in Shanxi Province
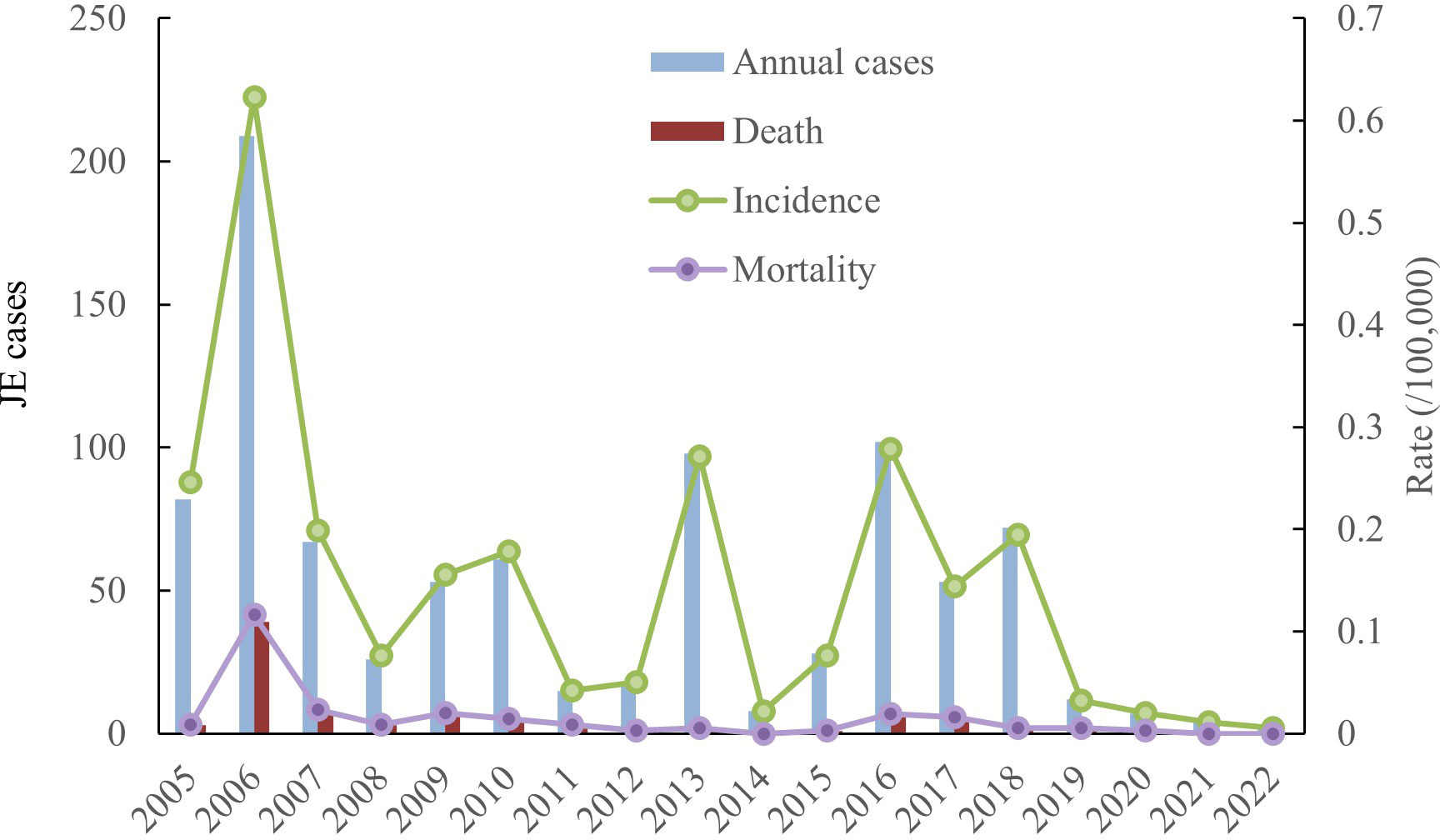
Incidence and mortality. A total of 917 cases and 90 deaths were documented between 2005 and 2022, with an annual incidence between 0.0057/100,000 and 0.623/100,000, and an annual mortality between 0 and 0.1162/100,000. The average annual incidence and mortality rates were 0.1459/100,000 and 0.0145/100, 000, respectively, with a fatality rate of 9.81%. The incidence of JE in Shanxi Province showed a decreasing trend between 2005 and 2022, with the highest number of JE cases in 2006 (Figure 1).
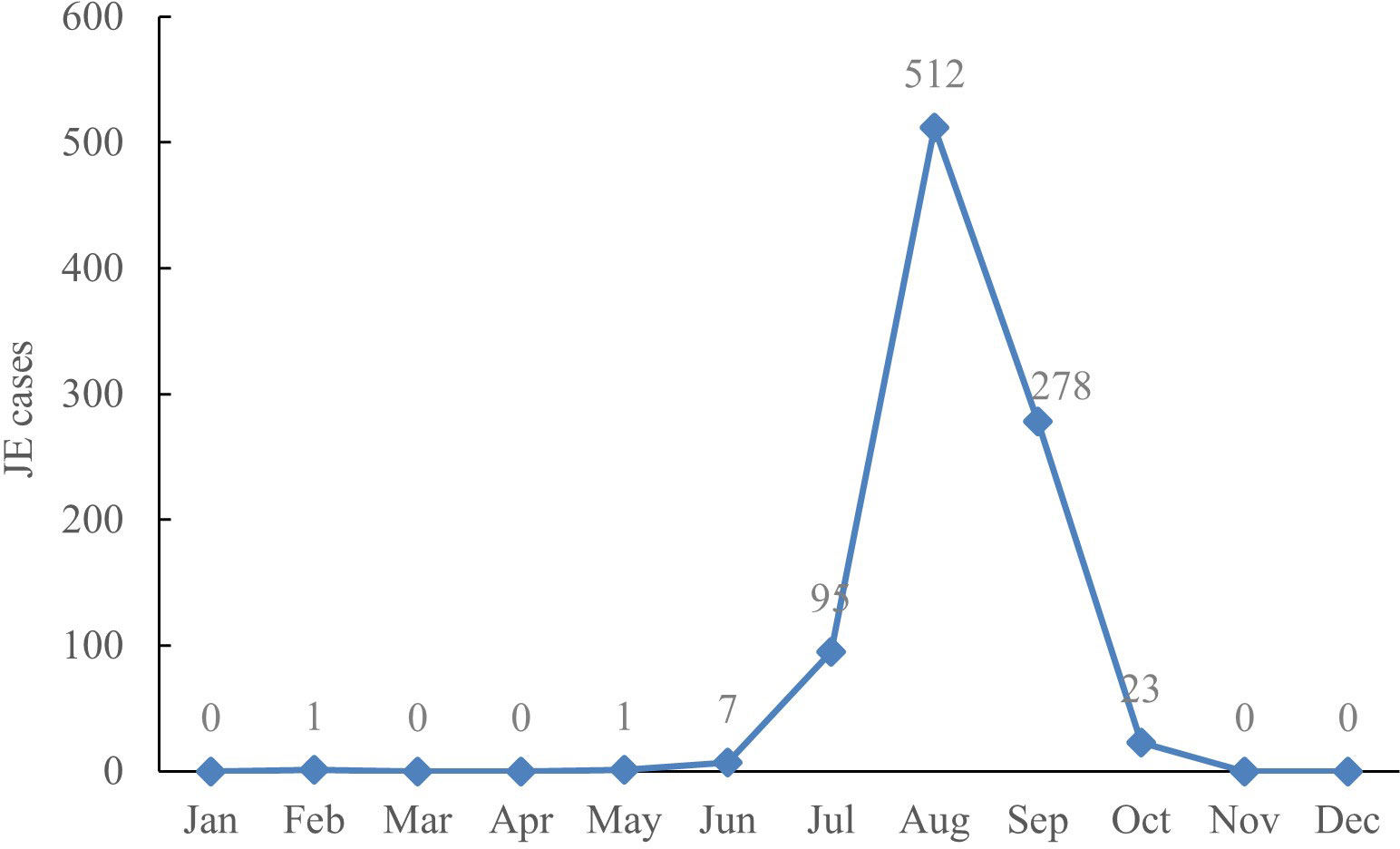
Seasonal pattern. The distribution of JE cases revealed clear seasonal patterns, with the highest wave from July to September, with a peak in August. There were 95 cases (10.36%) in July, 512 cases (55.83%) in August, 278 cases (30.32%) in September, and a total of 885 cases (96.51%) from July through September among JE cases between 2005 and 2022 (Figure 2).
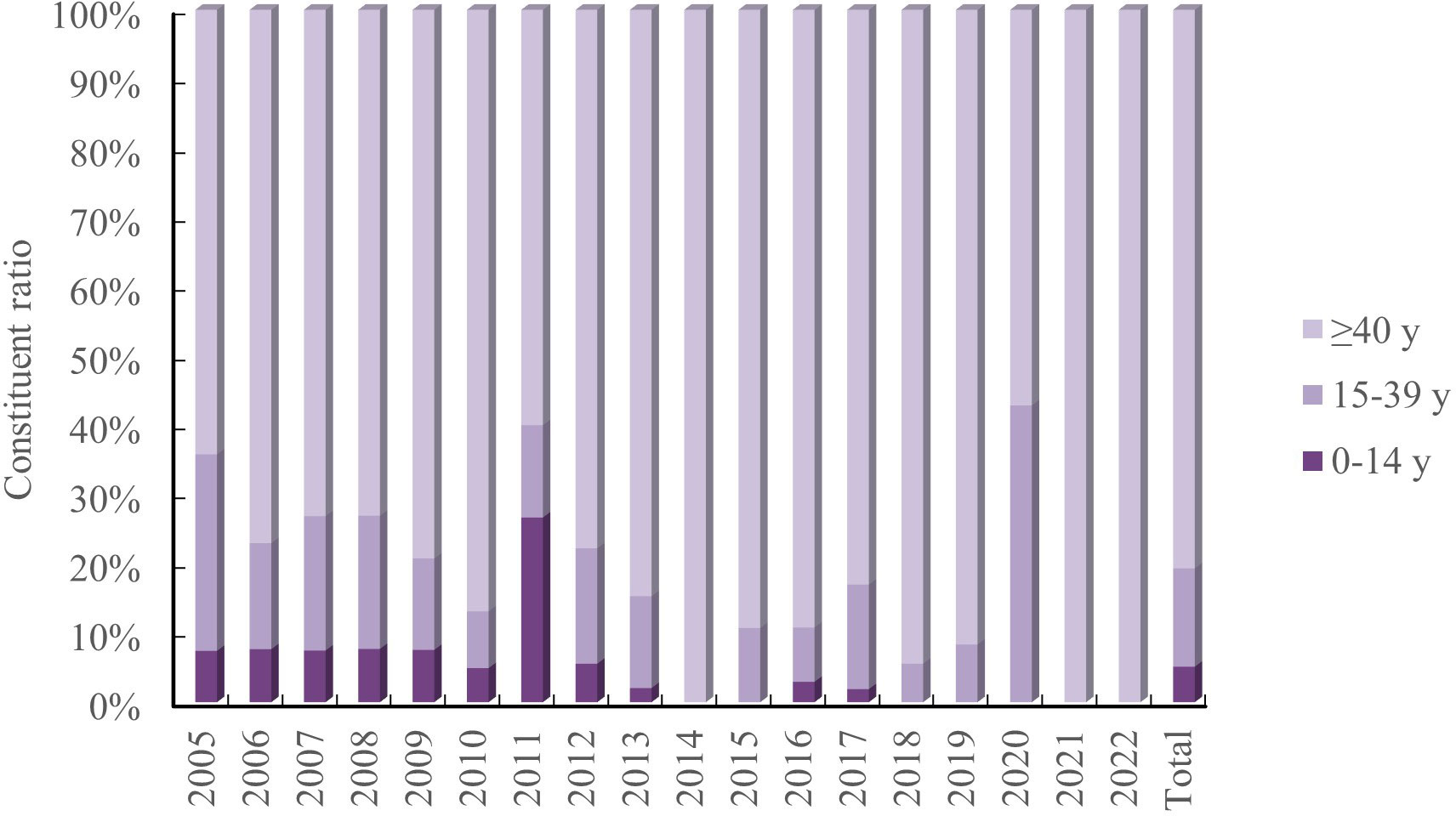
Age pattern. There were 47 cases (5.13%), 130 cases (14.18%), and 739 cases (80.59%) that were 0-14 years, 15-39 years, and ≥40 years, respectively, with 1 unknown case. The age group ≥ 40 years had the highest concentration of JE cases, with the age group 0-14 years gradually decreasing. There were no cases in the age group 0-14 years in 2014-2015 and 2018-2022, and all JE cases in 2014 and 2021-2022 were ≥40 years (Figure 3).
Geographical distribution. Shanxi Province has 117 counties, with 18 counties reporting no JE for over 10 years. Figure 4A depicts the annual distribution of JE cases by county. Liyi, Wanrong, Jincheng urban area (Zezhou), Yangcheng, and Hejin counties had the highest number of reported cases (Figure 4B).
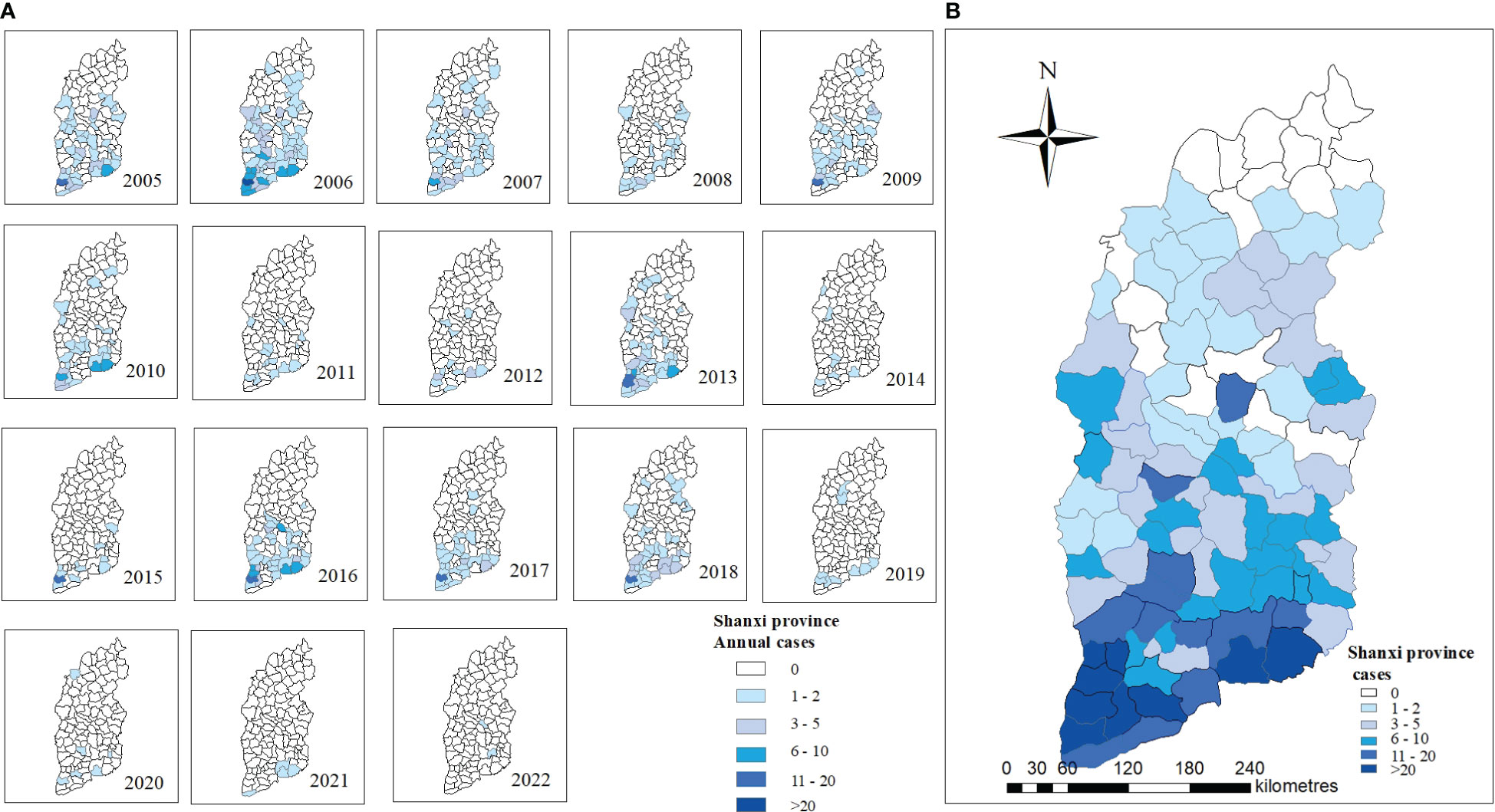
Figure 4 Spatiotemporal distribution of JE in Shanxi Province, 2005–2022. (A) Annual JE cases in 2005-2022. (B) Total JE cases in 2005-2022.
3.2 Spatial features of JE in Shanxi Province
The incidence of JE from 2005 to 2022 had a positive spatial correlation with significant clustering characteristics (Moran’s I=0.39, Z=8.13, P<0.001). LISA revealed significant spatial agglomeration areas, including high-incidence clusters in the south and southeast: Hejin County, Jishan County, Wanrong County, Liyi County, Yongji County, Ruicheng County, Yanhu District, Yangcheng County, Jincheng urban area (Zezhou County) (Figure 5).
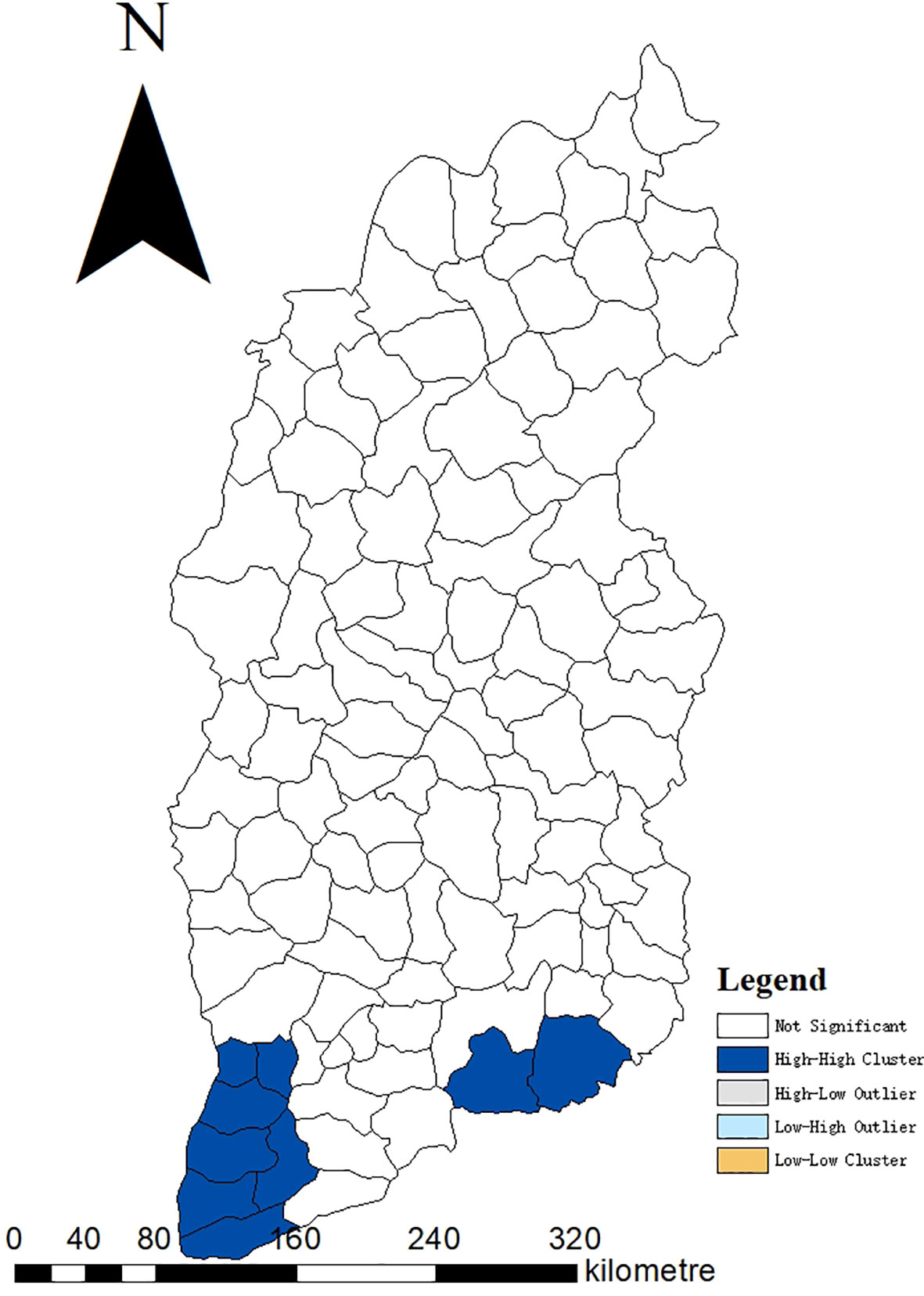
Figure 5 Moran LISA cluster map of JE cumulative cases in Shanxi Province, 2005–2022. LISA, Local Indicators of Spatial Association.
3.3 General information and clinical features
The study comprised 138 patients with JE (Table 1),79 (57.25%) of whom were male, the median age was 63 years, and the majority of patients were over 50 years old. The majority of the cases occurred in the summer. There were 101 cases with good outcomes and 37 cases with poor outcomes, including 56 men (55.45%) and 23 men (62.16%), respectively. Hypertension was the most prevalent underlying condition. Compared with the good outcomes group, the poor outcomes group was older (65 vs. 61, p<0.05), but there were no significant differences in gender and underlying diseases (p>0.05).
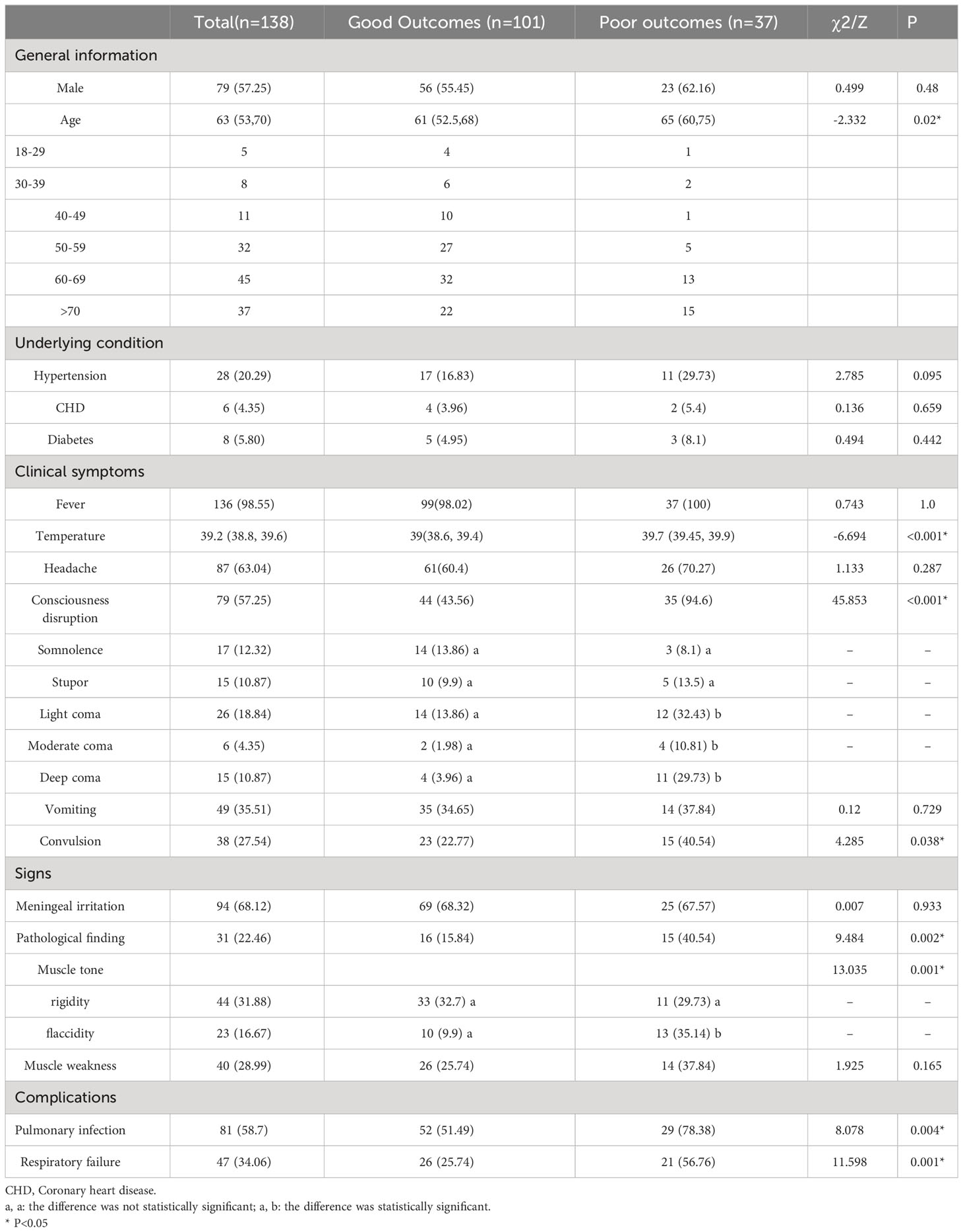
Table 1 General information and clinical features with a comparison between the good and poor outcomes groups.
Fever, with a median temperature of 39.2° C, was the most common symptom (98.55%), followed by headache (63.04%) and consciousness disruption (57.25%). Meningeal irritation was the main sign in 94 patients (68.12%); 31 presented with positive pathological findings, 23 had flaccid muscle tone, and 40 had muscle weakness. Temperature, consciousness disruption (coma), convulsions, positive pathological findings, and flaccid muscle tone were associated with poor outcomes. Pulmonary infection and respiratory failure were the most common clinical complications linked to poor outcomes. (Table 1)
3.4 Laboratory and imaging examination
The CSF was colorless and transparent, with increased pressure. The median CSF white blood cell (WBC) was 91*106/L, with slightly increased protein and reduced chloride. The CSF pressure, protein, and sugar levels in the poor outcomes group were significantly higher than in the good outcomes group (P< 0.05). WBC, neutrophils % (N%) in the poor outcomes group was higher than in the good outcomes group, but albumin (ALB) was lower (Table 2).
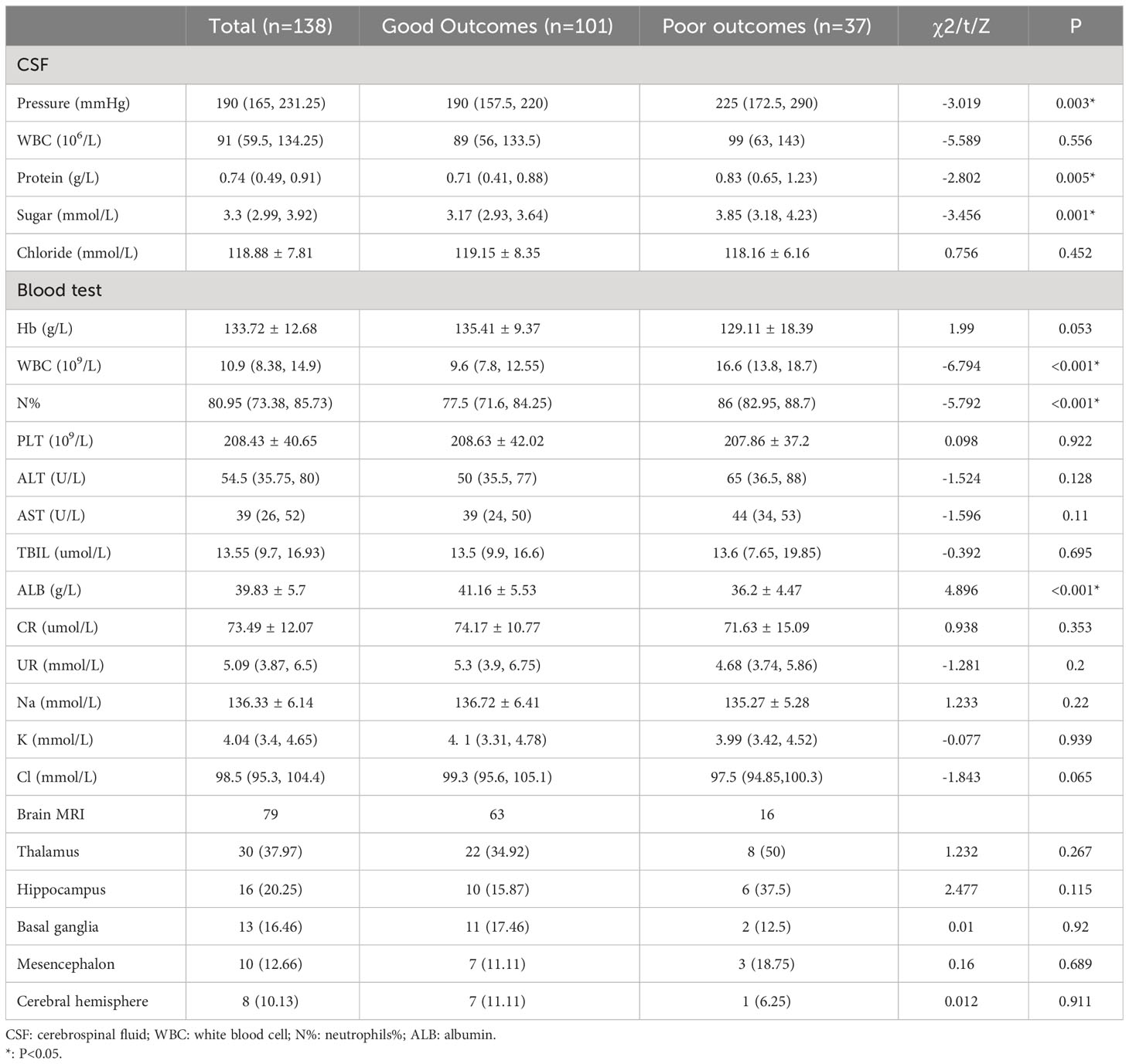
Table 2 Laboratory and imaging examination with a comparison between the good and poor outcomes groups.
As illustrated in Table 2, 79 patients underwent brain MRI, with 41 (51.9%) indicating inflammatory lesions. The thalamus was the most involved site, followed by the hippocampus and basal ganglia. Some patients involved two or more sites simultaneously. The sites implicated in the two groups differed, but the difference was not statistically significant.
3.5 Prognostic factor
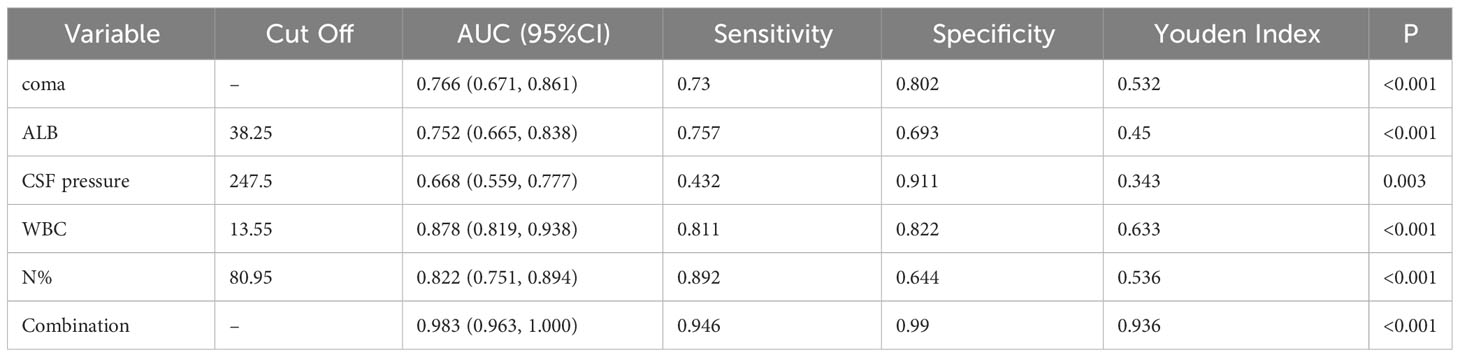
The aforementioned significant variables in Tables 1, 2 were included in the Logistic stepwise regression analysis, with the threshold value αin at 0.05 and αout at 0.10 to screen out five independent predictors of poor outcomes JE patients (Figure 6). CSF pressure, WBC, N%, and coma were risk factors for poor outcomes. For 1 unit increase in CSF pressure, WBC, N%, the risk of poor outcomes increased by 1.9%, 69.9%, and 31.8%, respectively, and the risk of poor outcomes increased by 9.935 times in patients with coma. ALB was a protective factor for poor outcomes, and 1 unit increase in ALB reduces the risk by 32.7%.
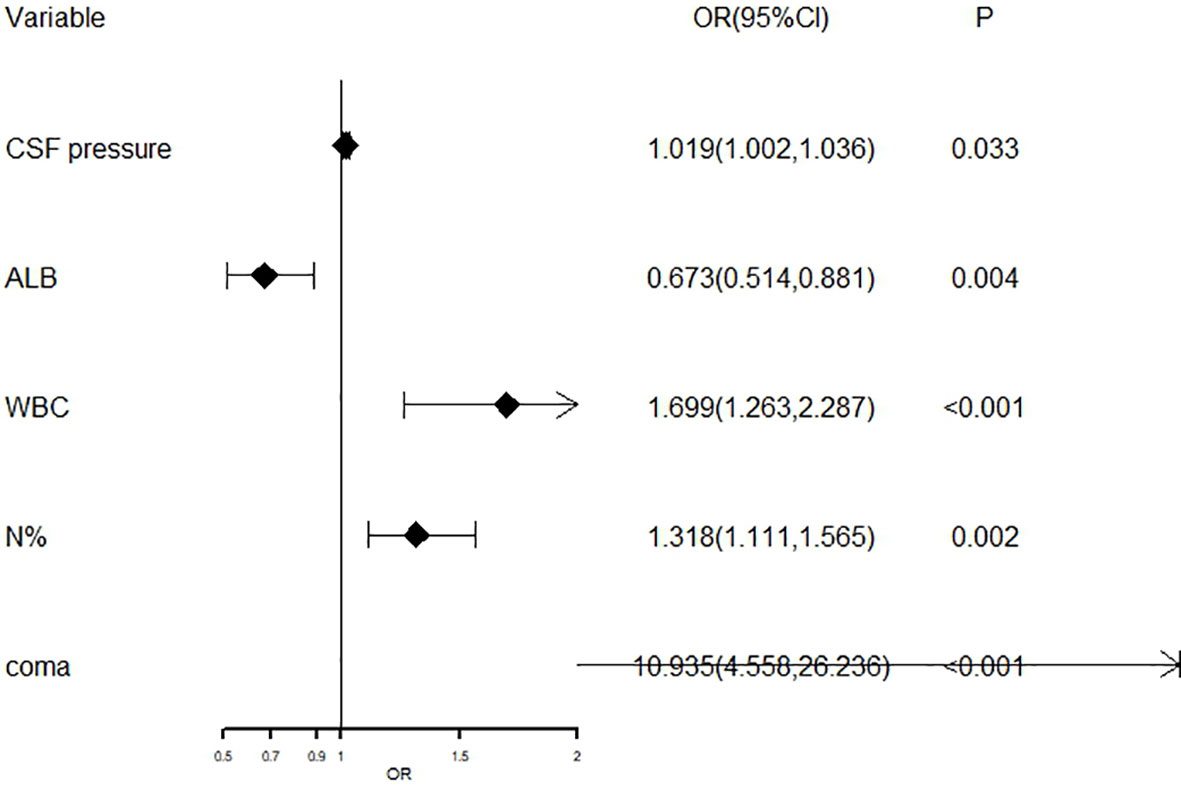
Figure 6 The forest plot for logistic regression analysis in poor outcomes of adult JE CSF, cerebrospinal fluid; ALB, albumin; WBC, white blood cell; N%, neutrophils%.
3.6 Evaluation predictive model
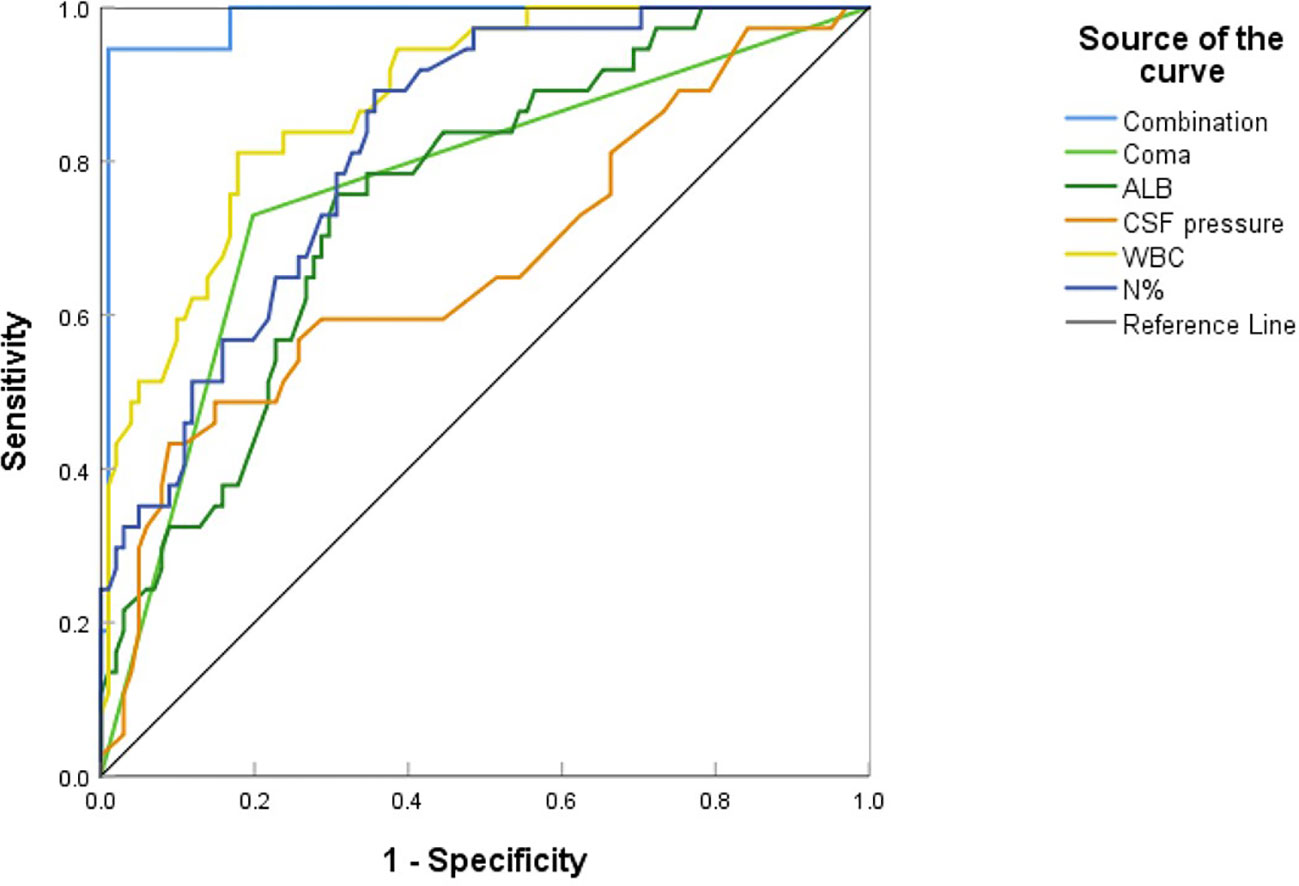
Multivariate analysis revealed that higher CSF pressure, higher WBC, higher N%, deep coma, and lower ALB were independent predictors of poor outcomes, generating a new prediction model: ln(p/1p)=0.019× (CSF pressure)+0.53× (WBC)+0.276× (N%)+2.392× (coma)-0.396× (ALB). The ROC curve was used to assess the prediction efficiency (Figure 7), sensitivity, specificity, and Youden index were calculated respectively, and the optimal critical value for prediction of each index was obtained from the maximum Youden index. The AUC of the combined prediction model was 0.983, the sensitivity was 94.6%, and the specificity was 99%, higher than that of CSF pressure (AUC=0.688), WBC (AUC=0.878), N% (AUC=0.822), coma (AUC=0.766), ALB (AUC=0.752) prediction alone (P <0.05) (Table 3), so the prediction model had the best prediction efficiency.
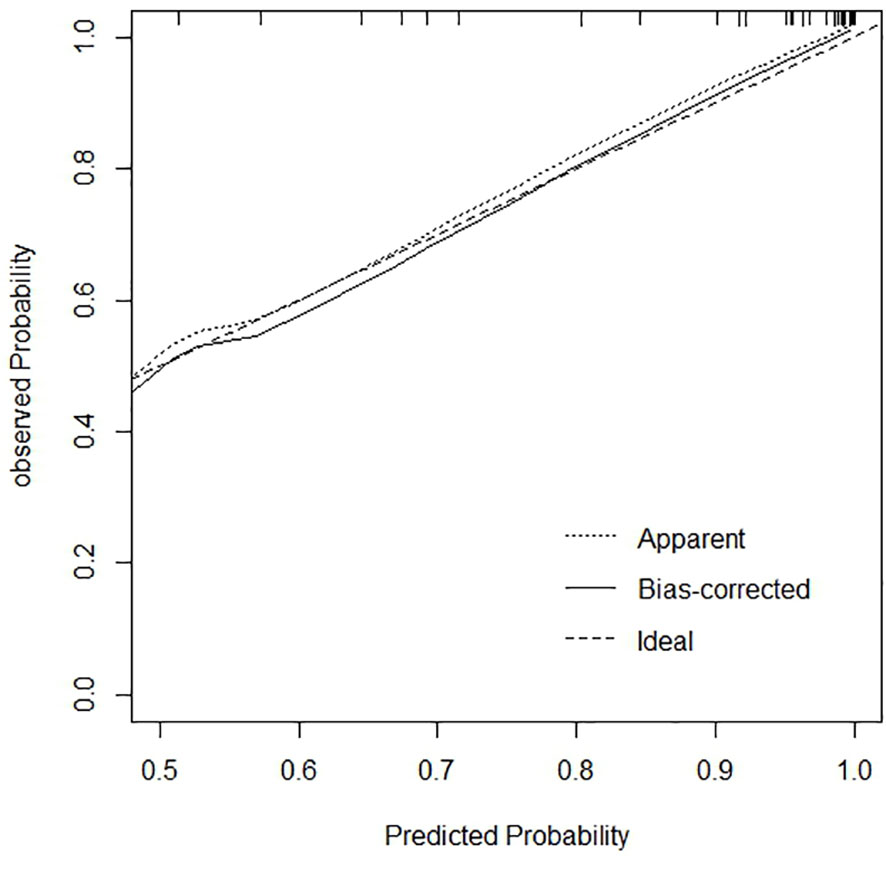
The calibration curve and Hosmer-Lemeshow goodness of fit test were drawn based on the bootstrape1000 repeated sampling verification to evaluate the calibration degree of the model (Figure 8). The predicted probability of poor JE outcomes was consistent with the actual probability, and the predicted curve matched the ideal curve. In the Hosmer-Lemeshow test, χ2 = 2.025, P=0.363 (P>0.05), indicating a good fit.
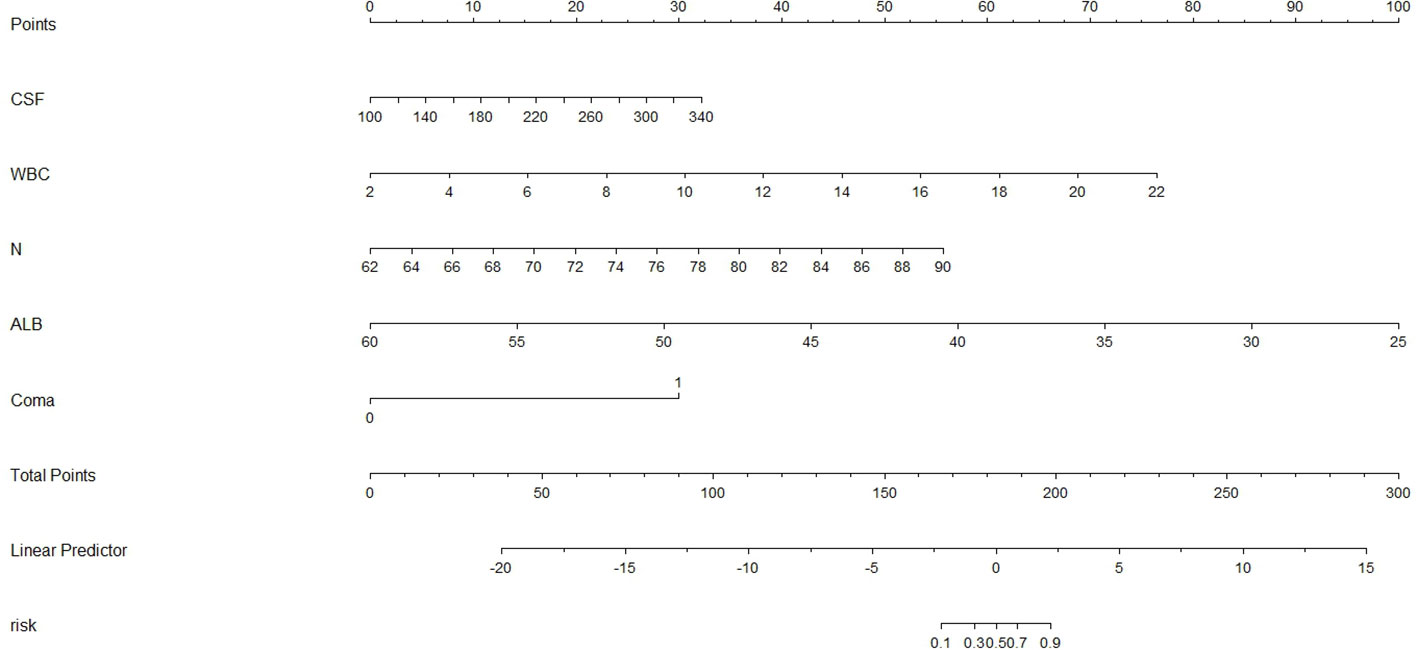
We constructed a nomogram for predicting poor JE outcomes using the above five predictors (Figure 9). According to the contribution degree of each variable, each value level of the variable corresponded to the upper score, from 0 to 100, then the score of each variable was combined to obtain the total score, and finally revealed the prediction probability of poor outcomes.
4 Discussion
This was the first study to examine the epidemiological characteristics of JE distribution in Shanxi Province during the last 18 years, and the prognosis factors of adult JE were investigated using clinical data to develop a prediction model. A total of 917 cases were recorded in Shanxi Province between 2005 and 2022, with an average annual incidence and mortality of 0.1459/100,000, 0.0145/100,000, and a fatality rate of 9.81%. These cases primarily occurred in August and September, with 739 cases (80.59%) involving people over the age of 40. Li and colleagues found that the proportions of JE for the above 40-year-old group in the six provinces north of the Yangtze River (Henan, Hebei, Shandong, Shanxi, Shaanxi, and Gansu) between 2004 and 2014 were higher than the national average (Li et al., 2016). Shanxi province accounted for over 50% of all JE cases in the over-40-year-old group, higher than the national average.
Shanxi Province has 18 counties with no recorded JE cases between 2005 and 2022, the majority of which were in northern Shanxi Province. JE prevention has been intensively pursued for many years; however, JE cases were found in most parts of Shanxi Province. The global spatial autocorrelation revealed a positive spatial correlation with significant clustering characteristics, indicating that disease burden was unevenly distributed across counties in Shanxi Province. LISA revealed the presence of significant spatial agglomeration areas, with high-incidence clusters in the south and southeast, primarily counties under the jurisdiction of Yuncheng City. Yuncheng City, located along the Yellow River and in the southernmost part of Shanxi Province, with high temperature and humidity, has a high prevalence of JE. Studies have demonstrated that temperature and relative humidity directly influence the distribution and population dynamics of mosquitoes. Temperature affects mosquito development and viral replication in an amplifying host and vector, and appropriate relative humidity allows mosquitoes to survive longer and spread further, increasing the risk of pathogen transmission (Song et al., 2020; Tu et al., 2021). A study in Liyi County, Yuncheng City, found that cotton field water by periodic flooding generates abundant favorable breeding sites for JE vector mosquitoes, and cotton field irrigation management was beneficial for JE control in this region (Liu et al., 2022). Furthermore, there was a tight association between pig number, vector infection rate, and the number of human JE cases (Borah et al., 2013). Pigsties near human dwellings serve as an amplifying host for JEV, resulting in JEV proliferation in the surrounding areas and an increased risk of JEV infection in humans. The high mortality caused by the JE outbreak in Yuncheng City in 2006 drew attention. Therefore, the pigsties and environment should be strengthened to minimize the incidence of adult JE in the high-incidence area.
Recently, there has been a great amount of support for vaccinating adults against JE. Supporters believe that it is urgent to carry out vaccination for adults based on the number of adult patients and the age of onset(Mao and Zhou, 2020). Their claim is unrealistic and unsustainable for all adults to be vaccinated, at least in Shanxi Province, because the hot spots for the JE epidemic were in the south and southeast. Therefore, adults at high risk of JEV exposure such as those working in rural agricultural areas or pig rearing businesses are recommended to receive the JE vaccine. Although the incidence and mortality of JE have shown a downtrend in Shanxi Province, China, the risk of an epidemic or an outbreak is still high. In recent years, we established a strong system for epidemic reporting, and the system has been running smoothly. However, most cases of JE are asymptomatic and go unreported, which makes the spatial distribution of the virus difficult to estimate. In a study that was reported in the south of Shanxi Province (Ren et al., 2017), the minimum infection rate of JEV in mosquito specimens collected from the courtyards of farmers’ households with pigsties was 7.39/1 000. In addition, agricultural changes; climate changes; JEV infection without encephalitis; changes in trip duration for JE case can also cause an outbreak or epidemic. This study suggested that the risk of JE epidemic occurring is still high and that prevention efforts in Shanxi Province should not be neglected.
Because of the rising proportion and poor prognosis of adult JE patients in Shanxi Province, this study analyzed clinical cases to identify prognosis factors of adult JE patients. There were somewhat more men than females in these clinical cases, but gender did not affect JE prognosis (Lo et al., 2019; Chen et al., 2022). These older patients had poor prognoses, possibly due to organ function decline, decreased immunity, and poor overall health (Li et al., 2022). The predominant clinical symptoms were fever, headache, vomiting, and consciousness disruption. Fever is the most common symptom (Gu et al., 2022), and patients with poor prognosis experienced elevated body temperatures. The consciousness disruption of is characterized by a partial or complete loss of conscious activity, which is related to the severity of brain damage. Coma is the complete loss of consciousness. Univariate analysis demonstrated that coma and convulsion are risk factors for poor JE prognosis, and recurrent convulsion and coma are indicators of nervous system involvement. Previous research evidence linked coma to poor prognosis of severe JE (Su et al., 2021; Chen et al., 2022). Dystonia is primarily caused by damage to the structure or network of the extrapyramidal system of the nervous system. Intracranial lesions in JE patients were located mainly in the thalamus, basal ganglia, and other parts, and muscle tone flaccidity is associated with poor prognosis, which is consistent with the findings of Lo et al. (2019). Because the above indicators of nervous system involvement imply that some patients have nontypical initial symptoms, we should pay close attention to nervous system examination to detect abnormal signs, identify early cases, and improve clinical prognosis.
Pulmonary infection and respiratory failure are the most common complications of JE, and univariate analysis revealed their potential link with poor prognosis. Most patients with consciousness disruption, long-term bed rest, poor cough ability, and low immunity increase the probability of lung infection. Respiratory failure is primarily caused by brain parenchymal lesions involving the respiratory center, necessitating mechanical ventilation, with ventilator-related pneumonia being the most prevalent complication. Patients with lung infection require additional supportive care and have poor prognoses (Ma and Jiang, 2013). Xiong et al. (2019) revealed that older JE patients had a higher incidence of acute secondary complications, including respiratory failure, hypoalbuminemia, and a poor prognosis. Therefore, these complications require early diagnosis, treatment, and prevention by ensuring smooth breathing and taking appropriate measures such as tracheal intubation and tracheotomy, combined with respiratory stimulants and glucocorticoids, as needed.
Nearly 50% of JE patients had increased CSF pressure and a poor prognosis. JEV infection can induce endothelial cells and astrocytes to produce mediators that regulate JEV production while destroying the integrity of the blood-brain barrier (Patabendige et al., 2018), increasing the CSF protein. A previous study discovered that CSF protein levels were linked to neurological symptoms, suggesting that abnormal intrathecal protein synthesis results in poor prognosis (Sarkar et al., 2015). Furthermore, CSF sugar levels in patients with JE were either normal or slightly elevated. Zheng (2020) demonstrated that CSF sugar was an independent factor of poor prognosis, indicating disease severity. JEV entrance into the body can induce oxidative stress reactions (such as reactive oxygen species, superoxide anion, and NO production) in neutrophils and glial cells, causing inflammation, immune activation, autophagy, and other cellular reactions (Sharma et al., 2018). Activation of the innate immune response inhibits viral spread and promotes the release of inflammatory mediators, and lectin expression on macrophages and neutrophils potentially exacerbates JEV-mediated neuroinflammation (Chen et al., 2012). An increase in WBC and N% was associated with poor prognosis. Su et al. demonstrated that a higher percentage of neutrophils and a lower percentage of lymphocytes on admission may be associated with poor prognosis (Su et al., 2021). Other research has linked a high WBC count to poor neurological prognosis (Kafle et al., 2017). Low ALB may be attributed to long-term bed rest, insufficient protein intake, malnutrition, or consumption status, resulting in decreased immunity; thus, a normal protein level is a protective factor for prognosis, which warrants adequate energy provision through nutritional support therapy.
Multivariate logistic regression analysis revealed that higher CSF pressure, higher WBC, higher N%, deep coma, and lower ALB were independent factors for poor JE outcomes. The prediction model had an AUC of 0.983, with 94.6% sensitivity and 99% specificity. Generally, the AUC between 0.5-0.7 implies poor accuracy, 0.7-0.9 moderate accuracy, and 0.9 high accuracy. In this view, the prediction model used in this investigation was highly accurate. Analysis of prognostic factors in adult JE in Taiwan revealed that CSF protein and dystonia were independent factors of poor prognosis (Lo et al., 2019). Another investigation of predictors of poor outcomes among JE survivors demonstrated that subjects who did not receive JE vaccination, were malnourished, had GCS ≤8 at admission, and required endotracheal intubation, had poorer outcomes with 77.8% sensitivity and 94.6% specificity (Srivastava et al., 2022). Sunwoo et al. (2017) demonstrated that midbrain injury and rapid disease deterioration were linked to severe neurological sequelae.
The calibration curve reflects the predicted probability of the prediction model consistency with the actual probability. Our prediction curve was consistent with the ideal curve, indicating that the prediction probability of the model concurred with reality, with high accuracy in predicting the poor prognosis of JE. Evaluating the goodness of fit of the logistic regression model is the key to the accuracy of estimated probabilities (Nattino et al., 2020). The Hosmer-Lemeshow test (P>0.05) revealed that the model was fitted and highly accurate. A visual nomogram was generated based on the five factors of JE poor prognosis, which can compute the total score according to the numerical value of each variable and then reveal the prediction probability of poor outcomes, providing clinicians with an early assessment of the risk of poor prognosis and make targeted prevention. Patients with increased CSF pressure, for example, should actively lower cranial pressure to prevent the occurrence of brain death and brain hernia. Patients with increased WBC and N% should consider early antibiotic usage to prevent and manage infection. These patients should also receive adequate nutritional support therapy. Early supportive treatment can limit the progression of JE and improve prognosis significantly. At the same time of basic treatment, strict control of body temperature, early invasive ventilator support therapy, and early consciousness disorder rehabilitation training can achieve significant curative effect (Gao et al., 2021). The comprehensive ICU treatment (hormones combined with anti-inflammatory, antiviral, and mild hypothermic cerebral protection therapies) can improve the survival rate(Gu et al., 2022). However, the effect of steroid use on outcome improvement remained inconsistent among studies. Lo et al. showed that no significant differences were observed between the good outcomes group and poor outcomes group in terms of steroid or antiviral treatments(Lo et al., 2019).
There are some limitations to this study. The number of JE patients in Shanxi Province has gradually declined in recent years due to economic growth, environmental improvement, mosquito control efforts, and increased public awareness of JE vaccination. Therefore, it is difficult to collect data on the long-term prognosis of patients after discharge due to the extended time range to collect enough cases retrospectively; thus, we used the GCS score at discharge to evaluate the prognosis. GCS score at admission has been linked to mortality, while GCS score at discharge is statistically significant for long-term prognosis. (Zhang et al., 2023). Second, due to the small sample size of the included studies and potential statistical errors in the model prediction procedure, the multivariate analysis of poor prognosis should be validated in the future using multi-sample and multi-center analyses. In addition, our study included limited influencing factors and some confounding factors that may have been overlooked. The different indicators and timely diagnosis and treatment influence patient prognosis in clinical practice.
5 Conclusion
Our study showed the epidemic dynamics and hotspots of JE in Shanxi Province. July to September was the primary epidemic season of JE and that JE cases were mainly in individuals over the age of 40. The age distribution of JE is steadily becoming more adult, with more severe complications and mortality and a higher disease burden, making adult JE a public health concern. Higher cerebrospinal fluid pressure, higher white blood cell counts, higher neutrophil percentage, deep coma and lower albumin were independent factors for poor outcomes of adult JE. The developed risk prediction model holds great promise in early prognosis assessment of patients, providing a basis for clinical decision-making and early clinical intervention.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
PZ : Writing – original draft. ZW: Writing – original draft. YL: Writing – review & editing. QW: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ashraf, U., Ding, Z., Deng, S., Ye, J., Cao, S., Chen, Z. (2021). Pathogenicity and virulence of Japanese encephalitis virus: Neuroinflammation and neuronal cell damage. Virulence 12, 968–980. doi: 10.1080/21505594.2021.1899674
Borah, J., Dutta, P., Khan, S. A., Mahanta, J. (2013). Epidemiological concordance of Japanese encephalitis virus infection among mosquito vectors, amplifying hosts and humans in India. Epidemiol. Infect. 141, 74–80. doi: 10.1017/S0950268812000258
Cao, L., Fu, S., Gao, X., Li, M., Cui, S., Li, X., et al. (2016). Low protective efficacy of the current Japanese encephalitis vaccine against the emerging genotype 5 Japanese encephalitis virus. PloS Negl. Trop. Dis. 10, e0004686. doi: 10.1371/journal.pntd.0004686
Chen, D.-D., Peng, X.-L., Cheng, H., Ma, J.-N., Cheng, M., Meng, L.-X., et al. (2022). Risk factors and a predictive model for the development of epilepsy after Japanese encephalitis. Seizure 99, 105–112. doi: 10.1016/j.seizure.2022.05.017
Chen, S.-T., Liu, R.-S., Wu, M.-F., Lin, Y.-L., Chen, S.-Y., Tan, D. T.-W., et al. (2012). CLEC5A regulates Japanese encephalitis virus-induced neuroinflammation and lethality. PloS Pathog. 8, e1002655. doi: 10.1371/journal.ppat.1002655
de Wispelaere, M., Frenkiel, M.-P., Desprès, P. (2015). A Japanese encephalitis virus genotype 5 molecular clone is highly neuropathogenic in a mouse model: impact of the structural protein region on virulence. J. Virol. 89, 5862–5875. doi: 10.1128/JVI.00358-15
Gao, L., He, W., Wang, L., Huang, C. (2021). Clinical characteristics of epidemic encephalitis B and the impact of early intervention on prognosis. J. Modern Med. Health 37, 400–403. doi: 10.3969/j.issn.1009-5519.2021.03.011
Gu, ,. H., Sun, L., Shen, X., Hu, W. (2022). A retrospective study of the clinical characteristics of Japanese encephalitis in adults. J. Integr. Neurosci. 21 (5), 125. doi: 10.31083/j.jin2105125
Hegde, N. R., Gore, M. M. (2017). Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vaccin Immunother. 13, 1–18. doi: 10.1080/21645515.2017.1285472
Hills, S. L., Netravathi, M., Solomon, T. (2023). Japanese encephalitis among adults: A review. Am. J. Trop. Med. Hyg 108, 860–864. doi: 10.4269/ajtmh.23-0036
Kafle, D. R., Subedi, M., Thapa, M. (2017). Outcome of patients with meningitis and encephalitis at tertiary care hospital in eastern Nepal. Kathmandu Univ Med. J. (KUMJ) 15, 40–44.
Li, X., Cui, S., Gao, X., Wang, H., Song, M., Li, M., et al. (2016). The spatio-temporal distribution of Japanese encephalitis cases in different age groups in mainland China 2004 - 2014. PloS Negl. Trop. Dis. 10, e0004611. doi: 10.1371/journal.pntd.0004611
Li, M. H., Fu, S. H., Chen, W. X., Wang, H. Y., Cao, Y. X., Liang, G. D. (2014). Molecular characterization of full-length genome of Japanese encephalitis virus genotype V isolated from Tibet, China. BioMed. Environ. Sci. 27, 231–239. doi: 10.3967/bes2014.046
Li, D., Zhang, X., Shi, T., Jin, N., Zhao, X., Meng, L., et al. (2022). A comparison of clinical manifestations of Japanese encephalitis between children and adults in Gansu Province, Northwest China, (2005-2020). Acta Trop. 231, 106449. doi: 10.1016/j.actatropica.2022.106449
Liu, M.-D., Li, C.-X., Cheng, J.-X., Zhao, T.-Y. (2022). Spatial statistical and environmental correlation analyses on vector density, vector infection index and Japanese encephalitis cases at the village and pigsty levels in Liyi County, Shanxi Province, China. Parasit Vectors 15, 171. doi: 10.1186/s13071-022-05305-8
Lo, S.-H., Tang, H.-J., Lee, S. S.-J., Lee, J.-C., Liu, J.-W., Ko, W.-C., et al. (2019). Determining the clinical characteristics and prognostic factors for the outcomes of Japanese encephalitis in adults: A multicenter study from southern Taiwan. J. Microbiol. Immunol. Infect. 52, 893–901. doi: 10.1016/j.jmii.2019.08.010
Ma, J., Jiang, L. (2013). Outcome of children with Japanese encephalitis and predictors of outcome in southwestern China. Trans. R Soc. Trop. Med. Hyg 107, 660–665. doi: 10.1093/trstmh/trt064
Mao, X., Zhou, H. (2020). The spatiotemporal distribution of Japanese Encephalitis cases in Yunnan Province, China, from 2007 to 2017. PloS One 15, e0231661. doi: 10.1371/journal.pone.0231661
Nattino, G., Pennell, M. L., Lemeshow, S. (2020). Assessing the goodness of fit of logistic regression models in large samples: A modification of the Hosmer-Lemeshow test. Biometrics 76, 549–560. doi: 10.1111/biom.13249
Oliveira, A. R. S., Strathe, E., Etcheverry, L., Cohnstaedt, L. W., McVey, D. S., Piaggio, J., et al. (2018). Assessment of data on vector and host competence for Japanese encephalitis virus: A systematic review of the literature. Prev. Vet. Med. 154, 71–89. doi: 10.1016/j.prevetmed.2018.03.018
Patabendige, A., Michael, B. D., Craig, A. G., Solomon, T. (2018). Brain microvascular endothelial-astrocyte cell responses following Japanese encephalitis virus infection in an in vitro human blood-brain barrier model. Mol. Cell Neurosci. 89, 60–70. doi: 10.1016/j.mcn.2018.04.002
Pham, D., Howard-Jones, A. R., Hueston, L., Jeoffreys, N., Doggett, S., Rockett, R. J., et al. (2022). Emergence of Japanese encephalitis in Australia: a diagnostic perspective. Pathology 54, 669–677. doi: 10.1016/j.pathol.2022.07.001
Rajaiah, P., Kumar, A. (2022). Japanese encephalitis virus in India: An update on virus genotypes. Indian J. Med. Res. 156, 588–597. doi: 10.4103/ijmr.IJMR_2606_19
Ren, X., Fu, S., Dai, P., Wang, H., Li, Y., Li, X., et al. (2017). Pigsties near dwellings as a potential risk factor for the prevalence of Japanese encephalitis virus in adult in Shanxi, China. Infect. Dis. Poverty 6, 100. doi: 10.1186/s40249-017-0312-4
Sarkar, A., Datta, S., Pathak, B. K., Mukhopadhyay, S. K., Chatterjee, S. (2015). Japanese encephalitis associated acute encephalitis syndrome cases in West Bengal, India: A sero-molecular evaluation in relation to clinico-pathological spectrum. J. Med. Virol. 87, 1258–1267. doi: 10.1002/jmv.24165
Sharma, M., Sharma, K. B., Chauhan, S., Bhattacharyya, S., Vrati, S., Kalia, M. (2018). Diphenyleneiodonium enhances oxidative stress and inhibits Japanese encephalitis virus induced autophagy and ER stress pathways. Biochem. Biophys. Res. Commun. 502, 232–237. doi: 10.1016/j.bbrc.2018.05.149
Shi, T., Meng, L., Li, D., Jin, N., Zhao, X., Zhang, X., et al. (2022a). Effect of different vaccine strategies for the control of Japanese encephalitis in mainland China from 1961 to 2020: A quantitative analysis. Vaccine 40, 6243–6254. doi: 10.1016/j.vaccine.2022.09.030
Shi, T., Meng, L., Li, D., Jin, N., Zhao, X., Zhang, X., et al. (2022b). Impact of the expanded program on immunization on the incidence of Japanese encephalitis in different regions of Mainland China: An interrupt time series analysis. Acta Trop. 233, 106575. doi: 10.1016/j.actatropica.2022.106575
Solomon, T., Thao, T. T., Lewthwaite, P., Ooi, M. H., Kneen, R., Dung, N. M., et al. (2008). A cohort study to assess the new WHO Japanese encephalitis surveillance standards. Bull. World Health Organ 86, 178–186. doi: 10.2471/blt.07.043307
Song, S., Yao, H., Yang, Z., He, Z., Shao, Z., Liu, K. (2020). Epidemic changes and spatio-temporal analysis of Japanese encephalitis in shaanxi province, China –2018. Front. Public Health 8. doi: 10.3389/fpubh.2020.00380
Srivastava, N., Deval, H., Mittal, M., Deoshatwar, A., Bondre, V. P., Kant, R., et al. (2022). Extent of disability among paediatric Japanese encephalitis survivors and predictors of poor outcome: a retrospective cohort study in North India. BMJ Open 12, e060795. doi: 10.1136/bmjopen-2022-060795
Su, Q., Xie, Z.-X., He, F., Liu, Z.-C., Song, X.-J., Zhao, F.-C., et al. (2021). Adults with severe Japanese encephalitis: a retrospective analysis of 9 cases in Linyi, China. Neurol. Sci. 42, 2811–2817. doi: 10.1007/s10072-020-04867-8
Sunwoo, J.-S., Lee, S.-T., Jung, K.-H., Park, K.-I., Moon, J., Jung, K.-Y., et al. (2017). Clinical characteristics of severe Japanese encephalitis: A case series from South Korea. Am. J. Trop. Med. Hyg 97, 369–375. doi: 10.4269/ajtmh.17-0054
Symptoms & Treatment (2022) Japanese encephalitis (CDC). Available at: https://www.cdc.gov/Japaneseencephalitis/symptoms/index.html (Accessed June 20, 2023).
Tu, T., Xu, K., Xu, L., Gao, Y., Zhou, Y., He, Y., et al. (2021). Association between meteorological factors and the prevalence dynamics of Japanese encephalitis. PloS One 16, e0247980. doi: 10.1371/journal.pone.0247980
Wang, X., Su, L., Sun, S., Hu, W., Mu, Q., Liang, X., et al. (2021). Long-term neurological sequelae and disease burden of Japanese encephalitis in gansu province, China. Ann. Glob Health 87, 103. doi: 10.5334/aogh.3343
Xiong, W., Lu, L., Xiao, Y., Li, J., Zhou, D. (2019). Mortality and disability due to Japanese encephalitis in elderly adults: evidence from an adult tertiary care center in west China. Front. Neurol. 10. doi: 10.3389/fneur.2019.00918
Yin, Z., Wang, X., Li, L., Li, H., Zhang, X., Li, J., et al. (2015). Neurological sequelae of hospitalized Japanese encephalitis cases in Gansu province, China. Am. J. Trop. Med. Hyg 92, 1125–1129. doi: 10.4269/ajtmh.14-0148
Zhang, F., Xu, G., Zhang, X., Li, Y., Li, D., Wang, C., et al. (2023). Clinical characteristics and short-term outcomes of Japanese encephalitis in pediatric and adult patients: a retrospective study in Northern China. Front. Neurol. 14. doi: 10.3389/fneur.2023.1135001
Keywords: Japanese encephalitis, spatiotemporal, prognostic, epidemiological, nomogram
Citation: Zheng P, Wen Z, Liu Y and Wang Q (2023) The spatiotemporal distribution and prognostic factors of Japanese encephalitis in Shanxi Province, China, 2005–2022. Front. Cell. Infect. Microbiol. 13:1291816. doi: 10.3389/fcimb.2023.1291816
Received: 19 September 2023; Accepted: 04 December 2023;
Published: 21 December 2023.
Edited by:
Hong Liu, Shandong University of Technology, ChinaReviewed by:
Luis Del Carpio-Orantes, Delegación Veracruz Norte, MexicoPrasad Liyanage, New York University, United States
Eugenia M. Bastos, National Center for Chronic Disease Prevention and Health Promotion, United States
Copyright © 2023 Zheng, Wen, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinying Wang, d2FuZ3F5ODExOEAxNjMuY29t
†These authors have contributed equally to this work
 Peiyu Zheng1,2†
Peiyu Zheng1,2† Yuan Liu
Yuan Liu Qinying Wang
Qinying Wang