
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 21 December 2023
Sec. Intestinal Microbiome
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1227450
Aims: This study aimed to investigate the association between the gut microbiota and the risk of stroke.
Methods: Faecal samples from 60 participants in South China, including 45 individuals with risk factors for stroke and 15 healthy controls, were collected and subjected to 16S rRNA sequencing. A bioinformatics analysis was performed to characterise the gut microbial diversity and taxonomic compositions at different risk levels (low, moderate, and high) of stroke. Functional prediction and correlation analyses between the microbiota and laboratory markers were performed to explore the potential mechanisms.
Results: A significant difference in beta diversity was observed between the participants from the stroke risk and healthy control groups. Linear discriminant effect size analysis revealed a large number of vascular beneficial bacteria enriched in the participants from the healthy control and low-risk groups, but a few vascular harmful bacteria were more abundant in the participants from the high-risk group than in those from the other groups. In addition, Anaerostipes, Clostridium_XlVb, and Flavonifractor, all of which belonged to the Firmicutes phylum, were enriched in the participants from the low-risk group, and their relative abundances gradually decreased as the stroke risk increased. Spearman’s analysis revealed that these outstanding microbiota correlated with the levels of triglycerides, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, white blood cells, neutrophils, and carotid intima-media thickness.
Conclusion: The preliminary evidence suggests that gut microbiota is associated with stroke risk. It potentially ameliorates atherosclerosis by targeting lipid metabolism and inflammation. This provides novel insights into the early screening of stroke risk and primary prevention.
Stroke remains the second leading cause of death and the third leading cause of death and disability worldwide (Feigin et al., 2022). From 1990 to 2019, the absolute number of stroke cases increased substantially (i.e. 70.0% increase in incident stroke rates), and over 12.2 million new stroke cases were reported each year, thereby necessitating urgent measures to reduce stroke incidence (GBD 2019 Stroke Collaborators, 2021). In China, stroke is the leading cause of disability-adjusted life year, and 3.94 million new stroke cases were recorded in 2019 (Wang et al., 2022). Traditionally, identification and modification of risk factors are essential for reducing stroke incidence (Pandian et al., 2018). However, considerable residual risks of stroke remain as conventional risk factors, which account for 90% risk of stroke worldwide and in China (Qi et al., 2020). Thus, treating the arteries instead of reducing the risk factors has been suggested (Spence and Hackam, 2010).
Atherosclerosis, characterised by lipid accumulation and inflammation of the large arteries, is a major cause of stroke (Björkegren and Lusis, 2022). Dysbiosis, which refers to microbial imbalance or dysfunction in the gut, has attracted considerable attention because of its role in atherosclerosis development (Tang et al., 2019). The gut microbiota and its metabolites may affect the risk factors of stroke, such as hypertension, obesity, and diabetes, or signal inflammation and immune responses during atherosclerosis and thrombosis (Wang et al., 2022). To be specific, a growing body of evidence suggests that Trimethylamine-N-oxide (TMAO), a small molecular compound derived from the metabolism of intestinal microorganisms, is associated with the elevated risk of stroke, as well as the severity and prognosis of stroke (Liu et al., 2023). Clinical evidence from dose-response meta-analyses also show the circulating TMAO is positively associated with an increased probability of risk factors in stroke, such as hypertension (Abbasalizad Farhangi and Vajdi, 2021), and inflammatory parameters involved in the pathological process of atherosclerosis, such as C-reactive protein (Farhangi and Vajdi, 2020). Besides, the mediation of the therapeutic effects and adverse events by the gut microbiome has been identified in the prevention and treatment of stroke such as statins and aspirin (Chakaroun et al., 2023).
The primary prevention strategy tailored to stroke risk stratification instead of individual risk factors has been developed, and included to clinical practice guidelines (Meschia et al., 2014; Arnett et al., 2019; Owolabi et al., 2022). However, few studies have investigated the association between the gut microbiome and stroke risk stratification. Thus, this study aimed to determine the association between the gut microbiota and stroke risk stratification.
This study was conducted in accordance with the Declaration of Helsinki (2013) and approved by The Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (institutional approval number No. BE2021-195). Each participant signed a written informed consent form.
All the participants were of Chinese descent long residing in China. Hence, the stroke risk factors identified by the Ministry of Health China Stroke Prevention Project Committee were adopted in this study (Chao et al., 2021). The participants of this study were recruited from a cross-sectional survey of a population with risk factors of stroke. The inclusion criteria were as follows: 1) age ≥40 years, 2) living in Guangdong Province for more than half a year, and 3) having one or more than one risk factors of stroke, including hypertension, diabetes mellitus, atrial fibrillation, smoking, dyslipidaemia, physical inactivity, overweight/obesity, and a family history of stroke, or a medical history of transient ischaemic attack (TIA) or stroke. Individuals aged ≥40 years and without any risk factor of stroke or a history of TIA/stroke were recruited from the same community-based population living in Guangdong Province for more than 6 months as healthy controls. The criteria for the individual risk factors are specified in Supplementary Table 1.
Participants were excluded if they met one of the following criteria: 1) diagnosis of a severe mental disorder or cognitive impairment affecting the face-to-face survey, 2) diagnosis of a major gastrointestinal disorder, and 3) ingestion of oral antibiotics, probiotics, coffee, wine or other fermented foods (e.g. traditional Asian pickled vegetables), spicy and greasy foods (e.g. Chinese hot pot), Chinese herbal medicine, and laxatives at least one month before stool collection.
A team of physicians from Guangdong Provincial Hospital of Chinese Medicine performed a face-to-face survey by using a health questionnaire that included demographics, medical history, medication usage, and behavioural risk factors. Subsequently, a team of nurses measured the heights and weights of the participants at the site and calculated their body mass indices (BMIs). Blood samples were collected after ≥10 h of fasting for analyses of the following clinical laboratory parameters: fasting glucose (GLU), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), white blood cell (WBC), absolute neutrophil (NEUT), and hypersensitive C-reactive protein (hs-CRP). The participants underwent electrocardiography and carotid ultrasonography.
In this study, the carotid intima-media thickness (IMT) was defined as the mean of the left and right IMTs of the common carotid artery (CCA) (Onut et al., 2012). A Doppler ultrasound (Philips, Netherlands) with a 3- to 12-MHz multifrequency linear probe was operated by a senior ultrasonographer in accordance with current clinical guidelines (The Ministry of Health China Stroke Prevention Project Committee, 2015; The Professional Committee of Vascular Ultrasound of Stroke Prevention and Treatment Expert et al., 2020). The IMT was scanned at the proximal far wall of the CCA (1–1.5 cm proximal to the carotid bifurcation and without plaque) (Stein et al., 2008). Subsequently, the vertical distance from the upper edge of the inner membrane to the upper edge of the outer membrane was measured.
The participants were primarily assessed using the Chinese National Stroke Risk Scorecard (CNSRS) (Chao et al., 2021) and divided into the following groups: healthy control group, participants without risk factors and a medical history of TIA/stroke; low-risk group, participants with <3 risk factors and without a medical history of hypertension, diabetes mellitus, or atrial fibrillation; moderate-risk group, participants with <3 risk factors and with hypertension, diabetes mellitus, or atrial fibrillation; and high-risk group, participants with ≥3 risk factors or a history of TIA/stroke.
Stroke risk was also stratified using the Framingham Stroke Risk Profile (FSRP) (D'Agostino et al., 1994), which is widely used to estimate the 10-year probability of incident stroke. FSRP included age, systolic blood pressure, cigarette smoking, cardiac disease (coronary heart disease, cardiac failure, intermittent claudication, atrial fibrillation, or left ventricular hypertrophy by electrocardiogram), diabetes mellitus, and anti-hypertensive medication use in the model, and its prediction was sex specific. The criteria in a previous study (Peng et al., 2020) were endorsed to define the risk level of FSRP. The participants were then divided into the following groups: low-risk group of FSRP, 10-Year Probability of stroke ≤ 5%; moderate-risk group, 5% < 10-Year Probability of stroke <15%; and high-risk group, 10-Year Probability of stroke ≥ 15%.
Stool samples were collected from the participants, stored at -80°C within 24 h, and then sent to BGI Genomics (Shenzhen, China) for sequencing. Bacterial DNA was extracted using the MagPure Stool DNA KF kit B (MD5115-01B, Magen, China) in accordance with the manufacturer’s instructions. The faecal microbiota composition was determined by sequencing the V4 region of the 16S rRNA gene on an Illumina HiSeq 2500 platform (BGI, Shenzhen, China) and generating 2×250 bp reads.
Raw reads were filtered to remove adaptors and low-quality and ambiguous bases, and paired-end reads were added to tags by using the Fast Length Adjustment of SHort reads program (FLASH, v1.2.11) (Magoč and Salzberg, 2011) to obtain the tags. The tags were clustered into operational taxonomical units (OTUs) with a cut-off value of 97% by using UPARSE software (v7.0.1090) (Edgar, 2013), and chimeric sequences were compared with those in the Gold database by using UCHIME (v4.2.40) (Edgar et al., 2011). OTU representative sequences were taxonomically classified using Ribosomal Database Project Classifier (v2.2) (Qiong et al., 2007), with a minimum confidence threshold of 0.6, and then trained on the Greengenes database v201305 by using QIIME v1.8.0 (Caporaso et al., 2010). All the tags were mapped back to the OTU sequences by using USEARCH_global (v7.0.1090) (Edgar, 2010) to obtain the OTU abundance statistical table for each sample.
The rarefaction curves for the observed species were plotted to determine whether the depth of sequencing adequately captured the full taxonomic diversity of all the samples (Supplementary Figure 1). Alpha and beta diversities were estimated at the OTU level by using MOTHUR (v1.31.2) (Schloss et al., 2009) and QIIME (v1.8.0) (Caporaso et al., 2010), respectively. The alpha diversity was determined by calculating the Chao1 (Chao, 1984), abundance-based coverage estimator (Ace) (Chao and Lee, 1992), Shannon (1948), and Simpson (1949) indices, whereas the beta diversity was evaluated by calculating the weighted and unweighted UniFrac distances (Lozupone et al., 2011). Linear discriminant analysis Effect Size (LEfSe) (Segata et al., 2011) analysis along with logarithmic linear discriminant analysis (LDA) scores > 2 and p values < 0.05 were considered in identifying the discriminative taxa. Kyoto Encyclopedia of Genes and Genomes (KEGG) (v77.1) functions were predicted using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (v2.3.0-b) (Wilkinson et al., 2018). The correlation between the outstanding gut microbiota and clinical characteristics was plotted using the R package pheatmap, and their correlation coefficients were calculated using Spearman’s correlation analysis. These results were visualised by preparing bar, box, and violin plots using R (version 4.2.2).
Statistical analyses were conducted using IBM SPSS Statistics for Windows version 26.0. Continuous variables are presented as mean (standard deviation or standard error of the mean) depending on the type of data distribution, and categorical variables are presented as numbers (percentages). For the continuous variables, either one-way ANOVA or Kruskal–Wallis H test was selected for multiple comparisons depending on the type of data distribution, and the Wilcoxon Rank Sum test was used for pairwise comparisons. For categorical data, the chi-square test was used for group comparisons. Spearman’s correlation analysis was used to explore the monotonic relationship between the continuous or ordinal data. Statistical significance was set at p < 0.05.
A total of 45 participants with stroke risk factors and 15 healthy controls were enrolled in the study between October 2021 and January 2022. The flowchart of the study is shown in Figure 1. The 60 participants had an average age of 52.58 years and a male/female ratio of 81.82%. None of the participants had a current or previous diagnosis of TIA/stroke. Only five participants had a diagnosis of cardiac disease or a family history of stroke, and physical inactivity was not identified by any of participants. No significant differences in age and cardiac disease were found between the participants from the healthy control group and CNSRS groups. However, no significant differences in overweight/obesity, dyslipidaemia, and family history of stroke were found between the participants from the FSRP groups. The demographic and clinical characteristics of the participants are detailed in Table 1.
The OTU dataset for the CNSRS groups and healthy control group comprised 769 OTUs that classified 140 genera, 54 families, and 13 phyla. No significant differences in alpha diversity indices (Chao1, Ace, Shannon, and Simpson) were identified between the CNSRS groups and healthy control group (Figure 2A). By contrast, significant differences in beta diversity were observed between the groups in terms of weighted (p < 0.0001) and unweighted (p = 0.0092) UniFrac distances (Figure 2B). The five dominant phyla in the microbiota of all participants were Bacteroidetes (69.0548% ± 2.2443%), Firmicutes (19.6341% ± 1.6876%), Proteobacteria (6.8188% ± 1.1084%), Fusobacteria (2.9961% ± 1.2420%), and Verrucomicrobia (0.9263% ± 0.7835%) (Figure 2C), and their relative abundances were not significantly different between the CNSRS groups and healthy control group (p > 0.05, Figure 2D).
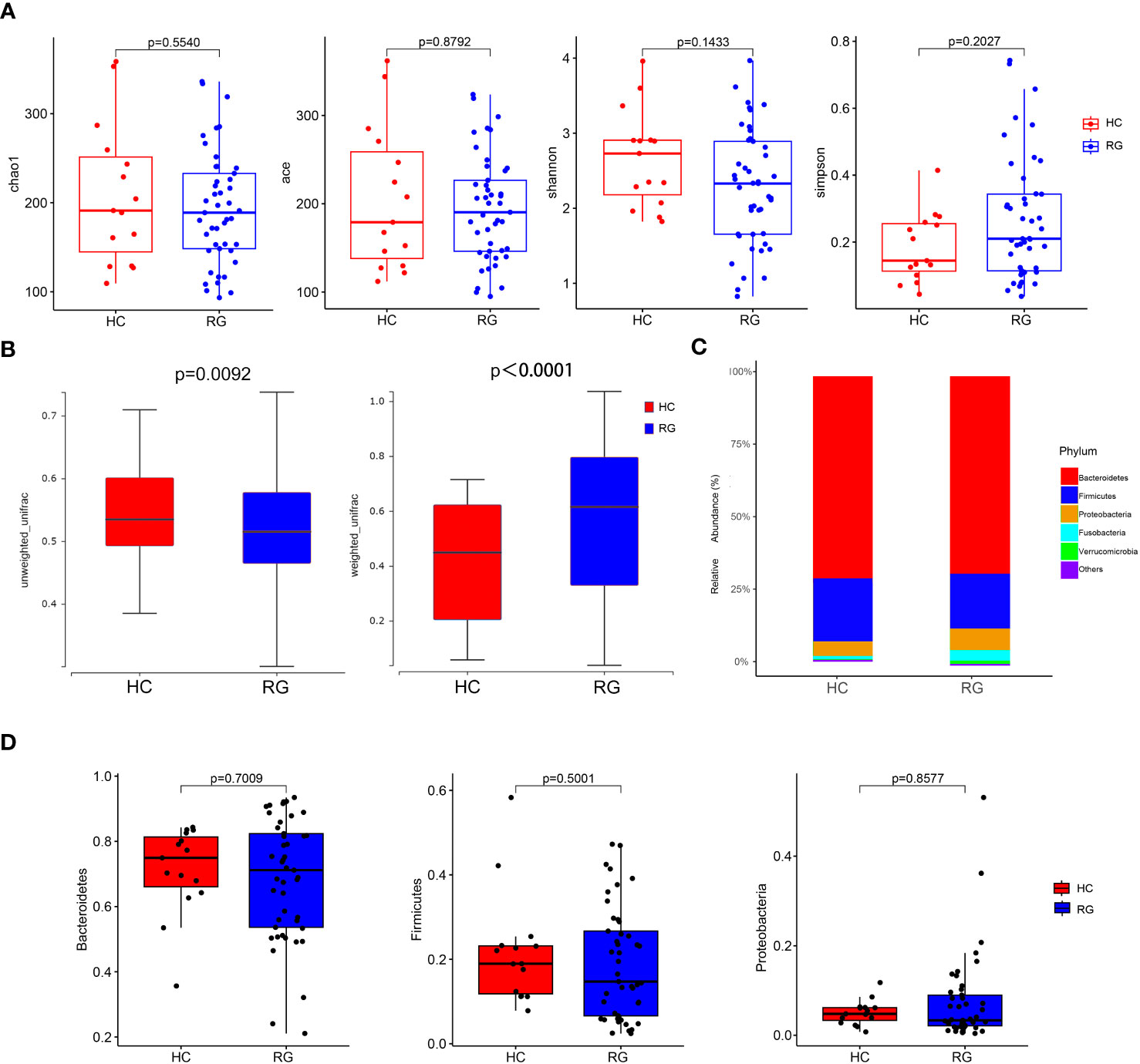
Figure 2 Comparison of microbial diversity and composition between the CNSRS risk groups and the healthy control group. (A) No significant difference was found in alpha diversity based on the Chao1, Ace, Shannon, and Simpson indices (p > 0.05). (B) A significant difference in beta diversity was found in terms of weighted (p < 0.0001) and unweighted UniFrac distances (p = 0.0092). (C) Relative abundance distribution of five dominant phyla of bacteria. (D) Relative abundances of Bacteroidetes, Firmicutes, and Proteobacteria did not differ significantly between the groups by the Wilcoxon test. The relative abundances of Fusobacteria and Verrucomicrobia were not detected in 95% samples and not significantly different between RG and HC (p = 0.1841, p = 0.4063, respectively). CNSRS, Chinese National Stroke Risk Scorecard; HC, healthy control group; RG, risk group.
The LEfSe analysis with an LDA score of more than 2 points identified one bacterial order (Actinomycetales), two bacterial families (Bacteroidaceae and Micrococcaceae), and nine bacterial genera (Bacteroides, Rothia, Synergistes, Fretibacterium, Ezakiella, Clostridium_XlVb, Flavonifractor, Ruminococcus 2, and Anaerostipes), which were all enriched in the participants from the healthy control group, and their relative abundances were significantly different between the participants from the healthy control and CNSRS groups (Figure 3). The flora with the highest abundance included Bacteroidaceae at the family level and Bacteroides at the genus level.
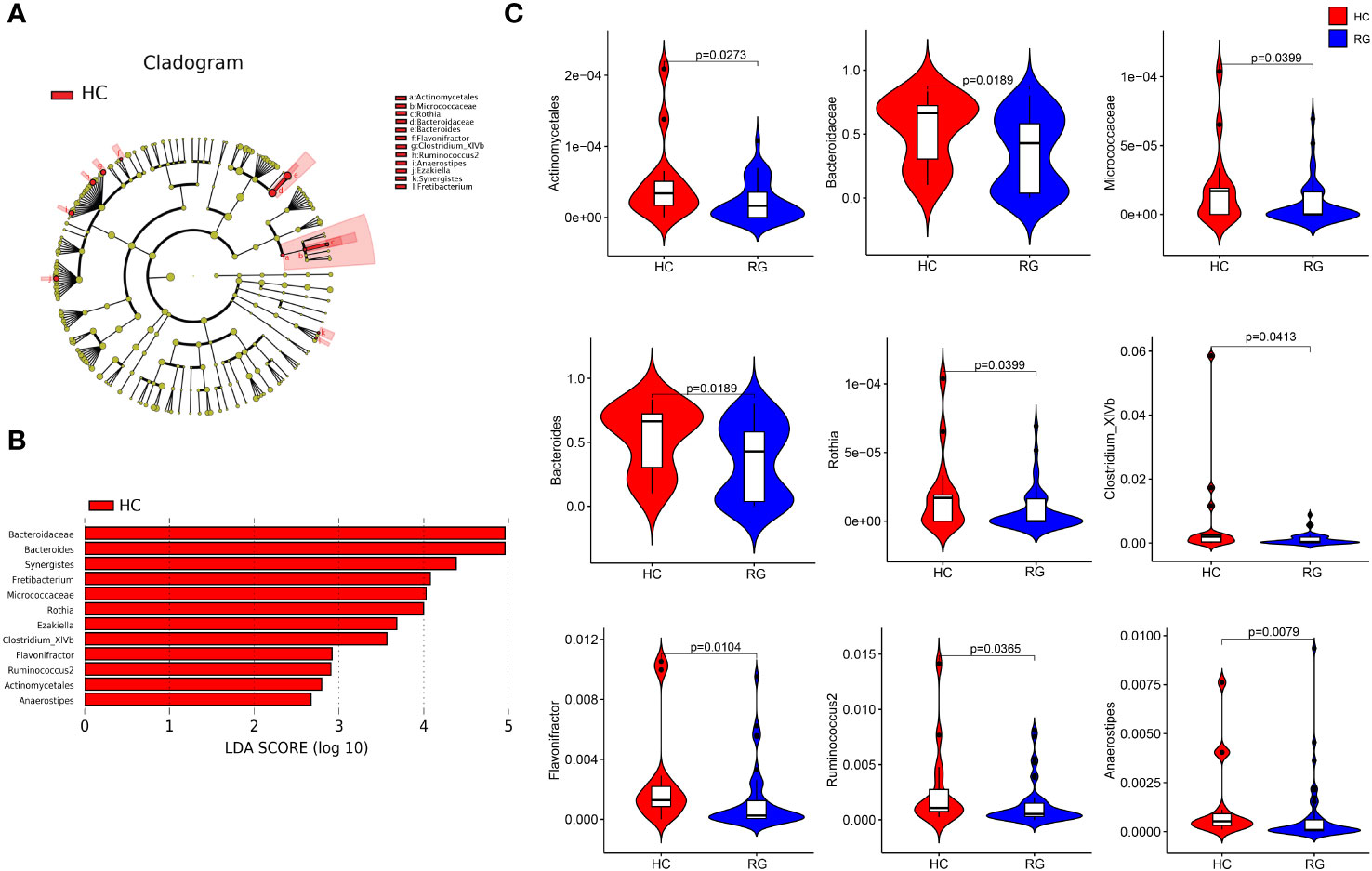
Figure 3 Discriminative taxa between the CNSRS risk groups and the healthy control group. (A) Cladogram showing the taxa with different abundance values based on LEfSe analysis. (B) Discriminative taxa between RG and HC were determined using LEfSe analysis along with LDA scores (log10) > 2 and p < 0.05. The red bar chart represents the bacteria that were more abundant in HC than RG. (C) Relative abundances of Bacteroidaceae, Bacteroides, Micrococcaceae, Rothia, Clostridium_XlVb, Flavonifractor, Ruminococcus 2, Actinomycetales, and Anaerostipes were significantly different between RG and HC (p < 0.05) as determined using the Wilcoxon test. The relative abundances of Synergistes, Fretibacterium and Ezakiella were not detected in more than 95% samples but were significantly different between RG and HC (p = 0.015, p = 0.003, p = 0.015, respectively). CNSRS, Chinese National Stroke Risk Scorecard; LEfSe, linear discriminant analysis effect size; LDA, linear discriminant analysis; HC, healthy control group; RG, risk group.
No significant difference in alpha diversity was detected among the three CNSRS groups (Figure 4A). Meanwhile, a significant difference in beta diversity was found among the three groups in terms of unweighted UniFrac distance (p = 0.0002, Figure 4B) but none in terms of weighted UniFrac distance (p = 0.4213, Figure 4B).
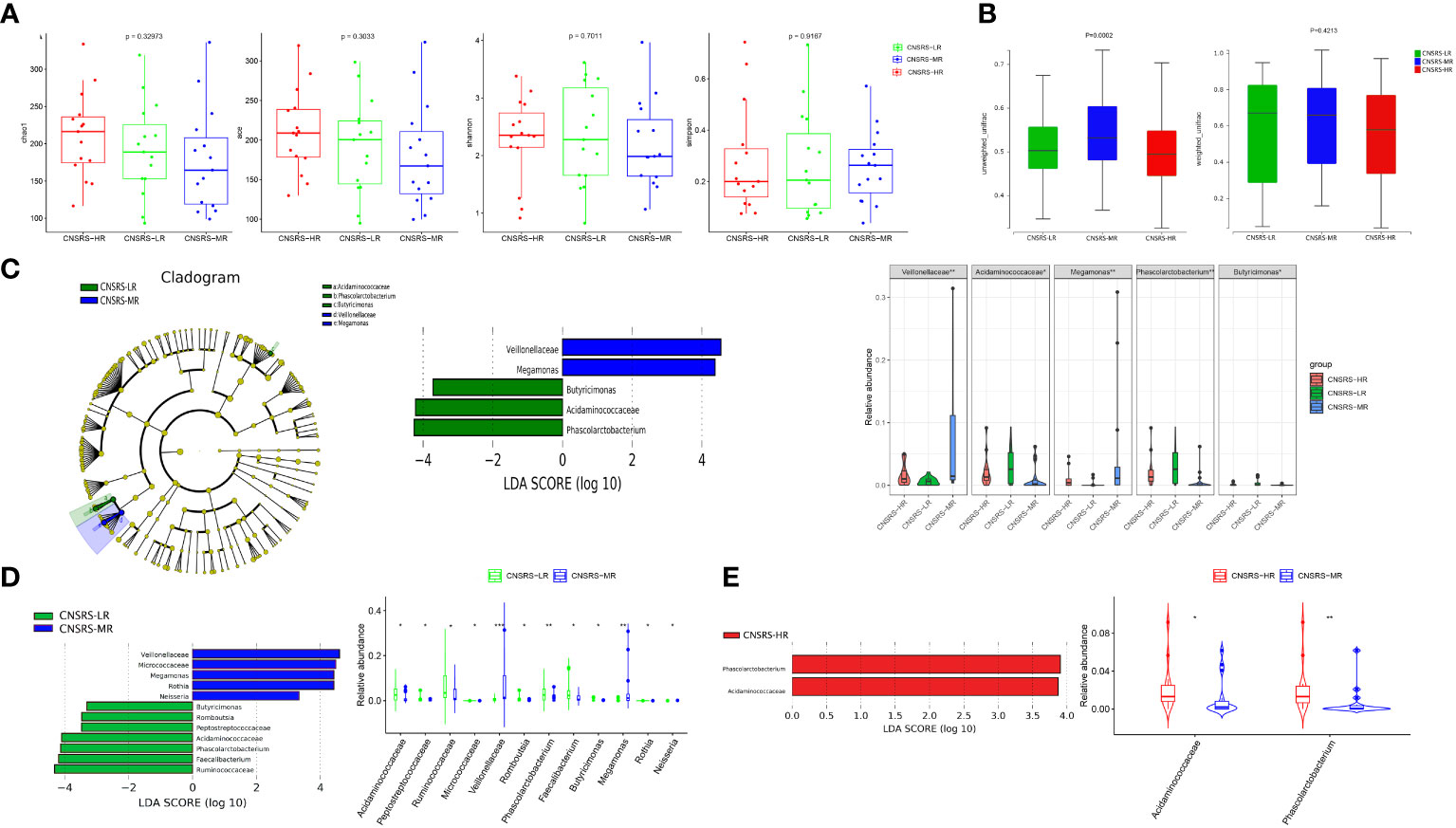
Figure 4 Microbial diversity and composition in different CNSRS groups. (A) No significant difference in alpha diversity was found in terms of the Chao1, Ace, Shannon, and Simpson indices (p > 0.05). (B) Significant difference in beta diversity was found among the participants from the three CNSRS groups in terms of unweighted UniFrac distances (p = 0.0002), whereas no apparent difference was found in terms of weighted UniFrac distances (p = 0.4213). (C) Cladogram showing the taxa with different abundance values based on LEfSe analysis. Discriminative taxa between groups were determined using LEfSe analysis along with LDA scores (log10) > 2 and p < 0.05. Green bar chart represents the bacteria enriched in LR, and the blue bar chart represents the bacteria enriched in MR. Kruskal–Wallis test results suggested that the relative abundances of Veillonellaceae, Acidaminococcaceae, Megamonas, Phascolarctobacterium, and Butyricimonas were significantly different among the participants from the three CNSRS groups. (D) LEfSe analysis along with LDA scores was applied in pairwise comparisons. The green bar chart represents the bacteria enriched in LR, and the blue bar chart represents the bacteria enriched in MR in the pairwise comparisons between LR and Wilcoxon Rank Sum test suggested that the relative abundances of 12 taxa were significantly different between the participants from LR and MR. (E) Red bar chart represents the bacteria enriched in HR in the pairwise comparisons between MR and HR. Wilcoxon Rank Sum test suggested that the relative abundances of two taxa were significantly different between the participants from LR and MR. CNSRS, Chinese National Stroke Risk Scorecard; LEfSe, linear discriminant analysis effect size; LDA, linear discriminant analysis; HR, high-risk group; LR, low-risk group; MR, moderate-risk group. p values: *p < 0.05, **p < 0.01, ***p < 0.001.
LEfSe analysis along with LDA scores identified two bacterial families (Veillonellaceae and Acidaminococcaceae) and three bacterial genera (Megamonas, Phascolarctobacterium, and Butyricimonas), which were statistically different among the three groups (Figure 4C). Acidaminococcaceae, Phascolarctobacterium and Butyricimonas were enriched in the participants from the low-risk group, whereas Veillonellaceae and Megamonas were enriched in the participants from the moderate-risk group.
Furthermore, pairwise comparisons between the participants from the low- and moderate-risk groups showed that Veillonellaceae, Megamonas, Micrococcaceae, Rothia, and Neisseria were enriched in the participants from the moderate-risk group, whereas Peptostreptococcaceae, Romboutsia, Acidaminococcaceae, Phascolarctobacterium, Ruminococcaceae, Faecalibacterium, and Butyricimonas were enriched in the participants from the low-risk group (Figure 4D). Pairwise comparisons between the participants from the moderate- and high-risk groups revealed that Acidaminococcaceae and Phascolarctobacterium were enriched in the participants from the high-risk group (Figure 4E).
Functional profiles of microbial communities predicted by PICRUSt identified 14 KEGG pathways in which the microbial abundance of the participants from the CNSRS groups was significantly lower than that of the healthy controls (p < 0.05). After removing pathways with low occurrence and abundance, 12 KEGG pathways remained. These pathways were related to the metabolism of cofactors, vitamins, lipids, terpenoids, polyketides, amino acids, and carbohydrates and to the endocrine system, immune system, replication, and repair. Figure 5A shows the matching relationships of KEGG pathways between levels 2 and 3 and the group comparison of microbial abundance for each pathway.
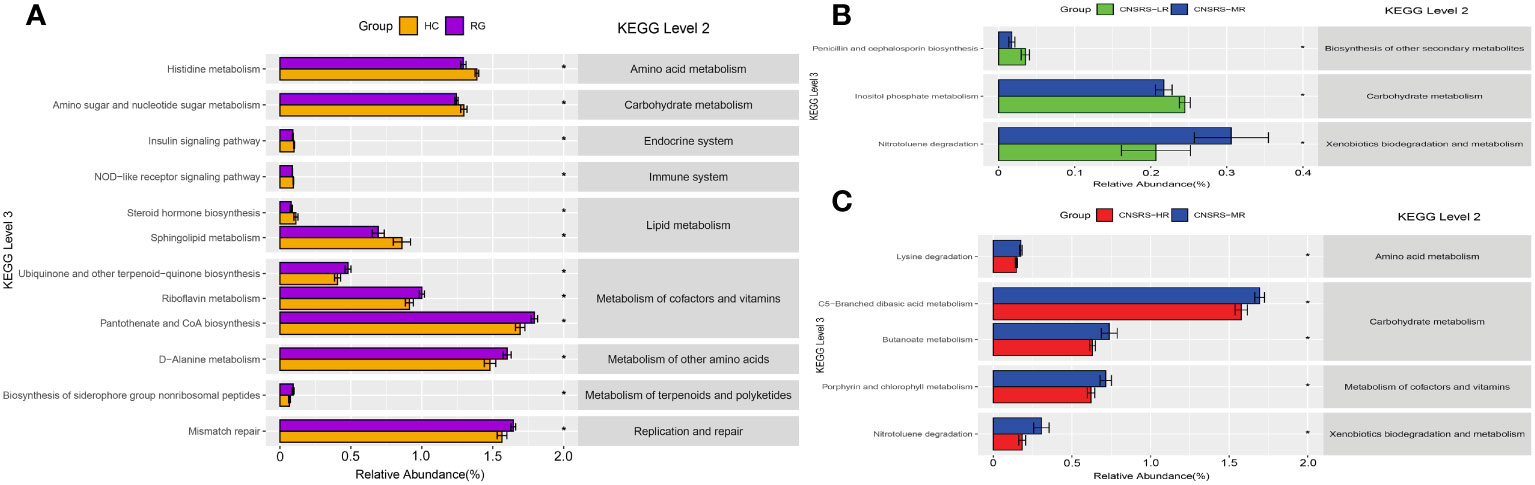
Figure 5 KEGG pathways predicted by PICRUSt and associations with CNSRS. (A) KEGG pathways with differences in abundance between RG and HC. (B) KEGG pathways with differences in abundance between LR and MR. (C) KEGG pathways with differences in abundance between MR and HR. CNSRS, Chinese National Stroke Risk Scorecard; KEGG, Kyoto Encyclopedia of Genes and Genomes; PICRUSt, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States; HC, healthy control group; HR, high-risk group; LR, low-risk group; MR, moderate-risk group; RG, risk group. Differential KEGG pathways with relative abundance were visualized as bar plot with standard error. *Wilcoxon Rank Sum test with p values less than 0.05.
Furthermore, pairwise comparisons between the participants from the low- and moderate-risk groups revealed three pathways in which the microbial abundance was significantly different (p < 0.05). These pathways were related to penicillin and cephalosporin biosyntheses (1 pathway), carbohydrate metabolism (1 pathway), and xenobiotic biodegradation and metabolism (1 pathway) (Figure 5B). Meanwhile, pairwise comparisons between the participants from the moderate- and high-risk groups revealed five outstanding pathways (p < 0.05). These pathways were involved in carbohydrate metabolism (2 pathways), amino acid metabolism (1 pathway), cofactor and vitamin metabolism (1 pathway), and xenobiotic biodegradation and metabolism (1 pathway) (Figure 5C).
No significant differences in either alpha or beta diversity were identified among the participants from the different FSRP groups. LEfSe analysis along with LDA scores suggested that Collinsella and Anaerostipes were enriched in the participants from the low-risk group, whereas Anaerofilum and Cloacibacillus were enriched in the participants from the high-risk group (Figure 6A). The relative abundances of these genera were significantly different among the three FSRP groups (Figure 6B).
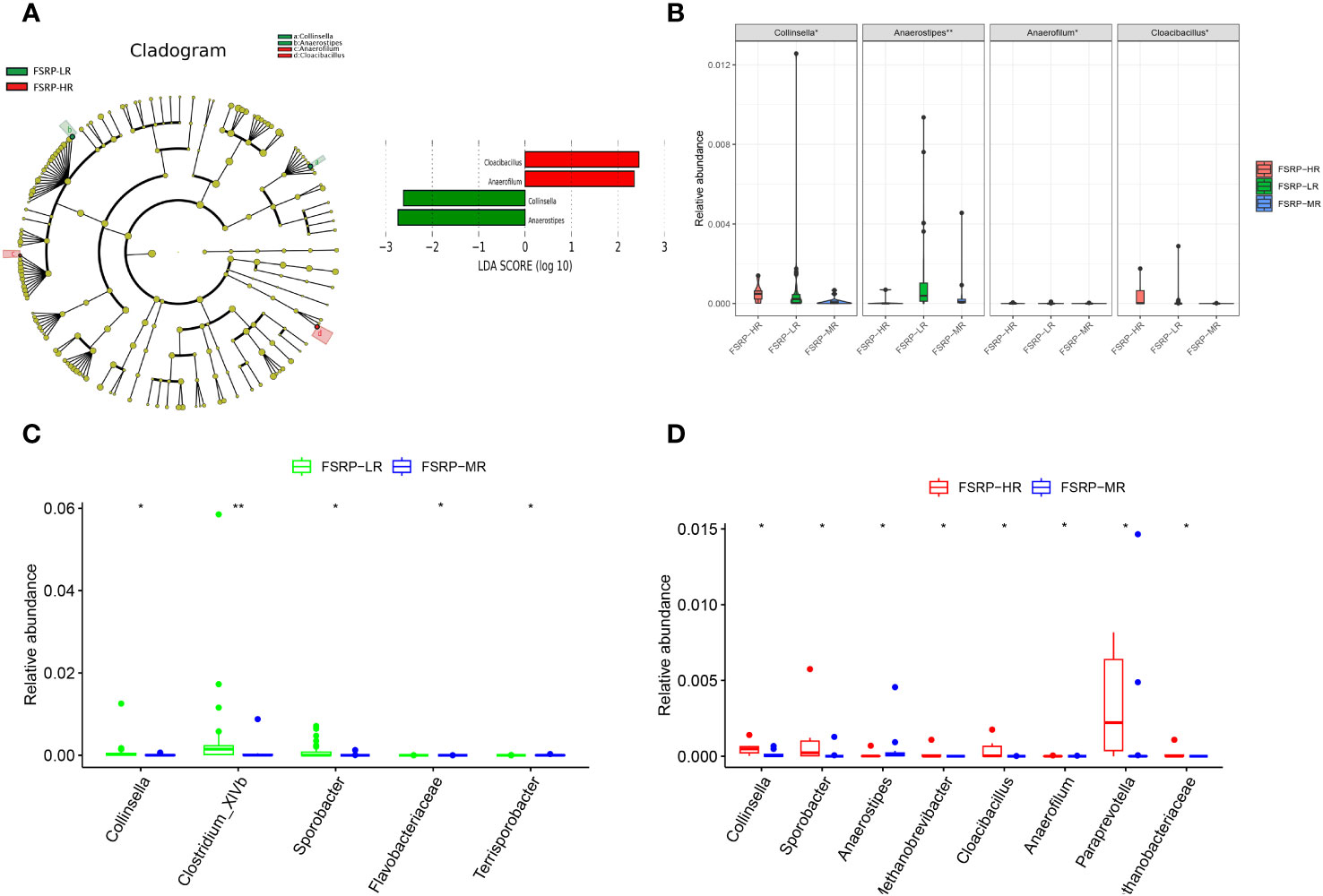
Figure 6 Discriminative taxa among different FSRP groups. (A) Cladogram reporting the taxa with different abundance values based on LEfSe analysis. Discriminative taxa between groups were determined using LEfSe analysis along with LDA scores (log10) > 2 and p < 0.05. The green bar chart represents the bacteria enriched in LR, and the red bar chart represents the bacteria enriched in HR. (B) Kruskal–Wallis test results suggested that the relative abundances of Collinsella, Anaerostipes, Anaerofilum and Cloacibacillus were significantly different among the three FSRP groups. (C) Wilcoxon Rank Sum test suggested that the relative abundances of five taxa were significantly different between LR and MR. (D) Wilcoxon Rank Sum test suggested that the relative abundances of eight taxa were significantly different between MR and HR. FSRP, Framingham Stroke Risk Profile; LEfSe, linear discriminant analysis effect size; LDA, linear discriminant analysis; HR, high-risk group; LR, low-risk group; MR, moderate-risk group. p values: *p < 0.05, **p < 0.01.
Furthermore, pairwise comparisons between the participants from the low- and moderate-risk groups suggested that Collinsella, Clostridium_XlVb, and Sporobacter were enriched in the participants from the low-risk group, whereas Flavobacteriaceae and Terrisporobacter were enriched in the participants from the moderate-risk group (Figure 6C). Pairwise comparisons between the participants from the moderate- and high-risk groups revealed that Methanobacteriaceae, Collinsella, Sporobacter, Cloacibacillus, Paraprevotella, Anaerofilum, and Methanobrevibacter were enriched in the participants from the high-risk group, whereas Anaerostipes was enriched in the participants from the moderate-risk group (Figure 6D). Although the between-group difference was statistically significant, the discriminative microbiota determined by pairwise comparisons was marginally abundant.
The KEGG pathways of microbial communities associated with the risk levels of FSRP were further investigated using PICRUSt. Pairwise comparisons between the participants from the low- and moderate-risk groups revealed two outstanding pathways related to the metabolism of terpenoids and polyketides (Figure 7A). Pairwise comparisons between the participants from the moderate- and high-risk groups showed two outstanding pathways associated with transcription and translation (Figure 7B). Pairwise comparisons between the participants from the low- and high-risk groups demonstrated 14 KEGG (level 3) pathways of significant difference. These pathways were involved in the metabolism of amino acids, lipids, cofactors, vitamins, terpenoids, and polyketides and in the biodegradation and metabolism of xenobiotics (Figure 7C).
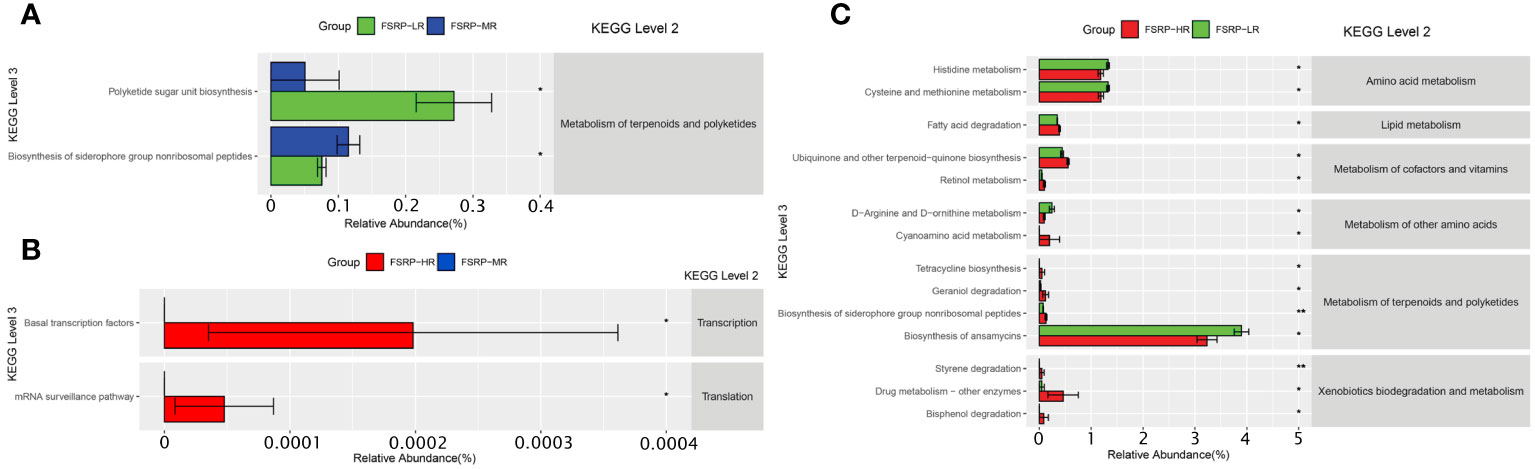
Figure 7 KEGG pathways predicted by PICRUSt and associations with FSRP groups. (A) KEGG pathways with differences in abundance between LR and MR. (B) KEGG pathways with differences in abundance between MR and HR. (C) KEGG pathways with differences in abundance between LR and HR. FSRP, Framingham Stroke Risk Profile; KEGG, Kyoto Encyclopedia of Genes and Genomes; PICRUSt, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States; HR, high-risk group; LR, low-risk group; MR, moderate-risk group. Differential KEGG pathways with relative abundance were visualized as a bar plot with standard error. *Wilcoxon Rank Sum test with p values less than 0.05, ** Wilcoxon Rank Sum test with p values less than 0.01.
Spearman rank-order analysis of the correlation between the discriminative gut microbiota and clinical characteristics (Figure 8) showed that Anaerostipes, Flavonifractor and Clostridium_XlVb were negatively correlated with IMT, TG, WBC, and NEUT, whereas Anaerostipes and Flavonifractor were positively correlated with HDL-C. Faecalibacterium was negatively correlated with TG and NEUT, and Ruminococcus2, which is under the same phylum (Firmicutes) as Faecalibacterium, showed a negative monotonic relationship with IMT. In addition, Bacteroidaceae and Bacteroides were negatively correlated with IMT and BMI, and Cloacibacillus were negatively correlated with hs-CRP. Correlation analysis also identified some gut microbiota specific to individual risk factors; for example, Peptostreptococcaceae and Romboutsia specifically benefited to the alteration of glucose, Fretibacterium negatively correlated with BMI, and Ruminococcaceae prevented the elevation of TG. In addition to beneficial bacteria, some pathogenic bacteria were identified; for example, Actinomycetales and Acidaminococcaceae contributed to the enhancement of LDL-C, whereas Megamonas and Anaerofilum contributed to the increase in WBC; Anaerofilum was also negatively correlated with hs-CRP.
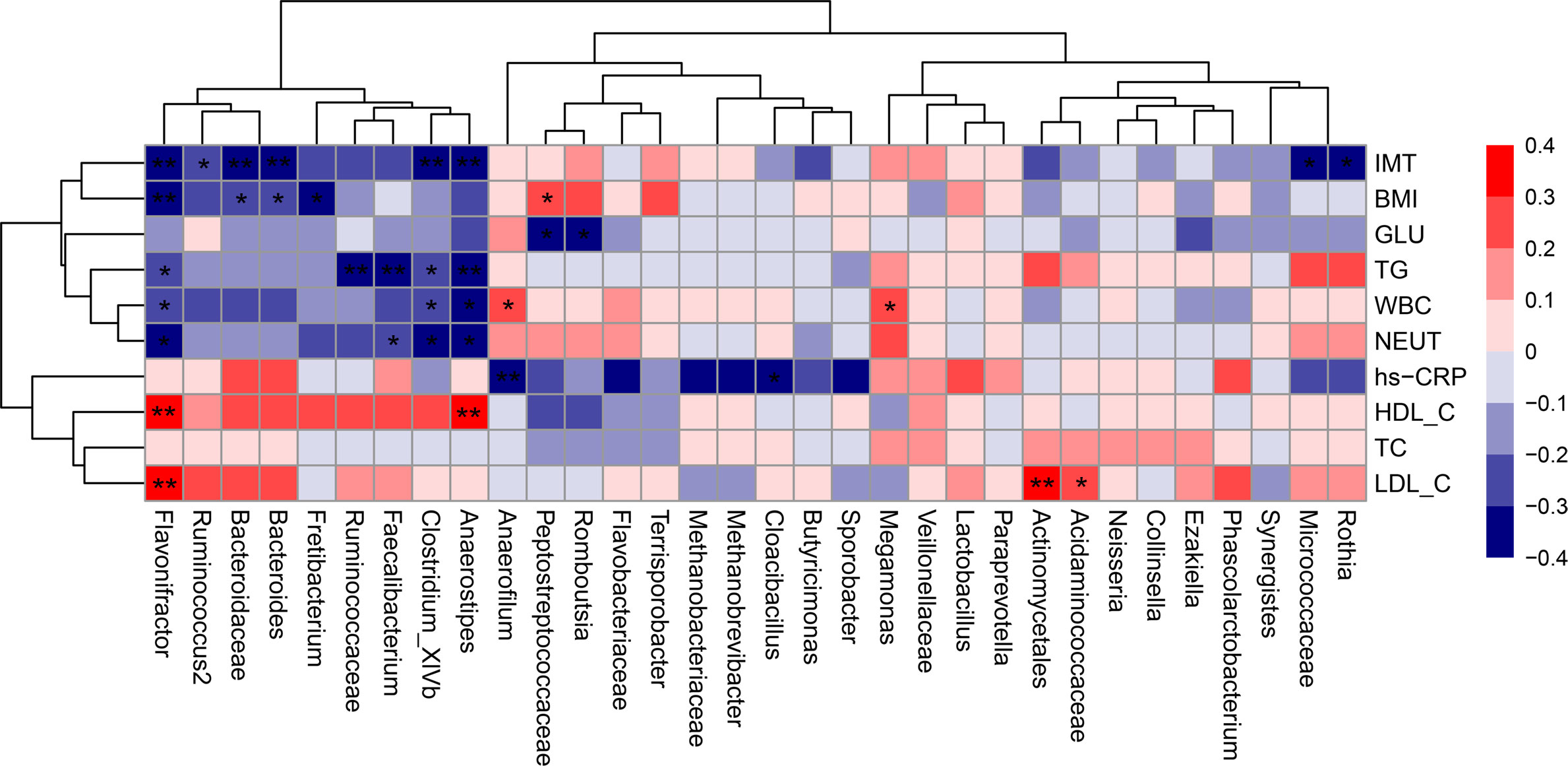
Figure 8 Correlation matrix for discriminative microbiota and clinical characteristics. Red cell indicates positive correlation, and blue indicates negative correlation in the Spearman correlation analysis. *p < 0.05, **p < 0.01. BMI, body mass index; GLU, fasting glucose; HDL-C, high-density lipoprotein cholesterol; hs-CRP, hypersensitive C-reactive protein; IMT, intima-media thickness; LDL-C, low-density lipoprotein cholesterol; NEUT, total neutrophils; TC, total cholesterol; TG, triglycerides; WBC, white blood cell.
In the present study, many vascular beneficial bacteria were enriched in the participants from the healthy control and low-risk stroke groups, whereas a few vascular harmful bacteria were more abundant in the participants from the high-risk group than in those from the other groups. However, Zeng et al. (2019) assessed southern participants with a mean age of 71 years based on CNSRS and found that opportunistic pathogens and lactate-producing bacteria are enriched in those participants with a high risk of stroke (Zeng et al., 2019). This result may be partially explained by the age difference between the participants of the two studies, as microbial diversity and composition are altered in elderly people (Vaiserman et al., 2017). Specifically, Rondanelli et al. (2015) reported that the population of Enterobacteriaceae increases in people aged more than 65 years (Rondanelli et al., 2015). This family was also detected in the study by Zeng et al. (2019) but not in the present study. Meanwhile, Rondanelli et al. (2015) found that the populations of Bacteroides and Clostridium are greatly reduced in elderly people (Rondanelli et al., 2015). These genera were enriched in the healthy controls of our study but not identified in the participants from the study by Zeng et al. (2019). These differences may be also ascribed to the sex difference between the studies because emerging evidence suggests that sex–microbiota–brain interactions are involved in the development of neurologic and psychiatric disorders (Shobeiri et al., 2022). Specifically, laboratory experiments showed that Lactobacillus is generally more enriched in females than in males (Shobeiri et al., 2022). Similarly, Zeng et al. (2019) found that Lactobacillus is enriched in a high-risk group composed of 58% females. However, Lactobacillus was not identified in the present study, where females only accounted for approximately 7% of the high-risk group. Our study also found that the participants in the low-risk group had an abundance of butyrate-producing bacteria, such as Faecalibacterium, Ruminococcaceae, Butyricimonas, and Peptostreptococcaceae. This finding is consistent with the results reported by Zeng et al. (2019). Both studies suggested that the accumulation of inflammation is correlated with the elevated stroke risk, considering that butyrate-producing bacteria function as anti-inflammatory and anti-atherosclerotic agents (Zhao et al., 2021).
The gut microbiota is a promising biomarker for predicting atherosclerotic cardiovascular diseases (Nayor et al., 2021). Our study revealed that Anaerostipes, Clostridium_XlVb, and Flavonifractor, all genera belonging to the Firmicutes phylum, were enriched in the participants from the low-risk group as assessed by CNSRS or FSRP, and their relative abundances gradually decreased as the stroke risk increased. In addition, the gut microbiota has a negative monotonic relationship with IMT, a widely used surrogate marker for atherosclerosis (Nezu et al., 2016). This finding deserves attention as it provides specific implications for developing novel microbial biomarkers for predicting stroke risk in future cohort studies. Furthermore, we found that Anaerostipes, Clostridium_XlVb, and Flavonifractor were negatively correlated with TG. Although elevated TG levels might not directly lead to atherosclerosis, triglyceride-rich lipoproteins (TGRL) and remnant lipoprotein particles (RLP) are causal risk factors for atherosclerotic cardiovascular events because of several reasons: 1) TGRL remnants can penetrate the endothelium and interact with macrophages, leading to foam cell formation and inflammation in arterial walls; 2) TGRL can be absorbed by circulating monocytes and become lipid-laden foamy monocytes, which infiltrate the arterial wall; and 3) TGRL and RLP can induce HDL remodelling and dysfunction, all of which promote vascular inflammation and atherosclerosis (Peng and Wu, 2022). The negative association between the gut microbiota and TG may contribute to the development of novel therapies, such as supplemental probiotics targeting hypertriglyceridaemia. In addition, Anaerostipes, Clostridium_XlVb, and Flavonifractor were positively correlated with HDL-C, whereas Flavonifractor was positively correlated with LDL-C. The benefits of these microbiota in atherosclerosis are supported by the traditional knowledge that HDL-C, the plasma lipoprotein responsible for reverse cholesterol transport, protects against atherosclerosis (Fernandes das Neves et al., 2021). However, the positive correlation of Flavonifractor with LDL-C was unexpected, considering that elevated LDL-C is a well-established risk factor for cardiovascular disease (Silverman et al., 2016). This finding may be explained by the fact that an increase in circulating oxidised LDL-C (oxLDL-C) is the primary contributor to the induction of foam cells in the arterial intima and that plasma oxLDL-C level is a better predictor of cardiovascular disease than LDL-C level (Zhang et al., 2019; Xu et al., 2022). We also found that Anaerostipes, Clostridium_XlVb, and Flavonifractor were negatively correlated with WBC and NEUT counts, which are universally available markers of chronic low-grade inflammation that are associated with carotid IMT and plaques presented in early and advanced atherosclerosis (Ortega et al., 2012; Willeit et al., 2016). Taken together, these findings suggest that Anaerostipes, Clostridium_XlVb, and Flavonifractor protect against atherosclerosis and reduce the risk of incident stroke by reducing lipid accumulation and attenuating the inflammatory responses of the arterial walls, which generally was consistent with the functional prediction results.
Additionally, the genus Bacteroides and its family Bacteroidaceae with a higher abundance in the healthy control group than in the stroke risk groups, were found negatively correlated with BMI, which were in line with the results of a previous study (Liu et al., 2017). Their negatively correlation with IMT might be attributed to their attenuating effect on atherosclerotic lesion by suppressing pro-inflammatory immune responses (Yoshida et al., 2018). Apart from the beneficial vascular microbiota, we also identified Megamonas and Anaerofilum, which were enriched in the participants from the high-risk group and positively correlated with WBC count. The identification of vascular harmful microbiota is supported by the previous findings that Megamonas enriched in a group of coronary artery diseases is positively correlated with cholesterol (Liu et al., 2019) and that the population of Anaerofilum increases in people consuming large amounts of ultra-processed foods, which promote inflammation possibly via diet–microbiome–host interactions (Zinöcker and Lindseth, 2018; Cuevas-Sierra et al., 2021).
The present study used CNSRS and FSRP in the assessment of stroke risk and characterised the gut microbiota by risk levels for the first time. The CNSRS, which was developed by Chinese government, has been used nationwide owing to its feasibility in the rapid determination of stroke risk and its inclusion of recently identified risk factors, such as obesity and dyslipidaemia (Chao et al., 2021). Using CNSRS, we found outstanding vascular beneficial bacteria, such as Phascolarctobacterium and Faecalibacterium, which ameliorate hyperlipidaemia and inflammation (Sokol et al., 2008; Tong et al., 2018; Oliver et al., 2022), and Bacteroides, which attenuates carotid atherosclerosis by altering BMI (Liu et al., 2017). These pieces of evidence can be directly generalised to the Chinese population, considering that obesity and metabolic conditions are leading risk factors for stroke (Ma et al., 2021; Pan et al., 2021). FSRP has long been used globally because it considers age, sex, systolic blood pressure, and anti-hypertensive medication to calculate stroke risk, and the 10-year stroke risk model is validated by a large-sample, long-follow-up cohort study (Peng et al., 2020). Using FSRP, we identified outstanding microbiota, such as Anaerostipes and Clostridium_XlVb, which reportedly lower blood pressure (Bier et al., 2018; Verhaar et al., 2020). In addition, Clostridium_XlVb produces indole-3-propionic acid, which reverses cholesterol transport for atherosclerosis (Xue et al., 2022). The mutual complementation of the two stroke risk instruments broadens our understanding of the microbial diversity and composition in terms of stroke risk levels and offers candidates for future development of probiotics in cardiovascular medicine.
This study had several limitations. Firstly, the evidence certainty on the association between the gut microbiota and the risk of stroke may be limited by the small-scale, cross-sectional, single-centre design of the present study. Future cohort studies with large sample sizes, multiple sites, and long-term follow-up are needed to validate these findings. Secondly, we did not conduct metagenomic sequencing and detect the microbial metabolites. The functional prediction and explanations of the mechanisms of actions of the enriched microbiota should be further elucidated. Thirdly, the carotid plaque scores were not calculated in the present study. Such scores should be calculated because they represent atherosclerotic progress and predict stroke risk more accurately than carotid IMT (Nezu and Hosomi, 2020; Raggi and Stein, 2020).
The preliminary evidence suggests that gut microbiota is associated with stroke risk stratification, and it ameliorates atherosclerotic progress possibly by regulating lipid metabolism and inflammation. This study provides novel insights into the microbial characteristics related to stroke risk levels in middle-aged individuals, which is critical to the early screening of stroke risk and primary prevention.
According to national legislation/guidelines, specifically the Administrative Regulations of the People’s Republic of China on Human Genetic Resources (http://www.gov.cn/zhengce/content/2019-06/10/content_5398829.htm, http://english.www.gov.cn/policies/latest_releases/2019/06/10/content_281476708945462.htm), no additional raw data is available at this time. Data of this project can be accessed after an approval application to the China National Genebank (CNGB, https://db.cngb.org/cnsa/). Please refer to https://db.cngb.org/, or email: Q05HQmRiQGNuZ2Iub3Jn for detailed application guidance. The accession code CNP0005062 should be included in the application.
XN conceived and designed the study, supervised its implementation, validated the data, and wrote the manuscript. HH led the study implementation, data analysis, and visualisation. ZK, RM, and MM participated in the survey, sample collection, and data entry. YC participated in participant recruitment and diagnosis ascertainment. XN and HH contributed to the interpretation of results. All the authors have read the manuscript and approved its submission.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Guangzhou Municipal Science and Technology Bureau, China (No. 202102010300, principal investigator: XN) and Traditional Chinese Medicine Bureau of Guangdong Province, China (No. 20225021, principal investigator: XN); and was additional supported by the internal funding from Guangzhou University of Chinese Medicine, China (No. 2021xk26) and State Key Laboratory of Dampness Syndrome of Chinese Medicine (No. SZ2021ZZ07).
We would like to thank BGI Genomics Co., Ltd., Shenzhen, China, for specimen processing and sequencing and the general support from the State Key Laboratory of Dampness Syndrome of Chinese Medicine, chaired by the Second Affiliated Hospital of Guangzhou University of Chinese Medicine, China. We are also grateful to the clinical staff and research postgraduates from the Department of Neurology, Guangdong Provincial Hospital of Chinese Medicine, for their assistance with the participant recruitment and physical examination.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1227450/full#supplementary-material
Supplementary Figure 1 | The rarefaction curves for observed species. HC, healthy control group; RG, risk group.
BMI, body mass index; GLU, fasting glucose; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; WBC, white blood cell; NEUT, absolute neutrophil; hs-CRP, hypersensitive C-reactive protein; TIA, transient ischaemic attack; IMT, intima-media thickness; CCA, common carotid artery; FSRP, Framingham Stroke Risk Profile; CNSRS, Chinese National Stroke Risk Scorecard; OTUs, operational taxonomical units; LDA, Logarithmic linear discriminant analysis; LEfSe, LDA effect size; KEGG, Kyoto Encyclopedia of Genes and Genomes; PICRUSt, phylogenetic investigation of communities by reconstruction of unobserved states;Ace, abundance-based coverage estimator; TGRL, triglyceride-rich lipoproteins; RLP, remnant lipoprotein particles.
Abbasalizad Farhangi, M., Vajdi, M. (2021). Gut microbiota-associated trimethylamine N-oxide and increased cardiometabolic risk in adults: a systematic review and dose-response meta-analysis. Nutr. Rev. 79 (9), 1022–1042. doi: 10.1093/nutrit/nuaa111
Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., et al. (2019). ACC/AHA guideline on the primary prevention of cardiovascular disease: A report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation 140 (11), e596–e646. doi: 10.1016/j.jacc.2019.03.009
Bier, A., Braun, T., Khasbab, R., Di Segni, A., Grossman, E., Haberman, Y., et al. (2018). A high salt diet modulates the gut microbiota and short chain fatty acids production in a salt-sensitive hypertension rat model. Nutrients. 10 (9), 1154. doi: 10.3390/nu10091154
Björkegren, J. L. M., Lusis, A. J. (2022). Atherosclerosis: recent developments. Cell. 185 (10), 1630–1645. doi: 10.1016/j.cell.2022.04.004
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7 (5), 335–336. doi: 10.1038/nmeth.f.303
Chakaroun, R. M., Olsson, L. M., Bäckhed, F. (2023). The potential of tailoring the gut microbiome to prevent and treat cardiometabolic disease. Nat. Rev. Cardiol. 20 (4), 217–235. doi: 10.1038/s41569-022-00771-0
Chao (1984). Non-parametric estimation of the number of classes in a population, Scand. J. Stat. 11, 265–270. doi: 10.2307/4615964
Chao, A., Lee, S. M. (1992). Estimating the number of classes via sample coverage. J. Am. Stat. Assoc. 87, 210–217. doi: 10.1080/01621459.1992.10475194
Chao, B. H., Yan, F., Hua, Y., Liu, J. M., Yang, Y., Ji, X. M., et al. (2021). Stroke prevention and control system in China: CSPPC-Stroke Program. Int. J. Stroke. 16 (3), 265–272. doi: 10.1177/1747493020913557
Cuevas-Sierra, A., Milagro, F. I., Aranaz, P., Martínez, J. A., Riezu-Boj, J. I. (2021). Gut microbiota differences according to ultra-processed food consumption in a spanish population. Nutrients. 13 (8), 2710. doi: 10.3390/nu13082710
D'Agostino, R. B., Wolf, P. A., Belanger, A. J., Kannel, W. B. (1994). Stroke risk profile: adjustment for antihypertensive medication. Framingham Study. Stroke. 25 (1), 40–43. doi: 10.1161/01.STR.25.1.40
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 26 (19), 2460–2461. doi: 10.1093/bioinformatics/btq461
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10 (10), 996–998. doi: 10.1038/nmeth.2604
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 27 (16), 2194–2200. doi: 10.1093/bioinformatics/btr381
Farhangi, M. A., Vajdi, M. (2020). Novel findings of the association between gut microbiota-derived metabolite trimethylamine N-oxide and inflammation: results from a systematic review and dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 60 (16), 2801–2823. doi: 10.1080/10408398.2020.1770199
Feigin, V. L., Brainin, M., Norrving, B., Martins, S., Sacco, R. L., Hacke, W., et al. (2022). World stroke organization (WSO): global stroke fact sheet 2022. Int. J. Stroke. 17 (1), 18–29. doi: 10.1177/17474930211065917
Fernandes das Neves, M., Batuca, J. R., Delgado Alves, J. (2021). The role of high-density lipoprotein in the regulation of the immune response: implications for atherosclerosis and autoimmunity. Immunology. 164 (2), 231–241. doi: 10.1111/imm.13348
GBD 2019 Stroke Collaborators (2021). Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20 (10), 795–820. doi: 10.1016/S1474-4422(21)00252-0
Liu, R., Hong, J., Xu, X., Feng, Q., Zhang, D., Gu, Y., et al. (2017). Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23 (7), 859–868. doi: 10.1038/nm.4358
Liu, Y., Qu, J., Xu, J., Gu, A., Deng, D., Jia, X., et al. (2023). Trimethylamine-N-oxide: a potential biomarker and therapeutic target in ischemic stroke. Front. Neurol. 14, 1156879. doi: 10.3389/fneur.2023.1156879
Liu, Z., Li, J., Liu, H., Tang, Y., Zhan, Q., Lai, W., et al. (2019). The intestinal microbiota associated with cardiac valve calcification differs from that of coronary artery disease. Atherosclerosis. 284, 121–128. doi: 10.1016/j.atherosclerosis.2018.11.038
Lozupone, C., Lladser, M. E., Knights, D., Stombaugh, J., Knight, R. (2011). UniFrac: an effective distance metric for microbial community comparison. ISME J. 5 (2), 169–172. doi: 10.1038/ismej.2010.133
Ma, Q., Li, R., Wang, L., Yin, P., Wang, Y., Yan, C., et al. (2021). Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 6 (12), e897–e906. doi: 10.1016/S2468-2667(21)00228-0
Magoč, T., Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 27 (21), 2957–2963. doi: 10.1093/bioinformatics/btr507
Meschia, J. F., Bushnell, C., Boden-Albala, B., Braun, L. T., Bravata, D. M., Chaturvedi, S., et al. (2014). Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 45 (12), 3754–3832. doi: 10.1161/STR.0000000000000046
Nayor, M., Brown, K. J., Vasan, R. S. (2021). The molecular basis of predicting atherosclerotic cardiovascular disease risk. Circ. Res. 128 (2), 287–303. doi: 10.1161/CIRCRESAHA.120.315890
Nezu, T., Hosomi, N. (2020). Usefulness of carotid ultrasonography for risk stratification of cerebral and cardiovascular disease. J. Atheroscler. Thromb. 27 (10), 1023–1035. doi: 10.5551/jat.RV17044
Nezu, T., Hosomi, N., Aoki, S., Matsumoto, M. (2016). Carotid intima-media thickness for atherosclerosis. J. Atheroscler. Thromb. 23 (1), 18–31. doi: 10.5551/jat.31989
Oliver, A., El Alaoui, K., Haunschild, C., Avelar-Barragan, J., Mendez Luque, L. F., Whiteson, K., et al. (2022). Fecal microbial community composition in myeloproliferative neoplasm patients is associated with an inflammatory state. Microbiol. Spectr. 10 (3), e0003222. doi: 10.1128/spectrum.00032-22
Onut, R., Balanescu, A. P., Constantinescu, D., Calmac, L., Marinescu, M., Dorobantu, P. M. (2012). Imaging Atherosclerosis by Carotid Intima-media Thickness in vivo: How to, Where and in Whom? Maedica (Bucur) 7 (2), 153–162.
Ortega, E., Gilabert, R., Nuñez, I., Cofán, M., Sala-Vila, A., de Groot, E., et al. (2012). White blood cell count is associated with carotid and femoral atherosclerosis. Atherosclerosis. 221 (1), 275–281. doi: 10.1016/j.atherosclerosis.2011.12.038
Owolabi, M. O., Thrift, A. G., Mahal, A., Ishida, M., Martins, S., Johnson, W. D., et al. (2022). Stroke Experts Collaboration Group. Primary stroke prevention worldwide: translating evidence into action. Lancet Public Health 7 (1), e74–e85. doi: 10.1016/S2468-2667(21)00230-9
Pan, X. F., Wang, L., Pan, A. (2021). Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 9 (6), 373–392. doi: 10.1016/S2213-8587(21)00045-0
Pandian, J. D., Gall, S. L., Kate, M. P., Silva, G. S., Akinyemi, R. O., Ovbiagele, B. I., et al. (2018). Prevention of stroke: a global perspective. Lancet. 392 (10154), 1269–1278. doi: 10.1016/S0140-6736(18)31269-8
Peng, S., Shen, Y., Wang, M., Zhang, J. (2020). Serum and CSF metabolites in stroke-free patients are associated with vascular risk factors and cognitive performance. Front. Aging Neurosci. 12, 193. doi: 10.3389/fnagi.2020.00193
Peng, X., Wu, H. (2022). Inflammatory links between hypertriglyceridemia and atherogenesis. Curr. Atheroscler. Rep. 24 (5), 297–306. doi: 10.1007/s11883-022-01006-w
Qi, W., Ma, J., Guan, T., Zhao, D., Abu-Hanna, A., Schut, M., et al. (2020). Risk factors for incident stroke and its subtypes in China: A prospective study. J. Am. Heart Assoc. 9 (21), e016352. doi: 10.1161/JAHA.120.016352
Qiong, W., George, M., Garrity, James, M., Tiedje, James, R. (2007). Cole. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy, Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Raggi, P., Stein, J. H. (2020). Carotid intima-media thickness should not be referred to as subclinical atherosclerosis: A recommended update to the editorial policy at Atherosclerosis. Atherosclerosis. 312, 119–120. doi: 10.1016/j.atherosclerosis.2020.09.015
Rondanelli, M., Giacosa, A., Faliva, M. A., Perna, S., Allieri, F., Castellazzi, A. M. (2015). Review on microbiota and effectiveness of probiotics use in older. World J. Clin. Cases. 3 (2), 156–162. doi: 10.12998/wjcc.v3.i2.156
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75 (23), 7537–7541. doi: 10.1128/AEM.01541-09
Segata, N., Izard, J., Waldron, L., Gevers, D., Miropolsky, L., Garrett, W. S., et al. (2011). Metagenomic biomarker discovery and explanation. Genome Biol. 12 (6), R60. doi: 10.1186/gb-2011-12-6-r60
Shannon, C. E. (1948). A mathematical theory of communication. Bell System Tech. J. 27 (3), 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Shobeiri, P., Kalantari, A., Teixeira, A. L., Rezaei, N. (2022). Shedding light on biological sex differences and microbiota-gut-brain axis: a comprehensive review of its roles in neuropsychiatric disorders. Biol. Sex Differ. 13 (1), 12. doi: 10.1186/s13293-022-00422-6
Silverman, M. G., Ference, B. A., Im, K., Wiviott, S. D., Giugliano, R. P., Grundy, S. M., et al. (2016). Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: A systematic review and meta-analysis. JAMA. 316 (12), 1289–1297. doi: 10.1001/jama.2016.13985
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermúdez-Humarán, L. G., Gratadoux, J. J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 105 (43), 16731–16736. doi: 10.1073/pnas.0804812105
Spence, J. D., Hackam, D. G. (2010). Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke. 41 (6), 1193–1199. doi: 10.1161/STROKEAHA.110.577973
Stein, J. H., Korcarz, C. E., Hurst, R. T., Lonn, E., Kendall, C. B., Mohler, E. R., et al. (2008). American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 21 (2), 93–111. doi: 10.1016/j.echo.2007.11.011
Tang, W. H. W., Bäckhed, F., Landmesser, U., Hazen, S. L. (2019). Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73 (16), 2089–2105. doi: 10.1016/j.jacc.2019.03.024
The Ministry of Health China Stroke Prevention Project Committee (2015). Chinese guidelines of vascular ultrasonography for stroke. Chin. J. Med. Ultrasound (Electronic Edition) 12 (08), 599–610. doi: 10.3877/cma.j.issn.1672-6448.2015.08.004
The Professional Committee of Vascular Ultrasound of Stroke Prevention and Treatment Expert, Committee of the National Health Commission; the Professional Committee of Superficial Organ and Peripheral Vascular Ultrasound of the Chinese Medical Ultrasound Engineering, the Professional Committee of Craniocerebral and Cervical Vascular Ultrasound of the Chinese Medical Ultrasound Engineering (2020). Expert consensus on some problems of cerebral and carotid vascular ultrasonography (Part of carotid). Chin. J. Cerebrovas. Dis. 17 (06), 346–353. doi: 10.3969/j.issn.1672-5921.2020.06.013
Tong, X., Xu, J., Lian, F., Yu, X., Zhao, Y., Xu, L., et al. (2018). Structural alteration of gut microbiota during the amelioration of human type 2 diabetes with hyperlipidemia by metformin and a traditional chinese herbal formula: a multicenter, randomized, open label clinical trial. mBio. 9 (3), e02392–e02317. doi: 10.1016/j.phrs.2020.105362
Vaiserman, A. M., Koliada, A. K., Marotta, F. (2017). Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 35, 36–45. doi: 10.1016/j.arr.2017.01.001
Verhaar, B. J. H., Collard, D., Prodan, A., Levels, J. H. M., Zwinderman, A. H., Bäckhed, F., et al. (2020). Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur. Heart J. 41 (44), 4259–4267. doi: 10.1093/eurheartj/ehaa704
Wang, Y. J., Li, Z. X., Gu, H. Q., Zhai, Y., Zhou, Q., Jiang, Y., et al. (2022). China Stroke Statistics: an update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc. Neurol. 7 (5), 415–450. doi: 10.1136/svn-2021-001374
Wang, J., Zhang, H., He, J., Xiong, X. (2022). The role of the gut microbiota in the development of ischemic stroke. Front. Immunol. 13, 845243. doi: 10.3389/fimmu.2022.845243
Wilkinson, T. J., Huws, S. A., Edwards, J. E., Kingston-Smith, A. H., Siu-Ting, K., Hughes, M., et al. (2018). CowPI: A rumen microbiome focussed version of the PICRUSt functional inference software. Front. Microbiol. 9, 1095. doi: 10.3389/fmicb.2018.01095
Willeit, P., Thompson, S. G., Agewall, S., Bergström, G., Bickel, H., Catapano, A. L., et al. (2016). Inflammatory markers and extent and progression of early atherosclerosis: Meta-analysis of individual-participant-data from 20 prospective studies of the PROG-IMT collaboration. Eur. J. Prev. Cardiol. 23 (2), 194–205. doi: 10.1177/2047487314560664
Xu, Q., Wu, Q., Li, H., Tian, X., Zuo, Y., Zhang, Y., et al. (2022). Joint high level of oxidized low-density lipoprotein and high-sensitivity C-reactive protein are associated with recurrent stroke and poor functional outcome in minor stroke or transient ischemic attack. J. Am. Heart Assoc. 11 (20), e027665. doi: 10.1161/JAHA.122.027665
Xue, H., Chen, X., Yu, C., Deng, Y., Zhang, Y., Chen, S., et al. (2022). Gut microbially produced indole-3-propionic acid inhibits atherosclerosis by promoting reverse cholesterol transport and its deficiency is causally related to atherosclerotic cardiovascular disease. Circ. Res. 131 (5), 404–420. doi: 10.1161/CIRCRESAHA.122.321253
Yoshida, N., Emoto, T., Yamashita, T., Watanabe, H., Hayashi, T., Tabata, T., et al. (2018). Bacteroides vulgatus and Bacteroides dorei Reduce Gut Microbial Lipopolysaccharide Production and Inhibit Atherosclerosis. Circulation. 138 (22), 2486–2498. doi: 10.1161/CIRCULATIONAHA.118.033714
Zeng, X., Gao, X., Peng, Y., Wu, Q., Zhu, J., Tan, C., et al. (2019). Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front. Cell Infect. Microbiol. 9, 4. doi: 10.3389/fcimb.2019.00004
Zhang, Q., Ai, Y., Dong, H., Wang, J., Xu, L. (2019). Circulating oxidized low-density lipoprotein is a strong risk factor for the early stage of coronary heart disease. IUBMB Life. 71 (2), 277–282. doi: 10.1002/iub.1966
Zhao, X., Oduro, P. K., Tong, W., Wang, Y., Gao, X., Wang, Q. (2021). Therapeutic potential of natural products against atherosclerosis: Targeting on gut microbiota. Pharmacol. Res. 163, 105362. doi: 10.1016/j.phrs.2020.105362
Keywords: gut microbiota, the risk of stroke, 16S rRNA sequencing, lipid metabolism, inflammation, atherosclerosis
Citation: Huang H, Kuang Z, Mo R, Meng M, Cai Y and Ni X (2023) The preliminary evidence on the association of the gut microbiota with stroke risk stratification in South Chinese population. Front. Cell. Infect. Microbiol. 13:1227450. doi: 10.3389/fcimb.2023.1227450
Received: 23 May 2023; Accepted: 24 November 2023;
Published: 21 December 2023.
Edited by:
Carlo Contini, University of Ferrara, ItalyReviewed by:
Ninfa Ramírez Durán, Autonomus University of the State of Mexico, MexicoCopyright © 2023 Huang, Kuang, Mo, Meng, Cai and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojia Ni, Z3JhY2UxOTg0MzI1QDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.