
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cell. Infect. Microbiol. , 08 December 2023
Sec. Clinical Microbiology
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1278921
This article is part of the Research Topic Vaginal Dysbiosis and Biofilms, volume II View all 12 articles
Bloodstream infection caused by anaerobic microorganisms continues to be associated with a high mortality risk, necessitating a rapid diagnosis and an appropriate treatment. As an anaerobic gram-positive organism associated with vaginal infections, Fannyhessea vaginae is a rare cause of invasive infections. In this case, a 32-year-old pregnant woman with bacterial vaginosis presented with bacteremia. The microbiological analysis of the blood cultures identified F. vaginae. The patient was treated empirically with 5 days of cefoperazone/sulbactam and recovered well. Here, we provide a review of the literature on F. vaginae infections, and the reported cases demonstrate the need for awareness of the different anaerobic species found in the vaginal tract and adaptation of empirical therapies, especially in pregnant women.
Anaerobes are the dominant organisms of the normal human microbiome. They inhabit mucosal membranes such as those in the female reproductive tract, gastrointestinal system, and oral cavity. Generally, these organisms play a crucial role in sustaining normal homeostasis in the human body. However, they can also serve as pathogens that cause invasive infections in human populations (Watanabe et al., 2021). Anaerobic bloodstream infections are responsible for up to 20% of bacteremic episodes with a high mortality rate, even higher in patients who are of advanced age and lack appropriate treatment (Dien Bard et al., 2020).
Fannyhessea vaginae, previously known as Atopobium vaginae, is a strict anaerobe first isolated from the vaginal flora of a healthy Swedish woman in 1999 (Jovita et al., 1999). In 2018, it was reclassified as F. vaginae (Nouioui et al., 2018). F. vaginae is a Gram-positive, elliptical- or rod-shaped coccobacillus that can appear as single elements, pairs, clumps, or short chains and is a part of the human vaginal microbiome. Many studies have emphasized that F. vaginae plays an important role in the pathophysiology of vaginal diseases (Mendling et al., 2019). F. vaginae is able to incorporate into Gardnerella vaginalis biofilms, a crucial marker of bacterial vaginosis (Castro et al., 2021). It has also been determined that high vaginal loads of F. vaginae in conjunction with Gardnerella spp. is linked to late miscarriage and preterm birth (Bretelle et al., 2015). Although F. vaginae can be detected in the normal vaginal microbiome (8%–25%), it is more prevalent in patients with bacterial vaginosis (50%–96%) (Mendling et al., 2019). However, F. vaginae is an uncommon cause of invasive infections.
Here, we describe a rare case of bacteremia caused by F. vaginae in a pregnant woman with bacterial vaginosis and hypothesize that an ascending infection of F. vaginae in the vagina caused this woman’s bacteremia. We also provide a review of previously published cases related to F. vaginae infections. The reported cases demonstrate that if a patient is febrile and exhibits symptoms of bloodstream infection, anaerobic species that are prevalent in the vaginal tract should be considered, especially for pregnant women with vaginal infections
The patient, a 32-year-old woman with uterine fibroids and resistant hypertension, was admitted for the delivery of her second child at a gestational age of 40 + 4 weeks on March 4, 2022. At the time of admission, the fetal membranes had not ruptured, and her body temperature was 36.3°C. Abdominal B-mode ultrasound indicated singleton pregnancy and multiple uterine fibroids (the largest was 17 × 8 mm). Chills occurred after oxytocin administration at admission, and her body temperature was 36.7°C. A baby boy was delivered by vaginal delivery, and his birth weight was 4,000 g. She had a first-degree perineal tear. After delivery, she was observed for >1 h, and her body temperature increased to 39°C. Emergency blood culture, blood analysis, and procalcitonin test were carried out. Her 4-h postpartum vaginal blood loss was 850 mL. Respiratory disease, hematologic disease, and urinary tract infection were ruled out. Prenatal vaginal discharge evaluation (posterior fornix swab) with a direct microscopic examination found gram-negative or -variable rods, and her Nugent score was 7. Hence, the patient was diagnosed with bacterial vaginosis, and cefoperazone/sulbactam was used for anti-infective treatment. After 5 days of antibiotic treatment, the patient was discharged. The patient appeared well at subsequent visits and seemed to have recovered completely.
In bilateral dual-bottle blood culture, the left anaerobic bottle was positive after 50 h of culture. The liquid in the positive blood culture bottle was aspirated and inoculated on blood agar plates, which were cultured in an aerobic environment and an anaerobic environment at 35°C. After 48 h, no bacterial growth was observed in the blood agar plate from the aerobic environment, and small grayish-white colonies could be observed in the plate from the anaerobic environment (Figure 1A). The Gram staining smear was positive, and the bacteria were elliptical or short rods in shape (Figure 1B). A single colony was selected, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Bruker, Germany, MALDI Biotyper 3.1) rapid identification result was F. vaginae, and the score was 2.010. Additionally, the 16S rRNA sequence (GenBank accession no. OR287194) analysis also indicated that this bacterium was F. vaginae.
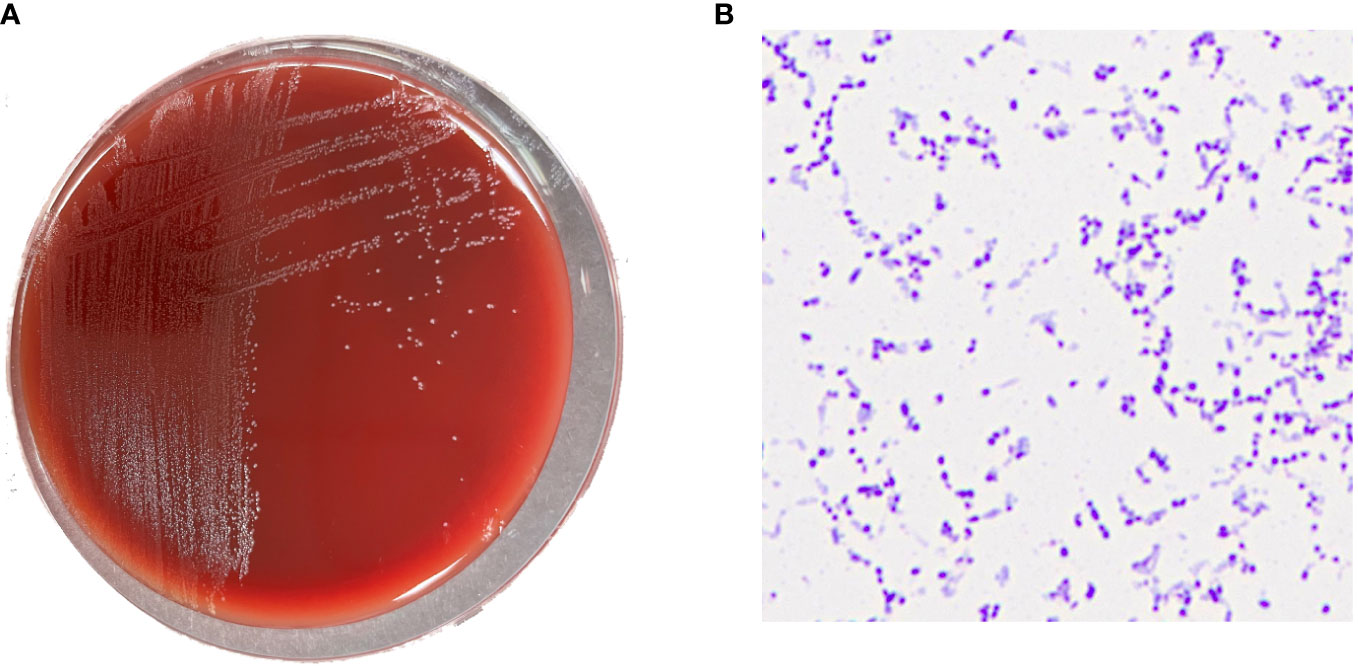
Figure 1 (A) Grayish white colonies of Fannyhessea vaginae after 48h of culture on blood agar plate under anaerobic conditions; (B) Gram staining showing gram-positive (F) vaginae bacteria appearing as elliptical or short rods.
Cefoperazone/sulbactam was used as an anti-infective treatment when the patient’s white blood cell count peaked at 34 × 109/L. The dose was 3.0 g, intravenous infusion for 8 h. At 5 days postpartum, antibiotic treatment was discontinued. The patient’s white blood cell count and procalcitonin continuously decreased until they returned to normal, and her temperature gradually returned to normal. Figure 2 displays the variation trend of body temperature, white blood cell count, and procalcitonin concentration.
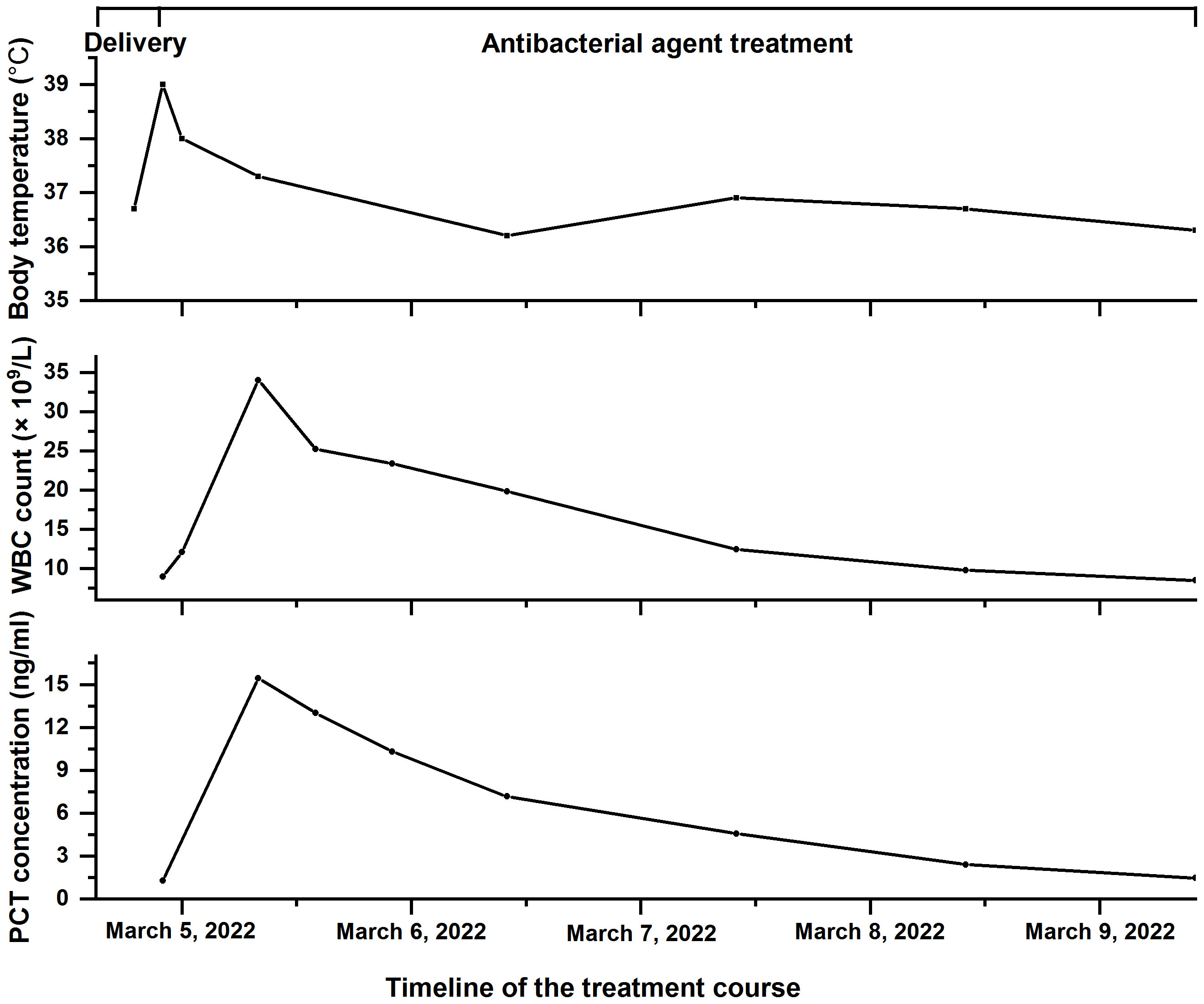
Figure 2 The timeline of the treatment course of the patient with Fannyhessea vaginae infection. Body temperature (top), white blood cell (WBC) count (middle), and procalcitonin (PCT) concentration (bottom) during hospitalization.
The minimum inhibitory concentration (MIC) breakpoints for anaerobic bacteria in the Clinical and Laboratory Standards Institute M100-S31 and European Committee on Antimicrobial Susceptibility Testing (Version 10.0) were used as a reference, one McFarland turbidity solution was prepared, and the E test strip was used for the drug sensitivity test. The results are shown in Table 1.
To the best of our knowledge, there are 10 previously published cases of F. vaginae infections, and a list of these cases is summarized in Table 2. These F. vaginae infections occurred in bloodstream infections (Knoester et al., 2011; Chan et al., 2012; Dauby et al., 2019; Taillandier et al., 2020), tubo-ovarian abscess (Geissdörfer et al., 2003), bacterial vaginosis (Burton et al., 2004), uterine endometritis (Yamagishi et al., 2011), subchorionic hematoma (Jacqmin et al., 2018), endocarditis (Mansell et al., 2018), and prosthetic joint infection (Massa et al., 2022). Our reported case revealed that anaerobic species found in the vaginal tract could cause bacteremia in pregnant women and cefoperazone/sulbactam was effective for antimicrobial treatment of F. vaginae infection.
In the last 20–30 years, rapid and precise species-level identification of anaerobes has aided clinicians in providing the best care for their patients, resulting in dramatically lower morbidity and mortality rates and hospital stays (Kovács et al., 2022). However, anaerobic bacteria continue to be among the most neglected and unrecognized pathogens because their cultivation necessitates substantial microbiological experience, and many hospitals (particularly in developing nations) may lack the equipment necessary to achieve anaerobiosis (Nagy et al., 2018). As an anaerobic, F. vaginae is found in the normal vaginal microbiota but is increasingly linked to bacterial vaginosis (Mendling et al., 2019). Recently, a prospective investigation linked F. vaginae to salpingitis and infertility, indicating this microorganism’s potential pathogenicity (Haggerty et al., 2016).
According to the Nugent score criteria for the identification of bacterial vaginosis via a direct microscopic examination, a Nugent score of 7 can be diagnosed as bacterial vaginosis as in our case report. This vaginal infection case allowed us to hypothesize that an ascending infection of F. vaginae from the vagina caused this woman’s bacteremia. Similar ascending bacteremia of F. vaginae has also been reported in other cases. For instance, a case reported of a pregnant woman who had F. vaginae transferred from her cervix to her uterus during chorionic villus sampling, causing an intrauterine infection that resulted in fetal mortality and bacteremia of the mother (Knoester et al., 2011). Another case described an intrapartum F. vaginae bacteremia that occurred spontaneously without any prior surgical trauma to the female genital tract; the case was characterized by an imbalanced vaginal microbiota with the proliferation of G. vaginalis and Candida albicans (Chan et al., 2012). In addition to bacteremia, other diseases have been documented as a result of ascending F. vaginae infections. A 33-year-old woman with bacterial vaginosis was clinically diagnosed with uterine endometritis due to an ascending F. vaginae infection (Yamagishi et al., 2011). Similarly, an 18-year-old patient lanced a vaginal cyst herself with a subcutaneous insulin cannula, resulting in infective endocarditis due to an ascending F. vaginae infection (Mansell et al., 2018). Therefore, disturbed vaginal microbiota is a significant cause of a variety of diseases in women, and more attention should be given to the vaginal microbiome of female patients with vaginal dysbiosis.
F. vaginae infections in pregnant patients have a clinical consequence that affects both the mother and the fetus. Although no maternal deaths had been documented, major morbidities such as the need for extensive surgery and consequent infertility, as well as the emotional toll of losing the fetus, might still occur (Table 2). In light of the severe consequences associated with F. vaginae infections, appropriate treatment should be initiated once the diagnosis is made.
The European Committee on Antimicrobial Susceptibility Testing states that the sensitivity of anaerobic bacteria to antimicrobial agents is exclusively measured using the MIC technique. However, because commercial automated identification and susceptibility testing systems are not commonly available, treatment of infections caused by these anaerobic microorganisms remains a challenge. Metronidazole is the most commonly used antimicrobial agent against anaerobic bacterial species. However, the results of susceptibility testing on metronidazole for F. vaginae are variable because some strains have high MIC values (De Backer et al., 2006). In our reported case, this F. vaginae strain was resistant to metronidazole (MIC >256 μg/mL), although the pathogen can be inhibited by low concentrations of clindamycin (MIC of 0.016 μg/mL), another commonly used antimicrobial agent for treating anaerobes.
According to the review of the literature (Table 2), penicillin-based antibiotics, such as amoxicillin and piperacillin, were mostly used in the treatment of F. vaginae infections in the reported cases. We also performed the susceptibility testing of the F. vaginae strain to penicillin-based antibiotics and found that this strain can be inhibited by low concentrations of ampicillin (MIC of 0.094 μg/mL), penicillin (MIC of 0.19 μg/mL), ampicillin/sulbactam (MIC of 0.75 μg/mL), and piperacillin/tazobactam (MIC of 0.38 μg/mL). However, in our case report, based on the understanding that cefoperazone/sulbactam appears in low levels in human milk and are not expected to cause adverse effects in breastfed infants (Matsuda et al., 1985; Lai et al., 2018), the patient was treated empirically with cefoperazone/sulbactam, and the retrospective antimicrobial susceptibility testing confirmed that cefoperazone/sulbactam (MIC of 0.38 μg/mL) was effective at inhibiting pathogen proliferation. In addition to our case report, it has been reported that other cephalosporin antibiotics, such as cefoxitin, are efficacious against F. vaginae infections (Geissdörfer et al., 2003). These results demonstrate that cephalosporin antibiotics are also an option for treating F. vaginae infections.
Incidence, morbidity, and death rates due to anaerobic bloodstream infections should be given more attention in patients. As an anaerobic bacterium, F. vaginae is found in normal vaginal microbiota; however, under certain conditions, it may cause life-threatening infections. If a patient with bacterial vaginosis is febrile and exhibits bloodstream infection symptoms during the postpartum period, it is vital to be mindful of bacterial vaginosis associated anaerobic species such as F. vaginae and to adapt the empirical therapy, as was the case here.
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (protocol code 2023-1-026). Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/ patient(s) for the publication of this case report.
PL: Writing – original draft. LW and RL: Data curation. XC: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Science and Technology Project of the Jinan Municipal Health Commission, grant numbers 2020-4-71 and 2022-1-37.
We would like to thank Yune Lu for her technical support. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bretelle, F., Rozenberg, P., Pascal, A., Favre, R., Bohec, C., Loundou, A., et al. (2015). High Atopobium vaginae and Gardnerella vaginalis vaginal loads are associated with preterm birth. Clin. Infect. Dis. 60, 860–867. doi: 10.1093/cid/ciu966
Burton, J. P., Devillard, E., Cadieux, P. A., Hammond, J.-A., Reid, G. (2004). Detection of Atopobium vaginae in postmenopausal women by cultivation-independent methods warrants further investigation. J. Clin. Microbiol. 42, 1829–1831. doi: 10.1128/JCM.42.4.1829-1831.2004
Castro, J., Rosca, A. S., Muzny, C. A., Cerca, N. (2021). Atopobium vaginae and Prevotella bivia are able to incorporate and influence gene expression in a pre-formed Gardnerella vaginalis biofilm. Pathogens 10 (2), 247. doi: 10.3390/pathogens10020247
Chan, J. F. W., Lau, S. K. P., Curreem, S. O. T., To, K. K. W., Leung, S. S. M., Cheng, V. C. C., et al. (2012). First report of spontaneous intrapartum Atopobium vaginae bacteremia. J. Clin. Microbiol. 50, 2525–2528. doi: 10.1128/JCM.00212-12
Dauby, N., Martiny, D., Busson, L., Cogan, A., Meghraoui, A., Argudín, M. A., et al. (2019). Atopobium vaginae intrapartum bacteremia: a case report with a literature review. Anaerobe 59, 212–214. doi: 10.1016/j.anaerobe.2018.09.010
De Backer, E., Verhelst, R., Verstraelen, H., Claeys, G., Verschraegen, G., Temmerman, M., et al. (2006). Antibiotic susceptibility of Atopobium vaginae. BMC Infect. Dis. 6, 51. doi: 10.1186/1471-2334-6-51
Dien Bard, J., Chang, T. P., Yee, R., Manshadi, K., Lichtenfeld, N., Choi, H. J., et al. (2020). The addition of anaerobic blood cultures for pediatric patients with concerns for bloodstream infections: prevalence and time to positive cultures. J. Clin. Microbiol. 58, e01844–e01819. doi: 10.1128/JCM.01844-19
Geissdörfer, W., Böhmer, C., Pelz, K., Schoerner, C., Frobenius, W., Bogdan, C. (2003). Tuboovarian abscess caused by Atopobium vaginae following transvaginal oocyte recovery. J. Clin. Microbiol. 41, 2788–2790. doi: 10.1128/JCM.41.6.2788-2790.2003
Haggerty, C. L., Totten, P. A., Tang, G., Astete, S. G., Ferris, M. J., Norori, J., et al. (2016). Identification of novel microbes associated with pelvic inflammatory disease and infertility. Sex Transm Infect. 92, 441–446. doi: 10.1136/sextrans-2015-052285
Jacqmin, H., deMunter, P., Verhaegen, J., Lewi, L. (2018). Atopobium vaginae bacteremia associated with a subchorionic hematoma. Clin. Microbiol. Newsl. 40, 83–85. doi: 10.1016/j.clinmicnews.2017.07.004
Jovita, M. R., Collins, M. D., Sjödén, B., Falsen, E. (1999). Characterization of a novel Atopobium isolate from the human vagina: description of Atopobium vaginae sp. nov. Int. J. Syst. Evol. Microbiol. 49, 1573–1576. doi: 10.1099/00207713-49-4-1573
Knoester, M., Lashley, LEELO, Wessels, E., Oepkes, D., Kuijper, E. J. (2011). First report of Atopobium vaginae bacteremia with fetal loss after chorionic villus sampling. J. Clin. Microbiol. 49, 1684–1686. doi: 10.1128/JCM.01655-10
Kovács, K., Nyul, A., Lutz, Z., Mestyán, G., Gajdács, M., Urbán, E., et al. (2022). Incidence and clinical characteristics of anaerobic bacteremia at a university hospital in Hungary: a 5-year retrospective observational study. Antibiotics 11, 1326. doi: 10.3390/antibiotics11101326
Lai, C. C., Chen, C. C., Lu, Y. C., Lin, T. P., Chuang, Y. C., Tang, H. J. (2018). Appropriate composites of cefoperazone-sulbactam against multidrug-resistant organisms. Infect. Drug Resist. 11, 1441–1445. doi: 10.2147/IDR.S175257
Mansell, J., Gourtsoyannis, Y., Draz, N., Buchanan, R. (2018). Infective endocarditis due to Atopobium vaginae: a rare association between genital infection and endocarditis of the tricuspid valve. BMJ Case Rep. 2018, bcr2018225871. doi: 10.1136/bcr-2018-225871
Massa, B., De Laere, E., Raes, R., Vervaeke, S., Van Hoecke, F. (2022). First report of a prosthetic joint infection with Fannyhessea (Atopobium) vaginae. Eur. J. Clin. Microbiol. Infect. Dis. 41, 1023–1027. doi: 10.1007/s10096-022-04461-0
Matsuda, S., Kashiwagura, T., Hirayama, H. (1985). Passage into the human milk and clinical evaluation of sulbactam/cefoperazone. Jpn J. Antibiot. 38 (2), 223–229.
Mendling, W., Palmeira-de-Oliveira, A., Biber, S., Prasauskas, V. (2019). An update on the role of Atopobium vaginae in bacterial vaginosis: what to consider when choosing a treatment? A mini review. Arch. Gynecol Obstet. 300, 1–6. doi: 10.1007/s00404-019-05142-8
Nagy, E., Boyanova, L., Justesen, U. S. (2018). How to isolate, identify and determine antimicrobial susceptibility of anaerobic bacteria in routine laboratories. Clin. Microbiol. Infect. 24, 1139–1148. doi: 10.1016/j.cmi.2018.02.008
Nouioui, I., Carro, L., García-López, M., Meier-Kolthoff, J. P., Woyke, T., Kyrpides, N. C., et al. (2018). Genome-based taxonomic classification of the phylum. Actinobacteria. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.02007
Taillandier, P., Roingeard, C., Violette, J., Leclère, F.-M., Faivre, S. (2020). Septic shock caused by Gardnerella vaginalis and Atopobium vaginae. IDCases 21, e00876. doi: 10.1016/j.idcr.2020.e00876
Watanabe, T., Hara, Y., Yoshimi, Y., Yokoyama-Kokuryo, W., Fujita, Y., Yokoe, M., et al. (2021). Application of MALDI-TOF MS to assess clinical characteristics, risk factors, and outcomes associated with anaerobic bloodstream infection: a retrospective observational study. Ann. Clin. Microbiol. Antimicrob. 20, 42. doi: 10.1186/s12941-021-00449-4
Keywords: anaerobic bloodstream infections, bacteremia, case report, Fannyhessea vaginae, literature review
Citation: Liu P, Wang L, Li R and Chen X (2023) A rare bacteremia caused by Fannyhessea vaginae in a pregnant woman: case report and literature review. Front. Cell. Infect. Microbiol. 13:1278921. doi: 10.3389/fcimb.2023.1278921
Received: 21 August 2023; Accepted: 27 November 2023;
Published: 08 December 2023.
Edited by:
Bingbing Xiao, Peking University, ChinaReviewed by:
Ming-Chi Li, National Cheng Kung University, TaiwanCopyright © 2023 Liu, Wang, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodi Chen, Y2hlbnhpYW9kaTIzNTJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.