
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 21 February 2023
Sec. Fungal Pathogenesis
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1133839
This article is part of the Research Topic Diversity and Molecular Diagnostics of Fungi and Oomycetes in Plants View all 18 articles
At present, 25 species are accepted in Haploporus and are distributed in Asia, Europe, North America, South America, Australia, and Africa. In this study, two new species, Haploporus ecuadorensis from Ecuador and H. monomitica from China, are described and illustrated based on morphological examination and phylogenetic analyses. H. ecuadorensis is characterized by annual, resupinate basidiomata with pinkish buff to honey yellow hymenophore when dry, round to angular pores of 2–4 per mm, a dimitic hyphal structure with generative hyphae bearing clamp connections, hyphae at dissepiment edge usually with one or two simple septa, the presence of dendrohyphidia and cystidioles, and oblong to ellipsoid basidiospores measuring 14.9–17.9 × 6.9–8.8 µm. Haploporus monomitica differs from other Haploporus species in that it has a monomitic hyphal system and strongly dextrinoid basidiospores. The differences between the new species and morphologically similar and phylogenetically related species are discussed. In addition, an updated key to 27 species of Haploporus is provided.
The genus Haploporus Bondartsev & Singer, belonging to Polyporaceae, Polyporales, Agaricomycetes, and Basidiomycota, was established by A. S. Bondartsev and R. Singer in 1944 and typified by Haploporus odorus (Sommerf.) Bondartsev & Singer (Singer, 1944). It is characterized by annual to perennial, resupinate to pileate basidiomata, a dimitic to trimitic hyphal system with clamp connections on the generative hyphae, cyanophilous skeletal hyphae, and thick-walled, cyanophilous, and ornamented basidiospores, causing a white rot of wood (Singer, 1944; Dai et al., 2002; Piatek, 2005; Li et al., 2007; Shen et al., 2016; Zhou et al., 2019; Zhou et al., 2021; Wu et al., 2022a).
In 1963, F. Kotlaba and Z. Pouzar proposed the genus Pachykytospora Kotl. & Pouzar (Kotlaba & Pouzar, 1963). However, most species of Pachykytospora, including P. alabamae (Berk. & Cooke) Ryvarden, P. nanospora A. David & Rajchenb, P. nepalensis T. Hatt., P. papyracea (Cooke) Ryvarden, P. thindii Natarajan & Koland, and P. tuberculosa (Fr.) Kotl. & Pouzar, have been transferred to Haploporus according to morphological characteristics and molecular phylogenetic analyses (Dai & Li, 2002; Piatek, 2003; Piatek, 2005; Shen et al., 2016; Zhou et al., 2021).
The genus Haploporus has been extensively studied in Australia, Brazil, China, Kenya, Sri Lanka, Sweden, and the USA (Lira et al., 2018; Zhou et al., 2019; Decock et al., 2021; Zhou et al., 2021). In the last decade, 16 species were described or combined in Haploporus, namely, H. angustisporus Meng Zhou & Y.C. Dai; H. bicolor Y.C. Dai, Meng Zhou, & Yuan; H. brasiliensis Nogueira-Melo & Ryvarden; H. crassus Meng Zhou & Y.C. Dai; H. cylindrosporus L.L. Shen, Y.C. Dai, & B.K. Cui; H. eichelbaumii (Henn.) Decock; H. gilbertsonii Meng Zhou, Vlasák, & Y.C. Dai; H. grandisporus Decock; H. longisporus Y.C. Dai, Meng Zhou, & Vlasák; H. microsporus L.L. Shen, Y.C. Dai, & B.K. Cui; H. pileatus Ryvarden; H. pirongia (G. Cunn.) Meng Zhou, Y.C. Dai, & T.W. May; H. punctatus Y.C. Dai, Meng Zhou, & Yuan; H. septatus L.L. Shen, Y.C. Dai, & B.K. Cui; H. srilankensis Y.C. Dai, Meng Zhou, & Yuan; and H. subpapyraceus L.L. Shen, Y.C. Dai, & B.K. Cui (Shen et al., 2016; Lira et al., 2018; Zhou et al., 2019; Decock et al., 2021; Zhou et al., 2021). Prior to our work, a total of 25 species was accepted in the genus (Dai et al., 2002; Hattori et al., 2002; Piatek, 2005; Li et al., 2007; Dai & Kashiwadani, 2009; Shen et al., 2016; Lira et al., 2018; Zhou et al., 2019; Decock et al., 2021; Zhou et al., 2021).
During a study on polypores from Ecuador and China, we collected specimens that morphologically fit the definition of Haploporus. After further examination and phylogenetic analysis, they formed two distinct lineages within Haploporus, and are morphologically different from the existing species in the genus. Thus, we describe them here as two new species.
The studied Haploporus specimens are deposited in the herbarium of the Institute of Microbiology, Beijing Forestry University (BJFC), the private herbarium of Josef Vlasák (JV), and the National Museum Prague of Czech Republic (PRM). For the morphological description, we followed the method from a previous study (Wu et al., 2022b). Color terms are from Anonymous (1969) and Petersen (1996).
The DNA was extracted from the dried specimens using a rapid plant genome extraction kit (Aidlab Biotechnologies Co., Ltd, Beijing, China), following the manufacturer’s protocol. The internal transcribed spacers (ITS), large subunit of nuclear ribosomal RNA gene (LSU), and small subunit mitochondrial rRNA gene (mtSSU) were amplified with primer pairs ITS 5 (5′‐GGA AGT AAA AGT CGT AAC AAG G‐3′) and ITS 4 (5′‐TCC TCC GCT TAT TGATAT GC‐3′; White et al., 1990), LR0R (5′‐ACC CGC TGA ACT TAA GC‐3′) and LR7 (5′‐TAC TAC CAC CAA GAT CT‐3′; http://www.biology.duke.edu/fungi/mycolab/primers.htm ), and MS1 (5′‐CAG CAG TCA AGA ATA TTA GTC AAT G‐3′) and MS2 5′‐GCG GAT TAT CGA ATT AAA TAA C‐3′; White et al., 1990), respectively. The PCR procedures were as follows: for ITS and mtSSU regions, an initial denaturation at 95°C for 3 min, followed by 34 cycles at 94°C for 40 s, 54°C for ITS and 55°C for mtSSU for 45 s and 72°C for 1 min, and a final extension of 72°C for 10 min; for the LSU region, an initial denaturation at 94°C for 1 min, followed by 34 cycles at 94°C for 30 s, 50°C for 1 min and 72°C for 1.5 min, and a final extension of 72°C for 10 min (Zhou et al., 2021; Zhao et al., 2022c). The PCR products were sequenced using BGI Tech Solutions (Beijing Liuhe Co., Ltd., Beijing, China). Finally, all the new sequences were submitted to GenBank, and the accession numbers are shown in Table 1.
The sequences generated were aligned with sequences downloaded from GenBank (Table 1) using MAFFT (version 7) and then manually adjusted (Katoh & Standley, 2013). A dataset of 34 specimens consisting of ITS, LSU, and mtSSU sequences was analyzed using Maximum Likelihood (ML), Maximum Parsimony (MP), and Bayesian Inference (BI) phylogenetic analyses using RAxML (version 8; Stamatakis, 2014), PAUP (version 4.0b10; Swofford, 2002), and MrBayes (version 3.2.7a; Ronquist et al., 2012), respectively, following Zhao et al, 2021; Zhao et al, 2022a; Zhao et al, 2022b). The ModelTest-NG (version 0.1.7; Darriba et al., 2020) determined the best models of ITS, LSU, and mtSSU sequences. The ML analysis was carried out with 1,000 bootstrap replications using the GTR + I + G substitution model. The MP analysis was conducted using 1,000 bootstrap replications with the heuristic search option. The BI analysis was performed for two million generations with random initial trees, using the GTR + I + G substitution model and the first 25% were set as burn-in.
The phylogenetic tree was visualized using FigTree version 1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/ ). Branches that received bootstrap support for ML, BP, and Bayesian Posterior Probabilities (BPP) greater than or equal to 50% (ML/BP) and 0.95 (BPP) were considered as significantly supported, respectively.
In this study, the combined ITS + LSU + mtSSU dataset included sequences from 37 specimens, representing 25 species of Haploporus and 2 species of Perenniporia Murrill as the outgroup (Table 1 and Figure 1). The aligned dataset had a length of 1,932 characters, of which 540 were constant characters, 122 were parsimony-uninformative characters, and 221 were parsimony-informative characters. The MP analysis yielded a tree with a length of 812, a consistency index of 0.5246, a homoplasy index of 0.4754, a retention index of 0.7551, and a rescaled consistency index of 0.3961. The best model for the ITS + LSU + mtSSU aligned dataset was GTR + I + G in the Bayesian analysis, and the average standard deviation of split frequencies is 0.00424. The phylograms of Bayesian analysis, MP analysis, and ML analysis are similar in topology, and the ML tree was chosen to represent the phylogenetic relationships (Figure 1).
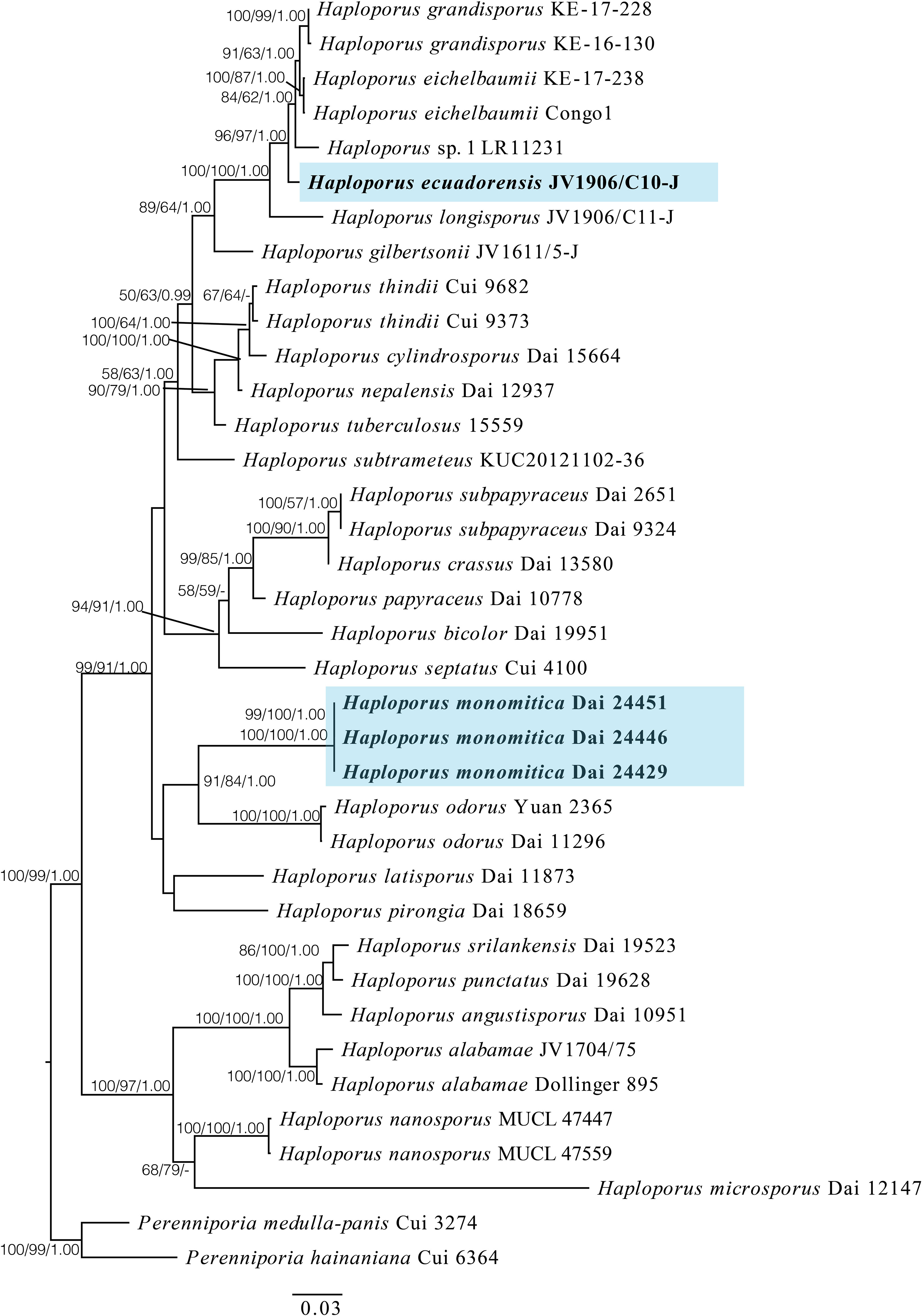
Figure 1 A maximum likelihood phylogenetic tree of Haploporus based on ITS, LSU, and mtSSU sequences, with two specimens of Perenniporia hainaniana and P. medulla-panis used as outgroups. The new species Haploporus ecuadorensis and H. monomitica are shaded. Maximum likelihood bootstrap values (≥50%)/maximum parsimony bootstrap values (≥50%)/Bayesian posterior probabilities (≥0.95) of each clade are indicated along branches. A scale bar below indicates the number of substitutions per site.
The phylogenetic tree suggests that the specimen of H. ecuadorensis forms an independent lineage in the Haploporus clade, and specimens of H. monomitica are closely related to H. odorus with strong support.
Haploporus ecuadorensis Y.C. Dai, Meng Zhou, & Vlasák, sp. nov. (Figures 2 , 3)
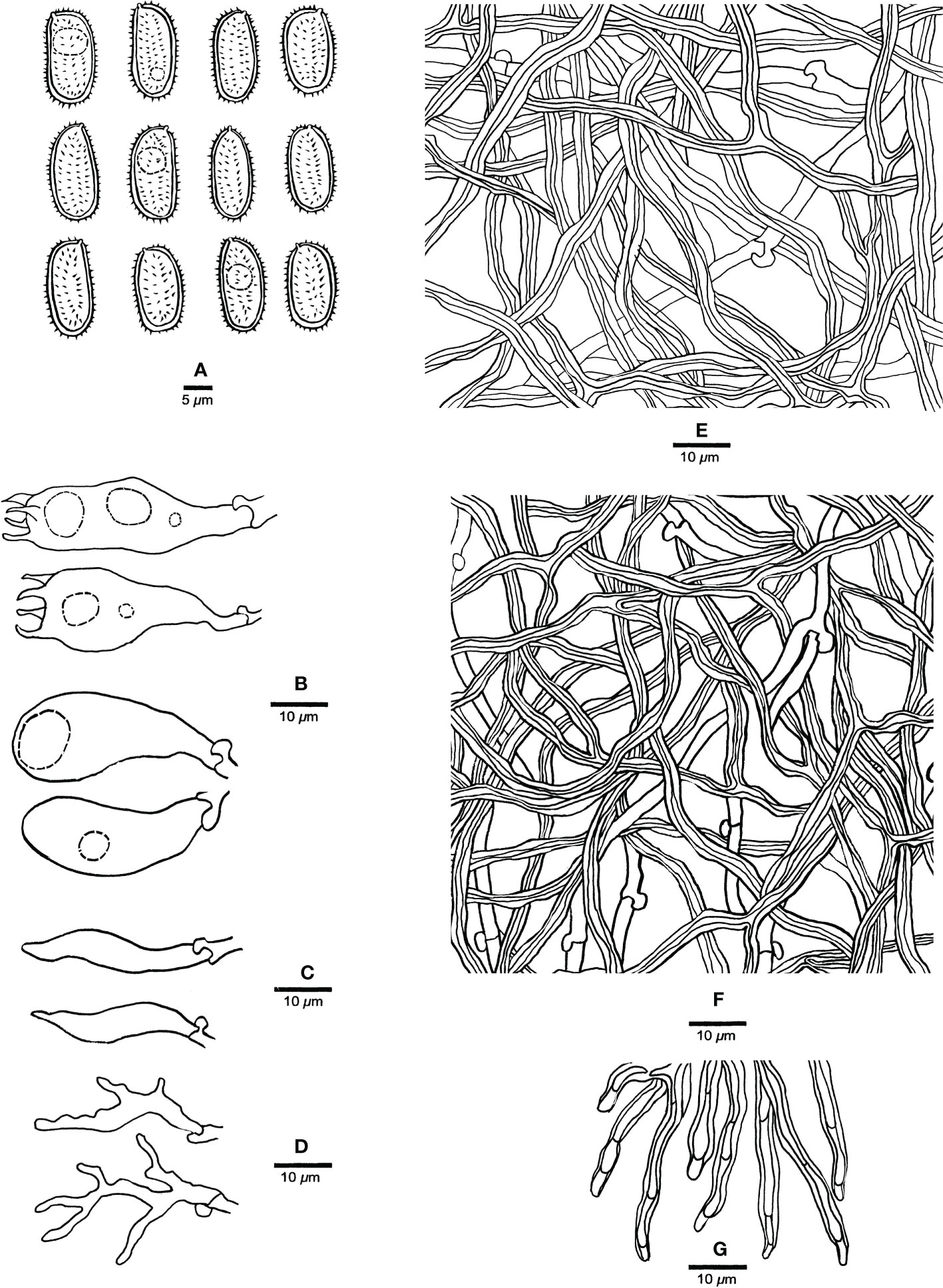
Figure 3 Microscopic characteristics of Haploporus ecuadorensis (Holotype, JV1906/C10-J). (A) Basidiospores. (B) Basidioles and basidia. (C) Cystidioles. (D) Dendrohyphidia. (E) Hyphae from subiculum. (F) Hyphae from tube trama. (G) Dissepiment hyphae. Scale bars: a = 5 μm, b–e = 10 μm.
MycoBank: MB847499
Etymology: ecuadorensis (Lat.): Refers to the occurrence of the species in Ecuador.
Type: Ecuador, Pichincha, Vicodin svah Volcán Pasochoa, on dead angiosperm branch, June 2019 JV1906/C10-J (Holotype PRM, isotypes BJFC 032988 and JV).
Basidiomata resupinate, annual, inseparable from the substrate, more or less corky when dry, up to 5 cm long, 1.5 cm wide, and 1.5 mm thick at the center. Hymenophore pinkish buff to honey yellow when dry, without distinct margin; pores angular to round, 2–4 per mm; dissepiments thick, entire. Subiculum paler than tubes, more or less corky, up to 0.5 mm thick. Tubes olivaceous buff, hard corky, up to 1.0 mm long.
Hyphal system dimitic; generative hyphae with clamp connections; skeletal hyphae thick-walled, frequently branched, neither amyloid nor dextrinoid in Melzer’s reagent, cyanophilous in Cotton Blue; tissues unchanged in 5% potassium hydroxide.
Subicular generative hyphae hyaline, thin-walled, sometimes branched, 2.2–3.3 µm in diameter; skeletal hyphae dominant, with a narrow to wide lumen, usually branched, flexuous, interwoven, 3–5.2 µm in diameter.
Tube tramal generative hyphae hyaline, thin-walled, usually branched, 1.6–3.2 µm in diameter; skeletal hyphae dominant, with a narrow lumen, usually branched, strongly flexuous, distinctly interwoven, 2.2–4 µm in diameter. Cystidioles fusiform with a sharp tip, thin-walled, hyaline, 23–34 × 4–6 µm. Basidia more or less capitate to pyriform, with four sterigmata, sometimes with a few small guttules, 40–45 × 13–15 µm, clamped at the base; basidioles capitate to pyriform, almost the same size as basidia. Dissepiment hyphae thick-walled with one or two simple septa. Dendrohyphidia present among hymenium, thin-walled, hyaline. Large and irregularly shaped crystals sometimes present among trama.
Basidiospores oblong to ellipsoid, thick-walled, tuberculate, hyaline, some with a guttule, neither amyloid nor dextrinoid in Melzer’s reagent, cyanophilous in Cotton Blue, (14.3–)14.9–17.9(–19) × (6.5–)6.9–8.8(–9) µm, arithmetic average length L = 15.94 µm, arithmetic average width W = 7.67 µm, and L/W ratio Q = 2.07 (n = 30/1).
Distribution and ecology: Haploporus ecuadorensis is distributed in tropical areas of Pichincha, Ecuador; it grows on dead angiosperm branch and causes a white rot.
Haploporus monomitica Y.C. Dai, sp. nov. (Figures 4, 5)

Figure 4 Basidiomata of Haploporus monomitica (Holotype, Dai 24446). Scale bar = 1 cm. Photo by Yu-Cheng Dai.
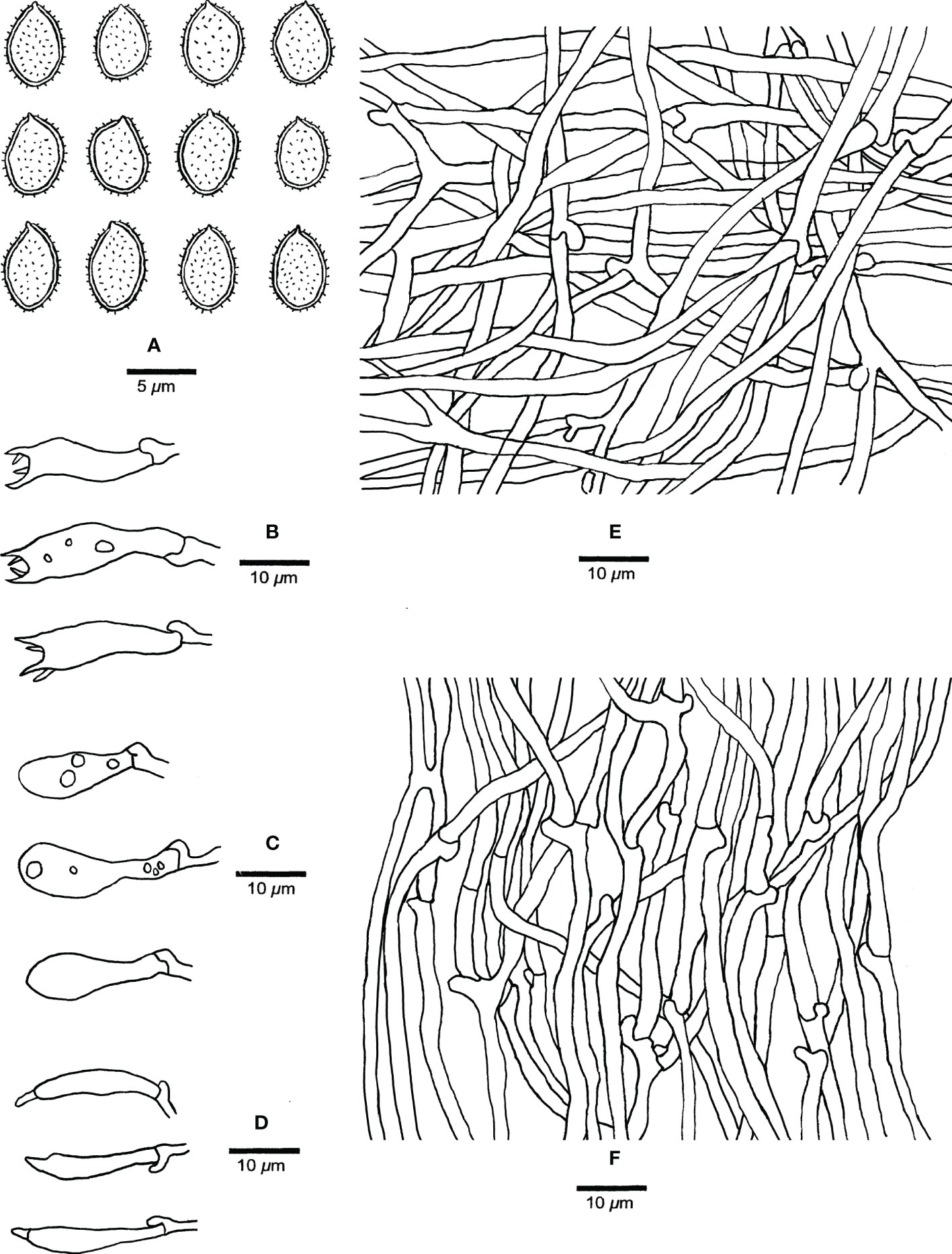
Figure 5 Microscopic structures of Haploporus monomitica (Holotype Dai 24446). (A) Basidiospores. (B) Basidia. (C) Basidioles. (D) Cystidioles. (E) Hyphae from subiculum. (F) Hyphae from trama.
MycoBank: MB838450
Etymology: monomitica (Lat.): refers to the species having a monomitic hyphal system.
Type: China, Beijing, Mentougou, Xiaolongmen National Forest Park, on fallen trunk of Quercus sp., 30 August 2022, Yu-Cheng Dai, Dai 24446 (Holotype BJFC 038932).
Basidiomata annual, resupinate, difficult to separate from the substrate, soft and white when fresh, become soft corky to fragile and white to cream when dry, up to 3 cm long, 1 cm wide, and 1 mm thick at the center. Sterile margin distinct, white, cottony, up to 1 mm; pores round to angular, 3–4 per mm; dissepiments thick, entire. Subiculum white, soft corky, up to 0.2 mm thick. Tubes concolorous with pores, fragile, up to 0.8 mm long.
Hyphal system monomitic; generative hyphae bearing clamp connections, hyaline, thin-walled, frequently branched, neither amyloid nor dextrinoid in Melzer’s reagent, cyanophilous in Cotton Blue; tissues unchanging in 5% potassium hydroxide.
Subicular generative hyphae hyaline, thin-walled, frequently branched, flexuous, interwoven, 2–3.3 µm in diameter.
Tube tramal generative hyphae hyaline, thin-walled, frequently branched, flexuous, interwoven, 2–3 µm in diameter. Cystidia absent; cystidioles present, clavate to fusiform, hyaline, thin-walled, 17–25 × 3–5 µm. Basidia clavate with 4-sterigmata and a basal clamp connection, 15–32 × 6–9 µm; basidioles pyriform, slightly smaller than basidia. Dendrohyphidia absent.
Basidiospores broadly ellipsoid, hyaline, thick-walled with echinulate ornamentation, dextrinoid in Melzer’s reagent, cyanophilous in Cotton Blue, (4.2–)4.9–6.5 × (3.0–)3.2–4.8(–5.0) µm, arithmetic average length L = 5.37 µm, arithmetic average width W = 3.90 µm, and L/W ratio Q = 1.32–1.43 (n =90/3).
Additional materials studied: China, Beijing, Mentougou, Xiaolongmen National Forest Park, on fallen trunk of Quercus sp., 30 August 2022, Yu-Cheng Dai, Dai 24429, Dai 24451.
Distribution and ecology: Haploporus monomitica is distributed in temperate area of Beijing, China; it grows on fallen trunk of Quercus, and causes a white rot.
In a combined ITS + LSU + mtSSU dataset-based phylogeny (Figure 1) Haploporus ecuadorensis forms an independent lineage that is closely related to H. grandisporus Decock, H. eichelbaumii (Henn.) Decock and H. sp. (Decock et al., 2021). Morphologically, H. eichelbaumii is different from H. ecuadorensis in that it has smaller basidiospores (11–14 × 5.3–6.5 µm vs. 14.9–17.9 × 6.9–8.8 µm; Decock et al., 2021). H. grandisporus is readily distinguished from H. ecuadorensis by larger pores (1.5–2.5 per mm vs. 2–4 per mm) and narrower basidiospores (14–17.5 × 6–7.3 µm vs. 14.9–17.9 × 6.9–8.8 µm; Decock et al., 2021). Haploporus sp. From Malawi is also an independent lineage within the Haploporus clade in a previous study (Decock et al., 2021). This taxon differs from H. ecuadorensis in that it has distinctly smaller pores (4–5 vs. 2–4 per mm Decock et al., 2021). In addition, there are more than 2% nucleotide difference in the ITS sequences between Haploporus sp. and H. ecuadorensis.
Haploporus ecuadorensis, H. crassus, H. pirongia, and H. septatus share thick-walled dissepiment hyphae with a simple septum or a few septa. Haploporuscrassus can be differentiated from H. ecuadorensis by its thick-walled basidia, the ventricose cystidioles occasionally with a simple septum, and the absence of dendrohyphidia (Zhou et al., 2019). Haploporus pirongia is distinguished from H. ecuadorensis by smaller basidiospores (11–14 × 5.2–7 µm vs. 14.9–17.9 × 6.9–8.8 µm; Zhou et al., 2019). Haploporus septatus is different from H. ecuadorensis in that it has dextrinoid skeletal hyphae in Melzer’s reagent and smaller pores and basidiospores (5–6 per mm vs. 2–4 per mm, 8.5–11 × 5–6 μm vs. 14.9–17.9 × 6.9–8.8 µm; Shen et al., 2016).
Haploporus longisporus resembles H. ecuadorensis in terms of resupinate basidiomata, similar pore dimension (2–3 per mm vs. 2–4 per mm), non-dextrinoid skeletal hyphae in Melzer’s reagent, and the presence of dendrohyphidia and cystidioles. Although both species have an overlapping distribution in Ecuador, H. longisporus is readily distinguished from H. ecuadorensis by bigger basidiospores (18.2–22 × 7–9 µm vs. 14.9–17.9 × 6.9–8.8 µm; Zhou et al., 2021).
Haploporus gilbertsonii was described from the USA recently (Zhou et al., 2021). It is similar to H. ecuadorensis in terms of resupinate basidiomata, similar pore dimension (2–3 per mm vs. 2–4 per mm; Zhou et al., 2019), non-dextrinoid skeletal hyphae in Melzer’s reagent, and the presence of cystidioles, but the former differs from the latter by the absence of dendrohyphidia and smaller basidiospores (12–15 × 6–8 µm vs. 14.9–17.9 × 6.9–8.8 µm; Zhou et al., 2021).
Our phylogeny shows that Haploporus monomitica forms a sister group to H. odorus with strong support (BP: 91%, MP: 84%, and BPP 1.0). However, H. odorus has pileate basidiomata with a strong fragrant odor, a dimitic hyphae system, non-dextrinoid or very weakly dextrinoid basidiospores, and grows exclusively on Salix (Niemelä, 2005; Zhou et al., 2021). Moreover, in Siberia and North America, the fungus grows on another member of the Salicaceae family, Populus tremula (Zmitrovich et al., 2019).
The dimitic or trimitic hyphal structure was mentioned in the previous definition of Haploporus (Ryvarden & Melo, 2014; Shen et al., 2016; Zhou et al., 2019; Decock et al., 2021; Zhou et al., 2021); however, a monomitic hyphal system is found in the new species Haploporus monomitica, and phylogenetically, it is nested in Haploporus. Therefore, the updated definition of the genus is as follows: basidiomata annual to perennial, resupinate to pileate, hyphal system monomitic, dimitic to trimitic with clamped generative hyphae, cyanophilous skeletal hyphae, thick-walled, cyanophilous, and ornamented basidiospores, and causing a white rot.
Like other genera of wood-decaying fungi having a rich diversity of species in tropical areas (Wu et al., 2017; Cui et al., 2019; Wu et al., 2020; Dai et al., 2021; Guan & Zhao, 2021; Wang et al., 2021; Wu et al., 2021; Ma et al., 2022), our result shows that a high diversity of Haploporus exists in neotropical areas.
1. Hyphal system monomitic.................................H. monomitica
1. Hyphal system dimitic to trimitic.............................................2
2. Basidiospores < 8 µm long..........................................................3
2. Basidiospores > 8 µm long..........................................................6
3. Pores 7–9 per mm.........................................................................4
3. Pores < 6 per mm..........................................................................5
4. Cystidioles absent...................................................H. nanosporus
4. Cystidioles present................................................H. microsporus
5. Pores 1–3 per mm; skeletal hyphae strongly dextrinoid...............................................................H. brasiliensis
5. Pores 4–5 per mm; skeletal hyphae weakly dextrinoid...............................................................H. odorus
6. Basidiomata annual to perennial................................................7
6. Basidiomata annual.......................................................................9
7. Skeletal hyphae dextrinoid...................................H. srilankensis
7. Skeletal hyphae non-dextrinoid..................................................8
8. Basidiospores cylindrical...............................................H. thindii
8. Basidiospores oblong ellipsoid to ellipsoid..................................
...........................................................................H. subtrameteus
9. Hyphal system trimitic...............................................................10
9. Hyphal system dimitic................................................................12
10. Skeletal hyphae dextrinoid...............................H. tuberculosus
10. Skeletal hyphae non-dextrinoid..............................................11
11. Basidiospores ovoid to ellipsoid...........................H. alabamae
11. Basidiospores oblong-ellipsoid to cylindrical.......H. pirongia
12. Cystidioles absent......................................................................13
12. Cystidioles present....................................................................15
13. Basidiomata pileate....................................................H. pileatus
13. Basidiomata resupinate............................................................14
14. Pores 4–5 per mm, basidiospores cylindrical, 10–11.5 × 4.5–5 µm..................................H. cylindrosporus
14. Pores 1.5–4 per mm, basidiospores ellipsoid to oblong, 10–15 × 5–6.8 µm.................................H. eichelbaumii
15. Dendrohyphidia present..........................................................16
15. Dendrohyphidia absent............................................................20
16. Pores 5–7 per mm.........................................................H. bicolor
16. Pores < 4 per mm.......................................................................17
17. Basidiospores cylindrical..........................................................18
17. Basidiospores ellipsoid to oblong...........................................19
18. Basidiospores 18.2–22 × 7–9 µm.......................H. longisporus
18. Basidiospores 13–15 × 5–6 µm...........................H. papyraceus
19. Hyphal system trimitic, skeletal hyphae dextrinoid.............................H. grandisporus
19. Hyphal system dimitic, skeletal hyphae non-dextrinoid.......................................................................H. ecuadorensis
20. Pores > 3 per mm.......................................................................21
20. Pores < 3 per mm.......................................................................25
21. Pores 5–6 per mm......................................................H. septatus
21. Pores 3–5 per mm......................................................................22
22. Skeletal hyphae non-dextrinoid................................H. crassus
22. Skeletal hyphae dextrinoid.......................................................23
23. Cystidioles without septum............................H. angustisporus
23. Cystidioles with a simple septum............................................24
24. Basidiospores 9–10.8 × 3.8–5 µm.........................H. punctatus
24. Basidiospores 9–12 × 5.5–8 µm...................H. subpapyraceus
25. Basidiospores 9–10 µm wide.................................H. latisporus
25. Basidiospores < 9 µm wide.......................................................26
26. Basidiospores 12–15 × 6–8 µm...........................H. gilbertsonii
26. Basidiospores 8.5–11.5 × 4.5–6.5 µm.................H. nepalensis
The datasets presented in this study can be found in GenBank Database. The names of the accession numbers can be found in the Table 1.
Y-CD, L-SB, HZ and JV collected specimens. X-WM, L-SB, MZ and HZ did the drawings, DNA sequencing, and data analyses, and drafted the paper. JV and Y-CD did the morphological descriptions and acquired funding. All authors contributed to the article and approved the submitted version.
This study was supported by the Fundamental Research Funds for the Central Non-profit Research Institution of the Chinese Academy of Forestry (Project No. CAFYBB2021MA007), the National Natural Science Foundation of China (Project No. 31800018; No. 32161143013), Investigation on ecosystem and biodiversity of Mentougou (Project No. 11010922210200001368-XM001) and Academy of Sciences of the Czechia RVO: 60077344.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anonymous (1969). Flora of British fungi. colour identification chart (London: Her Majesty’s Stationery Office).
Cui, B. K., Li, H. J., Ji, X., Zhou, J. L., Song, J., Si, J., et al. (2019). Species diversity, taxonomy and phylogeny of polyporaceae (Basidiomycota). in China. Fungal Diversity 97, 137–392. doi: 10.1007/s13225-019-00427-4
Dai, Y. C., Kashiwadani, H. (2009). Haploporus subtrameteus (Polyporaceae, basidiomycota) found in Japan. Mycoscience 50, 452–454. doi: 10.1007/S10267-009-0498-9
Dai, Y. C., Li, T. H. (2002). Megasporoporia major (Basidiomycota), a new combination. Mycosystema 21, 519–521.
Dai, Y. C., Niemelä, T., Kinnunen, J. (2002). The polypore genera Abundisporus and Perenniporia (Basidiomycota) in China, with notes on Haploporus. Annales Botanici Fennici 39, 169–182.
Dai, Y. C., Yang, Z. L., Cui, B. K., Wu, G., Yuan, H. S., Zhou, L. W., et al. (2021). Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 40, 770–805. doi: 10.13346/j.mycosystema.210036
Darriba, D., Posada, D., Kozlov, A. M., Stamatakis, A., Morel, B., Flouri, T. (2020). ModelTest-NG: a new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 37, 291–294. doi: 10.1093/molbev/msz189
Decock, C. A., Wagara, I., Balezi, A., Yombiyeni, P. (2021). Haploporus (Basidiomycota, polyporales) in sub-Saharan Africa: Poria eichelbaumii, a long-forgotten name, is reinstated in Haploporus and h. grandisporus sp. nov. is proposed. Mycological Prog. 20, 149–168. doi: 10.1007/s11557-020-01660-x
Guan, Q. X., Zhao, C. L. (2021). Two new corticioid species, Hyphoderma sinense and H. floccosum (Hyphodermataceae, polyporales), from southern China. Mycosystema 40, 447–461. doi: 10.13346/j.mycosystema.200382
Hattori, T., Adhikari, M. K., Suda, T., Doi, Y. (2002). A list of polypores (Basidiomycotina, aphyllophorales) collected in jumla, Nepal. Bull. Natl. Sci. Museum 28, 27–38.
Katoh, K., Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kotlaba, F., Pouzar, Z. (1963). A new genus of the polypores–Pachykytospora gen. nov. Ceská Mykol 17, 27–34.
Li, J., Dai, Y. C., Yuan, H. S. (2007). A new species of Haploporus (Basidiomycotina) from China. Mycotaxon 99, 181–187.
Lira, C., Nogueira-Melo, G., Ryvarden, L., Gibertoni, T. (2018). Two new species of Haploporus from Brazil. Synopsis Fungorum 38, 62–65.
Ma, H. X., Si, J., Dai, Y. C., Zhu, A. H., Cui, B. K., Fan, Y. G., et al. (2022). Diversity of wood-inhabiting macrofungi in hainan province, south China. Mycosystema 41, 695–712. doi: 10.13346/j.mycosystema.210424
Petersen, J. H. (1996). The Danish mycological society’s colour-chart (Greve.: Foreningen til Svampekundskabens Fremme), 1–6.
Piatek, M. (2003). Haploporus tuberculosus, a new polypore genus and species in Belarus, with a new combination in haploporus. Polish Botanical J. 48, 81–83.
Piatek, M. (2005). Taxonomic position and world distribution of Pachykytospora nanospora (Polyporaceae). Annales Botanici Fennici 42, 23–25.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Shen, L. L., Chen, J. J., Wang, M., Cui, B. K. (2016). Taxonomy and multi-gene phylogeny of Haploporus (Polyporales, basidiomycota). Mycological Prog. 15, 731–742. doi: 10.1007/s11557-016-1203-y
Singer, R. (1944). Notes on taxonomy and nomenclature of the polypores. Mycologia 36, 65–69. doi: 10.1080/00275514.1944.12017529
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Swofford, D. L. (2002). PAUP*: Phylogenetic analysis using parsimony (* and other methods); version 4.0b10 (MA, USA: Sinauer Associates: Sunderland).
Wang, K., Chen, S. L., Dai, Y. C., Jia, Z. F., Li, T. H., Liu, T. Z., et al. (2021). Overview of china’s nomenclature novelties of fungi in the new centur–2020). Mycosystema 40, 822–8332. doi: 10.13346/j.mycosystema.210483
White, T. J., Bruns, T., Lee, S., Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: guide to Methods Appl. 18, 315–322.
Wu, F., Chen, J. J., Ji, X. H., Vlasák, J., Dai, Y. C. (2017). Phylogeny and diversity of the morphologically similar polypore genera Rigidoporus, Physisporinus, Oxyporus, and Leucophellinus. Mycologia 109, 749–765. doi: 10.1080/00275514.2017.1405215
Wu, F., Man, X. W., Tohtirjap, A., Dai, Y. C. (2022a). A comparison of polypore funga and species composition in forest ecosystems of China, north America, and Europe. For. Ecosyst. 9, 1–7. doi: 10.1016/j.fecs.2022.100051
Wu, F., Tohtirjap, A., Fan, L. F., Zhou, L. W., Alvarenga, R. L. M., Gibertoni, T. B., et al. (2021). Global diversity and updated phylogeny of Auricularia (Auriculariales, basidiomycota). J. Fungi 7, 933. doi: 10.3390/jof7110933
Wu, F., Yuan, H. S., Zhou, L. W., Yuan, Y., Cui, B. K., Dai, Y. C. (2020). Polypore diversity in south China. Mycosystema 39, 653–682. doi: 10.13346/j.mycosystema.200087
Wu, F., Zhou, L. W., Vlasák, J., Dai., Y. C. (2022b). Global diversity and systematics of hymenochaetaceae with poroid hymenophore. Fungal Diversity 113, 1–192. doi: 10.1007/s13225-021-00496-4
Zhao, H., Nie, Y., Zong, T. K., Dai, Y. C., Liu, X. Y. (2022a). Three new species of Absidia (Mucoromycota) from China based on phylogeny, morphology and physiology. Diversity 14, 132. doi: 10.3390/d14020132
Zhao, H., Nie, Y., Zong, T. K., Wang, Y. J., Wang, M., Dai, Y. C., et al. (2022b). Species diversity and ecological habitat of Absidia (Cunninghamellaceae, mucorales) with emphasis on five new species from forest and grassland soil in China. J. Fungi 8, 471. doi: 10.3390/jof8050471
Zhao, H., Zhou, M., Liu, X.-Y., Wu, F., Dai, Y. C. (2022c). Phylogeny, divergence time estimation and biogeography of the genus Onnia (Basidiomycota, hymenochaetaceae). Fronter Microbiol. 13. doi: 10.3389/fmicb.2022.907961
Zhao, H., Zhu, J., Zong, T. K., Liu, X. L., Ren, L. Y., Lin, Q., et al. (2021). Two new species in the family cunninghamellaceae from China. Mycobiology 49, 142–150. doi: 10.1080/12298093.2021.1904555
Zhou, M., Dai, Y. C., Vlasák, J., Yuan, Y. (2021). Molecular phylogeny and global diversity of the genus Haploporus (Polyporales, basidiomycota). J. Fungi 7, 96. doi: 10.3390/jof7020096
Zhou, M., Wang, L., May, T. W., Vlasák, J., Chen, J. J., Dai, Y. C. (2019). Phylogeny and diversity of Haploporus (Polyporaceae, basidiomycota). MycoKeys 54, 77–98. doi: 10.3897/mycokeys.54.34362
Keywords: polyporaceae, wood-rotting fungi, taxonomy, fungi diversity, new taxa
Citation: Man X-W, Dai Y-C, Bian L-S, Zhou M, Zhao H and Vlasák J (2023) Two new species of Haploporus (Polyporales, Basidiomycota) from China and Ecuador based on morphology and phylogeny. Front. Cell. Infect. Microbiol. 13:1133839. doi: 10.3389/fcimb.2023.1133839
Received: 29 December 2022; Accepted: 06 February 2023;
Published: 21 February 2023.
Edited by:
Yusufjon Gafforov, Academy of Science of the Republic of Uzbekistan, UzbekistanReviewed by:
Sergey Volobuev, Komarov Botanical Institute (RAS), RussiaCopyright © 2023 Man, Dai, Bian, Zhou, Zhao and Vlasák. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heng Zhao, emhhb2hlbmcyMUBiamZ1LmVkdS5jbg==; Josef Vlasák, dmxhc2FrQHVtYnIuY2FzLmN6
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.