
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 16 August 2023
Sec. Clinical Microbiology
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1137664
This article is part of the Research Topic Diagnosis and the treatment of Gram-negative bacilli pneumonia in the ICU View all 5 articles
 Yahui Liu1,2†
Yahui Liu1,2† Lin Huang1,2†
Lin Huang1,2† Jing Cai3†
Jing Cai3† Haixing Zhu1,2
Haixing Zhu1,2 Junjie Li1,2
Junjie Li1,2 Youchao Yu1,2
Youchao Yu1,2 Yumin Xu4*
Yumin Xu4* Guochao Shi1,2*
Guochao Shi1,2* Yun Feng1,2*
Yun Feng1,2*Purpose: With advancements in medical technology and the growth of an aging society, the number of immunocompromised patients has increased progressively. Klebsiella pneumoniae (K. pneumoniae) is one of the most common opportunistic pathogens, causing a severe disease burden. We aimed to further clarify the differences in respiratory tract K. pneumoniae infections between immunocompromised and immunocompetent populations.
Methods: We retrospectively compared cases of respiratory tract K. pneumoniae infection in immunocompromised and immunocompetent patients admitted to Ruijin Hospital in Shanghai between January 2019 and August 2020 to clarify the differences between the two groups.
Results: We enrolled 400 immunocompromised patients and 386 immunocompetent patients. Compared to the immunocompetent group, immunocompromised patients were more likely to develop bacteremia and shock and to require mechanical ventilation support during hospitalization. Immunocompromised patients also had a greater probability of polymicrobial infection and a higher rate of antibacterial resistance to carbapenem, which resulted in a higher intensive care unit admission rate, 30-day case fatality rate (CFR), and 6-month CFR. Multivariate analysis indicated that immunocompromised patients with respiratory diseases (odds ratio [OR], 2.189; 95% confidence interval [CI], 1.103-4.344; P = 0.025) and cardiovascular diseases (OR, 2.008; 95% CI, 1.055-3.822; P = 0.034), using mechanical ventilation (OR, 3.982; 95% CI, 2.053-7.722; P = 0.000), or infected with multidrug-resistant K. pneumoniae (OR, 3.870; 95%, 1.577-9.498; P = 0.003) were more likely to have a higher 30-day CFR.
Conclusion: The disease burden of K. pneumoniae infection in immunocompromised patients is high. Immunocompromised patients who presented with respiratory diseases and cardiovascular diseases, used mechanical ventilation, or were infected with multidrug-resistant K. pneumoniae experienced a higher 30-day mortality rate.
Klebsiella pneumonia (K. pneumoniae) is one of the most commonly found Gram-negative bacilli in hospital environments, and is primarily considered an opportunistic pathogen that threatens vulnerable populations. However, with the advancement of life sciences and medical care, the average life expectancy has increased, and the number of immunosuppressed people has gradually grown, allowing infections caused by K. pneumoniae in the community to occur occasionally (Lin et al., 2010; Melot et al., 2015; Juan et al., 2019). Additionally, compared to other Gram-negative bacilli, reported resistance proportions to third-generation cephalosporins were higher in cases of K. pneumoniae infection (Antimicrobial resistance: global report on surveillance). Hypervirulent multidrug-resistant (MDR) strains of K. pneumoniae have also gradually emerged and spread widely (Zhang et al., 2016; Lee et al., 2017; Shankar et al., 2018). Infection by K. pneumoniae can cause sepsis, urinary infection, and pneumonia, resulting in a heavy disease burden on society (Zhang et al., 2016; Lee et al., 2017; Shankar et al., 2018). It was reported that the mortality rate for K. pneumoniae hospital-acquired pneumonia can exceed 50% sometimes (Antimicrobial resistance: global report on surveillance).
Owing to the unremitting efforts of clinicians and others, guidelines about clinical aspects of infectious diseases are well documented and continuously improved. However, due to the nature of the disease itself or the use of various immunosuppressive treatments, the causative pathogens, clinical characteristics, treatments, and infection prognosis in the immunocompromised population differ significantly from those of the immunocompetent population (Di Pasquale et al., 2019). Many of the existing guidelines are mostly based on immunocompetent populations and may not be applicable to immunocompromised populations (Ramirez et al., 2020). Therefore, we believe that it is of great significance to discover the characteristics of infection in immunocompromised people, especially the aspects that are different from those with normal immune function, and summarize the key points of treatment.
Considering the burden of K. pneumoniae infection in the immunocompromised population, it is crucial to obtain accurate and recent data concerning both the clinical and microbiological characteristics of K. pneumoniae infection in the immunocompromised population, as well as the predictors of intensive care unit (ICU) admission and the case-fatality rate (CFR). Our primary study objective was to identify the differences in respiratory tract infections by K. pneumoniae between immunocompetent and immunocompromised individuals. The secondary objective was to determine the risk factors of respiratory tract infection by K. pneumoniae in immunocompetent and immunocompromised populations so as to achieve early detection, early prevention, and an improved prognosis.
This retrospective cohort study was conducted at Ruijin Hospital, Shanghai Jiaotong University School of Medicine, China. Microbiology records were used to identify patients with respiratory tract infection caused by K. pneumoniae. The medical records of patients with respiratory tract infections involving K. pneumoniae were reviewed. A respiratory tract infection was defined by the following measures: (1) an acute pulmonary infiltrate evident on a chest X-ray or computerized tomography scan and compatible with pneumonia and (2) confirmatory findings of the clinical examination (Lin et al., 2010). Respiratory tract infection by K. pneumoniae was defined by the presence of as least one sputum culture sampled yielding a pathogen presumed to be the cause of the infection. The diagnosis was reconfirmed by two infectious disease specialists. For patients with multiple episodes of K. pneumoniae infection during the study period, only the first episode was included.
Clinical information of enrolled patients including demographic characteristics, comorbidities, immune function, surgery or invasive operation, biochemical indicators, microbiological characteristics, treatments, and outcome, were collected. Sputum samples were collected for culture, and pathogens were identified using standard microbiological procedures. Homogenized sputum samples were spread onto plates containing blood agar/chocolate agar (non-selective growth), Columbia CNA agar (selective Gram-positive), MacConkey agar (selective Gram-negative), or Brucella agar (for culture of anaerobes) and incubated at 35°C in the appropriate atmosphere. Susceptibility testing was performed by standard agar dilution methods. Results were interpreted according to the Clinical and Laboratory Stands Institute susceptibility breakpoints (CLSI, 2021). Bacterial colonies proceeded to the semi-quantitative steps. In order to report bacterial load, culture plates were divided into four quadrants that were then systematically streaked from the first to the last quadrant using an inoculation loop. After incubation, the number of quadrants with bacterial growth was assessed. A result of “negative” was recorded if no growth was observed on the plate, while “1+” was reported if growth was only observed in the first quadrant; “2+” was reported if growth was observed in the first and second quadrants; “3+” was reported if growth was observed in the first, second, and third quadrants; and “4+” was reported if growth was observed in all four quadrants of the plate, respectively. Improvement of K. pneumoniae infection was defined by a sputum culture turning negative from positive or positive sputum cultures being reduced by two plus signs “+”. Outcome variables included ICU admission, 30-day CFR, 6-month CFR and length of stay (LOS) (Lin et al., 2010; Juan et al., 2019; Montrucchio et al., 2022).
For this non-interventional study, a waiver for medical ethical approval was granted by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The study was performed in accordance with the ethical standards as laid out in the 1964 Declaration of Helsinki. No patient consent was required due to the retrospective nature of the study.
Participants were classified into the immunocompetent and immunocompromised groups. Patients were defined as immunocompromised when as least one of the following conditions were present: (1) asplenia; (2) active malignancy, or receiving cancer chemotherapy or radiotherapy during the last 3 months; (3) HIV infection with a CD4+ lymphocyte count < 200 cells/μL or percentage < 14%; (4) solid organ transplantation or hematopoietic stem cell transplantation; (5) receiving corticosteroid therapy with a ≥ 20 mg dose of prednisone or equivalent daily for ≥ 14 days or a > 700 mg cumulative dose of prednisone; (6) receiving biologic modulators; (7) receiving disease-modifying anti-rheumatic drugs or other immunosuppressive drugs; (8) liver cirrhosis; (9) severe burns; (10) primary immune deficiency diseases or acquired immune deficiency disorder; (11) hematological diseases, including aplastic anemia, lymphoma, acute or chronic leukemia, or multiple myeloma; and (12) neutropenia, defined by a neutrophil count < 500 cells/μL at complete blood cell count (Di Pasquale et al., 2019; Ramirez et al., 2020; Lin et al., 2022). The immunocompromised state had to be active at the time of the patient’s study inclusion. A neoplastic disease was defined as active if it required medical or surgical intervention within the last year or if no-treatable metastases were present at the time of study enrollment.
Outcome variables were compared between immunocompetent and immunocompromised patients. Propensity score matching was used to balance the baseline characteristics of immunocompetent and immunocompromised patients. Categorical variables are described as frequencies (percentages), while continuous variables are presented as mean and standard error of the mean (SEM) values or as median and interquartile range (IQR) values for data not normally distributed (Kolmogorov-Smirnov test). Categorical variables were analyzed using the chi-square test or Fisher’s exact test, as appropriate. Continuous variables were analyzed by t test or by non-parametric Mann-Whitney U test after verifying a non-normal distribution.
Univariate and multivariate logistic regression analyses were performed to identify variables predictive of disease severity in patients with K. pneumoniae infection. The following variables were analyzed: age, sex, presence of other bacterial infections, procalcitonin, C-reactive protein, neutrophil count, creatinine (Cr), alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transpeptidase (γGT), and respiratory support. Variables with P < 0.1 in the univariate analysis, after checking for collinearity, were selected via backward elimination for a multivariate logistic regression model. The fitness of the model was tested using the Hosmer-Lemeshow goodness-of-fit test. All statistical tests were two-tailed and P <0.05 was considered to indicate statistical significance. The analysis was completed using the SPSS version 25.0.0.2 statistical package (IBM Corporation, Armonk, NY, USA).
During the study period, data from 786 patients with respiratory infections by K. pneumoniae were collected. According to the definition of being immunocompromised, 386 (49.11%) of all enrolled patients were immunocompetent and 400 (50.89%) were immunocompromised. The prevalence of each risk factor for being immunocompromised is depicted in Figure 1A, with active malignancy (50.50%) and chronic steroid use (46.75%) being the most frequent risk factors. A total of 121 patients had more than one risk factor for being immunocompromised (Figure 1B).
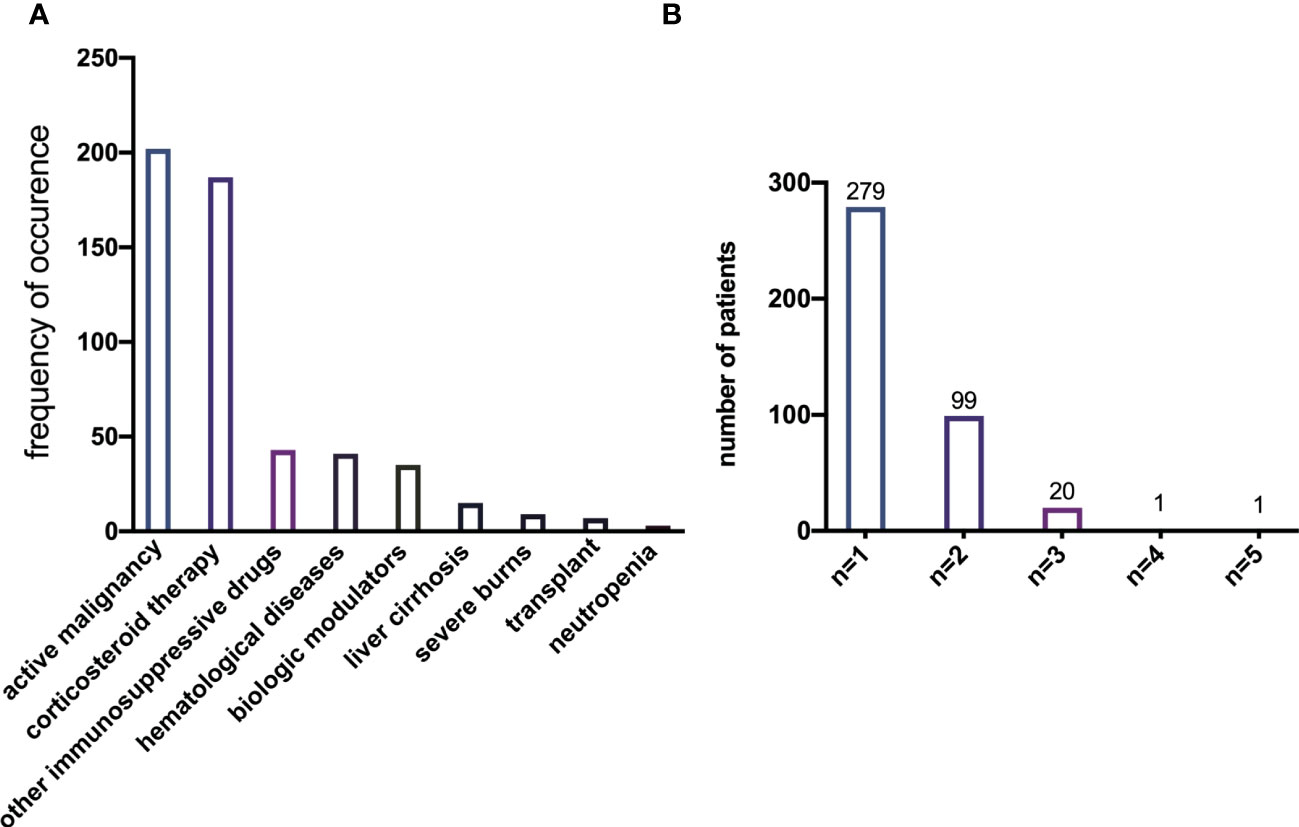
Figure 1 (A) Prevalence of each single risk factor for immunocompromise of K. pneumoniae infection. (B) Prevalence of the number of risk factors present simultaneously in a single patient.
Baseline characteristics of immunocompetent versus immunocompromised patients are shown in Table 1 and Supplementary Figure S1. There were no differences in sex (P = 0.7660) and age (P = 0.2048) between the groups. Forty patients in the immunocompromised group developed bacteremia during hospitalization. Compared to the immunocompetent group, more immunocompromised patients developed shock (P < 0.0001) and received mechanical ventilation support (P = 0.0183). In contrast, more immunocompetent patients underwent invasive operations or surgery (P < 0.0001). Additionally, more patients in the immunocompromised group had comorbid diseases, such as respiratory diseases (P = 0.0043), diabetes (P = 0.0265), malignancy (P < 0.0001), chronic kidney diseases (P = 0.0045), or chronic hepatic diseases (P < 0.0001).
Microbiological testing was performed in all the enrolled patients, and the microbiological findings are provided in Table 2. Immunocompromised patients were more likely to have polymicrobial respiratory infections, including K. pneumoniae (P < 0.0001) (Table 2; Supplemental Figure S2). As is known, K. pneumoniae is the main cause of infections initiated by carbapenem-resistant bacteria worldwide (Antimicrobial resistance: global report on surveillance). According to a Global Antimicrobial Resistance and Use Surveillance System (GLASS) report, some sources have reported greater than 50% resistance against carbapenem in K. pneumoniae isolates (Antimicrobial resistance: global report on surveillance). In our study, there were 214 (27.23%) K. pneumoniae isolates found to be resistant to carbapenem. Additionally, our results showed that the susceptibility rates for carbapenems (P = 0.0155), cefoperazone-sulbactam (P = 0.0003), and amikacin (P = 0.0053) were higher for immunocompromised isolates compared to immunocompetent isolates (Table 2). Besides, only seven (0.89%) polymyxin resistant K. pneumoniae isolates were identified, which is less than findings in studies by others (Podschun and Ullmann., 1998; CDC, 2013; Magill et al., 2014; Martin and Bachman, 2018; Asri et al., 2021). Moreover, MDR-K. pneumoniae was more frequently isolated from immunocompromised patients (16.75%) than from immunocompetent patients (10.62%) (P = 0.0126).
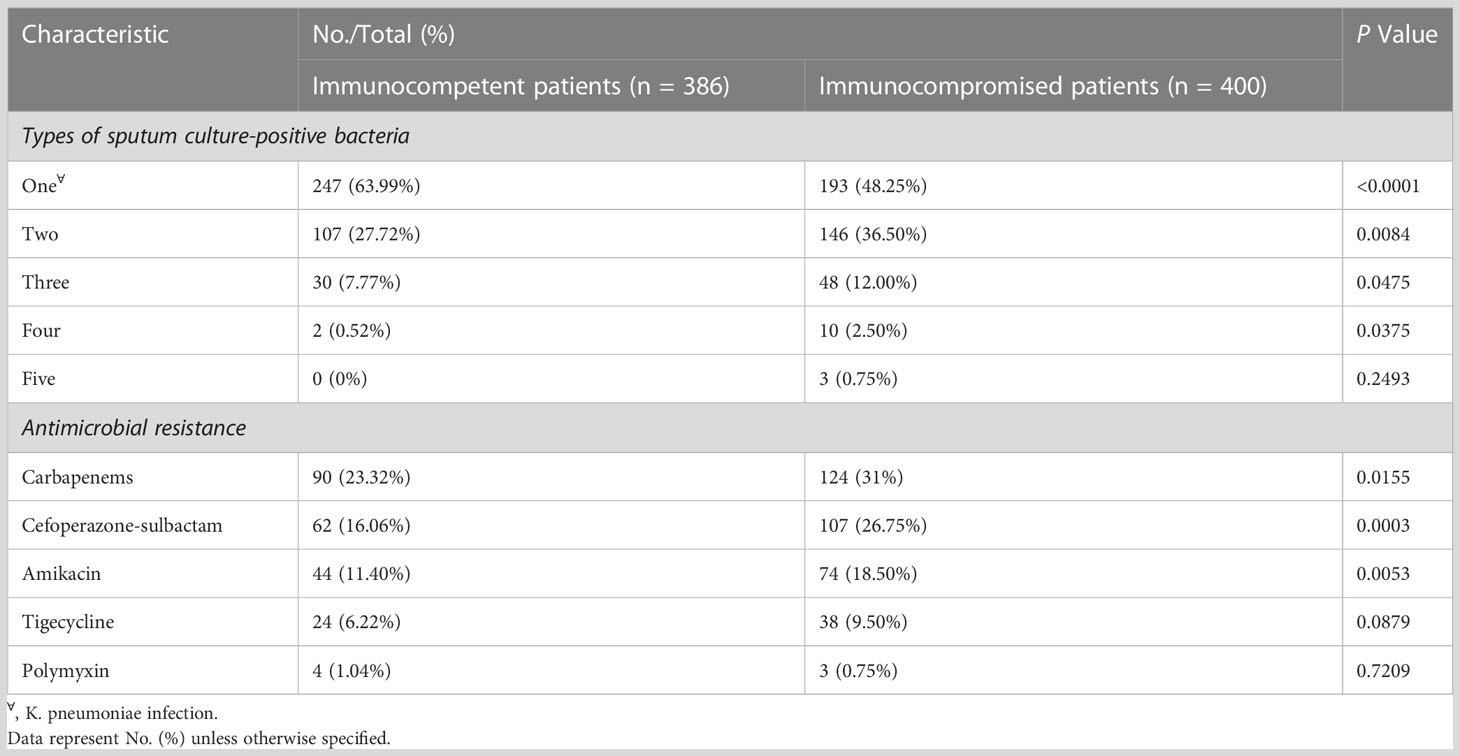
Table 2 Microbiological characteristics of the two study groups (immunocompetent vs immunocompromised).
The immunocompromised population has higher rates of antimicrobial resistance and polymicrobial infections. Consequently, there were some differences in case management between the two groups in this study (Table 3). The rate of ICU admission (P = 0.0125), LOS (P < 0.0001), 30-day CFR (P < 0.0001), and 6-month CFR (P < 0.0001) were all significantly higher in the immunocompromised group (Table 3). After propensity score matching, the immunocompromised patients retained a higher risk of death and boasted longer hospital stays (Supplemental Tables S1, S2).
As is shown in Table 4, the absolute neutrophil count (measured on the day or ± one day of sputum culture positive for K. pneumoniae) (P = 0.0012) and the γGT level (measured on the day of admission) (P = 0.0028) were higher in immunocompromised patients than in immunocompetent patients. The time of improvement in immunocompromised patients was 19.21 days, which was later than that in immunocompetent patients (14.70 days, P = 0.0010) (Table 4). Simultaneously, the levels of creatinine, alanine aminotransferase, aspartate aminotransferase, and γGT changed with statistical significance in both the immunocompetent and immunocompromised groups during treatment (Table 5), suggesting the importance the regularly monitoring the liver and renal functions of patients.
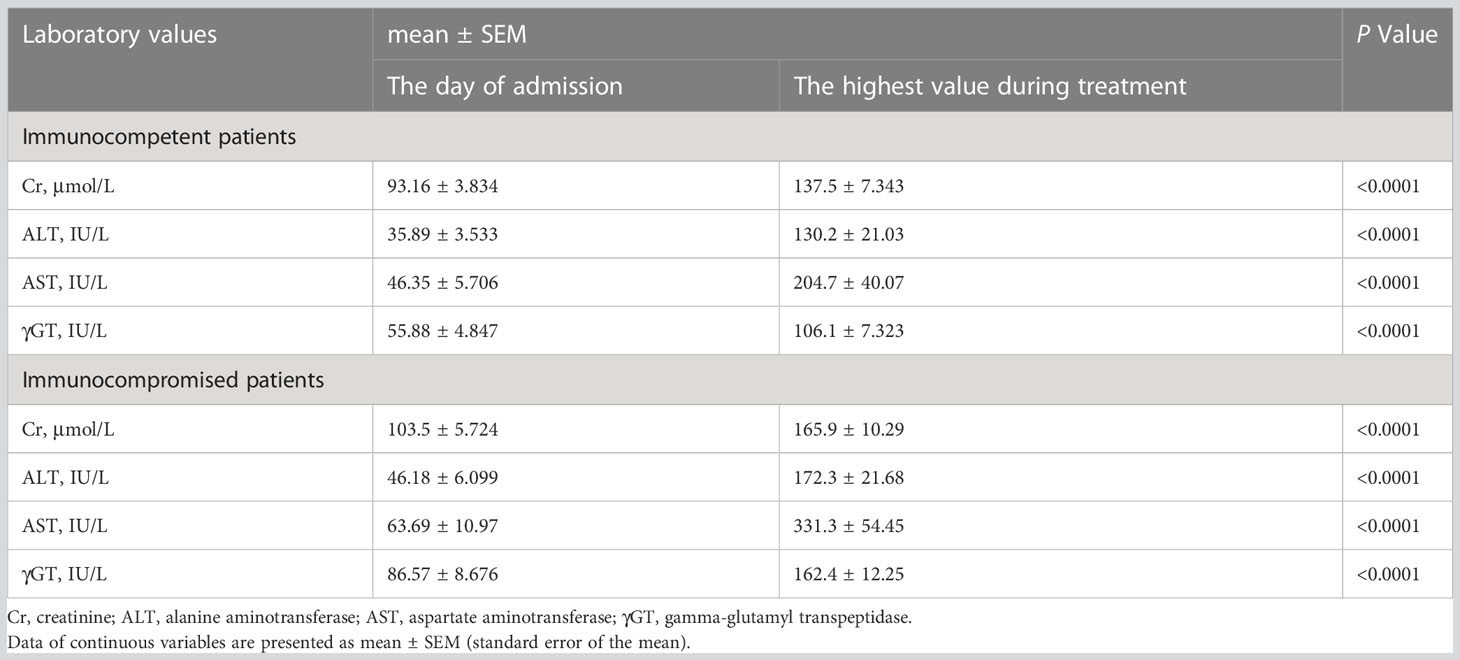
Table 5 Changes of laboratory values of the two study groups (immunocompetent vs immunocompromised) during treatment.
In the immunocompromised population, the univariate analysis indicated that age, polymicrobial infection, carbapenem resistance, combined diseases (nervous system diseases, respiratory diseases, cardiovascular diseases, chronic kidney diseases), bacteremia, immunosuppressive agent use, mechanical ventilation, ICU admission, shock, and MDR-K. pneumoniae infection corelated with a higher 30-day mortality rate. In the multivariate analysis, respiratory diseases, cardiovascular diseases, mechanical ventilation, and infection with MDR-K. pneumoniae were positive predictors of 30-day mortality in the immunocompromised group (Table 6). Considering the immunocompetent population, univariate analysis showed that age, polymicrobial infection, carbapenem resistance, combined diseases (nervous system diseases, respiratory diseases, cardiovascular diseases, chronic kidney diseases), mechanical ventilation, and ICU admission represented risk factors for 30-day mortality (Supplemental Table S3). However, only mechanical ventilation of immunocompetent patients has been recognized to increase 30-day mortality (Supplemental Table S3).
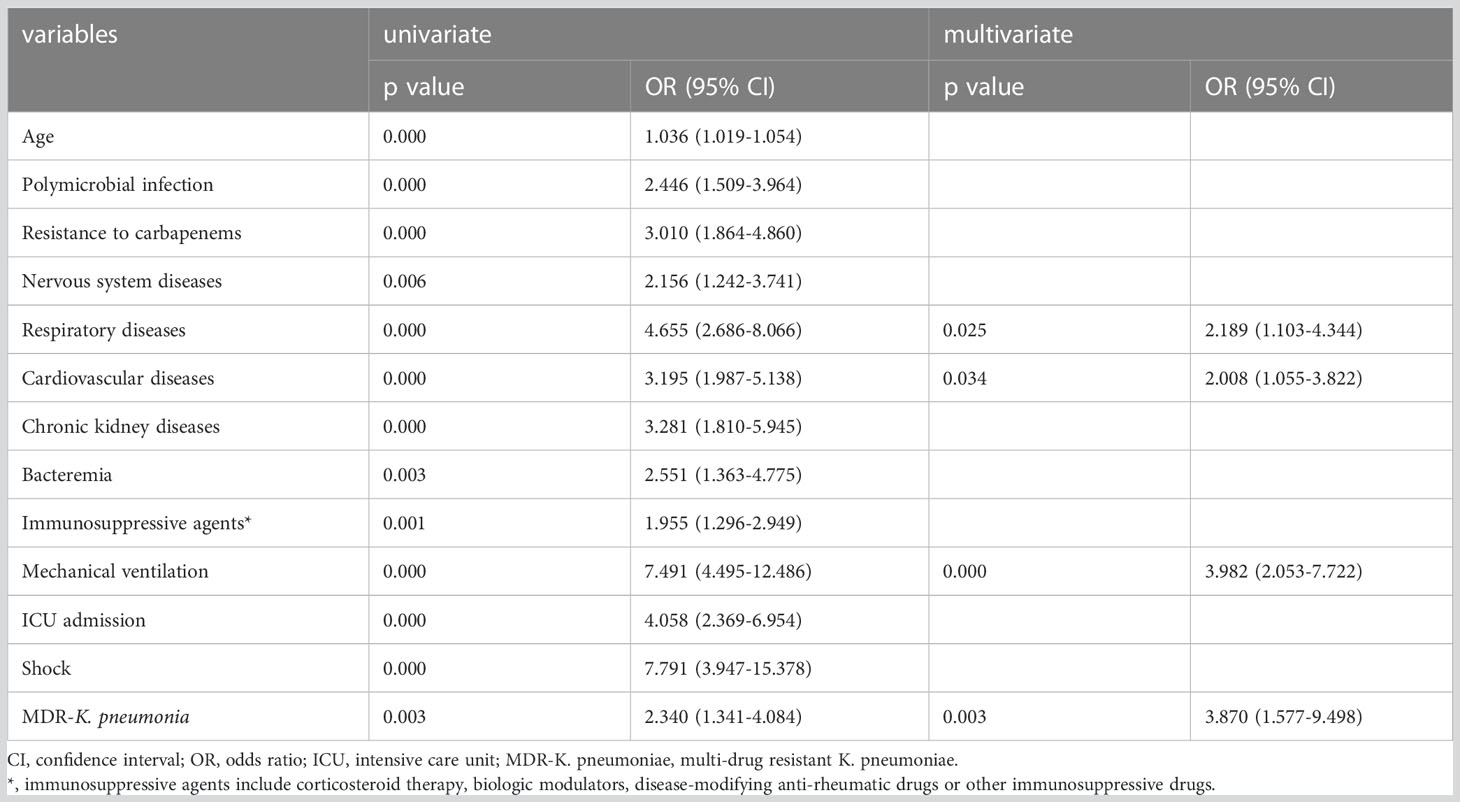
Table 6 Logistic regression analysis for variables associated with 30-day mortality of immunocompromised patients infected with K. pneumoniae.
Separately, univariate analysis indicated that age, polymicrobial infection, carbapenem resistance, combined diseases (nervous system diseases, respiratory diseases, cardiovascular diseases, chronic kidney diseases, chronic hepatic diseases), bacteremia, immunosuppressive agent use, mechanical ventilation, ICU admission, shock, and MDR-K. pneumoniae infection were associated with a higher 6-month mortality rate in the immunocompromised group (Supplemental Table S4). Separately, the multivariate analysis revealed that respiratory diseases, cardiovascular diseases, immunosuppressive agents, mechanical ventilation, and shock were positive predictors of 6-month mortality in the immunocompromised group (Supplemental Table S4). Polymicrobial infection, carbapenem resistance, combined diseases (nervous system diseases, respiratory diseases, cardiovascular diseases), immunosuppressive agent use, blood glucose level, mechanical ventilation, and ICU admission may trigger a higher 6-month mortality rate for immunocompetent patients in the univariate analysis (Supplemental Table S5). Finally, nervous system diseases, mechanical ventilation, and ICU admission were predictors of increased 6-month mortality among immunocompetent patients in the multivariate analysis (Supplemental Table S3). Predictors of ICU admission for both immunocompetent and immunocompromised patients are listed in Supplemental Table S6 and S7.
The main findings of the present study are as follows. A substantial number of patients hospitalized for K. pneumoniae infection in our study were immunocompromised due to various conditions, the most common of which were active malignancy and chronic steroid use. Immunocompromised patients are more likely to develop bacteremia and shock and to require mechanical ventilation support during hospitalization. Compared to immunocompetent patients, immunocompromised patients had a higher probability of polymicrobial infection and a higher rate of antibacterial resistance, which resulted in a worse prognosis with higher ICU admission and mortality rates. Patients in the immunocompromised group with K. pneumoniae respiratory infections took more time to improve compared to those in the immunocompetent group. Multivariate analysis indicated that respiratory diseases, cardiovascular diseases, using mechanical ventilation, and being infected with MDR-K. pneumoniae led immunocompromised patients to have a higher rate of 30-day mortality. Finally, it is necessary to monitor the liver and renal functions of patients during treatment.
A rapid increase in the drug resistance of K. pneumoniae has been witnessed over the past few decades, causing deleterious consequences for infected patients (Martin and Bachman, 2018). It was reported that K. pneumoniae has become the most common carbapenem-resistant Enterobacteriaceae worldwide because of the use of carbapenem for extended-spectrum β-lactamases infection (Martin and Bachman, 2018). In 2013, the U. S. Center for Disease Control declared carbapenem-resistant Enterobacteriaceae to be an urgent threat to public health in the United States, with K. pneumoniae present in about 80% of cases (CDC, 2013; Martin and Bachman, 2018). In our study, 27.23% (214/786) of the K. pneumoniae isolates were resistant to carbapenems, and 13.74% (108/786) of them were MDR-K. pneumoniae isolates, putting our finding in the mid-range level among studies of the same type (Antimicrobial resistance: global report on surveillance; Zhang et al., 2016; Lee et al., 2017; Asri et al., 2021; World Health Organization, 2021; Chen et al., 2022). Since we only enrolled patients with definitive sputum culture results, data on the resistance of K. pneumoniae may be slightly biased. However, we still believe this result is of great importance.
Accumulating evidence has demonstrated that resistance to K. pneumoniae has reached alarming levels in some areas of the world which indicates that many of the available treatment options for infections in some settings are becoming ineffective. Our results demonstrated that K. pneumoniae resistance in the immunocompromised group was significantly more serious than that in the immunocompetent group, which means that we need to perform screening cultures for high-risk patients to guide proper antibiotic use and prevent the misuse of ineffective antibiotics. Polymyxin is a type of antibiotic used to treat Gram-negative infections in the 1960s and 1970s (Jerke et al., 2016). Since then, the renal-toxicity and neurotoxicity of polymyxin have limited its clinical use; however, the emergence of greater K. pneumoniae resistance has led clinicians to turn to polymyxin instead as a drug of last resort (Jerke et al., 2016; Liu et al., 2016). Even so, polymyxin is not recommended as an empirical treatment option for K. pneumoniae infection unless the antibacterial susceptibility test demonstrates the bacteria are insensitive or resistant to other drugs, such as carbapenems and cefoperazone-sulbactam.
K. pneumoniae has been identified as the second most common of bloodstream infections (BSIs) caused by Gram-negative bacteria (Podschun and Ullmann., 1998; Magill et al., 2014). The mortality rate is significantly increased in immunocompromised patients with BSIs involving K. pneumoniae (Lin et al., 2022). The reported prevalence of bacteremia ranges from 6%-69%, depending on the pathogens and grade of immunocompromise (Bock et al., 2013; Opota et al., 2015; Taramasso et al., 2016; Ruiz-Giardin et al., 2019). In this study, we found that 48 (12%) immunocompromised patients developed bacteremia, which was associated with ICU admission, 30-day mortality, and 6-month mortality in a univariate analysis (Di Pasquale et al., 2019). Several possible reasons can account for the occurrence of bacteremia in immunosuppressed patients with K. pneumoniae infection. The use of invasive devices and procedures, such as mechanical ventilators and catheterization, and exposure to the hospital environment particularly the ICU are common BSIs risk factors (Montrucchio et al., 2022). Respiratory tract, urinary tract, and gastrointestinal colonization are also strongly associated with the risk of BSI. Additionally, various immunocompromised conditions increase the possibility of bacteremia by inhibiting bone marrow proliferation, reducing the activity of immune cells, and damaging the immune barrier. It was reported that the presence of corticosteroid catabolism enzymes in K. pneumoniae enhances the ability to use corticosteroids for their own nutrition source, suggesting that the intrinsic bioactivity of K. pneumoniae may increase the risk of infection (Chattopadhyay and Banerjee, 2019). Interventions to mitigate BSIs should target all these factors. It is of significance to perform larger-scale analyses or to design more rigorous trials to clarify the route, risk factors, and causative organisms of bacteremia in immunocompromised populations.
In agreement with previous studies, our results indicated that shock occurred more frequently in the immunocompromised group, which was also a risk factor for ICU admission, 30-day CFR, and 6-month CRF in prior univariate analyses (Cillóniz et al., 2011; Sousa et al., 2013; Zhang et al., 2016; Chen et al., 2022). Multivariate analysis has also demonstrated that shock represents a risk factor for ICU admission, 30-day CFR, and 6-month CRF. Thus, shock prevention as well as the timely recognition and treating shock are important to improve the prognosis. Mechanical ventilation is also a key predictor of ICU admission and CFR in both immunocompetent and immunocompromised groups since it implies that the patient developed respiratory failure or other conditions requiring respiratory system support.
Active malignancy and chronic steroid use were the leading immunocompromised factors in our results, which is consistent with findings of previous studies (Di Pasquale et al., 2019; Ramirez et al., 2020; Certan et al., 2022). Additionally, we suggest that patients may have more than one risk factor characteristic. Thus, clinical assessment should be comprehensive, taking into consideration risk factors for immunosuppression and their associated biological mechanisms.
Numerous studies have shown that the Streptococcus pneumoniae vaccine and influenza virus vaccine play an important role in clinical practice. Likewise, we believe that a K. pneumoniae vaccine is a feasible and promising strategy for K. pneumoniae infection. However, due to the complex drug resistance mechanism of K. pneumoniae, the development of its vaccine has been slow (Assoni et al., 2021). Although various vaccine candidates against K. pneumoniae have been proposed, there are no vaccines available on the market currently. Therefore, as far as the current situation is concerned, early diagnosis and proper treatment of K. pneumoniae infection are of great importance.
The current study has some limitations. First, we were not able to involve many investigators from more clinical centers, thus limiting the generalizability of our findings. However, our findings are valuable given the high prevalence of K. pneumoniae globally and the increase in the immunocompromised population. Another major limitation is the infeasibility of grading the severity of immunocompromised patients and, therefore, stratifying patients and defining the physio-pathological interactions between different risk factors, especially regarding the use of biological drugs and chronic steroids. Additionally, our study did not involve infections caused by fungi, viruses, and other pathogens. Previous studies have indicated that immunocompromised populations are vulnerable to various pathogens (Sousa et al., 2013; Di Pasquale et al., 2019; Certan et al., 2022). The prognosis of infectious diseases is the result of many factors related to the patient, pathogen, and treatment. The existence of other infectious pathogens, especially severe acute respiratory syndrome coronavirus 2, may have been an important confounding factor in our study that may have contributed to the mortality rate. This was a retrospective study and recent, rapidly evolving scenarios might have changed the situation, especially considering the coronavirus disease 2019 pandemic and the introduction of new antibiotics for multi-resistant pathogens. Future multicenter and prospective studies on patients with specific characteristics of immunosuppression could provide practical recommendations to confirm our data and better define the impact of each risk factor individually.
In conclusion, compared to the immunocompetent population, immunocompromised patients were more likely to develop bacteremia and shock and showed a greater probability of polymicrobial infection and a higher rate of resistance, resulting in worse prognosis with higher ICU admission and mortality rates. Multivariate analysis indicated that respiratory diseases, cardiovascular diseases, using mechanical ventilation, and infection with MDR-K. pneumoniae were more likely to cause a higher rate of 30-day mortality in the immunocompromised population. Based on this study, we conclude that rational use of anti-infective drugs, reduction of invasive procedures, strict implementation of aseptic techniques, and comprehensive intervention for comorbidities for hospitalized patients can significantly improve the outcomes.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine Ethics Committee. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Conception and design of the work: YF, YX, and GS. Data interpretation: YF and GS. Collecting data: YL, LH, JC, HZ, JL, and YY. Data analysis: YL, LH, and JC. Drafting the work and revising it critically for important intellectual content: YL, LH, JC, GS, and YF. All authors contributed to the article and approved the submitted version.
The present study was supported by the National Natural Science Foundation of China (No. 82170086), Shanghai Shenkang Hospital Development Center Clinical Science and Technology Innovation Project (SHDC12018102), Shanghai Municipal Key Clinical Specialty (shslczdzk02202), Shanghai Key Laboratory of Emergency Prevention, Diagnosis and Treatment of Respiratory Infectious Diseases (20dz2261100), and Shanghai Sailing Program (22YF1424800).
The authors also thank Shanghai Municipal Hospital Respiratory and Critical Care Medicine Specialist Alliance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2023.1137664/full#supplementary-material
Asri, N. A. M., Ahmad, S., Mohamud, R., Hanafi, M., Zaidi, N. F. M., Irekeola, A. A., et al. (2021). Global prevalence of nosocomial multidrug-resistant Klebsiella pneumoniae: a systematic review and meta-analysis. Antibiotics. 10 (12), 1508. doi: 10.3390/antibiotics10121508
Assoni, L., Girardello, R., Converso, T. R., Darrieux, M. (2021). Current stage in the development of Klebsiella pneumoniae vaccines. Infect. Dis. Ther. 10 (4), 2157–2175. doi: 10.1007/s40121-021-00533-4
Bock, A. M., Cao, Q., Ferrieri, P., Young, J. A. H., Weisdorf, D. J. (2013). Bacteremia in blood or marrow transplantation patients: clinical risk factors for infection and emerging antibiotic resistance. Biol. Blood Marrow Transplant. 19 (1), 102–108. doi: 10.1016/j.bbmt.2012.08.016
Centers for Disease Control and Prevention (U.S.). (2013). Antibiotic resistance threats in the United States (Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention). doi: 10.1016/j.medmal.2007.05.006
Certan, M., Garcia Garrido, H. M., Wong, G., Heijmans, J., Grobusch, M. P., Goorhuis, A. (2022). Incidence and predictors of community-acquired pneumonia in patients with hematological cancers between 2016 and 2019. Clin. Infect. Dis. 75 (6), 1046–1053. doi: 10.1093/cid/ciac005
Chattopadhyay, P., Banerjee, G. (2019). Corticosteroid catabolism by Klebsiella pneumoniae as a possible mechanism for increased pneumonia risk. Curr. Pharm. Biotechnol. 20 (4), 309–316. doi: 10.2174/1389201020666190313153841
Chen, I-R., Lin, S-N., Wu, X-N., Chou, S-H., Wang, F-D., Lin, Y-T. (2022). Clinical and microbiological characteristics of bacteremic pneumonia caused by Klebsiella pneumoniae. Front. Cell Infect. Microbiol. 12, 903682. doi: 10.3389/fcimb.2022.903682
Cillóniz, C., Ewig, S., Ferrer, M., Polverino, E., Gabarrús, A., Puig de la Bellacasa, J., et al. (2011). Community-acquired polymicrobial pneumonia in the intensive care unit: aetiology and prognosis. Crit. Care 15 (5), R209. doi: 10.1186/cc10444
CLSI. (2021). Performance standards for antimicrobial susceptibility testing, M100. 31st ed (Wayne, PA: Clinical and Laboratory Standards Institute).
Di Pasquale, M. F., Sotgiu, G., Gramegna, A., Radovanovic, D., Terraneo, S., Reyes, L. F., et al. (2019). Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin. Infect. Dis. 68 (9), 1482–1493. doi: 10.1093/cid/ciy723
Jerke, K. H., Lee, M. J., Humphries, R. M. (2016). Polymyxin susceptibility testing: a cold case reopened. Clin. Microbiol. Newsl. 38 (9), 69–77. doi: 10.1016/j.clinmicnews.2016.04.003
Juan, C-H., Chuang, C., Chen, C-H., Li, L., Lin., Y-T. (2019). Clinical characteristics, antimicrobial resistance and capsular types of community-acquired, healthcare-associated, and nosocomial Klebsiella pneumoniae bacteremia. Antimicrob. Resist. Infect. Control. 8 (1), 1–9. doi: 10.1186/s13756-018-0426-x
Lee, C-R., Lee, J. H., Park, K. S., Jeon, J. H., Kim, Y. B., Cha, C-J., et al. (2017). Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front. Cell Infect. Microbiol. 7, 483. doi: 10.3389/fcimb.2017.00483
Lin, Y. T., Jeng, Y. Y., Chen, T. L., Fung, C. P. (2010). Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001-2008. BMC Infect. Dis. 10 (1), 307. doi: 10.1186/1471-2334-10-307
Lin, H. X., Yang, L. L., Fang, J., Gao, Y. L., Zhu, H. X., Zhang, S. X., et al. (2022). Clinical characteristics of bloodstream infection in immunosuppressed patients: a 5-year retrospective cohort study. Front. Cell Infect. Microbiol. 12, 796656. doi: 10.3389/fcimb.2022.796656
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16 (2), 161–168. doi: 10.1016/S1473-3099(15)00424-7
Magill, S. S., Edwards, J. R., Bamberg, W., Beldavs, Z. G., Dumyati, G., Kainer, M. A., et al. (2014). Multistate point-prevalence survey of health care–associated infections. N Engl. J. Med. 370 (13), 1198–1208. doi: 10.1056/nejmoa1306801
Martin, R. M., Bachman, M. A. (2018). Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front. Cell Infect. Microbiol. 8, 4. doi: 10.3389/fcimb.2018.00004
Melot, B., Colot, J., Guerrier, G. (2015). Bacteremic community-acquired infections due to Klebsiella pneumoniae: clinical and microbiological presentation in New Caledonia, 2008-2013. Int. J. Infect. Dis. 41, 29–31. doi: 10.1016/j.ijid.2015.10.013
Montrucchio, G., Costamagna, A., Pierani, T., Petitti, A., Sales, G., Pivetta, E., et al. (2022). Bloodstream infections caused by carbapenem-resistant pathogens in intensive care units: risk factors analysis and proposal of a prognostic score. Pathogens. 11 (7), 718. doi: 10.3390/pathogens11070718
Opota, O., Croxatto, A., Prod’hom, G., Greub, G. (2015). Blood culture-based diagnosis of bacteraemia: State of the art. Clin. Microbiol. Infect. 21 (4), 313–322. doi: 10.1016/j.cmi.2015.01.003
Podschun, R., Ullmann, U. (1998). Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11, 589–603. doi: 10.1128/CMR.11.4.589
Ramirez, J. A., Musher, D. M., Evans, S. E., Dela Cruz, C., Crothers, K. A., Hage, C. A., et al. (2020). Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest. 158 (5), 1896–1911. doi: 10.1016/j.chest.2020.05.598
Ruiz-Giardin, J. M., Ochoa Chamorro, I., Velázquez Riós, L., Jaqueti Aroca, J., García Arata, M. I., SanMartín López, J. V., et al. (2019). Blood stream infections associated with central and peripheral venous catheters. BMC Infect. Dis. 19 (1), 841. doi: 10.1186/s12879-019-4505-2
Shankar, C., Veeraraghavan, B., Nabarro, L. E. B., Ravi, R., Ragupathi, N. K. D., Rupali, P. (2018). Whole genome analysis of hypervirulent Klebsiella pneumoniae isolates from community and hospital acquired bloodstream infection. BMC Microbiol. 18 (1), 6. doi: 10.1186/s12866-017-1148-6
Sousa, D., Justo, I., Domínguez, A., Manzur, A., Izquierdo, C., Ruiz, L., et al. (2013). Community-acquired pneumonia in immunocompromised older patients: incidence, causative organisms and outcome. Clin. Microbiol. Infect. 19 (2), 187–192. doi: 10.1111/j.1469-0691.2012.03765.x
Taramasso, L., Tatarelli, P., Di Biagio, A. (2016). Bloodstream infections in HIV-infected patients. Virulence. 7 (3), 320–328. doi: 10.1080/21505594.2016.1158359
World Health Organization (2021) Global antimicrobial resistance and use surveillance system (GLASS) report 2021. Available at: http://www.who.int/glass/resources/publications/early-implementation-report-2020/en/.
World Health Organization. (2014). Antimicrobial resistance: global report on surveillance. Available at: https://www.who.int/publications/i/item/9789241564748.
Zhang, Y., Zhao, C., Wang, Q., Wang, X., Chen, H., Li, H., et al. (2016). High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob. Agents Chemother. 60 (10), 6115–6120. doi: 10.1128/AAC.01127-16
Keywords: Klebsiella pneumoniae, immunocompromised patients, respiratory tract infection, sputum culture, case fatality rate
Citation: Liu Y, Huang L, Cai J, Zhu H, Li J, Yu Y, Xu Y, Shi G and Feng Y (2023) Clinical characteristics of respiratory tract infection caused by Klebsiella pneumoniae in immunocompromised patients: a retrospective cohort study. Front. Cell. Infect. Microbiol. 13:1137664. doi: 10.3389/fcimb.2023.1137664
Received: 04 January 2023; Accepted: 28 July 2023;
Published: 16 August 2023.
Edited by:
Adrien Bouglé, Hôpitaux Universitaires Pitié Salpêtrière, FranceReviewed by:
Rola Husni, Lebanese American University Medical Center Rizk Hospital, LebanonCopyright © 2023 Liu, Huang, Cai, Zhu, Li, Yu, Xu, Shi and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yumin Xu, eHltMTIxQDE2My5jb20=; Guochao Shi, c2hpZ3VvY2hhb0Bob3RtYWlsLmNvbQ==; Yun Feng, ZnkwMTA1N0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.