- 1Viral Hemorrhagic Fevers Research Unit, Chinese Academy of Sciences (CAS) Key Laboratory of Molecular Virology & Immunology, Institut Pasteur of Shanghai, Chinese Academy of Sciences (CAS), Shanghai, China
- 2University of Chinese Academy of Sciences, Beijing, China
Increased human activities around the globe and the rapid development of once rural regions have increased the probability of contact between humans and wild animals. A majority of bunyaviruses are of zoonotic origin, and outbreaks may result in the substantial loss of lives, economy contraction, and social instability. Many bunyaviruses require manipulation in the highest levels of biocontainment, such as Biosafety Level 4 (BSL-4) laboratories, and the scarcity of this resource has limited the development speed of vaccines for these pathogens. Meanwhile, new technologies have been created, and used to innovate vaccines, like the mRNA vaccine platform and bioinformatics-based antigen design. Here, we summarize current vaccine developments for three different bunyaviruses requiring work in the highest levels of biocontainment: Crimean-Congo Hemorrhagic Fever Virus (CCHFV), Rift Valley Fever Virus (RVFV), and Hantaan virus (HTNV), and provide perspectives and potential future directions that can be further explored to advance specific vaccines for humans and livestock.
1 Introduction
In 2019, the International Committee on Taxonomy of Viruses (ICTV) updated the classification list of Bunyaviruses from the family Bunyaviridae to the order Bunyavirales, to reflect the expanded diversity of these RNA viruses (Abudurexiti et al., 2019). According to the ICTV website (https://ictv.global/taxonomy), the order Bunyavirales includes 14 families, 4 subfamilies, 60 genera and 496 species as of September 2022. The bunyavirus genome consists of linear, segmented, negative-sense single stranded or ambisense RNA, except for non-enveloped plant tenuiviruses (Ter Horst et al., 2019). Generally, bunyavirus genomes are composed of the S segment encoding the nucleocapsid protein (NP), the M segment encoding the glycoprotein (GP), and the L segment encoding the RNA dependent RNA polymerase (RdRp). Some bunyaviruses are known to be highly virulent to humans and require manipulation in a high biocontainment laboratory, such as Crimean-Congo hemorrhagic fever virus (CCHFV) (Tsergouli et al., 2020; Temur et al., 2021), Rift Valley fever virus (RVFV) (Dar et al., 2013; McMillen and Hartman, 2021), and Hantaan virus (HTNV) (HTNV can be operated in a BSL-3 laboratory depending on viral concentration and animal species) (Knudsen et al., 1994; Dong et al., 2019; Meechan and Pots, 2020). These viruses belong to the families Nairoviridae, Phenuiviridae, and Hantaviridae, respectively. Due to globalization leading to increased travel and trade, human health is closely connected to animal and environmental health (Gruetzmacher et al., 2021). This can be partially linked to the changing environment and climate leading to the contraction of natural territories and the forced migration of wild animals and insects to human territories, resulting in increased chances for interaction of these wild species with humans and an elevated pathogen spillover risk.
Another factor to consider is outbreak preparedness for neglected pathogens that may pose substantial threats to humans due to their virulence and high mortality rates. Emerging viruses causing disease outbreaks that have garnered high-profile international attention since the 21st century include but are not limited to: SARS-CoV, H1N1 “swine” influenza, MERS-CoV, Ebola virus, Zika virus, and SARS-CoV-2 (Shi et al., 2017; Yang et al., 2022). These viruses were either neglected or unknown at the beginning of the outbreak, and there is a possibility that other poorly characterized pathogens with high lethality rates could potentially cause widespread pandemics in the future. Bunyaviruses, such as CCHFV, RVFV and HTNV are understudied hemorrhagic fever viruses with potentially devastating public health consequences and there are concerns that these viruses could be suitable agents for bioweapons development (Flick and Whitehouse, 2005; Rolin et al., 2013; Tian and Stenseth, 2019). In addition to surveillance efforts, medical countermeasures, such as vaccines, monoclonal antibodies and other small-molecule therapeutics are also needed as preventative and treatment options.
As a method of proactive protection against viral infection, an appropriate vaccine is usually the primary choice. In the development of vaccines, however, there are important considerations, including its safety, immunogenicity, and efficacy in animals and humans, its ease and costs of production, and whether transmission from vaccinated hosts to others is possible. Other logistical considerations include the scale of vaccination (such as endemic and other at-risk populations) and access to the highest biocontainment, such as BSL-4 laboratories, to test these vaccine candidates. Besides traditional vaccines, such as inactivated and subunit vaccines, new emerging technologies have facilitated the innovation of novel vaccines, such as microfluidic-based mRNA vaccines (Tarim et al., 2023), and silico-based antigen design (Hederman and Ackerman, 2023; Kuri and Goswami, 2023), which have enriched the variety of potential vaccine candidates. Here, we summarize research advances on prophylactic options currently in development for three highly pathogenic bunyaviruses: CCHFV, RVFV and HTNV, and discuss future directions towards the clinical development of these vaccine candidates.
2 Vaccines against CCHFV
CCHFV is a genetically diverse tick-borne pathogen with seven clades (Asia-1, Asia-2, Africa-1, Africa-2, Africa-3, Europe-1 and Europe-2), and can be found in Asia, Africa and Europe, south of 50 degrees northern latitude (Fillâtre et al., 2019). Carried by Hyalomma ticks, CCHFV could directly infect humans via tick bite, or indirectly infect humans via contact with the blood or tissue of viremia-phase livestock, which amplify the virus after infection from a CCHFV-positive tick (Hoogstraal, 1979; Bente et al., 2013; Spengler et al., 2016; Papa et al., 2017). Case fatality rates vary based on different regions and outbreaks. For instance, 15 deaths from 480 cases (3%) in Turkey during 2020, and 19 deaths from 37 cases (51%) in India during 2019 (Kuehnert et al., 2021), but the general case fatality rate is usually considered to be around 30% (Sanchez et al., 2002; Papa et al., 2017; Tipih et al., 2021; Hawman and Feldmann, 2023). The envelope glycoproteins Gn and Gc of CCHFV are known to be the main immunogen and possess neutralizing epitopes, but some of these neutralizing epitopes not always conserved due to the genetic diversity of CCHFV (Ahmed et al., 2005; Wampande et al., 2021). Meanwhile, as a more conserved domain, NP could also be used as a target antigen against CCHFV (Karaaslan et al., 2021). Non-structural proteins, such as the secreted GP-38 glycoprotein, was verified through protective antibody studies to be another potential target for CCHFV vaccine candidates (Karaaslan et al., 2021).
2.1 Inactivated vaccines
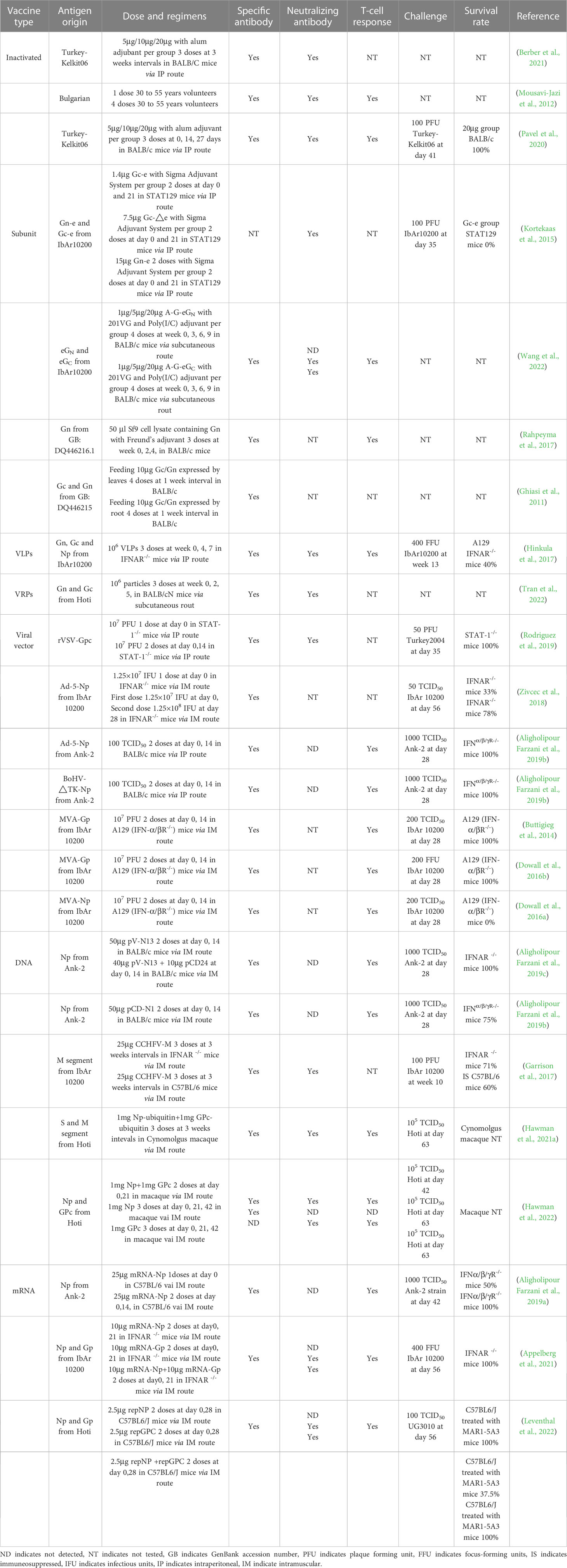
In 1974, Bulgaria approved a whole-virion CCHFV inactivated vaccine, prepared from viruses grown in newborn mouse brain tissue, and then inactivated by chloroform, heated at 58°C and adsorbed onto aluminum hydroxide (Mousavi-Jazi et al., 2012). At-risk populations such as butchers and animal slaughter workers in Bulgaria are immunized with this candidate, but the efficacy of the Bulgarian vaccine is yet to be demonstrated in clinical trials (Mousavi-Jazi et al., 2012). Subsequently, advances in technology showed that certain cell lines, such as SW-13, VeroE6 and Huh7 were capable of replicating CCHFV to different levels, which provided possibilities to produce CCHFV-inactivated vaccines via alternate means (Dai et al., 2021).
Berber et al. (2021) showed that both inactivated vaccine produced by VeroE6 culture (CCVax) and sucking mice brains (MBVax) induced humoral responses and dose-dependent protection, but better performance was observed with CCVax (Table 1). Although CCHFV-specific antibody levels could be sustained for at least ten months after the final administration, Mousavi-Jazi et al. (2012) verified that high levels of CCHFV-specific antibodies did not necessarily correlate with neutralization activity (Table 1), a result which was further demonstrated in a study comparing the virus neutralization and CCHFV-specific IgG titers from both VeroE6- and suckling mouse brain-produced inactivated vaccines (Table 1) (Pavel et al., 2020). Additionally, mouse brains and cell lines, and embryonated chicken eggs (ECE) could also be used for CCHFV cultivation (Xia et al., 2013), but so far ECE has not been used for producing inactivated vaccines, possibly because inactivated RVFV produced from chick embryo induced a lower neutralization index (1.2 logs) compared to VeroE6 cells (2.3 logs) and mouse brains (2.8 logs) (Randall et al., 1962). The above vaccines use formalin for inactivation, but a study showed that inactivation by β-propiolactone (BPL) may retain higher immunogenicity (Lim et al., 2018), potentially demonstrating a method for the development of more effective inactivated CCHFV vaccines.
2.2 Subunit vaccines
Subunit vaccines are based on the expression of recombinant proteins, in which segments of the viral antigen are generated, and contains advantages such as the possibility for high purity, minimal side effects, ease of large-scale production and low manufacturing costs. Adjuvants are used in conjunction to overcome shortcomings in this approach, such as low immunogenicity.
With the development of big data and artificial intelligence, bioinformatics is playing an increased role in the screening and design of suitable subunit vaccine targets. Using these in silico approaches, Sana et al. (2022) designed a subunit vaccine targeting all the seven genotypes of CCHFV, Nosrati et al. (2019) selected B/T cell epitopes based on parameters for antigenicity, allergenicity, toxicity, water solubility, hydrophobicity, population coverage, 3D structure and adjuvant compatibility, and Khan et al. (2021) constructed a 468-amino acid subunit vaccine, covering 98% of known CCHFV isolates. However, these subunit vaccine candidates have not yet been functionally evaluated in the laboratory, and as such the immunogenicity and efficacy is not known.
Traditional subunit vaccine approaches against CCHFV typically target the envelope glycoproteins Gn and Gc, or the more conserved NP. Golden et al. (2019) showed that the glycoprotein GP38, a region of the CCHFV GP, is not only secreted, but also becomes localized to the viral envelope and cellular plasma membranes, suggesting another potential target for vaccine development. A study comparing the efficacy of extracellular Gc (eGc), extracellular Gn (eGn), and conserved neutralizing antibody region of Gc (G-nAb), using a gram-positive enhancer matrix-protein anchor (GEM-PA) surface display system, found that these candidates could elicit humoral and cellular immunity in BALB/c mice, but specific neutralizing antibodies (nAbs) were only detected in the eGc group (Table 1) (Wang et al., 2022). Interesting, the eGn subunit vaccine generated by the Drosophila insect cell line could successfully elicit neutralizing antibodies (nAbs), but the levels were lower than that of the Gc ectodomain lacking the stem region (Table 1) (Kortekaas et al., 2015). These results suggest that different protein expression systems may also result in different immune responses. Rahpeyma et al. (2017) used a baculovirus expression system to generate Gn, showing that it was highly immunogenic and could elicit both high titers of antigen-specific antibodies and T-cell responses (Table 1), but it was not known whether these antibodies were neutralizing. Ghiasi et al. (2011) investigated the oral immunogenicity of a plant-derived G1 (Gc)/G2 (Gn) glycoprotein of the CCHFV with the aim of producing a CCHFV vaccine for livestock. From BALB/c mice studies, they found that BALB/c mice fed and boosted with transgenic plants expressing the CCHFV envelopes elicited strong levels of specific IgG and IgA antibodies in their serum and feces (Table 1), demonstrating that edible vaccines may be feasible against CCHFV, but these results need to be validated for protective efficacy in real livestock.
2.3 VLPs/VRPs vaccines
While straightforward to produce, the generation of inactivated vaccines for highly virulent pathogens require live virus to be grown to high titers, in which accidental laboratory release could result in a major public health incident. Virus-like particles (VLPs), which do not incorporate the viral genome of these pathogens and thus cannot replicate, addresses this weakness. Theoretically, VLPs maintain the structural integrity of the native virion, thus retaining the immunogenicity observed from inactivated vaccines. Viral replicon particles (VRPs) are VLPs that also include the complete S, L viral genome segments, but lack the M segment entirely. Thus, VRPs could enter host cells, but cannot produce nascent progeny particles.
Few VLPs vaccines against CCHFV have been reported thus far. In 2011, Zhou et al. (2011) successfully developed CCHFV VLPs via the expression of NP in insect cells using a recombinant baculovirus system, but did not test its immune response in animal models. In 2017, Zivcec et al. (2017) successfully used VLPs to screen nAbs against CCHFV, which supported that VLPs to have potential to induce efficient nAbs as a vaccine. In the same year, transcriptionally competent virus-like particles (tc-VLPs) elicited strong CCHFV-specific antibodies and nAbs, and found that the T cell response was biased towards Th2 after cytokine response analysis (Hinkula et al., 2017). However, the observation of significant weight loss, high RNA loads in the blood, spleen, and liver, as well as a protection rate of 40% in immunized mice (Table 1) (Hinkula et al., 2017), suggests that Th1-medidated responses may also play an important role in survival against CCHFV infection. Tran et al. (2022) developed a recombinant Kunjin strain of West Nile Virus (WNVKUN) replicon expressing CCHFV Gn and Gc, but found that this vaccine only induced seroconversion to WNV proteins and limited seroconversion of CCHFV-specific antibodies, without strongly nAbs against CCHFV (Table 1). Scholte et al. (2019) investigated a CCHFV VRPs vaccine, and showed that IFNAR-/- mice inoculated with this candidate at 32 days before challenge was fully protective (Table 1), and a follow-up study by Spengler et al. (2021) showed that mice inoculated with the VRPs vaccine was fully protective when administered 14 and 7 days before challenge, whereas mice given the vaccine at 3 days before challenge exhibited disease symptoms, but fully recovered with 100% survival.
2.4 Viral-vectored vaccines
Viral-vectored vaccines are recombinant viruses which expresses the target antigen via the insertion of the gene into the genome of the vaccine virus backbone. Since these are typically live vaccine viruses, this platform can induce strong antigen-specific T/B-cell responses and can be easily grown to high titers in vitro. Despite these advantages, drawbacks include a lowered safety profile, especially for immunocompromised patients, and the risk of viral integration into the human genome, except for platforms comprising of non-segmented, negative-strand RNA viral vectors or virus that replicate in the cytoplasm.
Vesicular stomatitis virus (VSV) is a popular viral vector for the development of vaccines against emerging pathogens. Several VSV-based vaccines have been developed, including against influenza virus (Furuyama et al., 2020), SARS-CoV-2 (Malherbe et al., 2021), and Ebola virus, which received clinical approval from the World Health Organization (WHO) in late 2019 (Suder et al., 2018). The feasibility of VSV particles expressing CCHFV glycoproteins was demonstrated by Shtanko et al. (2014), supporting the possibility of characterizing this vaccine. Suda et al. (2016) then showed that VSV expressing Gn and Gc have some N-glycosylated sites and are likely to bind to C-type lectins like DC-SIGN, suggesting that these recombinant VSV can enter cells. Rodriguez et al. (2019) verified that VSV expressing CCHFV GP can indeed induce strong levels of specific antibodies and nAbs, as well as protect mice after challenge (Table 1).
In the development of viral vector vaccines, Adenovirus (Ad) is a popular platform that has been tested in clinical trials for a wide variety of infectious and genetic diseases. A human Ad serotype 5 (Ad5) expressing CCHFV NP was developed and found to stimulate NP-specific antibodies and elicit 78% protection after 2 doses (Table 1) (Zivcec et al., 2018). In contrast, Farzani et al (Aligholipour Farzani et al., 2019b). found that the Ad5-based vaccine expressing NP vaccine could induce strong T-cell and humoral responses with no nAbs detected, but 100% of mice were protected after a CCHFV challenge (Table 1). After the comparison of above two Ad5-NP vaccines, it was suspected that the different development processes of the vaccines may have led to a difference in efficacy, because the Ad5-NP vaccine with 100% protection was generated by AdMax™HI-IQ Kit J, whereas the other study used Adeno-X Adenoviral System 3 (Zivcec et al., 2018; Aligholipour Farzani et al., 2019b). In any case, the feasibility of Ad-based vaccines against CCHFV need to be further explored.
Modified vaccinia virus Ankara (MVA) is another well-established vaccine platform which has been demonstrated to be safe in humans (von Krempelhuber et al., 2010; Frey et al., 2014; Greenberg et al., 2015). Buttigieg et al. (2014) first demonstrated that an MVA-based vaccine expressing CCHFV GP protects 100% of mice from CCHFV infection, but it is not certain whether this vaccine induces nAbs (Table 1). Dowall et al. (2016b) further verified that MVA-based vaccines stimulate both arms of the immune system, which were required to elicit protective effects against lethal CCHFV challenge (Table 1). An MVA-based vaccine expressing NP was shown to induce humoral and cellular responses, but in contrast to Ad5-NP, the vaccine failed to protect mice from challenge (Table 1) (Dowall et al., 2016a).
The Bovine Herpesvirus Type 4 (BoHV-4) virus-based vector vaccine has been successfully explored for different pathogens, such as Ebola virus (Rosamilia et al., 2016). Farzani et al (Aligholipour Farzani et al., 2019b). reported that BoHV4-ΔTK expressing CCHFV NP could provide complete protection against CCHFV in IFN α/β/γ R−/− mice, and in the T-cell and passive antibody transfer assay, both arms of the immune response were shown to be required for protection (Dowall et al., 2016b).
2.5 DNA vaccines
DNA vaccines are typically delivered into cells through electroporation, in which its advantages include its speed and ease of design and production, making this platform an ideal candidate for rapid development of vaccines during outbreaks, especially against pathogens with high mutation rates. However, concerns with DNA vaccines include the possibility of the DNA vaccine integrating into the host genome, and low immunogenicity (Schalk et al., 2006; Dowall et al., 2016b).
There are various studies on CCHFV DNA vaccines that have demonstrated promising results. A study with pV-N13 vaccination expressing NP with pCD24 adjuvant showed that it could induce strong T-cell responses and protect 100% of IFNAR-/-mice from CCHFV infection, despite the lack of nAbs (Table 1) (Aligholipour Farzani et al., 2019c). However, another study showed that an unadjuvanted pCD-N1 vaccine protected 75% of IFNα/β/γ-/- mice from challenge (Table 1) (Aligholipour Farzani et al., 2019b), suggesting that adjuvants are needed for optimal protection with DNA-vectored vaccines. Garrison et al. (2017) showed that an unadjuvanted DNA vaccine expressing CCHFV GP stimulated a balanced Th1/Th2 response, but was not sufficient for complete protection against CCHFV challenge, with only a 60% survival in mice (Table 1). However, a mixed DNA vaccine expressing CCHFV GP and NP showed a stronger Th1-immunity compared to Th2-immunity, and conferred 100% survival in mice (Hinkula et al., 2017). The mixed DNA vaccine with NP and GP precursor also protected cynomolgus macaques from CCHFV challenge (Table 1) (Hawman et al., 2021a), suggesting a crucial role for Th1 immunity in protection from CCHFV with DNA-vectored vaccines. Surprisingly, it was shown that DNA plasmids expressing NP and GPC stimulated Th2- and Th1-immunity (Table 1) (Hawman et al., 2022), perhaps due to the ubiquitin sequence playing an adjuvant role. Regardless, a mixed DNA vaccine with two doses eliciting both T- and B-cell responses was shown to be superior to even three immunizations with either NP or GPC alone (Hawman et al., 2022).
2.6 mRNA vaccines
Since the emergence of SARS-CoV-2, mRNA-based vaccines have been widely used in the human population. The advantages of mRNA vaccines include its good safety record, its speed and ease of design against emerging pathogens with high mutation rates, and good immunogenicity. However, mRNA vaccines are less stable than other types of vaccines, although a thermostable mRNA vaccine has been reported (Zhang et al., 2020), no commercial vaccines have been produced by this method so far. At the moment, mRNA vaccine still requires cold-chain storage and delivery, potentially contributing to logistical difficulties in less-developed regions with poor medical resources.
mRNA-vectored vaccines have also been tested against CCHFV since 2019. Farzani et al (Aligholipour Farzani et al., 2019a). found that an mRNA vaccine expressing NP could induce strong levels of IFN-γ, IL-4 and specific antibody but no nAbs, resulting in 100% protection in mice (Table 1). Appelberg et al. (2021) then compared 3 mRNA vaccines expressing either GnGc, NP or GnGc+NP, demonstrating that all candidates elicited T- and B-cell responses and completely protected IFNAR-/- mice from CCHFV challenge (Table 1). Leventhal et al. (2022) established an alphavirus-based replicon mRNA (repRNA) vaccine expressing either NP, GPC or NP+GPC, and showed that both NP and the NP+GPC combined vaccine could confer complete protection against CCHFV, but the survival in the GPC group was suboptimal at 40% in C57BL6/J mice treated with MAR1-5A3, which blocks type I IFN receptor to further block type I IFN signaling (Table 1).
3 Vaccines against RVFV
RVFV was first reported from Kenya during 1930 (Daubney and Garnham, 1931). Since then, the virus has been found from Eastern Africa to Southern Africa, West Africa, the Egyptian delta, and the Saudi Arabian peninsula (Weaver and Reisen, 2010), and could be imported by travel from these endemic regions, such as the discovery of a RVFV-infected patient in China during 2016 (Liu et al., 2016). The transmission of RVFV depends on mosquitoes, which are the reservoir hosts, and amplified by intermediate hosts including sheep, cattle, goats, and camels, subsequently infecting humans typically via direct contact (Linthicum et al., 2016). The case fatality rate in humans can range between 20–50% (Dar et al., 2013).
Similar to CCHFV, the surface glycoproteins Gn and Gc play an important role in virus attachment to initiate infection, and as the main immunogen, has the ability to elicit nAbs (Dessau and Modis, 2013; Wu et al., 2017; Wang et al., 2019). The RVFV NP (3.5% variable in the nucleotide sequence level) is known to be more conserved and could induce strong T-cell responses (Bird et al., 2007; Xu et al., 2013), but the L protein is the most conserved among 33 different RVFV strains (Bird et al., 2007). Currently circulating strains of RVFV are descended from an ancestral species discovered during the 19th century (Ikegami, 2012).
3.1 Inactivated vaccines
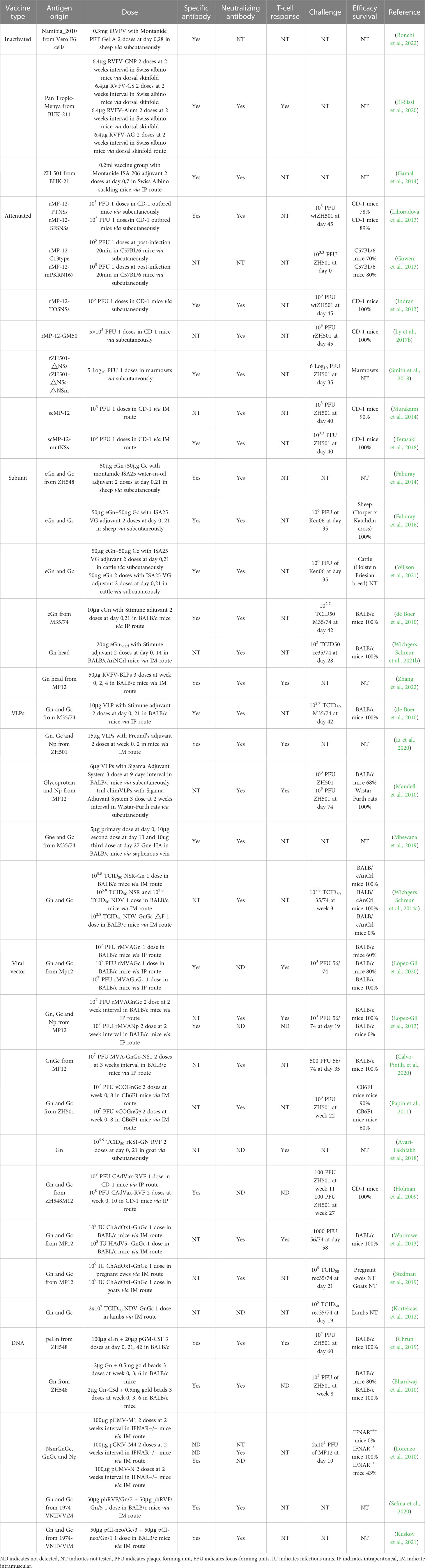
Randall et al. (1962) first developed formalin-inactivated RVFV candidate vaccines and indicated that the vaccine produced from mouse brains, which successfully elicited specific-RVFV antibodies, is better than those from primary monkey kidney cells and chicken eggs in both adult and sucking mice. The inactivated Entebbe strain of RVFV has been used by volunteers (Ikegami, 2019) but 6% of volunteers were reported to have mild side-effects such as headache, tiredness, and a general feeling of discomfort (Niklasson, 1982). To improve on this candidate, the United States Army Medical Research Institute of Infectious Diseases developed an inactivated RVFV vaccine by FRhL-2 cells called TSI-GSD-200 (Faburay et al., 2017). From its Phase I clinical trial results, although TSI-GSD-200 has better immunogenicity, mild and transient local reactions were still present, ranging from 5% at the lowest dose level to 43% at the highest, indicating safety concerns if the vaccine was to be administered to a larger population (Kark et al., 1982). Interestingly, there are long-lived RVFV-specific T-cell responses detected in volunteers vaccinated with the formalin-inactivated RVFV vaccine, suggesting that T-cell immunity may play a role in protection from RVFV infection (Harmon et al., 2020). Ronchi et al. (2022) formulated an inactivated Namibian field strain of RVFV with Montanide Pet Gel A and found after two doses, all animals seroconverted (Table 2). Due to the weak immunogenicity elicited by inactivated vaccines, different adjuvants were tested to determine which combination will elicit the strongest immune responses. Compared to alum, chitosan nanoparticles were found to be superior for inducing better immune responses against RVFV (Table 2) (El-Sissi et al., 2020). The foot and mouth disease (FMD)/RVF combined inactivated vaccine with oil adjuvant also elicited high antibody titers and strong T-cell responses, and could constitute a cheaper and more effective way to prevent RVFV in livestock (Table 2) (Gamal et al., 2014).
3.2 Attenuated vaccines
Live-attenuated vaccines are typically generated by the mutation or deletion of sequences, which limit the speed and scale of viral replication in the host. As such, while these types of vaccines stimulate more robust immune responses, disadvantages include safety concerns, such as reversion to virulence, and are typically not administered to immunocompromised patients.
The modified Smithburn vaccine was generated via serial passage of RVFV in a mouse brains and embryonated chicken eggs, and then passaged in BHK-21 cells to finally formulate the attenuated vaccine. The Smithburn animal vaccine has been sold in Eastern Africa and Saudi Arabia (Faburay et al., 2017), but alpacas have been found dead after vaccine administration (Anthony et al., 2021). Clone-13 virus was isolated from human with a 69% truncation in the non-structural protein NSs (Muller et al., 1995), the vaccine was proven safe in livestock such as sheep, goats and cattle (Sindato et al., 2021), but the occurrence of fetus malformations and stillbirths mean that the vaccine can induce serious side effects (Makoschey et al., 2016).
Through 12 serial passages of RVFV in the presence of 5-fluorouracil in MRC-5 cells, the attenuated MP-12 vaccine was obtained (Caplen et al., 1985), and was shown to protect 100% of mice from RVFV challenge after one dose (Morrill et al., 2022). MP-12 was found to be resistant to reversion to virulence (Ikegami et al., 2015), temperature changes (Nishiyama et al., 2016), and reassortment with other known RVFV isolates (Ly et al., 2017a). Although MP-12 is attenuated by multiple M- and L-segment mutations, NSs is found to be still functional (Billecocq et al., 2008). To further characterize mutations found in MP-12, 6 from 23 mutations of MP-12 were found via high-thoroughput sequencing to be derived from the parental RVFV ZH548 (Lokugamage et al., 2012). Continued passage of MP-12 on Vero cells showed that the re-amplification of rescued recombinant MP-12 (rMP-12) led to an increase in genetic subpopulations, affecting the viral phenotype via amino acid substitutions in the NSs gene, even though there was increased immunogenicity and the vaccine was shown to be safe (Ikegami, 2021).
From past studies, it is clear that the mutation of NSs is important for the attenuation of live RVFV vaccines. After replacement of the rMP-12 NSs with a modified NSs from the Clone-13 strain (C13), it was shown that rMP-12-C13type induced high nAbs and no viremia was detected (Lihoradova and Ikegami, 2012). The replacement of C13 NSs with the NSs of Punta Toro virus and sandfly fever sicilian virus showed that specific-NSs antibody was not induced but both candidates have better immune responses (Table 2) (Lihoradova et al., 2013). In contrast, the replacement of rMP-12 NSs with that from Toscana virus resulted in 100% protection but also enhanced the neuroinvasiveness of the vaccine candidate (Table 2) (Indran et al., 2013). Interestingly, through the modification of NSs, the rMP-C13type or rMP-12-mPKRN167 (a dominant-negative form of mouse dsRNA-dependent protein kinase in place of NSs) vaccine could protect 70% or 80% of mice at 20 min after a RVFV infection (Table 2) (Gowen et al., 2013). However, the truncated NSs protein seems to be related to the stimulation of a rapid protective innate immune response, suggesting that the functional inactivation of NSs plays an important role in the observed post-exposure efficacy (Fang et al., 2022). A study in which MP-12 was attenuated by the induction of 584 silent mutations within the N, NSs, M and L ORFs via reverse genetics showed that the protective efficacy remained 100% (Table 2) (Ly et al., 2017b).
Deletion vaccines based on the non-structural protein NSm were also explored as live-attenuated candidates, in which vaccinated livestock were protected from viremia and liver disease, with a significant decline in virus transmission among mosquitoes (Table 2) (Bird et al., 2008; Smith et al., 2018; Campbell et al., 2022; Ikegami et al., 2022). Serious side-effects, such as abortion or fetal malformation, were not observed (Weingartl et al., 2014).
Single-cycle replication-competent MP-12 (scMP-12), which lacks an endoplasmic reticulum retrieval signal and is defective for membrane fusion function, and RVFV-4s, which was generated by the splitting of the M segment open reading frame have shown better immunogenicity, protective efficacy and safety compared with MP-12 (Table 2) (Murakami et al., 2014; Wichgers Schreur et al., 2014b; Terasaki et al., 2018; Wichgers Schreur et al., 2021a; Wichgers Schreur et al., 2022). In addition, a favipiravir-mutagenized RVFV variant showed a strong attenuation, in which reverse genetics analysis showed that the mutations G924S and A1303T on segment were the main cause of attenuation (Borrego et al., 2022).
3.3 Subunit vaccines
The envelope glycoproteins Gn and Gc are a prime choice as target antigens for the development of RVFV vaccines (Sherman et al., 2009). Through expression of the Gn ectodomain (eGn) and the full-length Gc with the recombinant bacmid system, the antigens were formulated with Montanide ISA-25 VG adjuvant to generate a subunit vaccine. This candidate was shown to elicit strong nAbs after one dose and can completely protect sheep and cattle from RVFV challenge (Table 2) (Faburay et al., 2014; Faburay et al., 2016; Wilson et al., 2021). Although eGn expressed with the Drosophila system with Stimune adjuvant vaccine elicits lower levels of specific IgG compared to Montanide (Faburay et al., 2014), the mice were also completely protected (Table 2) (de Boer et al., 2010). Aside from GP-based vaccines, a recombinant RVFV NP with Alhydrogel adjuvant vaccine was evaluated in mice, and results showed that it elicited an early IFN-β as well as Th-2 responses, but cannot completely prevent RVFV infection (Jansen van Vuren et al., 2011).
Compared to free antigens, antigens immobilized on scaffolds, such as nanoparticles, utilize the Gn head domain bound to the lumazine synthase-based multimeric protein scaffold particles (MPSPs), were shown to improve immunogenicity and reduce mortality in BALB/c, as well as protecting lambs from viremia and clinical signs after immunization (Table 2) (Wichgers Schreur et al., 2021b). Through the insertion of the Gn head protein into Gram-positive enhancer matrix (GEM), which are non-living and non-genetically modified Gram-positive bacterial cells, a bacterium-like particle vaccine was constructed. Interestingly, while the results showed that IgG and nAbs were elicited after immunization, the immunogenicity was not dose-dependent. The induction of specific memory T-cells demonstrate the possibility for long-term protection (Table 2) (Zhang et al., 2022).
3.4 VLPs/RLPs vaccines
A study used the Drosophila insect protein expression system to generate VLPs of RVFV. VLPs composed of Gn and Gc, combined with the Stimune adjuvant, were shown to prevent RVFV infection in BALB/c mice (Table 2) (de Boer et al., 2010). In 2016, Li et al. (2016) used recombinant baculovirus to express RVFV NP and GP, which assembled into VLPs. They further explored the immunogenicity of these VLPs, and found that although T/B-cell responses were induced by VLPs, the responses were weaker than those stimulated with VLPs combined with Freund’s adjuvant (Table 2) (Li et al., 2020). Chimeric VLPs containing RVFV NP, Gn and Gc, meanwhile including gag from retroviruses were also tested against RVFV, in which the protective efficacy of chimeric VLPs is superior to VLPs with NP and VLPs without NP, which suggests gag enhances the uniformity and quantity of VLPs and may also serve as an adjuvant (Table 2) (Mandell et al., 2010).
Aside from traditional VLPs, protein fusion between eGn and the transmembrane domain and cytosolic tail (TMD/CT) of influenza hemagglutinin (HA) resulted in eGn-HA VLPs, which were found to elicit Gn-specific antibody (Table 2) (Mbewana et al., 2019), and provides a new prospect for VLPs development. Recombinant NDV expressing GnGc were used to infect stable cell lines with RVFV S and L genome segments to generate nonspreading RVFV (NSR), in which this strategy was shown to 100% protect mice from RVFV challenge (Table 2) (Wichgers Schreur et al., 2014a).
3.5 Viral vectored vaccines
Recombinant MVA vaccines expressing GnGc, Gn or Gc was developed and tested on mice. The results showed that these candidates did not elicit nAbs and passive transfer of immune serum to naïve mice did not result in protection. However, these vaccines induced strong T-cell responses, in which RVFV challenge resulted in protection efficacies of 100%, 60% and 80%, respectively, for rMVA-GnGc, rMVA-Gn and rMVA-Gc (Table 2) (López-Gil et al., 2020). In contrast, Lopez-Gil et al (López-Gil et al., 2013). demonstrated that the rMVA-NP vaccine did not induce IFN-γ and did not protect mice against a RVFV challenge (Table 2). The rMVA-GnGc(RVFV)-NS1(Bluetongue) demonstrated B-cell responses leading to survival against RVFV challenge in mice, but did not inhibit RVFV infection in vaccinated sheep (Table 2) (Calvo-Pinilla et al., 2020). Busquets et al. (2014) and Lorenzo et al. (2018) confirmed these results in sheep studies. From the above results, rMVA-GnGc is a promising potential candidate vaccine against RVFV.
Other poxvirus-vectored vaccines were also developed to investigate their efficacy against RVFV. A study used recombinant vaccinia viruses (rVACVs) to express RVFV GnGc or GnGc-IFNγ. Interestingly, the efficacy of the latter was lower, suggesting that the expression of IFN-γ may have played a role in reducing the replication of the recombinant pox-vectored vaccine (Table 2) (Papin et al., 2011). Based on KS1 recombination capripoxvirus (CPV) vector, a rKS1/RVFV-GnGc vaccine was developed, shown to induce high nAbs titers, and protected mice and sheep from RVFV challenge (Soi et al., 2010). To further understand the protective mechanism of the KS1/CPV-RVFV vaccine, Ayari-Fakhfakh et al. (2018) studied rKS1/CPV-Gn and found that the vaccine induced IL-4 and decreased IFN-γ in goats, suggesting the CD4+ T-cells are the main components of vaccine-induced rKS1/CPV-Gn immune response (Table 2).
The complex adenovirus (CAdVax) vector is a non-replicating system designed to avoid preexisting immune responses, which has hampered the efficacy of Ad5-vectored vaccines in the past. A CAdVax-GnGc vaccine was found to elicit strong GP-specific IgG antibody with 100% protection (Table 2) (Holman et al., 2009). Chimpanzee adenovirus vectors (ChAdOx) was another vector tested, and although ChAdOx1-GnGc was found to induce lower nAbs and T-cell response compared to Ad-5-GnGc, and both were found to have 100% protection from RVFV challenge (Table 2) (Warimwe et al., 2013). Warimwe et al (Stedman et al., 2019). further investigated ChAdOx1-GnGc efficacy and safety on sheep and goats, and showed that the candidates could inhibit RVFV infection in 100% of sheep and their fetuses, but not as protective in goat fetuses, suggesting differences in key mechanisms of protection against fetal RVFV infection between sheep and goats (Table 2).
Additionally, a RVFV pseudovirus based-VSV was constructed and successfully applied in neutralizing assay studies (Ma et al., 2019), suggesting the RVFV GP could inserted into VSV viral particles, and could be used for vaccine studies in the future. The Newcastle disease virus (NDV) vector and herpesvirus type 1 (EHV-1) vector expressing GnGc was shown to protect sheep from RVFV by nAbs (Table 2) (Kortekaas et al., 2012; Said et al., 2017).
3.6 DNA vaccines
Chrun et al. (2019) explored the effectiveness of a DNA vaccine expressing eGn (pscDEC-eGn) with GM-CSF adjuvant, in which the vaccine was shown to demonstrate complete protection from RVFV challenge, but the immunogenicity varied between different animal species, in which a decrese in the levels of specific IgG antibody led to a reduction in protection efficacy in mice (Table 2). Through comparison of the pTR600-eGn with pTR600-eGn with C3d adjuvant vaccine candidates, the latter was found to be better than the former on inducing specific IgG and nAbs. Although both vaccines did not elicit a detectable T-cell response, 80% and 100% protection was observed for pTR600-eGn and pTR600-eGn-C3d, respectively (Table 2) (Bhardwaj et al., 2010), suggesting that the B-cell response plays an important role on protection and that C3d could be a suitable adjuvant candidate.
Another study evaluated the protective efficacy of pCMV-NSmGnGc (0%), pCMV-GnGc (100%) and pCMV-NP (50%) against a RVFV challenge. It was found that a mixture of pCMV-NSmGnGc and pCMV-NP did not improve the protective efficacy of pCMV-NSmGnGc (Table 2) (Lorenzo et al., 2010), suggesting that the presence of NSm could impede the presentation process for the other antigens, and downregulate immune responses (Won et al., 2007).
Biodegradable alginate (ALG)/poly-L-lysine (PLL) microcapsules (MC), which are polycations used for fabrication of multilayer microcapsules, have been used to deliver DNA vaccines expressing Gn and Gc against RVFV, and results showed that this system enhanced specific-antibody and nAbs titers (Table 2) (Selina et al., 2020). The nanoparticles poly(N-vinylpyrrolidone) (PVP) can form complexes both with low molecular weight substances and polymers, and found to enhance humoral responses against Gn and Gc (Table 2) (Kuskov et al., 2021).
4 Vaccines against HTNV
In the 1950s, hemorrhagic fever with renal syndrome (HFRS) was reported. The causative pathogen, HTNV, was successfully isolated via serial passage in host rodents (Apodemus agrarius coreae) (Lee et al., 1978). HTNV sheds by host excreta and spreads to humans by aerosols (Jonsson et al., 2010). HTNV is mainly prevalent in China and Korea, which causes severe hemorrhagic fevers with a mortality rate ranging from 5% to 10% in humans (Cho et al., 2002). Four strains of HTNV, HTN76-118, AA57, KHF4 and KHF5, have been isolated from the host rodents and both caused hantavirus pulmonary syndrome-like disease in mice (Lee et al., 1978; Seto et al., 2012; Wei et al., 2022). Several vaccines against HTNV, based on the earliest 76-118 strain, have been developed and widely used, or are undergoing clinical evaluation.
4.1 Inactivated vaccines
Hantavax vaccine, the first vaccine developed against HTNV, was prepared from infected sucking mouse brains (Cho and Howard, 1999). Specific antibodies induced by Hantavax do not last for a long period and boosts are necessary to induce immune responses and maintain protection (Cho et al., 2002). Only 62% subjects showed seroconversion at 30 days after the first immunization and increased to 97% after a boost. The presence of nAbs was also boosted (from 13% to 75% of subjects) by the second immunization. However, only 50% of subjects were nAb-positive after 1 year (Cho et al., 2002). Current research on inactivated HTNV vaccines mainly focuses on the generation of robust long-term immune responses over a two-dose immunization strategy (Table 3) (Song et al., 2016; Song et al., 2020). Aside from Hantavax, two bivalent vaccines have also been developed against HTNV, one which also targets Seoul virus (SEOV) (Li et al., 2017) and the other also targeting Puumala virus (PUUV) (Cho et al., 2002). The HTNV-SEOV bivalent inactivated vaccine can induce high HTNV-NP specific IgG (over 1:1024) and last over 33 months after a three-dose immunization scheme.
4.2 VLPs vaccines
The Hepatitis B virus (HBV) core protein is a platform that has been used for several VLP-based vaccine development (Zuckerman et al., 2001; Geldmacher et al., 2004; Geldmacher et al., 2005), not only against HBV but also against other pathogens, by chemical or recombinant linkage to other antigens (Jegerlehner et al., 2002; Geldmacher et al., 2004; Geldmacher et al., 2005). In one study, Geldmacher et al. (2004) inserted the 120 amino-terminal aa of HTNV N protein (NP) into a carboxyl-terminal truncated HBV core protein. This recombinant protein can form a chimeric VLP on which the epitope of HTNV is partially exposed. Mice were immunized with 20μg VLP as a prime, followed with 10μg and 20μg boost at days 10 and 19, respectively. Both BALB/c and C57BL/6 mice had robust levels of specific antibodies against HTNV NP at day 28. The titer of HTNV NP-specific antibody is approximately 1:105 and 1:104 in BALB/c and C57BL/6 mice, respectively. Additionally, the induced antibody also had a strong cross-reaction to Dobrava virus (DOBV) and PUUV (Table 3). All IgG subclasses were induced, in which IgG1 is almost two orders of magnitude higher than IgG2a, indicating a Th2-biased response (Table 3) (Finkelman et al., 1990; Geldmacher et al., 2005).
However, the pre-existing immunity in humans against the HBV core protein may interfere the function of an HBV-based vaccine. Recent investigations on HTNV VLP-based vaccines are mainly focused on complete HTN-VLP and its modification, which consists of NP and GP (Gn and Gc) (Finkelman et al., 1990; Li et al., 2010; Ying et al., 2016; Dong et al., 2019). In one study, Li et al. (2010) generated HTN-VLP by co-expressing HTNV NP, Gn and Gc in Chinese hamster ovary (CHO) cells (Table 3). C57BL/6 mice were immunized with two doses of 120μg HTN-VLP with a two-week interval. The anti-NP IgG was boosted after the second immunization, while anti-GP protein antibody was not. IgG levels were similar with those generated with the inactivated bivalent vaccine (YOUERJIAN®) (Li et al., 2010), while nAbs were both similar (NT50 from 1:16 to 1:32). However, HTN-VLP vaccines induced significantly stronger Th1-type immune responses at day 28 compared to inactivated HTNV vaccines (Li et al., 2010). The HTN-VLP was modified in the later work (Ying et al., 2016; Dong et al., 2019), in which Ying et al. (2016) incorporated the HTNV GP with CD40 ligand (CD40L) and granulocyte macrophage colony-stimulating factor (GM-CSF), which can enhance antigen presentation as well as lymphocyte activation and maturation. The two vectors expressing HTNV NP and modified GP were co-transfected to Dhfr cells and HTN-VLPs was purified. The mice were immunized with three doses of 100μg recombinant HTN-VLP in a 2-week interval. The NP/GP specific and nAbs induced by CD40L-VLP and GM-CSF-VLP were higher than the modified VLP and inactive vaccine. Moreover, the recombinant HTN-VLP group has a higher ratio of IgG2a/IgG1, indicating that the modification of HTN-VLP can change the immunoglobulin response into specific subtypes and trend to a Th1-type immune response. ELISpot assay results also provided evidence that both CD40L-VLP and GM-CSF-VLP can significantly increase the amount of splenocytes secreting IL-2 and IFN-γ, but not IL-4 and IL-10 (Table 3). The immune responses induced by the recombinant HTN-VLP were shown to protect mice against HTNV challenge and significantly decreased HTNV antigen levels in all organs. The CD40L-VLP animals are still able to maintain a nAbs titer of 1:80 at 6 months after the last immunization, which was almost two times higher than other vaccines (Dong et al., 2019).
4.3 Peptide vaccines
Peptide antigens are internalized and proteolyzed by antigen presenting cells and presented on the major histocompatibility complex (MHC) of the cell surface as the epitope to be recognized by T-cell receptors (Malonis et al., 2020). These epitopes are short peptides which can stimulate T-cells and activate cellular immune responses. Based on this, several peptide vaccines against plasmodium, Hepatitis C Virus (HCV), influenza virus and HIV-1 have been developed (Malonis et al., 2020).
Tang et al. (2017) predicted seven HTNV GP 9mer epitopes by Bioinformatics and Molecular Analysis Section and SYFPEITHI database, and synthesized the HTNV peptide/HLA-A*0201 tetramers as HTNV peptide vaccine candidates (Table 3). These epitopes were shown to be recognized by CD8+ T-cell from HFRS patients. Each epitope was prepared as a separate vaccine for mice immunization. The peptide vaccines were found to protect mice against HTNV challenge by inducing a protective cytotoxic T-cell (CTL) response, which significantly decreased the viral RNA load in the liver, spleen, and kidneys. However, the effect of peptide vaccines was not superior to the inactivated HTNV vaccine. Therefore, the authors modified the peptide vaccine and synthesized a linear multi-epitope peptide instead (Table 3) (Ma et al., 2020). The new peptide vaccine linked the HTNV CTL epitope (VV9) with pan HLA-DR-binding epitope and significantly increased the number of HTNV-specific IFN-γ secreting CD8+ T-cells. The protective effect of the vaccine was further improved by the modification, which reduced the viral RNA load in spleen. However, the activation of CD4+ T-cells and long-term protection were not tested with these candidates.
5 Prospects and perspectives
Despite the discovery of CCHFV, RVFV and HTNV over half a century ago, there remains a dearth of approved specific vaccines for human and livestock use. Aside from limited access to high biosafety level laboratories, which is required for experimental manipulation with live versions of these viruses, other factors complicating the rapid development of specific prophylaxis for these pathogens include: 1) the genetic diversity of these viruses due to the high mutation rates associated with RNA viruses and the segmented nature of bunyaviruses allowing for recombination, 2) the lack of a robust, immunocompetent animal model accurately recapitulating the hallmark disease symptoms observed in humans, and 3) the safety and efficacy of vaccines need to be better characterized.
An ideal vaccine is one that could protect humans from several (or all) genotypes of a specific pathogen. For instance, the seven CCHFV genotypes differ as much as 20%, 31% and 22% in the S, M and L segments, respectively, at the nucleotide sequence level (Bente et al., 2013). The increased geographical distribution of ticks carrying CCHFV, due to global warming, potentially facilitates the spread of different CCHFV subtypes to other regions (Kariwa et al., 2012; Fillâtre et al., 2019; Garrison et al., 2019). As such, a candidate vaccine effective against multiple genotypes of CCHFV would be welcomed. However, few studies have been completed thus far on the cross-protection of CCHFV vaccines, so in order to better prevent future outbreaks of CCHFV, investigating cross-reactive and potentially protective immune responses between CCHFV subtypes should be a topic of focus in the future. From a diagnostics viewpoint, it has been shown that the NP of CCHFV has the potential to detect different genotypes of CCHFV due to a more conserved sequence, which potentially supports cross-protection (Rangunwala et al., 2014; Shrivastava et al., 2021). Leventhal et al. showed that CCHFV NP from Hoti strain could protect 100% of mice from a challenge with the UG3010 strain (Leventhal et al., 2022).
In contrast, for 33 different strains of RVFV, NP shown 3.5% variable and 2.8% changes in amino acid of Gn and Gc (Ikegami, 2012), multiple studies have investigated the cross protection of RVFV vaccines, but the results were suboptimal (Table 2) and further vaccine optimization and investigations are needed. Considering that HTNV is more conserved and that the amino acid similarity of S, M and L segments between the A9 strain and 76-118 strain are 96.7%, 95.4% and 95.6%, respectively, also specify the biggest divergence of HTNV (Kariwa et al., 2012), it may be possible to develop a cross-protective vaccine against HTNV. Regardless, the ability of candidate vaccines to stimulate cross-protective immune responses against multiple pathogen genotypes should be a field of investigation in the future.
Regarding animal models, CCHFV does not cause disease and death in most immunocompetent, small adult animals such as mice, rats and hamster, so type I IFN signal pathway-related knockout mice have been used instead. Disease leading to partial to full lethality have been observed after a CCHFV challenge (Garrison et al., 2019), but the drawback of such animal models is the lack of ability to generate a complete immune response, which does not allow the effect of the vaccination to be fully characterized. For instance, CCHFV is sensitive to type I IFN (Rodriguez et al., 2022), but type I IFN effects induced by the vaccine cannot be evaluated in this model. To address this weakness, a mouse-adapted variant of CCHFV (MA-CCHFV) was developed by serial passaging, in which challenge with MA-CCHFV resulted in symptoms similar to human CCHF such as hepatitis, weight loss and lethargy (Hawman et al., 2021b). The non-human primate (NHP) cynomolgus macaque model was also developed by infection of animals with the Kosova Hoti strain, and shown that similar hallmark signs of human Crimean-Cango hemorrhagic fever disease, such as shock syndrome and coagulopathy (Haddock et al., 2018). So far, however, no clinical trials have approved after developing these two animal model.
In contrast, animal models for RVFV and HTNV are more well-characterized, because immunocompetent animals are susceptible to infection with these viruses. For example, RVFV infection in BALB/c mice could result in acute hepatic necrosis and meningoencephalitis and 100% death via intraperitoneal, subcutaneous, and aerosol challenge (Ross et al., 2012). In marmosets, RVFV infection could cause viremia, weight loss, kidney disease and 25-100% mortality (Smith et al., 2012). Through studies in these animal models, some vaccines have advanced to clinical trials, such as the ChAdOx1-based RVFV vaccine (Stedman et al., 2019). HTNV is known to be lethal for wild-type mice (in which the LD50 for C57BL/6 mice is approximately 60 PFU), in which infection causes encephalitis, pneumonia, and hepatitis (Wichmann et al., 2002; Wei et al., 2022). An activated HTNV vaccine (Hantavax) was eventually advanced to and completed Phase III clinical trials (Song et al., 2020). Therefore, the development of immunocompetent animal models is crucial for advancing vaccine candidates beyond the laboratory.
In order for vaccines to be approved for human use, safety and efficacy is a key consideration. Whether the vaccine causes side-effects, the rate of seroconversion, the strength of immunity over time needs to be evaluated in the long-term, with supporting data from a large number of experimental and clinical studies. This is especially important when using new emerging technologies such as the mRNA vaccine platform and silico-based antigen design. Billions of people were immunized with the mRNA vaccine during the SARS-CoV-2 pandemic, in which the data shows that mRNA vaccines appear to be safe and reliable (Fang et al., 2022). However, this vaccine platform will need to be characterized over time to generate data on long-term safety, immunogenicity and efficacy, especially when multiple boosters are required to maintain protection. Currently, vaccine candidates against the bunyaviruses discussed in this manuscript have not been evaluated for these parameters. In the future, it will be important to consider the points made above in order to produce optimal, well-characterized vaccines suitable for human use against these highly virulent bunyaviruses threatening human and animal health.
Author contributions
TC: writing (first draft)-introduction, CCHFV, RVFV, discussion sections, and make tables. ZD: writing (first draft) HTNV and discussion sections. JL: writing-review outline, supervision, and reference support. GW: conceptualization, supervision, funding acquisition, and writing-review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Ministry of Science and Technology of China (Grant No. 2021YFC0863400, 2022YFE0114700); and G4 funding from Institut Pasteur, Fondation Merieux, and Chinese Academy of Sciences to GW; and the International Affairs Department of the Institut Pasteur of Paris.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abudurexiti, A., Adkins, S., Alioto, D., Alkhovsky, S. V., Avšič-Županc, T., Ballinger, M. J., et al. (2019). Taxonomy of the order bunyavirales: update 2019. Arch. Virol. 164, 1949–1965. doi: 10.1007/s00705-019-04253-6
Ahmed, A. A., McFalls, J. M., Hoffmann, C., Filone, C. M., Stewart, S. M., Paragas, J., et al. (2005). Presence of broadly reactive and group-specific neutralizing epitopes on newly described isolates of Crimean-Congo hemorrhagic fever virus. J. Gen. Virol. 86, 3327–3336. doi: 10.1099/vir.0.81175-0
Aligholipour Farzani, T., Földes, K., Ergünay, K., Gurdal, H., Bastug, A., Ozkul, A. (2019a). Immunological analysis of a CCHFV mRNA vaccine candidate in mouse models. Vaccines (Basel) 7, 115. doi: 10.3390/vaccines7030115
Aligholipour Farzani, T., Földes, K., Hanifehnezhad, A., Yener Ilce, B., Bilge Dagalp, S., Amirzadeh Khiabani, N., et al. (2019b). Bovine herpesvirus type 4 (BoHV-4) vector delivering nucleocapsid protein of Crimean-Congo hemorrhagic fever virus induces comparable protective immunity against lethal challenge in IFNα/β/γR-/- mice models. Viruses 11, 237. doi: 10.20944/preprints201901.0317.v1
Aligholipour Farzani, T., Hanifehnezhad, A., Földes, K., Ergünay, K., Yilmaz, E., Hashim Mohamed Ali, H., et al. (2019c). Co-Delivery effect of CD24 on the immunogenicity and lethal challenge protection of a DNA vector expressing nucleocapsid protein of Crimean Congo hemorrhagic fever virus. Viruses 11, 75. doi: 10.3390/v11010075
Anthony, T., van Schalkwyk, A., Romito, M., Odendaal, L., Clift, S. J., Davis, A. S. (2021). Vaccination with rift valley fever virus live attenuated vaccine strain smithburn caused meningoencephalitis in alpacas. J. Vet. Diagn. Invest. 33, 777–781. doi: 10.1177/10406387211015294
Appelberg, S., John, L., Pardi, N., Végvári, Á., Bereczky, S., Ahlén, G., et al. (2021). Nucleoside-modified mRNA vaccines protect IFNAR mice against Crimean Congo hemorrhagic fever virus infection. J. Virol. 96, 3. doi: 10.1128/JVI.01568-21
Ayari-Fakhfakh, E., Ghram, A., Albina, E., Cêtre-Sossah, C. (2018). Expression of cytokines following vaccination of goats with a recombinant capripoxvirus vaccine expressing rift valley fever virus proteins. Vet. Immunol. Immunopathol. 197, 15–20. doi: 10.1016/j.vetimm.2018.01.001
Bente, D. A., Forrester, N. L., Watts, D. M., McAuley, A. J., Whitehouse, C. A., Bray, M. (2013). Crimean-Congo Hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 100, 159–189. doi: 10.1016/j.antiviral.2013.07.006
Berber, E., Çanakoğlu, N., Tonbak, Ş., Ozdarendeli, A. (2021). Development of a protective inactivated vaccine against Crimean-Congo hemorrhagic fever infection. Heliyon 7, e08161. doi: 10.1016/j.heliyon.2021.e08161
Bhardwaj, N., Heise, M. T., Ross, T. M. (2010). Vaccination with DNA plasmids expressing gn coupled to C3d or alphavirus replicons expressing gn protects mice against rift valley fever virus. PloS Negl. Trop. Dis. 4, e725. doi: 10.1371/journal.pntd.0000725
Billecocq, A., Gauliard, N., Le May, N., Elliott, R. M., Flick, R., Bouloy, M. (2008). RNA Polymerase I-mediated expression of viral RNA for the rescue of infectious virulent and avirulent rift valley fever viruses. Virology 378, 377–384. doi: 10.1016/j.virol.2008.05.033
Bird, B. H., Albariño, C. G., Hartman, A. L., Erickson, B. R., Ksiazek, T. G., Nichol, S. T. (2008). Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J. Virol. 82, 2681–2691. doi: 10.1128/JVI.02501-07
Bird, B. H., Khristova, M. L., Rollin, P. E., Ksiazek, T. G., Nichol, S. T. (2007). Complete genome analysis of 33 ecologically and biologically diverse rift valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J. Virol. 81, 2805–2816. doi: 10.1128/JVI.02095-06
Borrego, B., Moreno, S., López-Valiñas, Á., de la Losa, N., Weber, F., Núñez, J. I., et al. (2022). Identification of single amino acid changes in the rift valley fever virus polymerase core domain contributing to virus attenuation. Front. In Cell. Infect. Microbiol. 12, 875539. doi: 10.3389/fcimb.2022.875539
Busquets, N., Lorenzo, G., López-Gil, E., Rivas, R., Solanes, D., Galindo-Cardiel, I., et al. (2014). Efficacy assessment of an MVA vectored rift valley fever vaccine in lambs. Antiviral Res. 108, 165–172. doi: 10.1016/j.antiviral.2014.05.020
Buttigieg, K. R., Dowall, S. D., Findlay-Wilson, S., Miloszewska, A., Rayner, E., Hewson, R., et al. (2014). A novel vaccine against Crimean-Congo haemorrhagic fever protects 100% of animals against lethal challenge in a mouse model. PloS One 9, e91516. doi: 10.1371/journal.pone.0091516
Calvo-Pinilla, E., Marín-López, A., Moreno, S., Lorenzo, G., Utrilla-Trigo, S., Jiménez-Cabello, L., et al. (2020). A protective bivalent vaccine against rift valley fever and bluetongue. NPJ Vaccines 5, 70. doi: 10.1038/s41541-020-00218-y
Campbell, C. L., Snell, T. K., Bennett, S., Wyckoff, J. H., Heaslip, D., Flatt, J., et al. (2022). Safety study of rift valley fever human vaccine candidate (DDVax) in mosquitoes. Transbound Emerg. Dis. 69, 2621–2633. doi: 10.1111/tbed.14415
Caplen, H., Peters, C. J., Bishop, D. H. (1985). Mutagen-directed attenuation of rift valley fever virus as a method for vaccine development. J. Gen. Virol. 66 (Pt 10), 2271–2277. doi: 10.1099/0022-1317-66-10-2271
Cho, H. W., Howard, C. R. (1999). Antibody responses in humans to an inactivated hantavirus vaccine (Hantavax). Vaccine 17, 2569–2575. doi: 10.1016/S0264-410X(99)00057-2
Cho, H.-W., Howard, C. R., Lee, H.-W. (2002). Review of an inactivated vaccine against hantaviruses. Intervirology 45, 328–333. doi: 10.1159/000067925
Chrun, T., Lacôte, S., Urien, C., Richard, C.-A., Tenbusch, M., Aubrey, N., et al. (2019). A DNA vaccine encoding the gn ectodomain of rift valley fever virus protects mice via a humoral response decreased by DEC205 targeting. Front. Immunol. 10, 860. doi: 10.3389/fimmu.2019.00860
Dai, S., Wu, Q., Wu, X., Peng, C., Liu, J., Tang, S., et al. (2021). Differential cell line susceptibility to Crimean-Congo hemorrhagic fever virus. Front. Cell. Infect. Microbiol. 11, 648077. doi: 10.3389/fcimb.2021.648077
Dar, O., Hogarth, S., McIntyre, S. (2013). Tempering the risk: rift valley fever and bioterrorism. Trop. Med. Int. Health 18, 1036–1041. doi: 10.1111/tmi.12108
Daubney, J. R. H. R., Garnham, P. C. (1931). Enzootic hepatitis or rift valley fever. an undescribed virus disease of sheep cattle and man from east africa. J. Pathol. Bacteriol. 34, 545–579. doi: 10.1002/path.1700340418
de Boer, S. M., Kortekaas, J., Antonis, A. F., Kant, J., van Oploo, J. L., Rottier, P. J. M., et al. (2010). Rift valley fever virus subunit vaccines confer complete protection against a lethal virus challenge. Vaccine 28, 2330–2339. doi: 10.1016/j.vaccine.2009.12.062
Dessau, M., Modis, Y. (2013). Crystal structure of glycoprotein c from rift valley fever virus. Proc. Natl. Acad. Sci. U. S. A. 110, 1696–1701. doi: 10.1073/pnas.1217780110
Dong, Y., Ma, T., Zhang, X., Ying, Q., Han, M., Zhang, M., et al. (2019). Incorporation of CD40 ligand or granulocyte-macrophage colony stimulating factor into hantaan virus (HTNV) virus-like particles significantly enhances the long-term immunity potency against HTNV infection. J. Med. Microbiol. 68, 480–492. doi: 10.1099/jmm.0.000897
Dowall, S. D., Buttigieg, K. R., Findlay-Wilson, S. J. D., Rayner, E., Pearson, G., Miloszewska, A., et al. (2016a). A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum. Vaccines Immunotherapeutics 12, 519–527. doi: 10.1080/21645515.2015.1078045
Dowall, S. D., Graham, V. A., Rayner, E., Hunter, L., Watson, R., Taylor, I., et al. (2016b). Protective effects of a modified vaccinia Ankara-based vaccine candidate against Crimean-Congo haemorrhagic fever virus require both cellular and humoral responses. PloS One 11, e0156637. doi: 10.1371/journal.pone.0156637
El-Sissi, A. F., Mohamed, F. H., Danial, N. M., Gaballah, A. Q., Ali, K. A. (2020). Chitosan and chitosan nanoparticles as adjuvant in local rift valley fever inactivated vaccine. 3 Biotech. 10, 88. doi: 10.1007/s13205-020-2076-y
Faburay, B., LaBeaud, A. D., McVey, D. S., Wilson, W. C., Richt, J. A. (2017). Current status of rift valley fever vaccine development. Vaccines 5, 29. doi: 10.3390/vaccines5030029
Faburay, B., Lebedev, M., McVey, D. S., Wilson, W., Morozov, I., Young, A., et al. (2014). A glycoprotein subunit vaccine elicits a strong rift valley fever virus neutralizing antibody response in sheep. Vector Borne Zoonotic Dis. (Larchmont N.Y.) 14, 746–756. doi: 10.1089/vbz.2014.1650
Faburay, B., Wilson, W. C., Gaudreault, N. N., Davis, A. S., Shivanna, V., Bawa, B., et al. (2016). A recombinant rift valley fever virus glycoprotein subunit vaccine confers full protection against rift valley fever challenge in sheep. Sci. Rep. 6, 27719. doi: 10.1038/srep27719
Fang, E., Liu, X., Li, M., Zhang, Z., Song, L., Zhu, B., et al. (2022). Advances in COVID-19 mRNA vaccine development. Signal Transduct. Target Ther. 7, 94. doi: 10.1038/s41392-022-00950-y
Fillâtre, P., Revest, M., Tattevin, P. (2019). Crimean-Congo Hemorrhagic fever: an update. Med. Mal Infect. 49, 574–585. doi: 10.1016/j.medmal.2019.09.005
Finkelman, F. D., Holmes, J., Katona, I. M., Urban, J. F., Beckmann, M. P., Park, L. S., et al. (1990). Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 8, 303–333. doi: 10.1146/annurev.iy.08.040190.001511
Flick, R., Whitehouse, C. A. (2005). Crimean-Congo Hemorrhagic fever virus. Curr. Mol. Med. 5, 753–760. doi: 10.2174/156652405774962335
Frey, S. E., Winokur, P. L., Hill, H., Goll, J. B., Chaplin, P., Belshe, R. B. (2014). Phase II randomized, double-blinded comparison of a single high dose (5×10(8) TCID50) of modified vaccinia Ankara compared to a standard dose (1×10(8) TCID50) in healthy vaccinia-naïve individuals. Vaccine 32, 2732–2739. doi: 10.1016/j.vaccine.2014.02.043
Furuyama, W., Reynolds, P., Haddock, E., Meade-White, K., Quynh Le, M., Kawaoka, Y., et al. (2020). A single dose of a vesicular stomatitis virus-based influenza vaccine confers rapid protection against H5 viruses from different clades. NPJ Vaccines 5, 4. doi: 10.1038/s41541-019-0155-z
Gamal, W. M., Soliman, E. M. M., El-Manzalawy, M. A. (2014). Tracing the antibody mediated acquired immunity by foot and mouth disease and rift valley fever combined vaccine in pregnant ewes and their lambs. Vet. World 7, 922–928. doi: 10.14202/vetworld.2014.922-928
Garrison, A. R., Shoemaker, C. J., Golden, J. W., Fitzpatrick, C. J., Suschak, J. J., Richards, M. J., et al. (2017). And death in two lethal mouse models. PloS Negl. Trop. Dis. 11 (9), e0005908. doi: 10.1371/journal.pntd.0005908
Garrison, A. R., Smith, D. R., Golden, J. W. (2019). Animal models for Crimean-Congo hemorrhagic fever human disease. Viruses 11, 590. doi: 10.3390/v11070590
Geldmacher, A., Skrastina, D., Borisova, G., Petrovskis, I., Krüger, D. H., Pumpens, P., et al. (2005). A hantavirus nucleocapsid protein segment exposed on hepatitis b virus core particles is highly immunogenic in mice when applied without adjuvants or in the presence of pre-existing anti-core antibodies. Vaccine 23, 3973–3983. doi: 10.1016/j.vaccine.2005.02.025
Geldmacher, A., Skrastina, D., Petrovskis, I., Borisova, G., Berriman, J. A., Roseman, A. M., et al. (2004). An amino-terminal segment of hantavirus nucleocapsid protein presented on hepatitis b virus core particles induces a strong and highly cross-reactive antibody response in mice. Virology 323, 108–119. doi: 10.1016/j.virol.2004.02.022
Ghiasi, S. M., Salmanian, A. H., Chinikar, S., Zakeri, S. (2011). Mice orally immunized with a transgenic plant expressing the glycoprotein of Crimean-Congo hemorrhagic fever virus. Clin. Vaccine Immunol. 18, 2031–2037. doi: 10.1128/CVI.05352-11
Golden, J. W., Shoemaker, C. J., Lindquist, M. E., Zeng, X., Daye, S. P., Williams, J. A., et al. (2019). GP38-targeting monoclonal antibodies protect adult mice against lethal Crimean-Congo hemorrhagic fever virus infection. Sci. Adv. 5, eaaw9535. doi: 10.1126/sciadv.aaw9535
Gowen, B. B., Bailey, K. W., Scharton, D., Vest, Z., Westover, J. B., Skirpstunas, R., et al. (2013). Post-exposure vaccination with MP-12 lacking NSs protects mice against lethal rift valley fever virus challenge. Antiviral Res. 98, 135–143. doi: 10.1016/j.antiviral.2013.03.009
Greenberg, R. N., Hurley, M. Y., Hurley, Y., Dinh, D. V., Mraz, S., Vera, J. G., et al. (2015). Open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PloS One 10, e0138348. doi: 10.1371/journal.pone.0138348
Gruetzmacher, K., Karesh, W. B., Amuasi, J. H., Arshad, A., Farlow, A., Gabrysch, S., et al. (2021). The Berlin principles on one health - bridging global health and conservation. Sci. Total Environ. 764, 142919. doi: 10.1016/j.scitotenv.2020.142919
Haddock, E., Feldmann, F., Hawman, D. W., Zivcec, M., Hanley, P. W., Saturday, G., et al. (2018). A cynomolgus macaque model for Crimean-Congo haemorrhagic fever. Nat. Microbiol. 3, 556–562. doi: 10.1038/s41564-018-0141-7
Harmon, J. R., Barbeau, D. J., Nichol, S. T., Spiropoulou, C. F., McElroy, A. K., fever virus vaccination induces long-lived, R. V. (2020). Antigen-specific human T cell responses. NPJ Vaccines 5, 17. doi: 10.1038/s41541-020-0166-9
Hawman, D. W., Ahlén, G., Appelberg, K. S., Meade-White, K., Hanley, P. W., Scott, D., et al. (2021a). A DNA-based vaccine protects against Crimean-Congo haemorrhagic fever virus disease in a cynomolgus macaque model. Nat. Microbiol. 6, 187–195. doi: 10.1038/s41564-020-00815-6
Hawman, D. W., Feldmann, H. (2023). Crimean-Congo Haemorrhagic fever virus. Nat. Rev. Microbiol. 1–15. doi: 10.1038/s41579-023-00871-9
Hawman, D. W., Meade-White, K., Leventhal, S., Appelberg, S., Ahlén, G., Nikouyan, N., et al. (2022). Accelerated DNA vaccine regimen provides protection against Crimean-Congo hemorrhagic fever virus challenge in a macaque model. Mol. Ther. J. Am. Soc. Gene Ther. 31 (2), 387–397. doi: 10.1016/j.ymthe.2022.09.016
Hawman, D. W., Meade-White, K., Leventhal, S., Feldmann, F., Okumura, A., Smith, B., et al. (2021b). Immunocompetent mouse model for Crimean-Congo hemorrhagic fever virus. Elife 10, e63906. doi: 10.7554/eLife.63906
Hederman, A. P., Ackerman, M. E. (2023). Leveraging deep learning to improve vaccine design. Trends Immunol. 44, 5. doi: 10.1016/j.it.2023.03.002
Hinkula, J., Devignot, S., Åkerström, S., Karlberg, H., Wattrang, E., Bereczky, S., et al. (2017). Immunization with DNA plasmids coding for Crimean-Congo hemorrhagic fever virus capsid and envelope proteins and/or virus-like particles induces protection and survival in challenged mice. J. Virol. 91 (10), e02076–16. doi: 10.1128/JVI.02076-16
Holman, D. H., Penn-Nicholson, A., Wang, D., Woraratanadharm, J., Harr, M.-K., Luo, M., et al. (2009). A complex adenovirus-vectored vaccine against rift valley fever virus protects mice against lethal infection in the presence of preexisting vector immunity. Clin. Vaccine Immunol. 16, 1624–1632. doi: 10.1128/CVI.00182-09
Hoogstraal, H. (1979). The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 15, 307–417. doi: 10.1093/jmedent/15.4.307
Ikegami, T. (2012). Molecular biology and genetic diversity of rift valley fever virus. Antiviral Res. 95, 293–310. doi: 10.1016/j.antiviral.2012.06.001
Ikegami, T. (2019). Candidate vaccines for human rift valley fever. Expert Opin. Biol. Ther. 19, 1333–1342. doi: 10.1080/14712598.2019.1662784
Ikegami, T. (2021). Development of a simian RNA polymerase I promoter-driven reverse genetics for the rescue of recombinant rift valley fever virus from vero cells. J. Virol. 95 (7), e02004–20. doi: 10.1128/JVI.02004-20
Ikegami, T., Hill, T. E., Smith, J. K., Zhang, L., Juelich, T. L., Gong, B., et al. (2015). Rift valley fever virus MP-12 vaccine is fully attenuated by a combination of partial attenuations in the s, m, and l segments. J. Virol. 89, 7262–7276. doi: 10.1128/JVI.00135-15
Ikegami, T., Jurado-Cobena, E., Alkan, C., Smith, J. K., Zhang, L., Kalveram, B., et al. (2022). Evaluations of rationally designed rift valley fever vaccine candidate RVax-1 in mosquito and rodent models. NPJ Vaccines 7, 109. doi: 10.1038/s41541-022-00536-3
Indran, S. V., Lihoradova, O. A., Phoenix, I., Lokugamage, N., Kalveram, B., Head, J. A., et al. (2013). Rift valley fever virus MP-12 vaccine encoding toscana virus NSs retains neuroinvasiveness in mice. J. Gen. Virol. 94, 1441–1450. doi: 10.1099/vir.0.051250-0
Jansen van Vuren, P., Tiemessen, C. T., Paweska, J. T. (2011). Anti-nucleocapsid protein immune responses counteract pathogenic effects of rift valley fever virus infection in mice. PloS One 6, e25027. doi: 10.1371/journal.pone.0025027
Jegerlehner, A., Tissot, A., Lechner, F., Sebbel, P., Erdmann, I., Kündig, T., et al. (2002). A molecular assembly system that renders antigens of choice highly repetitive for induction of protective b cell responses. Vaccine 20, 3104–3112. doi: 10.1016/S0264-410X(02)00266-9
Jonsson, C. B., Figueiredo, L. T. M., Vapalahti, O. (2010). A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 23, 412–441. doi: 10.1128/CMR.00062-09
Karaaslan, E., Çetin, N. S., Kalkan-Yazıcı, M., Hasanoğlu, S., Karakeçili, F., Özdarendeli, A., et al. (2021). Immune responses in multiple hosts to nucleocapsid protein (NP) of Crimean-Congo hemorrhagic fever virus (CCHFV). PloS Negl. Trop. Dis. 15, e0009973. doi: 10.1371/journal.pntd.0009973
Kariwa, H., Yoshikawa, K., Tanikawa, Y., Seto, T., Sanada, T., Saasa, N., et al. (2012). Isolation and characterization of hantaviruses in far East Russia and etiology of hemorrhagic fever with renal syndrome in the region. Am. J. Trop. Med. Hyg. 86, 545–553. doi: 10.4269/ajtmh.2012.11-0297
Kark, J. D., Aynor, Y., Peters, C. J. (1982). A rift valley fever vaccine trial. i. side effects and serologic response over a six-month follow-up. Am. J. Epidemiol. 116, 808–820. doi: 10.1093/oxfordjournals.aje.a113471
Khan, M. S. A., Nain, Z., Syed, S. B., Abdulla, F., Moni, M. A., Sheam, M. M., et al. (2021). Computational formulation and immune dynamics of a multi-peptide vaccine candidate against Crimean-Congo hemorrhagic fever virus. Mol. Cell Probes 55, 101693. doi: 10.1016/j.mcp.2020.101693
Knudsen, R. C., Mathews, H. M., Richmond, J. Y., Craven, R. B., Garner, J. S., Karabatsos, N., et al. (1994). Laboratory management of agents associated with hantavirus pulmonary syndrome; interim biosafety guidelines. 43 (RR-7), 1–7. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/00031653.htm.
Kortekaas, J., Antonis, A. F. G., Kant, J., Vloet, R. P. M., Vogel, A., Oreshkova, N., et al. (2012). Efficacy of three candidate rift valley fever vaccines in sheep. Vaccine 30, 3423–3429. doi: 10.1016/j.vaccine.2012.03.027
Kortekaas, J., Vloet, R. P., McAuley, A. J., Shen, X., Bosch, B. J., de Vries, L., et al. (2015). Crimean-Congo Hemorrhagic fever virus subunit vaccines induce high levels of neutralizing antibodies but no protection in STAT1 knockout mice. Vector Borne Zoonotic Dis. 15, 759–764. doi: 10.1089/vbz.2015.1855
Kuehnert, P. A., Stefan, C. P., Badger, C. V., Ricks, K. M. (2021). Crimean-Congo Hemorrhagic fever virus (CCHFV): a silent but widespread threat. Curr. Trop. Med. Rep. 8, 141–147. doi: 10.1007/s40475-021-00235-4
Kuri, P. R., Goswami, P. (2023). Current update on rotavirus in-silico multiepitope vaccine design. ACS Omega 8, 190–207. doi: 10.1021/acsomega.2c07213
Kuskov, A., Selina, O., Kulikov, P., Imatdinov, I., Balysheva, V., Kryukov, A., et al. (2021). Amphiphilic poly(-vinylpyrrolidone) nanoparticles loaded with DNA plasmids encoding gn and gc glycoproteins of the rift valley fever virus: preparation and evaluation. ACS Appl. Bio Mater. 4, 6084–6092. doi: 10.1021/acsabm.1c00426
Lee, H. W., Lee, P. W., Johnson, K. M. (1978). Isolation of the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 137, 298–308. doi: 10.1093/infdis/137.3.298
Leventhal, S. S., Meade-White, K., Rao, D., Haddock, E., Leung, J., Scott, D., et al. (2022). Replicating RNA vaccination elicits an unexpected immune response that efficiently protects mice against lethal Crimean-Congo hemorrhagic fever virus challenge. EBioMedicine 82, 104188. doi: 10.1016/j.ebiom.2022.104188
Li, Y., Han, L., Zhao, Y., Zheng, X., Wang, H., Gai, W., et al. (2020). Immunogenicity assessment of rift valley fever virus virus-like particles in BALB/c mice. Front. Vet. Sci. 7, 62. doi: 10.3389/fvets.2020.00062
Li, C., Liu, F., Liang, M., Zhang, Q., Wang, X., Wang, T., et al. (2010). Hantavirus-like particles generated in CHO cells induce specific immune responses in C57BL/6 mice. Vaccine 28, 4294–4300. doi: 10.1016/j.vaccine.2010.04.025
Li, Y. T., Wang, C. L., Zheng, X. X., Wang, H. L., Zhao, Y. K., Gai, W. W., et al. (2016). Development and characterization of rift valley fever virus-like particles. Genet. Mol. Res. 15, 1. doi: 10.4238/gmr.15017772
Li, Z., Zeng, H., Wang, Y., Zhang, Y., Cheng, L., Zhang, F., et al. (2017). The assessment of hantaan virus-specific antibody responses after the immunization program for hemorrhagic fever with renal syndrome in northwest China. Hum. Vaccines Immunotherapeutics 13, 802–807. doi: 10.1080/21645515.2016.1253645
Lihoradova, O., Ikegami, T. (2012). Modifying the NSs gene to improve live-attenuated vaccine for rift valley fever. Expert Rev. Vaccines 11, 1283–1285. doi: 10.1586/erv.12.106
Lihoradova, O. A., Indran, S. V., Kalveram, B., Lokugamage, N., Head, J. A., Gong, B., et al. (2013). Characterization of rift valley fever virus MP-12 strain encoding NSs of punta toro virus or sandfly fever Sicilian virus. PloS Negl. Trop. Dis. 7, e2181. doi: 10.1371/journal.pntd.0002181
Lim, H., In, H. J., Lee, J.-A., Sik Yoo, J., Lee, S.-W., Chung, G. T., et al. (2018). The immunogenicity and protection effect of an inactivated coxsackievirus A6, A10, and A16 vaccine against hand, foot, and mouth disease. Vaccine 36, 3445–3452. doi: 10.1016/j.vaccine.2018.05.005
Linthicum, K. J., Britch, S. C., Anyamba, A. (2016). Rift valley fever: an emerging mosquito-borne disease. Annu. Rev. Entomol. 61, 395–415. doi: 10.1146/annurev-ento-010715-023819
Liu, W., Sun, F.-J., Tong, Y.-G., Zhang, S.-Q., Cao, W.-C. (2016). Rift valley fever virus imported into China from Angola. Lancet Infect. Dis. 16, 1226. doi: 10.1016/S1473-3099(16)30401-7
Lokugamage, N., Freiberg, A. N., Morrill, J. C., Ikegami, T. (2012). Genetic subpopulations of rift valley fever virus strains ZH548 and MP-12 and recombinant MP-12 strains. J. Virol. 86, 13566–13575. doi: 10.1128/JVI.02081-12
López-Gil, E., Lorenzo, G., Hevia, E., Borrego, B., Eiden, M., Groschup, M., et al. (2013). A single immunization with MVA expressing GnGc glycoproteins promotes epitope-specific CD8+-T cell activation and protects immune-competent mice against a lethal RVFV infection. PloS Negl. Trop. Dis. 7, e2309. doi: 10.1371/journal.pntd.0002309
López-Gil, E., Moreno, S., Ortego, J., Borrego, B., Lorenzo, G., Brun, A. (2020). MVA vectored vaccines encoding rift valley fever virus glycoproteins protect mice against lethal challenge in the absence of neutralizing antibody responses. Vaccines 8, 82. doi: 10.3390/vaccines8010082
Lorenzo, G., López-Gil, E., Ortego, J., Brun, A. (2018). Efficacy of different DNA and MVA prime-boost vaccination regimens against a rift valley fever virus (RVFV) challenge in sheep 12 weeks following vaccination. Vet. Res. 49, 21. doi: 10.1186/s13567-018-0516-z
Lorenzo, G., Martín-Folgar, R., Hevia, E., Boshra, H., Brun, A. (2010). Protection against lethal rift valley fever virus (RVFV) infection in transgenic IFNAR(-/-) mice induced by different DNA vaccination regimens. Vaccine 28, 2937–2944. doi: 10.1016/j.vaccine.2010.02.018
Ly, H. J., Lokugamage, N., Nishiyama, S., Ikegami, T. (2017a). Risk analysis of inter-species reassortment through a rift valley fever phlebovirus MP-12 vaccine strain. PloS One 12, e0185194. doi: 10.1371/journal.pone.0185194
Ly, H. J., Nishiyama, S., Lokugamage, N., Smith, J. K., Zhang, L., Perez, D., et al. (2017b). Attenuation and protective efficacy of rift valley fever phlebovirus rMP12-GM50 strain. Vaccine 35, 6634–6642. doi: 10.1016/j.vaccine.2017.10.036
Ma, J., Chen, R., Huang, W., Nie, J., Liu, Q., Wang, Y., et al. (2019). In vitro and in vivo efficacy of a rift valley fever virus vaccine based on pseudovirus. Hum. Vaccines Immunotherapeutics 15, 2286–2294. doi: 10.1080/21645515.2019.1627820
Ma, Y., Tang, K., Zhang, Y., Zhang, C., Cheng, L., Zhang, F., et al. (2020). Protective CD8 T-cell response against hantaan virus infection induced by immunization with designed linear multi-epitope peptides in HLA-A2.1/K transgenic mice. Virol. J. 17, 146. doi: 10.1186/s12985-020-01421-y
Makoschey, B., van Kilsdonk, E., Hubers, W. R., Vrijenhoek, M. P., Smit, M., Wichgers Schreur, P. J., et al. (2016). Rift valley fever vaccine virus clone 13 is able to cross the ovine placental barrier associated with foetal infections, malformations, and stillbirths. PloS Negl. Trop. Dis. 10, e0004550. doi: 10.1371/journal.pntd.0004550
Malherbe, D. C., Kurup, D., Wirblich, C., Ronk, A. J., Mire, C., Kuzmina, N., et al. (2021). A single dose of replication-competent VSV-vectored vaccine expressing SARS-CoV-2 S1 protects against virus replication in a hamster model of severe COVID-19. NPJ Vaccines 6, 91. doi: 10.1038/s41541-021-00352-1
Malonis, R. J., Lai, J. R., Vergnolle, O. (2020). Peptide-based vaccines: current progress and future challenges. Chem. Rev. 120, 3210–3229. doi: 10.1021/acs.chemrev.9b00472
Mandell, R. B., Koukuntla, R., Mogler, L. J. K., Carzoli, A. K., Freiberg, A. N., Holbrook, M. R., et al. (2010). A replication-incompetent rift valley fever vaccine: chimeric virus-like particles protect mice and rats against lethal challenge. Virology 397, 187–198. doi: 10.1016/j.virol.2009.11.001
Mbewana, S., Meyers, A. E., Rybicki, E. P. (2019). Chimaeric rift valley fever virus-like particle vaccine candidate production in nicotiana benthamiana. Biotechnol. J. 14, e1800238. doi: 10.1002/biot.201800238
McMillen, C. M., Hartman, A. L. (2021). Rift valley fever: a threat to pregnant women hiding in plain sight? J. Virol. 95 (9) e01394–19. doi: 10.1128/JVI.01394-19
Meechan, P. J., Potts, J. (2020). Biosafety in microbiological and biomedical laboratories. Available at: https://www.cdc.gov/labs/BMBL.html.
Morrill, J. C., Peters, C. J., Bettinger, G. E., Palermo, P. M., Smith, D. R., Watts, D. M. (2022). Rift valley fever MP-12 vaccine elicits an early protective immune response in mice. Vaccine. 40 (50), 7255–7261. doi: 10.1016/j.vaccine.2022.10.062
Mousavi-Jazi, M., Karlberg, H., Papa, A., Christova, I., Mirazimi, A. (2012). Healthy individuals' immune response to the Bulgarian Crimean-Congo hemorrhagic fever virus vaccine. Vaccine 30, 6225–6229. doi: 10.1016/j.vaccine.2012.08.003
Muller, R., Saluzzo, J. F., Lopez, N., Dreier, T., Turell, M., Smith, J., et al. (1995). Characterization of clone 13, a naturally attenuated avirulent isolate of rift valley fever virus, which is altered in the small segment. Am. J. Trop. Med. Hyg. 53, 405–411. doi: 10.4269/ajtmh.1995.53.405
Murakami, S., Terasaki, K., Ramirez, S. I., Morrill, J. C., Makino, S. (2014). Development of a novel, single-cycle replicable rift valley fever vaccine. PloS Negl. Trop. Dis. 8, e2746. doi: 10.1371/journal.pntd.0002746
Niklasson, B. (1982). Rift valley fever virus vaccine trial: study of side-effects in humans. Scand. J. Infect. Dis. 14, 105–109. doi: 10.3109/inf.1982.14.issue-2.06
Nishiyama, S., Lokugamage, N., Ikegami, T. (2016). The l, m, and s segments of rift valley fever virus MP-12 vaccine independently contribute to a temperature-sensitive phenotype. J. Virol. 90, 3735–3744. doi: 10.1128/JVI.02241-15
Nosrati, M., Behbahani, M., Mohabatkar, H. (2019). Towards the first multi-epitope recombinant vaccine against Crimean-Congo hemorrhagic fever virus: a computer-aided vaccine design approach. J. BioMed. Inform 93, 103160. doi: 10.1016/j.jbi.2019.103160
Papa, A., Tsergouli, K., Tsioka, K., Mirazimi, A. (2017). Crimean-Congo Hemorrhagic fever: tick-Host-Virus interactions. Front. Cell Infect. Microbiol. 7, 213. doi: 10.3389/fcimb.2017.00213
Papin, J. F., Verardi, P. H., Jones, L. A., Monge-Navarro, F., Brault, A. C., Holbrook, M. R., et al. (2011). Recombinant rift valley fever vaccines induce protective levels of antibody in baboons and resistance to lethal challenge in mice. Proc. Natl. Acad. Sci. U. S. A. 108, 14926–14931. doi: 10.1073/pnas.1112149108
Pavel, S. T. I., Yetiskin, H., Kalkan, A., Ozdarendeli, A. (2020). Evaluation of the cell culture based and the mouse brain derived inactivated vaccines against Crimean-Congo hemorrhagic fever virus in transiently immune-suppressed (IS) mouse model. PloS Negl. Trop. Dis. 14, e0008834. doi: 10.1371/journal.pntd.0008834
Rahpeyma, M., Samarbaf-Zadeh, A., Makvandi, M., Ghadiri, A. A., Dowall, S. D., Fotouhi, F. (2017). Expression and characterization of codon-optimized Crimean-Congo hemorrhagic fever virus gn glycoprotein in insect cells. Arch. Virol. 162, 1951–1962. doi: 10.1007/s00705-017-3315-3
Randall, R., Gibbs, C. J., Aulisio, C. G., Binn, L. N., Harrison, V. R. (1962). The development of a formalin-killed rift valley fever virus vaccine for use in man. J. Immunol. 89, 660–671. doi: 10.4049/jimmunol.89.5.660
Rangunwala, A., Samudzi, R. R., Burt, F. J. (2014). Detection of IgG antibody against Crimean-Congo haemorrhagic fever virus using ELISA with recombinant nucleoprotein antigens from genetically diverse strains. Epidemiol. Infect. 142, 2147–2154. doi: 10.1017/S0950268813002987
Richmond, J. Y., McKinney, R. W. (2009). Biosafety in microbiological and biomedical laboratories (US Government Printing Office). https://www.cdc.gov/labs/BMBL.html.
Rodriguez, S. E., Cross, R. W., Fenton, K. A., Bente, D. A., Mire, C. E., Geisbert, T. W. (2019). Vesicular stomatitis virus-based vaccine protects mice against Crimean-Congo hemorrhagic fever. Sci. Rep. 9, 7755. doi: 10.1038/s41598-019-44210-6
Rodriguez, S. E., Hawman, D. W., Sorvillo, T. E., O'Neal, T. J., Bird, B. H., Rodriguez, L. L., et al. (2022). Immunobiology of Crimean-Congo hemorrhagic fever. Antiviral Res. 199, 105244. doi: 10.1016/j.antiviral.2022.105244
Rolin, A. I., Berrang-Ford, L., Kulkarni, M. A. (2013). The risk of rift valley fever virus introduction and establishment in the united states and European union. Emerg. Microbes Infect. 2, e81. doi: 10.1038/emi.2013.81
Ronchi, G. F., Testa, L., Iorio, M., Pinoni, C., Bortone, G., Dondona, A. C., et al. (2022). Immunogenicity and safety studies of an inactivated vaccine against rift valley fever. Acta Trop. 232, 106498. doi: 10.1016/j.actatropica.2022.106498
Rosamilia, A., Jacca, S., Tebaldi, G., Tiberti, S., Franceschi, V., Macchi, F., et al. (2016). BoHV-4-based vector delivering Ebola virus surface glycoprotein. J. Transl. Med. 14, 325. doi: 10.1186/s12967-016-1084-5
Ross, T. M., Bhardwaj, N., Bissel, S. J., Hartman, A. L., Smith, D. R. (2012). Animal models of rift valley fever virus infection. Virus Res. 163, 417–423. doi: 10.1016/j.virusres.2011.10.023
Said, A., Elmanzalawy, M., Ma, G., Damiani, A. M., Osterrieder, N. (2017). An equine herpesvirus type 1 (EHV-1) vector expressing rift valley fever virus (RVFV) gn and gc induces neutralizing antibodies in sheep. Virol. J. 14, 154. doi: 10.1186/s12985-017-0811-8
Sana, M., Javed, A., Babar Jamal, S., Junaid, M., Faheem, M. (2022). Development of multivalent vaccine targeting m segment of Crimean Congo hemorrhagic fever virus (CCHFV) using immunoinformatic approaches. Saudi J. Biol. Sci. 29, 2372–2388. doi: 10.1016/j.sjbs.2021.12.004
Sanchez, A. J., Vincent, M. J., Nichol, S. T. (2002). Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J. Virol. 76, 7263–7275. doi: 10.1128/JVI.76.14.7263-7275.2002
Schalk, J. A. C., Mooi, F. R., Berbers, G. A. M., van Aerts, L.A.G.J.M., Ovelgönne, H., Kimman, T. G. (2006). Preclinical and clinical safety studies on DNA vaccines. Hum. Vaccin. 2, 45–53. doi: 10.4161/hv.2.2.2620
Scholte, F. E. M., Spengler, J. R., Welch, S. R., Harmon, J. R., Coleman-McCray, J. D., Freitas, B. T., et al. (2019). Single-dose replicon particle vaccine provides complete protection against Crimean-Congo hemorrhagic fever virus in mice. Emerg. Microbes Infect. 8, 575–578. doi: 10.1080/22221751.2019.1601030
Selina, O., Imatdinov, I., Balysheva, V., Akasov, R., Kryukov, A., Balyshev, V., et al. (2020). Microencapsulated plasmids expressing gn and gc glycoproteins of rift valley fever virus enhance humoral immune response in mice. Biotechnol. Lett. 42, 529–536. doi: 10.1007/s10529-020-02816-1
Seto, T., Nagata, N., Yoshikawa, K., Ichii, O., Sanada, T., Saasa, N., et al. (2012). Infection of hantaan virus strain AA57 leading to pulmonary disease in laboratory mice. Virus Res. 163, 284–290. doi: 10.1016/j.virusres.2011.10.016
Sherman, M. B., Freiberg, A. N., Holbrook, M. R., Watowich, S. J. (2009). Single-particle cryo-electron microscopy of rift valley fever virus. Virology 387, 11–15. doi: 10.1016/j.virol.2009.02.038
Shi, W., Li, J., Zhou, H., Gao, G. F. (2017). Pathogen genomic surveillance elucidates the origins, transmission and evolution of emerging viral agents in China. Sci. China Life Sci. 60, 1317–1330. doi: 10.1007/s11427-017-9211-0
Shrivastava, N., Kumar, J. S., Yadav, P., Shete, A. M., Jain, R., Shrivastava, A., et al. (2021). Development of double antibody sandwich ELISA as potential diagnostic tool for rapid detection of Crimean-Congo hemorrhagic fever virus. Sci. Rep. 11, 14699. doi: 10.1038/s41598-021-93319-0
Shtanko, O., Nikitina, R. A., Altuntas, C. Z., Chepurnov, A. A., Davey, R. A. (2014). Crimean-Congo Hemorrhagic fever virus entry into host cells occurs through the multivesicular body and requires ESCRT regulators. PloS Pathog. 10, e1004390. doi: 10.1371/journal.ppat.1004390
Sindato, C., Karimuribo, E. D., Swai, E. S., Mboera, L. E. G., Rweyemamu, M. M., Paweska, J. T., et al. (2021). Safety, immunogenicity and antibody persistence of rift valley fever virus clone 13 vaccine in sheep, goats and cattle in Tanzania. Front. Vet. Sci. 8, 779858. doi: 10.3389/fvets.2021.779858
Smith, D. R., Bird, B. H., Lewis, B., Johnston, S. C., McCarthy, S., Keeney, A., et al. (2012). Development of a novel nonhuman primate model for rift valley fever. J. Virol. 86, 2109–2120. doi: 10.1128/JVI.06190-11
Smith, D. R., Johnston, S. C., Piper, A., Botto, M., Donnelly, G., Shamblin, J., et al. (2018). Attenuation and efficacy of live-attenuated rift valley fever virus vaccine candidates in non-human primates. PloS Negl. Trop. Dis. 12, e0006474. doi: 10.1371/journal.pntd.0006474
Soi, R. K., Rurangirwa, F. R., McGuire, T. C., Rwambo, P. M., DeMartini, J. C., Crawford, T. B. (2010). Protection of sheep against rift valley fever virus and sheep poxvirus with a recombinant capripoxvirus vaccine. Clin. Vaccine Immunol. 17, 1842–1849. doi: 10.1128/CVI.00220-10
Song, J. Y., Jeong, H. W., Yun, J. W., Lee, J., Woo, H. J., Bae, J.-Y., et al. (2020). Immunogenicity and safety of a modified three-dose priming and booster schedule for the hantaan virus vaccine (Hantavax): a multi-center phase III clinical trial in healthy adults. Vaccine 38, 8016–8023. doi: 10.1016/j.vaccine.2020.10.035
Song, J. Y., Woo, H. J., Cheong, H. J., Noh, J. Y., Baek, L. J., Kim, W. J. (2016). Long-term immunogenicity and safety of inactivated hantaan virus vaccine (Hantavax™) in healthy adults. Vaccine 34, 1289–1295. doi: 10.1016/j.vaccine.2016.01.031
Spengler, J. R., Estrada-Peña, A., Garrison, A. R., Schmaljohn, C., Spiropoulou, C. F., Bergeron, É., et al. (2016). A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antiviral Res. 135, 31–47. doi: 10.1016/j.antiviral.2016.09.013
Spengler, J. R., Welch, S. R., Scholte, F. E. M., Rodriguez, S. E., Harmon, J. R., Coleman-McCray, J. D., et al. (2021). Viral replicon particles protect IFNAR mice against lethal Crimean-Congo hemorrhagic fever virus challenge three days after vaccination. Antiviral Res. 191, 105090. doi: 10.1016/j.antiviral.2021.105090
Stedman, A., Wright, D., Wichgers Schreur, P. J., Clark, M. H. A., Hill, A. V. S., Gilbert, S. C., et al. (2019). Safety and efficacy of ChAdOx1 RVF vaccine against rift valley fever in pregnant sheep and goats. NPJ Vaccines 4, 44. doi: 10.1038/s41541-019-0138-0
Suda, Y., Fukushi, S., Tani, H., Murakami, S., Saijo, M., Horimoto, T., et al. (2016). Analysis of the entry mechanism of Crimean-Congo hemorrhagic fever virus, using a vesicular stomatitis virus pseudotyping system. Arch. Virol. 161, 1447–1454. doi: 10.1007/s00705-016-2803-1
Suder, E., Furuyama, W., Feldmann, H., Marzi, A., de Wit, E. (2018). The vesicular stomatitis virus-based Ebola virus vaccine: from concept to clinical trials. Hum. Vaccines Immunotherapeutics 14, 2107–2113. doi: 10.1080/21645515.2018.1473698
Tang, K., Cheng, L., Zhang, C., Zhang, Y., Zheng, X., Zhang, Y., et al. (2017). Novel identified HLA-A*0201-Restricted hantaan virus glycoprotein cytotoxic T-cell epitopes could effectively induce protective responses in HLA-A2.1/K transgenic mice may associate with the severity of hemorrhagic fever with renal syndrome. Front. Immunol. 8, 1797. doi: 10.3389/fimmu.2017.01797
Tarim, E. A., Anil Inevi, M., Ozkan, I., Kecili, S., Bilgi, E., Baslar, M. S., et al. (2023). Microfluidic-based technologies for diagnosis, prevention, and treatment of COVID-19: recent advances and future directions. BioMed. Microdevices 25, 10. doi: 10.1007/s10544-023-00649-z
Temur, A. I., Kuhn, J. H., Pecor, D. B., Apanaskevich, D. A., Keshtkar-Jahromi, M. (2021). Epidemiology of Crimean-Congo hemorrhagic fever (CCHF) in Africa-underestimated for decades. Am. J. Trop. Med. Hyg. 104, 1978–1990. doi: 10.4269/ajtmh.20-1413
Terasaki, K., Juelich, T. L., Smith, J. K., Kalveram, B., Perez, D. D., Freiberg, A. N., et al. (2018). A single-cycle replicable rift valley fever phlebovirus vaccine carrying a mutated NSs confers full protection from lethal challenge in mice. Sci. Rep. 8, 17097. doi: 10.1038/s41598-018-35472-7
Ter Horst, S., Conceição-Neto, N., Neyts, J., Rocha-Pereira, J. (2019). Structural and functional similarities in bunyaviruses: perspectives for pan-bunya antivirals. Rev. Med. Virol. 29, e2039. doi: 10.1002/rmv.2039
Tian, H., Stenseth, N. C. (2019). The ecological dynamics of hantavirus diseases: from environmental variability to disease prevention largely based on data from China. PloS Negl. Trop. Dis. 13, e0006901. doi: 10.1371/journal.pntd.0006901
Tipih, T., Heise, M., Burt, F. J. (2021). Immunogenicity of a DNA-based sindbis replicon expressing Crimean-Congo hemorrhagic fever virus nucleoprotein. Vaccines 9, 1491. doi: 10.3390/vaccines9121491
Tran, P.-T.-H., Höglund, U., Larsson, O., Appelberg, S., Mirazimi, A., Johansson, M., et al. (2022). Enhanced seroconversion to West Nile virus proteins in mice by West Nile kunjin replicon virus-like particles expressing glycoproteins from Crimean-Congo hemorrhagic fever virus. Pathogens 11, 233. doi: 10.3390/pathogens11020233
Tsergouli, K., Karampatakis, T., Haidich, A. B., Metallidis, S., Papa, A. (2020). Nosocomial infections caused by Crimean-Congo haemorrhagic fever virus. J. Hosp. Infect. 105, 43–52. doi: 10.1016/j.jhin.2019.12.001
von Krempelhuber, A., Vollmar, J., Pokorny, R., Rapp, P., Wulff, N., Petzold, B., et al. (2010). A randomized, double-blind, dose-finding phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE. Vaccine 28, 1209–1216. doi: 10.1016/j.vaccine.2009.11.030
Wampande, E. M., Waiswa, P., Allen, D. J., Hewson, R., Frost, S. D. W., Stubbs, S. C. B. (2021). Phylogenetic characterization of Crimean-Congo hemorrhagic fever virus detected in African blue ticks feeding on cattle in a Ugandan abattoir. Microorganisms 9, 438. doi: 10.3390/microorganisms9020438
Wang, Q., Ma, T., Wu, Y., Chen, Z., Zeng, H., Tong, Z., et al. (2019). Neutralization mechanism of human monoclonal antibodies against rift valley fever virus. Nat. Microbiol. 4, 1231–1241. doi: 10.1038/s41564-019-0411-z
Wang, Q., Wang, S., Shi, Z., Li, Z., Zhao, Y., Feng, N., et al. (2022). GEM-PA-Based subunit vaccines of Crimean Congo hemorrhagic fever induces systemic immune responses in mice. Viruses 14, 1664. doi: 10.3390/v14081664
Warimwe, G. M., Lorenzo, G., Lopez-Gil, E., Reyes-Sandoval, A., Cottingham, M. G., Spencer, A. J., et al. (2013). Immunogenicity and efficacy of a chimpanzee adenovirus-vectored rift valley fever vaccine in mice. Virol. J. 10, 349. doi: 10.1186/1743-422X-10-349
Weaver, S. C., Reisen, W. K. (2010). Present and future arboviral threats. Antiviral Res. 85, 328–345. doi: 10.1016/j.antiviral.2009.10.008
Wei, Z., Shimizu, K., Sarii, R. S., Muthusinghe, D. S., Lokupathirage, S. M. W., Nio-Kobayashi, J., et al. (2022). Pathological studies on hantaan virus-infected mice simulating severe hemorrhagic fever with renal syndrome. Viruses 14, 2247. doi: 10.3390/v14102247
Weingartl, H. M., Nfon, C. K., Zhang, S., Marszal, P., Wilson, W. C., Morrill, J. C., et al. (2014). Efficacy of a recombinant rift valley fever virus MP-12 with NSm deletion as a vaccine candidate in sheep. Vaccine 32, 2345–2349. doi: 10.1016/j.vaccine.2013.12.064
Wichgers Schreur, P. J., Mooij, P., Koopman, G., Verstrepen, B. E., Fagrouch, Z., Mortier, D., et al. (2022). Safety and immunogenicity of four-segmented rift valley fever virus in the common marmoset. NPJ Vaccines 7, 54. doi: 10.1038/s41541-022-00476-y
Wichgers Schreur, P. J., Oreshkova, N., Harders, F., Bossers, A., Moormann, R. J. M., Kortekaas, J. (2014a). Paramyxovirus-based production of rift valley fever virus replicon particles. J. Gen. Virol. 95, 2638–2648. doi: 10.1099/vir.0.067660-0
Wichgers Schreur, P. J., Oreshkova, N., Moormann, R. J. M., Kortekaas, J. (2014b). Creation of rift valley fever viruses with four-segmented genomes reveals flexibility in bunyavirus genome packaging. J. Virol. 88, 10883–10893. doi: 10.1128/JVI.00961-14
Wichgers Schreur, P. J., Oymans, J., Kant, J., van de Water, S., Kollár, A., Dehon, Y., et al. (2021a). A single vaccination with four-segmented rift valley fever virus prevents vertical transmission of the wild-type virus in pregnant ewes. NPJ Vaccines 6, 8. doi: 10.1038/s41541-020-00271-7
Wichgers Schreur, P. J., Tacken, M., Gutjahr, B., Keller, M., van Keulen, L., Kant, J., et al. (2021b). Vaccine efficacy of self-assembled multimeric protein scaffold particles displaying the glycoprotein gn head domain of rift valley fever virus. Vaccines 9, 301. doi: 10.3390/vaccines9030301
Wichmann, D., Gröne, H.-J., Frese, M., Pavlovic, J., Anheier, B., Haller, O., et al. (2002). Hantaan virus infection causes an acute neurological disease that is fatal in adult laboratory mice. J. Virol. 76, 8890–8899. doi: 10.1128/JVI.76.17.8890-8899.2002
Wilson, W. C., Faburay, B., Trujillo, J. D., Ragan, I., Sunwoo, S.-Y., Morozov, I., et al. (2021). Preliminary evaluation of a recombinant rift valley fever virus glycoprotein subunit vaccine providing full protection against heterologous virulent challenge in cattle. Vaccines 9, 748. doi: 10.3390/vaccines9070748
Won, S., Ikegami, T., Peters, C. J., Makino, S. (2007). NSm protein of rift valley fever virus suppresses virus-induced apoptosis. J. Virol. 81, 13335–13345. doi: 10.1128/JVI.01238-07
Wu, Y., Zhu, Y., Gao, F., Jiao, Y., Oladejo, B. O., Chai, Y., et al. (2017). Structures of phlebovirus glycoprotein gn and identification of a neutralizing antibody epitope. Proc. Natl. Acad. Sci. U. S. A. 114, E7564–E7573. doi: 10.1073/pnas.1705176114
Xia, H., Zhao, J., Li, Y., Yin, S., Tang, S., Zhang, Z., et al. (2013). Infection and propagation of Crimean-Congo hemorrhagic fever virus in embryonated chicken eggs. Virus Res. 173, 344–349. doi: 10.1016/j.virusres.2013.01.008
Xu, W., Watts, D. M., Costanzo, M. C., Tang, X., Venegas, L. A., Jiao, F., et al. (2013). The nucleocapsid protein of rift valley fever virus is a potent human CD8+ T cell antigen and elicits memory responses. PloS One 8, e59210. doi: 10.1371/journal.pone.0059210
Yang, J., Gong, Y., Zhang, C., Sun, J., Wong, G., Shi, W., et al. (2022). Co-Existence and co-infection of influenza a viruses and coronaviruses: public health challenges. Innovation (Camb.) 3, 100306. doi: 10.1016/j.xinn.2022.100306
Ying, Q., Ma, T., Cheng, L., Zhang, X., Truax, A. D., Ma, R., et al. (2016). Construction and immunological characterization of CD40L or GM-CSF incorporated hantaan virus like particle. Oncotarget 7, 63488–63503. doi: 10.18632/oncotarget.11329
Zhang, N. N., Li, X. F., Deng, Y. Q., Zhao, H., Huang, Y. J., Yang, G., et al. (2020). A thermostable mRNA vaccine against COVID-19. Cell 182, 1271–1283.e16. doi: 10.1016/j.cell.2020.07.024
Zhang, S., Yan, F., Liu, D., Li, E., Feng, N., Xu, S., et al. (2022). Bacterium-like particles displaying the rift valley fever virus gn head protein induces efficacious immune responses in immunized mice. Front. Microbiol. 13, 799942. doi: 10.3389/fmicb.2022.799942
Zhou, Z.-R., Wang, M.-L., Deng, F., Li, T.-X., Hu, Z.-H., Wang, H.-L. (2011). Production of CCHF virus-like particle by a baculovirus-insect cell expression system. Virol. Sin. 26, 338–346. doi: 10.1007/s12250-011-3209-6
Zivcec, M., Guerrero, L. I. W., Albariño, C. G., Bergeron, É., Nichol, S. T., Spiropoulou, C. F. (2017). Identification of broadly neutralizing monoclonal antibodies against Crimean-Congo hemorrhagic fever virus. Antiviral Res. 146, 112–120. doi: 10.1016/j.antiviral.2017.08.014
Zivcec, M., Safronetz, D., Scott, D. P., Robertson, S., Feldmann, H. (2018). Nucleocapsid protein-based vaccine provides protection in mice against lethal Crimean-Congo hemorrhagic fever virus challenge. PloS Negl. Trop. Dis. 12, e0006628. doi: 10.1371/journal.pntd.0006628
Keywords: bunyaviruses, Crimean-Congo Hemorrhagic Fever Virus, Rift Valley Fever Virus, Hantaan virus, vaccines
Citation: Chen T, Ding Z, Lan J and Wong G (2023) Advances and perspectives in the development of vaccines against highly pathogenic bunyaviruses. Front. Cell. Infect. Microbiol. 13:1174030. doi: 10.3389/fcimb.2023.1174030
Received: 25 February 2023; Accepted: 03 May 2023;
Published: 18 May 2023.
Edited by:
Wei Ye, Air Force Medical University, ChinaReviewed by:
Jianzhong Wang, Jilin Agriculture University, ChinaSergio E. Rodriguez, Centers for Disease Control and Prevention (CDC), United States
Copyright © 2023 Chen, Ding, Lan and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gary Wong, Z2FyeWNrd29uZ0BpcHMuYWMuY24=
 Tong Chen
Tong Chen Zhe Ding
Zhe Ding Jiaming Lan
Jiaming Lan Gary Wong
Gary Wong