
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol., 10 March 2023
Sec. Intestinal Microbiome
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1121947
This article is part of the Research TopicHelicobacter pylori Prevention, Individualized Diagnosis, and Eradication as well as the Construction of Precise Prevention, Early Diagnosis, and Clinical Treatment for Gastric CancerView all 9 articles
As a confirmed carcinogen, Helicobacter pylori (H. pylori) is the main cause of inflammatory diseases of the upper digestive tract and even gastric cancer. There is a high prevalence of H. pylori infection among the elderly population, which may cause adverse clinical outcomes. Particularly noteworthy is that guidelines or expert consensus presently available on H. pylori infection overlook the management of the elderly population as a special group. A brief overview of H. pylori in the elderly is as follows. The detection of H. pylori infection can be divided into invasive and non-invasive techniques, and each technique has its advantages and shortcomings. There may be more side effects associated with eradication treatment in elderly individuals, especially for the frail population. Physical conditions and risk-benefit assessments of the elderly should be considered when selecting therapeutic strategies for H. pylori eradication. Unless there are competing factors, elderly patients should receive H. pylori eradication regimens to finally reduce the formation of gastric cancer. In this review, we summarize the latest understanding of H. pylori in the elderly population to provide effective managements and treatment measures.
The challenge of population aging is becoming increasingly intense in several developed and developing countries, such as China, Japan, and the United States (Zhao et al., 2019). An Italian study showed that about 40% of more than 3,000 people over 60 years old had gastrointestinal symptoms due to the presence of disordered digestive function in the elderly population (Pilotto et al., 2011). The functional decline of the upper gastrointestinal tract is mainly characterized by the atrophic gastric mucosa and reduced digestive enzyme activity, and infection [e.g., Helicobacter pylori (H. pylori)], non-steroidal anti-inflammatory drugs (NSAIDs) and other factors can lead to the further deterioration (Huang et al., 2021a).
Higher prevalence and prolonged accumulation of H. pylori infection in the elderly lead more easily to atrophic gastritis, intestinal metaplasia, and even gastric cancer (Weck and Brenner, 2006; Weck et al., 2007; Toyokawa et al., 2010). Due to a decline in physical function from underlying diseases, complications of some diseases like renal insufficiency and more severe drug adverse effects, different eradication therapy regimens, and ideal drug dosing, especially antibiotics, are still not well defined for the elderly (Zendehdel and Roham, 2020). It is well known that currently updated guidelines or expert consensus on H. pylori infection are either unmentioned or understated about assessments of risks and benefits, therapeutic strategies, and treatment-related adverse effects among the elderly (Sugano et al., 2015; Liu W. Z. et al., 2018; Malfertheiner et al., 2022). As can be seen, the concern about H. pylori infection among older adults is still relatively low. In this review we presented the most recent advancements in H. pylori infection in the elderly, involving several aspects, such as epidemiology, diagnosis, treatment, and adverse events.
It is estimated that approximately 50% of the world’s population is infected with H. pylori usually acquired in childhood (Suerbaum and Michetti, 2002). The infection rate of H. pylori in the elderly having higher education, dominated by significant mental work or living in more economically developed regions is relatively lower (Nurgalieva et al., 2002; Matsuhisa et al., 2015). The correlation between some living habits (e.g., tea drinking, smoking, alcohol intake) and H. pylori infection is uncertain. An early epidemiological survey showed the prevalence of H. pylori infection among the elderly was 83.4% (84.7% in males and 82.1% in females) in Beijing, China (Zhang et al., 2005). However, the H. pylori infection rate in Beijing was 46.5% (507/1090) from a recent investigation, with a significantly higher rate in males than in females (51.8% vs 42.5%). And, the total infection rate increased gradually with age (Zhu et al., 2020). Several early studies in other countries revealed that the infection rate of H. pylori in the elderly with peptic ulcer disease is 58%-78%, but only 40% to 56% of whom were tested for H. pylori infection, and 50% to 73% of whom with a positive test were subsequently treated with antibiotics (Roll et al., 1997; Ofman et al., 2000; Pilotto, 2001). Studies also confirmed the infection was more common in the elderly population, with a prevalence ranging from 68% to 86.5% in hospitalized patients, most of whom came from nursing homes (Pilotto et al., 1996; Regev et al., 1999). However, there is updated evidence of a descending trend in H. pylori infection rate in both adults and children from some countries (Bures et al., 2012; Tonkic et al., 2012).
Concerning antibiotic resistance, a recent study revealed that the overall resistance rates of clarithromycin, levofloxacin and metronidazole in the Chinese population were 17.76%, 19.66% and 95.5%, respectively, and the resistance rates of clarithromycin and levofloxacin were highest in the elderly (Ji et al., 2016). Similarly, another study showed that middle-aged and elderly patients exhibited higher resistance rates to clarithromycin, azithromycin, levofloxacin, and moxifloxacin compared to patients younger than 40 years old (Liu D. S. et al., 2018). The infection continues if left untreated and this cumulative effect is even more pronounced in older people. Taken together, drug resistance and high prevalence of H. pylori infection among elderly patients characterize its epidemiology. Thus, an increased focus is needed on diagnosing and treating H. pylori infection in the elderly.
Modalities for evaluating the H. pylori infection can be divided into invasive and non-invasive tests (Yilmaz et al., 2006). Invasive methods for directly detecting H. pylori in biopsy samples during endoscopy involve rapid urease test (RUT), histology and bacterial culture (Kim S. E. et al., 2020). The C-urea breath test (UBT), serological blood test, and stool antigen test (SAT) are the most commonly used non-invasive tests (Vonkeman et al., 2012). There are advantages, disadvantages, and limitations to each approach.
The UBT still remains the gold standard in non-invasive methods dependent of urease activity of H. pylori (Toyoshima et al., 2018). In comparison with 14C-UBT, 13C-UBT most widely used in the elderly is a stable isotope with high safety and no radioactivity hazards (Xie et al., 2020). The greatest advantage of UBT makes it possible to take samples throughout the entire stomach and avoids the appearance of false negatives upon focal distribution of H. pylori in the stomach. Studies demonstrated that 13C-UBT had a sensitivity of 100%, a specificity of 95.7%, an accuracy of 98% for the diagnosis of H. pylori in the elderly, and 14C-UBT obtained a sensitivity of 91.4%, a specificity of 93.8% for the elderly (Pilotto et al., 2000b; Atli et al., 2012). However, the sensitivity and accuracy of 13C-UBT for elderly participants are lower than those of non-elderly patients (Choi et al., 2011). As can be seen that UBT is an accurate, practical and convenient test method (Gisbert and Pajares, 2005). Eisdorfer I et al. (Eisdorfer et al., 2018) indicated that the average UBT values increased significantly with age (28.6‰ in young group and 32.3‰ in elderly group). The possible explanations are not only the high H. pylori load or density in the elderly but also are reduced basal metabolic rate or impaired gastric emptying (Liu et al., 1995; Chang et al., 2002; Roberts and Dallal, 2005; Perets et al., 2019). Although a uniform cut-off value of UBT for all age groups is conducted by manufacturers, a higher threshold value of UBT might be recommended to determine the presence of H. pylori infection in the elderly population (Perets et al., 2019).
Based on the immune system triggered by H. pylori infection, the serological blood test is used to detect specific antibodies, with the 74.4% sensitivity, 59% specificity and 67% diagnostic accuracy in the elderly (Pilotto et al., 1996). In the participants over 60 years, the specificity of the serological blood test is lower than in those < 40 years (Choi et al., 2011). H. pylori antibodies can still remain positive six months after eradication due to the possibility of antibodies persisting in the blood for a prolonged period. Positive antibody test results cannot distinguish the presence of H. pylori current infection, so this method is mainly used for epidemiological investigation, rather than for follow-up after eradication (Koyama et al., 2016). False-negative serological results may occur in elderly patients with immune deficiency or protein malnutrition due to the lack of antibody response (Cizginer et al., 2014). In fact, it can also be used to observe the former presence of H. pylori in the elderly with atrophic gastritis.
H. pylori, which is excreted with the stool following renewal and shedding of gastric mucosa epithelial cells, can be confirmed by detection of fecal antigens. SAT remains unaffected by atrophic gastritis, ulcer or intestinal metaplasia in the elderly population. It is also applicable to detect H. pylori after gastrectomy. The UBT tends to appear false negative results, whereas SAT is not affected by decreased gastric acid secretion of remnant stomach upon reduced gastral cavity (Best et al., 2018). The diagnostic accuracy of SAT is similar to that of UBT according to the Maastricht IV/Florence consensus report (Malfertheiner et al., 2012). Several studies suggested SAT showed high specificity and accuracy for the elderly, but its sensitivity remained unsatisfactory (Inelmen et al., 2004; Inelmen et al., 2005; Han et al., 2020). Relatively, SAT appears to have a higher sensitivity for the diagnosis of individuals younger than 60 years than elderly individuals aged 60 and over, suggesting a slight tendency for decreasing sensitivity with increasing age (Choi et al., 2011). This might be because constipation commonly occurs among the elderly population, which contributes to longer H. pylori transport time in the intestinal tract so as to lead to degradation of bacterial antigens (Salles-Montaudon et al., 2002). In addition, SAT appears to be more advantageous in the elderly reluctant to receive the UBT or with suboptimal breathing cooperation (e.g., severe pulmonary fibrosis and chronic obstructive pulmonary disease) (Konstantopoulos et al., 2001; Alzoubi et al., 2020).
The main strength of the invasive methods is a lesion can be identified in the stomach by a gastroscopy examination, especially in the context of increased risk of gastric cancer and gastric mucosa with dysplasia in the elderly. Endoscopy-based H. pylori detection method should be preferred in the elderly with alarm symptoms (e.g., emaciation, anemia) to avoid missing important diseases (Zendehdel and Roham, 2020). The RUT with several advantages of rapidness, convenience and high accuracy can be used as a rapid detection method of H. pylori in elderly patients undergoing gastroscopy (Uotani and Graham, 2015). In spite of its high specificity and accuracy, the RUT is considered to have a lower sensitivity in older patients compared to younger patients (57% vs 75%) (Rogge et al., 1995; Abdalla et al., 1998). The possible reason is that H. pylori is focally distributed within the stomach and false negative results may occur when the bacterial count in the biopsy specimen is less than 1 × 104 (Godbole et al., 2020). Multi-point biopsy in the stomach can improve the positive rate (Hsu et al., 2010). The RUT is also not recommended for follow-up after H. pylori eradication (Malfertheiner et al., 2017).
Histology is effective for evaluating H. pylori-associated gastritis (Malfertheiner et al., 2017). Choi J et al. (Choi et al., 2011) showed that the sensitivity, specificity, and accuracy of histology for the diagnosis of H. pylori in elderly participants were lower than those 40-60 years and those under 40 years. Moreover, according to the Sydney system for histological diagnosis and classification of chronic gastritis, objective evaluation indices for H. pylori are taken into account. The tissue biopsy and histological staining are recommended for elderly patients with suspected lesions upon gastroscopy to determine the presence of cancerous lesions and/or H. pylori infection (Zhao and Chi, 2022). The most conventional staining is hematoxylin-eosin staining, which can be false negative for H. pylori when the specimen contains little bacterial count (Wang et al., 2010). Notably, special stainings with high contrast (e.g., Giemsa staining, immunohistochemical staining) contribute to diagnose H. pylori infection (Wang et al., 2010; Batts et al., 2013; Kocsmar et al., 2017).
H. pylori is a microaerophilic bacterium and its isolation culture depends on stringent culture conditions. The positive rate of cultures is affected by improper storage and transport of specimens, as well as too low concentrations of inoculum and so on (Leszczynska et al., 2010; Gong et al., 2015). Thus, the bacterial culture is mainly used for the epidemiological investigation and antimicrobial susceptibility testing in the resistant elderly population where H. pylori eradication failed (Rojas-Rengifo et al., 2019). H. pylori culture taken from gastric mucosal tissue samples under appropriate conditions has a satisfactory specificity but a somewhat lower sensitivity (70%-90%) (Wang et al., 2015). Moreover, the molecular biology technique is not a conventional method for the diagnosis of H. pylori infection (Liu W. Z. et al., 2018), but it with high sensitivity and specificity is superior to bacterial culture and susceptibility testing, which has important clinical value for guiding the choices of antibiotics in the elderly (Figure 1) (Liu W. Z. et al., 2018; Marrero et al., 2021).
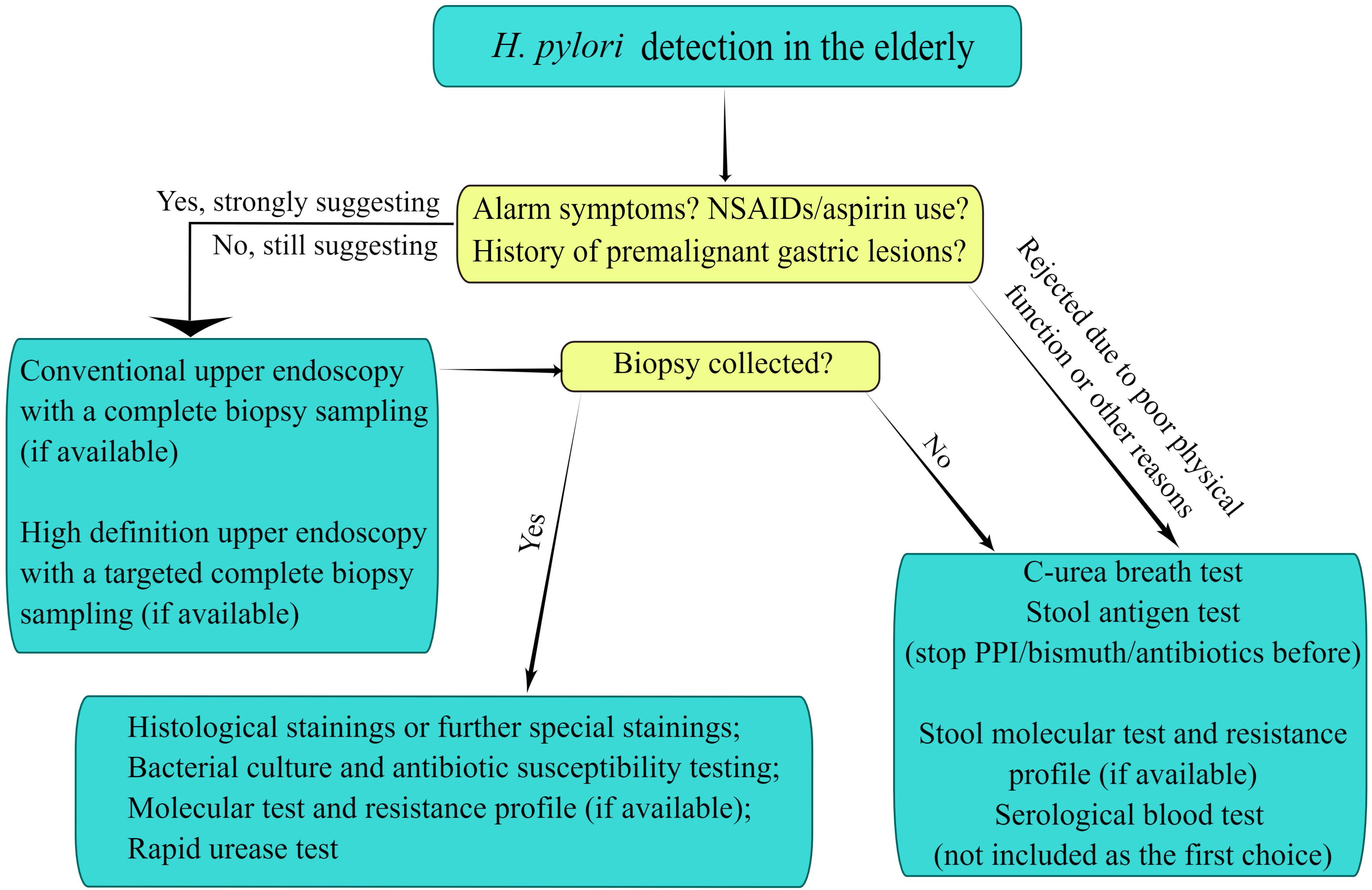
Figure 1 Diagnostic evaluation to detect Helicobacter pylori infection in the elderly. The figure is drawn by Figdraw.
H. pylori infection in the elderly induces the inflammatory response of gastric mucosa, abnormal secretion of gastrointestinal hormones and gastric acid to affect gastroduodenal motility and sensitivity, which is associated with dyspeptic symptoms (Suzuki and Moayyedi, 2013). H. pylori infection is the main factor of peptic ulcer, which can be promoted to heal, with a decrease in the relapse rate by eradicating H. pylori (Gisbert et al., 2012). Moreover, benign diseases of the digestive system associated with H. pylori infection in the elderly also include functional dyspepsia, non-atrophic gastritis, gastroesophageal reflux disease, and even chronic atrophic gastritis (Pilotto and Franceschi, 2014). Gastric mucosa-associated lymphoid tissue lymphoma (MALT) and gastric cancer are also associated with H. pylori infection in the elderly. Nagy P et al. (Nagy et al., 2016) demonstrated that 60%~80% of patients with H. pylori-positive locally gastric MALT achieved remission after eradicating H. pylori, suggesting that eradication of H. pylori is the first-line therapy for localized gastric MALT. Cancer of the stomach ranks fourth on the global cancer list, with the highest incidence in the elderly population (Zeng et al., 2020). H. pylori has been identified as a type I carcinogenic factor of gastric cancer, which is associated with 90% of non-cardia gastric cancer (Herrero et al., 2014; Wang et al., 2018). One study from Japan showed that 2.9% of the H. pylori-infected subjects developed gastric cancer following 7.8 years, whereas none of the non-infected subjects progressed to gastric cancer. Notably, histological findings characterized by severe gastric atrophy, intestinal metaplasia, and corpus-predominant gastritis contribute to the development of gastric cancer (Uemura et al., 2001).
H. pylori infection in the elderly also is associated with several extra-digestive diseases, such as iron-deficiency anemia (IDA), idiopathic thrombocytopenic purpura (ITP), vitamin B12 deficiency (Stasi et al., 2009; Yuan et al., 2010). A meta-analysis suggested that improved anemia and iron status could be attributable to the eradication of H. pylori in patients with IDA, especially in patients with moderate or severe anemia (Yuan et al., 2010). It has been reported that approximately 50% of H. pylori-positive ITP patients showed complete remission following the eradication of H. pylori, with improved platelet counts (Stasi et al., 2009). Vitamin B12 deficiency is common in the elderly, and may be related to gastrectomy, proton pump inhibitors (PPIs), H. pylori infection, and other factors. The eradication of H. pylori can only play an auxiliary role (Carmel et al., 2001). Besides the above-mentioned extra-gastric diseases, H. pylori infection has also been reported to be associated with cardiovascular diseases, Alzheimer’s disease, Parkinson’s disease, stroke, etc. (Liu W. Z. et al., 2018). However, these associations are still inconsistent and need to be further clarified.
Due to a decrease in gastric mucosal barrier function in the elderly, H. pylori eradication is beneficial in remission of the disease and repairing the damaged mucosa (Cui et al., 2016). The incidence rates of chronic atrophic gastritis and intestinal metaplasia are higher in the elderly than in young and middle-aged people (Liu et al., 2019). A retrospective study enrolling 73237 patients confirmed that the cumulative incidence rate of gastric cancer decreased apparently after H. pylori eradication and the benefits were more marked in the elderly (Leung et al., 2018). Higher proportion of older people are often on acetyl salicylic acid (aspirin) and/or nonsteroidal anti-inflammatory drugs (NSAIDs) against cardiovascular disease and/or gout, and the clinical benefits are obvious by H. pylori eradication (Hernandez-Diaz and Garcia, 2006). Due to deteriorating physical condition, serious comorbidities and renal dysfunction, elderly patients are more likely to experience adverse drug reactions. Thus, a comprehensive risk-benefit assessment and individualized treatment should be performed to eradicate H. pylori in the elderly (Norgard et al., 2009; Liu W. Z. et al., 2018). In this paper, we compiled studies of different treatment regimens for H. pylori eradication in the elderly (Table 1).
The standard triple therapy containing a PPI and two antibiotics is widely accepted since the first Maastricht Consensus (Malfertheiner et al., 1997). Three early randomized controlled trials have shown that the standard triple regimen (pantoprazole/rabeprazole, amoxicillin and clarithromycin) had effective H. pylori eradication rates [intention-to-treat (ITT): 79.0%-85.7%; per-protocol (PP): 82.8%-94.0%] in the elderly aged over 60 (Pilotto et al., 2000a; Pilotto et al., 2001; Zullo et al., 2005). The efficacies of clarithromycin 250mg twice daily and 5-day regimen were comparable to 500mg twice daily and 7-day regimen, respectively, but the lower dose of clarithromycin 250mg twice daily and 5-day regimen minimized adverse events and costs (Pilotto et al., 2001; Zullo et al., 2005). However, pantoprazole-based triple therapy was less effective in the prevention of gastroduodenal injury in H. pylori-positive elderly patients taking NSAIDs than pantoprazole monotherapy for one month (Pilotto et al., 2000a). Multiple studies (Chen et al., 2014; Heo et al., 2014; Chuah et al., 2016; Durazzo et al., 2021; Ang et al., 2022; Park et al., 2012; Tai et al., 2015; Tsujimae et al., 2016; Zhou et al., 2016; Kobayashi et al., 2019; Kim J. L. et al., 2020) on H. pylori eradication have compared elderly individuals to those of non-elderly population, then from which we observe that the increase with age does not affect efficacy of some PPI-based triple therapies (involving amoxicillin + clarithromycin/metronidazole/levofloxacin, amoxicillin-clavulanate + levofloxacin) for H. pylori eradication. But from overall presented literature data (Table 1), the efficacy of PPI-based triple therapies in the elderly population remains controversial within the context of the higher resistance rates for antibiotics (e.g., clarithromycin, metronidazole and levofloxacin). Possibly, since differences are found in the antibiotic resistance rates from different regions, the eradication rate of H. pylori with PPI-based triple therapies shows obvious fluctuation (approximately 40%-95%) in the elderly, with the eradication rate lower in Chinese Taiwan and higher in Singapore and Japan (Chen et al., 2014; Heo et al., 2014; Chuah et al., 2016; Durazzo et al., 2021; Ang et al., 2022; Park et al., 2012; Tai et al., 2015; Tsujimae et al., 2016; Kobayashi et al., 2019; Kim J. L. et al., 2020).
Moreover, the eradication rate of clarithromycin resistance-guided (tailored) triple therapy is significantly higher than those of empirical triple plus bismuth therapy and concomitant therapy, and the efficacy of triple therapy containing amoxicillin-clavulanate also is more favorable, both for young and elderly people (Chen et al., 2014; Zhou et al., 2016). Interestingly, these eradication therapies are more effective among the elderly than in the young, when stratifying individuals by age. Clinicians are often reluctant to treat patients with advanced age, possibly due to unfounded concerns about adverse effects. However, Kobayashi S et al. (Kobayashi et al., 2019) showed that there was no significant difference in the eradication rate and the frequency of adverse event among the super-elderly (over age 75), elderly (aged 65-74) and younger groups (under age 65). The vonoprazan, as a new potassium-competitive acid blocker, produces a stronger and more lasting inhibitory effect of intragastric acid secretion than traditional PPIs (Kagami et al., 2016). Ang D et al. (Ang et al., 2022) demonstrated that 7-day vonoprazan-based triple therapy was as effective as 14-day PPI-based triple therapy, and old age did not influence the efficacies. Interestingly, Tsujimae M et al. (Tsujimae et al., 2016) further confirmed that the efficacy of vonoprazan-based triple regimen was superior to PPI-based triple regimen in patients younger than 70 upon the same timing of administration but both of regimens were as effective in patients aged over 70.
Due to the increase of antimicrobial resistance globally, the bismuth quadruple therapy has been recommended as the empiric primary treatment regimen in “Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection (Liu W. Z. et al., 2018)”. Tetracycline with the low resistance rate may exhibit higher eradication rate. A study from Italy showed tetracycline-containing bismuth quadruple therapy had an excellent H. pylori eradication rate of more than 90% in the elderly (Dore et al., 2006). Another study has shown that 14-day triple plus bismuth therapy was as well effective as triple plus berberine therapy for both the elderly and the young. Similarly, old age did not influence the efficacies (Zhang et al., 2017). But the study by Zhou L et al. showed that the eradication rate of 10-day triple plus bismuth therapy was less efficient than concomitant therapy (77.1% vs 93.5%) for the elderly in a setting with higher rates of resistance to clarithromycin and metronidazole (Zhou et al., 2016). Thus, clarithromycin resistance-guided bismuth quadruple therapy (tailored therapy) could be a good alternative to improve eradication efficacy compared to empiric treatment (Gao and Fan, 2022; Kim J. L. et al., 2020). Moreover, given not drug-resistant bismuth, high safety with the short-term application of bismuth, and wide antibiotic selections after eradication failure, bismuth quadruple therapy appears to be more advantageous for first eradication treatment than non-bismuth quadruple therapy (Ford et al., 2008; Dore et al., 2016). In short, the combination of bismuth and the triple therapy improves the eradication rate of H. pylori in the elderly, also dependent of the cure rate of the resistant strain and the resistance rate from endemic areas (Dore et al., 2016). If a high rate of resistance exists, triple plus bismuth therapy cannot achieve the ideal eradication rate, which needs to switch to a non-bismuth regimen or susceptible antibiotic to eradicate H. pylori (Malfertheiner et al., 2012).
Sequential and hybrid therapies may be more effective for the eradication of H. pylori. Several studies have shown that the efficacy of sequential therapy with eradication rate higher than 90% was superior to standard triple therapy in the elderly (Zullo et al., 2005; Park et al., 2012; Chuah et al., 2016). Moreover, hybrid therapy also exhibits similar excellent efficacy to sequential therapy (Oh et al., 2014; Heo et al., 2015). Overall, we observe in these studies that the elderly does not affect the efficacies of sequential and hybrid therapies compared to the non-elderly. The mechanism by which sequential therapy and hybrid therapy exhibit an effective action is that the administration of amoxicillin within the initial 5 or 7 days of treatment may significantly reduce the density and number of resistant bacteria to increase the efficacy of subsequent clarithromycin (Gatta et al., 2009). Furthermore, the resistance to nitroimidazole does not seem to influence the eradication efficacy of non-bismuth quadruple therapy (Zullo et al., 2000; Treiber et al., 2002). However, one study from Korea showed suboptimal efficacy of sequential therapy in the elderly though significantly higher than standard triple therapy, which was probably related to high antibiotics resistance (Lim et al., 2013). The efficacy of sequential therapy may be reduced in the presence of single drug resistance to metronidazole or clarithromycin, so this regimen is less recommended in Chinese adults (Zhou et al., 2014; Liu W. Z. et al., 2018).
Concomitant therapy shows shorter treatment duration and less complex drug administration as compared to sequential therapy and hybrid therapy. Practically, this can make treatment more convenient and improve patient compliance and physician preferences towards healthcare (Hsu et al., 2014). Heo J et al. (Heo et al., 2015) confirmed that concomitant therapy has similar efficacy compared to hybrid therapy in the elderly, and their efficacies did not decrease with the age. When bismuth quadruple therapy is not available, it is recommended to administer concomitant therapy in high-prevalence areas of clarithromycin resistance by the Maastricht IV/Florence Consensus Report (Malfertheiner et al., 2012). Several studies have confirmed that concomitant therapy exhibited excellent H. pylori eradication rates regardless of old age (Heo et al., 2015; Tai et al., 2015; Zhou et al., 2016). And, two studies from Chinese mainland and Chinese Taiwan suggested that the efficacy of concomitant therapy was superior to standard triple therapy and bismuth quadruple therapy in the elderly (Tai et al., 2015; Zhou et al., 2016). However, one study from Korea showed that older patients aged over 70 had a significantly lower eradication rate than patients younger than 70 (45.5% vs 86.6%) (Lim et al., 2013). Considering too few subjects older than 70 years, this result remains questionable. A Randomized controlled trial with larger sample size is needed to investigate the effect of old age on the efficacy of concomitant therapy. When the high prevalence of dual resistance to clarithromycin and metronidazole is anticipated, concomitant therapy is actually double therapy consisted of PPI + amoxicillin and also difficult to eradicate H. pylori (Graham and Dore, 2016; Zhou et al., 2016).
High-dose PPI plus amoxicillin has gained much attention due to the low amoxicillin resistance and simple composition of drug. High-dose dual therapy as salvage therapy offers better efficacy as compared with bismuth quadruple therapy or triple therapy (Miehlke et al., 2006; Yang et al., 2015). Potent inhibitory gastric acid secretion and sufficient amoxicillin dosage may be the effective factors. High-dose dual therapy appears to be equally effective with current mainstream regimens for the eradication of H. pylori based on a meta-analysis (C. P. Gao et al., 2020). However, this effectiveness has rarely been validated in the elderly. Although the daily dose of dual therapy exhibits pronounced increase but it remains within the recommended safe range. Due to the reduced use of antibiotics and bismuth, the incidence of side effects is significantly reduced (Huang et al., 2021b). But this regimen is conducted in only the elderly patients without penicillin allergy and renal insufficiency. Gao W et al. (W. Gao et al., 2020) showed that the eradication rate of high-dose rabeprazole plus amoxicillin was as high as 90.5% and 91.5% in elderly and non-elderly patients with or without multiple complications, respectively. Therefore, high-dose PPI plus amoxicillin may be a more likely alternative regimen for eradication of H. pylori.
Since the researchers give little importance to H. pylori infection in the elderly, few studies for adverse events and/or adherence are available for analysis. Based on the available literature data, the incidence of adverse events with triple therapy in the elderly is approximately 10% or lower, which suggests that the triple therapy for the eradication of H. pylori in elderly patients is safe and well tolerated (Pilotto et al., 2001; Zullo et al., 2005; Kobayashi et al., 2019). Adverse events mainly include diarrhea, abdominal pain, vomiting, urticaria and so on. Diarrhea predominates among them and side effects are rarely a reason for patients to discontinue treatment. Pilotto A et al. (Pilotto et al., 2001) showed that older women were more susceptible to therapeutic side effects than older men. Moreover, Kobayashi S et al. (Kobayashi et al., 2019) indicted that there was no significant difference in the incidence of adverse events among younger, elderly and super-elderly patients. That said, increasing age did not seem to affect the risk of adverse events in terms of triple therapy.
Concerning triple plus bismuth therapy, adverse events are more common among the elderly. The major cause is bismuth-induced black stool, which is not clinically significant. Furthermore, other common adverse events include dysgeusia and diarrhea, and no one withdraws because of severe side effects according to the study by Gao C et al. (Gao and Fan, 2022). Only constipation is more prevalent among elderly patients than younger patients (5.6% vs 1.2%) (Gao and Fan, 2022). Another Italian study suggested that 23.7% of elderly patients receiving tetracycline-containing bismuth quadruple therapy complained of side effects without affecting their daily life, but another 9.7% of patients discontinued treatment due to severe side effects or subjective willingness (Dore et al., 2006). Interestingly, 23.7% of elderly patients reported significant improvements in bowel symptoms such as abdominal bloating and abnormal stool consistency. Regarding high-dose PPI plus amoxicillin therapy, one recent study showed the incidence of adverse events in non-elderly and elderly people was 13.4% and 9.5%, respectively, and 5.2% of elderly people and 7.3% of non-elderly people discontinued treatment due to adverse events (W. Gao et al., 2020). Symptoms such as rash, abdominal pain, and diarrhea were the most common adverse events and disappeared upon stopping treatment. Furthermore, Pea F et al. (Pea, 2015) showed that serious adverse events are most likely to appear in the frail elderly population due to drug-drug interactions and/or comorbidities. But antibiotics were used for the treatment of a broad spectrum of diseases and were not validated for the treatment for H. pylori in their study (Pea, 2015).
The elderly is prone to antibiotic resistance due to previous overuse of antibiotics and long-term accumulation (Nguyen et al., 2019). In the elderly population with chronic respiratory or urinary tract infection, (fluoro)quinolone antibiotics are more commonly prescribed (Tandan et al., 2020). Lyu T et al. (Lyu et al., 2020) showed that middle-aged and elderly patients had higher secondary resistance rates to metronidazole and levofloxacin in southern China. It is suggested to conduct drug susceptibility testing for H. pylori in the elderly population to select appropriate antibiotics. With increasing age, the decrease in liver and kidney functions in the elderly contributes to the development of adverse drug reactions related to the mitigation of drug metabolism. Moreover, the decline in the function of the gastric mucosa defense system in the elderly leads to increased sensitivity to various damages and more noticeable drug reactions. Drug-drug interactions would trigger severe side effects in the elderly with multiple comorbidities (Tomita et al., 2019). Commonly used PPIs (for example, omeprazole) can interact with clopidogrel used in cardiovascular diseases; antibiotics (amoxicillin, clarithromycin, etc) for H. pylori can also interact with cardiovascular medications (such as statins, warfarin, etc) (Abrignani et al., 2021; Anrys et al., 2021). These factors mentioned above may result in elderly individuals not adhering to their dosage regimens. The elderly patients with frequent use of aspirin/NSAIDs or malabsorption of vitamin B12 also are considered candidates for H. pylori eradication (Malfertheiner et al., 2017). An individualized risk-benefit assessment should be undertaken in the elderly with the different physical conditions and benefits of eradication of H. pylori. Patients are subject to alterations of the intestinal flora in a short time due to H. pylori eradication, when necessary, supplementary microbial agents can be administered during or after H. pylori eradication to reduce gastrointestinal adverse effects (Malaty et al., 1999; Oh et al., 2016). More convenient regimens containing fewer drugs can increase medication adherence and reduce adverse reaction in the elderly. The vonoprazan-based or tetracycline-containing bismuth quadruple therapies, antibiotic resistance-guided regimens, and even currently hot high-dose dual therapy provide a new direction for the eradication of H. pylori in the elderly. The effectiveness and practicability of these regimens for the elderly merit further concern and investigation in the future.
As is well known, guidelines or expert consensus presently available on H. pylori infection overlook the management of the elderly population as a special group. We discussed the recent advances of H. pylori in the elderly population via epidemiology, diagnosis and treatment, etc. In summary, the choice of treatment strategies of H. pylori infection should be combined with the individualized characteristics and risk-benefit assessment of the elderly. To decrease the risk of gastric cancer, older patients should be received H. pylori eradication therapy unless with the presence of competing factors. There is an urgent need to explore effective treatment options due to the decreasing efficacy of triple therapy. Once failure treatment occurs in non-bismuth quadruple therapies, the choice of antibiotics will be confined. Thus, given the superiority of bismuth in quadruple therapies, bismuth quadruple therapy may be considered the empirical and preferable treatment regimen for H. pylori eradication in the elderly. If the antibiotic susceptibility testing against H. pylori is performed, the elderly patient should receive susceptibility-based regimens; if there is no susceptibility testing, elderly patients should be treated with the best locally available regimens (if available), and vonoprazan-based or tetracycline-containing bismuth quadruple therapies (if local antibiotic resistance profile is not available). Moreover, the currently hot high-dose dual therapy provides a new direction for the eradication of H. pylori in the future. At the same time, we strongly recommend conventional or high-definition upper endoscopy with a complete biopsy sampling for elderly patients with alarming symptoms (Figure 2). More details of the management of H. pylori infection in the elderly need to be further studied.
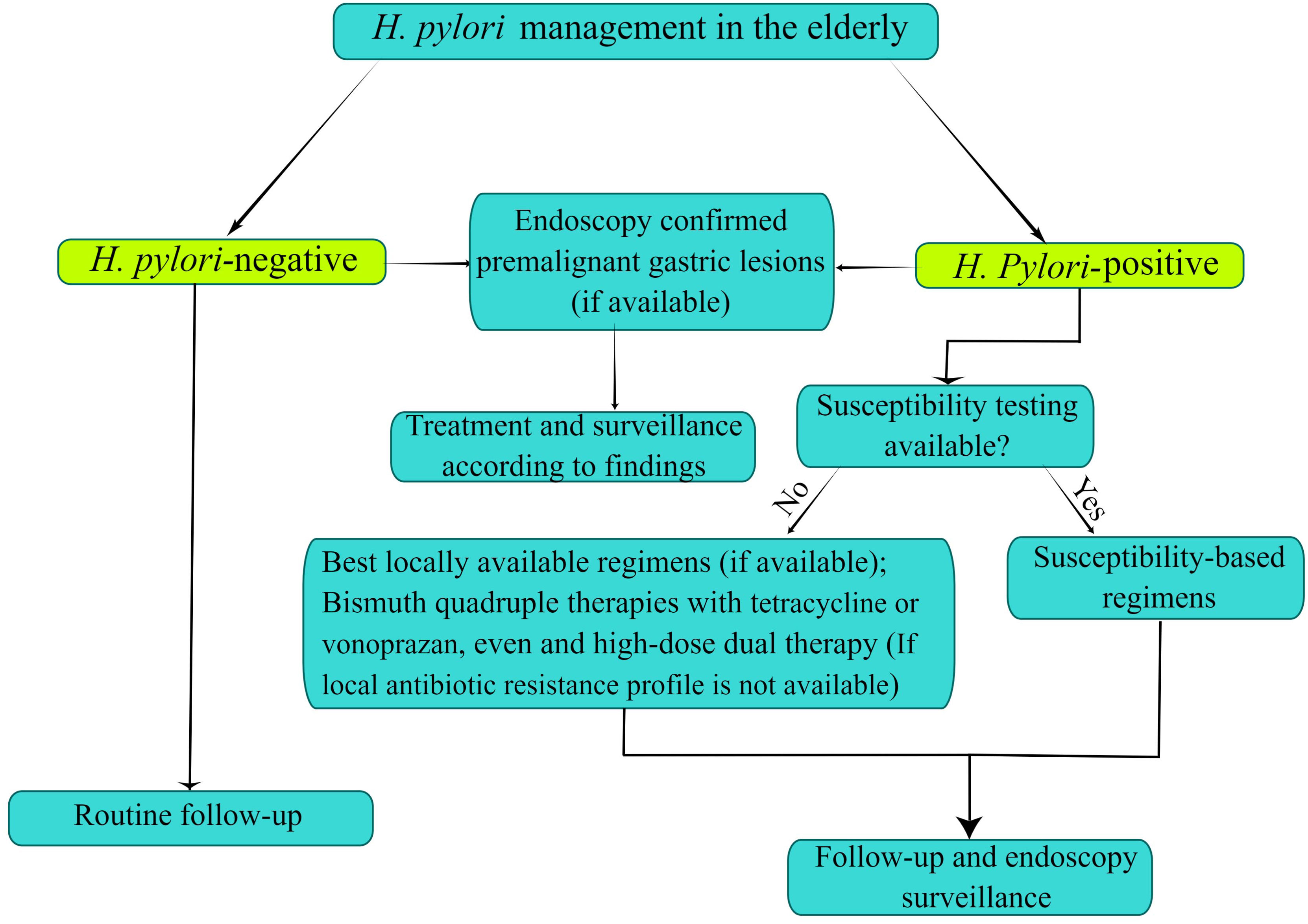
Figure 2 Flowchart of Helicobacter pylori infection control and management in the elderly. The figure is drawn by Figdraw.
HG: Writing-original draft. H-MX: Designing some contents of the manuscript. D-KZ: Writing and revising the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Key Talent Project of Gansu Province (grant number 2022RCXM071); and Key R & D Program of Gansu Province (grant number 20YF8FA078).
We appreciate the contributions of all the doctors, coworkers, and friends involved in this study and thank the editors and reviewers for their help with this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdalla, A. M., Sordillo, E. M., Hanzely, Z., Perez-Perez, G. I., Blaser, M. J., Holt, P. R., et al. (1998). Insensitivity of the clotest for h. pylori, especially in the elderly. Gastroenterology. 115 (1), 243–244.
Abrignani, M. G., Gatta, L., Gabrielli, D., Milazzo, G., De Francesco, V., De Luca, L., et al. (2021). Gastroprotection in patients on antiplatelet and/or anticoagulant therapy: A position paper of national association of hospital cardiologists (anmco) and the italian association of hospital gastroenterologists and endoscopists (aigo). Eur. J. Intern. Med. 85, 1–13. doi: 10.1016/j.ejim.2020.11.014
Alzoubi, H., Al-Mnayyis, A., Al, R. I., Aqel, A., Abu-Lubad, M., Hamdan, O., et al. (2020). The use of (13)c-urea breath test for non-invasive diagnosis of helicobacter pylori infection in comparison to endoscopy and stool antigen test. Diagnostics 10 (7), 448. doi: 10.3390/diagnostics10070448
Ang, D., Koo, S. H., Chan, Y. H., Tan, T. Y., Soon, G. H., Tan, C. K., et al. (2022). Clinical trial: Seven-day vonoprazan- versus 14-day proton pump inhibitor-based triple therapy for first-line helicobacter pylori eradication. Aliment Pharmacol. Ther. 56 (3), 436–449. doi: 10.1111/apt.17070
Anrys, P., Petit, A. E., Thevelin, S., Sallevelt, B., Drenth, C., Soiza, R. L., et al. (2021). An international consensus list of potentially clinically significant drug-drug interactions in older people. J. Am. Med. Dir Assoc. 22 (10), 2121–2133. doi: 10.1016/j.jamda.2021.03.019
Atli, T., Sahin, S., Arslan, B. U., Varli, M., Yalcin, A. E., Aras, S. (2012). Comparison of the c14 urea breath test and histopathology in the diagnosis of helicobacter pylori in the elderly. J. Pak. Med. Assoc. 62 (10), 1061–1065.
Batts, K. P., Ketover, S., Kakar, S., Krasinskas, A. M., Mitchell, K. A., Wilcox, R., et al. (2013). Appropriate use of special stains for identifying helicobacter pylori: Recommendations from the rodger c. haggitt gastrointestinal pathology society. Am. J. Surg. Pathol. 37 (11), e12–e22. doi: 10.1097/PAS.0000000000000097
Best, L. M., Takwoingi, Y., Siddique, S., Selladurai, A., Gandhi, A., Low, B., et al. (2018). Non-invasive diagnostic tests for helicobacter pylori infection. Cochrane Database Syst. Rev. 3, D12080. doi: 10.1002/14651858.CD012080.pub2
Bures, J., Kopacova, M., Koupil, I., Seifert, B., Skodova, F. M., Spirkova, J., et al. (2012). Significant decrease in prevalence of helicobacter pylori in the czech republic. World J. Gastroenterol. 18 (32), 4412–4418. doi: 10.3748/wjg.v18.i32.4412
Carmel, R., Aurangzeb, I., Qian, D. (2001). Associations of food-cobalamin malabsorption with ethnic origin, age, helicobacter pylori infection, and serum markers of gastritis. Am. J. Gastroenterol. 96 (1), 63–70. doi: 10.1111/j.1572-0241.2001.03453.x
Chang, M. C., Chang, Y. T., Sun, C. T., Wu, M. S., Wang, H. P., Lin, J. T. (2002). Quantitative correlation of helicobacter pylori stool antigen (hpsa) test with 13c-urea breath test (13c-ubt) by the updated sydney grading system of gastritis. Hepatogastroenterology. 49 (44), 576–579.
Chen, M. C., Lei, W. Y., Lin, J. S., Yi, C. H., Wu, D. C., Hu, C. T. (2014). Levofloxacin-amoxicillin/clavulanate-rabeprazole versus a standard seven-day triple therapy for eradication of helicobacter pylori infection. BioMed. Res. Int. 2014, 158520. doi: 10.1155/2014/158520
Choi, J., Kim, C. H., Kim, D., Chung, S. J., Song, J. H., Kang, J. M., et al. (2011). Prospective evaluation of a new stool antigen test for the detection of helicobacter pylori, in comparison with histology, rapid urease test, (13)c-urea breath test, and serology. J. Gastroenterol. Hepatol. 26 (6), 1053–1059. doi: 10.1111/j.1440-1746.2011.06705.x
Chuah, S. K., Liang, C. M., Lee, C. H., Chiou, S. S., Chiu, Y. C., Hu, M. L., et al. (2016). A randomized control trial comparing 2 levofloxacin-containing second-line therapies for helicobacter pylori eradication. Med. (Baltimore) 95 (19), e3586. doi: 10.1097/MD.0000000000003586
Cizginer, S., Ordulu, Z., Kadayifci, A. (2014). Approach to helicobacter pylori infection in geriatric population. World J. Gastroint Pharmacol. Ther. 5 (3), 139–147. doi: 10.4292/wjgpt.v5.i3.139
Cui, Y., Sun, Z., Li, X., Leng, C., Zhang, L., Fu, X., et al. (2016). Expression and clinical significance of cyclooxygenase-2 and interleukin-32 in primary gastric b-cell lymphoma. Oncol. Lett. 11 (1), 693–698. doi: 10.3892/ol.2015.3950
Dore, M. P., Lu, H., Graham, D. Y. (2016). Role of bismuth in improving helicobacter pylori eradication with triple therapy. Gut. 65 (5), 870–878. doi: 10.1136/gutjnl-2015-311019
Dore, M. P., Maragkoudakis, E., Pironti, A., Tadeu, V., Tedde, R., Realdi, G., et al. (2006). Twice-a-day quadruple therapy for eradication of helicobacter pylori in the elderly. Helicobacter. 11 (1), 52–55. doi: 10.1111/j.0083-8703.2006.00370.x
Durazzo, M., Ferro, A., Fagoonee, S., Staiano, M. T., Saracco, G. M., Pellicano, R. (2021). Helicobacter pylori eradication with a clarithromycin-based triple therapy in elderly patients. Panminerva Med. 63 (3), 332–335. doi: 10.23736/S0031-0808.21.04500-6
Eisdorfer, I., Shalev, V., Goren, S., Chodick, G., Muhsen, K. (2018). Sex differences in urea breath test results for the diagnosis of helicobacter pylori infection: A large cross-sectional study. Biol. Sex Differ. 9 (1), 1. doi: 10.1186/s13293-017-0161-7
Ford, A. C., Malfertheiner, P., Giguere, M., Santana, J., Khan, M., Moayyedi, P. (2008). Adverse events with bismuth salts for helicobacter pylori eradication: Systematic review and meta-analysis. World J. Gastroenterol. 14 (48), 7361–7370. doi: 10.3748/wjg.14.7361
Gao, C., Fan, Y. H. (2022). Effect and safety of helicobacter pylori eradication treatment based on molecular pathologic antibiotic resistance in chinese elderly people. Infect. Drug Resist. 15, 3277–3286. doi: 10.2147/IDR.S371113
Gao, W., Ye, H., Deng, X., Wang, C., Xu, Y., Li, Y., et al. (2020). Rabeprazole-amoxicillin dual therapy as first-line treatment for h pylori eradication in special patients: A retrospective, real-life study. Helicobacter. 25 (5), e12717. doi: 10.1111/hel.12717
Gao, C. P., Zhang, D., Zhang, T., Wang, J. X., Han, S. X., Graham, D. Y., et al. (2020). Ppi-amoxicillin dual therapy for helicobacter pylori infection: An update based on a systematic review and meta-analysis. Helicobacter. 25 (4), e12692. doi: 10.1111/hel.12692
Gatta, L., Vakil, N., Leandro, G., Di Mario, F., Vaira, D. (2009). Sequential therapy or triple therapy for helicobacter pylori infection: Systematic review and meta-analysis of randomized controlled trials in adults and children. Am. J. Gastroenterol. 104 (12), 3069–3079,1080. doi: 10.1038/ajg.2009.555
Gisbert, J. P., Calvet, X., Cosme, A., Almela, P., Feu, F., Bory, F., et al. (2012). Long-term follow-up of 1,000 patients cured of helicobacter pylori infection following an episode of peptic ulcer bleeding. Am. J. Gastroenterol. 107 (8), 1197–1204. doi: 10.1038/ajg.2012.132
Gisbert, J. P., Pajares, J. M. (2005). 13c-urea breath test in the management of helicobacterpylori infection. Dig Liver Dis. 37 (12), 899–906. doi: 10.1016/j.dld.2005.09.006
Godbole, G., Megraud, F., Bessede, E. (2020). Review: diagnosis of helicobacter pylori infection. Helicobacter. 25 Suppl 1, e12735. doi: 10.1111/hel.12735
Gong, Y. N., Li, Y. M., Yang, N. M., Li, H. Z., Guo, F., Lin, L., et al. (2015). Centralized isolation of helicobacter pylori from multiple centers and transport condition influences. World J. Gastroenterol. 21 (3), 944–952. doi: 10.3748/wjg.v21.i3.944
Graham, D. Y., Dore, M. P. (2016). Helicobacter pylori therapy: A paradigm shift. Expert Rev. Anti Infect. Ther. 14 (6), 577–585. doi: 10.1080/14787210.2016.1178065
Han, Y., Dai, W., Meng, F., Gan, X., Liu, M., Deng, X., et al. (2020). Diagnosis of helicobacter pylori infection in the elderly using an immunochromatographic assay-based stool antigen test. Microbiologyopen. 9 (9), e1102. doi: 10.1002/mbo3.1102
Heo, J., Jeon, S. W., Jung, J. T., Kwon, J. G., Kim, E. Y., Lee, D. W., et al. (2014). A randomised clinical trial of 10-day concomitant therapy and standard triple therapy for helicobacter pylori eradication. Dig Liver Dis. 46 (11), 980–984. doi: 10.1016/j.dld.2014.07.018
Heo, J., Jeon, S. W., Jung, J. T., Kwon, J. G., Lee, D. W., Kim, H. S., et al. (2015). Concomitant and hybrid therapy for helicobacter pylori infection: A randomized clinical trial. J. Gastroenterol. Hepatol. 30 (9), 1361–1366. doi: 10.1111/jgh.12983
Hernandez-Diaz, S., Garcia, R. L. (2006). Cardioprotective aspirin users and their excess risk of upper gastrointestinal complications. BMC Med. 4, 22. doi: 10.1186/1741-7015-4-22
Herrero, R., Park, J. Y., Forman, D. (2014). The fight against gastric cancer - the iarc working group report. Best Pract. Res. Clin. Gastroenterol. 28 (6), 1107–1114. doi: 10.1016/j.bpg.2014.10.003
Hsu, W. H., Wang, S. S., Kuo, C. H., Chen, C. Y., Chang, C. W., Hu, H. M., et al. (2010). Dual specimens increase the diagnostic accuracy and reduce the reaction duration of rapid urease test. World J. Gastroenterol. 16 (23), 2926–2930. doi: 10.3748/wjg.v16.i23.2926
Hsu, P. I., Wu, D. C., Chen, W. C., Tseng, H. H., Yu, H. C., Wang, H. M., et al. (2014). Randomized controlled trial comparing 7-day triple, 10-day sequential, and 7-day concomitant therapies for helicobacter pylori infection. Antimicrob. Agents Chemother. 58 (10), 5936–5942. doi: 10.1128/AAC.02922-14
Huang, Q., Jia, X., Chu, Y., Zhang, X., Ye, H. (2021a). Helicobacter pylori infection in geriatric patients: Current situation and treatment regimens. Front. Med. 8. doi: 10.3389/fmed.2021.713908
Huang, Q., Shi, Z., Cheng, H., Ye, H., Zhang, X. (2021b). Efficacy and safety of modified dual therapy as the first-line regimen for the treatment of helicobacter pylori infection: A meta-analysis of randomized controlled trials. J. Clin. Gastroenterol. 55 (10), 856–864. doi: 10.1097/MCG.0000000000001448
Inelmen, E. M., Gasparini, G., Sergi, G., Enzi, G. (2005). Evaluation of helicobacter pylori with a stool antigen assay in frail, elderly patients. Scand. J. Gastroenterol. 40 (7), 794–799. doi: 10.1080/00365520510015638
Inelmen, E. M., Maccari, T., Enzi, G., Gasparini, G., Fuson, F., Davanzo, B., et al. (2004). Helicobacter pylori infection diagnosis in hospitalised elderly patients: The stool antigen test (hpsa) in comparison with other methods. Aging Clin. Exp. Res. 16 (5), 349–355. doi: 10.1007/BF03324563
Ji, Z., Han, F., Meng, F., Tu, M., Yang, N., Zhang, J. (2016). The association of age and antibiotic resistance of helicobacter pylori: A study in jiaxing city, zhejiang province, china. Med. (Baltimore) 95 (8), e2831. doi: 10.1097/MD.0000000000002831
Kagami, T., Sahara, S., Ichikawa, H., Uotani, T., Yamade, M., Sugimoto, M., et al. (2016). Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to cyp2c19 genotype. Aliment Pharmacol. Ther. 43 (10), 1048–1059. doi: 10.1111/apt.13588
Kim, J. L., Cho, S. J., Chung, S. J., Lee, A., Choi, J., Chung, H., et al. (2020). Empiric versus clarithromycin resistance-guided therapy for helicobacter pylori based on polymerase chain reaction results in patients with gastric neoplasms or gastric mucosa-associated lymphoid tissue lymphoma: A randomized controlled trial. Clin. Transl. Gastroenterol. 11 (9), e194. doi: 10.14309/ctg.0000000000000194
Kim, S. E., Memon, A., Kim, B. Y., Jeon, H., Lee, W. K., Kang, S. C. (2020). Gastroprotective effect of phytoncide extract from pinus koraiensis pinecone in helicobacter pylori infection. Sci. Rep. 10 (1), 9547. doi: 10.1038/s41598-020-66603-8
Kobayashi, S., Joshita, S., Yamamoto, C., Yanagisawa, T., Miyazawa, T., Miyazawa, M., et al. (2019). Efficacy and safety of eradication therapy for elderly patients with helicobacter pylori infection. Med. (Baltimore) 98 (30), e16619. doi: 10.1097/MD.0000000000016619
Kocsmar, E., Szirtes, I., Kramer, Z., Szijarto, A., Bene, L., Buzas, G. M., et al. (2017). Sensitivity of helicobacter pylori detection by giemsa staining is poor in comparison with immunohistochemistry and fluorescent in situ hybridization and strongly depends on inflammatory activity. Helicobacter. 22 (4), e12387. doi: 10.1111/hel.12387
Konstantopoulos, N., Russmann, H., Tasch, C., Sauerwald, T., Demmelmair, H., Autenrieth, I., et al. (2001). Evaluation of the helicobacter pylori stool antigen test (hpsa) for detection of helicobacter pylori infection in children. Am. J. Gastroenterol. 96 (3), 677–683. doi: 10.1111/j.1572-0241.2001.03603.x
Koyama, T., Kuriyama, N., Ozaki, E., Matsui, D., Watanabe, I., Miyatani, F., et al. (2016). Serum albumin to globulin ratio is related to cognitive decline via reflection of homeostasis: A nested case-control study. BMC Neurol. 16 (1), 253. doi: 10.1186/s12883-016-0776-z
Leszczynska, K., Namiot, A., Namiot, Z., Leszczynska, J. K., Jakoniuk, P., Chilewicz, M., et al. (2010). Patient factors affecting culture of helicobacter pylori isolated from gastric mucosal specimens. Adv. Med. Sci. 55 (2), 161–166. doi: 10.2478/v10039-010-0028-1
Leung, W. K., Wong, I., Cheung, K. S., Yeung, K. F., Chan, E. W., Wong, A., et al. (2018). Effects of helicobacter pylori treatment on incidence of gastric cancer in older individuals. Gastroenterology. 155 (1), 67–75. doi: 10.1053/j.gastro.2018.03.028
Lim, J. H., Lee, D. H., Choi, C., Lee, S. T., Kim, N., Jeong, S. H., et al. (2013). Clinical outcomes of two-week sequential and concomitant therapies for helicobacter pylori eradication: A randomized pilot study. Helicobacter. 18 (3), 180–186. doi: 10.1111/hel.12034
Liu, L., Lang, J., Jin, Y., Chen, Y., Chang, W., Yao, Y., et al. (2019). The value of pepsinogen in gc screening: A systematic review and meta-analysis. Gastroenterol. Res. Pract. 2019, 7087232. doi: 10.1155/2019/7087232
Liu, H. Y., Lu, Y. F., Chen, W. J. (1995). Predictive equations for basal metabolic rate in chinese adults: A cross-validation study. J. Am. Diet Assoc. 95 (12), 1403–1408. doi: 10.1016/S0002-8223(95)00369-X
Liu, D. S., Wang, Y. H., Zeng, Z. R., Zhang, Z. Y., Lu, H., Xu, J. M., et al. (2018). Primary antibiotic resistance of helicobacter pylori in chinese patients: A multiregion prospective 7-year study. Clin. Microbiol. Infect. 24 (7), 780–785. doi: 10.1016/j.cmi.2017.11.010
Liu, W. Z., Xie, Y., Lu, H., Cheng, H., Zeng, Z. R., Zhou, L. Y., et al. (2018). Fifth chinese national consensus report on the management of helicobacter pylori infection. Helicobacter. 23 (2), e12475. doi: 10.1111/hel.12475
Lyu, T., Cheung, K. S., Ni, L., Guo, J., Mu, P., Li, Y., et al. (2020). High prevalence and risk factors of multiple antibiotic resistance in patients who fail first-line helicobacter pylori therapy in southern china: A municipality-wide, multicentre, prospective cohort study. J. Antimicrob. Chemother. 75 (11), 3391–3394. doi: 10.1093/jac/dkaa315
Malaty, H. M., Graham, D. Y., Wattigney, W. A., Srinivasan, S. R., Osato, M., Berenson, G. S. (1999). Natural history of helicobacter pylori infection in childhood: 12-year follow-up cohort study in a biracial community. Clin. Infect. Dis. 28 (2), 279–282. doi: 10.1086/515105
Malfertheiner, P., Megraud, F., O'Morain, C., Bell, D., Bianchi, P. G., Deltenre, M., et al(1997). Current european concepts in the management of helicobacter pylori infection--the maastricht consensus report. The european helicobacter pylori study group (EHPSG). Eur J Gastroenterol Hepatol 9 (1), 1–2. doi: 10.1097/00042737-199701000-00002
Malfertheiner, P., Megraud, F., O'Morain, C. A., Atherton, J., Axon, A. T., Bazzoli, F., et al. (2012). Management of helicobacter pylori infection–the maastricht iv/ florence consensus report. Gut. 61 (5), 646–664. doi: 10.1136/gutjnl-2012-302084
Malfertheiner, P., Megraud, F., O'Morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2017). Management of helicobacter pylori infection-the maastricht v/florence consensus report. Gut. 66 (1), 6–30. doi: 10.1136/gutjnl-2016-312288
Malfertheiner, P., Megraud, F., Rokkas, T., Gisbert, J. P., Liou, J. M., Schulz, C., et al. (2022). Management of helicobacter pylori infection: The maastricht vi/florence consensus report. Gut. doi: 10.1136/gutjnl-2022-327745
Marrero, R. R., Cunningham, S. A., Mandrekar, J. N., Polo, E. T., Patel, R. (2021). Clinical evaluation of a real-time pcr assay for simultaneous detection of helicobacter pylori and genotypic markers of clarithromycin resistance directly from stool. J. Clin. Microbiol. 59 (5), e03040–20. doi: 10.1128/JCM.03040-20
Matsuhisa, T., Yamaoka, Y., Uchida, T., Duger, D., Adiyasuren, B., Khasag, O., et al. (2015). Gastric mucosa in mongolian and japanese patients with gastric cancer and helicobacter pylori infection. World J. Gastroenterol. 21 (27), 8408–8417. doi: 10.3748/wjg.v21.i27.8408
Miehlke, S., Hansky, K., Schneider-Brachert, W., Kirsch, C., Morgner, A., Madisch, A., et al. (2006). Randomized trial of rifabutin-based triple therapy and high-dose dual therapy for rescue treatment of helicobacter pylori resistant to both metronidazole and clarithromycin. Aliment Pharmacol. Ther. 24 (2), 395–403. doi: 10.1111/j.1365-2036.2006.02993.x
Nagy, P., Johansson, S., Molloy-Bland, M. (2016). Systematic review of time trends in the prevalence of helicobacter pylori infection in china and the usa. Gut Pathog. 8, 8. doi: 10.1186/s13099-016-0091-7
Nguyen, C. T., Davis, K. A., Nisly, S. A., Li, J. (2019). Treatment of helicobacter pylori in special patient populations. Pharmacotherapy. 39 (10), 1012–1022. doi: 10.1002/phar.2318
Norgard, N. B., Mathews, K. D., Wall, G. C. (2009). Drug-drug interaction between clopidogrel and the proton pump inhibitors. Ann. Pharmacother 43 (7), 1266–1274. doi: 10.1345/aph.1M051
Nurgalieva, Z. Z., Malaty, H. M., Graham, D. Y., Almuchambetova, R., Machmudova, A., Kapsultanova, D., et al. (2002). Helicobacter pylori infection in kazakhstan: Effect of water source and household hygiene. Am. J. Trop. Med. Hyg. 67 (2), 201–206. doi: 10.4269/ajtmh.2002.67.201
Ofman, J. J., Etchason, J., Alexander, W., Stevens, B. R., Herrin, J., Cangialose, C., et al. (2000). The quality of care for medicare patients with peptic ulcer disease. Am. J. Gastroenterol. 95 (1), 106–113. doi: 10.1111/j.1572-0241.2000.01514.x
Oh, B., Kim, J. W., Kim, B. S. (2016). Changes in the functional potential of the gut microbiome following probiotic supplementation during helicobacter pylori treatment. Helicobacter. 21 (6), 493–503. doi: 10.1111/hel.12306
Oh, D. H., Lee, D. H., Kang, K. K., Park, Y. S., Shin, C. M., Kim, N., et al. (2014). Efficacy of hybrid therapy as first-line regimen for helicobacter pylori infection compared with sequential therapy. J. Gastroenterol. Hepatol. 29 (6), 1171–1176. doi: 10.1111/jgh.12518
Park, H. G., Jung, M. K., Jung, J. T., Kwon, J. G., Kim, E. Y., Seo, H. E., et al. (2012). Randomised clinical trial: A comparative study of 10-day sequential therapy with 7-day standard triple therapy for helicobacter pylori infection in naive patients. Aliment Pharmacol. Ther. 35 (1), 56–65. doi: 10.1111/j.1365-2036.2011.04902.x
Pea, F. (2015). Antimicrobial treatment of bacterial infections in frail elderly patients: The difficult balance between efficacy, safety and tolerability. Curr. Opin. Pharmacol. 24, 18–22. doi: 10.1016/j.coph.2015.06.006
Perets, T. T., Gingold-Belfer, R., Leibovitzh, H., Itskoviz, D., Schmilovitz-Weiss, H., Snir, Y., et al. (2019). Optimization of (13) c-urea breath test threshold levels for the detection of helicobacter pylori infection in a national referral laboratory. J. Clin. Lab. Anal. 33 (2), e22674. doi: 10.1002/jcla.22674
Pilott, A., Fabrello, R., Franceschi, M., Scagnelli, M., Soffiati, F., Di Mario, F., et al. (1996). Helicobacter pylori infection in asymptomatic elderly subjects living at home or in a nursing home: Effects on gastric function and nutritional status. Age Ageing 25 (3), 245–249. doi: 10.1093/ageing/25.3.245
Pilotto, A. (2001). Helicobacter pylori-associated peptic ulcer disease in older patients: Current management strategies. Drugs Aging 18 (7), 487–494. doi: 10.2165/00002512-200118070-00002
Pilotto, A., Di Mario, F., Franceschi, M., Leandro, G., Battaglia, G., Germana, B., et al. (2000a). Pantoprazole versus one-week helicobacter pylori eradication therapy for the prevention of acute nsaid-related gastroduodenal damage in elderly subjects. Aliment Pharmacol. Ther. 14 (8), 1077–1082. doi: 10.1046/j.1365-2036.2000.00804.x
Pilotto, A., Di Mario, F., Franceschi, M., Leandro, G., Soffiati, G., Scagnelli, M., et al. (1996). Cure of helicobacter pylori infection in the elderly: Effects of eradication on gastritis and serological markers. Aliment Pharmacol. Ther. 10 (6), 1021–1027. doi: 10.1046/j.1365-2036.1996.88260000.x
Pilotto, A., Franceschi, M. (2014). Helicobacter pylori infection in older people. World J. Gastroenterol. 20 (21), 6364–6373. doi: 10.3748/wjg.v20.i21.6364
Pilotto, A., Franceschi, M., Leandro, G., Bozzola, L., Rassu, M., Soffiati, G., et al. (2001). Cure of helicobacter pylori infection in elderly patients: Comparison of low versus high doses of clarithromycin in combination with amoxicillin and pantoprazole. Aliment Pharmacol. Ther. 15 (7), 1031–1036. doi: 10.1046/j.1365-2036.2001.01008.x
Pilotto, A., Franceschi, M., Leandro, G., Rassu, M., Zagari, R. M., Bozzola, L., et al. (2000b). Noninvasive diagnosis of helicobacter pylori infection in older subjects: Comparison of the 13c-urea breath test with serology. J. Gerontol A Biol. Sci. Med. Sci. 55 (3), M163–M167. doi: 10.1093/gerona/55.3.m163
Pilotto, A., Maggi, S., Noale, M., Franceschi, M., Parisi, G., Crepaldi, G. (2011). Association of upper gastrointestinal symptoms with functional and clinical charateristics in elderly. World J. Gastroenterol. 17 (25), 3020–3026. doi: 10.3748/wjg.v17.i25.3020
Regev, A., Fraser, G. M., Braun, M., Maoz, E., Leibovici, L., Niv, Y. (1999). Seroprevalence of helicobacter pylori and length of stay in a nursing home. Helicobacter. 4 (2), 89–93. doi: 10.1046/j.1523-5378.1999.98640.x
Roberts, S. B., Dallal, G. E. (2005). Energy requirements and aging. Public Health Nutr. 8 (7A), 1028–1036. doi: 10.1079/phn2005794
Rogge, J. D., Wagner, D. R., Carrico, R. J., Glowinski, E. A., Mahoney, S. J., Boguslaski, R. C., et al. (1995). Evaluation of a new urease reagent strip for detection of helicobacter pylori in gastric biopsy specimens. Am. J. Gastroenterol. 90 (11), 1965–1968.
Rojas-Rengifo, D. F., Mendoza, B., Jaramillo, C., Rodriguez-Urrego, P. A., Vera-Chamorro, J. F., Alvarez, J., et al. (2019). Helicobacter pylori culture as a key tool for diagnosis in colombia. J. Infect. Dev. Ctries 13 (8), 720–726. doi: 10.3855/jidc.10720
Roll, J., Weng, A., Newman, J. (1997). Diagnosis and treatment of helicobacter pylori infection among california medicare patients. Arch. Intern. Med. 157 (9), 994–998. doi: 10.1001/archinte.1997.00440300102008
Salles-Montaudon, N., Dertheil, S., Broutet, N., Gras, N., Monteiro, L., De Mascarel, A., et al. (2002). Detecting helicobacter pylori infection in hospitalized frail older patients: The challenge. J. Am. Geriatr Soc 50 (10), 1674–1680. doi: 10.1046/j.1532-5415.2002.50459.x
Stasi, R., Sarpatwari, A., Segal, J. B., Osborn, J., Evangelista, M. L., Cooper, N., et al. (2009). Effects of eradication of helicobacter pylori infection in patients with immune thrombocytopenic purpura: A systematic review. Blood. 113 (6), 1231–1240. doi: 10.1182/blood-2008-07-167155
Suerbaum, S., Michetti, P. (2002). Helicobacter pylori infection. N Engl. J. Med. 347 (15), 1175–1186. doi: 10.1056/NEJMra020542
Sugano, K., Tack, J., Kuipers, E. J., Graham, D. Y., El-Omar, E. M., Miura, S., et al. (2015). Kyoto Global consensus report on helicobacter pylori gastritis. Gut. 64 (9), 1353–1367. doi: 10.1136/gutjnl-2015-309252
Suzuki, H., Moayyedi, P. (2013). Helicobacter pylori infection in functional dyspepsia. Nat. Rev. Gastroenterol. Hepatol. 10 (3), 168–174. doi: 10.1038/nrgastro.2013.9
Tai, W. C., Liang, C. M., Lee, C. H., Chiu, C. H., Hu, M. L., Lu, L. S., et al. (2015). Seven-day nonbismuth containing quadruple therapy could achieve a grade "a" success rate for first-line helicobacter pylori eradication. BioMed. Res. Int. 2015, 623732. doi: 10.1155/2015/623732
Tandan, M., Zimmerman, S., Sloane, P. D., Ward, K., Daniels, L. M., Wretman, C. J. (2020). Which nursing home residents with pneumonia are managed on-site and which are hospitalized? results from 2 years' surveillance in 14 us homes. J. Am. Med. Dir Assoc. 21 (12), 1862–1868. doi: 10.1016/j.jamda.2020.07.028
Tomita, N., Mizokami, F., Kisara, S., Arai, H. (2019). Transdisciplinary approach for potentially harmful drug-drug interaction detection as a part of a comprehensive medication review and geriatric assessment. Geriatr Gerontol Int. 19 (5), 462–463. doi: 10.1111/ggi.13638
Tonkic, A., Tonkic, M., Lehours, P., Megraud, F. (2012). Epidemiology and diagnosis of helicobacter pylori infection. Helicobacter. 17 Suppl 1, 1–8. doi: 10.1111/j.1523-5378.2012.00975.x
Toyokawa, T., Suwaki, K., Miyake, Y., Nakatsu, M., Ando, M. (2010). Eradication of helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: A long-term prospective cohort study. J. Gastroenterol. Hepatol. 25 (3), 544–547. doi: 10.1111/j.1440-1746.2009.05995.x
Toyoshima, O., Nishizawa, T., Arita, M., Kataoka, Y., Sakitani, K., Yoshida, S., et al. (2018). Helicobacter pylori infection in subjects negative for high titer serum antibody. World J. Gastroenterol. 24 (13), 1419–1428. doi: 10.3748/wjg.v24.i13.1419
Treiber, G., Wittig, J., Ammon, S., Walker, S., van Doorn, L. J., Klotz, U. (2002). Clinical outcome and influencing factors of a new short-term quadruple therapy for helicobacter pylori eradication: A randomized controlled trial (maclor study). Arch. Intern. Med. 162 (2), 153–160. doi: 10.1001/archinte.162.2.153
Tsujimae, M., Yamashita, H., Hashimura, H., Kano, C., Shimoyama, K., Kanamori, A., et al. (2016). A comparative study of a new class of gastric acid suppressant agent named vonoparazan versus esomeprazole for the eradication of helicobacter pylori. Digestion. 94 (4), 240–246. doi: 10.1159/000454762
Uemura, N., Okamoto, S., Yamamoto, S., Matsumura, N., Yamaguchi, S., Yamakido, M., et al. (2001). Helicobacter pylori infection and the development of gastric cancer. N Engl. J. Med. 345 (11), 784–789. doi: 10.1056/NEJMoa001999
Uotani, T., Graham, D. Y. (2015). Diagnosis of helicobacter pylori using the rapid urease test. Ann. Transl. Med. 3 (1), 9. doi: 10.3978/j.issn.2305-5839.2014.12.04
Vonkeman, H. E., Deleest, H., van Delaar, M., Vanbaarlen, J., Steen, K., Lems, W., et al. (2012). Assessment of helicobacter pylori eradication in patients on nsaid treatment. BMC Gastroenterol. 12, 133. doi: 10.1186/1471-230X-12-133
Wang, Y. K., Kuo, F. C., Liu, C. J., Wu, M. C., Shih, H. Y., Wang, S. S., et al. (2015). Diagnosis of helicobacter pylori infection: Current options and developments. World J. Gastroenterol. 21 (40), 11221–11235. doi: 10.3748/wjg.v21.i40.11221
Wang, Z., Lv, Z., Ding, H., Xu, Q., Sun, L., Jing, J., et al. (2018). Role of serum ebv-vca igg detection in assessing gastric cancer risk and prognosis in northern chinese population. Cancer Med. 7 (11), 5760–5774. doi: 10.1002/cam4.1792
Wang, X. I., Zhang, S., Abreo, F., Thomas, J. (2010). The role of routine immunohistochemistry for helicobacter pylori in gastric biopsy. Ann. Diagn Pathol. 14 (4), 256–259. doi: 10.1016/j.anndiagpath.2010.05.002
Weck, M. N., Brenner, H. (2006). Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol. Biomarkers Prev. 15 (6), 1083–1094. doi: 10.1158/1055-9965.EPI-05-0931
Weck, M. N., Stegmaier, C., Rothenbacher, D., Brenner, H. (2007). Epidemiology of chronic atrophic gastritis: Population-based study among 9444 older adults from germany. Aliment Pharmacol. Ther. 26 (6), 879–887. doi: 10.1111/j.1365-2036.2007.03430.x
Xie, S., Wang, S., Xue, L., Middleton, D., Guan, C., Hao, C., et al. (2020). Helicobacter pylori is associated with precancerous and cancerous lesions of the gastric cardia mucosa: Results of a large population-based study in china. Front. Oncol. 10. doi: 10.3389/fonc.2020.00205
Yang, J. C., Lin, C. J., Wang, H. L., Chen, J. D., Kao, J. Y., Shun, C. T., et al. (2015). High-dose dual therapy is superior to standard first-line or rescue therapy for helicobacter pylori infection. Clin. Gastroenterol. Hepatol. 13 (5), 895–905. doi: 10.1016/j.cgh.2014.10.036
Yilmaz, O., Sen, N., Kupelioglu, A. A., Simsek, I. (2006). Detection of h. pylori infection by elisa and western blot techniques and evaluation of anti caga seropositivity in adult turkish dyspeptic patients. World J. Gastroenterol. 12 (33), 5375–5378. doi: 10.3748/wjg.v12.i33.5375
Yuan, W., Li, Y., Yang, K., Ma, B., Guan, Q., Wang, D., et al. (2010). Iron deficiency anemia in helicobacter pylori infection: Meta-analysis of randomized controlled trials. Scand. J. Gastroenterol. 45 (6), 665–676. doi: 10.3109/00365521003663670
Zendehdel, A., Roham, M. (2020). Role of helicobacter pylori infection in the manifestation of old age-related diseases. Mol. Genet. Genomic Med. 8 (4), e1157. doi: 10.1002/mgg3.1157
Zeng, Z., Zhang, X., Li, D., Li, J., Yuan, J., Gu, L., et al. (2020). Expression, location, clinical implication, and bioinformatics analysis of rnaset2 in gastric adenocarcinoma. Front. Oncol. 10. doi: 10.3389/fonc.2020.00836
Zhang, D., Ke, L., Ni, Z., Chen, Y., Zhang, L. H., Zhu, S. H., et al. (2017). Berberine containing quadruple therapy for initial helicobacter pylori eradication: An open-label randomized phase iv trial. Medicine 96 (32), e7697. doi: 10.1097/MD.0000000000007697
Zhang, M., Tang, Z., Zhou, Y. Z., Cai, L., Tang, X. (2005). [seroepidemiology study on helicobacter pylori (caga, vaca) infection of elderly people in beijing]. Zhonghua Liu Xing Bing Xue Za Zhi 26 (9), 687–689.
Zhao, Q., Chi, T. (2022). Deep learning model can improve the diagnosis rate of endoscopic chronic atrophic gastritis: A prospective cohort study. BMC Gastroenterol. 22 (1), 133. doi: 10.1186/s12876-022-02212-1
Zhao, X., Li, X., Ma, Y., Zhu, Y. (2019). The design of a homocysteine fluorescent probe based on rhodamine b and its responsiveness in the serum of cerebral infarction patients. Exp. Ther. Med. 18 (4), 2675–2680. doi: 10.3892/etm.2019.7833
Zhou, L., Zhang, J., Chen, M., Hou, X., Li, Z., Song, Z., et al. (2014). A comparative study of sequential therapy and standard triple therapy for helicobacter pylori infection: A randomized multicenter trial. Am. J. Gastroenterol. 109 (4), 535–541. doi: 10.1038/ajg.2014.26
Zhou, L., Zhang, J., Song, Z., He, L., Li, Y., Qian, J., et al. (2016). Tailored versus triple plus bismuth or concomitant therapy as initial helicobacter pylori treatment: A randomized trial. Helicobacter. 21 (2), 91–99. doi: 10.1111/hel.12242
Zhu, H. M., Li, B. Y., Tang, Z., She, J., Liang, X. Y., Dong, L. K., et al. (2020). Epidemiological investigation of helicobacter pylori infection in elderly people in beijing. World J. Clin. Cases 8 (11), 2173–2180. doi: 10.12998/wjcc.v8.i11.2173
Zullo, A., Gatta, L., De Francesco, V., Hassan, C., Ricci, C., Bernabucci, V., et al. (2005). High rate of helicobacter pylori eradication with sequential therapy in elderly patients with peptic ulcer: A prospective controlled study. Aliment Pharmacol. Ther. 21 (12), 1419–1424. doi: 10.1111/j.1365-2036.2005.02519.x
Keywords: Helicobacter pylori, eradication therapy, elderly, side effect, drug resistance
Citation: Gong H, Xu H-M and Zhang D-K (2023) Focusing on Helicobacter pylori infection in the elderly. Front. Cell. Infect. Microbiol. 13:1121947. doi: 10.3389/fcimb.2023.1121947
Received: 12 December 2022; Accepted: 28 February 2023;
Published: 10 March 2023.
Edited by:
Bangshun He, Nanjing Medical University, ChinaReviewed by:
Abbas Yadegar, Shahid Beheshti University of Medical Sciences, IranCopyright © 2023 Gong, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Kui Zhang, emhhbmdkazg2MTZAMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.