- 1Shanghai Public Health Clinical Center, Fudan University, Shanghai, China
- 2School of Laboratory Medicine and Life Science, Wenzhou Medical University, Wenzhou, China
- 3Department of Infectious Diseases and Public Health, Central Hospital of Xiangtan, Xiangtan, China
Objective: To compare the diagnostic performance of laboratory assays on the ultrasound-guided core needle biopsy samples for diagnosis of extra-pulmonary tuberculosis (EPTB) in HIV-positive and HIV-negative patients.
Methods: A total of 217 patients suspected to have EPTB underwent lesion biopsy from 2017 to 2020. Results of laboratory tests on the biopsy and non-biopsy samples were collected with clinical data for retrospective analysis of test utility. The calculated diagnostic accuracy of the tests was stratified according to the specimen types and HIV status.
Results: The cohort contained 118 patients with a final positive diagnosis of extrapulmonary tuberculosis (EPTB group, 54.4%) and 99 finally diagnosed as without TB (non-EPTB group, 45.6%). The risk factor for EPTB was HIV co-infection (OR 2.22, 95% CI 1.17-4.28, p = 0.014). In biopsy samples, GeneXpert (Xpert) showed higher sensitivity (96.6% [91.6-98.7], p < 0.0001) than culture (56.1% [47.0-64.9]). Regardless of HIV status, Xpert had the highest sensitivity (>95%) and specificity (nearly 100%) of any methods. In non-biopsy samples, only T-SPOT.TB (T-SPOT) showed higher sensitivity than culture (90.9% [62.3-99.5] vs 35.3% [17.3-58.7], p = 0.0037). Furthermore, the sensitivities of Xpert were lower in non-biopsy samples (60.0% [23.1-92.9], p = 0.022) than in biopsy samples (100% [86.7-100]). Even in smear-negative biopsy samples, Xpert still had higher sensitivity than culture and retained high specificity (100% [95.7-100]).
Conclusion: Superior performance of Xpert in diagnosing EPTB was observed regardless of HIV status and specimen types. Nevertheless, the biopsy samples still substantially facilitated the accurate diagnosis of extrapulmonary tuberculosis.
1 Introduction
Tuberculosis (TB) is a communicable disease caused by the mycobacterium tuberculosis complex (MTBC). Globally, an estimated 10.6 million people (range, 9.9-11.0 million) fell ill with TB in 2021, an increase of 4.5% from 10.1 million (95% UI: 9.5-10.7 million) in 2020; and about 1.6 million died from TB in the same year, up from a best estimate of 1.5 million in 2020 (WHO, 2022).
Extrapulmonary tuberculosis (EPTB) refers to TB occurring in parts of the body other than the lungs (e.g., lymph nodes, meninges, abdomen, pleura, genitourinary tract, skin, joints, and bones) (Golden and Vikram, 2005). As per the Global TB Report 2020, EPTB constituted 16% of the 7.5 million notified TB cases in 2019, ranging from 8% in the Western Pacific Region to 24% in the Eastern Mediterranean Region (WHO, 2020). In China, EPTB accounted for approximately 24% of TB cases, with a maximum of 33% in the western region (Li et al., 2022). In the context of WHO’s End TB Strategy, timely diagnosis and treatment of EPTB is a challenge we have to face.
The main risk factors associated with EPTB vary widely and include human immunodeficiency virus (HIV) co-infection, female sex, age (young children or over 65 years of age), and diabetes (Shivakoti et al., 2017; Ohene et al., 2019; Pang et al., 2019; Banta et al., 2020). Due to the absence of typical TB symptoms, EPTB is often misdiagnosed as other diseases, such as cancers (Xiang et al., 2021) and inflammatory diseases (Aisenberg et al., 2005; Jain, 2011). Laboratory diagnosis plays a decisive role in the diagnosis of EPTB. However, studies comparing various laboratory assays based on biopsy samples are limited, probably because biopsy samples are not readily available (Norbis et al., 2014; Park and Kon, 2021).
This study analyzed the records from laboratory investigations of specimens from suspected extrapulmonary tuberculosis patients in an infectious disease hospital from 2017 to 2020 to compare the accuracy of different methods of laboratory diagnosis.
2 Methods
2.1 Study population and specimens
This study was conducted in Shanghai Public Health Clinical Center, one of the designated National Tuberculosis Hospitals in China. Patients with suspected EPTB (WHO, 2021a) who had undergone biopsy between July 01 2017 and September 30 2020 were enrolled. The inclusion criteria were patients with lymph node enlargement and typical symptoms of TB (fever, wasting, night sweats, etc.), or a positive PPD/TSPOT.TB test, or suspicion of TB on imaging, and willing to receive puncture procedures. The exclusion criteria were the patient refusing the biopsy or patients with contraindications to puncture, such as coagulation dysfunction. The biopsy samples were collected by an ultrasound-guided core needle biopsy. For the non-biopsy samples, we collected data from the hospital’s Laboratory Examination Control System by matching the patient’s ID and the exact test date. Demographic information (sex, age, HIV status, and diagnosis) and anatomical locations of EPTB were recorded upon enrollment. The results of pathological and microbiological tests were included.
2.2 Clinical definition and classification
The culture (combined with the MPB64 test) and Xpert results were used as a microbiological reference standard. Patients were eventually classified into the EPTB group (culture (4 cases), Xpert (54 cases), or culture-Xpert (60 cases) positive) and non-EPTB group [culture and Xpert negative (99 cases)].
2.3 Laboratory methods
Biopsy samples were collected by ultrasound-guided biopsy in the Ultrasound Intervention Department and sent to the Laboratory and Pathology Departments for diagnostic tests and histological examinations. Non-biopsy samples were collected and tested routinely in the Laboratory Department. An optimized sample pre-treatment process was used to concentrate mycobacteria in the specimens and thus improve the accuracy of the assays (Rickman and Moyer, 1980; Peterson et al., 1999). Briefly, Large-volume liquid specimens were first centrifuged at 3000-3800g for 15 min, the supernatant was discarded and digested with 2-4% NaOH for 15-20 min. Solid samples were digested directly with 2-4% NaOH. After digestion, the samples were neutralized with sterile PBS, then centrifuged at 3000-3800g for 15 min and the supernatant was discarded. The digested samples were mixed with 0.1-1mL of PBS and used for subsequent assays. Routine tests included culture (BACTEC MGIT 960 rapid culture method), smear (Auramine O staining kit, Zhuhai Baso Biotechnology Co.), and Xpert (Gene X-Pert MTB/RIF, Cepheid, USA). T-SPOT.TB (Oxford Immunotec Ltd, UK), was carried out using kits based upon the hospital’s programmatic laboratory procedures. Species identification was carried out with an MPB64 monoclonal antibody assay (Hangzhou Genesis Biodetection & Biocontrol Co., Ltd, Hangzhou, China) based on positive cultures. Next-generation sequencing is done by Shanghai Simple Gene Medical Laboratory (Kindstar Globalgene Technology, Inc. Shanghai, China) when required.
The pathological tissues were fixed with 4.0% formaldehyde, routinely dehydrated and paraffin-embedded, and serially sectioned at a thickness of 4 μm. HE stain and acid-fast stain (Zhuhai Baso Biotechnology Co. Zhuhai, China) were performed in sections for routine microscopic diagnosis. EPTB positive was identified when there was typical epithelioid granuloma formation, caseation, and positive acid-fast staining.
2.4 Statistical analysis
We used R studio version 4.0.0 to process the data and GraphPad Prism version 8.0 for all analyses. The baseline table was performed using the R‐based tableone package (version 0.13.2). The χ² test (including McNemar’s test) was used to calculate differences in diagnostic accuracy metrics; the Mann-Whitney U test was used to calculate differences in non-parametric data; the two-sample proportion test (Chi-square test) was used to compare, for example, sensitivity across two groups.
3 Results
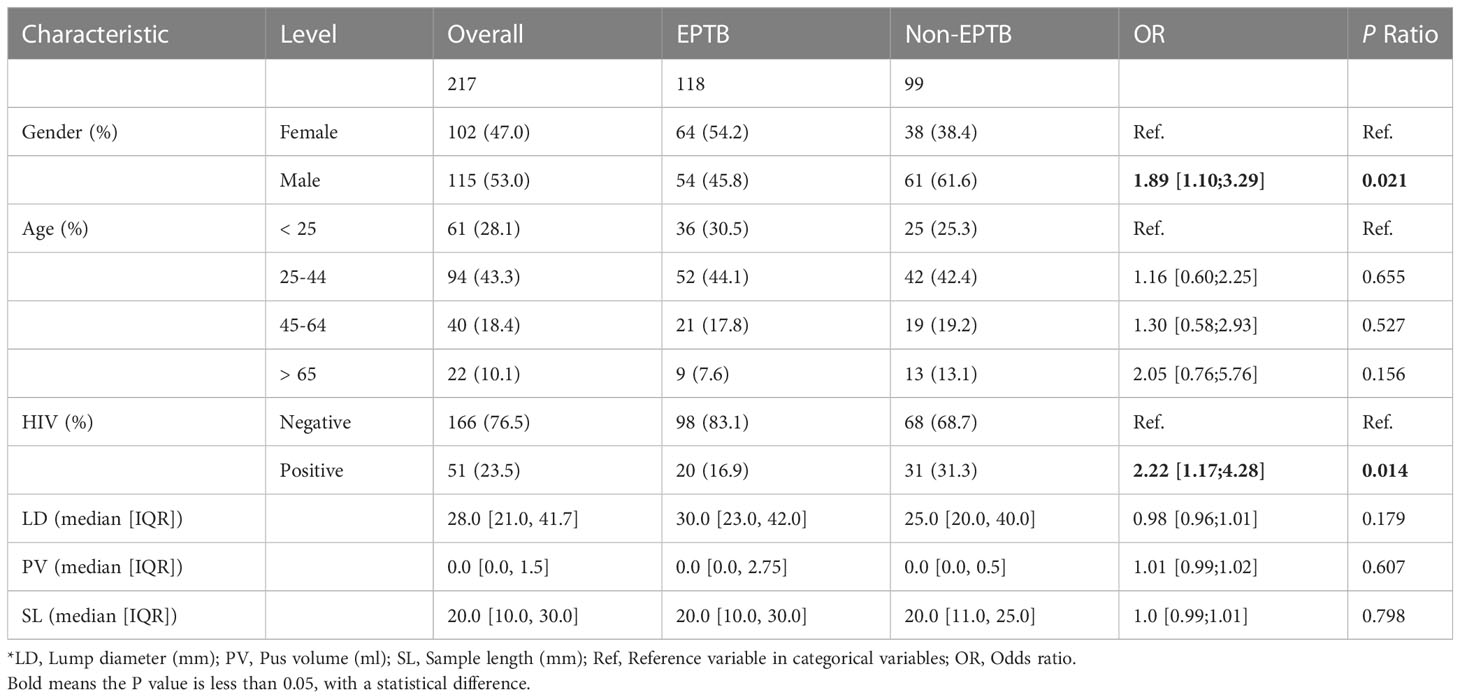
In this study, we enrolled 217 cases of suspected EPTB, including 118 (54.4%) cases that had been confirmed as EPTB patients and 99 (45.6%) cases that had been finally diagnosed as non-EPTB patients (Figure 1). The locations of the biopsy were the neck (134 cases), axillary (25 cases), musculoskeletal (14 cases), abdominal (13 cases), chest (9 cases), supraclavicular (9 cases), limb (6 cases), testicular or epididymal (5 cases), fossailiaca (1 case) and face (1 case). In addition, data from 53 non-biopsy samples (mostly sputum) were retrieved based on patient ID and sampling date. As shown in Figure 2, culture, smear, Xpert, Hematoxylin-eosin staining (HE), and Acid Fast Bacteria (AFB) Stain were performed on biopsy samples. For non-biopsy samples, culture, Xpert, and TSPOT assays were done.
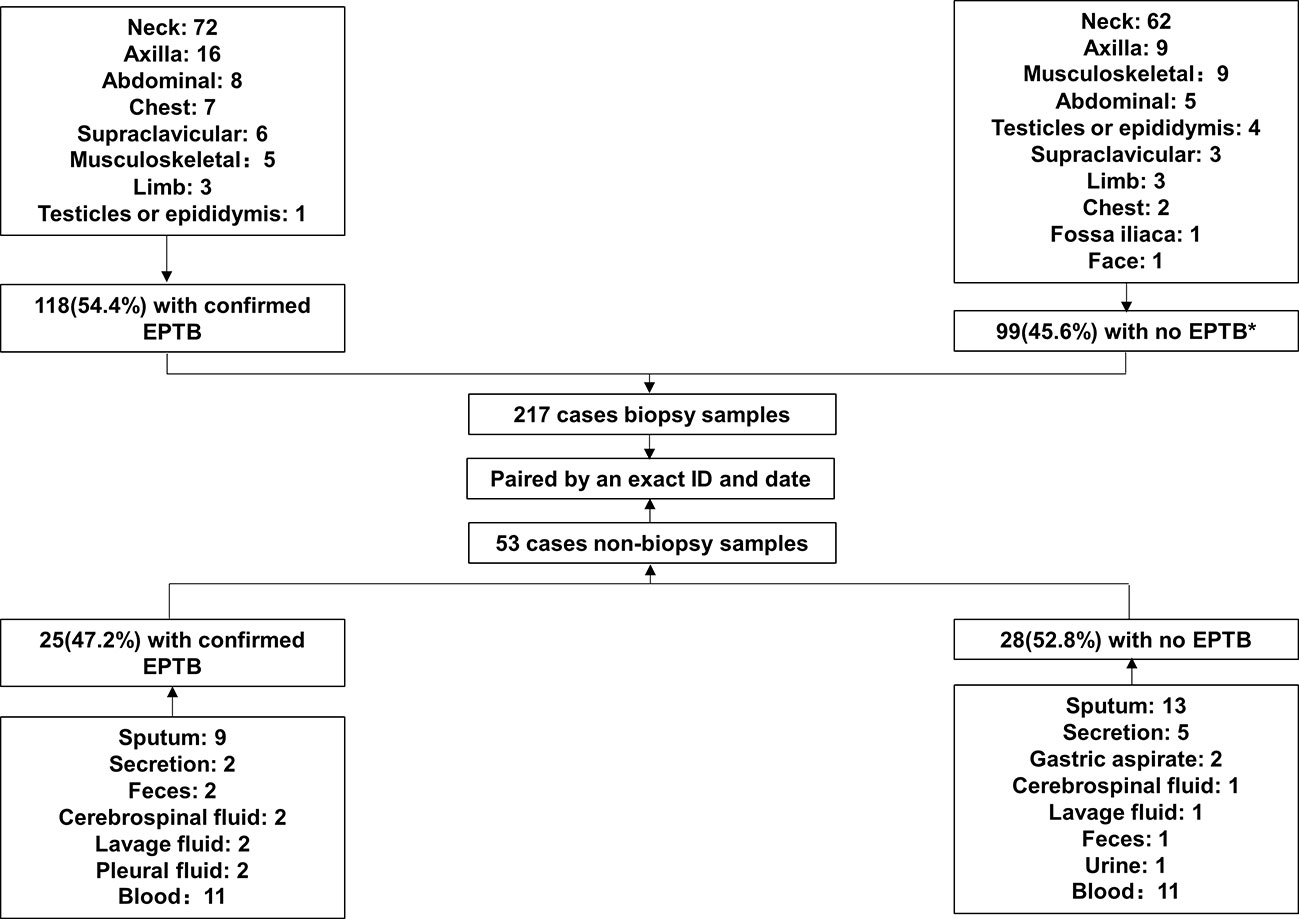
Figure 1 Study profile.* 26 cases of lymphadenitis, 2 cases of NTM infection, 3 cases of BCG infection, 8 cases of tumor, 4 cases of Penicillium marneffei infection, 3 cases of Staphylococcus aureus infection, and 53 cases of other non-TB diseases.
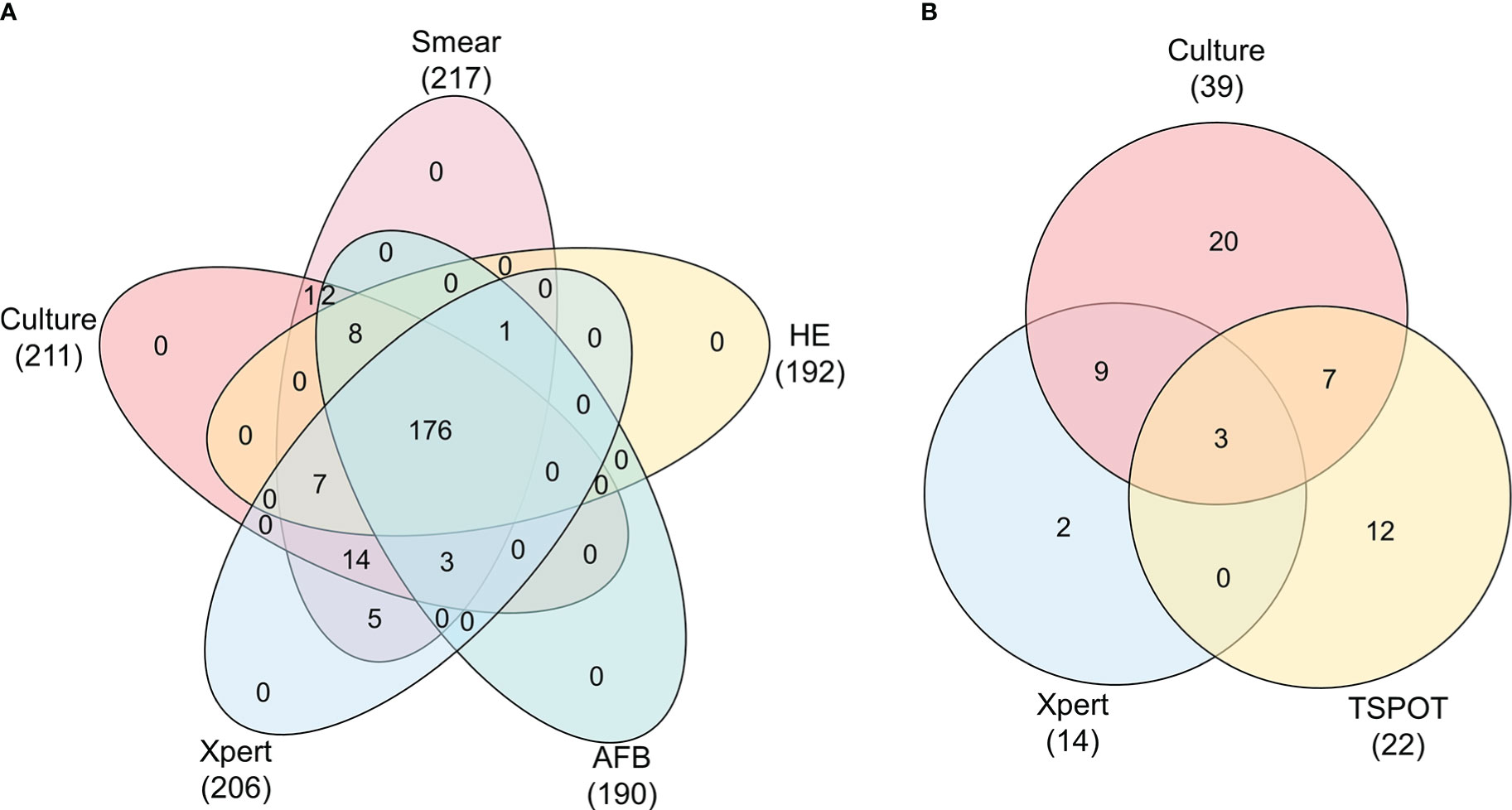
Figure 2 Venn diagram showing the relationship between tests of biopsy and non-biopsy samples. Data are shown stratified according to: (A) Veen diagram of culture, smear, HE, AFB, Xpert tests in biopsy samples, (B) Veen diagram of culture, Xpert, TSPOT tests in non-biopsy samples.
Males were more likely to have EPTB than females (OR 1.89, 95%CI 1.10-3.29, p = 0.021). Using patients < 25 years of age as a control group, we found that patients exhibited an increased risk of extrapulmonary TB with increasing age (OR 1.16, 95% CI 0.60-2.25 for patients 25-44 years of age; OR 1.30, 95% CI 0.58-2.93 for patients 45-64 years of age; OR 2.05, 95% CI 0.76-5.76 for patients > 65 years of age). As expected, HIV-positive patients were more frequently affected by EPTB than HIV-negative ones (OR 2.22, 95% CI 1.17-4.28, p = 0.014). However, the lump diameter, pus volume, and length of patients’ biopsy samples were not related to the likelihood of a positive diagnosis of EPTB (Table 1).
Firstly, we compared the diagnostic accuracy of conventional assays with 217 biopsy samples (Table 2). In biopsy samples, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the culture were56.1% [47.0-64.9], 96.9% [91.3-99.2], 95.5% [87.6-98.8] and 57.2% [32.6-72.6], respectively. Notably, Xpert had higher sensitivity (96.6% [91.6-98.7] vs 56.1% [47.0-64.9]; p < 0.0001), specificity (100% [95.8-100] vs 96.9% [91.3-99.2]; p = 0.096, and PPV (100% [96.7-100] vs 95.5% [87.6-98.8]; p = 0.023), and NPV (95.7% [89.4-98.3] vs 57.2% [32.6-72.6]; p < 0.0001) when compared with culture (Table 2). The histological assays (HE and AFB) had a better sensitivity performance (HE 92.4% [85.7-96.1], p < 0.0001; AFB 81.7% [73.2-88.0], p < 0.0001) and NPV (HE 88.6% [79.0-94.1], p = 0.0003; AFB 76.8% [66.6-84.6], p = 0.07) than culture, but poorer performance in specificity (HE 71.3% [61.0-79.7], p < 0.0001; AFB 73.3% [63.1-81.5], p < 0.0001), and PPV (HE 79.5% [71.5-85.7], p = 0.003; AFB 78.7% [70.1-85.4], p = 0.0024), consistent with a previous report (sensitivity: 95.6%, specificity: 64.6%, PPV: 74.1%, NPV: 93.2%)(Bennani et al., 2019). Unexpectedly, the sensitivity and NPV of the smear were slightly higher than that of the culture, but the difference was not significant (Table 2).
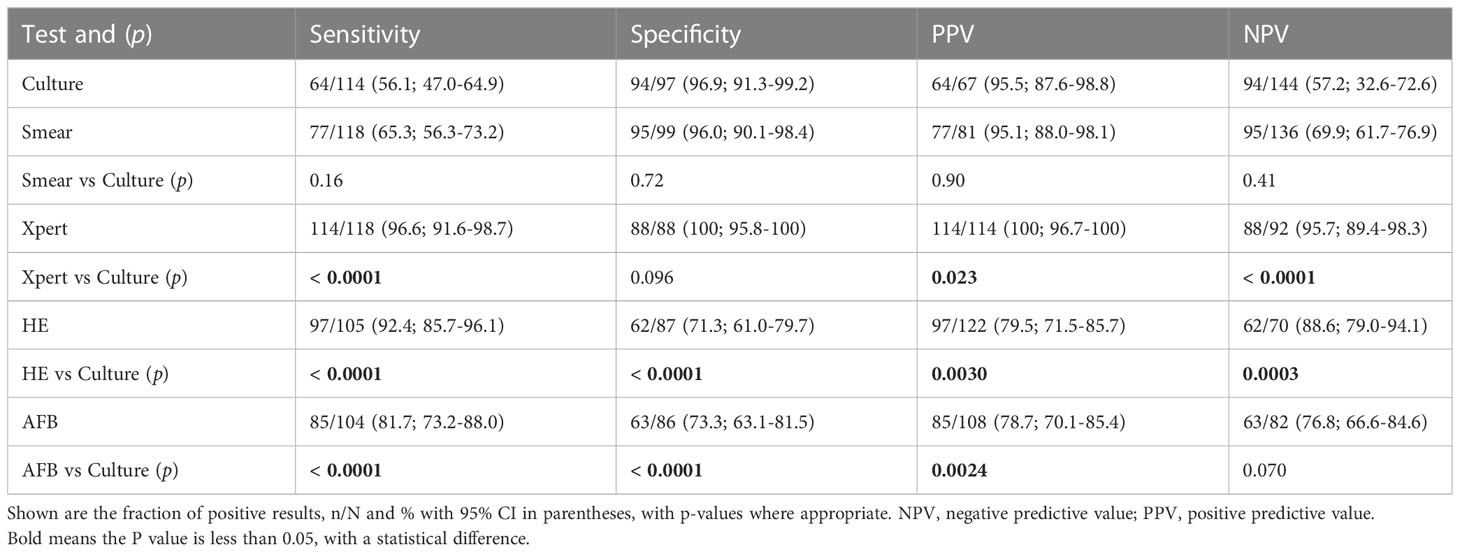
Table 2 Diagnostic utility of culture, smear, Xpert, HE, and AFB in the examination of biopsy samples.
By HIV status, culture had greater sensitivity (70.0% [48.1-85.5] vs 53.2% [43.2-63.0], p = 0.17) and NPV (82.9% [67.3-91.9] vs 59.6% [50.3-68.4], p = 0.012) in HIV-positive patients than HIV-negative ones. Both specificity (93.6% [79.3-98.9] vs 98.5% [91.9-99.9], p = 0.50) and PPV (87.5% [64.0-97.8] vs 98.0% [89.7-99.9], p = 0.28) of culture were lower in HIV-positive patients than in HIV-negative patients. Similar trends were observed for the smear and histological methods, but the specificity (HE: 54.2% [35.1-72.1] vs 77.8% [66.1-86.3], p = 0.056; AFB: 53.9% [35.5-71.2] vs 81.7% [70.1-89.4], p = 0.016) and PPV (smear: 83.3% [60.8-94.2] vs 98.4% [91.5-99.9], p = 0.033; HE: 62.1% [44.0-77.3] vs 85.0% [76.3-90.8], p = 0.016; AFB: 58.6% [40.7-74.5] vs 86.1% [76.8-92.0], p = 0.0047) of the assays for HIV-positive patients were significantly lower than for HIV-negative patients, because HIV-positive patients were more likely to be infected by non-tuberculous mycobacteria (NTM)(Álvaro-Meca et al., 2015) (3.8% in this study). Remarkably, Xpert had the highest sensitivity, specificity, PPV, and NPV values of all assays and did not differ significantly between HIV-positive and negative patients (Figure 3; Supplementary Table S1).
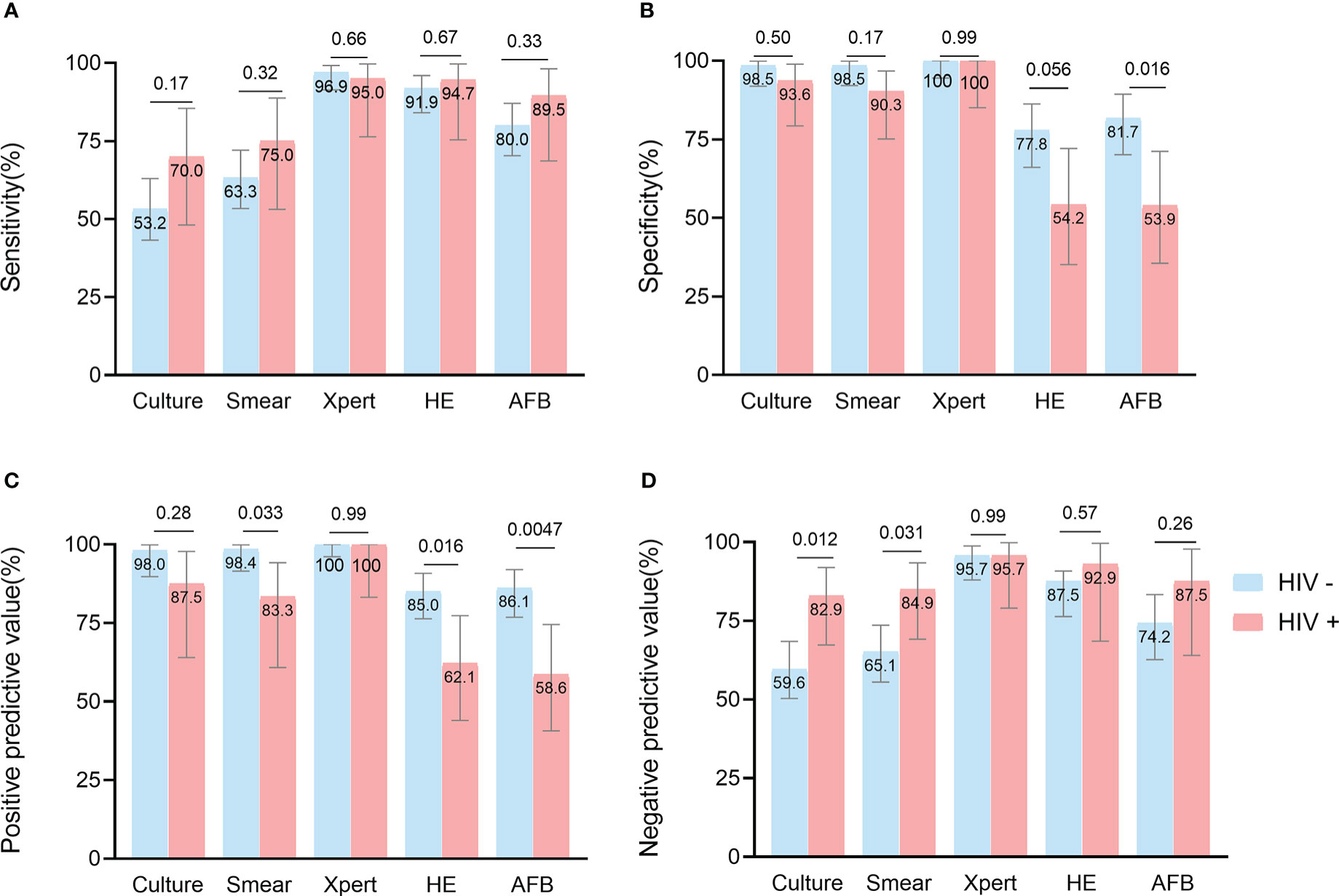
Figure 3 Head-to-head comparison of test accuracy in biopsy samples, by HIV status. Data are shown stratified according to: (A) sensitivity, (B) specificity, (C) positive predictive value, (D) negative predictive value.
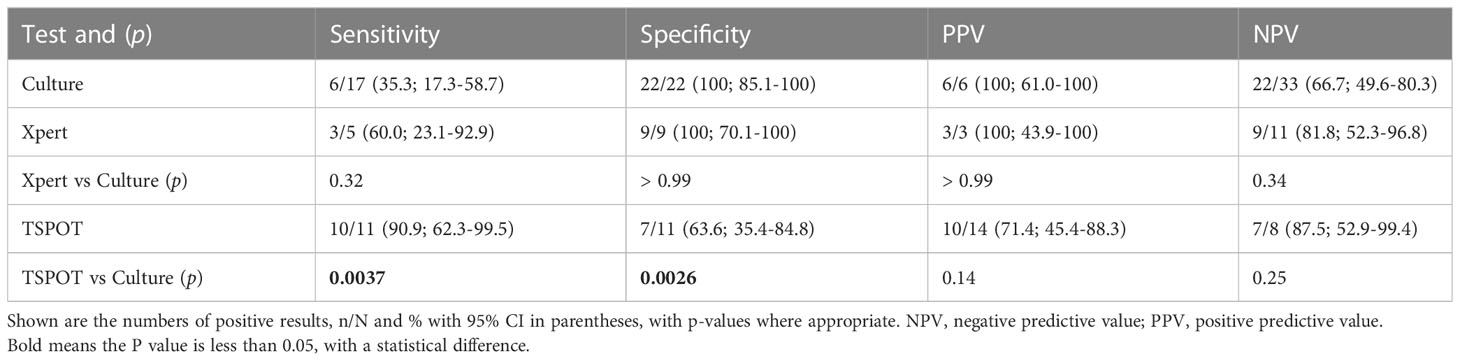
In 53 non-biopsy samples (Table 3), TSPOT had higher sensitivity than culture (90.9% [62.3-99.5] vs 35.3% [17.3-58.7], p = 0.0037), but lower specificity (63.6% [35.4-84.8] vs 100% [85.1-100], p = 0.0026). The sensitivity and specificity of Xpert and culture did not differ significantly, most likely due to the small sample size (Table 3). We also compared the performance of different assays between biopsy samples and non-biopsy samples. The Xpert showed higher sensitivity (100% [86.7-100] vs 60.0% [23.1-92.9], p = 0.022) and NPV (100% [86.2-100] vs 81.8% [52.3-96.8], p = 0.032) in biopsy samples than in non-biopsy samples, but not higher specificity or PPV. The same trend was observed for culture, but not significantly (Figure 4; Supplementary Table S2).
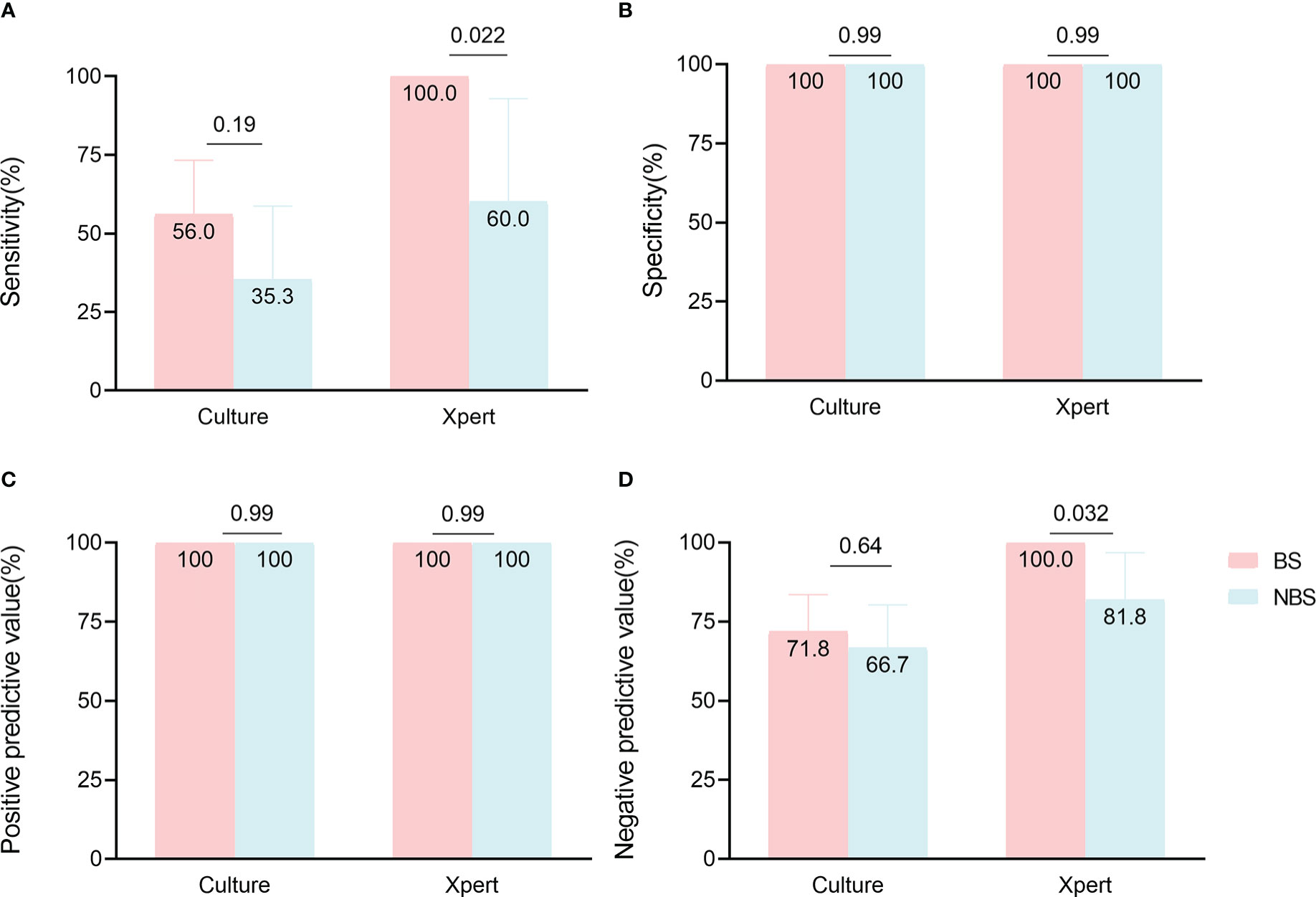
Figure 4 Comparison of culture and Xpert accuracy in paired biopsy samples (BS) and non-biopsy samples (NBS). Data are shown stratified according to (A) sensitivity, (B) specificity, (C) positive predictive value, (D) negative predictive value.
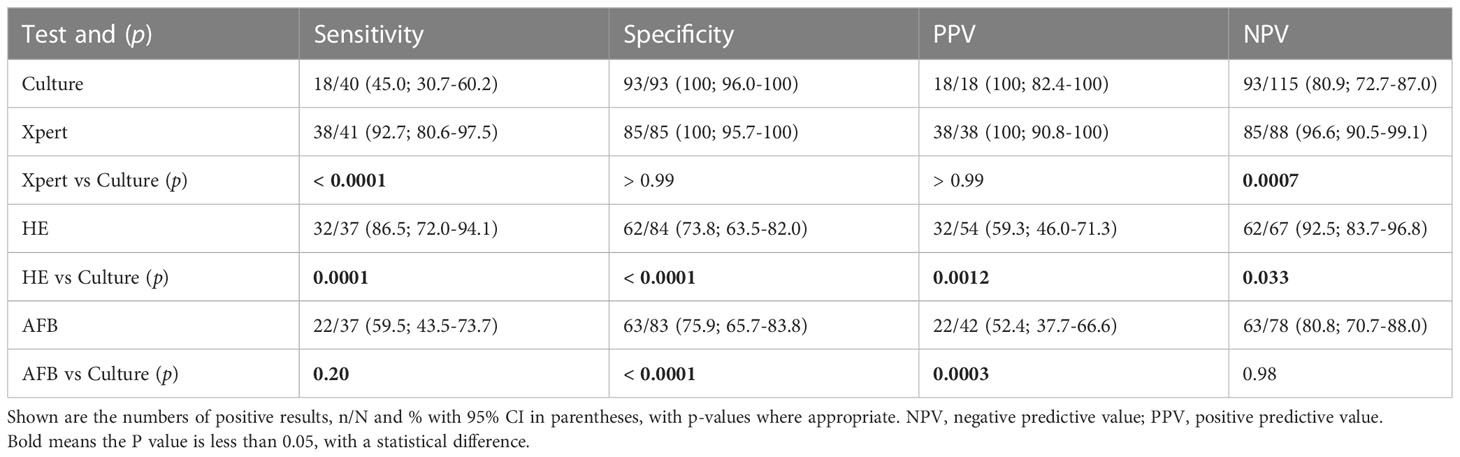
In smear-negative biopsy samples (Table 4), the Xpert had significantly higher sensitivity (92.7% [80.6-97.5] vs 45.0% [30.7-60.2], p < 0.0001) and NPV (96.6% [90.5-99.1] vs 80.9% [72.7-87.0], p = 0.0007) than culture, and comparable specificity and PPV to culture. The sensitivity (86.5% [72.0-94.1] vs 45.0% [30.7-60.2], p = 0.0001) and NPV (92.5% [83.7-96.8] vs 80.9% [72.7-87.0], p = 0.033) of HE were also higher than culture, while the specificity (HE: 73.8% [63.5-82.0] vs 100% [96.0-100], p < 0.0001; AFB: 75.9% [65.7-83.8] vs 100% [96.0-100], p < 0.0001) and PPV (HE: 59.3% [46.0-71.3] vs 100% [82.4-100], p = 0.0012; AFB: 52.4% [37.7-66.6] vs 100% [82.4-100], p = 0.0003) of histological methods were lower than culture (Table 4).
4 Discussion
In this retrospective analysis, we used biopsy samples and non-biopsy samples from patients with presumptive EPTB to determine the diagnostic accuracy, sensitivity, and specificity of the assays. Our key finding was that Xpert performed better than other laboratory assays regardless of the HIV status of the patients or the types of specimens. Overall, the biopsy samples provided more realistic pictures of the patient’s conditions and a more accurate diagnosis of EPTB than non-biopsy samples.
From the demographic aspects, several studies have reported similar findings that HIV co-infection and age (> 65 years old) contribute to EPTB infection (Lakoh et al., 2020; Winter et al., 2020; Barreto-Duarte et al., 2021), consistent with our results. However, we found that males were more likely to have EPTB than females (OR 1.89, 95%CI 1.10-3.29, p = 0.021), which was not consistent with some previous studies (Peto et al., 2009; Pang et al., 2019). This may be attributed to a higher proportion of HIV-positive men than women (34.8% vs 10.8%, p < 0.0001; Supplementary Table S3), although the relationship between gender and EPTB is controversial in current studies (Liu et al., 2020; Barreto-Duarte et al., 2021).
Culture is the gold standard for TB diagnosis, but culture cannot distinguish between MTB, BCG, and NTM, and its specificity is compromised (96.9% [91.3-99.2] in this study) when used in populations susceptible to NTM disease (e.g. HIV-positive patients) (Álvaro-Meca et al., 2015). Unexpectedly, the sensitivity (%65.3 [56.3-73.2] vs 56.1% [47.0-64.9], p = 0.16) of the smear was slightly higher than that of the culture. Compared to direct smears, centrifugally concentrated specimens can increase the sensitivity of the smear by 10-30%(Perera and Arachchi, 1999; Peterson et al., 1999), and the Auramine O staining used in this study had a higher sensitivity (66-85.9% vs 30-60%) than Ziehl-Neelsen staining (Marais et al., 2008; Laifangbam et al., 2009; Hooja et al., 2011; Runa et al., 2011; Assefa et al., 2021; Gulati et al., 2021), but the NaOH used to digest the specimens may have reduced the viability of mycobacteria or even killed mycobacteria (Mtafya et al., 2019; Stephenson et al., 2021). Auramine O staining is not able to distinguish between dead or live bacteria, but culture only detects viable bacteria, thus NaOH used in sample pre-treatment may result in lower sensitivity of culture than smear. Obtaining appropriate specimens for histological examinations was recommended for a patient with suspected EPTB (Hopewell et al., 2006; Migliori et al., 2018). In general, histopathology is highly sensitive (86%-95% reported; HE: 92.4% [85.7-96.1] and AFB: 81.7% [73.2-88.0] in this study), but not very specific (64%-92% reported; HE: 71.3% [61.0-79.7] and AFB: 73.3% [63.1-81.5] in this study), for the diagnosis of tuberculosis (Bennani et al., 2019; Shen et al., 2022; Tahseen et al., 2022).
Xpert was recommended by the World Health Organization as a rapid initial diagnostic test for tuberculosis (WHO, 2021b). For the diagnosis of EPTB, Xpert showed different performance in various types of samples (Scott et al., 2014; Kohli et al., 2021), with excellent performance in lymph node tissue and aspirates (sensitivity: 80-100%; specificity:90-100%) (Ablanedo-Terrazas et al., 2014; Scott et al., 2014; Tadesse et al., 2019), as demonstrated in this study (sensitivity: 96.6%[91.6-98.7]; specificity:100%[95.8-100]). HIV-positive patients are more likely to have comorbidities such as tumors (Lerner et al., 2020), and opportunistic infections (fungal infections, NTM infections, etc.) (Limper et al., 2017), and this may affect the specificity of detection of MTB (decreased specificity in this study: culture 5%; smear 8%; HE 23%; AFB 27%). However, Xpert maintained high sensitivity (>95%) and specificity (nearly 100%) in both HIV-positive and -negative patients, consistent with previous reports (sensitivity: >80%; specificity: 97-99%) (Horne et al., 2019; Tomaz et al., 2021). Finally, we evaluated the performance of different assays in smear-negative samples, and Xpert still had higher sensitivity (92.7% [80.6-97.5]) and specificity (100% [95.7-100]) compared to culture. This was slightly higher than the reported sensitivity (70-85%) (Bankar et al., 2018; Rakotoarivelo et al., 2018; Horne et al., 2019), perhaps due to the different choice of the reference standard (culture and Xpert were used in this study).
We found little statistical difference in the sensitivity, specificity, and predictive values of the non-biopsy samples-based Xpert compared to culture, mainly due to the small sample size. However, in agreement with previous studies, TSPOT showed a high sensitivity (70-100%) compared to culture and Xpert (Zhou et al., 2015; Li et al., 2020), predicting that TSPOT can be used as a powerful screening method for EPTB (Antel et al., 2020). Furthermore, the culture and Xpert performed better with biopsy samples than with non-biopsy samples, suggesting that biopsy is important for the accurate diagnosis of EPTB.
There are several limitations to this study. The smaller sample size of non-biopsy samples may affect the methodological comparison between non-biopsy samples and biopsy samples. The small number of samples assayed by various methods in non-biopsy samples was not conducive to evaluating the diagnostic accuracy of the method, for instance, the sensitivity of Xpert may be underestimated. In addition, we did not exclude patients with both pulmonary and extrapulmonary TB (42 cases), which may affect the comparison of assays between biopsy and non-biopsy samples.
In summary, our study compared the diagnostic accuracy of commonly used EPTB diagnostic methods across HIV status and sample types, highlighting the superiority of Xpert in different clinical settings and the critical contribution of biopsy samples in the diagnosis of EPTB. Further clinical studies evaluating the performance of the different laboratory assays in extrapulmonary samples and HIV populations are warranted to help clinicians choose the best diagnostic methods when faced with various dilemmas.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Public Health Clinical Center (2019-S030-02). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
Project concept conceived (X-YF and HZ), experiments performed (J-CX and W-FG), sampling (XM and XS), data analysis (J-CX, XM, W-FG, X-YF, and HZ), and paper writing (J-CX and X-YF). All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Key Research and Development Program of China (2022YFC2302900, 2021YFC2301503), National Natural and Science Foundation of China (82171815, 82171739), Shanghai Municipal Health Bureau (2022XD060) and Shanghai Science and Technology Commission (20Y11903400, 18411970800).
Acknowledgments
We are thankful to Dr. Douglas B. Lowrie for his critical reading and improvement on this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1154939/full#supplementary-material
References
Ablanedo-Terrazas, Y., Alvarado-de la Barrera, C., Hernández-Juan, R., Ruiz-Cruz, M., Reyes-Terán, G. (2014). Xpert MTB/RIF for diagnosis of tuberculous cervical lymphadenitis in HIV-infected patients. Laryngoscope 124 (6), 1382–1385. doi: 10.1002/lary.24478
Aisenberg, G. M., Jacobson, K., Chemaly, R. F., Rolston, K. V., Raad, I. I., Safdar, A. (2005). Extrapulmonary tuberculosis active infection misdiagnosed as cancer: Mycobacterium tuberculosis disease in patients at a comprehensive cancer center, (2001-2005). Cancer 104 (12), 2882–2887. doi: 10.1002/cncr.21541
Álvaro-Meca, A., Rodríguez-Gijón, L., Díaz, A., Gil, Á., Resino, S. (2015). Trends in nontuberculous mycobacterial disease in hospitalized subjects in Spain, (1997-2010) according to HIV infection. HIV Med. 16 (8), 485–493. doi: 10.1111/hiv.12251
Antel, K., Oosthuizen, J., Malherbe, F., Louw, V. J., Nicol, M. P., Maartens, G., et al. (2020). Diagnostic accuracy of the xpert MTB/Rif ultra for tuberculosis adenitis. BMC Infect. Dis. 20 (1), 33. doi: 10.1186/s12879-019-4749-x
Assefa, G., Desta, K., Araya, S., Girma, S., Mihret, A., Hailu, T., et al. (2021). Diagnostic efficacy of light-emitting diode (LED) fluorescence based microscope for the diagnosis of tuberculous lymphadenitis. PloS One 16 (7), e0255146. doi: 10.1371/journal.pone.0255146
Bankar, S., Set, R., Sharma, D., Shah, D., Shastri, J. (2018). Diagnostic accuracy of xpert MTB/RIF assay in extrapulmonary tuberculosis. Indian J. Med. Microbiol. 36 (3), 357–363. doi: 10.4103/ijmm.IJMM_18_173
Banta, J. E., Ani, C., Bvute, K. M., Lloren, J. I. C., Darnell, T. A. (2020). Pulmonary vs. extra-pulmonary tuberculosis hospitalizations in the US [1998-2014]. J. Infect. Public Health 13 (1), 131–139. doi: 10.1016/j.jiph.2019.07.001
Barreto-Duarte, B., Araújo-Pereira, M., Nogueira, B. M. F., Sobral, L., Rodrigues, M. M. S., Queiroz, A. T. L., et al. (2021). Tuberculosis burden and determinants of treatment outcomes according to age in Brazil: A nationwide study of 896,314 cases reported between 2010 and 2019. Front. Med. 8. doi: 10.3389/fmed.2021.706689
Bennani, K., Khattabi, A., Akrim, M., Mahtar, M., Benmansour, N., Essakalli Hossyni, L., et al. (2019). Evaluation of the yield of histopathology in the diagnosis of lymph node tuberculosis in Morocco 2017: Cross-sectional study. JMIR Public Health Surveill 5 (4), e14252. doi: 10.2196/14252
Golden, M. P., Vikram, H. R. (2005). Extrapulmonary tuberculosis: An overview. Am. Fam Physician 72 (9), 1761–1768.
Gulati, H. K., Mawlong, M., Agarwal, A., Ranee, K. R. (2021). Comparative evaluation of clinical, cytological and microbiological profile in abdominal vs. cervical lymph nodal tuberculosis with special emphasis on utility of auramine-O staining. J. Cytol 38 (4), 191–197. doi: 10.4103/joc.Joc_61_20
Hooja, S., Pal, N., Malhotra, B., Goyal, S., Kumar, V., Vyas, L. (2011). Comparison of ziehl neelsen & auramine O staining methods on direct and concentrated smears in clinical specimens. Indian J. Tuberc 58 (2), 72–76.
Hopewell, P. C., Pai, M., Maher, D., Uplekar, M., Raviglione, M. C. (2006). International standards for tuberculosis care. Lancet Infect. Dis. 6 (11), 710–725. doi: 10.1016/s1473-3099(06)70628-4
Horne, D. J., Kohli, M., Zifodya, J. S., Schiller, I., Dendukuri, N., Tollefson, D., et al. (2019). Xpert MTB/RIF and xpert MTB/RIF ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 6 (6), Cd009593. doi: 10.1002/14651858.CD009593.pub4
Jain, A. (2011). Extra pulmonary tuberculosis: a diagnostic dilemma. Indian J. Clin. Biochem. 26 (3), 269–273. doi: 10.1007/s12291-010-0104-0
Kohli, M., Schiller, I., Dendukuri, N., Yao, M., Dheda, K., Denkinger, C. M., et al. (2021). Xpert MTB/RIF ultra and xpert MTB/RIF assays for extrapulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst. Rev. 1 (1), Cd012768. doi: 10.1002/14651858.CD012768.pub3
Laifangbam, S., Singh, H. L., Singh, N. B., Devi, K. M., Singh, N. T. (2009). A comparative study of fluorescent microscopy with ziehl-neelsen staining and culture for the diagnosis of pulmonary tuberculosis. Kathmandu Univ Med. J. 7 (27), 226–230. doi: 10.3126/kumj.v7i3.2728
Lakoh, S., Jiba, D. F., Adekanmbi, O., Poveda, E., Sahr, F., Deen, G. F., et al. (2020). Diagnosis and treatment outcomes of adult tuberculosis in an urban setting with high HIV prevalence in Sierra Leone: A retrospective study. Int. J. Infect. Dis. 96, 112–118. doi: 10.1016/j.ijid.2020.04.038
Lerner, A. M., Eisinger, R. W., Fauci, A. S. (2020). Comorbidities in persons with HIV: The lingering challenge. Jama 323 (1), 19–20. doi: 10.1001/jama.2019.19775
Li, S., Lin, L., Zhang, F., Zhao, C., Meng, H., Wang, H. (2020). A retrospective study on xpert MTB/RIF for detection of tuberculosis in a teaching hospital in China. BMC Infect. Dis. 20 (1), 362. doi: 10.1186/s12879-020-05004-8
Li, T., Yan, X., Du, X., Huang, F., Wang, N., Ni, N., et al. (2022). Extrapulmonary tuberculosis in China: A national survey. Int. J. Infect. Dis. 128, 69–77. doi: 10.1016/j.ijid.2022.12.005
Limper, A. H., Adenis, A., Le, T., Harrison, T. S. (2017). Fungal infections in HIV/AIDS. Lancet Infect. Dis. 17 (11), e334–e343. doi: 10.1016/s1473-3099(17)30303-1
Liu, Y., Jiang, Z., Chen, H., Jing, H., Cao, X., Coia, J. E., et al. (2020). Description of demographic and clinical characteristics of extrapulmonary tuberculosis in Shandong, China. Hippokratia 24 (1), 27–32.
Marais, B. J., Brittle, W., Painczyk, K., Hesseling, A. C., Beyers, N., Wasserman, E., et al. (2008). Use of light-emitting diode fluorescence microscopy to detect acid-fast bacilli in sputum. Clin. Infect. Dis. 47 (2), 203–207. doi: 10.1086/589248
Migliori, G. B., Sotgiu, G., Rosales-Klintz, S., Centis, R., D'Ambrosio, L., Abubakar, I., et al. (2018). ERS/ECDC statement: European union standards for tuberculosis care 2017 update. Eur. Respir. J. 51 (5), 1702678. doi: 10.1183/13993003.02678-2017
Mtafya, B., Sabiiti, W., Sabi, I., John, J., Sichone, E., Ntinginya, N. E., et al. (2019). Molecular bacterial load assay concurs with culture on NaOH-induced loss of mycobacterium tuberculosis viability. J. Clin. Microbiol. 57 (7), e01992-18. doi: 10.1128/jcm.01992-18
Norbis, L., Alagna, R., Tortoli, E., Codecasa, L. R., Migliori, G. B., Cirillo, D. M. (2014). Challenges and perspectives in the diagnosis of extrapulmonary tuberculosis. Expert Rev. Anti Infect. Ther. 12 (5), 633–647. doi: 10.1586/14787210.2014.899900
Ohene, S. A., Bakker, M. I., Ojo, J., Toonstra, A., Awudi, D., Klatser, P. (2019). Extra-pulmonary tuberculosis: A retrospective study of patients in Accra, Ghana. PloS One 14 (1), e0209650. doi: 10.1371/journal.pone.0209650
Pang, Y., An, J., Shu, W., Huo, F., Chu, N., Gao, M., et al. (2019). Epidemiology of extrapulmonary tuberculosis among inpatients, China 2008-2017. Emerg. Infect. Dis. 25 (3), 457–464. doi: 10.3201/eid2503.180572
Park, M., Kon, O. M. (2021). Use of xpert MTB/RIF and xpert ultra in extrapulmonary tuberculosis. Expert Rev. Anti Infect. Ther. 19 (1), 65–77. doi: 10.1080/14787210.2020.1810565
Perera, J., Arachchi, D. M. (1999). The optimum relative centrifugal force and centrifugation time for improved sensitivity of smear and culture for detection of mycobacterium tuberculosis from sputum. Trans. R Soc. Trop. Med. Hyg 93 (4), 405–409. doi: 10.1016/s0035-9203(99)90135-9
Peterson, E. M., Nakasone, A., Platon-DeLeon, J. M., Jang, Y., de la Maza, L. M., Desmond, E. (1999). Comparison of direct and concentrated acid-fast smears to identify specimens culture positive for mycobacterium spp. J. Clin. Microbiol. 37 (11), 3564–3568. doi: 10.1128/jcm.37.11.3564-3568.1999
Peto, H. M., Pratt, R. H., Harrington, T. A., LoBue, P. A., Armstrong, L. R. (2009). Epidemiology of extrapulmonary tuberculosis in the united states 1993-2006. Clin. Infect. Dis. 49 (9), 1350–1357. doi: 10.1086/605559
Rakotoarivelo, R., Ambrosioni, J., Rasolofo, V., Raberahona, M., Rakotosamimanana, N., Andrianasolo, R., et al. (2018). Evaluation of the xpert MTB/RIF assay for the diagnosis of smear-negative pulmonary and extrapulmonary tuberculosis in Madagascar. Int. J. Infect. Dis. 69, 20–25. doi: 10.1016/j.ijid.2018.01.017
Rickman, T. W., Moyer, N. P. (1980). Increased sensitivity of acid-fast smears. J. Clin. Microbiol. 11 (6), 618–620. doi: 10.1128/jcm.11.6.618-620.1980
Runa, F., Yasmin, M., Hoq, M. M., Begum, J., Rahman, A. S., Ahsan, C. R. (2011). Molecular versus conventional methods: Clinical evaluation of different methods for the diagnosis of tuberculosis in Bangladesh. J. Microbiol. Immunol. Infect. 44 (2), 101–105. doi: 10.1016/j.jmii.2010.05.001
Scott, L. E., Beylis, N., Nicol, M., Nkuna, G., Molapo, S., Berrie, L., et al. (2014). Diagnostic accuracy of xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for south Africa. J. Clin. Microbiol. 52 (6), 1818–1823. doi: 10.1128/jcm.03553-13
Shen, Y., Fang, L., Ye, B., Xu, X., Yu, G., Zhou, L. (2022). The role of core needle biopsy pathology combined with molecular tests in the diagnosis of lymph node tuberculosis. Infect. Drug Resist. 15, 335–345. doi: 10.2147/idr.S350570
Shivakoti, R., Sharma, D., Mamoon, G., Pham, K. (2017). Association of HIV infection with extrapulmonary tuberculosis: A systematic review. Infection 45 (1), 11–21. doi: 10.1007/s15010-016-0960-5
Stephenson, D., Perry, A., Nelson, A., Robb, A. E., Thomas, M. F., Bourke, S. J., et al. (2021). Decontamination strategies used for AFB culture significantly reduce the viability of mycobacterium abscessus complex in sputum samples from patients with cystic fibrosis. Microorganisms 9 (8), 1597. doi: 10.3390/microorganisms9081597
Tadesse, M., Abebe, G., Bekele, A., Bezabih, M., Yilma, D., Apers, L., et al. (2019). Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a diagnostic evaluation study. Clin. Microbiol. Infect. 25 (8), 1000–1005. doi: 10.1016/j.cmi.2018.12.018
Tahseen, S., Ambreen, A., Ishtiaq, S., Khanzada, F. M., Safdar, N., Sviland, L., et al. (2022). The value of histological examination in the diagnosis of tuberculous lymphadenitis in the era of rapid molecular diagnosis. Sci. Rep. 12 (1), 8949. doi: 10.1038/s41598-022-12660-0
Tomaz, A. P. O., Raboni, S. M., Kussen, G. M. B., da Silva Nogueira, K., Lopes Ribeiro, C. E., Costa, L. M. D. (2021). The xpert® MTB/RIF diagnostic test for pulmonary and extrapulmonary tuberculosis in immunocompetent and immunocompromised patients: Benefits and experiences over 2 years in different clinical contexts. PloS One 16 (3), e0247185. doi: 10.1371/journal.pone.0247185
Winter, J. R., Smith, C. J., Davidson, J. A., Lalor, M. K., Delpech, V., Abubakar, I., et al. (2020). The impact of HIV infection on tuberculosis transmission in a country with low tuberculosis incidence: a national retrospective study using molecular epidemiology. BMC Med. 18 (1), 385. doi: 10.1186/s12916-020-01849-7
WHO (2020). Global tuberculosis report 2020. Geneva, Switzerland. Available at: http://www.who.int/tb/publications/global_report/en/
WHO. (2021a). WHO consolidated guidelines on tuberculosis. Module 2: Screening - systematic screening for tuberculosis disease. Geneva, Switzerland. Available at: https://www.who.int/publications/i/item/9789240022614
WHO (2021b). WHO consolidated guidelines on tuberculosis: module 3: diagnosis: rapid diagnostics for tuberculosis detection. Geneva, Switzerland. Available at: https://www.who.int/publications/i/item/9789240029415
WHO (2022). Global tuberculosis report 2022. Geneva, Switzerland. Available at: http://www.who.int/tb/publications/global_report/en/
Xiang, Y., Huang, C., He, Y., Zhang, Q. (2021). Cancer or tuberculosis: A comprehensive review of the clinical and imaging features in diagnosis of the confusing mass. Front. Oncol. 11. doi: 10.3389/fonc.2021.644150
Keywords: extrapulmonary tuberculosis (EPTB), diagnosis, biopsy, Xpert, human immunodeficiency virus (HIV)
Citation: Xu J-C, Shi X, Ma X, Gu W-f, Fang Z-x, Zhang H and Fan X-Y (2023) Diagnosis of extrapulmonary tuberculosis by ultrasound-guided biopsy: A retrospective comparison study. Front. Cell. Infect. Microbiol. 13:1154939. doi: 10.3389/fcimb.2023.1154939
Received: 31 January 2023; Accepted: 07 March 2023;
Published: 22 March 2023.
Edited by:
Amit Singh, Central University of Punjab, IndiaReviewed by:
Parul Singh, All India Institute of Medical Sciences Gorakhpur, IndiaGuoliang Zhang, Shenzhen Third People’s Hospital, China
Copyright © 2023 Xu, Shi, Ma, Gu, Fang, Zhang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Yong Fan, eHlmYW4wMDhAZnVkYW4uZWR1LmNu; Hui Zhang, WmhhbmcuaHVpQHpzLWhvc3BpdGFsLnNoLmNu
†These authors have contributed equally to this work
 Jin-Chuan Xu
Jin-Chuan Xu Xia Shi1†
Xia Shi1† Xiao-Yong Fan
Xiao-Yong Fan