
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 02 November 2023
Sec. Parasite and Host
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1277956
This article is part of the Research Topic Babesia: Biology, Interactions, and Mechanisms of Pathogenesis in Ticks and Its Hosts Volume II View all 6 articles
 Sezayi Ozubek1*
Sezayi Ozubek1* Mehmet Can Ulucesme1
Mehmet Can Ulucesme1 Reginaldo G. Bastos2,3
Reginaldo G. Bastos2,3 Heba F. Alzan3,4
Heba F. Alzan3,4 Jacob M. Laughery3
Jacob M. Laughery3 Carlos E. Suarez2,3
Carlos E. Suarez2,3 Munir Aktas1
Munir Aktas1Babesiosis is an acute and persistent tick-borne disease caused by protozoan parasites of the genus Babesia. These hemoparasites affect vertebrates globally, resulting in symptoms such as high fever, anemia, jaundice, and even death. Advancements in molecular parasitology revealed new Babesia species/genotypes affecting sheep and goats, including Babesia aktasi n. sp., which is highly prevalent in goats from Turkiye’s Mediterranean region. The objective of this study was to investigate the pathogenesis of B. aktasi infection in immunosuppressed (n=7) and non-immunosuppressed (n=6) goats. These animals were experimentally infected with fresh B. aktasi infected blood, and their clinical signs, hematological and serum biochemical parameters were monitored throughout the infection. The presence of parasites in the blood of immunosuppressed goats was detected by microscopic examination between 4 and 6 days after infection, accompanied by fever and increasing parasitemia. Goats that succumbed acute disease exhibited severe clinical signs, such as anemia, hemoglobinuria, and loss of appetite. However, the goats that survived showed milder clinical signs. In the non-immunosuppressed group, piroplasm forms of B. aktasi were observed in the blood within 2-5 days after inoculation, but with low (0.01-0.2%) parasitemia. Although these goats showed loss of appetite, typical signs of babesiosis were absent except for increased body temperature. Hematological analysis revealed significant decreases in the levels of red blood cells, leukocytes and platelet values post-infection in immunosuppressed goats, while no significant hematological changes were observed in non-immunosuppressed goats. In addition, serum biochemical analysis showed elevated transaminase liver enzymes levels, decreased glucose, and lower total protein values in the immunosuppressed group post-infection. Babesia aktasi, caused mild disease with minor clinical symptoms in non-immunosuppressed goats. However, in immunosuppressed goats, it exhibited remarkable pathogenicity, leading to severe clinical infections and death. In conclusion, this study provides valuable insights into the pathogenicity of the parasite and will serve as a foundation for future research aimed at developing effective prevention and control strategies against babesiosis in small ruminants. Further research is required to investigate the pathogenicity of B. aktasi in various goat breeds, other potential hosts, the vector ticks involved, and its presence in natural reservoirs.
Babesiosis, caused by protozoa of the genus Babesia, is one of the most common and economically important tick-borne diseases of domestic and wild ruminants worldwide (Homer et al., 2000). Babesiosis is an acute and chronic disease that can vary in severity depending on factors such as age, species, immunological status, presence of other pathogens, and genetic factors of the host. Typically, Babesia parasites invade and destroy vertebrate host erythrocytes and cause acute babesiosis characterized by high fever, anemia, icterus, hemoglobinuria, tachycardia, jaundice, weakness, lethargy, loss of appetite, abdominal pain, and a high mortality (Friedhoff, 1988). In contrast, chronic infections typically do not exhibit clinical signs but may negatively affect livestock production (Friedhoff, 1988; Homer et al., 2000). The disease is primarily transmitted by ticks belonging to the Rhipicephalus, Dermacentor, Ixodes, Hyalomma and Haemaphysalis genera (Guglielmone et al., 2010; Dantas-Torres, 2018; Gray et al., 2019). Currently, more than 100 species of Babesia have been identified worldwide that can infect humans, domestic and wild mammals, and most recently birds, and this number is likely to increase due to new research in other vertebrate hosts (Martínez-García et al., 2021). Of interest are the species Babesia ovis, B. motasi, and B. crassa that can cause babesiosis in sheep and goats (Liu et al., 2007; Ozubek and Aktas, 2017a; Schnittger et al., 2022), with B. ovis identified as the primary etiological agent of clinical babesiosis in these animals (Yeruham et al., 1998; Yeruham et al., 2001). Significant advances have been made in the field of molecular parasitology in the past two decades, leading to an increased interest in better defining blood protozoa belonging to the piroplasmid lineage. This has resulted in the identification of new Babesia species or genotypes that affect sheep and goats, including Babesia sp. Xinjiang, Babesia lengau-like, and B. motasi-like (B. motasi Lintan, B. motasi Tianzhu, B. motasi Hebei, and B. motasi Ningxian) (Liu et al., 2007; Guan et al., 2009; Niu et al., 2009; Bosman et al., 2010). The subspecies of Babesia motasi can be distinguished into two subtypes (B. motasi Lintanensis and B. motasi Hebeinensis) based on their distinct morphological, serological, pathogenic, genetic, and virulent characteristics (Wang et al., 2019; Wang et al., 2020; Wang et al., 2023). Using the PCR-based reverse line blot (RLB) method, we have recently discovered a new Babesia species in goats in Turkiye’s Mediterranean region, which is clearly distinct from the ovine Babesia species described to date (Ozubek and Aktas, 2017b). After being isolated from a naturally infected goat and having its genetic and morphological traits characterized, this newly identified Babesia species was named Babesia aktasi n. sp. (Ozubek et al., 2023). Following that, a large-scale survey in the Mediterranean region revealed that B. aktasi n. sp. has a high prevalence (22.5%) in goats (Ulucesme et al., 2023a). Comparisons of pathogenesis and virulence of Babesia species require systematic animal experiments and often the use of spleen-intact (non-immunosuppressed) and splenectomized (immunosuppressed) animals (Hasherni-Fesharki and Uilenberg, 1981; Habela et al., 1990; Guan et al., 2009). It is well known that the spleen plays an important role in the clearance of Babesia-infected erythrocytes. Thus, consistently, splenectomy reduces the host’s capacity to control the parasite, thereby allowing detection and sometimes significant expansion of parasite populations that were previously undetectable and potentially clinically relevant (Bach et al., 2005; Buffet et al., 2011; Lewis et al., 2019). This is particularly important for Babesia species such as Babesia sp. Xinjiang (Guan et al., 2009), Babesia sp. BQ1-Lintan (Guan et al., 2010) and B. crassa (Hasherni-Fesharki and Uilenberg, 1981) which have low pathogenicity, where comparing non-immunosuppressed and immunosuppressed animals is crucial. In a study with Theileria haneyi, which is a novel species infecting horses also known for its low pathogenicity, spleen intact and splenectomised groups were formed in experimental infections (Sears et al., 2022).
In fact, some studies have shown that dexamethasone treatment, in addition to splenectomy, leads to better results by further suppressing the immune system (Guan et al., 2001; Guan et al., 2009). In the in vivo isolation study, B. aktasi n. sp. was obtained from an apparently non-immunosuppressed goat through the suppression of its immune system. Subsequently, the isolated parasite was inoculated into another goat that had also undergone splenectomy and dexamethasone injection. Following inoculation, the second goat experienced a significant increase in body temperature and reached a parasitemia level of 10% on day three post-infection. Due to the severe acute symptoms of babesiosis, the goat was humanely euthanized four days post-infection (Ozubek et al., 2023). This previous study did not systematically investigate the pathogenicity of B. aktasi n. sp., highlighting the necessity for further research to enhance our understanding of this newly identified Babesia species. Conducting pathogenicity studies is essential to gain a comprehensive understanding of how B. aktasi n. sp. affects non-immunosuppressed goats, and its specific impact on immunosuppressed individuals. These studies can contribute significantly to our knowledge of the disease and help develop control interventions against small ruminant babesiosis. Therefore, this study was conducted to assess clinical, hematological, and biochemical alterations of goats experimentally infected with B. aktasi n. sp.
This study was carried out according to the regulations of animal and welfare issued by the Turkish legislation for the protection of animals. All animal experiments were approved by the Firat University, Animal Experiment Ethic Committee, protocol number 2018/100.
Babesia aktasi n. sp., previously isolated from a naturally infected goat in the Mediterranean region of Turkiye, was utilized in this study (Ozubek et al., 2023). Briefly, one goat that was determined to be infected with B. aktasi n. sp. by nested PCR-based RLB in our previous field survey was splenectomized to increase circulating parasitemia and allow direct observation of the parasites in peripheral blood smears. When parasitemia reached 1.9%, 20 ml of blood from this goat was injected into another immunosuppressed goat that was free of tick-borne pathogens. After the detection of parasitemia (5% PPE) in the infected goat, 60 ml of blood was collected and divided into 5 ml aliquots, which were cryopreserved in 10% dimethyl sulfoxide (DMSO) solution. This B. aktasi n. sp. stabilate was used for experimental infections in the current study (Ozubek et al., 2023).
Fourteen 6–8-month-old native local breed male goats were used in this study. Prior to the experiments, animals were shown to be negative for Babesia, Theileria and Anaplasma species by microscopy of peripheral blood smears and nested PCR-based RLB (Sevinc et al., 2007; Ulucesme et al., 2023a). During the experiment, the goats were housed in a closed pen at the veterinary medicine animal unit and received feed and water ad libitum. To prevent possible tick infestations, flumethrin 1% pour-on (Flugon® 1%, Vetaş, Turkiye) was applied to the goats according to the manufacturer’s recommendation, and acaricide application was continued at 21-day intervals throughout the experiment. The goats were divided into two groups: in group I, animals (n = 7) were immunosuppressed through a combination of splenectomy and dexamethasone application (Vetakort® 4 mg, Vetas, Turkiye; 20 mg/day for 3 days), while in group II, animals (n = 6) were spleen-intact and not immunosuppressed prior to B. aktasi n. sp. infection (Guan et al., 2009). Animals in group I underwent splenectomy performed at the Firat University Veterinary Hospital and were placed in separate compartments to ensure recovery for 2 weeks before proceeding with the experiment. The splenectomy was performed using standard surgical, anesthetic, and analgesic procedures (Sevinc et al., 2007).
Experimental infections were performed using freshly infected blood, 15 ml of B. aktasi n. sp. stabilate (5% PPE) (Ozubek et al., 2023). Stabilates were thawed and immediately administered intravenously to an immunosuppressed goat (No.1). When parasitemia level reached 5%, 15 ml and 30 ml of infected fresh blood taken from the donor goat were intravenously inoculated into goats in group I and group II, respectively (Guan et al., 2009) (Figure 1). Following the inoculation, the goats were monitored daily for signs of clinical babesiosis (fever, anemia, icterus and hemoglobinuria). Additionally, a few drops of blood from the ear tip of the goats were drawn daily, and peripheral blood smears were prepared for microscopic examination. Five milliliters of whole blood samples in anticoagulant-coated (EDTA) vacuum tubes were collected from the jugular vein of each goat, and used for DNA isolation and hematological analysis. Blood samples were also collected in non-anticoagulant tubes, and sera were separated for assessment of serum biochemical parameters.
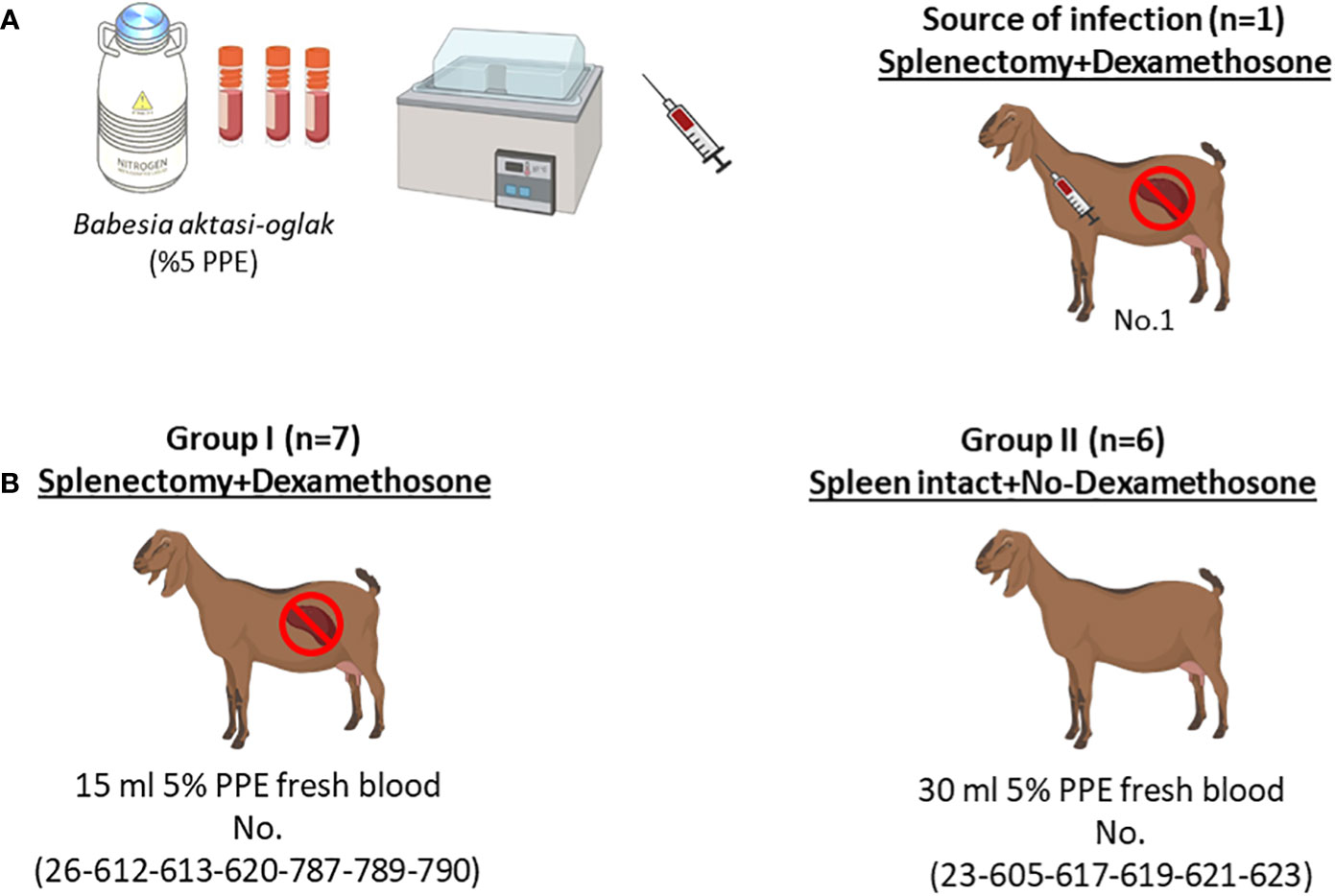
Figure 1 Schematic representation of the experimental infection. (A) Infection of the splenectomized goat (No.1) with (B) aktasi stabilate to generate the fresh infected blood for experimental infection. (B) Experimental infection of goats in group I and group II with fresh infected blood obtained from No.1.
Blood smears were stained with a 10% Giemsa solution or Diff-Quick stain (DiffPlus, Biyosistem Medikal, Turkiye) and screened under 100X objective to detect intraerythrocytic piroplasm. The level of parasitemia was calculated by examining at least 20 microscopic fields as previously described (Sevinc et al., 2007).
Hemogram (RBC: red blood cell count; WBC: white blood cell count; HCT: hematocrit; HB: hemoglobin; PLT: platelet count; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration) (Mindray Medical Electronics Co., Shenzhen, China) and serum biochemical parameters (Albumin, ALT: alanine aminotransferase, AST: aspartate aminotransferase, Creatinine, GGT: gamma glutamyl aminotransferase, Glucose, Total bilirubin, Total protein) (Fujifilm Corporation, Tokyo, Japan) were measured at four different time points tailored to each individual animal’s unique progression: first, before the start of the experiment, and subsequently, three additional times following the appearance and subsequent disappearance of piroplasm forms in each animal. Due to variations in the duration of piroplasm presence among different animals, the specific days of evaluation were adapted accordingly for each case.
Genomic DNA was isolated from 200 µl of EDTA anticoagulated blood samples from the goats using with the PureLinkTM Genomic DNA Mini Kit (Invitrogen Corporation, Carlsbad, USA) according to the manufacturer’s instructions, and stored in −20°C until use. For the detection of B. aktasi n. sp. DNA, a nested PCR assay was performed using two universal primers Nbab1F/Nbab1R (Oosthuizen et al., 2008) and RLBF2/RLBR2 (Georges et al., 2001).
The data analysis and graphs for this study were generated using GraphPad Prism software version 8 (GraphPad Software, San Diego, CA). The software was used to display the temperature, PCV, parasitemia rate, hemogram-serum biochemistry values as the mean and standard deviation of group I, group II and negative control samples. To compare the means between the two groups, a two-tailed t test was used, with a significance level of p<0.05 considered to be significant. Figure 1 was created with BioRender.com (www.biorender.com)
Parasites were detected in peripheral blood between days 4 and 6 after experimental infection of immunosuppressed goats with B. aktasi n. sp. (Figure 2). As the infection progressed, all the goats developed a fever, with peak temperature ranging from 40.3°C to 42.2°C, which correlated with increased and peak parasitemia (Figure 3; Table 1). Examination of peripheral blood smears using light microscopy revealed parasitemia rates ranging from 6.5% to 41.8% (Figure 2; Table 1). Only 2 out of the 7 experimentally infected immunosuppressed goats were able to survive acute disease. Goats displaying severe clinical signs of babesiosis (26, 612, 613, 620, and 787), including symptoms such as hemoglobinuria, icterus, loss of appetite, and immobility, experienced a significant drop in hematocrit levels, falling below 10%. These goats also exhibited elevated parasitemia levels, reaching as high as 41.8%. In consideration of the severe babesiosis symptoms they were experiencing, these goats were humanely euthanized.
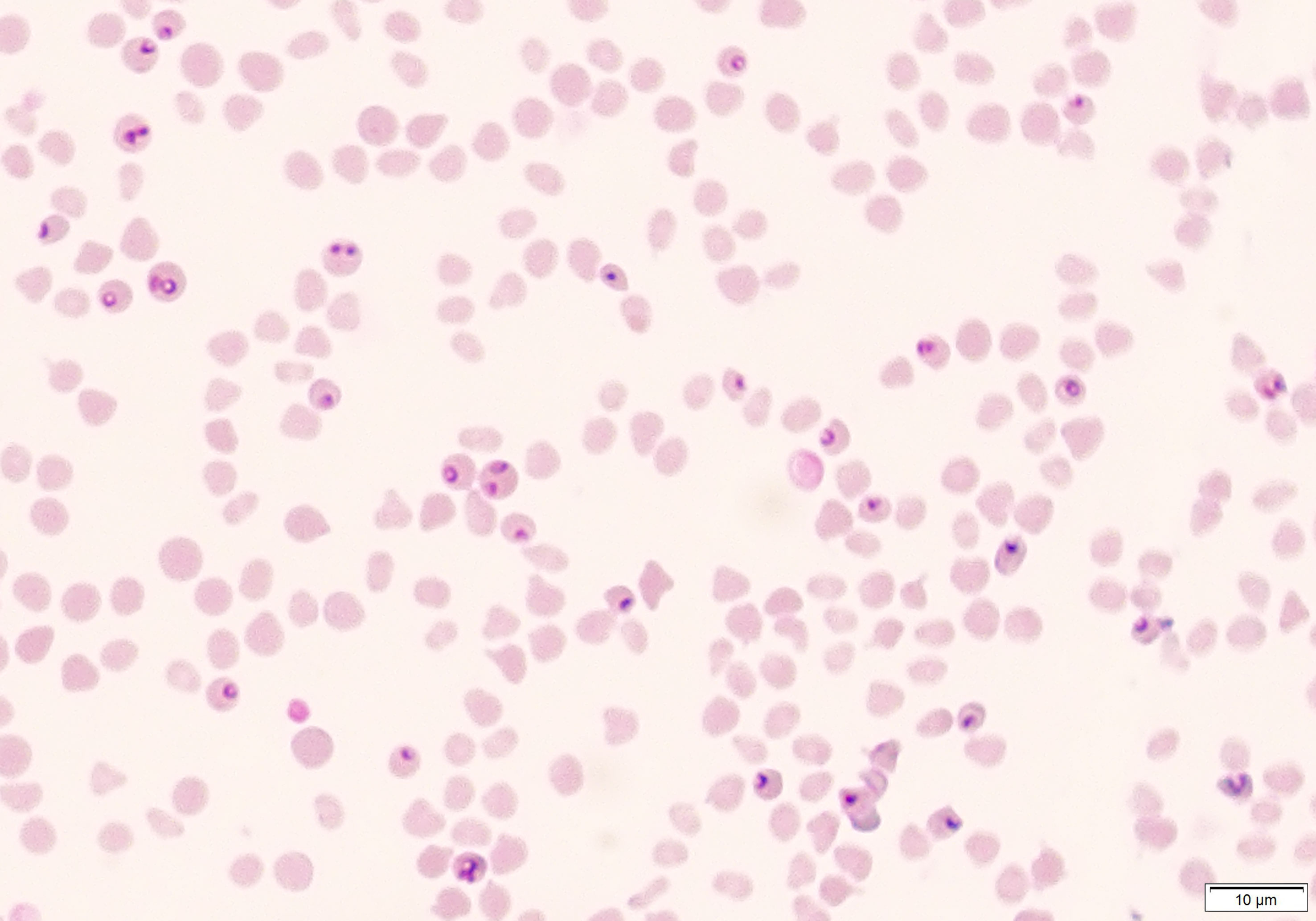
Figure 2 Microscopic visualization of blood smear obtained from immunosuppressed sheep (Animal ID. 787) after experimental infection with (B) aktasi n. sp.
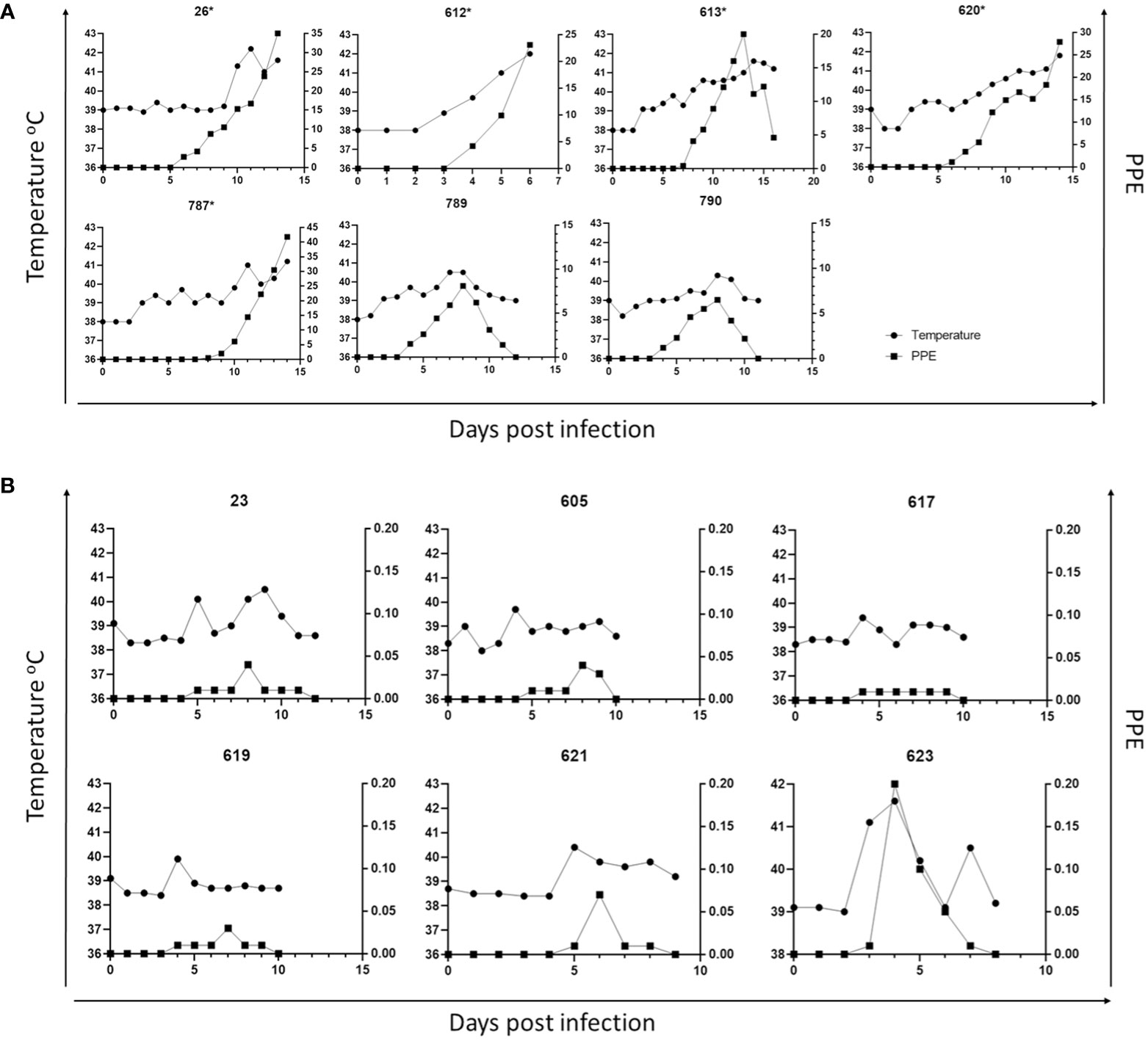
Figure 3 Parasitemia (PPE) and body temperature of goat in immunosuppressed (A) and non-immunosuppressed (B) groups. (* Animals humanely euthanized due to severe babesiosis caused by B. aktasi n. sp.).
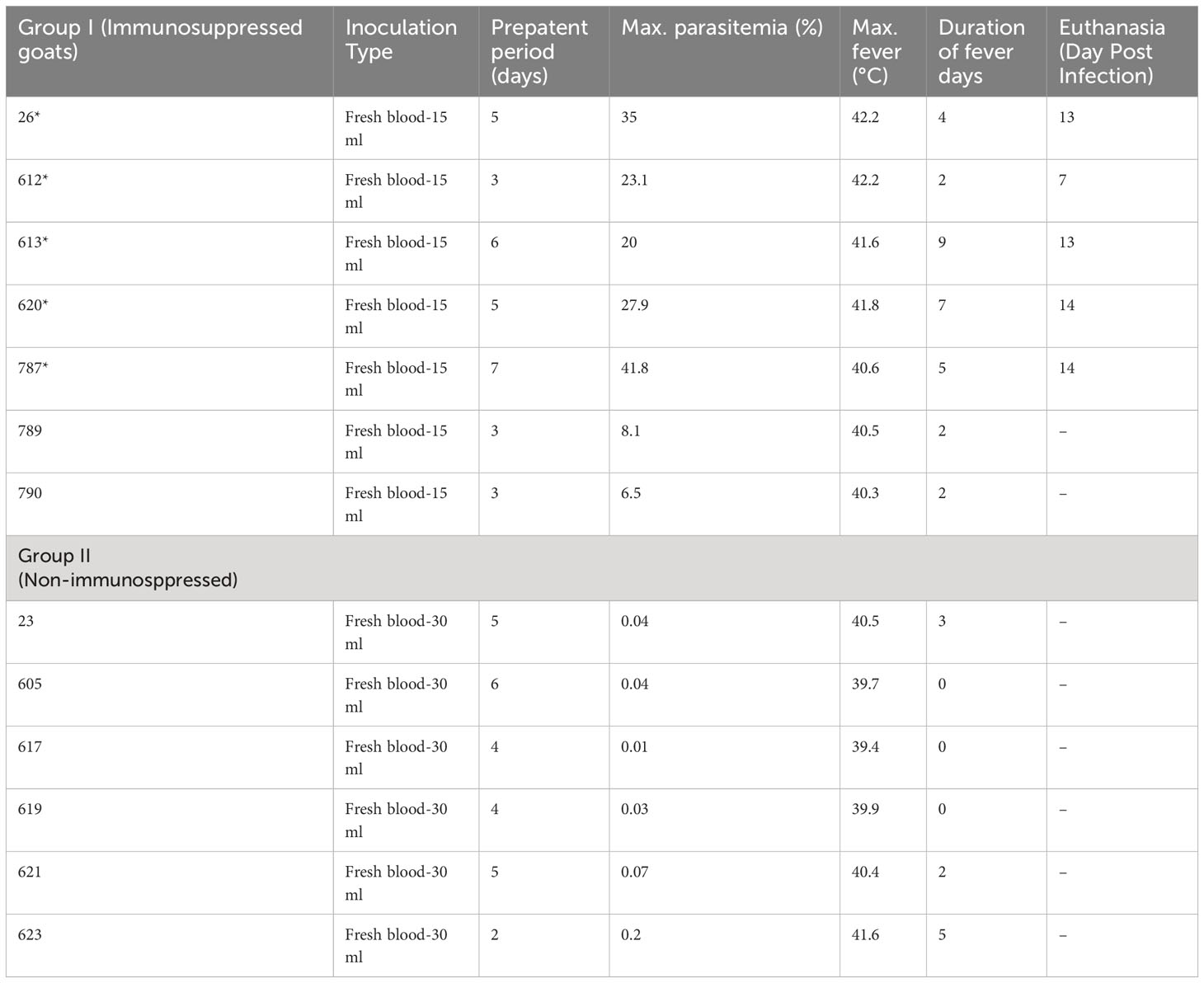
Table 1 Amount of infected blood inoculated and changes in infection parameters in group I (Splenectomy+Dexamethasone) and group II (Spleen-intact+No-Dexamethasone) goats (*humanely euthanized due to severe babesiosis caused by B. aktasi n. sp.).
Babesia aktasi n. sp. piroplasms were identified in peripheral blood of the non-immunosuppressed group of infected goats between days 2 and 5 post-inoculation, and observed until day 6 post-infection. Four out of six infected goats (23, 619, 621, 623) exhibited an increase in body temperature between days 2 and five post-infection (Table 1; Figure 3). Parasitemia ranged from 0.01% to 0.2% in peripheral blood. All the goats among the non-immunosuppressed group showed clinical signs, such as loss of appetite, stagnation, and prostration for 2-3 days, however typical symptoms of acute babesiosis, such as anemia, icterus, and hemoglobinuria, were not observed, except for an increased body temperature (Table 1; Figure 3). Notable clinical signs were observed in both immunosuppressed (group I) and non-immunosuppressed (group II) goats even though group I received only half of the parasite dose compared to animals in group II.
Values of RBC, WBC, HCT, HB, PLT, and MCHC in group I showed statistically significant decreases compared to the pre-infection levels (Table 2, Figure 4). Group II did not show any statistically significant differences in hematological and serum biochemistry parameters before and after infection. (Table 2). Animals in the group I had elevated levels of ALT and AST. Additionally, a decrease in glucose and total protein values was observed compared to pre-infection levels in this group (Table 2, Figure 5).
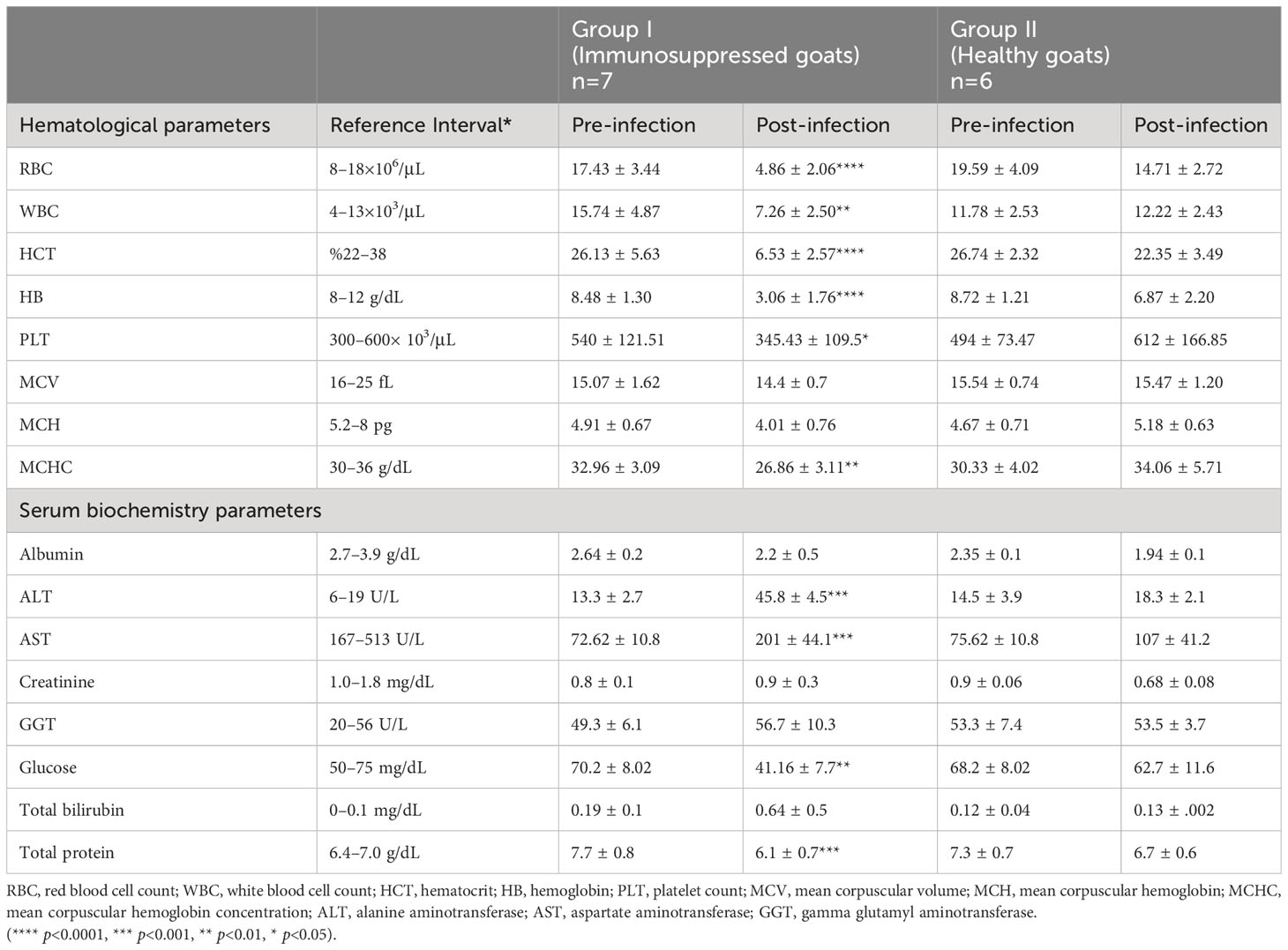
Table 2 Comparison of hematological and serum biochemistry parameters between group I (immunosuppressed goats) and group II (non-immunosuppressed goats).
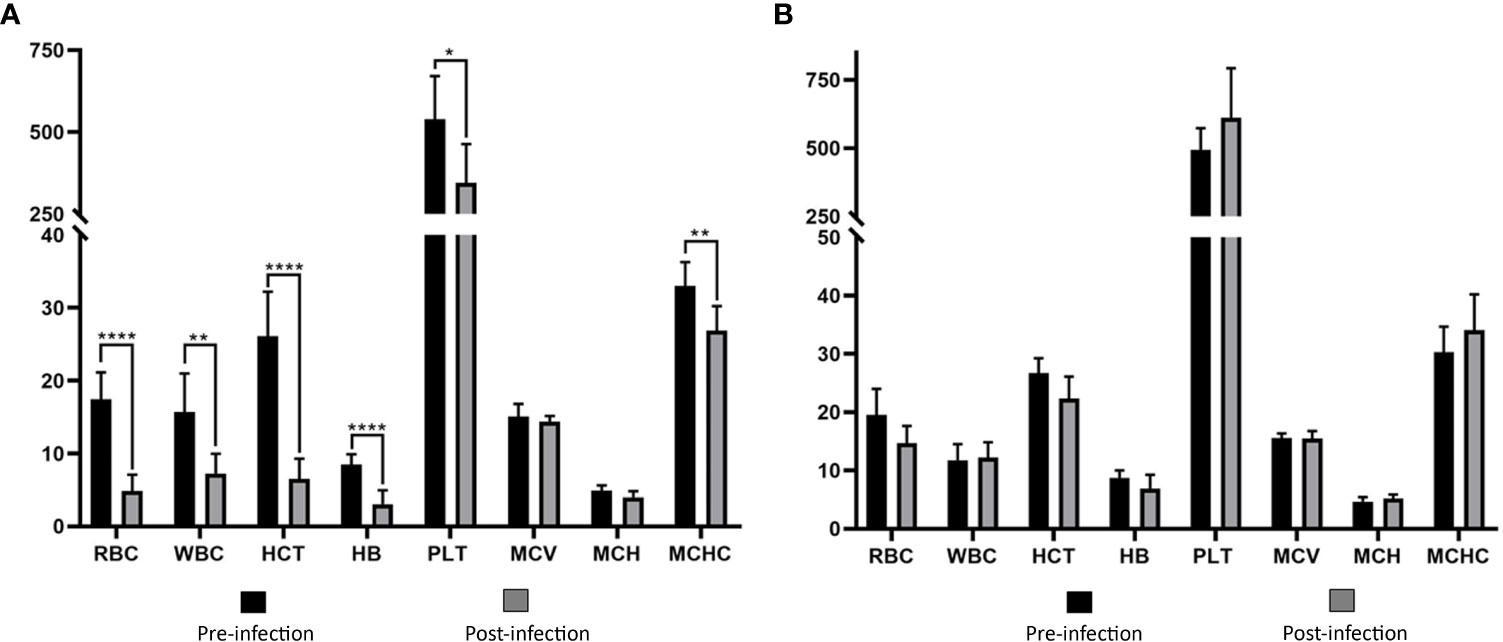
Figure 4 Comparison of hematological parameters in immunosuppressed (A) and non-immunosuppressed (B) goats before and after infection. Statistical significance is indicated as follows: **** p<0.0001, ** p<0.01, * p<0.05. RBC, red blood cell count (106/μL); WBC, white blood cell count (103/μL); HCT, hematocrit (%); HB, hemoglobin (g/dL); PLT, platelet count (103/μL); MCV, mean corpuscular volume (fL); MCH, mean corpuscular hemoglobin (pg); MCHC, mean corpuscular hemoglobin concentration (g/dL).
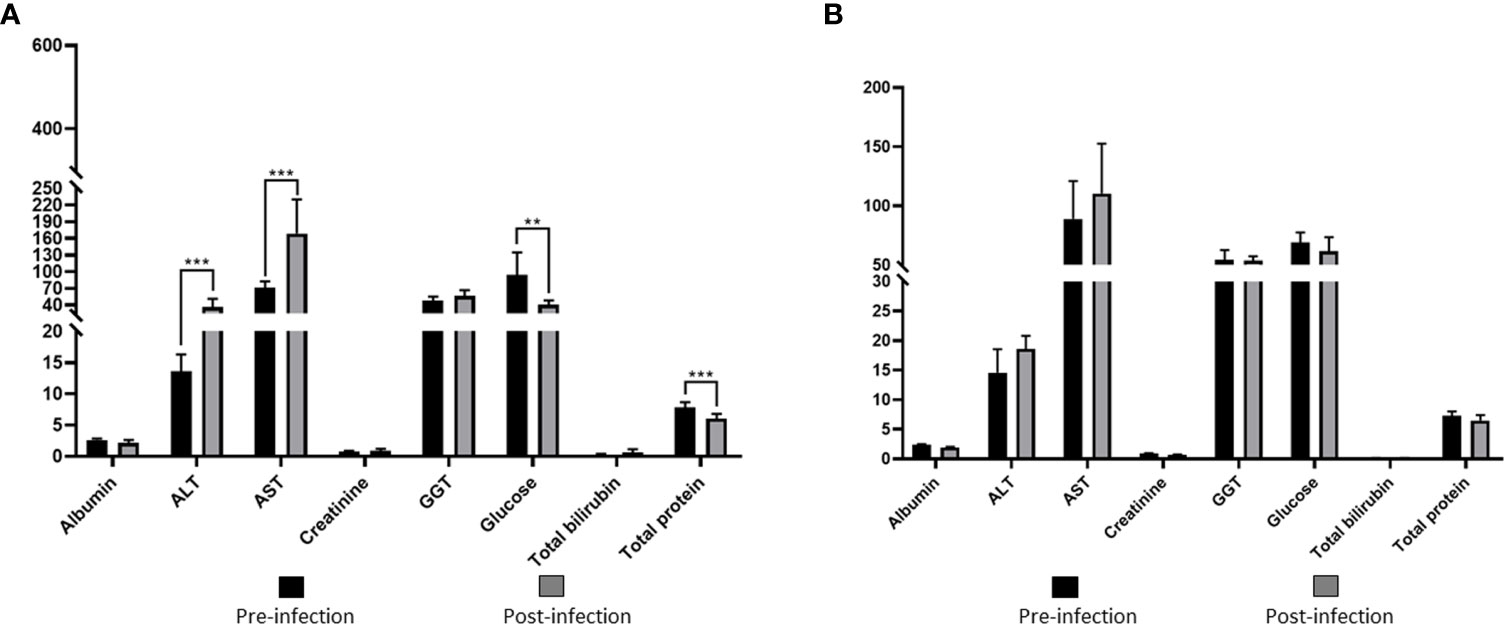
Figure 5 Comparison of serum biochemical parameters in immunosuppressed (A) and non-immunosuppressed (B) goats before and after infection. Statistical significance is indicated as follows: *** p<0.001; ** p<0.01. Albumine (g/dL); ALT, alanine aminotransferase (U/L); AST, aspartate aminotransferase (U/L); Creatinine (mg/dL); GGT, gamma glutamyl aminotransferase (U/L); Total bilirubin (mg/dL); Total protein (g/dL).
Small ruminant babesiosis holds significant economic importance in the Middle East, Southern Europe, as well as in certain African and Asian countries (Yin et al., 1997; Uilenberg, 2006; Stuen, 2020; Schnittger et al., 2022; Stevanović et al., 2022), but despite its substantial impact, ovine babesiosis is still considered a neglected disease (Uilenberg, 2006; Yin et al., 2007; Stevanović et al., 2022). Furthermore, there is a lack of research on the pathogenesis of babesiosis in goats, regardless of its potential to cause fatal infections in sheep (Sevinc et al., 2013; Schnittger et al., 2022). Several parameters need to be compared to assess the pathogenicity of Babesia and Theileria species, including anemia, fever, time to peak parasitemia, duration of parasitemia, as well as hematological and serum biochemistry values (Guan et al., 2001; Bai et al., 2002; Guan et al., 2002; Shkap et al., 2007; Rahbari et al., 2008; Guan et al., 2009; Guan et al., 2010; Sevinc et al., 2013; Niu et al., 2017; Sears et al., 2022). This present study was carried out to compare the pathogenicity of B. aktasi n. sp. among immunosuppressed and non-immunosuppressed goats. As the behavior of B. aktasi in field conditions remains unknown, we have relied on previously published pathogenicity studies to design the experimental infections for our study. In determining the preferred amount of blood for the challenge dose, we have taken experimental infections conducted with Babesia sp. Xinjiyang, a novel pathogen discovered in China which infects small ruminants, as a reference (Guan et al., 2009). In this study, as commonly preferred in experimental Babesia infections in small ruminants, we opted for using young animals (under 1 year of age) that were free from tick-borne pathogens (Hasherni-Fesharki and Uilenberg, 1981; Rahbari et al., 2008; Guan et al., 2009; Guan et al., 2010). Given the distinct characteristics of various Babesia species, the parasitemia level in the provided blood varies across different studies, making it impractical to rely on blood parasitemia levels as a reference point. Furthermore, due to the diversity among Babesia species, animals that died due to infection in various studies exhibited differing levels of parasitemia. Therefore, it is challenging to establish a connection in terms of these parameters between pathogenicity studies conducted with different Babesia species, and meaningful correlations may not be relevant. Experimental studies in sheep and goats have confirmed the essential role of the spleen in controlling parasitemia of Babesia species, and consequently, removal of the spleen usually leads to uncontrolled parasite proliferation and even death (Wright and Goodger, 2018). Small ruminant Babesia species exhibit diverse pathogenicity and respond differently to splenectomy. For instance, experimental infection with B. ovis revealed a critical outcome, as all splenectomized lambs died due to severe babesiosis in two separate studies (Habela et al., 1990; Rahbari et al., 2008). In contrast, experimental infections with B. motasi (Uilenberg et al., 1980; Lewis et al., 1981) and B. crassa (Hasherni-Fesharki and Uilenberg, 1981) in sheep and goats did not result in any mortality, even after splenectomy. Regarding Babesia sp. Xinjiang, while splenectomized lambs survived acute infection, splenectomy combined with dexamethasone treatment resulted in death (Guan et al., 2009). In this study, severe clinical babesiosis was observed in immune suppressed goats and five out of seven animals had to be humanely euthanized on days 7 through 14 post-infection due to severe babesiosis.
Splenectomy and dexamethasone are often employed together as well-established approach in experimental studies on B. ovata (Fujinaga, 1982), B. bigemina (Ravindran et al., 2006), Babesia sp. Xinjiang (Guan et al., 2009) to suppress the immune system. Experimental infection of goats with Babesia sp. Lintan revealed that splenectomy alone did not result in any clinical signs despite the detection of high parasitemia. In the same study, when dexamethasone was administered to splenectomized goats, high parasitemia and clinical manifestations of babesiosis were observed, but no fatalities were recorded among the animals (Guan et al., 2010). These findings highlight that splenectomy alone may not be enough to trigger clinical signs in certain Babesia species. However, the addition of dexamethasone treatment significantly increased the likelihood of observing clinical manifestations of babesiosis (Guan et al., 2009). Therefore, in this study, the application of dexamethasone alongside splenectomy was chosen to suppress the immune system. Our main goal was to achieve a thorough suppression of the immune system, aligning with previous research that has shown enhanced results when both splenectomy and dexamethasone are used simultaneously. By employing this immunosuppression method, we could observe the genuine effects of the parasite. This approach was selected to improve the accuracy of our assessment regarding the parasite’s impact on the host. Considering the synergistic effects of splenectomy and dexamethasone in immune suppression, we found it to be the most suitable approach for our research objectives and the focal point of our study.
In this current study, four euthanized goats in the immune suppressed group exhibited severe clinical signs, such as high fever, high parasitemia, anemia, hemoglobinuria, lethargy, anorexia, rapid breathing. Our findings align with previous studies, indicating that immune suppression in lambs infected with B. ovis led to fever, accompanied by 70% parasitemia (Habela et al., 1990; Rahbari et al., 2008). However, in the case of experimental infections with B. motasi, the observed increase of temperature was mild, and the parasitemia remained at a moderate level, peaking at 6.7% (Lewis et al., 1981). Notably, splenectomized goats did not display any signs of parasitemia or clinical manifestations in an experimental infection study based on the B. motasi Ameland strain, (Lewis et al., 1981). Similar to B. motasi, experimental infections with B. crassa showed mild fever, ranging from 40.2°C to 41.5°C, and moderate parasitemia, reaching up to 14% (Hasherni-Fesharki and Uilenberg, 1981). These findings highlight the importance of the spleen in the immune response against Babesia infections and demonstrate how different Babesia species can lead to different clinical manifestations and parasitemia, depending on the presence or absence of the spleen (Bach et al., 2005; Buffet et al., 2011; Lewis et al., 2019; Sears et al., 2022). Interestingly, the two surviving goats also displayed similar clinical signs, but their parasitemia levels were relatively low, ranging from 6.5% to 8.1%, compared to the goats that were euthanized. Except for displaying low levels of parasitemia and a slight increase in body temperature, the group of non-immunosuppressed goats did not exhibit the typical clinical manifestations of babesiosis. The absence of babesiosis specific clinical signs, such as icterus, anemia and hemoglobinuria in spleen-intact goats suggests that the animal effectively controlled the infection and limited the progression of acute disease. Experimental infections conducted on spleen intact sheep and goats have demonstrated that various Babesia species can cause clinical babesiosis. For instance, in an experimental infection study with B. ovis, spleen-intact lambs exhibited significant parasitemia and a high mortality rate due to severe babesiosis (Habela et al., 1990; Rahbari et al., 2008). Similarly, Babesia sp. BQ1-Ningxian caused a low level of parasitemia in lambs but resulted in a high fever and mortality rate (Niu et al., 2017). In contrast, experimental infections with B. motasi and B. crassa in spleen-intact lambs have been reported to result in low parasitemia and mild fever (Hasherni-Fesharki and Uilenberg, 1981; Lewis et al., 1981). Additionally, experimental infection with B. motasi (Welsh strain) in spleen intact goats resulted in mild anemia, a modest 1% parasitemia, and mild fever (Lewis et al., 1981). Conversely, lambs infected with Babesia sp. Xinjiang did not exhibit any fever or symptoms (Guan et al., 2001). Interestingly, despite the high parasitemia in spleen-intact lambs experimentally infected with Babesia sp. BQ1-Lintan, no clinical signs were observed (Guan et al., 2010). Overall, the level of parasitemia can provide some insight into the pathogenicity of Babesia species, but it is not the sole determinant. Other factors, including host factors and parasite characteristics, also contribute to the clinical manifestations and severity of babesiosis (Yeruham et al., 1998; Sevinc et al., 2013). In this study, a comprehensive and objective clinical comparison was conducted to evaluate the effects of experimental infection in spleen intact goats. Our findings indicate that B. aktasi exhibits relatively low pathogenicity in spleen-intact goats, resulting in only mild fever and low parasitemia. This parallels the characteristics of certain other small ruminant Babesia species, even though some of these species can induce severe clinical infections and even mortality in spleen-intact goats.
Interpretation of clinical signs of acute babesiosis, combined with hemogram and serum biochemistry analysis, involves examination of blood tests to assess the health status of animals (Turgut, 2000). Severe clinical infection was observed in splenectomized goats in this study, resulting in a significant decrease in RBC, HCT, HB, and PLT values compared to their pre-infection levels. This decline can be attributed to the destruction of erythrocytes caused by parasite replication (Esmaeilnejad et al., 2012; Sevinc et al., 2013; Salem and Farag, 2014; Sevinc et al., 2014; Kage et al., 2019). According to hematocrit and HCT values, splenectomized goats developed severe anemia (Tvedten, 2022). In this study, MCV was normal, indicating normocytic RBC. However, MCHC showed a statistically significant decrease, indicating reduced hemoglobin concentration within RBC (hypochromic). This led to the classification of the anemia as normocytic hypochromic, where the red blood cells had normal size but lower than expected hemoglobin content. Normocytic hypochromic anemia is commonly associated with typical forms of iron deficiency (Turgut, 2000; Polizopoulou, 2010). Although we did not determine iron concentrations after experimental infection in this study, significant decreases were observed in this concentration in cattle infected with T. annulata, B. bigemina (Lotfollahzadeh et al., 2012) and B. ovis (Voyvoda et al., 1997) compared to uninfected animals. Various studies on anemia types in sheep babesiosis have reported macrocytic hypochromic anemia in splenectomized animals infected with B. ovis (Rahbari et al., 2008) and B. motasi (Alani and Herbert, 1988), and microcytic hypochromic anemia in spleen-intact sheep infected with B. ovis (Rahbari et al., 2008). However, natural infection with B. ovis in sheep has been associated with normocytic-normochromic anemia (Sevinc et al., 2013). In this study, thrombocytopenia, which is a significant hematological manifestation of babesiosis in dogs and sheep, was also observed. However, the decrease in HCT values, although statistically significant, was evaluated within the reference range established for goats (Table 2). Likewise, a significant statistical increase in the levels of ALT and AST was observed in the immunosuppressed group following experimental infection. However, the increase in AST levels remained within the reference range, similar to the HCT values (Table 2). Our results agree with previous reports and indicate that animals with babesiosis may experience elevated ALT levels, indicating potential disruptions in liver function associated with the disease. In particular, certain species of Babesia can cause liver tissue damage and potentially suppress its overall function (Rogers, 1971; Schneider et al., 2011; Sevinc et al., 2013; Esmaeilnejad et al., 2021)
In conclusion, B. aktasi n. sp. isolated from goats caused mild disease with minor clinical symptoms in non-immunosuppressed goats but displayed significant pathogenicity, resulting in severe clinical infections in immunosuppressed goats. A previous study revealed a 22.5% prevalence of B. aktasi n. sp. in clinically healthy goats, suggesting its limited pathogenicity in this host, while no evidence of B. aktasi n. sp. infection was found in sheep (Ulucesme et al., 2023a). Further experimental infection studies are necessary to investigate the pathogenicity of the parasite in sheep. Another study conducted in the same geographic area identified Rhipicephalus bursa as the predominant tick species collected from goats and suggested this tick species as the primary vector responsible for transmitting the parasite (Ulucesme et al., 2023b). This previous study was limited to local goat breeds, emphasizing the need for additional studies to encompass different breeds to expand our understanding. Future studies focused on investigating specific tick species responsible for transmitting B. aktasi n. sp., and in the identification of other potential hosts apart from goats that can be susceptible to infection are also needed. There is also a need for studies involving experimental infections caused by tick bites, as the tick may add additional factors that may affect the virulence of the parasite and/or the host’s susceptibility to infection. Given the dense population of mountain goats in the previous study area, it is essential to conduct further research to determine the natural reservoir of B. aktasi n. sp. Understanding the vector species involved in the transmission of B. aktasi n. sp. will provide valuable insights into the biology, epidemiology, and ecology of this pathogen.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The animal study was approved by Firat University, Animal Experiment Ethic Committee, protocol number 2018/100. The study was conducted in accordance with the local legislation and institutional requirements.
SÖ: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MU: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. RB: Formal Analysis, Writing – review & editing. HA: Writing – review & editing. JL: Writing – review & editing. CS: Writing – review & editing. MA: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Scientific and Technological Council of Turkiye (TUBITAK) Grant Program (project number: 118O871).
We are grateful to Aleyna Karoglu and Zeliha Irem Turk for the excellent technical and administrative support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alani, A. J., Herbert, I. V. (1988). The morphometrics of Babesia motasi (Wales) and its transmission by Haemaphysalis punctata (Canestrini and Fanzago 1877) to sheep. Vet. Parasitol. 30, 87–95. doi: 10.1016/0304-4017(88)90155-0
Bach, O., Baier, M., Pullwitt, A., Fosiko, N., Chagaluka, G., Kalima, M., et al. (2005). Falciparum malaria after splenectomy: a prospective controlled study of 33 previously splenectomized Malawian adults. Trans. R. Soc Trop. Med. Hyg. 99, 861–867. doi: 10.1016/j.trstmh.2005.03.008
Bai, Q., Liu, G., Liu, D., Ren, J., Li, X. (2002). Isolation and preliminary characterization of a large Babesia sp. from sheep and goats in the eastern part of Gansu Province, China. Parasitol. Res. 88, S16–S21. doi: 10.1007/s00436-001-0563-6
Bosman, A.-M., Oosthuizen, M. C., Peirce, M. A., Venter, E. H., Penzhorn, B. L. (2010). Babesia lengau sp. nov., a novel Babesia species in cheetah (Acinonyx jubatus, Schreber 1775) populations in South Africa. J. Clin. Microbiol. 48, 2703–2708. doi: 10.1128/JCM.02266-09
Buffet, P. A., Safeukui, I., Deplaine, G., Brousse, V., Prendki, V., Thellier, M., et al. (2011). The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood 117, 381–392. doi: 10.1182/blood-2010-04-202911
Dantas-Torres, F. (2018). Species concepts: what about ticks? Trends Parasitol. 34, 1017–1026. doi: 10.1016/j.pt.2018.09.009
Esmaeilnejad, B., Dalir-Naghadeh, B., Tavassoli, M., Asri-Rezaei, S., Mahmoudi, S., Rajabi, S., et al. (2021). Assessment of hepatic oxidative damage, paraoxonase-1 activity, and lipid profile in cattle naturally infected with Babesia bigemina. Trop. Anim. Health Prod. 53, 219. doi: 10.1007/s11250-021-02662-x
Esmaeilnejad, B., Tavassoli, M., Asri-Rezaei, S. (2012). Investigation of hematological and biochemical parameters in small ruminants naturally infected with Babesia ovi. Vet. Res. Forum. (Urmia, Iran), 31.
Friedhoff, K. T. (1988). “Transmission of babesia,” in Babesiosis of domestic animals and man (Florida: CRC Press). pp. 23–53.
Fujinaga, T. (1982). Effect of splenectomy and dexamethasone administration on cattle experimentally infected with Babesia ovata. Nihon Juigaku Zasshi. Japanese J. Of Veterinary Sci. 44 (1), 71–80. doi: 10.1292/jvms1939.44.71
Georges, K., Loria, G., Riili, S., Greco, A., Caracappa, S., Jongejan, F., et al. (2001). Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 99, 273–286. doi: 10.1016/S0304-4017(01)00488-5
Gray, J. S., Estrada-Peña, A., Zintl, A. (2019). Vectors of babesiosis. Annu. Rev. Entomol. 64, 149–165. doi: 10.1146/annurev-ento-011118-111932
Guan, G., Ma, M., Moreau, E., Liu, J., Lu, B., Bai, Q., et al. (2009). A new ovine Babesia species transmitted by Hyalomma anatolicum anatolicum. Exp. Parasitol. 122, 261–267. doi: 10.1016/j.exppara.2009.05.001
Guan, G., Moreau, E., Liu, J., Hao, X., Ma, M., Luo, J., et al. (2010). Babesia sp. BQ1 (Lintan): Molecular evidence of experimental transmission to sheep by Haemaphysalis qinghaiensis and Haemaphysalis longicornis. Parasitol. Int. 59, 265–267. doi: 10.1016/j.parint.2009.12.002
Guan, G., Yin, H., Luo, J., Lu, W., Zhang, Q., Gao, Y., et al. (2002). Transmission of Babesia sp. to sheep with field-collected Haemaphysalis qinghaiensis. Parasitol. Res. 88, S22–S24. doi: 10.1007/s00436-001-0564-5
Guan, G., Yin, H., Luo, J., Lu, W., Zhang, Q., Ma, M., et al. (2001). Morphology and pathogenicity initial investigate of Babesia sp. Chin. J. Vet. Sci. Tech. 31, 35–36.
Guglielmone, A. A., Robbins, R. G., Apanaskevich, D. A., Petney, T. N., Estrada Peña, A., Horak, I. G., et al. (2010). The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa. 2528, 1–28. doi: 10.11646/zootaxa.2528.1.1
Habela, M., Reina, D., Nieto, C., Navarrete, I. (1990). Antibody response and duration of latent infection in sheep following experimental infection with Babesia ovis. Vet. Parasitol. 35, 1–10. doi: 10.1016/0304-4017(90)90111-N
Hasherni-Fesharki, R., Uilenberg, G. (1981). Babesia crassa n. sp.(Sporozoa, Babesiidae) of domestic sheep in Iran. Vet. Q. 3, 1–8. doi: 10.1080/01652176.1981.9693787
Homer, M. J., Aguilar-Delfin, I., Telford, S. R., Krause, P. J., Persing, D. H. (2000). Babesiosis. Clin. Microbiol. Rev. 13, 451–469doi: 10.1128/cmr.13.3.451
Kage, S., Mamatha, G., Lakkundi, J. N., Shivashankar, B., D’Souza, P. E. (2019). Detection of incidence of Babesia spp. in sheep and goats by parasitological diagnostic techniques. J. Parasitol. Dis. 43, 452–457. doi: 10.1007/s12639-019-01109-3
Lewis, D., Holman, M., Purnell, R., Young, E., Herbert, I., Bevan, W. (1981). Investigations on Babesia motasi isolated from Wales. Res. Vet. Sci. 31, 239–243. doi: 10.1016/S0034-5288(18)32501-3
Lewis, S. M., Williams, A., Eisenbarth (2019). Structure and function of the immune system in the spleen. Sci. Immunol. 4, eaau6085. doi: 10.1126/sciimmunol.aau6085
Liu, A., Yin, H., Guan, G., Schnittger, L., Liu, Z., Ma, M., et al. (2007). At least two genetically distinct large Babesia species infective to sheep and goats in China. Vet. Parasitol. 147, 246–251. doi: 10.1016/j.vetpar.2007.03.032
Lotfollahzadeh, S., Rahmani, M., Mohri, M., Madadgar, O. (2012). Changes in serum iron concentration and hepatic enzyme activities in cattle infected with Theileria annulata and Babesia bigemina. Comp. Clin. Pathol. 21, 829–832. doi: 10.1007/s00580-011-1185-8
Martínez-García, G., Santamaría-Espinosa, R. M., Lira-Amaya, J. J., Figueroa, J. V. (2021). Challenges in tick-borne pathogen detection: the case for Babesia spp. identification in the tick vector. Pathogens 10, 92. doi: 10.3390/pathogens10020092
Niu, Q., Liu, Z., Yang, J., Gao, S., Pan, Y., Guan, G., et al. (2017). Genetic characterization and molecular survey of Babesia sp. Xinjiang infection in small ruminants and ixodid ticks in China. Infect. Genet. Evol. 49, 330–335. doi: 10.1016/j.meegid.2017.01.025
Niu, Q., Luo, J., Guan, G., Liu, Z., Ma, M., Liu, A., et al. (2009). Differentiation of two ovine Babesia based on the ribosomal DNA internal transcribed spacer (ITS) sequences. Exp. Parasitol. 121, 64–68. doi: 10.1016/j.exppara.2008.09.021
Oosthuizen, M. C., Zweygarth, E., Collins, N. E., Troskie, M., Penzhorn, B. L. (2008). Identification of a novel Babesia sp. from a sable antelope (Hippotragus Niger Harris 1838). J. Clin. Microbiol. 46, 2247–2251. doi: 10.1128/JCM.00167-08
Ozubek, S., Aktas, M. (2017a). Molecular and parasitological survey of ovine piroplasmosis, including the first report of Theileria annulata (Apicomplexa: Theileridae) in sheep and goats from Turkey. J. Med. Entomol. 54, 212–220. doi: 10.1093/jme/tjw134
Ozubek, S., Aktas, M. (2017b). Molecular evidence of a new Babesia sp. in goats. Vet Parasitol. 233, 1–8. doi: 10.1016/j.vetpar.2016.11.016
Ozubek, S., Ulucesme, M. C., Aktas, M. (2023). Discovery of a Novel Species Infecting Goats: Morphological and Molecular Characterization of Babesia aktasi n. sp. Pathogens 12, 113. doi: 10.3390/pathogens12010113
Polizopoulou, Z. S. (2010). Haematological tests in sheep health management. Small Rumin. Res. 92, 88–91. doi: 10.1016/j.smallrumres.2010.04.015
Rahbari, S., Nabian, S., Khaki, Z., Alidadi, N., Ashrafihelan, J. (2008). Clinical, haematologic and pathologic aspects of experimental ovine babesiosis in Iran. Iran. J. Vet. Res. 9, 59–64. doi: 10.22099/IJVR.2008.523
Ravindran, R., Mishra, A. K., Rao, J. R. (2006). Methodology for raising high parasitaemic Babesia bigemina infection in donor bovine calves. J. Appl. Anim. Res. 29 (1), 59–60. doi: 10.1080/09712119.2006.9706571
Rogers, R. J. (1971). Observations on the pathology of Babesia Argentina infections in cattle. Aust. Vet. J. 47, 242–247. doi: 10.1111/j.1751-0813.1971.tb02142.x
Salem, N., Farag, H. (2014). Clinical, hematologic, and molecular findings in naturally occurring Babesia canis vogeli in Egyptian dogs. Vet. Med. Int 2014, 270345. doi: 10.1155/2014/270345
Schneider, D. A., Yan, H., Bastos, R. G., Johnson, W. C., Gavin, P. R., Allen, A. J., et al. (2011). Dynamics of bovine spleen cell populations during the acute response to Babesia bovis infection: an immunohistological study. Parasite Immunol. 33, 34–44. doi: 10.1111/j.1365-3024.2010.01249.x
Schnittger, L., Ganzinelli, S., Bhoora, R., Omondi, D., Nijhof, A. M., Florin-Christensen, M. (2022). The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: Species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 121, 1207–1245. doi: 10.1007/s00436-022-07424-8
Sears, K. P., Knowles, D. P., Fry, L. M. (2022). Clinical Progression of Theileria haneyi in splenectomized horses reveals decreased virulence compared to Theileria equi. Pathogens 11, 254. doi: 10.3390/pathogens11020254
Sevinc, F., Sevinc, M., Ekici, O. D., Yildiz, R., Isik, N., Aydogdu, U. (2013). Babesia ovis infections: Detailed clinical and laboratory observations in the pre-and post-treatment periods of 97 field cases. Vet. Parasitol. 191, 35–43. doi: 10.1016/j.vetpar.2012.07.025
Sevinc, F., Sevinc, M., Koc, Y., Alkan, F., Ekici, O. D., Yildiz, R., et al. (2014). The effect of 12 successive blood passages on the virulence of Babesia ovis in splenectomized lambs: A preliminary study. Small Rumin. Res. 116, 66–70. doi: 10.1016/j.smallrumres.2013.10.010
Sevinc, F., Turgut, K., Sevinc, M., Ekici, O. D., Coskun, A., Koc, Y., et al. (2007). Therapeutic and prophylactic efficacy of imidocarb dipropionate on experimental Babesia ovis infection of lambs. Vet. Parasitol. 149, 65–71. doi: 10.1016/j.vetpar.2007.07.014
Shkap, V., Rasulov, I., Abdurasulov, S., Fish, L., Leibovitz, B., Krigel, Y., et al. (2007). Babesia bigemina: Attenuation of an Uzbek isolate for immunization of cattle with live calf-or culture-derived parasites. Vet. Parasitol. 146, 221–226. doi: 10.1016/j.vetpar.2007.02.018
Stevanović, O., Radalj, A., Subić, I., Jovanović, N. M., Sladojević, Ž., Amović, M., et al. (2022). The presence of Malignant ovine babesiosis in Bosnia and Herzegovina indicates a possible emerging risk for Balkan region. Comp. Immunol. Microbiol. Infect. Dis. 90, 101893. doi: 10.1016/j.cimid.2022.101893
Stuen, S. (2020). Haemoparasites—Challenging and wasting infections in small ruminants: A review. Animals 10, 2179. doi: 10.3390/ani10112179
Turgut, K. (2000). Veterinary clinic laboratory diagnosis. Ankara Bahcıvanlar Basim Evi. pp, 19–366.
Tvedten, H. (2022). Classification and laboratory evaluation of anemia. Schalm’s Veterinary Hematol., 198–208. doi: 10.1002/9781119500537.ch25
Uilenberg, G. (2006). Babesia—a historical overview. Vet. Parasitol. 138, 3–10. doi: 10.1016/j.vetpar.2006.01.035
Uilenberg, G., Rombach, M., Perié, N., Zwart, D. (1980). Blood parasites of sheep in the Netherlands. II. Babesia motasi (Sporozoa, Babesiidae). Vet. Q. 2, 3–14. doi: 10.1080/01652176.1980.9693752
Ulucesme, M. C., Ozubek, S., Aktas, M. (2023b). Molecular prevalence and genetic diversity based on msp1a gene of Anaplasma ovis in Goats from Türkiye. Life (Basel) 13, 1101. doi: 10.3390/life13051101
Ulucesme, M. C., Ozubek, S., Karoglu, A., Turk, Z. I., Olmus, I., Irehan, B., et al. (2023a). Small Ruminant Piroplasmosis: high prevalence of Babesia aktasi n. sp. in goats in Türkiye. Pathogens 12, 514. doi: 10.3390/pathogens12040514
Voyvoda, H., Sekin, S., Kaya, A., Bi̇ldi̇k, A. (1997). Modifications of seru iron, copper concentration (SI, Cu), total and latent iron-binding capacity (TIBC, LIBC), and transferrin saturation (TS) in natural Babesia ovis infection of sheep. Turkish J. Vet. Anim. Sci. 21, 31–37. doi: 10.55730/1300-0128.4055
Wang, J., Chen, K., Ren, Q., Zhang, S., Yang, J., Wang, Y., et al. (2023). Comparative genomics reveals unique features of two Babesia motasi subspecies: Babesia motasi lintanensis and Babesia motasi hebeiensis. Int. J. Parasitol. 53, 265–283. doi: 10.1016/j.ijpara.2023.02.005
Wang, X., Wang, J., Liu, J., Liu, A., He, X., Xiang, Q., et al. (2020). Insights into the phylogenetic relationships and drug targets of Babesia isolates infective to small ruminants from the mitochondrial genomes. Parasitol. Vectors 13, 1–11. doi: 10.1186/s13071-020-04250-8
Wang, X., Wang, J., Liu, J., Liu, A., He, X., Xu, J., et al. (2019). Comparative analysis of apicoplast genomes of Babesia infective to small ruminants in China. Parasitol. Vectors 12, 1–11. doi: 10.1186/s13071-019-3581-x
Wright, I. G., Goodger, B. V. (2018). Pathogenesis of babesiosis. Babesiosis Domest. Anim. man 99-118. doi: 10.1201/9781351070027-6
Yeruham, I., Hadani, A., Galker, F. (1998). Some epizootiological and clinical aspects of ovine babesiosis caused by Babesia ovis--a review. Vet. Parasitol. 74, 153–163. doi: 10.1016/S0304-4017(97)00143-X
Yeruham, I., Hadani, A., Galker, F. (2001). The effect of the ovine host parasitaemia on the development of Babesia ovis (Babes 1892) in the tick Rhipicephalus bursa (Canestrini and Fanzago 1877). Vet. Parasitol. 96, 195–202. doi: 10.1016/S0304-4017(00)00433-7
Yin, H., Lu, W., Luo, J. (1997). Babesiosis in China. Trop. Anim. Health Prod. 29, 11S–15S. doi: 10.1007/BF02632908
Keywords: Babesia aktasi n. sp., experimental infection, goat, immunosuppression, pathogenicity
Citation: Ozubek S, Ulucesme MC, Bastos RG, Alzan HF, Laughery JM, Suarez CE and Aktas M (2023) Experimental infection of non-immunosuppressed and immunosuppressed goats reveals differential pathogenesis of Babesia aktasi n. sp.. Front. Cell. Infect. Microbiol. 13:1277956. doi: 10.3389/fcimb.2023.1277956
Received: 15 August 2023; Accepted: 18 October 2023;
Published: 02 November 2023.
Edited by:
David R. Allred, University of Florida, United StatesReviewed by:
Dana Mordue, New York Medical College, United StatesCopyright © 2023 Ozubek, Ulucesme, Bastos, Alzan, Laughery, Suarez and Aktas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sezayi Ozubek, c296dWJla0BmaXJhdC5lZHUudHI=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.