- 1Institute of Microbiology, School of Ecology and Nature Conservation, Beijing Forestry University, Beijing, China
- 2National Institute of Occupational Health and Poison Control, Chinese Center for Disease Control and Prevention, Beijing, China
Cortinarius is a globally distributed agaricoid genus that has been well studied in Europe and America with over 1,000 described species. However, as part of an ongoing effort to investigate the diversity of Cortinarius section Anomali in China, the resource investigation and classification research are still limited, and the species diversity has not been clarified by far. During the re-examination of the Chinese Cortinarius specimens, C. cinnamomeolilacinus, C. subclackamasensis, and C. tropicus, belonging to the sect. Anomali, were described in China as new to science based on morphological examination and phylogenetic analysis. The three new species are described and illustrated in detail according to the Chinese materials. The phylogenetic analysis based on internal transcribed spacer sequences confirmed the placement of the three species in the Cortinarius sect. Anomali clade. Phylogenetically related and morphologically similar species to these three new species are discussed.
1 Introduction
Cortinarius (Pers.) Gray, established based on Cortinarius violaceus (L.) Gray, is the largest genus of Agaricales with a worldwide distribution (Clements and Shear, 1931; Garnica et al., 2016; Varga et al., 2019). It is mainly marked by a fugacious veil enveloping the basidiocarp and a cortina, which initially covers the lamellae, but later vanishes in expanding basidiocarps (Stensrud et al., 2014). Macroscopically, members of this genus are highly variable, with their basidiocarps, lamellae, and basidiospores varying considerably in size, shape, or color (Peintner et al., 2004; Frøslev et al., 2006; Niskanen et al., 2009; Niskanen et al., 2013; Liimatainen et al., 2014; Liimatainen et al., 2015; Niskanen et al., 2016). Cortinarius species are widely reported in temperate and subtropical forests and form mycorrhizal associations with ectotrophic trees, such as plants of the Cistaceae, Fagaceae, Malvaceae, Nothofagaceae, Pinaceae, and Salicaceae families (Singer, 1986; Frøslev et al., 2006; Soop et al., 2018). With the advances in taxonomy and molecular biology techniques, increases have been detected in the number of species in the genus Cortinarius. To date, more than 5,000 scientific names in the genus have been published as listed in the Index Fungorum (http://www.indexfungorum.org/Names/names.asp, 2023), and about 2,000 species are estimated in the Dictionary of Fungi, 10th edition (Ammirati et al., 2007; Kirk et al., 2008; Brandrud et al., 2014).
Owing to the considerable morphological variations in this genus, the subdivision of Cortinarius into subgeneric units has caused some problems (Peintner et al., 2004). Morphologically, Cortinarius has been divided into several different subgenera and infrageneric sections by various taxonomists, which results in taxonomic chaos and indicates that morphology alone is insufficient for recognizing natural units in this group of fungi (Garnica et al., 2005; Garnica et al., 2009). In recent research, phylogenetic analyses of the genus have contributed to the delimitation of taxonomic entities within the genus (Peintner et al., 2003; Suarez-Santiago et al., 2009; Dima et al., 2016; Dima et al., 2021).
Cortinarius sect. Anomali Konrad & Maubl., a species-rich group, is established based on Cortinarius anomalus (Fr.) Fr. It is characterized by a telamonioid/sericeocyboid appearance, often with yellowish to brownish universal veil remnants on the stipe, typically young bluish lamellae, and subglobose to broadly ellipsoid or rarely ellipsoid, verrucose spores (Dima et al., 2016; Dima et al., 2021). Anomali was originally placed by Brandrud et al. (1989) as a section of subgenus Telamonia Melot but not belong to the subgenus Telamonia s. str. based on later phylogenetic data (Høiland and Holst-Jensen, 2000; Peintner et al., 2004; Garnica et al., 2005; Niskanen et al., 2009). Later, many species were added or transferred to sect. Anomali, causing confusions in the classification of sect. Anomali. No consensus on the content or placement of this section have been reached to date (Bidaud et al., 1992; Bidaud et al., 1994; Consiglio et al., 2005; Consiglio et al., 2006; Consiglio, 2012; Dima et al., 2016). For instances, C. spilomeus (Fr.) Fr. and C. bolaris (Pers.) Fr. have been included in the section, and sometimes have been separated in another sect. Spilomei (Moënne-Locc. & Reumaux) Consiglio, D. Antonini & M. Antonini (Dima et al., 2016). Some phylogenetic studies show that sect. Anomali is a monophyletic group in the genus Cortinarius, without any traditional subgenera (Dima et al., 2016; Dima et al., 2021). The classification of sect. Anomali has been studied previously and systematically in Europe and North America, but rarely in Asia and Africa (Kauffman, 1905; Murrill, 1946; Dima et al., 2016; Ammirati et al., 2017). In China, about 163 Cortinarius species, including dozens of new species, have been described in the past 10 years, with only three species belonging to the sect. Anomali (Wei and Yao, 2013; Xie et al., 2019; Luo and Bau, 2021; Xie et al., 2021; Xie, 2022). On this basis, more taxa of the genus are waiting to be discovered in China.
In this study, we conducted taxonomic and phylogenetic studies of Cortinarius sect. Anomali in China. Three new species were found during the intensive fieldwork and are described here based on their morphological and ecological characteristics, as well as phylogenetic evidence.
2 Materials and methods
2.1 Morphological studies
All specimens have been deposited in the National Institute of Occupational Health and Poison Control, Chinese Center for Disease Control (NIOHP, China CDC). Macro-morphological descriptions were based on field notes and dried specimens. Microscopic features were examined and described in 5% KOH, Congo Red, or Melzer’s reagent and observed under a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan) with a magnification of up to ×1,000. Thirty basidiospores were measured per collection (excluding apiculus and ornamentation), and the averages (av. X) and quotients (av. Q = L/B) were calculated. Color terms are cited from Anonymous (1969) as well as Kornerup and Wanscher (1978).
2.2 DNA extraction and sequencing
A Phire® Plant Direct PCR Kit (Finnzymes Oy, Finland) was used to obtain PCR products from dried specimens, according to the manufacturer’s instructions and as described previously by Li et al. (2015), with some modifications. The following primer pairs were used to amplify the internal transcribed spacer (ITS): ITS5 (5′‐GGA AGT AAA AGT CGT AAC AAG G‐3′) and ITS4 (5′‐TCC TCC GCT TAT TGATAT GC‐3′) (White et al., 1990). The PCR procedure was as follows: initial denaturation at 98 °C for 5 min, followed by 35 cycles at 98 °C for 5 s, 58 °C for 5 s, and 72 °C for 5 s, and a final extension of 72 °C for 10 min. The PCR products were purified and sequenced by Sangon Biotech, China. The newly generated sequences from this study have been deposited in GenBank and are listed in Table 1.
2.3 Phylogenetic analysis
New sequences generated in this study and additional sequences retrieved from GenBank (Table 1) were aligned using BioEdit 7.0.5.3 (Hall, 1999) and ClustalX 1.83 (Thompson et al., 1997), followed by manual adjustments. Sequences of Cortinarius bolaris (sect. Bolares) were used as outgroups (Hughes et al., 2009; Dima et al., 2021). The maximum likelihood (ML) and Bayesian inference (BI) methods were used for the phylogenetic analysis. The best-fit model was selected by ModelFinder (Kalyaanamoorthy et al., 2017), adopting Akaike information criterion (AIC). The ML analysis was carried out using RAxML 8.2.12 (Stamatakis, 2006; Silvestro and Michalak, 2012), and the BI tree reconstruction was reconstructed using MrBayes 3.2.5 (Ronquist et al., 2012). Four Markov chains were run for two runs from random starting trees for 10 million generations, and the trees were sampled every 1,000 generations. The burn-in was set to discard 25% of the trees. A majority rule consensus tree of all remaining trees was calculated. The sequence alignment was deposited at TreeBase (submission ID: 30414). Branches that received bootstrap supports for ML and Bayesian posterior probabilities (BPP) greater than or equal to 75% (ML) and 0.95 (BPP) were considered significantly supported.
3 Results
3.1 Phylogeny
The ITS dataset comprised 229 fungal collections representing approximately 81 taxa of the genus Cortinarius. ModelFinder suggested that GTR + I + G was the best-fit model of nucleotide evolution for BI. The Bayesian analysis resulted in a concordant topology with an average standard deviation of split frequencies of 0.005575. The ML and BI analyses resulted in nearly identical topologies, and thus only the ML tree is presented with the bootstrap supports for ML and BPP when they were greater than or equal to 50% and 0.90, respectively.
Our phylogeny, which is inferred from ITS sequences (Figure 1), is similar to those of Dima et al (Dima et al., 2016; Dima et al., 2021). The phylogenetic analysis showed five sections, and each section formed separate monophyletic lineages with strong statistical support. Section Anomali formed a distinct highly supported clade (ML = 99 and BPP = 1) and was separated from other sections. Three new species, namely, C. cinnamomeolilacinus (ML = 98 and BPP = 1), C. subclackamasensis (ML = 86 and BPP = 0.99), and C. tropicus (ML = 100 and BPP = 1), nested within the sect. Anomali clade, and formed an independent lineage with high statistical supports, respectively. It is worth noting that collections of C. cinnamomeolilacinus had genetic distances in our phylogeny. However, there were only four base pair differences between them, which were below 1.5% nucleotide differences in the ITS regions. Morphologically, there were no obvious differences of these C. cinnamomeolilacinus collections.
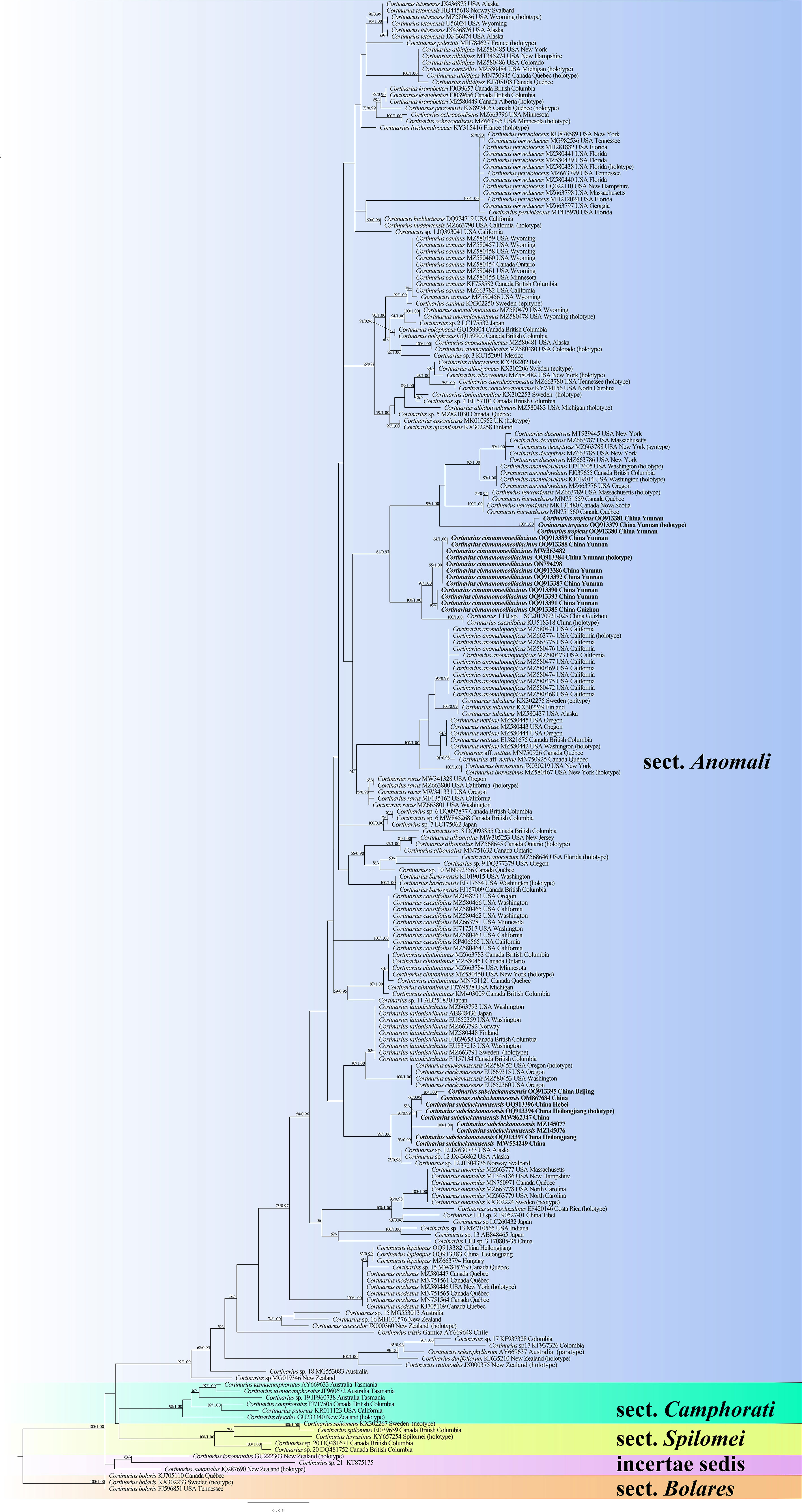
Figure 1 Maximum likelihood (ML) tree illustrating the phylogeny of Cortinarius section Anomali based on ITS sequences. Branches are labeled with ML bootstrap > 50% and Bayesian posterior probabilities (BPP) > 0.90, respectively. New species are highlighted in bold.
3.2 Taxonomy
Cortinarius cinnamomeolilacinus Q.Y. Zhang, Jing Si & Hai J. Li, sp. nov. Figure 2.
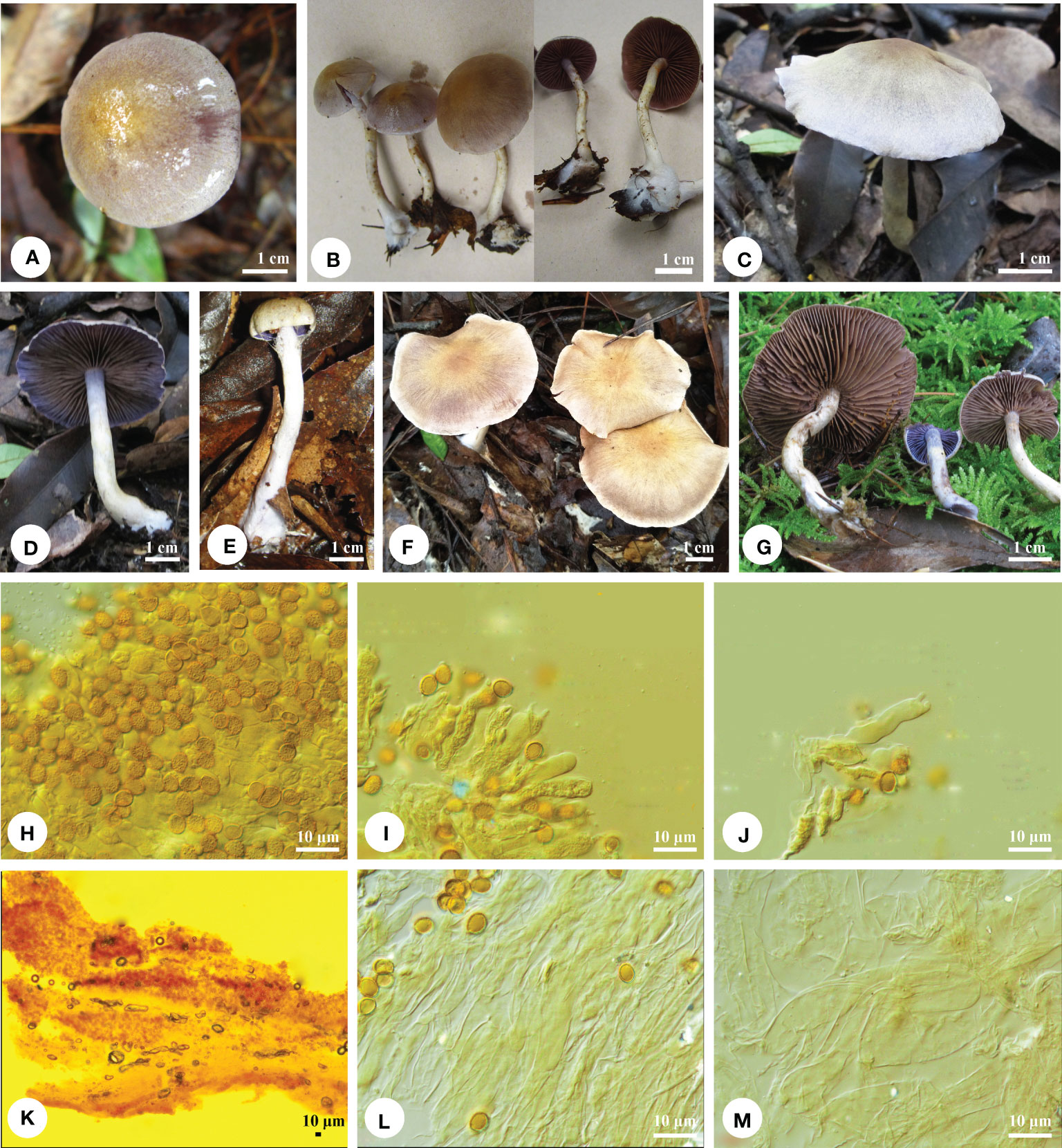
Figure 2 Basidiomata and microscopic structures of Cortinarius cinnamomeolilacinus. (A-G) Basidiomata (A, B) TCWH007; (C, D) Li 180825-21; (E) LLLJ20170805-002; (F) tcmb005; (G) 180825-21), (H) Basidiospores, (I-J) Basidia and basidioles, (K) Pileipellis, (L) Hypodermium of pileipellis, and (M) Context hyphae.
MycoBank: 848613
Diagnosis. — This species is characterized by its small basidiomata, more or less hygrophanous, lilac pileal surface with cinnamon buff to brownish cinnamon center, and pale gray to whitish toward margin, subglobose to broadly ellipsoid basidiospores (7−8.5 × 5.8−6.8 μm); it is gregarious on ground dominant with Fagaceae or Pinaceae.
Type. — China, Yunnan Province, Baoshan, Tengchong, Wuhe Town, Lushan, Alt: 1989 m, N: 24°54′06.98″, E: 98°36′30.37″, on ground dominant with Fagaceae, 8 August 2016, TCWH 007 (holotype), and GenBank accession number for ITS: OQ913384.
Etymology. — cinnamomeolilacinus refers to its more or less lilac pileal surface with a cinnamon center.
Habitat and distribution. — Scattered or gregarious on ground dominant with Fagaceae or Pinaceae. Currently, it is only found in tropical Guizhou and Yunnan (nine collections) in summer and autumn.
Macrostructures. — Basidiomata small. Pileus 15−52 mm in diam., hemispheric when young, then convex to plano-convex, some with a low umbo, margin narrowly when young, surface smooth to finely felty, color evenly pale silvery gray to lilac [15A(2−4)], the disc slightly more brownish, cinnamon buff, cinnamon to brownish cinnamon [5B(4−5)], pale gray to whitish (1A1−1B1) toward margin, even to rugulose, hygrophanous. Lamellae adnate with decurrent tooth to slightly adnexed, close, violet (16A4−16A5) when young, then grayish violet [16B4−16C5], pale silvery gray to pale drab gray (16B2−16D2), finally pale brown to cinnamon [6D(4−8)]. Stipe 30- to 70- mm long, apex 4−6 mm in diam., and base 5−10 mm in diam., usually clavate to cylindrical, even or slightly bulbous at base, fragile, shiny, covered with white fibrillose, apex violet [16A(4−5)] when young, later pale brownish (6B2−6D2), other part pale silvery gray [15A(2−3)], pale white veil (1A2) usually sparse, forming yellow [3A(3−4)] floccose-girdles on the stipe, often becoming pale yellow [4B(3−5)], sometimes indistinct, basal mycelium white (1A1). Context in pileus solid, firm, sometimes hollow in stipe, pale silvery gray [16B4−16C5] when fresh, finally pale brown to cinnamon in age [6D(4−8)]. Odor and taste of context strongly fungoid.
Microstructures. — Basidiospores [150/5/5] (6.8−) 7−8.5 (−8.8) × (5.5−) 5.8−6.8 (−7) μm, av. 7.7 × 6.1 μm, Q = 1.22−1.29, av. Q = 1.26, subglobose to broadly ellipsoid, buff to cinnamon-buff, coarsely verrucose, non-dextrinoid. Basidia 4-spored, 29−33 × 6−7 μm, clavate, colorless, or yellowish. Lamella trama hyphae smooth, parallel, 5- to 12- μm wide. Lamellae edge fertile, with some small clavate sterile cells. Pileipellis duplex: Epicutis thin to ± well developed, hyphae ± cylindrical, moderately to strongly interwoven, 5- to 8- μm wide, hyaline or yellowish, smooth to encrusted; hypocutis ± cellular or hyphae more interwoven and radially arranged, cylindrical to enlarged, hyaline to yellowish, smooth to encrusted, (3.5) 4−18 (20) μm wide. Clamp connections present.
Remarks. — Cortinarius albomalus Liimat. & Niskanen and C. cinnamomeolilacinus have similar basidiomata. However, C. albomalus has smaller basidiospores (6.5−7.5 × 5.5−6 µm) and is distributed in North America (Dima et al., 2021; Liimatainen and Niskanen, 2021). Cortinarius pastoralis Soop, H. Lindstr., Dima, Niskanen, Liimat. & Kytöv. and C. cinnamomeolilacinus share pale buff or brownish pilei, but C. pastoralis has larger basidiospores (8.5−9 × 7−8 μm; Dima et al., 2016). Cortinarius albocyaneus Fr. is similar to C. cinnamomeolilacinus with grayish ochraceous to whitish pilei, but C. albocyaneus has larger basidiomata (40−70 mm) and basidiospores (8.5−9.0 × 6.0−7.5 μm), is found in Europe, and is common in northeastern North America (Dima et al., 2021). Cortinarius anomalovelatus Ammirati, Berbee, E. Harrower, Liimat. & Niskanen has grayish-to-white basidiomata and subglobose to broadly ellipsoid basidiospores, which are similar to those of C. cinnamomeolilacinus, but C. anomalovelatus has a heavier universal veil and a grayish-blue to violet pileal surface, and is usually found in western North America (Dima et al., 2021).
Additional specimens (paratypes) examined. — China, Guizhou Province, Liupanshui, Lingshan Temple, on ground of Fagaceae, Alt: 1929 m, N: 26°37′35.94″, E: 104°48′54.95″, October 15, 2016, Li 161015-10; Yunnan Province, Baoshan, Longling County, Longjiang Town, Laohuangtian, on ground dominant with Fagaceae, Alt: 1773 m, N: 24°41′31.01″, E: 98°42′55.24″, 5 August 2017, LLLJ 20170805-002; Longyang District, Wafang Town, Dapingdi, on ground of Pinus yunnanensis, Alt: 1921.9 m, N: 25°21′35.82″, E: 99°4′45.73″, 6 August 2018, LYWF015; Tengchong, Mangbang Town, Hongdoushu, on ground dominant with Fagaceae, Alt: 1772 m, N: 24°54′52″, E: 98°37′59″, 8 August 2018, tcmb 005; Menglian Town, Xiamenglian Village, on ground of mixed forests composed of Fagaceae and P. yunnanensis, 5 August 2014, Li 140805-18; Chuxiong Yi Autonomous Prefecture, Zixi Mountain, near King Baotou, on ground of mixed forest composed of Fagaceae and P. yunnanensis, Alt: 1926 m, N: 25°1′3″, E: 101°24′7″, 25 August 2018, Li 180825-21; Yuxi, Huaning County, on ground dominant with Fagaceae, 8 September 2013, Li 130908-29; Zhaotong, Weixin County, Miaogou Town, Zhashigou Village, on ground dominant with Fagaceae, Alt: 1450 m, N: 27°47′6″, E: 104°49′18″, 22 September 2017, WX 20170922065.
Cortinarius subclackamasensis Q.Y. Zhang, Jing Si & Hai J. Li, sp. nov. Figure 3.
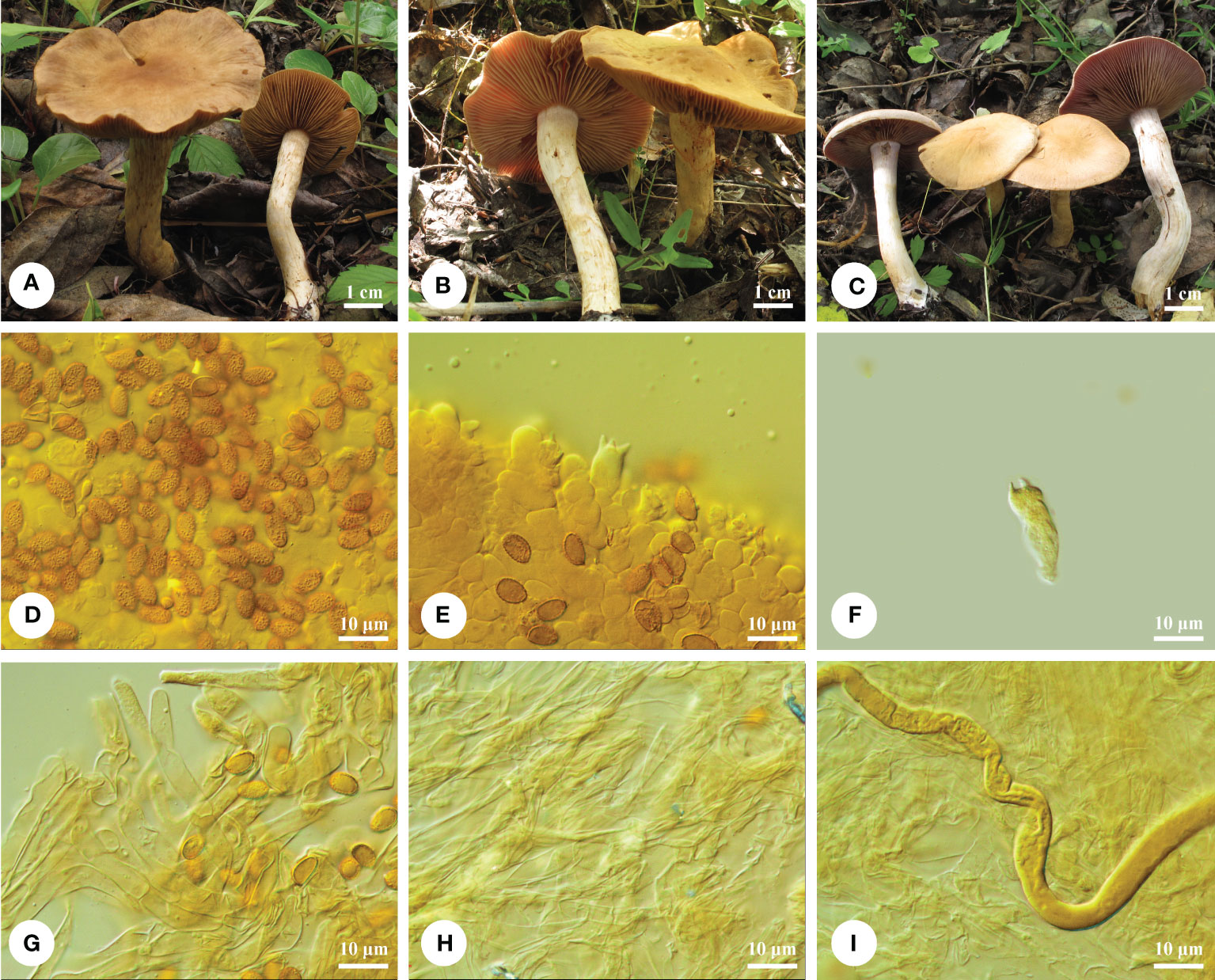
Figure 3 Basidiomata and microscopic structures of Cortinarius subclackamasensis. (A-C) Basidiomata (A Li 170818-16; B Li 170818-02; C Li 170818-01), (D) Basidiospores, (E) Hymenium in trama, (F) One basidium, (G) Epicutis of pileipellis, (H) Hypodermium of pileipellis, and (I) Context hyphae.
MycoBank: 848614
Diagnosis. — This species notably has small- to medium-sized basidiomata, buff-yellow and plano-convex pilei, ellipsoid to oblong ellipsoid basidiospores (9.5−10.8 × 5.8−6.8 μm); it is gregarious on ground of Salix or other deciduous trees and distributed in temperate China.
Type. — China, Heilongjiang Province, Huzhong, Huzhong Forest Farm, near Dongfanghong Line 31, on ground of Salix, 18 August 2017, Li 170818-16 (holotype), GenBank accession number for ITS: OQ913394.
Etymology. — Subclackamasensis refers to its morphological similarity to C. clackamasensis.
Habitat and distribution. — Scattered or gregarious on ground of Salix or other deciduous trees. Currently, it is found in Northeast and North China in summer.
Macrostructures. — Basidiomata small to medium sized. Pileus 30−60 mm in diam., hemispheric to subhemispheric when young, becoming plano-convex, then depressed at center and wrinkled, margin sharp sometimes waves; surface moist to hygrophanous when young, with fibrous veil remnants; buff yellow (4A4), to light honey yellow (4/5B4), somewhat lighter olivaceous buff (4C4) at margin, finely radially striate with age. Lamellae adnexed-emarginate, moderately crowded to crowded, vinaceous (9F6) or peach (6A6) when young, somewhat darkening on manipulation, edge even, somewhat lighter. Stipe 40- to 60- mm long, 5- to 10- mm thick above, qual to somewhat clavate, white, and cream with age. Universal veil usually sparse, thin, and somewhat glossy, cream (4A2/3) to light yellow (4A4), flocculose or forming a week girdle on the stipe. Context rather thick, especially in pileus center, brittle, weakly light honey yellow (4/5B4) with age. Odor and taste of context strongly fungoid.
Microstructures. — Basidiospores [90/3/3] 9.5−10.8 (−11) × (5.5−) 5.8−6.8 μm, av. 9.9 × 6.3 μm, Q = 1.56−1.60, av. Q = 1.59, ellipsoid to oblong ellipsoid, weekly to moderately, but distinctly verrucose, indextrinoid to weakly dextrinoid. Basidia 4-spored, 22−25 × 8−11 μm, clavate, colorless or yellowish. Lamella trama hyphae smooth, parallel, 4- to 12- μm wide. Lamellae edge fertile, with some small clavate sterile cells. Pileipellis duplex: Epicutis about 27- to 32 -μm thick, hyphae (6−) 11- to 16- μm wide, in upper part loosely entangled; hypocutis distinct well-developed, colorless or yellowish, irregular, interwoven, with some intracellular pigments, 18−28.5 μm in diam. Clamp connections present.
Remarks. — Phylogenetically, C. latiodistributus Dima, Ammirati, Niskanen, Kytöv., Liimat. & Brandrud, C. clackamasensis Ammirati, Liimat., Niskanen & Dima, and Cortinarius sp. 12 are closely related to C. subclackamasensis. Cortinarius latiodistributus has violet to pallid brown pilei, and shorter basidiospores (7−9.5 μm). Cortinarius clackamasensis has wider basidiospores (9−11 × 6.5−7.5 μm, av. 9.7 × 6.5 μm, Q = 1.4−1.5), grows on the ground under mixed conifers composed of Picea, Pinus, and Abies, and is distributed in the US (Dima et al., 2021). Similar to C. subclackamasensis, C. clintonianus Peck, and C. anomalopacificus Bojantchev, Liimat., Niskanen, Dima & Ammirati have yellowish and plano-convex basidiomata. However, the basidiospores in C. clintonianus and C. anomalopacificus are shorter (6.7−8.1 × 5.6−6.3 μm vs. 6.5−7 × 5−6 μm; Dima et al., 2021).
Additional specimens (paratypes) examined. — China, Beijing, Mentougou District, Xiaolongmen National Forest Park, 30 August 2017, BJMTG20170830-34; Hebei Province, Baoding, Fuping County, Dongxiaguan Town, Zhujiaying Village, Tianshengqiao, 22 August 2019, 20190822-11; Heilongjiang Province, Huzhong, Huzhong forest farm, near Dongfanghong Line 31, on ground of Salix, 18 August 2017, Li 170818-01 and Li 170818-02.
Cortinarius tropicus Q.Y. Zhang, Jing Si & Hai J. Li, sp. nov. Figure 4.

Figure 4 Basidiomata and microscopic structures of Cortinarius tropicus. (A-E) Basidiomata (tcqushi 006, Holotype), (F) Basidiospores, (G-H) Basidia and basidioles, (I) Epicutis of pileipellis, (J) Hypodermium of pileipellis, and (K) Context hyphae.
MycoBank: 848616
Diagnosis. — This species notably has small basidiomata, violet to dark violet pileal surface with fibrillose disc and nearly glabrous, cream to pale violet margin, subglobose to broadly ellipsoid basidiospores (6−8 × 5−6 μm); it is scattered or gregarious on ground dominant with Fagaceae.
Type. — China, Yunnan Province, Baoshan, Tengchong, Qushi Town, Qingqiao Village, Alt: 1490 m, N: 25°17′17″, E: 98°35′26″, on ground dominant with Fagaceae, 6 August 2016, tcqushi 006 (holotype), GenBank accession number for ITS: OQ913379.
Etymology. — tropicus refers to its tropical distribution in Southwest China.
Habitat and distribution. — Scattered or gregarious on ground dominant with Fagaceae. Currently, it is only found in tropical Yunnan (three collections) in summer.
Macrostructures. — Basidiomata small. Pileus 20−45 mm in diam., hemispheric to subhemispheric when young, becoming obtusely conical, conico-convex, broadly umbonate, pale brown [5D(4−5)] at center and gradually paler toward margin when young, violet (17B8) to dark violet (17F8) when mature, disc grayish violet (15E5) to dark violet (17F8), margin cream (4A2/3) to pale violet [16A(2−3)], fibrillose at disc and nearly glabrous near margin, somewhat hygrophanous, margin decurved. Lamellae adnexed-emarginate, subclose, purplish to pale violet lilac [16D(4−6)], finally ± pale brown to cinnamon (16D3-16E3). Stipe 50- to 80- mm long, apex 3 to 5 mm in diam., base 5−10 mm in diam., enlarged, narrowly clavate, surface grayish (16B2) with dark violet [16F(3−4)] streaks or silvery violet (16A3) on upper half and watery brownish below, veil usually sparse, forming floccose-girdles on the stipe, often at first brownish (6B2-6C2) then becoming pale yellow [4A(2-3)], sometimes indistinct, and basal mycelium whitish. Context in pileus solid, firm, violet to silvery violet [16C(4−6)] when fresh, finally pale brown to cinnamon (16D4−16F4) in age. Odor and taste of context strongly fungoid.
Microstructures. — Basidiospores [90/3/3] 6−8 × 5−6 μm, av. 7.4 × 5.9 μm, Q = 1.21−1.29, av. Q = 1.25, subglobose to broadly ellipsoid, buff to cinnamon-buff, coarsely verrucose, non-dextrinoid to slightly dextrinoid. Basidia 4-spored, 30−42 × 7−11 μm, clavate, colorless or yellowish. Lamella trama hyphae hyaline, smooth, parallel, 5- to 13- μm wide. Pileipellis duplex: Epicutis well developed, hyaline or yellow to ochraceous, smooth, cylindrical, radially arranged, ± interwoven, 4−10 μm wide; hypocutis distinct, colorless or yellowish, smooth to slightly encrusted, cylindrical to enlarged, radially oriented, ± interwoven, (4)6- to 15(17)- μm wide. Clamp connections present.
Remarks. — Cortinarius perviolaceus Murrill is easily confused with C. tropicus because of its violet pilei, but C. perviolaceus has smaller basidiomata (8−22 mm vs. 20−45 mm) and wider basidiospores (6−6.5 μm vs. 5−6 μm; Dima et al., 2021). Cortinarius anomalus has similar pilei with blue tinge when young and larger basidiospores (8−9 × 6−7 μm; Dima et al., 2016). Cortinarius anomalovelatus, C. deceptivus Kauffman, and C. harvardensis L. Nagy, Dima & Ammirati formed a sister group with C. tropicus. Compared with the new species, C. anomalovelatus has wider basidiospores (6.3−7 μm vs. 5−6 μm), C. deceptivus produces larger basidiospores (7.8−9.3 × 6−7.4 μm), and C. harvardensis has bluish to violet pileus, lamellae, and stipe, and slightly bigger basidiospores (7.5−8.5 × 5.5−6.5 μm; Dima et al., 2021).
Additional specimens (paratypes) examined. — CHINA. Yunnan Province, Dehong Dai and Jingpo Autonomous Prefecture, Mang City, Jiangdong Town, Daxinzhai, Alt: 1619 m, N: 24°27′54″, E: 98°18′56″, on ground dominant with Fagaceae, 28 July 2015, Li 150728-56 and Li 150728-63.
4 Discussion
Cortinarius, the largest agaric genus worldwide, contains important ectomycorrhizal fungi, among which sect. Anomali represents a monophyletic, species-rich group of this genus (Dima et al., 2016; Garnica et al., 2016; Soop et al., 2019; Dima et al., 2021; Liimatainen and Niskanen, 2021; Xie, 2022). Species recognition based on morphology is difficult in Cortinarius lineages due to overlapping characteristics and variations within species. Notably, the basidiospore size helps identify some species when used in correlation with other characteristics (Frøslev et al., 2006; Niskanen et al., 2013; Liimatainen et al., 2014; Liimatainen et al., 2015; Niskanen et al., 2016).
According to our phylogenetic analysis, sect. Anomali indicated a widely distributed lineage of Cortinarius in both the northern and southern Hemispheres. Furthermore, certain patterns of distribution correlated with ecology and plant hosts. Cortinarius albocyaneus exhibited regional patterns of distribution with conifers in northern Michigan and northern Europe. Several species, including Cortinarius brevissimus Peck, C. caeruleoanomalus Dima, Matheny, K. Hughes & Ammirati, C. deceptivus, C. harvardensis, C. modestus Rob. Henry, C. perrotensis A. Paul, Matheny & Lebeuf, and C. perviolaceus, occurred in hardwood, mixed hardwood conifer, and/or conifer forests in eastern North America. Cortinarius rarus Bojantchev, Ammirati, Parker, Liimat., Niskanen & Dima displayed regional patterns of distribution associated with mountain conifer forests (Dima et al., 2016; Dima et al., 2021).
Current studies related to this genus have indicated significant regional variations. Classical European species were examined and typified by Dima et al. (2016) before species from other parts of the world were studied. In sect. Anomali, more than 50 binomials were introduced in the last century, mainly from Europe, with fewer from elsewhere, but only about 20% of these names have been in general use (Dima et al., 2021). China is geographically located in East Asia and has a land area comparable with that of the entire Europe, various climate types ranging from the temperate to the tropical climate, as well as a complex and diverse habitat, which provides an ideal place for the survival of Cortinarius species. However, the resource investigation and taxonomic research on Cortinarius have not yet been extensively carried out in China. Currently, there have been reports of a total of 163 taxa of the genus in China, with only three species in the sect. Anomali, viz. C. caninus (Fr.) Fr., C. albocyaneus, and C. tabularis (Fr.) Fr. Therefore, the collection of more samples from China and exploration of multigene phylogeny are urgently needed to systematically elucidate the diversity of Cortinarius s.l.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/nuccore/OQ913379,OQ913380,OQ913381,OQ913382,OQ913383,OQ913384,OQ913385,OQ913386,OQ913387,OQ913388,OQ913389,OQ913390,OQ913391,OQ913392,OQ913393,OQ913394,OQ913395,OQ913396,OQ913397,OQ920003,OQ920004,OQ920005.
Author contributions
Q-YZ, CJ, H-MZ, Z-YM, Y-ZZ, J-QL, JS, and H-JL designed the research and contributed to data analysis and interpretation. Q-YZ, CJ, H-MZ and Z-YM prepared the samples and drafted the manuscript. Y-ZZ, J-QL, Z-YM, JS, and H-JL conducted the molecular experiments and analyzed the data. JS and H-JL discussed the results and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The research was supported by the National Natural Science Foundation of China (Nos. 32270016, 32270021, and 32070016).
Acknowledgments
The authors would like to express their deep appreciations to Xian-Hu Kang, Xi-Shang Li, Chao Guo, Guang-Jie Liu, Cheng-Sheng Li, Dan Jiang, Ping Wang, Wen-Song Chen, and Zhi-Wen Liu (Yunnan CDC, China) for helping during field collections.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ammirati, J., Garnica, S., Halling, R. E., Mata, M., Mueller, G. M., Carranza, J. (2007). New Cortinarius species associated with Quercus and Comarostaphylis in Costa Rica. Can. J. Bot. 85, 794–812. doi: 10.1139/B07-067
Ammirati, J. F., Niskanen, T., Liimatainen, K., Dimitar, B., Peintner, U., Kuhnert-Finkernagel, R., et al. (2017). Spring and early summer species of Cortinarius, subgenus Telamonia, section Colymbadini and /Flavobasilis, in the mountains of western north America. Mycologia 109, 443–458. doi: 10.1080/00275514.2017.1349468
Anonymous (1969). Flora of British fungi. colour identification chart (London: Her Majesty’s Stationery Office).
Bidaud, A., Moënne-Loccoz, P., Reumaux, P. (1992). Atlas des cortinaires. pars IV (France: Fédération Mycologique Dauphiné-Savoie).
Bidaud, A., Reumaux, P., Moënne-Loccoz, P. (1994). Novitates – validates – genre Cortinarius. Docums. Mycol. 24, 39–45.
Brandrud, T. E., Dima, B., Schmidt-Stohn, G., Bellù, F., Frøslev, T. G., Oertel, B., et al. (2014). Cortinarius subgenus Phlegmacium section Multiformes in Europe. J. Des. J.E.C. 16, 162–199.
Brandrud, T. E., Lindström, H., Marklund, H., Melot, J., Muskos, S. (1989). Cortinarius flora photographica I (Swedish version). Sweden, Matfors, Cortinarius HB. pp. 1–60.
Consiglio, G. (2012). Il genere cortinarius in italia. parte sesta. Luglio, Associazione Micologica Bresadola.
Consiglio, G., Antonini, D., Antonini, M. (2005). Il genere cortinarius in Italia. parte terza. Italy, Trento, Associazione Micologica Bresadola, pp. 1–44.
Consiglio, G., Antonini, D., Antonini, M. (2006). Il genere cortinarius in italia. Luglio, Associazione Micologica Bresadola, Fondazione Centro Studi Micologici.
Dima, B., Liimatainen, K., Niskanen, T., Bojantchev, D., Harrower, E., Papp, V., et al. (2021). Type studies and fourteen new north American species of Cortinarius section Anomali reveal high continental species diversity. Mycol. Prog. 20, 1399–1439. doi: 10.1007/s11557-021-01738-0
Dima, B., Lindstrom, H., Liimatainen, K., Olson, A., Soop, K., Kytovuori, I., et al. (2016). Typification of friesian names in Cortinarius sections Anomali, Spilomei, and Bolares, and description of two new species from northern Europe. Mycol. Prog. 15, 903–919. doi: 10.1007/s11557-016-1217-5
Frøslev, T. G., Brandrud, T. E., Jeppesen, T. S. (2006). New species and combinations in Cortinarius subgenus Phlegmacium section Calochroi. Mycotaxon 97, 367–377.
Garnica, S., Schon, M. E., Abarenkov, K., Riess, K., Liimatainen, K., Niskanen, T., et al. (2016). Determining threshold values for barcoding fungi: lessons from Cortinarius (Basidiomycota), a highly diverse and widespread ectomycorrhizal genus. FEMS Microbiol. Ecol. 92, 4. doi: 10.1093/femsec/fiw045
Garnica, S., Weiss, M., Oertel, B., Ammirati, J., Oberwinkler, F. (2009). Phylogenetic relationships in Cortinarius, section Calochroi, inferred from nuclear DNA sequences. BMC Evol. Biol. 9, 1. doi: 10.1186/1471-2148-9-1
Garnica, S., Weiss, M., Oertel, B., Oberwinkler, F. (2005). A framework for a phylogenetic classification in the genus Cortinarius (Basidiomycota, agaricales) derived from morphological and molecular data. Can. J. Bot. 83, 1457–1477. doi: 10.1139/b05-107
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Høiland, K., Holst-Jensen, A. (2000). Cortinarius phylogeny and possible taxonomic implications of ITS rDNA sequences. Mycologia 92, 694–710. doi: 10.1080/00275514.2000.12061210
Hughes, K. W., Petersen, R. H., Lickey, E. B. (2009). Using heterozygosity to estimate a percentage DNA sequence similarity for environmental species' delimitation across basidiomycete fungi. New Phytol. 182, 795–798. doi: 10.1111/j.1469-8137.2009.02802.x
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., Jermiin, L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. doi: 10.1038/nmeth.4285
Kauffman, C. H. (1905). The genus Cortinarius: a preliminary study. Bull. Torr. Bot. Club. 32, 301–325. doi: 10.2307/2478812
Kirk, P. M., Cannon, P. E., Minter, D. W., Stalpers, J. A. (2008). Dictionary of the fungi. 10 edition (Wallingford: CABI International).
Kornerup, A., Wanscher, J. H. (1978). Methuen Handbook of color. 3rd Ed. Ed. Methuen, E. (London: Co., Ltd.).
Li, H. J., Xie, J. W., Zhang, S., Zhou, Y. J., Ma, P. B., Zhou, J., et al. (2015). Amanita subpallidorosea, a new lethal fungus from China. Mycol. Prog. 14, 43. doi: 10.1007/s11557-015-1055-x
Liimatainen, K., Niskanen, T., Ammirati, J., Kytovuori, I., Dima, B. (2015). Cortinarius, subgenus Telamonia, section Disjungendi, cryptic species in north America and Europe. Mycol. Prog. 14, 1016. doi: 10.1007/s11557-014-1016-9
Liimatainen, K., Niskanen, T., Dima, B., Kytovuori, I., Ammirati, J. F., Froslev, T. G. (2014). The largest type study of agaricales species to date: bringing identification and nomenclature of Phlegmacium (Cortinarius, agaricales) into the DNA era. Persoonia 33, 98–140. doi: 10.3767/003158514X684681
Luo, Y., Bau, T. (2021). Cortinarius jiaoheensis (Cortinariaceae), a new species of Cortinarius subgenus Telamonia section Flexipedes, from northeast China. Phytotaxa 494, 113–121. doi: 10.11646/phytotaxa.494.1.7
Niskanen, T., Kytovuori, I., Liimatainen, K., Lindstrom, H. (2013). The species of Cortinarius, section Bovini, associated with conifers in northern Europe. Mycologia 105, 977–993. doi: 10.3852/12-320
Niskanen, T., Liimatainen, K., Kytovuori, I. (2009). Cortinarius sect. Brunnei (Basidiomycota, agaricales) in north Europe. Mycol. Res. 113, 182–206. doi: 10.1016/j.mycres.2008.10.006
Niskanen, T., Liimatainen, K., Kytövuori, I., Lindström, H., Dentinger, B., Ammirati, J. F. (2016). Cortinarius subgenus Callistei in north America and Europe–type studies, diversity, and distribution of species. Mycologia 108, 1018–1027. doi: 10.3852/16-033
Peintner, U., Moncalvo, J. M., Vilgalys, R. (2004). Toward a better understanding of the infrageneric relationships in Cortinarius (Agaricales, basidiomycota). Mycologia 96, 1042–1058. doi: 10.1080/15572536.2005.11832904
Peintner, U., Moser, M., Thomas, K. A., Manimohan, P. (2003). First records of ectomycorrhizal Cortinarius species (Agaricales, basidiomycetes) from tropical India and their phylogenetic position based on rDNA ITS sequences. Mycol. Res. 107, 485–494. doi: 10.1017/S0953756203007585
Ronquist, F., Teslenko, M., van der Mark, P., Avres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice, across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Silvestro, D., Michalak, I. (2012). RaxmlGUI: a graphical front-end for RAxML. Org. Divers. Evol. 12, 335–337. doi: 10.1007/s13127-011-0056-0
Singer, R. (1986). The agaricales in modern taxonomy. 4th ed (Germany: Koenigstein, Koeltz Scientific Books).
Soop, K., Dima, B., Cooper, J. A., Park, D., Oertel, B. (2019). A phylogenetic approach to a global supraspecific taxonomy of Cortinarius (Agaricales) with an emphasis on the southern mycota. Persoonia 42, 261–290. doi: 10.3767/persoonia.2019.42.10
Soop, K., Wallace, M., Dima, B. (2018). New Cortinarius (Agaricales) species described from new Zealand. New Z. J. Bot. 56, 163–182. doi: 10.1080/0028825X.2018.1436574
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinf. (Oxford England) 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Stensrud, Ø., Orr, R. J. S., Reier-Røberg, K., Schumacher, T., Orr, R., Høiland, K. (2014). Phylogenetic relationships in Cortinarius with focus on north European species. Karstenia 54, 57–71. doi: 10.29203/ka.2014.464
Suarez-Santiago, V. N., Ortega, A., Peintner, U., Lopez-Flores, I. (2009). Study on Cortinarius subgenus Telamonia section Hydrocybe in Europe, with especial emphasis on Mediterranean taxa. Mycol. Res. 113, 1070–1090. doi: 10.1016/j.mycres.2009.07.006
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882. doi: 10.1093/nar/25.24.4876
Varga, T., Krizsan, K., Cs, F., Dima, B., Sanchez-Garcia, M., Sanchez-Ramirez, S., et al. (2019). Megaphylogeny resolves global patterns of mushroom diversification. Nat. Ecol. Evol. 3, 668–678. doi: 10.1038/s41559-019-0834-1
Wei, T. Z., Yao, Y. J. (2013). Cortinarius korfii: a new species from China. Mycosystema 32, 557–562. doi: 10.13346/j.mycosystema.2013.03.017
White, T. J., Bruns, T. D., Lee, S., Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR protocols: a guide to methods and applications. Eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J., White, T. J. (US: New York Academic Press), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Xie, M. L. (2022). Taxonomic, molecular phylogenetic and biogeographic studies of cortinarius in china. PhD thesis (China: Northeast Normal University), 1–314.
Xie, M. L., Li, D., Wei, S. L., Ji, R. Q., Li, Y. (2019). Cortinarius subcaesiobrunneus sp. nov., (Cortinariaceae, agaricales) a new species from northwest China. Phytotaxa 392, 217–224. doi: 10.11646/phytotaxa.392.3.4
Keywords: fungi diversity, morphology, new taxa, phylogeny, taxonomy
Citation: Zhang Q-Y, Jin C, Zhou H-M, Ma Z-Y, Zhang Y-Z, Liang J-Q, Si J and Li H-J (2023) Enlargement of the knowledge of Cortinarius section Anomali (Agaricales, Basidiomycota): introducing three new species from China. Front. Cell. Infect. Microbiol. 13:1215579. doi: 10.3389/fcimb.2023.1215579
Received: 02 May 2023; Accepted: 23 May 2023;
Published: 12 June 2023.
Edited by:
Yusufjon Gafforov, Academy of Science of the Republic of Uzbekistan, UzbekistanReviewed by:
Ming Zhang, Guangdong Academy of Science, ChinaLu-Sen Bian, Chinese Academy of Forestry, China
Yu-Guang Fan, Hainan Medical University, China
Copyright © 2023 Zhang, Jin, Zhou, Ma, Zhang, Liang, Si and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Si, amluZ3NpMTc4OEAxMjYuY29t; Hai-Jiao Li, bGloYWlqaWFvNzE1QDEyNi5jb20=
 Qiu-Yue Zhang
Qiu-Yue Zhang Can Jin1
Can Jin1 Yi-Zhe Zhang
Yi-Zhe Zhang Jia-Qi Liang
Jia-Qi Liang Jing Si
Jing Si Hai-Jiao Li
Hai-Jiao Li