- 1Shanghai Institute of Immunology, Department of Immunology and Microbiology, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Microbiology and Immunology, Kunming Medical University, Kunming, China
- 3National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention (Chinese Center for Tropical Diseases Research), World Health Organization (WHO) Collaborating Centre for Tropical Diseases, National Health Commission (NHC) Key Laboratory of Parasite and Vector Biology; National Center for International Research on Tropical Diseases, Shanghai, China
- 4School of Global Health, Chinese Center for Tropical Diseases Research, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Introduction: Clonorchis sinensis infection results in various complications in the liver and biliary systems and is a neglected tropical disease in Eastern Asia. In this study, we report that C. sinensis calcium-binding protein Cs16 activates host immune cells and induces immunopathology in liver.
Methods: Immunohistochemistry was used to detect the localization of Cs16 in C. sinensis adult worms. ELISA was used to detect the serum levels of anti-Cs16 IgG antibody in infected humans and mice. Bile duct injection model was used to figure out the role of Cs16 in vivo. RT-qPCR and ELISA were used to detect the cytokine production from Cs16-treated BMMs in vitro. Seahorse assay was used to detect the metabolic pathway of Cs16-treated BMMs in vitro.
Result: Cs16 localizes in the tegument and gut of C. sinensis. Humans and mice with C. sinensis infection exhibited increased levels of anti-Cs16-specific antibody. Using the bile duct injection technique, we found that Cs16 induced obvious inflammation and hepatic necrosis in vivo. Cs16 treatment caused the upregulation of inflammatory cytokines in innate immune cells. Moreover, Cs16-treated monocytes relied more on the glycolytic metabolic pathway.
Discussion: Our findings suggest that Cs16 is a potential pathogenic factor derived from C. sinensis adult worm. By reprogramming the metabolic pathway of innate immune cells, Cs16 triggers pro-inflammatory responses in the liver, and therefore, Cs16 is a potential target for the prevention and treatment of clonorchiasis.
1 Introduction
Clonorchis sinensis is one of the regionally epidemic zoonotic parasites, affecting more than 15 million people around the world, and is mainly endemic in China, South Korea, Vietnam, and part of Russia (Yoshida, 2012; Lee et al., 2018). The adult flukes living in the biliary trees of the liver induce chronic inflammation, periductal fibrosis, and even the adenomatous hyperplasia of biliary epithelial cells, all of which are closely related to the occurrence of cholangiocarcinoma (Kim et al., 2016). Therefore, C. sinensis was listed as a Group 1 carcinogen by the International Agency for Research on Cancer (Bouvard et al., 2009).
The pathogenesis of C. sinensis infection encompasses several factors, including mechanical obstruction and injury by the worms, and immunopathology caused by infection-related inflammation (van Tong et al., 2017). Inflammation in clonorchiasis can be induced by secondary bacterial infection and toxic effects of the worms’ tegumental proteins or excretory–secretory products (ESPs) (Qian et al., 2015). For example, ESPs from C. sinensis trigger increases in free radicals and NF-κB-mediated inflammation (Nam et al., 2012). It is a key issue to identify important molecules contributing to C. sinensis infection-related pathology, which might serve as drug and vaccine targets.
Elongation factor hand (EF-hand) structure proteins are ubiquitously expressed in most of helminths and bind with Ca2+, exerting diverse biological functions (Thomas and Timson, 2016). These calcium-binding proteins induce host immune response and specific antibody production. For example, SjTP22.4 is a calcium-binding tegumental protein of Schistosoma japonicum. SjTP22.4 immunization may relieve the symptom of schistosomiasis by inducing host IgG production (Zhang et al., 2012). Our previous research showed that S. japonicum egg specific protein SjE16.7 can recruit myeloid innate immune cells and promote the formation of inflammatory hepatic granuloma (Wu et al., 2014; Fang et al., 2015). Cs16 is a C. sinensis 16-kDa calcium-binding protein, which has an EF-hand motif and is a homologue of SjE16.7. In this study, we report that Cs16 serves as a pathogenic factor of C. sinensis. Distributed in the tegument and gut of worm, Cs16 activates host immunocytes by regulating cell metabolism and then triggers inflammation in the liver.
2 Materials and methods
2.1 Ethics statement
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University School of Medicine (project number A-2019-053, 069).
Human subject research was approved by the Ethics Committee of the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention in Shanghai, China (reference no. 2011-005). All individuals or their guardians for those aged <18 years have provided written informed consent.
2.2 Mice
Six- to eight-week-old male C57BL/6 or BALB/c mice were purchased from Shanghai Laboratory Animal Center, Chinese Academy of Sciences. Mice were housed under specific pathogen-free conditions and fed autoclaved food and water.
2.3 Cells
Bone marrow-derived monocytes (BMMs) were harvested from tibias and femurs of mice. Seeded on the petri dishes, cells were cultured in Dulbecco′s Modified Eagle′s Medium (DMEM, Thermo Fisher Scientific, USA) containing 10% heat-inactivated FBS, 2 mM L-glutamine, and 100 U/mL penicillin/streptomycin supplemented with 50 ng/mL M-CSF (R&D Systems, USA). Four days later, cells were dissociated with 5 mM EDTA·2Na and considered as BMMs. For further experiments, BMMs were stimulated with 5 μg/mL Cs16 or 5 μg/mL CsAWA for 0, 24, and 48 h, respectively, unless noted otherwise.
CCLP, RBE, and HuCCT1 human cholangiocarcinoma cell lines were cultured in DMEM containing 10% heat-inactivated FBS, 2 mM L-glutamine, and 100 U/mL penicillin/streptomycin.
2.4 C. sinensis infection
C. sinensis metacercariae were collected from Pseudorasbora parva from a clonorchiasis endemic area in China through the digestion method and 6-week-old BALB/c mice were infected with 50 metacercariae of C. sinensis by gastric gavage. Mice were then sacrificed after 28 weeks post-infection and sera were obtained for anti-CsAWA IgG and anti-Cs16 IgG antibody detection.
2.5 Preparation of C. sinensis adult worm antigen
C. sinensis adult worm antigen (CsAWA) was prepared as described previously (Kan et al., 2022). In brief, C. sinensis adult worms suspended in PBS were lysed by sonication in an ice-chilled water bath. Then, the lysed homogenate was centrifuged at 15,000 g for 20 min at 4°C. The supernatant was dialyzed against PBS at 4°C overnight and used as CsAWA. Protein concentration was measured by the BCA Protein Assay Kit (Sangon Biotech, China).
2.6 Expression and purification of recombinant Cs16
The eukaryotic expression system has been successfully established in our own lab and recombinant Cs16 from yeast expression system was prepared as described previously (PU et al., 2018). Briefly, to express the eukaryotic Cs16 in vitro, a pair of primers designed for target gene amplification was listed below: 5′-TCCGGAATTCCATCATCATCATCATCATATGCGATTTAGTTCACATGAGC-3′ (Forward) and 5′-TTGCGGCCGCTTATTCAGTATCTCCTGAACC-3′ (Reverse). The vector pPIC9K (Invitrogen, USA) subcloned with the Cs16 coding gene was transformed in Pichia pastoris strain GS115. The generated protein was fused with His-tag at the N terminal for affinity purification. The recombinant His-Cs16 collected from the yeast culture supernatant was purified by Ni-NTA Superflow Cartridges (QIAGEN, Germany) according to the manufacturer’s instructions. The molecular weight and purity of recombinant proteins were identified by SDS-PAGE. Protein concentration was measured by the BCA Protein Assay Kit. Denatured Cs16 was obtained with a 95°C, 30-min treatment.
2.7 Preparation of polyclonal antibody
Cs16 antiserum was prepared in C57BL/6 mice. Cs16 (50 μg) was formulated with either Freund′s complete (primary) or Freund′s incomplete (two boosts at 2-week intervals) adjuvant (Sigma-Aldrich, USA) and injected subcutaneously into mice. Mice were sacrificed 2 weeks after the final antigen immunization. Immunoglobulins from sera were first precipitated with ammonium sulfate and then purified using Protein A/G PLUS-Agarose Beads (Santa Cruz Biotechnology, USA) according to the manufacturer’s instructions.
2.8 Immunohistochemical assay
To determine the localization of Cs16 in C. sinensis adult worms, worms were fixed in 4% PFA, embedded in paraffin, and sliced at 3- to 5-μm thicknesses. Paraffin sections were deparaffinized and rehydrated, and then the heat-induced antigen retrieval method was used. Sections were blocked with 3% BSA for 45 min and then incubated with mouse anti-Cs16 polyclonal antibody diluted 1:50 in PBS overnight at 4°C. Identical concentration of control antibody from normal mouse sera was used as negative control. Sections were washed three times with PBS and then incubated with HRP-conjugated anti-mouse IgG (Cell Signaling Technology, USA) for 1 h at room temperature in the dark. After washing, sections were developed using the DAB (Diaminobenzidine) substrate system (Maxim, China). Counterstaining was done with hematoxylin. Sections were examined with an Olympus BX51 microscope (Olympus, Japan). Positive area was calculated with ImageJ software (NIH, USA).
2.9 Enzyme-linked immunosorbent assay
Antibody reactivity of humans or mice sera against Cs16 or CsAWA was determined by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated with 100 μL of 100 μg/mL Cs16 or 2.5 μg/mL CsAWA overnight at 4°C. Sera were diluted 1:100 and HRP-conjugated goat anti-human IgG (Sigma-Aldrich, USA, 1:5,000 dilution) or goat anti-mouse IgG (Invitrogen, USA, 1:5,000 dilution) was used as the secondary antibody. Next, reactions were developed using 3,3′,5,5′-tetramethylbenzidine (TMB) substrates and stopped with 2 N H2SO4. The optical densities were read at 450 nm in a microwell reader system (Biotek, USA).
The levels of IL-1β, IL-6, and TNF-α in the supernatant of BMMs were measured by commercial ELISA kits (R&D Systems, USA) according to the manufacturer’s instructions.
2.10 Bile duct injection model
The bile duct injection technique was performed according to the protocol (Berntsen et al., 2018). In brief, 8-week-old male C57BL/6 mice were administered general anesthesia with pentobarbital sodium. For the surgery, median laparotomy was performed with a 15- to 20-mm vertical incision of the skin and peritoneum in line with the linea alba caudally from the sternum. The common bile duct (CBD) was firstly clamped. Fine-precision surgical equipment were subsequently used for the gall bladder catheterization; forceps with marked holding strength that close flat and have gently blunted tips to avoid tissue puncture were used for grasping the gall bladder. The gall bladder incision was made with the aid of sharp microscissors and to guide the catheter through the small incision using serrated, slightly curved forceps. The 20-μL fluid containing 20 μg of Cs16 or CsAWA was injected into the biliary tree. The control mice were injected with equal volume of PBS. After injection, the bulldog clamp was removed from the CBD. A loop of nonabsorbable 7-0 suture was made at the cystic bile duct directly in front of the catheter that was then removed by gentle retraction while performing a functional cholecystectomy by closing the suture completely. Approximately 50 μL of sterile physiological saline preheated to 37°C was administered in the abdominal cavity to avoid drying of the internal organs before the peritoneal and skin incisions were closed by suture and clips, respectively. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured by Beckman-Coulter chemistry analyzer AU5800.
2.11 Histology and scoring
PFA-fixed liver tissues were embedded in paraffin, cut in 3- to 5-μm sections, and deparaffinized. Serial sections were stained with hematoxylin–eosin (H&E). Sections were examined with an Olympus BX51 microscope (Olympus, Japan). Mouse histological activity index (mHAI) of liver sections was scored according to the protocol (Goodman, 2007).
2.12 Cytometric bead array
Key serum cytokines for mouse inflammation, including IL-1β, IL-6, IL-10, TNF-α, and IFN-β were measured using a LEGENDplex™ Mouse Inflammation Panel Kit (BioLegend, USA). The data were acquired on a BD FACSCanto™ II flow cytometer, analyzed using LEGENDplex™ Data Analysis Software, and calculated by standard curves according to the manufacturer’s instructions.
2.13 Cell proliferation assay
Cell proliferation was quantified using Cell Counting Kit-8 (CCK-8, YEASEN, China) according to the manufacturer’s instructions. Briefly, cells were seeded into 96-well plates and incubated with 10 μL per well of the reagent for 1 h at 37°C and proliferation was quantified by measuring the absorbance at 450 nm using a microplate reader.
2.14 RNA isolation and real-time quantitative PCR
Total RNA was extracted from BMMs or liver tissues with the High Pure RNA Isolation Kit (Roche, Germany) in accordance with the manufacturer’s instructions. RNA quality (nucleic acid concentration, A260/280 and A260/230 ratios) was examined using a NanoDrop2000 (Thermo Fisher Scientific, USA) microvolume UV-Vis spectrophotometer. RNA (1 μg) was used for first-strand cDNA synthesis. Mouse complementary DNA (cDNA) was reverse-transcribed with the RevertAid First Strand cDNA Synthesis Kit (Invitrogen, USA) and real-time quantitative PCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems, USA) using FastStart Universal SYBR Green Master Mix (Roche, Germany). Thermocycler conditions comprised an initial holding at 50°C for 2 min and a subsequent holding at 95°C for 10 min, which was followed by a two-step PCR program at 95°C for 15 s and 60°C for 60 s for 40 cycles. Relative gene expression was normalized to β-actin. Primer sequences are listed in Supplementary Table 1.
2.15 Measurement of nitric oxide production
To determine nitric oxide (NO) production, BMMs were stimulated with 5 μg/mL Cs16 or CsAWA for 48 h. NO was measured in the supernatant of the cultured cells as nitrite using an NO assay kit (Beyotime, China), according to the manufacturer’s instructions. Briefly, a standard curve was prepared with standard nitrite solutions in DMEM. The standard solutions or cell supernatants were reacted with nitrate reductase for 30 min in a 96-well plate, and then Griess reagent I and Griess reagent II were added. After incubation at room temperature for 10 min, the absorbance at 540 nm was read in a microplate reader (Thermo Fisher Scientific, USA).
2.16 ATP assay
BMMs seeded on the 96-well plates were lysed with ATP lysis buffer (Beyotime Biotechnology, China) on ice. Cell lysates were added to a reaction mixture containing luciferase and luciferin for bioluminescence quantification. Relative light unit (RLU) was measured by a luminometer Gen5 (Biotek, USA). ATP level was normalized to total protein content by BCA assay.
2.17 Oxygen consumption and extracellular acidification measurement
Extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were determined using a Seahorse XFe96 Extracellular Flux Analyzer (Agilent Technologies, USA) with commercial kits. BMMs were seeded in specialized cell culture microplates at a density of 1×105 cells/well. One day prior to the measurement, a Seahorse XFe96 cartridge was loaded with 200 μL of XF Calibrant solution per well and incubated overnight at 37°C in a CO2-free atmosphere. One hour before the measurement, cells were resuspended with XF base medium and incubated at 37°C in a CO2-free atmosphere. The ports of the Seahorse cartridge were loaded with glucose, oligomycin, and 2-DG for the glycolysis stress test and oligomycin, FCCP, rotenone/antimycin A for the mitochondrial stress test. After sensor calibration, assays were run as detailed in the manufacturer’s manuals recording ECAR and OCR over time. Metabolic parameters were derived from the XF Wave software. All experiments were performed in quadruplicates. Raw values were normalized to the total protein content for each well.
2.18 Statistical analyses
The data were presented as mean ± s.e.m. from two or more independent experiments. Statistical analyses were performed using GraphPad Prism 8 software. Statistical significance was calculated using the unpaired, two-tailed Student’s t-test or one-way ANOVA followed by post-hoc Tukey′s test as detailed in the figure legends. p-values < 0.05 were considered to indicate a significant difference. Asterisks used to indicate significance correspond to the following: *p < 0.05, **p < 0.01, ***p < 0.005.
3 Results
3.1 Cs16 is expressed in C. sinensis and recognized by the host immune system
To identify the characteristics of Cs16, we prepared recombinant Cs16 from P. pastoris and anti-Cs16-specific antibodies from mice. Then, we probed the sites of Cs16 expression within adult C. sinensis. Cs16 exhibited ubiquitous expression through all tissues, particularly in the gut and tegument of adult worms (Figure 1), suggesting that it could be involved in the interaction between worms and hosts.
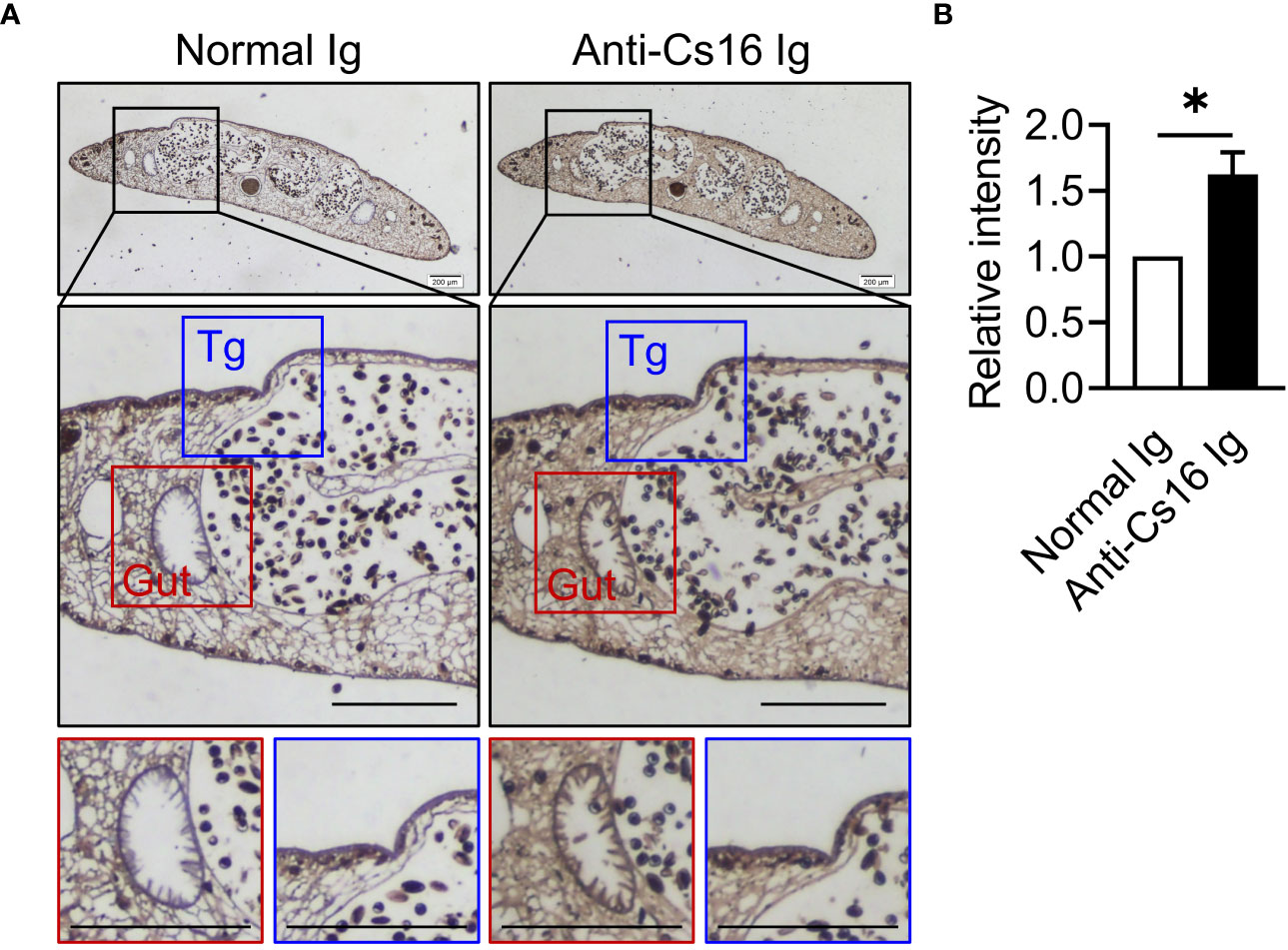
Figure 1 Immunolocalization of Cs16 in adult C. sinensis. (A) The representative immunohistochemical images of Cs16 in C. sinensis adult worms. Left panel: normal Ig (negative control) as primary antibody. Right panel: anti-Cs16 Ig as primary antibody. Scale bar, 200 μm. Tg, tegument. (B) The relative intensity of positive area. The data were shown as the mean ± s.e.m., *p < 0.05, unpaired two-tailed Student’s t-test.
We then asked the antigenicity of Cs16, whether this protein provokes immune responses during infection with C. sinensis. To address this question, mice were infected with C. sinensis metacercariae. The antibody production against C. sinensis adult extract (CsAWA) and Cs16 was detected by ELISA. The ELISA results showed that the infected mice developed specific antibodies against Cs16 as CsAWA, while sera from control mice without infection revealed no specific antibodies (Figures 2A, B). Similar phenotypes were observed in C. sinensis-infected people (Figures 2C, D), implying that Cs16 can be recognized by the host immune system and induce immune responses.

Figure 2 Serum levels of anti-Cs16 and anti-CsAWA IgG in C. sinensis-infected mice and human. (A, B) Serum anti-Cs16 and anti-CsAWA IgG levels of control mice (n = 5) and C. sinensis-infected mice (n = 4). (C, D) Serum anti-Cs16 and anti-CsAWA IgG levels of human with healthy control (n = 23) and C. sinensis-infected people (n = 24). CsAWA, C. sinensis adult worm antigen. The data were shown as the mean ± s.e.m., *p < 0.05, **p < 0.01, ***p < 0.005, unpaired two-tailed Student’s t-test.
3.2 Cs16 results in inflammation and acute hepatic injury in vivo
Since C. sinensis adults live in bile ducts, we used bile duct injection technique to explore the role of Cs16 in vivo (Figure 3A). Results showed that liver tissues of both Cs16-treated and CsAWA-treated mice exhibited more inflammatory cell infiltration and severe necrosis of liver parenchyma than PBS-treated mice (Figure 3B). Meanwhile, serum ALT and AST levels, which reflect the extent of liver damage, were significantly increased after Cs16 and CsAWA treatment (Figure 3C). Elevated inflammatory cytokines secreted by inflammatory cells, such as Il-1β, Il-6, and Tnf-α, were also observed in liver tissues of both Cs16-treated and CsAWA-treated mice (Figure 3D). Meanwhile, serum IL-1β and IL-6 levels were increased after Cs16 or CsAWA injected into the bile ducts of mice (Figure 3E). The above results indicated that Cs16 can promote inflammation and hepatic injury in vivo.
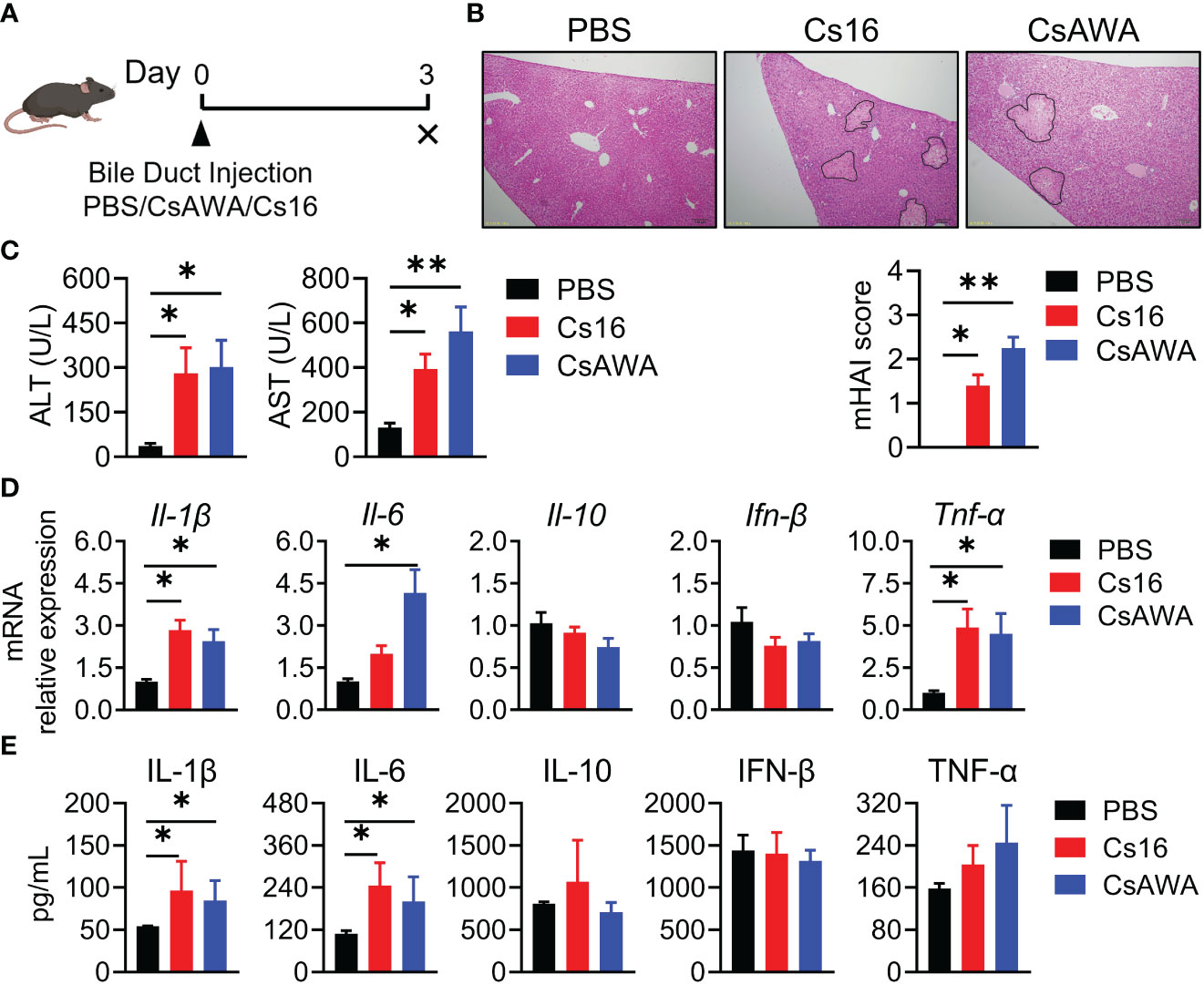
Figure 3 Pathology of bile duct injection with Cs16 and CsAWA. (A) Schematic diagram of the bile duct injection technique. (B) Representative images of liver tissues after Cs16 and CsAWA injection (upper panel) and mouse hepatic activity index (mHAI, lower panel). (C) Serum levels of ALT (left panel) and AST (right panel) in mice injected with PBS, Cs16, and CsAWA for 72 h. (D) Transcript levels of Il-1β, Il-6, Il-10, Ifn-β, and Tnf-α in the liver of mice injected with PBS, Cs16, or CsAWA for 72 h. (E) Serum levels of IL-1β, IL-6, IL-10, IFN-β, and TNF-α in mice injected with PBS, Cs16, or CsAWA for 72 h. CsAWA, C. sinensis adult worm antigen. ALT, alanine aminotransferase. AST, aspartate aminotransferase. The data were shown as the mean ± s.e.m., PBS, n = 5; Cs16, n = 5; CsAWA, n = 4. *p < 0.05, **p < 0.01, one-way ANOVA followed by post-hoc Tukey’s test.
3.3 Cs16 promotes inflammatory cytokine secretion of BMMs
We then test the roles of Cs16 in vitro. Cs16 has no cytotoxicity under the concentration of 5 μg/mL, and the proliferation of cholangiocytes and immunocytes was not influenced (Supplementary Figures 1, 2). Given the pro-inflammatory property of SjE16.7 and the phylogenetic similarity between SjE16.7 and Cs16, we detected the effects of Cs16 on inflammatory cytokine production. BMMs were challenged with Cs16. Enhanced inflammatory cytokine release such as Il-1β, Il-6, Ifn-β, and Nos2, an inflammatory enzyme, in transcriptional levels was found in cells treated with Cs16 for 48 h. Similarly, TNF-α, IL-6, and NO levels in the supernatant of the cultured cells were increased after Cs16 treatment (Figure 4). These results indicated that Cs16 activates BMMs and induces a release of pro-inflammatory cytokines.
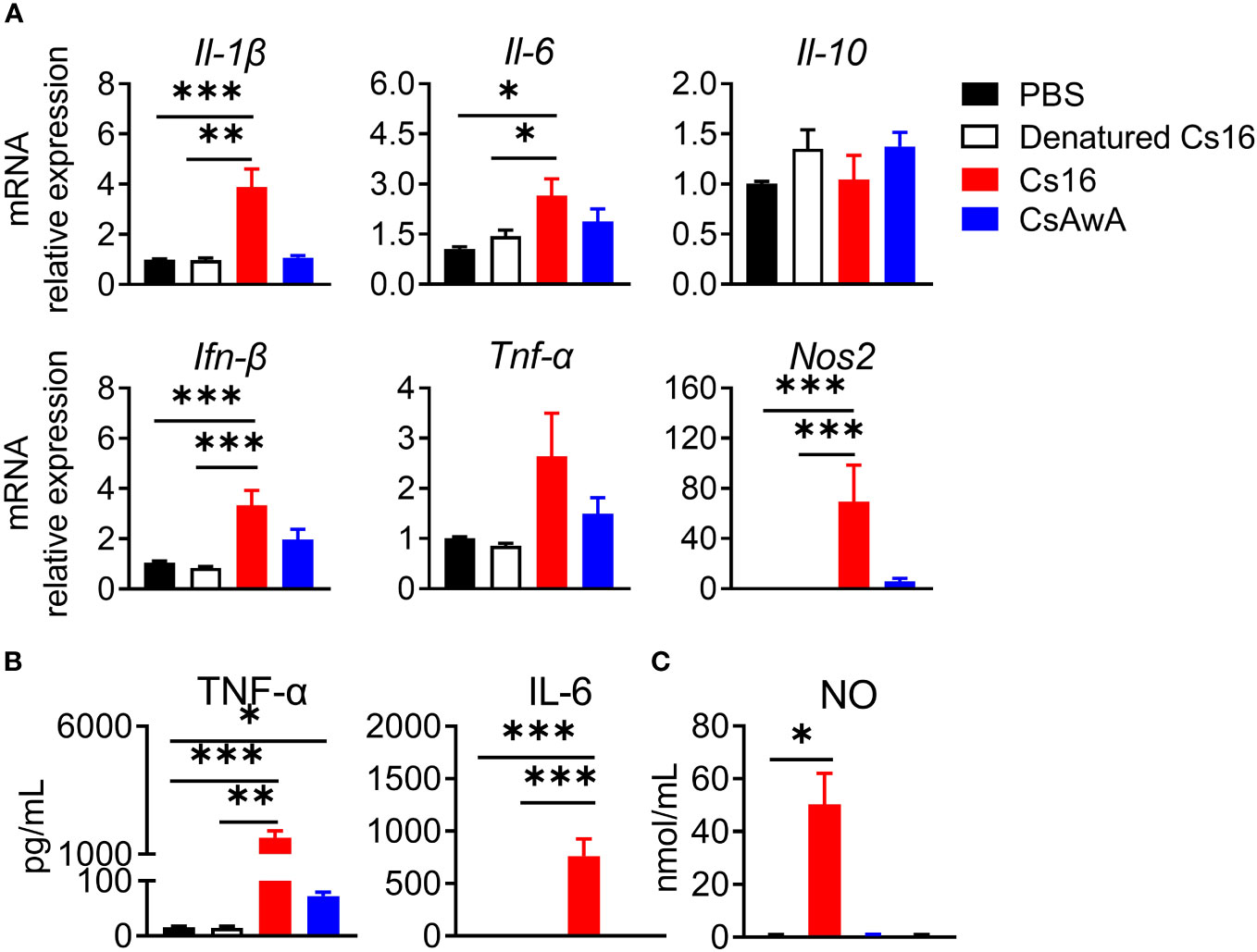
Figure 4 Inflammatory cytokine levels in the supernatant of BMMs after Cs16 and CsAWA treatment. (A) Transcript levels of Il-1β, Il-6, Il-10, Ifn-β, Tnf-α, and Nos2 in BMMs treated with Cs16 or CsAWA for 48 h. (B) TNF-α (left panel) and IL-6 (right panel) levels in the supernatant of BMMs treated with Cs16 or CsAWA for 48 h. (C) NO levels in the supernatant of BMMs treated with Cs16 or CsAWA for 48 h. CsAWA, C. sinensis adult worm antigen. The data were representative of two or three independent experiments (biological replicates) and shown as the mean ± s.e.m., *p < 0.05, **p <0.01, ***p < 0.005, one-way ANOVA followed by post-hoc Tukey’s test.
3.4 Cs16-treated BMMs prefer glycolysis for energy generation
Immune cell activation favors the ATP production. We detected the effects of Cs16 on cellular ATP levels. The results showed that BMMs stimulated with Cs16 or CsAWA produced more ATP than controls (Figure 5A). It is well known that the main source of ATP derives from glycolysis or oxidative phosphorylation. Therefore, we assessed the ECAR and OCR of cells treated with Cs16 and CsAWA. Results showed that ECAR was significantly increased after Cs16 or CsAWA stimulation (Figure 5B); meanwhile, there was no difference between control and antigen stimulation in OCR (Figure 5C). The above results suggested that BMMs stimulated with Cs16 relied more on glycolysis for energy metabolism.
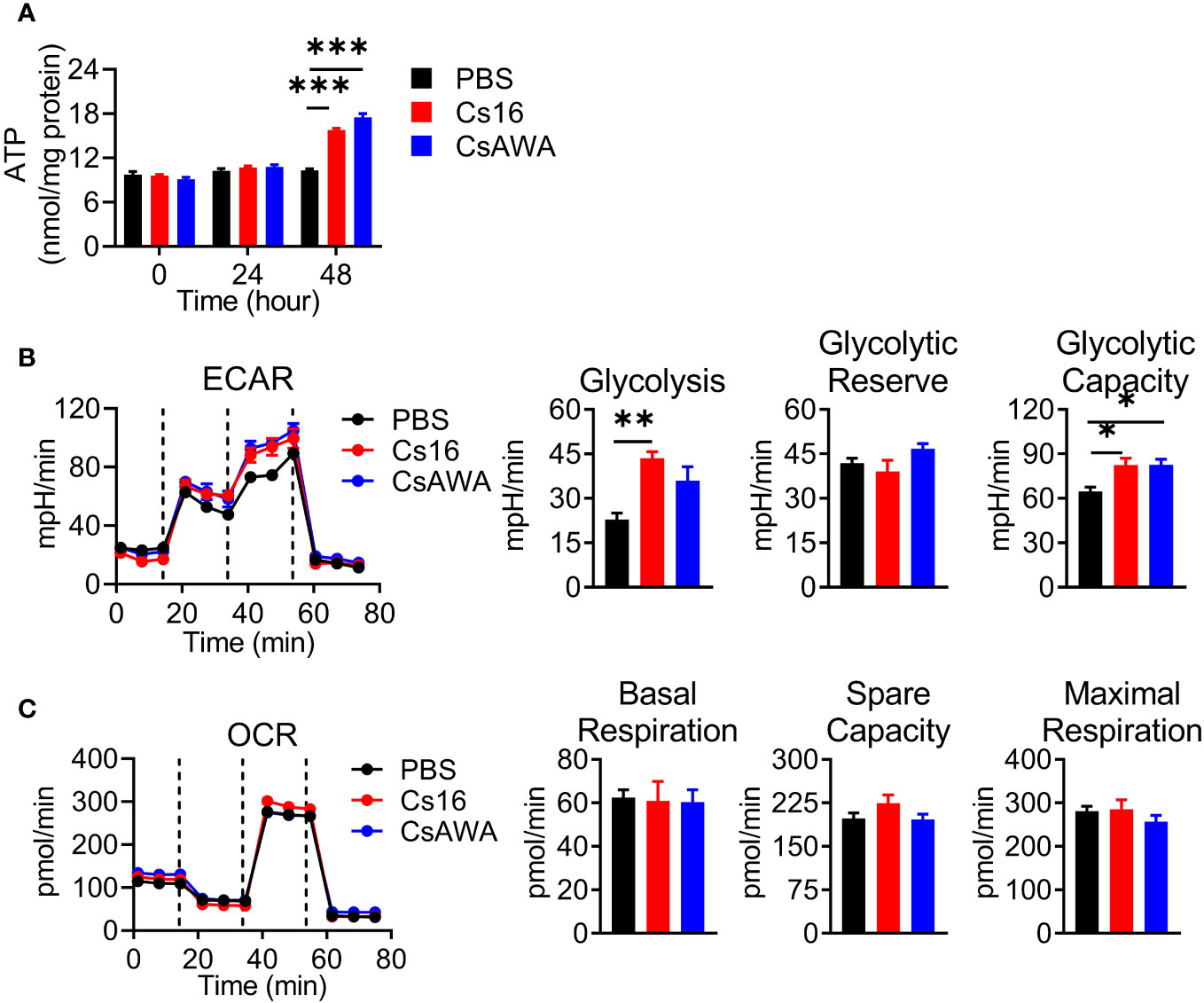
Figure 5 Cellular ATP, ECAR, and OCR levels after Cs16 and CsAWA treatment. (A) ATP levels in BMMs treated with Cs16 or CsAWA for 48 h. (B) Dynamic curves of ECAR (left panel), glycolysis, glycolytic reserve, and glycolytic capacity (right panel) in BMMs treated with Cs16 or CsAWA for 48 h. (C) Dynamic curves of OCR (left panel), basal respiration, spare capacity, and maximal respiration (right panel) in BMMs treated with Cs16 or CsAWA for 48 h. CsAWA, C. sinensis adult worm antigen. The data were representative of two or three independent experiments (biological replicates) and shown as the mean ± s.e.m., *p < 0.05, **p < 0.01, ***p < 0.005, one-way ANOVA followed by post-hoc Tukey’s test.
4 Discussion
Clonorchiasis is one of the most important neglected tropical diseases in Eastern Asia (Lai et al., 2016). C. sinensis adults, which dwell in bile ducts, cause hepatic and biliary disease with biliary epithelial hyperplasia, periductal fibrosis, and cystic changes in the ducts and even facilitate the development of cholangiocarcinoma (Na et al., 2020). However, the component of C. sinensis that plays a role in the pathogenesis is not well explored. As a C. sinensis adult worm-derived protein, Cs16 can induce host immune responses. This conclusion is based on the following observations: (a) Cs16 localizes in the tegument and gut of C. sinensis adult worms; (b) the levels of anti-Cs16 IgG antibody are increased in both humans and mice with C. sinensis infection; (c) Cs16 activates BMMs and promotes inflammatory cytokines release; and (d) Cs16 induces acute hepatic injury in vivo.
Cs16 protein has low molecular weight and a conserved calcium-binding motif called the EF-hand, which is similar to S100 protein families in humans (Sreejit et al., 2020). Several S100 proteins are considered as damage-associated molecular pattern molecules (DAMPs), and associated with infection, cellular stress, tissue damage, and cancer (Lim et al., 2011). It has been reported that S100 proteins induce inflammatory responses during bacteria and virus infection (Tsai et al., 2014; Zackular et al., 2015; Kozlyuk et al., 2019). Bai et al. found that inhibition of S100A9 alleviates acute liver injury, which is accompanied by M2-like macrophages activation (Bai et al., 2021). In our study, we observed Cs16 promotes glycolytic pathway (a characteristic of M1-like macrophages) and acute liver injury, indicating the pro-inflammatory role of Cs16. Host immune responses after C. sinensis infection is a dynamic process. Kim et al. reported that hepatic macrophages preferentially differentiated into M1 macrophages during the early stages of C. sinensis infection, but the numbers of M2 macrophages increased during the fibrotic and cirrhotic stage of infection (Kim et al., 2017). It is of interest to investigate the roles of Cs16 in chronic infection.
In this study, we used the bile duct injection model and examined inflammatory cytokines after antigen treatment for 72 h, which is usually considered to be in the acute immune response stage (4–96 h post-infection or stimulation). Monocytes and macrophages are key components of the innate immune system and are involved in the development of acute inflammatory disorders. In addition, it has been reported that monocytes play important roles in cholangitis (Kunzmann et al., 2020). In this study, we found that Cs16 activates BMMs and induces inflammatory cytokine production in vitro; thus, we propose that Cs16 causes acute hepatic injury by activating BMMs. Besides monocytes, Cs16 may also activate other cells and contribute to inflammation. It needs to be further studied. Excretory–secretory proteins (ESPs) of liver flukes are critical for the pathogenesis, nutrient metabolism, etiology, and immune response of liver cancer. Our data showed that Cs16 localizes in the gut and tegument of C. sinensis adults and anti-Cs16 IgG is detected in infected mouse or human sera, suggesting that Cs16 may secrete from adults and be recognized by the host. However, Shi et al. identified the ESPs of C. sinensis after culture for 6 h, and results showed that there exist some kind of calcium-binding proteins, in which Cs16 is not listed (Shi et al., 2020). It might be due to the fact that ESPs obtained from the in vitro culture of C. sinensis adult may not fully mimic the true microenvironment in vivo. The appropriate method to check whether Cs16 belongs to ESPs or not is worth exploring in the future.
Calcium-binding proteins are a heterogeneous and wide group of proteins that participate in numerous cellular functions (Yanez et al., 2012). Chung et al. reported that CsCa8 was derived from C. sinensis adult worms and possessed an EF-hand structure, which localized in the oral and ventral suckers of the adult worm (Chung et al., 2013). Kim et al. reported a 21.6-kDa tegumental protein from C. sinensis (Kim et al., 2012). Both of them are calcium-binding proteins, but their effects on host immune systems are not well elucidated. Cs16 derived from a C. sinensis adult can be recognized by hosts, and then induces pro-inflammatory immune responses via altering metabolic pathways of immunocytes. We reported that Cs16 can activate host immune cells and induces hepatic injury, indicating that Cs16 is a potential pathogenic factor of C. sinensis and can be a target for diagnosis and treatment of clonorchiasis (Figure 6).
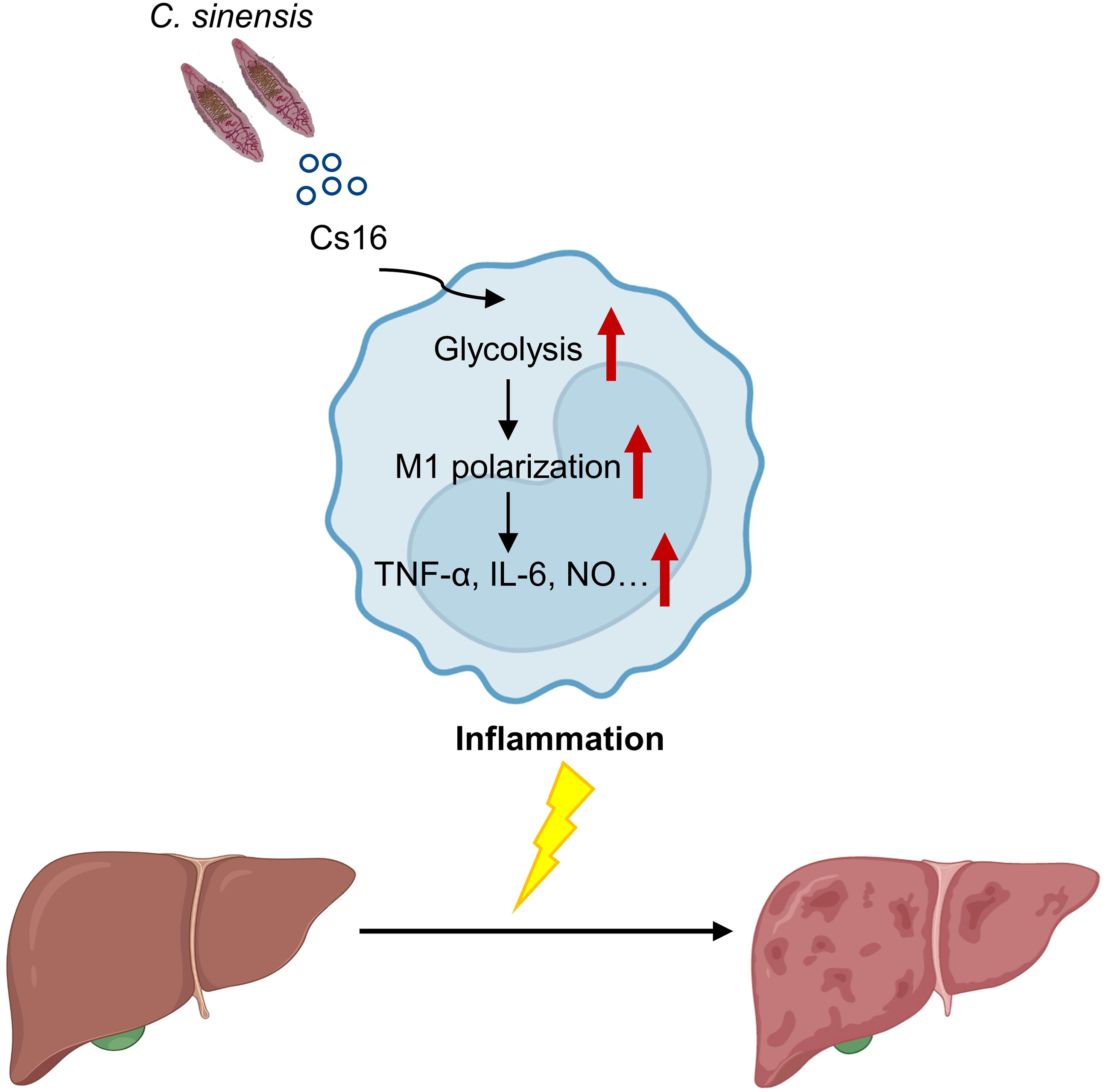
Figure 6 A proposed working model for Cs16 in regulating the metabolic pathway of BMMs and promoting hepatic injury.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of the National Institute of Parasitic Diseases, Chinese Center for Disease Control and Prevention in Shanghai, China (reference no.2011-005). All individuals or their guardians for those aged <18 years have provided written informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. The animal study was approved by the Institutional Animal Care and Use Committee (IACUC) of Shanghai Jiao Tong University School of Medicine (project number A-2019-053, 069). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
QL: Data curation, Investigation, Methodology, Writing – original draft. XL: Data curation, Formal Analysis, Investigation, Writing – review & editing, Methodology. SK: Investigation, Writing – review & editing. T-JZ: Writing – review & editing, Resources. CL: Writing – review & editing, Investigation. X-YD: Investigation, Writing – review & editing. XW: Methodology, Writing - review & editing. H-BY: Methodology, Writing - review & editing. C-YW: Methodology, Writing – review & editing, Resources. G-JC: Supervision, Writing – review & editing. M-BQ: Resources, Supervision, Writing – review & editing, Methodology, Project administration. MY: Supervision, Writing – review & editing, Resources. Z-JW: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing, Methodology.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (81971486 and 82272362).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1280358/full#supplementary-material
Supplementary Figure 1 | Growth curves of BMMs treated with Cs16 and CsAWA measured by CCK-8 assay. The data were representative of two or three independent experiments (biological replicates) and shown as the mean ± s.e.m.
Supplementary Figure 2 | Growth curves of CCLP, RBE, HuCCT1 cells treated with Cs16 and CsAWA measured by CCK-8 assay. The data were representative of two or three independent experiments (biological replicates) and shown as the mean ± s.e.m.
Supplementary Table 1 | Information for qPCR primers.
References
Bai, L., Kong, M., Duan, Z., Liu, S., Zheng, S., Chen, Y. (2021). M2-like macrophages exert hepatoprotection in acute-on-chronic liver failure through inhibiting necroptosis-S100A9-necroinflammation axis. Cell Death Dis. 12 (1), 93. doi: 10.1038/s41419-020-03378-w
Berntsen, N. L., Fosby, B., Valestrand, L., Tan, C., Reims, H. M., Schrumpf, E., et al. (2018). Establishment of a surgical bile duct injection technique giving direct access to the bile ducts for studies of the murine biliary tree. Am. J. Physiol. Gastrointest Liver Physiol. 314 (3), G349–G359. doi: 10.1152/ajpgi.00124.2017
Bouvard, V., Baan, R., Straif, K., Grosse, Y., Secretan, B., El Ghissassi, F., et al. (2009). A review of human carcinogens–Part B: biological agents. Lancet Oncol. 10 (04), 321–322. doi: 10.1016/s1470-2045(09)70096-8
Chung, E. J., Kim, T. Y., Hong, S. J., Yong, T. S. (2013). An EF-handed Ca(2+)-binding protein of Chinese liver fluke Clonorchis sinensis. Parasitol. Res. 112 (12), 4121–4128. doi: 10.1007/s00436-013-3603-0
Fang, Y., Wu, C., Chen, Q., Wu, J., Yang, Y., Guo, X., et al. (2015). SjE16.7 activates macrophages and promotes Schistosoma japonicum egg-induced granuloma development. Acta Trop. 149, 49–58. doi: 10.1016/j.actatropica.2015.05.016
Goodman, Z. D. (2007). Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J. Hepatol. 47 (4), 598–607. doi: 10.1016/j.jhep.2007.07.006
Kan, S., Li, Q., Li, H. M., Yao, Y. H., Du, X. Y., Wu, C. Y., et al. (2022). Clonorchis sinensis infection modulates key cytokines for essential immune response impacted by sex. PloS Negl. Trop. Dis. 16 (9), e0010726. doi: 10.1371/journal.pntd.0010726
Kim, E. M., Kwak, Y. S., Yi, M. H., Kim, J. Y., Sohn, W. M., Yong, T. S. (2017). Clonorchis sinensis antigens alter hepatic macrophage polarization in vitro and in vivo. PloS Negl. Trop. Dis. 11 (5), e0005614. doi: 10.1371/journal.pntd.0005614
Kim, T. S., Pak, J. H., Kim, J. B., Bahk, Y. Y. (2016). Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: a brief review. BMB Rep. 49 (11), 590–597. doi: 10.5483/bmbrep.2016.49.11.109
Kim, Y. J., Yoo, W. G., Lee, M. R., Kim, D. W., Lee, W. J., Kang, J. M., et al. (2012). Identification and characterization of a novel 21.6-kDa tegumental protein from Clonorchis sinensis. Parasitol. Res. 110 (5), 2061–2066. doi: 10.1007/s00436-011-2681-0
Kozlyuk, N., Monteith, A. J., Garcia, V., Damo, S. M., Skaar, E. P., Chazin, W. J. (2019). S100 proteins in the innate immune response to pathogens. Methods Mol. Biol. 1929, 275–290. doi: 10.1007/978-1-4939-9030-6_18
Kunzmann, L. K., Schoknecht, T., Poch, T., Henze, L., Stein, S., Kriz, M., et al. (2020). Monocytes as potential mediators of pathogen-induced T-helper 17 differentiation in patients with primary sclerosing cholangitis (PSC). Hepatology 72 (4), 1310–1326. doi: 10.1002/hep.31140
Lai, D. H., Hong, X. K., Su, B. X., Liang, C., Hide, G., Zhang, X., et al. (2016). Current status of Clonorchis sinensis and clonorchiasis in China. Trans. R Soc. Trop. Med. Hyg 110 (1), 21–27. doi: 10.1093/trstmh/trv100
Lee, Y. I., Seo, M., Kim, S. B. (2018). Infections of soil-transmitted helminth in refugees from North Korea. Korean J. Parasitol. 56 (3), 291–294. doi: 10.3347/kjp.2018.56.3.291
Lim, S. Y., Raftery, M. J., Geczy, C. L. (2011). Oxidative modifications of DAMPs suppress inflammation: the case for S100A8 and S100A9. Antioxid Redox Signal 15 (8), 2235–2248. doi: 10.1089/ars.2010.3641
Na, B. K., Pak, J. H., Hong, S. J. (2020). Clonorchis sinensis and clonorchiasis. Acta Trop. 203, 105309. doi: 10.1016/j.actatropica.2019.105309
Nam, J. H., Moon, J. H., Kim, I. K., Lee, M. R., Hong, S. J., Ahn, J. H., et al. (2012). Free radicals enzymatically triggered by Clonorchis sinensis excretory-secretory products cause NF-kappaB-mediated inflammation in human cholangiocarcinoma cells. Int. J. Parasitol. 42 (1), 103–113. doi: 10.1016/j.ijpara.2011.11.001
Pu, J.-b., Tang, L.-l., Yin, C.-n., Du, X.-y., Wu, J.-h., Zhao, W., et al. (2018). Recombinant expression of calcium-binding protein from Clonorchis sinensis and preliminary evaluation of its application in immunodiagnosis. Chin. J. Parasitol. Parasitic Dis. 35, 275–279.
Qian, M.-B., Utzinger, J., Keiser, J., Zhou, X.-N. (2015). Clonorchiasis. Lancet 387 (10020), 800–810. doi: 10.1016/s0140-6736(15)60313-0
Shi, Y., Yu, K., Liang, A., Huang, Y., Ou, F., Wei, H., et al. (2020). Identification and analysis of the tegument protein and excretory-secretory products of the carcinogenic liver fluke clonorchis sinensis. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.555730
Sreejit, G., Flynn, M. C., Patil, M., Krishnamurthy, P., Murphy, A. J., Nagareddy, P. R. (2020). S100 family proteins in inflammation and beyond. Adv. Clin. Chem. 98, 173–231. doi: 10.1016/bs.acc.2020.02.006
Thomas, C. M., Timson, D. J. (2016). A mysterious family of calcium-binding proteins from parasitic worms. Biochem. Soc. Trans. 44 (04), 1005–1010. doi: 10.1042/BST20150270
Tsai, S. Y., Segovia, J. A., Chang, T. H., Morris, I. R., Berton, M. T., Tessier, P. A., et al. (2014). DAMP molecule S100A9 acts as a molecular pattern to enhance inflammation during influenza A virus infection: role of DDX21-TRIF-TLR4-MyD88 pathway. PloS Pathog. 10 (1), e1003848. doi: 10.1371/journal.ppat.1003848
van Tong, H., Brindley, P. J., Meyer, C. G., Velavan, T. P. (2017). Parasite infection, carcinogenesis and human Malignancy. EBioMedicine 15, 12–23. doi: 10.1016/j.ebiom.2016.11.034
Wu, C., Chen, Q., Fang, Y., Wu, J., Han, Y., Wang, Y., et al. (2014). : Schistosoma japonicum egg specific protein SjE16.7 recruits neutrophils and induces inflammatory hepatic granuloma initiation. PloS Negl. Trop. Dis. 8 (2), e2703. doi: 10.1371/journal.pntd.0002703
Yanez, M., Gil-Longo, J., Campos-Toimil, M. (2012). Calcium binding proteins. Adv. Exp. Med. Biol. 740, 461–482. doi: 10.1007/978-94-007-2888-2_19
Yoshida, Y. (2012). Clonorchiasis–a historical review of contributions of Japanese parasitologists. Parasitol. Int. 61 (1), 5–9. doi: 10.1016/j.parint.2011.06.003
Zackular, J. P., Chazin, W. J., Skaar, E. P. (2015). Nutritional immunity: S100 proteins at the host-pathogen interface. J. Biol. Chem. 290 (31), 18991–18998. doi: 10.1074/jbc.R115.645085
Keywords: Clonorchis sinensis, inflammation, metabolism, calcium binding protein, monocytes
Citation: Li Q, Li X, Kan S, Zhu T-J, Li C, Du X-Y, Wang X, Yan H-B, Wu C-Y, Chen G-J, Qian M-B, Yan M and Wang Z-J (2023) Clonorchis sinensis calcium-binding protein Cs16 causes acute hepatic injury possibly by reprogramming the metabolic pathway of bone marrow-derived monocytes. Front. Cell. Infect. Microbiol. 13:1280358. doi: 10.3389/fcimb.2023.1280358
Received: 20 August 2023; Accepted: 25 September 2023;
Published: 18 October 2023.
Edited by:
Xing He, Second Military Medical University, ChinaReviewed by:
Xi Sun, Sun Yat-sen University, ChinaYadong Zheng, Zhejiang Agriculture and Forestry University, China
Copyright © 2023 Li, Li, Kan, Zhu, Li, Du, Wang, Yan, Wu, Chen, Qian, Yan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Men-Bao Qian, cWlhbm1iQG5pcGQuY2hpbmFjZGMuY24=; Min Yan, eWFubWluMDMxMDYwNkAxNjMuY29t; Zhao-Jun Wang, emp3YW5nQHNqdHUuZWR1LmNu
†These authors have contributed equally to this work
 Qi Li
Qi Li Xiao Li1,2†
Xiao Li1,2† Shuo Kan
Shuo Kan Chen-Yun Wu
Chen-Yun Wu Guang-Jie Chen
Guang-Jie Chen Men-Bao Qian
Men-Bao Qian Min Yan
Min Yan Zhao-Jun Wang
Zhao-Jun Wang