
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Cell. Infect. Microbiol. , 21 November 2023
Sec. Fungal Pathogenesis
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1269543
This article is part of the Research Topic Update on diagnostics, treatment, and prognosis on invasive fungal infections View all 7 articles
Candida and Aspergillus spp. were identified as the most common causes of invasive fungal infections in COVID-19 patients (Ezeokoli and Pohl, 2020). The mortality rate of invasive Candida infections was twice as high in COVID-19 patients as in non-COVID-19 patients (Seagle et al., 2022). Therefore, prevention of the complications of fungal infections in COVID-19 is a crucial issue with implications for prognosis.
Yazdanpanah S et al. analyzed risk factors for COVID-19-associated candidiasis (Yazdanpanah et al., 2023). They used multiple logistic regression to show that heart failure, bacterial co-infection, and empiric antifungal use were significant risk factors for Candida co-infection in hospitalized COVID-19 patients (Yazdanpanah et al., 2023). In their study, azoles were used for empirical antifungal treatment, and in some cases, azoles were used in combination with the echinocandin antifungal agent caspofungin. The antifungal effects seem to differ among these drugs. While some Candida strains were resistant to azoles, caspofungin was effective against all isolated strains in that study (Yazdanpanah et al., 2023). In addition, the study found higher odds of in-hospital mortality in Candida culture-positive patients (RR: 2.18, 95% CI: 0.9, 5.2) (Yazdanpanah et al., 2023), but no subgroup analysis classified by empiric therapy was performed. We believe that this study should elucidate whether empirical caspofungin treatment can improve prognosis in a cohort of COVID-19 patients with risk factors for fungal infection complications.
Caspofungin is an echinocandin antifungal drug that exerts its antifungal activity by targeting β-D-glucan synthase in the membrane of fungal cells, an enzyme that is absent in the human body (Patil and Majumdar, 2017). The mechanism by which caspofungin inhibits the main protease of SARS-CoV-2 suggests that echinocandin-based antifungals including caspofungin inhibit the intracellular viral replication process of SARS-CoV-2 (Liu and Wang, 2020; Nakajima et al., 2023). In addition to these effects, our research has shown host immunomodulatory effects (Itoh et al., 2021). Caspofungin inhibited inflammatory cytokine and chemokine production in THP-1 cells stimulated with LPS or zymosan. In this model, investigation of signaling pathways revealed that caspofungin inhibited the activation of spleen tyrosine kinase (Syk) and its downstream signaling molecules (Figure 1A) (Itoh et al., 2021). Binding to the ATP-binding site of Syk and inhibition of its kinase activity as an ATP-competitive inhibitor, or inhibition of the association of Syk with a substrate protein, is generally accepted as the mechanism of Syk inhibition. However, the mechanism by which caspofungin inhibits Syk has not yet been elucidated. Additionally, it has not been determined whether or not caspofungin acts on signaling pathways other than those involving Syk. Immune cell receptors involved in Syk activation include C-type lectin receptors (Dectin-1, Dectin-2, Mincle) and toll-like receptor (TLR) 4 (Chaudhary et al., 2007; Mócsai et al., 2010). The TLR 4-mediated signaling pathway has been found to be activated in COVID-19 patients (Sohn et al., 2020), and thus inhibition of the Syk pathway may suppress the overproduction of proinflammatory cytokines.
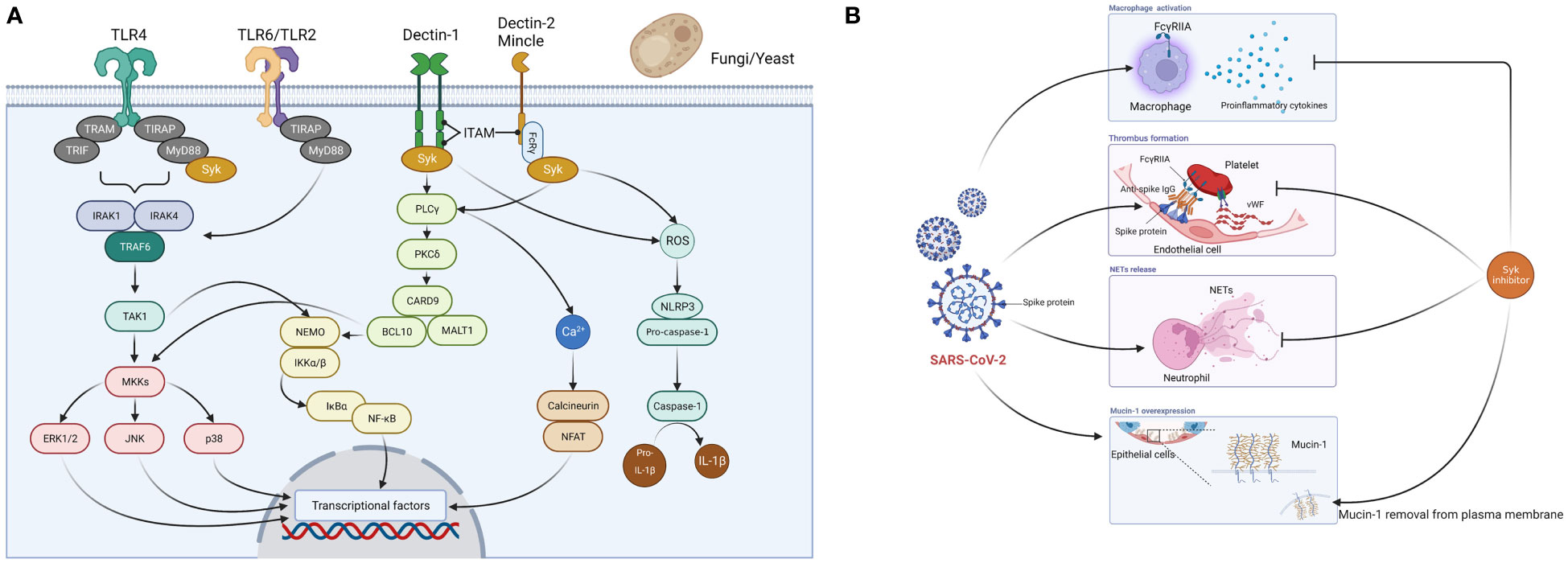
Figure 1 Spleen tyrosine kinase (Syk)-mediated mechanism of action of caspofungin. (A) Fungi (yeast-like fungi) are recognized by PRRs (TLR2, TLR4, TLR6, Dectin-1, Dectin-2, Mincle) expressed on host immune cells such as monocytes and macrophages. When pathogens are recognized by Dectin-1, phosphorylation of immunoreceptor tyrosine-based activation motif (ITAM)-like motifs occur and Syk is recruited. There are three major pathways downstream of the Syk signaling pathway. First, several proteins, including phospholipase C-γ (PLCγ), protein kinase C-δ (PKCδ), caspase recruitment domain-containing protein 9 (CARD9), B-cell lymphoma 10 (BCL10), and mucosa-associated lymphoid tissue lymphoma translocation protein 1 (MALT1) activate NF-kB essential modulator (NEMO) and IkB kinase (IKK) complexes, which then induce NF-kB signaling. This subsequently triggers the 3MAPK (p38, JNK, ERK1/2) pathway and induces activator protein 1 expression. Second, PLCγ induces an increase in Ca2+ and triggers the calcineurin and NFAT pathways. Third, Syk-dependent reactive oxygen species (ROS) production activates the nucleotide-binding domain and leucine-rich repeat-containing protein 3 (NLRP3) inflammasome, which triggers caspase-1 activation and cleaves pro-IL-1b to generate IL-1b. When Dectin-2 and Mincle cooperate with the Fc-γ receptor, Syk is recruited to ITAM and triggers PKCδ-CARD9-dependent NF-kB activation. TLR2/TLR6 heterodimers recruit MyD88 protein and activate a series of kinases [IL-1 receptor-related kinase (IRAK)1, IRAK4, TNF receptor-associated factor 6 (TRAF6), and transforming growth factor-β-activated kinase 1 (TAK1)] to phosphorylate the NEMO-IKK complex, which subsequently triggers NF-kB. Fungal (mannan) recognition by TLR4 is a relatively minor signaling pathway, and the major ligand for TLR4 is LPS. TLR4 associates with MyD88 via its intracellular domain and activates IRAK, which activates TRAF6 and the MAPK and IkBα pathways. Syk cooperates with TLR4 and the adaptor molecule MyD88 and plays an important role in LPS-triggered signaling. These cascades ultimately activate the production of inflammatory cytokines and chemokines. (B) Syk inhibitors suppress macrophage activation and overproduction of proinflammatory cytokines, thrombus formation, and the release of neutrophil extracellular traps (NETs) caused by SARS-CoV-2, and remove overexpressed mucin-1 from the cell membrane in lung epithelial cells. PRRs, pattern recognition receptors; TIR, Toll/interleukin-1 receptor; TIRAP, TIR domain-containing adaptor protein; TLR, toll-like receptor; TRAM, TRIF-related adaptor molecule; TRIF, TIR domain-containing adaptor protein inducing interferon-β. Figures created with BioRender (https://biorender.com/).
Here we summarize the relationship between COVID-19 and Syk inhibitors. R406, a Syk inhibitor, inhibits both the mainly FcγRIIA-dependent release of proinflammatory cytokines by macrophages and thrombus formation induced by the anti-spike immune complex (Bye et al., 2021; Hoepel et al., 2021). R406 inhibits the release of neutrophil extracellular traps (NETs) when healthy neutrophils are stimulated with plasma from COVID-19 patients (Strich et al., 2021). NETs have been found in the lungs of deceased COVID-19 patients and are promoters of immune thrombosis (Papayannopoulos, 2018; Middleton et al., 2020; Veras et al., 2020). Furthermore, NETs are associated with COVID-19 severity (Zuo et al., 2020). R406 also inhibits mucin-1, a transmembrane protein of the lung epithelium associated with acute respiratory distress syndrome (ARDS) (Kost-Alimova et al., 2020) (Figure 1B). Caspofungin, which inhibits the Syk signaling pathway, may therefore reduce the severity of COVID-19 via the same mechanism described prior.
In a SARS-CoV-2-specific chimeric antigen receptor (CAR)-T-cell model established to mimic the cytokine storm in COVID-19 patients, caspofungin suppressed inflammatory cytokine production (Xia et al., 2023). It also suppressed lethal inflammation, ameliorated severe pneumonia, and reduced mortality in a SARS-CoV-2-infected Syrian hamster model (Xia et al., 2023). T lymphocytes generally do not express Syk in their T cell receptors (TCRs) but do express ZAP-70, which contributes to T cell activation (Mócsai et al., 2010). However, Syk inhibitors (R406 and GS-9973) suppress TCR-stimulated phosphorylation of ZAP-70 (Colado et al., 2017). Thus, we believe that the inhibitory effect of caspofungin on Syk suppressed the COVID-19 cytokine storm in in vitro and in vivo models.
Caspofungin is an antifungal agent with activity against Candida and Aspergillus, which are common pathogens associated with COVID-19 (Ezeokoli and Pohl, 2020). Caspofungin inhibits the proliferation of SARS-CoV-2 directly (Liu and Wang, 2020; Nakajima et al., 2023). Additionally, caspofungin has an inhibitory effect on host immune cells against Syk (Itoh et al., 2021), which promotes the suppression of COVID-19 severity by inhibiting inflammatory cytokines, immunothrombosis, and ARDS. Moreover, caspofungin has fewer side effects than azoles and amphotericin B because it targets the β-D-glucan synthase, which is not generally found in the human body (Patil and Majumdar, 2017). Because fungal infections can be difficult to diagnose, even with limited tests (antigen and culture tests), and because treatment of patients with fungal infection complications can be delayed, leading to severe disease, patients at risk must receive empirical administration (early administration). On the other hand, there is a risk of overtreating COVID-19 patients who are not at high risk for fungal infections, which could expose patients to unnecessary drug-related adverse effects (mostly liver dysfunction) and increased drug cost burden. In conclusion, we believe that empirical administration of caspofungin to COVID-19 patients may prevent worsening of prognosis by preventing fungal infection complications in addition to reducing the severity of COVID-19, but is especially recommended in hospitalized COVID-19 patients with risk factors for fungal infection complications, such as heart failure and bacterial co-infection. Further studies are warranted to clarify the prognostic benefit of empiric administration of caspofungin to COVID-19 patients.
KI: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. HT: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. YM: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. HI: Conceptualization, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bye, A. P., Hoepel, W., Mitchell, J. L., Jégouic, S., Loureiro, S., Sage, T., et al. (2021). Aberrant glycosylation of anti-SARS-CoV-2 spike IgG is a prothrombotic stimulus for platelets. Blood 138, 1481–1489. doi: 10.1182/blood.2021011871
Chaudhary, A., Fresquez, T. M., Naranjo, M. J. (2007). Tyrosine kinase Syk associates with toll-like receptor 4 and regulates signaling in human monocytic cells. Immunol. Cell Biol. 85, 249–256. doi: 10.1038/sj.icb7100030
Colado, A., Almejún, M. B., Podaza, E., Risnik, D., Stanganelli, C., Elías, E. E., et al. (2017). The kinase inhibitors R406 and GS-9973 impair T cell functions and macrophage-mediated anti-tumor activity of rituximab in chronic lymphocytic leukemia patients. Cancer Immunol. Immunother. 66, 461–473. doi: 10.1007/s00262-016-1946-y
Ezeokoli, O. T., Pohl, C. H. (2020). Opportunistic pathogenic fungal co-infections are prevalent in critically ill COVID-19 patients: Are they risk factors for disease severity? S Afr Med. J. 110, 1081–1085. doi: 10.7196/SAMJ.2020.v110i11.15248
Hoepel, W., Chen, H.-J., Geyer, C. E., Allahverdiyeva, S., Manz, X. D., de Taeye, S. W., et al. (2021). High titers and low fucosylation of early human anti-SARS-CoV-2 IgG promote inflammation by alveolar macrophages. Sci. Transl. Med. 13, eabf8654. doi: 10.1126/scitranslmed.abf8654
Itoh, K., Shigemi, H., Chihara, K., Sada, K., Yamauchi, T., Iwasaki, H. (2021). Caspofungin suppresses zymosan-induced cytokine and chemokine release in THP-1 cells: possible involvement of the spleen tyrosine kinase pathway. Transl. Res. 227, 53–63. doi: 10.1016/j.trsl.2020.07.005
Kost-Alimova, M., Sidhom, E.-H., Satyam, A., Chamberlain, B. T., Dvela-Levitt, M., Melanson, M., et al. (2020). A high-content screen for mucin-1-reducing compounds identifies fostamatinib as a candidate for rapid repurposing for acute lung injury. Cell Rep. Med. 1, 100137. doi: 10.1016/j.xcrm.2020.100137
Liu, X., Wang, X.-J. (2020). Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicines. J. Genet. Genomics 47, 119–121. doi: 10.1016/j.jgg.2020.02.001
Middleton, E. A., He, X.-Y., Denorme, F., Campbell, R. A., Ng, D., Salvatore, S. P., et al. (2020). Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 136, 1169–1179. doi: 10.1182/blood.2020007008
Mócsai, A., Ruland, J., Tybulewicz, V. L. J. (2010). The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 10, 387–402. doi: 10.1038/nri2765
Nakajima, S., Ohashi, H., Akazawa, D., Torii, S., Suzuki, R., Fukuhara, T., et al. (2023). Antiviral activity of micafungin and its derivatives against SARS-coV-2 RNA replication. Viruses 15, 452. doi: 10.3390/v15020452
Papayannopoulos, V. (2018). Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147. doi: 10.1038/nri.2017.105
Patil, A., Majumdar, S. (2017). Echinocandins in antifungal pharmacotherapy. J. Pharm. Pharmacol. 69, 1635–1660. doi: 10.1111/jphp.12780
Seagle, E. E., Jackson, B. R., Lockhart, S. R., Georgacopoulos, O., Nunnally, N. S., Roland, J., et al. (2022). The landscape of candidemia during the COVID-19 pandemic. Clin Infect Dis 74, 802–811. doi: 10.1093/cid/ciab562
Sohn, K. M., Lee, S. G., Kim, H. J., Cheon, S., Jeong, H., Lee, J., et al. (2020). COVID-19 patients upregulate toll-like receptor 4-mediated inflammatory signaling that mimics bacterial sepsis. J. Korean Med. Sci. 35, e343. doi: 10.3346/jkms.2020.35.e343
Strich, J. R., Ramos-Benitez, M. J., Randazzo, D., Stein, S. R., Babyak, A., Davey, R. T., et al. (2021). Fostamatinib inhibits neutrophils extracellular traps induced by COVID-19 patient plasma: A potential therapeutic. J. Infect. Dis. 223, 981–984. doi: 10.1093/infdis/jiaa789
Veras, F. P., Pontelli, M. C., Silva, C. M., Toller-Kawahisa, J. E., de Lima, M., Nascimento, D. C., et al. (2020). SARS-CoV-2-triggered neutrophil extracellular traps mediate COVID-19 pathology. J. Exp. Med. 217, e20201129. doi: 10.1084/jem.20201129
Xia, L., Yuan, L.-Z., Hu, Y.-H., Liu, J.-Y., Hu, G.-S., Qi, R.-Y., et al. (2023). A SARS-CoV-2-specific CAR-T-cell model identifies felodipine, fasudil, imatinib, and caspofungin as potential treatments for lethal COVID-19. Cell Mol. Immunol. 20, 351–364. doi: 10.1038/s41423-023-00985-3
Yazdanpanah, S., Ahmadi, M., Zare, Z., Nikoupour, H., Arabsheybani, S., Jabrodini, A., et al. (2023). Assessment of risk factors and clinical outcomes in hospitalized COVID-19 patients with candida spp. Co-infections: species distribution and antifungal susceptibility patterns of isolates. Mycopathologia 188, 9–20. doi: 10.1007/s11046-022-00694-x
Keywords: COVID-19, SARS-CoV-2, co-infection, fungal infections, caspofungin, spleen tyrosine kinase
Citation: Itoh K, Tsutani H, Mitsuke Y and Iwasaki H (2023) Implications of empirical administration of caspofungin in COVID-19 complicated fungal infections. Front. Cell. Infect. Microbiol. 13:1269543. doi: 10.3389/fcimb.2023.1269543
Received: 30 July 2023; Accepted: 08 November 2023;
Published: 21 November 2023.
Edited by:
Jon Salmanton-Garcia, University Hospital of Cologne, GermanyReviewed by:
Atif Khurshid Wani, Lovely Professional University, IndiaCopyright © 2023 Itoh, Tsutani, Mitsuke and Iwasaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuhiro Itoh, a2l0b2hAdS1mdWt1aS5hYy5qcA==
†ORCID: Kazuhiro Itoh, orcid.org/0000-0001-5574-7118
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.