- 1State Key Laboratory of Oral Diseases and National Clinical Research Center for Oral Diseases, Sichuan University, Chengdu, China
- 2Department of Cariology and Endodontics, West China Hospital of Stomatology, Sichuan University, Chengdu, China
As one of the most common oral diseases in kids, early childhood caries affects the health of children throughout the world. Clinical investigations show the copresence of Candida albicans and Streptococcus mutans in ECC lesions, and mechanistic studies reveal co-existence of C. albicans and S. mutans affects both of their cariogenicity. Clearly a comprehensive understanding of the interkingdom interaction between these two microorganisms has important implications for ECC treatment and prevention. To this end, this review summarizes advances in our understanding of the virulence of both C. albicans and S. mutans. More importantly, the synergistic and antagonistic interactions between these two microbes are discussed.
1 Introduction
Early childhood caries is defined as “the presence of one or more decayed (non-cavitated or cavitated lesions), missing (due to caries) or filled tooth surfaces in any primary tooth” in a child under the age of six (American Academy of Pediatric Dentistry, 2021). Although significant progress against ECC has been made in pediatric dentistry, it still affects kids throughout the world. According to 72 global studies which measured the prevalence of ECC in preschoolers between 1998 and 2018, the average prevalence of ECC in kids at 1-year-old was 17%, while the prevalence of ECC in toddlers at 2-year-old sharply rose to 36% (Tinanoff et al., 2019). Worse still, the prevalence of ECC increases with the development of children. Specifically, the prevalence of ECC in 3, 4 and 5-year-old preschoolers was 43%, 55% and 63%, respectively (Tinanoff et al., 2019). ECC impacts children and their parents or caregivers in different ways. Apart from dental pain and abscess, children with ECC suffer a higher risk of hospitalization and emergency room visits, treatment costs, school time loss, poor learning ability and life quality, low growth parameters (weight, height), sleep disorders, self-esteem setbacks and negative social interactions (Sachdev et al., 2016; Vieira-Andrade et al., 2016; Xiao et al., 2018a; Pierce et al., 2019).
ECC is a multifactorial disease. The prerequisites of ECC include the synchronized appearance of susceptible hosts, cariogenic microbes, and cariogenic substrate from food present for a sufficient length of time. Microbes are the initial factor of ECC. For quite a long time, oral microbiologists focused on the role of oral bacteria in the pathogenesis of ECC. Streptococcus mutans, a gram-positive facultative anaerobe, has long been taken as the main etiological factor of ECC. It is important to keep in mind that, the oral cavity is colonized by microbes from different domains, including eukaryotic cells, prokaryotic cells, archaea, and viruses. Although bacteria-targeting studies provide important clues to understand how microbes destroy the hard tissues of mammalian teeth, the roles of other microorganisms should not be ignored. In recent years, Candida albicans, a eukaryote and an opportunistic fungus, has been frequently detected in children with ECC. Studies revealed that the detection of C. albicans is positively related to the severity and incidence of ECC (Cui et al., 2021), suggesting its role in the occurrence of ECC. Importantly however, within the oral cavity, S. mutans and C. albicans actively interact with each other, and their inter-kingdom interactions mediate both of their cariogenicity (Falsetta et al., 2014; Kim et al., 2017; Xiao et al., 2018b; Sridhar et al., 2020). In this review, the virulence of S. mutans and C. albicans is present first, and then their complicated interactions as well as the influence of these interspecies interactions on the occurrence of ECC are discussed.
2 Streptococcus mutans
S. mutans is naturally present in the human oral cavity. To survive in the harsh environment of the human mouth, S. mutans, as well as other oral microbes, forms a highly organized microbial community termed as biofilm (Marsh and Zaura, 2017). Biofilm is a complex structure composed of aggregated microbial cells and microbially produced extracellular polymeric substances (EPS) (Marsh and Zaura, 2017). The construction of biofilm begins when saliva components selectively adsorb to the tooth surfaces, forming a thin acellular homogeneous organic membrane termed as acquired enamel pellicle. Acquired enamel pellicle provides binding sites for oral microorganisms including S. mutans. The surface proteins P1 (also known as PAc, SpaP, Ag I/II protein) of S. mutans can selectively bind to salivary lectins in acquired enamel pellicle, and this binding process enable the initial colonization of S. mutans. After initial binding, microbes including S. mutans start to proliferate and produce EPS, forming a stable three-dimensional community that contains channels to effectively distribute nutrients, oxygen and signaling molecules. S. mutans significantly promotes biofilm formation through sucrose-dependent and -independent ways. The sucrose-dependent mechanism mainly relies on extracellular glucose transferase secreted by itself. There are three extracellular glucose transferases secreted by S. mutans, including GtfB, GtfC, and GtfD. GtfB mostly produces viscous water-insoluble polysaccharides from sucrose, while GtfC synthesizes a mixture of insoluble and soluble polysaccharides from sucrose. GtfD predominantly catalyzes sucrose to be soluble polysaccharides (Bowen and Koo, 2011; Krzyściak et al., 2014). Water-insoluble polysaccharides are the major component of EPS. Water-insoluble polysaccharides work as glue to facilitate bacterial adherence and accumulation. They also constitute the protective barrier for residing microbes, and provide the biofilm with mechanical stability (Cugini et al., 2019).
S. mutans promote the occurrence of dental caries including ECC by generating organic acids through carbohydrate metabolization. Carbohydrates including sugars from the environment are transported into S. mutans cells mainly through the phosphoenolpyruvate dependent phosphotransferase system (PEP-PTS). This system catalyzes the transportation and phosphorylation of monosaccharides, disaccharides, amino sugars, polyols, and other sugar derivatives. The bacteria ferment the phosphorylated sugar into pyruvate by glycolysis. Pyruvate then is catalyzed to be organic acids such as lactic acid and formic acid through a series of branched chain pathways. These organic acids lower the pH of the local microenvironment, which is the direct cause of tooth surface demineralization. Majority of sucrose (> 95%) is internalized through the PEP-PTS system, and the rest is extracellularly metabolized by Gtfs and fructose transferases (Ftfs) (Vadeboncoeur and Pelletier, 1997; Lemos et al., 2019). Other two binding protein-dependent carbohydrate transport systems, the multiple-sugar metabolism system (Msm) and maltose/maltodextrin ABC transporter, are also involved in the sucrose uptake of S. mutans (Zeng and Burne, 2013).
Acid resistance is a significant survival advantage and virulence factor of S. mutans. S. mutans utilizes a series of adaptation mechanisms to respond to the acid-damage. One of these mechanisms is called acid tolerance response. ATR helps S. mutans maintain the cytoplasm at a neutral level compared to extracellular space when the environment becomes acidic (Baker et al., 2017). First, the F1F0-ATPase system within the S. mutans cells serves as the proton pump and the primary mechanism to maintain pH homeostasis. In an acidic condition, the F1F0-ATPase system is activated, consequently, protons are pumped out of the cell (Bender et al., 1986). Second, in response to the acidification of its environment, S. mutans increases the proportion of monounsaturated membrane fatty acids. The monounsaturated membrane fatty acids decrease the permeability of extracellular protons (Fozo and Quivey, 2004; Matsui and Cvitkovitch, 2010). S. mutans also secretes cardiolipin (Macgilvray et al., 2012), an important acid resistant substance. In terms of alkali production to cope with acid stress, S. mutans is urease and ADS negative, both of which are utilized by oral streptococci to synthesize neutralizing molecules urea and/or ammonia. Instead, S. mutans has an agmatine deiminase system (AgDS) encoded by the agmatine-inducible aguBDAC operon. The AgDS catalyzes ammonia, CO2, and ATP production (Chakraborty and Burne, 2017). Although AgDS does not seem to have a significant effect on environmental alkalization, the ammonia produced internally may contribute to the neutralization of cytoplasmic pH. In addition, Malolactic fermentation (MLF) could be also helpful for the alkalization of cytoplasm. MLF converts malate to less acidic lactate and CO2 (Chakraborty and Burne, 2017). Interestingly, this fermentation process can also lead to ATP synthesis through the reversible action of the F1−F0-ATPase (Chakraborty and Burne, 2017). In addition, when growing at low pH, the branched-chain amino acid (BCAA) biosynthesis of S. mutans was up-regulated. Pyruvate, the key metabolic intermediate, can be redirected to BCAA biosynthesis, therefore reducing acid end products, and maintaining the intracellular pH to alleviate acid stress (Len et al., 2004). Meanwhile, amino acids biosynthetic genes such as ilvC and ilvE related to the biosynthesis/degradation of branched-chain amino acids and the production of branched-chain fatty acids were identified as being up-regulated (Santiago et al., 2012). Last but not the least, S. mutans encodes DNA/protein repairing enzymes, proteases and chaperones that can fix protein and DNA damages in an acidic environment.
3 Candida albicans
Candida is the most detected fungal species in the oral cavity, especially C. albicans (Witherden et al., 2017; Delaney et al., 2019). It is a polymorphic organism that grows in yeast or filamentous fungal filament or pseudo-hyphae. The ability to switch between yeast and filamentous forms is critical for its virulence. External environmental signals and internal regulation are involved in the regulation of yeast filamentous transformation of C. albicans. External signals include temperature, pH, CO2 concentration, serum, and malnutrition. Acidic pH, low temperature and rich nutritional conditions are conducive to C. albicans into yeast form (Lopes and Lionakis, 2022). Internal regulation includes signaling pathways and the phenotype conversion system called as white -opaque transition (Huang, 2012; Tao et al., 2022). C. albicans can adhere to the tooth enamel, and initial adhesion occurs through a strong interaction between yeast cell wall-associated adhesins and the salivary pellicle (Gunaratnam et al., 2021). Children with ECC had higher rates of C. albicans in saliva, dental plaque and infected dentin samples compared to kids without carious lesions (Moalic et al., 2001). Children with oral C. albicans have a higher risk for ECC (> 5 times) than children without C. albicans (Xiao et al., 2018b). The detection frequency of C. albicans in ECC was higher than that in caries cases not belonging to ECC and caries-free groups (Carvalho et al., 2006). The total loads of C. albicans and S. mutans in the supragingival dental plaque of children with ECC increase with the percentage of active carious lesions and the severity of dental caries (Sridhar et al., 2020). These studies, combined with others suggested there is a strong correlation between C. albicans and ECC.
It has been proved that C. albicans can produce acid (Klinke et al., 2011). C. albicans metabolized carbohydrates such as glucose from food, which causes the reduction of the pH in the growing environment from pH 7 to 4 (Fakhruddin et al., 2021). When the pH is below 5.5, the acidification of S. mutans decreased greatly and stopped at pH 4.2 (De Soet et al., 1991). However, C. albicans still maintains its acid production ability even at pH 4.0 (Klinke et al., 2009). The main organic acid synthesized by C. albicans were pyruvate and acetate (Klinke et al., 2009). C. albicans has a greater ability to dissolve hydroxyapatite, which is about 20 times higher than that of S. mutans (Nikawa et al., 2003).
C. albicans has a high affinity to acquired enamel pellicle. Scanning electron microscopy showed that C. albicans initially combined with the in-situ pellicles on enamel, indicating that yeast cells attached to the enamel surface were in close contact with the salivary pellicle (Rocha et al., 2018; Gunaratnam et al., 2021). C. albicans adheres to hydroxyapatite through electrostatic interaction (Nikawa et al., 2003). In recent years, two different patterns of Candida colonization have been found. One is the establishment of a mycelial network with bacteria, and the other is forming a spatial arrangement with Streptococcus (Dige and Nyvad, 2019).
Secretory aspartyl proteases (Sap) is a vital virulence factor of C. albicans. Sap1 may play important roles in the development of severe early childhood caries (S-ECC) (Li et al., 2014). C. albicans is the main producer of hydrolase, secreting enzymes such as protease, hemolysin, phospholipase, collagenase and so on, et al. C. albicans also has significantly higher protease activity and phospholipase activity. The study also showed that the phospholipase activity of isolated C. albicans was may be positively correlated with protease activity.
4 Interactions between S. mutans and C. albicans
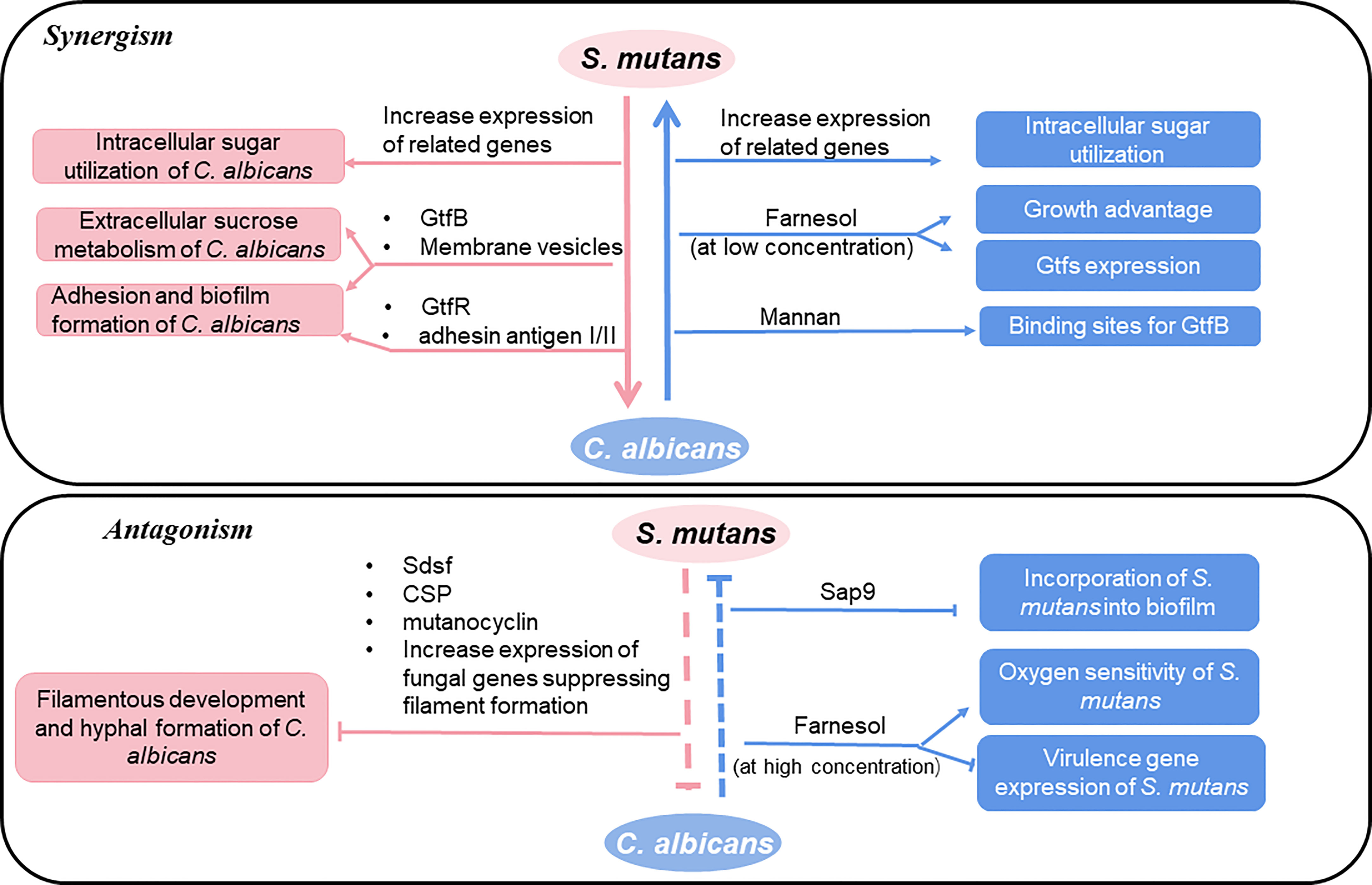
The copresence of S. mutans and C. albicans is frequently observed in oral samples from ECC patients (Xiao et al., 2018a). In recent years, the way how S. mutans and C. albicans interacts with each other as well as the effect of these interactions on the progress of dental caries including ECC has been intensively explored as summarized in Figure 1.
4.1 Synergism in metabolic activities
C. albicans promotes the carbohydrate intake of S. mutans. The glucose metabolic rate of S. mutans co-cultured with C. albicans was higher than those in the pure culture group (He et al., 2017; Oliveira et al., 2021). Further mechanism studies show that C. albicans affects the transcription of S. mutans genes associated with the transportation and metabolization of carbohydrates (He et al., 2017; Oliveira et al., 2021). Comparing to C. albicans or S. mutans single-species biofilms, dual species biofilms exhibited unique transcriptome profiles and most up-regulated genes were related to carbohydrate transport and metabolic/catabolic processes (Xiao et al., 2022). Specifically, when coexistence, many up-regulated genes of S. mutans are these participating in carbohydrate metabolism, galactose metabolism and glycolysis/gluconeogenesis (Xiao et al., 2022). Meanwhile, coculturing of C. albicans and S. mutans is a double win in terms of sugar utilization. Genes of C. albicans associating with carbohydrate metabolism were also significantly enhanced by co-culturing, including those involved in sugar transport, aerobic respiration, pyruvate breakdown, and the glyoxylate cycle (He et al., 2017; Oliveira et al., 2021). Moreover, a new cross-feeding mechanism between these two species were identified to be mediated by GtfB. GtfB secreted by S. mutans enhances C. albicans carbohydrate utilization (Ellepola et al., 2019). Membrane vesicles (MVs) are subcellular parts secreted by S. mutans cells. MVs containing the Gtf enzyme can locate in the extracellular matrix of C. albicans biofilm, which contributes to the sucrose metabolism of C. albicans (Wu et al., 2020).
4.2 Synergism through signal molecules
The quorum sensing molecule farnesol secreted by C. albicans is generally considered as a bacteriostatic substance (Fernandes et al., 2018). However, farnesol can promote the growth of S. mutans, the expression of gtfs, the formation of biofilm and biological colonies and the activity of transferrin (Kim et al., 2017). It is worth noting that the effect of farnesol on S. mutans is concentration-dependent. This molecule inhibits a series of life activities of S. mutans at a high concentration (> 100μM) (Kim et al., 2017). The specific action mechanism of farnesol on S. mutans is still unknown, and further study is needed.
4.3 Synergism during biofilm formation
The C. albicans-S. mutans dual biofilm is characterized by the interweaving of expanded S. mutans microcolonies with Candida yeasts, hyphae, and pseudo-hyphae to form a three-dimensional inter-kingdom superstructure (Negrini et al., 2022). Compared to C. albicans or S. mutans single species biofilm, dual-species biofilm shows increased biomass, viable cells, EPS and protein content, acid resistance, oxidation, and antibacterial stress resistance, and larger microcolonies as well as much more complex 3D structure (Falsetta et al., 2014; Lobo et al., 2019). The unique physical and chemical properties of C. albicans-S. mutans dual biofilm are resulted from the inter-kingdom synergistic interactions between these two microbes.
Polysaccharides are the major EPS components of biofilm playing important roles in the colonization of microorganisms. Polysaccharides produced by S. mutans are mainly β-1,3-glucan and β-1,6-glucan through the activity of Gtfs (Gregoire et al., 2011), and α-mannan is the most abundant EPS component of C. albicans biofilm, followed by β-1,6-glucan and β-1,3-glucan (Pierce et al., 2017). S. mutans-secreted Gtfs can bind strongly and stably to the mannan layer of C. albicans. This binding converts C. albicans into a de facto glucan producer and consequently promotes the assembly of the EPS-rich matrix scaffold. At present, it is known that three kinds of Gtfs can bind to the cell surface of C. albicans in vitro, among which GtfB has the strongest affinity. The binding of Gtfs to C. albicans affects the physical and chemical properties of dual biofilm significantly because, first, polysaccharides present in the biofilm directly affect the formation and size of the microcolony (Xiao et al., 2012). Second, the production of this 3D EPS-matrix contributes to form an intricate network of exopolysaccharide-enmeshed bacterial-islets (microcolonies) through localized cell-to-matrix interactions. Third, as a diffusion-limiting barrier, the EPS-matrix prevents acid within the biofilm from diffusing outward, thus prolonging and intensifying the acid attack. It helps to create spatial heterogeneities and acidic regions at specific locations at the surface of biofilm attachment (despite exposure to buffered neutral pH) (Xiao et al., 2017; Kim et al., 2018). Fourth, EPS-producing bacterial GtfB exoenzymes can directly modulate antifungal drug tolerance both at a single-cell level and within multicellular biofilms, even if C. albicans is defective in producing its own protective matrices (Liu et al., 2022). In addition, the binding of Gtfs to C. albicans enables the fungus to colonize EPS-coated surfaces readily, therefore recruiting more fungal cells into the biofilm. Finally, it enhances fungal-bacterial coherence (Falsetta et al., 2014). Consistent with this, mutants of S. mutans lacking glucosyltransferase gene showed the ability to seriously disrupt the colonization, accumulation, and formation of cospecies biofilms of C. albicans (Carvalho et al., 2006; Wan et al., 2021). BCR1 is the key gene to develop the biofilm of C. albicans, whose major functional downstream targets include HWP1, ALS1, and ALS3 genes that encode cell surface proteins. Interestingly, GtfB augments the C. albicans counterpart in mixed-species biofilms through a BCR1-independent mechanism (Ellepola et al., 2017). In addition, GtfR of S. mutans can provide adhesion conditions to increase the biomass of C. albicans and biofilm matrix (Souza et al., 2020). Membrane vesicles (MVs) are subcellular parts secreted by cells and are of significance in disease progression and intercellular communication. MVs containing the Gtf enzyme can locate in the extracellular matrix of C. albicans biofilm, which contributes to the sucrose metabolism of C. albicans (Wu et al., 2020). S. mutans can also mediate the recruitment of C. albicans into biofilm through its adhesin antigen I/II. S. mutans antigen I/II promotes the adhesion of both S. mutans and C. albicans (Yang et al., 2018). Antigen I/II mediated process is independent of the streptococcal receptors of C. albicans (such as Als1 and Als3 proteins).
5 Antagonism between C. albicans and S. mutans
Not only “peace” but also “war” exists between C. albicans and S. mutans. The S. mutans-C. albicans association has pleiotropic effects that could be both cooperative and antagonistic. S. mutans exerts an inhibitory effect on the morphogenesis, pathogenicity and biofilm formation of C. albicans (Vílchez et al., 2010; Barbosa et al., 2016). S. mutans inhibits the hyphal formation of C. albicans through streptococcus diffusible signal factor (Sdsf) and competence stimulating peptide (CSP). Sdsf is a fatty acid signaling molecule and an intermediate product of unsaturated fatty acid synthesis. It decreases the expression of HWP1 and SAP5 and therefore suppresses the transformation of C. albicans from yeast to hypha at a concentration that does not affect fungal growth (Lee et al., 2017). CSP not only inhibits the formation of germ tube but stimulates the transformation of mycelium to yeast (Satala et al., 2021). When mixed culturing, S. mutans also increases the expression of fungal genes which are known to suppress filament formation such as tup1 and nrg1 (Jarosz et al., 2009). CSP is a quorum sensing molecule produced by S. mutans at the beginning of the exponential growth stage. S. mutans affect the filamentous development of C. albicans also by secreting a secondary metabolite mutanocyclin (a tetra acid). It is well-known that the conserved camp/protein kinase A (PKA) pathway plays a central role in many life activities of C. albicans (Tao et al., 2022). Mutanocyclin can metabolize the subunit TPK2 in C. albicans in a dose-dependent manner by regulating the PKA pathway to inhibit the growth of filaments, and the inactivation of TPK2 also leads to an increase in the sensitivity of C. albicans to mutanomycin. Meanwhile, mutanocylin shows a global impact on the transcriptional profile of C. albicans, which mainly regulates cell wall components through the Ras1 camp/PKA signaling pathway and EFG1 as well as the subset of filamentous regulators regulated by SFL1. In addition, anchor protein genes related to cell wall biogenesis and remodeling may be involved in the regulation of mutanocyclin response, and anchor protein plays a key role in mutanocyclin regulated inflammation (Dutton et al., 2016).
C. albicans also has an inhibitory effect on S. mutans. For example, farnesol, the signaling molecule secreted by C. albicans at higher concentrations increases the oxygen sensitivity of S. mutans and down-regulated the expression of bacterial virulence-related genes including luxS, brpA, ffh, recA, nth, smx, comC, comYB as well as genes encoding bacteriocin. In addition, farnesol inhibits biofilm formation of biofilm (Rocha et al., 2018) and water-soluble EPS production (Wang et al., 2020). From this aspect, C. albicans suppress the cariogenic ability of S. mutans in specific situations. C. albicans that have the deletion of Sap9 (encoding a secreted aspartyl protease) caused an increase in the incorporation of S. mutans into dual species biofilms, suggesting that Sap9 may act as a key module to influence the competition between C. albicans and S. mutans (Dutton et al., 2016).
6 Summary and prospective
S. mutans has long been taken as the key etiological factor of dental caries, and the oral opportunistic fungus C. albicans also played an important role in promoting S. mutans to cause dental caries. At the same time, the studies related to ECC also reflect the correlation between them, but the specific interaction is unknown. Most of the released studies focus on gene regulation, adhesion, and metabolism interactions between these two microbes. With the innovation and development of technology, we need to explore the cariogenic mechanism and interaction of S. mutans and C. albicans in order to expand more ideas for the prevention and treatment of dental caries, formulate more detailed treatment strategies, and improve better oral health.
Author contributions
YLu and YLin drafted the manuscript. ML and JH designed, edited, and added valuable insights to the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Sichuan Science and Technology Program 2021YFH0188 (ML), the Science and Technology Department of Sichuan Province under Grant 2020YJ0240 (JH), the National Natural Science Foundation of China grant 81400501 (ML).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
American Academy of Pediatric Dentistry (2021). “Policy on early childhood caries (ECC): consequences and preventive strategies,” in The reference manual of pediatric dentistry (Chicago, III: American Academy of Pediatric Dentistry), 81–84.
Baker, J. L., Faustoferri, R. C., Quivey, R. G., Jr (2017). Acid-adaptive mechanisms of Streptococcus mutans-the more we know, the more we don't. Mol. Oral. Microbiol. 32, 107–117. doi: 10.1111/omi.12162
Barbosa, J. O., Rossoni, R. D., Vilela, S. F., De Alvarenga, J. A., Velloso Mdos, S., Prata, M. C., et al. (2016). Streptococcus mutans can modulate biofilm formation and attenuate the virulence of Candida albicans. PloS One 11, e0150457. doi: 10.1371/journal.pone.0150457
Bender, G. R., Sutton, S. V., Marquis, R. E. (1986). Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect. Immun. 53, 331–338. doi: 10.1128/iai.53.2.331-338.1986
Bowen, W. H., Koo, H. (2011). Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45, 69–86. doi: 10.1159/000324598
Carvalho, F. G. D., Silva, D. S., Hebling, J., Spolidorio, L. C., Spolidorio, D. M. P. (2006). Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch. Oral. Biol. 51, 1024–1028. doi: 10.1016/j.archoralbio.2006.06.001
Chakraborty, B., Burne, R. A. (2017). Effects of arginine on Streptococcus mutans growth, virulence gene expression, and stress tolerance. Appl. Environ. Microbiol. 83, e00496–e00417. doi: 10.1128/AEM.00496-17
Cugini, C., Shanmugam, M., Landge, N., Ramasubbu, N. (2019). The role of exopolysaccharides in oral biofilms. J. Dent. Res. 98, 739–745. doi: 10.1177/0022034519845001
Cui, Y., Wang, Y., Zhang, Y., Pang, L., Zhou, Y., Lin, H., et al. (2021). Oral mycobiome differences in various spatial niches with and without severe early childhood caries. Front. Pediatr. 9. doi: 10.3389/fped.2021.748656
Delaney, C., O'donnell, L. E., Kean, R., Sherry, L., Brown, J. L., Calvert, G., et al. (2019). Interkingdom interactions on the denture surface: implications for oral hygiene. Biofilm 1, 100002. doi: 10.1016/j.bioflm.2019.100002
De Soet, J. J., Van Loveren, C., Lammens, A. J., Pavicić, M. J., Homburg, C. H., Ten Cate, J. M., et al. (1991). Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 25, 116–122. doi: 10.1159/000261353
Dige, I., Nyvad, B. (2019). Candida species in intact in vivo biofilm from carious lesions. Arch. Oral. Biol. 101, 142–146. doi: 10.1016/j.archoralbio.2019.03.017
Dutton, L. C., Jenkinson, H. F., Lamont, R. J., Nobbs, A. H. (2016). Role of Candida albicans secreted aspartyl protease Sap9 in interkingdom biofilm formation. Pathog. Dis. 74, ftw005. doi: 10.1093/femspd/ftw005
Ellepola, K., Liu, Y., Cao, T., Koo, H., Seneviratne, C. J. (2017). Bacterial GtfB augments Candida albicans accumulation in cross-kingdom biofilms. J. Dent. Res. 96, 1129–1135. doi: 10.1177/0022034517714414
Ellepola, K., Truong, T., Liu, Y., Lin, Q., Seneviratne, C. J. (2019). Multi-omics analyses reveal synergistic carbohydrate metabolism in Streptococcus mutans-candida albicans mixed-species biofilms. Infect. Immun. 87, e00339–e00319. doi: 10.1128/IAI.00339-19
Fakhruddin, K. S., Samaranayake, L. P., Egusa, H., Ngo, H. C., Pesee, S. (2021). Profuse diversity and acidogenicity of the candida-biome of deep carious lesions of severe early childhood caries (S-ECC). J. Oral. Microb. 13, 1964277. doi: 10.1080/20002297.2021.1964277
Falsetta, M. L., Klein, M. I., Colonne, P. M., Scott-Anne, K., Gregoire, S., Pai, C. H., et al. (2014). Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect. Immun. 82, 1968–1981. doi: 10.1128/IAI.00087-14
Fernandes, R. A., Monteiro, D. R., Arias, L. S., Fernandes, G. L., Delbem, A., Barbosa, D. B. (2018). Virulence factors in Candida albicans and Streptococcus mutans biofilms mediated by farnesol. Indian J. Microbiol. 58, 138–145. doi: 10.1007/s12088-018-0714-4
Fozo, E. M., Quivey, R. G., Jr (2004). The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. J. Bacteriol 186, 4152–4158. doi: 10.1128/JB.186.13.4152-4158.2004
Gregoire, S., Xiao, J., Silva, B. B., Gonzalez, I., Agidi, P. S., Klein, M. I., et al. (2011). Role of glucosyltransferase b in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl. Environ. Microbiol. 77, 6357–6367. doi: 10.1128/AEM.05203-11
Gunaratnam, G., Dudek, J., Jung, P., Hannig, M. (2021). Quantification of the adhesion strength of Candida albicans to tooth enamel. Microorganisms 9, 2213. doi: 10.3390/microorganisms9112213
He, J., Dongyeop, K., Zhou, X., Sang-Joon, A., Burne, R. A., Richards, V. P., et al. (2017). RNA-Seq reveals enhanced sugar metabolism in Streptococcus mutans Co-cultured with Candida albicans within mixed-species biofilms. Front. Microbiol. 8. doi: 10.3389/fmicb
Huang, G. (2012). Regulation of phenotypic transitions in the fungal pathogen Candida albicans. Virulence 3, 251–261. doi: 10.4161/viru.20010
Jarosz, L. M., Deng, D. M., van der Mei, H. C., Crielaard, W., Krom, B. P. (2009). Streptococcus mutans competence-stimulating peptide inhibits Candida albicans hypha formation. Eukaryot Cell 8, 1658–1664. doi: 10.1128/EC.00070-09
Kim, D., Liu, Y., Benhamou, R. I., Sanchez, H., Simón-Soro, Á., Li, Y., et al. (2018). Bacterial-derived exopolysaccharides enhance antifungal drug tolerance in a cross-kingdom oral biofilm. Isme J. 12, 1427–1442. doi: 10.1038/s41396-018-0113-1
Kim, D., Sengupta, A., Niepa, T. H., Lee, B. H., Weljie, A., Freitas-Blanco, V. S., et al. (2017). Candida albicans stimulates Streptococcus mutans microcolony development via cross-kingdom biofilm-derived metabolites. Sci. Rep. 7, 41332. doi: 10.1038/srep41332
Klinke, T., Guggenheim, B., Klimm, W., Thurnheer, T. (2011). Dental caries in rats associated with Candida albicans. Caries Res. 45, 100–106. doi: 10.1159/000324809
Klinke, T., Kneist, S., De Soet, J. J., Kuhlisch, E., Mauersberger, S., Forster, A., Klimm, W. (2009). Acid production by oral strains of Candida albicans and Lactobacilli. Caries Res. 43, 83–91. doi: 10.1159/000204911
Krzyściak, W., Jurczak, A., Kościelniak, D., Bystrowska, B., Skalniak, A. (2014). The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 33, 499–515. doi: 10.1007/s10096-013-1993-7
Lee, K. H., Park, S. J., Choi, S. J., Park, J. Y. (2017). Proteus Vulgaris and Proteus mirabilis decrease Candida albicans biofilm formation by suppressing morphological transition to its hyphal form. Yonsei Med. J. 58, 1135–1143. doi: 10.3349/ymj.2017.58.6.1135
Lemos, J. A., Palmer, S. R., Zeng, L., Wen, Z. T., Kajfasz, J. K., Freires, I. A., et al. (2019). The biology of Streptococcus mutans. Microbiol. Spectr. 7, 10. doi: 10.1128/microbiolspec.GPP3-0051-2018
Len, A. C. L., Harty, D. W. S., Jacques, N. A. (2004). Proteome analysis of Streptococcus mutans metabolic phenotype during acid tolerance. Microbiology 150, 1353–1366. doi: 10.1099/mic.0.26888-0
Li, W., Yu, D., Gao, S., Lin, J., Chen, Z., Zhao, W. (2014). Role of Candida albicans-secreted aspartyl proteinases (Saps) in severe early childhood caries. Int. Jo Mol. Sci. 15, 10766–10779. doi: 10.3390/ijms150610766
Liu, Y., Wang, Z., Zhou, Z., Ma, Q., Li, J., Huang, J., et al. (2022). Candida albicans CHK1 gene regulates its cross-kingdom interactions with Streptococcus mutans to promote caries. Appl. Microbiol. Biotechnol. 106, 7251–7263. doi: 10.1007/s00253-022-12211-7
Lobo, C. I. V., Rinaldi, T. B., Christiano, C. M. S., De Sales Leite, L., Barbugli, P. A., Klein, M. I. (2019). Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. J. Oral. Microbiol. 11, 1581520. doi: 10.1080/20002297.2019.1581520
Lopes, J. P., Lionakis, M. S. (2022). Pathogenesis and virulence of Candida albicans. Virulence 13, 89–121. doi: 10.1080/21505594.2021.2019950
Macgilvray, M. E., Lapek, J. D., Friedman, A. E., Quivey, R. G. (2012). Cardiolipin biosynthesis in Streptococcus mutans is regulated in response to external pH. Microbiol. (Reading) 158, 2133–2143. doi: 10.1099/mic.0.057273-0
Marsh, P. D., Zaura, E. (2017). Dental biofilm: ecological interactions in health and disease. J. Clin. Periodontol. 44, S12–S22. doi: 10.1111/jcpe.12679
Matsui, R., Cvitkovitch, D. (2010). Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 5, 403–417. doi: 10.2217/fmb.09.129
Moalic, E., Gestalin, A., Quinio, D., Gest, P. E., Zerilli, A., Le Flohic, A. M. (2001). The extent of oral fungal flora in 353 students and possible relationships with dental caries. Caries Res. 35, 149–155. doi: 10.1159/000047447
Negrini, T. C., Ren, Z., Miao, Y., Kim, D., Simon-Soro, Á., Liu, Y., et al. (2022). Dietary sugars modulate bacterial-fungal interactions in saliva and inter-kingdom biofilm formation on apatitic surface. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.993640
Nikawa, H., Yamashiro, H., Makihira, S., Nishimura, M., Hamada, T. (2003). In vitro cariogenic potential of Candida albicans. Mycoses 46, 471–478. doi: 10.1046/j.0933-7407.2003.00888.x
Oliveira, B., Filho, A., Burne, R. A., Lin, Z. (2021). The route of sucrose utilization by Streptococcus mutans affects intracellular polysaccharide metabolism. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.636684
Pierce, A., Singh, S., Lee, J., Grant, C., Cruz De Jesus, V., Schroth, R. J. (2019). The burden of early childhood caries in Canadian children and associated risk factors. Front. Public Health 7. doi: 10.3389/fpubh.2019.00328
Pierce, C. G., Vila, T., Romo, J. A., Montelongo-Jauregui, D., Wall, G., Ramasubramanian, A., et al. (2017). The Candida albicans biofilm matrix: composition, structure and function. J. Fungi (Basel) 3 (1), 14. doi: 10.3390/jof3010014
Rocha, G. R., Florez Salamanca, E. J., De Barros, A. L., Lobo, C. I. V., Klein, M. I. (2018). Effect of tt-farnesol and myricetin on in vitro biofilm formed by Streptococcus mutans and Candida albicans. BMC Complement Altern. Med. 18, 61. doi: 10.1186/s12906-018-2132-x
Sachdev, J., Bansal, K., Chopra, R. (2016). Effect of comprehensive dental rehabilitation on growth parameters in pediatric patients with severe early childhood caries. Int. J. Clin. Pediatr. Dent. 9, 15–20. doi: 10.5005/jp-journals-10005-1326
Santiago, B., Macgilvray, M., Faustoferri, R. C., Quivey, R. G. (2012). The branched-chain amino acid aminotransferase encoded by ilvE is involved in acid tolerance in Streptococcus mutans. J. Bact. 194, 2010–2019. doi: 10.1128/JB.06737-11
Satala, D., Gonzalez-Gonzalez, M., Smolarz, M., Surowiec, M., Kulig, K., Wronowska, E., et al. (2021). The role of Candida albicans virulence factors in the formation of multispecies biofilms with bacterial periodontal pathogens. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.765942
Souza, J. G. S., Bertolini, M., Thompson, A., Mansfield, J. M., Dongari-Bagtzoglou, A. (2020). Role of glucosyltransferase r in biofilm interactions between Streptococcus oralis and. Candida albicans. ISME J. 14, 1–16. doi: 10.1038/s41396-020-0608-4
Sridhar, S., Suprabha, B. S., Shenoy, R., Suman, E., Rao, A. (2020). Association of Streptococcus mutans, Candida albicans and oral health practices with activity status of caries lesions among 5-Year-Old children with early childhood caries. Oral. Health Prev. Dent. 18, 911–919. doi: 10.3290/j.ohpd.a45411
Tao, L., Wang, M., Guan, G., Zhang, Y., Hao, T., Li, C., et al. (2022). Streptococcus mutans suppresses filamentous growth of Candida albicans through secreting mutanocyclin, an unacylated tetramic acid. Virulence 13, 542–557. doi: 10.1080/21505594.2022.2046952
Tinanoff, N., Baez, R. J., Diaz Guillory, C., Donly, K. J., Feldens, C. A., Mcgrath, C., et al. (2019). Early childhood caries epidemiology, aetiology, risk assessment, societal burden, management, education, and policy: global perspective. Int. J. Paediatr. Dent. 29, 238–248. doi: 10.1111/ipd.12484
Vadeboncoeur, C., Pelletier, M. (1997). The phosphoenolpyruvate:sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol. Rev. 19, 187–207. doi: 10.1111/j.1574-6976.1997.tb00297.x
Vieira-Andrade, R. G., Gomes, G. B., De Almeida Pinto-Sarmento, T. C., Firmino, R. T., Pordeus, I. A., Ramos-Jorge, M. L., et al. (2016). Oral conditions and trouble sleeping among preschool children. J. Public Health-UK 24, 395–400. doi: 10.1007/s10389-016-0734-7
Vílchez, R., Lemme, A., Ballhausen, B., Thiel, V., Schulz, S., Jansen, R., et al. (2010). Streptococcus mutans inhibits Candida albicans hyphal formation by the fatty acid signaling molecule trans-2-decenoic acid (SDSF). Chembiochem 11, 1552–1562. doi: 10.1002/cbic.201000086
Wan, S. X., Tian, J., Liu, Y., Dhall, A., Koo, H., Hwang, G. (2021). Cross-kingdom cell-to-Cell interactions in cariogenic biofilm initiation. J. Dent. Res. 100, 74–81. doi: 10.1177/0022034520950286
Wang, X., He, H., Liu, J., Xie, S., Han, J. (2020). Inhibiting roles of farnesol and HOG in morphological switching of Candida albicans. Am. J. Transl. Res. 12, 6988–7001.
Witherden, E. A., Shoaie, S., Hall, R. A., Moyes, D. L. (2017). The human mucosal mycobiome and fungal community interactions. JoF 3, 56. doi: 10.3390/jof3040056
Wu, R., Tao, Y., Cao, Y., Zhou, Y., Lin, H. (2020). Streptococcus mutans membrane vesicles harboring glucosyltransferases augment Candida albicans biofilm development. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.581184
Xiao, J., Grier, A., Faustoferri, R. C., Alzoubi, S., Gill, A. L., Feng, C., et al. (2018a). Association between oral Candida and bacteriome in children with severe ECC. J. Dent. Res. 97, 1468–1476. doi: 10.1177/0022034518790941
Xiao, J., Hara, T., Anderson, Kim, D., Zero, T., Domenick, et al. (2017). Biofilm three-dimensional architecture influences in situ pH distribution pattern on the human enamel surface. Int. J. Oral. Sci. 9, 74–79. doi: 10.1038/ijos.2017.8
Xiao, J., Huang, X., Alkhers, N., Alzamil, H., Alzoubi, S., Wu, T. T., et al. (2018b). Candida albicans and early childhood caries: a systematic review and meta-analysis. Caries Res. 52, 102–112. doi: 10.1159/000481833
Xiao, J., Klein, M. I., Falsetta, M. L., Lu, B., Delahunty, C. M., Yates, J. R., et al. (2012). The exopolysaccharide matrix modulates the interaction between 3D architecture and virulence of a mixed-species oral biofilm. PloS Pathog. 8, e1002623. doi: 10.1371/journal.ppat.1002623
Xiao, J., Zeng, Y., Rustchenko, E., Huang, X., Wu, T. T., Falsetta, M. L. (2022). Dual transcriptome of Streptococcus mutans and Candida albicans interplay in biofilms. J. Oral. Microbiol. 15, 2144047. doi: 10.1080/20002297.2022.2144047
Yang, C., Scoffield, J., Wu, R., Deivanayagam, C., Zou, J., Wu, H. (2018). Antigen I/II mediates interactions between Streptococcus mutans and Candida albicans. Mol. Oral. Microbiol. 33, 283–291. doi: 10.1111/omi.12223
Keywords: early-childhood caries, Candida albicans, Streptococcus mutans, interkingdom interaction, symbiosis, antagonism
Citation: Lu Y, Lin Y, Li M and He J (2023) Roles of Streptococcus mutans-Candida albicans interaction in early childhood caries: a literature review. Front. Cell. Infect. Microbiol. 13:1151532. doi: 10.3389/fcimb.2023.1151532
Received: 26 January 2023; Accepted: 21 April 2023;
Published: 16 May 2023.
Edited by:
Jin Xiao, University of Rochester Medical Center, United StatesReviewed by:
Maryam Roudbary, Iran University of Medical Sciences, IranCopyright © 2023 Lu, Lin, Li and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinzhi He, aGVqaW56aGlAc2N1LmVkdS5jbg==; Mingyun Li, bGltaW5neXVuQHNjdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yifei Lu1†
Yifei Lu1† Mingyun Li
Mingyun Li Jinzhi He
Jinzhi He