
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 14 September 2023
Sec. Microbiome in Health and Disease
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1166366
This article is part of the Research TopicThe Influence of Gut Microbiota and Their Metabolites on Urogenital System DiseasesView all 5 articles
 Qi Wang1,2†
Qi Wang1,2† Pengjiao Wang1,2†
Pengjiao Wang1,2† Minyan Yuan1,2
Minyan Yuan1,2 Min Zhang1,2
Min Zhang1,2 Shuo Zhang2,3
Shuo Zhang2,3 Xiaodong Sun1,2
Xiaodong Sun1,2 Leyuan Shang1,2
Leyuan Shang1,2 Yujie Liu1,2
Yujie Liu1,2 Yanni Zhao1,2
Yanni Zhao1,2 Nan Jiang4
Nan Jiang4 Xiuli Gao1,2*
Xiuli Gao1,2*Background: Mixed vaginitis is the infection of the vagina by at least two different pathogens at the same time, both of which contribute to an abnormal vaginal environment leading to signs and symptoms. Baicao Fuyanqing suppository (BCFYQ) is a Miao ethnomedicine, used to treat various vaginitis. The aim of this study was to investigate the efficacy and possible mechanism of BCFYQ in the treatment of mixed vaginitis based on 16S rRNA high-throughput sequencing and metabonomics.
Methods: Escherichia coli and Candida albicans were used to establish mixed vaginitis model in SD rats. Three groups of low, medium and high doses (0.18/0.36/0.64 g.kg-1) were established, and administered vaginally once a day for 6 consecutive days. After the last administration, vaginal pH and IL-1β, IL-2, IL-13 and IgA levels were measured, and the vaginal tissue was examined pathologically. In addition, the vaginal flora was characterised by 16S rRNA, and endogenous metabolites in the vaginal tissue were detected by UHPLC-Q-Exactive MS.
Results: Compared with the model group, BCFYQ can reduce the vaginal pH of rats, make it close to the normal group and improve the damaged vaginal epithelial tissue. The results of ELISA showed that BCFYQ decreased the levels of IL-1 β and IL-2 and increased the levels of IL-13 and IgA (P<0.05). In addition, BCFYQ may increase the abundance of vaginal flora, especially Lactobacillus. The differential metabolite enrichment pathway suggests that the therapeutic mechanism of BCFYQ is mainly related to lipid metabolism and amino acid metabolism.
Conclusion: Our research shows that BCFYQ has a good therapeutic effect on mixed vaginitis. It repairs the damaged vaginal mucosa by regulating the vaginal flora and lipid metabolism disorders to improve the local immune function of the vagina and inhibit the growth and reproduction of pathogens.
Vaginitis is a common and diverse gynecological disease. It is a general term for a variety of vaginal mucosal inflammatory diseases caused by different pathogens invading the vagina. It is mainly divided into aerobic vaginosis (AV), bacterial vaginosis (BV), trichomonad vaginitis(TV) and Vulvovaginal Candidiasis (VVC). Mixed vaginitis is the infection of the vagina by at least two different pathogens at the same time, both of which contribute to an abnormal vaginal environment leading to signs and symptoms (Tumietto et al., 2019). Clinically more frequent mixed infection with bacteria and Candida albicans (Benyas and Sobel, 2022). Vaginitis, if not treated in time, leads to a decline in a woman’s local immune function and subsequent infection with other gynecological diseases such as cervicitis, pelvic inflammatory disease, cervical erosion, etc. Currently, antibiotics are the main treatment for vaginitis. However, they also kill the symbiotic bacteria, leading to an imbalance of the vaginal flora and also to antibiotic resistance of pathogens (Stojanović et al., 2012). Therefore, the search for effective alternative therapies with few side effects has become a new field of research.
The female vaginal microecology is one of the most important microecological systems in the human body, and it is directly or indirectly related to various gynecological diseases such as vaginitis. More and more researchers are advocating the treatment of vaginitis by improving the vaginal microecology. In recent years, probiotics used to combat vaginal dysbiosis have shown great promise in restoring normal vaginal flora, including preventing the loss of beneficial bacteria (Das et al., 2022). Therefore, in this study, vaginal microbial flora was used as a vital indicator, and high-throughput sequencing of 16S rRNA gene to investigate whether BCFYQ could treat vaginitis by improving vaginal microecology.
Traditional Chinese medicine suppositories for the treatment of vaginitis first appeared in the Western Han Dynasty in China. Studies have shown that traditional Chinese medicine suppositories have unique effects in the treatment of various gynecological inflammations, have fewer side effects, do not easily to cause drug resistance, and have advantages in restoring normal physiological functions of patients and eliminating potential causes (Ma et al., 2017). BCFYQ is a traditional Chinese medicine suppository developed from traditional Miao ethnomedicine (registration number: Z20026597). Its main ingredients are Sophora flavescens (0.64 g), Stemona japonica (0.32 g), Cnidium monnieri (L.) Cuss. (0.32 g), Callicarpa macrophylla (0.32 g), Agrimonia Pilosa (0.32 g), Alum (0.01 g), Borneol (0.01 g), Camphor (0.06 g), and Boric acid (0.05 g). Sophora flavescens has been used for thousands of years to prevent and treat common ailments such as eczema, diarrhoea, vaginal itching and colitis (He et al., 2015). Studies have shown that the main active ingredient of Stemona japonica is alkaloid, which has the functions of anthelmintic, insecticidal, antitussive and anti-asthmatic, antitumor and antibacterial (But et al., 2012). Recent studies have shown that Cnidium monnieri (L.) Cuss. has a variety of pharmacological activities, mainly in anti-inflammatory, antioxidation and antibacterial itching (Tsai et al., 2015; Z. Wang and Shen, 2017). Callicarpa macrophylla has a variety of biological activities such as analgesic, anti-inflammatory, antipyretic, antifungal, antiarthritic, and antitumor (Chung et al., 2014; Feng et al., 2017; Z. H. Wang et al., 2019). Agrimonia pilosa has a long history of medicinal use and is also widely used for gynecological disorders (Y. M. Lee et al., 2012). TIAN Wen-yang’s review summarised the clinical studies on BCFYQ in the treatment of vaginitis, among which there were 2 literatures on the use of BCFYQ alone in the treatment of instructions for the indication of internal vaginitis, and they used case series observation and case control study methods respectively. The results showed that the drug alone was effective in treating vaginitis, and the efficacy was better than metronidazole suppository (Wen-yang, 2016). However, there is currently no research on the therapeutic effect of BCFYQ on mixed vaginitis, and its mechanism of action in the treatment of vaginitis has not yet been elucidated.
In this study, we investigate the therapeutic effect of BCFYQ on mixed vaginitis by modeling mixed vaginitis rats with Escherichia coli and Candida albicans. Finally, combined with tissue metabolomics and 16S rRNA high-throughput sequencing methods, we sought to determine its mechanism of action in the treatment of vaginitis.
BCFYQ (Guizhou Changsheng Pharmaceutical Co., Ltd., batch number: 06210401); Estradiol Benzoate Injection (Shanghai Quanyu Biotechnology Animal Pharmaceutical Co., Ltd., batch number: 210301).
Escherichia coli (BNCC133264) and Candida albicans (BNCC186382) used in this study were purchased from Beina Chuanglian Biotechnology Co., Ltd., Escherichia coli was cultured in nutrient broth medium and placed in a 37°C incubator; Candida albicans was cultured in YM medium and placed in a 28°C incubator. Before use, were diluted to a concentration of 2~3×108 CFU/mL, respectively, and then mixed to prepare a bacterial suspension (Escherichia coli: Candida albicans, 1:1).
Female Sprague-Dawley (SD) rats, weighing 180-200 g, SPF grade, were obtained from the Animal Center of Guizhou Medical University (No.2000350). Animal experiments were approved by the Animal Experiment Center of Guizhou Medical University and the Animal Ethics Committee of Guizhou Medical University, and were conducted according to the guidelines of the “National Laboratory Animal Welfare and Ethical Requirements for Animal Experiments” (License No. SYXK (Qian) 2018-0001). Briefly, 40 rats were housed in an animal room with a relative humidity of 60 ± 5% and a 12 h light/dark cycle at 22 ± 2°C. After 1 week of adaptive feeding, 32 SD female rats were randomly selected and injected subcutaneously with 0.1 mL of 2 mg/mL estradiol benzoate hormone for 6 days to induce false estrus. 32 SD female rats were injected daily for 6 days with 20 μL of the mixed bacterial suspension through the vaginal opening, and then randomly divided into the model group and three treatment groups (Low dose group: 0.184 g/kg; Medium dose group: 0.364 g/kg; High dose group: 0.64 g/kg). The treatment group was given a 6-days course, the Model group was given the same amount of normal saline, and the Normal group (no infection and no medication) was established. There were eight rats in each group.
Six days after treatment, the vaginal pH of all rats was measured using pH test paper (Hangzhou Shisan Technology Co., Ltd.).
In a sterile environment, rat vaginal secretions were collected with sterile cotton swabs, immediately frozen in liquid nitrogen, and stored at -80°C.The vagina of each rat was lavaged with 0.5 mL of 0.9% sterile saline, and the vaginal lavage fluid was collected, immediately frozen with liquid nitrogen, and stored at -80°C.
After the experiment, the rats were sacrificed in a sterile environment, and the vaginal tissues were separated. Part of this tissue was immediately stored in liquid nitrogen and then at −80°C until further use for expression studies. The remainder of the tissue was fixed in buffered formalin (4%) for histopathological studies.
The frozen vaginal lavage fluid was placed at room temperature (24 ± 2°C) for 30 min. It was centrifuged at 1000 g for 10 minutes at 4°C. The supernatant was collected and set aside. According to the operating steps of the ELISA kit (Xinbosheng, Neobioscience, Shenzhen, China), the double antibody sandwich method was used to detect interleukin-1β (IL-1β), interleukin-2 (IL-2), interleukin-13 (IL-13) and secretory immunoglobulin A (sIgA) in vaginal lavage fluid.
Vaginal tissues fixed in 4% formalin were collected, fixed tissues were embedded in paraffin, sectioned at 3-5 μm thickness, and stained with hematoxylin-eosin (H&E) for histopathological examination (Gilbert et al., 2013). Histological examination was performed under a light microscope (BX43, Olympus, Japan).
Using OMEGAD kits (Omega Bio-Tek, Norcross, GA, USA), extract genomic DNA from cotton swab samples according to the manufacturer’s instructions. The purity and concentration of DNA were detected by Thermo NanoDrop One. Concentration and purity were measured using the NanoDrop One (Thermo Fisher Scientific, MA, USA). PCR amplification of the V3-V4 region of the bacterial 16S rRNA gene was performed using forward primer 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and reverse primer 806R (5′-GGACTACHVGGGTWTCTAAT-3′). PCR products were mixed in equidensity ratios according to the GeneTools analysis Software (version4.03.05.0, SynGene). The mixture of PCR products was then purified using the E.Z.N.A. Gel Extraction Kit (Omega, USA). The library was sequenced on an Illumina Nova6000 platform and 250 bp paired-end reads were generated (Guangdong Magigene Biotechnology Co., Ltd., Guangzhou, China).
Based on the tissue sample processing method established by Velenosi et al. (Velenosi et al., 2016), we slightly modified it. Briefly, 100 mg of vaginal tissue was collected, 500 μL of water, acetonitrile and methanol (1:2:2) was added, vortexed and mixed for 5 min, ultrasonicated for 10 min, placed on ice for 30 minutes and centrifuge at 4°C for 15 minutes at 26775g.The supernatant was passed through a 0.22 μm filter. Samples were analyzed by UHPLC-Q-Exactive MS. The quality control (QC) sample used to assess the analytical variance of the data was mixed from each biological sample in the same volume.
Normally distributed data are expressed as mean ± standard deviation (X ± SD). Data were analysed and visualised using SPSS 19.0 (IBM, Chicago, USA) and GraphPad Prism 8.0 (GraphPad Software, CA, USA). Statistical analysis was performed using one-way analysis of variance (ANOVA). P values less than 0.05 indicate statistical significance, where *P<0.05, **P<0.01. The differences between indices of alpha diversity analysis were analyzed by Kruskal-Wallis rank sum test. Use R to construct heat map and PCoA.
After 6 days of administration, the vaginal pH values of the three administration groups were significantly different from that of the Model group, with statistical significance (Table 1, P<0.05), and returned to the level of the Normal group. This indicates that BCFYQ can restore the vaginal pH to normal level in rats with mixed vaginitis.
Table 1 Data were analyzed using one-way analysis of variance (ANOVA). Results are expressed as mean x ± SD(N=8), compared with Normal group #P<0.05,compared with Model group *P<0.05.
Pathological studies of vaginal tissue were performed by H&E staining. The results showed that under the infection of Escherichia coli and Candida albicans, vaginal epithelial tissue showed edema and hyperplasia, a large number of epithelial cells were shed, and a large number of inflammatory cells were infiltrated. Compared with the Model group, the administration groups of the three doses had different degrees of improvement, and they approached the Normal group (Figure 1).
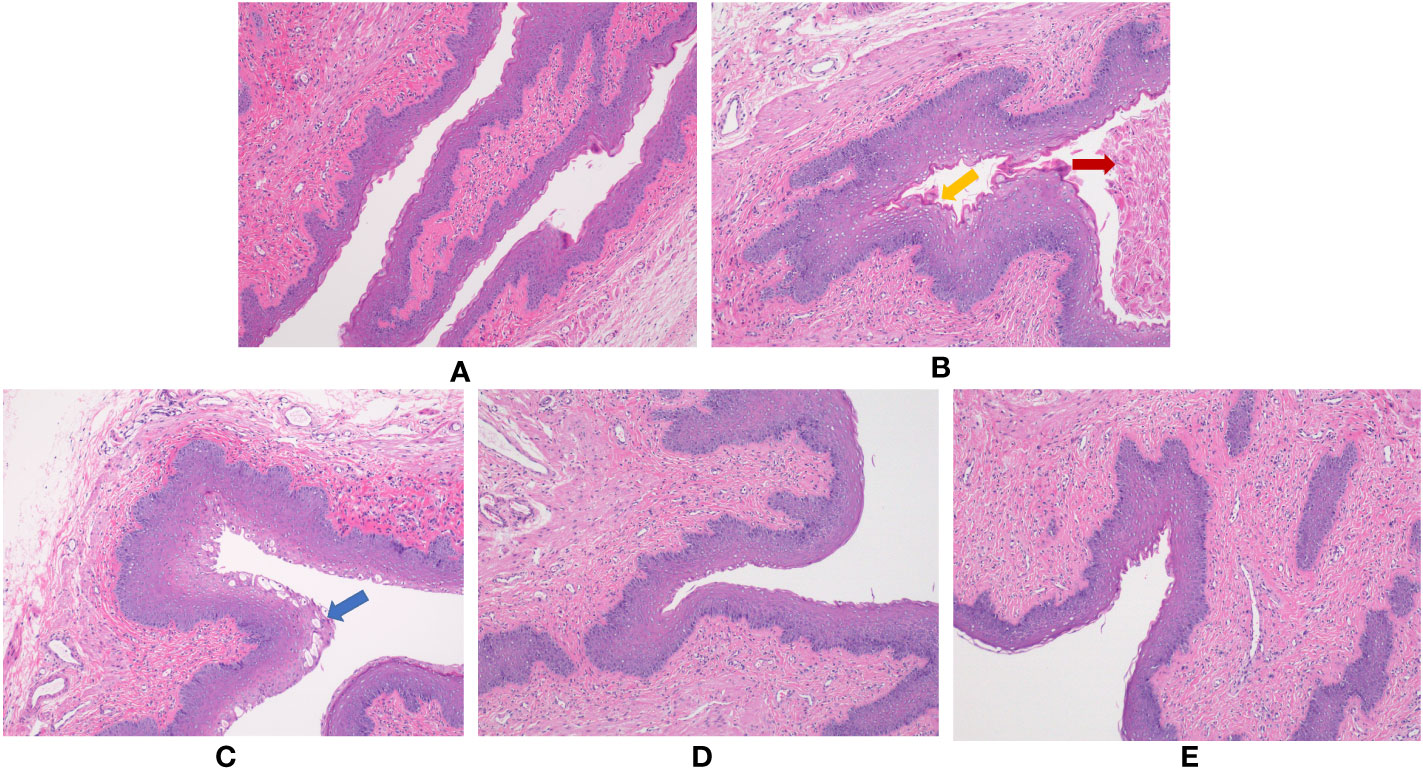
Figure 1 Effect of BCFYQ on vaginal tissue in rats with mixed vaginitis. (A) Normal group. (B) Model group. (C) Low-dose group. (D) Medium-dose group. (E) High-dose group. The red arrow indicates shedding of vaginal epithelial cells. The yellow arrow indicates edema and hyperplasia of vaginal epithelial tissue. The blue arrow indicates ulceration of vaginal epithelial tissue. All images were taken at 100x magnification.
The results showed that the pro-inflammatory cytokines IL-1β and IL-2 in the Model group were significantly increased compared with those in the Normal group (56.48 ± 9.45 vs. 45.71 ± 5.68, P<0.01; 1200.45 ± 92.44 vs. 1057.59 ± 121.14, P<0.05). The levels of pro-inflammatory cytokines IL-1β and IL-2 were decreased in the BCFYQ-treated group compared to the Model group, with the effect being more pronounced in the Medium-dose group. (40.03 ± 9.00, P <0.01; 858.25 ± 76.00, P <0.01) (Figures 2A, B). The level of IL-13 in the Model group was significantly decreased compared with that in the Normal group (33.89 ± 1.08 vs. 42.35 ± 3.12, P<0.01), and the level of IL-13 in the treatment group was increased, and there was a significant difference between the Middle-dose group and the Model group (39.48 ± 4.55, P<0.01) (Figure 2C).
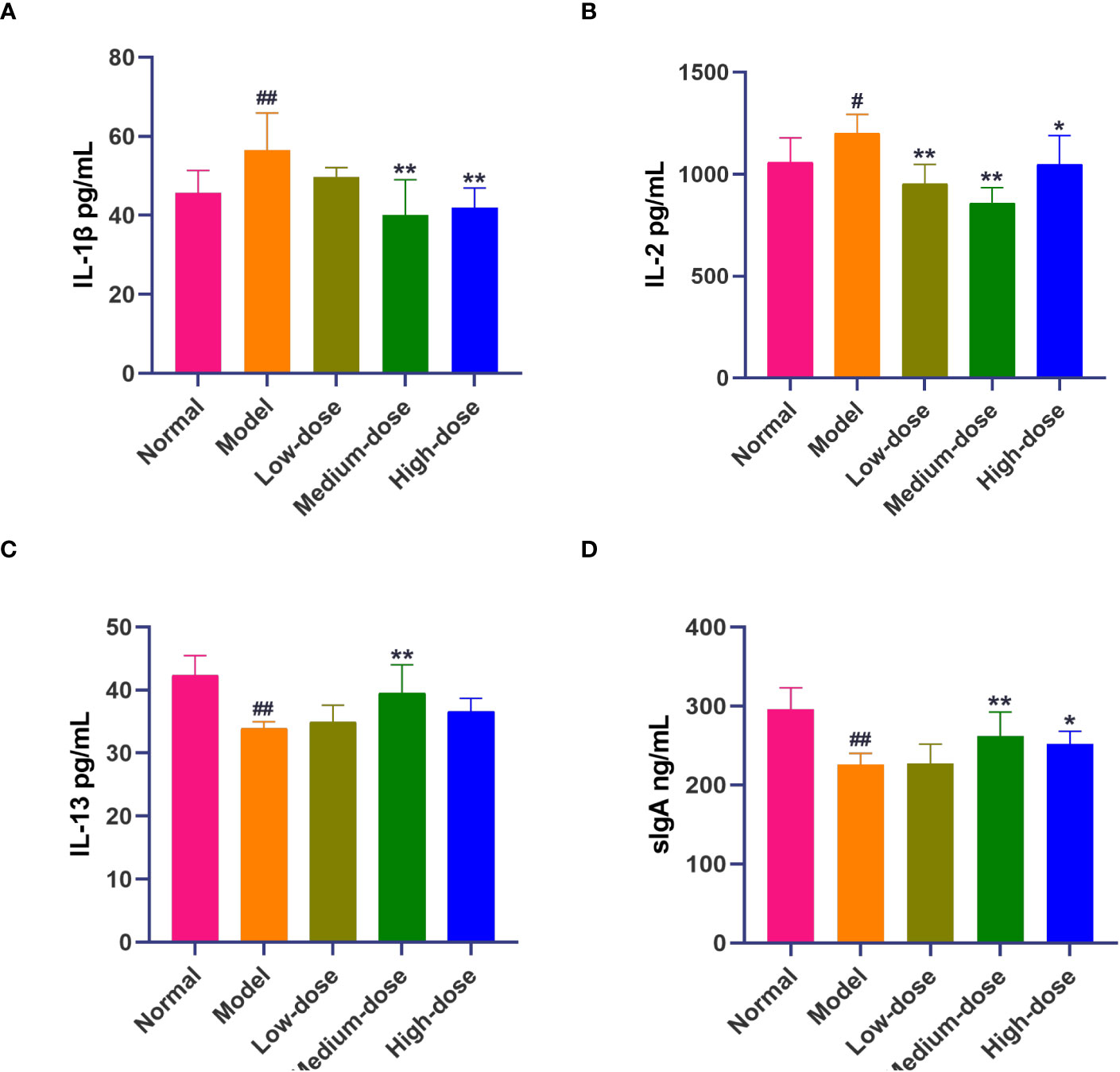
Figure 2 Effect of BCFYQ on cytokine levels in vaginal lavage fluid of rats with mixed vaginitis. (A) Differences in IL-1β levels. (B) Differences in IL-2 levels. (C) Differences in IL-13 levels. (D) Differences in sIgA levels. Each value is presented as the mean ± SD (n=8). Compared with the Normal group, #P<0.05, ##P<0.01; compared with the Model group, *P<0.05, **P<0.01.
The level of secreted IgA (sIgA) in the Model group was significantly lower than that in the Normal group (226.28 ± 13.84 vs. 295.78 ± 27.3, P<0.05). In the treatment group, the Middle-dose group and the High-dose group were significantly higher than the Model group (262.29 ± 30.34, P<0.01; 252.21 ± 15.84, P<0.05) (Figure 2D).
Six samples were randomly selected from Normal, Model and BCFYQ groups for detection. To verify whether the amount of sequencing in this study was sufficient to reflect the diversity of the original microorganisms, the 16S rRNA sequencing results were analyzed for α-diversity (e.g., Chao1, Richness) based on the 97% similarity level. The Chao index reflects community richness, while Richness index reflects species richness. The statistical analysis of α diversity index showed that the community richness and species richness of the Normal group and the BCFYQ group were higher than those of the Model group (Figures 3A, B). Beta diversity refers to the comparison of biodiversity among different samples, which is represented by Principal Coordinate Analysis (PCoA) and hierarchical cluster analysis (Figures 3C, D). It can be seen that, the vaginal microbiota composition distribution between BCFYQ group and Model group is clearly separated, indicating that BCFYQ group can significantly improve the vaginal microbiota in mixed vaginitis rats. It is worth noting that the microbial composition distribution of the BCFYQ group and the Normal group almost overlapped, indicating that the BCFYQ group had a tendency to restore the disturbed vaginal flora of the mixed vaginitis rats to the normal level.
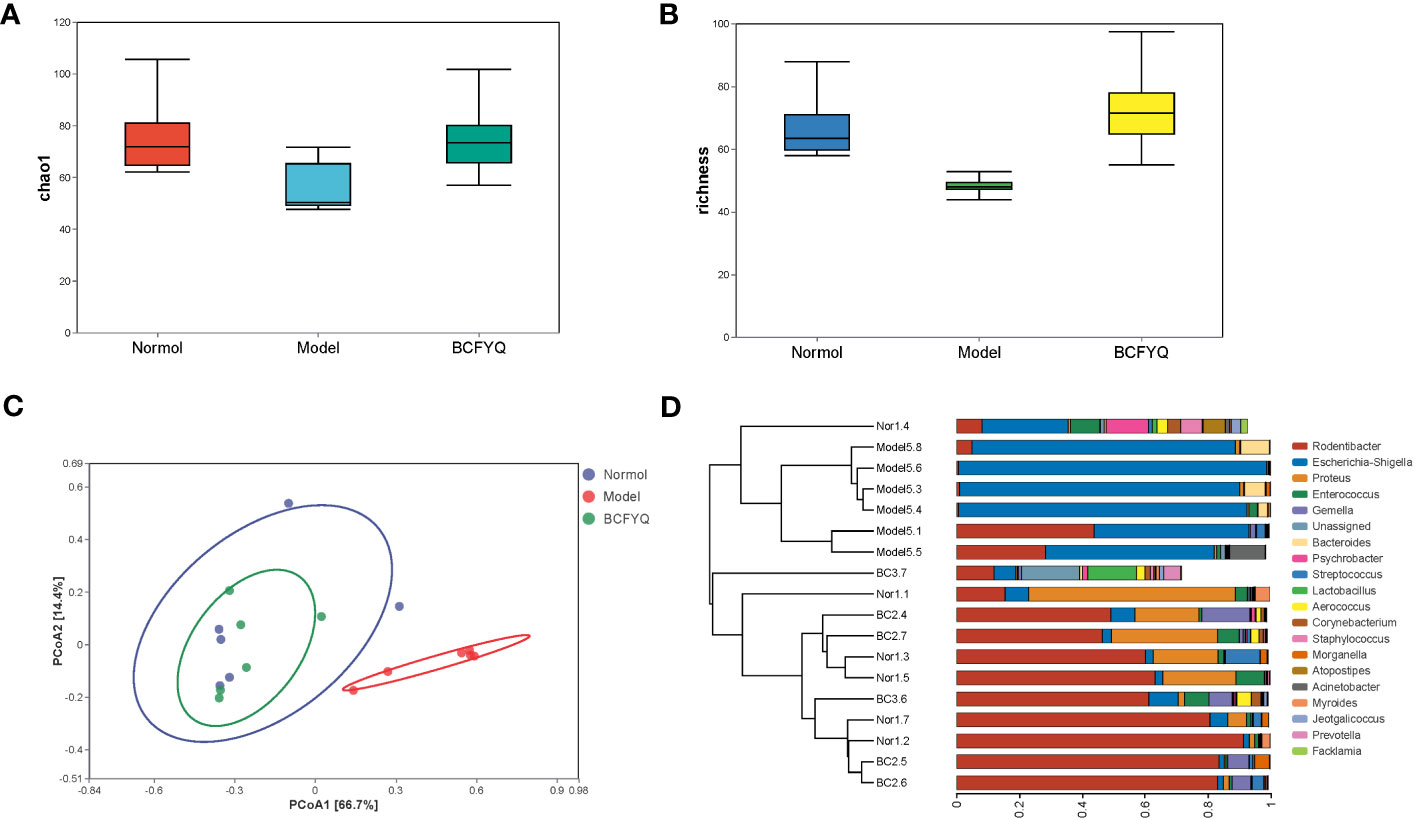
Figure 3 Analysis of alpha diversity. (A) The α-diversity indexes of Chao. (B) The α-diversity indexes of Richness. (C) Principal component analysis (PCoA) of vaginal flora microbiota based on Normal, Model and BCFYQ grouping. (D) Hierarchical clustering Analysis at Genus level.
Figures 4A, B show the results of phylum and genus level species annotation analysis for the three groups revealed by 16S rRNA sequencing. The results showed the top 4 most abundant bacteria at phylum level and the top 20 bacteria at genus level. At the phylum level, Proteobacteria and Firmicutes were the most important parts of the rat vaginal flora, accounting for the main relative abundance in all samples. Compared with the Model group, the BCFYQ group inhibited Proteobacteria and Bacteroides, and increased the abundance of Firmicutes and Actinomycetes. As can be seen from Figure 4E, compared with the Normal group, the BCFYQ group improved the Model group and restored the flora abundance to the level of the Normal group. We selected the top 20 genera at the genus level. The remaining species are grouped into Others, while Unassigned represents those species that are not classified and annotated. Figure 4B shows that Escherichia-Shigella was absolutely dominant in the Model group with a relative abundance of 0.8, while the sum of other bacteria only reached about 0.2, indicating that the vaginal flora of the rats in the Model group was disturbed by modelling. On the contrary, the BCFYQ group significantly reduced Escherichia-Shigella, Bacteroides and Acinetobacter compared to the Model group, but increased the relative abundance of Lactobacillus and Streptococcus (Figure 4D).
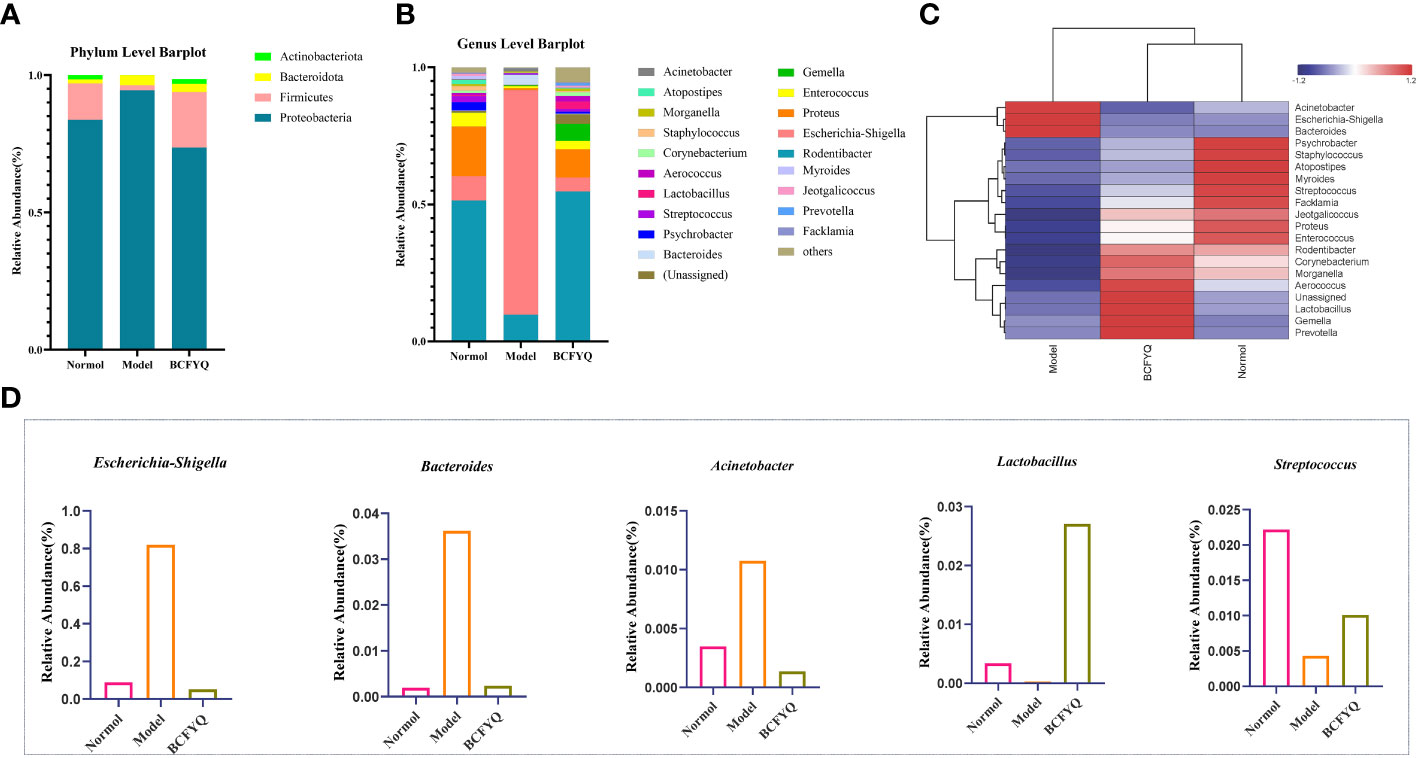
Figure 4 Analysis of beta diversity. (A) Relative percentages of community richness at the phylum level for the three groups. (B) Relative percentages of community richness at the genus level for the three groups. (C) Heat map analysis of communities at the genus level. (D) Comparison of relative abundance of Escherichia-Shigella, Bacteroides, Acinetobacter, Lactobacillus, and Streptococcus in the three groups.
According to the species annotation and abundance information of all samples at the genus level, the top 20 genera in abundance were selected, and clustering was performed at the species and sample levels according to their abundance information in each group of samples. Plot as a heatmap to identify species that aggregate at higher or lower levels in the sample (Figure 4C). Our results showed that Escherichia-Shigella, Acinetobacter and Bacteroides were more abundant in the Model group than other groups, with Z>1. Lactobacillus, Blautia, Coprococcus and Prevotella were more abundant in BCFYQ group with Z>1. The abundance of Psychrobacter, Staphylococcus, and Streptococcus in the Normal group was higher than that in the other groups, Z>1. This is consistent with our previous observation that both the diversity and richness of the BCFYQ and Normal groups were better than those of the Model group. The genera with higher abundance in the Model group were all opportunistic pathogens. This also indicates that the Model group was successfully infected and disrupted its flora.
As the vaginal flora changes, it will inevitably affect the endogenous metabolites of the vaginal tissue. We further explored the beneficial mechanism of BCFYQ by screening differential metabolites. Pairwise comparisons were established using the supervised mode OPLS-DA, and differences in metabolic profiles between the Model group and the BCFYQ group were observed. The OPLS-DA score plot showed that the BCFYQ-treated samples could be well separated from the Model group (Figure 5A). We created a heatmap by converting the concentration values of the 30 differential metabolites to Z-scores by a normalized Z-score transformation (Figure 5B). The results showed that BCFYQ had a significant effect on vaginal tissue metabolism in mixed vaginitis. Among them, several metabolites such as acrylic acid, arachidonic acid, LysoPC (P-18:0), and amylcinnamaldehyde were significantly decreased after BCFYQ treatment. In addition, the treatment group increased levels of metabolites such as betaine, phosphocholine, dethiobiotin, and N-ribosylnicotinamide. We can preliminarily judge that the therapeutic effect of BCFYQ should be closely related to amino acid metabolism and lipid metabolism. After using Metaboanalyst to analyze the metabolic pathways of the above potential markers, the related pathways with influence value>0.1 were obtained according to the pathway topology analysis. The results showed that there were 7 significant pathways, which were related to several pathways including Lactose Synthesis, Phospholipid Biosynthesis, and Betaine Metabolism (Figure 5C).
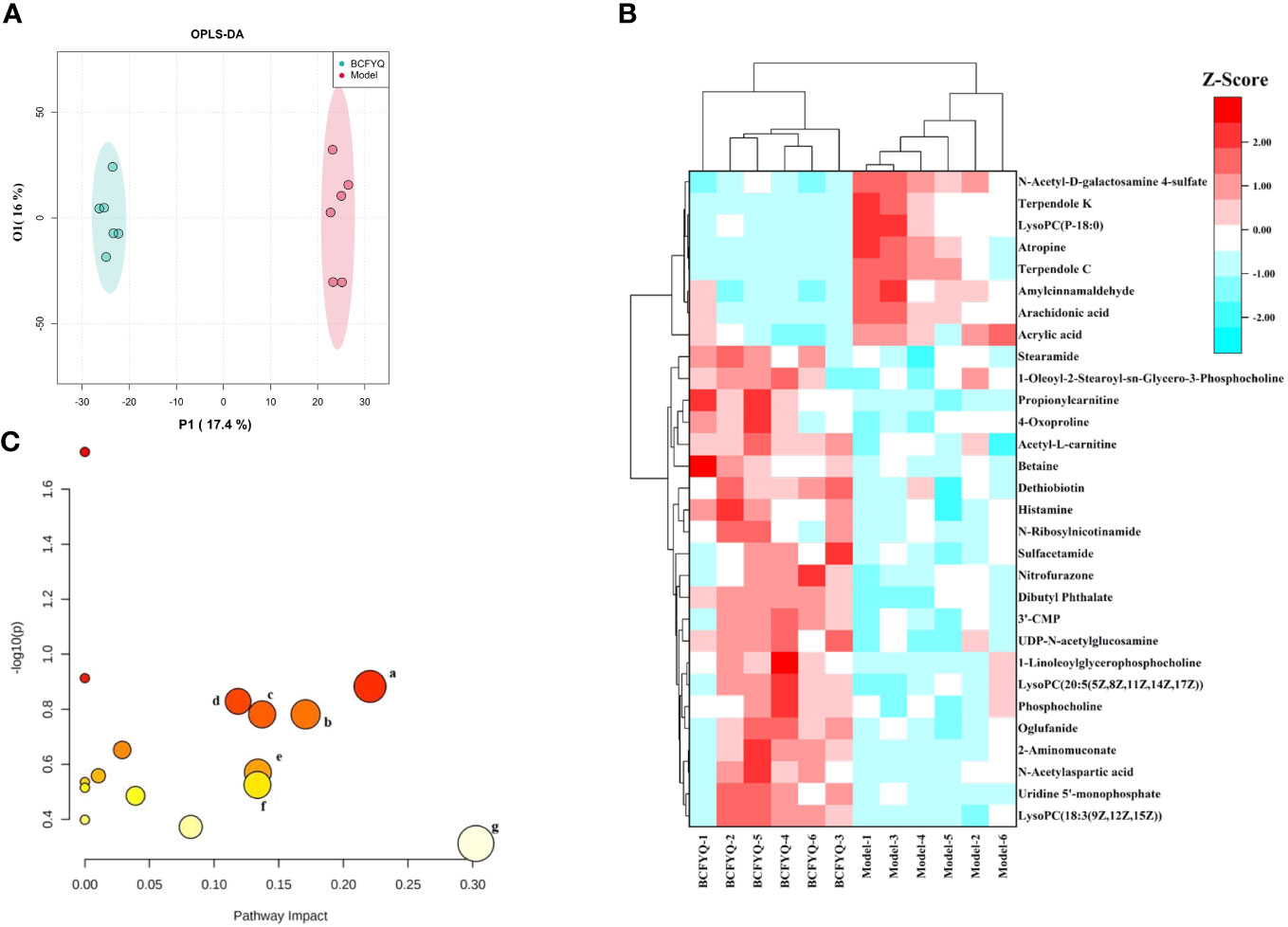
Figure 5 Effects of BCFYQ on vaginal metabolites in rats with mixed vaginitis. (A) OPLS-DA plots with the scores of the two principal components. (B) Heatmap of 30 differential metabolites. A standardized Z-score conversion was applied to convert concentration values to Z-scores prior to heatmap analysis. (C) Metabolic pathways of BCFYQ in the treatment of mixed vaginitis in rats (Impact value > 1). (A) Lactose Synthesis. (B) Phosphatidylcholine Biosynthesis. (C) Betaine Metabolism. (D) Alpha Linolenic Acid and Linoleic Acid Metabolism. (E) Amino Sugar Metabolism. (F) Histidine Metabolism. (G) Arachidonic Acid Metabolism.
We used Spearman rank correlation analysis to analyze the correlations between 30 differential metabolites and 29 altered vaginal microbiota (Figure 6). It can be seen from the figure that a variety of metabolites are significantly associated with vaginal flora. The results showed that Lactobacillus was negatively correlated with acrylic acid, and positively correlated with acetyl-L-carnitine and Dethiobiotin. Escherichia-Shigella was negatively correlated with Phosphocholine, Propionylcarnitine and Dethiobiotin, and positively correlated with amylcinnamaldehyde and arachidonic acid. We also observed that most of the metabolites were negatively correlated with Escherichia-Shigella and positively correlated with other vaginal flora. The results further suggest that BCFYQ alters vaginal endogenous metabolites by improving vaginal flora, thereby improving its inflammatory response or enhancing immune response.
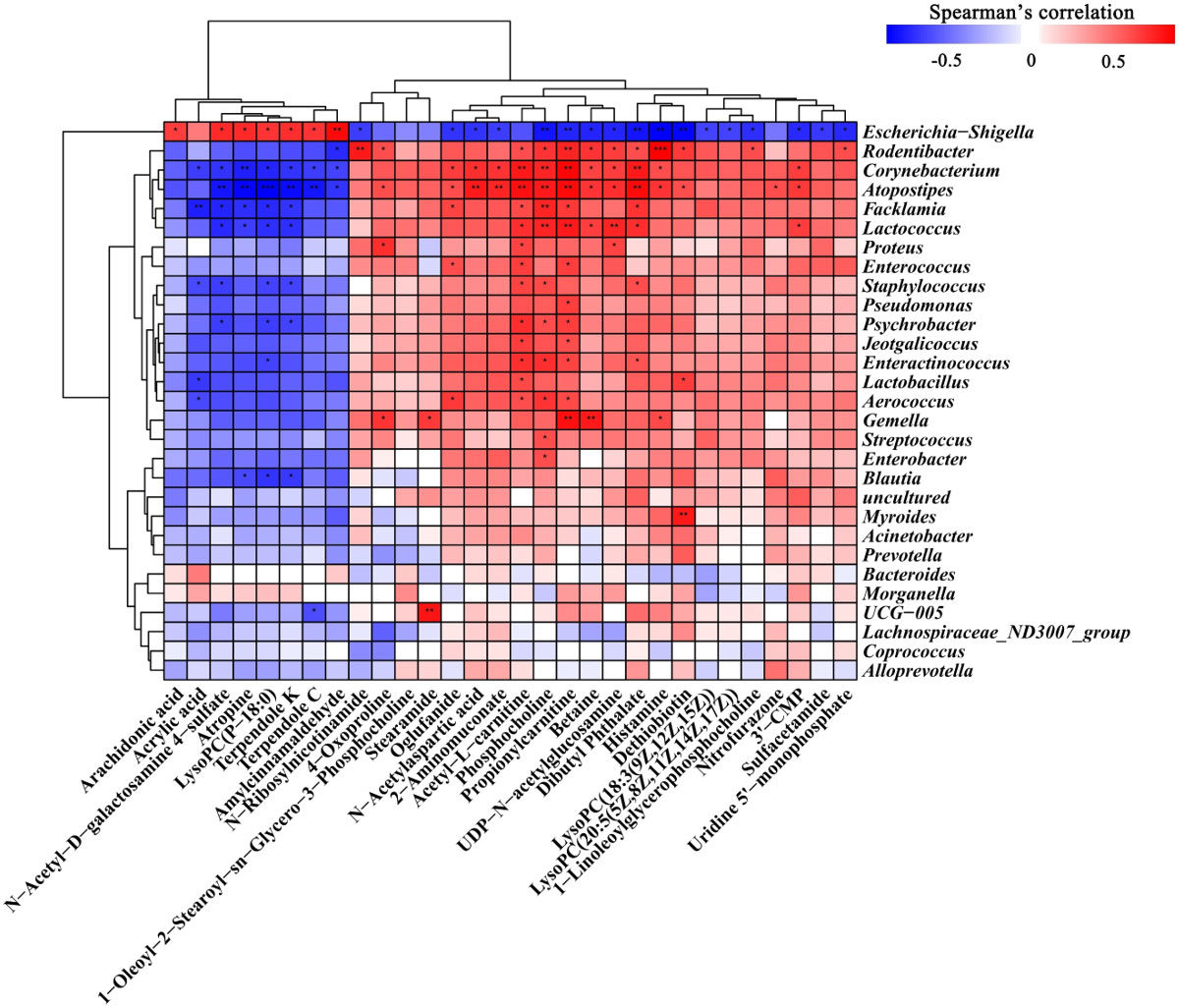
Figure 6 Spearman correlation between 29 altered vaginal microbiota and 30 metabolites. Red means positive correlation, blue means negative correlation. No identification means that the relevant confidence is less than 95%, which is not statistically significant. Sig < 0.05 is expressed by *, sig < 0.01 is expressed by **, and sig < 0.001 is expressed by ***, suggesting that there is a significant correlation between them.
According to a literature review evaluating the prevalence of mixed vaginitis (Rivers et al., 2011), the proportion of mixed vaginitis ranges from 4.44% to 35.06%. Currently, the mixed infection rates associated with AV are most notable among Chinese women. The mixed infection rates of AV+BV, AV+VVC and AV+TV are 36.9%, 38.1% and 25%, respectively (Fan et al., 2013). Therefore, the mixed vaginitis model used in this study is a mixed infection of Escherichia coli and Candida albicans. Estrogen is widely used to establish mouse models of bacterial infection. In addition to providing a high degree of stability, these models can stimulate successful colonization of the administered strains (Jerse et al., 2011). The pathogenesis of mixed vaginitis has not been fully elucidated. Some researchers have suggested that the pathogenesis of mixed vaginitis may be related to the mixed biofilm formed by bacteria and fungi (Beaussart et al., 2013). Lohse et al. (Lohse et al., 2018) proposed that the microbial populations in the host interact with each other in a synergistic or antagonistic form, so the pathogenesis of mixed vaginitis is also related to the imbalance of vaginal microbiota. Antibiotics are commonly used in clinical treatment of mixed vaginitis, but while killing pathogens, they will also kill beneficial bacteria, causing vaginal flora imbalance and leading to disease recurrence. Therefore, it has become a difficult problem to find drugs that are effective in treating vaginitis with less side effects. This study selected BCFYQ, a traditional Chinese medicine preparation, to explore its efficacy in the treatment of mixed vaginitis, and to make a basic exploration of its therapeutic mechanism.
Sophora flavescens, one of the main components of BCFYQ, has a strong inhibitory effect on bacteria and fungi by affecting the formation of biofilms, destroying cell walls, interfering in protein synthesis, and inhibiting bacterial division (Z. Li et al., 2022). Sophora flavescens is often prepared as a gel preparation alone to treat bacterial vaginosis, and it has been shown to have good anti-inflammatory activity (H. D. Zhao et al., 2016). In addition, Stemona japonica, Cnidium monnieri (L.) Cuss., Callicarpa macrophylla and Agrimonia pilosa have certain antibacterial and anti-inflammatory effects. It is worth noting that BCFYQ also contains boric acid. Some studies have reported that boric acid can destabilize the mycelial cytoskeleton and inhibit the invasive growth of Candida albicans (Pointer et al., 2015). Boric acid is commonly used as a pH regulator in industrial and pharmaceutical studies, so we infer that this is one of the reasons for lowering vaginal pH.
Studies have shown that the local immunity of the vagina plays a more important role than the systemic immunity, and its occurrence is related to local immune dysfunction (Budilovskaya et al., 2020). Th1/Th2 polarization is a key link in the regulation of immune response, and cytokines are the most important factors affecting the differentiation of Th cells. Th cells are mainly divided into Th1 and Th2. IL-2 is a pro-inflammatory factor secreted by Th1 cells, which can enhance the lethality of natural killer cells and mononuclear macrophages. When the cellular immune function of the body changes, the concentration of IL-2 will change. IL-13 is an anti-inflammatory molecule secreted by Th2 cells, which can stimulate the proliferation and differentiation of human B cells and secrete immunoglobulin, and plays an important role in humoral immunity. Our ELISA results showed that the secretion of IL-2 increased and the secretion of IL-13 decreased in rats infected with mixed bacteria. This suggests that mixed vaginitis may be related to Th1/Th2 immune imbalance. After BCFYQ treatment, the levels of IL-2 and IL-13 tended to return to normal levels. We can speculate that BCFYQ can reduce inflammation by inhibiting the secretion of proinflammatory factor IL-2 and promoting the secretion of anti-inflammatory factor IL-13, so as to re-balance Th1/Th2 immunity. IL-1β is one of the important inflammatory mediators, as well as a pyrogenic component, which plays a role in heating and mediating inflammation. It mainly plays a regulatory role in the activation of cellular immunity. Our results showed that BCFYQ did reduce the increase of IL-1β caused by infection, which was consistent with the results of Marconi et al. (Marconi et al., 2013).
Although some microorganisms, such as viruses and fungi, are controlled by cellular immunity, sIgA can also attach to the mucosa of the reproductive tract and play an important role in controlling these pathogens (Giraldo et al., 2006). If bacteria and extracellular parasites need to be removed, antibodies (immunoglobulins) are produced, constituting a humoral response (Brodsky, 1991). The results showed that sIgA secretion was significantly reduced in the infected rats compared with the Normal group, indicating that changes in vaginal mucosal tissue would impair IgA secretion, which would lead to changes in local immune function and a significant increase in this mucosal infection (Janković et al., 1995). After BCFYQ treatment, the content of sIgA tended to increase, and the middle dose returned to the normal level. This suggests that BCFYQ can enhance local humoral immunity in the vagina. In conclusion, BCFYQ plays an important role in anti-mixed bacterial vaginal infection through local cellular and humoral immunity.
The vaginal mucosa is the first line of defense for microbial contact, and vaginal epithelial cells increase immune responses by providing a physical barrier. The defense mechanism against this infection is demonstrated by the rapid shedding of epithelial cells, the presence of pattern recognition receptors, and the release of inflammatory cytokines (Balakrishnan et al., 2022). This was consistent with our H&E staining results. The Model group had a large number of vaginal epithelial cells shedding and accompanied by a large number of inflammatory cells invasion, while the three groups of rats after BCFYQ treatment had different degrees of improvement, among which the middle dose had the best effect. This indicates that BCFYQ has a healing effect on the damaged vaginal mucosa after mixed bacterial infection. BCFYQ restores immune function and reduces inflammation by repairing the vaginal mucosa.
The vaginal microbiota of healthy women of childbearing age is composed of aerobic and anaerobic bacteria, mainly Lactobacillus (Larsen and Monif, 2001). A large number of studies have shown that the disorder of vaginal microecology is one of the important factors leading to most gynecological diseases, among which different kinds of vaginitis are the most likely to be caused (Han et al., 2019). We characterized the vaginal microbiota of rats in the three groups by 16S rRNA high-throughput sequencing, and it could be seen that the community abundance and species richness of vaginal microbiota of rats in the Model group was reduced. Among them, pathogenic bacteria such as Escherichia-Shigella were the dominant bacteria, which occupied a dominant position in the whole vaginal microbiota, while beneficial bacteria such as Lactobacillus significantly decreased. This shows that our model is successful. Our results showed that the vaginal microbiota of rats treated with BCFYQ returned to a normal state, and the richness of microbiota at both phylum and genus levels was significantly increased. What is more, treatment with BCFYQ can promote the growth and reproduction of Lactobacillus in the vagina. Lactobacillus has been reported to enhance vaginal immunity to pathogens (Greifová et al., 2017). Therefore, the proliferation of Lactobacillus can not only maintain the vaginal low pH environment, conducive to the growth of beneficial bacteria. It also enhances the immune function of the vagina. We also observed elevated relative abundance of Bacteroides and Acinetobacter in the Model group. It has been reported that Bacteroides are beneficial bacteria in the intestinal tract, but if they escape to other parts of the body outside the intestinal tract, they act as pathogenic bacteria, leading to abscesses and other infections, such as reproductive tract infections(Zafar and Saier, 2021). Acinetobacter is a conditionally pathogenic bacterium that easily causes infection when the body’s resistance decreases. Acinetobacter is one of the major opportunistic pathogens causing nosocomial infections, which can cause skin infections, genitourinary tract infections and so on (C. M. Lee et al., 2022).
The beneficial effects of BCFYQ on host metpabolism may be related to metabolites produced by vaginal microbes, and we found that BCFYQ altered 30 metabolites. It is worth mentioning that we screened out 30 differential metabolites. Among them, phosphorylcholine and betaine, as metabolites of biogenic amine precursors, have anti-inflammatory properties (Rowley et al., 2010; G. Zhao et al., 2018). The results of cluster analysis showed that the content of these two metabolites increased after BCFYQ treatment compared with the Model group. Through our pathway enrichment analysis, betaine was associated with betaine metabolism, methionine metabolism, glycine and serine metabolism. Studies have shown that betaine directly affects the concentration of homocysteine by promoting the formation of methionine from homocysteine, and attenuates the stress response induced by homocysteine. At the same time, betaine converts homocysteine into methionine, which plays an important role in antioxidant effects (Glier et al., 2014). In addition, betaine exerts an anti-inflammatory effect by inhibiting the NF-κB signaling pathway and reduces the levels of pro-inflammatory factors such as IL-1β (Veskovic et al., 2019). This is consistent with our cytokine assay results. Arachidonic acid (AA) can be metabolized to prostaglandins (PGs) by the cyclooxygenase pathway. Among them, PGE2 has the effect of inducing inflammation, fever and pain. Our results showed that AA was significantly aggregated in the Model group, while its levels were significantly reduced after BCFYQ treatment. Interestingly, IL-1 can stimulate phospholipase A2 gene expression and protein synthesis in different cells, and increase its activity, thereby decomposing membrane phospholipids to produce AA, resulting in the generation and release of PGE2 (Schalkwijk et al., 1992; Pfeilschifter et al., 1993). It can be seen that the relationship between inflammation and metabolites is not a single relationship, but plays a complementary role.
Our results showed that the concentration of phosphatidylcholine in the BCFYQ group was significantly higher than that in the Model group, and pathway enrichment analysis showed that phosphatidylcholine biosynthesis had a significant effect on this pathway. Phosphatidylcholine, the major phospholipid component of eukaryotic cell membranes, is generally known for its anti-inflammatory properties (Treede et al., 2007). And there is increasing evidence that choline metabolites derived from their synthesis and catabolism contribute to proliferative growth and programmed cell death (Ridgway, 2013). In addition, we also observed some metabolite changes related to amino acid metabolism, such as UDP-N-acetylglucosamine and N-Acetylaspartic acid. But overall, BCFYQ treatment of mixed vaginitis was more related to lipid metabolism.
We found that Dethiobiotin was positively correlated with Lactobacillus and negatively correlated with Escherichia-Shigella in Spearman’s rank correlation analysis. Dethiobiotin is a sulfur-containing water-soluble vitamin that acts as a cofactor in various key cellular metabolic reactions. It has been reported in the literature that Dethiobiotin can maintain the integrity and soundness of epithelial tissue structure and has a protective effect on epithelial mucosal tissue. In addition, it is necessary for the reproduction of yeast, Lactobocillus and other bacteria. Studies have shown that some chronic inflammatory diseases are associated with biotin deficiency, and biotin deficiency can enhance the inflammatory response of CD4+ T cells (Rodriguez-Melendez et al., 2003; Elahi et al., 2018). Therefore, we preliminarily concluded that BCFYQ increased the content of Dethiobiotin in vaginal epithelium, which provided better conditions for the growth and reproduction of Lactobacillus. At the same time, Dethiobiotin can also repair the damaged vaginal epithelial mucosa caused by mixed bacterial infection, and enhance the local immunity and resistance.
Lactic acid, the major end product of Lactobacillus-mediated fermentation (O'Hanlon et al., 2013), is a key metabolite for maintaining low vaginal pH and homeostasis in the vaginal microenvironment. Moreover, it has antibacterial and immunomodulatory activities (Aldunate et al., 2015). The results of our vaginal flora showed that BCFYQ significantly increased the abundance of Lactobacillus. It is worth noting that the relative abundance of Streptococcus is higher in the BCFYQ group than in the Model group. Streptococcus belongs to the order Lactobacillus, which can produce lactic acid by fermenting glucose (Köhler, 2007). However, the lack of experimental results is that lactate was not found in the differential metabolites we screened, which may be a problem of sample size. However, it is undeniable that Lactobacillus and its metabolites are one of the key mediators of BCFYQ in the treatment of mixed vaginitis.Mixed biofilms formed by bacteria and fungi reduce susceptibility to antimicrobial agents and increase the spread of antimicrobial resistance (Muzny and Schwebke, 2015). Studies have shown that metabolites of boric acid and Lactobacillus contained in BCFYQ have inhibitory effects on biofilm formation (Ventolini, 2015). We speculate that this is also a key point of BCFYQ in the treatment of mixed vaginitis, which is worthy of our further study.
Meng Li et al. investigated the effect of Fufang FuRong Effervescent Suppository on vaginal microecological composition and dynamics in mixed vaginitis(M. Li et al., 2022). Similar to BCFYQ, both products are vaginal suppositories derived from traditional Chinese medicine and both contain Sophora flavescens and Stemona japonica. Although there is a large difference in the species of bacteria at the genus level, this may be due to the ethnic differences in the vaginal flora between humans and rats. But their results show that the FuRong group can significantly increase the abundance of Lactobacillus, which is consistent with our results. The findings of the current study have several limitations. First of all, BCFYQ is a traditional Chinese medicine compound, the drug composition is complex, it is difficult to determine the main antibacterial drugs. The active components of BCFYQ still need to be elucidated. Secondly, the recurrence rate of mixed vaginitis is high (Parsapour et al., 2017), and single drug treatment is difficult to ensure no recurrence of the disease at present. Some studies have conducted intervention experiments on vaginitis recurrence (De Seta et al., 2014), which has a great enlightenment for us. In conclusion, subsequent thorough investigation of downstream mechanisms is essential for future research.
BCFYQ is a multi-component traditional Chinese medicine preparation, which has significant therapeutic effect on mixed vaginitis. BCFYQ can improve vaginal flora, inhibit the growth of harmful bacteria, but also protect and promote the growth and reproduction of beneficial bacteria such as Lactobacillus. Secondly, BCFYQ can repair the damaged vaginal epithelial tissue by affecting the lipid metabolism pathway. This effect can improve the cellular and humoral immune function and reduce the inflammatory response. It is suggested that BCFYQ has a significant advantage in the treatment of mixed vaginitis (Figure 7). This study explored the mechanism of BCFYQ in the treatment of mixed vaginitis, which provided a basis for our further research.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.ncbi.nlm.nih.gov/, PRJNA1009968.
The animal study was approved by the Animal Experiment Center of Guizhou Medical University and the Animal Ethics Committee of Guizhou Medical University. The study was conducted in accordance with the local legislation and institutional requirements.
Participated in research design: QW, PW and XG. Conducted experiments: QW, PW, MY, MZ, SZ, XS, LS, YL, YZ, NJ and XG. Performed data analysis: QW, PW and XG. Wrote or contributed to the writing of the manuscript: QW and PW. All authors contributed to the article and approved the submitted version.
This work was financially supported by the Science and Technology Department of Guizhou Province ([2021]414).
The mechanism diagram of this study was drawn by Figdraw.
Author NJ was employed by Changsheng Pharmaceutical Co. Ltd.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BCFYQ, Baicao Fuyanqing suppository; ELISA, Enzyme Linked Immunosorbent Assay; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-13, interleukin-13; sIgA, secreted immunoglobulin A; H&E, Hematoxylin and Eosin; AV, Aerobic Vaginitis; BV, Bacterial Vaginosis; VVC, Vulvovaginal Candidiasis.
Aldunate, M., Srbinovski, D., Hearps, A. C., Latham, C. F., Ramsland, P. A., Gugasyan, R., et al. (2015). Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 6. doi: 10.3389/fphys.2015.00164
Balakrishnan, S. N., Yamang, H., Lorenz, M. C., Chew, S. Y., Than, L. T. L. (2022). Role of vaginal mucosa, host immunity and microbiota in vulvovaginal candidiasis. Pathogens 11 (6), 618. doi: 10.3390/pathogens11060618
Beaussart, A., Herman, P., El-Kirat-Chatel, S., Lipke, P. N., Kucharíková, S., Van Dijck, P., et al. (2013). Single-cell force spectroscopy of the medically important Staphylococcus epidermidis-Candida albicans interaction. Nanoscale 5 (22), 10894–10900. doi: 10.1039/c3nr03272h
Benyas, D., Sobel, J. D. (2022). Mixed vaginitis due to bacterial vaginosis and candidiasis. J. Low Genit. Tract. Dis. 26 (1), 68–70. doi: 10.1097/lgt.0000000000000641
Brodsky, F. (1991). Cellular and molecular immunology. Eds. A. K., Abbas, A. H., Lichtman, J. S., Pober Peking University Medical, China.
Budilovskaya, O. V., Shipitsina, E. V., Spasibova, E. V., Pereverzeva, N. A., Vorob'eva, N. E., Tsypurdeeva, N. D., et al. (2020). Differential expression of local immune response genes in the vagina: implication for the diagnosis of vaginal infections. Bull. Exp. Biol. Med. 168 (5), 646–650. doi: 10.1007/s10517-020-04771-3
But, P. H., Shaw, P. C., Lin, G. E., Jiang, R. W., Xu, Y. T. (2012). Authentication and quality assessment of the antitussive herb baibu (radix stemonae). Advances in Botanical Research 62, 1–33. doi: 10.1016/B978-0-12-394591-4.00001-5
Chung, P. Y., Chung, L. Y., Navaratnam, P. (2014). Potential targets by pentacyclic triterpenoids from Callicarpa farinosa against methicillin-resistant and sensitive Staphylococcus aureus. Fitoterapia 94, 48–54. doi: 10.1016/j.fitote.2014.01.026
Das, S., Bhattacharjee, M. J., Mukherjee, A. K., Khan, M. R. (2022). Recent advances in understanding of multifaceted changes in the vaginal microenvironment: implications in vaginal health and therapeutics. Crit. Rev. Microbiol. 49 (2), 256–282. doi: 10.1080/1040841x.2022.2049696
De Seta, F., Parazzini, F., De Leo, R., Banco, R., Maso, G. P., De Santo, D., et al. (2014). Lactobacillus plantarum P17630 for preventing Candida vaginitis recurrence: a retrospective comparative study. Eur. J. Obstet. Gynecol. Reprod. Biol. 182, 136–139. doi: 10.1016/j.ejogrb.2014.09.018
Elahi, A., Sabui, S., Narasappa, N. N., Agrawal, S., Lambrecht, N. W., Agrawal, A., et al. (2018). Biotin Deficiency Induces Th1- and Th17-Mediated Proinflammatory Responses in Human CD4(+) T Lymphocytes via Activation of the mTOR Signaling Pathway. J. Immunol. 200 (8), 2563–2570. doi: 10.4049/jimmunol.1701200
Fan, A., Yue, Y., Geng, N., Zhang, H., Wang, Y., Xue, F. (2013). Aerobic vaginitis and mixed infections: comparison of clinical and laboratory findings. Arch. Gynecol. Obstet. 287 (2), 329–335. doi: 10.1007/s00404-012-2571-4
Feng, S. X., Yi, B., Zhang, M., Xu, J., Lin, H., Xu, W. T. (2017). Iridoid glycosides from Callicarpa nudiflora Hook. Nat. Prod. Res. 31 (2), 181–189. doi: 10.1080/14786419.2016.1224872
Gilbert, N. M., Lewis, W. G., Lewis, A. L. (2013). Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PloS One 8 (3), e59539. doi: 10.1371/journal.pone.0059539
Giraldo, P. C., Gonçalves, A. K., Eleutério Junior, J. (2006). Secretory immunoglobulin A: a protective factor in the genital mucosa. Braz. J. Infect. Dis. 10 (4), 232–234. doi: 10.1590/s1413-86702006000400002
Glier, M. B., Green, T. J., Devlin, A. M. (2014). Methyl nutrients, DNA methylation, and cardiovascular disease. Mol. Nutr. Food Res. 58 (1), 172–182. doi: 10.1002/mnfr.201200636
Greifová, G., Májeková, H., Greif, G., Body, P., Greifová, M., Dubničková, M. (2017). Analysis of antimicrobial and immunomodulatory substances produced by heterofermentative Lactobacillus reuteri. Folia Microbiol. (Praha) 62 (6), 515–524. doi: 10.1007/s12223-017-0524-9
Han, C., Li, H., Han, L., Wang, C., Yan, Y., Qi, W., et al. (2019). Aerobic vaginitis in late pregnancy and outcomes of pregnancy. Eur. J. Clin. Microbiol. Infect. Dis. 38 (2), 233–239. doi: 10.1007/s10096-018-3416-2
He, X., Fang, J., Huang, L., Wang, J., Huang, X. (2015). Sophora flavescens Ait.: Traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 172, 10–29. doi: 10.1016/j.jep.2015.06.010
Janković, L., Jelić, S., Filipović-Ljesković, I., Ristović, Z. (1995). Salivary immunoglobulins in cancer patients with chemotherapy-related oral mucosa damage. Eur. J. Cancer B Oral. Oncol. 31b (3), 160–165. doi: 10.1016/0964-1955(95)00011-6
Jerse, A. E., Wu, H., Packiam, M., Vonck, R. A., Begum, A. A., Garvin, L. E. (2011). Estradiol-treated female mice as surrogate hosts for neisseria gonorrhoeae genital tract infections. Front. Microbiol. 2. doi: 10.3389/fmicb.2011.00107
Köhler, W. (2007). The present state of species within the genera Streptococcus and Enterococcus. Int. J. Med. Microbiol. 297 (3), 133–150. doi: 10.1016/j.ijmm.2006.11.008
Larsen, B., Monif, G. R. (2001). Understanding the bacterial flora of the female genital tract. Clin. Infect. Dis. 32 (4), e69–e77. doi: 10.1086/318710
Lee, Y. M., Kim, J. B., Bae, J. H., Lee, J. S., Kim, P. S., Jang, H. H., et al. (2012). Estrogen-like activity of aqueous extract from Agrimonia pilosa Ledeb. in MCF-7 cells. BMC Complement Altern. Med. 12, 260. doi: 10.1186/1472-6882-12-260
Lee, C. M., Kim, C. J., Kim, S. E., Park, K. H., Bae, J. Y., Choi, H. J., et al. (2022). Risk factors for early mortality in patients with carbapenem-resistant Acinetobacter baumannii bacteraemia. J. Glob. Antimicrob. Resist. 31, 45–51. doi: 10.1016/j.jgar.2022.08.010
Li, Z., Lin, M., Li, Y., Shao, J., Huang, R., Qiu, Y., et al. (2022). Total flavonoids of Sophora flavescens and kurarinone ameliorated ulcerative colitis by regulating Th17/Treg cell homeostasis. J. Ethnopharmacol. 297, 115500. doi: 10.1016/j.jep.2022.115500
Li, M., Zeng, Z., Feng, H., Cao, Y., Zhang, Q., Lv, T., et al. (2022). Accurate 16S absolute quantification sequencing revealed vaginal microecological composition and dynamics during mixed vaginitis treatment with fufang fuRong effervescent suppository. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.883798
Lohse, M. B., Gulati, M., Johnson, A. D., Nobile, C. J. (2018). Development and regulation of single- and multi-species Candida albicans biofilms. Nat. Rev. Microbiol. 16 (1), 19–31. doi: 10.1038/nrmicro.2017.107
Ma, K., Luo, S. P., Li, M., Zhang, H. X., Xu, L. M., Zhao, R. H., et al. (2017). [Advantages and evidences research on Chinese medicine for treatment of pelvic inflammatory disease]. Zhongguo Zhong Yao Za Zhi 42 (8), 1449–1454. doi: 10.19540/j.cnki.cjcmm.2017.0041
Marconi, C., Donders, G. G., Bellen, G., Brown, D. R., Parada, C. M., Silva, M. G. (2013). Sialidase activity in aerobic vaginitis is equal to levels during bacterial vaginosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 167 (2), 205–209. doi: 10.1016/j.ejogrb.2012.12.003
Muzny, C. A., Schwebke, J. R. (2015). Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections. Clin. Infect. Dis. 61 (4), 601–606. doi: 10.1093/cid/civ353
O'Hanlon, D. E., Moench, T. R., Cone, R. A. (2013). Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PloS One 8 (11), e80074. doi: 10.1371/journal.pone.0080074
Parsapour, R., Majlessi, F., Rahimiforoushani, A., Sadeghi, R. (2017). Determination of factors affecting relapse of vaginitis among reproductive-aged women: An experimental study. Electron Physician 9 (1), 3499–3507. doi: 10.19082/3499
Pfeilschifter, J., Schalkwijk, C., Briner, V. A., van den Bosch, H. (1993). Cytokine-stimulated secretion of group II phospholipase A2 by rat mesangial cells. Its contribution to arachidonic acid release and prostaglandin synthesis by cultured rat glomerular cells. J. Clin. Invest. 92 (5), 2516–2523. doi: 10.1172/jci116860
Pointer, B. R., Boyer, M. P., Schmidt, M. (2015). Boric acid destabilizes the hyphal cytoskeleton and inhibits invasive growth of Candida albicans. Yeast 32 (4), 389–398. doi: 10.1002/yea.3066
Ridgway, N. D. (2013). The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. Biol. 48 (1), 20–38. doi: 10.3109/10409238.2012.735643
Rivers, C. A., Adaramola, O. O., Schwebke, J. R. (2011). Prevalence of bacterial vaginosis and vulvovaginal candidiasis mixed infection in a southeastern american STD clinic. Sex Transm. Dis. 38 (7), 672–674. doi: 10.1097/OLQ.0b013e31820fc3b8
Rodriguez-Melendez, R., Lewis, B., McMahon, R. J., Zempleni, J. (2003). Diaminobiotin and desthiobiotin have biotin-like activities in Jurkat cells. J. Nutr. 133 (5), 1259–1264. doi: 10.1093/jn/133.5.1259
Rowley, T. J., McKinstry, A., Greenidge, E., Smith, W., Flood, P. (2010). Antinociceptive and anti-inflammatory effects of choline in a mouse model of postoperative pain. Br. J. Anaesth. 105 (2), 201–207. doi: 10.1093/bja/aeq113
Schalkwijk, C. G., de Vet, E., Pfeilschifter, J., van den Bosch, H. (1992). Interleukin-1 beta and transforming growth factor-beta 2 enhance cytosolic high-molecular-mass phospholipase A2 activity and induce prostaglandin E2 formation in rat mesangial cells. Eur. J. Biochem. 210 (1), 169–176. doi: 10.1111/j.1432-1033.1992.tb17405.x
Stojanović, N., Plećaš, D., Plešinac, S. (2012). Normal vaginal flora, disorders and application of probiotics in pregnancy. Arch. Gynecol. Obstet. 286 (2), 325–332. doi: 10.1007/s00404-012-2293-7
Treede, I., Braun, A., Sparla, R., Kühnel, M., Giese, T., Turner, J. R., et al. (2007). Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 282 (37), 27155–27164. doi: 10.1074/jbc.M704408200
Tsai, Y. F., Yu, H. P., Chung, P. J., Leu, Y. L., Kuo, L. M., Chen, C. Y., et al. (2015). Osthol attenuates neutrophilic oxidative stress and hemorrhagic shock-induced lung injury via inhibition of phosphodiesterase 4. Free Radic. Biol. Med. 89, 387–400. doi: 10.1016/j.freeradbiomed.2015.08.008
Tumietto, F., Posteraro, B., Sanguinetti, M. (2019). Looking for appropriateness in the cure of mixed vaginitis: the role of fenticonazole as an empiric treatment. Future Microbiol. 14, 1349–1355. doi: 10.2217/fmb-2019-0189
Velenosi, T. J., Hennop, A., Feere, D. A., Tieu, A., Kucey, A. S., Kyriacou, P., et al. (2016). Untargeted plasma and tissue metabolomics in rats with chronic kidney disease given AST-120. Sci. Rep. 6, 22526. doi: 10.1038/srep22526
Ventolini, G. (2015). Vaginal Lactobacillus: biofilm formation in vivo - clinical implications. Int. J. Womens Health 7, 243–247. doi: 10.2147/ijwh.S77956
Veskovic, M., Mladenovic, D., Milenkovic, M., Tosic, J., Borozan, S., Gopcevic, K., et al. (2019). Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur. J. Pharmacol. 848, 39–48. doi: 10.1016/j.ejphar.2019.01.043
Wang, Z., Shen, Y. (2017). The natural product osthole attenuates yeast growth by extensively suppressing the gene expressions of mitochondrial respiration chain. Curr. Microbiol. 74 (3), 389–395. doi: 10.1007/s00284-016-1191-9
Wang, Z. H., Xu, H. J., Zhai, Y. Y., Yang, L. P., Li, Q., Huang, M., et al. (2019). Three new labdane-type diterpenoids from Callicarpa macrophylla Vahl. Nat. Prod. Res. 33 (20), 2904–2910. doi: 10.1080/14786419.2018.1509336
Wen-yang, T. (2016). Pharmaceutical and clinical comprehensive evaluation of baicaofuyanqing suppository. Chin. J. Pharmacovigilance 13 (11), 686–690.
Zafar, H., Saier, M. H., Jr. (2021). Gut Bacteroides species in health and disease. Gut Microbes 13 (1), 1–20. doi: 10.1080/19490976.2020.1848158
Zhao, H. D., Feng, X. L., Zhao, Y., Li, N. (2016). [Randomized controlled study: Sophora flavescens gel in treatment of cervical HPV infection]. Zhongguo Zhong Yao Za Zhi 41 (21), 4072–4075. doi: 10.4268/cjcmm20162129
Keywords: Chinese medicine suppository, mixed vaginitis, vaginal flora, Lactobacillus, lipid metabolism
Citation: Wang Q, Wang P, Yuan M, Zhang M, Zhang S, Sun X, Shang L, Liu Y, Zhao Y, Jiang N and Gao X (2023) Efficacy and mechanism of Baicao Fuyanqing suppository on mixed vaginitis based on 16S rRNA and metabolomics. Front. Cell. Infect. Microbiol. 13:1166366. doi: 10.3389/fcimb.2023.1166366
Received: 15 February 2023; Accepted: 22 August 2023;
Published: 14 September 2023.
Edited by:
Kunjie Wang, Sichuan University, ChinaReviewed by:
Namarta Kalia, University of California, San Diego, United StatesCopyright © 2023 Wang, Wang, Yuan, Zhang, Zhang, Sun, Shang, Liu, Zhao, Jiang and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiuli Gao, Z2FveGxAZ21jLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.