
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 30 June 2023
Sec. Clinical Microbiology
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1167638
Background: In recent years, with the continuous development of treatments for hematological malignancies (HMs), the remission and survival rates of patients with HMs have been significantly improved. However, because of severe immunosuppression and long-term recurrent neutropenia during treatment, the incidence and mortality of bloodstream infection (BSI) were all high in patients with HMs. Therefore, we analyzed pathogens’ distribution and drug-resistance patterns and developed a nomogram for predicting 30-day mortality in patients with BSIs among HMs.
Methods: In this retrospective study, 362 patients with positive blood cultures in HMs were included from June 2015 to June 2020 at West China Hospital of Sichuan University. They were randomly divided into the training cohort (n = 253) and the validation cohort (n = 109) by 7:3. A nomogram for predicting 30-day mortality after BSIs in patients with HMs was established based on the results of univariate and multivariate logistic regression. C-index, calibration plots, and decision curve analysis were used to evaluate the nomogram.
Results: Among 362 patients with BSIs in HMs, the most common HM was acute myeloid leukemia (48.1%), and the most common pathogen of BSI was gram-negative bacteria (70.4%). The final nomogram included the septic shock, relapsed/refractory HM, albumin <30g/l, platelets <30×109/l before BSI, and inappropriate empiric antibiotic treatment. In the training and validation cohorts, the C-indexes (0.870 and 0.825) and the calibration plots indicated that the nomogram had a good performance. The decision curves in both cohorts showed that the nomogram model for predicting 30-day mortality after BSI was more beneficial than all patients with BSIs or none with BSIs.
Conclusion: In our study, gram-negative bacterial BSIs were predominant in patients with HMs. We developed and validated a nomogram with good predictive ability to help clinicians evaluate the prognosis of patients.
Hematological malignancies (HMs) are diseases originating from or implicating the hematologic system and hematopoietic tissue. The treatment of HMs includes chemotherapy, immunotherapy, targeted therapy, and hematopoietic stem cell transplantation (Auberger et al., 2020). In recent years, with the continuous development of treatments for HMs, the remission and survival rates of patients with HMs have significantly improved. However, because of severe immunosuppression and long-term recurrent neutropenia during treatment, the incidence (20.8%-24.1%) and mortality (10%-32%) of bloodstream infection (BSI) were all high in patients with HMs (Nørgaard et al., 2006; Åttman et al., 2015; Trecarichi et al., 2015; Tang et al., 2018; Chen et al., 2021; Okamoto et al., 2021; Zhao et al., 2022). Meanwhile, compared with solid tumors, patients with HMs had a higher risk for BSIs (Marin et al., 2014). A 14-year longitudinal surveillance study showed that the incidence of BSIs in patients with HMs was three times higher than that of other cancer (Schelenz et al., 2013).
BSI has many unfavorable effects on patients with HMs, such as the increase in mortality, the addition of medical bills, and the prolongation of the hospitalization period (Liao et al., 2021). Therefore, timely and effective control of BSI is vital for patients with HMs. On the one hand, antibiotic therapy is crucial to patients with BSIs, and no other treatment can replace the efficacy of antibiotics. Doctors usually treat patients with an empiric antibiotic to control BSIs and then adjust the antibiotic based on the drug sensitivity test results, as it takes some time to isolate the pathogens by blood culture. Investigating pathogens’ distribution and drug-resistance patterns contribute to choosing the appropriate empiric antibiotics (Kadri et al., 2021).
On the other hand, it was essential to identify the risk factors for mortality in patients with BSIs, and then conduct early intervention and management (Timsit et al., 2020). Studies have explored the risk factors of prognosis for patients with BSIs in hematological diseases (Tumbarello et al., 2012; Tang et al., 2018; Albasanz-Puig et al., 2021; Shi et al., 2022; Zhao et al., 2022). These risk factors included age >60, relapsed or uncontrol malignancies, nosocomial infection, prolonged neutropenia, profound neutropenia, inappropriate empiric antibiotics, albumin <30g/l, BSI with multiple drug-resistant (MDR) bacteria, placement of the central venous catheter (CVC), placement of the urinary catheter, decreased white blood cell, and so on. Many prediction models were applied to assess outcomes for patients at high risk of various diseases (Woo et al., 2016; Yang et al., 2020; Asami et al., 2022; Zhu et al., 2022). Nevertheless, there were few models for the prognosis of patients with BSIs in HMs (Tang et al., 2018; Zhao et al., 2022). Meanwhile, these models may not apply to patients with BSIs in this region due to regional differences.
West China Hospital of Sichuan University is one of the largest diagnosis and treatment centers for difficult miscellaneous diseases in Western China, and its data are representative. Therefore, based on data from our hospital, we analyzed pathogens’ distribution and drug-resistance patterns and established a nomogram model for predicting 30-day mortality in patients with BSIs among HMs, to provide evidence for the prevention and treatment of patients with BSIs in HMs.
This retrospective study was conducted at the hematology department (215 beds) of West China Hospital, a national center for diagnosing and treating complex and critical diseases in Western China. The subjects in this study covered patients with positive blood cultures (bacteria or fungi) in HMs from June 2015 to June 2020. West China Hospital of Sichuan University Ethics Committee approved this study and waived informed consent because this study was retrospectively designed. We followed the following inclusion criteria to screen patients: (1) positive blood culture; (2) patient diagnosed with HM; (3) complete clinical data. The exclusion criteria were as follows: (1) patient diagnosed with non-HM; (2) incomplete clinical data; (3) patient with multiple positive blood cultures with the same pathogen during the same hospital stay; they were counted once.
Data were extracted from the Hospital Information System (HIS) of West China Hospital of Sichuan University. The details were as follows: ID number, gender, age, temperature, pathogens of BSIs, results of drug sensitivity test, blood pressure, state of disease, hematopoietic stem cell transplantation, nosocomial infection, length of stay before BSI, concurrent infection, white blood cell count, neutrophil count, platelet count, albumin level, procalcitonin (PCT), C-reactive protein (CRP), prothrombin time, activated partial thromboplastin time, D-dimer, invasive operation (urinary catheter, CVC), or peripherally inserted central catheter (PICC)), empiric antibiotic therapy, chemotherapy regimen, and outcomes at 30-day after BSI.
Patients with positive blood culture (excluding pollution) who had a fever (>38°C) and at the same time one or more symptoms, including chill, low oxygen saturation, hypotension, cold moist limbs, and disturbance of consciousness, were identified as true BSI (Amanati et al., 2021). Septic shock was defined as the vasopressor requirement for maintaining mean arterial pressure ≥65mmHg or serum lactate level ≥2mmol/l (Singer et al., 2016). Disseminated intravascular coagulation (DIC) was diagnosed with the Chinese DIC scoring system (Luo et al., 2019). MDR bacteria were defined as resistant to three or more antibacterial agents (Magiorakos et al., 2012). Empiric antibiotic therapy was defined as one or more antibiotics the patient received within 48 hours of being diagnosed with BSI (time to draw a positive blood culture specimen). Empiric antibiotic therapy was deemed appropriate if at least one of the empiric antibiotics was sensitive in vitro drug sensitivity test; otherwise, it was considered inappropriate empiric antibiotic therapy. Absolute neutrophil counts <0.5×109/l and <0.1×109/l were defined as neutropenia and profound neutropenia.
A database was established with Excel, and statistical analysis was performed with IBM SPSS 26.0. All cases were randomly divided into a training cohort (n =253) and a validation cohort (n =109) by 7:3. Continuous variables were expressed with mean ± standard deviation or median (IQR) according to the distribution pattern, and t-test or Mann-Whitney U test was used to the comparison between two cohorts. Frequency and proportion were applied to represent classified variables, and the comparison between two cohorts was conducted by Chi-square test or Fisher’s exact test. The 30-day mortality risk factors were analyzed by binary logistic regression. Variables with p<0.05 in the univariate logistic regression analysis were included in the multivariate logistic regression analysis. Based on the result of multivariate logistic regression analysis, we used the package of rms in R version 4.1.1 (http://www.r-project.org/) to plot the nomogram. The C-index was used to evaluate the discrimination of the nomogram. If the C-index is larger, the discrimination of the nomogram model is better. The accuracy (the extent to which the nomogram predicted 30-day mortality in accordance with actual mortality) of the nomogram was assessed by calibration plots. Calibration at a 45° diagonal is best, and above or below this diagonal indicates under-prediction or over-prediction. Decision curve analysis (DCA) was conducted to evaluate the net clinical benefit by quantifying net benefits at different threshold probabilities.
A total of 362 patients who had HMs with BSIs were included in the final analysis, 174 with acute myeloid leukemia, 74 with acute lymphocytic leukemia, 58 with non-Hodgkin lymphoma, and 56 with other HMs. All patients were randomly divided into the training cohort (n = 253) and the validation cohort (n = 109) by 7:3. The detailed characteristics of all patients are shown in Table 1. In all patients with an average age of 42.4 years, the male accounted for 64.6%, while the female accounted for 35.4%. The state of 108 patients with HMs was relapsed/refractory. A minority (6.9%) received hematopoietic stem cell transplantation, and a majority (86.7%) had concurrent pulmonary infections. Neutropenia existed in 79.3% of patients, and secondary DIC occurred in 20%. BSIs with MDR bacteria occurred in 204 patients. 35.1% of all patients received inappropriate empiric antibiotic therapy. 30-day mortality in patients with HMs after BSIs was 29%. Most variables were balanced between the training and validation cohorts, except for the PCT (p=0.002) (Table 1).
Three hundred sixty-two pathogens were isolated from blood culture, including gram-negative bacteria (70.4%), gram-positive bacteria (24%), and fungi (5.5%) (Figure 1). The most common gram-negative bacterium was Escherichia coli, accounting for 25.1%, followed by Klebsiella pneumoniae (16.0%) and Pseudomonas aeruginosa (11.1%). The common gram-positive bacteria were coagulase-negative staphylococci (7.7%), Enterococcus faecium (7.5%), and Staphylococcus aureus (4.7%). Seven strains of methicillin-resistant Staphylococcus aureus (MRSA) and 21 strains of methicillin-resistant coagulase-negative staphylococcus (MRCNS) were detected in all of Staphylococcus aureus and coagulase-negative staphylococci. The most common fungus was Candida tropicalis, accounting for about 2.8%. Although the proportion of gram-negative bacteria, gram-positive bacteria, and fungi fluctuated from 2015 to 2020, gram-negative bacteria were still dominant (Figure 1).
The results of the drug sensitivity test for the common pathogens are exhibited in Figure 2. Escherichia coli had the lowest rate of antibiotic resistance to amikacin (4.3%), followed by meropenem (8.7%) and imipenem (9.8%). The rates of antibiotic resistance of Klebsiella pneumoniae to amikacin, imipenem, and meropenem were 6.8%, 20.6%, and 22.4%, respectively. Pseudomonas aeruginosa had the lowest rates of antibiotic resistance to amikacin and ciprofloxacin, which were 2.5%. MRSA had no antibiotic resistance to tigecycline, linezolid, and vancomycin. MRCNS was completely sensitive to tigecycline and vancomycin. Enterococcus faecium had no resistance to tigecycline and linezolid. Fungal resistance was low to caspofungin (5%) and micafungin (5%). Meanwhile, no fungus resistant to amphotericin B was found in this study.
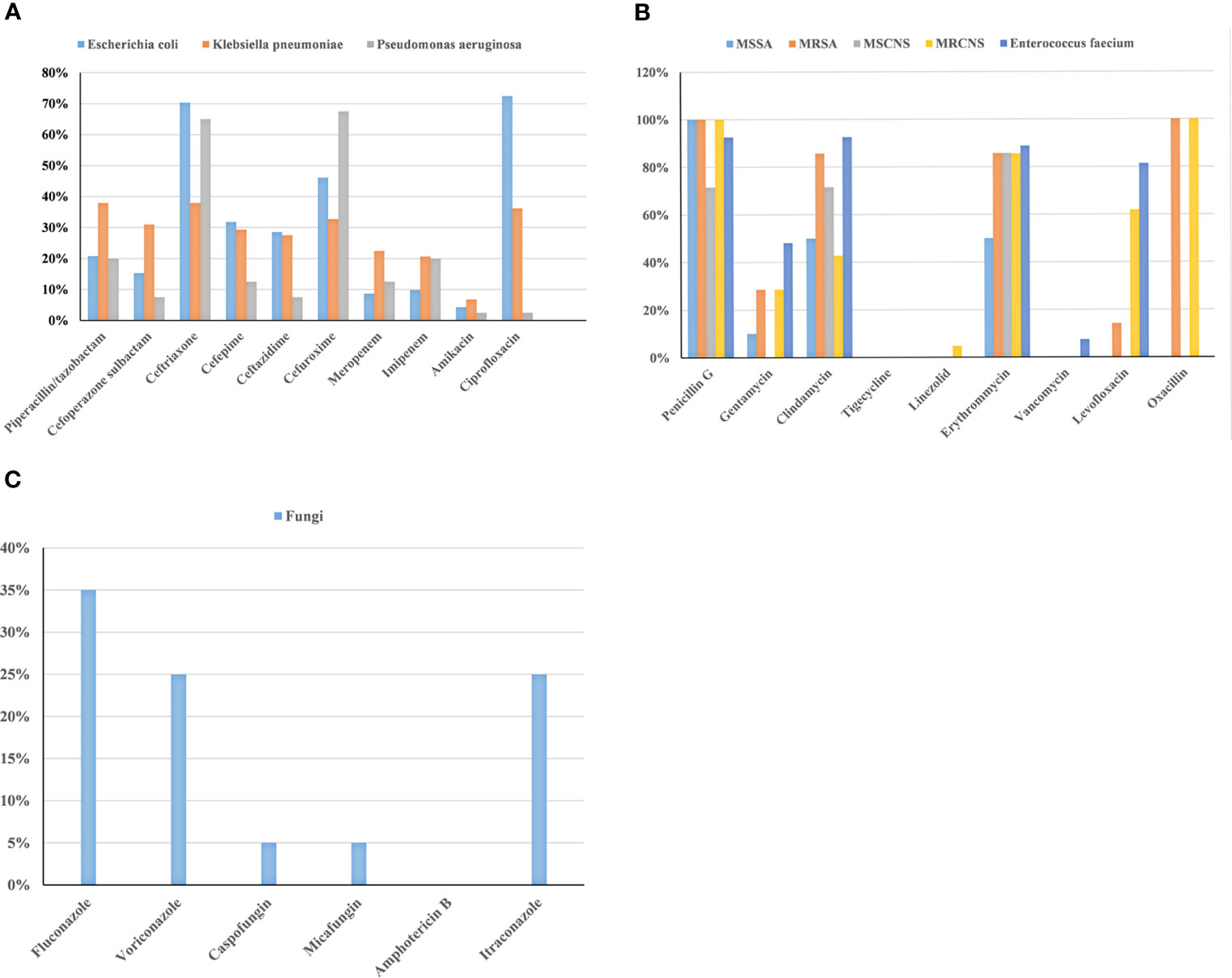
Figure 2 Analysis of drug-resistance in BSIs with gram-negative bacteria (A), gram-positive bacteria (B), and fungi (C) among patients with HMs.
In the training cohort, univariate logistic regression analysis showed that septic shock, relapsed/refractory HM, length of stay before BSI, concurrent pulmonary infection, concurrent perianal infection, neutropenia, albumin <30g/l, platelets <30×109/l before BSI, platelets <30×109/l after BSI, placement of the urinary catheter, secondary DIC, inappropriate empiric antibiotic treatment, combined empiric antibiotic treatment, and chemotherapy regimen containing glucocorticoid were associated with 30-day mortality after BSI (p<0.05 for all, Table 2). Further multivariate logistic regression analysis revealed that septic shock (p<0.001, OR=6.548, 95%CI: 3.042-14.092), relapsed/refractory HM (p=0.008, OR=2.811, 95%CI: 1.317-6.002), albumin <30g/l (P<0.001, OR=7.640, 95%CI: 3.529-16.542), platelets <30×109/l before BSI (P<0.001, OR=9.761, 95%CI: 3.511-27.132), and inappropriate empiric antibiotic treatment (P=0.002, OR=3.213, 95%CI: 1.522-6.785) were independent risk factors for 30-day mortality after BSI in patients with HMs (Table 2). Based on the result of multivariate logistic regression analysis, we established a visual nomogram for predicting 30-day mortality after BSI in patients with HMs (Figure 3).
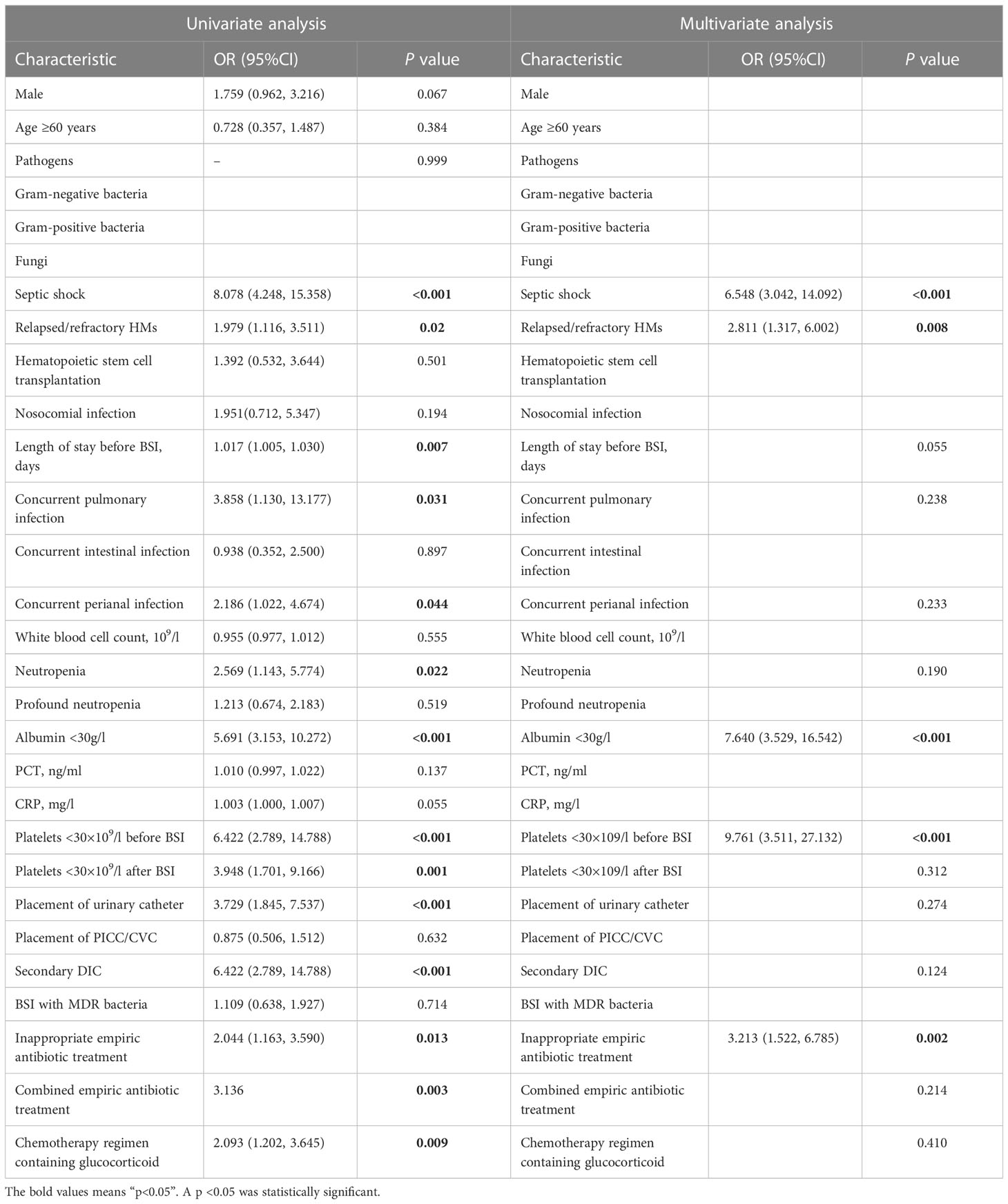
Table 2 Univariate and multivariate logistic regression analyses of 30-day mortality in the training cohort.
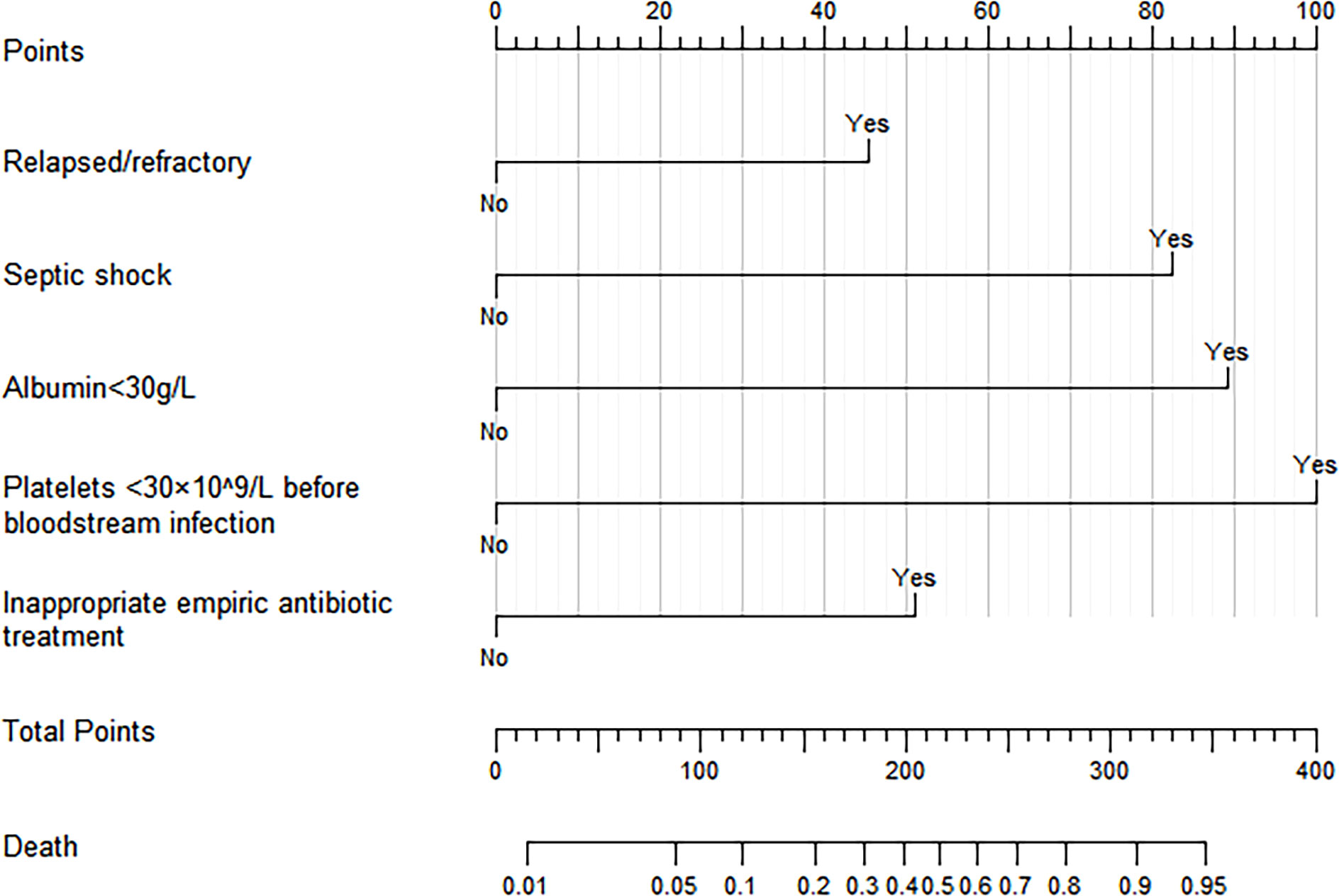
Figure 3 Nomogram for predicting 30-day mortality after BSIs in patients with HMs. (The “Yes” or “No” of each variable corresponded to the score on the “Points” axis, then the individual scores were added together to obtain the total score, the total score on the “Total points” axis corresponded to the dot of “Death” axis, which was the predicted probability of 30-day mortality).
The C-indexes of the nomogram were 0.870 (95%CI: 0.820-0.921) and 0.825 (95%CI: 0.737-0.912) in the training and validation cohorts, which indicated that the model had good discrimination. As shown in Figure 4, the calibration plots of the nomogram in both cohorts suggested that 30-day mortality after BSI predicted by the nomogram model was consistent with the actual mortality. Decision curves were used to evaluate the net clinical benefit of the nomogram model. The decision curves of the nomogram in the training and validation cohorts showed that the model for predicting 30-day mortality after BSI was more beneficial than all patients with BSIs or none with BSIs (Figure 5).
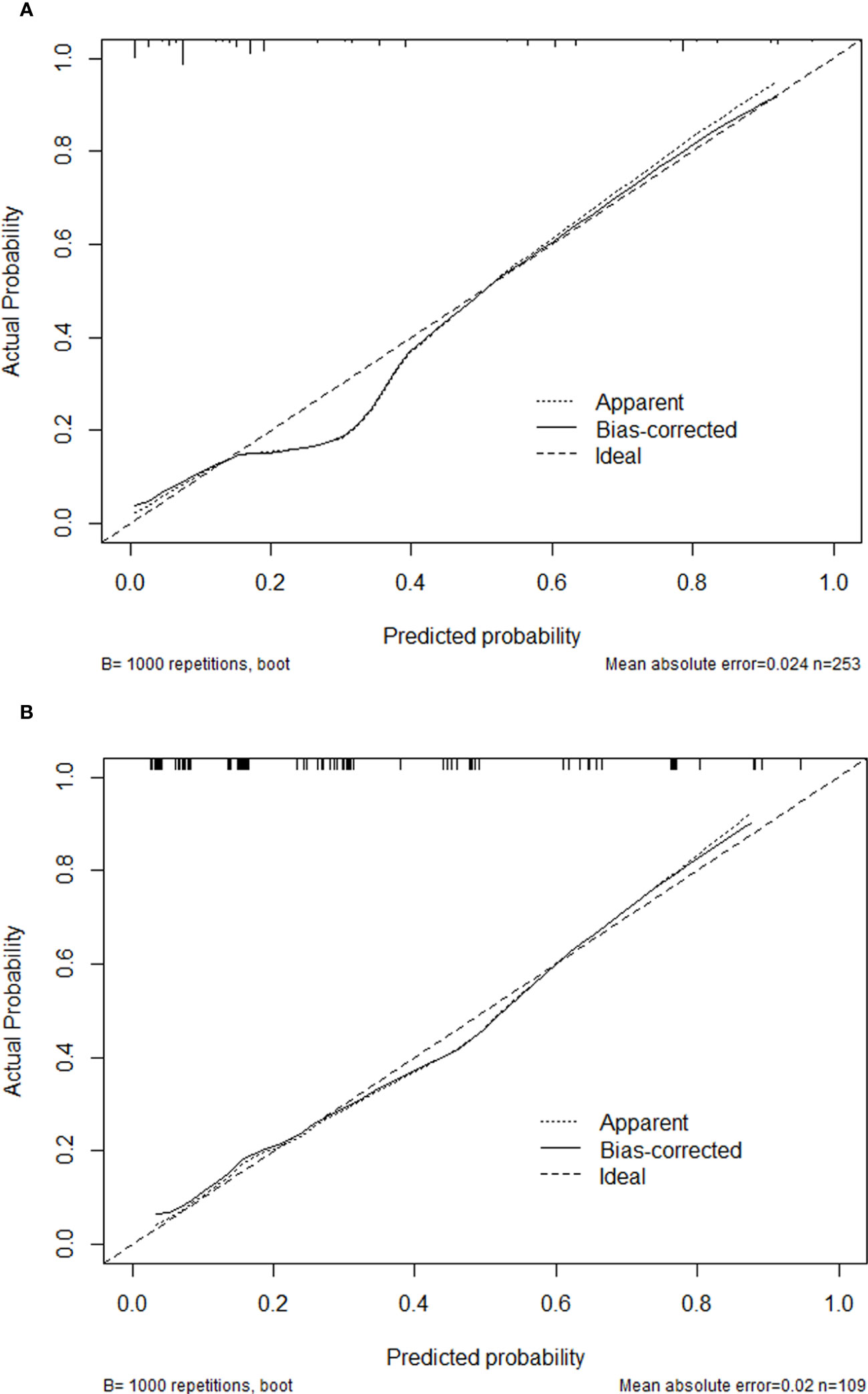
Figure 4 Calibration plots of the nomogram in the training cohort (A) and the validation cohort (B).
BSI is a common infection in patients with HMs. BSI negatively impacts patients with HMs, which may lead to death (Kochanek et al., 2019). Based on the complexity and high mortality of BSIs in HMs, we retrospectively analyzed the hospital data to identify pathogens’ distribution and antibiotic resistance. Meanwhile, we developed a nomogram for predicting the 30-day mortality of patients with BSIs in HMs. Our study represented a large-scale, single-center experience, and the results deepened our understanding of BSIs in patients with HMs.
Acute leukemia (acute myeloid leukemia and acute lymphocytic leukemia) was the most common HMs with BSIs in our study. The reason may be that patients with acute leukemia frequently received intensive chemotherapy and had long-term neutropenia (Garcia-Vidal et al., 2018). Neutropenia was often associated with life-threatening infections during cytotoxic chemotherapy (Kuo et al., 2017). Research showed that the rate of infections was 86.9% in patients with acute leukemia during the induction chemotherapy (Kato et al., 2018). Therefore, active infection control measures were necessary for patients with acute leukemia.
In the early days, researchers generally observed that gram-positive bacteria were the most common in BSIs (Ortega et al., 2005; Safdar et al., 2006; Huttunen et al., 2015). This may be associated with the prophylactic use of fluoroquinolone (Kern et al., 2018). However, this trend has reversed in recent years, and BSIs with gram-negative bacteria were more prevalent than gram-positive bacteria (Chen et al., 2017; Mimura et al., 2020; Haddad et al., 2021). Our study also confirmed the changing trend. We collected 362 pathogens, mainly gram-negative bacteria (70.4%), the most common being Escherichia coli. A retrospective study showed that BSIs with gram-negative bacteria accounted for 65% from 2007 to 2017, of which Escherichia coli was the most common (Haddad et al., 2021). A study from China suggested that gram-negative bacteria accounted for 64.7%, gram-positive bacteria accounted for 27.7%, and fungi accounted for 7.7% of BSIs in patients with HMs (Chen et al., 2021). In the present study, BSIs with gram-positive bacteria accounted for 24%, and coagulase-negative staphylococci were the most common, similar to other reports (Tang et al., 2018; Amanati et al., 2021). Most BSIs with gram-positive bacteria may be caused by long-term or repeated placement of PICC and CVC (van den Bosch et al., 2021). Candida was the primary pathogen of fungal BSIs, but the positive rate of fungal blood culture was low. This may be related to the difficulty of fungal culture and the lack of specific detection methods (Pappas et al., 2018). Therefore, blood cultures should be drawn actively and repeated for patients with fever if necessary. Moreover, new detection methods, such as second-generation sequencing, help detect the fungus (Peri et al., 2022). When patients have a poor response to antibiotics, they should be alert to fungal infections and be treated with antifungal agents as soon as necessary (Freifeld et al., 2011). The distribution of pathogens in our study was distinct from a study from Spain (Garcia-Vidal et al., 2018). This may be due to differences in region and habits of medication.
With the extensive use of antibiotics, bacterial drug resistance is becoming more serious. The mechanisms of bacterial resistance include the production of inactive enzymes and modifying enzymes, the change of target sites, the expression of efflux pumps, and the reduced permeability of the bacterial outer membrane (Blair et al., 2015). Identifying patterns of local bacterial resistance is a prerequisite for selecting appropriate antibiotics. Our study found that Escherichia coli and Klebsiella pneumoniae were sensitive to carbapenem antibiotics and amikacin, and Pseudomonas aeruginosa was sensitive to amikacin. This result was consistent with other studies (Trecarichi et al., 2015; Zhao et al., 2020; Shi et al., 2022). The antibiotics above may be appropriate for the common BSIs with gram-negative bacteria in clinical practice. Compared to our study showing a low resistance rate of Pseudomonas aeruginosa to ciprofloxacin (2.5%), other studies have shown various resistance in Pseudomonas aeruginosa to ciprofloxacin (2.4%-80.3%) (Sligl et al., 2015; Trecarichi et al., 2015; Chen et al., 2017; Zhao et al., 2020). The inconsistent results among the studies may be due to regional differences and sample sizes. Common gram-positive bacteria were susceptible to tigecycline, linezolid, and vancomycin. In the present study, MRSA, MRCNS, and vancomycin-resistant Enterococcus faecium were also detected (Haddad et al., 2021). With the application of antibiotics, the emergence of extended-spectrum β-lactamase-producing bacteria and MDR bacteria has become one of the thorniest problems. Therefore, it is necessary to have clear indications for the use of antibiotics. At the same time, the use of antibiotics must be cautious, and the distribution of local pathogens and drug-resistance results should be considered. Fungal infections were not uncommon in patients with HMs. In our hospital, Candida tropicalis was the most common. Amphotericin B, caspofungin, and micafungin were effective for patients with fungal BSIs in HMs. Xiao et al. found that the mortality of patients with fungal BSIs in HMs was 40.5%, much higher than that of patients with common fungal infections in HMs (Xiao et al., 2020). Therefore, it is vital to understand fungi’s distribution and drug resistance of fungi for treating fungal BSIs.
BSI was a common complication associated with high mortality in a patient with HM. The 30-day mortality was 29% in our study population, and other studies reported mortality ranging from 10% to 32% (Nørgaard et al., 2006; Åttman et al., 2015; Tang et al., 2018; Chen et al., 2021). Some studies about the risk factors for the poor prognosis of patients with BSIs in HMs were published (Tumbarello et al., 2012; Trecarichi et al., 2016; Tang et al., 2018; Shi et al., 2022). Nomograms are commonly used to evaluate the prognosis of tumor patients (Balachandran et al., 2015). We developed a nomogram for predicting the 30-day mortality of patients with BSIs in HMs. According to the evaluation with the c-indexes, calibration plots, and decision curves, the nomogram had satisfactory discrimination, consistency, and clinical benefit. The nomogram showed that septic shock, relapsed/refractory HM, albumin <30g/l, platelets <30×109/l before BSI, and inappropriate empiric antibiotic treatment were independent risk factors for 30-day mortality after BSI in patients with HMs.
A notable finding in our study was the identification of a previously unnoted risk factor for 30-day mortality, namely platelets <30×109/l before BSI. Patients with HMs developed thrombocytopenia due to myelosuppression. After BSI, especially progression to sepsis, platelet levels in patients with HMs may decrease further (Ghimire et al., 2021). Platelets are essential in primary hemostasis and are also involved in angiogenesis, tissue repair, and inflammation (Vinholt, 2019). Menard et al. found that thrombocytopenia was associated with major bleeding events and increased mortality in patients with septic shock (Menard et al., 2019). Thus, patients with BSIs in HMs with platelets less than 30×109/l had a higher risk of death at 30 days, possibly because they were more prone to major bleeding. Therefore, attention should be paid to the patients with BSIs in HMs who had low platelet levels, and appropriate platelet transfusion should be given to improve the prognosis of patients.
Septic shock is characterized by systemic hypoperfusion, often leading to organ failure and high mortality (Cecconi et al., 2018). Our study and others confirmed that septic shock was an independent risk factor for death in patients with HMs after BSI (Criscuolo et al., 2019; Chen et al., 2020; Royo-Cebrecos et al., 2022). Patients with low oxygen saturation, hypotension, or disturbance of consciousness need to be identified early and intervened as soon as possible, including fluid resuscitation and vasopressors. The association between the state of HM and mortality had been previously determined (Tang et al., 2018; Chen et al., 2021). We also found that relapsed/refractory HM was an independent risk factor for 30-day mortality after BSI in patients with HMs. Albumin is synthesized by the liver and reflects liver function and nutritional status. The study by Tang et al. suggested that albumin <30g/l was closely related to 30-day mortality in patients with HMs and BSIs (Tang et al., 2018). The same finding was confirmed in our study. Monitoring the albumin level and, if necessary, infusion of albumin is of great significance for the prognosis of patients with BSIs in HMs.
Empiric antibiotic therapy is one of the primary measures of anti-infection treatment. For patients with BSIs in HMs, the use of antibiotics is best based on the drug sensitivity, but it takes a while to wait for the blood culture results. The infection may further aggravate or even become life-threatening if antibiotics are not given in time. Previous studies have shown that inappropriate empiric antibiotic therapy was a risk factor for mortality in patients with BSIs among HMs (Tang et al., 2018; Tang et al., 2020; Zhao et al., 2022). This was also confirmed in the present study. Empiric antibiotic therapy for patients with BSIs in HMs should be individualized, considering previous antibiotic use, current epidemiology, and appropriate guidelines (Freifeld et al., 2011; Chinese Society of Hematology et al., 2020). Other factors such as neutropenia, age, placement of the urinary catheter, and nosocomial infection were not ultimately included in the nomogram in our study, possibly because of differences in the population and region and the retrospective nature of the study (Tumbarello et al., 2012; Tang et al., 2018; Shi et al., 2022).
Our study has some limitations. First, our study is retrospective, with limited samples and biases, and we only conducted internal validation. External validation and prospective studies are needed to optimize our model. Second, our study was based on diagnostic, laboratory testing, and therapeutic regimens at a single center, and there may be regional differences in findings.
In conclusion, our study suggested that gram-negative bacterial BSIs were predominant in patients with HMs. Meanwhile, we found that septic shock, relapsed/refractory HM, albumin <30g/l, platelets <30×109/l before BSI, and inappropriate empiric antibiotic treatment were independent risk factors for 30-day mortality after BSI in patients with HMs. Based on these factors, the nomogram was developed and validated to have good predictive ability. It can be used to evaluate the prognosis of patients promptly for clinicians.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
West China Hospital of Sichuan University Ethics Committee approved this study (2022-1930).
JW collected clinical data, interpreted the results, wrote, and revised the manuscript. MW and AZ participated in collecting data, data statistics. HZ and MM revised the manuscript. XL checked statistical methods. TN participated in the study design and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Incubation Program for Clinical Trials (No. 19HXFH030), Achievement Transformation Project (No. CGZH21001), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC21007), Translational Research Grant of NCRCH (No. 2021WWB03), Chengdu Science and Technology Program (No. 2022-YF05-01444-SN), Key Research and Development Program of Sichuan Province (No. 2023YFS0031), and National Key Research and Development Program of China (No. 2022YFC2502600, 2022YFC2502603).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Albasanz-Puig, A., Gudiol, C., Puerta-Alcalde, P., Ayaz, C. M., Machado, M., Herrera, F., et al. (2021). Impact of the inclusion of an aminoglycoside to the initial empirical antibiotic therapy for gram-negative bloodstream infections in hematological neutropenic patients: a propensity-matched cohort study (AMINOLACTAM study). Antimicrob. Agents Chemother. 65 (8), e0004521. doi: 10.1128/AAC.00045-21
Amanati, A., Sajedianfard, S., Khajeh, S., Ghasempour, S., Mehrangiz, S., Nematolahi, S., et al. (2021). Bloodstream infections in adult patients with malignancy, epidemiology, microbiology, and risk factors associated with mortality and multi-drug resistance. BMC Infect. Dis. 21 (1), 636. doi: 10.1186/s12879-021-06243-z
Asami, Y., Hiranuma, K., Takayanagi, D., Matsuda, M., Shimada, Y., Kato, M. K., et al. (2022). Predictive model for the preoperative assessment and prognostic modeling of lymph node metastasis in endometrial cancer. Sci. Rep. 12 (1), 19004. doi: 10.1038/41598-022-23252-3
Åttman, E., Aittoniemi, J., Sinisalo, M., Vuento, R., Lyytikäinen, O., Kärki, T., et al. (2015). Etiology, clinical course and outcome of healthcare-associated bloodstream infections in patients with hematological malignancies: a retrospective study of 350 patients in a Finnish tertiary care hospital. Leuk. Lymphoma 56 (12), 3370–3377. doi: 10.3109/10428194.2015.1032967
Auberger, P., Tamburini-Bonnefoy, J., Puissant, A. (2020). Drug resistance in hematological malignancies. Int. J. Mol. Sci. 21 (17), 6091. doi: 10.3390/ijms21176091
Balachandran, V. P., Gonen, M., Smith, J. J., DeMatteo, R. P. (2015). Nomograms in oncology: more than meets the eye. Lancet Oncol. 16 (4), e173–e180. doi: 10.1016/S1470-2045(14)71116-7
Blair, J. M., Webber, M. A., Baylay, A. J., Ogbolu, D. O., Piddock, L. J. (2015). Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 13 (1), 42–51. doi: 10.1038/nrmicro3380
Cecconi, M., Evans, L., Levy, M., Rhodes, A. (2018). Sepsis and septic shock. Lancet (London England) 392 (10141), 75–87. doi: 10.1016/S0140-6736(18)30696-2
Chen, S., Lin, K., Li, Q., Luo, X., Xiao, M., Chen, M., et al. (2021). A practical update on the epidemiology and risk factors for the emergence and mortality of bloodstream infections from real-world data of 3014 hematological malignancy patients receiving chemotherapy. J. Cancer 12 (18), 5494–5505. doi: 10.7150/jca.50802
Chen, C. Y., Tien, F. M., Sheng, W. H., Huang, S. Y., Yao, M., Tang, J. L., et al. (2017). Clinical and microbiological characteristics of bloodstream infections among patients with haematological malignancies with and without neutropenia at a medical centre in northern Taiwan, 2008-2013. Int. J. Antimicrob. Agents 49 (3), 272–281. doi: 10.1016/j.ijantimicag.2016.11.009
Chen, X. C., Xu, J., Wu, D. P. (2020). Clinical characteristics and outcomes of breakthrough candidemia in 71 hematologic malignancy patients and/or allogeneic hematopoietic stem cell transplant recipients: a single-center retrospective study from China, 2011-2018. Clin. Infect. Dis. 71 (Suppl 4), S394–S3s9. doi: 10.1093/cid/ciaa1523
Chinese Society of Hematology, Chinese Medical Association, Chinese Medical Doctor Association, Hematology Branch. (2020). [Chinese guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever (2020)]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi 41 (12), 969–978. doi: 10.3760/cma.j.issn.0253-2727.2020.12.001
Criscuolo, M., Marchesi, F., Candoni, A., Cattaneo, C., Nosari, A., Veggia, B., et al. (2019). Fungaemia in haematological malignancies: SEIFEM-2015 survey. Eur. J. Clin. Invest. 49 (5), e13083. doi: 10.1111/eci.13083
Freifeld, A. G., Bow, E. J., Sepkowitz, K. A., Boeckh, M. J., Ito, J. I., Mullen, C. A., et al. (2011). Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 52 (4), e56–e93. doi: 10.1093/cid/cir073
Garcia-Vidal, C., Cardozo-Espinola, C., Puerta-Alcalde, P., Marco, F., Tellez, A., Agüero, D., et al. (2018). Risk factors for mortality in patients with acute leukemia and bloodstream infections in the era of multiresistance. PloS One 13 (6), e0199531. doi: 10.1371/journal.pone.0199531
Ghimire, S., Ravi, S., Budhathoki, R., Arjyal, L., Hamal, S., Bista, A., et al. (2021). Current understanding and future implications of sepsis-induced thrombocytopenia. Eur. J. Haematol. 106 (3), 301–305. doi: 10.1111/ejh.13549
Haddad, S., Jabbour, J. F., Hindy, J. R., Makki, M., Sabbagh, A., Nayfeh, M., et al. (2021). Bacterial bloodstream infections and patterns of resistance in patients with haematological malignancies at a tertiary centre in Lebanon over 10 years. J. Global Antimicrob. Resist. 27, 228–235. doi: 10.1016/j.jgar.2021.09.008
Huttunen, R., Åttman, E., Aittoniemi, J., Outinen, T., Syrjänen, J., Kärki, T., et al. (2015). Nosocomial bloodstream infections in a Finnish tertiary care hospital: a retrospective cohort study of 2175 episodes during the years 1999-2001 and 2005-2010. Infect. Dis. (London England) 47 (1), 20–26. doi: 10.3109/00365548.2014.956791
Kadri, S. S., Lai, Y. L., Warner, S., Strich, J. R., Babiker, A., Ricotta, E. E., et al. (2021). Inappropriate empirical antibiotic therapy for bloodstream infections based on discordant in-vitro susceptibilities: a retrospective cohort analysis of prevalence, predictors, and mortality risk in US hospitals. Lancet Infect. Dis. 21 (2), 241–251. doi: 10.1016/S1473-3099(20)30477-1
Kato, H., Fujita, H., Akiyama, N., Kimura, S. I., Hiramoto, N., Hosono, N., et al. (2018). Infectious complications in adults undergoing intensive chemotherapy for acute myeloid leukemia in 2001-2005 using the Japan adult leukemia study group AML201 protocols. Support. Care Cancer 26 (12), 4187–4198. doi: 10.1007/s00520-018-4292-0
Kern, W. V., Weber, S., Dettenkofer, M., Kaier, K., Bertz, H., Behnke, M., et al. (2018). Impact of fluoroquinolone prophylaxis during neutropenia on bloodstream infection: data from a surveillance program in 8755 patients receiving high-dose chemotherapy for haematologic malignancies between 2009 and 2014. J. Infect. 77 (1), 68–74. doi: 10.1016/j.jinf.2018.05.004
Kochanek, M., Schalk, E., von Bergwelt-Baildon, M., Beutel, G., Buchheidt, D., Hentrich, M., et al. (2019). Management of sepsis in neutropenic cancer patients: 2018 guidelines from the infectious diseases working party (AGIHO) and intensive care working party (iCHOP) of the German society of hematology and medical oncology (DGHO). Ann. Hematol. 98 (5), 1051–1069. doi: 10.1007/s00277-019-03622-0
Kuo, F. C., Wang, S. M., Shen, C. F., Ma, Y. J., Ho, T. S., Chen, J. S., et al. (2017). Bloodstream infections in pediatric patients with acute leukemia: emphasis on gram-negative bacteria infections. J. Microbiol. Immunol. Infect. = Wei mian yu gan ran za zhi 50 (4), 507–513. doi: 10.1016/j.jmii.2015.08.013
Liao, W. C., Chung, W. S., Lo, Y. C., Shih, W. H., Chou, C. H., Chen, C. Y., et al. (2021). Changing epidemiology and prognosis of nosocomial bloodstream infection: a single-center retrospective study in Taiwan. J. Microbiol. Immunol. Infect. 55 (6 Pt 2), 1293–1300. doi: 10.1016/j.jmii.2021.09.015
Luo, L., Wu, Y., Niu, T., Han, Y., Feng, Y., Ding, Q., et al. (2019). A multicenter, prospective evaluation of the Chinese society of thrombosis and hemostasis scoring system for disseminated intravascular coagulation. Thromb. Res. 173, 131–140. doi: 10.1016/j.thromres.2018.11.022
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M. E., Giske, C. G., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18 (3), 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
Marin, M., Gudiol, C., Ardanuy, C., Garcia-Vidal, C., Calvo, M., Arnan, M., et al. (2014). Bloodstream infections in neutropenic patients with cancer: differences between patients with haematological malignancies and solid tumours. J. Infect. 69 (5), 417–423. doi: 10.1016/j.jinf.2014.05.018
Menard, C. E., Kumar, A., Houston, D. S., Turgeon, A. F., Rimmer, E., Houston, B. L., et al. (2019). Evolution and impact of thrombocytopenia in septic shock: a retrospective cohort study. Crit. Care Med. 47 (4), 558–565. doi: 10.1097/CCM.0000000000003644
Mimura, W., Fukuda, H., Akazawa, M. (2020). Antimicrobial utilization and antimicrobial resistance in patients with haematological malignancies in Japan: a multi-centre cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 19 (1), 7. doi: 10.1186/s12941-020-00348-0
Nørgaard, M., Larsson, H., Pedersen, G., Schønheyder, H. C., Sørensen, H. T. (2006). Risk of bacteraemia and mortality in patients with haematological malignancies. Clin. Microbiol. Infect. 12 (3), 217–223. doi: 10.1111/j.1469-0691.2005.01298.x
Okamoto, A., Kanda, Y., Kimura, S. I., Oyake, T., Tamura, K. (2021). Predictive and risk factor analysis for bloodstream infection in high-risk hematological patients with febrile neutropenia: post-hoc analysis from a prospective, large-scale clinical study. Int. J. Hematol. 114 (4), 472–482. doi: 10.1007/s12185-021-03183-x
Ortega, M., Rovira, M., Almela, M., Marco, F., de la Bellacasa, J. P., Martínez, J. A., et al. (2005). Bacterial and fungal bloodstream isolates from 796 hematopoietic stem cell transplant recipients between 1991 and 2000. Ann. Hematol. 84 (1), 40–46. doi: 10.1007/s00277-004-0909-0
Pappas, P. G., Lionakis, M. S., Arendrup, M. C., Ostrosky-Zeichner, L., Kullberg, B. J. (2018). Invasive candidiasis. Nat. Rev. Dis. Primers 4, 18026. doi: 10.1038/nrdp.2018.26
Peri, A. M., Harris, P. N. A., Paterson, D. L. (2022). Culture-independent detection systems for bloodstream infection. Clin. Microbiol. Infect. 28 (2), 195–201. doi: 10.1016/j.cmi.2021.09.039
Royo-Cebrecos, C., Laporte-Amargós, J., Peña, M., Ruiz-Camps, I., Puerta-Alcalde, P., Abdala, E., et al. (2022). Pseudomonas aeruginosa bloodstream infections in patients with cancer: differences between patients with hematological malignancies and solid tumors. Pathog. (Basel Switzerland) 11 (10), 1132. doi: 10.3390/pathogens11101132
Safdar, A., Rodriguez, G. H., Balakrishnan, M., Tarrand, J. J., Rolston, K. V. (2006). Changing trends in etiology of bacteremia in patients with cancer. Eur. J. Clin. Microbiol. Infect. Dis. 25 (8), 522–526. doi: 10.1007/s10096-006-0173-4
Schelenz, S., Nwaka, D., Hunter, P. R. (2013). Longitudinal surveillance of bacteraemia in haematology and oncology patients at a UK cancer centre and the impact of ciprofloxacin use on antimicrobial resistance. J. Antimicrob. Chemother. 68 (6), 1431–1438. doi: 10.1093/jac/dkt002
Shi, N., Kang, J., Wang, S., Song, Y., Yin, D., Li, X., et al. (2022). Bacteriological profile and antimicrobial susceptibility patterns of gram-negative bloodstream infection and risk factors associated with mortality and drug resistance: a retrospective study from shanxi, China. Infect. Drug Resist. 15, 3561–3578. doi: 10.2147/IDR.S370326
Singer, M., Deutschman, C. S., Seymour, C. W., Shankar-Hari, M., Annane, D., Bauer, M., et al. (2016). The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315 (8), 801–810. doi: 10.1001/jama.2016.0287
Sligl, W. I., Dragan, T., Smith, S. W. (2015). Nosocomial gram-negative bacteremia in intensive care: epidemiology, antimicrobial susceptibilities, and outcomes. Int. J. Infect. Dis.: IJID 37, 129–134. doi: 10.1016/j.ijid.2015.06.024
Tang, Y., Cheng, Q., Yang, Q., Liu, J., Zhang, D., Cao, W., et al. (2018). Prognostic factors and scoring model of hematological malignancies patients with bloodstream infections. Infection 46 (4), 513–521. doi: 10.1007/s15010-018-1151-3
Tang, Y., Wu, X., Cheng, Q., Li, X. (2020). Inappropriate initial antimicrobial therapy for hematological malignancies patients with gram-negative bloodstream infections. Infection 48 (1), 109–116. doi: 10.1007/s15010-019-01370-x
Timsit, J. F., Ruppé, E., Barbier, F., Tabah, A., Bassetti, M. (2020). Bloodstream infections in critically ill patients: an expert statement. Intensive Care Med. 46 (2), 266–284. doi: 10.1007/s00134-020-05950-6
Trecarichi, E. M., Pagano, L., Candoni, A., Pastore, D., Cattaneo, C., Fanci, R., et al. (2015). Current epidemiology and antimicrobial resistance data for bacterial bloodstream infections in patients with hematologic malignancies: an Italian multicentre prospective survey. Clin. Microbiol. Infect. 21 (4), 337–343. doi: 10.1016/j.cmi.2014.11.022
Trecarichi, E. M., Pagano, L., Martino, B., Candoni, A., Di Blasi, R., Nadali, G., et al. (2016). Bloodstream infections caused by klebsiella pneumoniae in onco-hematological patients: clinical impact of carbapenem resistance in a multicentre prospective survey. Am. J. Hematol. 91 (11), 1076–1081. doi: 10.1002/ajh.24489
Tumbarello, M., Trecarichi, E. M., Caira, M., Candoni, A., Pastore, D., Cattaneo, C., et al. (2012). Derivation and validation of a scoring system to identify patients with bacteremia and hematological malignancies at higher risk for mortality. PloS One 7 (12), e51612. doi: 10.1371/journal.pone.0051612
van den Bosch, C. H., van Woensel, J., van de Wetering, M. D. (2021). Prophylactic antibiotics for preventing gram-positive infections associated with long-term central venous catheters in adults and children receiving treatment for cancer. Cochrane Database System. Rev. 10 (10), Cd003295. doi: 10.1002/14651858.CD003295.pub4
Vinholt, P. J. (2019). The role of platelets in bleeding in patients with thrombocytopenia and hematological disease. Clin. Chem. Lab. Med. 57 (12), 1808–1817. doi: 10.1515/cclm-2019-0380
Woo, Y., Son, T., Song, K., Okumura, N., Hu, Y., Cho, G. S., et al. (2016). A novel prediction model of prognosis after gastrectomy for gastric carcinoma: development and validation using Asian databases. Ann. Surg. 264 (1), 114–120. doi: 10.1097/SLA.0000000000001523
Xiao, H., Tang, Y., Cheng, Q., Liu, J., Li, X. (2020). Risk prediction and prognosis of invasive fungal disease in hematological malignancies patients complicated with bloodstream infections. Cancer Manage. Res. 12, 2167–2175. doi: 10.2147/CMAR.S238166
Yang, Z., Hou, Y., Lyu, J., Liu, D., Chen, Z. (2020). Dynamic prediction and prognostic analysis of patients with cervical cancer: a landmarking analysis approach. Ann. Epidemiol. 44, 45–51. doi: 10.1016/j.annepidem.2020.01.009
Zhao, Y., Lin, Q., Liu, L., Ma, R., Chen, J., Shen, Y., et al. (2020). Risk factors and outcomes of antibiotic-resistant pseudomonas aeruginosa bloodstream infection in adult patients with acute leukemia. Clin. Infect. Dis. 71 (Suppl 4), S386–Ss93. doi: 10.1093/cid/ciaa1522
Zhao, Y., Lin, Q., Zhang, T., Zhen, S., Wang, J., Jiang, E., et al. (2022). Pseudomonas aeruginosa bloodstream infection in patients with hematological diseases: clinical outcomes and prediction model of multidrug-resistant infections. J. Infect. 86 (1), 66–117. doi: 10.1016/j.jinf.2022.08.037
Keywords: bloodstream infection, hematological malignancy, nomogram, model, microbiology, 30-day mortality, risk factor
Citation: Wang J, Wang M, Zhao A, Zhou H, Mu M, Liu X and Niu T (2023) Microbiology and prognostic prediction model of bloodstream infection in patients with hematological malignancies. Front. Cell. Infect. Microbiol. 13:1167638. doi: 10.3389/fcimb.2023.1167638
Received: 18 February 2023; Accepted: 14 June 2023;
Published: 30 June 2023.
Edited by:
Juan Carlos Rodriguez Diaz, Hospital General Universitario de Alicante, SpainReviewed by:
İlhami Çelik, Department of Infectious Diseases, TürkiyeCopyright © 2023 Wang, Wang, Zhao, Zhou, Mu, Liu and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Niu, dGluZ25pdUBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.