- 1Department of Pharmacy, Shaoxing Hospital of Traditional Chinese Medicine Affiliated to Zhejiang Chinese Medical University, Shaoxing, Zhejiang, China
- 2Department of Traditional Chinese Medicine, Hangzhou Linping District Hospital of Integrated Chinese and Western Medicine, Hangzhou, China
- 3Department of Clinical Laboratory, The People’s Hospital of Zhangqiu Area, Jinan, China
- 4School of Medical Technology and Information Engineering, Zhejiang Chinese Medical University, Hangzhou, China
- 5Core Facility, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, China
- 6Department of Clinical Laboratory, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, China
- 7Department of Pharmacy, Jiaxing Hospital of Traditional Chinese Medicine, Jiaxing, China
- 8Department of Urology, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, China
The emergence of carbapenemase-producing Acinetobacter spp. has been widely reported and become a global threat. However, carbapenem-resistant A. johnsonii strains are relatively rare and without comprehensive genetic structure analysis, especially for isolates collected from human specimen. Here, one A. johnsonii AYTCM strain, co-producing NDM-1, OXA-58, and PER-1 enzymes, was isolated from sputum in China in 2018. Antimicrobial susceptibility testing showed that it was resistant to meropenem, imipenem, ceftazidime, ciprofloxacin, and cefoperazone/sulbactam. Whole-genome sequencing and bioinformatic analysis revealed that it possessed 11 plasmids. blaOXA-58 and blaPER-1 genes were located in the pAYTCM-1 plasmid. Especially, a complex class 1 integron consisted of a 5′ conserved segment (5′ CS) and 3′ CS, which was found to carry sul1, arr-3, qnrVC6, and blaPER-1 cassettes. Moreover, the blaNDM-1 gene was located in 41,087 conjugative plasmids and was quite stable even after 70 passages under antibiotics-free conditions. In addition, six prophage regions were identified. Tracking of closely related plasmids in the public database showed that pAYTCM-1 was similar to pXBB1-9, pOXA23_010062, pOXA58_010030, and pAcsw19-2 plasmids, which were collected from the strains of sewage in China. Concerning the pAYTCM-3 plasmids, results showed that strains were collected from different sources and their hosts were isolated from various countries, such as China, USA, Japan, Brazil, and Mexico, suggesting that a wide spread occurred all over the world. In conclusion, early surveillance is warranted to avoid the extensive spread of this high-risk clone in the healthcare setting.
Introduction
Acinetobacter spp. are ubiquitous in nature and are usually identified in the hospital environment, and some of these species have been reported in a variety of nosocomial infections (Wong et al., 2017). The most common species to cause infections is A. baumannii, followed by A. calcoaceticus and A. lwoffii. However, A. johnsonii, a kind of potentially opportunistic pathogen in Acinetobacter spp., generally distributed in natural or nosocomial environments, such as agricultural soil (Wang et al., 2019; Jia et al., 2021).
Carbapenems are the main antimicrobial agents for the treatment of infections with multidrug-resistant Acinetobacter spp., including A. johnsonii (Tang et al., 2020). However, the problem of carbapenem resistance is being increasingly reported, which has contributed to a huge challenge for clinicians (Bonnin et al., 2014). The carbapenem resistance mechanism was usually mediated via enzymatic inactivation (such as carbapenemases), efflux pump overexpression, and target site modification (i.e., altered penicillin-binding proteins) (Mohd Rani et al., 2017; Castanheira et al., 2023). Upon previous studies, more than 210 β-lactamases have been identified in Acinetobacter spp. with class D β-lactamases being the most widespread carbapenemase (Mohd Rani et al., 2017), including OXA-23, OXA-24, and OXA-58 (Liu et al., 2021). Moreover, several insertion sequence (IS) elements such as ISAba1 and ISAba3 could increase the expression of class D β-lactamase genes (including blaOXA-58-like and blaOXA-23-like genes) when they were found upstream of these IS elements (Mohd Rani et al., 2017).
Considering the increasing resistance to carbapenems and almost all other antimicrobial agents, Acinetobacter spp. are important resistant microorganisms with a global public health threat, which are associated with severe nosocomial infections including pneumonia, urinary tract, bloodstream, and wound infections (Gonzalez-Villoria and Valverde-Garduno, 2016). However, limited knowledge concerning the carbapenem resistance was known in A. johnsonii strains. Until now, researchers only reported some genome sequences and described the features of A. johnsonii strains which are isolated from the environment, especially in hospital sewage (Feng et al., 2016; Zong et al., 2020). However, little is known about this species which was collected from a patient source in the hospital. Here, we investigated the genetic characteristics of one carbapenem-resistant A. johnsonii, co-producing NDM-1, OXA-58, and PER-1 in a patient’s sputum in 2018 in China. To the best of our knowledge, this is the first comprehensive description of one carbapenem-resistant A. johnsonii from a patient source.
Materials and methods
Bacterial isolation and identification of the A. johnsonii AYTCM strain
A flowchart is shown in Figure S1 (Behzadi and Gajdacs, 2021). A. johnsonii AYTCM strain was isolated from sputum in China in 2018. Isolate identification was conducted using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS, Bruker Daltonik GmbH, Bremen, Germany) and further confirmed by PCR and 16S rRNA (GenBank ID: NR_164627.1) gene-based sequencing with specific primers 27F (5′-agagtttgatcctggctcag-3′) and 1492R (5′-ggttaccttgttacgactt-3′) (Zong et al., 2020).
Minimum inhibitory concentration measurement
Antimicrobial susceptibility testing (AST) was performed by the broth microdilution method and interpreted based on the recommendations of Clinical and Laboratory Standards Institute (CLSI) 2021 guidelines and European Committee on Antimicrobial Susceptibility Testing (EUCAST) 2021 breakpoint tables for tigecycline. The antimicrobial agents used in this study were shown as follows: ceftazidime (CAZ), cefoperazone/sulbactam (CFS), imipenem (IPM), meropenem (MEM), ciprofloxacin (CIP), amikacin (AMI), colistin (COL), tigecycline (TGC), and cefiderocol (CFDC). Escherichia coli ATCC 25922 served as the quality control strain.
Mating experiments
To determine whether the plasmids carrying blaNDM-1, blaOXA-58, and blaPER-1 were transferable, conjugation experiments using E. coli J53 (sodium azide resistant) as the recipient strain were carried out using the filter mating method (Yang et al., 2021). Transconjugants were screened on Mueller–Hinton (MH) agar plates containing sodium azide (100 mg/L) and meropenem (2 mg/L). The identity of putative transconjugants was confirmed via PCR and MALDI-TOF MS.
Stability experiments of plasmids carrying blaNDM-1, blaOXA-58, or blaPER-1 genes
A. johnsonii AYTCM strain was grown overnight at 37°C in 2 mL of Luria broth (LB) without antibiotics, followed by serial passage of 2-µL overnight culture into the 2-mL LB (1:1,000) each day, with a yield 10 generations, lasting for 7 days (Tian et al., 2022). On the last day, samples were collected and streaked onto antibiotic-free MHA plates. Colonies were selected randomly, and the presence of blaNDM-1, blaOXA-58, or blaPER-1 genes was confirmed by PCR with specific primers.
Whole-genome sequencing, assembly, quality control, and annotation
Genomic DNA was extracted from A. johnsonii AYTCM strain using Qiagen Mini Kit (Qiagen, Germany) and Gentra® Puregene® Yeast/Bact. Kit (Qiagen, Germany) for Illumina and Nanopore sequencing, respectively. For trimming, quality control, and quality assessment of raw reads, fastp v 0.20.1 was used (Chen et al., 2018). De novo assembly of the reads of Illumina and MinION was constructed using Unicycler v0.4.8 (Wick et al., 2017). The assembly sequence was assessed via QUAST v 5.0.2 (Gurevich et al., 2013). Genome sequence annotation was conducted using the National Center for Biotechnology Information (NCBI) Prokaryotic Genome Annotation Pipeline (PGAP) (http://www.ncbi.nlm.nih.gov/genome/annotation_prok/) and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST) server (Overbeek et al., 2014; Tatusova et al., 2016). Annotation function was further compared with A. johnsonii C6 (accession no. FUUY00000000) and MB44 (accession no. LBMO00000000) strains (Tian et al., 2016; Kaas et al., 2017).
Bioinformatics analysis
Antimicrobial resistance genes were identified using the ABRicate program (https://github.com/tseemann/abricate) based on the ResFinder database (http://genomicepidemiology.org/) (Zankari et al., 2012). Bacterial virulence factors were identified using the virulence factor database (VFDB, http://www.mgc.ac.cn/VFs/) (Liu et al., 2022a). Average nucleotide identity (ANI) analysis with A. johnsonii C6 (accession no. FUUY00000000) and MB44 (accession no. LBMO00000000) strains was conducted using an ANI calculator (http://enve-omics.ce.gatech.edu/ani/index) (Luis and Konstantinos, 2016), and genome-based phylogenetic reconstruction with A. johnsonii, A. baumannii, A. pittii, and A. seifertii strains was further performed using the BacWGSTdb server (Marquez-Ortiz et al., 2017; Feng et al., 2021). Insertion sequences (ISs) were identified with ISfinder (Siguier et al., 2006). Conjugation transfer elements, including the origin site of DNA transfer (oriT), type IV secretion system (T4SS), type IV coupling protein (T4CP), and relaxase-related encoding genes, were predicted using oriTfinder with default parameter settings (Li et al., 2018). PHAge Search Tool (PHAST) was utilized for the prediction of bacteriophages (Zhou et al., 2011). Typing of plasmids was performed based on a previous description (Lam et al., 2023). The plasmid structure was visualized using DNAPlotter (https://www.sanger.ac.uk/tool/dnaplotter/) (Carver et al., 2009). Plasmid comparisons were conducted using the Circoletto tool (http://tools.bat.infspire.org/circoletto/) (Darzentas, 2010). Similar plasmids in Acinetobacter spp., Providencia rettgeri, and Klebsiella pneumoniae were tracked using the BacWGSTdb server (Marquez-Ortiz et al., 2017; Feng et al., 2021).
Results
Genome annotations and subsystem categories
Genome was annotated using PGAP and RAST. Based on PGAP annotation, there are 3,980 genes in total, of which 3,731 are protein-coding genes, 136 are pseudo genes, and the remaining 113 are predicted RNA-coding genes. Compared with the PGAP server, 4,182 genes, including 109 RNA-coding genes, belonged to 293 subsystems when annotated using RAST. The statistics of the subsystem is shown (Figure 1). Most of them belonged to metabolism (427), amino acids and derivatives (252), and carbohydrates (130). Additionally, 14 CDS were sorted into “Phages, transposable elements, plasmids” and only 2 and 1 CDS belonged to “cell division and cell cycle” and “dormancy and sporulation,” respectively. Functional comparison showed that most subsystems were metabolism among three A. johnsonii strains. However, a huge difference was found in “Phages, Prophages, Transposable elements, Plasmids”. There are two CDS that belonged to “Phages, Prophages, Transposable elements, Plasmids” in A. johnsonii C6 and MB44 strains. However, 14 subsystems of this function were identified in A. johnsonii AYTCM strain.
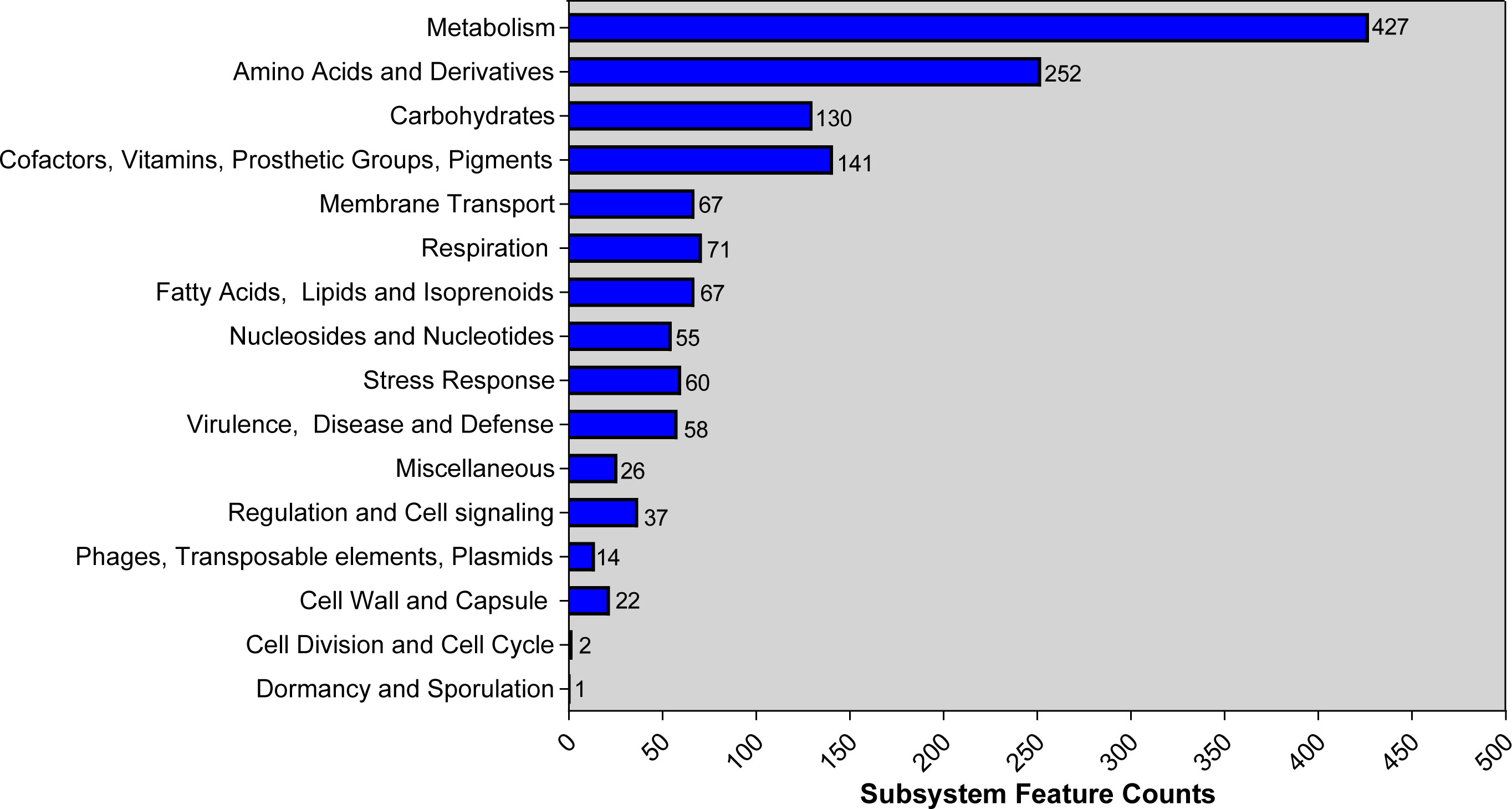
Figure 1 RAST annotation of A. johnsonii AYTCM strain. The number of each subsystem category is shown on the right of column.
MICs, antimicrobial resistance, and virulence profiles
Antimicrobial susceptibility testing revealed that A. johnsonii AYTCM strain possessed a multidrug-resistant (MDR) profile and the meropenem and imipenem MICs are all >128 mg/L. Furthermore, it exhibited resistance to ceftazidime (>128 mg/L), ciprofloxacin (>32 mg/L), and cefoperazone/sulbactam (128 mg/L) but still remained susceptible to tigecycline (1 mg/L) and cefiderocol (<0.03 mg/L). The MICs of colistin and amikacin are 2 mg/L and 32 mg/L, respectively, which were defined as intermediate.
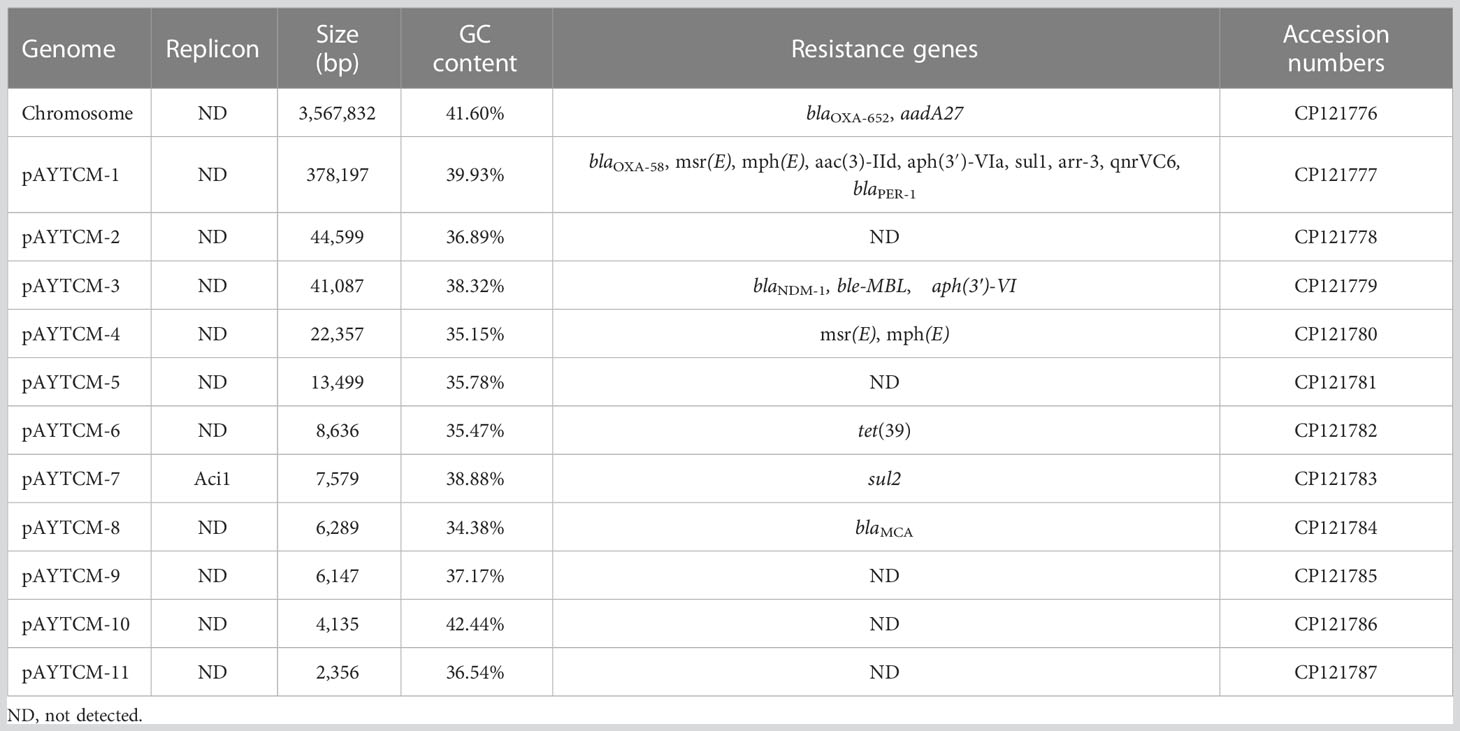
Analysis of the genome of A. johnsonii AYTCM strain revealed that, in addition to co-harboring chromosomal blaOXA-652 and aadA27, a series of other antibiotic resistance genes were identified, including blaOXA-58, blaNDM-1, blaPER-1, msr(E), mph(E), aac(3)-IId, aph(3′)-VIa, sul1, arr-3, qnrVC6, ble-MBL, aph(3′)-VI, tet(39), sul2, and blaMCA (Table 1). However, only two virulence factors, two-component regulatory system bfmRS involved in Csu expression and lpxC-encoding lipopolysaccharide (LPS), were found in AYTCM strain.
ANI, core-genome phylogeny, lipooligosaccharide outer core, and capsular polysaccharide (KL)
According to the ANI analysis, the result showed that 95.82% two-way ANI between A. johnsonii AYTCM and A. johnsonii C6 and 95.86% ANI were found between A. johnsonii AYTCM and A. johnsonii MB44 and only 79.89% two-way ANI between A. johnsonii AYTCM and A. baumannii ATCC 17978. Core-genome phylogeny analysis showed a close genetic relationship among A. johnsonii AYTCM, C6, and MB44 strains. However, a huge diversity was observed among A. baumannii, A. pittii, and other A. seifertii strains based on the phylogenetic tree (Figure S2A). Similar results of SNP difference are shown in Figure S2B.
Kaptive revealed that AYTCM strain contains OC locus 1c (OCL-1c), matching the 92.01% nucleotide identity. The K locus in A. johnsonii AYTCM strain is KL19, to which it matches with an overall nucleotide identity of 72.75%.
Transfer ability and stability of plasmids in the A. johnsonii AYTCM strain
Mating assays were performed to explore the transfer ability of blaNDM-1, blaOXA-58, and blaPER-1 genes; results showed that only blaNDM-1 could transfer to the recipient strain. The stability assays revealed that all three resistance genes were quite stable even after 70 passages under antibiotics-free conditions.
Genome characterization of the chromosome and 11 plasmids
Hybrid assembly of the short and long reads generated a 3,567,832-bp size circular chromosome with a GC content of 41.60% (Table 1). One intrinsic resistance gene, blaOXA-652, was identified in the chromosome. Of note, A. johnsonii AYTCM strain carries 11 plasmids, namely, pAYTCM-1 to pAYTCM-11, with sizes between 2,356 bp and 378,197 bp and GC contents ranging from 34.38% to 42.44% (Table 1). Apart from pAYTCM-2, pAYTCM-5, pAYTCM-9, pAYTCM-10, and pAYTCM-11, various kinds of resistance genes were found in other plasmids. Analysis of rep genes showed that only pAYTCM-7 possessed one identified name with Aci1.
Genetic context characterization of pAYTCM-1 multidrug-resistant plasmid
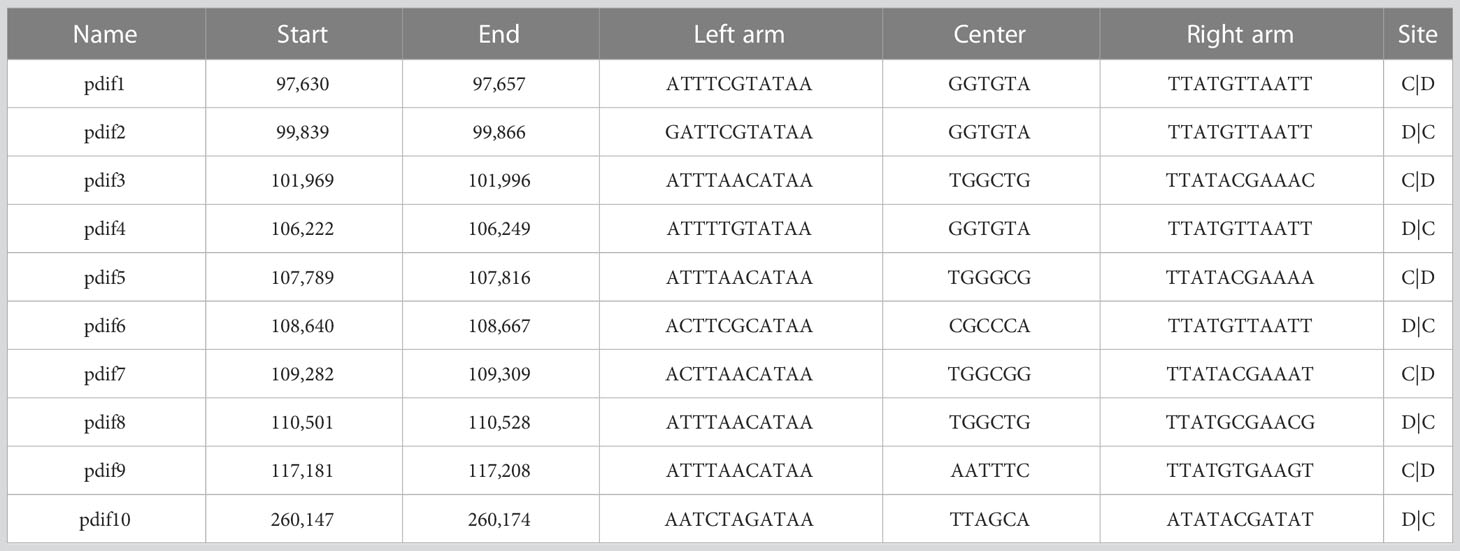
pAYTCM-1 is a huge 378,197-bp multidrug-resistant plasmid with an average GC content of 39.93%. It comprises different regions, including type IV secretion system (T4SS) region, class 1 integron region, and mercury resistance region (Figure 2A). blaOXA-58 and blaPER-1 genes were located in the pAYTCM-1 plasmid. Concerning the genetic context of blaOXA-58, three intact and one truncated ISAjo2 were located upstream or downstream. 9-bp TSD sequences were observed in the upstream and downstream of ISAjo2 genetic elements. Nevertheless, the TSD sequences were all different (Figure 2B). Importantly, a complex class 1 integron complex consisted of a 5′ conserved segment (5′ CS) and 3′ CS, which was found to carry sul1, arr-3, qnrVC6, and blaPER-1 cassettes (Figure 2C). Of note, 10 XerC and XerD-like binding sites (pdif sites) were found in the pAYTCM-1 plasmid (Table 2). In addition, no oriT was identified in the pAYTCM-1 plasmid and no transconjugants were obtained via conjugation. Moreover, results of the Circoletto tool showed that there were many similar segments between pAYTCM-1 and pXBB1-9 (GenBank accession number: CP010351) plasmids (Figure 3). Genetic structure comparison revealed that 98% coverage and 99.91% identity were identified between pAYTCM-1 and pXBB1-9 plasmids, which was found in the A. johnsonii XBB1 isolate from a hospital sewage in 2010 in Chengdu, western China.
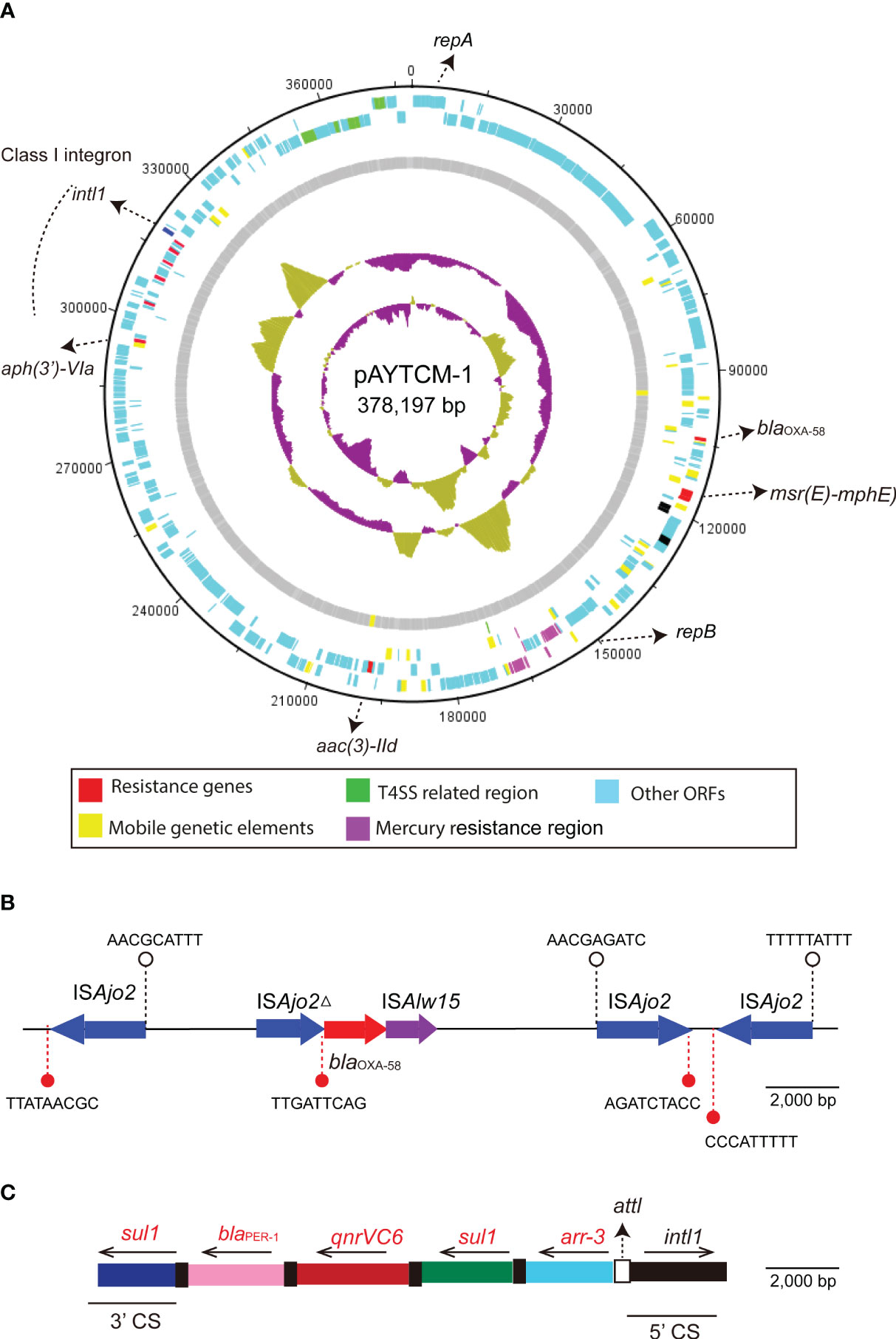
Figure 2 Circular map and genetic environment of the pAYTCM-1 plasmid. (A) Circular map of the pAYTCM-1 plasmid. Different filled boxes indicate various open reading frames (ORFs). The GC content and GC skew are shown in the inner rings. Resistance genes (red filled boxes), mobile genetic elements (yellow filled boxes), T4SS region (green filled boxes), and mercury resistance region (purple filled boxes). Light blue represents other ORFs. (B) Genetic environment of the blaOXA-58 gene. The red filled arrow indicates the position of the blaOXA-58 gene. Blue filled arrows indicate ISAjo2 and ISAjo2Δ. Purple filled arrow indicates ISAlw15. Arrows’ directions indicate the ORF directions. 9-bp target site duplications (TSD) are shown upstream and downstream of ISAjo2 and ISAjo2Δ using white or red filled circles, respectively. (C) Structure of the class 1 integron containing blaPER-1. intl1 is shown as a black filled box. attl is shown as a white filled box. The 5′ conserved segment (5′ CS) and 3′ CS of class 1 integron are labeled. The various kinds of resistance genes were shown as different colors with the names labeled above with the orientation indicated by thin black arrows.
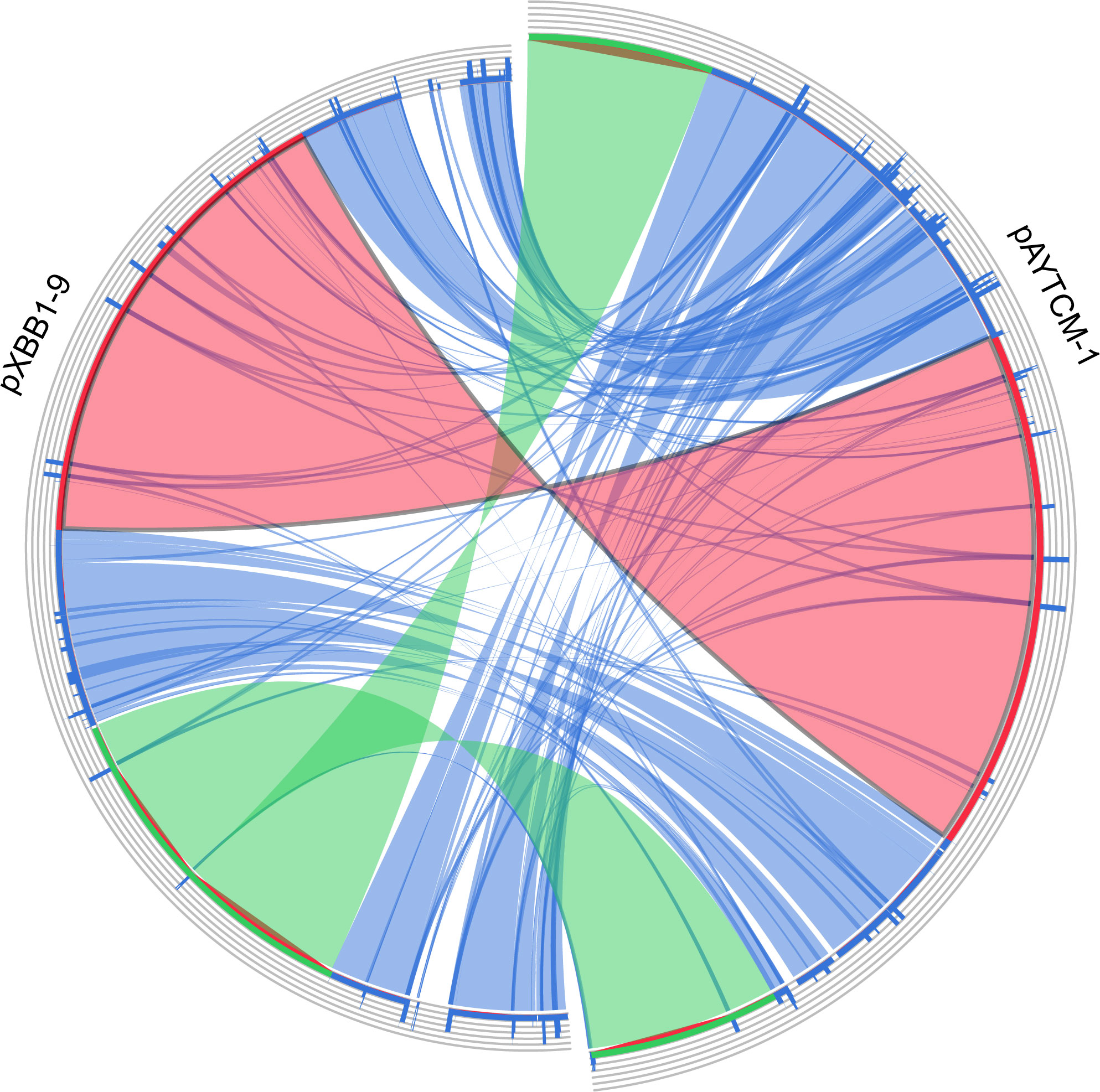
Figure 3 Plasmid comparison with pXBB1-9 using Circoletto. Ribbons represent the alignments produced by BLAST, their width the alignment length, and the colors the alignment bitscore in four quartiles: blue for the first 25% of the maximum bitscore, green for the next 25%, orange for the third, and finally red for the top bitscores of between 75% and 100%.
Genetic features of blaNDM-1-carrying plasmid pAYTCM-3
The blaNDM-1 carbapenem gene was located in 41,087 plasmids with the GC content of 38.32%. Genetic context analysis revealed that blaNDM-1 was ISAba14-aph(3′)-VI-ISAba125-blaNDM-1-ble-MBL (Figure 4). Moreover, a T4SS region, T4CP, a gene encoding relaxase, and a 38-bp oriT region (AGGGATTCATAAGGGAATTATTCCCTTATGTGGGGCTT) were identified. pAYTCM-3 could transfer to E. coli J53 via conjugation.
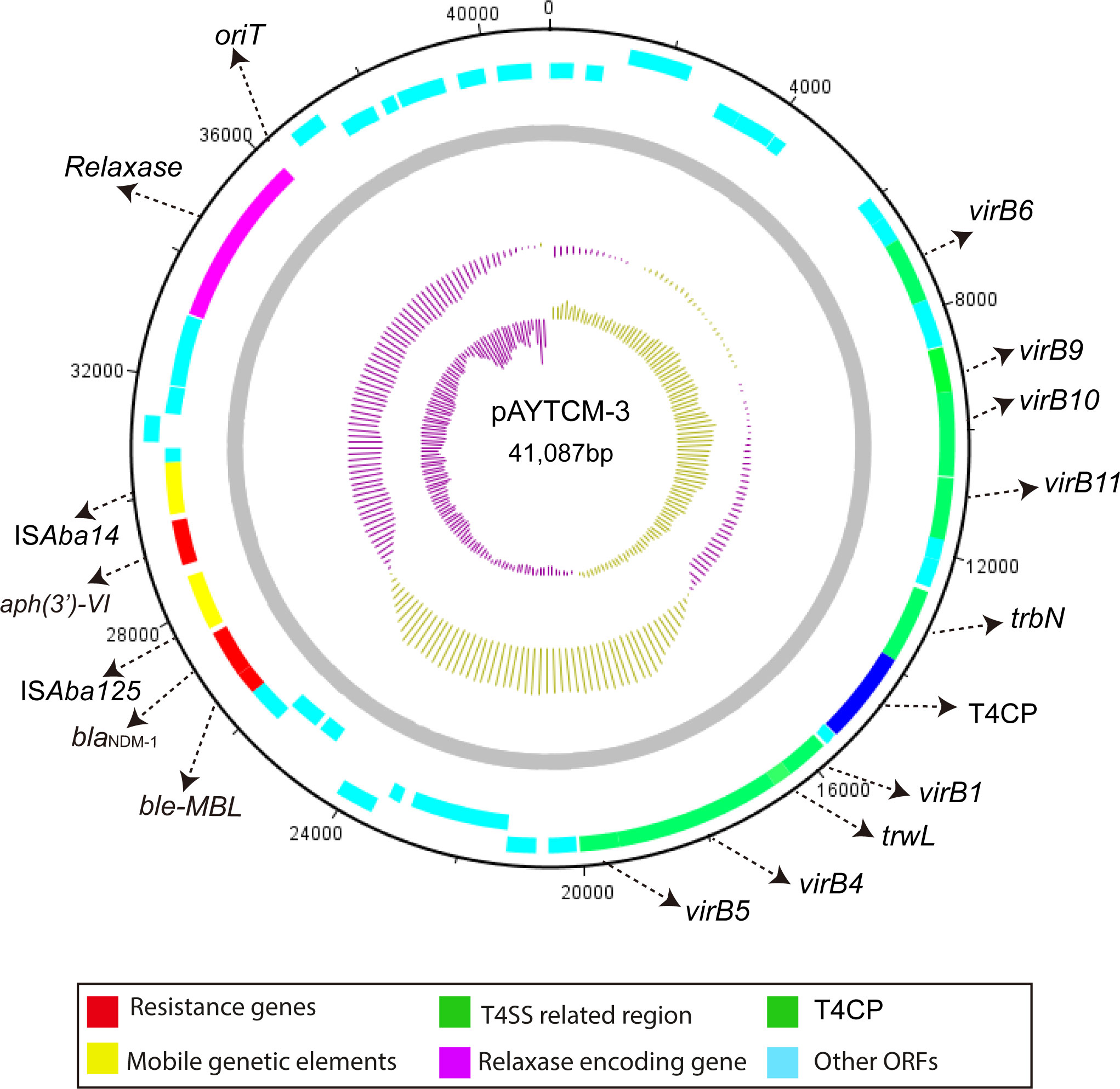
Figure 4 Circular map of pAYTCM-3. Different filled boxes indicate various open reading frames (ORFs). The GC content and GC skew are shown in the inner rings. Resistance genes are shown as a red filled box. Mobile genetic elements are shown as a yellow filled box. The T4SS region and T4CP are indicated as a green filled box. The relaxase-encoding gene is shown in the purple filled box. Light blue represents other ORFs. Moreover, the position of oriT was also labeled.
Prophage regions in the chromosome
Prophage regions were predicted by the PHASTER tool; results showed two intact, two questionable, and two incomplete regions in the chromosome (Figure 5A). Based on the PHASTER tool, regions 4 and 5 were predicted to be intact due to the score of >90. In addition, regions 1 and 6 were classified as questionable due to the scores of 70–90. However, regions 2 and 3 were shown as incomplete due to the low scores. Gene functions of the two intact and two questionable prophage regions are shown, including attachment, phage integration, and cell lysis (Figure 5B).
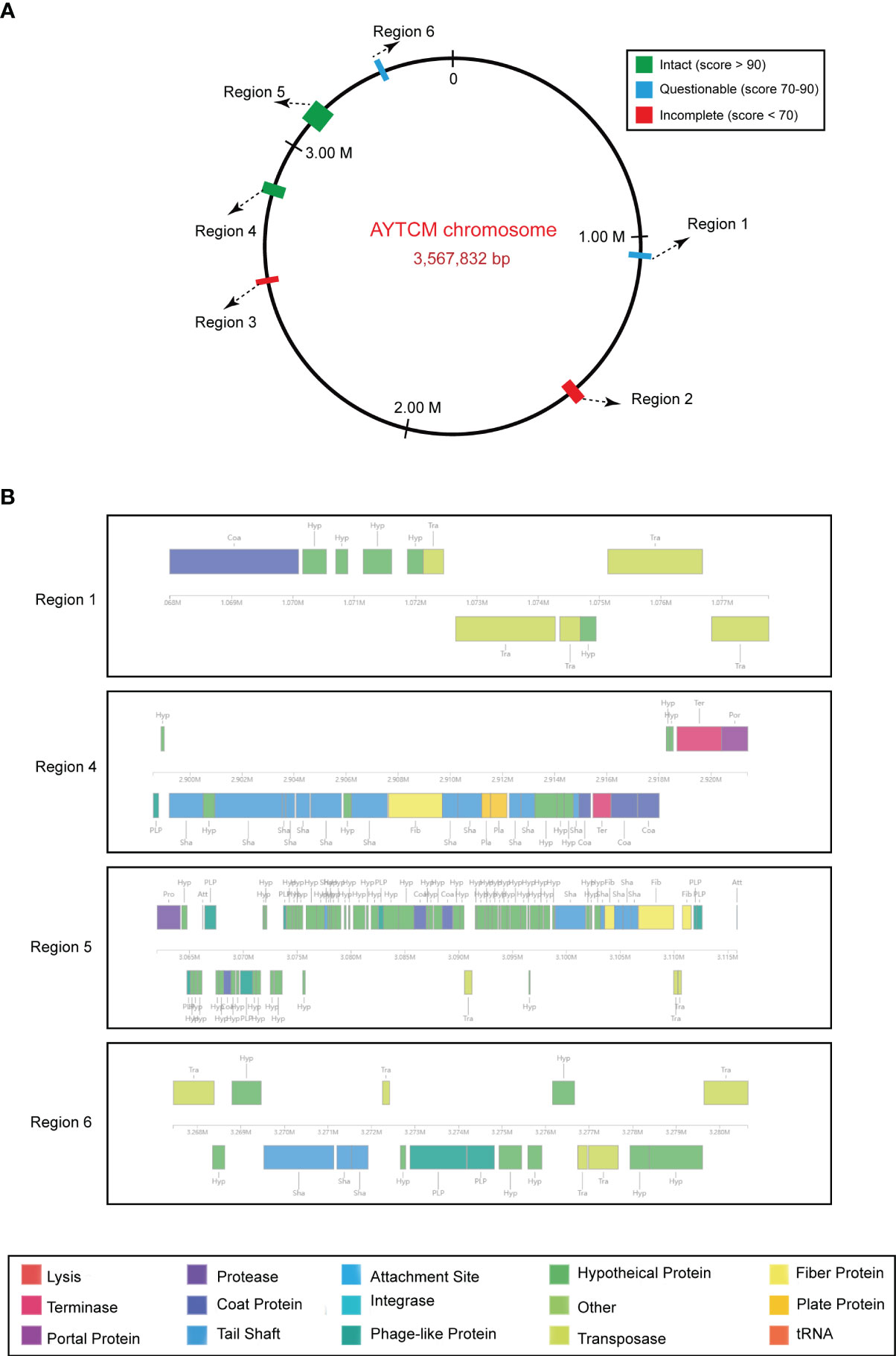
Figure 5 Predicted prophage regions within the A johnsonii AYTCM chromosome. (A) Six prophage regions positions in the chromosome. Green filled boxes mean the intact prophage regions (score > 90), filled boxes mean the questionable prophage regions (score 70–90), filled boxes mean the incomplete prophage regions (score < 70). (B) Structure of two intact and two questionable prophage regions. Genes are colored based on predicted functions.
Track and characteristics of closely related plasmids in the public database
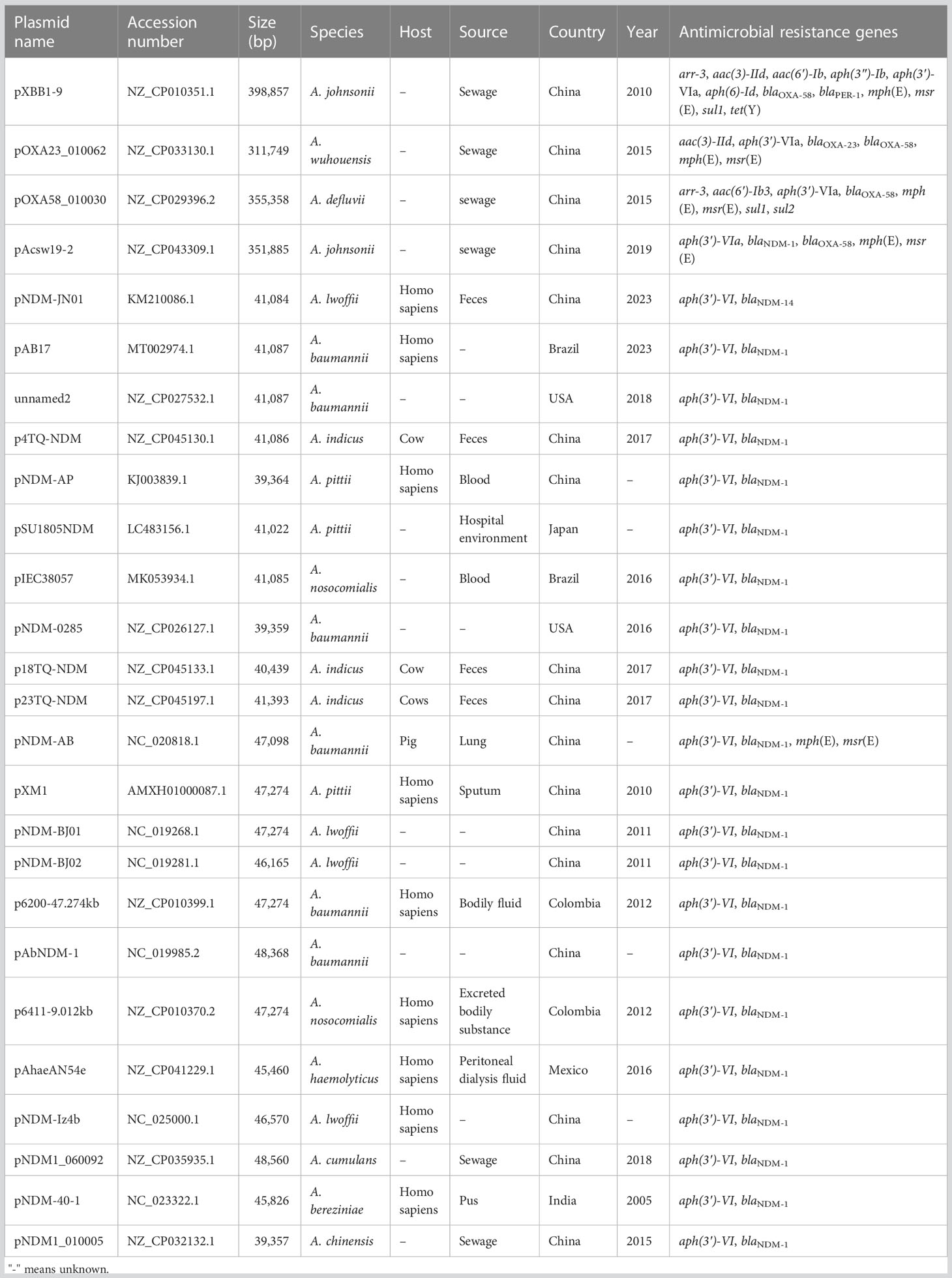
To track the closely related plasmids from different countries, a wide search was performed via the BacWGSTdb server. Data showed that pAYTCM-1 was similar to pXBB1-9, pOXA23_010062, pOXA58_010030, and pAcsw19-2 plasmids (Table 3). Their sizes are all >300 kb, and they were collected from the strains of sewage in China. However, the species were various, including A. johnsonii, A. wuhouensis, and A. defluvii.
Concerning the closely related plasmids of pAYTCM-3, results showed that hosts, also carrying the blaNDM-1-related plasmid, were collected from several different sources, including feces, blood, sputum, pus, sewage, and hospital environment, from 2005 to 2023. These blaNDM-1-harboring plasmids were all collected in Acinetobacter spp. and not in P. rettgeri and K. pneumoniae. Their hosts were isolated from various countries, such as China, USA, Japan, Brazil, and Mexico.
Discussion
Emergence of carbapenemase-producing Acinetobacter spp. has become dominant in several countries, and it is being increasingly considered a quite important nosocomial pathogen and poses a huge challenge to the healthcare setting (Mohd Rani et al., 2017). Class D β-lactamases (mainly OXA-23), commonly named as OXA, are responsible for carbapenem resistance in Acinetobacter spp. species (Zong et al., 2020). However, the reports of other β-lactamases (e.g., NDM-1) are relatively rare, especially for those with a high resistance level. In this work, NDM-1 and OXA-58 were found in our strain, which leads to a high-level carbapenem resistance. To promote better understanding regarding the genomic features of our A. johnsonii strain, whole-genome sequencing and further RAST software were used to classify the different CDS into subsystems based on their function. Consistent with other A. pittii strains, the majority of the CDS belong to the function of “Metabolism” (Chapartegui-Gonzalez et al., 2022).
Mobile genetic elements (MGEs), including ISs, integrons, and transposons, play a particularly important role in the movement and dissemination of resistance genes (Gorbunova et al., 2021). Concerning the acquisition of the blaOXA-58 gene, many copies of ISAjo2 were identified in the pAYTCM-1 plasmid and located upstream and downstream of blaOXA-58. Nevertheless, considering the various 9-bp TSD sequences of ISAjo2, we failed to find direct evidence to conclude that blaOXA-58 was embedded into the plasmid via different ISAjo2. Interestingly, XerC/XerD-like recombinase sites (pdif sites) were considered as a new approach for the transfer of carbapenem resistance genes, such as blaOXA-24, blaOXA-72, and blaOXA-58 (Merino et al., 2010; Kuo et al., 2016; Liu et al., 2021). Here, 10 pdif sites were identified in the pAYTCM-1 plasmid. Furthermore, we observed that the blaOXA-58 gene was flanked by two pdif sites. Consequently, the blaOXA-58 gene might have been introduced by pdif site-mediated specific recombination. This is consistent with previous research (Feng et al., 2016). Moreover, considering the high coverage and identity with pXBB1-9 (Feng et al., 2016), we deduced that the pAYTCM-1 plasmid may come from a hospital environment-related A. johnsonii isolate XBB1 strain and underwent slight evolution. Another finding in this study is that blaPER-1 was also located in the pAYTCM-1 plasmid. Liu et al. reported that the production of PER-1 in A. baumannii is the key mechanism of cefiderocol resistance (Liu et al., 2022b). However, the MIC of cefiderocol was low and considered as susceptible in our A. johnsonii AYTCM strain. We inferred that the resistance in A. baumannii was caused by species specificity.
In our previous study, we reported that blaNDM-1 was located in the chromosome, which was mediated by two ISAba125-based Tn125 composite transposons, highlighting the importance of Tn125-mediated transfer of blaNDM-1 resistance determinants (Tian et al., 2022). However, we could not find the composite Tn125 transposon in the A. johnsonii AYTCM strain due to that only one copy of ISAba125 was identified. In addition, two studies from Krahn et al. and Abouelfetouh et al. showed that prophages may play a key role in the carbapenem resistance genes, such as blaNDM-1 and blaOXA-23 (Krahn et al., 2016; Abouelfetouh et al., 2022). In addition, a study demonstrated the presence of resistance genes (including mcr-1 and vanA) in the phage fraction and its role on the acquisition and transfer of these resistance genes (Pires et al., 2023). However, the blaNDM-1 gene is not part of any of the prophages. Hence, the relationship of these prophages and the blaNDM-1 gene should be further confirmed through induced experiments. Concerning the blaNDM-1-harboring plasmids, we discovered that they were located in diverse sources and hosts and in various countries. These data indicated that a wide spread of blaNDM-1-bearing plasmids has occurred all over the world. However, these plasmids usually transferred among different Acinetobacter species. Concerning the various resistance plasmids in A. johnsonii AYTCM strain, it is revealed that our strain has great potential to capture plasmids that contribute to its resistance. Since our strain is of patient origin, there may be a great possibility that this strain will emerge and further spread between patients and the environment in the hospital. More importantly, Lam et al. reported that the Aci1 plasmid usually was found in extensively and pan-resistant A. baumannii isolates which belong to global clones GC1 and GC2 (Lam et al., 2023). Here, the Aci1 plasmid has been identified in A. johnsonii strain, further suggesting that the Aci1 plasmid has transferred among various Acinetobacter species.
Apart from resistance determinants, virulence factors should also be paid attention in bacteria. However, the low content of virulence factors in A. johnsonii AYTCM strain is in clear contrast to the high number of resistance genes. Thus, in the surveillance of A. johnsonii, researchers should probably pay more attention to the antimicrobial resistance when compared with virulence. This is a different aspect from the hypervirulent carbapenem-resistant K. pneumoniae (Pu et al., 2023).
Conclusion
This study is the first comprehensive description for the complete genome characteristics of a carbapenem-resistant A. johnsonii, co-producing NDM-1, OXA-58, and PER-1 from a patient source. The A. johnsonii isolate AYTCM carried 11 plasmids, which revealed great genome plasticity for this species, which possesses huge potential to capture resistance plasmids. Moreover, the Aci1 plasmid has been identified in A. johnsonii strain using the current plasmid typing system. However, other eight plasmids failed to type. Therefore, the rep genes for the plasmid typing system need to be further explored. Early surveillance of this kind of carbapenem-resistant isolate is warranted to avoid the extensive spread of this high-risk clone in the healthcare setting.
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://www.ncbi.nlm.nih.gov/; PRJNA953498.
Ethics statement
This study was approved by the local Ethics Committees of the Hospital with a waiver of informed consent since this study mainly focused on bacterial genome and the retrospective nature of the study.
Author contributions
CT and JS designed the experiments, analyzed the data, and wrote the initial manuscript. CT, LR, DH, SW, LF, YZ, and YB performed the majority of the experiments. JS collected the bacteria. XF, TM, and JY supervised this study and reviewed and edited the paper. All authors read and approved the final version of the manuscript.
Funding
This work was supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2023KY1270, 2022RC278); Natural Science Foundation of Zhejiang Province (LQ19H160002), Quzhou technology projects, China (2019K36); and Zhejiang Province Traditional Chinese Medicine Science and Technology Project (2023ZL729).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1227063/full#supplementary-material
References
Abouelfetouh, A., Mattock, J., Turner, D., Li, E., Evans, B. A. (2022). Diversity of carbapenem-resistant Acinetobacter baumannii and bacteriophage-mediated spread of the Oxa23 carbapenemase. Microb. Genom 8. doi: 10.1099/mgen.0.000752
Behzadi, P., Gajdacs, M. (2021). Writing a strong scientific paper in medicine and the biomedical sciences: a checklist and recommendations for early career researchers. Biol. Futur. 72, 395–407. doi: 10.1007/s42977-021-00095-z
Bonnin, R. A., Docobo-Perez, F., Poirel, L., Villegas, M. V., Nordmann, P. (2014). Emergence of OXA-72-producing Acinetobacter pittii clinical isolates. Int. J. Antimicrob. Agents 43, 195–196. doi: 10.1016/j.ijantimicag.2013.10.005
Carver, T., Thomson, N., Bleasby, A., Berriman, M., Parkhill, J. (2009). DNAPlotter: circular and linear interactive genome visualization. Bioinformatics 25, 119–120. doi: 10.1093/bioinformatics/btn578
Castanheira, M., Mendes, R. E., Gales, A. C. (2023). Global epidemiology and mechanisms of resistance of Acinetobacter baumannii-calcoaceticus complex. Clin. Infect. Dis. 76, S166–S178. doi: 10.1093/cid/ciad109
Chapartegui-Gonzalez, I., Lazaro-Diez, M., Ramos-Vivas, J. (2022). Genetic resistance determinants in clinical Acinetobacter pittii genomes. Antibiotics (Basel) 11. doi: 10.3390/antibiotics11050676
Chen, S., Zhou, Y., Chen, Y., Gu, J. (2018). fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890. doi: 10.1093/bioinformatics/bty560
Darzentas, N. (2010). Circoletto: visualizing sequence similarity with Circos. Bioinformatics 26, 2620–2621. doi: 10.1093/bioinformatics/btq484
Feng, Y., Yang, P., Wang, X., Zong, Z. (2016). Characterization of Acinetobacter johnsonii isolate XBB1 carrying nine plasmids and encoding NDM-1, OXA-58 and PER-1 by genome sequencing. J. Antimicrob. Chemother. 71, 71–75. doi: 10.1093/jac/dkv324
Feng, Y., Zou, S., Chen, H., Yu, Y., Ruan, Z. (2021). BacWGSTdb 2.0: a one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 49, D644–D650.
Gonzalez-Villoria, A. M., Valverde-Garduno, V. (2016). Antibiotic-resistant Acinetobacter baumannii increasing success remains a challenge as a nosocomial pathogen. J. Pathog. 2016, 7318075. doi: 10.1155/2016/7318075
Gorbunova, V., Seluanov, A., Mita, P., Mckerrow, W., Fenyo, D., Boeke, J. D., et al. (2021). The role of retrotransposable elements in ageing and age-associated diseases. Nature 596, 43–53. doi: 10.1038/s41586-021-03542-y
Gurevich, A., Saveliev, V., Vyahhi, N., Tesler, G. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29, 1072–1075. doi: 10.1093/bioinformatics/btt086
Jia, J., Guan, Y., Li, X., Fan, X., Zhu, Z., Xing, H., et al. (2021). Phenotype profiles and adaptive preference of Acinetobacter johnsonii isolated from Ba River with different environmental backgrounds. Environ. Res. 196, 110913. doi: 10.1016/j.envres.2021.110913
Kaas, R. S., Mordhorst, H., Leekitcharoenphon, P., Dyring Jensen, J., Haagensen, J., Molin, S., et al. (2017). Draft genome sequence of Acinetobacter johnsonii C6, an environmental isolate engaging in interspecific metabolic interactions. Genome Announc 5. doi: 10.1128/genomeA.00155-17
Krahn, T., Wibberg, D., Maus, I., Winkler, A., Bontron, S., Sczyrba, A., et al. (2016). Intraspecies Transfer of the Chromosomal Acinetobacter baumannii blaNDM-1 Carbapenemase Gene. Antimicrob. Agents Chemother. 60, 3032–3040. doi: 10.1128/AAC.00124-16
Kuo, H. Y., Hsu, P. J., Chen, J. Y., Liao, P. C., Lu, C. W., Chen, C. H., et al. (2016). Clonal spread of blaOXA-72-carrying Acinetobacter baumannii sequence type 512 in Taiwan. Int. J. Antimicrob. Agents 48, 111–113. doi: 10.1016/j.ijantimicag.2016.04.020
Lam, M. M. C., Koong, J., Holt, K. E., Hall, R. M., Hamidian, M. (2023). Detection and Typing of Plasmids in Acinetobacter baumannii Using rep Genes Encoding Replication Initiation Proteins. Microbiol. Spectr. 11, e0247822. doi: 10.1128/spectrum.02478-22
Li, X., Xie, Y., Liu, M., Tai, C., Sun, J., Deng, Z., et al. (2018). oriTfinder: a web-based tool for the identification of origin of transfers in DNA sequences of bacterial mobile genetic elements. Nucleic Acids Res. 46, W229–W234. doi: 10.1093/nar/gky352
Liu, X., Lei, T., Yang, Y., Zhang, L., Liu, H., Leptihn, S., et al. (2022b). Structural basis of PER-1-mediated cefiderocol resistance and synergistic inhibition of PER-1 by cefiderocol in combination with Avibactam or Durlobactam in Acinetobacter baumannii. Antimicrob. Agents Chemother. 66, e0082822. doi: 10.1128/aac.00828-22
Liu, H., Moran, R. A., Chen, Y., Doughty, E. L., Hua, X., Jiang, Y., et al. (2021). Transferable Acinetobacter baumannii plasmid pDETAB2 encodes OXA-58 and NDM-1 and represents a new class of antibiotic resistance plasmids. J. Antimicrob. Chemother. 76, 1130–1134. doi: 10.1093/jac/dkab005
Liu, B., Zheng, D., Zhou, S., Chen, L., Yang, J. (2022a). VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res. 50, D912–D917. doi: 10.1093/nar/gkab1107
Luis, M. R., Konstantinos, T. K. (2016). The enveomics collection: A toolbox for specialized analyses of microbial genomes and metagenomes. PeerJ. Prepr 4, e1900v1.
Marquez-Ortiz, R. A., Haggerty, L., Olarte, N., Duarte, C., Garza-Ramos, U., Silva-Sanchez, J., et al. (2017). Genomic epidemiology of NDM-1-encoding plasmids in Latin American clinical isolates reveals insights into the evolution of multidrug resistance. Genome Biol. Evol. 9, 1725–1741. doi: 10.1093/gbe/evx115
Merino, M., Acosta, J., Poza, M., Sanz, F., Beceiro, A., Chaves, F., et al. (2010). OXA-24 carbapenemase gene flanked by XerC/XerD-like recombination sites in different plasmids from different Acinetobacter species isolated during a nosocomial outbreak. Antimicrob. Agents Chemother. 54, 2724–2727. doi: 10.1128/AAC.01674-09
Mohd Rani, F., Ni, A. R., Ismail, S., Alattraqchi, A. G., Cleary, D. W., Clarke, S. C., et al. (2017). Acinetobacter spp. Infections in Malaysia: A review of antimicrobial resistance trends, mechanisms and epidemiology. Front. Microbiol. 8 2479. doi: 10.3389/fmicb.2017.02479
Overbeek, R., Olson, R., Pusch, G. D., Olsen, G. J., Davis, J. J., Disz, T., et al. (2014). The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 42, D206–D214. doi: 10.1093/nar/gkt1226
Pires, J., Santos, R., Monteiro, S. (2023). Antibiotic resistance genes in bacteriophages from wastewater treatment plant and hospital wastewaters. Sci. Total Environ. 892, 164708. doi: 10.1016/j.scitotenv.2023.164708
Pu, D., Zhao, J., Lu, B., Zhang, Y., Wu, Y., Li, Z., et al. (2023). Within-host resistance evolution of a fatal ST11 hypervirulent carbapenem-resistant Klebsiella pneumoniae. Int. J. Antimicrob. Agents 61, 106747. doi: 10.1016/j.ijantimicag.2023.106747
Siguier, P., Perochon, J., Lestrade, L., Mahillon, J., Chandler, M. (2006). ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34, D32–D36. doi: 10.1093/nar/gkj014
Tang, L., Shen, W., Zhang, Z., Zhang, J., Wang, G., Xiang, L., et al. (2020). Whole-Genome Analysis of Two Copies of bla (NDM-1) Gene Carrying Acinetobacter johnsonii Strain Acsw19 Isolated from Sichuan, China. Infect. Drug Resist. 13, 855–865. doi: 10.2147/IDR.S236200
Tatusova, T., Dicuccio, M., Badretdin, A., Chetvernin, V., Nawrocki, E. P., Zaslavsky, L., et al. (2016). NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 44, 6614–6624. doi: 10.1093/nar/gkw569
Tian, S., Ali, M., Xie, L., Li, L. (2016). Draft Genome Sequence of Acinetobacter johnsonii MB44, Exhibiting Nematicidal Activity against Caenorhabditis elegans. Genome Announc 4. doi: 10.1128/genomeA.01772-15
Tian, C., Xing, M., Fu, L., Zhao, Y., Fan, X., Wang, S. (2022). Emergence of uncommon KL38-OCL6-ST220 carbapenem-resistant Acinetobacter pittii strain, co-producing chromosomal NDM-1 and OXA-820 carbapenemases. Front. Cell Infect. Microbiol. 12, 943735. doi: 10.3389/fcimb.2022.943735
Wang, W., Chen, X., Yan, H., Hu, J., Liu, X. (2019). Complete genome sequence of the cyprodinil-degrading bacterium Acinetobacter johnsonii LXL_C1. Microb. Pathog. 127, 246–249. doi: 10.1016/j.micpath.2018.11.016
Wick, R. R., Judd, L. M., Gorrie, C. L., Holt, K. E. (2017). Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PloS Comput. Biol. 13, e1005595. doi: 10.1371/journal.pcbi.1005595
Wong, D., Nielsen, T. B., Bonomo, R. A., Pantapalangkoor, P., Luna, B., Spellberg, B. (2017). Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin. Microbiol. Rev. 30, 409–447. doi: 10.1128/CMR.00058-16
Yang, X., Dong, N., Liu, X., Yang, C., Ye, L., Chan, E. W., et al. (2021). Co-conjugation of virulence plasmid and KPC plasmid in a clinical Klebsiella pneumoniae strain. Front. Microbiol. 12, 739461. doi: 10.3389/fmicb.2021.739461
Zankari, E., Hasman, H., Cosentino, S., Vestergaard, M., Rasmussen, S., Lund, O., et al. (2012). Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 2640–2644. doi: 10.1093/jac/dks261
Zhou, Y., Liang, Y., Lynch, K. H., Dennis, J. J., Wishart, D. S. (2011). PHAST: a fast phage search tool. Nucleic Acids Res. 39, W347–W352. doi: 10.1093/nar/gkr485
Keywords: Acinetobacter johnsonii, carbapenem resistance, NDM-1, OXA-58, PER-1, integron
Citation: Tian C, Song J, Ren L, Huang D, Wang S, Fu L, Zhao Y, Bai Y, Fan X, Ma T and Ying J (2023) Complete genetic characterization of carbapenem-resistant Acinetobacter johnsonii, co-producing NDM-1, OXA-58, and PER-1 in a patient source. Front. Cell. Infect. Microbiol. 13:1227063. doi: 10.3389/fcimb.2023.1227063
Received: 22 May 2023; Accepted: 31 July 2023;
Published: 25 August 2023.
Edited by:
Luis Esau Lopez Jacome, Instituto Nacional de Rehabilitación, MexicoReviewed by:
Ulises Garza-Ramos, National Institute of Public Health, MexicoJossue Mizael Ortiz-Álvarez, National Council of Science and Technology (CONACYT), Mexico
Copyright © 2023 Tian, Song, Ren, Huang, Wang, Fu, Zhao, Bai, Fan, Ma and Ying. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junjie Ying, NjUyODY4MTMzQHFxLmNvbQ==; Tianhong Ma, MTU5OTAzMTIyMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Chongmei Tian
Chongmei Tian Jianqin Song2†
Jianqin Song2† Siwei Wang
Siwei Wang