- Department of Intensive Care Unit, Hangzhou Red-Cross Hospital, Hangzhou, Zhejiang, China
Background: Varicella-zoster virus (VZV) is a common and widespread human-restricted pathogen. It is famous for its dermatological manifestations, such as varicella and herpes zoster. Patients with aplastic anemia-paroxysmal nocturnal hemoglobinuria (AA-PNH) syndrome complicated with fatal disseminated varicella zoster virus infection are very rare and in danger.
Patient concerns: A 26-year-old man with a history of AA-PNH syndrome was receiving cyclosporine and corticosteroid treatment in the hematology department. During his hospitalization in our hospital, he developed fever, abdominal pain, and lower back pain, and his face, penis, trunk, and limbs developed itchy rash. Subsequently, the patient had to undergo cardiopulmonary resuscitation because of sudden cardiac arrest, and be transferred to ICU for treatment. It was presumed that the cause is unknown severe sepsis. The patient’s condition quickly progressed to multiple organ failure, accompanied by liver, respiratory, and circulatory failure, and signs of disseminated intravascular coagulation. Unfortunately, the patient died after 8 h of active treatment. Finally, we collected all the evidence and concluded that the patient died of AA-PNH syndrome combined with poxzoster virus.
Conclusion: AA-PNH syndrome patients treated with steroids and immunosuppressants are prone to various infections, considering that herpes virus infection with chickenpox and rash as the initial manifestations is characterized by rapid progress and often accompanied by serious complications. It is more difficult to distinguish it from AA-PNH syndrome with skin bleeding points. If it is not identified in time, it may delay the treatment opportunity, make the condition worse, and cause serious adverse prognosis. Therefore, clinicians need to pay attention to it.
Introduction
Aplastic anemia (AA) is a syndrome characterized by bone marrow hypoplasia and failure and peripheral blood pancytopenia. At lower clonal abundance, paroxysmal nocturnal hemoglobinuria (PNH) cells can also be found in bone marrow failure disorders, such as AA, and in apparently healthy individuals (Parker, 2016; Hill et al., 2017). In the first-named condition referred to as AA-PNH, the disease is dominated by grossly reduced blood cell production rather than hemolysis (Young, 2018). Most patients need to use immunosuppressive therapy such as hormone and cyclosporine. The risk of concurrent infection increases sharply, and medical evaluation is required.
Varicella-zoster virus (VZV) is a kind of pathogenic human alphaherpesvirus (Zerboni et al., 2014). VZV usually causes primary infection in children. After a period of incubation, a widely distributed water blister appears, which is called varicella (also known as chickenpox) (Gershon et al., 2015); in individuals with normal immune function, only skin rash and flu-like symptoms, which are usually self-limiting. In special populations, such as immunocompromised individuals and pregnant women, varicella zoster virus infection may lead to serious or even fatal diseases, often due to bacterial superinfection (Tommasi and Breuer, 2022).
We report a case of 26-year-old man with a history of AA-PNH syndrome complicated with a typical separated VZV Infection, which was confirmed by clinical manifestations and laboratory evaluation. Although the outstanding condition of the patient in this case has been actively treated, the prognosis is poor.
Case report
Case presentation
Three months ago (July 17, 2022), a 26-year-old man was found with thrombocytopenia during physical examination, with a platelet count of 29 × 109/L. At that time, the patient could see gingival bleeding when brushing his teeth. However, the patient did not pay enough attention. After 4 days, the patient went to the hematology department of our hospital. He completed blood routine test and bone marrow biopsy. A complete blood count disclosed pancytopenia (Hb: 9.8 g/dl; reticulocytes: 3.5 × 109/L; leukocytes: 2.2 × 109/L; neutrophils: 1.0 × 109/L; and platelets: 30 × 109/L). Blood marrow aspiration showed persistent severe hypoplasia with 25% cellularity, Megakaryocytes scattered occasionally. Cytofluorometric analysis was positive for the PNH clone (Babushok, 2021). AA-PNH syndrome was considered according to the test results. After communicating with the patient, the patient began to receive Traditional Chinese Medicine, hormone, and cyclosporine treatment. During the regular follow-up of the outpatient department, there was no significant fluctuation in various indicators.
On November 26, 2022, the patient went to the hospital again because of the persistent stabbing pain in the waist and back 2 days ago. Physical examination revealed that patient had discomfort and anemia appearance, unpalpable superficial lymph nodes, abdominal distension, abdominal muscle tension, tenderness, no shifting dullness, and limb edema. Eczema and swelling appeared on the glans and prepuce, accompanied by pruritus. Laboratory analyses showed Hb: 11.5 g/dl; reticulocytes: 3.1 × 109/L; leukocytes: 2.7 × 109/L; neutrophils: 1.8 × 109/L; and platelets: 14 × 109/L. C-reactive protein level was 0.4 mg/L. The liver function test showed that direct bilirubin (7.6 μmol/L), glutamic-oxalic transaminase (152 U/L), and glutamic-pyruvic transaminase (199 U/L) were slightly increased. Renal function test, urine examination, and coagulation profile were normal, and blood culture was sterile. Antinuclear antibodies titer was negative. Abdominal ultrasound only showed fatty liver. Renal ultrasound showed multiple strong echoes in the collection system of both kidneys, with the maximum diameter of about 0.4 cm. Cardiac ultrasound showed no obvious abnormality. Symptomatic treatment was carried out for the pain of the patient, and specialist consultation was invited for the perineal symptoms.
Days 3 after admission (November 28, 2022), the patient developed stubborn high fever with the highest temperature of 38.9°C, accompanied by rapid heart rate and tachypnea. At this time, the abdominal pain still persists and cannot be relieved. Abdominal computed tomography (CT) scan showed that fatty liver, gas accumulation, and expansion of colon, and more contents in intestinal cavity. Laboratory analyses showed Hb: 10.6 g/dl; leukocytes: 2.4 × 109/L; neutrophils: 1.5 × 109/L; and procalcitonin was 0.16 ng/ml. Considering that the patient had enterogenous infection and intestinal bleeding, he was empirically given anti-infection treatment.
Days 4 after admission (November 29, 2022), at 9:30 a.m., the patient developed unconsciousness and difficulty breathing (blood pressure 90/40mmHg, pulse 39/min, respiratory rate 22/min, temperature 38.8°C, oxygen saturation 81% on nasal catheter oxygen inhalation 6L/min). After about 1 minute, the patient had cardiac arrest and was immediately rescued. After successful resuscitation, he was transferred to the intensive care unit for further treatment. At 11:30 a.m., the patient was in a coma condition, with ventilator-assisted breathing. His whole body had diffuse rashes at different stages of development, including vesicles, pustules, and crusted vesicles (Figure 1). As can be seen on the bedside electrocardiographic monitor: blood pressure was 87/53 mmHg (norepinephrine 11 μg/kg/min), HR (140–150)/min (atrial fibrillation), respiratory rate 15/min, temperature 35.2 °C; oxygen saturation cannot be measured (Fi O2 100%). Laboratory analysis revealed Hb: 1.6 g/dl; leukocytes: 0.8 × 109/L; neutrophils: 0.6 × 109/L; and platelets: 9 × 109/L (21U platelets were infused on November 28, 2022); procalcitonin was 0.26 ng/ml. Cardiac troponin I was 1.78 ng/ml. In addition, acute hepatic insufficiency (direct bilirubin 13.6 μmol/L, glutamic-oxalic transaminase 7360 U/L and glutamic-pyruvic transaminase 9780 U/L), acute renal insufficiency (creatinine 240.2 μmol/L) and blood coagulation disorder occurred (D-dimer > 80,000 μg/L, plasma protamine paracoagulation (3P) test was positive). Multiple organ failure caused by infection was first considered. We gave empirical anti-infection, blood product infusion, and life support treatment. However, it failed to save the life of the patient, and the clinical death was announced 8 h later. The day after the patient died, we had obtained the results of metagenomic next-generation sequencing (mNGS) (Han et al., 2019); only human herpesvirus type 3 can be seen in the blood of the patient, and no bacteria, fungi, and parasites were found, along with the appearance of lesions in the context of immunosuppression. A clinical diagnosis of disseminated VZV infection was made.
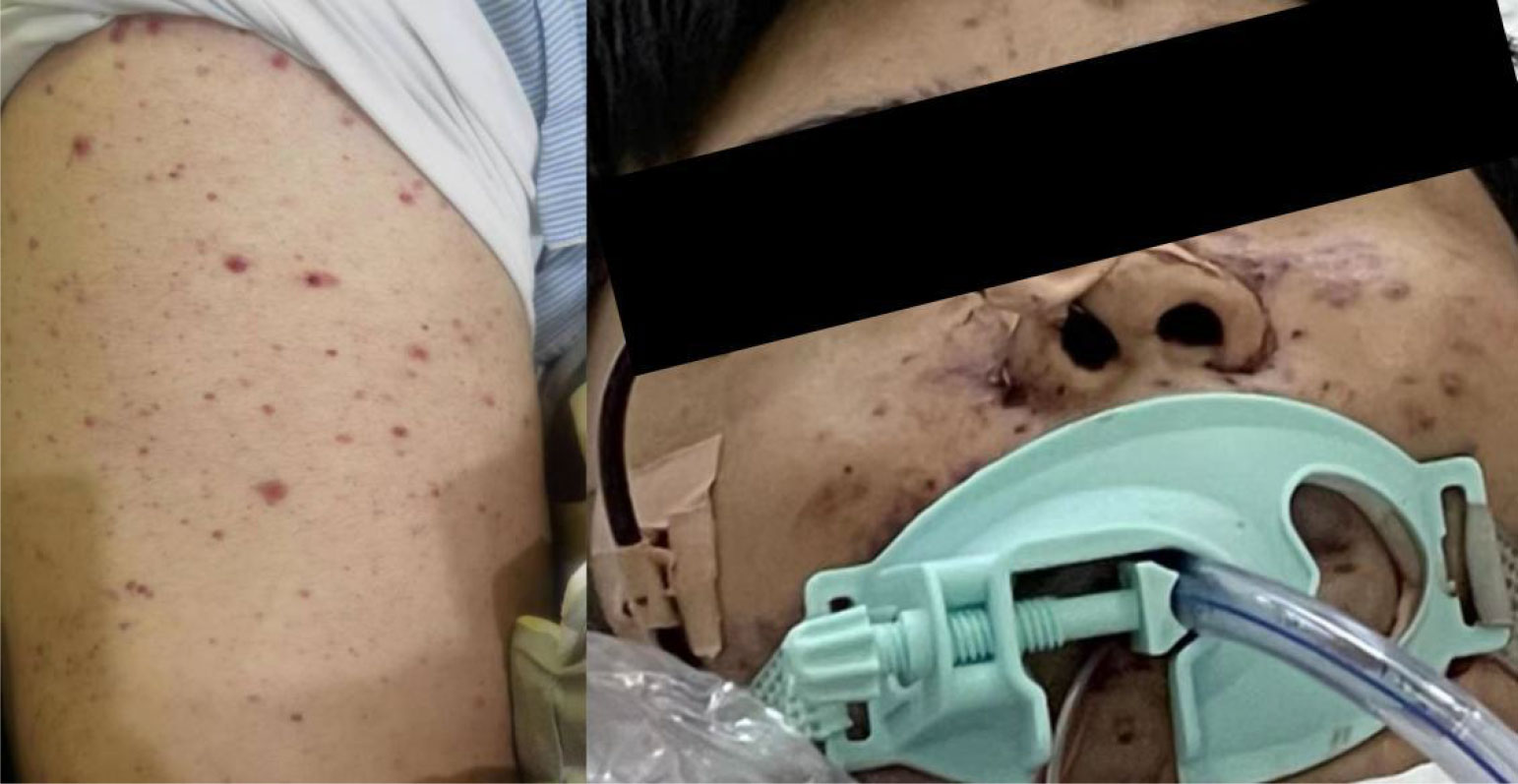
Figure 1 Representative clinical images from presentation showing a diffuse rash at different stages of development, including papules, vesicles, pustules, and crusted vesicles.
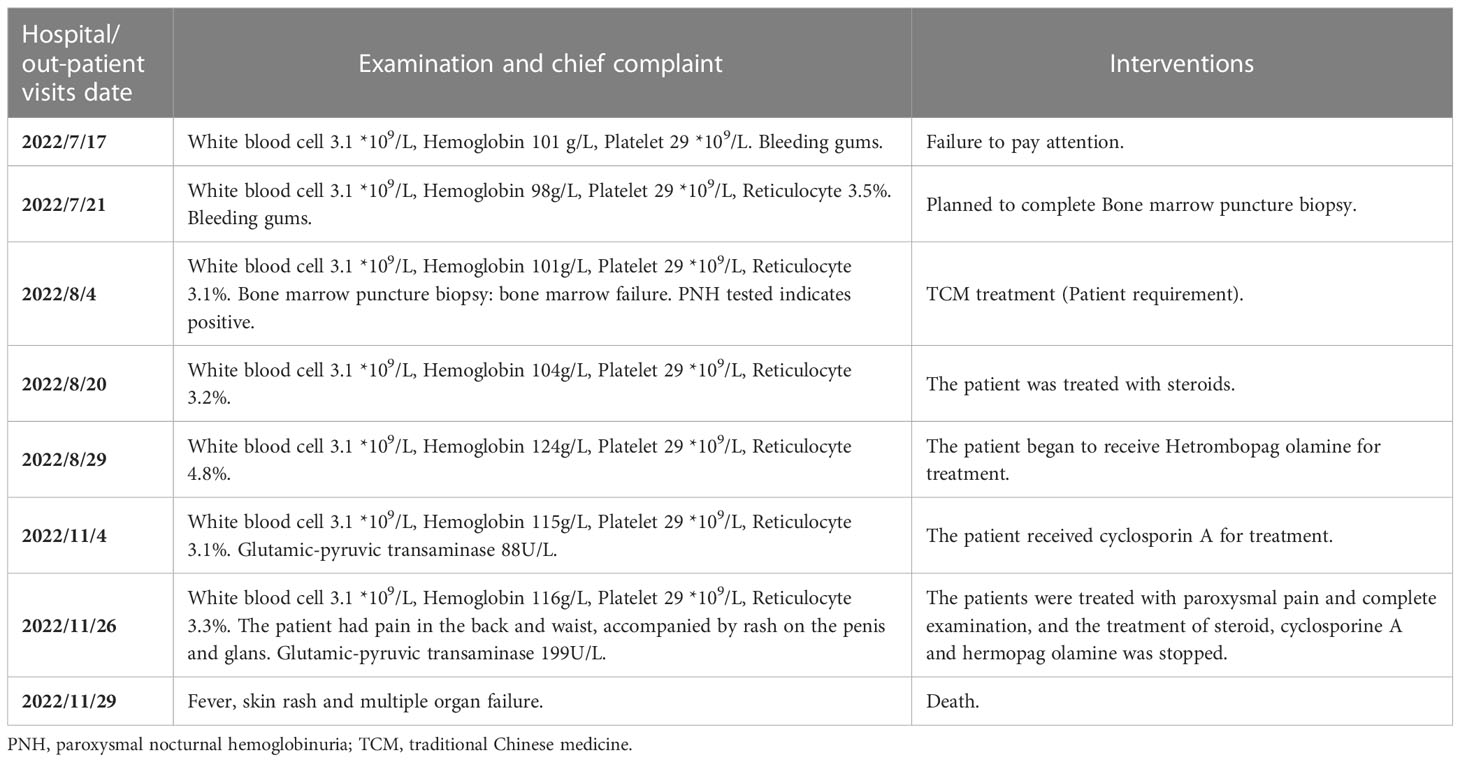
Table 1 shows a timeline for our patient’s past medical history and follow-up visits as well as interventions.
Discussion
This case reflects one important clinical learning point. Disseminated VZV infection can affect patients with AA-PNH syndrome who receive cyclosporine A and steroid. AA-PNH syndrome is a rare immune mediated hematopoietic disease with significant incidence rate and mortality. AA-PNH syndrome is treated according to AA principles (Young, 2018; Alashkar et al., 2020). AA is considered to be an immune-mediated disease. Immunosuppressive therapy and bone marrow transplantation are the main means of treatment for AA patients. As mentioned in our report, the patient was being treated with cyclosporine A and corticosteroids (Peslak et al., 2017; Young, 2018). We report a patient who was previously infected with VZV, but was re-infected with VZV during the immunosuppressive treatment of AA-PNH syndrome (Zawitkowska et al., 2020). The patient failed to be diagnosed and treated in time, and finally died. People with specific immunity to varicella zoster virus still have the chance to be infected again. With the aging of human host, the cell-mediated immunity to this virus in human body gradually decreases, and the virus reactivation becomes more frequent (Kennedy and Gershon, 2018; Laing et al., 2018).
It is generally believed that chickenpox is usually associated with mild diseases. Varicella zoster virus infection can be divided into primary infection or reactivation, the latter usually manifesting as rash and acute neuritis. Nevertheless, immunocompromised patients may have more severe and atypical manifestations, such as encephalitis, aseptic meningitis, pneumonia and hepatitis, along with disseminated visceral and cutaneous involvement (Vassia et al., 2020; Lenfant et al., 2022). Rash is the main clinical manifestation of our patients. Skin lesions often develop from papules to vesicles to scabs within a few days. However, severe rash takes longer to heal; accompanying symptoms include discomfort, fever, and fatigue, usually lasting for about a week. More serious cases may even be complicated with skin bacterial superinfection, encephalitis, and pneumonia. Adults and immunocompromised patients are more prone to severe infections than healthy children (Gershon et al., 2015; Nagel and Gilden, 2014). Severe or even fatal chickenpox, moreover, often occurs in patients with impaired immune function due to diseases or drugs, such as corticosteroids or cancer chemotherapy (Petrun et al., 2015; Lewis et al., 2017; Kennedy and Gershon, 2018; Loftus et al., 2019; Pannu and Manikandan, 2019).
The skin of patients with AA-PNH syndrome is prone to bleeding points, especially when the platelet level is significantly decreased. Our patient had skin “macula” at the time of last hospitalization, and our attending physician suspected it was a bleeding point after physical examination. However, it is possible that these “macula” are also mixed with some rashes caused by human herpes zoster virus. If we can identify and make a clear diagnosis at an early stage and give the patients antiviral treatment in time, the prognosis may not be known. It is worth considering that it is also unclear whether the abdominal pain of the patient is caused by early visceral disseminated varicella zoster virus infection, although abdominal CT scan did not find characteristic imaging manifestations (Furuto et al., 2019; Agatsuma et al., 2021). At present, clinical treatment found that most patients with varicella zoster virus infection had good prognosis after standard antiviral treatment (Andrei and Snoeck, 2021), and similar conclusions were also observed in immunocompromised patients (Andrei and Snoeck, 2021).
Conclusion
AA-PNH syndrome patients treated with steroids and immunosuppressants are prone to various infections. Therefore, it is necessary to dynamically evaluate the benefits and risks of using steroid drugs or immunosuppressive drugs based on the patient’s situation in a timely manner. Considering that herpes virus infection with chickenpox and rash as the initial manifestations is characterized by rapid progress and often accompanied by serious complications. It is more difficult to distinguish it from AA-PNH syndrome with skin bleeding points. If it is not identified in time, it may delay the treatment opportunity, make the condition worse, and cause serious adverse prognosis. Therefore, clinicians need to pay attention to it.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Hangzhou Red Cross Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JW and ZY: data curation, funding acquisition, and writing original draft. DR and ZL: investigation. KF, ZS, and ZL: resources and writing review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Guiding project of Hangzhou Science and Technology Bureau (No. 20220919Y033).
Acknowledgments
We are grateful to the patients and their families for their invaluable contributions to this study. We wish to thank the help given by the physicians who participated in this study and the professionals involved in sample collection and culture maintenance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agatsuma, N., Tsujioka, K., Nishikawa, Y., Nakatani, Y., Yamashita, Y. (2021). Gastrointestinal varicella-zoster virus infection. Cleve Clin. J. Med. 88, 592–593. doi: 10.3949/ccjm.88a.20151
Alashkar, F., Saner, F. H., Vance, C., Schmücker, U., Herich-Terhürne, D., Dührsen, U., et al. (2020). Pregnancy in classical paroxysmal nocturnal hemoglobinuria and aplastic anemia-paroxysmal nocturnal hemoglobinuria: A high-risk constellation. Front. Med. (Lausanne) 7, 543372. doi: 10.3389/fmed.2020.543372
Andrei, G., Snoeck, R. (2021). Advances and perspectives in the management of varicella-zoster virus infections. Molecules 26(4):1132. doi: 10.3390/molecules26041132
Babushok, D. V. (2021). When does a PNH clone have clinical significance? Hematol. Am. Soc. Hematol. Educ. Program 2021, 143–152. doi: 10.1182/hematology.2021000245
Furuto, Y., Kawamura, M., Namikawa, A., Takahashi, H., Shibuya, Y. (2019). Successful management of visceral disseminated varicella zoster virus infection during treatment of membranous nephropathy: a case report. BMC Infect. Dis. 19, 625. doi: 10.1186/s12879-019-4193-y
Gershon, A. A., Breuer, J., Cohen, J. I., Cohrs, R. J., Gershon, M. D., Gilden, D., et al. (2015). Varicella zoster virus infection. Nat. Rev. Dis. Primers 1, 15016. doi: 10.1038/nrdp.2015.16
Han, D., Li, Z., Li, R., Tan, P., Zhang, R., Li, J. (2019). mNGS in clinical microbiology laboratories: on the road to maturity. Crit. Rev. Microbiol. 45 (5-6), 668–685. doi: 10.1080/1040841X.2019.1681933
Hill, A., DeZern, A. E., Kinoshita, T., Brodsky, R. A. (2017). Paroxysmal nocturnal haemoglobinuria. Nat. Rev. Dis. Primers 3, 17028. doi: 10.1038/nrdp.2017.28
Kennedy, P., Gershon, A. A. (2018). Clinical features of varicella-zoster virus infection. Viruses 10 (11), 609. doi: 10.3390/v10110609
Laing, K. J., Ouwendijk, W., Koelle, D. M., Verjans, G. (2018). Immunobiology of varicella-zoster virus infection. J. Infect. Dis. 218, S68–S74. doi: 10.1093/infdis/jiy403
Lenfant, T., L'Honneur, A. S., Ranque, B., Pilmis, B., Charlier, C., Zuber, M., et al. (2022). Neurological complications of varicella zoster virus reactivation: Prognosis, diagnosis, and treatment of 72 patients with positive PCR in the cerebrospinal fluid. Brain Behav. 12, e2455. doi: 10.1002/brb3.2455
Lewis, D. J., Schlichte, M. J., Dao, H. J. (2017). Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis 100, 321;324;330.
Loftus, M. J., Yong, M. K., Wilson, S., Peleg, A. Y. (2019). Fatal disseminated visceral varicella zoster virus infection in a renal transplant recipient. Transpl Infect. Dis. 21, e13062. doi: 10.1111/tid.13062
Nagel, M. A., Gilden, D. (2014). Update on varicella zoster virus vasculopathy. Curr. Infect. Dis. Rep. 16, 407. doi: 10.1007/s11908-014-0407-z
Pannu, A. K., Manikandan, G. (2019). Disseminated varicella infection. N Engl. J. Med. 381, e21. doi: 10.1056/NEJMicm1814626
Parker, C. J. (2016). Update on the diagnosis and management of paroxysmal nocturnal hemoglobinuria. Hematol. Am. Soc. Hematol. Educ. Program 2016, 208–216. doi: 10.1182/asheducation-2016.1.208
Peslak, S. A., Olson, T., Babushok, D. V. (2017). Diagnosis and treatment of aplastic anemia. Curr. Treat Options Oncol. 18, 70. doi: 10.1007/s11864-017-0511-z
Petrun, B., Williams, V., Brice, S. (2015). Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol. Online J. 21(3):13030/qt3cz2x99b. doi: 10.5070/D3213022343
Tommasi, C., Breuer, J. (2022). The biology of varicella-zoster virus replication in the skin. Viruses 14 (5), 982. doi: 10.3390/v14050982
Vassia, V., Croce, A., Ravanini, P., Leutner, M., Saglietti, C., Fangazio, S., et al. (2020). Unusual presentation of fatal disseminated varicella zoster virus infection in a patient with lupus nephritis: a case report. BMC Infect. Dis. 20, 538. doi: 10.1186/s12879-020-05254-6
Zawitkowska, J., Lejman, M., Szmydki-Baran, A., Zaucha-Prażmo, A., Czyżewski, K., Dziedzic, M., et al (2020). Varicella-zoster virus infection in the pediatric population with acute lymphoblastic leukemia in Poland. J. Med. Virol. 92 (12), 3645-3649. doi: 10.1002/jmv.26008
Keywords: disseminated varicella zoster infection, varicella zoster virus, aplastic anemia, AA-PNH syndrome, case report
Citation: Wang J, Yang Z, Ren D, Shi Z, Fang K and Li Z (2023) Disseminated varicella-zoster virus infection in an aplastic anemia- paroxysmal nocturnal hemoglobinuria syndrome patient: A case report. Front. Cell. Infect. Microbiol. 13:1163872. doi: 10.3389/fcimb.2023.1163872
Received: 11 February 2023; Accepted: 29 March 2023;
Published: 19 April 2023.
Edited by:
Slobodan Paessler, University of Texas Medical Branch at Galveston, United StatesReviewed by:
Muhsin Jamal, Abdul Wali Khan University Mardan, PakistanManish Sharma, Emory University, United States
Copyright © 2023 Wang, Yang, Ren, Shi, Fang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhihui Li, bGl6aGlodWlAemNtdS5lZHUuY24=
 Jie Wang
Jie Wang Zheng Yang
Zheng Yang Zhanli Shi
Zhanli Shi Zhihui Li
Zhihui Li