
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 16 June 2023
Sec. Virus and Host
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1151899
This article is part of the Research Topic Unsolved Challenges in Hepatitis B and Hepatitis C: From Prevention to Treatment View all 20 articles
 Jun Li1†
Jun Li1† Xiao-Qin Dong2†
Xiao-Qin Dong2† Li-Hua Cao3
Li-Hua Cao3 Zhan-Qing Zhang4
Zhan-Qing Zhang4 Wei-Feng Zhao5
Wei-Feng Zhao5 Qing-Hua Shang6
Qing-Hua Shang6 Da-Zhi Zhang7
Da-Zhi Zhang7 An-Lin Ma8
An-Lin Ma8 Qing Xie9
Qing Xie9 Hong-Lian Gui9
Hong-Lian Gui9 Guo Zhang10
Guo Zhang10 Ying-Xia Liu11
Ying-Xia Liu11 Jia Shang12
Jia Shang12 Shi-Bin Xie13
Shi-Bin Xie13 Yi-Qi Liu1
Yi-Qi Liu1 Chi Zhang1
Chi Zhang1 Gui-Qiang Wang1,14,15*
Gui-Qiang Wang1,14,15* Hong Zhao1,15* and China HepB Related Fibrosis Assessment Research Group
Hong Zhao1,15* and China HepB Related Fibrosis Assessment Research GroupIntroduction: The clinical significance of persistent positive in Hepatitis B Virus (HBV) DNA level in patients receiving antiviral therapy is not well known. We investigated factors associated with persistent viremia (PV) in patients with chronic hepatitis B (CHB) given 78-week entecavir.
Methods: A total of 394 treatment-naïve CHB patients who had undergone liver biopsy at baseline and week 78 of treatment were analyzed in this prospective multicentre study. We identified patients with PV (above the lower limit of quantification, 20 IU/ml) after 78 weeks of entecavir therapy. Stepwise, forward, multivariate regression analyses of specified baseline parameters were apllied to identify factors associated with PV. Futhermore, we assessed the incidence of hepatocellular carcinoma (HCC) in all patients using models of the risk of HCC development.
Results: Of the 394 patients, 90 (22.8%) still with PV after 78-week antiviral treatment. Factors associated significantly with PV (vs complete virological response, CVR) were HBV DNA level ≥8 log10 IU/mL (OR, 3.727; 95% CI, 1.851-7.505; P < 0.001), Anti-HBc level < 3 log10 IU/mL (OR, 2.384; 95% CI, 1.223-4.645; P=0.011), and HBeAg seropositivity (OR, 2.871; 95% CI, 1.563-5.272; P < 0.001). Patients with PV were less likely to have fibrosis progression and HCC development than those with the CVR. Of the 11 HBeAg-positive patients with HBV DNA level ≥8 log10 IU/mL and Anti-HBc level < 3 log10 IU/mL at baseline, 9 (81.8%) had persistent positivity in HBV DNA level and 0 had fibrosis progression at week 78 of treatment.
Discussion: In conclusion, HBV DNA level ≥8 log10 IU/mL, Anti-HBc level < 3 log10 IU/mL and HBeAg seropositivity at baseline contribute to PV in patients with CHB receiving 78-week antiviral treatment. In addition, the rate of fibrosis progression and the risk of HCC development in patients with PV were kept low. The complete protocol for the clinical trial has been registered at clinicaltrials.gov (NCT01962155 and NCT03568578).
Chronic hepatitis B (CHB) affects more than 250 million people worldwide and causes annual mortality of nearly 1 million from cirrhosis, hepatocellular carcinoma and other diseases associated with hepatitis B virus (HBV) infection (World Health Organization, 2016; Polaris Observatory Collaborators, 2018). Nucleos(t)ide analogues (NAs) approved for CHB treatment have been demonstrated to reduce HBV disease progression, reverse liver fibrosis and decrease the risk of hepatocellular carcinoma (HCC) development. Entecavir (ETV) and tenofovir disoproxil fumarate (TDF) are clinically used as first-line nucleos(t)ide analogue (NA) antivirals for the treatment of patients with CHB, and both have reasonably improved the rates of virological suppression, biochemical and serological response. Both drugs display high genetic barriers with very minimal resistance and high rates of viral suppression (Tenney et al., 2009; Chang et al., 2010; Yang et al., 2013).
In controlled clinical trials, most but not all patients with CHB experience undetectable serum HBV DNA levels on oral antiviral therapy. Reported rates of undetectable serum HBV DNA (<300 copies/mL) range from 67% to 97% among hepatitis B e antigen–positive (HBeAg+) patients (Chang et al., 2006; Gish et al., 2007; Pan et al., 2012; Marcellin et al., 2013) and 90% to 97% among HBeAg-negative (HBeAg-) patients (Lai et al., 2006; Marcellin et al., 2013). Precisely why a proportion of patients do not achieve an undetectable serum HBV DNA level despite apparently effective antiviral treatment has not been fully explored. Both the stability of the HBV covalently closed circular DNA (cccDNA) in the hepatocyte nucleus (Allweiss and Dandri, 2017), and the resistance of HBV to NAs (Sinn et al., 2011) are believed to be some mechanisms.
Some reports suggested that persistent positive in HBV DNA level after NA therapy was associated with a higher risk of hepatocellular carcinoma (HCC) occurrence and fibrosis progression (Kim et al., 2017; Sun et al., 2020). However, it had also been reported that low-level viremia (LLV, <2,000 IU/mL) during treatment was not a predictive factor for HCC and cirrhotic complications in patients with treatment-naïve CHB and good adherence to ETV treatment (Lee et al., 2020). And Lee et al. suggested that episodic LLV among untreated patients with compensated cirrhosis did not increase the risk of disease progression compared with maintained virological response status (Lee et al., 2022). As a result, it remains unclear whether it is more beneficial to continue original NAs or to switch/add another NA in order to prevent liver-related events including fibrosis progression and HCC development in persistent viremia (PV, above the lower limit of quantification, 20 IU/ml) patients. The AASLD 2018 hepatitis B guidance suggested that persons with persistent LLV on ETV or TDF monotherapy should continue monotherapy, but the quality and certainty of this evidence was very low (Terrault et al., 2018).
Therefore, the objectives of this study were to identify factors associated with PV and investigate whether patients with PV were associated with HCC development and fibrosis progression, so as to further explore the next treatment options for patients with PV, by analyzing data collected from a well-characterized cohort of CHB patients that have been treated with ETV for 78 weeks.
This multi-center, prospective, longitudinal study included 780 Chinese treatment-naïve patients with CHB who were consecutively admitted from 24 teaching hospitals located in Mainland China between October 2013 and May 2021. Patients recruited in the cohort study were those with hepatitis B surface antigen (HBsAg) positive for at least 6 months and negative of other forms of chronic liver diseases (CLD), decompensate liver cirrhosis or HCC. Patients who had received treatments with either bicyclol or antiviral drugs within 26 weeks before the recruitment were excluded. The specific inclusion and exclusion criteria had been described previously (Deng et al., 2015). Demographic data were collected at baseline. Clinical data, including blood test results and liver stiffness measurement (LSM) were recorded at the time of liver biopsy at baseline and every 26 weeks of follow-up in local hospitals. Paired liver biopsies were performed at baseline and week 78. The study was approved by the Ethics Committee of Peking University First Hospital and the other 23 teaching hospitals. All patients gave informed consent for research use of their clinical data and liver biopsy specimens. All authors had access to the study data and reviewed and approved the final manuscript.
Blood specimens were routinely obtained on the same day of liver biopsy in local hospitals. Serum HBV DNA quantitation was detected at central laboratory using Roche COBAS TaqMan platform (lower limit of detection 20 IU/mL) according to manufacturer’s instructions. Levels of HBV serological markers (HBsAg/anti-HBs, HBeAg/anti-HBe) were measured by relevant Roche Elecsys® assays (Roche Diagnostics, Penzberg, Germany). Quantitative detection of anti-hepatitis B virus core antibody (Anti-HBc) was performed using Sandwich enzyme-linked immunosorbent assays (Jia et al., 2014). The level of HBV DNA, HBsAg, and Anti-HBc were expressed as log10 IU/mL.
LSM was performed on fasting patients at baseline and week 78 using 1-dimensional ultrasound TE (FibroScan®, Echosens, Paris, France) in local hospitals. All operators who had no knowledge of the patients’ clinical data were trained according to the manufacturer’s recommendations. LSM values are expressed in units of kilopascals (kPa). Only a procedure with at least ten valid measurements, an interquartile range (IQR)/median value (IQR/M) <30% and a success rate >60% was considered reliable (Sandrin et al., 2003).
The two noninvasive indexes for fibrosis were calculated at baseline and week 78 based on the following formulas:
Ultrasonographic-guided liver biopsies were performed at baseline (on day before starting antiviral therapy) and week 78 in each institute. A minimum of 20 mm of liver biopsy with at least 11 portal tracts was considered adequate for diagnosis. All liver biopsies were blindly and independently reviewed by 2 hepatopathologists from Beijing You An Hospital affiliated to Capital Medical University. When discrepancies occurred, final decision was made by the third experienced hepatopathologist who was also responsible for reassessment of 10% samples by random drawing. Necro-inflammation and fibrosis were assessed with the Ishak scoring system (Ishak et al., 1995). Fibrosis was scored as follows: F0-1, no/mild fibrosis; F≥2, moderate fibrosis; F≥3, significant fibrosis; F≥4, advanced fibrosis; and F≥5, cirrhosis. Histological inflammation grading was performed using the modified histology activity index (HAI), and the scored as follows: HAI 0-4, zero to mild inflammation; HAI 5-18, moderate to severe inflammation. Histological improvement was defined as ≥2-point decrease in the HAI score and without concurrent worsening of the fibrosis score 78 weeks after the therapy initiation. Fibrosis improvement was defined as ≥1-point decrease in the Ishak fibrosis score, whereas ≥1-point increase was considered as fibrosis progression. Inflammation improvement was defined as ≥2-point decrease in the HAI score.
Statistical analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Patients’ characteristics were summarized as median (range), or numbers of cases and percentages, as appropriate. Continuous variables were compared using Student t test or Mann-Whitney test, categorical variables were compared using Chi-squared test or Fisher’s exact test. Univariate and multivariate logistic regression analysis were used to identify independent predictors associated with persistent positivity in HBV DNA level after 78-week antiviral therapy. Factors significant in univariate analyses were included in the multivariate model using the forward selection procedure; predictors were retained in the model if the P value was less than 0.10. All statistical tests were two-sided, and P<0.05 was considered statistical significance.The original data was shown in https://github.com/Xiaoqind/Factors-associated-with-PV.
Of 780 Chinese treatment-naïve CHB patients who met the eligible criteria and with qualified liver biopsy at baseline, 504 (64.6%) patients received entecavir-based therapy and were prospectively followed to 78 weeks for second liver biopsy. Ishak fibrosis scores was available at all two time-points (baseline and week 78) for 394 patients (Supplementary Figure 1). In total, 90 patients (22.8%) had PV at week 78.
Baseline demographic and disease characteristics of patients with PV and patients with complete virological responses (CVR, below the lower limit of quantification, 20 IU/ml) at week 78 are summarized in Table 1. Compared with the group that achieved CVR, patients with PV were younger. A higher proportion of patients with PV were HBeAg positive and this group had higher baseline HBV DNA and HBsAg levels, and lower Anti-HBc level compared with the CVR group. Baseline liver biopsy analysis showed that patients with PV had less fibrosis than patients with CVR (Ishak fibrosis score>3: 43.3% vs 56.6%; P=0.027), but the prevalence of necroinflammation was similar (HAI ≥5: 77.8% vs 70.7%; P=0.189). There was no difference between the two groups in the terms of sex, BMI, PLT, ALT, ALB, TBIL, AFP, CR, FPG, FIB-4, APRI, LSM, patients with family history of HCC or hepatitis B.
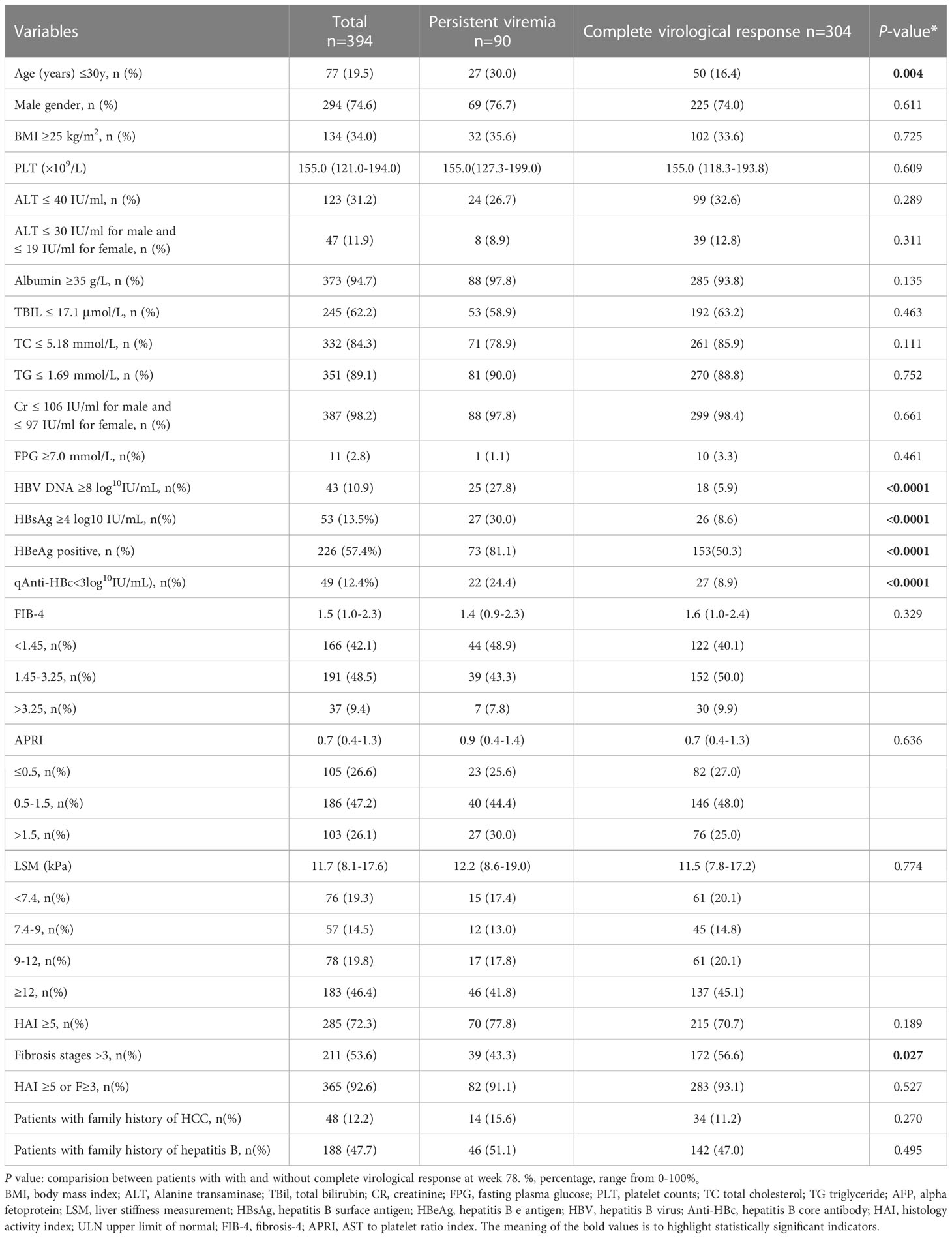
Table 1 Baseline demographics and disease characteristics of 394 patients with and without complete virological response at week 78 (univariate analysis).
Furthermore, according to different baseline HBV DNA levels, we analyzed the virological responses in all patients after 78-week antiviral therapy (Figure 1). Among patients with baseline HBV DNA level <2 log10 IU/mL, none has showed PV. As baseline HBV DNA levels increased, the proportion of patients with PV also increased after 78 weeks of treatment (when the HBV DNA levels were between 2-3, 3-4, 4-5, 5-6, 6-7,7-8, and ≥8, there were 4.3%, 10.6%, 10.3%, 17.9%, 27.1%, 29.0% and 58.1% patients occurred PV, respectively). Similarly, in patients with PV, the higher the baseline HBV DNA level was, the greater the proportion of patients has developed PV (when the HBV DNA levels were between 2-3, 3-4, 4-5, 5-6, 6-7, 7-8, and ≥8, the proportions were 1.1%, 5.6%, 6.7%, 15.6%, 21.1%, 22.2% and 27.8%, respectively.) (Figure 2).
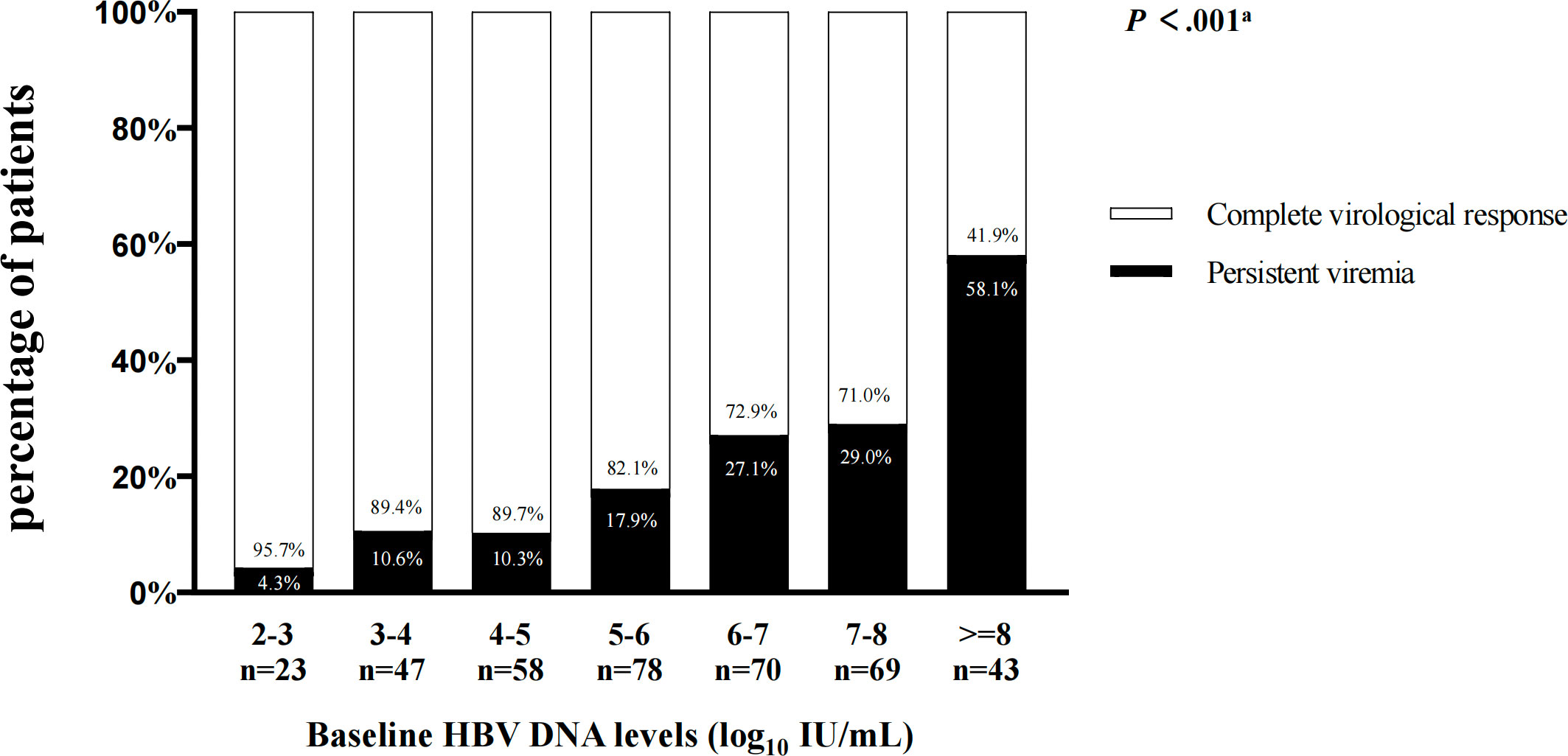
Figure 1 The virological responses in patients after 78-week antiviral therapy according to different HBV DNA levels at baseline. aComparisons by the Chi-squared test (between 2-3, 3-4, 4-5, 5-6, 6-7 ,7-8, and ≥8 groups).

Figure 2 The proportion of patients in persistent viremia group according to different HBV DNA levels at baseline.
In a multivariate analysis (Table 2), 3 baseline variables were identified to be associated independently with PV at week 78: baseline HBV DNA level ≥8 log10 IU/mL (odds ratio [OR], 3.727; 95% confidence interval [CI], 1.851-7.505; P<0.001), baseline Anti-HBc level<3 log10 IU/mL (OR, 2.384; 95% CI, 1.223-4.645; P=0.011), and baseline HBeAg seropositivity (OR, 2.871; 95% CI, 1.563-5.272; P<0.001). As shown in Table 2, among patients with baseline HBV DNA level ≥8 log10 IU/mL, 58.1% of patients had PV at week 78 compared with 18.5% of those with CVR. Similarly, 44.8% of patients with Anti-HBc level<3 log10 IU/mL had PV at week 78 compared with 19.7% of those with CVR. In HBeAg+ patient, 32.3% had persistence viremia and 10.1% achieved CVR at week 78 (both P<0.001). Among HBeAg+ patients with HBV DNA level ≥8 log10 IU/mL and Anti-HBc level<3 log10 IU/mL at baseline, 81.8% had PV at week 78 compared with 10.1% of HBeAg- patients with HBV DNA level<8 log10 IU/mL and Anti-HBc level ≥3 log10 IU/mL (P<0.001) (Figure 3). Of the 11 HBeAg-positive patients with baseline HBV DNA level ≥8 log10 IU/mL and Anti-HBc level<3 log10 IU/mL, none had shown fibrosis progression at week 78 of treatment (P<0.001).

Figure 3 Effects of baseline HBV DNA level ≥8 log10 IU/mL, Anti-HBc level<3 log10 IU/mL and HBeAg status on persistent viremia at week 78. aComparisons between the related two groups (HBV DNA level ≥8 log10 IU/mL vs <8 log10 IU/mL; Anti-HBc level<3 log10 IU/mL vs ≥3log10 IU/mL; HBeAg+, HBV DNA level ≥8 log10 IU/mL and Anti-HBc level<3 log10 IU/mL vs HBeAg- , HBV DNA level<8 log10 IU/mL and Anti-HBc level ≥3 log10 IU/mL ).
On-treatment serum immunological, biochemical, and histologic responses associated with PV patients are summarized in Table 3. Compared with patients with CVR, patients with PV were less likely to achieve HBeAg loss (21.9% vs 35.9%; P=0.034); however, no differences in the reduction of HBsAg level were shown. Significant differences in histologic responses were observed, where patients with PV were less likely to have a worse LSM value (0% vs 5.9%, P=0.038) and Ishak fibrosis score (11.1% vs 21.4%, P=0.029) than patients with CVR at week 78. In addition, there was no significant difference between the two groups in obtaining normal ALT levels (92.2% vs 91.4%, P=0.816) and histological improvement (58.9% vs 50.3%, P=0.153) after 78-week antiviral therapy.
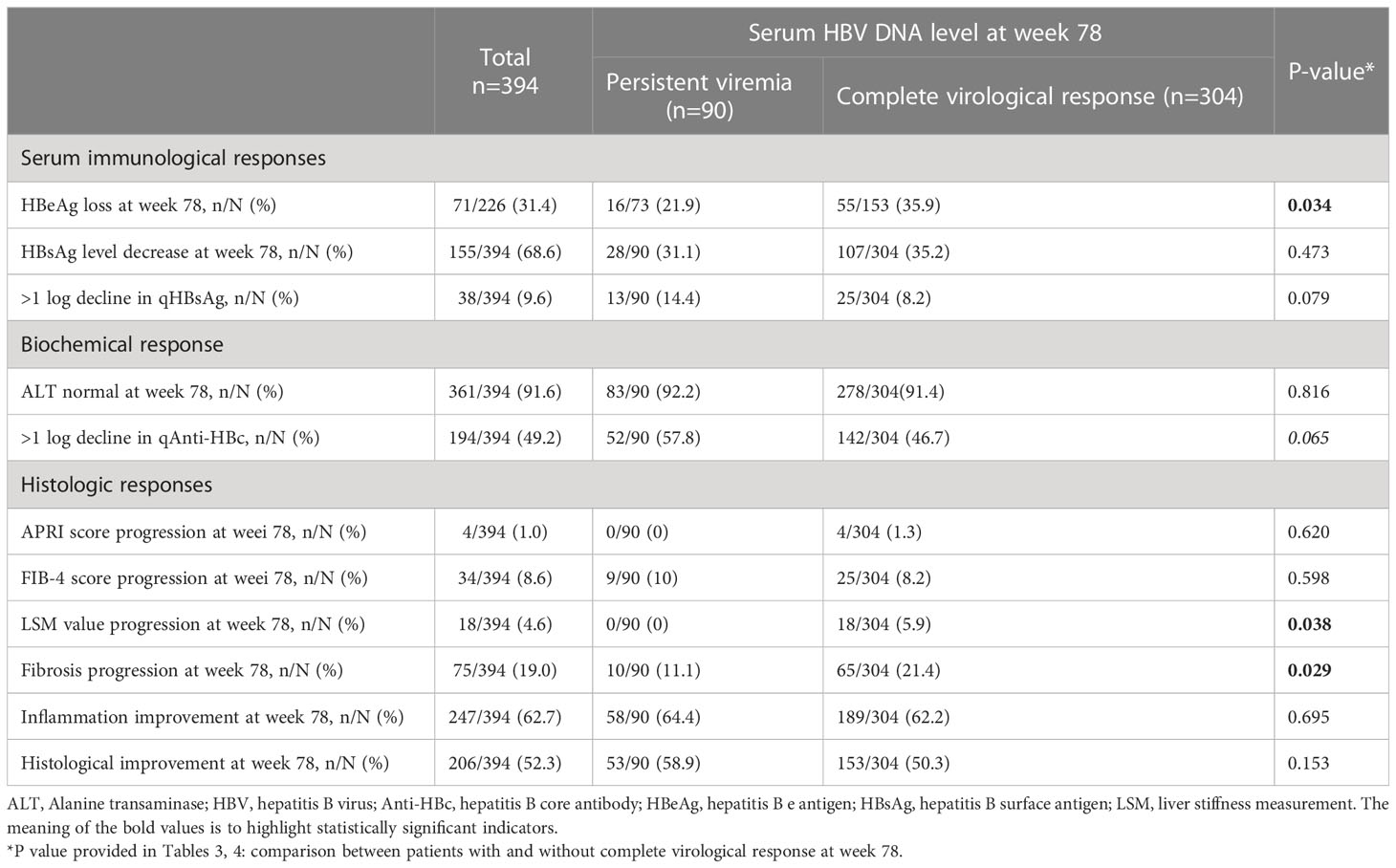
Table 3 Different treatment responses between persistent viremia group and complete virological response group (univariate analysis).
Furthermore, we divided the patients with fibrosis progression into 3 groups based on baseline Ishak fibrosis score: group 1 with Ishak fibrosis score between 0-2 (33 patients); group 2 with Ishak fibrosis score between 3-4 (39 patients); group 3 with Ishak fibrosis score between 5-6 (3 patients). There were 5 (15.2%), 5 (12.8%) and 0 (0%) patients in groups 1, 2 and 3, respectively, with PV (Supplementary Figure 2).
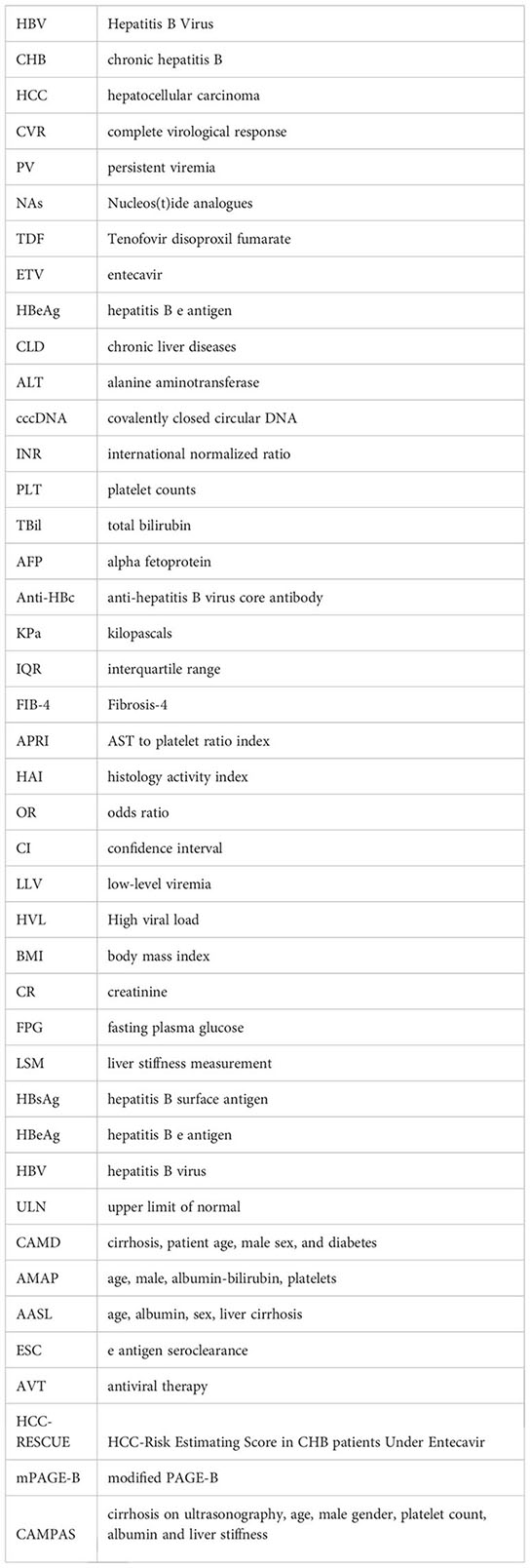
Considering that some models evaluating the risk of HCC development which suitable for the untreated patients with chronic HBV infection include the serum HBV DNA level as a constituent variable, we selected the models which suitable for the treated patients to avoid overestimating the incidence of HCC. According to the CAMD score (Hsu et al., 2018), more patients with PV were observed in the low-risk group (CAMD score, <8; 73.3% vs 56.9%), whilst less patients with PV were in the high-risk group (CAMD score, >13; 4.4% vs 8.9%), compared to the patients with CVR (P =0.018). Although there was no significant difference according to the AMAP score (Fan et al., 2020), AASL-HCC score (Yu et al., 2019), HCC-ESCAVT score (Lim et al., 2020), CAMPA score (Lee et al., 2020), HCC-RESCUE score (Sohn et al., 2017), and mPAGE-B HCC score (Kim et al., 2018), more patients with PV were in the low-risk group than patients with CVR (Table 4).
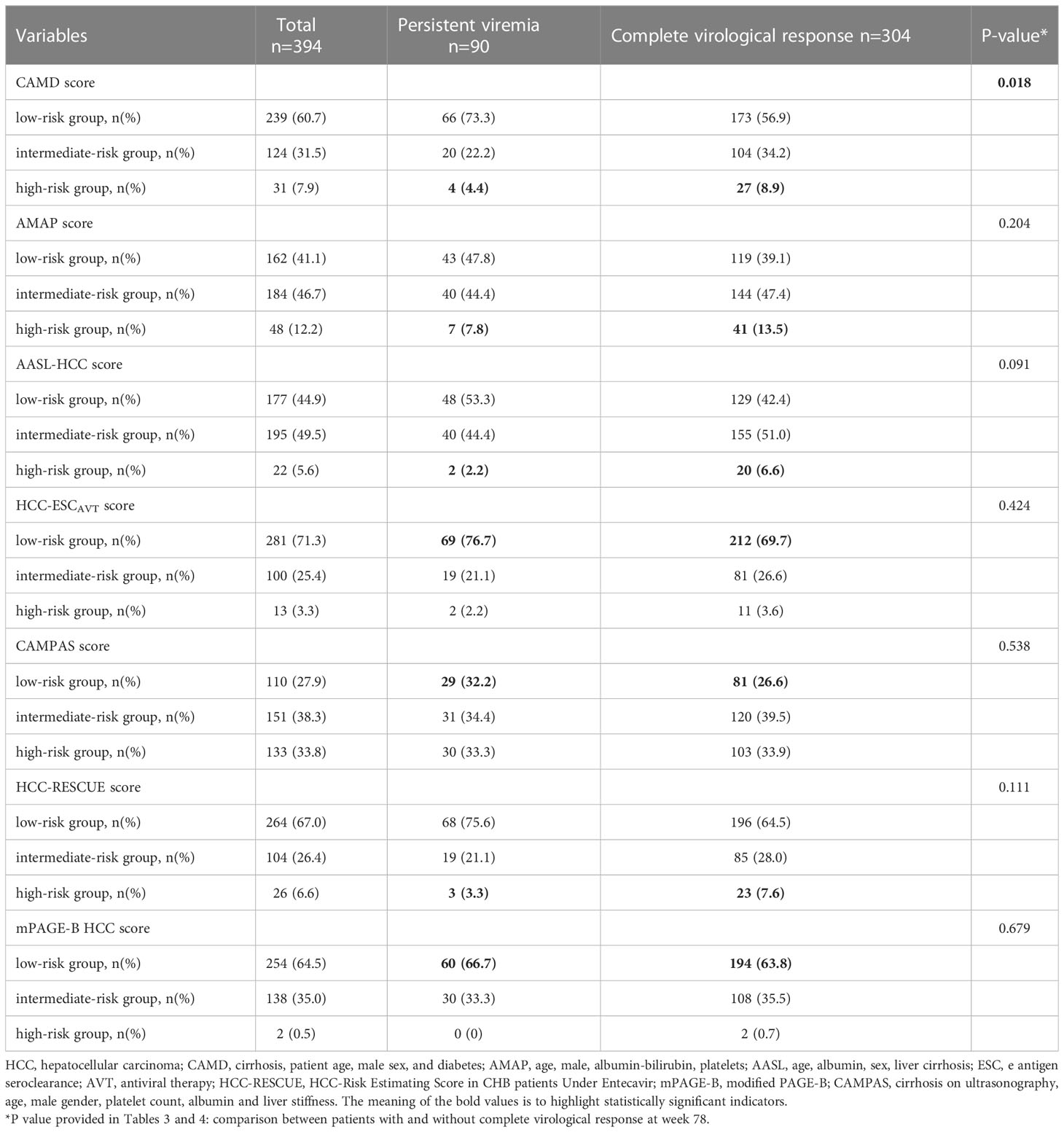
Table 4 Risk of HCC development in 394 patients with and without complete virological response at different HCC risk prediction models.
In the era of NAs therapy, most patients with CHB achieve CVR and the majority experience fibrosis regression including the reversal of cirrhosis (Chang et al., 2006; Lai et al., 2006; Chang et al., 2010; Xu et al., 2015). However, despite these highly beneficial outcomes, a small number of patients still fail to achieve CVR, and the clinical significance of a PV with respect to the outcome of CHB is not clear. In this analysis of predictive factors for PV after 78 weeks of ETV treatment in a well-characterized cohort of CHB patients, we found that HBV DNA level ≥8 log10 IU/mL, Anti-HBc level<3 log10 IU/mL, and HBeAg seropositivity are independently predictive of failure to achieve CVR. Furthermore, of the 11 HBeAg-positive patients with HBV DNA level ≥8 log10 IU/mL and Anti-HBc level<3 log10 IU/mL at baseline, 9 (81.8%) had persistent positive in HBV DNA level and 0 had fibrosis progression at week 78 of treatment. Of the 90 patients with PV, only 10 (11.1%) had fibrosis progression and 0-7 (0-7.8%) were with high risk of HCC occurrence according to different HCC risk scores.
Higher levels of HBV DNA are associated with an increased risk for HCC and cirrhosis (Chen et al., 2011), but its impact on virological response is debatable. Yuen et al. reported that 100% of treatment-naïve HBV patients who received entecavir for 3 years with baseline HBV DNA <8 log10 copies/mL had undetectable HBV DNA (<12 IU/mL), whereas only 75% of patients with baseline HBV DNA ≥8 log10 copies/mL did (Yuen et al., 2011). Gordon et al. reported that equal virologic responsiveness between CHB patients with high viral load (HVL) (HBV DNA ≥9 log10 copies/mL) and with non-HVL, 98.3% of HVL and 99.2% of non-HVL patients achieving HBV DNA <400 copies/mL by week 240 (Gordon et al., 2013). Chan et al. reported that 55% of HBeAg-positive patients with high levels of HBV DNA (mean baseline level of HBV DNA of 8.41 log10 IU/mL) and normal levels of ALT treated with TDF had levels of HBV DNA <69 IU/mL at week 192 (Chan et al., 2014). In our analysis, only 77.2% of treatment-naïve CHB patients who received entecavir for 78 weeks achieve CVR, 81.5% of patients with baseline HBV DNA <8 log10 IU/mL achieved CVR (HBV DNA <20 IU/mL), whilst the CVR rates were only 41.9% in patients with baseline HBV DNA ≥8 log10 IU/mL. The poor efficacy of ETV may be related to previous using of lamivudine (LAM) and telbivudine (LdT), but the proportion of such patients was similar between the two groups. This finding may be attributed to the short time courses of antiviral therapy or some patients may be in immune-tolerant phase. Larger longitudinal studies are warranted to explore this factor further and its potential effect on the virological response to antiviral treatment in CHB patients.
Previous studies have shown that rates of CVR in clinical trials of CHB patients were different between HBeAg+ and HBeAg- patients, and the former tended to have lower rates of CVR compared to the latter (Chang et al., 2006; Lai et al., 2006; Gish et al., 2007; Pan et al., 2012; Marcellin et al., 2013). Consequently, the observed associations between HBeAg-positive and a PV state is not entirely surprising. Among 875 treatment-naive chronic hepatitis B virus (HBV) mono-infected patients, 377 patients with low-level viremia (LLV; <2,000 IU/mL), Kim et al. reported that HBeAg status was the only significant factor associated with LLV (Kim et al., 2017), which coincided with our results.
Our finding that Anti-HBc level at baseline is associated independently with failure to achieve CVR on NAs therapy is novel and may relate to the intensity of liver inflammation. It remains controversial of the association between ALT levels and liver inflammation. Some studies have suggested that substantial CHB patients with normal ALT levels exhibit severe liver damage. Chao et al. reported that approximately one fifth (20.7%) of CHB patients with ALT ≤ 40 IU/L may have significant hepatic fibrosis. The corresponding proportion was 27.8% even when the newer ULN of 30 IU/L (males) and 19 IU/L (females) was applied (Chao et al., 2014).
Recent studies proposed that serum anti-HBc levels could serve as a promising marker for predicting the severity of liver inflammation and exhibited a high diagnostic accuracy in CHB patients with normal ALT (Song et al., 2015; Zhou et al., 2017). In that studies, Anti-HBc can accurately reflect the inflammation of the liver. In our analysis, the levels of ALT at baseline were comparable between the two groups, but the level of Anti-HBc in patients with PV was lower than patients in CVR (3.6 vs 3.9 log10 IU/mL, P<0.001), which indicated that Anti-HBc is a more responsive indicator of liver inflammation than ALT. Of 49 patients with Anti-HBc level<3 log10 IU/mL, 22 (44.9%) failed to achieve CVR, which suggested that some of patients with low levels of Anti-HBc may be in the immune tolerance phase.
Evidence has emerged that incomplete virologic suppression, particularly intrahepatic viral transcriptional activity, was associated with abnormal liver histopathology in a cross-sectional study (Wang et al., 2017). Sun et al. suggested that detectable low-level HBV DNA was associated with fibrosis progression in patients with chronic HBV infection during 78 weeks of entecavir therapy (Sun et al., 2020). But in our study, patients with PV were less likely to have a worse LSM value (0% vs 5.9%, P=0.038) and fibrosis progression (11.1% vs 21.4%, P=0.029) than patients with CVR during 78 weeks of entecavir therapy. Furthermore, Patients with PV were more likely to have a low-risk of HCC development than those with CVR. High viral load (HVL) (HBV DNA≥8 log10 copies/mL), positive HBeAg, slightly liver inflammation (Anti-HBc <3 log10 IU/mL), low-risk of fibrosis progression and HCC development, all these suggested that these particular PV patients may be in the immune tolerance phase (Sarin et al., 2016; European Association for the Study of the Liver, 2017; Terrault et al., 2018). Even with potent drugs like entecavir, their virological response was unsatisfactory. Hence, the initiation of antiviral therapy could be delayed or waiting for stronger and more effective drugs may be alternative choice. Further prospective studies with larger sample size and longer follow-up are needed to confirm this finding and may provide some hint to optimization of histological and longterm clinical outcomes of antiviral therapy.
Our study has several limitations. First, the definition of PV was based on a single serum HBV DNA measurement at week 78. Although HBV DNA was measured at week 0, 26, 52 and 78 in local hospitals, respectively, we selected week 0 and 78 data detected at central laboratory to ensure the consistency of the results. Second, the follow-up time was relatively short. There was a possibility that PV observed at week 78 may turn to CVR after longer follow-up. Third, the development of drug-associated mutations was not investigated. However, considering the good compliance of patients and the extremely low resistance rate of entecavir, HBV mutations are unlikely to be the main cause of PV during antiviral therapy. Finally, our study used only ETV as a first choice for treatment of naïve CHB patients. Other NAs may have differential results. Therefore, the result of present study applies only to the patients using ETV.
In conclusion, our study suggested that HBV DNA level ≥8 log10 IU/mL, Anti-HBc level<3 log10 IU/mL and HBeAg seropositivity at baseline contributed to persistent positivity in HBV DNA level in patients with CHB receiving 78-week antiviral treatment. The risk of HCC development and the rate of fibrosis progression in these patients were low. Maybe these particular PV patients were in the immune tolerance phase, and the initiation of antiviral therapy could be delayed or waiting for stronger and more effective drugs may be another choice. Further well-designed prospective studies with large-scale and longer follow-up are needed to confirm this finding and may provide some hint to judge true immune tolerance phase.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The study was approved by the Ethics Committee of Peking University First Hospital and other 23 teaching hospitals. The patients/participants provided their written informed consent to participate in this study.
JL, X-QD, HZ and G-QW designed the experiments; HZ and G-QW provided the overall principle and direction of the study; X-QD and JL gathered and analyzed data, and drafted the manuscript; X-QD, Y-QL, and CZ done the laboratory examination. L-HC, Z-QZ, W-FZ, Q-HS, D-ZZ, A-LM, QX, H-LG, GZ, Y-XL, JS, S-BX and China HepB Related Fibrosis Assessment Research Group had participated in acquisition of data, revision of the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This study was supported by China Mega-Project for Infectious Diseases (grant numbers 2017ZX10203202, 2013ZX10002005) and China Mega-Project for Innovative Drugs (grant numbers 2016ZX09101065).
We gratefully acknowledge the members of China HepB-Related Fibrosis Assessment Research Group for assisting patient recruitment and data acquisition. We gratefully acknowledge Dr. Xiaomeng Wang from the University of Manchester for her critical reading of the manuscript and suggestions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1151899/full#supplementary-material
Allweiss, L., Dandri, M. (2017). The role of cccDNA in HBV maintenance. Viruses 21, 156. doi: 10.3390/v9060156
Chan, H. L., Chan, C. K., Hui, A. J., Chan, S., Poordad, F., Chang, T. T., et al. (2014). Effects of tenofovir disoproxil fumarate in hepatitis b e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis b virus DNA. Gastroenterology 146, 1240–1248. doi: 10.1053/j.gastro.2014.01.044
Chang, T. T., Gish, R. G., de Man, R. A., Gadano, A., Sollano, J., Chao, Y. C., et al. (2006). A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis b. N. Engl. J. Med. 354, 1001–1010. doi: 10.1056/NEJMoa051285
Chang, T. T., Liaw, Y. F., Wu, S. S., Schiff, E., Han, K. H., Lai, C. L., et al. (2010). Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis b. Hepatology 52, 886–893. doi: 10.1002/hep.23785
Chao, D. T., Lim, J. K., Ayoub, W. S., Nguyen, L. H., Nguyen, M. H. (2014). Systematic review with meta-analysis: the proportion of chronic hepatitis b patients with normal alanine transaminase ≤ 40 IU/L and significant hepatic fibrosis. Aliment. Pharmacol. Ther. 39, 349–358. doi: 10.1111/apt.12590
Chen, C. F., Lee, W. C., Yang, H. I., Chang, H. C., Jen, C. L., Iloeje, U. H., et al. (2011). Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 141, 1240–1248.e1-2. doi: 10.1053/j.gastro.2011.06.036
Deng, Y. Q., Zhao, H., Ma, A. L., Zhou, J. Y., Xie, S. B., Zhang, X. Q., et al. (2015). Selected cytokines serve as potential biomarkers for predicting liver inflammation and fibrosis in chronic hepatitis b patients with normal to mildly elevated aminotransferases. Med. (Baltimore) 94, e2003. doi: 10.1097/MD.0000000000002003
European Association for the Study of the Liver (2017). Electronic address:ZWFzbG9mZmljZUBlYXNsb2ZmaWNlLmV1; European association for the study of the liver. EASL 2017 clinical practice guidelines on the management of hepatitis b virus infection. J. Hepatol. 67, 370–398. doi: 10.1016/j.jhep.2017.03.021
Fan, R., Papatheodoridis, G., Sun, J., Innes, H., Toyoda, H., Xie, Q., et al. (2020). aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 73, 1368–1378. doi: 10.1016/j.jhep.2020.07.025
Gish, R. G., Lok, A. S., Chang, T. T., de Man, R. A., Gadano, A., Sollano, J., et al. (2007). Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis b. Gastroenterology 133, 1437–1444. doi: 10.1053/j.gastro.2007.08.025
Gordon, S. C., Krastev, Z., Horban, A., Petersen, J., Sperl, J., Dinh, P., et al. (2013). Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis b with high baseline viral load. Hepatology 58, 505–513. doi: 10.1002/hep.26277
Hsu, Y. C., Yip, T. C., Ho, H. J., Wong, V. W., Huang, Y. T., El-Serag, H. B., et al. (2018). Development of a scoring system to predict hepatocellular carcinoma in asians on antivirals for chronic hepatitis b. J. Hepatol. 69, 278–285. doi: 10.1016/j.jhep.2018.02.032
Ishak, K., Baptista, A., Bianchi, L., Callea, F., De Groote, J., Gudat, F., et al. (1995). Histological grading and staging of chronic hepatitis. J.Hepatol 22, 696–699. doi: 10.1016/0168-8278(95)80226-6
Jia, W., Song, L. W., Fang, Y. Q., Wu, X. F., Liu, D. Y., Xu, C., et al. (2014). Antibody to hepatitis b core antigen levels in the natural history of chronic hepatitis b: a prospective observational study. Med. (Baltimore) 93, e322. doi: 10.1097/MD.0000000000000322
Kim, J. H., Kim, Y. D., Lee, M., Jun, B. G., Kim, T. S., Suk, K. T., et al. (2018). Modified PAGE-b score predicts the risk of hepatocellular carcinoma in asians with chronic hepatitis b on antiviral therapy. J. Hepatol. 69, 1066–1073. doi: 10.1016/j.jhep.2018.07.018
Kim, J. H., Sinn, D. H., Kang, W., Gwak, G. Y., Paik, Y. H., Choi, M. S., et al. (2017). Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology 66, 335–343. doi: 10.1002/hep.28916
Lai, C. L., Shouval, D., Lok, A. S., Chang, T. T., Cheinquer, H., Goodman, Z., et al. (2006). Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis b. N. Engl. J. Med. 354, 1011–1020. doi: 10.1056/NEJMoa051287
Lee, S. B., Jeong, J., Park, J. H., Jung, S. W., Jeong, I. D., Bang, S. J., et al. (2020). Low-level viremia and cirrhotic complications in patients with chronic hepatitis b according to adherence to entecavir. Clin. Mol. Hepatol. 26, 364–375. doi: 10.3350/cmh.2020.0012
Lee, H. W., Park, S. Y., Lee, M., Lee, E. J., Lee, J., Kim, S. U., et al. (2020). An optimized hepatocellular carcinoma prediction model for chronic hepatitis b with well-controlled viremia. Liver. Int. 40, 1736–1743. doi: 10.1111/liv.14451
Lee, H. W., Park, S. Y., Lee, Y. R., Lee, H., Lee, J. S., Kim, S. U., et al. (2022). Episodic detectable viremia does not affect prognosis in untreated compensated cirrhosis with serum hepatitis b virus DNA <2,000 IU/mL. Am. J. Gastroenterol. 117, 288–294. doi: 10.14309/ajg.0000000000001497
Lim, T. S., Lee, H. W., Lee, J. I., Kim, I. H., Lee, C. H., Jang, B. K., et al. (2020). Predictive score for hepatocellular carcinoma after hepatitis b e antigen loss in patients treated with entecavir or tenofovir. J. Viral. Hepat. 27, 1052–1060. doi: 10.1111/jvh.13316
Marcellin, P., Gane, E., Buti, M., Afdhal, N., Sievert, W., Jacobson, I. M., et al. (2013). Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis b: a 5-year open-label follow-up study. Lancet 381, 468–475. doi: 10.1016/S0140-6736(12)61425-1
Pan, C. Q., Tong, M., Kowdley, K. V., Hu, K. Q., Chang, T. T., Lai, C. L., et al. (2012). High rates of viral suppression after long-term entecavir treatment of Asian patients with hepatitis b e antigen-positive chronic hepatitis b. Clin. Gastroenterol. Hepatol. 10, 1047–1050.e1. doi: 10.1016/j.cgh.2012.03.016
Polaris Observatory Collaborators (2018). Global prevalence, treatment, and prevention of hepatitis b virus infection in 2016: a modelling study. Lancet Gastroenterol. Hepatol. 3, 383–403. doi: 10.1016/S2468-1253(18)30056-6
Sandrin, L., Fourquet, B., Hasquenoph, J. M., Yon, S., Fournier, C., Mal, F., et al. (2003). Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound. Med. Biol. 29, 1705–1713. doi: 10.1016/j.ultrasmedbio.2003.07.001
Sarin, S. K., Kumar, M., Lau, G. K., Abbas, Z., Chan, H. L., Chen, C. J., et al. (2016). Asian-Pacific clinical practice guidelines on the management of hepatitis b: a 2015 update. Hepatol. Int. 10, 1–98.
Sinn, D. H., Lee, H. I., Gwak, G. Y., Choi, M. S., Koh, K. C., Paik, S. W., et al. (2011). Virological response to adefovir monotherapy and the risk of adefovir resistance. World J. Gastroenterol. 17, 3526–3530. doi: 10.3748/wjg.v17.i30.3526
Sohn, W., Cho, J. Y., Kim, J. H., Lee, J. I., Kim, H. J., Woo, M. A., et al. (2017). Risk score model for the development of hepatocellular carcinoma in treatment-naïve patients receiving oral antiviral treatment for chronic hepatitis b. Clin. Mol. Hepatol. 23, 170–178. doi: 10.3350/cmh.2016.0086
Song, L. W., Liu, P. G., Liu, C. J., Zhang, T. Y., Cheng, X. D., Wu, H. L., et al. (2015). Quantitative hepatitis b core antibody levels in the natural history of hepatitis b virus infection. Clin. Microbiol. Infect. 21, 197–203. doi: 10.1016/j.cmi.2014.10.002
Sterling, R. K., Lissen, E., Clumeck, N., Sola, R., Correa, M. C., Montaner, J., et al. (2006). Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 43, 1317–1325. doi: 10.1002/hep.21178
Sun, Y., Wu, X., Zhou, J., Meng, T., Wang, B., Chen, S., et al. (2020). Persistent low level of hepatitis b virus promotes fibrosis progression during therapy. Clin. Gastroenterol. Hepatol. 18, 2582–2591.e6. doi: 10.1016/j.cgh.2020.03.001
Tenney, D. J., Rose, R. E., Baldick, C. J., Pokornowski, K. A., Eggers, B. J., Fang, J., et al. (2009). Long-term monitoring shows hepatitis b virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 49, 1503–1514. doi: 10.1002/hep.22841
Terrault, N. A., Lok, A. S., McMahon, B. J., Chang, K. M., Hwang, J. P., Jonas, M. M., et al. (2018). Update on prevention, diagnosis, and treatment of chronic hepatitis b: AASLD 2018 hepatitis b guidance. Hepatology 67, 1560–1599. doi: 10.1002/hep.29800
Wai, C. T., Greenson, J. K., Fontana, R. J., Kalbfleisch, J. D., Marrero, J. A., Conjeevaram, H. S., et al. (2003). A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis c. Hepatology 38, 518–526. doi: 10.1053/jhep.2003.50346
Wang, J., Yu, Y., Li, G., Shen, C., Meng, Z., Zheng, J., et al. (2017). Relationship between serum HBV-RNA levels and intrahepatic viral as well as histologic activity markers in entecavir-treated patients. J. Hepatol. S0168-8278, 32261–32264. doi: 10.1016/j.jhep.2017.08.021
World Health Organization (2016). Combating hepatitis b and c to reach elimination by 2030 (Geneva: World Health Organization).
Xu, Y., Zhang, Y. G., Wang, X., Qi, W. Q., Qin, S. Y., Liu, Z. H., et al. (2015). Long-term antiviral efficacy of entecavir and liver histology improvement in Chinese patients with hepatitis b virus-related cirrhosis. World. J. Gastroenterol. 21, 7869–7876. doi: 10.3748/wjg.v21.i25.7869
Yang, S. C., Lee, C. M., Hu, T. H., Wang, H., Lu, S. N., Hung, C. H., et al. (2013). Virological response to entecavir reduces the risk of liver disease progression in nucleos(t)ide analogue-experienced HBV-infected patients with prior resistant mutants. J. Antimicrob. Chemother. 68, 2154–2163. doi: 10.1093/jac/dkt147
Yu, J. H., Suh, Y. J., Jin, Y. J., Heo, N. Y., Jang, J. W., You, C. R., et al. (2019). Prediction model for hepatocellular carcinoma risk in treatment-naive chronic hepatitis b patients receiving entecavir/tenofovir. Eur. J. Gastroenterol. Hepatol. 31, 865–872. doi: 10.1097/MEG.0000000000001357
Yuen, M. F., Seto, W. K., Fung, J., Wong, D. K., Yuen, J. C., Lai, C. L. (2011). Three years of continuous entecavir therapy in treatment-naïve chronic hepatitis b patients: VIRAL suppression, viral resistance, and clinical safety. Am. J. Gastroenterol. 106, 1264–1271. doi: 10.1038/ajg.2011.45
Keywords: chronic hepatitis B, persistant viremia, HBV DNA, anti-hepatitis B virus core antibody, fibrosis, carcinoma
Citation: Li J, Dong X-Q, Cao L-H, Zhang Z-Q, Zhao W-F, Shang Q-H, Zhang D-Z, Ma A-L, Xie Q, Gui H-L, Zhang G, Liu Y-X, Shang J, Xie S-B, Liu Y-Q, Zhang C, Wang G-Q, Zhao H and China HepB Related Fibrosis Assessment Research Group (2023) Factors associated with persistent positive in HBV DNA level in patients with chronic Hepatitis B receiving entecavir treatment. Front. Cell. Infect. Microbiol. 13:1151899. doi: 10.3389/fcimb.2023.1151899
Received: 26 January 2023; Accepted: 12 May 2023;
Published: 16 June 2023.
Edited by:
Ming Yue, Nanjing Medical University, ChinaReviewed by:
Yuan Gu, Incyte Corporation, United StatesCopyright © 2023 Li, Dong, Cao, Zhang, Zhao, Shang, Zhang, Ma, Xie, Gui, Zhang, Liu, Shang, Xie, Liu, Zhang, Wang, Zhao and China HepB Related Fibrosis Assessment Research Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Zhao, bWlubWluMjAwMUAxMjYuY29t; emhhb2hvbmdfcHVmaEBiam11LmVkdS5jbg==; Gui-Qiang Wang, am9objEzMTIxMkBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.