- 1One Health Center of Excellence, University of Florida, Gainesville, FL, United States
- 2Section of Microbiology, Department of Medical and Surgical Sciences, Alma Mater Studiorum University of Bologna, Bologna, Italy
- 3Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, FL, United States
- 4Johns Hopkins University, SAIS Europe, Bologna, Italy
- 5Institute of Food and Agricultural Sciences, University of Florida, Gainesville, FL, United States
Circular Health is a novel approach to address complex health issues that is based on the expansion of the One Health Paradigm. Circular health recognizes the need for a multidisciplinary convergence effort to complement the biomedical dimension of health. Antimicrobial resistance (AMR) is one of the greatest global concerns for public health that is likely on the rise, given the extensive use of antibiotics during the early Covid-19 years. Prior to the Covid-19 pandemic, an expert group chaired by Jim O’Neill published “The Review on Antimicrobial Resistance”, which contains a final report and recommendations on how to tackle AMR. The report, for the first time, considers AMR from a multi-perspective viewpoint highlighting how it cannot be successfully addressed unless there is a converging approach encompassing many dimensions of the problem. In this perspective, we propose to include the recommendations from that seminal report and other more recent reviews which include the lessons learnt from the Covid-19 pandemic, into the operational framework of the sustainable development goals (SDGs). AMR represents a perfect case study to explore how the SDG roadmap has the potential of becoming the driving force and implementation tool to address complex health issues by pursuing the optimization of resources and actions via a convergent and multi-stakeholder approach. The implementation of health-related policies through the whole spectrum of the SDGs could be both a novel and a well-established framework to inform multi-dimensional policies for more sustainable health in the future.
Introduction
Antimicrobial resistance (AMR) is recognized as a leading cause of death around the world, one of the top ten global health priorities, and a threat to development (FAO et al., 2022). It requires urgent multisectoral action to achieve the Sustainable Development Goals (SDGs) (WHO, 2015). According to the United Nations Environment Programme (UNEP) it is imperative to tackle the environmental dimensions of AMR to maintain global progress towards the SDGs (United Nations Environment Programme, 2023). Thus, fighting AMR is essential not only to keep people and animals healthy, but also to achieve sustainability. In this paper we describe a new conceptual model, Circular Health, which seeks to address major health issues (such as AMR) by including relevant recommendations within the SDGs, thus achieving a convergence of efforts. For the reasons mentioned above, we used fighting AMR as a case study for our Circular Health model.
Our starting point was a seminal report that analyzed the magnitude and potential impact of AMR and the numerous steps necessary to address it published as the “Review on Antimicrobial Resistance” (O’Neill, 2016). However, after a fruitful beginning, global efforts sparked by that report have been completely sidelined by the efforts needed to manage the Covid-19 pandemic. For example, from March to October 2020, almost 80% of patients infected with Covid-19 in the USA received antibiotic treatment and estimates about increasing deaths caused by AMR are worrisome (CDC, 2022). A recent paper concludes that in many countries of the world the AMR issue is worsening (Tomczyk et al., 2021).
Hence, AMR is growing fast, and novel roadmaps should be developed to comprehensively address AMR by using existing consensus documents and frameworks to empower such activities. The 2030 Agenda for Sustainable Development is an existing framework that may be used to seek a broader convergence of AMR related efforts. Here we propose an example of how to implement a series of recommendations taken from the O’Neill report and selected others from more recent studies by including them in selected targets pertaining to each SDG, excluding, for obvious reasons, SDG 3 - Health and Well-being.
The aim of this paper is to exemplify how countries can contribute to fight AMR by implementing, expanding, or focusing on actions that are already part of the SDG roadmap.
SDG1 no poverty
Poverty can be a lack of money, but also a lack of access (to resources, services, technology, and rights). Both types of poverty contribute to AMR. Poorer people are more susceptible to infectious diseases treated with antimicrobials (Do et al., 2021) and so is their livestock (McKune et al., 2021). Equally, as it is discussed in Aliro et al. (2021), lack of access to basic services such as health care and veterinary services promotes self-medication that greatly contributes to the development of AMR, especially when antimicrobials are cheap and available without prescriptions. For these reasons, reducing poverty would contribute to reducing AMR by itself. Moreover, access to health care and veterinary services should be explicitly listed as basic services in target 1.4, and the ability to live in healthy environments should be one of the dimensions of poverty of target 1.2.
SDG2 zero hunger
The intensification of agriculture driven by increasing demand, especially for animal-source foods, is increasing antimicrobial use in livestock (Founou et al., 2021). The general balance of achieving sustainability while increasing production, already addressed in target 2.4, should include the role of antibiotics: they are important for maintaining the health and welfare of livestock and the economic stability of farms (Founou et al., 2021; Tandon and Bouzanis, 2021) but their misuse negatively affects human and animal health (Founou et al., 2021). Our recommendation is to explicitly include a rational use of antibiotics in food-producing animals as part of the meaning of “sustainable” in target 2.4 and develop specific monitoring indicators.
SDG4 quality education
Lack of awareness and poor education are the main drivers of improper use and disposal of antimicrobials, in turn, one of the main causes of AMR. While certain categories of health care workers have inadequate or incorrect knowledge about how to appropriately manage antimicrobials and on the potential consequences of AMR (Ogunnigbo et al., 2022) most medical students show interest in learning more about “antimicrobial stewardship” after learning of the problem (Abbo et al., 2013). For this reason, we recommend extending the scope of target 4.7 to specifically include the importance of education on antimicrobial stewardship, AMR, and the appropriate and sustainable use of these drugs from primary through high school and in university programs in life sciences. The educational approach should be creative and interactive, especially for younger children, including the use of cartoons, posters and storytelling to influence AMR knowledge, beliefs and attitudes of young students and their parents (Appiah et al., 2022).
SDG5 gender equality
Women represent 80% of caregivers worldwide (Sharma et al., 2016), thus playing a major role in the acquisition, administration, management, and disposal of drugs (including antibiotics and other antimicrobials); they are more susceptible to urinary tract infections and reproductive health issues treated with antimicrobials; and they are also the majority in those professions, such as teaching and health care, with more frequent exposure to infectious diseases (WHO, 2018). The World Health Organization (WHO) reports that AMR mitigation strategies with a gender focus are more people-centered and effective and thus it is reasonable to empower women to better understand AMR, especially for future generations, with a focus on how to contribute to its prevention (WHO, 2018). We propose to develop programs to empower women to understand and combat AMR in the framework of targets 5.4, 5.5, 5.6, 5.b, for example building upon existing initiatives such as HPV (human papillomavirus) prevention campaigns to increase awareness about AMR in collaboration with public health institutions, pharmacies, health care providers and drug manufacturers, using a combination of digital or conventional tools.
SDG6 clean water and sanitation
Unsafe drinking water is one of the main sources of bacterial infections (Tandon and Bouzanis, 2021; Kaiser et al., 2022) and it is more common in low-resource settings, especially in low- and middle-income countries (LMICs) (Tandon and Bouzanis, 2021). When combined with self-medication and inappropriate disposal practices it is more likely to cause and amplify AMR. In addition, SDG6 includes equitable sanitation, hygiene for all and an end to open defecation, all essential to combat AMR. Promoting proper sewage systems also reduces the threat that water related to human uses, especially if not properly sanitized, poses for the environment, and the plants and animals found in it (Kaiser et al., 2022).
SDG7 affordable and clean energy
Reliable access to electricity is fundamental to provide the highest level of care (Irwin et al., 2020). Indeed, electricity is required to run diagnostic services (O’Neill, 2016; Kaprou et al., 2021), store vaccines (Fanelli et al., 2022), which are instrumental to fighting AMR, and to administer alternatives to antimicrobial treatment such as hemofiltration devices (Kumar et al., 2021). Electricity is also necessary to efficiently run surveillance systems that require laboratory equipment and information technology (IT) infrastructure. Our recommendation is to expand target 7.1 to also measure the share of hospitals and health care facilities with access to reliable and affordable electricity. Moreover, target 7.b could be adjusted to prioritize investments in electrifying hospitals, diagnostic laboratories and health care centers.
SDG8 decent work and economic growth
Wealthier countries, especially when experiencing rapid economic growth, tend to have a higher consumption of antimicrobials (Malik and Bhattacharyya, 2019). Since AMR has in turn a negative impact on economic growth (Dadgostar, 2019), it is important to expand target 8.1 to highlight the importance of developing best management practices for antimicrobials to achieve sustainable growth. Wealthier countries have the economic power to invert this tendency and pave the way for LMICs.
Health care is also one of the high value-added and labor-intensive sectors described in target 8.2. Investing in AMR mitigation strategies is necessary to increase the sector’s productivity and quality of services. At the same time, promoting better jobs and innovative enterprises in health care (target 8.5 and 8.3) is essential to address AMR. This positive feedback effect could be promoted by appropriately amending targets 8.2, 8.3, and 8.5. Moreover, managing the impact of AMR through the lens of economic policy could have beneficial effects given the number of proven tools already available to policymakers (Roope et al., 2019; Booth et al., 2020).
SDG9 industry innovation and infrastructure
Scientific and technological innovation together with infrastructure development are the foundations of many policies to address AMR. The O’Neill report (O’Neill, 2016) highlights the importance of innovation to develop new antibiotics, vaccines, alternative treatments, rapid diagnostic tools, and to improve the global surveillance infrastructure. It also promotes the creation of a global innovation fund to support research on AMR. From an infrastructure point of view, target 9.4 should be expanded to include processing of antimicrobial waste (Booth et al., 2020; Kotwani et al., 2021). From a science and technology perspective, target 9.5 could be amended to include a focus on “anti-AMR” research and technology. Finally, target 9.3 could be amended to prioritize incubating start-up companies working on AMR, to generate the range of innovative ideas and technologies needed to address such a complex issue.
SDG10 reduced inequalities
The global volume of antibiotic consumption (40.2 billion DDD -defined daily dose- in 2018) increased 46% since 2000 (Browne et al., 2021). However, this consumption was inequitable between high-income countries (HICs) and LMICs, with respective rates of 20.6 DDD and 13.1 DDD per 1000 population/day. The lowest rate was estimated for Sub-Saharan Africa despite high prevalence of sepsis.
Proper access to antibiotics for refugees and migrants is significantly influenced by the health systems of the host countries as reported by the WHO in 2022 (WHO, 2022a). Resistant bacteria often cause outbreaks in refugee camps, (Tokajian et al., 2018) posing a risk also for hosting communities. The concept of safe migration addressed by target 10.7, and specifically by the indicator 10.7.4, should be expanded to explicitly include proactive anti-AMR policies, especially in the context of refugee camps.
Promoting better public health practices to prevent infections, especially in poorer countries, will reduce antimicrobial consumption overall, including their misuse (Mendelson, 2022). Similarly, antimicrobial consumption can be reduced in wealthier countries by promoting greater equality of access and treatment among lower-income, undocumented, and/or uninsured people who are more likely to be exposed to infectious diseases and to self-medicate due to lack of access to health care providers (Nadimpalli et al., 2021). Reducing inter-country economic and health inequalities should be specifically included in target 10.b, while target 10.2 should be expanded to add inclusive access to health care and improved public health interventions.
SDG11 sustainable cities and communities
A growing share of humanity, about 55% in 2020, are urbanite, highlighting the importance of building sustainable cities (United Nations Human Settlements Programme (UN-Habitat) 2020). Sustainability should include monitoring and promoting a healthy microbiome of the built environment (Bruno et al., 2022) because, on one hand, it exerts selective pressures on microorganisms to develop AMR, especially in health care settings (Tarricone et al., 2020; Bruno et al., 2022) but, on the other hand, it can also be a source of a healthy and balanced microbiome that helps city dwellers to fend off and/or control the growth of harmful microorganisms (Tarricone et al., 2020). The importance of a healthy microbiome could be included in target 11.7 as another important environmental factor of sustainable and healthy cities.
SDG12 responsible consumption and production
Consumption behaviors and production practices of antimicrobials are among the main causes of AMR. From a consumption perspective, target 12.8 should be expanded to clearly promote increasing public awareness on how to properly use antimicrobials, how to dispose of them, and the risks associated with their misuse (Allison et al., 2017). This should go hand-in-hand with policies to control access and to reduce over-prescription. From a production perspective, target 12.4 should be expanded to include management of wastewater from factories producing antimicrobials or their ingredients: a major driver of AMR according to Kotwani et al. (2021).
SDG13 climate action
There is a positive association between global warming and AMR (Kaba et al., 2020). Higher temperatures are associated with higher bacterial growth rates, increased gene transfer, and emergence of new pathogens (Kaba et al., 2020). Livestock farming, rising temperatures, and water and environmental contaminants were identified as factors linking AMR and climate change (Magnano San Lio et al., 2023). Some countries (e.g., Ireland) have divested from fossil fuels and invested into the Global Innovation Fund for AMR research, to tackle the climate crisis and AMR at the same time (Turner, 2018). Here we encourage the recognition of such a link on a global scale, so that the fight against both threats can be synergistic. Guidelines on AMR mitigation linked to livestock farming and environmental contamination should be updated and integrated in target 13.2 to encompass both climate change and AMR actions within national policies, strategies, and planning, providing solutions to reach a common goal.
SDG14 life below water
Many human activities are drivers of AMR in marine life. Medicated feeds, a common practice in aquaculture, are an important source of AMR (Shah et al., 2014), and ocean plastics can accumulate and serve as vehicles for resistant bacteria, exacerbating their impact and spread (Moore and Millar, 2020). Contaminant discharge, particularly improper antibiotic disposal, contributes to increasing the load and density of resistant pathogens in coastal ecosystems, and consequent accumulation in marine species that can harbor resistant pathogens (Wallace et al., 2013). These drivers of AMR should be explicitly incorporated in targets 14.1, 14.2, and 14.6. Actions may include targeted screening of antimicrobials in municipal and agricultural wastewater, aquaculture, and marine debris to monitor misuse, estimate its threat, and tailor appropriate ocean AMR mitigation measures.
SDG15 life on land
Protection of natural ecosystems can be significant in addressing AMR. On one hand, natural habitats can accumulate drugs discharged from hospitals, health care settings and agriculture, resulting in antimicrobial agents exerting selective pressure on environmental microorganisms (Radhouani et al., 2014). On the other hand, terrestrial wildlife can harbor and spread resistant pathogens (Greig et al., 2015) whose resistant genes can commonly and easily be exchanged between microbes affecting wildlife, humans, and domestic animals (Vittecoq et al., 2016). We invite to specifically include in the targets of SDG15 the benefit of reducing human encroachment on wild habitats on mitigating AMR drivers as well as developing monitoring plans for environmental disposal of antibiotics focused on local wildlife and the species-specific potential for antimicrobial accumulation and exchange.
SDG16 peace, justice and strong institutions
Antibiotic misuse is one of the most important components of AMR and dysfunctional public institutions and corruption seem to account for some of the remarkable between-region variation in antibiotic consumption. Poor governance and corruption correlate better than antibiotic usage volumes with resistance rates (Collignon et al., 2015). A strong positive correlation between measures of corruption, in the health sector and in the society at large, has been shown in Europe (Rönnerstrand and Lapuente, 2017) as well as in several African countries (Harant, 2022). Furthermore, when the quality of governance is low, antibiotic stewardship programs are less effective, as well as food and water safety controls. Achieving targets 16.5 and 16.6 can contribute to controlling AMR. Moreover, increasing transparency can enable stakeholders, such as civil society, to demand accountability for results and be empowered as individuals to fight AMR.
SDG17 partnership for the goals
AMR is a highly complex problem that in a globalized world is not bound to specific geographic areas. Therefore, fighting AMR requires an interdisciplinary and multi-stakeholder response in which every country participates (de Kraker et al., 2016). This means that wealthier countries have an interest in supporting economically and technologically those countries where a higher burden of infectious diseases and less awareness of AMR are paired with weaker governance and health systems (Gandra et al., 2020). This should be strengthened by creating South-South partnerships in collaboration with HICs partners. These collaborations need to address multiple issues such as inadequate laboratory infrastructure (Opintan et al., 2015) and surveillance systems (Hay et al., 2018), lack of comprehensive population-based surveillance (Gandra et al., 2020), and access to technology for data generation, analysis, sharing and dissemination (Pokharel et al., 2019). SDG17 should be expanded accordingly to increase international investments to support research and development of vaccines and newer drugs, as well as the transfer of technology and resources to promote: i) development and uptake of diagnostic tests; ii) improved surveillance systems; iii) computerized data repositories to collect and share surveillance data; iv) global efforts to improve antimicrobial stewardship through science- and evidence-based knowledge.
Conclusion
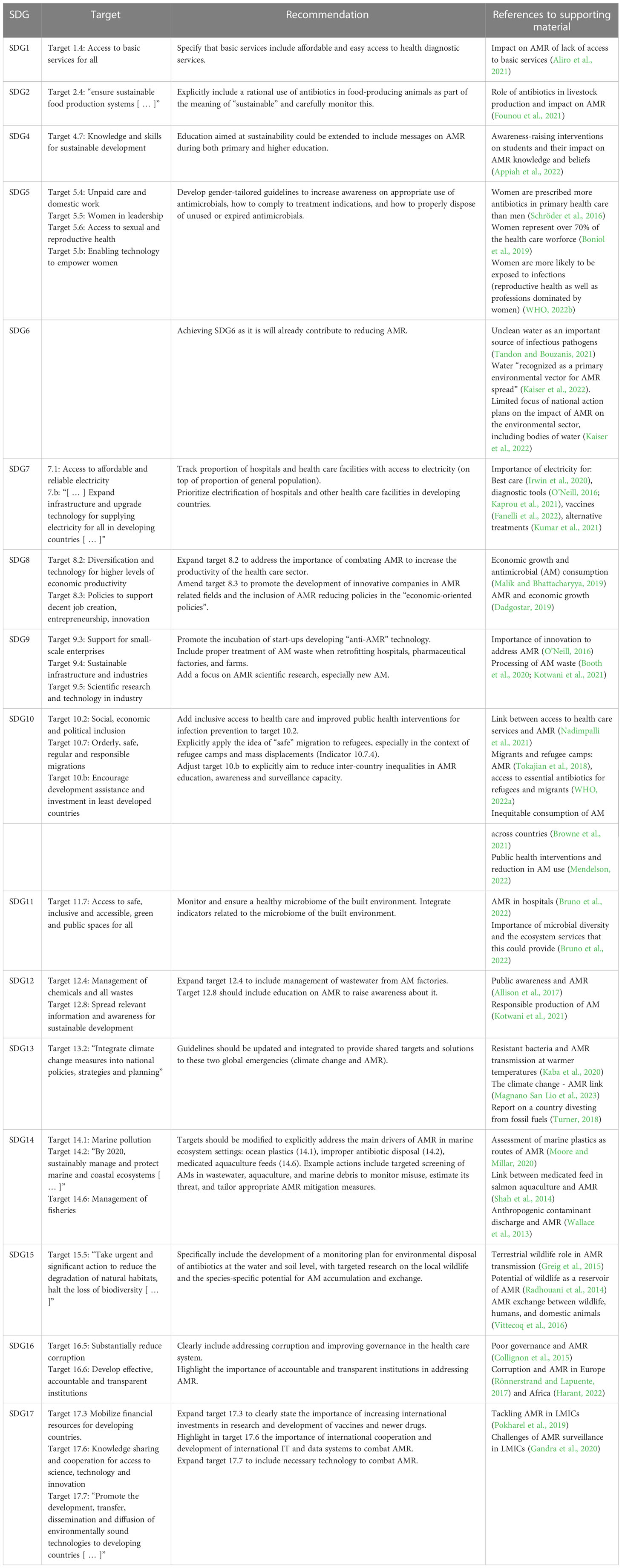
In this paper we propose a novel approach based on a convergence model to fight AMR, which exploits the existing SDG roadmap. Such a model is based on a circular approach to the problem that seeks to highlight win-win scenarios by enforcing existing objectives and targets to fortify the effort to combat AMR in a multidisciplinary synergistic effort (Table 1; Figure 1). If AMR is truly considered to be a global health priority and a hurdle to achieving sustainability, then it should also become a priority within the SDG roadmap. Efficiently fighting AMR with a Circular Health approach requires addressing this issue not only through the actions pertaining to SDG 3 - Health and Well-being, but also through the objectives of every SDG. Our examples can be a starting point to address AMR as a top health priority by funneling convergent and synergistic activities that are already part of the SDG roadmap or that can be included in a revised version of the roadmap. Such an effort could significantly advance health as a system by focusing on a major priority such as AMR.

Figure 1 Mapping on the SDGs framework of the recommendations to fight AMR presented in O'Neill, 2016. Tackling drug-resistant infections globally: Final report and recommendations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
IC conceived the contents and outline of the manuscript. All authors were assigned specific sections of the manuscript and identified the literature relevant for their sections. LM wrote the first draft of the manuscript assembling and synthetizing the materials provided by all authors. AP curated the bibliography. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbo, L. M., Cosgrove, S. E., Pottinger, P. S., Pereyra, M., Sinkowitz-Cochran, R., Srinivasan, A., et al. (2013). Medical students’ perceptions and knowledge about antimicrobial stewardship: how are we educating our future prescribers? Clin. Infect. Dis. 57 (5), 631–638. doi: 10.1093/cid/cit370
Aliro, T., Chenais, E., Odongo, W., Okello, D. M., Masembe, C., Ståhl, K. (2021). Prevention and control of African swine fever in the smallholder pig value chain in northern Uganda: thematic analysis of stakeholders’ perceptions. Front. Vet. Sci. 8, 707819. doi: 10.3389/fvets.2021.707819
Allison, D. G., Higginson, P., Martin, S. (2017). Antibiotic resistance awareness: a public engagement approach for all pharmacists. Int. J. Pharm. Pract. 25 (1), 93–96. doi: 10.1111/ijpp.12287
Appiah, B., Asamoah-Akuoko, L., Samman, E., Koduah, A., Kretchy, I. A., Ludu, J. Y., et al. (2022). The impact of antimicrobial resistance awareness interventions involving schoolchildren, development of an animation and parents engagements: a pilot study. Antimicrob. Resist. Infect. Control. 11 (1), 26. doi: 10.1186/s13756-022-01062-6
Boniol, M., McIsaac, M., Xu, L., Wuliji, T., Diallo, K., Campbell, J. (2019). Gender equity in the health workforce: analysis of 104 countries. (World Health Organization). Available at: https://apps.who.int/iris/handle/10665/311314.
Booth, A., Aga, D. S., Wester, A. L. (2020). Retrospective analysis of the global antibiotic residues that exceed the predicted no effect concentration for antimicrobial resistance in various environmental matrices. Environ. Int. 141, 105796. doi: 10.1016/j.envint.2020.105796
Browne, A. J., Chipeta, M. G., Haines-Woodhouse, G., Kumaran, E. P. A., Hamadani, B. H. K., Zaraa, S., et al. (2021). Global antibiotic consumption and usage in humans, 2000-18: a spatial modelling study. Lancet Planet. Health 5 (12), e893–e904. doi: 10.1016/S2542-5196(21)00280-1
Bruno, A., Fumagalli, S., Ghisleni, G., Labra, M. (2022). The microbiome of the built environment: the nexus for urban regeneration for the cities of tomorrow. Microorganisms 10 (12), 2311. doi: 10.3390/microorganisms10122311
CDC. (2022). COVID-19: U.S. impact on antimicrobial resistance, special report 2022 (Atlanta, GA: U.S. Department of Health and Human Services).
Collignon, P., Athukorala, P. C., Senanayake, S., Khan, F. (2015). Antimicrobial resistance: the major contribution of poor governance and corruption to this growing problem. PloS One 10 (3), e0116746. doi: 10.1371/journal.pone.0116746
Dadgostar, P. (2019). Antimicrobial resistance: implications and costs. Infect. Drug Resist. 12, 3903–3910. doi: 10.2147/IDR.S234610
de Kraker, M. E., Stewardson, A. J., Harbarth, S. (2016). Will 10 million people die a year due to antimicrobial resistance by 2050? PloS Med. 13 (11), e1002184. doi: 10.1371/journal.pmed.1002184
Do, N. T. T., Vu, H. T. L., Nguyen, C. T. K., Punpuing, S., Khan, W. A., Gyapong, M., et al. (2021). Community-based antibiotic access and use in six low-income and middle-income countries: a mixed-method approach. Lancet Glob. Health 9 (5), e610–e6e9. doi: 10.1016/S2214-109X(21)00024-3
Fanelli, A., Mantegazza, L., Hendrickx, S., Capua, I. (2022). Thermostable vaccines in veterinary medicine: state of the art and opportunities to be seized. Vaccines (Basel) 10 (2), 245. doi: 10.3390/vaccines10020245
FAO, UNEP, WHO, WOAH. (2022). One health joint plan of action (2022-2026). working together for the health of humans, animals, plants and the environment (Rome: Food and Agriculture Organization of the United Nations, United Nations Environment Programme, World Health Organization, World Organisation for Animal Health).
Founou, L. L., Founou, R. C., Essack, S. Y. (2021). Antimicrobial resistance in the farm-to-plate continuum: more than a food safety issue. Future Sci. OA 7 (5), FSO692. doi: 10.2144/fsoa-2020-0189
Gandra, S., Alvarez-Uria, G., Turner, P., Joshi, J., Limmathurotsakul, D., van Doorn, H. R. (2020). Antimicrobial resistance surveillance in low- and middle-income countries: progress and challenges in eight south Asian and southeast Asian countries. Clin. Microbiol. Rev. 33 (3). doi: 10.1128/CMR.00048-19
Greig, J., Rajić, A., Young, I., Mascarenhas, M., Waddell, L., LeJeune, J. (2015). A scoping review of the role of wildlife in the transmission of bacterial pathogens and antimicrobial resistance to the food chain. Zoonoses Public Health 62 (4), 269–284. doi: 10.1111/zph.12147
Harant, A. (2022). Assessing transparency and accountability of national action plans on antimicrobial resistance in 15 African countries. Antimicrob. Resist. Infect. Control. 11 (1), 15. doi: 10.1186/s13756-021-01040-4
Hay, S. I., Rao, P. C., Dolecek, C., Day, N. P. J., Stergachis, A., Lopez, A. D., et al. (2018). Measuring and mapping the global burden of antimicrobial resistance. BMC Med. 16 (1), 78. doi: 10.1186/s12916-018-1073-z
Irwin, B. R., Hoxha, K., Grépin, K. A. (2020). Conceptualising the effect of access to electricity on health in low- and middle-income countries: a systematic review. Glob. Public Health 15 (3), 452–473. doi: 10.1080/17441692.2019.1695873
Kaba, H. E. J., Kuhlmann, E., Scheithauer, S. (2020). Thinking outside the box: association of antimicrobial resistance with climate warming in Europe - a 30 country observational study. Int. J. Hyg. Environ. Health 223 (1), 151–158. doi: 10.1016/j.ijheh.2019.09.008
Kaiser, R. A., Taing, L., Bhatia, H. (2022). Antimicrobial resistance and environmental health: a water stewardship framework for global and national action. Antibiotics 11 (1), 63. doi: 10.3390/antibiotics11010063
Kaprou, G. D., Bergšpica, I., Alexa, E. A., Alvarez-Ordóñez, A., Prieto, M. (2021). Rapid methods for antimicrobial resistance diagnostics. Antibiotics (Basel). 10 (2), 209. doi: 10.3390/antibiotics10020209
Kotwani, A., Joshi, J., Kaloni, D. (2021). Pharmaceutical effluent: a critical link in the interconnected ecosystem promoting antimicrobial resistance. Environ. Sci. Pollut. Res. Int. 28 (25), 32111–32124. doi: 10.1007/s11356-021-14178-w
Kumar, M., Sarma, D. K., Shubham, S., Kumawat, M., Verma, V., Nina, P. B., et al. (2021). Futuristic non-antibiotic therapies to combat antibiotic resistance: a review. Front. Microbiol. 12, 609459. doi: 10.3389/fmicb.2021.609459
Magnano San Lio, R., Favara, G., Maugeri, A., Barchitta, M., Agodi, A. (2023). How antimicrobial resistance is linked to climate change: an overview of two intertwined global challenges. Int. J. Environ. Res. Public Health 20 (3), 1681. doi: 10.3390/ijerph20031681
Malik, B., Bhattacharyya, S. (2019). Antibiotic drug-resistance as a complex system driven by socio-economic growth and antibiotic misuse. Sci. Rep. 9 (1), 9788. doi: 10.1038/s41598-019-46078-y
McKune, S., Serra, R., Touré, A. (2021). Gender and intersectional analysis of livestock vaccine value chains in kaffrine, Senegal. PloS One 16 (7), e0252045. doi: 10.1371/journal.pone.0252045
Mendelson, M. (2022). BSAC vanguard series: inequality and antibiotic resistance. J. Antimicrob. Chemother. 77 (2), 277–278. doi: 10.1093/jac/dkab426
Moore, J. E., Millar, B. C. (2020). The day the agar stopped working: what emerging antimicrobial resistance (AMR) means for microbiology laboratory testing-potential effects on infectious disease reporting. Clin. Microbiol. Infect. 26 (8), 973–975. doi: 10.1016/j.cmi.2020.04.035
Nadimpalli, M. L., Chan, C. W., Doron, S. (2021). Antibiotic resistance: a call to action to prevent the next epidemic of inequality. Nat. Med. 27 (2), 187–188. doi: 10.1038/s41591-020-01201-9
Ogunnigbo, O., Nabiryo, M., Atteh, M., Muringu, E., Olaitan, O. J., Rutter, V., et al. (2022). Exploring the antimicrobial stewardship educational needs of healthcare students and the potential of an antimicrobial prescribing app as an educational tool in selected African countries. Antibiotics 11 (5), 691. doi: 10.3390/antibiotics11050691
O’Neill, J. (2016). Tackling drug-resistant infections globally: final report and recommendations. (London UK). Available at: https://amr-review.org/ (Accessed May 23, 2023).
Opintan, J. A., Newman, M. J., Arhin, R. E., Donkor, E. S., Gyansa-Lutterodt, M., Mills-Pappoe, W. (2015). Laboratory-based nationwide surveillance of antimicrobial resistance in Ghana. Infect. Drug Resist. 8, 379–389. doi: 10.2147/IDR.S88725
Pokharel, S., Raut, S., Adhikari, B. (2019). Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 4 (6), e002104. doi: 10.1136/bmjgh-2019-002104
Radhouani, H., Silva, N., Poeta, P., Torres, C., Correia, S., Igrejas, G. (2014). Potential impact of antimicrobial resistance in wildlife, environment and human health. Front. Microbiol. 5, 23. doi: 10.3389/fmicb.2014.00023
Rönnerstrand, B., Lapuente, V. (2017). Corruption and use of antibiotics in regions of Europe. Health Policy 121 (3), 250–256. doi: 10.1016/j.healthpol.2016.12.010
Roope, L. S. J., Smith, R. D., Pouwels, K. B., Buchanan, J., Abel, L., Eibich, P., et al. (2019). The challenge of antimicrobial resistance: what economics can contribute. Science 364 (6435). doi: 10.1126/science.aau4679
Schröder, W., Sommer, H., Gladstone, B. P., Foschi, F., Hellman, J., Evengard, B., et al. (2016). Gender differences in antibiotic prescribing in the community: a systematic review and meta-analysis. J. Antimicrob. Chemother. 71 (7), 1800–1806. doi: 10.1093/jac/dkw054
Shah, S. Q., Cabello, F. C., L’abée-Lund, T. M., Tomova, A., Godfrey, H. P., Buschmann, A. H., et al. (2014). Antimicrobial resistance and antimicrobial resistance genes in marine bacteria from salmon aquaculture and non-aquaculture sites. Environ. Microbiol. 16 (5), 1310–1320. doi: 10.1111/1462-2920.12421
Sharma, N., Chakrabarti, S., Grover, S. (2016). Gender differences in caregiving among family - caregivers of people with mental illnesses. World J. Psychiatry 6 (1), 7–17. doi: 10.5498/wjp.v6.i1.7
Tandon, P., Bouzanis, K. (2021). Antimicrobial Resistance: a bottleneck in the achievement of the united nations’ sustainable development goals. Global Health: Annual Review 1 (6), 41–44.
Tarricone, R., Rognoni, C., Arnoldo, L., Mazzacane, S., Caselli, E. (2020). A probiotic-based sanitation system for the reduction of healthcare associated infections and antimicrobial resistances: a budget impact analysis. Pathogens 9 (6), 502. doi: 10.3390/pathogens9060502
Tokajian, S., Moghnieh, R., Salloum, T., Arabaghian, H., Alousi, S., Moussa, J., et al. (2018). Extended-spectrum β-lactamase-producing escherichia coli in wastewaters and refugee camp in Lebanon. Future Microbiol. 13, 81–95. doi: 10.2217/fmb-2017-0093
Tomczyk, S., Taylor, A., Brown, A., de Kraker, M. E. A., El-Saed, A., Alshamrani, M., et al. (2021). Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: a global survey. J. Antimicrob. Chemother. 76 (11), 3045–3058. doi: 10.1093/jac/dkab300
Turner, A. (2018). Tackling antimicrobial resistance and climate change. Lancet 392 (10163), 2435–2436. doi: 10.1016/S0140-6736(18)32413-9
United Nations Environment Programme. (2023). Bracing for Superbugs: Strengthening environmental action in the One Health response to antimicrobial resistance. (Geneva: United Nations Environment Programme). Available at: https://www.unep.org/resources/superbugs/environmental-action.
United Nations Human Settlements Programme (UN-Habitat). (2020). World Cities Report 2020. Unpacking the Value of Sustainable Urbanization. doi: 10.18356/c41ab67e-en
Vittecoq, M., Godreuil, S., Prugnolle, F., Durand, P., Brazier, L., Renaud, N., et al. (2016). Antimicrobial resistance in wildlife. J. Appl. Ecol. 53, 519–529. doi: 10.1111/1365-2664.12596
Wallace, C. C., Yund, P. O., Ford, T. E., Matassa, K. A., Bass, A. L. (2013). Increase in antimicrobial resistance in bacteria isolated from stranded marine mammals of the Northwest Atlantic. Ecohealth 10 (2), 201–210. doi: 10.1007/s10393-013-0842-6
WHO. (2015). Global action plan on antimicrobial resistance (WHO Document Production Services, Geneva, Switzerland: WHO Library Cataloguing-in-Publication Data Global Action Plan on Antimicrobial Resistance).
WHO. (2018). Tackling antimicrobial resistance (AMR) together: working paper 5.0: enhancing the focus on gender and equity. (Geneva: World Health Organization)
WHO. (2022a). Capturing the evidence on access to essential antibiotics in refugee and migrant populations (Geneva: World Health Organization. Global Evidence Review on Health and Migration (GEHMseries). Available at: https://www.who.int/publications/i/item/9789240057807.
WHO. (2022b). The fight against antimicrobial resistance requires a focus on gender. Available at: https://www.who.int/europe/publications/i/item/WHO-EURO-2021-3896-43655-61363.
Keywords: antimicrobial resistance (AMR), sustainable development goals (SDGs), sustainability, interdisciplinarity, one health (OH), circular health
Citation: Mantegazza L, De Pascali AM, Munoz O, Manes C, Scagliarini A and Capua I (2023) Circular Health: exploiting the SDG roadmap to fight AMR. Front. Cell. Infect. Microbiol. 13:1185673. doi: 10.3389/fcimb.2023.1185673
Received: 13 March 2023; Accepted: 10 May 2023;
Published: 22 June 2023.
Edited by:
Luther King Abia Akebe, University of KwaZulu-Natal, South AfricaReviewed by:
Francesca Prestinaci, National Institute of Health (ISS), ItalyLuria Leslie Founou, Centre of Expertise and Biological Diagnostic of Cameroon (CEDBCAM), Cameroon
Copyright © 2023 Mantegazza, De Pascali, Munoz, Manes, Scagliarini and Capua. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilaria Capua, aWNhcHVhQGpodS5lZHU=
 Luca Mantegazza
Luca Mantegazza Alessandra Mistral De Pascali
Alessandra Mistral De Pascali Olga Munoz
Olga Munoz Costanza Manes
Costanza Manes Alessandra Scagliarini
Alessandra Scagliarini Ilaria Capua
Ilaria Capua