- 1Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Immunotherapy Research Center for Hematologic Diseases of Hubei Province, Wuhan, Hubei, China
- 3Department of Pathology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 4Department of Nuclear Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
High response rates in B-cell malignancies have been achieved with chimeric antigen receptor (CAR) T-cell therapy. Emerging reports indicate a risk of active tuberculosis (TB) with novel immunotherapy for tumors. However, studies of TB in patients post CAR T-cell therapy are limited. In this case series study, we describe five patients with active TB post CD19/CD22 target CAR T-cell therapy alone or following autologous stem cell transplantation (ASCT). One of the patients developed active TB within the first 30 days post CAR T-cell therapy, and fever was the dominant presenting symptom; extrapulmonary manifestations of active TB were common in the other four patients and manifested after the first 30 days of CAR T-cell therapy. Four of the five patients improved with anti-TB treatment, but one patient with isoniazid resistance died of central nervous system TB infection. Our study provides the first series report of active TB following CD19/CD22 target CAR T-cell therapy.
1 Introduction
Chimeric antigen receptor (CAR) T-cell therapy has revolutionized the management of relapsed/refractory (r/r) B-cell malignancy. Despite the efficacy of CD19-targeted CAR (CAR19) T-cell therapy, growing numbers of infectious complications have recently been reported in patients receiving this treatment (Hill et al., 2018; Park et al., 2018; Wudhikarn et al., 2020; Logue et al., 2021). The incidence of overall infection in institutional cohorts of patients treated with CAR19 T cells within the first 90 days post CAR T-cell infusion ranges from 23% to 45% (Hill et al., 2018; Wudhikarn et al., 2020; Logue et al., 2021). CAR T-cell recipients are considered as being at increased risk for infection due to underlying malignancy, extensive prior antitumor therapies, and pre-CAR T-cell infusion lymphodepletion chemotherapy (Hill et al., 2018). Furthermore, cytokine release syndrome (CRS), the most common CAR T-cell-related toxicity requiring treatment with corticosteroids and/or tocilizumab, may increase risk of infection (Park et al., 2018). Moreover, CAR19 T cells deplete normal CD19+ B cells in most patients, which contributes to hypogammaglobulinemia.
Our previous work together with other trials suggest that CAR T-cell therapy is safe and effective for r/r B-cell malignancy (Wang et al., 2020; Yan et al., 2022). However, the infection etiologic spectrum of CAR T-cell recipients post CAR T-cell therapy has not been fully clarified. The majority of infection events are bacterial in the first 28 days after CAR T-cell therapy, with respiratory viral infections predominating at later time points (Hill et al., 2018). Severe infection complications, including opportunistic infections involving, e.g., Pneumocystis jirovecii, Varicella Zoster virus, Aspergillus, Fusarium, and Mucorales, have been reported (Haidar et al., 2020; Logue et al., 2021). Furthermore, there are emerging reports that risk of active tuberculosis (TB) is increased by PD-1 blockade (Morelli et al., 2022), a novel immunotherapy revolutionizing cancer treatment. Nevertheless, there are scarce data on active TB post CAR T-cell therapy.
In this study, we present five cases of active TB post CD19/CD22 target CAR (CAR19/22) T-cell therapy alone or following autologous stem cell transplantation (ASCT). In view of these cases, we emphasize the need for early screening and diagnosis, comprehensive supportive care, including long-term monitoring and prophylaxis, and treatment management for TB in the era of CAR T-cell therapy.
2 Methods
We performed a retrospective electronic medical records review of 427 patients who received CAR T-cell therapy in two trials at Tongji Hospital from January 2017 to December 2021. We included patients diagnosed with active TB after CAR T-cell therapy. Active TB was defined as microbiological tests demonstrating TB in any clinical specimen based on acid-fast bacillus (AFB) staining, mycobacterial culture, Xpert MTB/Rif, or polymerase chain reaction (PCR) for TB, accompanied by clinical and/or radiographic evidence of current disease (Zumla et al., 2013). All five cases of active TB were confirmed. Of the five TB cases, two occurred after CAR19/22 T-cell cocktail therapy (ChiCTR-OPN-16008526) and three after CAR19/22 T-cell cocktail therapy in tandem with ASCT (ChiCTR-OPN-16009847) (shown in Supplemental Figure 1).
Clinical, laboratory, imaging, and treatment data for the confirmed cases were collected from the medical record system, including a thorough clinical history of malignancy, history of prior therapy, dose of CAR T-cell infusion, adverse events, and responses after infusion of CAR T-cells. Additionally, TB-related clinical manifestations (e.g., cough, fever, night sweats, weight loss), microbiology studies of TB (e.g., AFB staining, mycobacterial cultures, Xpert MTB/Rif, PCR, T-SPOT.TB test), other relevant laboratory results correlating with immune status and concomitant presence of other infection (e.g., white blood cell (WBC) count, levels of immunoglobulin, inflammatory markers including interleukin-6 (IL-6), high-sensitivity C-reactive protein (hsCRP), and ferritin, lymphocyte subsets), radiographs (e.g., chest X-rays, chest computerized tomography (CT), cranial magnetic resonance imaging (MRI), positron emission tomography/computed tomography (PET/CT)), the process of treatment, and outcome of the patients were recorded for further analysis.
The study was carried out in accordance with the Declaration of Helsinki and approved by the ethics committee of Tongji Medical College, Huazhong University of Science and Technology.
3 Results
3.1 Baseline characteristics
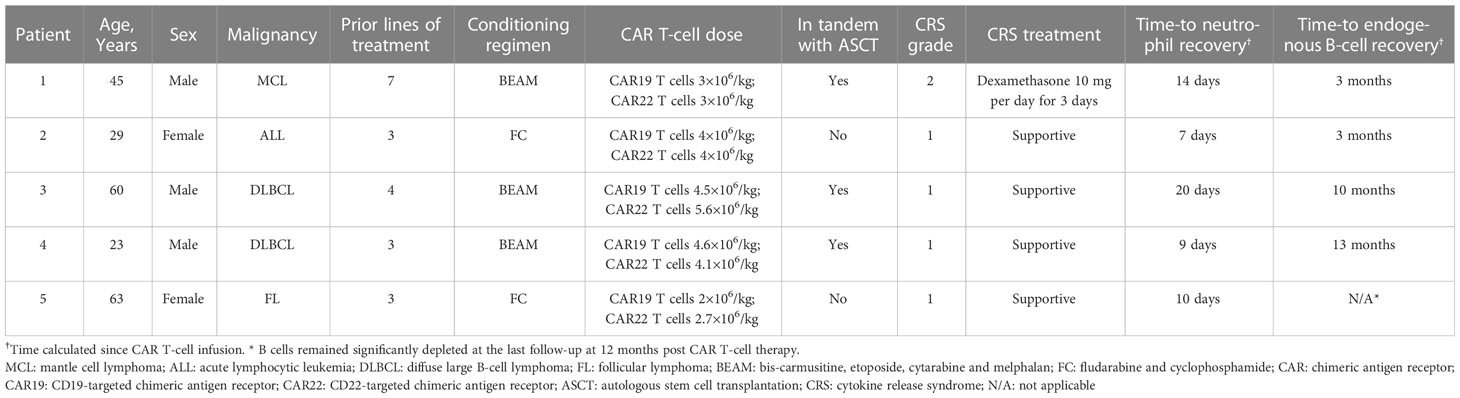
The baseline characteristics of the patients are shown in Table 1. Four patients received CAR T-cell therapy for relapsed or refractory lymphoma and one patient for acute lymphoblastic leukemia. All patients received at least 3 lines of antitumor treatment regimens before CAR T-cell therapy. CRS of grade 1-2 developed and was characterized by fever with or without hypotension and resolved with short-term glucocorticoids or supportive therapy. None of the patients received tocilizumab. No immune effector cell-associated neurotoxicity syndrome (ICANS) was observed. Although neutropenia resolved within three weeks, B-cell aplasia and hypoglobulinemia continued during active TB (Supplemental Table 1), and intravenous immunoglobulin was given. CD4+ T cells decreased from normal in the five patients and four patients had a CD4+ T cell count of ≤100 cells/μL during active TB (Supplemental Table 1). All patients denied a history of TB or previous known exposure. Because testing for latent TB is not routinely performed among CAR T-cell therapy recipients, the T-SPOT.TB test at baseline was only performed for patient 5, with a positive result. None of the patients had received isoniazid prophylaxis. No coinfection with cytomegalovirus, Epstein-Barr virus, or hepatitis B virus was detected prior to or concomitant with TB presentation in any of the patients based on manifestations and routine specific viral nucleic acid testing.
3.2 Clinical presentations and treatment
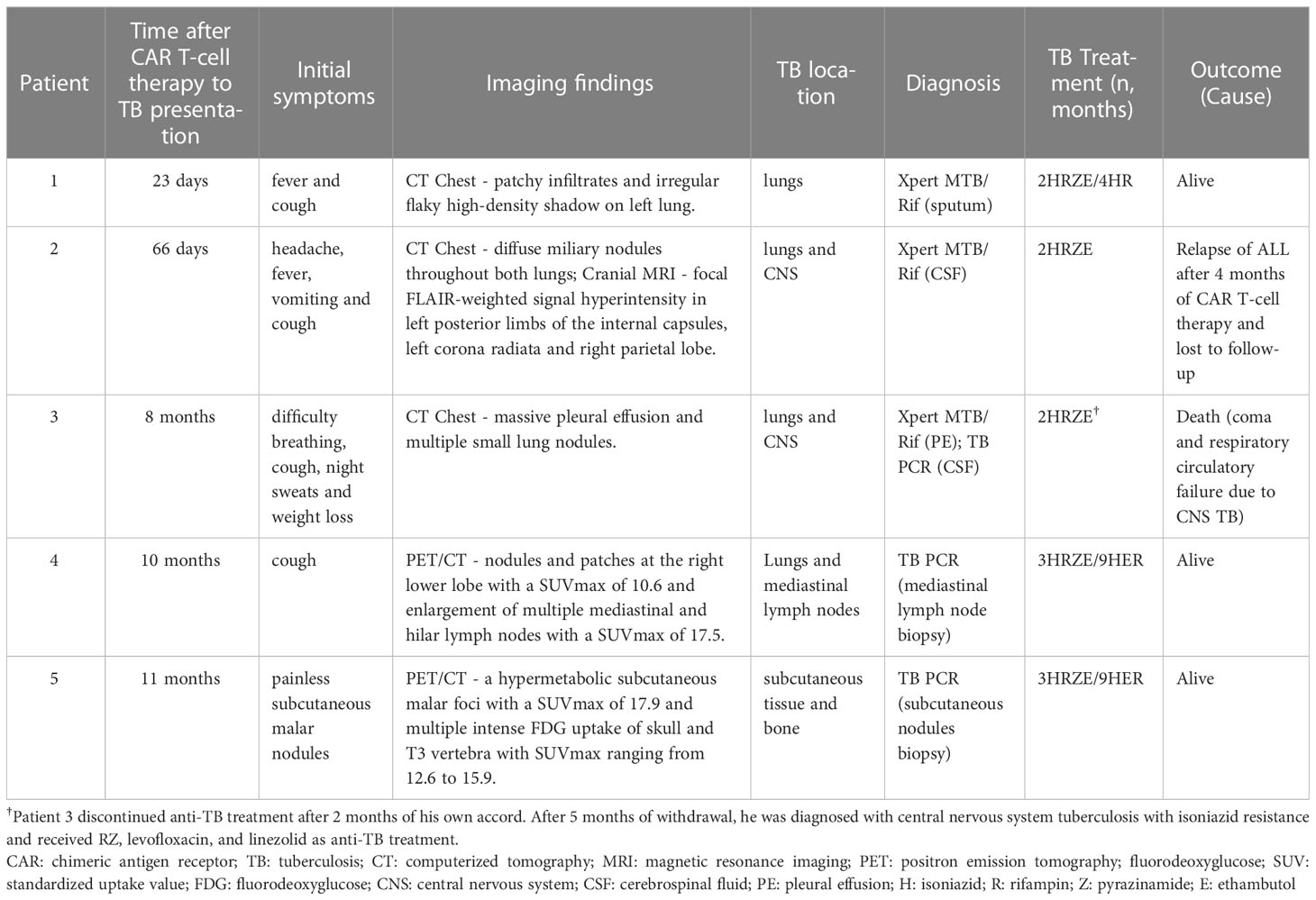
The clinical presentations of the patients are described in Table 2. Patient 1 manifested active TB within the first 30 days after CAR T-cell therapy. He developed a fever up to 39°C on day 4 and had persistent fever along with new hypotension in the ensuing 72 hours; IL-6 peaked at 1044 pg/mL on day 7. Due to grade 2 CRS, he received dexamethasone 10 mg per day for three days and was transitioned from levofloxacin to meropenem and demethovancomycin intravenously. His symptoms improved on day 10, and IL-6 gradually returned to baseline levels on day 12. His neutropenia resolved before day 14. However, low-grade afternoon fever lasted, and the patient developed cough with bloody sputum on day 23. CT scanning demonstrated patchy infiltrates and irregular flaky high-density shadows on his left lung. Xpert MTB/Rif of sputum was positive for rifampin-sensitive Mycobacterium tuberculosis (MTB). He had a good response to standard anti-TB therapy, with a normal body temperature.
In our case series, extrapulmonary manifestations of TB occurred after the first 30 days of CAR T-cell therapy. Patient 2 presented with symptoms of headache, fever, vomiting, and cough from day 66 after CAR T-cell therapy, and a cerebrospinal fluid (CSF) sample was positive for Xpert MTB/Rif at this time. Cranial magnetic resonance imaging (MRI) showed focal FLAIR-weighted signal hyperintensity in the left posterior limbs of the internal capsules, left corona radiata, and right parietal lobe (Figure 1A). CT revealed disseminated miliary nodules in bilateral lung fields (Figure 1C). Patient 5 presented with painless subcutaneous nodules on the right malar region after 11 months of CAR T-cell therapy, which displayed intense fluorodeoxyglucose (FDG) uptake on [(18)F]-FDG positron emission tomography/computed tomography (PET/CT) scanning (Figure 1D). Pathology of the nodule indicated necrotizing granuloma (Figure 1E), and MTB DNA was detectable by PCR. Although patient 3 was diagnosed with TB based on Xpert MTB/Rif of pleural effusion, he discontinued anti-TB treatment after 2 months of his own accord. At 5 months after withdrawal, he was admitted to the ICU of a local hospital with a 1-day history of coma after headache and fever for 20 days. Analysis of CSF samples was positive for MTB DNA by PCR and revealed a -15C>T mutation in the inhA promoter (Vilchèze and Jacobs, 2014), which has a strong association with isoniazid resistance. His CSF culture was negative.
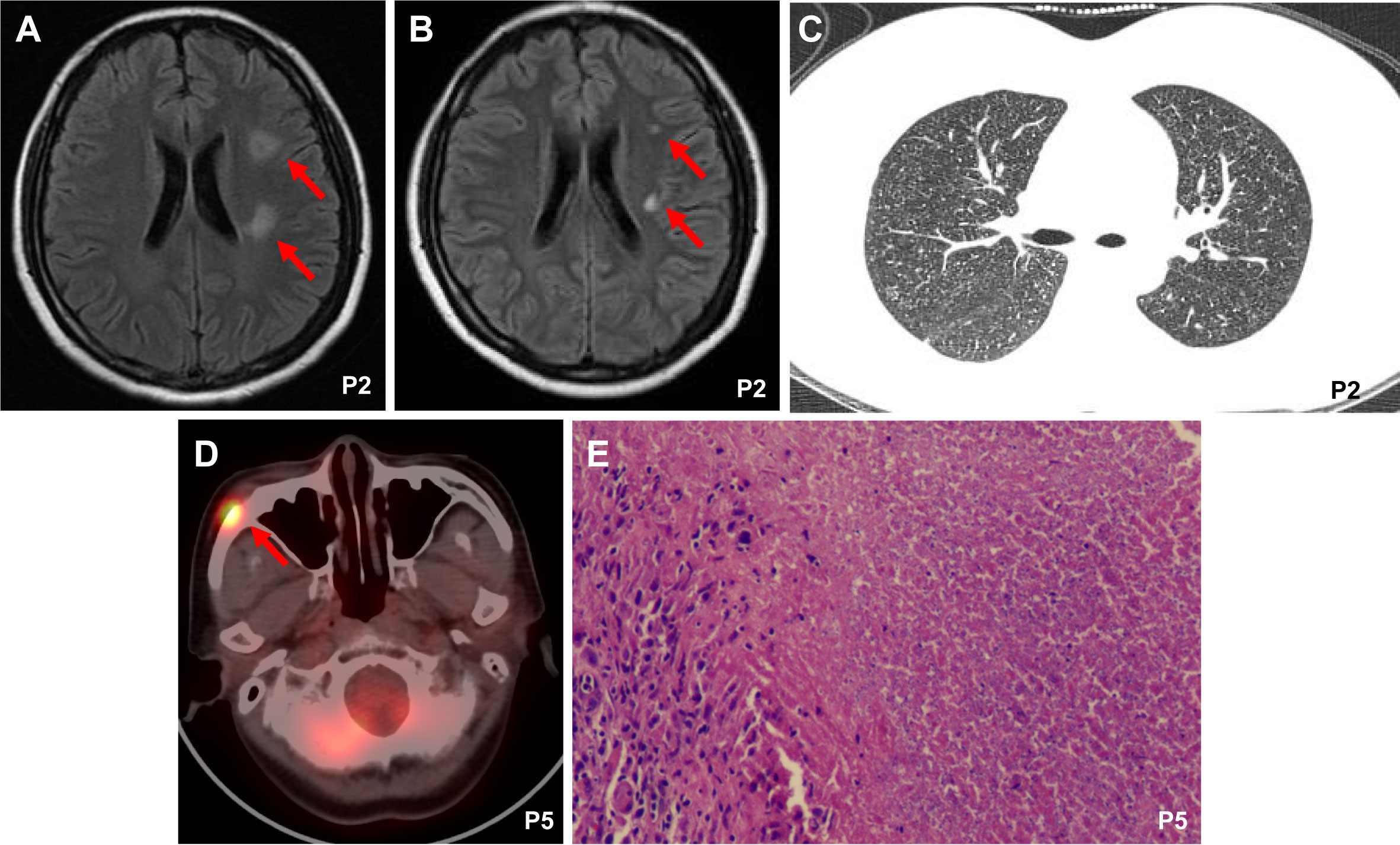
Figure 1 Imaging findings and tissue biopsy. (A) Cranial magnetic resonance imaging (MRI) showed focal FLAIR-weighted signal hyperintensity in patient 2 after 2 months of CAR19/22 T-cell cocktail therapy. (B) The MRI image of patient 2 after anti-TB therapy for one month. (C) Chest computerized tomography (CT) images showed disseminated miliary nodules in bilateral lung fields in patient 2 after 2 months of CAR19/22 T-cell cocktail therapy. (D) Positron emission tomography/computed tomography (PET/CT) scanning of patient 5 after 11 months of CAR19/22 T-cell cocktail therapy. (E) Pathology of the subcutaneous nodules on the right malar region in patient 5 revealed necrotizing granuloma. P, Patient.
Patient 4 developed mild cough at 10 months after CAR T-cell therapy. PET/CT scanning showed newly emerged nodules and patches at the right lower lobe and enlargement of multiple mediastinal and hilar lymph nodes with intense FDG uptake at routine follow-up examinations of CAR T-cell therapy. Metastatic lymph node samples from patient 4 obtained via endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) were pathologically diagnosed as chronic granulomatous inflammation. The MTB PCR results were positive.
All patients received first-line quadruple drug intensive therapy with isoniazid (H), rifampin (R), pyrazinamide (Z), and ethambutol (E) for two months and continuation therapy for at least 4 months, without TB recurrence, except for patient 2 and patient 3. Patient 2 showed improvement of both symptoms and imaging after treatment for one month (Figure 1B). However, acute lymphoblastic leukemia recurred after 4 months of CAR T-cell therapy and 2 months of anti-TB treatment, and the patient was lost to follow-up. Patient 3 received RZ, levofloxacin, and linezolid as anti-TB treatment and glycerin to control increased intracranial pressure after drug resistance was detected. Unfortunately, the patient experienced respiratory circulatory failure and eventually died at 12 days after admission.
4 Discussion
While wide-scale application of CAR T-cell therapy for B-cell malignancies has shown unprecedented success, adverse effects of CAR T-cell therapy are now appearing, including infectious complications. Indeed, CAR T-cell recipients are at increased risk of infection due to immune deficiencies caused by previous immunosuppression, lymphodepleting chemotherapy, treatment with tocilizumab and/or steroids, on-target effects of hypogammaglobulinemia, and protracted cytopenia (Bupha-Intr et al., 2021). The most common infections are bacterial, viral, and fungal infection (Hill et al., 2018; Park et al., 2018; Vora et al., 2020). Although many recommendations are based on other hematological treatment modalities, screening, monitoring, and prophylaxis might not be sufficient for CAR T-cell recipients, and there is a lack of clinical trials to define how to screen, monitor, and prevent infection effectively.
TB accounts for millions of deaths worldwide and is very harmful, particularly in patients with immune deficiencies (Pai et al., 2016). Immune deficiencies increase risk of active TB and mortality due to high incidences of extrapulmonary and disseminated TB (Meintjes et al., 2019; Bergeron et al., 2022). In addition, diagnosing TB is difficult owing to the high incidences of extrapulmonary TB and the low sensitivity of the tuberculin skin test (TST) and interferon gamma release assays (IGRA) for immunocompromised patients (Meintjes et al., 2019; Bergeron et al., 2022). Overall, risk of active TB increases soon after infection with human immunodeficiency virus (HIV), and one in five HIV-related deaths are due to TB (Zumla et al., 2013; Pai et al., 2016). In this study, we describe five cases of active TB in CAR T-cell recipients with B-cell malignancies. To our knowledge, this is the first report of a case series in adults, though one TB case in a child with acute lymphoblastic leukemia following CAR T-cell therapy has recently been reported (Grasa et al., 2022). Symptoms and chest imaging indicative of active TB were screened before CAR T-cell therapy in our center. This study indicates that more proactive screening and prophylaxis for TB may be necessary prior to CAR T-cell therapy. Currently, there is a lack of consensus on the best strategy for TB screening and prophylaxis in CAR T-cell recipients. Nonetheless, there are some recommendations based on cancer patients, including that screening for TB, including TST and IGRA, should be performed prior to CAR T-cell therapy in patients with risk factors for exposure, particularly in TB-endemic regions (Hill and Seo, 2020). Prophylaxis for active TB is controversial, and there are few data regarding patients with hematological malignancies (Bergeron et al., 2022). The optimal duration and time to start prophylaxis should be determined based on large-sample, prospective clinical trials.
The link between CAR-T cells and infection and its mechanism are unclear. We collected lymphocyte subsets and levels of immunoglobulins during active TB. Notably, CD4+ T-cell lymphopenia was observed in most CAR T-cell recipients in our case series, accompanied by B-cell aplasia and hypoglobulinemia. Some studies have found profound and prolonged CD4 T-cell immunosuppression for more than one year in CAR T-cell recipients, with a long-term risk of infectious complications (Logue et al., 2021; Strati et al., 2021). Protective immune responses against Mycobacterium tuberculosis are largely mediated by CD4+ Th1 cells, which secrete IFN-γ (O’Garra et al., 2013). CD4+ T-cell lymphopenia is a risk factor for progression from latent TB to active TB (Pai et al., 2016), which may be associated with an increased risk of active TB in CAR T-cell recipients. Furthermore, CD4 counts of less than 200 per cubic millimeter may increase the presentation of extrapulmonary TB (Zumla et al., 2013), which was observed in four of the five patients in this study. A reduction in CD4 counts may suggest the timing of TB prophylactic treatment.
There are limitations to our study. We retrospectively collected CAR T-cell recipients with positive TB microbiological tests at a single center, with only a small sample size of patients with active TB. This brings difficulties with regard to calculating the exact risk of TB in CAR T-cell recipients. As commercial use of CAR T-cell products and an increasing number of CAR T-cell clinical trials are ongoing, more data should be collected to assess the risk of TB in CAR T-cell recipients. The timing and role of TB prophylactic treatment or preemptive treatment after CAR T-cell therapy should also be defined in detail.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PZ, JW, and YC analyzed the data and wrote the manuscript. LH and MZ managed the patients. PZ, CZ, and DW collected the data. JW and YC revised the manuscript and were in charge of the final approval of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Key Research Program (2021YFA1101504, to YC) and the National Natural Science Foundation of China (82070217, to JW).
Acknowledgments
The authors would like to thank all members of the study team, the patients, and their families. We would also like to thank the work of Bio-RAID Company for the preparation of CAR T cells.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1147454/full#supplementary-material
References
Bergeron, A., Mikulska, M., De Greef, J., Bondeelle, L., Franquet, T., Herrmann, J. L., et al. (2022). Mycobacterial infections in adults with haematological malignancies and haematopoietic stem cell transplants: guidelines from the 8th European conference on infections in leukaemia. Lancet Infect. Dis. 22 (12), e359–ee69. doi: 10.1016/S1473-3099(22)00227-4
Bupha-Intr, O., Haeusler, G., Chee, L., Thursky, K., Slavin, M., Teh, B. (2021). CAR-T cell therapy and infection: a review. Expert Rev. Anti Infect. Ther. 19 (6), 749–758. doi: 10.1080/14787210.2021.1855143
Grasa, C., Ochoa, B., Baquero-Artigao, F., San Roman, S., Galan-Gomez, V., Del Rosal, T., et al. (2022). Disseminated tuberculosis infection in a CAR T-cell recipient. Pediatr. Blood Cancer 69 (7), e29615. doi: 10.1002/pbc.29615
Haidar, G., Dorritie, K., Farah, R., Bogdanovich, T., Nguyen, M. H., Samanta, P. (2020). Invasive mold infections after chimeric antigen receptor-modified T-cell therapy: a case series, review of the literature, and implications for prophylaxis. Clin. Infect. Dis. 71 (3), 672–676. doi: 10.1093/cid/ciz1127
Hill, J. A., Li, D., Hay, K. A., Green, M. L., Cherian, S., Chen, X., et al. (2018). Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 131 (1), 121–130. doi: 10.1182/blood-2017-07-793760
Hill, J. A., Seo, S. K. (2020). How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for b-cell malignancies. Blood 136 (8), 925–935. doi: 10.1182/blood.2019004000
Logue, J. M., Zucchetti, E., Bachmeier, C. A., Krivenko, G. S., Larson, V., Ninh, D., et al. (2021). Immune reconstitution and associated infections following axicabtagene ciloleucel in relapsed or refractory large b-cell lymphoma. Haematologica 106 (4), 978–986. doi: 10.3324/haematol.2019.238634
Meintjes, G., Brust, J. C. M., Nuttall, J., Maartens, G. (2019). Management of active tuberculosis in adults with HIV. Lancet HIV 6 (7), e463–ee74. doi: 10.1016/S2352-3018(19)30154-7
Morelli, T., Fujita, K., Redelman-Sidi, G., Elkington, P. T. (2022). Infections due to dysregulated immunity: an emerging complication of cancer immunotherapy. Thorax 77 (3), 304–311. doi: 10.1136/thoraxjnl-2021-217260
O’Garra, A., Redford, P. S., McNab, F. W., Bloom, C. I., Wilkinson, R. J., Berry, M. P. (2013). The immune response in tuberculosis. Annu. Rev. Immunol. 31, 475–527. doi: 10.1146/annurev-immunol-032712-095939
Pai, M., Behr, M. A., Dowdy, D., Dheda, K., Divangahi, M., Boehme, C. C., et al. (2016). Tuberculosis. Nat. Rev. Dis. Primers 2, 16076. doi: 10.1038/nrdp.2016.76
Park, J. H., Romero, F. A., Taur, Y., Sadelain, M., Brentjens, R. J., Hohl, T. M., et al. (2018). Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory b-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin. Infect. Dis. 67 (4), 533–540. doi: 10.1093/cid/ciy152
Strati, P., Varma, A., Adkins, S., Nastoupil, L. J., Westin, J., Hagemeister, F. B., et al. (2021). Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with large b-cell lymphoma. Haematologica 106 (10), 2667–2672. doi: 10.3324/haematol.2020.254045
Vilchèze, C., Jacobs, W. R., Jr. (2014). Resistance to isoniazid and ethionamide in mycobacterium tuberculosis: genes, mutations, and causalities. Microbiol. Spectr. 2 (4), Mgm2–0014-2013. doi: 10.1128/microbiolspec.MGM2-0014-2013
Vora, S. B., Waghmare, A., Englund, J. A., Qu, P., Gardner, R. A., Hill, J. A. (2020). Infectious complications following CD19 chimeric antigen receptor T-cell therapy for children, adolescents, and young adults. Open Forum Infect. Dis. 7 (5), ofaa121. doi: 10.1093/ofid/ofaa121
Wang, N., Hu, X., Cao, W., Li, C., Xiao, Y., Cao, Y., et al. (2020). Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed b-cell malignancies. Blood 135 (1), 17–27. doi: 10.1182/blood.2019000017
Wudhikarn, K., Palomba, M. L., Pennisi, M., Garcia-Recio, M., Flynn, J. R., Devlin, S. M., et al. (2020). Infection during the first year in patients treated with CD19 CAR T cells for diffuse large b cell lymphoma. Blood Cancer J. 10 (8), 79. doi: 10.1038/s41408-020-00346-7
Yan, N., Wang, N., Wang, G., Huang, L., Li, C., Wang, D., et al. (2022). CAR19/22 T cell cocktail therapy for b-ALL relapsed after allogeneic hematopoietic stem cell transplantation. Cytotherapy 24 (8), 841–849 doi: 10.1016/j.jcyt.2022.01.011
Keywords: immunocompromised, host immunity, tuberculosis, extrapulmonary tuberculosis, chimeric antigen receptor T-cell
Citation: Zhang P, Huang L, Zheng M, Zhang C, Wan D, Wei J and Cao Y (2023) Case Report: Active tuberculosis infection in CAR T-cell recipients post CAR T-cell therapy: a retrospective case series. Front. Cell. Infect. Microbiol. 13:1147454. doi: 10.3389/fcimb.2023.1147454
Received: 18 January 2023; Accepted: 26 April 2023;
Published: 11 May 2023.
Edited by:
Alejandro Martin-Quiros, University Hospital La Paz, SpainReviewed by:
Jennifer T. Grier, University of South Carolina, United StatesKeith Sacco, Phoenix Children’s Hospital, United States
Copyright © 2023 Zhang, Huang, Zheng, Zhang, Wan, Wei and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Cao, Y2FveWFuZ2VtbWFAMTYzLmNvbQ==; Jia Wei, amlhd2VpQHRqaC50am11LmVkdS5jbg==
†These authors have contributed equally to this work
 Peiling Zhang
Peiling Zhang Liang Huang
Liang Huang Miao Zheng1,2
Miao Zheng1,2 Dongyi Wan
Dongyi Wan Jia Wei
Jia Wei Yang Cao
Yang Cao