- 1Department of Infectious Diseases, Children’s Hospital of Fudan University, Shanghai, China
- 2Department of Clinical Laboratory, Children’s Hospital of Fudan University, Shanghai, China
- 3Department of Infectious Diseases, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 4Department of Clinical Laboratory, Children’s Hospital of Chongqing Medical University, Chongqing, China
- 5Department of Infectious Diseases, Children’s Hospital of Zhejiang University School of Medicine, Hangzhou, China
- 6Department of Infectious Diseases, Qilu Children’s Hospital of Shandong University, Jinan, China
- 7Department of Infectious Diseases, Shenzhen Children’s Hospital, Shenzhen, China
- 8Department of Infectious Diseases, Shanghai Children’s Medical Center of Shanghai Jiaotong University School of Medicine, Shanghai, China
- 9Department of Infectious Diseases, Xi’an Children’s Hospital, Xi’an, China
- 10Department of Clinical Laboratory, Xi’an Children’s Hospital, Xi’an, China
- 11Department of Pediatric Infectious Diseases, Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
- 12Department of Clinical Laboratory, Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
- 13Department of Gastroenterology and Infectious Diseases, Children’s Hospital of Shanghai Jiaotong University School of Medicine, Shanghai, China
- 14Department of Pediatrics, Bethune First Hospital of Jilin University, Changchun, China
- 15Department of Infectious Diseases, Kaifeng Children’s Hospital, Kaifeng, China
Introduction: Methicillin-resistant Staphylococcus aureus (MRSA) poses a serious threat to public health worldwide. In December 2015, the Infectious Disease Surveillance of Pediatrics (ISPED) program was organized to monitor bacterial epidemiology and resistance trends in children.
Methods: This retrospective study was conducted from January 2016–December 2021 on patients at eleven ISPED-group hospitals.
Results: From 2016–2021, a total of 13024 MRSA isolates were obtained from children. The most common age group for patients with MRSA infection was less than 3 years old, and newborns were an important group affected by MRSA infection. MRSA was most commonly isolated from the lower respiratory, an abscess, a secretion, or blood in neonates and from the lower respiratory, an abscess, or the upper respiratory in non-neonates. All isolates were susceptible to vancomycin and linezolid and resistant to penicillin; additionally, 76.88%, 54.97%, 22.30%, 5.67%, 5.14%, 3.63%, and 1.42% were resistant to erythromycin, clindamycin, tetracycline, levofloxacin, sulfamethoxazole-trimethoprim (TMP-SMX), gentamicin, and rifampin, respectively. Between 2016 and 2021, a significant increase was seen in the levofloxacin- and TMP-SMX-resistance rates (from 5.45% to 7.14% and from 4.67% to 6.50%, respectively) among MRSA isolates, along with a significant decrease in the rates of resistance to erythromycin (from 82.61% to 68.08%), clindamycin (from 60.95% to 46.82%), tetracycline (from 25.37% to 17.13%), gentamicin (from 4.53% to 2.82%), and rifampin (from 1.89% to 0.41%).
Discussion: The antibiotic-resistance rates varied among MRSA isolated from different sources. Because of the high antibiotic resistance rate to clindamycin, this antibiotic is not recommended for empirical treatment of MRSA infections, especially in osteomyelitis.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA), which was first identified in 1961, poses a serious threat to public health worldwide owing to its significant resistance to antibiotics. Among community-associated S. aureus infections in children, 63.6% were found to be due to MRSA (Wang et al., 2017). Previous studies in children found that MRSA was responsible for 45.8%–75.6% of S. aureus pneumonia cases (Doudoulakakis et al., 2017; Song et al., 2017), 44% of S. aureus bacteraemia cases (Kumarachandran et al., 2017), and 32.8% of cases with an S. aureus central nervous system infection (Vallejo et al., 2017). Recently, in a study of bloodstream infections in China, the percentage of cases due to MRSA in non-intensive care unit (ICU) patients increased significantly from 8.4% in 1998–2002 to 68.3% in 2013–2017 (Tian et al., 2019).
MRSA can cause invasive, life-threatening systemic infections, e.g., in severe sepsis and necrotizing pneumonia, which are particularly problematic in children (Liu et al., 2011; Lim et al., 2014). The high antibiotic resistance of MRSA is very concerning because it can lead to treatment failure in clinical practice. Currently, MRSA infections remain prevalent and account for significant morbidity and mortality worldwide. MRSA infections in children have been associated with a longer duration of bacteraemia, longer length of hospital stay, higher likelihood of complications, and greater mortality rate compared with methicillin-sensitive S. aureus (MSSA) infections (Burke et al., 2009; Hamdy et al., 2019).
Given the heavy burden associated with MRSA infections, there is an urgent need to understand the distribution and antimicrobial susceptibilities of MRSA. Here, to investigate the profiles of MRSA infection and MRSA drug resistance in children, we compared the distribution and antimicrobial susceptibilities of MRSA isolates in cases from eleven hospitals within the Infectious Diseases Surveillance of Pediatrics (ISPED) group of China over a six-year period (2016–2021).
Patients and methods
Surveillance population
This retrospective study was conducted across eleven hospitals within the ISPED group of China from January 2016 to December 2021. We reviewed the medical records of patients who were younger than 18 years and had any clinical culture that yielded an isolate of MRSA. We collected the following data from the patient medical records: sex, age, infection site, and antibiotic resistance profile. Our analysis included a total of 13024 MRSA clinical isolates obtained from the following eleven hospitals: Children’s Hospital of Chongqing Medical University, Children’s Hospital of Fudan University, Children’s Hospital of Zhejiang University School of Medicine, Qilu Children’s Hospital of Shandong University, Shenzhen Children’s Hospital, Shanghai Children’s Medical Center of Shanghai Jiaotong University School of Medicine, Xi’an Children’s Hospital, Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University, Children’s Hospital of Shanghai Jiaotong University School of Medicine, Bethune First Hospital of Jilin University, and Kaifeng Children’s Hospital.
Antimicrobial susceptibility testing (AST)
The antibiotic susceptibility testing was performed in each participating site. Antimicrobial susceptibility testing (AST) was performed using the Kirby-Bauer method or automated systems. AST was conducted for penicillin, oxacillin, erythromycin, clindamycin, levofloxacin, sulfamethoxazole-trimethoprim (TMP-SMX), gentamicin, rifampin, and minocycline. MRSA were identified based on their resistance to oxacillin. The standard strains ATCC 25922, ATCC 29213, and ATCC 29212 were used as quality-control strains for the antimicrobial susceptibility tests. The AST breakpoint criteria of the Clinical and Laboratory Standards Institute (CLSI) were adopted (Clinical and Laboratory Standards Institute, 2019).
Statistical analysis
Statistical significance was calculated by applying the χ2 test, or the Fisher’s exact test in the case of small sample sizes, using the SPSS (Version 20) statistics program. Statistical significance in this study was defined as a P-value of < 0.05.
Results
Distribution of MRSA
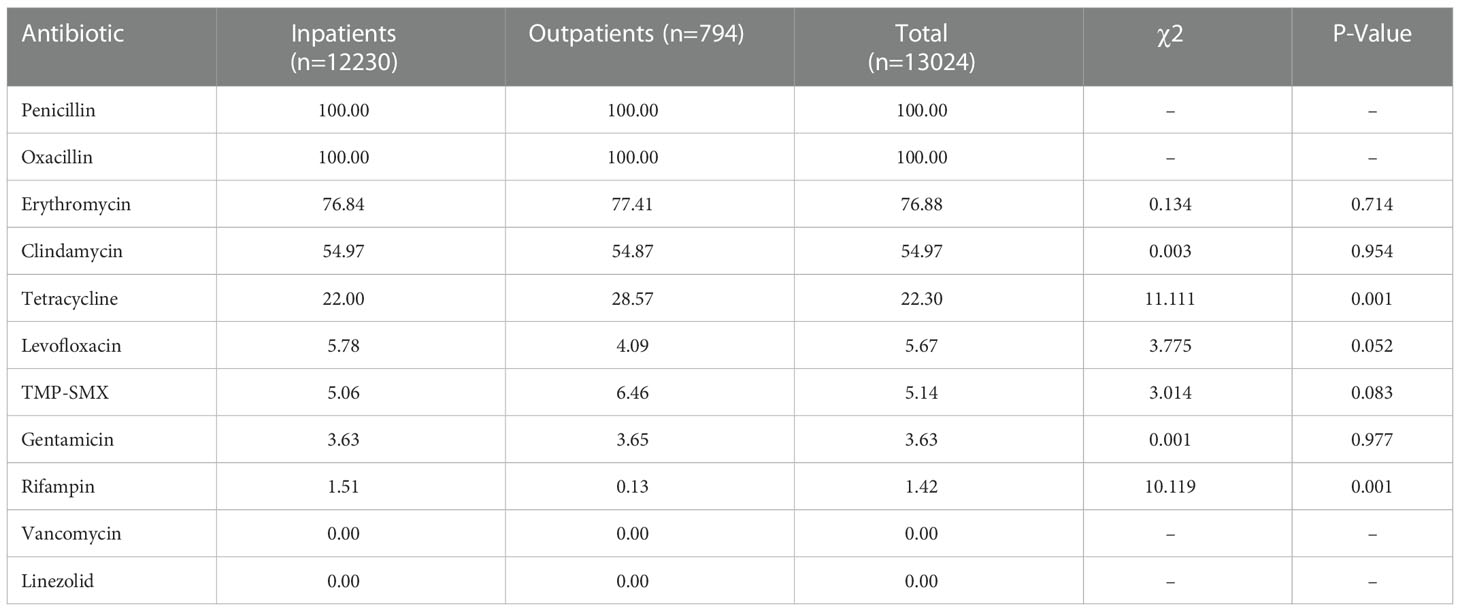
From 2016 to 2021, the yearly total number of S. aureus isolates obtained ranged from 4231 to 8561. During these years, the respective proportions of S. aureus isolates that were MRSA isolates were 31.50%, 36.80%, 34.10%, 34.41%, 35.00%, and 32.31%. A total of 13024 MRSA isolates, of which 6.10% (794) were collected from outpatients and 93.90% (12230) were collected from inpatients, were obtained from children in this study. The distribution of MRSA in inpatients was distinct from that in outpatients; MRSA isolates were detected much more commonly from inpatients than from outpatients (34.85% vs. 25.56%, χ2 = 109.564, P = 0.000).
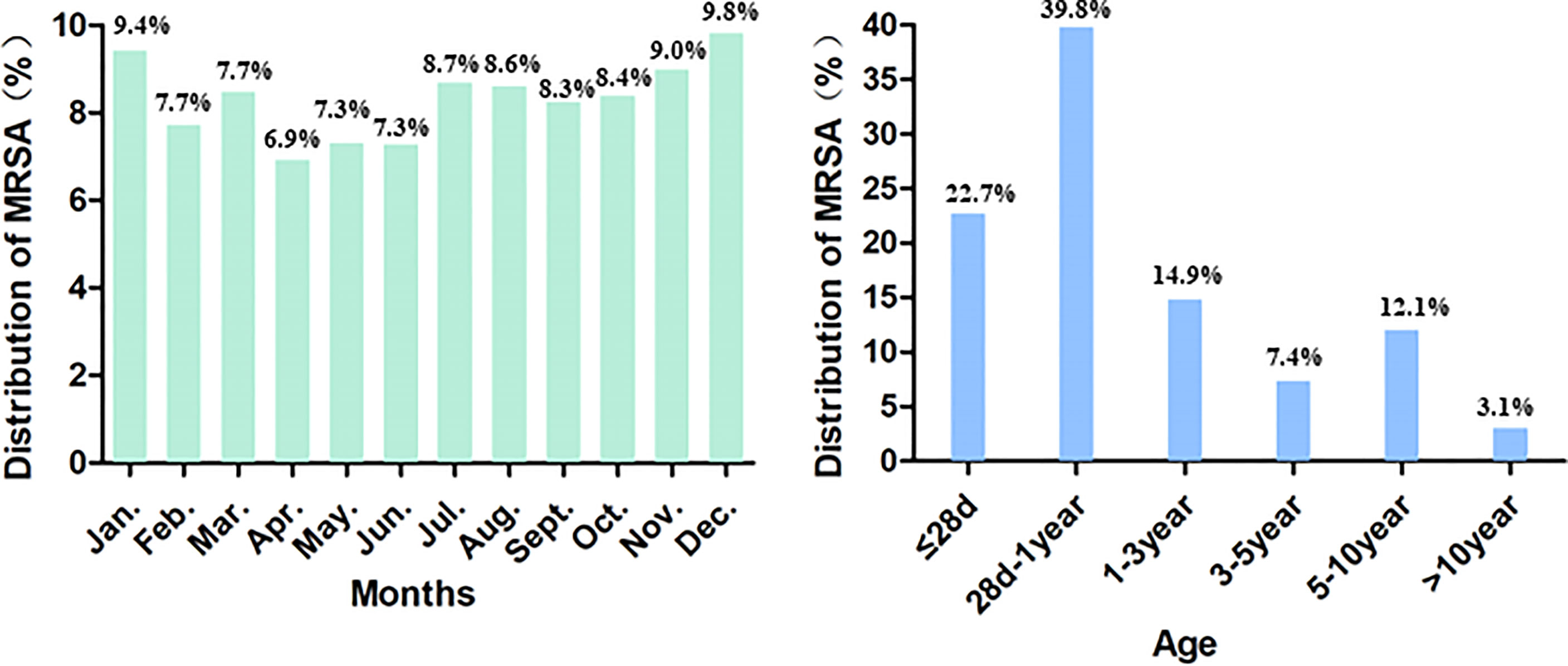
Among the included 13024 cases, 7763 (59.61%) of the patients were male. The median patient age was 5 months (range: 1 day to 17 years), and 2902 (22.73%) of the patients were newborn infants (aged ≤28 days). MRSA cases were detected much more commonly during the months of January, November, and December (Figure 1).
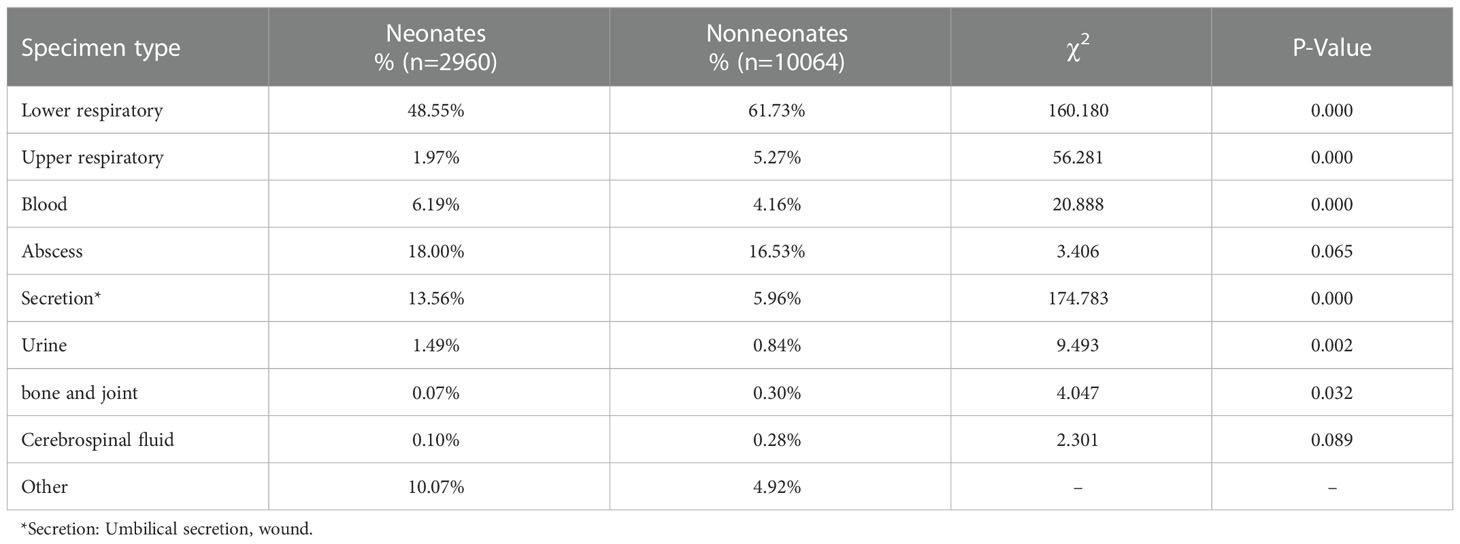
MRSA was most commonly isolated from the lower respiratory (58.28%), followed by from an abscess (16.78%), a secretion (7.61%), blood (4.64%), the upper respiratory (4.45%), urine (0.98%), a bone or joint (0.27%), the central nervous system (0.24%), and other sources. Notably, compared with those in non-neonates, the constituent proportions of MRSA isolates obtained from blood, secretions, and urine were higher, while those obtained from respiratory sites and bone or joint sites were lower, in neonates (Table 1).
Bacterial identification and AST
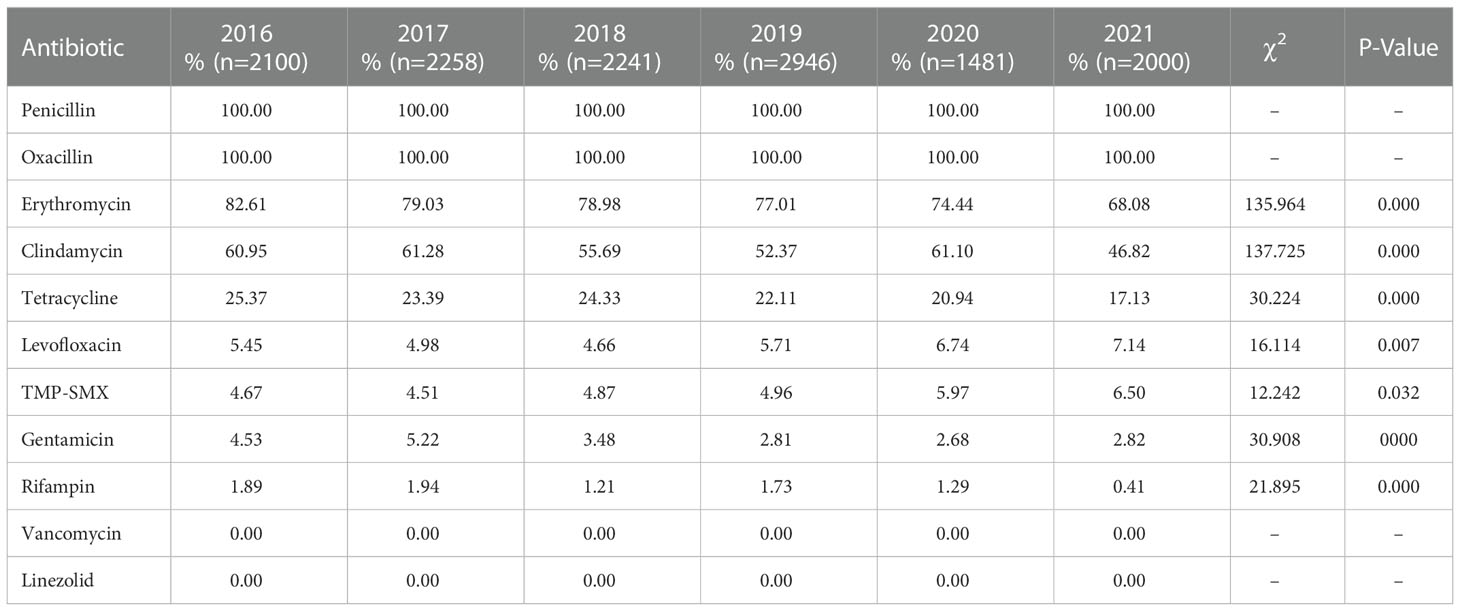
The antimicrobial resistance profiles of the 13024 MRSA isolates from this study are provided in Table 2 and Table 3. All study isolates were susceptible to vancomycin and linezolid and were resistant to penicillin; additionally, 76.88% of the study isolates were resistant to erythromycin, 54.97% to clindamycin, 22.3% to tetracycline, 5.67% to levofloxacin, 5.14% to TMP-SMX, 3.63% to gentamicin, and 1.42% to rifampin. The rifampin-resistance rate of MRSA isolates derived from inpatients was significantly higher than those of MRSA isolates derived from outpatients (1.51% vs. 0.13%, χ2 = 10.119, P = 0.001), while tetracycline-resistance rate of inpatients-derived MRSA isolates was lower than that of outpatients-derived strains (22.00% vs. 28.57%, χ2 = 11.111, P = 0.001). Changes in the antibiotic resistance rates among the MRSA isolates from 2016 to 2021 were observed, with an increase in the rates of resistance to levofloxacin (from 5.45% to 7.14%) and TMP-SMX (from 4.67% to 6.50%) and a decrease in the rates of resistance to erythromycin (from 82.61% to 68.08%), clindamycin (from 60.95% to 46.82%), tetracycline (from 25.37% to 17.13%), gentamicin (from 4.53% to 2.82%), and rifampin (from 1.89% to 0.41%) (Table 3, Figure 2).
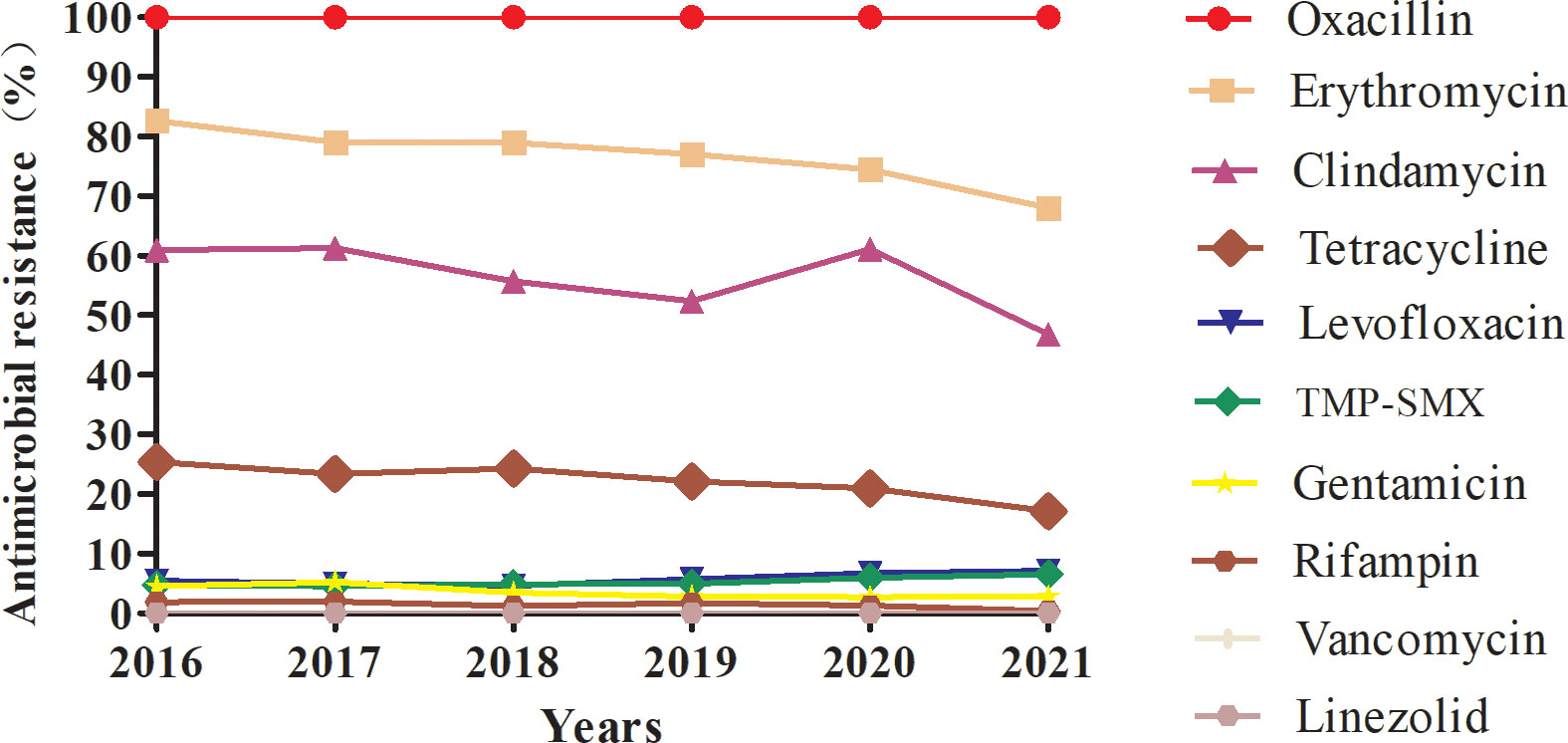
Figure 2 Profile of resistance to ten main antimicrobials (%) of MRSA isolates collected from 2016 to 2021.
The activity of the tested antimicrobials against the 13024 MRSA isolates obtained from different infection sites is summarized in Table 4. The erythromycin-resistance rates of blood-derived (79.62%) and abscess-derived (79.29%) MRSA isolates were significantly higher than that of urine-derived isolates (70.87%; χ2 = 4.645 and 5.089, P = 0.031 and 0.024, respectively). The clindamycin-resistance rates of bone and joint-derived (72.73%) and urine-derived (63.49%) MRSA isolates were significantly higher than that of lower respiratory-derived isolates (54.61%; χ2 = 4.351 and 3.942, P = 0.037 and 0.047, respectively). The tetracycline-resistance rate of bone and joint-derived MRSA isolates was significantly higher than those of MRSA isolates derived from other sources (50.00% vs. 20.42%–31.82%, χ2 = 63.809, P = 0.000). The levofloxacin-resistance rate of blood-derived MRSA isolates (8.07%) was significantly higher than those of abscess-derived (4.48%) and secretion-derived (5.15%) isolates (χ2 = 11.548 and 5.098, P = 0.001 and 0.024, respectively). The TMP-SMX-resistance rates of secretion-derived (6.15%) and blood-derived (6.12%) MRSA isolates were significantly higher than that of upper respiratory-derived isolates (3.50%; χ2 = 5.144 and 4.352, P = 0.023 and 0.037, respectively).
Discussion
We observed the antimicrobial resistance trends of MRSA isolates obtained from children participating in ISPED over the six-year period from 2016 to 2021. Despite the reduction in the proportion of MRSA among S. aureus infections that was noted over the recent 10-year span (Kramer et al., 2019), the incidence of MRSA has remained high in some areas (Takemura and Mochizuki, 2018), and MRSA remains a predominant cause of infection. The proportions of MRSA among S. aureus infections in adults, according to the China Antimicrobial Surveillance Network (CHINET) report, decreased from 69.94% in 2005 to 31.00% in 2020 (Hu et al., 2016; Hu et al., 2017; Hu et al., 2018; Hu et al., 2020a; Hu et al., 2020b; Hu et al., 2021). In contrast, the proportions of MRSA among paediatric S. aureus infections found in our study remained in the range of 31.50%–36.80% over the past six years. Here, MRSA cases were detected much more commonly in the months of January, November, and December, during which the weather in China becomes colder. The seasonal nature of MRSA infections varied based on specimen source, with wound infections more prevalent in warmer months, and respiratory infections more prevalent during colder months (Delorme et al., 2017; Marcelin et al., 2017). The most common age group of patients with MRSA infection is <5 years old (Perovic et al., 2015; Ilczyszyn et al., 2016). In the present study, the median patient age was only 5 months old. The most common age stage of patients participating in our study was 28 d–1 year (39.8%), and 77.4% of study participants were aged less than 3 years old, which is younger compared with the participants studied in other reports (Milstone et al., 2010). In addition, 22.73% of the patients in our study were newborn infants, and newborns are also an important group affected by MRSA infections.
MRSA is a particularly threatening pathogen in children because it causes infections of multiple organ systems. Previous research has found that the most common sites of MRSA infection are the respiratory tract (36.3%–48.0%), followed by the blood (15%–35.7%), wound or intravenous sites (25.2%–44%), and the urinary tract (2.8%–28%), while the least common source of MRSA isolates is puncture fluid (containing hydrothorax, ascites, pericardial fluid, cerebrospinal fluid, or articular cavity fluid; 21.32%) (Rahimi and Shokoohizadeh, 2016; Huang et al., 2019; Samadi et al., 2019). MRSA is an important cause of bloodstream infections (BSI), and in 2009–2017, the MRSA detection rate was 40%–50% among BSI-associated S. aureus (Zhang et al., 2022). Additionally, S. aureus was reported to be the main pathogen (67.5%) in paediatric osteomyelitis, and the proportion of MRSA among the cases due to S. aureus was 44% (Chen et al., 2021). In the present work, the most common clinical sources of MRSA infection were the respiratory tract (62.73%), followed by an abscess (16.78%), a secretion (7.61%), and blood (4.64%), and the constituent proportions isolated from blood, secretions, and urine were higher in neonates compared with those in non-neonates. An increasing incidence of MRSA infections was observed among neonates (Dolapo et al., 2014). Because immunologically immature children are more susceptible to MRSA infection, neonates are more likely to develop MRSA BSIs compared with non-neonates. In neonates with MRSA infection, the proportion of umbilical infection is high, and BSI may be associated with umbilical cord infection. Therefore, it is important to monitor the MRSA epidemiology and resistance trends in neonates.
MRSA antibiotic resistance has been reported in many countries. The significant antibiotic resistance of MRSA is a particular concern because it can lead to treatment failure in clinical practice. Several studies of MRSA antimicrobial susceptibility have revealed their high rates of resistance to erythromycin, clindamycin, levofloxacin, and ciprofloxacin (Decousser et al., 2018; Liang et al., 2019). Similarly, among MRSA isolates from paediatric patients, the highest percentage of isolates was resistant to erythromycin (62%), followed by those resistant to clindamycin (14%–57%), TMP-SMX (3%–24%), gentamicin (24%), rifampicin (12%), or minocycline (10%), while all isolates were susceptible to both vancomycin and linezolid (Milstone et al., 2010; Miguel et al., 2019). In the CHINET surveillance report of MRSA resistance trends between 2005 and 2020, decreases were observed in the percentages of isolates resistant to clindamycin (from 90.1% to 58.6%), erythromycin (from 92.7% to 78.9%), levofloxacin (from 83.3% to 32.6%), gentamicin (from 77.3% to 20.7%), rifampin (from 34.9% to 8.2%), and TMP-SMX (from 36.3% to 6.4%) (Hu et al., 2016; Hu et al., 2019; Hu et al., 2020; Hu et al., 2021). In the present study, our MRSA isolates had high rates of resistance to erythromycin and clindamycin but low rates of resistance to levofloxacin, TMP-SMX, gentamicin, and rifampin, and all these isolates were susceptible to vancomycin and linezolid. Changes in the antibiotic-resistance profile of MRSA isolates from 2016 to 2021 were noted, with an increase in the levofloxacin- and TMP-SMX-resistance rates (from 5.45% to 7.14% and from 4.67% to 6.50%, respectively), and a decrease in the erythromycin-, tetracycline-, gentamicin-, and rifampin-resistance rates. The decreased rates of resistance to these antibiotics may be related to their decreased use in recent years. In our monitoring, the clindamycin-resistance rate remained at 50%–60% from 2016 to 2020, and although this rate decreased to 47% in 2021, it remained close to 50%.
MRSA isolates obtained from different clinical sources may exhibit different levels of antimicrobial susceptibility. A study in Chicago suggested that MRSA isolates obtained from the blood are more likely to be drug resistant compared with MRSA skin and soft tissue isolates (Acree et al., 2017). Liang et al. reported that the rates of gentamicin and ciprofloxacin resistance were significantly higher in MRSA isolates derived from the respiratory tract than in those isolated from the skin and soft tissue or the blood (Liang et al., 2019). Resistance to antibiotics was prevalent among MRSA isolates cultured from patients with ocular infections at US centres, with 72.7% of isolates resistant to fluoroquinolones and 92.9% resistant to azithromycin (Asbell et al., 2020). We found that the erythromycin-resistance rates of blood- and abscess-derived MRSA isolates were higher than that of MRSA isolates obtained from other clinical sources. Additionally, the rates of clindamycin and tetracycline resistance among bone and joint-derived MRSA isolates were significantly higher than those obtained from other clinical sources.
An understanding of the distribution and antimicrobial susceptibilities of MRSA strains will be crucial for guiding antibiotic treatment in MRSA cases. Presently, vancomycin, linezolid, and tigecycline are the most active agents against MRSA. Based on our monitoring results, erythromycin is not recommended for treating respiratory infections, bacteraemia, or abscesses in children. Intravenous vancomycin is recommended for the treatment of children with invasive MRSA infections. It has been reported that MRSA strains outside of China have retained high susceptibility to clindamycin (Aglua et al., 2018). Greenberg evaluated the effectiveness of clindamycin in infants. They found that 76% of the infants who had MRSA bacteraemia cleared the infection after clindamycin treatment (Greenberg et al., 2020). Clindamycin should be considered for inclusion in the initial antibiotic regimen for treating osteomyelitis and septic arthritis because patients whose initial antibiotic regimens included vancomycin had a longer hospitalization compared with those initiated on a treatment regimen of clindamycin without vancomycin (Weiss et al., 2020). However, we found a particularly high rate of clindamycin resistance among bone and joint-derived MRSA isolates (72.73%), and clindamycin is not suitable to be recommended for the empirical treatment of osteomyelitis. Because of the low number of bone and joint-derived isolates in our study, it needs for more studies. The use of antibiotic treatment is a particular concern owing to the limitation that some antibiotic classes are not suitable for use among neonates, e.g., TMP-SMX, rifampin, gentamicin. Vancomycin and linezolid are recommended for use in treating MRSA infections in neonates, and clindamycin may be considered for the treatment of patients with susceptible isolates who have non-invasive MRSA infections. Although the levofloxacin-resistance rate was low compared to other antibiotics, levofloxacin is not recommended for use in children due to serious side effect.
Conclusions
The most common age group of patients with MRSA infection is less than 3 years old, and newborns are an important group affected by MRSA infections. MRSA isolates had high rates of resistance to erythromycin and clindamycin but low rates of resistance to levofloxacin, TMP-SMX, gentamicin, and rifampin. No isolate was found to be resistant to vancomycin or linezolid. Changes in the antibiotic resistance rates among the MRSA isolates obtained from 2016 to 2021 were observed, with an increase in the levofloxacin- and TMP-SMX-resistance rates and a decrease in the erythromycin-, clindamycin-, tetracycline-, gentamicin-, and rifampin-resistance rates. The clindamycin-resistance rate of bone and joint-derived MRSA isolates was higher than that of isolates derived from other clinical sources. In areas where clindamycin-resistant MRSA strains are a concern, empirical vancomycin therapy is suggested for the treatment of paediatric osteomyelitis, and clindamycin is not recommended for the treatment of osteomyelitis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by the Ethics Committee of the Children’s Hospital of Fudan University (No. (2020)321). The need for Informed Consent was waived by the Ethics Committee of the Children’s Hospital of Fudan University due to the retrospective nature of the study
Author contributions
All authors have contributed to the manuscript. XW designed and wrote the draft. XW, CW, LH, HX, CJ, YinC, JD, AL, HD, HC, YipC, JY, TZ, QC, JH and YH collected and analyzed the data, and helped with the data interpretation. HY reviewed the manuscript for its intellectual content and revised the entire work. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Key Development Program of Children’s Hospital of Fudan University, grant number EK2022ZX05.
Acknowledgments
We would like to thank all investigators of the Group of Infectious Diseases Surveillance of Paediatrics (ISPED) of China who have participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acree, M. E., Morgan, E., David, M. Z. (2017). S. aureus infections in Chicago 2006-2014: Increase in CA MSSA and decrease in MRSA incidence. Infect. Control Hosp Epidemiol. 38, 1226–1234. doi: 10.1017/ice.2017.177
Aglua, I., Jaworski, J., Drekore, J., Urakoko, B., Poka, H., Michael, A., et al. (2018). Methicillin-resistant staphylococcus aureus in Melanesian children with haematogenous osteomyelitis from the central highlands of Papua new Guinea. Int. J. Pediatr. 6, 8361–8370. doi: 10.22038/ijp.2018.31279.2763
Asbell, P. A., Sanfilippo, C. M., Sahm, D. F., Decory, H. H. (2020). Trends in antibiotic resistance among ocular microorganisms in the united states from 2009 to 2018. JAMA Ophthalmol. 138, 439–450. doi: 10.1001/jamaophthalmol.2020.0155
Burke, R. E., Halpern, M. S., Baron, E. J., Gutierrez, K. (2009). Pediatric and neonatal staphylococcus aureus bacteremia: Epidemiology, risk factors, and outcome. Infect. Control Hosp Epidemiol. 30, 636–644. doi: 10.1086/597521
Chen, J., Lin, H., Wei, H., Hsu, Y. L., Lai, H. C., Low, Y. Y., et al. (2021). Clinical characteristics and outcomes of culture-negative versus culture-positive osteomyelitis in children treated at a tertiary hospital in central Taiwan. J. Microbiol. Immunol. 54, 1061–1069. doi: 10.1016/j.jmii.2020.08.005
Clinical and Laboratory Standards Institute (2019). Performance standards for antimicrobial susceptibility testing. CLSI document M100-S29, 29th informational supplement. Available at: USA,www.clsi.org.
Decousser, J., Woerther, P., Soussy, C., Fines-Guyon, M., Dowzicky, M. J. (2018). The tigecycline evaluation and surveillance trial; assessment of the activity of tigecycline and other selected antibiotics against gram-positive and gram-negative pathogens from France collected between 2004 and 2016. Antimicrob Resist Infect Control. 7, 68. doi: 10.1186/s13756-018-0360-y
Delorme, T., Garcia, A., Nasr, P. (2017). A longitudinal analysis of methicillin-resistant and sensitive staphylococcus aureus incidence in respect to specimen source, patient location, and temperature variation. Int. J. Infect. Dis. 54, 50–57. doi: 10.1016/j.ijid.2016.11.405
Dolapo, O., Dhanireddy, R., Talati, A. J. (2014). Trends of staphylococcus aureus bloodstream infections in a neonatal intensive care unit from 2000-2009. BMC Pediatr. 14, 12. doi: 10.1186/1471-2431-14-121
Doudoulakakis, A. G., Bouras, D., Drougka, E., Kazantzi, M., Michos, A., Charisiadou, A., et al. (2016). Community-associated staphylococcus aureus pneumonia among Greek children: Epidemiology, molecular characteristics, treatment, and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 35, 1177–1785. doi: 10.1007/s10096-016-2651-7
Greenberg, R. G., Wu, H., Maharaj, A., Cohen-Wolkowiez, M., Tomashek, K. M., Osborn, B. L., et al. (2020). A pharmacoepidemiologic study of the safety and effectiveness of clindamycin in infants. Pediatr. Infect. Dis. J. 39, 204–210. doi: 10.1097/INF.0000000000002524
Hamdy, R. F., Dona, D., Jacobs, M. B., Gerber, J. S. (2019). Risk factors for complications in children with staphylococcus aureus bacteremia. J. Pediatr. 208, 214–220. doi: 10.1016/j.jpeds.2018.12.002
Huang, L., Zhang, R., Hu, Y., Zhou, H. W., Cao, J. M., Lv, H. Y., et al. (2019). Epidemiology and risk factors of methicillin-resistant staphylococcus aureus and vancomycin-resistant enterococci infections in zhejiang China from 2015 to 2017. Antimicrob. Resist. Infect. Control. 8, 90. doi: 10.1186/s13756-019-0539-x
Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X. F., Xu, Y. C., et al. (2016). Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance 2005-2014. Clin. Microbiol. Infect. 22, S9–14. doi: 10.1016/j.cmi.2016.01.001
Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X. F., Xu, Y. C., et al. (2017). CHINET surveillance of bacterial resistance in China: 2016 report. Chin. J. Infect. Chemother. 17, 481–491. doi: 10.16718/j.1009-7708.2017.05.001
Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X. F., Xu, Y. C., et al. (2018). CHINET surveillance of bacterial resistance in China: 2017 report. Chin. J. Infect. Chemother. 18, 241–251. doi: 10.16718/j.1009-7708.2018.03.001
Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X. F., Xu, Y. C., et al. (2020a). CHINET surveillance of bacterial resistance in China: 2018 report. Chin. J. Infect. Chemother. 20, 1–10. doi: 10.16718/j.1009-7708.2020.01.001
Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X. F., Xu, Y. C., et al. (2020b). CHINET surveillance of bacterial resistance in China: 2019 report. Chin. J. Infect. Chemother. 20, 233–243. doi: 10.16718/j.1009-7708.2020.03.001
Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X. F., Xu, Y. C., et al. (2021). CHINET surveillance of bacterial resistance in China: 2020 report. Chin. J. Infect. Chemother. 21, 377–387. doi: 10.16718/j.1009-7708.2021.04.001
Ilczyszyn, W. M., Sabat, A. J., Akkerboom, V., Szkarlat, A., Klepacka, J., Sowa-Sierant, I., et al. (2016). Clonal structure and characterization of staphylococcus aureus strains from invasive infections in paediatric patients from south Poland: Association between age, spa types, clonal complexes, and genetic markers. PloS One 11, e01519373. doi: 10.1371/journal.pone.0151937
Kramer, T. S., Schroeder, C., Behnke, M., Aghdassi, S. J., Geffers, C., Gastmeier, R., et al. (2019). Decrease of methicillin resistance in staphylococcus aureus in nosocomial infections in Germany-a prospective analysis over 10 years. J. Infection. 78, 215–219. doi: 10.1016/j.jinf.2018.12.005
Kumarachandran, G., Johnson, J. K., Shirley, D. A., Graffunder, E., Heil, E. L. (2017). Predictors of adverse outcomes in children with staphylococcus aureus bacteremia. J. Pediatr. Pharmacol. Ther. 22, 218–226. doi: 10.5863/1551-6776-22.3.218
Liang, Y., Tu, C., Tan, C., Ahmed, M., Dai, M., Xia, Y., et al. (2019). Antimicrobial resistance, virulence genes profiling and molecular relatedness of methicillin-resistant staphylococcus aureus strains isolated from hospitalized patients in guangdong province, China. Infect. Drug Resist. 12, 447–459. doi: 10.2147/IDR.S192611
Lim, W. H., Lien, R., Huang, Y., Lee, W., Lai, J. (2014). Community-associated methicillin-resistant staphylococcus aureus necrotizing pneumonia in a healthy neonate. J. Microbiol. Immunol. Infect. 47, 555–557. doi: 10.1016/j.jmii.2012.07.001
Liu, C., Bayer, A., Cosgrove, S. E., Daum, R. S., Fridkin, S. K., Gorwitz, R. J., et al. (2011). Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52, 285–292. doi: 10.1093/cid/cir034
Marcelin, J. R., Challener, D. W., Tan, E. M., Lahr, B. D., Baddour, L. M. (2017). Incidence and effects of seasonality on nonpurulent lower extremity cellulitis after the emergence of community-acquired methicillin-resistant staphylococcus aureus. Mayo Clin. Proc. 92, 1227–1233. doi: 10.1016/j.mayocp.2017.04.008
Miguel, C. P. V., Mejias, A., Leber, A., Sanchez, P. (2019). A decade of antimicrobial resistance in staphylococcus aureus: A single center experience. PloS One 14, e02120292. doi: 10.1371/journal.pone.0212029
Milstone, A. M., Carroll, K. C., Ross, T., Shangraw, K. A., Perl, T. M. (2010). Community-associated methicillin-resistant staphylococcus aureus strains in pediatric intensive care unit. Emerg. Infect. Dis. 16, 647–655. doi: 10.3201/eid1604.090107
Perovic, O., Iyaloo, S., Kularatne, R., Lowman, W., Bosman, N., Wadula, J., et al. (2015). Prevalence and trends of staphylococcus aureus bacteraemia in hospitalized patients in south Africa 2010 to 2012: Laboratory-based surveillance mapping of antimicrobial resistance and molecular epidemiology. PloS One 10, e0145429. doi: 10.1371/journal.pone.0145429
Rahimi, F., Shokoohizadeh, L. (2016). Characterization of methicillin resistant staphylococcus aureus strains among inpatients and outpatients in a referral hospital in Tehran, Iran. Microb. Pathog. 97, 89–93. doi: 10.1016/j.micpath.2016.06.006
Samadi, R., Ghalavand, Z., Mirnejad, R., Nikmanesh, B., Eslami, G. (2019). Antimicrobial resistance and molecular characteristics of methicillin-resistant staphylococcus aureus isolates from children patients in Iran. Infect. Drug Resist. 12, 3849–3857. doi: 10.2147/IDR.S229394
Song, Z., Gu, F., Guo, X., Ni, Y., He, P., Han, L. (2017). Antimicrobial resistance and molecular characterization of staphylococcus aureus causing childhood pneumonia in shanghai. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00455
Takemura, H., Mochizuki, T. (2018). Comparison between local and national epidemiology of antimicrobial resistance using the JANIS data. J. Infect. Chemother. 24, 868–872. doi: 10.1016/j.jiac.2018.08.016
Tian, L., Zhang, Z., Sun, Z. (2019). Antimicrobial resistance trends in bloodstream infections at a large teaching hospital in China: A 20-year surveillance study, (1998-2017). Antimicrob. Resist. Infect. Control. 8, 86. doi: 10.1186/s13756-019-0545-z
Vallejo, J. G., Cain, A. N., Mason, E. O., Kaplan, S. L., Hulten, K. G. (2017). Staphylococcus aureus central nervous system infections in children. Pediatr. Infect. Dis. J. 36, 947–951. doi: 10.1097/INF.0000000000001603
Wang, H., Huang, C., Huang, Y. (2017). Clinical features and molecular characteristics of childhood community-associated methicillin-resistant staphylococcus aureus infection in a medical center in northern Taiwan 2012. BMC Infect. Dis. 17, 470. doi: 10.1186/s12879-017-2560-0
Weiss, L., Lansell, A., Figueroa, J., Suchdev, P. S., Kirpalani, A. (2020). Declining prevalence of methicillin-resistant staphylococcus aureus septic arthritis and osteomyelitis in children: Implications for treatment. Antibiotics-Basel. 9, 101. doi: 10.3390/antibiotics9030101
Keywords: methicillin-resistant Staphylococcus aureus, antimicrobial resistance, children, infectious disease surveillance of pediatrics (ISPED), neonates
Citation: Wu X, Wang C, He L, Xu H, Jing C, Chen Y, Lin A, Deng J, Cao Q, Deng H, Cai H, Chen Y, Yang J, Zhang T, Huang Y, Hao J and Yu H (2023) Antimicrobial resistance profile of methicillin-resistant Staphylococcus aureus isolates in children reported from the ISPED surveillance of bacterial resistance, 2016–2021. Front. Cell. Infect. Microbiol. 13:1102779. doi: 10.3389/fcimb.2023.1102779
Received: 19 November 2022; Accepted: 04 January 2023;
Published: 19 January 2023.
Edited by:
Fann Wu, Columbia University Irving Medical Center, United StatesReviewed by:
Nazan Tuna, Namik Kemal University, TürkiyeXiaotian Zheng, Akron Children’s Hospital, United States
Copyright © 2023 Wu, Wang, He, Xu, Jing, Chen, Lin, Deng, Cao, Deng, Cai, Chen, Yang, Zhang, Huang, Hao and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Yu, eXVodWk0NzU2QHNpbmEuY29t
 Xia Wu
Xia Wu Chuanqing Wang
Chuanqing Wang Leiyan He2
Leiyan He2 Hongmei Xu
Hongmei Xu Yinghu Chen
Yinghu Chen Jikui Deng
Jikui Deng Qing Cao
Qing Cao Huiling Deng
Huiling Deng Ting Zhang
Ting Zhang Hui Yu
Hui Yu