
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 22 May 2023
Sec. Clinical Microbiology
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1183736
This article is part of the Research Topic Antimicrobial resistance in pediatric infectious diseases: antimicrobial resistance, resistance mechanisms and antimicrobial use View all 18 articles
 Ruiqi Xiao1†
Ruiqi Xiao1† Ying Li2†
Ying Li2† Xiaowei Liu3†
Xiaowei Liu3† Yijun Ding4
Yijun Ding4 Jidong Lai5
Jidong Lai5 Yangfang Li6
Yangfang Li6 Wenqing Kang7
Wenqing Kang7 Peicen Zou1
Peicen Zou1 Jie Wang4
Jie Wang4 Yue Du2
Yue Du2 Jinjing Zhang4
Jinjing Zhang4 Yajuan Wang2*
Yajuan Wang2*Background: Escherichia coli is one of the most common pathogens causing neonatal infections. Recently, the incidence and drug resistance of E. coli have increased, posing a major threat to neonatal health. The aim of this study was to describe and analyze the antibiotic resistance and multilocus sequence typing (MLST) characteristics of E. coli derived from infants admitted to neonatal intensive care units (NICUs) across China.
Methods: In this study, 370 strains of E. coli from neonates were collected. E. coli isolated from these specimens were subjected to antimicrobial susceptibility testing (by broth microdilution method) and MLST.
Results: The overall resistance rate was 82.68%, with the highest rate of methicillin/sulfamethoxazole (55.68%) followed by cefotaxime (46.22%). Multiple resistance rate was 36.74%, 132 strains (35.68%) had extended-spectrum β-lactamase (ESBL) phenotype and 5 strains (1.35%) had insensitivity to the tested carbapenem antibiotics. The resistance of E. coli isolated from different pathogenicity and different sites of infections varied, strains derived from sputum were significantly more resistant to β-lactams and tetracyclines. Currently, the prevalence spectrum in NICUs was dominated by ST1193, ST95, ST73, ST69 and ST131 across China. And the multidrug resistance of ST410 was the most severe. ST410 had the highest resistance rate to cefotaxime (86.67%), and its most common multidrug resistance pattern was β-lactams + aminoglycosides + quinolones + tetracyclines + sulfonamides.
Conclusions: Substantial proportions of neonatal E. coli isolates were severely resistant to commonly administered antibiotics. MLST results can suggest the prevalent characteristics of antibiotic resistance in E. coli with different ST types.
Newborns admitted to the Neonatal Intensive Care Units (NICUs), and particularly those born preterm, are at high risk of infection for several reasons, including relative immunocompromise from an immature immune system, prolonged hospitalization, and frequent use of invasive devices and antibiotics (Collins et al., 2018). Infectious diseases are also the main causes of neonatal morbidity and mortality (Zhang et al., 2019; GBD 2019 Diseases and Injuries Collaborators, 2020). Globally, 2.6 million newborns still die each year, with preterm birth and infections the two leading causes. Neonatal sepsis and meningitis were responsible for an estimated 420,000 deaths annually, accounting for 16% of neonatal mortality (Khan et al., 2017).
Escherichia coli is one of the most well-adapted and pathogenically versatile bacterial organisms (Riley, 2020). It is the main pathogen causing neonatal meningitis and sepsis especially in developing countries (Tan, 2020; van der Flier, 2021; Wen et al., 2021; Hallmaier-Wacker et al., 2022), also a common pathogen of ventilator associated pneumonia (VAP) in hospitals(Scamardo et al., 2020). According to the data from the Neonatal Monitoring Network released by NICHD (the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network) in 2020 (Stoll et al., 2020), the most frequent pathogens of early onset neonatal sepsis were E. coli (36.6%) and Group B streptococcus (30.2%), and E. coli mainly occurred in premature infants (51.9%). Besides, a retrospective cohort study in China found that the complications and mortality of E. coli meningitis were higher than those of other pathogens (Collaborative Study Group for Neonatal Bacterial Meningitis, 2018).
At the same time, bacterial antimicrobial resistance (AMR) further increases the difficulty of treatment and the speed of transmission of infection—has emerged as one of the leading public health threats of the 21st century (Antimicrobial Resistance Collaborators, 2022). Recent surveillance data from the 2000s indicate that antibiotic resistance to all major antibiotic classes exists among E. coli strains. These include the production of extended-spectrum-beta-lactamases (ESBLs) (including TEM, SHV, CMY, and CTX-M types), production of carbapenemases (including KPC, NDM, VIM, OXA-48 and IMP types), resistance to fluoroquinolones, aminoglycosides and trimethoprim-sulfamethoxazole and recently also plasmid-mediated colistin resistance (Paitan, 2018). A multicenter cohort study in the United States showed that the majority of neonatal E. coli isolates were insensitive to commonly used antibiotics, with 66.8% of isolates insensitive to ampicillin and 16.8% insensitive to aminoglycosides (Flannery et al., 2021).
One major challenge to tackling AMR is understanding the true burden of resistance and its epidemiological distribution. Neonatal clinicians must balance concerns of inadequate empirical coverage for suspected infection with the risks of indiscriminate antibiotic use. Currently, there is a paucity of contemporary, large-scale, neonatal-specific antibiotic susceptibility data for E. coli in China. Therefore, this study retrospectively analyzed the antimicrobial resistance characteristics of 370 clinical isolates of E. coli among infants admitted to NICUs across China, aiming to provide reasonable guidance for the use of antibiotics in neonatal infections.
Patients hospitalized in the NICUs between November 2015 and October 2020, aged ≤28 days, with E. coli-positive cultures from any of the specimens described below were included in this study. Specimens were collected from patients who matched these conditions: blood was collected from sepsis, cerebrospinal fluid was collected from meningitis, sputum was collected from lower respiratory tract infection, gastric fluid was collected from early-onset sepsis, ear secretions, umbilical cord secretions were collected for a routine test from neonates without systemic symptoms. A total of 370 E. coli strains isolated from clinical culture-positive specimens across China were ultimately included. The specimens were stored in a refrigerator at -20°C. The study was conducted in accordance with the Declaration of Helsinki, met all ethical requirements, and was approved by the ethics committee of Beijing Children’s Hospital.
MacConkey agar (CM00078) and chromogenic E. coli media (EC166) (Beijing Land Bridge Technology Co., Ltd., Beijing, China) were used to isolate and identify E. coli strains. The E. coli strains were stored in the refrigerator at -80°C. The antimicrobial susceptibility tests was performed by the broth dilution method according to the instructions of the Sensititre TM Gram Negative GNX2F Plate (Thermo Fisher Scientific, USA) and included 21 antimicrobial agents, namely Imipenem, Ertapenem, Doripenem, Meropenem, Aztreonam, Ceftazidime, Cefotaxime, Cefepime, Ticarcillin/clavulanic acid, Piperacillin/tazobactam, Tobramycin, Gentamicin, Amikacin, Levofloxacin, Ciprofloxacin, Doxycycline, Tigecycline, Minocycline, Trimethoprim/sulfamethoxazole, Colistin, and Polymyxin B. Antimicrobial susceptibility testing results were classified as susceptible (S), intermediate (I), or resistant (R), in accordance with Clinical and Laboratory Standards Institute (CLSI), 2023 standards (Clinical and Laboratory Standards Institute. CLSI M100-ED33: 2023 Performance Standards for Antimicrobial Susceptibility Testing, 33rd Edition (2023). M100-ED33). ESBL phenotype, defined as any isolate with at least 1 nonsusceptibility result to cefotaxime, ceftazidime, or cefepime; and carbapenem-resistant Enterobacteriaceae, defined as any isolate with at least 1 nonsusceptibility result to imipenem, meropenem, doripenem, or ertapenem sodium. Definitions of ESBL and carbapenem-resistant Enterobacteriaceae were based on updated Centers for Disease Control and Prevention (CDC) definition (Flannery et al., 2021; Centers for Disease Control and Prevention, Antibiotic/antimicrobial resistance (AR/ARM): biggest threats and data: 2019. AR threats report. https://www.cdc.gov/DrugResistance/Biggest-Threats.html). E. coli ATCC 25922 (American Type Culture Collection, Manassas, VA, USA) was used for routine quality control.
DNA was extracted using bacterial genomic DNA extraction kits (Tiangen Biotech Co., Ltd., Beijing, China). Briefly, after the bacteria were collected by centrifugation, the cell wall was removed by lysozyme digestion. DNA was released from the cell after adding the lysate and proteinase K. Then the binding solution was added to adjust the optimal binding conditions. Next, the solution was transferred to the purification column and centrifuged. DNA was bound to the filter membrane, and impurities such as proteins were filtered out into the filtrate. Residual contaminants and enzyme inhibitors were removed after two washing steps, and the DNA was finally eluted with a small amount of buffer.
MLST was performed on all isolates. Seven housekeeping genes were targeted as follows: adk, fumC, gyrB, icd, mdh, purA, and recA. The genes were amplified using polymerase chain reaction (PCR) and sent to Tiangen Biotech Co., Ltd., Beijing, China for sequencing. Allelic patterns of these genes were used to determine the sequence type. Sequence data were analyzed based on the E. coli MLST database (https://pubmlst.org/organisms/escherichia-spp).
Data analysis was performed by SPSS 27.0 (IBM SPSS, Chicago, IL, USA). The χ 2 test was performed for comparing antibiotic and multidrug resistance proportions of E. coli strains. Differences with P < 0.05 were considered statistically significant.
Among the 370 E. coli strains isolated from newborns aged less than 28 days, 104 were from blood, 31 from cerebrospinal fluid, 96 from sputum, 45 from gastric juice, and 94 from other secretions (ear secretions, umbilical cord secretions). The strains were divided into three groups according to the source, the invasive infection group (n=135) deriving from blood and cerebrospinal fluid, the respiratory tract infection (RTI) group (n=96) consisted of strains from sputum, and the others (n=139) being isolated from gastric juice, ear secretions, and umbilical cord secretions.
In all the E. coli isolates, 315 were resistant to at least one antimicrobial drug (the total resistance rate was 82.68%), and the resistance rate of trimethoprim/sulfamethoxazole was the highest (55.68%, 206/370), followed by cefotaxime (46.22%; 171/370), ciprofloxacin (35.41%; 131/370). No E. coli strains were found to be resistant to polymyxin B. We defined multidrug resistance in E. coli as resistance to at least three distinct antibiotic families and estimated this rate at ∼36.74% (136/370) across all isolates. More details about antimicrobial resistance rates were presented in Table 1. And a total of 132 (35.68%) E. coli strains had the ESBL phenotype and 5 (1.35%) strains had insensitivity to the carbapenem antibiotics tested (Table 2).
According to distinct pathogenic characteristics and various isolation sites, we divided the strains into RTI group, invasive infection group, and others for comparison. E. coli isolated from sputum had generally higher resistance to commonly used antibiotics. Almost all those of the β-lactams and tetracyclines were statistically different between the three groups, with aztreonam, ceftazidime, cefotaxime, cefepime, ticarcillin/clavulanic acid, piperacillin/tazobactam, doxycycline, and minocycline were the most significant (P<0.001, Table 3).
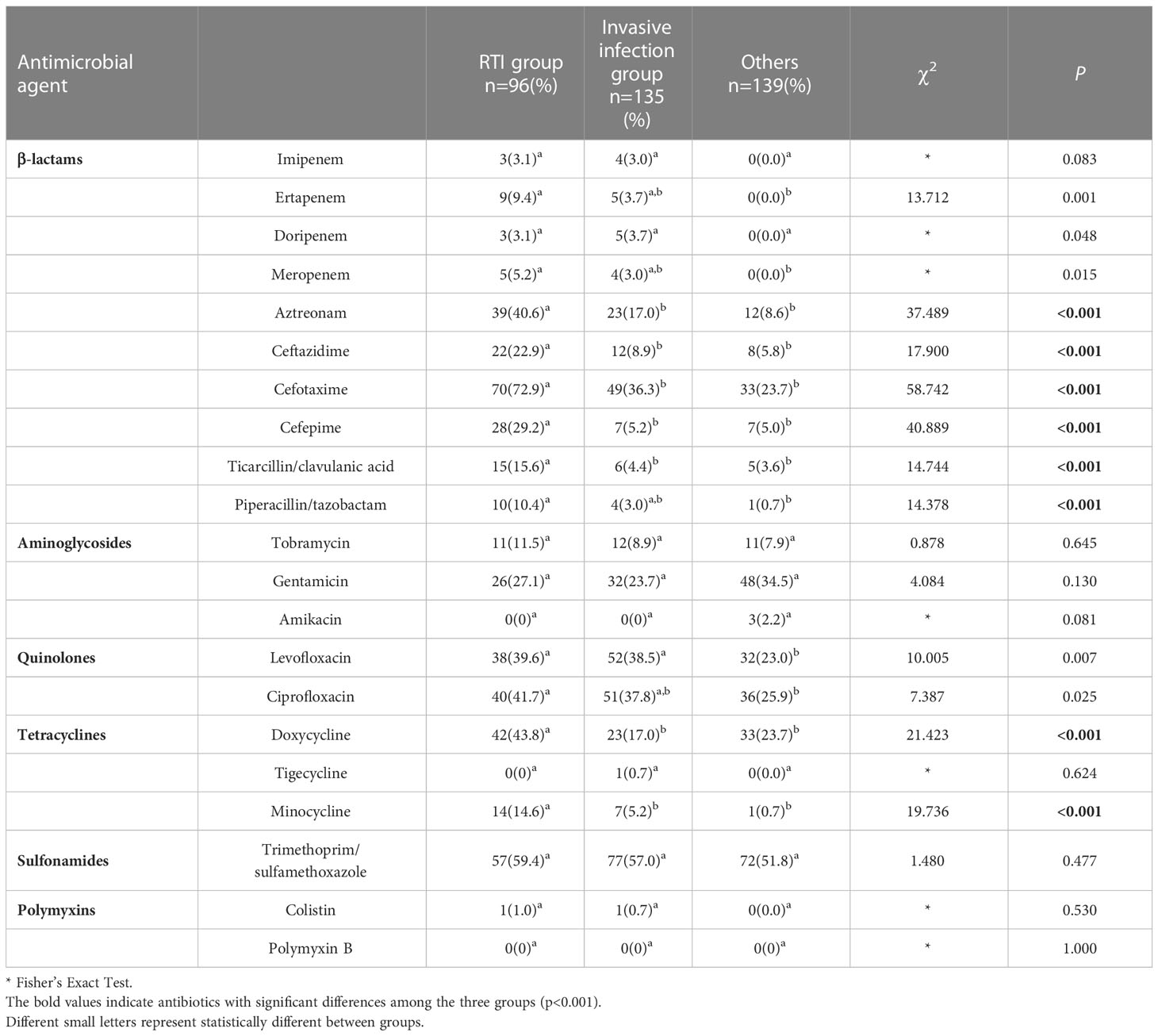
Table 3 Comparison of resistance between respiratory tract infection (RTI) group, invasive infection group and others.
Of the 370 E. coli neonatal isolates in this study, 313 strains(84.60%) were assigned to 85 known STs, and the remaining 57 strains were unknown ST. Among known STs, the most common sequence type was ST1193 (16.76%; 62/370), followed by ST95 (8.92%; 33/370), ST73 (6.49%; 24/370), ST69 (6.22%; 23/370), ST131 (5.42%; 20/370) and ST410 (4.05%; 15/370). ST410 had the highest multidrug resistance rate (80.00%; 13/16). The multidrug resistance rates of ST410, ST1193 and ST131 were much higher than those of ST95 and ST73 (Table 4).
ST410, ST1193 and ST131 with the top three multidrug resistance rates were all sensitive to amikacin, and were highly sensitive to tigecycline, polymyxin B and colistin. ST410 isolates demonstrated the highest resistance rate to cefotaxime (86.67%; 13/15), and they were found to have serious resistance to β-lactam, quinolones and sulfonamides. The most common multidrug resistance pattern of ST410 was β-lactams + aminoglycosides + quinolones + tetracyclines + sulfonamides (Figure 1A). ST1193 exhibited a 90.32% resistance rate to levofloxacin and ciprofloxacin and the common patterns of resistance were β-lactams + quinolones + sulfonamides, aminoglycosides + quinolones + sulfonamides (Figure 1B). Among 20 ST131 isolates, 14 (70.00%) were resistant to gentamicin, with 12 resistant to ceftazidime (60.00%), and the most frequent multidrug resistance pattern was β-lactams + aminoglycosides + sulfonamides (Figure 1C) (Table 5).
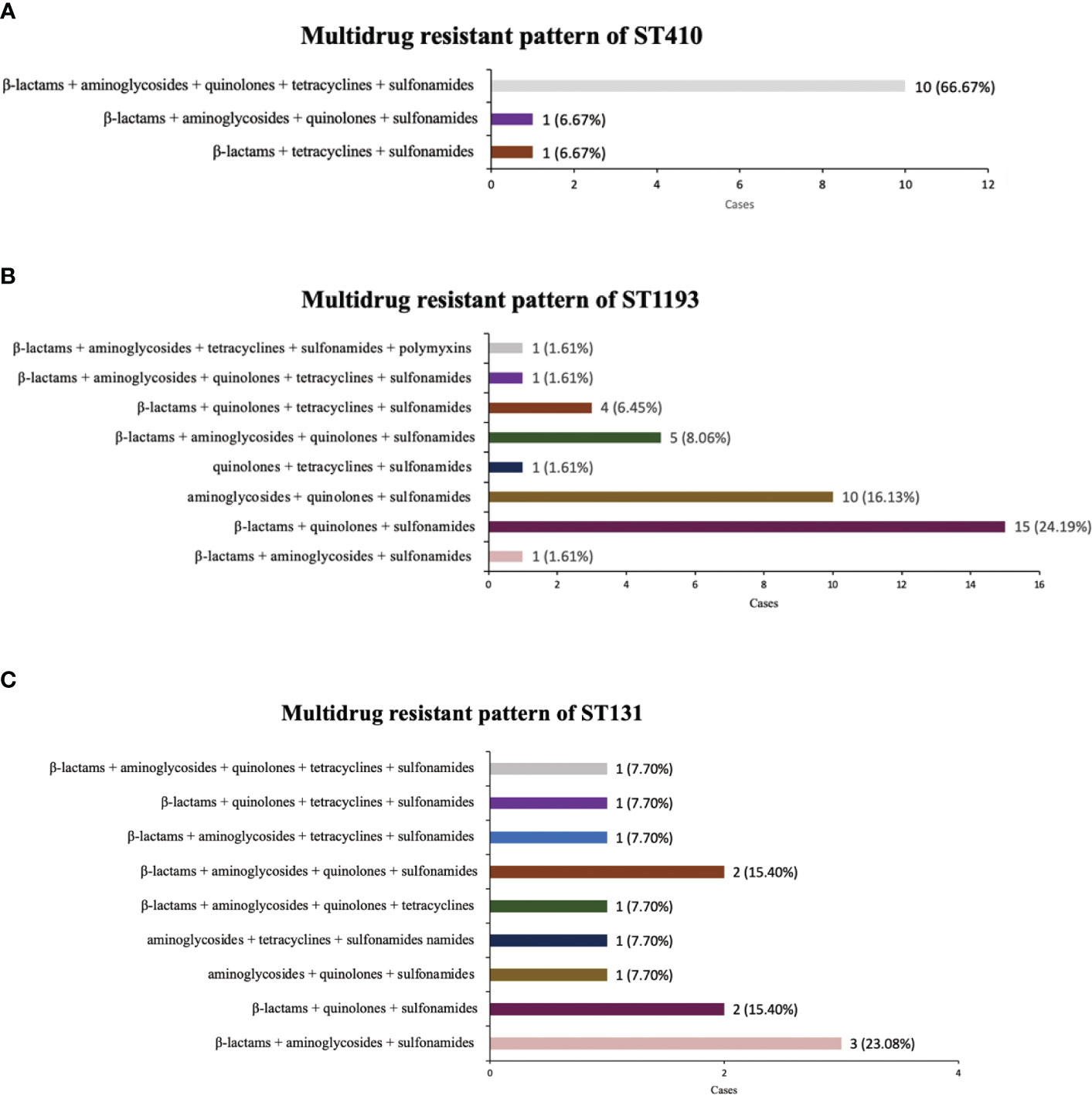
Figure 1 Multi-antibiotics resistance patterns of ST410, ST1193, ST131. (A) Composition of antibiotics resistance patterns of ST410. (B) Composition of antibiotics resistance patterns of ST1193. (C) Composition of antibiotics resistance patterns of ST131.
E. coli is a gram-negative bacillus and resident of the normal intestinal microbiota. However, some pathogenic E. coli strains are capable of causing human disease, and can be broadly divided into two groups, extraintestinal pathogenic E. coli (ExPEC) and intestinal pathogenic E. coli (InPEC) (Pokharel et al., 2023). In terms of morbidity and mortality, ExPEC has a great impact on neonatal health, and it has become a common causative agent of neonatal infections, especially invasive infections (Ejiofor et al., 2018). Several pandemics of E. coli strains, which are highly virulent and antibiotic resistant, have occurred in recent years (Yair and Gophna, 2018). In addition, selection pressures exerted by antibiotic use, overuse, and misuse are driving a gradual increase in antibiotic resistance and leading to the emergence of multidrug resistant bacterial strains, further accelerating the rate of spread of infection and the difficulty of treatment (Wu et al., 2021).
The emergence of multidrug-resistant E. coli has been reported in many countries. And the parallel increase in incidence and frequency of multidrug resistance has raised increasing concerns about the treatment of E. coli infections (Dunn et al., 2019). E. coli tested in this study generally had a high rate of resistance to commonly used antibiotics, with the highest rate of resistance for methotrexate/sulfamethoxazole (55.68%; 206/370), followed by cefotaxime (46.22%; 171/370), and ciprofloxacin (35.41%; 131/370). The Pediatric Surveillance of Infectious Diseases (ISPED) reported bacterial epidemiology and drug resistance in Chinese children in 2020: E. coli resistance to methotrexate/sulfamethoxazole was 54.0%, ceftazidime 49.0%, and ciprofloxacin 41.5%, which is broadly consistent with our findings (Fu et al., 2021). Methotrexate/sulfamethoxazole have been used for decades as effective and inexpensive antimicrobial agents in animals and humans, but extensively resistance has spread widely and rapidly due to the horizontal spread of sul1 and sul2 genes and expressing dihydropteroate synthases highly resistant to sulfonamide. It is rarely used at present (Sköld, 2001). Notably, the total of 370 neonatal E. coli isolates had a multidrug resistance rate of 36.74%, with 35.68% having ESBL phenotype and 5 strains (1.35%) resistant to carbapenem antibiotics. In a cohort study by Flannery et al. of neonatal E. coli samples admitted to multicenter NICUs across the United States from 2009 to 2017 (Flannery et al., 2021), cefazolin resistance was 17.1% and ciprofloxacin was 10.2%, with only 5.0% of isolates meeting ESBL phenotype criteria and no resistance to carbapenems was observed. This difference may be due to the different sources of specimens, but it also shows that the form of drug resistance is more severe in China compared to developed countries.
ESBL and CRE have become more prevalent in pediatric patients and are strongly associated with a poorer clinical prognosis, but surveillance data and evidence of medication use for the neonatal population are currently inadequate (Chiotos et al., 2020). Emergence of ESBL-producing Enterobacterales and CRE in neonatal settings is particularly worrisome because such infections may be resistant to most or all conventional antibiotics (Flannery et al., 2022). Horizontal transfer of plasmid-borne ESBL genes causes resistance of E. coli to β-lactam antibiotics such as cephalosporin, and cephalosporin treatment failure is a serious problem in infection control worldwide. (Dunn et al., 2019; Ibrahim et al., 2023). A minority of E. coli strains were found to be resistant to carbapenem antibiotics such as ertapenem, donipenem, meropenem and imipenem in our study. Carbapenem resistance can result from several mechanisms including, porins coupled with ESBL production, membrane permeability changes via mutations in efflux pumps or by hydrolysis of the beta-lactam ring by dedicated carbapenemase enzymes (Paitan, 2018). Treatment options for multidrug-resistant E. coli (especially ESBL and CRE) infections in neonates are severely limited. Therefore, there is an urgent need to focus on surveillance, prevention, and management of ESBL and CRE to improve the accuracy of diagnosis of neonatal infections and the rationality of antibiotic use.
We found that E. coli with different pathogenicity or isolation sites also differed in drug resistance characteristics. E. coli isolated from sputum were generally more resistant to commonly administered antibiotics than those isolated from other sites –even blood and cerebrospinal fluid. Among them, aztreonam, ceftazidime, cefotaxime, cefepime, ticarcillin/clavulanic acid, piperacillin/tazobactam, doxycycline, and minocycline were the most significant(P < 0.001). In an adult epidemiological survey conducted in France, pneumonia-specific E. coli were also found to be more resistant than commensal isolates to all antimicrobial drugs tested except amikacin and more resistant to cefotaxime and cefoxitin compared to bacteremia isolates (La Combe et al., 2019). E. coli is one of the most genetically versatile microorganisms and has the high plasticity of the genome which gives it a tremendous capacity for evolution, resulting in the acquisition of drug resistance genes and virulence factors (Pokharel et al., 2023). Several studies have found that E. coli strains containing all virulence genomes had a lower resistance phenotype than that observed in non-virulent E. coli strains, and this may be related to the regulation of the bacterial genome (Čurová et al., 2020). As we know, meningitis-associated E. coli crosses the blood-brain barrier requiring a combination of virulence factors, such as OmpA, Ibe, CNF1 (Yang et al., 2023). We therefore hypothesize that the reason why strains causing invasive infections were instead less resistant than those separated from sputum may be related to the complex regulation between virulence and drug resistance. Moreover, the clinical use of antibiotics has had a selective effect on drug-resistant bacteria (Davies and Davies, 2010). Hospitalized neonates are often in need of respiratory support, which, combined with their immature immune development, makes them highly susceptible to pneumonia. Neonatal pneumonia can be fatal and challenging to diagnose, so empirical antibiotic therapy is often initiated early, further leading to a preferential proliferation of antibiotic-resistant strains in the respiratory tract (Vishnu Bhat and Adhisivam, 2018).
ExPEC strains are comprised of many lineages. MLST is a nucleic acid sequencing-based genotyping method that has been widely used in the study of E. coli and the identification of ExPEC-related clonal complexes or lineages. Different ST types have different drug resistance and virulence characteristics (Vanstokstraeten et al., 2022). Manges et al. (2019) using meta-analysis described the type, evolution, distribution and characteristics of ExPEC, which showed that ST131 had the highest proportion, and other major lineages included ST69, ST95, ST10, ST405, ST73, ST410, and ST1193. Among the 370 strains of E. coli in the current study, ST1193 (16.76%) was the most prevalent, followed by ST95 (8.92%). In some regions, ST1193 had emerged as a new virulent clone of fluoroquinolone-resistant E. coli in several countries (Pitout et al., 2022), and it was also isolated from blood and cerebrospinal fluid specimens of Chinese newborns (Ding et al., 2021). In the bacteremia E. coli isolates from newborns in the United States, ST95 and ST131 prevailed; ST1193 emerged recently (Cole et al., 2019), with some similarity to our study, suggesting that there may be an epidemic spectrum in the neonatal population that differs from the adult population.
In this study, we also statistically compared the drug resistance characteristics of isolates from different STs. It is noteworthy that ST410, ST1193 and ST131 had significantly higher multidrug resistance rates than ST95 and ST73, and there was a certain pattern in their resistance profile. ST410 had the most serious multidrug-resistant situation, with very high resistance to β-lactams and quinolones, and its most common multidrug resistance pattern was β-lactams + aminoglycosides + quinolones + tetracyclines + sulfonamides. ST1193 showed the most significant resistance to quinolones (90.32%), in agreement with Johnson et al. (2019), and the common multidrug resistance pattern was β-lactams + quinolones + sulfonamides. ST131 isolate had the highest rate of resistance to gentamicin (70%). Usually, ST131 are reported to produce ESBLs, such as CTX-M-15, and almost all are resistant to fluoroquinolones (Nicolas-Chanoine et al., 2014). The difference may be due to the small number of ST131 E. coli isolated in this study, which was not representative enough.
There is an enormous public health burden due to E. coli multidrug-resistant high-risk clones such as ST1193, ST131 and ST410. These clones have played pivotal roles in the global spread of AMR. It is notable that ST410, as an emerging multidrug-resistant clone, should raise more serious concerns and be monitored more closely. The results of this study showed the higher level of resistance to fluoroquinolones, cephalosporins, and carbapenems in ST410 compared to other ST types. ST410 belongs to 2 clades namely antimicrobial susceptible ST410-A and ST410-B. Clade B is divided into the following subclades: ST410-B1, ST410-B2 that is associated with fluoroquinolone resistance and blaCTX-M-15, while ST410-B3 is linked with fluoroquinolone resistance, blaCTX-M-15 and blaOXA-181 (Roer et al., 2018). Genomic analysis of ST410 by Chen et al. (2022) revealed that ST410-B2 and ST410-B3, which are resistant to fluoroquinolones, contained identical quinolone resistance determining region (QRDR) mutations, and that the acquisition of these QRDR mutations may be due to a single multiple allele homologous recombination event. And the prevalence of the plasmid-mediated fluoroquinolone resistance determinant aac(6)-Ib-cr) was high among ST410 isolates (>90%), especially among the ST410-B3 subclade. Furthermore, the most common ESBLs type in ST410 was CTX-M-15, and the CTX-M-15 genes was mainly carried on the IncF plasmids to move within and between different strains or clones (Pitout et al., 2023). OXA-181 and NDM-5 were the most frequent carbapenemases in ST410 and specifically linked with the ST410-B3 subclade (Roer et al., 2018). The OXA-181 genes were located on near identical broad-host range IncX3 plasmids and NDM-5 genes were located on mosaic narrow-host range IncFII plasmids (i.e. F1:A1:B49) that contained various AMR genes including blaCTX-M-15. ST410 high-risk clones acquired MDR determinants (i.e., fluoroquinolone resistance, CTX-M enzyme, carbapenemase) in a stepwise pattern, acting as “hoarders and transmitters” of AMR genes through horizontal and vertical transmission, which together lead to a high level of resistance and risk in ST410. The specimens in our study were obtained from tertiary care hospitals across China, and contained detailed antibiotic susceptibility data for 370 neonatal-specific E. coli isolates, which makes the analysis of the prevalence of E. coli in Chinese neonates very representative. Nevertheless, this study has some limitations. We only analyzed the antibiotic resistance and susceptibility characteristics of E. coli and lacked further classification and analysis of clinical symptoms and diagnostic information. Records on antibiotic dose or frequency of administration and detailed patient-level data, such as maternal antibiotic exposure, gestational age, and mode of delivery, were not available; therefore, we were unable to include these variables in the adjusted analysis.
In conclusion, the results of this study suggest that resistance of E. coli clinically isolated from neonates hospitalized in NICUs across China was severe, with a substantial proportion of isolates found to be insensitive to commonly used antibiotics. Particular attention should be paid to the monitoring and management of ESBL-type E. coli and strains resistant to carbapenem antibiotics. The resistance phenotype of E. coli varied by pathogenicity and by site, with lower respiratory tract infection such as neonatal pneumonia was generally more resistant to antibiotics. Currently, the main prevalent sequence types in the neonatal population in China were ST1193 and ST95, but multidrug resistance was most severe with ST410. Different MLST types existed with different antibiotic resistance patterns, suggesting that the antibiotic resistance characteristics of E. coli can be inferred from MLST results.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the ethics committee of Beijing Children’s Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
RX drafted the manuscript and did the statistical analysis. YL and XL aggregated and analyzed the data. YJD, JL, YFL and WK completed the data curation, investigation, validation. PZ, JW performed experimental operation and searched for literature research. YD, JZ analyzed the data and edited the manuscript. All authors contributed to the article and approved the submitted version. YW conceptualized and designed the study.
This work was funded by the National Natural Science Foundation of China (Grant No. 81872676).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Antimicrobial Resistance Collaborators (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655. doi: 10.1016/S0140-6736(21)02724-0
Centers for Disease Control and Prevention Antibiotic/antimicrobial resistance (AR/ARM): biggest threats and data: 2019. AR threats report. Available at: https://www.cdc.gov/DrugResistance/Biggest-Threats.html (Accessed December 17, 2019).
Chen, L., Peirano, G., Kreiswirth, B. N., Devinney, R., Pitout, J. D. D. (2022). Acquisition of genomic elements were pivotal for the success of escherichia coli ST410. J. Antimicrob. Chemother. 77, 3399–3407. doi: 10.1093/jac/dkac329
Chiotos, K., Hayes, M., Gerber, J. S., Tamma, P. D. (2020). Treatment of carbapenem-resistant enterobacteriaceae infections in children. J. Pediatr. Infect. Dis. Soc. 9, 56–66. doi: 10.1093/jpids/piz085
Clinical and Laboratory Standards Institute. CLSI M100-ED33:. 2023 Performance Standards for Antimicrobial Susceptibility Testing, 33rd Edition (2023). M100-ED33. Available at: https://clsi.org/standards/products/microbiology/documents/m100/.
Cole, B. K., Ilikj, M., McCloskey, C. B., Chavez-Bueno, S. (2019). Antibiotic resistance and molecular characterization of bacteremia escherichia coli isolates from newborns in the united states. PloS One 14, e0219352. doi: 10.1371/journal.pone.0219352
Collaborative Study Group for Neonatal Bacterial Meningitis (2018). [A multicenter epidemiological study of neonatal bacterial meningitis in parts of south China]. Zhonghua Er Ke Za Zhi 56, 421–428. doi: 10.3760/cma.j.issn.0578-1310.2018.06.004
Collins, A., Weitkamp, J.-H., Wynn, J. L. (2018). Why are preterm newborns at increased risk of infection? Arch. Dis. Child Fetal Neonatal. Ed. 103, F391–F394. doi: 10.1136/archdischild-2017-313595
Čurová, K., Slebodníková, R., Kmeťová, M., Hrabovský, V., Maruniak, M., Liptáková, E., et al. (2020). Virulence, phylogenetic background and antimicrobial resistance in escherichia coli associated with extraintestinal infections. J. Infect. Public Health 13, 1537–1543. doi: 10.1016/j.jiph.2020.06.032
Davies, J., Davies, D. (2010). Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 74, 417–433. doi: 10.1128/MMBR.00016-10
Ding, Y., Zhang, J., Yao, K., Gao, W., Wang, Y. (2021). Molecular characteristics of the new emerging global clone ST1193 among clinical isolates of escherichia coli from neonatal invasive infections in China. Eur. J. Clin. Microbiol. Infect. Dis. 40, 833–840. doi: 10.1007/s10096-020-04079-0
Dunn, S. J., Connor, C., McNally, A. (2019). The evolution and transmission of multi-drug resistant escherichia coli and klebsiella pneumoniae: the complexity of clones and plasmids. Curr. Opin. Microbiol. 51, 51–56. doi: 10.1016/j.mib.2019.06.004
Ejiofor, O. S., Ajunwa, O. M., Ezeudu, C. E., Emechebe, G. O., Okeke, K. N., Ifezulike, C. C., et al. (2018). The bacteriology and its virulence factors in neonatal infections: threats to child survival strategies. J. Pathog. 2018, 4801247. doi: 10.1155/2018/4801247
Flannery, D. D., Akinboyo, I. C., Mukhopadhyay, S., Tribble, A. C., Song, L., Chen, F., et al. (2021). Antibiotic susceptibility of escherichia coli among infants admitted to neonatal intensive care units across the US from 2009 to 2017. JAMA Pediatr. 175, 168–175. doi: 10.1001/jamapediatrics.2020.4719
Flannery, D. D., Chiotos, K., Gerber, J. S., Puopolo, K. M. (2022). Neonatal multidrug-resistant gram-negative infection: epidemiology, mechanisms of resistance, and management. Pediatr. Res. 91, 380–391. doi: 10.1038/s41390-021-01745-7
Fu, P., Xu, H., Jing, C., Deng, J., Wang, H., Hua, C., et al. (2021). Bacterial epidemiology and antimicrobial resistance profiles in children reported by the ISPED program in China 2016 to 2020. Microbiol. Spectr. 9, e0028321. doi: 10.1128/Spectrum.00283-21
GBD 2019 Diseases and Injuries Collaborators (2020). Global burden of 369 diseases and injuries in 204 countries and territories 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet 396, 1204–1222. doi: 10.1016/S0140-6736(20)30925-9
Hallmaier-Wacker, L. K., Andrews, A., Nsonwu, O., Demirjian, A., Hope, R. J., Lamagni, T., et al. (2022). Incidence and aetiology of infant gram-negative bacteraemia and meningitis: systematic review and meta-analysis. Arch. Dis. Child 107, 988–994. doi: 10.1136/archdischild-2022-324047
Ibrahim, D. R., Dodd, C. E. R., Stekel, D. J., Meshioye, R. T., Diggle, M., Lister, M., et al. (2023). Multidrug-resistant ESBL-producing e. coli in clinical samples from the UK. Antibiotics (Basel) 12, 169. doi: 10.3390/antibiotics12010169
Johnson, T. J., Elnekave, E., Miller, E. A., Munoz-Aguayo, J., Flores Figueroa, C., Johnston, B., et al. (2019). Phylogenomic analysis of extraintestinal pathogenic escherichia coli sequence type 1193, an emerging multidrug-resistant clonal group. Antimicrob. Agents Chemother. 63, e01913-18. doi: 10.1128/AAC.01913-18
Khan, A. M., Morris, S. K., Bhutta, Z. A. (2017). Neonatal and perinatal infections. Pediatr. Clin. North Am. 64, 785–798. doi: 10.1016/j.pcl.2017.03.008
La Combe, B., Clermont, O., Messika, J., Eveillard, M., Kouatchet, A., Lasocki, S., et al. (2019). Pneumonia-specific escherichia coli with distinct phylogenetic and virulence profiles, France 2012-2014. Emerg. Infect. Dis. 25, 710–718. doi: 10.3201/eid2504.180944
Manges, A. R., Geum, H. M., Guo, A., Edens, T. J., Fibke, C. D., Pitout, J. D. D. (2019). Global extraintestinal pathogenic escherichia coli (ExPEC) lineages. Clin. Microbiol. Rev. 32, e00135-18. doi: 10.1128/CMR.00135-18
Nicolas-Chanoine, M.-H., Bertrand, X., Madec, J.-Y. (2014). Escherichia coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 27, 543–574. doi: 10.1128/CMR.00125-13
Paitan, Y. (2018). Current trends in antimicrobial resistance of escherichia coli. Curr. Top. Microbiol. Immunol. 416, 181–211. doi: 10.1007/82_2018_110
Pitout, J. D. D., Peirano, G., Chen, L., DeVinney, R., Matsumura, Y. (2022). Escherichia coli ST1193: following in the footsteps of e. coli ST131. Antimicrob. Agents Chemother. 66, e0051122. doi: 10.1128/aac.00511-22
Pitout, J. D., Peirano, G., DeVinney, R. (2023). The contributions of multidrug resistant clones to the success of pandemic extra-intestinal pathogenic escherichia coli. Expert Rev. Anti Infect. Ther. 21, 343–353. doi: 10.1080/14787210.2023.2184348
Pokharel, P., Dhakal, S., Dozois, C. M. (2023). The diversity of escherichia coli pathotypes and vaccination strategies against this versatile bacterial pathogen. Microorganisms 11, 344. doi: 10.3390/microorganisms11020344
Riley, L. W. (2020). Distinguishing pathovars from nonpathovars: escherichia coli. Microbiol. Spectr. 8. doi: 10.1128/microbiolspec.AME-0014-2020
Roer, L., Overballe-Petersen, S., Hansen, F., Schønning, K., Wang, M., Røder, B. L., et al. (2018). Escherichia coli sequence type 410 is causing new international high-risk clones. mSphere 3, e00337-18. doi: 10.1128/mSphere.00337-18
Scamardo, M. S., Dolce, P., Esposito, E. P., Raimondi, F., Triassi, M., Zarrilli, R. (2020). Trends, risk factors and outcomes of healthcare-associated infections in a neonatal intensive care unit in Italy during 2013-2017. Ital. J. Pediatr. 46, 34. doi: 10.1186/s13052-020-0799-3
Sköld, O. (2001). Resistance to trimethoprim and sulfonamides. Vet. Res. 32, 261–273. doi: 10.1051/vetres:2001123
Stoll, B. J., Puopolo, K. M., Hansen, N. I., Sánchez, P. J., Bell, E. F., Carlo, W. A., et al. (2020). Early-onset neonatal sepsis 2015 to 2017, the rise of escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 174, e200593. doi: 10.1001/jamapediatrics.2020.0593
Tan, L. E. (2020). Gram negative organisms and viral infections in neonatal sepsis. BMJ 371, m4248. doi: 10.1136/bmj.m4248
van der Flier, M. (2021). Neonatal meningitis: small babies, big problem. Lancet Child Adolesc. Health 5, 386–387. doi: 10.1016/S2352-4642(21)00092-4
Vanstokstraeten, R., Crombé, F., Piérard, D., Castillo Moral, A., Wybo, I., De Geyter, D., et al. (2022). Molecular characterization of extraintestinal and diarrheagenic escherichia coli blood isolates. Virulence 13, 2032–2041. doi: 10.1080/21505594.2022.2147735
Vishnu Bhat, B., Adhisivam, B. (2018). Can we reduce the duration of antibiotic therapy for neonatal pneumonia? Indian J. Pediatr. 85, 952–953. doi: 10.1007/s12098-018-2750-9
Wen, S. C. H., Ezure, Y., Rolley, L., Spurling, G., Lau, C. L., Riaz, S., et al. (2021). Gram-negative neonatal sepsis in low- and lower-middle-income countries and WHO empirical antibiotic recommendations: a systematic review and meta-analysis. PloS Med. 18, e1003787. doi: 10.1371/journal.pmed.1003787
Wu, D., Ding, Y., Yao, K., Gao, W., Wang, Y. (2021). Antimicrobial resistance analysis of clinical escherichia coli isolates in neonatal ward. Front. Pediatr. 9. doi: 10.3389/fped.2021.670470
Yair, Y., Gophna, U. (2018). Pandemic bacteremic escherichia coli strains: evolution and emergence of drug-resistant pathogens. Curr. Top. Microbiol. Immunol. 416, 163–180. doi: 10.1007/82_2018_109
Yang, R., Wang, J., Wang, F., Zhang, H., Tan, C., Chen, H., et al. (2023). Blood-brain barrier integrity damage in bacterial meningitis: the underlying link, mechanisms, and therapeutic targets. Int. J. Mol. Sci. 24, 2852. doi: 10.3390/ijms24032852
Keywords: neonate, neonatal infection, Escherichia coli, antimicrobial resistance, MLST, epidemiology
Citation: Xiao R, Li Y, Liu X, Ding Y, Lai J, Li Y, Kang W, Zou P, Wang J, Du Y, Zhang J and Wang Y (2023) Antibiotic susceptibility of Escherichia coli isolated from neonates admitted to neonatal intensive care units across China from 2015 to 2020. Front. Cell. Infect. Microbiol. 13:1183736. doi: 10.3389/fcimb.2023.1183736
Received: 10 March 2023; Accepted: 08 May 2023;
Published: 22 May 2023.
Edited by:
Mogens Kilian, Aarhus University, DenmarkCopyright © 2023 Xiao, Li, Liu, Ding, Lai, Li, Kang, Zou, Wang, Du, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yajuan Wang, Y3hzd3lqQHZpcC5zaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.