
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 13 July 2023
Sec. Clinical Microbiology
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1179509
This article is part of the Research Topic Antimicrobial resistance in pediatric infectious diseases: antimicrobial resistance, resistance mechanisms and antimicrobial use View all 18 articles
Background: Skin and Soft Tissue Infections (SSTIs) Surveillance Network of S. aureus In Pediatrics in China was established in 2009 to routinely report epidemiological changes. We aimed to monitor the present antibiotic sensitivity and molecular characteristics of S. aureus and methicillin-resistant S. aureus (MRSA) from SSTIs in children nationwide and track the changes over the past decade.
Methods: Patients diagnosed with SSTIs from the dermatology departments of 22 tertiary pediatric hospitals in seven geographical regions of China were recruited continuously from May 2019 to August 2021. S. aureus was isolated, and its sensitivity to 15 antimicrobials was evaluated using the broth microdilution method. The molecular characteristics of the MRSA isolates were determined through multilocus sequence typing (MLST) and staphylococcal cassette chromosome mec (SCCmec) typing. The presence of the Panton–Valentine leukocidin gene (pvl) was determined.
Results: The detection rate of S. aureus was 62.57% (1379/2204), among which MRSA accounted for 14.79% (204/1379), significantly higher than the result in previous study in 2009-2011 (2.58%, 44/1075). Compared with previous study, the sensitivity to cephalosporins and fusidic acid decreased to varying degrees, while that to chloramphenicol, ciprofloxacin, clindamycin, erythromycin, gentamicin, penicillin, and tetracycline increased significantly. The sensitivity to mupirocin, trimethoprim/sulfamethoxazole (TRISUL), and rifampicin still maintained at a high level (97.90%, 99.35% and 96.66% respectively). The leading multidrug resistance pattern of MRSA and methicillin-sensitive S. aureus (MSSA) were erythromycin-clindamycin-tetracycline (55.84%; 43/77) and erythromycin-clindamycin-chloramphenicol (27.85%, 44/158) respectively. 12 high-level mupirocin-resistant strains were detected, and notable differences in geographical distribution and seasonal variation were observed. The main types of MRSA were ST121 (46.08%, 94/204), followed by ST59 (19.61%, 40/204). SCCmec V (65.69%, 134/204) and SCCmec IV (31.86%, 65/204) were dominant epidemic types. ST121-V, ST59-IV, and ST22-V were the most prevalent clones nationwide. The detection rate of pvl had increased markedly from 9.09% (4/44) in 2009-2011 to 22.55% (46/204) in 2019-2021 (P<0.05).
Conclusion: The antibiotic sensitivity and molecular characteristics of S. aureus from pediatric SSTIs has changed significantly over the past decade. To standardize medical care, provide timely and reasonable clinical treatment, and effectively manage infection control, Chinese pediatric SSTIs guidelines are urgently needed.
Staphylococcus aureus is the main pathogen that causes skin and soft tissue infections (SSTIs), such as pustules, folliculitis, boils, and abscesses, in pediatric patients (Lowy, 1998) as well as fatal infections, such as necrotizing fasciitis and toxic shock syndrome (Burnham and Kollef, 2018). Methicillin-resistant S. aureus (MRSA) has attracted considerable attention owing to its drug resistance and virulence (Lee et al., 2018). Community-associated MRSA (CA-MRSA) mainly causes SSTIs in young and healthy people in the community (Turner et al., 2019). Over the past decade, CA-MRSA has been considered to be the main cause of the increased burden of MRSA diseases. Some CA-MRSA strains have been increasingly involved in nosocomial infections and have even become dominant strains in medical settings (Elston and Barlow, 2009). The resistance rate of CA-MRSA is increasing, not only to β-lactam antibiotics but also to non-β-lactam antibiotics (Guo et al., 2020). The spectrum of CA-MRSA invasive diseases is expanding and is increasingly becoming the focus of global infection (Hassoun et al., 2017). Epidemiological information on MRSA is important for clinical decision-making and public health monitoring. Furthermore, classification of MRSA is an important part of describing epidemiological trends and formulating infection control strategies (Mediavilla et al., 2012).
To our knowledge, few studies on the antibiotic sensitivity of S. aureus and molecular characteristics of MRSA from SSTIs in China have been conducted (Xiao et al., 2019; Zhao et al., 2022). Furthermore, results from different regions differ, and national studies related to children are rare. A national SSTIs surveillance network of S. aureus in pediatrics, established in 2009 and led by the Department of Dermatology, Beijing Children’s Hospital, is the only available nationwide epidemiological surveillance network with regular investigation in China (Liu et al., 2016). Currently, 22 children’s hospitals have joined. The present study aimed to track the changes in antibiotic sensitivity of S. aureus as well as the molecular characteristics and epidemiology of MRSA in children diagnosed with SSTIs in China.
This was a multi-center, cross-sectional epidemiological study on children diagnosed with SSTIs in the dermatology departments of 22 tertiary pediatric hospitals. According to their geographical location, the hospitals were divided into seven groups: North, Middle, East, South, Northeast, Northwest, and Southwest China. All patients who met the criteria for SSTIs were recruited continuously from the dermatology departments from May 2019 to August 2021. The inclusion criteria were as follows: 1) no history of major congenital malformations or severe chronic diseases, 2) no history of surgery or hospitalization within the past year, 3) no history of dialysis or deep catheterization, and 4) no history of antibiotic use within the past week. Information, including sex, age, predisposing factors, disease type, specimen collection time, and basic medical history, was collected. Patients who could not provide general information were excluded. The enrolled patients were treated according to routine treatment, and the swab specimens of infection sites from the non-repetitive participants were collected continuously.
The isolated strains were identified using traditional microbial identification methods, coagulase and catalase tests, and latex slide agglutination test (Oxoid Ltd., Basingstoke, UK). All three tests were positive for S. aureus (Weist et al., 2006).
In addition to resistance to cefoxitin and oxacillin, MRSA was further identified through polymerase chain reaction (PCR) amplification of the nuc and mecA genes according to the method described previously (Sahebnasagh et al., 2014). ATCC 29213 and ATCC35601 were used as a negative and positive control, respectively, for the mecA gene.
The antibiotic susceptibility profiles of the S. aureus isolates were determined using the Sensititre® Antimicrobial Susceptibility Testing System (Thermo Scientific, UK), following the manufacturer’s instructions. The minimum inhibitory concentration (MIC) to 15 antibiotics (cefazolin, ceftriaxone, cefuroxime, chloramphenicol, ciprofloxacin, clindamycin, erythromycin, fusidic acid, gentamicin, mupirocin, penicillin, rifampicin, tetracycline, TRISUL, and vancomycin) were detected using the broth microdilution method (Novy et al., 2014). S. aureus ATCC 29213 and ATCC 35601 were used for quality control. The antimicrobial sensitivity breakpoints were interpreted according to the current Clinical and Laboratory Standards Institute (CLSI) breakpoints for S. aureus (CLSI, 2019), while sensitivity to cefazolin, ceftriaxone, and cefuroxime was interpreted according to a previous version of CLSI (Clinical and Laboratory Standards Institute/NCCLS, 2005). An E-test ((bioMérieux, Marcy-L’Étoile, France) was further performed on the isolates classified as mupirocin-resistant through broth microdilution.
DNA was extracted for PCR using a bacterial genomic DNA extraction kit (Tiangen Biochemical Technology, China). Multilocus sequence typing (MLST) was performed on MRSA isolates using the method described previously (Enright et al., 2000). Sequences of seven housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi, and yqiL) were compared with known alleles from the MLST database (https://pubmlst.org/organisms/staphylococcus-aureus). Allelic profiles and sequence types (STs) were determined using the database.
The isolates were also subjected to staphylococcal cassette chromosome mec (SCCmec) typing, which is based on multiplex PCR with 10 primers (Omuse et al., 2016). SCCmec types I-V were assigned according to the combination of the cassette chromosome recombinase (ccr) type and mec class. Isolates that could not be assigned to any expected type were defined as non-typable.
pvl was amplified using PCR as described previously (Hesje et al., 2011) with minor modifications. MRSA N315 was used as a negative control, while ATCC 25923 was used as a positive control.
A database including the age, sex and disease patterns of the patients, antimicrobial resistance and molecular characteristics of the corresponding isolate was constructed in Microsoft Excel 2003. GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA, United States) was used to create graphs. All susceptibility data were analyzed using WHONET software (version 5.6). JMP® 11 Statistical Discovery Software (S.A.S. Institute Inc., Cary, North Carolina) was used for statistical analysis. Categorical variables were analyzed using the chi-squared (χ2) or Fisher’s exact test. Significance was set at P < 0.05.
The initial data of S. aureus and MRSA collection as well as the distribution of them are summarized here. From May 2019 to August 2021, 2204 patients with SSTIs were enrolled in 22 children’s hospitals in 19 provinces of seven geographic regions. The overall positive rate of S. aureus was 62.57% (1379/2204). The detection rate of S. aureus was the highest at 75.77% (519/685) in South China and was the lowest at 46.49% (159/342) in North China. The proportion of MRSA was 14.79% (204/1379) nationally, with significantly different proportions across China ranging from 12.73% (21/165) in Middle China to 17.24% (5/29) in North West China. Single institutional prevalence ranged from 4.35% (1/23) to 30.77% (8/26) (P < 0.05). The distribution of enrolled patients and the number of positive S. aureus and MRSA isolates in each geographic region are shown in Figure 1.
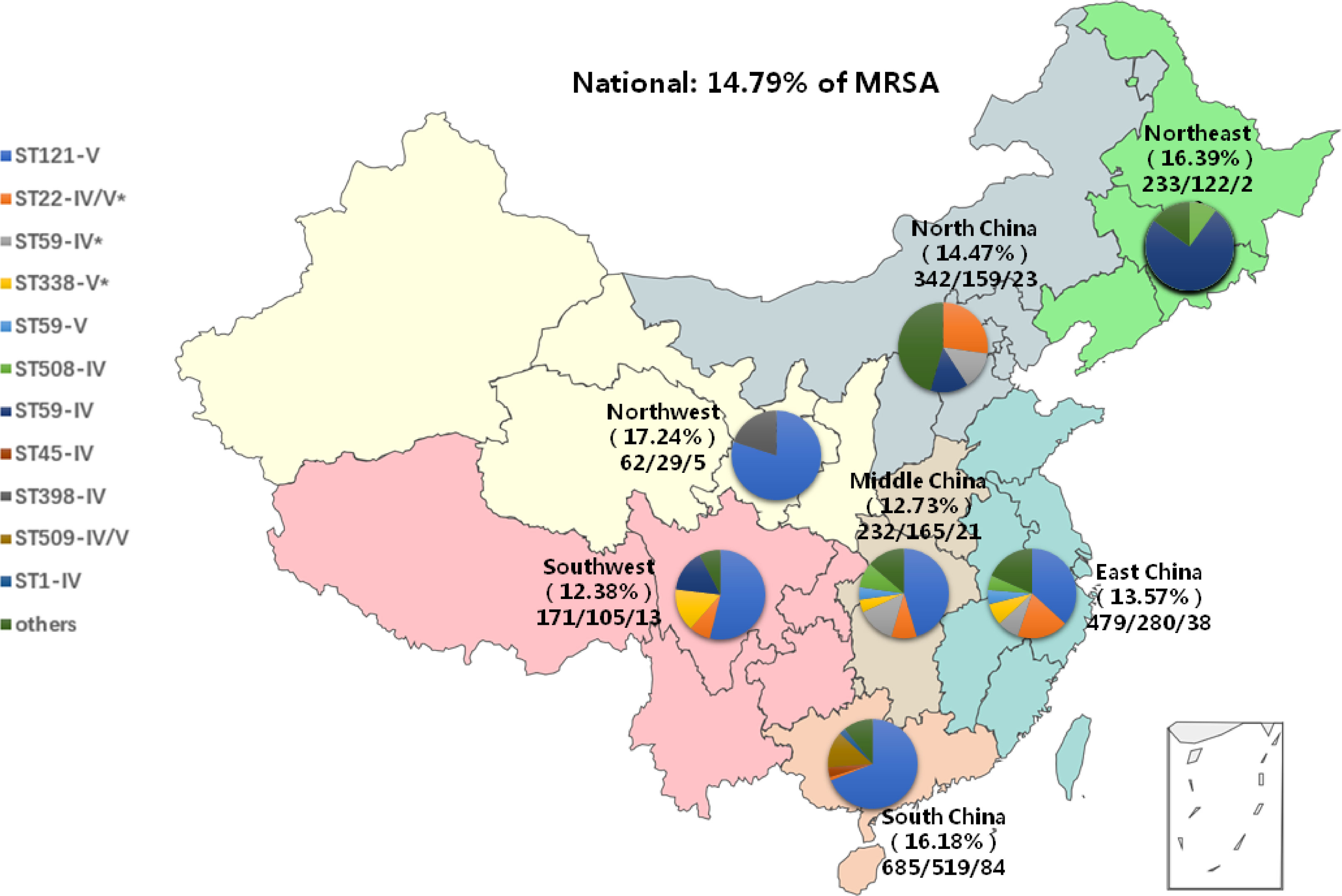
Figure 1 The distribution of specimens, S. aureus and MRSA as well as prevalent clones, by region. All seven geographical regions were included as follows: Northeast, green, North China, gray; East China, blue; Northwest, yellow; Middle China, khaiki; Southwest, pink; South China, orange. *pvl-positive clones contained.
The clinical features of patients from whom the strains were collected are analyzed as follows. The age of patients ranged from 3 days to 18 years old, with 77.16% (1064/1379) aged 1–6 years. Of the patients, 58.45% (806/1379) were males and 41.55% (573/1379) were females. Detailed clinical features are presented in Table 1.
Great changes on infection patterns had occurred during the past decade. The top three primary infections in this study were impetigo (39.38%; 543/1379), furuncles (19.87%; 274/1379), and folliculitis (12.76%; 176/1379). Compared with our study conducted in 2009-2011 (Liu et al., 2016), the distribution of deep infections such as folliculitis and furuncle in 2019-2021 increased significantly from 1.20% (21/1749) to 12.76% (176/1379) and from 0.57% (10/1749) to 19.87% (274/1379) with P<0.001, respectively. The distribution of superficial infections such as impetigo and staphylococcal scalded skin syndrome had decreased from 79.87% (1397/1749) to 39.38% (543/1379) and from 4.69% (82/1749) to 0.80% (11/1379) with P<0.001, respectively. The detailed composition of infections caused by S. aureus in 2009-2011 and 2019-2021 is shown in Figure 2.
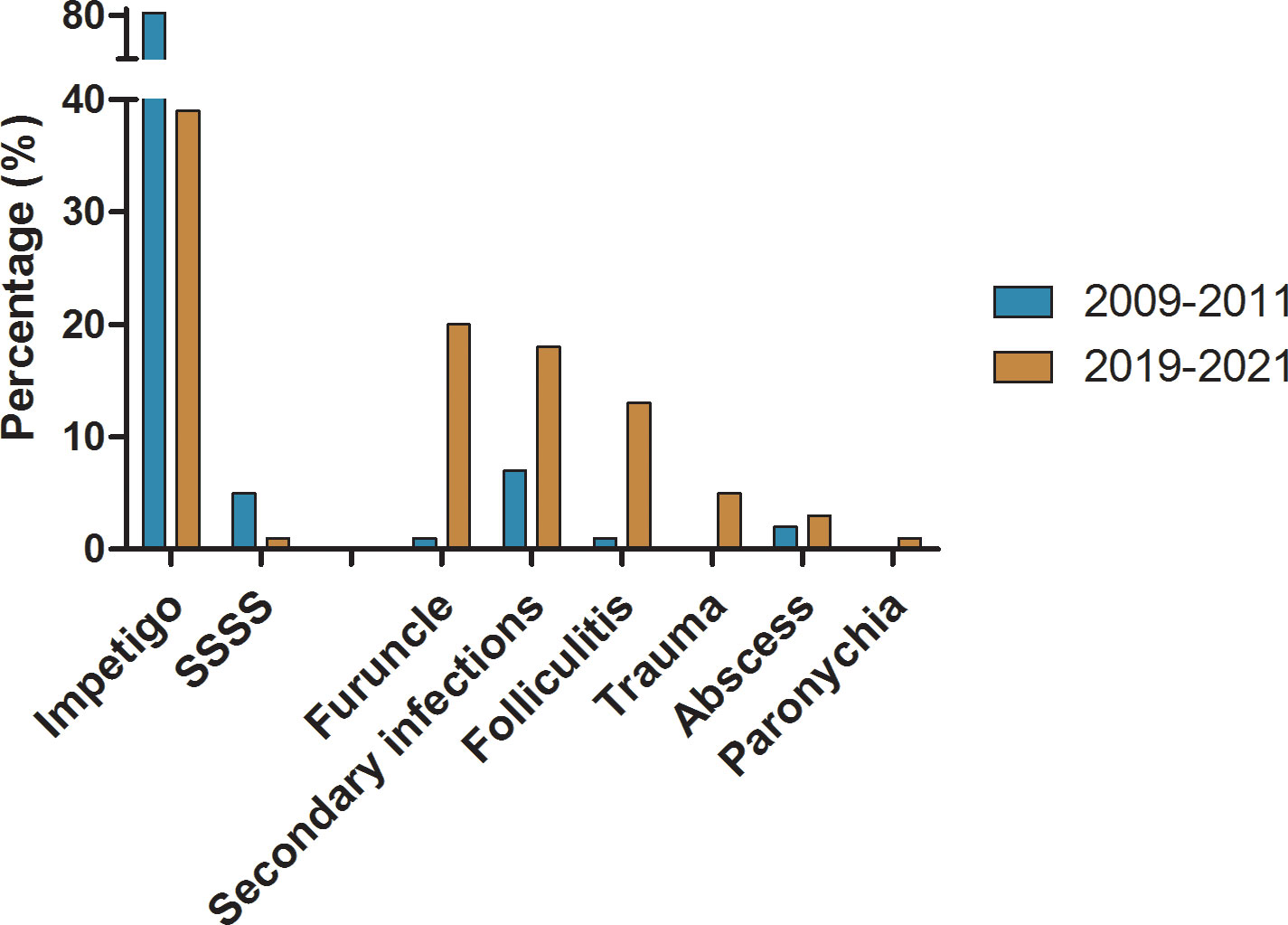
Figure 2 The comparation on distribution of major infection pattern caused by S. aureus in 2009-2011 and 2019-2021 respectively.
The results of the antimicrobial susceptibility test on strains collected in this present study are shown in Table 2. The resistance rates of MRSA to ciprofloxacin, clindamycin, erythromycin, and tetracycline were significantly higher than those of MSSA (P<0.05).
S. aureus with resistance to three or more classes of antimicrobial agents were defined as multidrug-resistant (MDR). In this study, MDR was observed in 37.75% (77/204) of MRSA strains and 13.45% (158/1175) of MSSA strains. The predominant resistance patterns of MRSA to non-β-lactam antibiotics were erythromycin-clindamycin-tetracycline (55.84%; 43/77), followed by erythromycin-clindamycin-tetracycline-chloramphenicol (18.18%; 14/77). The resistance patterns of MSSA were very different from that of MRSA, the most common profiles of which were erythromycin-clindamycin-chloramphenicol (27.85%, 44/158) and erythromycin- clindamycin-tetracycline (22.15%, 35/158). Among different STs of MRSA strains, the proportion of MDR in ST121 was the highest (49.35%; 38/77), followed by ST59 (22.08%; 17/77) and ST338 (6.49%; 5/77).
A total of 12 high-level mupirocin-resistant (MuH) strains (MIC ≥ 512 μg/mL) were detected, including nine MSSA strains and three MRSA strains. The differences in ST distributions of MuH strains were irregular, while notable in the differences in geographical distribution and the seasonal variation. MuH strains mainly distributed in South China (66.67%, 8/12)、East China (16.67%, 2/12) and Middle China (16.67%, 2/12). Of the hospitals, the isolates were predominantly separated from Shenzhen Children’s Hospital (41.67%, 5/12), which was geographically assigned to South China. The infection patterns were mainly secondary infection, including secondary infection of eczema (5/12), trauma (2/12) and herpes (1/12). The infections caused by MuH isolates mainly occured in autumn (8/12), followed by summer (4/12). The children mainly aged >3y (66.67%, 8/12).
The changes of resistance patterns of S. aureus collected in this study with strains collected in 2009-2011were analyzed. The current resistance rates of S. aureus to cefazolin, ceftriaxone, cefuroxime, and fusidic acid had increased significantly (P<0.05), and that to chloramphenicol, ciprofloxacin, clindamycin, erythromycin, gentamicin, rifampicin, tetracycline, and TRISUL decreased significantly (P<0.0001), while no significant difference was found in resistance to vancomycin and mupirocin. Besides, the resistance rate to penicillin decreased from 96.8% to 94.13% (P=0.0004). The comparison of the antimicrobial sensitivity of S. aureus isolates between 2009-2011 and 2019-2021 is shown in Figure 3.
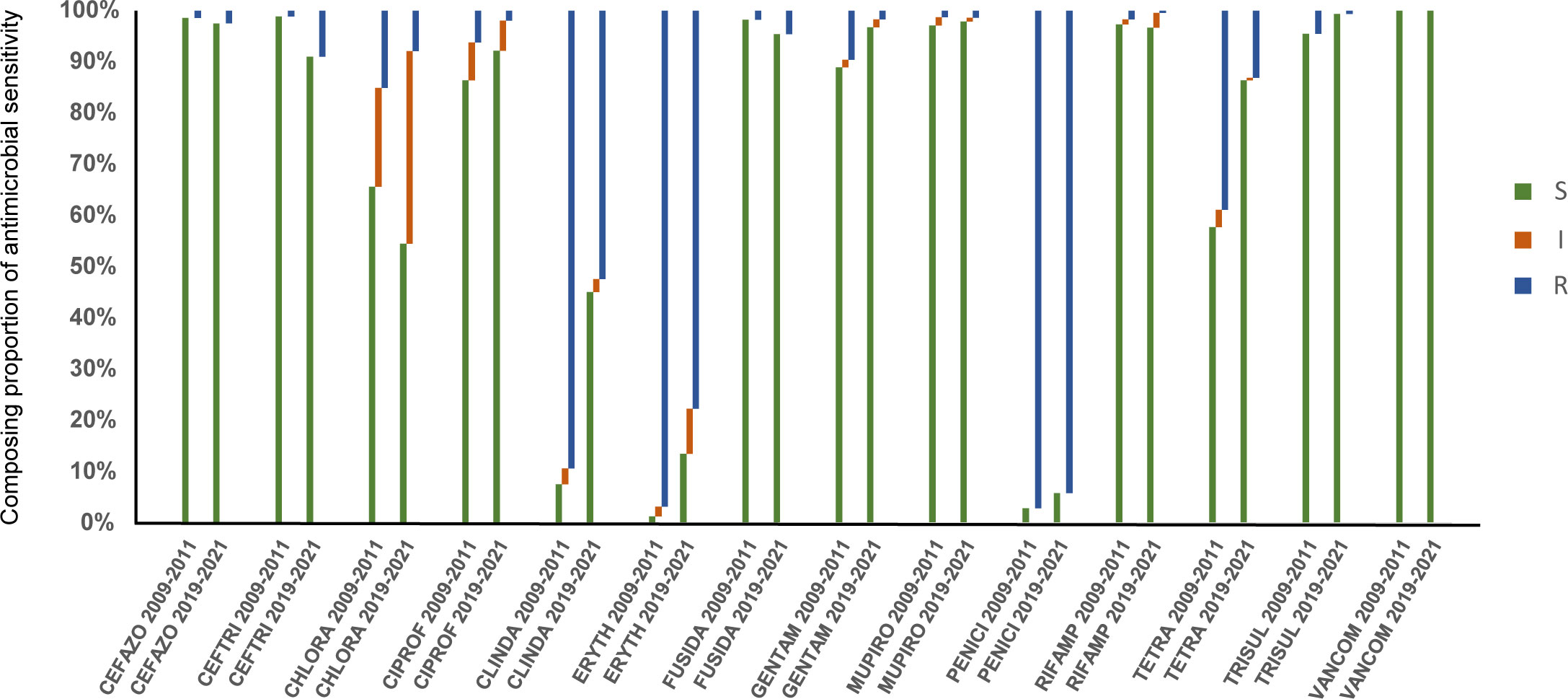
Figure 3 Comparison of antimicrobial susceptible, intermediate and resistant rates of S. aureus isolates in 2009-2011 and 2019-2021. S, susceptible; I, intermediate; R, resistant.
Overall, 25 STs were detected, of which ST121 accounted for 46.08% (94/204), followed by ST 59 (19.61%, 40/204) and ST22 (9.80%, 20/204). In SCCmec classification, 65.69% (134/204) were type V, and 25.98% (53/204) were type IV. ST121-V, ST59-IV, and ST22-V were the most prevalent clones nationwide. The molecular biological characteristics of MRSA isolates and the dominant MRSA clones by region are summarized in Table 3; Figure 1.
ST121 was the most prevalent type of MRSA strains, which all typed as SCCmec V and pvl negative. ST121 strains were mainly distributed in Northwest and South China with a positive rate of 80.00% (4/5) and 69.05% (58/84), respectively, while they were not detected in Northeast China. Compared with non-ST121 isolates, ST121 isolates had a significantly higher resistance rate to clindamycin and lower resistance rates to cefazolin, cefuroxime, and ciprofloxacin. The detection rate of ST59 was second to that of ST121. ST59, which was opposite to ST121, was mainly distributed in Northeast and North China, with a positive rate of 80.00% (16/20) and 40.91% (9/22), respectively. Inconsistent with ST121, ST59 strains were mainly typed as SCCmec IV (77.50%, 31/40). The positive rate of pvl was 35.00% (14/40), significantly higher than that of ST121 (0.00%, 0/94). The resistance rate of ST59 isolates to ciprofloxacin, cefazolin, and cefuroxime was significantly higher than that of ST121 isolates (P<0.05), and there was no significant difference in the resistance rate to other antibiotics.
The detection rate of pvl had increased markedly from 9.09% (4/44) in 2009-2011 to 22.55% (46/204) in 2019-2021 (P<0.05). Infection patterns caused by pvl-positive and pvl-negative MRSA strains in this study are shown in Figure 4A. pvl-positive MRSA strains mainly caused furuncle (41.30%,19/46) and folliculitis (21.74%,10/46), higher than pvl-negative strains with P<0.0001 and P=0.073 respectively, while pvl-negative MRSA strains mainly caused impetigo (44.94%,71/158) and secondary infection (25.95%,41/158), higher than pvl-positive strains with P<0.0001 and P=0.067 respectively. Among the pvl-positive strains, ST22 (41.30%, 19/46), ST59 (30.43%, 14/46) and ST338 (15.22%, 7/46) were the most common types as shown in Figure 4B, which were significantly higher than the ratio of pvl-negative strains with P<0.05 respectively. In contrast, among pvl-negative strains, ST121 (59.49%,94/158) was the most prevalent ST, the ratio of which was significantly higher than that of pvl-positive strains (P<0.0001).
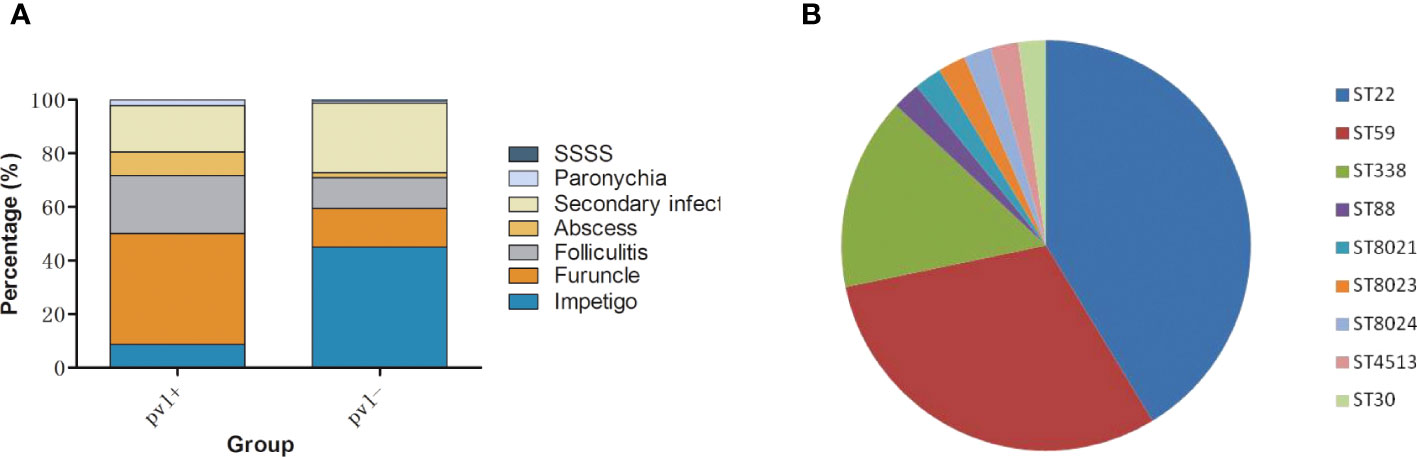
Figure 4 Clinical and molecular characteristics of pvl-positive MRSA strains. (A), infection patterns caused by pvl-positive and pvl-negative MRSA strains. (B), ST distribution of pvl-positive MRSA strains.
This study was the second to conduct national multicenter epidemiological monitoring of S. aureus for SSTIs in pediatrics since the surveillance network was established in China in 2009. The epidemiological trends of SSTIs in 22 tertiary children’s hospitals in seven geographical regions of mainland China were investigated. Compared with the study conducted 10 years ago, we presented four major findings: 1) the resistance profiles of S. aureus isolates had changed considerably; 2) the prevalence of CA-MRSA and its pvl-positive strains, increased significantly; 3) the proportion of deep infections increased significantly; and 4) ST121-V was the dominant clone, with the percentage increased.
In this study, MRSA accounted for 14.79%, significantly higher than in 2009-2011 (2.58%). It was reported that antimicrobial agents should be chosen to target MRSA and MSSA if MRSA accounts for >10% of S. aureus among SSTIs (David and Daum, 2017). Either clindamycin or TRISUL was recommended because of the low cost and activity against community-associated MRSA and MSSA strains of each of these drugs (Williams et al., 2011). According to previous studies, one of these antimicrobials should be used in addition to incision and drainage for a skin infection (Miller et al., 2015; Daum et al., 2017). In this study, we identified a low resistance rate of S. aureus to TRISUL (0.4%–2%). The long-term use of TRISUL remains a suitable option for treating complex hyperimmunoglobulin E syndrome and chronic granulomatous disease (Hashemi et al., 2017). Clindamycin was reported to be effective at treating infections caused by susceptible CA-MRSA isolates (Miller et al., 2015). However, in this study, though significantly decreased compared with that in 2009-2011, the resistance rate of clindamycin was still higher than 50%, indicating that it is not a good choice for treating SSTIs in children in China.
For empiric or targeted therapy for S. aureus, an anti-staphylococcal β-lactam drug was the most appropriate choice (David and Daum, 2010). Furthermore, it was reported that cephalosporins and penicillin are most commonly used in China (Li et al., 2016). Therefore, the MICs of the clinical strains to penicillin as well as cephalosporins was detected to track changes in sensitivity. The resistance rates of S. aureus to penicillin, though decreased significantly, remained at a high level (94.13%). On the contrary, the sensitivity to cephalosporins was maintained at a high level (90.65%–97.53%). According to this result, cephalosporins might be a suitable alternative to penicillin for empiric therapy.MDR to non-β-lactam antibiotics was detected in both MRSA and MSSA. The presence of MDR strains in outpatients with SSTIs can lead to persistent or recurrent MRSA infections (Lee et al., 2017).
Clinically, most SSTIs can be controlled only with topical antibiotics. Mupirocin is a topical antibiotic that has been extensively used for treating MRSA skin and soft-tissue infections, decreasing certain types of surgical site infections and eliminating nasal colonisation of MRSA among patients and medical staff. In the present study, the sensitivity to mupirocin was still high, consistent with previous results (Liu et al., 2016; Chen et al., 2020). However, high-level mupirocin strains were detected in both MRSA and MSSA strains in this study. Dadashi et al. reported that the incidence of high-level mupirocin resistance in MRSA was the highest in Asia (12.1%), followed by Europe (8.0%) and the USA (5.9%) (Dadashi et al., 2020). In China, it was reported that mupA mainly accounted for high level mupirocin resistance (Jin et al., 2018; Guo et al., 2023). The mupA gene is typically located on mobile genetic elements and is plasmid mediated, which maybe the reasons for transmission of clones (Liu et al., 2010). In this study, high-level mupirocin resistant strains were mainly isolated from South China, which were generally with higher economical levels than others. Easy access to antibiotics without prescriptions, a high rate of antibiotic misuse, and the frequency of empiric treatment in these regions may lead to the situation. The results suggested that the rational use of mupirocin should be strengthened, and drug resistance should be further monitored.
Fusidic acid was also an important choice for SSTIs. The worldwide resistance rate of S. aureus, especially MRSA, to fusidic acid was reported to be 0.3%–64% (Gajdács, 2019). In the present study, the resistance rate of S. aureus to fusidic acid increased from 1.8% to 4.57%, which was still low, similar to that identified previously (Gu et al., 2016). The resistance rate in MSSA (4.85%) was higher than that in MRSA (2.94%), consistent with previous study (Zhanel et al., 2021). The increased detection rate of fusidic acid-resistant strains suggests that the drug should be used in moderation.
Based on the above results of antimicrobial sensitivity, we call for the timely introduction of guidelines for the treatment of SSTIs in children in China to develop scientific and effective diagnosis and treatment programs.
MRSA has been the focus of global SSTIs (Mediavilla et al., 2012). Recently, an upward trend in the incidence of pvl-positive MRSA was observed in Europe and Japan (Bouchiat et al., 2017; Nakaminami et al., 2020). Concern was raised as pvl-positive MRSA strains usually cause deep infections such as furuncles and abscesses (Shallcross et al., 2013). Compared with the study conducted 10 years ago, the detection rate of MRSA in the present study had increased by 5.65-fold (14.79% vs. 2.58%; P<0.0001), and the positive rate of pvl had increased by 2.3-fold (22.55% vs. 9.8%; P<0.05). In addition, the infection spectrum had changed, as deep infections including folliculitis, furuncle, and abscesses increased significantly, while superficial infections decreased. Therefore, according to our surveillance, there was an increasing trend in the prevalence of pvl-positive MRSA among SSTI isolates in Chinese children, which was probably connected with the increase in deep infections. Attention should be paid to the surveillance of pvl-positive MRSA in the future.
MLST is a universal method for understanding the molecular epidemiology of MRSA (Enright et al., 2000). Previous studies demonstrated that the most prevalent clones of CA-MRSA from SSTIs had unique geographic distribution, as ST8 was mostly reported in the USA (Otter and French, 2010), while ST80 was mainly in Europe, and ST59 was mainly in the Asia–Pacific region (Deurenberg and Stobberingh, 2008). In mainland of China, Taiwan, and Hong Kong, ST59 was reported as the most prevalent ST of MRSA strains from SSTIs (Yu et al., 2015), while ST121 was rarely dominant for clinical infections (Chuang and Huang, 2013; Wang et al., 2019). The epidemiological data hint that most ST121 strains were MSSA (Goering et al., 2008; Rao et al., 2015). However, in the present survey, ST121 (46.08%; 94/204) was the dominant ST in MRSA strains, followed by ST59, which was consistent with the results of our previous study (Liu et al., 2016). This was probably due to the differences in the population. In the present study, the enrolled children were at preschool age (1–6 years). It was reported that among preschool children, ST121 was the most prevalent clone in China (Fan et al., 2009). Besides, they were all outpatients who had no history of hospitalization. Therefore, the study was more representative of infections from the community.
In conclusion, tremendous changes in the antibiotic sensitivity of S. aureus from SSTIs in Chinese children had been observed compared with the results obtained 10 years ago. The incidence of MRSA as well as the positive rate of pvl had increased significantly, with ST121, ST59, and ST22 being the main epidemic types. With the significant changes, further research tracking sensitivity to antibiotics as well as the molecular epidemiological characteristics of MRSA is needed. Moreover, to standardize medical care, help clinicians make evidence-based treatment decisions, and effectively manage infection control, guidelines for SSTIs in pediatrics in China are urgently needed.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
This project (2019-k-123) was approved by the Research Ethics Committee in Beijing Children’s Hospital, China on May 21, 2019.
YL, KY, YY and LM designed the study. WS, YL, QW, LY and WG conducted the experiments. WS, YL and QW collected and analyzed the data, interpreted the results, and drafted the manuscript. All authors contributed to the article and approved the submitted version.
National Nature Science Foundation of China (31900132, 81903668), The Special Fund of the Pediatric Medical Coordinated Development Center of Beijing Hospitals Authority (XTZD20180502); Beijing Hospital Authority (QM20191202); BINC Nutrition and Care of Maternal & Child Funding Project (2019BINC-MCF116).
Thanks for the support of Chinese Dermatologist Association. We also thank the following hospitals and their staff members for participating in this study: Beijing Children’s Hospital, Children’s Hospital Affiliated to Capital Institute of Pediatrics, Shenzhen Children’s Hospital, Dalian Children’s Hospital of Dalian Medical University, Children’s Hospital of Changchun, Harbin Children’s Hospital, Inner Mongolia maternal and Child Health Hospital, Xuzhou Children’s Hospital, Children’s Hospital Affiliated to Zhejiang University, Anhui Children’s Hospital, Nanjing Children’s Hospital, Children’s Hospital Affiliated to Shandong University, Hangzhou Children’s Hospital, Xi’an Children’s Hospital, Hubei Maternal and Child Health Hospital, Hunan Children’s Hospital, Zhengzhou Children’s Hospital, Chengdu Children’s Hospital, Kunming Children’s Hospital, Guangzhou Women and Children’s Medical Center, Hainan Maternal and Child Health Hospital, Guangxi Liuzhou Maternal and Child Health Hospital.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bouchiat, C., Curtis, S., Spiliopoulou, I., Bes, M., Cocuzza, C., Codita, I., et al. (2017). MRSA infections among patients in the emergency department: a European multicentre study. J. Antimicrob. Chemother. 72 (2), 372–375. doi: 10.1093/jac/dkw431
Burnham, J. P., Kollef, M. H. (2018). Treatment of severe skin and soft tissue infections: a review. Curr. Opin. Infect. Dis. 31 (2), 113–119. doi: 10.1097/QCO.0000000000000431
Chen, W., He, C., Yang, H., Shu, W., Cui, Z., Tang, R., et al. (2020). Prevalence and molecular characterization of methicillin-resistant Staphylococcus aureus with mupirocin, fusidic acid and/or retapamulin resistance. BMC Microbiol. 20 (1), 183. doi: 10.1186/s12866-020-01862-z
Chuang, Y. Y., Huang, Y. C. (2013). Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Asia. Lancet Infect. Dis. 13 (8), 698–708. doi: 10.1016/S1473-3099(13)70136-1
Clinical and Laboratory Standards Institute/NCCLS (2005). “Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement,” in CLSI/NCCLS document M100-S15 (940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA: Clinical and Laboratory Standards Institute).
CLSI (2019). “Performance standards for antimicrobial susceptibility testing,” in CLSI supplement M100, 29th ed, vol. 2019. (Wayne, PA: Clinical and Laboratory Standards Institute).
Dadashi, M., Hajikhani, B., Darban-Sarokhalil, D., van Belkum, A., Goudarzi, M. (2020). Mupirocin resistance in Staphylococcus aureus: a systematic review and meta-analysis. J. Glob Antimicrob. Resist. 20, 238–247. doi: 10.1016/j.jgar.2019.07.032
Daum, R. S., Miller, L. G., Immergluck, L., Fritz, S., Creech, C. B., Young, D., et al. (2017). A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl. J. Med. 376 (26), 2545–2555. doi: 10.1056/NEJMoa1607033
David, M. Z., Daum, R. S. (2010). Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 23 (3), 616–687. doi: 10.1128/CMR.00081-09
David, M. Z., Daum, R. S. (2017). Treatment of Staphylococcus aureus infections. Curr. Top. Microbiol. Immunol. 409, 325–383. doi: 10.1007/82_2017_42
Deurenberg, R. H., Stobberingh, E. E. (2008). The evolution of Staphylococcus aureus. Infect. Genet. Evol. 8 (6), 747–763. doi: 10.1016/j.meegid.2008.07.007
Elston, J. W., Barlow, G. D. (2009). Community-associated MRSA in the united kingdom. J. Infect. 59 (3), 149–155. doi: 10.1016/j.jinf.2009.07.001
Enright, M. C., Day, N. P., Davies, C. E., Peacock, S. J., Spratt, B. G. (2000). Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38 (3), 1008–1015. doi: 10.1128/JCM.38.3.1008-1015.2000
Fan, J., Shu, M., Zhang, G., Zhou, W., Jiang, Y., Zhu, Y., et al. (2009). Biogeography and virulence of Staphylococcus aureus. PloS One 4 (7), e6216. doi: 10.1371/journal.pone.0006216
Gajdács, M. (2019). The continuing threat of methicillin-resistant Staphylococcus aureus. Antibio. (Basel). 8 (2), 52. doi: 10.3390/antibiotics8020052
Goering, R. V., Shawar, R. M., Scangarella, N. E., O'Hara, F. P., Amrine-Madsen, H., West, J. M., et al. (2008). Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates from global clinical trials. J. Clin. Microbiol. 46 (9), 2842–2847. doi: 10.1128/JCM.00521-08
Gu, F. F., Chen, Y., Dong, D. P., Song, Z., Guo, X. K., Ni, Y. X., et al. (2016). Molecular epidemiology of Staphylococcus aureus among patients with skin and soft tissue infections in two Chinese hospitals. Chin. Med. J. (Engl). 129 (19), 2319–2324. doi: 10.4103/0366-6999.190673
Guo, Y., Song, G., Sun, M., Wang, J., Wang, Y. (2020). Prevalence and therapies of antibiotic- resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00107
Guo, Y., Xu, L., Wang, B., Rao, L., Xu, Y., Wang, X., et al. (2023). Dissemination of methicillin-resistant Staphylococcus aureus sequence type 764 isolates with mupirocin resistance in China. Microbiol. Spectr. 11 (1), e0379422. doi: 10.1128/spectrum.03794-22
Hashemi, H., Mohebbi, M., Mehravaran, S., Mazloumi, M., Jahanbani-Ardakani, H., Abtahi, S. H. (2017). Hyperimmunoglobulin e syndrome: genetics, immunopathogenesis, clinical findings, and treatment modalities. J. Res. Med. Sci. 22, 53. doi: 10.4103/jrms.JRMS_1050_16
Hassoun, A., Linden, P. K., Friedman, B. (2017). Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit. Care 21 (1), 211. doi: 10.1186/s13054-017-1801-3
Hesje, C. K., Sanfilippo, C. M., Haas, W., Morris, T. W. (2011). Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolated from the eye. Curr. Eye Res. 36 (2), 94–102. doi: 10.3109/02713683.2010.534229
Jin, Y, Li, M., Shang, Y., Liu, L, Shen, X., Lv, Z., et al. (2018). Sub-Inhibitory concentrations of mupirocin strongly inhibit alpha-toxin production in high-level mupirocin-resistant MRSA by down-regulating agr, saeRS, and sarA. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00993
Lee, G. C., Dallas, S. D., Wang, Y., Olsen, R. J., Lawson, K. A., Wilson, J., et al. (2017). Emerging multidrug resistance in community-associated Staphylococcus aureus involved in skin and soft tissue infections and nasal colonization. J. Antimicrob. Chemother. 72 (9), 2461–2468. doi: 10.1093/jac/dkx200
Lee, A. S., de Lencastre, H., Garau, J., Kluytmans, J., Malhotra-Kumar, S., Peschel, A., et al. (2018). Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers. 4, 18033. doi: 10.1038/nrdp.2018.33
Li, X., Chen, Y., Gao, W., Ouyang, W., Wei, J., Wen, Z. (2016). Epidemiology and outcomes of complicated skin and soft tissue infections among inpatients in southern China from 2008 to 2013. PloS One 11 (2), e0149960. doi: 10.1371/journal.pone.0149960
Liu, Q. Z., Wu, Q., Zhang, Y. B., Liu, M. N., Hu, F. P., Xu, X. G., et al. (2010). Prevalence of clinical meticillin-resistant Staphylococcus aureus (MRSA) with high-level mupirocin resistance in shanghai and wenzhou, China. Int. J. Antimicrob. Agents. 35 (2), 114–118. doi: 10.1016/j.ijantimicag.2009.09.018
Liu, Y., Xu, Z., Yang, Z., Sun, J., Ma, L. (2016). Characterization of community-associated Staphylococcus aureus from skin and soft-tissue infections: a multicenter study in China. Emerg. Microbes Infect. 5 (12), e127. doi: 10.1038/emi.2016.128
Lowy, F. D. (1998). Staphylococcus aureus infections. N Engl. J. Med. 339 (8), 520–532. doi: 10.1056/NEJM199808203390806
Mediavilla, J. R., Chen, L., Mathema, B., Kreiswirth, B. N. (2012). Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr. Opin. Microbiol. 15 (5), 588–595. doi: 10.1016/j.mib.2012.08.003
Miller, L. G., Daum, R. S., Creech, C. B., Young, D., Downing, M. D., Eells, S. J., et al. (2015). Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl. J. Med. 372 (12), 1093–1103. doi: 10.1056/NEJMoa1403789
Nakaminami, H., Ozawa, K., Sasai, N., Ikeda, M., Nemoto, O., Baba, N., et al. (2020). Current status of panton-valentine leukocidin-positive methicillin-resistant Staphylococcus aureus isolated from patients with skin and soft tissue infections in Japan. J. Dermatol. 47 (11), 1280–1286. doi: 10.1111/1346-8138.15506
Novy, P., Kloucek, P., Rondevaldova, J., Havlik, J., Kourimska, L., Kokoska, L. (2014). Thymoquinone vapor significantly affects the results of Staphylococcus aureus sensitivity tests using the standard broth microdilution method. Fitoterapia 94, 102–107. doi: 10.1016/j.fitote.2014.01.024
Omuse, G., Van Zyl, K. N., Hoek, K., Abdulgader, S., Kariuki, S., Whitelaw, A., et al. (2016). Molecular characterization of Staphylococcus aureus isolates from various healthcare institutions in Nairobi, Kenya: a cross sectional study. Ann. Clin. Microbiol. Antimicrob. 15 (1), 51. doi: 10.1186/s12941-016-0171-z
Otter, J. A., French, G. L. (2010). Molecular epidemiology of community-associated meticillin-resistant Staphylococcus aureus in Europe. Lancet Infect. Dis. 10 (4), 227–239. doi: 10.1016/S1473-3099(10)70053-0
Rao, Q., Shang, W., Hu, X., Rao, X. (2015). Staphylococcus aureus ST121: a globally disseminated hypervirulent clone. J. Med. Microbiol. 64 (12), 1462–1473. doi: 10.1099/jmm.0.000185
Sahebnasagh, R., Saderi, H., Owlia, P. (2014). The prevalence of resistance to methicillin in Staphylococcus aureus strains isolated from patients by PCR method for detection of mecA and nuc genes. Iran J. Public Health 43 (1), 84–92.
Shallcross, L. J., Fragaszy, E., Johnson, A. M., Hayward, A. C. (2013). The role of the panton-valentine leucocidin toxin in staphylococcal disease: a systematic review and meta-analysis. Lancet Infect. Dis. 13 (1), 43–54. doi: 10.1016/S1473-3099(12)70238-4
Turner, N. A., Sharma-Kuinkel, B. K., Maskarinec, S. A., Eichenberger, E. M., Shah, P. P., Carugati, M., et al. (2019). Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat. Rev. Microbiol. 17 (4), 203–218. doi: 10.1038/s41579-018-0147-4
Wang, X., Shen, Y., Huang, W., Zhou, Y. (2019). Characterisation of community-acquired Staphylococcus aureus causing skin and soft tissue infections in a children's hospital in shanghai, China. Epidemiol. Infect. 147, e323. doi: 10.1017/S0950268819002127
Weist, K., Cimbal, A. K., Lecke, C., Kampf, G., Rüden, H., Vonberg, R. P. (2006). Mar;Evaluation of six agglutination tests for Staphylococcus aureus identification depending upon local prevalence of meticillin-resistant S. aureus (MRSA). J. Med. Microbiol. 55 (Pt 3), 283–290. doi: 10.1099/jmm.0.46225-0
Williams, D. J., Cooper, W. O., Kaltenbach, L. A., Dudley, J. A., Kirschke, D. L., Jones, T. F., et al. (2011). Comparative effectiveness of antibiotic treatment strategies for pediatric skin and soft-tissue infections. Pediatrics 128 (3), e479–e487. doi: 10.1542/peds.2010-3681
Xiao, N., Yang, J., Duan, N., Lu, B., Wang., L. (2019). Community-associated Staphylococcus aureus PVL+ ST22 predominates in skin and soft tissue infections in Beijing, China. Infect. Drug resist. 12, 2495–2503. doi: 10.2147/IDR.S212358
Yu, F., Liu, Y., Lv, J., Qi, X., Lu, C., Ding, Y., et al. (2015). Antimicrobial susceptibility, virulence determinant carriage and molecular characteristics of Staphylococcus aureus isolates associated with skin and soft tissue infections. Braz. J. Infect. Dis. 19 (6), 614–622. doi: 10.1016/j.bjid.2015.08.006
Zhanel, G. G., Adam, H. J., Baxter, M., Lagace-Wiens, P. R. S., Karlowsky, J. A. (2021). In vitro activity and resistance rates of topical antimicrobials fusidic acid, mupirocin and ozenoxacin against skin and soft tissue infection pathogens obtained across Canada (CANWARD 2007-18). J. Antimicrob. Chemother. 76 (7), 1808–1814. doi: 10.1093/jac/dkab098
Keywords: SSTIs, Staphylococcus aureus, MRSA, antimicrobial sensitivity, molecular epidemiology, China
Citation: Su W, Liu Y, Wang Q, Yuan L, Gao W, Yao KH, Yang YH and Ma L (2023) Antibiotic susceptibility and clonal distribution of Staphylococcus aureus from pediatric skin and soft tissue infections: 10-year trends in multicenter investigation in China. Front. Cell. Infect. Microbiol. 13:1179509. doi: 10.3389/fcimb.2023.1179509
Received: 24 March 2023; Accepted: 19 June 2023;
Published: 13 July 2023.
Edited by:
Mogens Kilian, Aarhus University, DenmarkReviewed by:
William R. Schwan, University of Wisconsin–La Crosse, United StatesCopyright © 2023 Su, Liu, Wang, Yuan, Gao, Yao, Yang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Ma, YmNoX21hbGVlbkBhbGl5dW4uY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.