- 1Department of Thoracic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2Department of Respiration, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Objective: Mucormycosis has emerged as an increasingly important cause of morbidity and mortality in immunocompromised patients, but the effective drugs for the treatment are limited. Hence, the study aimed to summarize the characteristics of mucormycosis in patients with hematological malignancies, and investigate the efficacy and safety of Amphotericin B Colloidal Dispersion (ABCD) in treating mucormycosis.
Methods: In this study, patients with mucormycosis complicated by hematological malignancies who received ABCD at the First Affiliated Hospital of Zhengzhou University from April 2021 to May 2022 were retrospectively enrolled. The clinical data of the enrolled patients were collected, and then, the drug response at 2 weeks, 4 weeks, and the end of treatment; the survival rate at 4, 8, and 12 weeks; and the laboratory-related indicators and adverse events (AEs) associated with ABCD were evaluated.
Results: In total, 9 patients with mucormycosis complicated by hematological malignancies were enrolled. The main symptoms were fever, cough, and chest pain. In addition, reversed halo signs (RHS) were found on chest CTs. The responses to ABCD at 2 weeks, 4 weeks, and the end of treatment were 100% (9/9), 77.8% (7/9), and 77.8% (7/9), respectively. The survival rates of the patients at 4, 8, and 12 weeks were 77.8% (7/9), 66.7% (6/9), and 66.7% (6/9), respectively. Among laboratory-related indicators, white blood cell (WBC) counts were significantly increased from baseline after 1 and 2 weeks of ABCD treatment (P<0.05), whereas neutrophil counts were only increased significantly from baseline at 2 weeks post-treatment (P<0.05). The most common AEs were infusion-related AEs manifesting as fever, chills, and pruritus. Moreover, none of the patients suffered from renal injury once again.
Conclusion: ABCD is a promising treatment strategy for patients with mucormycosis complicated by hematologic malignancies, showing remarkable efficacy and safety.
Introduction
Mucormycosis is a rare disease with high morbidity and mortality, which is difficult to diagnose (Cornely et al., 2019). Hematological malignancies are the main underlying diseases associated with mucormycosis. The available data suggest that mucormycosis has the highest mortality rate among all types of fungal infections (Bretagne et al., 2022), whereas hematologic malignancies ranked the first in the mortality of all primary diseases among mucormycosis patients (Roden et al., 2005; Muthu et al., 2021). Mucormycosis has thus become one of the most important causes of death in patients with hematologic malignancies. Although surgical debridement combined with antifungal drug therapy can improve the survival rate of patients with mucormycosis, compared to that with antifungal drugs alone, unfortunately, many patients with hematologic malignancies are not eligible for surgery owing to susceptibility to infection and bleeding. Therefore, systemic antifungal drug therapy remains the most important treatment for many patients with hematologic malignancies (Muthu et al., 2021). In addition, compared to that with early treatment, delaying amphotericin B (AmB)-based first-line therapy was shown to lead to a 2-fold increase in the mortality rate at 12 weeks after diagnosis (Chamilos et al., 2008). Thus, timely and effective treatment is critical for improving prognosis.
The main drugs recommended for this are liposomal amphotericin B (L-AmB), isavuconazole, and posaconazole based on the revised consensus definitions of IFD from EORTC/MSGERC. L-AmB is preferred when all three drugs are available (Cornely et al., 2019). Unfortunately, L-AmB made in China is different from L-AmB in other countries, and there is no significant improvement in nephrotoxicity compared with AmB (Caillot et al., 2018), and even worse, few patients can afford isavuconazole and posaconazole because of their high price, and they have not been included in China’s medical insurance system. Consequently, AmB and Amphotericin B Colloidal Dispersion (ABCD) are the main drugs presently used to treat mucormycosis in China. Sodium deoxycholate has been used as a co-solvent of the traditional AmB, but this readily causes renal insufficiency and severe hypokalemia, severely limiting the dosage and duration of medication and affecting its antifungal effect (Ullmann et al., 2006). Moreover, it usually takes months to receive antifungal treatment for patients with mucormycosis complicated by hematologic malignancies. Hence, AmB is far from meeting clinical demands. ABCD is a disc-shaped colloidal nanoparticle dispersion formed with AmB and sodium cholesterol-sulfate at a molar ratio of 1:1. Sodium cholesterol sulfate can combine with AmB, thereby reducing the combination of AmB with cholesterol in the human cell membrane (Herbrecht et al., 2001). In addition, ABCD can be quickly absorbed by reticuloendothelial system organs, such as the liver, spleen, and lung, after entering the blood, thus avoiding damage to the renal tubules; therefore, nephrotoxicity is low (Oppenheim et al., 1995). Adverse reactions to ABCD mainly comprise an infusion reaction (White et al., 1998). Since ABCD (CSPC Ouyi Pharmaceutical Group Co., Ltd.) got successful marketing authorisation by the National Medical Products Administration in China on March 30, 2021, as a polyene drug with minimal renal toxicity, it has been increasingly used for antifungal therapy. However, to our knowledge, there are no relevant research reports on the efficacy and safety of ABCD in China for patients with mucormycosis complicated by hematologic malignancies.
Therefore, for the first time, we summarized the clinical characteristics of 9 patients with mucormycosis complicated by hematologic malignancies and treated with ABCD. Moreover, we assessed the efficacy and safety of ABCD for the treatment of mucormycosis, expecting to provide evidence for clinical application.
Patients and methods
Study design
In this study, data of patients diagnosed with mucormycosis complicated by hematologic malignancies, who had received ABCD treatment for more than 7 days with complete laboratory data, at the First Affiliated Hospital of Zhengzhou University from April 2021 to May 2022 were retrospectively collected. The mucormycosis diagnosis was in accordance with the revised consensus definitions of IFD from EORTC/MSGERC (Cornely et al., 2019), and the patients included were classified into three categories, “proven” “probable” and “possible”. Specifically, 2 cases were proven, 6 cases were probable, and 1 case was possible. All included patients were diagnosed by a respiratory specialist, a hematologist, and a radiologist. This study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2021-KY-0286). The requirement for informed consent was waived owing to the retrospective nature of the study. The hospital electronic database was searched to collect clinical data of patients, including demographic, primary morbidity, clinical manifestations, imaging features, laboratory examination, diagnosis processes, treatment measures, and adverse events (AEs).
Evaluation of clinical response
The response to ABCD at 2 weeks, 4 weeks, and the end of treatment and the survival rate at 4, 8, and 12 weeks post-treatment were evaluated according to the 2008 Mycoses Study Group and European Organization for Research and Treatment of Cancer Consensus Criteria (De Pauw et al., 2008). Based on the basis of a composite of clinical, radiological, and mycological criteria, the general criteria for global responses to antifungal therapy were classified as follows: 1) complete response (CR), survival within the prespecified period of observation, resolution of all attributable symptoms and signs of disease and radiological abnormalities, and mycological evidence of eradication of disease; 2) partial response (PR), survival within the prespecified period of observation, improvement in attributable symptoms and signs of disease and radiological abnormalities, and evidence of clearance of cultures or reduction of fungal burden. As assessed by a quantitative and validated laboratory marker; 3) stable response, survival within the prespecified period of observation and minor or no improvement in fungal disease, but no evidence of progression, as determined on the basis of a composite of clinical, radiological, and mycological criteria; 4) progression of fungal disease, evidence of progressive fungal disease based on a composite of clinical, radiological, and mycological criteria (Chamilos et al., 2008); death, death during the prespecified period of evaluation, regardless of attribution. CR and PR were defined as success, whereas the remaining states were considered failure.
Laboratory indicators, including routine blood tests, liver function, renal function, and electrolytes, were assessed. Drug safety was evaluated based on the AEs recorded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 5.0). Clinical AEs caused by ABCD were defined as parameters that changed from normal to abnormal or aggravated compared to those at baseline, which occurred during treatment with the drugs.
Statistical analysis
All data analyses were performed using SPSS version 25.0 (SPSS Inc. IBM Corp). Continuous data were tested for normality using the Shapiro-Wilk method. If the normality criteria were satisfied, data were described as the mean ± standard deviation, and the comparisons were performed using paired-samples Student’s t-tests. Otherwise, the median and interquartile ranges (IQR) were used. The categorical data were expressed as percentages (%) and frequencies. Missing values were not included in the percentage calculations unless otherwise noted. Effect sizes were reported with 95% confidence intervals. Statistical significance was defined as P<0.05 for all analyses.
Results
Baseline characteristics
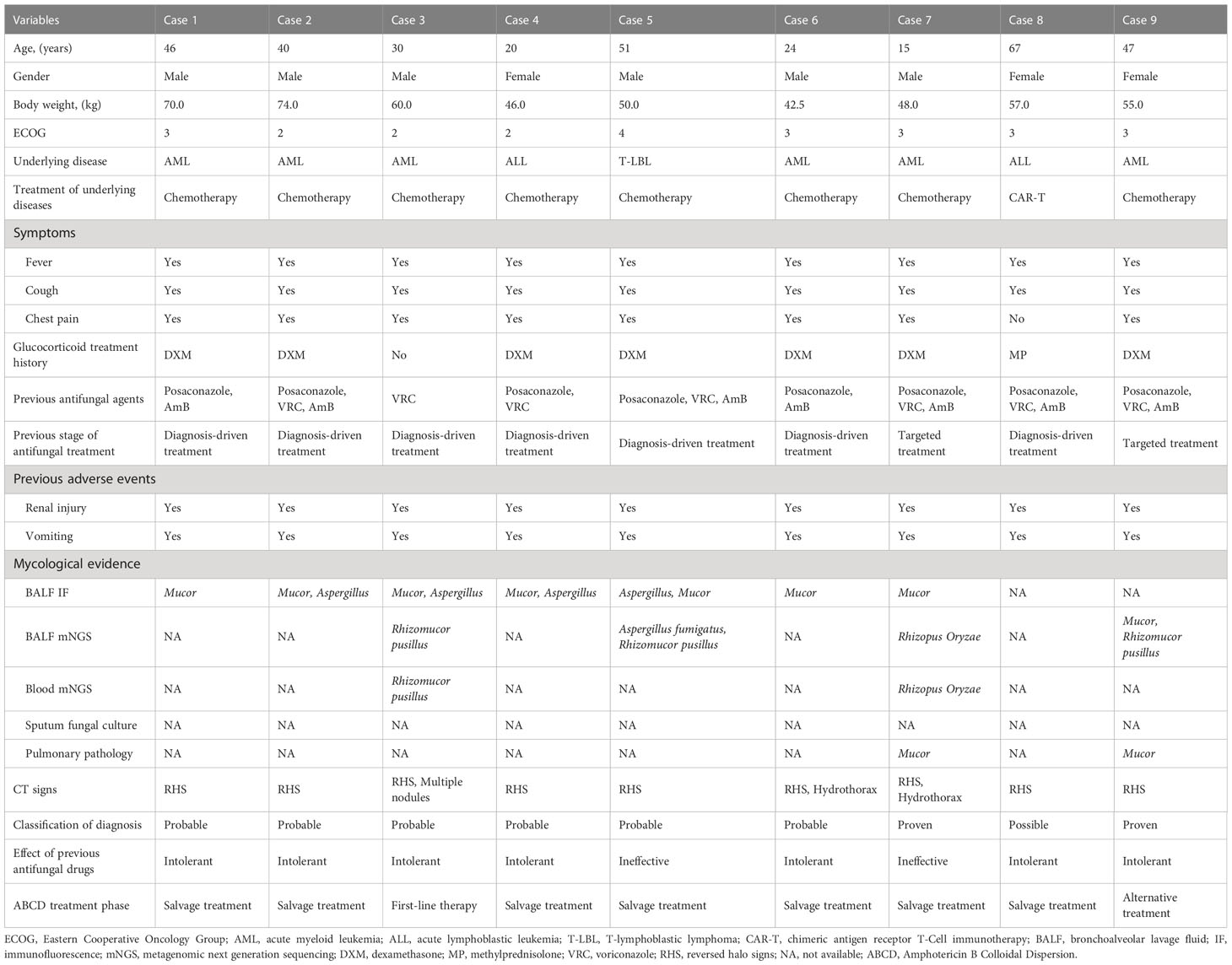
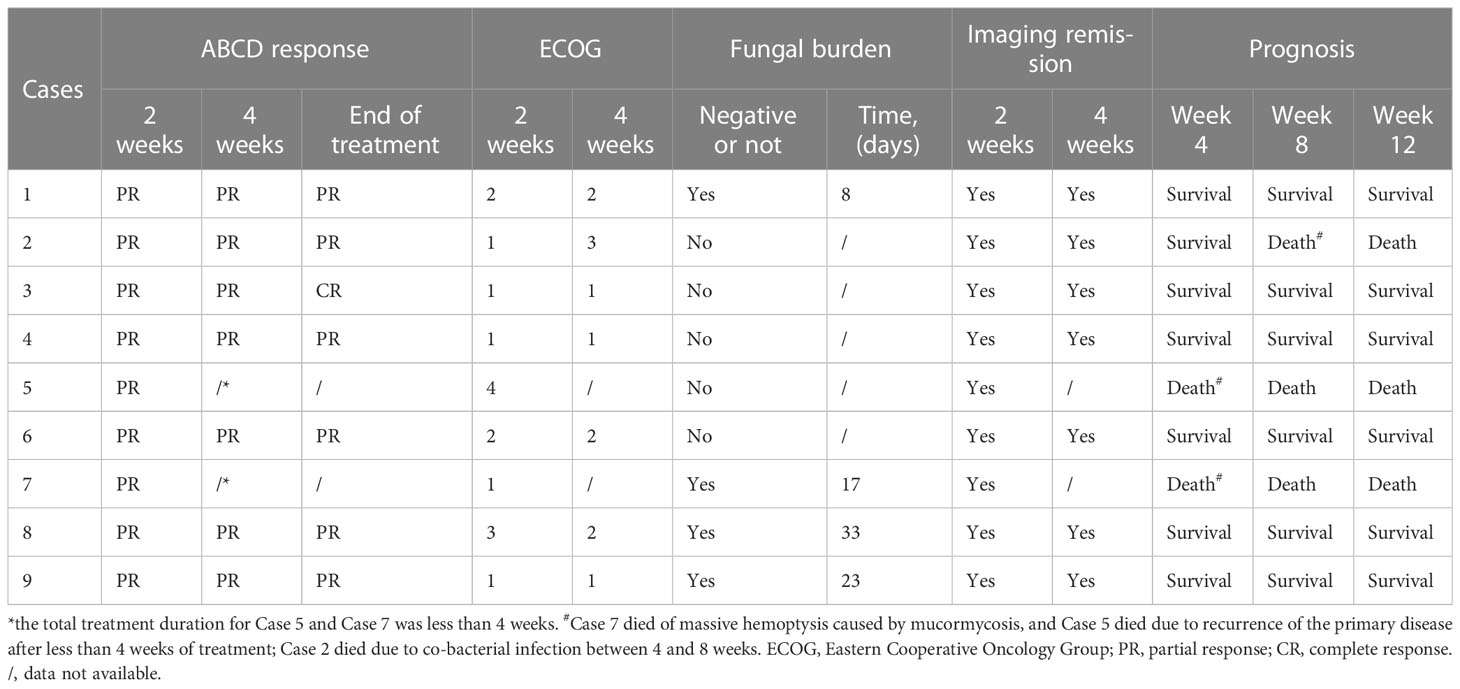
There were 12 patients diagnosed with hematological malignancies complicated by mucormycosis, of which, 2 were excluded because they were treated for fewer than 7 days with ABCD and one was excluded as they were lost to follow-up. A total of 9 cases were included in the analysis (Figure 1). Among the 9 patients, 6 were males and 3 were females, with a median age of 40 years. The underlying diseases were acute myeloid leukemia (6 cases), acute lymphoblastic leukemia (2 cases) and T-lymphoblastic lymphoma (1 case). According to the revised consensus definitions of IFD from EORTC/MSGERC (Cornely et al., 2019), 2 were proven cases, 6 were probable cases and 1 was possible case among the 9 patients. Additionally, 6 cases were pulmonary mucormycosis and 3 cases were disseminated mucormycosis (pulmonary and cerebral mucormycosis). Fever, cough, and chest pain were the main symptoms. All cases had reversed halo signs (RHS) on chest CT, of which, Case 3 had multiple nodules in the lungs and Cases 6 and 7 simultaneously had pleural effusion. Before the administration of ABCD, 7 patients received AmB and posaconazole combined antifungal therapy, 1 patient received posaconazole combined with voriconazole therapy, and 1 patient received voriconazole as antifungal monotherapy. However, all patients had varying degrees of renal damage, nausea, and vomiting. Among the 9 cases, Case 3 received ABCD as the first-line therapy, Case 9 was treated with ABCD as an alternative treatment, and the remaining 7 cases received ABCD as salvage treatment. Detailed information was shown in Table 1.
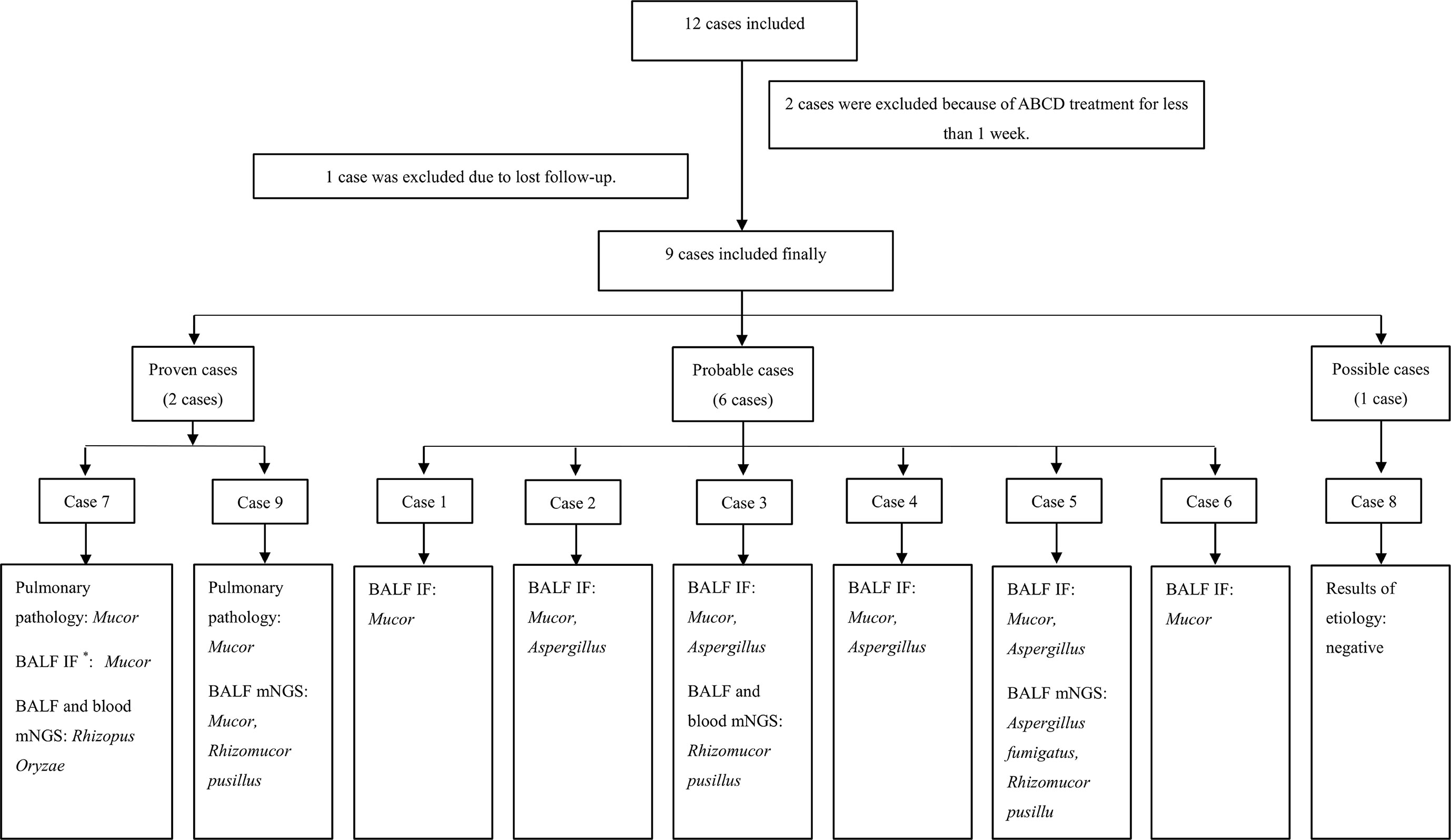
Figure 1 Flow chart of patients’ inclusion and diagnosis. *BALF immunofluorescen staining of Mucor and Aspergillus: Mucor, broad undivided, right-angled branching hyphae; Aspergillus, widely septate, branching hyphae at acute angles. ABCD, Amphotericin B Colloidal Dispersion; BALF, bronchoalveolar lavage fluid; IF, immunofluorescen staining; mNGS, metagenomic next generation sequencing.
Bronchoscopy was performed in all cases. Cases 7 and 9 were proven by biopsy using fiberoptic bronchoscopy, which indicated Mucor infection. In the probable cases, Mucor filaments were detected via immunofluorescence in the bronchoalveolar lavage fluid (BALF), including 4 cases of both Aspergillus and Mucor filaments. Case 8 was the only possible case in which mycological evidence were all negative in the present study. Furthermore, among all the 9 cases, BALF metagenomic next generation sequencing (mNGS) for Cases 3, 7, 5, and 9 suggested Mucor, including 1 case (Case 5) suggesting mixed infection of Aspergillus fumigatus and Rhizomucor pusillus. Additionally, peripheral blood mNGS and BALF mNGS were used for Cases 3 and 7 simultaneously, and the results were consistent between these two methods. Sputum fungal culture was negative in all cases. Detailed information was shown in Table 1.
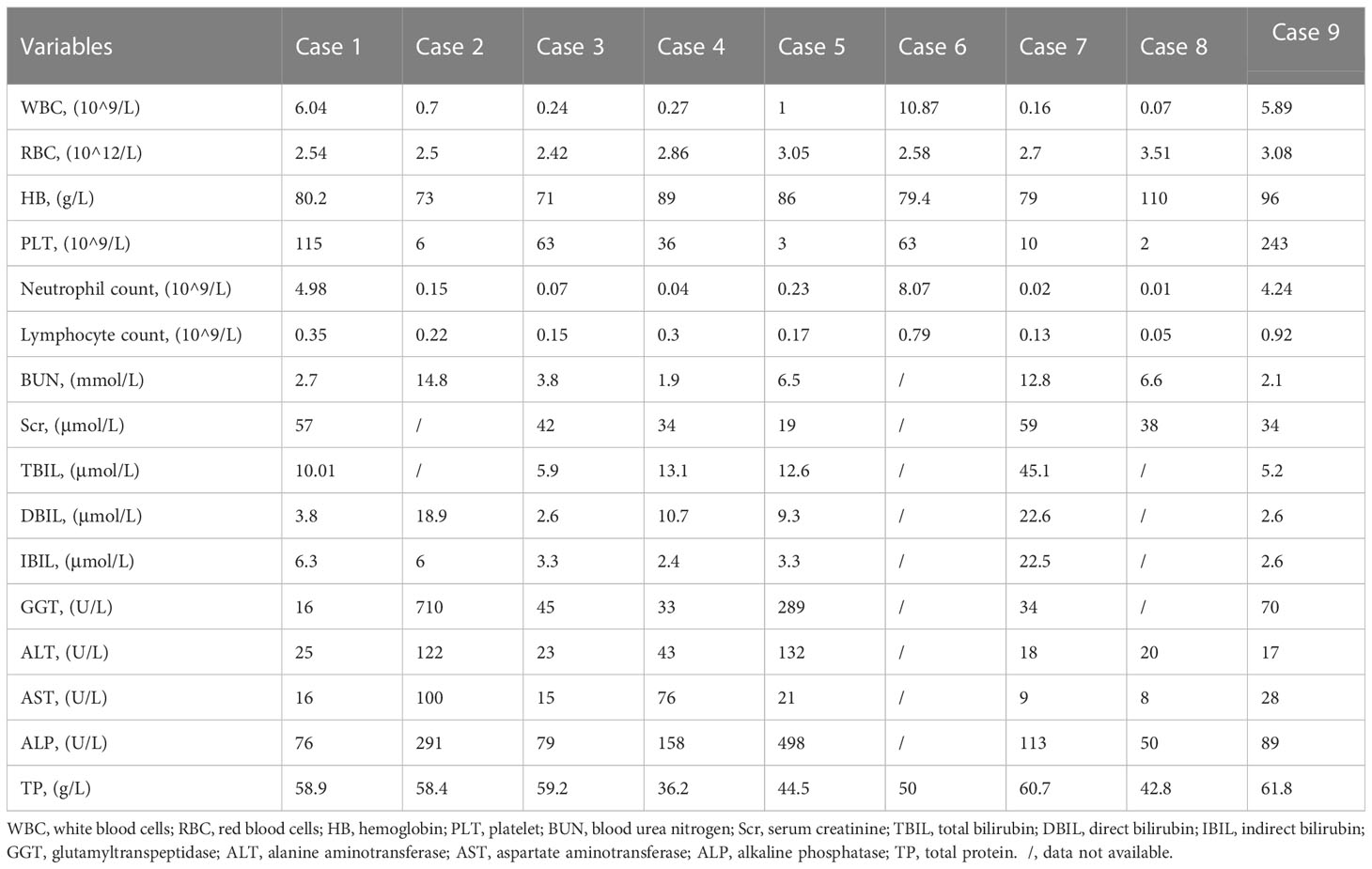
As shown in Table 2, before ABCD treatment, 8 of the 9 patients had mild-to-moderate anemia, 6 had severe neutropenia when diagnosed with mucormycosis, and 3 had extremely severe thrombocytopenia. In terms of renal function, 2 patients (Cases 2 and 7) had elevated urea nitrogen. Regarding liver function, the elevated levels of total, direct, and indirect bilirubin were observed in Case 7. Glutamyl transpeptidase (GGT) levels were significantly increased in 2 cases (Cases 2 and 5) and slightly increased in Case 9. The levels of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were significantly increased in 2 cases (Cases 2 and 5), and the levels of aspartate aminotransferase (AST) were significantly increased in Case 2.
ABCD therapy
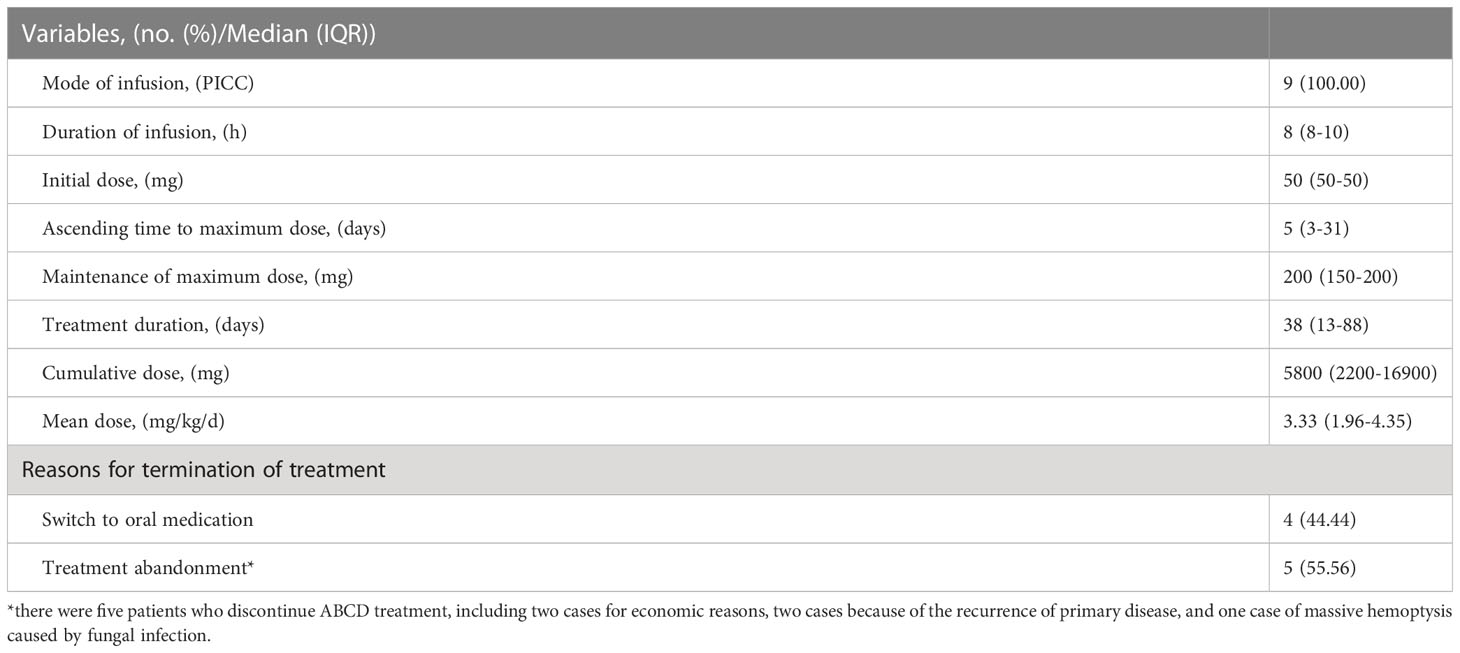
In all included cases, ABCD was infused via a peripherally inserted central venous catheter (PICC) for 8-10 h. The dose on the first day was 50 mg, which was increased to 150-200 mg within 2-4 days. The median duration of treatment was 33 (13-88) days. Taken together, the accumulated dose for the nine patients was 5800 (2200-16900) mg, and the mean dose was 3.33 (1.96-4.35) mg/kg/d. Among the nine cases, four were treated with posaconazole enteric-coated tablets or suspension maintenance after effective ABCD treatment, and 5 discontinued with ABCD for economic reasons (2 cases; Cases 1 and 6), primary disease recurrence (2 cases; Cases 2 and 5), and death resulting from massive hemoptysis caused by IFD (1 case; Case 7) (Table 3). The administration of ABCD in each patient is shown in Supplementary Table 1.
Adjuvant therapy
All patients were treated with promethazine and dexamethasone prior to ABCD to prevent possible infusion-related AEs, which occurred in 8 cases after 0.5-1 h of ABCD treatment on the first day. After stopping the infusion of ABCD and giving methylprednisolone, all patient symptoms related to infusion-related AEs were relieved, and then continued to receive ABCD treatment.
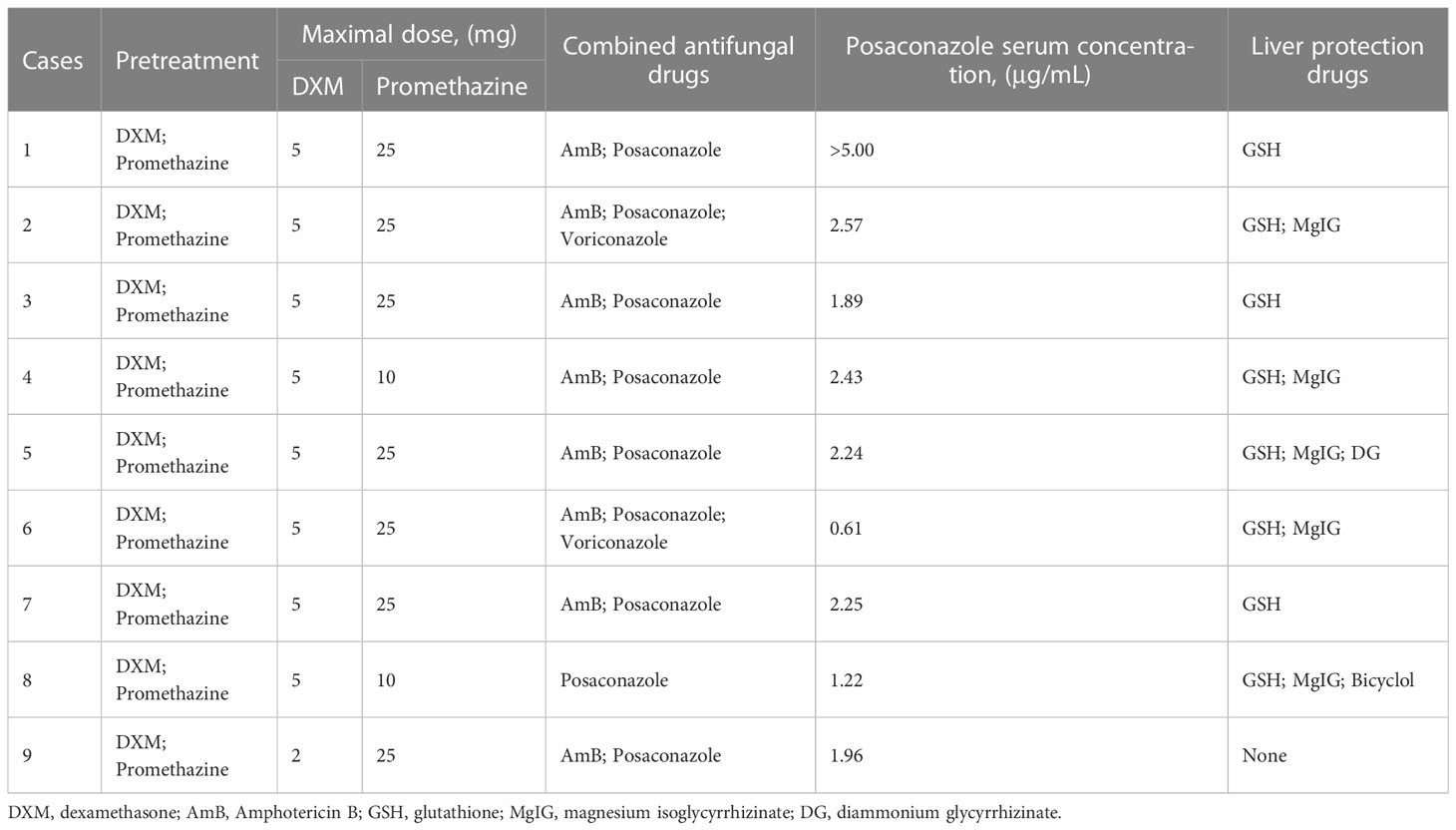
After the infusion-related AEs, ALT, AST, total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), procalcitonin (PCT), and C-reactive protein (CRP) were elevated transitionally in 8 patients. Therefore, glutathione (GSH), magnesium isoglycyrrhizinate (MgIG), and other hepatoprotective drugs were administrated. Liver function in 6 patients returned to normal. In the absence of other new infections, PCT and CRP levels returned to pre-ABCD levels in approximately 7 days. All patients were treated with antifungal combination therapy via the oral administration of AmB atomization and posaconazole tablets or suspensions while using ABCD treatment. In addition, the posaconazole concentration for Case 1 was higher than 5.00 μg/mL, that for Case 6 was 0.61 μg/mL, and that for the other 7 cases was 1.00-3.00 μg/mL (Table 4).
Efficacy analysis
As shown in Table 5, at 2 weeks of ABCD treatment, all 9 patients achieved PR with a drug response of 100% (9/9). At 4 weeks of treatment, 7 patients achieved PR, with a drug response of 77.8% (7/9). At the end of treatment, 6 patients achieved PR and 1 achieved CR (Case 3), with a drug response of 77.8% (7/9). Figure 2 showed the findings of chest CT scans before and after ABCD treatment in cases 3 and 4. In Case 3, before ABCD treatment, multiple nodules and RHS were seen in both lungs (Figure 2A); after 6 weeks of ABCD treatment, both lung lesions were significantly reduced (Figure 2B). In Case 4, before ABCD treatment, RHS were observed in the upper and lower lobes of the right lung (Figure 2C); after 6 weeks of ABCD treatment, the lesions were significantly reduced, and cavity was formed in the upper lobe of the right lung, while low-density necrosis and small cavity was found in the lesions in the lower lobe of the right lung (Figure 2D).
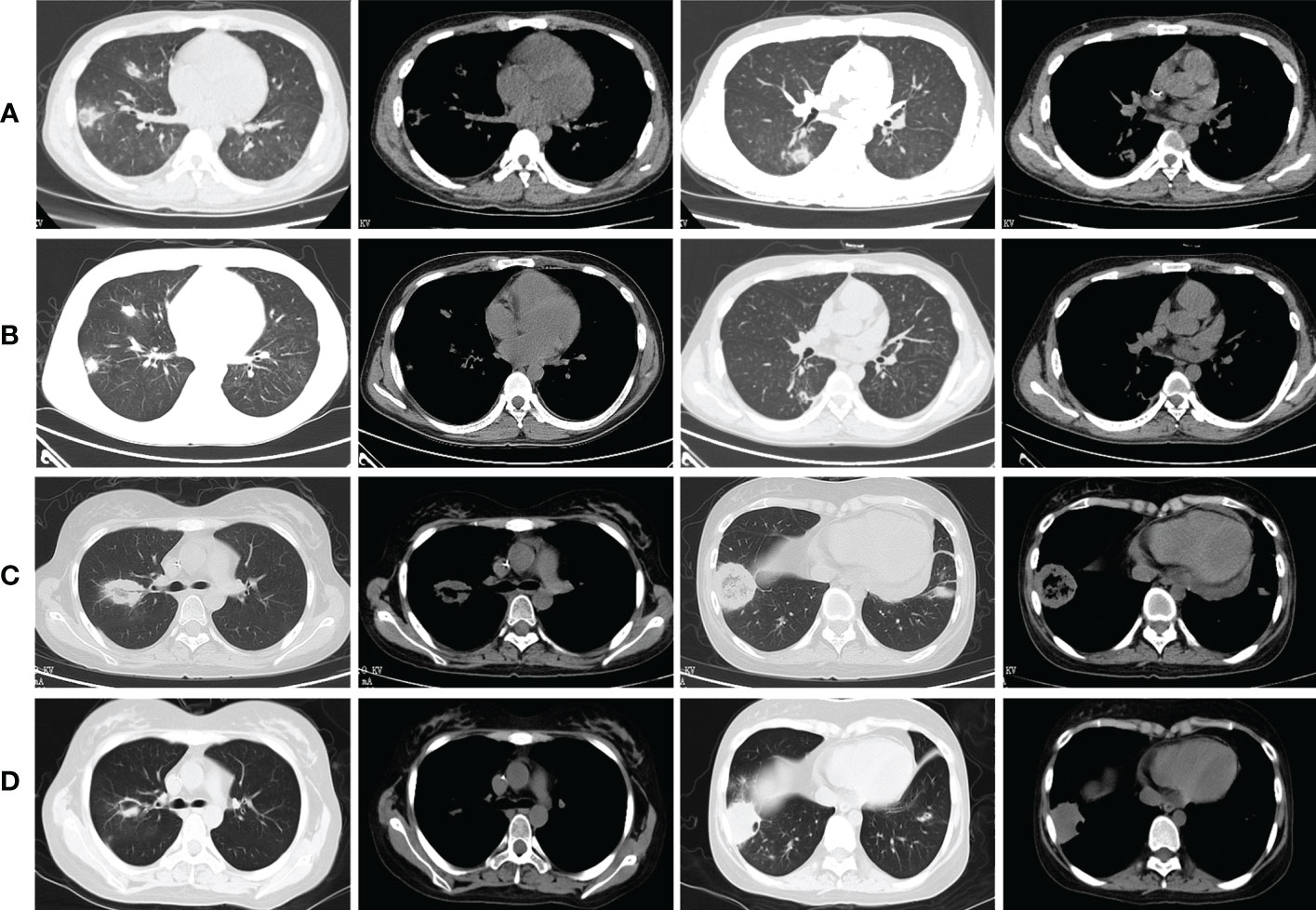
Figure 2 Findings of chest CT scans before and after ABCD treatment in cases 3 and 4. (A) Before ABCD treatment, multiple nodules and reversed halo signs were seen in both lungs; (B) After 6 weeks of ABCD treatment, both lung lesions were significantly reduced; (C) Before ABCD treatment, reversed halo signs were observed in the upper and lower lobes of the right lung; (D) After 6 weeks of ABCD treatment, the lesions were significantly reduced, and cavity was formed in the upper lobe of the right lung, while low-density necrosis and small cavity was found in the lesions in the lower lobe of the right lung.
Survival rates of patients at 4, 8, and 12 weeks were 77.8% (7/9), 66.7% (6/9), and 66.7% (6/9), respectively. Notably, Case 5 and Case 7 both died within 4 weeks after ABCD treatment due to recurrence of underlying disease and massive hemoptysis caused by IFD, respectively. Case 2 died resulted from co-bacterial infection between 4 and 8 weeks of post-treatment.
Laboratory data analysis
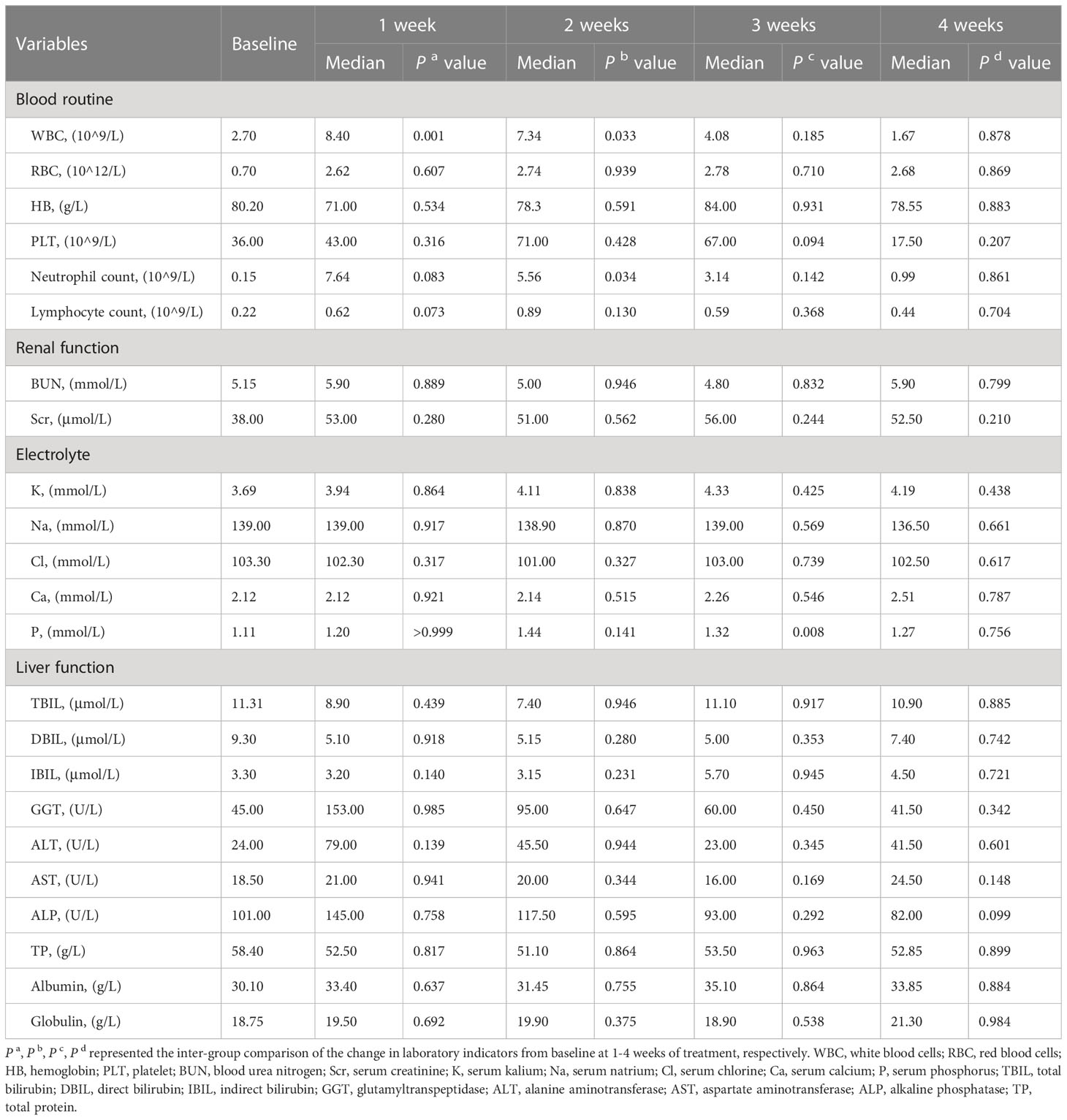
As shown in Table 6, at 1 week of ABCD treatment, white blood cells (WBC) levels were increased significantly from baseline (P<0.001). After 2 weeks of treatment, WBC and neutrophil levels were increased significantly from baseline (P<0.05). However, there were no significant differences in other laboratory indicators, such as red blood cells (RBCs), hemoglobin (HB), platelets (PLTs), urea nitrogen, creatinine (Scr), blood kalium, and liver function before and after ABCD treatment (P>0.05).
Drug safety analysis
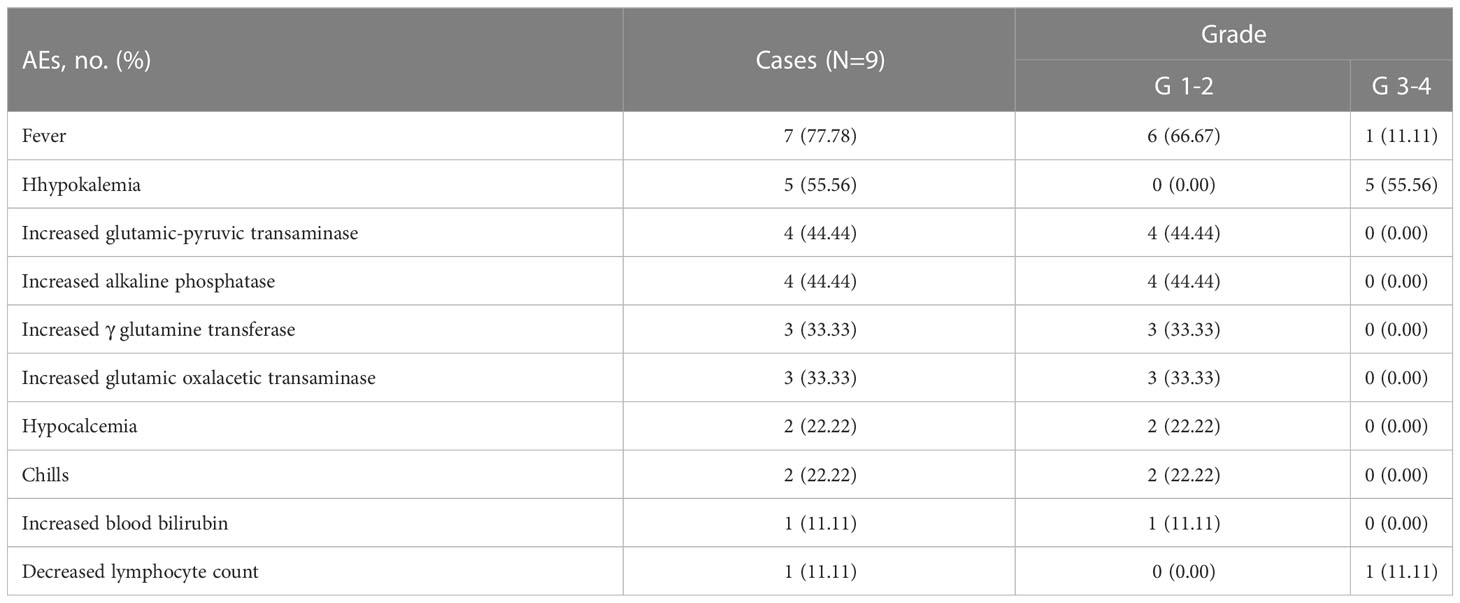
ABCD treatment-related AEs were shown in Table 7. Most of the AEs were grade 1-2, including 6 cases of fever, 2 cases of chills, 4 cases of elevated liver function indexes and 2 cases of hypocalcemia. Grade 3-4 AEs included fever (1 case), hypokalemia (5 cases), and decreased lymphocyte count (1 case). After symptomatic treatment, those parameters in all patients returned to normal.
Discussion
To our knowledge, this is the first study of ABCD in the treatment of hematologic malignancies associated with mucormycosis in the last 10 years. Our study confirmed that ABCD showed satisfactory safety and efficacy for the treatment of mucormycosis complicated by hematologic malignancies and could be used for this application. In the present study, a total of 9 patients with mucormycosis complicated by hematologic malignancies were included, all of whom received antifungal therapy with ABCD. The overall responses of ABCD at 2 weeks, 4 weeks, and the end of treatment were 100% (9/9), 77.8% (7/9), and 77.8% (7/9), respectively. The survival rates at 4, 8, and 12 weeks were 77.8% (7/9), 66.7% (6/9), and 66.7% (6/9), respectively. The most common adverse event was an infusion reaction, followed by hypokalemia and liver insufficiency.
At present, the incidence of mucormycosis is increasing and the incidence of mucormycosis varies across countries and regions. In developed countries, hematologic malignancies have become the main cause of mucormycosis, whereas in developing countries, diabetes and ketoacidosis are still the main causes (Prakash and Chakrabarti, 2019). In our center, the main populations affected by mucormycosis are those with hematologic malignancies and those receiving hematopoietic stem cell transplantation (HSCT). To date, it is still difficult to diagnose patients with mucormycosis complicated by hematological malignancies because there are no specific serological markers. In addition, owing to the characteristics of patients with hematological malignancies, PLT numbers are often lower than 50×109/L with poor coagulation functions. It is less likely for these patients to tolerate CT-guided percutaneous lung puncture or bronchoscopy biopsy to obtain histopathological results. Therefore, in this study, only 2 out of 9 cases were proven to have mucormycosis through histopathology. Moreover, previous studies have shown that RHS is a specific imaging-based manifestation of pulmonary mucormycosis (Jung et al., 2015; Bourcier et al., 2017), which is a focal circular area of ground-glass changes surrounded by ring consolidation. In addition, in a clinical study of 21 patients receiving allo-HSCT complicated by pulmonary mucormycosis, RHS were seen both at the beginning of infection and after antifungal therapy (Bao and Liu, 2022). Therefore, in our study, all patients started empirical treatment for RHS based on CT imaging before obtaining the results of etiology.
In our study, the main therapeutic drugs used to treat mucormycosis were posaconazole suspensions or tablets, AmB, and ABCD, which are also the main drugs used to treat mucormycosis in China. The revised consensus definitions of IFD from EORTC/MSGERC (Cornely et al., 2019) recommend L-AmB as the first-line antifungal therapy; However, L-AmB made in China is different from L-AmB in other countries, and there is no significant improvement in nephrotoxicity between L-AmB made in China and AmB. Therefore, before the launch of ABCD in China, the most important drugs for mucormycosis treatment were posaconazole suspensions or tablets and AmB. Of these cases, 8 were treated with a combination of AmB and posaconazole as first-line therapy. Although current relevant research results suggest that antifungal combination therapy does not improve the prognosis of mucormycosis (Kyvernitakis et al., 2016), AmB combined with triazole drugs were still used as the main treatment plan for most patients with mucormycosis in our center before ABCD was marketed (Ma et al., 2021), owing to adverse reactions to AmB. In the present study, creatinine and urea nitrogen levels returned to normal and hypokalemia was corrected after ABCD treatment. Furthermore, both the drug responses and survival rates were significantly higher than those in our previous study (Ma et al., 2021) and other studies (Hammond et al., 2011; Lanternier et al., 2012), probably because of the timely application of AmB and ABCD. Unfortunately, in this study, 2 patients (22.22%) stopped ABCD early for economic reasons. But fortunately, ABCD entered the China’s National Reimbursement Drug List (NRDL) in July 2021, so the price of ABCD is significantly lower than that previously, greatly reducing financial pressure on patients.
In this study, the median treatment duration of ABCD was 38 (13-88) days and the median cumulative dose was 5800 (2,200-16,900) mg. Previous studies have shown that ABCD is as effective as AmB for the treatment of invasive aspergillosis, but its nephrotoxicity was lower and the median time to onset of nephrotoxicity was posterior to AmB (301 days vs. 22 days) (White et al., 1997; Bowden et al., 2002). Our research also showed that even mucormycosis patients with renal injury caused by AmB could still use ABCD as an alternative or salvage treatment, and with an extension of the administration time, renal injury did not occur again, which is consistent with previous research results (Yoshida et al., 2021). In our research, the main adverse reactions related to the application of ABCD were infusion-related AEs, hypokalemia, liver function damage, and hypocalcemia. Except for the infusion-related AEs, other adverse reactions improved after symptomatic treatment. Similar to that in other studies, infusion-related AEs were common, usually most frequent during the first infusion, and the intensity and frequency decreased with subsequent administration (Le et al., 2017). In this study, 9 patients were administered promethazine and dexamethasone as pre-treatment before ABCD to prevent the infusion-related AEs. In cases of infusion-related AEs, methylprednisolone injection was used for treatment. However, 7 patients still experienced a serious infusion reaction on the first day of infusion. As expected, 6 patients had an infusion reaction only on the first day, whereas that in Case 4 lasted for nearly 20 days, necessitating pre-treatment with dexamethasone (0.5 mg) every day for 14 days. After stopping the infusion of ABCD and giving methylprednisolone, all patients’ symptoms related to infusion-related AEs were relieved, and then ABCD treatment was continued. Similarly, a previous study enumerated the infusion-related AEs of 170 patients from 21 locations around the world. In total, 1230 ABCD infusion-related AEs occurred (mean dose of 2.8 mg/kg/d), 90% of the infusion-related AEs (1105/1230) were pre-dosed, and the overall incidence rate ratio (IRR) was 12%, with that of pre-dosed cases (11%) lower than that of non-pre-dosed cases (22%). Corticosteroids are related to a reduction in the IRR (Paterson et al., 2008). Nevertheless, the incidence of infusion-related AEs in the 9 cases included in our study was significantly higher than that in previous reports, which might be due to the biased results caused by the small sample size. According to our research results, although pretreatment could only prevent some infusion-related AEs, if proper treatment is provided, related symptoms quickly disappear. Therefore, it is not necessary to stop ABCD due to an infusion reaction. Furthermore, owing to the infusion-related AEs and lack of clinical experience, not all patients were administered the targeted dose on the first day of treatment, but this was increased to the targeted dose within 2-4 days. In our experience, the infusion reaction is controllable, and thus, it is worthwhile to try to provide a therapeutic dose on the first day to achieve a rapid bactericidal effect.
There are some limitations to our study. First, this was a single-center retrospective study. Owing to the rarity of mucormycosis, the results could be biased because of the small sample size. Second, some laboratory indicator results were unavailable. Because patients with hematological malignancies have few platelets, poor coagulation function, and cannot tolerate biopsy, only 2 of the 9 cases were proven based on pathological results, whereas the rest were probable or possible cases. More larger-scale, multicenter studies of mucormycosis in the real world should be done.
In conclusion, our study confirmed that ABCD is a favorable alternative with remarkable efficacy and safety for mucormycosis complicated by hematologic malignancies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2021-KY-0286). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and Design: XM. Administrative support: XM. Materials and samples collection: JL. Data collection and collation: JL, XM. Data analysis and interpretation: JL, XM. Manuscript writing: JL, XM. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1147624/full#supplementary-material
References
Bao, J., Liu, C. (2022). Clinical manifestations of pulmonary mucormycosis in recipients of allogeneic hematopoietic stem cell transplantation: a 21-case series report and literature review Can Respir J. 2022, 1237125. doi: 10.1155/2022/1237125
Bourcier, J., Heudes, P. M., Morio, F., Gastinne, T., Chevallier, P., Rialland-Battisti, F., et al. (2017). Prevalence of the reversed halo sign in neutropenic patients compared with non-neutropenic patients: data from a single-centre study involving 27 patients with pulmonary mucormycosis (2003-2016). Mycoses 60, 526–533. doi: 10.1111/myc.12624
Bowden, R., Chandrasekar, P., White, M. H., Li, X., Pietrelli, L., Gurwith, M., et al. (2002). A double-blind, randomized, controlled trial of amphotericin b colloidal dispersion versus amphotericin b for treatment of invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 35, 359–366. doi: 10.1086/341401
Bretagne, S., Sitbon, K., Desnos-Ollivier, M., Garcia-Hermoso, D., Letscher-Bru, V., Cassaing, S., et al. (2022). Active surveillance program to increase awareness on invasive fungal diseases: the French RESSIF network (2012 to 2018). mBio 13, e0092022. doi: 10.1128/mbio.00920-22
Caillot, D., Legouge, C., Lafon, I., Ferrant, E., Pagès, P. B., Plocque, A., et al. (2018). [Retrospective study of 25 cases of pulmonary mucormycosis in acute leukaemia]. Rev. Des. maladies respiratoires 35, 452–464. doi: 10.1016/j.rmr.2017.11.009
Chamilos, G., Lewis, R. E., Kontoyiannis, D. P. (2008). Delaying amphotericin b-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin. Infect. Dis. 47, 503–509. doi: 10.1086/590004
Cornely, O. A., Alastruey-Izquierdo, A., Arenz, D., Chen, S. C. A., Dannaoui, E., Hochhegger, B., et al. (2019). Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect. Dis. 19, e405–ee21. doi: 10.1016/s1473-3099(19)30312-3
De Pauw, B., Walsh, T. J., Donnelly, J. P., Stevens, D. A., Edwards, J. E., Calandra, T., et al. (2008). Revised definitions of invasive fungal disease from the European organization for research and treatment of Cancer/Invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) consensus group. Clin. Infect. Dis. 46, 1813–1821. doi: 10.1086/588660
Hammond, S. P., Baden, L. R., Marty, F. M. (2011). Mortality in hematologic malignancy and hematopoietic stem cell transplant patients with mucormycosis, 2001 to 2009. J. Antimicrob. Chemother. 55, 5018–5021. doi: 10.1128/aac.00536-11
Herbrecht, R., Letscher-Bru, V., Bowden, R. A., Kusne, S., Anaissie, E. J., Graybill, J. R., et al. (2001). Treatment of 21 cases of invasive mucormycosis with amphotericin b colloidal dispersion. Eur. J. Clin. Microbiol. Infect. Dis. 20, 460–466. doi: 10.1007/s100960100528
Jung, J., Kim, M. Y., Lee, H. J., Park, Y. S., Lee, S. O., Choi, S. H., et al. (2015). Comparison of computed tomographic findings in pulmonary mucormycosis and invasive pulmonary aspergillosis. Clin. Microbiol. Infect. 21, 684 e11–8. doi: 10.1016/j.cmi.2015.03.019
Kyvernitakis, A., Torres, H. A., Jiang, Y., Chamilos, G., Lewis, R. E., Kontoyiannis, D. P. (2016). Initial use of combination treatment does not impact survival of 106 patients with haematologic malignancies and mucormycosis: a propensity score analysis. Clin. Microbiol. Infect. 22, 811.e1–811.e8. doi: 10.1016/j.cmi.2016.03.029
Lanternier, F., Dannaoui, E., Morizot, G., Elie, C., Garcia-Hermoso, D., Huerre, M., et al. (2012). A global analysis of mucormycosis in France: the RetroZygo study (2005-2007). Clin. Infect. Dis. 54 Suppl 1, S35–S43. doi: 10.1093/cid/cir880
Le, T., Kinh, N. V., Cuc, N. T. K., Tung, N. L. N., Lam, N. T., Thuy, P. T. T., et al. (2017). A trial of itraconazole or amphotericin b for HIV-associated talaromycosis. New Engl. J. Med. 376, 2329–2340. doi: 10.1056/NEJMoa1613306
Ma, X., Li, A., Cao, W., Li, H., Zhang, S., Li, L., et al. (2021). Characteristics of mucormycosis in hematological patients and a death prediction model. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.784974
Muthu, V., Agarwal, R., Dhooria, S., Sehgal, I. S., Prasad, K. T., Aggarwal, A. N., et al. (2021). Has the mortality from pulmonary mucormycosis changed over time? a systematic review and meta-analysis. Clin. Microbiol. Infect. 27, 538-549. doi: 10.1016/j.cmi.2020.12.035
Oppenheim, B. A., Herbrecht, R., Kusne, S. (1995). The safety and efficacy of amphotericin b colloidal dispersion in the treatment of invasive mycoses. Clin. Infect. Dis. 21, 1145–1153. doi: 10.1093/clinids/21.5.1145
Paterson, D. L., David, K., Mrsic, M., Cetkovsky, P., Weng, X. H., Sterba, J., et al. (2008). Pre-medication practices and incidence of infusion-related reactions in patients receiving AMPHOTEC: data from the patient registry of amphotericin b cholesteryl sulfate complex for injection clinical tolerability (PRoACT) registry. J. Antimicrob. Chemother. 62, 1392–1400. doi: 10.1093/jac/dkn394
Prakash, H., Chakrabarti, A. (2019). Global epidemiology of mucormycosis. J. Fungi (Basel) 5, 26. doi: 10.3390/jof5010026
Roden, M., Zaoutis, T., Buchanan, W., Knudsen, T., Sarkisova, T., Schaufele, R., et al. (2005). Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41, 634–653. doi: 10.1086/432579
Ullmann, A. J., Sanz, M. A., Tramarin, A., Barnes, R. A., Wu, W., Gerlach, B. A., et al. (2006). Prospective study of amphotericin b formulations in immunocompromised patients in 4 European countries. Clin. Infect. Dis. 43, e29–e38. doi: 10.1086/505969
White, M. H., Anaissie, E. J., Kusne, S., Wingard, J. R., Hiemenz, J. W., Cantor, A., et al. (1997). Amphotericin b colloidal dispersion vs. amphotericin b as therapy for invasive aspergillosis. Clin. Infect. Dis. 24, 635–642.
White, M. H., Bowden, R. A., Sandler, E. S., Graham, M. L., Noskin, G. A., Wingard, J. R., et al. (1998). Randomized, double-blind clinical trial of amphotericin b colloidal dispersion vs. amphotericin b in the empirical treatment of fever and neutropenia. Clin. Infect. Dis. 27, 296–302. doi: 10.1086/514672
Keywords: amphotericin B colloidal dispersion, hematological malignancies, mucormycosis, efficacy, safety
Citation: Liu J and Ma X (2023) Amphotericin B colloidal dispersion: an effective drug for the treatment of mucormycosis in China. Front. Cell. Infect. Microbiol. 13:1147624. doi: 10.3389/fcimb.2023.1147624
Received: 19 January 2023; Accepted: 02 May 2023;
Published: 17 May 2023.
Edited by:
Gill Diamond, University of Louisville, United StatesReviewed by:
Çağrı Ergin, Pamukkale University, TürkiyeAiming Pang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2023 Liu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxu Ma, ZmNjbWF4eEB6enUuZWR1LmNu
 Juntao Liu
Juntao Liu Xiaoxu Ma
Xiaoxu Ma