
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 18 May 2023
Sec. Fungal Pathogenesis
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1189600
This article is part of the Research Topic Diversity and Molecular Diagnostics of Fungi and Oomycetes in Plants View all 18 articles
Two new species of Scytinostroma viz. S. acystidiatum and S. macrospermum, are described from southwest China. Phylogeny based on ITS + nLSU dataset demonstrates that samples of the two species form two independent lineages and are different in morphology from the existing species of Scytinostroma. Scytinostroma acystidiatum is characterized by resupinate, coriaceous basidiomata with cream to pale yellow hymenophore, a dimitic hyphal structure with generative hyphae bearing simple septa, the absence of cystidia, and amyloid, broadly ellipsoid basidiospores measuring 4.7–7 × 3.5–4.7 μm. Scytinostroma macrospermum is characterized by resupinate, coriaceous basidiomata with cream to straw yellow hymenophore, a dimitic hyphal structure with generative hyphae bearing simple septa, numerous cystidia embedded or projecting from hymenium, and inamyloid, ellipsoid basidiospores measuring 9–11 × 4.5–5.5 μm. The differences between the new species and morphologically similar and phylogenetically related species are discussed.
The genus Scytinostroma Donk (Russulales, Basidiomycota), typified by S. portentosum (Berk. & M.A. Curtis) Donk, was established by Donk (1956). It is traditionally characterized by resupinate, coriaceous basidiomata, smooth to tuberculate hymenophore and a dimitic hyphal structure with simple septa or clamps on generative hyphae, filiform and dichotomously branched skeletal hyphae which are dextrinoid and cyanophilous, and subglobose to ellipsoid, thin-walled, variably amyloid basidiospores, and a white-rotting ecology (Donk, 1956; Rattan, 1974; Bernicchia and Gorjón, 2010; Wang et al., 2020; Tabish and Daniel, 2021).
The genus accommodated seven species derived from Corticium Fr. (without gloeocystidia) and Gloeocystidium P. Karst. (with gloeocystidia) when it was established. Later, Scytinostroma was gradually recognized by taxonomists, and the number of new species and new combinations has been increasing continuously (Donk, 1956; Gilbertson, 1962; Boidin, 1967; Rattan, 1974; Boidin and Lanquetin, 1977; Lanquetin, 1984; Boidin and Lanquetin, 1987; Boidin and Gilles, 1988; Hjortstam, 1990; Stalpers, 1996). So far, 36 species have been accepted in Scytinostroma worldwide (Nakasone, 2008; Liu, 2019; Wang et al., 2020). Recently, molecular phylogenetic studies demonstrated that Scytinostroma nested in Peniophoraceae within Russulales; furthermore, Scytinostroma was polyphyletic and formed four stable clades, as well as related to Gloiothele Bres., Vararia P. Karst., and Dichostereum Pilát (Nakasone and Micales, 1988; Larsson and Larsson, 2003; Miller et al., 2006; Larsson, 2007). Morphologically, Scytinostroma species are separated from other corticioid fungi of Russulales mainly by their tough and leathery texture of the basidiomata, as well as dextrinoid and dichotomously branched skeletal hyphae (Rattan, 1974; Liu, 2019).
During investigations on the diversity of wood-rotting fungi from China, two unknown corticioid specimens were collected from southwest China, and their morphology corresponded to the concepts of Scytinostroma. To confirm their affinity, phylogenetic analyses based on the ITS+ nLSU rDNA sequences were carried out. The two newly sequenced samples from Guizhou and Chongqing formed two well-supported lineages clustered with two sequences from Korea (KJ668461, Jang et al., 2016) and Japan (LC327052, Ogura-Tsujita et al., 2018), respectively. Based on morphological and phylogenetic evidences, we hereby propose two new species of Scytinostroma.
The studied specimens are deposited in the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC). Macro-morphological descriptions are based on field notes and dried specimens. Color terms followed Petersen (1996). Microscopic structures and abbreviations used in this study followed Wu et al. (2020) and Liu et al. (2022).
A CTAB rapid plant genome extraction kit (Aidlab Biotechnologies, Co., Ltd., Beijing, China) was used to obtain DNA products from voucher specimens, according to the manufacturer’s instructions with some modifications (Yuan et al., 2021; Yuan et al., 2022). The following primer pairs were used to amplify the DNA: ITS5 (5′-GGA AGT AAA AGT CGT AAC AAG G-3′) and ITS4 (5′-TCC TCC GCT TAT TGATAT GC-3′) for the internal transcribed spacer (ITS) regions (White et al., 1990); LR0R (5′-ACC CGC TGA ACT 6 TAA GC-3′) and LR7 (5′-TAC TAC CAC CAA GAT CT-3′) for nuclear large subunit (nLSU) rDNA (Vilgalys and Hester, 1990).
The procedures for DNA extraction and polymerase chain reaction (PCR) used in this study were the same as described by Wu et al. (2022b). The PCR products were purified and sequenced by Beijing Genomics Institute (BGI), China. All newly generated sequences in this study were deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and listed in Table 1.
Phylogenetic analyses were performed with the Maximum Parsimony (MP), Maximum Likelihood (ML), and Bayesian Inference (BI) methods. New sequences generated in this study, along with reference sequences retrieved from GenBank (Table 1), were aligned by MAFFT 7 (Katoh et al., 2019; http://mafft.cbrc.jp/alignment/server/) using the “G-INS-i” strategy and manually adjusted in BioEdit (Hall, 1999). Unreliably aligned sections were removed before the analyses, and efforts were made to manually inspect and improve the alignment. The data matrix was edited in Mesquite v3.70. Confertobasidium olivaceoalbum (Bourdot & Galzin) Jülich and Metulodontia nivea (P. Karst.) Parmasto were selected as outgroups (Larsson and Larsson, 2003).
MP topology and bootstrap (BT) values obtained from 1,000 replicates were computed in PAUP* version 4.0b10 (Swofford, 2002). All characters were equally weighted, and the gaps were treated as missing data. Trees were inferred using the heuristic search option with tree-bisection reconnection (TBR) branch swapping and 1,000 random sequence additions. Max-trees were set to 5,000, branches of zero length were collapsed, and all parsimonious trees were saved. Clade robustness was assessed by a BT analysis with 1,000 replicates (Felsenstein, 1985). Descriptive tree statistics, such as tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC), and homoplasy index (HI) were calculated for each Maximum Parsimonious Tree (MPT) generated.
RAxML 7.2.8 was used to construct ML trees for the combined dataset with the GTR+I+G model of site substitution, including estimation of Gamma-distributed rate heterogeneity and a proportion of invariant sites (Stamatakis, 2006). The branch support was evaluated with a bootstrapping method of 1000 replicates (Hillis and Bull, 1993).
The BI was conducted with MrBayes 3.2.6 in two independent runs, each of which had four chains for 5 million generations and started from random trees (Ronquist and Huelsenbeck, 2003). Trees were sampled every 1,000 generations. The first 25% of the sampled trees were discarded as burn-in, and the remaining ones were used to reconstruct a majority rule consensus and calculate Bayesian Posterior Probabilities (BPP) of the clades.
Branches that received BT supports for Maximum Parsimony (BP) and Maximum Likelihood (BS) greater than or equal to 75%, and BPP greater than or equal to 0.95 were considered as significantly supported. FigTree v1.4.4 and Treeview (Page, 1996) were used to visualize the resulting tree.
Two ITS and two nLSU sequences were generated in this study and were deposited in GenBank. Their accession numbers are specified in the phylogenetic tree (Figure 1). The final ITS + nLSU dataset included 60 sequences representing 28 species and resulted in an alignment of 1,826 characters. Maximum parsimony analysis yielded one equally parsimonious tree (TL = 2833, CI = 0.502, HI = 0.846, RI = 0.424, and RC = 0.498). BI analysis and ML analysis resulted in a similar topology to the MP analysis, with an average standard deviation of split frequencies of 0.002601 (BI).
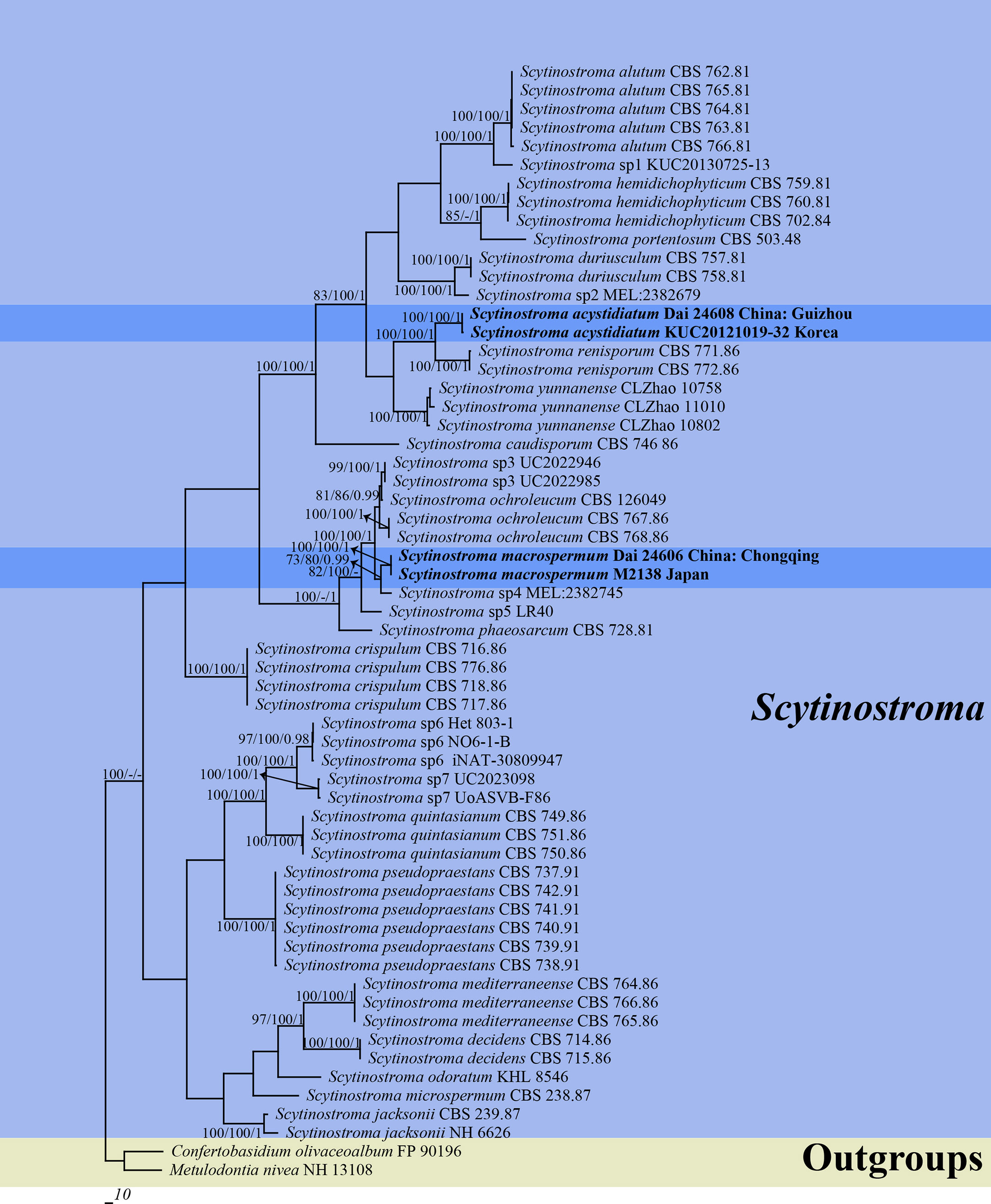
Figure 1 Phylogeny of Scytinostroma by Maximum Parsimony (MP) analysis based on combined ITS + nLSU dataset. Branches are labeled with bootstrap supports for Maximum Parsimony (BP) > 70%, Maximum Likelihood bootstrap (BS) > 70%, and Bayesian Posterior Probabilities (BPP) > 0.95, respectively. The new species are in bold.
The phylogeny (Figure 1) inferred from the ITS + nLSU dataset demonstrated that two new species, Scytinostroma acystidiatum and S. macrospermum, clustered in the Scytinostroma clade. Moreover, Scytinostroma acystidiatum clustered with one sample from Korea (KUC20121019-32) formed an independent lineage with a robust support (BP = 100%, BS = 100%, and BPP = 1.00) and then closely related to S. renisporum Boidin, Lanq. & Gilles. S. macrospermum clustered with one sample from Japan (M2138), forming an independent lineage with a strong support (BP = 100%, BS = 100%, and BPP = 1.00).
Scytinostroma acystidiatum Q.Y. Zhang, L.S. Bian & Q. Chen, sp. nov., Figures 2, 3

Figure 2 Basidiomata of Scytinostroma acystidiatum (Holotype, Dai 24608). Scale bar = 1.0 cm. Photo by: Qiu-Yue Zhang.
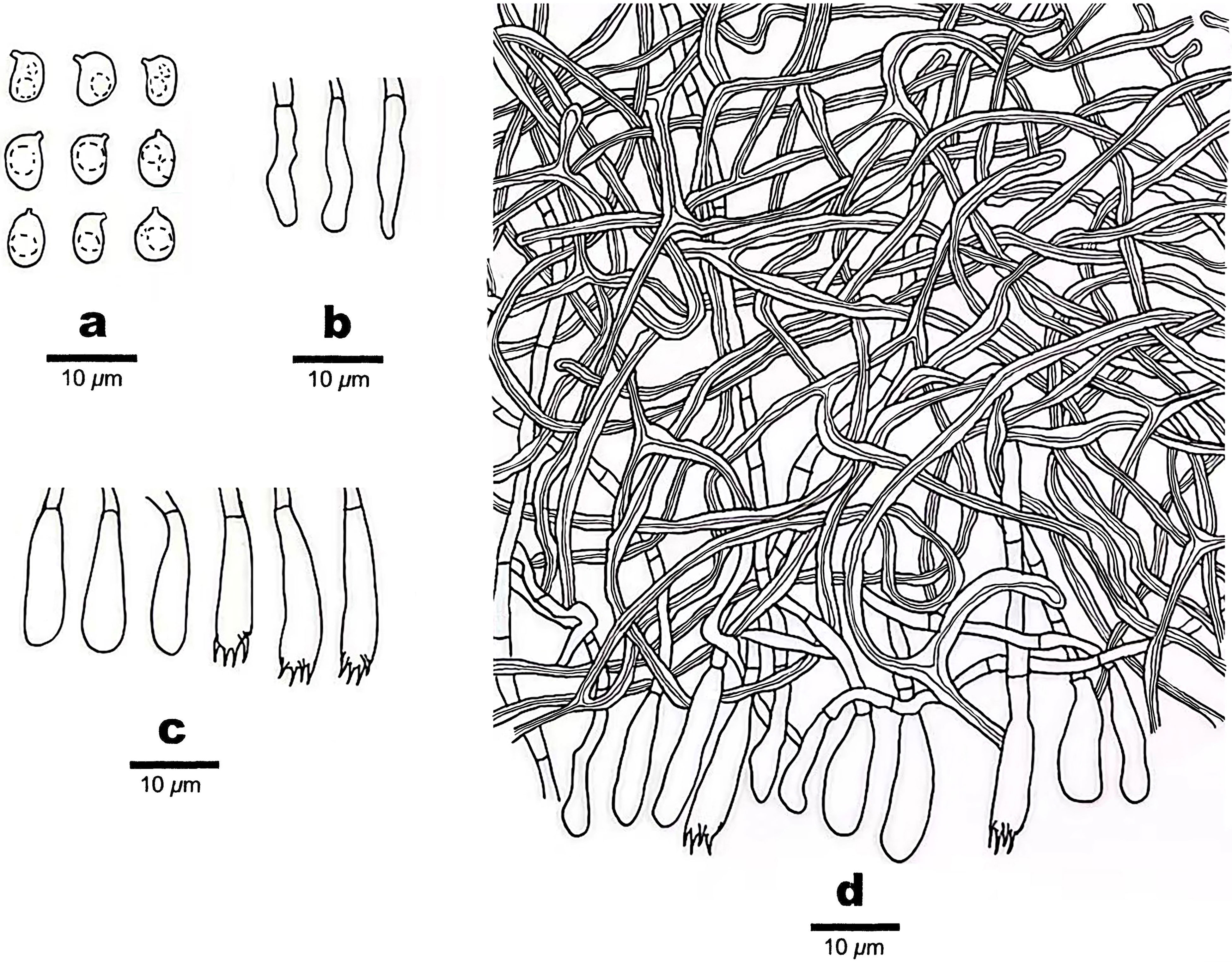
Figure 3 Microscopic structures of Scytinostroma acystidiatum (Holotype, Dai 24608). (A) Basidiospores. (B) Cystidioles. (C) Basidia and basidioles. (D) A section of basidiomata. Drawings by: Qiu-Yue Zhang.
MycoBank no.: 848524
Type — China, Guizhou Province, Tongren, Fanjingshan, on fallen angiosperm branch, 13 July 2022, Dai 24608 (BJFC039842).
Etymology — Acystidiatum (Lat.): refers to the species lacking cystidia.
Basidiomata —Annual, resupinate, coriaceous, not separable from substrate, up to 7 cm long, 2 cm wide, and less than 0.1 mm thick at center. Hymenial surface smooth to locally tuberculate, cream to pale yellow; margin concolorous with hymenial surface, thinning out, and adnate.
Hyphal structure —Hyphal system dimitic; generative hyphae infrequent, simple septate, hyaline, thin-walled, rarely branched, 2–3 μm in diameter, IKI–, CB–; skeletal hyphae dominant, frequently dichotomously branched, tortuous, interwoven, thick-walled, dextrinoid, cyanophilous, 1–2.5 μm in diameter; tissues unchanged in KOH.
Hymenium —Cystidia absent; cystidioles present, clavate, some gradually tapering to the apex, thin-walled, hyaline, smooth, 12–18 × 2–4 μm; basidia clavate, with a basal simple septum and four sterigmata, thin-walled, smooth, 13–21 × 3.5–5 μm; basidioles similar to basidia in shape, but slightly smaller.
Spores —Basidiospores broadly ellipsoid with an apiculus, hyaline, thin-walled, smooth, occasionally with one or two guttules, amyloid, acyanophilous, (4.5–)4.7–7 × (3–)3.5–4.7(–5) μm, L = 5.68 μm, W = 4.02 μm, Q = 1.41 (n = 30/1).
Scytinostroma macrospermum Q.Y. Zhang, L.S. Bian & Q. Chen, sp. nov., Figures 4, 5

Figure 4 Basidiomata of Scytinostroma macrospermum (Holotype, Dai 24606). Scale bar = 1.0 cm. Photo by: Qiu-Yue Zhang.
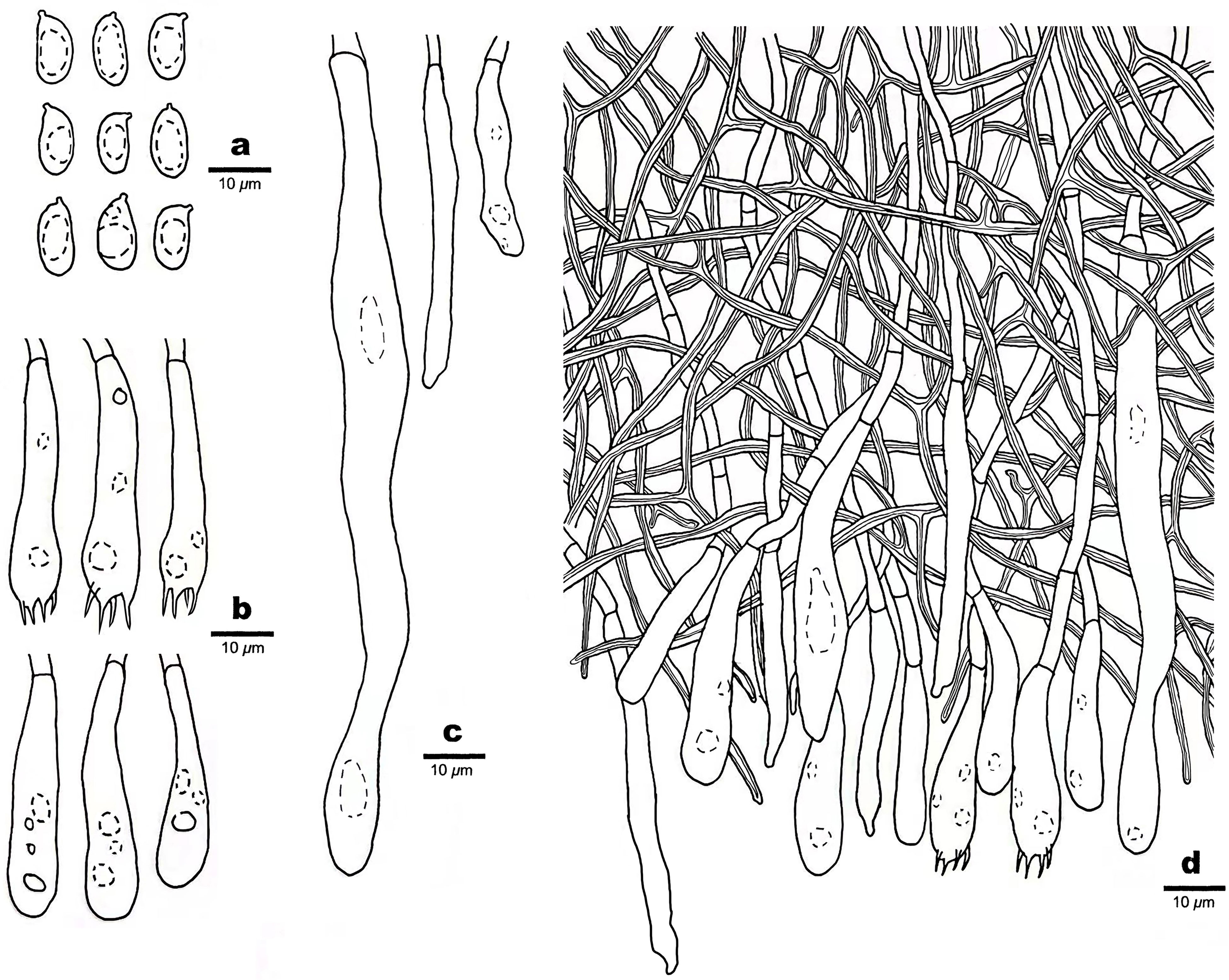
Figure 5 Microscopic structures of Scytinostroma macrospermum (Holotype, Dai 24606). (A) Basidiospores. (B) Basidia and basidioles. (C) Cystidia. (D) A section of basidiomata. Drawings by: Qiu-Yue Zhang.
MycoBank no.: 848525
Type —China, Chongqing, Jiangjin District, Simianshan National Scenic Spot, on rotten angiosperm wood, 10 July 2022, Dai 24606 (BJFC039838).
Etymology — Macrospermum (Lat.): refers to the species having large basidiospores.
Basidiomata —Annual, resupinate, coriaceous, not separable from substrate, up to 13.5 cm long, 3 cm wide, and less than 0.2 mm thick at center. Hymenial surface smooth to locally tuberculate, cream to straw yellow; margin concolorous with hymenial surface, slightly fimbricate.
Hyphal structure —Hyphal system dimitic; generative hyphae infrequent, simple septate, thin-walled, hyaline, rarely branched, 1.5–3 μm in diameter, IKI–, CB–; skeletal hyphae dominant, frequently dichotomously branched, interwoven, thick-walled, dextrinoid, cyanophilous, 1–3 μm in diameter; tissues unchanged in KOH.
Hymenium —Cystidia numerous, narrowly fusoid to cylindrical, thin-walled, smooth, 25–107 × 2.5–10 μm, embedded or projecting from hymenium up to 25 µm; basidia clavate, with a basal simple septum and four sterigmata, thin-walled, smooth, with some guttules, 30–45 × 6–8 μm; basidioles dominant, similar to basidia in shape, but slightly smaller.
Spores —Basidiospores ellipsoid with an apiculus, hyaline, thin-walled, smooth, occasionally with one or two guttules, inamyloid, acyanophilous, 9–11(–12) × (4–)4.5–5.5(–6) μm, L = 9.89 μm, W = 4.94 μm, Q = 2.00 (n = 30/1).
Two new species, Scytinostroma acystidiatum and S. macrospermum, are described in this study based on morphological characteristics and phylogenetic analyses. The ITS + nLSU-based phylogeny (Figure 1) shows the phylogenetic positions of the two new species in the genus Scytinostroma. In detail, the sequence of KUC20121019-32 from Korea, clustered together with Scytinostroma acystidiatum, and shares less than 1.5% sequence (ITS) dissimilarity (Jang et al., 2016). The sample KUC20121019-32 was collected in Odaesan National Park, South Korea, which has geographical proximity (eastern Asia) and a similar climate (subtropical climate) to Guizhou, China. So, we treat KUC20121019-32 as Scytinostroma acystidiatum. In addition, Scytinostroma acystidiatum grouped with S. renisporum with strong support (100% BP, 100% BS, 1.00 BPP, Figure 1). Scytinostroma renisporum is morphologically distinguished from S. acystidiatum by its membranaceous to paper-like basidiomata and larger gloeocystidia measuring 20–35 × 6–10 µm (Boidin and Lanquetin, 1987).
Morphologically, Scytinostroma alutum Lanq., S. arachnoideum (Peck) Gilb., S. cystidiatum Boidin, S. hemidichophyticum Pouzar, S. portentosum (Berk. & M.A. Curtis) Donk, and S. yunnanense C.L. Zhao are similar to S. acystidiatum by sharing amyloid basidiospores. However, S. alutum differs from S. acystidiatum by its resupinate to effuse-reflexed basidiomata with cracked hymenophore, larger basidia (40–65 × 5–6 µm vs. 13–21 × 3.5–5 μm), and bigger basidiospores (5.3–7.2 × 5.7–7.3 μm vs. 4.7–7 × 3.5–4.7 μm; Lanquetin, 1984). Scytinostroma arachnoideum is separated from S. acystidiatum by its cottony basidiomata with white rhizomorphs and smaller basidiospores (3.5–4.5 × 3–3.5 μm vs. 4.7–7 × 3.5–4.7 μm; Gilbertson, 1962). Scytinostroma cystidiatum, S. hemidichophyticum, and S. portentosum are separated from S. acystidiatum by the presence of cystidia (Donk, 1956; Boidin, 1960; Pouzar, 1966). S. yunnanense differs from S. acystidiatum by its white to cream basidiomata and shorter basidiospores (4.5–5.5 μm vs. 4.7–7 μm in length; Wang et al., 2020).
Phylogenetically, the sequence of M2138 from Japan, clustered together with Scytinostroma macrospermum and formed an independent lineage with less than 1.5% sequence (ITS) dissimilarity (Ogura-Tsujita et al., 2018). The sample M2138 was collected in Kagoshima, Japan, which has geographical proximity (eastern Asia) and a similar climate (subtropical climate) to Chongqing, China. So, we treat M2138 as Scytinostroma macrospermum (Figure 1). Morphologically, Scytinostroma ochroleucum (Bres. & Torrend) Donk resembles S. macrospermum by resupinate, cream-colored to pale ochraceous basidiomata, but the former is different from the latter by its larger basidia (35–85 × 6.5–9 µm vs. 30–45 × 6–8 μm), and larger basidiospores (9–14 × 5–7 µm vs. 9–11 × 4.5–5.5 μm; Donk, 1956). Scytinostroma phaeosarcum Boidin & Lanq. resembles S. macrospermum by the approximately same size of basidiospores (8–10 × 4.5–5.5 μm), while S. phaeosarcum differs from S. macrospermum by its basidiomata becoming brown when bruised and thinner basidia (3–5 μm vs. 6–8 μm in width; Boidin and Lanquetin, 1977). In addition, Scytinostroma macrospermum is similar to S. decidens Boidin, Gilles & Lanq., S. jacksonii Boidin and S. mediterraneense Boidin & Lanq. by sharing large cystidia (> 100 μm in length) and inamyloid basidiospores. However, the latter three species distinctly differ from S. macrospermum by their obviously narrower basidiospores (2.5–3.5 μm in width vs. 4.5–5.5 μm in width, Boidin, 1981; Boidin and Lanquetin, 1987; Nakasone and Micales, 1988).
Wood-rotting fungi as an important group within the Basidiomycota are known for their ecological role in the forest ecosystem in terms of decaying living and dead trees and recycling nutrients in forest ecosystems (Dai et al., 2007; Yuan et al., 2021; Yuan et al., 2022). However, the diversity and taxonomy of these fungi remain not well known, and many new species have been described recently because of the application of molecular phylogeny (Dai et al., 2021; Mao et al., 2023; Wang et al., 2021; Wang et al., 2022; Wu et al., 2022a; Wu et al., 2022b; Zhou et al., 2021). Similarly, despite numerous species of Scytinostroma have been described, many unknown species or unnamed sequences still exist (Scytinostroma sp., Figure 1). Consequently, with the application of molecular phylogeny, the diversity and systematics will be outlined by further studies based on more samples worldwide.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, OQ629350, OQ629351, OQ689126, OQ689127.
Q-YZ, H-GL, L-SB, and QC designed the research and contributed to data analysis and interpretation. Q-YZ prepared the samples and drafted the manuscript. H-GL, L-SB and QC discussed the results and edited the manuscript. All authors contributed to the article and approved the submitted version.
The research was financed by the National Natural Science Foundation of China (Project nos. 32100014, 31800018) and Fundamental Research Funds for the Central Non-profit Research Institution of the Chinese Academy of Forestry (Project No. CAFYBB2021MA007).
The authors would like to express their deep appreciations to Prof. Yu-Cheng Dai (Beijing Forestry University, China) for allowing us to study his specimens.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bernicchia, A., Gorjón, S. P. (2010). Fungi europaei 12: corticiaceae s.l (Lomazzo: Edizioni Candusso), 1–1007.
Boidin, J. (1960). Le genre stereum pers. s.l. au Congo belge. Bull. du Jardin Botanique l'État à Bruxelles 30, 283–355. doi: 10.2307/3667306
Boidin, J. (1967). Basidiomycètes lachnocladiaceae résupinés de la republique centrafricaine. Cahiers la Maboké 5, 23–35.
Boidin, J. (1981). Nouvelles espèces del lachnocladiaceae du Canada (Basidiomycetes). Naturaliste Canadien 108, 199–203.
Boidin, J., Gilles, G. (1988). Basidiomycètes aphyllophorales de l’Île de la réunion XI - compléments aux genres traités antérieurement (2e partie). Bull. la Société Mycologique France 104, 179–190.
Boidin, J., Lanquetin, P. (1977). Scytinostroma albo-cinctum (Berk. & br.) et phaeosarcum nov. sp. (Basidiomycètes, lachnocladiaceae). Kew Bull. 31, 621–628. doi: 10.2307/4119412
Dai, Y. C., Cui, B. K., Yuan, H. S., Li, B. D. (2007). Pathogenic wood-decaying fungi in China. For. Pathol. 37, 105–120. doi: 10.1111/j.1439-0329.2007.00485.x
Dai, Y. C., Yang, Z. L., Cui, B. K., Wu, G., Yuan, H. S., Zhou, L. W., et al. (2021). Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 40, 770–805. doi: 10.13346/j.mycosystema.210036
Felsenstein, J. (1985). Confidence intervals on phylogenetics: an approach using bootstrap. Evolution 39, 783–791. doi: 10.2307/2408678
Gilbertson, R. L. (1962). Resupinate hydnaceous fungi of north America 1. type studies of species described by peck. Mycologia 54, 658–677. doi: 10.1080/00275514.1962.12025047
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids symposium Ser. 41, 95–98.
Hillis, D. M., Bull, J. J. (1993). An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst. Biol. 42, 182–192. doi: 10.1093/sysbio/42.2.182
Hjortstam, K. (1990). Corticioid fungi described by M.J. Berkeley 2. species from Cuba. Mycotaxon 39, 415–423.
Jang, Y., Jang, S., Lee, J., Lee, H., Lim, Y. W., Kim, C., et al. (2016). Diversity of wood-inhabiting polyporoid and corticioid fungi in odaesan national park, Korea. Mycobiology 44, 217–236. doi: 10.5941/MYCO.2016.44.4.217
Katoh, K., Rozewicki, J., Yamada, K. D. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166. doi: 10.1093/bib/bbx108
Lanquetin, P. (1984). Scytinostroma aluta nov. sp. Bull. Mensuel la Société Linnéenne Lyon 53, 187–189. doi: 10.3406/linly.1984.10642
Larsson, K. H. (2007). Re-thinking the classification of corticioid fungi. Mycol. Res. 111, 1040–1063. doi: 10.1016/j.mycres.2007.08.001
Larsson, E., Larsson, K. H. (2003). Phylogenetic relationships of russuloid basidiomycetes with emphasis on Aphyllophoralean taxa. Mycol. Prog. 95, 1037–1065. doi: 10.1080/15572536.2004.11833020
Liu, S. L. (2019). “Taxonomy and phylogeny of Vararia and related genera in China (Doctoral dissertation),” (China: Beijing Forestry University).
Liu, Z. B., Wu, Y. D., Zhao, H., Lian, Y. P., Wang, Y. R., Wang, C. G., et al. (2022). Outline, divergence times, and phylogenetic analyses of trechisporales (Agaricomycetes, basidiomycota). Front. Microbiol. 13. doi: 10.3389/fmicb.2022.818358
Mao, W. L., Wu, Y. D., Liu, H. G., Yuan, Y., Dai, Y. C. (2023). A contribution to Porogramme (Polyporaceae, agaricomycetes) and related genera. IMA fungus 14, 5. doi: 10.1186/s43008-023-00110-z
Miller, S. L., Larsson, E., Larsson, K. H., Verbeken, A., Nuytinck, J. (2006). Perspectives in the new russulales. Mycologia 98, 960–970. doi: 10.1080/15572536.2006.11832625
Nakasone, K. K. (2008). Type studies of corticioid hymenomycetes described by bresadola. Cryptogamie Mycol. 29, 231–257.
Nakasone, K. K., Micales, J. A. (1988). Scytinostroma galactinum species complex in the united states. Mycologia 80, 546–559. doi: 10.1080/00275514.1988.12025577
Ogura-Tsujita, Y., Gebauer, G., Xu, H., Fukasawa, Y., Umata, H., Tetsuka, K., et al. (2018). The giant mycoheterotrophic orchid Erythrorchis altissima is associated mainly with a divergent set of wood-decaying fungi. Mol. Ecol. 27, 1324–1337. doi: 10.1111/mec.14524
Otto, E., Held, B., Redford, S., Blanchette, R. A. (2021). Detecting Heterobasidion irregulare in Minnesota and assessment of indigenous fungi on pines. Forests 12, 57. doi: 10.3390/f12010057
Page, R. D. M. (1996). Treeview: application to display phylogenetic trees on personal computers. Bioinformatics 12, 357–358. doi: 10.1093/bioinformatics/12.4.357
Petersen, J. H. (1996). Farvekort. the Danish mycological society’s color-chart (Greve: Foreningen til Svampekundskabens Fremme).
Pouzar, Z. (1966). Scytinostroma hemidichophyticum pouz. spec. nov. a new species of resupinate hymenomycetes. Ceská Mykologie 20, 217–220.
Rattan, S. S. (1974). Scytinostroma in India with notes on extralimital species. Trans. Br. Mycological Soc. 63, 1–12. doi: 10.1016/S0007-1536(74)80129-4
Ronquist, F., Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Rosenthal, L. M., Larsson, K. H., Branco, S., Chung, J. A., Glassman, S. I., Liao, H. L., et al. (2017). Survey of corticioid fungi in north American pinaceous forests reveals hyperdiversity, underpopulated sequence databases, and species that are potentially ectomycorrhizal. Mycologia 109, 115–127. doi: 10.1080/00275514.2017.1281677
Stalpers, J. A. (1996). The aphyllophoraceous fungi 2. keys to the species of the hericiales. Stud. Mycol. 40, 1–185.
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Swofford, D. L. (2002). PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0 beta (Sunderland, MA: Sinauer). doi: 10.1002/0471650129.dob0522
Tabish, M., Daniel, S. (2021). Scytinostroma portentosum (Berk. and curt.) donk from West Bengal, India on a new host. Curr. Sci. 120, 30.
Vilgalys, R., Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Vu, D., Groenewald, M., De Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., et al. (2019). Large-Scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 92, 135–154. doi: 10.1016/j.simyco.2018.05.001
Wang, Y. R., Dai, Y. C., Liu, H. G., Vlasák, J., Buchanan, P., Yuan, Y., et al. (2022). A new contribution to Megasporoporia sensu lato, six new species and three new combinations. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1046777
Wang, H., He, X., Zhao, C. L. (2020). Scytinostroma yunnanense sp. nov. (Russulales, basidiomycota) evidenced by morphological characteristics and phylogenetic analyses in China. Phytotaxa 451, 145–153. doi: 10.11646/phytotaxa.451.2.4
Wang, Y. R., Wu, Y. D., Vlasák, J., Yuan, Y., Dai, Y. C. (2021). Phylogenetic analysis demonstrating four new species in Megasporoporia sensu lato (Polyporales, basidiomycota). Mycosphere 12, 1012–1037. doi: 10.5943/mycosphere/12/1/11
White, T. J., Bruns, T., Lee, S., Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics,” in PCR protocols: a guide to methods and applications. Eds. Innis, M. A., Gelfand, D. H., Sninsky, J. J., White, T. J. (New York, NY: Academic Press), 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wu, F., Man, X. W., Tohtirjap, A., Dai, Y. C. (2022a). A comparison of polypore funga and species composition in forest ecosystems of China, north America, and Europe. For. Ecosyst. 9, 100051. doi: 10.1016/j.fecs.2022.100051
Wu, F., Yuan, Y., Chen, J. J., Cui, B. K., Zhou, M., Dai, Y. C. (2020). Terrestriporiaceae fam. nov., a new family of russulales (Basidiomycota). Mycosphere 11, 2755–2766. doi: 10.5943/mycosphere/11/1/21
Wu, F., Zhou, L. W., Vlasák, J., Dai, Y. C. (2022b). Global diversity and systematics of hymenochaetaceae with poroid hymenophore. Fungal Divers. 113, 1–192. doi: 10.1007/s13225-021-00496-4
Yuan, Y., Chen, J. J., Korhonen, K., Martin, F., Dai, Y. C. (2021). An updated global species diversity and phylogeny in the forest pathogenic genus Heterobasidion (Basidiomycota, russulales). Front. Microbiol. 11. doi: 10.3389/fmicb.2020.596393
Yuan, Y., Wu, Y. D., Wang, Y. R., Zhou, M., Qiu, J. Z., Li, D. W., et al. (2022). Two new forest pathogens in Phaeolus (Polyporales, basidiomycota) on Chinese coniferous trees were confirmed by molecular phylogeny. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.942603
Keywords: new taxa, Peniophoraceae, phylogeny, taxonomy, wood-rotting fungi
Citation: Zhang Q-Y, Liu H-G, Bian L-S and Chen Q (2023) Two new species of Scytinostroma (Russulales, Basidiomycota) in Southwest China. Front. Cell. Infect. Microbiol. 13:1189600. doi: 10.3389/fcimb.2023.1189600
Received: 19 March 2023; Accepted: 28 April 2023;
Published: 18 May 2023.
Edited by:
Yusufjon Gafforov, Academy of Science of the Republic of Uzbekistan, UzbekistanReviewed by:
Bálint Dima, Eötvös Loránd University, HungaryCopyright © 2023 Zhang, Liu, Bian and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu-Sen Bian, YXBiaWFubHVzZW5AMTI2LmNvbQ==; Qian Chen, Y2hlbnFpYW4zMTUwQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.