- 1Pediatric Intensive Care Unit, Beijing Children’s Hospital, Capital Medical University, Beijing, China
- 2Department of Scientific Affairs, Hugobiotech Co., Ltd., Beijing, China
Background: Cryptosporidium infections in humans typically result in symptoms such as abdominal pain and diarrhea. When the diarrhea is severe, it can cause serious complications and even be life-threatening, especially in patients with compromised immune systems.
Case presentation: Here, we reported the use of metagenomic next-generation sequencing (mNGS) to assist in the diagnosis and treatment of a 10-year-old boy with severe Cryptosporidium infection. Despite the absence of any history of immunocompromise, the infection still resulted in severe symptoms, including shock, as well as damage to his pancreas and kidneys. The mNGS tests detected the presence of Cryptosporidium parvum when conventional methods failed. The patient received anti-parasite treatment along with supportive care to manage the condition. With disease surveillance based on regular clinical tests and sequential mNGS tests, the child recovered from the severe conditions.
Conclusion: Our study emphasized the importance of recognizing the potential severity of Cryptosporidium infection, even among individuals with normal immune systems. Timely diagnosis and ongoing monitoring are essential for patient prognosis.
Introduction
Cryptosporidiosis, caused by Cryptosporidium spp., is a parasitic disease that typically infects the epithelial cells of the digestive tract in both humans and animals, leading to symptoms like abdominal pain and diarrhea. The first documented case of human self-limited enteritis caused by Cryptosporidium infection was reported in 1976 (Nime et al., 1976). Since then, Cryptosporidium infection has been widely reported in over 90 countries and regions worldwide (Liu et al., 2020), with Cryptosporidium hominis and Cryptosporidium parvum being responsible for more than 90% of human Cryptosporidium infections (Feng et al., 2018). Patients with compromised immune systems who are infected with Cryptosporidium may suffer from severe gastroenteritis (Hassanein et al., 2012; Gerace et al., 2019), resulting in copious amounts of watery diarrhea that can cause significant fluid loss and life-threatening complications. Conversely, individuals with normal immune function typically experience asymptomatic infection or mild self-limiting diarrhea as their primary clinical symptom (Ryan et al., 2014). Here, we present a rare case of C. parvum infection in a healthy child that resulted in severe dehydration, hypovolemic shock, rhabdomyolysis, acute kidney injury, and pancreatitis due to the presence of massive watery stools and vomiting.
Case presentation
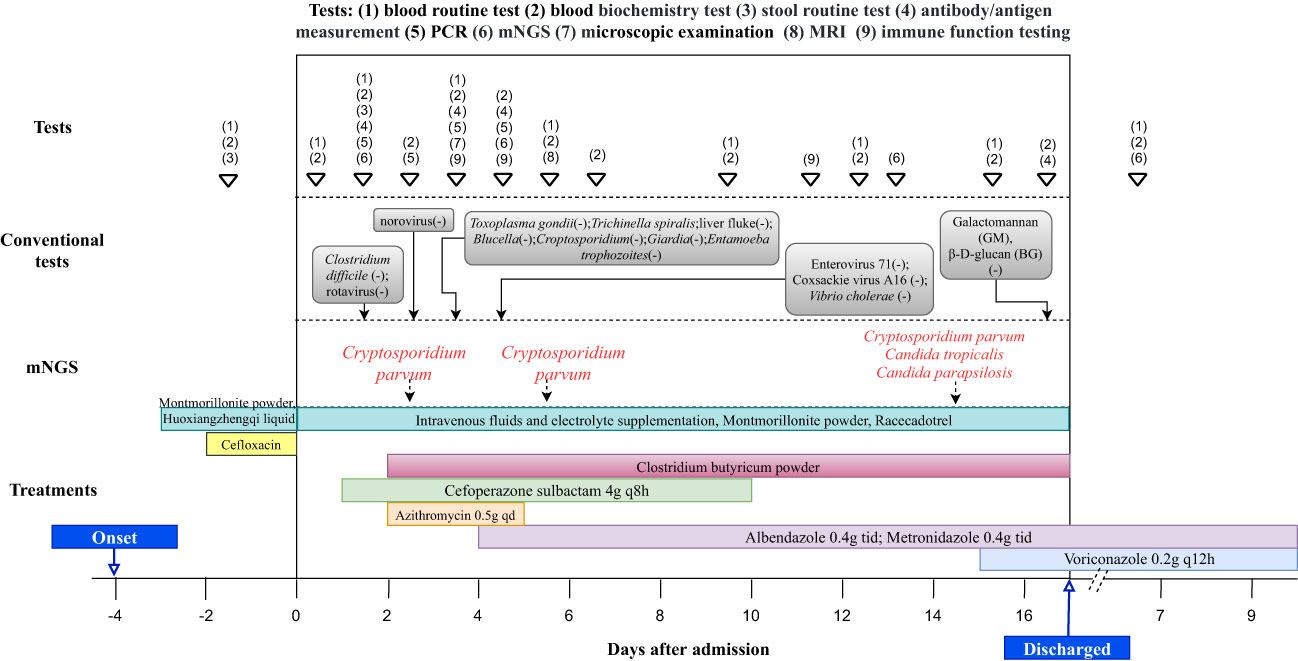
We reported a case of a ten-year-old boy from Zhangjiakou City in Hebei Province, who presented with persistent symptoms including nausea, abdominal pain, vomiting and severe diarrhea, accompanied by intermittent fever. The child was firstly admitted to a local hospital, where routine examination revealed elevated levels of multiple indicators, as well as the presence of blood and white blood cells in his stool (Table 1). Initial treatment involved cefloxacin administration and fluid rehydration (Figure 1), but his symptoms did not improve. On the fourth day after the onset of symptoms, he exhibited a decreased level of consciousness, prompting his transfer to the Pediatric Intensive Care Unit (PICU) at Beijing Children’s Hospital Affiliated with Capital Medical University.
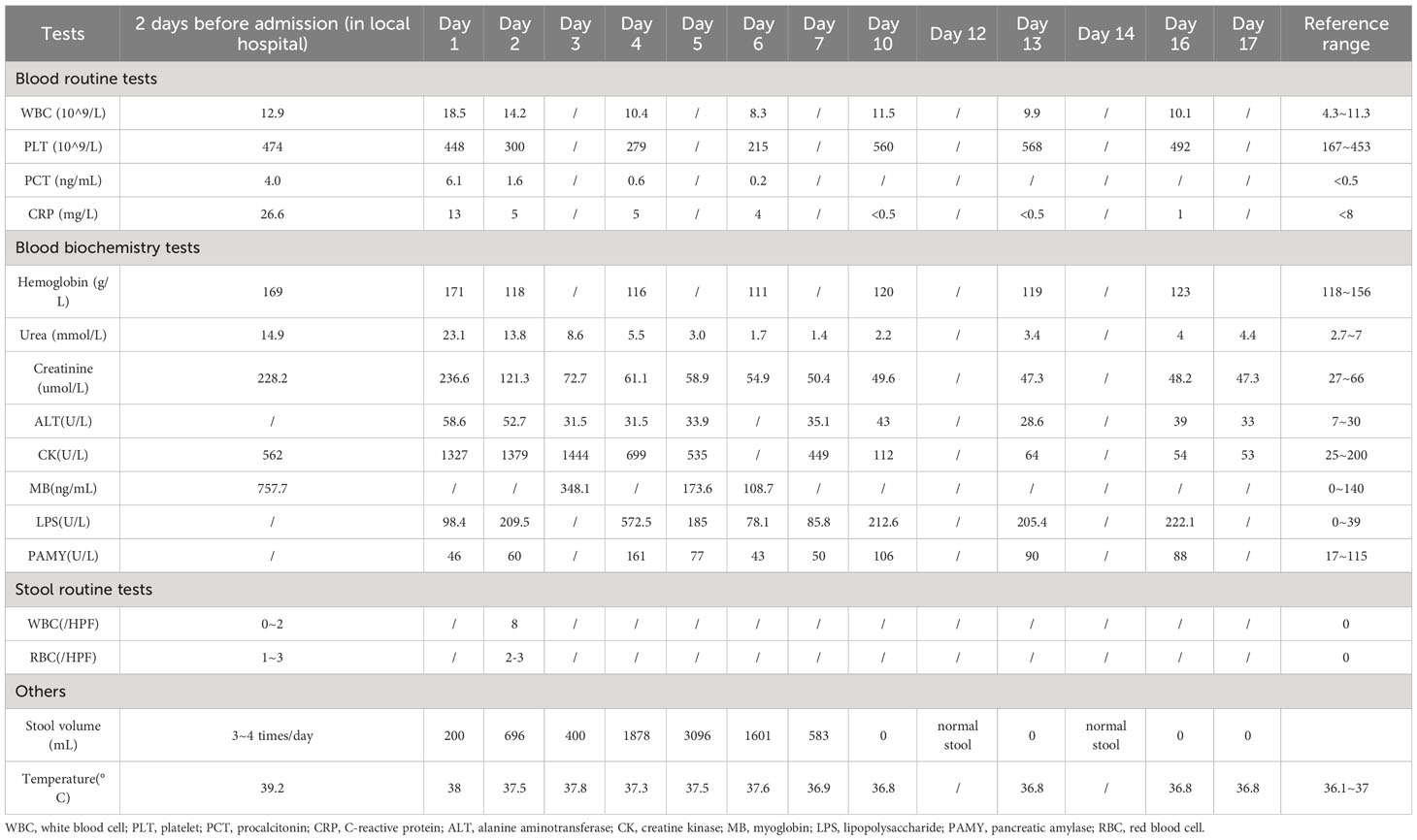
Upon admission to the PICU, the child had a fever of 38°C and presented with intermittent episodes of confusion, disorganized speech and shock. His physical examination revealed a heart rate of 138 beats per minute, respiratory rate of 26 breaths per minute, and blood pressure of 134/90 mmHg. Clinical signs of dehydration were observed, including dry and less elastic skin, dry lips, as well as sunken eye sockets. His extremities exhibited coldness, and the capillary refill time (CRT) was measured at 4 seconds. Routine blood tests showed leukocytosis (18.5×109/L) and thrombocytosis (448×109/L), as well as elevated levels of C-reactive protein (CRP, 13 mg/L), hemoglobin (171 g/L) and procalcitonin (6.1 ng/mL). Blood biochemical results indicated remarkably high levels of urea (23.1 mmol/L), creatinine (236.6 umol/L), creatine kinase (1,327 U/L) (Table 1). Subsequent stool test results revealed 8 white blood cells per high power field.
There were no obvious abnormalities in the patient’s immunoglobulin and lymphocyte counts and proportions. Antishock therapy was administered to him via intravenous fluids and electrolyte supplementation, resulting in the correction of his shock within 6 hours of admission. In view of the elevated levels of creatine kinase and serum myoglobin, a diagnose of rhabdomyolysis was made and the child was treated with hydration and alkalization. Due to severe dehydration caused by vomiting and diarrhea, he received a fluid supplement. In addition, racecadotrel was administered orally to reduce intestinal fluid secretion, montmorillonite powder was given orally to protect the intestinal mucosa, and probiotics were prescribed orally to regulate the intestinal flora. Infectious diarrhea was highly suspected, and cefoperazone sulbactam (4g q8h) was given as empiric therapy (Figure 1). The child’s pancreatic enzyme levels were observed to be elevated on day 4 after admission, and subsequent pancreatic magnetic resonance imaging (MRI) indicated abnormalities in pancreatic morphology and filling. This led to a diagnosis of pancreatitis, and somatostatin was administered to inhibit the secretion of pancreatic enzymes.
The antigen test for rotavirus was negative, and negative PCR results ruled out the presence of norovirus and Clostridium difficile. Metagenomic next-generation sequencing (mNGS) tests were performed with the consent of the family. Given the possibility of a systemic infection, the whole blood samples of the child were collected on Day 2 after admission, and sent for nucleic acid extraction, library construction and high-throughput sequencing (Hugo-Biotech Company, Beijing). After the sequence of human genome was excluded, mNGS identified 3,178 specific reads belong to C. parvum, accounting for 1.9% of nucleotide sequence coverage (Figure 2A). The test results were obtained on Day 3, and the patient was promptly initiated on intravenous infusion of azithromycin (0.5g qd) for anti-parasitic treatment. After 2 days, the medication was transitioned to oral administration of metronidazole (0.4g tid) and albendazole (0.4g tid) tablets. During this period, the patient’s peripheral blood cultures were negative. Additionally, tests for IgG antibodies against various pathogens, including Toxoplasma gondii、Trichinella spiralis、Dengue fever and liver fluke, were negative. The Brucella tiger red test was also negative, as were tests for Cryptosporidium and Giardia antigens (the colloidal gold method) in stool samples. Furthermore, PCR results of Entamoeba trophozoites and cysts, Enterovirus 71, Coxsackie virus A16, and Vibrio cholerae slide agglutination test were all negative. Microscopy examination did not reveal any fungi or parasites.
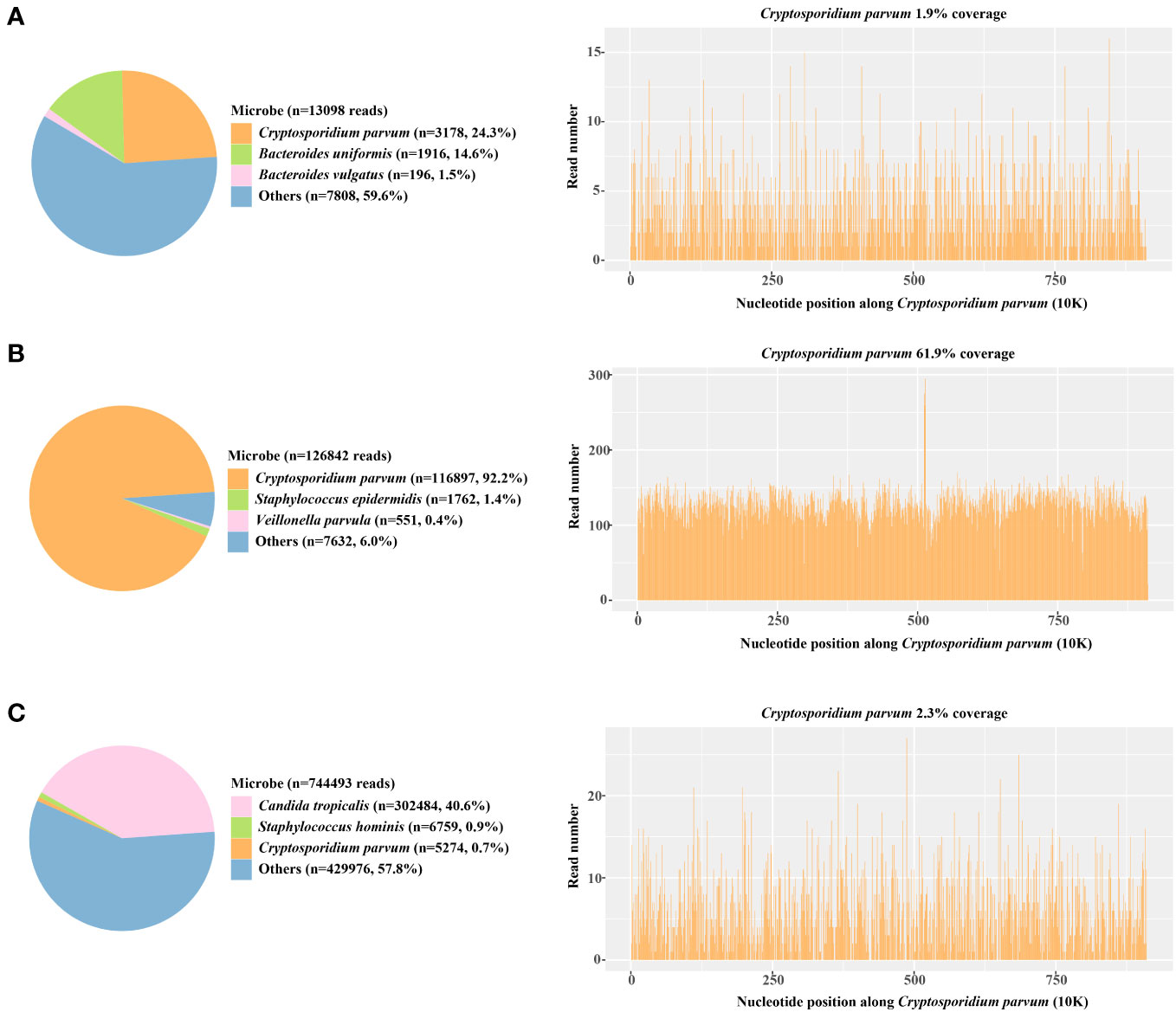
Figure 2 The proportion of detected Cryptosporidium parvum sequences in the total microbial sequences and the genome coverage (A) Day 3; blood samples (B) Day 6; anal swabs (C) Day 15; anal swabs.
On Day 5 after admission, anal swabs of the child and his parents were collected for further detection using mNGS. The results of child’s tests showed that 92.2% (116,897) of pathogenic reads corresponded to C. parvum with a coverage of 61.9%, and neither parent was detected with any pathogen (Figure 2B). On day 10, the child’s temperature and inflammatory markers returned to normal levels, and cefoperazone sulbactam was discontinued. Additionally, his vomiting and gastrointestinal symptoms were relieved, and the levels of pancreatic enzymes, creatinine and creatine kinase were lower than before. On Day 15, mNGS identified 5,274 specific reads of C. parvum in the child’s anal swabs samples, indicating a decrease in pathogen nucleic acid load. However, Candida tropicalis and Candida parapsilosis (302,484 and 5,320 specific reads) were also detected in this test (Figure 2C). This was considered to be caused by an imbalance in intestinal flora, and oral voriconazole (0.2g q12h) was given to prevent opportunistic infections. On Day 17, the patient’s temperature was normal without vomiting or diarrhea, and he was discharged. After discharge, he continued to take albendazole, metronidazole, and voriconazole tablets orally for 10 days, and no fever, diarrhea, or vomiting recurred. On the 7th day after discharged, his blood routine test, liver and kidney function, and pancreatic enzyme levels returned to normal, and re-examination using mNGS showed no presence of pathogens.
Discussion
Cryptosporidium oocysts are common in the environment, and in China, the primary risk factors for infection include consumption of contaminated water or food, contact with infected animals, and seasonal outbreaks of Cryptosporidium (usually during summer or autumn) (Feng and Xiao, 2017). The faecal-oral route is the primary mode of transmission for this parasite. The child in this report lived in Hebei Province, where cryptosporidiosis has rarely been reported. He fell ill in the summer, and denied any exposure with domestic animals. He had spent a short time in a rural village in Hebei Province and drank from a well, but he lived and ate with his parents, who were not infected with Cryptosporidium. Despite having no history of immune dysfunction, the child experienced severe symptoms including shock, and damage to the pancreas and kidneys after contracting the infection.
Cryptosporidium is known to be able to cause severe gastroenteritis (Bouzid et al., 2013; Gerace et al., 2019), with immunocompromised individuals experiencing more serious complications. The development of kidney injury in Cryptosporidium infection may be explained by the mechanism of direct cytopathic injury as observed in cases of nematode infections (Prasad and Patel, 2018). Previous reports have described pancreatitis associated with Cryptosporidium in both immunocompromised and immunocompetent patients (Miller et al., 1992; Norby et al., 1998). The pathogenesis of parasite-induced pancreatitis may involve worm obstruction of the pancreatic duct, the common bile duct, or the formation of abscesses (Rawla et al., 2017). A comprehensive understanding of the inflammatory syndromes and complications associated with Cryptosporidium infection is crucial for guiding appropriate disease management. Furthermore, our case also restated that Cryptosporidium can pose a serious threat even to healthy individuals, emphasizing the need for vigilance towards cryptosporidiosis.
Serology tests for Cryptosporidium usually exhibits high sensitivity and specificity (Ungar, 1990; Agnamey et al., 2011; El-Moamly and El-Sweify, 2012). However, false negative results may occur when the concentration of oocysts in samples is low (El-Moamly and El-Sweify, 2012). In this study, the intermittent shedding pattern observable in C. parvum infection (Jokipii and Jokipii, 1986; Chappell et al., 1996) may account for the initial negative findings obtained by immunochromatographic assay. PCR testing has been shown to have excellent performance for Cryptosporidium diagnosis (Helmy et al., 2014), and qPCR can serve as an accurate quantification method for assessing pathogenic load during disease progression (Clementi et al., 1995; Caldas et al., 2012). Nevertheless, designing primers for PCR requires expertise and can be time-consuming. Multiplex PCR panels targeted towards enteric pathogens, such as FilmArray (Golan Shaposhnik et al., 2019) and TaqMan Array Card (Liu et al., 2013), have been increasingly used in the early workup of a severely ill patient with diarrhea. However, these panels are not currently widely accessible in Chinese hospitals. Hence, in this case, mNGS was selected for pathogen detection to avoid diagnostic delays.
mNGS has emerged as a crucial technique for identifying unexplained infections, with the potential to detect all pathogen sequences in a single test without requiring specific amplification or primers. Its use is increasingly widespread in clinical settings to diagnose various infectious diseases (Chiu and Miller, 2019; Gu et al., 2021). In this study, mNGS was able to rapidly detect Cryptosporidium in the child’s blood samples within two days, enabling adjustments in the clinical treatment plan based on the detection results. This highlights the potential of mNGS to provide timely and accurate diagnosis. Additionally, semi-quantitative mNGS reads have been reported to reflect disease progression and therapeutic effectiveness (Ai et al., 2018; Zhang et al., 2020; Chen et al., 2022). Shedding of Cryptosporidium is known to be prolonged after recovering from initial symptomatic illness (Johansen et al., 2022). The presence of Cryptosporidium reads identified by mNGS on Day 15 in this case provides additional confirmation of this phenomenon.
The application of mNGS in pathogen detection also has certain limitations. In comparison to multiplex PCR panels, mNGS incurs higher costs and longer turnaround time (Chiu and Miller, 2019; Ramachandran and Wilson, 2020). The abundance of data generated by mNGS necessitates intricate bioinformatics analysis and interpretation, demanding specialized personnel and advanced computational resources. Additionally, the heightened sensitivity of mNGS technology may result in the introduction of noise and false-positive outcomes (Jing et al., 2021). This could arise from exogenous DNA contamination, cross-contamination during sample handling, or errors during sequencing procedures (Chiu and Miller, 2019). These constraints presently hinder mNGS from emerging as a forefront diagnostic tool in clinical settings, but ongoing technological advancements are continuously improving its potential. Additionally, although not utilized in this particular study, it is crucial to emphasize the important role of multiplex PCR panels in the diagnosis and surveillance of Cryptosporidium infection.
At present, there is currently no specific drug treatment for cryptosporidiosis. Nitazoxanide has been proven effective and is the only drug approved by the Food and Drug Administration to treat cryptosporidiosis, but it is less effective for AIDS patients and malnourished children (Amadi et al., 2002; White, 2004). Azithromycin (Dionisio et al., 1998), paromomycin (Marshall and Flanigan, 1992), and acetylated spiramycin (Huang et al., 2015) have also shown some efficacy. Due to the unavailability of nitazoxanide in China, azithromycin was used as an antiparasitic for three days. Given the rapid improvement in inflammation and pancreatitis markers by day 4, it is likely that azithromycin had a beneficial effect on this patient. However, despite this improvement, the patient’s gastrointestinal symptoms did not alleviate, and the volume of diarrhea continued to increase. Unfortunately, we were unable to add paromomycin and acetylated spiramycin to the treatment regimen due to the unavailability of these medications within a short timeframe.
After consulting with the Institute of Tropical Medicine at Friendship Hospital Beijing, albendazole and metronidazole were prescribed. Although this treatment strategy is typically used for Giardia infection (Morch et al., 2008; Rossignol, 2010), the clinical experience of the institute suggested that it can be effective against Cryptosporidium infection as well. Efficacy of metronidazole and albendazole in treating Cryptosporidium infection has been reported in mice (Fayer and Fetterer, 1995; Abouel-Nour et al., 2016), leading us to hypothesize their potential benefits in this case. It is worth noting that the child’s normal immune system likely contributed to the favorable outcome once adequate hydration was achieved. Additionally, probiotics have been shown to prevent further intestinal damage in children with diarrhea caused by Cryptosporidium and can affect intestinal function, immune response, and clinical outcomes (Pickerd and Tuthill, 2004; Sindhu et al., 2014).
During the child’s anti-parasitic treatment, a regimen of cefoperazone sulbactam was also administered. Although no bacterial pathogens were identified, we cannot rule out the possibility of bacterial infection in this case. Upon admission, the patient’s procalcitonin and CRP levels were significantly elevated. Early antibiotic treatment (cefloxacin) prior to transfer to our hospital may have resulted in the omission of other potential bacterial co-pathogens, which could have aggravated the condition and contributed to pancreatitis development. The parasite-bacteria interaction within the human host often have negative consequences, and reports have suggested that superimposed bacterial infections in parasitic diseases can lead to pancreatitis (Singh et al., 2004; Ashour and Othman, 2020). Previous research has demonstrated the superiority of mNGS over traditional tests in patients who have received empirical treatment. However, the efficacy of mNGS may decline with prolonged medication (Zhang et al., 2020). In addition, it is possible that the blood sample used for the initial mNGS test had a relatively low bacterial load, as it was not directly collected from the infection site, thus potentially affecting the detection sensitivity.
Conclusion
By utilizing mNGS, we identified Cryptosporidium parvum in a healthy boy, which resulted in a life-threatening condition. Our study underscored the potential for severe consequences associated with Cryptosporidium infection in immunocompetent patients, emphasizing the importance of timely diagnosis and regular monitoring of disease progression in the management of cryptosporidiosis.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://ngdc.cncb.ac.cn/?lang=zh, PRJCA018717.
Ethics statement
The studies involving humans were approved by the Clinical Research Ethics Committee of Beijing Children’s Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
XL: Formal Analysis, Investigation, Writing – original draft. JW: Formal Analysis, Investigation, Writing – original draft. JL: Investigation, Writing – original draft. XML: Data curation, Methodology, Writing – original draft. YG: Methodology, Supervision, Writing – review & editing. SQ: Conceptualization, Supervision, Validation, Writing – review & editing. XJ: Conceptualization, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Beijing Municipal Science and Technology Commission (No. Z211100002921063).
Conflict of interest
JW, XML, and YG were employed by Hugobiotech Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abouel-Nour, M. F., El-Shewehy, D. M., Hamada, S. F., Morsy, T. A. (2016). The efficacy of three medicinal plants; garlic, ginger and mirazid and a chemical drug metronidazole against cryptosporidium parvum: ii-histological changes. J. Egypt Soc. Parasitol. 46 (1), 185–200. doi: 10.12816/0026164
Agnamey, P., Sarfati, C., Pinel, C., Rabodoniriina, M., Kapel, N., Dutoit, E., et al. (2011). Evaluation of four commercial rapid immunochromatographic assays for detection of Cryptosporidium antigens in stool samples: a blind multicenter trial. J. Clin. Microbiol. 49 (4), 1605–1607. doi: 10.1128/jcm.02074-10
Ai, J. W., Zhang, H. C., Cui, P., Xu, B., Gao, Y., Cheng, Q., et al. (2018). Dynamic and direct pathogen load surveillance to monitor disease progression and therapeutic efficacy in central nervous system infection using a novel semi-quantitive sequencing platform. J. Infect. 76 (3), 307–310. doi: 10.1016/j.jinf.2017.11.002
Amadi, B., Mwiya, M., Musuku, J., Watuka, A., Sianongo, S., Ayoub, A., et al. (2002). Effect of nitazoxanide on morbidity and mortality in Zambian children with cryptosporidiosis: a randomised controlled trial. Lancet. 360 (9343), 1375–1380. doi: 10.1016/S0140-6736(02)11401-2
Ashour, D. S., Othman, A. A. (2020). Parasite-bacteria interrelationship. Parasitol. Res. 119 (10), 3145–3164. doi: 10.1007/s00436-020-06804-2
Bouzid, M., Hunter, P. R., Chalmers, R. M., Tyler, K. M. (2013). Cryptosporidium pathogenicity and virulence. Clin. Microbiol. Rev. 26 (1), 115–134. doi: 10.1128/CMR.00076-12
Caldas, S., Caldas, I. S., Diniz Lde, F., Lima, W. G., Oliveira Rde, P., Cecílio, A. B., et al. (2012). Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta tropica 123 (3), 170–177. doi: 10.1016/j.actatropica.2012.05.002
Chappell, C. L., Okhuysen, P. C., Sterling, C. R., DuPont, H. L. (1996). Cryptosporidium parvum: intensity of infection and oocyst excretion patterns in healthy volunteers. J. Infect. Dis. 173 (1), 232–236. doi: 10.1093/infdis/173.1.232
Chen, L., Zhao, Y., Wei, J., Huang, W., Ma, Y., Yang, X., et al. (2022). Metagenomic next-generation sequencing for the diagnosis of neonatal infectious diseases. Microbiol. Spectr 10 (6), e0119522. doi: 10.1128/spectrum.01195-22
Chiu, C. Y., Miller, S. A. (2019). Clinical metagenomics. Nat. Rev. Genet. 20 (6), 341–355. doi: 10.1038/s41576-019-0113-7
Clementi, M., Menzo, S., Manzin, A., Bagnarelli, P. (1995). Quantitative molecular methods in virology. Arch. Virol. 140 (9), 1523–1539. doi: 10.1007/bf01322527
Dionisio, D., Orsi, A., Sterrantino, G., Meli, M., Di Lollo, S., Ibba Manneschi, L., et al. (1998). Chronic cryptosporidiosis in patients with AIDS: stable remission and possible eradication after long-term, low dose azithromycin. J. Clin. Pathol. 51 (2), 138–142. doi: 10.1136/jcp.51.2.138
El-Moamly, A. A., El-Sweify, M. A. (2012). ImmunoCard STAT! cartridge antigen detection assay compared to microplate enzyme immunoassay and modified Kinyoun's acid-fast staining technique for detection of Cryptosporidium in fecal specimens. Parasitol. Res. 110 (2), 1037–1041. doi: 10.1007/s00436-011-2585-z
Fayer, R., Fetterer, R. (1995). Activity of benzimidazoles against cryptosporidiosis in neonatal BALB/c mice. J. Parasitol. 81 (5), 794–795.
Feng, Y., Ryan, U. M., Xiao, L. (2018). Genetic diversity and population structure of cryptosporidium. Trends Parasitol. 34 (11), 997–1011. doi: 10.1016/j.pt.2018.07.009
Feng, Y., Xiao, L. (2017). Molecular epidemiology of cryptosporidiosis in China. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.01701
Gerace, E., Lo Presti, V. D. M., Biondo, C. (2019). Cryptosporidium infection: epidemiology, pathogenesis, and differential diagnosis. Eur. J. Microbiol. Immunol. (Bp) 9 (4), 119–123. doi: 10.1556/1886.2019.00019
Golan Shaposhnik, E., Abozaid, S., Grossman, T., Marva, E., On, A., Azrad, M., et al. (2019). The Prevalence of Cryptosporidium among Children Hospitalized because of Gastrointestinal Symptoms and the Efficiency of Diagnostic Methods for Cryptosporidium. Am. J. Trop. Med. hygiene 101 (1), 160–163. doi: 10.4269/ajtmh.19-0057
Gu, W., Deng, X., Lee, M., Sucu, Y. D., Arevalo, S., Stryke, D., et al. (2021). Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat. Med. 27 (1), 115–124. doi: 10.1038/s41591-020-1105-z
Hassanein, S. M., Abd-El-Latif, M. M., Hassanin, O. M., Abd-El-Latif, L. M., Ramadan, N. I. (2012). Cryptosporidium gastroenteritis in Egyptian children with acute lymphoblastic leukemia: magnitude of the problem. Infection. 40 (3), 279–284. doi: 10.1007/s15010-011-0230-5
Helmy, Y. A., Krucken, J., Nockler, K., von Samson-Himmelstjerna, G., Zessin, K. H. (2014). Comparison between two commercially available serological tests and polymerase chain reaction in the diagnosis of Cryptosporidium in animals and diarrhoeic children. Parasitol. Res. 113 (1), 211–216. doi: 10.1007/s00436-013-3645-3
Huang, M. Z., Li, J., Guan, L., Li, D. Q., Nie, X. M., Gui, R., et al. (2015). Therapeutic effects of acetylspiramycin and garlicin on cryptosporidiosis among drug users. Int. J. Parasitol. Drugs Drug Resist. 5 (3), 185–190. doi: 10.1016/j.ijpddr.2015.09.002
Jing, C., Chen, H., Liang, Y., Zhong, Y., Wang, Q., Li, L., et al. (2021). Clinical evaluation of an improved metagenomic next-generation sequencing test for the diagnosis of bloodstream infections. Clin. Chem. 67 (8), 1133–1143. doi: 10.1093/clinchem/hvab061
Johansen, ØH., Abdissa, A., Bjørang, O., Zangenberg, M., Sharew, B., Alemu, Y., et al. (2022). Oocyst shedding dynamics in children with cryptosporidiosis: a prospective clinical case series in Ethiopia. Microbiol. Spectr 10 (4), e0274121. doi: 10.1128/spectrum.02741-21
Jokipii, L., Jokipii, A. M. (1986). Timing of symptoms and oocyst excretion in human cryptosporidiosis. N Engl. J. Med. 315 (26), 1643–1647. doi: 10.1056/nejm198612253152604
Liu, A., Gong, B., Liu, X., Shen, Y., Wu, Y., Zhang, W., et al. (2020). A retrospective epidemiological analysis of human Cryptosporidium infection in China during the past three decades (1987-2018). PLoS Negl. Trop. Dis. 14 (3), e0008146. doi: 10.1371/journal.pntd.0008146
Liu, J., Gratz, J., Amour, C., Kibiki, G., Becker, S., Janaki, L., et al. (2013). A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J. Clin. Microbiol. 51 (2), 472–480. doi: 10.1128/jcm.02658-12
Marshall, R. J., Flanigan, T. P. (1992). Paromomycin inhibits Cryptosporidium infection of a human enterocyte cell line. J. Infect. Dis. 165 (4), 772–774. doi: 10.1093/infdis/165.4.772
Miller, T. L., Winter, H. S., Luginbuhl, L. M., Orav, E. J., McIntosh, K. (1992). Pancreatitis in pediatric human immunodeficiency virus infection. J. Pediatr. 120 (2 Pt 1), 223–227. doi: 10.1016/s0022-3476(05)80431-7
Morch, K., Hanevik, K., Robertson, L. J., Strand, E. A., Langeland, N. (2008). Treatment-ladder and genetic characterisation of parasites in refractory giardiasis after an outbreak in Norway. J. Infect. 56 (4), 268–273. doi: 10.1016/j.jinf.2008.01.013
Nime, F. A., Burek, J. D., Page, D. L., Holscher, M. A., Yardley, J. H. (1976). Acute enterocolitis in a human being infected with the protozoan Cryptosporidium. Gastroenterology. 70 (4), 592–598.
Norby, S. M., Bharucha, A. E., Larson, M. V., Temesgen, Z. (1998). Acute pancreatitis associated with Cryptosporidium parvum enteritis in an immunocompetent man. Clin. Infect. Dis. 27 (1), 223–224. doi: 10.1086/517686
Pickerd, N., Tuthill, D. (2004). Resolution of cryptosporidiosis with probiotic treatment. Postgrad Med. J. 80 (940), 112–113. doi: 10.1136/pmj.2003.014175
Prasad, N., Patel, M. R. (2018). Infection-induced kidney diseases. Front. Med. (Lausanne) 5. doi: 10.3389/fmed.2018.00327
Ramachandran, P. S., Wilson, M. R. (2020). Metagenomics for neurological infections - expanding our imagination. Nat. Rev. Neurol. 16 (10), 547–556. doi: 10.1038/s41582-020-0374-y
Rawla, P., Bandaru, S. S., Vellipuram, A. R. (2017). Review of infectious etiology of acute pancreatitis. Gastroenterol. Res. 10 (3), 153–158. doi: 10.14740/gr858w
Rossignol, J. F. (2010). Cryptosporidium and Giardia: treatment options and prospects for new drugs. Exp. Parasitol. 124 (1), 45–53. doi: 10.1016/j.exppara.2009.07.005
Ryan, U., Fayer, R., Xiao, L. (2014). Cryptosporidium species in humans and animals: current understanding and research needs. Parasitology. 141 (13), 1667–1685. doi: 10.1017/S0031182014001085
Sindhu, K. N., Sowmyanarayanan, T. V., Paul, A., Babji, S., Ajjampur, S. S., Priyadarshini, S., et al. (2014). Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 58 (8), 1107–1115. doi: 10.1093/cid/ciu065
Singh, T. N., Singh, H. R., Devi Kh, S., Singh, N. B., Singh, Y. I. (2004). Pulmonary paragonimiasis. Indian J. chest Dis. Allied Sci. 46 (3), 225–227.
Ungar, B. L. (1990). Enzyme-linked immunoassay for detection of Cryptosporidium antigens in fecal specimens. J. Clin. Microbiol. 28 (11), 2491–2495. doi: 10.1128/jcm.28.11.2491-2495.1990
White, C. A., Jr. (2004). Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Rev. Anti Infect. Ther. 2 (1), 43–49. doi: 10.1586/14787210.2.1.43
Keywords: Cryptosporidium parvum, mNGS, healthy boy, PICU, pancreatitis
Citation: Liu X, Wang J, Liu J, Li X, Guan Y, Qian S and Jia X (2023) Cryptosporidiosis diagnosed using metagenomic next-generation sequencing in a healthy child admitted to pediatric intensive care unit: a case report. Front. Cell. Infect. Microbiol. 13:1269963. doi: 10.3389/fcimb.2023.1269963
Received: 02 August 2023; Accepted: 12 October 2023;
Published: 27 October 2023.
Edited by:
Ye Zhang, Fourth Military Medical University, ChinaReviewed by:
Shannon Moonah, University of Florida, United StatesKurt Hanevik, University of Bergen, Norway
Jie Liu, Qingdao University, China
Copyright © 2023 Liu, Wang, Liu, Li, Guan, Qian and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinlei Jia, YWxlaXBpY3VAbWUuY29t
†These authors have contributed equally to this work
 Xiaoyi Liu
Xiaoyi Liu Jing Wang
Jing Wang Jun Liu1
Jun Liu1 Xuming Li
Xuming Li Yuanlin Guan
Yuanlin Guan Suyun Qian
Suyun Qian Xinlei Jia
Xinlei Jia