
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 12 May 2023
Sec. Fungal Pathogenesis
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1152552
This article is part of the Research Topic Emerging Fungal Pathogens: Perspectives View all 7 articles
 Mahdieh Sharifi1
Mahdieh Sharifi1 Parisa Badiee2
Parisa Badiee2 Mahdi Abastabar3,4
Mahdi Abastabar3,4 Hamid Morovati5
Hamid Morovati5 Iman Haghani3,4
Iman Haghani3,4 Mahta Noorbakhsh6
Mahta Noorbakhsh6 Rasoul Mohammadi1,7*†
Rasoul Mohammadi1,7*†Objective: Opportunistic fungal infections by Candida species arise among cancer patients due to the weakened immune system following extensive chemotherapy. Prophylaxis with antifungal agents have developed the resistance of Candida spp. to antifungals. Accurate identification of yeasts and susceptibility patterns are main concerns that can directly effect on the treatment of patients.
Methods: Over a period of three years, 325 cancer patients suspected to Candida infections were included in the current investigation. The clinical isolates were molecularly identified by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). All strains, were examined for in vitro susceptibility to the amphotericin B, itraconazole, fluconazole, and anidulafungin according to the CLSI M27 document.
Results: Seventy-four cancer patients had Candida infections (22.7%). Candida albicans was the most common species (83.8%). Antifungal susceptibility results indicated that 100% of the Candida isolates were sensitive to amphotericin B; however, 17.6%, 9.4%, and 5.4% of clinical isolates were resistant to anidulafungin, fluconazole, and itraconazole, respectively.
Conclusion: The findings of the present work shows a warning increase in resistance to echinocandins. Since all fluconazole resistance isolates were obtained from candidemia, we recommend amphotericin B as the first line therapy for this potentially fatal infection.
Candidiasis is an opportunistic fungal infection closely connected to malignancies and the complications of their treatment. Incidence of Candida infections has been showed to be ranging from 7 to 52% among cancer patients (Lone et al., 2014). The cytotoxic anti-cancer drugs have severe effects on mucosal immune defense, leading to Candida colonization. Candida may impel some types of cancer including oral squamous cell carcinoma (OSCC) by using of carcinogenic compounds production such as nitrosamines and N−nitrosobenzylmethylamine (Krogh et al., 1987). Although C. albicans is the most prevalent species, but non-albicans Candida species including C. tropicalis, Pichia kudriavzevii (C. krusei), Meyerozyma guilliermondii (C. guilliermondii), C. glabrata, C. parapsilosis, and Kluyveromyces marxianus (C. kefyr) with a reduced susceptibility to echinocandins and triazoles become a consequential clinical challenge (Eddouzi et al., 2013). Prophylaxis with azoles and echinocandins among vulnerable populations, have been connected to a shift from C. albicans to non-albicans Candida species in some countries (Cleveland et al., 2012; Pfaller et al., 2014a). Since there are main differences in species distributions and drug susceptibilities in various regions, we aimed to determine Candida distribution and antifungal susceptibility of clinical isolates among cancer patients in Isfahan, Iran.
A total of 325 suspected cases referred to 4 university hospitals (Al-Zahra, Seyed-al-Shohada, Imam Hossein, and Amin) were included in this study from April 2018 to June 2021 based on clinical manifestations. Patients who had not received haematological/oncological treatment within the past 12 months were excluded from the study. All types of Candida infections were included. Written informed consent for participation provided by all patients.
Boiling method was used to extract genomic DNA (Silva et al., 2012). Briefly, a loopful of fresh colony was suspended in 80 µL of double distilled water and boiled for 20 min, then centrifuged for 8 min at 6000 rpm, and then the supernatant (containing DNA) was used for PCR. The ITS1-5.8S-ITS2 region was amplified using the universal primers ITS1 (5-TCCGTAGGTGAACCTGCGG-3) and ITS4 (5- TCCTCCGCTTATTGATATGC-3) (White et al., 1990) in a final volume of 25 μl, containing 2 μl of extracted DNA, 0.4 mM of dNTPs, 1.5 mM of MgCl2, 30 pmol of each primer, 1.25 U of Taq DNA polymerase, and 2.5 μl of 10× PCR buffer. The following program was set for PCR: 1 cycle at 95°C for 5 min, followed by 30 cycles of 1 min at 94°C, 45 sec at 55°C, and 45 sec at 72°C, with a final extension step at 72°C for 7 min. Digestion of PCR products were performed with 1U of restriction enzyme MspI (Fermentas, Vilnius, Lithuania) in a final reaction volume of 15 μl containing 3.5 μl water, 1.5 μl buffer, and 10 μl PCR product at 37°C for 20 min (FastDigest™). PCR amplicons and RFLP products were run onto 1.5% and 2% agarose gel electrophoresis, respectively. The products were stained with SYBR Safe DNA gel stain (1:10,000 dilution in Tris/Borate/EDTA) and then photographed.
According to the clinical and laboratory standard institute (CLSI) document M27 (Clinical and Laboratory Standards Institute (M27), 2017), minimum inhibitory concentrations (MICs) of antifungals viz. fluconazole (Sigma-Aldrich, Germany), amphotericin B (Sigma-Aldrich, Germany), itraconazole (Janssen Research Foundation, Beerse, Belgium), and anidulafungin (Cayman Chemical, USA) were assessed. Final concentrations for antifungal agents were as follows: itraconazole and amphotericin B (0.0313–16 μg/ml), fluconazole (0.125–64 μg/ml), and anidulafungin (0.015–8 μg/ml). A serial dilution of each antifungal was prepared in RPMI1640 medium (with L-Glutamine, without bicarbonate) (Sigma Chemical Co., St. Louis, MO, USA). Compared to a McFarland standard; no. 0.5 = 1–5×106 CFU/ml, the desired final inoculum size in the wells was 0.5×103 to 2.5×103 CFU/ml after 100 μl inoculation. Microplates were incubated at 35°C, and the MICs were visually determined after 24 h. The MIC endpoint for fluconazole, itraconazole, and anidulafungin have been described as the level which inhibited a significant growth of fungus (50%) compared to drug-free growth control, while for amphotericin B, 100% growth inhibition is considered. Table 1 shows interpretive breakpoints for in vitro susceptibility testing of Candida species according to M27 and M60 documents, Borman et al., and Mroczyńska, et al. (Clinical and Laboratory Standards Institute (M27), 2017; Clinical and Laboratory Standards Institute (M60), 2017; Borman et al., 2020; Mroczyńska and Brillowska-Dąbrowska, 2020).
The relationship among Candida infections and age of patients, gender, and type of cancer was adjusted using Fisher’s exact test and Mann–Whitney U−test. A P-value less than 0.05 was considered statistically significant. The MIC range, MIC50 (the minimum concentration of antifungal agent at which 50% of isolates are inhibited) and MIC90 (the minimum concentration of antifungal agent at which 90% of isolates are inhibited) were determined.
Over the investigation period, 74 cases of Candida infection were detected. Forty-eight patients (64.8%) had solid organ tumors and 26 patients (35.2%) had haematological malignancies (Figure 1). Age range was between 1-89 years with median age of 47.3. Gender ratio was 43 males per 31females. The most Candida species were isolated from urine (n=24), bronchoalveolar lavage (BAL) (n=15), blood (n=13), and esophageal biopsies (n=7) (Table 2). Candida albicans was the most prevalent species (83.8%) followed by C. glabrata (5.4%), C. tropicalis (4%), C. parapsilosis (4%), C. krusei (1.3%), and C. famata (1.3%) (Figure 2; Table 2). The prevalence of C. albicans was significantly higher than other species (P = 0.029). In solid organ tumor group, the type of cancer was not statistically different (P = 0.085); however, in patients with haematological malignancies, leukemia was significantly more common among patients (P = 0.043) (Table 3). Antifungal susceptibility results are shown in Table 4. Briefly, our findings indicated that all clinical isolates (100%) were sensitive to amphotericin B. The lowest activity was observed in anidulafungin against Candida spp. In addition, 83.8% and 81.1% of isolates were sensitive to fluconazole and itraconazole, respectively.
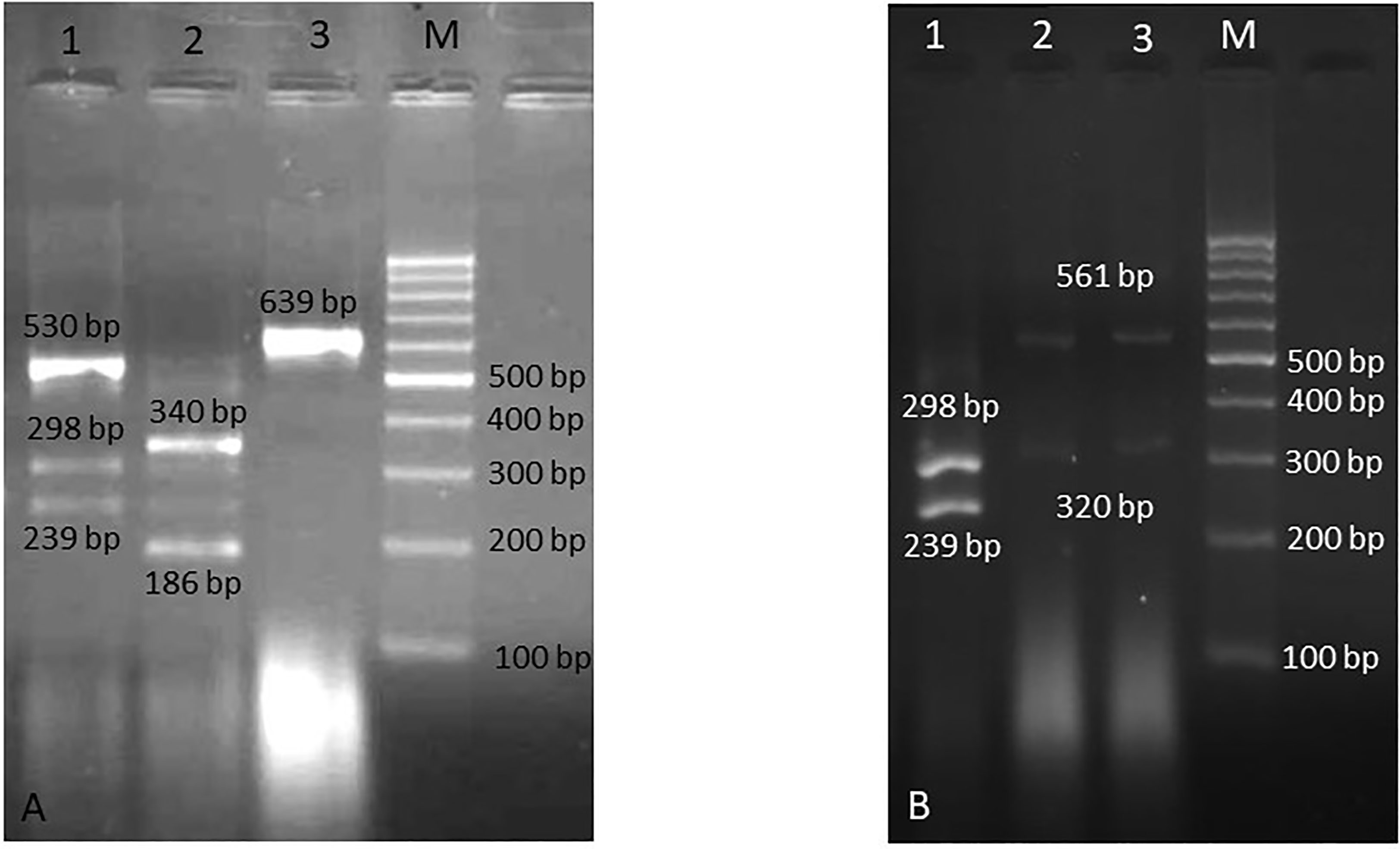
Figure 2 Agarose gel electrophoresis of RFLP products. (A) Lane 1: C. albicans and C. parapsilosis (mixed), lane 2: C. tropicalis, lane 3: C. famata, and M is 100 bp DNA size marker, (B) Lane 1: C. albicans, lanes 2, 3: C. glabrata, and M is 100 bp DNA size marker.
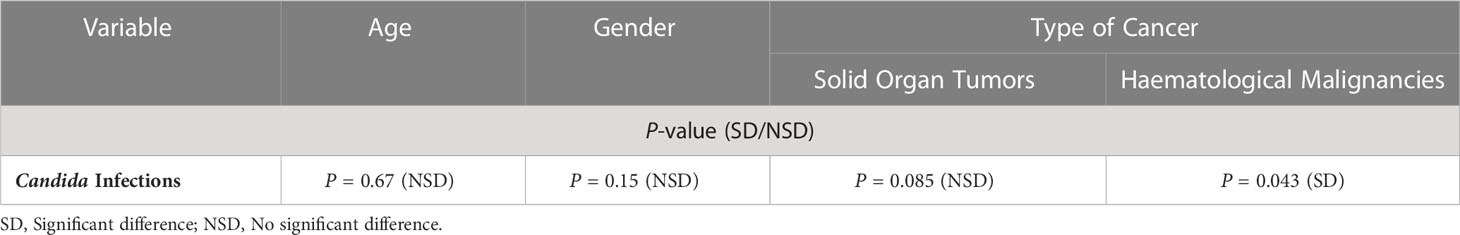
Table 3 Statistical analysis for Candida infections and variables of age, gender and type of cancer.
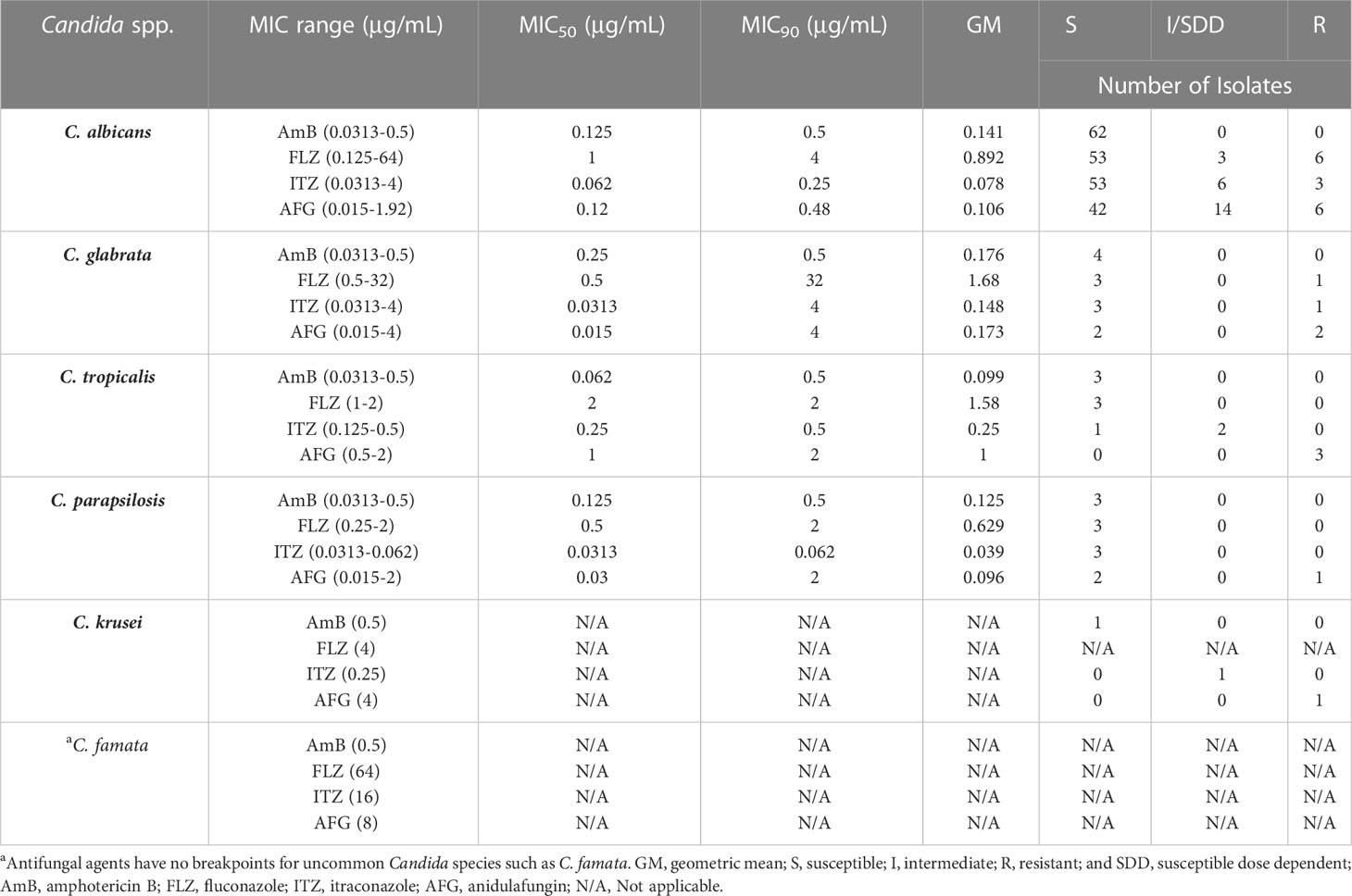
Table 4 MIC range, MIC50, MIC90, and geometric mean of the antifungals against Candida spp. and susceptibility pattern of clinical isolates.
Candida infections can be considered as an important sign of immunosuppression in patients with malignancies. Most cancer patients are neutropenic (diminution of blood neutrophils to less than 1500/mm3) due to the cytotoxic chemotherapy, hematological disorders, and acute leukemia (Walsh and Gamaletsou, 2013). This condition is one of the main predisposing factors for invasive fungal infections (IFIs) such as invasive aspergillosis and candidiasis (Spiess et al., 2007). The incidence of fungal infections among cancer patients is connected to the immune system status, antifungal resistance rates, and type of malignancy (Bhatt et al., 2011). In the present study, the most clinical samples were obtained from urine (32.4%). Candiduria in cancer patients should be considered as a marker for disseminated candidiasis and appropriate antifungal therapy is needed (Georgiadou et al., 2013). All patients with candiduria were treated with fluconazole; however, in three patients (12.5%), amphotericin B were added to their regimen due to the positive blood culture. The presence of Candida in the upper respiratory tract of cancer patients is usual, and cannot be evaluated as invasive pulmonary Candida infection. Only histopathological examinations can prove the infection (Kontoyiannis et al., 2002), nevertheless, none of the patients with pulmonary symptoms (n=15) underwent pathology examination, and this is one of the main limitations of our study. Candidemia is a fatal fungal infection nearly related to cancer and the difficulties of its treatment. Here, we isolated 13 Candida strains (17.5%) from patients with bloodstream infections (BSIs). Interestingly, except for one case, all BSIs were caused by C. albicans, and all fluconazole-resistant isolates were obtained from the blood samples. Gastroesophageal biopsies were in the fourth place of clinical specimens that were collected endoscopically. Gastroesophageal candidiasis have been shown to be increasing in cancer patients who consuming acid suppressing therapy (AST) and proton pump inhibitors (PPIs) (Daniell, 2016), because these drugs change the gastric pH, which can encourage Candida colonization of the esophagus (patients with reflux) and gastrointestinal tract (Shah et al., 2015). In agreement, all patients were taking PPIs (omeprazole; n=6, pantoprazole; n=3, and lansoprazole; n=2) in this investigation. Although stomach and esophageal cancers were the most prevalent malignancies reported by Kolahdoozan et al. in 2010 (Kolahdoozan et al., 2010); however, leukemia and breast cancers were the most common types of cancers in our study (Figure 1). Nine out of 13 patients with candidemia had leukemia (69.2%). Candida albicans is the most common opportunistic yeast in the clinical setting, which causes a widespread of infections ranging from mucocutaneous lesions to lethal deep-tissue infections (Pu et al., 2017). We also revealed C. albicans as the most frequent species in this survey (83.8%) which was isolated from all specimens except nail infection. In vitro antifungal activities of four antifungal agents were assessed for clinical isolates, and we found that 17.6% of Candida spp. were resistant to anidulafungin, 9.4% to fluconazole, and 5.4% to itraconazole. None of them were resistant to amphotericin B. Our results are in line with Hamzehee et al. (2019) which reported a 9.52% and 4.7% resistance to fluconazole and itraconazole, respectively. Unlike the study of Sharifynia et al. (2016), who reported 62.5% resistance to amphotericin B, in the present study, all isolates were sensitive to amphotericin B, which were consistent with the results of the Roy study (Roy et al., 2013). Infectious Diseases Society of America (IDSA) proposes the echinocandins (micafungin, caspofungin, and anidulafungin) as the newest class of antifungals for disseminated Candida infections as initial therapy in both neutropenic and non-neutropenic patients (Pappas et al., 2016), nevertheless, fluconazole is still broadly consumed due to its availability for both parenteral and enteral administration and low cost of the drug. Our findings showed that resistance to anidulafungin was more than fluconazole, and 6 out of 12 resistant isolates (50%) were obtained from candidemia. Unlike the study of Mohamed et al. (2018), which the most common strains were non-albicans (70.7%), in the present study, 83.8% of the clinical isolates were C. albicans. This variation in different geographical areas is related to many factors such as patient demographic features, various antifungal therapy practices, chronic underlying diseases, and use of indwelling catheters (Yapar, 2014). Candida tropicalis is main reported non-albicans Candida species in Asia and tropical regions (Tan et al., 2016) compared to the United States and Europe, where C. glabrata is the most leading non-albicans species (Falagas et al., 2010); however, C. glabrata was the most prevalent non-albicans Candida species in the current study. Increased number of C. glabrata isolates in Iran (Aslani et al., 2018; Mardani et al., 2020), may be related to the overuse of fluconazole as prophylaxis and treatment.
Although prophylaxis with azoles and echinocandins is one of the main factors in shifting the etiologic agents from albicans to non-albicans (Pfaller et al., 2014a), but our results revealed that C. albicans was still the leading cause of infection. The echinocandin anidulafungin is broadly used as first-line antifungal therapy for invasive candidiasis and candidemia (Pfaller et al., 2014b); nevertheless, the antifungal resistance was predominantly restricted to anidulafungin (17.6%) and fluconazole (9.4%) in the present study. Among non-albicans Candida species, all C. tropicalis were resistant to anidulafungin, and C. glabrata showed the most resistance to antifungal agents. Since the resistance to echinocandins and other antifungal agents is still infrequent, it alarms for an uninterrupted inspection and exacerbation of antifungal stewardship policies to decrease acquired resistance among clinical isolates.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
This research was approved by the Ethics committee of Isfahan University of Medical Sciences (no. IR.MUI.MED.REC.1399.1056). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Writing original draft preparation: RM. Patient’s follow-up and data collection: MS, MN and RM. Identification of the fungi: RM, MS and HM. Antifungal susceptibility testing: MS, PB, MA and IH. Reviewing and editing the manuscript: RM, HM and PB. All authors contributed to the article and approved the submitted version.
The Project was financially supported by a grant from the Isfahan University of Medical Sciences (no. 399985).
The authors would like to thank the laboratory personnel of Al-Zahra, Seyed Al-Shohada, Amin, and Imam Hossein Children’s hospitals for their cooperation to collect clinical specimens.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aslani, N., Janbabaei, G., Abastabar, M., Meis, J. F., Babaeian, M., Khodavaisy, S., et al. (2018). Identification of uncommon oral yeasts from cancer patients by MALDI-TOF mass spectrometry. BMC Infect. Dis. 18, 24. doi: 10.1186/s12879-017-2916-5
Bhatt, V. R., Viola, G. M., Ferrajoli, A. (2011). Invasive fungal infections in acute leukemia. Ther. Adv. Hematol. 2, 231–247. doi: 10.1177/2040620711410098
Borman, A. M., Muller, J., Walsh-Quantick, J., Szekely, A., Patterson, Z., Palmer, M. D., et al. (2020). MIC distributions for amphotericin b, fluconazole, itraconazole, voriconazole, flucytosine and anidulafungin and 35 uncommon pathogenic yeast species from the UK determined using the CLSI broth microdilution method. J. Antimicrob. Chemother. 75, 1194–1205. doi: 10.1093/jac/dkz568
Cleveland, A. A., Farley, M. M., Harrison, L. H., Stein, B., Hollick, R., Lockhart, S. R., et al. (2012). Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008-2011. Clin. Infect. Dis. 55 (10), 1352–1361. doi: 10.1093/cid/cis697
Clinical and Laboratory Standards Institute (M27) (2017). Reference method for broth dilution antifungal susceptibility testing of yeasts. 4th ed. Ed. Wayne, P. A. (USA: Clinical and Laboratory Standards Institute).
Clinical and Laboratory Standards Institute (M60) (2017). Reference method for broth dilution antifungal susceptibility testing of yeasts. 1st ed. Ed. Wayne, P. A. (USA: Clinical and Laboratory Standards Institute).
Daniell, H. W. (2016). Acid suppressing therapy as a risk factor for candida esophagitis. Dis. Esophagus 29, 479–483. doi: 10.1111/dote.12354
Eddouzi, J., Lohberger, A., Vogne, C., Manai, M., Sanglard, D. (2013). Identification and antifungal susceptibility of a large collection of yeast strains isolated in Tunisian hospitals. Med. Mycol. 51, 737–746. doi: 10.3109/13693786.2013.800239
Falagas, M. E., Roussos, N., Vardakas, K. Z. (2010). Relative frequency of albicans and the various non-albicans candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int. J. Infect. Dis. 14, e954–e966. doi: 10.1016/j.ijid.2010.04.006
Georgiadou, S. P., Tarrand, J., Sipsas, N. V., Kontoyiannis, D. P. (2013). Candiduria in haematologic malignancy patients without a urinary catheter: nothing more than a frailty marker? Mycoses 56, 311–314. doi: 10.1111/myc.12024
Hamzehee, S., Kalantar-Neyestanaki, D., Afshari, S. A. K., Mousavi, S. A. A. (2019). Molecular identification of candida species, assessment of the antifungal susceptibility and the genetic relationship of candida albicans isolated from immunocompromised patients in kerman, Iran. Gene Rep. 17, 100484. doi: 10.1016/j.genrep.2019.100484
Kolahdoozan, S., Sadjadi, A., Radmard, A. R., Khademi, H. (2010). Five common cancers in Iran. Arch. Iran Med. 13, 143–146.
Kontoyiannis, D. P., Reddy, B. T., Torres, H. A., Luna, M., Lewis, R. E., Tarrand, J., et al. (2002). Pulmonary candidiasis in patients with cancer: an autopsy study. Clin. Infect. Dis. 34, 400–403. doi: 10.1086/338404
Krogh, P., Hald, B., Holmstrup, P. (1987). Possible mycological etiology of oral mucosal cancer: catalytic potential of infecting Candida aibicans and other yeasts in production of N - nitrosobenzylmethylamine. Carcinogenesis 8 (10), 1543–1548. doi: 10.1093/carcin/8.10.1543
Lone, M. S., Bashir, G., Bali, N., Sajad, S., Aejaz, S., Bashir, H., et al. (2014). Oral Candida colonization and infection in cancer patients and their antifungal susceptibility in a tertiary care hospital Int. J. Adv. Res. 2, 541–550.
Mardani, M., Abolghasemi, S., Darvishnia, D., Lotfali, E., Ghasemi, R., Rabiei, M. M., et al. (2020). Oral candidiasis in hematological malignancy patients: identification and antifungal susceptibility patterns of isolates. Jundishapur J. Microbiol. 13, 1–6. doi: 10.5812/jjm.103290
Mohamed, N. A., Pathmanathan, S. G., Hussin, H., Zaini, A. B. (2018). Distribution and antifungal susceptibility pattern of candida species at a tertiary hospital in Malaysia. J. Infect. Dev. Ctries 12, 102–108. doi: 10.3855/jidc.9634
Mroczyńska, M., Brillowska-Dąbrowska, A. (2020). Review on current status of echinocandins use. Antibiotics (Basel) 9, 227. doi: 10.3390/antibiotics9050227
Pappas, P. G., Kauffman, C. A., Andes, D. R., Clancy, C. J., Marr, K. A., Ostrosky-Zeichner, L., et al. (2016). Clinical practice guideline for the management of candidiasis: 2016 update by the infectious diseases society of America. Clin. Infect. Dis. 62, e1–50. doi: 10.1093/cid/civ933
Pfaller, M. A., Andes, D. R., Diekema, D. J., Horn, D. L., Reboli, A. C., Rotstein, C., et al. (2014a). Epidemiology and outcomes of invasive candidiasis due to non-albicans species of candida in 2,496 patients: data from the prospective antifungal therapy (PATH) registry 2004-2008. PloS One 9, e101510. doi: 10.1371/journal.pone.0101510
Pfaller, M. A., Espinel-Ingroff, A., Bustamante, B., Canton, E., Diekema, D. J., Fothergill, A., et al. (2014b). Multicenter study of anidulafungin and micafungin MIC distributions and epidemiological cutoff values for eight candida species and the CLSI M27-A3 broth microdilution method. Antimicrob. Agents Chemother. 58, 916–922. doi: 10.1128/AAC.02020-13
Pu, S., Niu, S., Zhang, C., Xu, X., Qin, M., Huang, S., et al. (2017). Epidemiology, antifungal susceptibilities, and risk factors for invasive candidiasis from 2011 to 2013 in a teaching hospital in southwest China. J. Microbiol. Immunol. Infect. 50, 97–103. doi: 10.1016/j.jmii.2015.01.005
Roy, R., Sharma, G., Barman, S., Ch, S. (2013). Trend of candida infection and antifungal resistance in a tertiary care hospital of north east India. Afr. J. Microbiol. Res. 7, 3112–3116. doi: 10.5897/AJMR12.2257
Shah, M. D. N., Cavanagh, Y., Shulik, O., Patel, P., DeBari, V. A., Baddoura, W. (2015). Proton pump inhibitors and corticosteroids as synergistic risk factors for candida esophagitis. J. Adv. Med. Med. Res. 10, 1–6. doi: 10.9734/BJMMR/2015/20171
Sharifynia, S., Badali, H., Sharifi Sorkherizi, M., Shidfar, M. R., Hadian, A., Shahrokhi, S., et al. (2016). In vitro antifungal susceptibility profiles of candida albicans complex isolated from patients with respiratory infections. Acta Med. Iran 54, 376–381.
Silva, G., Bernardi, T. L., Schaker, P. D. C., Menegotto, M., Valente, P. (2012). Rapid yeast DNA extraction by boiling and freeze-thawing without using chemical reagents and DNA purification. Braz. Arch. Biol. Technol. 55, 319–327. doi: 10.1590/S1516-89132012000200020
Spiess, B., Seifarth, W., Hummel, M., Frank, O., Fabarius, A., Zheng, C., et al. (2007). DNA Microarray-based detection and identification of fungal pathogens in clinical samples from neutropenic patients. J. Clin. Microbiol. 45, 3743–3753. doi: 10.1128/JCM.00942-07
Tan, T. Y., Hsu, L. Y., Alejandria, M. M., Chaiwarith, R., Chinniah, T., Chayakulkeeree, M., et al. (2016). Antifungal susceptibility of invasive candida bloodstream isolates from the Asia-pacific region. Med. Mycol. 54, 471–477. doi: 10.1093/mmy/myv114
Walsh, T. J., Gamaletsou, M. N. (2013). Treatment of fungal disease in the setting of neutropenia. Hematol. Am. Soc. Hematol. Educ. Program 2013, 423–427. doi: 10.1182/asheducation-2013.1.423
White, T. J., Bruns, T., Lee, S., Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: guide to Methods applications 18, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Keywords: Candida infections, malignancy, antifungal susceptibility, molecular identification, epidemiology
Citation: Sharifi M, Badiee P, Abastabar M, Morovati H, Haghani I, Noorbakhsh M and Mohammadi R (2023) A 3-year study of Candida infections among patients with malignancy: etiologic agents and antifungal susceptibility profile. Front. Cell. Infect. Microbiol. 13:1152552. doi: 10.3389/fcimb.2023.1152552
Received: 27 January 2023; Accepted: 27 April 2023;
Published: 12 May 2023.
Edited by:
Danielly Corrêa Moreira, Oswaldo Cruz Foundation (Fiocruz), BrazilReviewed by:
Nivea Pereira De Sa, Stony Brook University, United StatesCopyright © 2023 Sharifi, Badiee, Abastabar, Morovati, Haghani, Noorbakhsh and Mohammadi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rasoul Mohammadi, RHIucmFzb3VsX21vaGFtbWFkaUB5YWhvby5jb20=
†ORCID: Rasoul Mohammadi, orcid.org/0000-0002-8220-4511
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.