
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 19 June 2023
Sec. Fungal Pathogenesis
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1216277
This article is part of the Research Topic Diversity and Molecular Diagnostics of Fungi and Oomycetes in Plants View all 18 articles
Phylogenetic and morphological analyses on Rigidoporus were carried out. The genus Rigidoporus (Hymenochaetales, Basidiomycota), typified by R. microporus (Fr.) Overeem. (synonym Polyporus micromegas Mont.), was established by Murrill in 1905. The genus is mainly characterized by annual to perennial, resupinate, effused-reflexed to pileate or stipitate basidiomata with azonate or concentrically zonate and sulcate upper surface, a monomitic to pseudo-dimitic hyphal structure, simple-septate generative hyphae, and ellipsoid to globose basidiospores. Phylogeny on species of the genus is reconstructed with two loci DNA sequences including the internal transcribed spacer regions and the large subunit. Three new species in Rigidoporus are described and illustrated from Asia, and one new combination in the genus is proposed. The main morphological characteristics of the currently accepted species of Rigidoporus are provided.
The genus Rigidoporus Murrill (Hymenochaetales, Basidiomycota), typified by Rigidoporus microporus (Fr.) Overeem. (synonym Polyporus micromegas Mont.), was established by Murrill (1905). This type of species is an important parasite on cultivated tropical trees, widely distributed in the tropical zone (Ryvarden and Melo, 2017). Traditionally, the genus is characterized by a fawn, reddish to dark tube layer, which is very hard in dried material contrasting with corky to waxy consistency of the context, a monomitic or pseudo-dimitic hyphal system with simple-septate, often in part strongly sclerified generative hyphae, ovoid to globose basidiospores, inamyloid and indextrinoid walls of hyphae and spores, and causing a white rot (Murrill, 1905; Pouzar, 1966; Ryvarden and Melo, 2017).
Later, Rigidoporus was often recognized as a confusing genus with Oxyporus (Bourdot & Galzin) Donk, which was established by Donk (1933) for species with similar hyphae and spores but tubes of waxy consistency. Pouzar (1966) treated Oxyporus as a subgenus of Rigidoporus. However, light-colored basidiomata with thick-walled, encrusted, hymenial cystidia are mostly present in species of Oxyporus, while basidiomata with colored pore surface, thick-walled encrusted hyphoid cystidia, and mammillate cystidioles are mostly in Rigidoporus (Ryvarden and Melo, 2017).
Rigidoporus is also morphologically similar to Physisporinus P. Karst., but this similarity is evidently of only a superficial character. Many taxa had been described in Rigidoporus according to morphology (Ryvarden, 1972a; Ryvarden, 1972b; Ryvarden, 1983; Dai, 1998; Núñez and Ryvarden, 1999; Buchanan and Ryvarden, 2000; Núñez et al., 2001; Vampola and Vlasák, 2012; Ryvarden, 2015), but phylogenetic studies demonstrated that they should be classified into Physisporinus belonging to Polyporales (Wu et al., 2017).
Wu et al. (2017) revealed that Rigidoporus belongs to Hymenochaetales Oberw., and Oxyporus was considered as a synonym of Rigidoporus. Currently, Rigidoporus and Oxyporus are merged as one genus based on phylogenetic and morphological studies and absorbed into Oxyporaceae Zmitr. & Malysheva (Zmitrovich and Malysheva, 2014; Wu et al., 2017). Then, Wang et al. (2023) reactivated Rigidoporaceae Jülich and Oxyporaceae as its synonyms. However, only 14 species have been accepted in Rigidoporus based on phylogenetic analyses until now (Wu et al., 2017; Yuan et al., 2020), and many lack molecular data. In this study, a comprehensive study about Rigidoporus is displayed including phylogenetic and morphological analyses. Phylogeny based on a two-gene dataset (ITS + nLSU) is carried out. Three new species that occur in Asia in the genus are described and illustrated, and one new combination in the genus is proposed. The main morphological characteristics of the currently accepted species of Rigidoporus are provided.
The studied specimens are deposited in the fungoria of the Institute of Microbiology, Beijing Forestry University (BJFC) and the Institute of Applied Ecology, Chinese Academy of Sciences (IFP), Museum Vysociny Jihlava, Czech Republic (MJ), herbarium of V.N. Karazin National University, Kharkiv, Ukraine (CWU), the private fungorium of Josef Vlasák (JV), which will be later deposited at the National Museum Prague of Czech Republic (PRM). Morphological descriptions are conducted based on field notes and fungoria specimens. The microscopic analysis refers to Miettinen et al. (2018) and Wu et al. (2022). Sections were studied at a magnification of up to ×1,000 using a Nikon Eclipse 80i microscope and phase contrast illumination. Microscopic features and measurements were made from slide preparations stained with Cotton Blue and Melzer’s reagent. Spores were measured from sections cut from the tubes. To represent variations in the size of spores, 5% of measurements were excluded from each end of the range and are given in parentheses. In the description, KOH = 5% potassium hydroxide, IKI = Melzer’s reagent, IKI− = neither amyloid nor dextrinoid, CB = Cotton Blue, CB+ = cyanophilous in Cotton Blue, CB− = acyanophilous in Cotton Blue, L = arithmetic average of spore length, W = arithmetic average of spore width, Q = L/W ratios, and n = number of basidiospores/measured from given number of specimens. Color terms are recognized from Anonymous (1969) and Petersen (1996).
A Hexadecyl trimethyl ammonium bromide (CTAB) rapid plant genome extraction kit-DN14 (Aidlab Biotechnologies Co., Ltd, Beijing) was used to obtain DNA from dried specimens and to perform the polymerase chain reaction (PCR) according to the manufacturer’s instructions with some modifications (Li et al., 2014; Shen et al., 2019). Two DNA gene fragments, i.e., internal transcribed spacer (ITS) and large subunit nuclear ribosomal RNA gene (nLSU), were amplified using the primer pairs ITS5/ITS4 and LR0R/LR7 (White et al., 1990; Hopple and Vilgalys, 1999) (http://www.biology.duke.edu/fungi/mycolab/primers.htm). The PCR procedure for ITS was as follows: initial denaturation at 95°C for 3 min, followed by 34 cycles of denaturation at 94°C for 40 s, annealing at 54°C for 45 s, and extension at 72°C for 1 min, and a final extension of 72°C for 10 min. The PCR procedure for nLSU was as follows: initial denaturation at 94°C for 1 min, followed by 34 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 1 min, and extension at 72°C for 1.5 min, and a final extension at 72°C for 10 min. All sequences analyzed in this study are listed in Table 1.
In this study, one combined matrix was reconstructed for phylogenetic analysis and a two-gene dataset (ITS + nLSU) was used to determine the phylogenetic position of new species. The sequence alignments and retrieved topologies were deposited in TreeBase (http://www.treebase.org), under accession ID: 30342 (Reviewer access URL: http://purl.org/phylo/treebase/phylows/study/TB2:S30342?x-access-code=32520482eef1e868d55c54893e12e2e3&format=html). Sequences of Exidiopsis calcea KHL 11075, obtained from GenBank, were used as the outgroup (Wu et al., 2017). Phylogenetic analyses were carried out using the approaches of Han et al. (2016) and Zhu et al. (2019). Maximum likelihood (ML) and Bayesian inference (BI) analyses were performed based on the two datasets. The best-fit evolutionary model was selected by hierarchical likelihood ratio tests (HLRTs) and Akaike information criterion (AIC) in MrModeltest 2.2 (Nylander, 2004) after scoring 24 models of evolution in PAUP* v.4.0b10 (Swofford, 2002).
Sequences were analyzed using ML with RAxML-HPC2 through the CIPRES Science Gateway (www.phylo.org; Miller et al., 2009). Branch support (BT) for ML analysis was determined by 1,000 bootstrap replicates. Bayesian phylogenetic inference and Bayesian posterior probabilities (BPPs) were computed with MrBayes v.3.1.2 (Ronquist and Huelsenbeck, 2003). Four Markov chains were run for 1,000,000 generations (two-gene dataset) until the split deviation frequency value was less than 0.01, and trees were sampled every 100 generations. The first 25% of the sampled trees were discarded as burn-in, and the remaining ones were used to reconstruct a majority rule consensus and calculate the BPP of the clades. All trees were viewed in FigTree v.1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/). Branches that received bootstrap supports for ML [≥75% (ML-BS)], and BPP (≥0.95 BPP) were considered as significantly supported. The ML bootstrap (ML) ≥ 50% and BBP (BPP) ≥ 0.90 are displayed on topologies from ML analyses, respectively.
The combined two-gene dataset (ITS + nLSU) included sequences from 98 samples representing 45 taxa. The dataset had an aligned length of 2,244 characters, of which 1,296 (58%) characters were constant, 174 (8%) were variable and parsimony uninformative, and 774 (34%) were parsimony informative. The phylogenetic reconstruction performed with ML and BI analyses for two combined datasets showed similar topology and few differences in statistical support. The best model-fit applied in the Bayesian analysis was GTR + I + G, lset nst = 6, rates = invgamma, and prset statefreqpr = dirichlet (1, 1, 1, 1). Bayesian analysis resulted in a nearly congruent topology with an average standard deviation of split frequencies as 0.005047 to ML analysis, and thus, only the ML tree is provided (Figure 1). The phylogeny reveals that Rigidoporus belongs to Hymenochaetales and Physisporinus belongs to Polyporales. There form three independent lineages in Rigidoporus as new species.
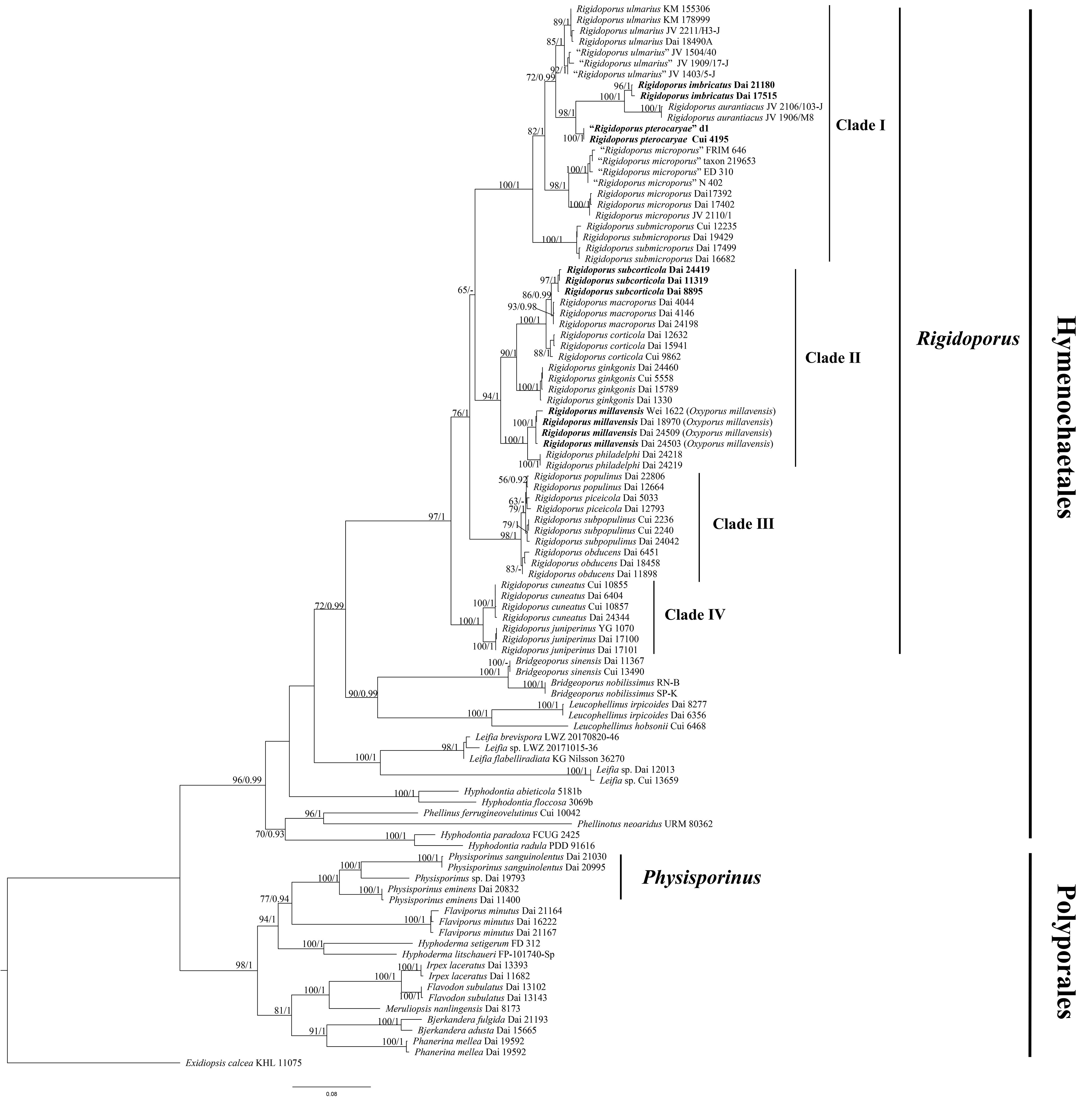
Figure 1 Maximum likelihood (ML) analysis of Rigidoporus based on the dataset of ITS + nLSU. ML bootstrap values higher than 50% and Bayesian posterior probability values more than 0.90 are shown. New taxa are in bold.
Rigidoporus Murrill, Bull. Torrey bot. Club 32(9): 478 (1905) — MycoBank: MB18478
Type species. — Rigidoporus microporus (Sw.) Overeem, Icon. Fung. Malay. 5: 1 (1924).
Synonymy. — Polyporus micromegas Mont., Ann. Sci. Nat., Bot., sér. 2 17: 128 (1842).
Basidiomata annual to perennial, resupinate, effused-reflexed to pileate or stipitate, soft to corky when fresh, becoming soft corky to hard when dry. Pileal surface cinnamon to fawn, glabrous or velutinate, azonate or concentrically zonate and sulcate. Pore surface white, fawn, orange to brown black. Hyphal system monomitic to pseudo-dimitic; generative hyphae simple septate, thin- to thick-walled, sometimes subsolid. Hyphoid or hymenial cystidia mostly present, thin- to thick-walled, usually apically encrusted; mammillate cystidioles sometimes present. Basidiospores ovoid, ellipsoid to globose, hyaline, thin- to thick-walled, sometimes with one big guttule, IKI−, CB− or weakly CB+. Causing a white rot.
Notes. — Rigidoporus is a cosmopolitan genus growth on both angiosperm and gymnosperm woods (Wu et al., 2022). Up to now, 54 taxa in Rigidoporus and 18 taxa in Oxyporus are recorded in Index Fungorum (http://www.indexfungorum.org/), and among them, 18 species have available DNA sequences (Table 2). In this study, three new species are described and illustrated.
Rigidoporus imbricatus Chao G. Wang, Jing Si & Y.C. Dai, sp. nov. — MycoBank: MB848605; Figures 2, 3.
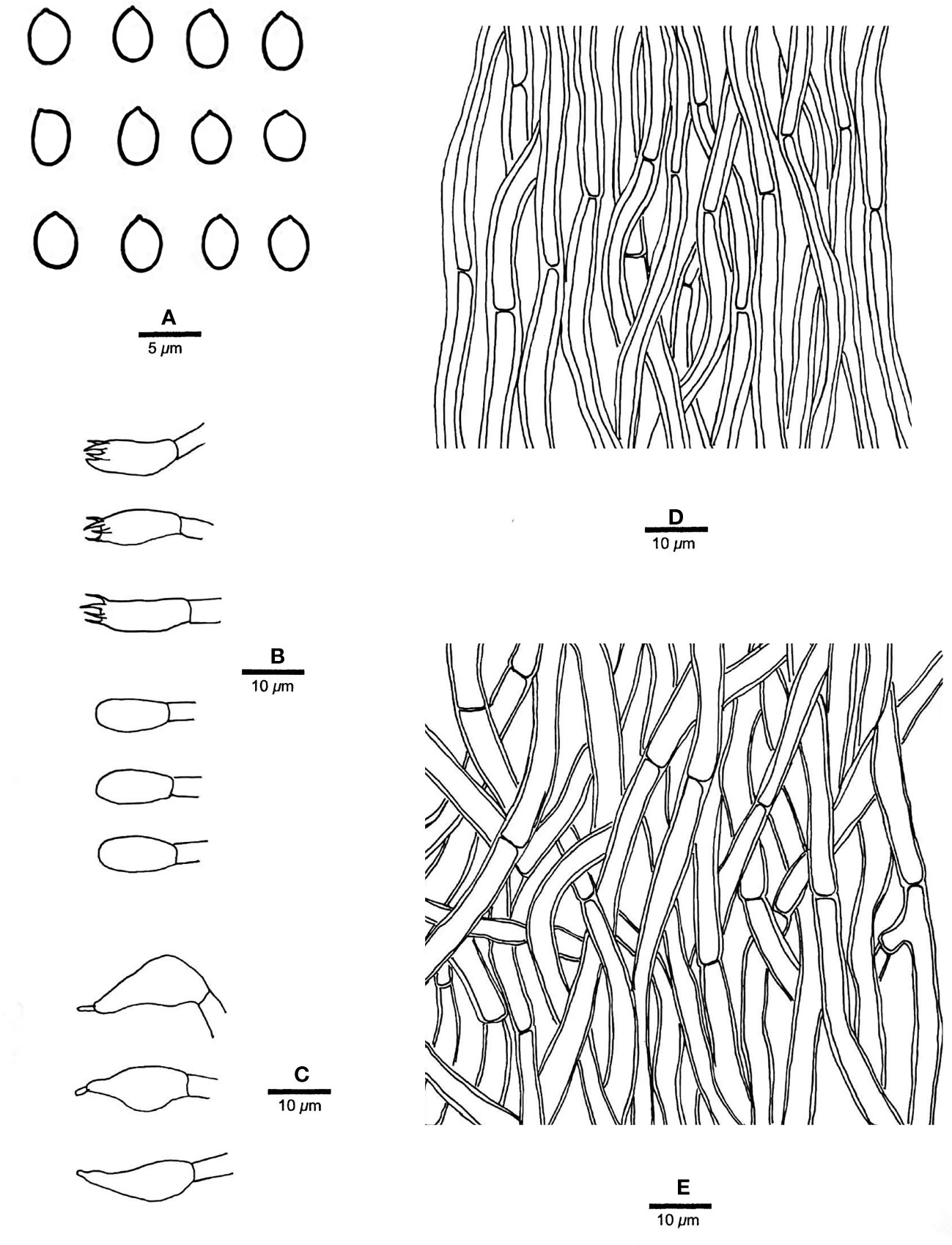
Figure 3 Microscopic structures of R. imbricatus (drawn from the holotype, Dai 17515). (A) Basidiospores; (B) Basidia and basidioles; (C) Cystidioles; (D) Hyphae from trama; (E) Hyphae from context. — Scale bars: a = 5 µm; b−e = 10 µm.
Etymology. — Imbricatus (Lat.), refers to the species having distinct imbricate basidiomata.
Holotype. — China. Yunnan Province, Mengla County, Wangtianshu Nature Reserve, on fallen angiosperm trunk, 18.VI.2017, Dai 17515 (BJFC025047).
Additional specimen examined. — Malaysia, Selangor, Kota Damansara, Community Forest Reserve, on dead angiosperm tree, 7.XII.2019, Dai 21180 (BJFC032834).
Fruiting body. — Basidiomata annual, effused-reflexed to pileate, corky, without odor or taste when fresh, becoming hard corky upon drying, up to 5 cm long, 4 cm wide when resupinate; pilei applanate to flabelliform, projecting up to 6 cm, 12 cm wide and 14 mm thick at base. Pileal surface yellowish brown to brownish orange when fresh, cinnamon to fawn, glabrous, concentrically zonate and sulcate, sometimes tuberculate when dry; margin blunt. Pore surface clay pink to flesh pink when fresh, becoming orange brown upon bruising, eventually honey-yellow to grayish brown when dry; sterile margin distinct, white when fresh, buff yellow when dry, up to 2.5 mm wide; pores round to angular, 7−9 per mm; dissepiments thin, entire to slightly lacerate. Context buff, corky when dry, up to 6 mm thick. Tubes stratified, cream near the context part, concolorous with pore surface near the pores part, hard corky when dry, up to 8 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae simple septate, hyaline to yellowish, smooth, IKI−, CB−; tissues becoming black in KOH.
Context. — Contextual hyphae thick-walled with a wide lumen, rarely branched, rarely simple septate, straight to slightly flexuous, interwoven, 4−6 µm in diam.
Tubes. — Tramal hyphae distinctly thick-walled with a wide lumen, unbranched, straight to slightly flexuous, subparallel along the tubes, strongly agglutinated, 3−5.5 µm in diam. Cystidia absent; cystidioles ventricose with a pointed tip, thin-walled, smooth, 15−17 × 5−8 µm; basidia broadly clavate to barrel-shaped, bearing four sterigmata and a simple basal septum, 12−13 × 5−6 µm; basidioles of similar shape to basidia, but smaller.
Spores. — Basidiospores broadly ellipsoid to subglobose, hyaline, thin-walled, smooth, IKI−, CB−, (3.2−)3.4−4 × (2.7−)2.8−3.2(−3.5) µm, L = 3.64 µm, W = 3.05 µm, Q = 1.17−1.21 (n = 60/2).
Notes. — R. imbricatus is characterized by annual and imbricate basidiomata, concentrically zonate and sulcate upper surface, clay pink to flesh pink pore surface when fresh, ventricose cystidioles, broadly ellipsoid to subglobose, thin-walled basidiospores measuring 3.4−4 × 2.8−3.2 µm, and occurrence in tropical Asia.
R. imbricatus, R. aurantiacus Ryvarden & Iturr., and R. pterocaryae are phylogenetically related (Figure 1) but different in morphology. R. aurantiacus has deep-orange to ochraceous pore surface, globose basidiospores measuring 3 × 4 µm, and occurrence in South America (Ryvarden and Iturriaga, 2003). R. pterocaryae has azonate and smooth upper surface and larger thick-walled basidiospores (6.8−7.2 × 5.8−6.8 µm vs. 3.4−4 × 2.8−3.2 µm).
R, dimiticus (Corner) T. Hatt., R. malayanus (Corner) T. Hatt., and R. pendulus Ryvarden were originally described from Southeast Asia and have effused-reflexed to pileate or stipitate basidiomata. However, R. dimiticus has a dimitic hyphal system and larger basidiospores (4−4.7 × 3.5−4 µm vs. 3.4−4 × 2.8−3.2 µm; Hattori, 2001a); R. malayanus has effused-reflexed basidiomata with azonate upper surface, whitish pore surface, angular pores of 1−3 per mm and larger basidiospores (5−6 × 3−3.5 µm vs. 3.4−4 × 2.8−3.2 µm; Hattori, 2003); R. pendulus has violaceous pore surface when fresh, thick-walled hyphoid cystidia encrusted with crystals and globose basidiospores (3.4−4 µm vs. 3.4−4 × 2.8−3.2 µm; Ryvarden, 1990). Thus, they are all different from R. imbricatus.
Rigidoporus pterocaryae Chao G. Wang, Jing Si & Y.C. Dai, sp. nov. — MycoBank: MB848606; Figures 4, 5.
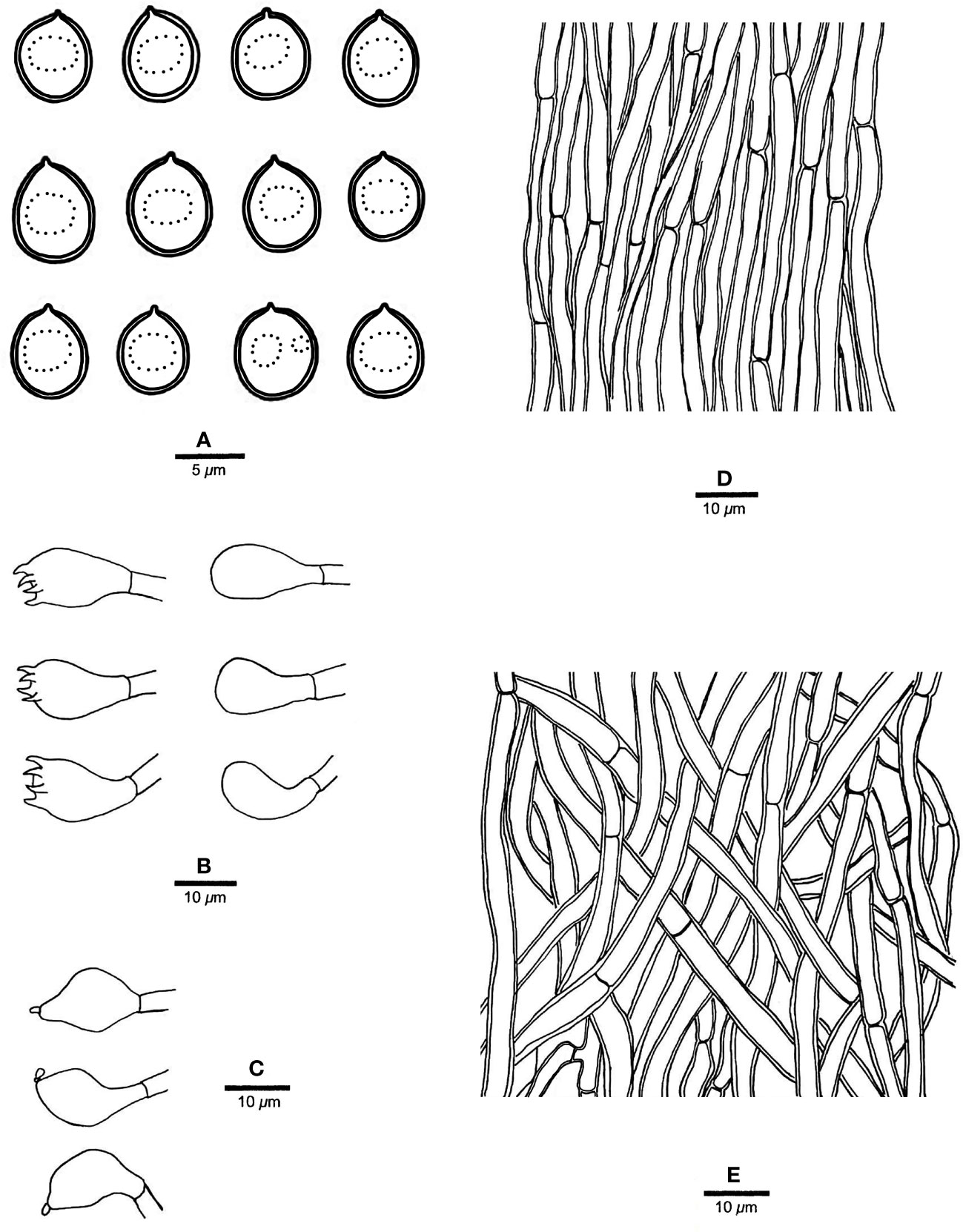
Figure 5 Microscopic structures of R. pterocaryae (drawn from the holotype, Cui 4195). (A) Basidiospores; (B) basidia and basidioles; (C) cystidioles; (D) hyphae from trama; and (E) hyphae from context. — Scale bars: a = 5 µm; b−e = 10 µm.
Etymology. — pterocaryae (Lat.), refers to the species growth on Pterocarya sp.
Holotype. — China. Fujian Province, Wuyishan, Wuyi Palace, on living tree of Pterocarya, 29.VIII.2006, Cui 4195 (BJFC002245).
Fruiting body. — Basidiomata annual, pileate, corky, without odor or taste when fresh, becoming hard corky upon drying. Pilei petaloid, projecting up to 6 cm, 6.5 cm wide and 9 mm thick at base. Pileal surface honey yellow, glabrous, azonate, tuberculate when dry; margin irregularly lobed to slightly petaloid, blunt, slightly recurved when dry. Pore surface buff, honey yellow to fawn when dry, shiny; sterile margin distinct, cream when dry, up to 2.5 mm wide; pores round to angular, 7−9 per mm; dissepiments thin, slightly lacerate. Context curry yellow, corky when dry, up to 4 mm thick. Tubes concolorous with pore surface, hard corky when dry, up to 5 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae simple septate, hyaline to yellowish, smooth, IKI−, CB+; tissues becoming black in KOH.
Context. — Contextual hyphae slightly thick- to thick-walled with a wide lumen, rarely branched, moderately simple septate, straight to flexuous, interwoven, 3−5 µm in diam.
Tubes. — Tramal hyphae slightly thick-walled with a wide lumen, unbranched, straight to slightly flexuous, subparallel along the tubes, agglutinated, 2.5−4 µm in diam. Cystidia absent; cystidioles ventricose with a pointed tip, thin-walled, smooth, 16−17 × 8−9 µm; basidia broadly barrel-shaped, bearing four sterigmata and a simple basal septum, 12−15 × 10−11 µm; basidioles of similar shape to basidia, but smaller. Rhomboid or irregular hyaline crystals present among hymenium.
Spores. — Basidiospores subglobose, hyaline, thick-walled, smooth, with one big or two small guttules, IKI−, CB−, (6.2−)6.8−7.5(−7.8) × (5.5−)5.8−7(−7.2) µm, L = 7.2 µm, W = 6.21 µm, Q = 1.15 (n = 30/1).
Notes. — R. pterocaryae is characterized by annual and pileate basidiomata, petaloid pilei, azonate and smooth upper surface, buff, honey yellow to fawn pore surface when dry, ventricose cystidioles, subglobose, thick-walled basidiospores measuring 6.8−7.5 × 5.8−7 µm, and growth on Pterocarya in China.
R. pterocaryae and R. ulmarius (Sowerby) Imazeki are similar in morphology, and share pileate basidiomata, azonate upper surface, subglobose, thick-walled, and almost the same size of basidiospores (this study). However, the latter has concentrically sulcate upper surface, and phylogenetically, they are distantly related (Figure 1).
One sequence of sample d1 from China was identified as R. ulmarius in GenBank (GenBank accession NO. KC414238). In our phylogenetic analysis, it nested together with R. pterocaryae.
Rigidoporus subcorticola Chao G. Wang, Jing Si & Y.C. Dai, sp. nov. — MycoBank: MB848607; Figures 6, 7.
Figure 7 Microscopic structures of R. subcorticola (drawn from the holotype, Dai 24419). (A) Basidiospores; (B) basidia and basidioles; (C) cystidia and cystidioles; (D) hyphae from trama; and (E) hyphae from context. — Scale bars: a = 5 µm; b−e = 10 µm.
Etymology. — subcorticola (Lat.), refers to the species being similar to R. corticola.
Holotype. — China. Beijing, Xiaolongmen Forest Park, on fallen angiosperm trunk, 30.VIII.2022, Dai 24419 (BJFC039662).
Additional specimens examined. — China. Heilongjiang Province, Ning’an County, Jingpo Lake Forest Park, on Pinus koraiensis, Dai 8895 (IFP 011351); Henan Province, Neixiang County, Baotianman Nature Reserve, on Juglans sp., 23.IX.2009, Dai 11319 (BJFC007465).
Fruiting body. — Basidiomata annual, resupinate to effused-reflexed, soft, without odor or taste when fresh, becoming soft corky upon drying, up to 8 cm long and 2 cm wide when resupinate; pilei flabelliform, projecting up to 1.7 cm, 3 cm wide, 3 mm thick at base. Pileal surface white to cream when fresh, becoming cream to pinkish buff, glabrous, azonate and tuberculate upon drying; margin slightly acute. Pore surface white to cream when fresh, becoming cream to buff yellow upon drying; sterile margin white to cream when fresh, buff when dry, thinning out, somewhat incised, up to 1 mm wide; pores angular, 2−4 per mm; dissepiments thin, lacerate. Context cream, soft corky when dry, up to 0.4 mm thick. Tubes concolorous with pore surface, soft corky when dry, up to 0.8 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae simple septate, hyaline, IKI−, CB+; tissues unchanged in KOH.
Context. — Contextual hyphae thick-walled with a wide lumen, occasionally branched, straight to flexuous, interwoven, 3−6 µm in diam.
Tubes. — Tramal hyphae thick-walled with a wide lumen, occasionally branched, straight to flexuous, subparallel along the tubes, 2−3.5 µm in diam. Cystidia present, arising from tramal hyphae and projecting from the hymenium, thick-walled, apically encrusted with crown crystals, 15−16 × 4.5−5 µm; cystidioles fusoid to ventricose, thin-walled, smooth or encrusted with tiny crystals, 12−15 × 5−6 µm; basidia barrel-shaped, with four sterigmata and a simple basal septum, 14−16 × 5.5−7 µm; basidioles of similar shape to basidia.
Spores. — Basidiospores oblong ellipsoid to ellipsoid, hyaline, thin-walled, smooth, IKI−, weakly CB+, 5−5.8(−6) × 3−4 µm, L = 5.44 µm, W = 3.48 µm, Q = 1.56 (n = 30/1).
Notes. — R. subcorticola is characterized by annual, resupinate to effused-reflexed basidiomata, azonate and tuberculate upper surface, cream to buff yellow pore surface when dry, big angular pores of 2−4 per mm, thick-walled and encrusted hymenial cystidia, fusoid to ventricose cystidioles, oblong ellipsoid to ellipsoid basidiospores measuring 5−5.8 × 3−4 µm, and often occur in the north temperate zone.
R. subcorticola and R. macroporus (Y.C. Dai & Y.L. Wei) F. Wu et al. are closely related in our phylogeny (Figure 1), share cream to buff yellow pore surface, clavate to ventricose cystidioles, and ellipsoid basidiospores, as well as are distributed in China. However, the later has resupinate basidiomata, thin-walled tramal hyphae and larger basidiospores (7−8 × 3.5−4.1 µm vs. 5−5.8 × 3−4 µm; Dai et al., 2004). R. subcorticola and R. corticola (Fr.) Pouzarare are very similar in morphology and share resupinate to effused-reflexed basidiomata with light-colored pore surface and ellipsoid basidiospores (5−6 × 3.5−4.5 µm vs. 5−5.8 × 3−4 µm; Ryvarden and Gilbertson, 1994). However, the latter has slightly zonate and somewhat radially wrinkled upper surface. The three species form different independent lineages in Rigidoporus (Figure 1).
Rigidoporus millavensis (Bourdot & Galzin) Chao G. Wang, Jing Si & Y.C. Dai, comb. et stat. nov. — MycoBank: MB848608; Figure 8.

Figure 8 Basidiomata of two species: (A) R. millavensis (Dai 24509) and (B) R. philadelphi (Dai 24218).
Basionym. — Poria mucida subsp. millavensis Bourdot & Galzin, Bull. trimest. Soc. mycol. Fr. 41(2): 238 (1925)
≡ Oxyporus millavensis (Bourdot & Galzin) Ryvarden & Melo, Syn. Fung. (Oslo) 31: 293 (2014).
Specimens examined. — China. Gansu Province, Zhangye, Qilianshan Nature Reserve, on the dead tree of Juniperus przewalskii, Dai 18970 (BJFC027439); Inner Mongolia, Alxa County, Helanshan Nature Reserve, on the fallen branch of Picea, Dai 24509 (BJFC039751); on the fallen trunk of Picea, Dai 24503 (BJFC039745); Xinjiang, Xinyuan County, Nalati Nature Reserve, on the fallen trunk of Populus euphratica, Wei 1622 (BJFC010351).
Descriptions for one known species and one uncertain species
Rigidoporus microporus — Figures 9 and 10
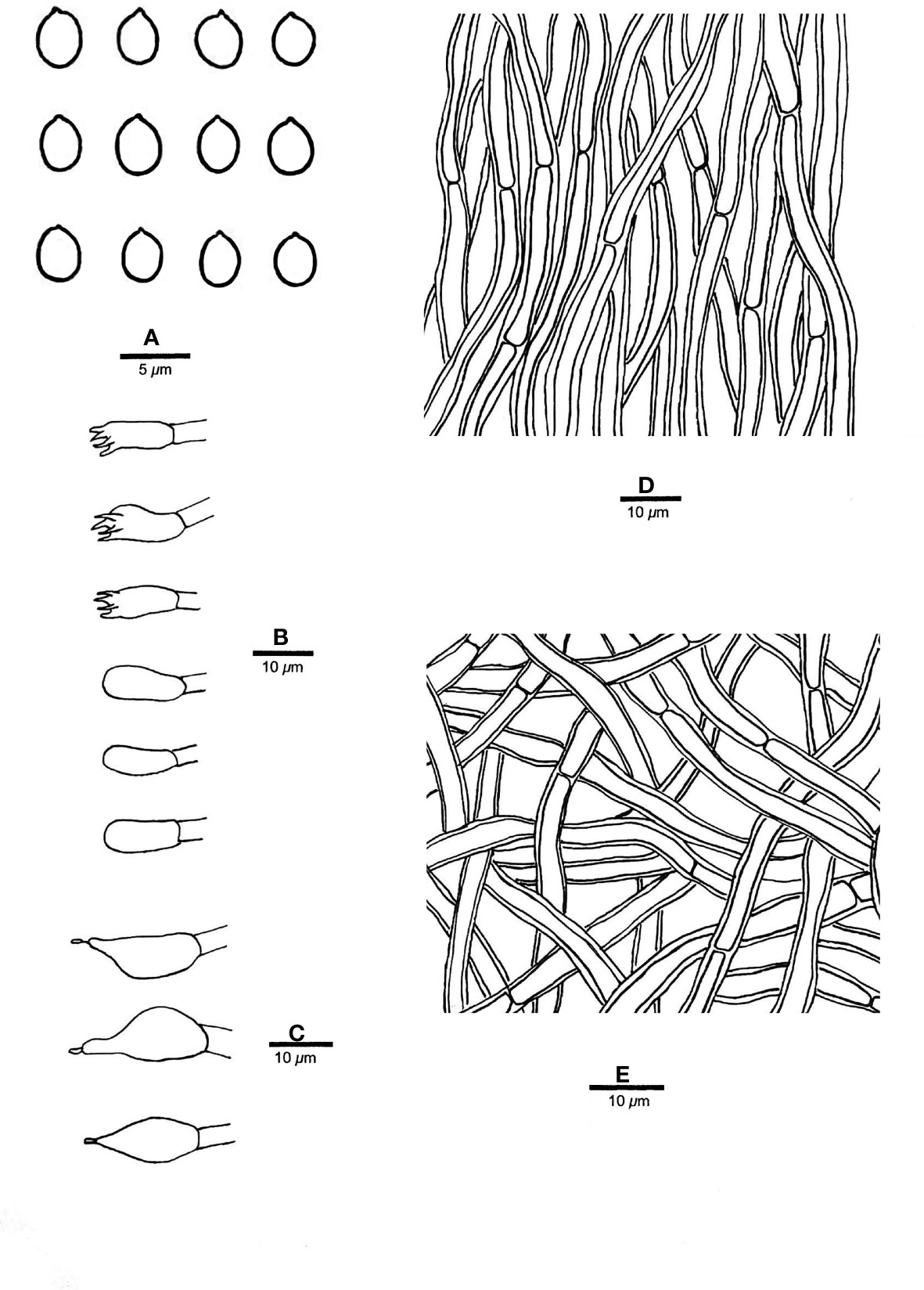
Figure 10 Microscopic structures of R. microporus (drawn from sample Dai 17402). (A) Basidiospores; (B) basidia and basidioles; (C) cystidioles; (D) hyphae from trama; and (E) hyphae from context. — Scale bars: a = 5 µm; b−e = 10 µm.
Specimens examined. — Brazil. Manaus, parque Municipal Cachoeira das Orquídeas, on a fallen angiosperm trunk, 12.V.2017, Dai 17402 (BJFC024937); Dai 17392 (BJFC024928).
Fruiting body. — Basidiomata annual to perennial, resupinate, effused-reflexed to pileate, corky, without odor or taste when fresh, becoming hard corky upon drying, up to 6 cm long, 5 cm wide when resupinate; pilei flabelliform, projecting up to 6 cm, 7.5 cm wide and 4 mm thick at base. Pileal surface cinnamon to fawn, glabrous, concentrically zonate and sulcate when dry; margin acute. Pore surface fawn to reddish brown when dry; sterile margin thinning out, pinkish buff when dry; pores round to angular, 12−14 per mm; dissepiments thin, entire to lacerate. Context buff, corky when dry, up to 1 mm thick. Tubes honey yellow to fawn when dry, paler than pore surface, hard corky when dry, up to 3 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae simple septate, hyaline, smooth, IKI−, weakly CB+; tissues becoming black in KOH.
Context. — Contextual hyphae thick-walled with a wide lumen, unbranched, rarely simple septate, straight to slightly flexuous, interwoven, 3.5−5.5 µm in diam.
Tubes. — Tramal hyphae distinctly thick-walled with a wide lumen, unbranched, straight to slightly flexuous, subparallel along the tubes, strongly agglutinated, 3.5−5 µm in diam. Cystidia absent; cystidioles ventricose with a pointed tip, thin-walled, smooth, 10−15 × 7−8 µm; basidia broadly clavate to barrel-shaped, bearing four sterigmata and a simple basal septum, 10−12 × 4.5−6 µm; basidioles of similar shape to basidia, but smaller. Rhomboid or irregular crystals sometimes present among hymenium.
Spores. — Basidiospores broadly ellipsoid to subglobose, hyaline, thin-walled, smooth, IKI−, CB−, (3.6−)3.8−4.3 × (3−)3.2−3.8(−4) µm, L = 3.98 µm, W = 3.42 µm, Q = 1.16−1.17 (n = 60/2).
“Rigidoporus ulmarius” — Figures 11 and 12
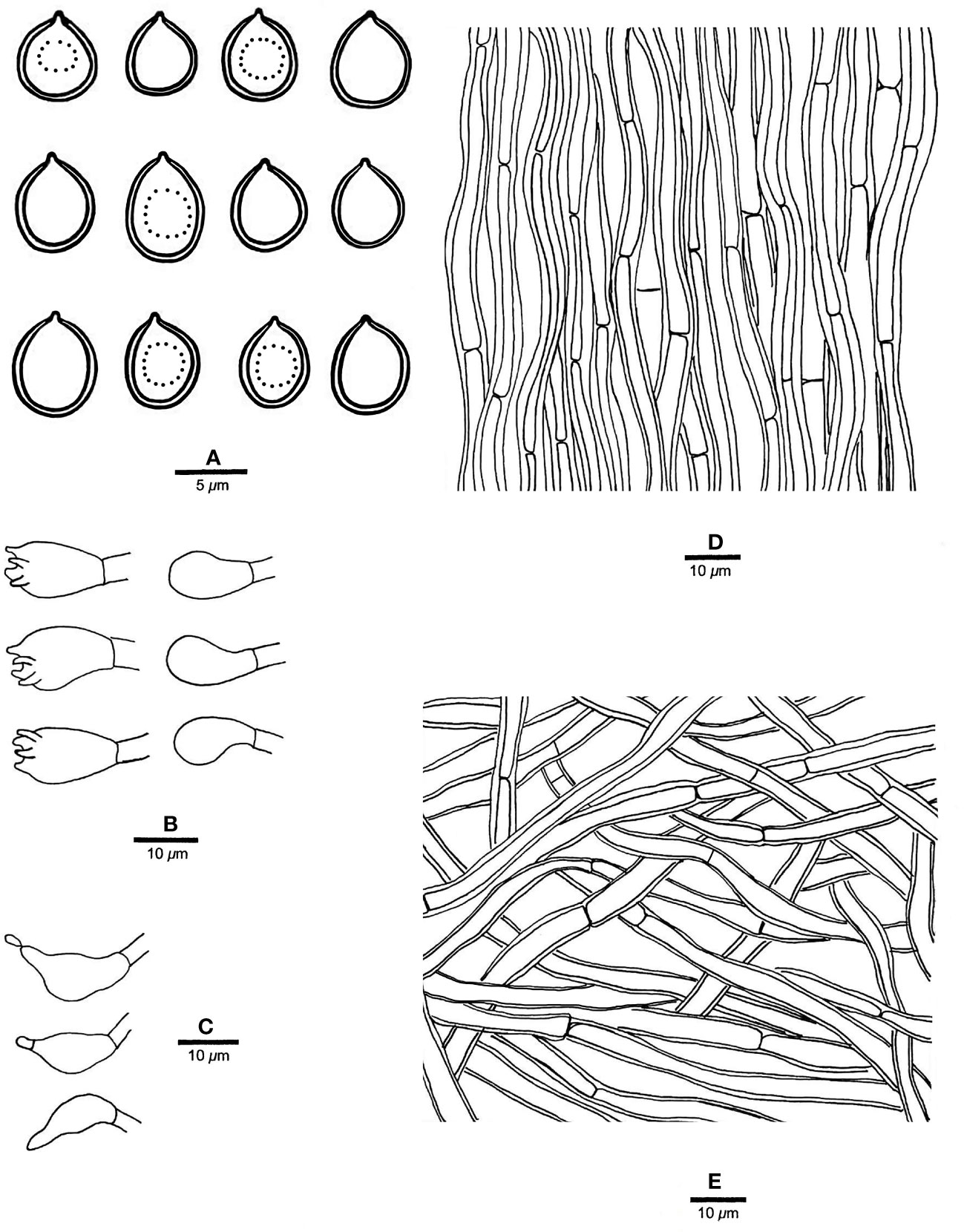
Figure 12 Microscopic structures of “Rigidoporus ulmarius” (drawn from sample JV 1909/17-J). (A) Basidiospores; (B) basidia and basidioles; (C) cystidioles; (D) hyphae from trama; and (E) hyphae from context. — Scale bars: a = 5 µm; b−e = 10 µm.
Specimen examined. — French Guiana. Roura, Amazon Lodge, 2.IX.2019, JV 1909/17-J (JV, isotype in BJFC033010).
Fruiting body. — Basidiomata annual, pileate, corky, without odor or taste when fresh, becoming hard corky upon drying. Pilei flabelliform, projecting up to 8 cm, 15 cm wide and 7 mm thick at base. Pileal surface buff to cinnamon buff, glabrous, azonate, distinctly radially wrinkled to ribbed and slightly squamose when dry; margin acute, incurved when dry. Pore surface clay buff to fawn when dry; sterile margin indistinct, almost absent; pores angular, 8−10 per mm; dissepiments thin, slightly lacerate. Context ochraceous, corky when dry, up to 3 mm thick. Tubes concolorous with pore surface, distinctly deeper-colored than the context, hard corky when dry, up to 4 mm long.
Hyphal structure. — Hyphal system monomitic; generative hyphae simple septate, yellowish, smooth, IKI−, CB−; tissues unchanged in KOH.
Context. — Contextual hyphae thick-walled with a wide lumen, unbranched, moderately simple septate, more or less flexuous, interwoven, 3−6 µm in diam.
Tubes. — Tramal hyphae distinctly thick-walled with a wide lumen, unbranched, flexuous, subparallel along the tubes, 3.5−6 µm in diam. Cystidia absent; cystidioles ventricose with a pointed tip, thin-walled, smooth, 14−17 × 6−8 µm; basidia broadly barrel-shaped, bearing four sterigmata and a simple basal septum, 15−17 × 9−10 µm; basidioles of similar shape to basidia but smaller. Rhomboid or irregular hyaline crystals present among hymenium.
Spores. — Basidiospores subglobose, sometimes collapsed, hyaline, slightly thick-walled, smooth, sometimes with one big guttule, IKI−, CB+, (5.5−)6−7 × 5−6.2(−6.3) µm, L = 6.36 µm, W = 5.66 µm, Q = 1.12 (n = 30/1).
In the present study, 18 species of Rigidoporus with available sequences were divided into four clades. Clade I includes six species, viz., R. aurantiacus Ryvarden & Iturriaga, R. imbricatus, R. microporus, R. pterocaryae, R. submicroporus F. Wu et al., and R. ulmarius (Figure 1), and these species share pileate and hard corky basidiomata with azonate, tuberculate or concentrically zonate, and sulcate upper surface (except R. submicroporus), slightly dark-colored (cinnamon to fawn or reddish brown) pore surface when dry, and sometimes thick-walled basidiospores. R. corticola, R. ginkgonis (Y.C. Dai) F. Wu et al., R. macroporus, R. millavensis, R. philadelphi (Parmasto) Pouzar, and R. subcorticola form a subclade as Clade II (Figure 1), and all have resupinate or resupinate to effused-reflexed, soft corky basidiomata with light-colored (white to cream or buff) pore surface. Clade III is composed of R. obducens (Pers.) Pouzar, R. piceicola (B.K. Cui & Y.C. Dai) F. Wu et al., R. populinus (Schumach.) Pouzar, and R. subpopulinus (B.K. Cui & Y.C. Dai) F. Wu et al. (Figure 1), and species in this clade have resupinate or resupinate to effused-reflexed basidiomata and thick-walled cystidia encrusted with coarse crystals. Clade IV contains two taxa, i.e., R. cuneatus (Murrill) F. Wu et al. and R. juniperinus Gafforov et al., but these two species are not similar in morphology. R. cuneatus has resupinate to effused-reflexed basidiomata with white to isabelline pore surface, angular to irregular pores of 1−3 per mm, thin-walled hymenial cystidia encrusted with crystals, and globose basidiospores measuring 3−5 µm (Yuan et al., 2020). R. juniperinus has resupinate basidiomata with white to pale ochraceous pore surface, round to angular pores of 4−5 per mm, thin-walled hymenial cystidia encrusted with crystals, thick-walled encrusted hyphoid cystidia, and broadly ellipsoid to globose basidiospores measuring 4.2−4.5 × 2.9−3 µm (Yuan et al., 2020). Clades I, II, and III above represent traditional genera Rigidoporus (s. str.), Chaetoporus P. Karst., and Oxyporus Donk, which were however united in Rigidoporus by Wu et al. (2017).
Boletus ulmarius Sowerby was originally described by Sowerby (1797) growing on rotted Ulmus campestris from the UK and then combined as R. ulmarius by Imazeki (1952). It is characterized by perennial, pileate basidiomata with concentrically sulcate or irregularly tuberculate upper surface, pinkish buff to orange brown pore surface when fresh, angular pores of 5−6 per mm, a monomitic hyphal system, fusoid cystidioles, subglobose and thick-walled basidiospores measuring 6−8 × 5−6.5 µm, and mostly growth on angiosperm wood in the north temperate zone (Gerber and Loguercio-Leite, 1997; Ryvarden and Melo, 2017). The Chinese sample Dai 18490A nests together with two British samples KM 178999 and KM 155306 and Hawaiian sample JV 2211/H3-J, forming an independent lineage with a robust support, and is treated as R. ulmarius in our phylogeny (Figure 1). Morphologically, the Chinese sample Dai 18490A also has pileate basidiomata, slightly zonate, concentrically sulcate and radially wrinkled upper surface, round to angular pores of 5−7 per mm, and subglobose, thick-walled basidiospores measuring 5.5−7 × 5−6.5 µm. The Chinese sample Cui 4195 and American sample JV1909/17-J are very similar with R. ulmarius by pileate basidiomata with azonate upper surface, and subglobose, thick-walled and almost the same size of basidiospores (6.8−7.5 × 5.8−7 µm in Cui 4195, 6−7 × 5−6.2 µm in JV1909/17-J). However, they have smaller pores (7−9 per mm in Cui 4195, 8−10 per mm in JV1909/17-J, vs. 5−6 per mm in Dai 18490A) and form respectively independent lineages distinctive from Dai 18490A etc. Polyporus cytisinus Berk., Po. actinobolus Mont., Po. sublinguaeformis Schulzer, Po. geotropus Cooke, Placodes incanus Quél., and Po. fraxineus Lloyd are listed as synonyms of R. ulmarius in Index Fungorum (http://www.indexfungorum.org/). Unfortunately, we did not study the types of these taxa, and for the time being, we treat the three American samples JV 1909/17-J, JV 1403/5-J, and JV 1504/40 as “Rigidoporus ulmarius” in our study.
Boletus microporus Sw. was originally described by Swartz (1788) from Jamaica. It is characterized by occasionally resupinate but mostly pileate basidiomata with concentrically zonate and sulcate upper surface, bright orange to reddish-brown pore surface, the absence of cystidia, ventricose smooth cystidiols, and broadly ellipsoid to subglobose basidiospores measuring 3.5−5 × 3.5−4 µm. Three America samples Dai 17402, Dai 17392, and JV 2110/1 form an independent lineage in Rigidoporus, and they also have completely resupinate or effused-reflexed to pileate basidiomata, concentrically zonate and sulcate upper surface, broadly ellipsoid to subglobose basidiospores measuring 3.8−4.3 × 3.2−3.8 µm, which is in accordance with the descriptions of R. microporus. In addition, four sequences of R. microporus (GenBank: KJ559458, KJ559468, AB697722, and HQ400709) deposited in GenBank form another lineage in our study, and they are from Africa (Nigeria and Cameroon) and Southeast Asia (Indonesia and Malaysia). Many synonyms of R. microporus are listed in Index Fungorum (http://www.indexfungorum.org/), and in order to avoid conflict with them, four Asian and African samples FRIM 646, taxon 219653, ED 310, and N 402 are regarded as “Rigidoporus microporus” in our study. O. mollis Gibertoni & Ryvarden, R. amazonicus Ryvarden, R. grandisporus Ryvarden et al., and R. mariae Gibertoni et al. were also originally described from South America (Brazil). However, O. mollis has bigger pores than three America samples (5–6 per mm vs. 12–14 per mm; Gibertoni et al., 2012), the other three species have stipitate basidiomata (Ryvarden, 1987; Gomes-Silva et al., 2014).
O. millavensis (Bourdot & Galzin) Ryvarden & Melo is morphologically similar and phylogenetically related to R. philadelphi (Figure 1). In addition, Ryvarden and Melo (2017) thought that they are synonymous species. P. mucida subsp. millavensis Bourdot & Galzin (1925) as the basionym of O. millavensis was originally described from France growth on rotting wood of Juniperus and Pinus. It also has resupinate basidiomata with grayish white to ochraceous pore surface, lacerate dissepiments, two types of smooth cystidia, and ovoid, broadly ellipsoid to subglobose basidiospores measuring 4.5−6.5 × 3.5−4.7 µm (Michel et al., 2005). Chaetoporus philadelphi Parmasto was originally described by Parmasto (1959) growth on the bark of Philadelphus coronarius from Estonia and then combined as R. philadelphi by Pouzar (1966). It is characterized by annual, resupinate basidiomata with white to pale-yellow pore surface, reticulated, angular pores of 2−3 per mm, encrusted or smooth cystidia, and broadly ellipsoid basidiospores measuring 4.5−5.5 × 3.8−4.5 µm (Parmasto, 1959). Four Chinese samples Dai 24503, Dai 24509, Dai 18970, and Wei 1622 and two Chinese samples Dai 24218 and Dai 24219 form two independent lineages nested in Rigidoporus, and all these six samples are very similar by resupinate basidiomata with cream, buff-yellow to gray pore surface, angular pores of 2−3 per mm, lacerate dissepiments, numerous small crystals, two type cystidia, and broadly ellipsoid and almost the same size of basidiospores (4.8−5.8 × 4−4.8 µm in the former four samples; 5.2−6.2 × 4.5−5.6 µm in the latter two samples), which is in accordance with the descriptions of R. millavensis and R. philadelphi. However, the former grows on both gymnosperm and angiosperm woods (Juniperus, Picea, and Populus), while the latter only grows on angiosperm wood. In addition, there are 14-base pair differences between them, which accounts for > 2% nucleotide differences in the ITS regions (610 bp in total). Thus, the four Chinese samples Dai 24503, Dai 24509, Dai 18970, and Wei 1622 are treated as R. millavensis and the two samples Dai 24218 and Dai 24219 are treated as R. philadelphi in this study.
Three new species of Rigidoporus are described in the present paper, and most of them are from tropical Asia. Most of these new species have rather similar morphology as existing species but formed independent lineages in phylogeny; thus, we deal with as new species. The same phenomenon was found in several other polypore genera (Yuan et al., 2021; Wang et al., 2022; Yuan et al., 2022; Si et al., 2023; Zhang et al., 2023; Zhou et al., 2023), and it seems that more new taxa will be found after further investigation and phylogenetic analyses.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, OQ930240-OQ930284; OQ924520-OQ924549.
C-GW, JV, CJ, and JS designed the research and contributed to data analysis and interpretation. C-GW and JV prepared the samples. C-GW, JV, and JS conducted the molecular experiments and analyzed the data. C-GW prepared the samples and drafted the manuscript. JV and JS discussed the results and edited the manuscript. All authors contributed to the article and approved the submitted version.
The research is supported by the National Natural Science Foundation of China (Nos. 32070016 and 32270016).
The authors would like to express their deep appreciations to Prof. Yu-Cheng Dai (Beijing Forestry University, China) for forwarding specimens on loan, and to Prof. Tatiana B. Gibertoni (Recife, Brasil) for help in field trip to Manaus, Brazil.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anonymous (1969). Flora of British fungi. colour identification chart (Edinburgh, UK: Her Majesty’s Stationery Office).
Bourdot, H., Galzin, A. (1925). Hyménomycètes de France (14. porés). Bull. Soc Mycol. France 41, 98–255.
Buchanan, P. K., Ryvarden, L. (1988). Type studies in the Polyporaceae - 18. Species described by G.H. Cunninghamn. Mycotaxo 31, 1–38.
Buchanan, P. K., Ryvarden, L. (2000). An annotated checklist of polypore and polypore-like fungi recorded from new Zealand. New Z. J. Bot. 38, 265–323. doi: 10.1080/0028825X.2000.9512683
Corner, E. J. H. (1992). Additional resupinate non-xanthochroic polypores from Brazil and malesia. Nova Hedwig. 55, 119–152.
Cui, B. K., Dai, Y. C. (2009). Oxyporus piceicola sp. nov. with a key to species of the genus in China. Mycotaxon 109, 307–313. doi: 10.5248/109.307
Cui, B. K., Huang, M. Y., Dai, Y. C. (2006). A new species of Oxyporus (Basidiomycota, aphyllophorales) from Northwest China. Mycotaxon 96, 207–210.
Dai, Y. C. (1998). Changbai wood-rotting fungi 9. three new species and other species in Rigidoporus, Skeletocutis and Wolfiporia (Basidiomycota, aphyllophorales). Ann. Bot. Fenn. 35, 143–154.
Dai, Y. C., Wei, Y. L., Wang, Z. (2004). Wood-inhabiting fungi in southern China 2. polypores from sichuan province. Ann. Bot. Fenn. 41, 319–329.
Domanski, S. (1974). Mala flora grzybów. tom I: basidiomycetes (Podstawczaki), aphyllophorales (Bezblaszkowe). bondarzewiaceae, fistulinaceae, ganodermataceae, polyporaceae. Państwowe Wydawn. Naukowe 1, 1–316.
Donk, M. A. (1933). Revisie van de nederlandse heterobasidiomyceteae (uitgez. uredinales en ustilaginales) en homobasidiomyceteae-aphyllophraceae: 2. Mededelingen van het botanisch Museum en Herbarium van Rijksuniversiteit Utrecht 9, 1–278.
Gerber, A. L., Loguercio-Leite, C. (1997). New records of polypores (Aphyllophorales) from southern Brazil. Mycotaxon 62, 305–318.
Gibertoni, T. B., Martins-Junior, A., Ryvarden, L., Sotão, H. M. (2012). Oxyporus mollis sp. nov. (Agaricomycetes) from the Eastern Brazilian Amazonia. Nova Hedwig. 94, 175–179. doi: 10.1127/0029-5035/2012/0094-0175
Gomes-Silva, A. C., Medeiros, P. S., Soares, A. M. S., Sotão, H. M. P., Ryvarden, L., Gibertoni, T. B. (2014). Two new species of Rigidoporus (Meripilaceae) from Brazil and new records from the Brazilian Amazonia. Phytotaxa 156, 191–200. doi: 10.11646/phytotaxa.156.4.1
Han, M. L., Chen, Y. Y., Shen, L. L., Song, J., Vlasák, J., Dai, Y. C., et al. (2016). Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 80, 343–373. doi: 10.1007/s13225-016-0364-y
Hattori, T. (2001a). Type studies of the polypores described by E.J.H. corner from Asia and West pacific areas 3. species described in Trichaptum, Albatrellus, Boletopsis, Diacanthodes, Elmerina, Fomitopsis and Gloeoporus. Mycoscience 42, 423–431. doi: 10.1007/BF02464338
Hattori, T. (2001b). Type studies of the polypores described by e. j. h. corner from Asia and West pacific areas 2. species described in Gloeophyllum, Heteroporus, Microporellus, Oxyporus, Paratrichaptum, and Rigidoporus. Mycoscience 42, 19–28. doi: 10.1007/BF02463971
Hattori, T. (2003). Type studies of the polypores described by E.J.H. corner from Asia and West pacific areas. 5. species described in tyromyces (2). Mycoscience 44, 265–276. doi: 10.1007/s10267-003-0114-3
Hopple, J. S., Vilgalys, R. (1999). Phylogenetic relationships in the mushroom genus Coprinus and dark-spored allies based on sequence data from the nuclear gene coding for the large ribosomal subunit RNA: divergent domains, outgroups, and monophyly. Mol. Phylogenet. Evol. 13, 1–19. doi: 10.1006/mpev.1999.0634
Imazeki, R. (1952). A contribution to the fungous flora of Dutch new Guinea. Bull. Govern. For. Exp. Station Meguro 57, 87–128.
Johansen, I., Ryvarden, L. (1979). Studies in the aphyllophorales of Africa 7. some new genera and species in the polyporacea. Trans. Bri. Mycol. Soc 72, 189–199. doi: 10.1016/S0007-1536(79)80031-5
Læssøe, T., Ryvarden, L. (2010). Studies in Neotropical polypores 26. some new and rarely recorded polypores from Ecuador. Synop. Fungorum 27, 34–58.
Li, H. J., Cui, B. K., Dai, Y. C. (2014). Taxonomy and multi-gene phylogeny of Datronia (Polyporales, basidiomycota). Persoonia 32, 170–182. doi: 10.3767/003158514X681828
Lindblad, I., Ryvarden, L. (1999). Studies in neotropical polypores. 3. new and interesting basidiomycetes (Poriales) from Costa Rica. Mycotaxon 71, 335–359.
Michel, H., Duhem, B., Trichies, G. (2005). Nouveau regard sur Poria millavensis. Bull. Soc Mycol. France 121, 29–46.
Miettinen, O., Vlasák, J., Rivoire, B., Spirin, V. (2018). Postia caesia complex (Polyporales, basidiomycota) in temperate northern hemisphere. Fungal System. Evol. 1, 101–129. doi: 10.3114/fuse.2018.01.05
Miller, M. A., Holder, M. T., Vos, R., Midford, P. E., Liebowitz, T., Chan, L., et al. (2009). “The CIPRES portals,” in CIPRES. Available at: http://www.phylo.org/sub_sections/portalhttp://www.webcitation.org/5imQlJeQa.
Murrill, W. A. (1905). The polyporaceae of north America-12. a synopsis of the white and bright-colored pileate species. Bull. Torrey Bot. Club 32, 469–493. doi: 10.2307/2478463
Núñez, M., Parmasto, E., Ryvarden, L. (2001). New and interesting polypores from East Russia. Fungal Divers. 6, 107–114.
Núñez, M., Ryvarden, L. (1999). New and interesting polypores from Japan. Fungal Divers. 3, 107–121.
Nylander, J. A. A. (2004). MrModeltest v2 (Uppsala: Evolutionary Biology Centre, Uppsala University).
Parmasto, E. (1959). De speciebus et formis novis polyporacearum in RSS estonica inventis. botanicheskie materialy otdela sporovyh rastenij botanicheskogo instituti imeni V.L. Komarova 12, 237–242.
Petersen, J. H. (1996). The Danish mycological society’s colour-chart (Greve: Foreningen til Svampekundskabens Fremme), 1–6.
Pouzar, Z. (1966). Studies in the taxonomy of polypores 2. Folia Geobot. Phytotaxa 1, 356–375. doi: 10.1007/BF02854587
Ronquist, F., Huelsenbeck, J. P. (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Ryvarden, L. (1972a). Studies in the aphyllophorales of canary islands with a note on the genus Perenniporia. Norw. J. Bot. 19, 139–144.
Ryvarden, L. (1972b). A critical checklist of the polyporaceae in tropical East Africa. Norw. J. Bot. 19, 229–238.
Ryvarden, L. (1983). Type-studies in the polyporaceae 14. species described by patouillard, either alone or with other authors. Occa. Papers Farlow Herb. Cryptog. Bot. 18, 1–39. doi: 10.5962/p.305853
Ryvarden, L. (1988). Type studies in the polyporaceae. 19. species described by M.C. Cooke. Mycotaxon 31, 45–58.
Ryvarden, L. (1990). Aphyllophorales, mycoflora of noth sulawesi, Indonesia. Mem. New York Bot. Garden 59, 155–165.
Ryvarden, L. (2015). Neotropical Polypores part 3. polyporaceae obba-wrightoporia. Synop. Fungorum 36, 513–522.
Ryvarden, L. (2018). Studies in African aphyllophorales 25. new poroid species from East and central Africa. Synop. Fungorum 38, 25–32.
Ryvarden, L. (2019). Studies in African aphyllophorales 32. some new African polypores. Synop. Fungorum 39, 59–71.
Ryvarden, L., Iturriaga, T. (2003). Studies in neotropical polypores 10. new polypores from Venezuela. Mycologia 95, 1066–1077. doi: 10.1080/15572536.2004.11833021
Ryvarden, L., Iturriaga, T. (2010). Studies in Neotropical polypores 29. some new and interesting species from the Andes region in Venezuela. Synop. Fungorum 27, 78–91.
Ryvarden, L., Johansen, I. (1980). A preliminary polypore flora of East Africa (Oslo: Fungiflora). doi: 10.2307/3759822
Shen, L. L., Wang, M., Zhou, J. L., Xing, J. H., Cui, B. K., Dai, Y. C. (2019). Taxonomy and phylogeny of Postia. multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, basidiomycota) and related genera. Persoonia 42, 101–126. doi: 10.3767/persoonia.2019.42.05
Si, J., Zhang, Y. Z., Liang, J. Q., Li, H. J. (2023). Morphology and phylogeny identify two new species and one new subspecies of Podoscypha from yunnan province, southwest China. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1151365
Sowerby, J. (1797). Coloured figures of English fungi or mushrooms. J. Davis 1, 1–54. doi: 10.5962/bhl.title.6342
Swofford, D. L. (2002). PAUP*: phylogenetic analysis using parsimony (*and other methods). version 4.0b10 (Sunderland, MA: Sinauer Associates). doi: 10.1002/0471650129.dob0522
Teixeira, A. R. (1992). New combinations and new names in the polyporaceae. Rev. Bras. Botânica 15, 125–127.
Vampola, P., Vlasák, J. (2012). Rigidoporus pouzarii, a new polypore species related to Rigidoporus crocatus. Czech Mycol. 64, 3–11. doi: 10.33585/cmy.64102
Wang, Y. R., Dai, Y. C., Liu, H. G., Vlasák, J., Buchanan, P., Yuan, Y., et al. (2022). A new contribution to Megasporoporia sensu lato: six new species and three new combinations. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1046777
Wang, X. W., Liu, S. L., Zhou, L. W. (2023). An updated taxonomic framework of Hymenochaetales (Agaricomycetes, Basidiomycota). Mycosphere 14, 452–496. doi: 10.5943/mycosphere/14/1/6
White, T. J., Bruns, T., Lee, S., Taylor, J. (1990). ““Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,”,” in PCR protocols: a guide to methods and applications. Eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J., White, J. T. (New York Academic Press: US), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wu, F., Chen, J. J., Ji, X. H., Vlasák, J., Dai, Y. C. (2017). Phylogeny and diversity of the morphologically similar polypore genera Rigidoporus, Physisporinus, Oxyporus, and Leucophellinus. Mycologia 109, 749–765. doi: 10.1080/00275514.2017.1405215
Wu, F., Zhou, L. W., Vlasák, J., Dai, Y. C. (2022). Global diversity and systematics of hymenochaetaceae with poroid hymenophore. Fungal Divers. 113, 1–192. doi: 10.1007/s13225-021-00496-4
Yuan, Y., Chen, J. J., Korhonen, K., Martin, F., Dai, Y. C. (2021). An updated global species diversity and phylogeny in the forest pathogenic genus Heterobasidion (Basidiomycota, russulales). Front. Microbiol. 11. doi: 10.3389/fmicb.2020.596393
Yuan, H. S., Dai, Y. C. (2012). Wood-inhabiting fungi in southern China 5. polypores from guangxi autonomous region. Ann. Bot. Fenn. 49, 341–351. doi: 10.5735/085.049.0605
Yuan, H. S., Lu, X., Dai, Y. C., Hyde, K. D., Kan, Y. H., Kušan, I., et al. (2020). Fungal diversity notes 1277–1386: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 104, 1–266. doi: 10.1007/s13225-020-00461-7
Yuan, Y., Wu, Y. D., Wang, Y. R., Zhou, M., Qiu, J. Z., Li, D. W., et al. (2022). Two new forest pathogens in Phaeolus (Polyporales, basidiomycota) on Chinese coniferous trees were confirmed by molecular phylogeny. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.942603
Zhang, Q. Y., Liu, Z. B., Liu, H. G., Si, J. (2023). Two new corticioid species of phanerochaetaceae (Polyporales, basidiomycota) from southwest China. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1105918
Zhou, H. M., Bau, T., Si, J. (2023). Morphological and phylogenetic evidence reveal three new Pseudohydnum (Auriculariales, basidiomycota) species from north China. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1139449
Zhu, L., Song, J., Zhou, J. L., Si, J., Cui, B. K. (2019). Species diversity, phylogeny, divergence time and biogeography of the genus Sanghuangporus (Basidiomycota). Front. Microbiol. 10. doi: 10.3389/fmicb.2019.00812
Keywords: Oxyporaceae, polypore, taxonomy, wood-decaying fungi, phylogeny
Citation: Wang C-G, Vlasák J, Jin C and Si J (2023) Phylogeny and diversity of Rigidoporus (Hymenochaetales, Basidiomycota), including three new species from Asia. Front. Cell. Infect. Microbiol. 13:1216277. doi: 10.3389/fcimb.2023.1216277
Received: 03 May 2023; Accepted: 31 May 2023;
Published: 19 June 2023.
Edited by:
Yusufjon Gafforov, Academy of Sciences Republic of Uzbekistan (UzAS), UzbekistanReviewed by:
Viktor Papp, Hungarian University of Agricultural and Life Sciences, HungaryCopyright © 2023 Wang, Vlasák, Jin and Si. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Si, amluZ3NpMTc4OEAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.