
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 29 June 2023
Sec. Fungal Pathogenesis
Volume 13 - 2023 | https://doi.org/10.3389/fcimb.2023.1079535
This article is part of the Research TopicEmerging Fungal Pathogens: PerspectivesView all 7 articles
Objective: Malassezia furfur (M. furfur) is a lipophilic, conditionally pathogenic yeast that mainly causes skin infections, but the reports of related invasive infections are increasing. The aim of this study is to provide clinical data to assist physicians in the management of patients with invasive infections caused by M. furfur.
Methods: A case of pulmonary infection caused by M. furfur in a hematopoietic stem cell transplant patient for aplastic anemia was reported. In addition, the literature on invasive infection by M. furfur published in PubMed and Web of Science in English until 31 July 2022 was reviewed.
Results: Clinical data analysis of 86 patients (from 37 studies and our case) revealed that most of them were preterm (44.2%), followed by adults (31.4%). M. furfur fungemia occurred in 79.1% of the 86 patients, and 45 of them were clearly obtained from catheter blood. Other patients developed catheter-related infections, pneumonia, peripheral thromboembolism, endocarditis, meningitis, peritonitis and disseminated infections. Thirty-eight preterm infants had underlying diseases such as very low birth weight and/or multiple organ hypoplasia. The remaining patients had compromised immunity or severe gastrointestinal diseases. 97.7% of patients underwent invasive procedures and 80.2% received total parenteral nutrition (TPN). Fever, thrombocytopenia and leukocytosis accounted for 55.8%, 38.4% and 24.4% of patients with M. furfur invasive infections, respectively. 69.8% of the patients received antifungal therapy, mainly amphotericin B (AmB) or azoles. Of 84 patients with indwelling catheters, 58.3% underwent the removal of catheters. TPN were discontinued in 30 of 69 patients. The all-cause mortality of 86 patients was 27.9%.
Conclusions: M. furfur can cause a variety of invasive infections. These patients mostly occur in premature infants, low immunity and severe gastrointestinal diseases. Indwelling catheters and TPN infusion are major risk factors. AmB, l-AmB and azoles are the most commonly used agents, and simultaneous removal of the catheter and termination of TPN infusion are important for the treatment of M. furfur invasive infections.
Malassezia are yeast species belonging to Basidiomycota, Ustilaginomycotina, and class Malasseziomycetes, which are commonly found on the skin surfaces of human body and other warm-blooded animals (Theelen et al., 2021). Currently, the Malassezia genus comprises 18 species with numerous functionally distinct strains based on morphology, microstructure, physiological biochemistry and molecular biology (Vijaya Chandra et al., 2021). As a conditionally pathogenic fungus, it is well known for causing a variety of skin diseases such as tinea versicolor, seborrheic dermatitis/dandruff, atopic dermatitis, folliculitis and psoriasis (Abdillah and Ranque, 2021). Besides these skin disorders, invasive infections caused by Malassezia have been increasingly reported in recent years.
Malassezia furfur (M. furfur) is the most encountered species from bloodstream infections in the Malassezia genus, followed by M. pachydermatis and M. sympodialis, especially in premature neonates and immunocompromised hosts (Rhimi et al., 2020). Apart from fungemia, pneumonia, peritonitis, meningitis and disseminated infection caused by M. furfur have been continuously reported worldwide in recent years (Shek et al., 1989; Johnson et al., 1996; Rosales et al., 2004; Baker et al., 2016). However, the epidemiology of these infections caused by M. furfur has not been well investigated. Due to its lipid-dependent nature, M. furfur infections are likely to be underestimated in routine clinical cultures (Theelen et al., 2018). The non-specific clinical manifestations of these infections make the diagnosis of M. furfur even more difficult.
Here, we describe a case of pulmonary infection caused by M. furfur in a patient undergoing hematopoietic stem cell transplantation (HSCT) for aplastic anemia (AA). As the largest tertiary medical center in the region, M. furfur is isolated from respiratory specimen for the first time in our hospital, which arises our great interest. In order to improve the understanding of M. furfur among clinicians and laboratory personnel, this article reviews the cases of invasive infections caused by it. In addition, the latest advances in the pathogenesis, diagnosis and treatment of M. furfur invasive infections were summarized and discussed.
A 17-year-old male underwent HSCT in our hematology department 7 months ago for AA. Immunosuppressant and hormone therapy were routinely administered after transplantation. The patient developed fever 3 days before admission, and a chest computed tomography (CT) examination at the local hospital indicated severe infection of both lungs. At admission, laboratory tests revealed a normal white blood cell count (WBC) but with a markedly reduced platelet count (56×109/L) and low hemoglobin (105 g/L). His C-reactive protein (CRP) was markedly elevated (52.1 mg/L), and the level of galactomannan was normal. Multi-row CT chest scan showed multiple patchy ground-glass shadows in the lower lobe of both lungs, considering the possibility of infectious lesions.
Blood cultures drawn from peripheral vein and central venous catheter (CVC) were negative. No abnormality was observed in sputum culture. Due to the aggravation of infection, bronchoscopy with bronchoalveolar lavage was performed. After centrifugation of bronchoalveolar lavage fluid (BALF), gram staining and Gomori methenamine silver (GMS) staining revealed some bottle-shaped and budding yeast cells (Figures 1A, B). The BALF was cultured to sabouraud dextrose agar (SDA) and columbia blood agar medium and incubated at 35°C. Dust-like colonies grew on the SDA after 3 days of incubation (Figure 2A) but not on blood agar. After 5 days of culture, small creamy and fragile colonies were observed on SDA plate (Figure 2B). Gram staining of the colonies showed a culster of budding yeast cells, with a collarette-like structure seen at the junction between the budding and the mother spore (Figure 1C). The colonies were transferred to another SDA and CHROMagar™ Candida covered with a thin layer of sterile olive oil for subculturing. After 3 days of incubation, the morphology of colonies on the SDA covered with olive oil (Figure 2C) were cheese-colored, raised, smooth or rough, and pink on CHROMagar™ Candida covered with olive oil (Figure 2D). The colonies were identified as M. furfur by Vitek 2 Compact microbial identification system (BioMérieux) and matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) with a 99.9% confidence level. Furthermore, the second-generation sequencing results of BALF showed that the number of sequences of M. furfur was as high as 5830.

Figure 1 (A) After centrifugation of bronchoalveolar lavage fluid, gram staining of the precipitate revealed gram-positive and ‘bottle-shaped’ budding yeast cells (black arrows indicated). (B) Bronchoalveolar lavage fluid staining with gomori methenamine silver showed dark brown and budding spores (black arrows indicated). (C) Gram staining of the colonies showed ‘bottle-shaped’ budding yeast cells, with a collarette-like thickening seen at the junction between the budding and the mother spores.
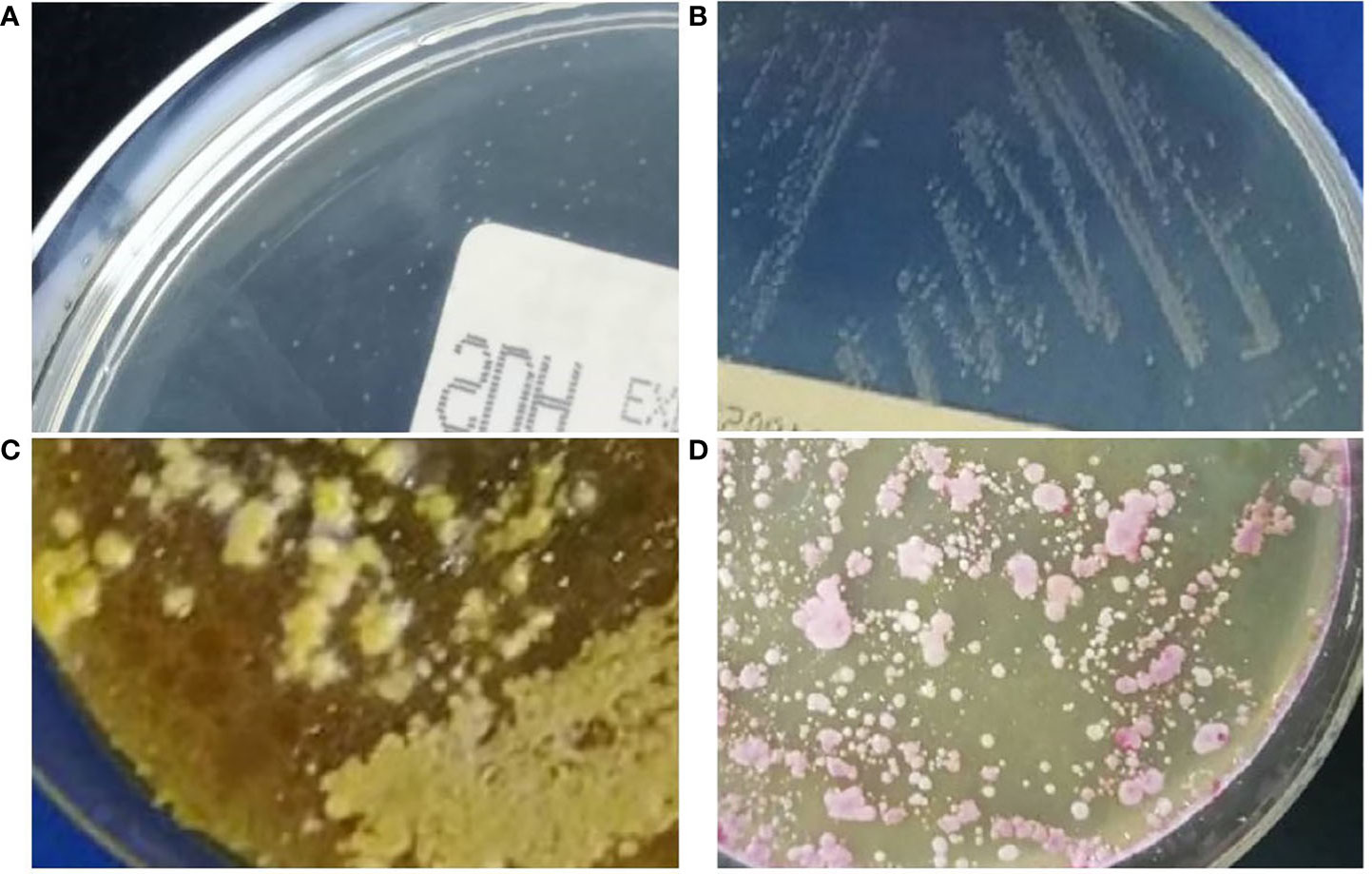
Figure 2 (A) Dust-like colonies were found in bronchoalveolar lavage fluid cultured on sabouraud dextrose agar plate for 3 days at 35°C. (B) Small creamy and fragile colonies were observed on the sabouraud dextrose agar plate after 5 days at 35°C. (C) After 3 days of incubation on sabouraud dextrose agar covered with sterile olive oil at 35°C, the morphology of colonies was cheesy, raised, smooth or rough. (D) The colonies appeared pink on CHROMagar™ Candida with sterile olive oil supplementation after 3 days at 35°C.
Originally, pneumocystis carinii infection was highly suspected clinically, given the patient’s condition. Biapenem, sulfamethoxazole-trimethoprim and caspofungin were administered as anti-microbial therapy. When the pathogen was confirmed as M. furfur, caspofungin was replaced with voriconazole (VOR, 200mg every 12 hours) for antifungal treatment. After 14 days of treatment, the patient had no fever, and the symptoms of chest distress and asthma were improved. The results of chest CT reexamination showed that the infection was better than before. The patient was transferred from the intensive care unit to the hematology department and continued oral VOR (200mg twice daily) for 14 days. At follow-up 3 months later, the patient continued to show clinical improvement with no signs of infection.
We conducted literature searches in PubMed and Web of Science, and the literature was published until 31 July 2022. The query was ‘Malassezia furfur’, and article type choosed ‘Case report’. All relevant, available full texts published in English were extracted and the details of clinical data were retrieved.
A total of 37 articles including 85 patients were obtained through retrieval (Figure 3) and included in the data analysis together with the case of this study. The clinical data of 86 patients with M. furfur invasive infections were shown in Table 1. The geographic distribution, demographic characteristics, site of infection, underlying disease, clinical feature, laboratory data, treatment and patient outcome were summarized and analyzed.
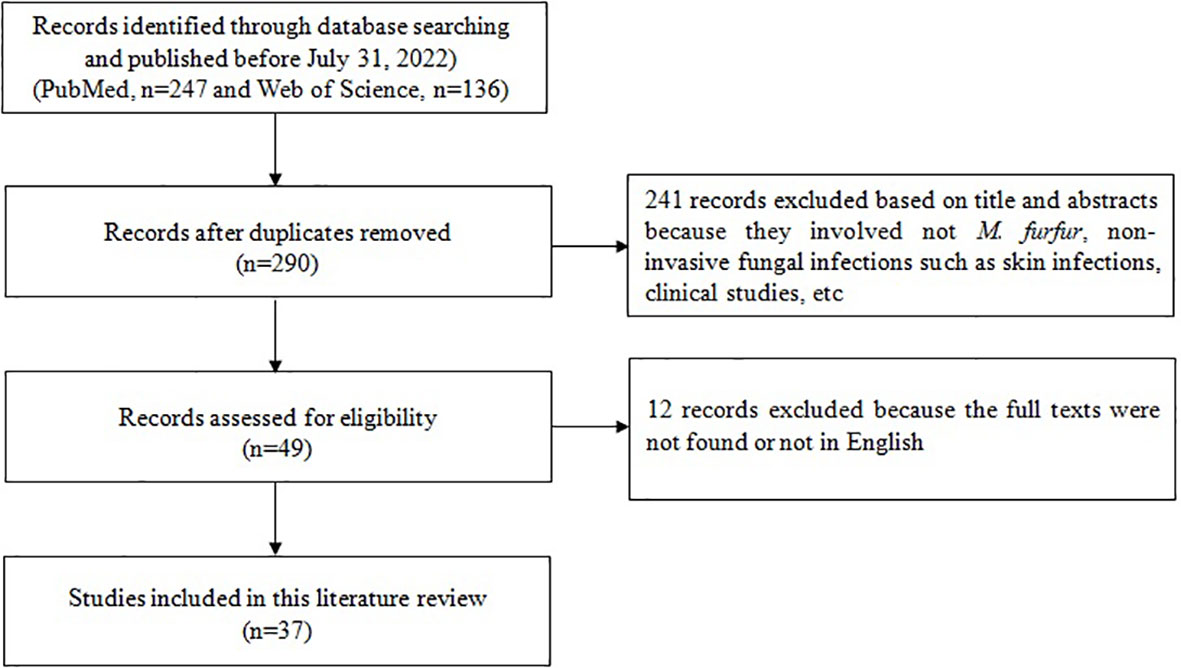
Figure 3 Flow-chart of articles related to case reports of invasive infection caused by Malassezia furfur.
The majority of 86 patients in this study were reported from USA with 68 patients (80%), 6 from Belgium, 3 each from China and Portugal, 2 in Australia, and 1 each from France, UK, Italy and Japan. 79.1% of the patients were isolated from the blood culture, of which 45 were definitely drawn from catheter blood. In addition, mycotic thrombosis around the indwelling catheter tip or leading to catheter occlusion, peripheral thromboembolism, pneumonia, endocardial neoplasms, meningitis, peritonitis, pulmonary vasculitis, inflammation of alveolar, bronchial and bronchiolitis, and disseminated infections (spread to heart, kidney, liver, spleen and pancreas) caused by M. furfur were reported.
62.0% were male in 79 patients with known gender. The majority of 86 patients were premature neonates (44.2%), followed by adults older than 18 years (31.4%) and infants younger than 1 year (12.8%). Among 38 premature infants, 78.9% were less than 32 weeks of gestational age and all had very low birth weight (VLBW, birth weight was less than 1500g). These premature infants were born with multiple organ hypoplasia. In addition, 12 patients had hematological malignancy and half of which underwent stem cell transplantation (SCT), and 11 patients had solid malignant tumor. Other patients reported in the literature presented with a variety of underlying diseases such as Crohn’s disease, Hirschsprung disease, short bowel syndrome, bowel surgery, and HIV infection.
97.7% of patients underwent invasive procedures, and predominantly with central venous access devices (CVADs). All 38 premature infants had CVADs and received total parenteral nutrition (TPN). Among the 48 patients of non-premature infants, 31 patients (64.6%) received intralipid emulsions, and 20 of them were statistically found to be caused by feeding intolerance, gastrointestinal disease and/or intestinal resection. The most common presentations were fever (55.8%), bradycardia (15.1%), apnea (14.0%), and respiratory distress (8.1%). Laboratory examination showed thrombocytopenia in 38.4% patients and leukocytosis in 24.4% patients. Leukopenia was found in 15 patients, and further analysis revealed that 6 of them were premature, and the other 9 had immunodeficiency disease or had received chemoradiotherapy or SCT for malignancy.
69.8% of the patients received anti-microbial therapy. Of 84 patients with indwelling catheters, 58.3% underwent the removal of catheters. Parenteral lipid emulsions were discontinued in 30 of 69 patients. Amphotericin B (AmB) or liposomal-AmB (l-AmB) was the most commonly used antifungal agent, accounting for 76.7% (46/60). 17 patients were treated with azole drugs, most of which were combined with AmB or sequential therapy. Of the 26 patients who did not receive antifungal therapy, 80.8% had catheters removed (partially simultaneous termination of TPN) and 34.6% terminated TPN. The all-cause mortality of 86 patients was 27.9%. Among the 24 patients who died, 15 cases of bacteremia, 5 of pneumonia and 4 of disseminated infection were caused by M. furfur. All deaths had CVADs, and 20 patients received TPN infusion. There were 22 deaths that were treated with antifungal therapy, 17 of which were treated with AmB or l-AmB. Only 6 of these 22 patients had the catheter removed.
The overall incidence of invasive fungal infection (IFI) was approximately 6 cases per 100 000 persons per year, with a mortality of 27.6% (von Lilienfeld-Toal et al., 2019). Recent studies have reported higher morbidity and mortality in neonates and immunocompromised patients, especially those with cancer and stem cell or solid-organ transplant (Bassetti et al., 2021; Puerta-Alcalde and Garcia-Vidal, 2021; Weimer et al., 2022). Candida and Aspergillus were generally considered to be the most common pathogens of invasive fungal disease (IFD). However, increasingly emerging opportunistic fungal (such as Malassezia) infections were being recognized and might play an important role in IFD (Miceli et al., 2011). The majority of invasive infections caused by Malassezia genus reported in recent years were associated with the lipid-dependent M. furfur. A 12-month study of fungal bloodstream infections in critical care patients showed a higher prevalence of M. furfur (2.1%) than candida spp. (1.4%) in patients with very complex underlying diseases or premature infants (Iatta et al., 2014). The severity of invasive infection caused by M. furfur could range from localized organ infection to fulminant fungemia with death (Devlin, 2006). Due to improper diagnosis, invasive infections caused by M. furfur were likely to be underestimated. In this study, we provided an overview of the epidemiology, risk factors, pathogenesis, clinical feature, diagnosis, treatment and clinical outcome of M. furfur invasive infections.
Several retrospective studies found that host immunosuppression played a dominant role in the development of IFI (Jenks et al., 2020). Congenital immune deficiency, prolonged neonatal intensive care units (NICU) stay, multiple courses of antibiotics and surgical treatment might increase the risk of M. furfur infection in premature infants. M. furfur colonization has been reported to occur in rates of 36.8% to 64% of infants hospitalized in NICU (Nguyen et al., 2001). Furthermore, there has been a nosocomial outbreak of systemic M. furfur infection in neonatal nurseries, with evidence of human-to-human transmission (Devlin, 2006). The presence and duration (>10 days) of granulocytopenia, treatment with glucocorticoids, chemoradiotherapy, and exposure to broad-spectrum antibiotics that caused changes in endogenous microbiota might be predisposing conditions for M. furfur infection. Limon et al. found that Malassezia was strongly associated with the inflammatory bowel disease, especially Crohn’s disease (Limon et al., 2019). In addition, Malassezia probably colonized internal organs in immunocompromised HIV patients (Abdillah and Ranque, 2021). The correlation of M. furfur infection with these underlying diseases deserved further investigation.
The risk of opportunistic fungal infection was increased by invasive procedures such as deep venous catheterization, mechanical ventilation and percutaneous drainage that breached the skin barrier and served as a port of entry and a nidus for pathogen infection (Jenks et al., 2020). Biofilms formation of M. furfur played an important role in its pathogenesis. It underwent multistep growth processes, including contacting with suitable substrates, adhesion to the biotic or abiotic surface by reversible hydrophobic interactions, production of polysaccharide material, biofilms maturation and dissemination (Inigo and Del Pozo, 2018). The correlation between hydrophobicity, adherence and biofilms formation in M. furfur species was examined and confirmed, as reported by other authors for Candida albicans (Borghi et al., 2011; Vavala et al., 2013). Human tissues, indwelling medical devices, other implanted foreign bodies and mucosal surfaces provided the substrate for biofilms colonization and infection (Boisvert et al., 2016). Once adhered to the surfaces, pathogens were extremely difficult to be eradicated, leading to a large number of nosocomial infections. The increased use of medical devices such as catheters and prosthetics contributed to the recurrent invasive infections and increased mortality rates among patients (Lagree and Mitchell, 2017).
Malassezia yeasts could produce a variety of enzymes, such as lipases and phospholipases that utilized lipids and proteases that utilized proteins (Vijaya Chandra et al., 2021). These enzymes might play complex roles in the human hosts, on one hand were involved in the pathogenesis, and on the other hand had a protective function (Rhimi et al., 2020). Studies found that M. furfur strains causing fungemia showed very high lipolytic enzyme activity, which were considered as virulence factors since they might be related to the colonization, proliferation, invasion and persistence in host tissues (Petrokilidou et al., 2019). However, Malassezia yeasts lacked fatty acid synthase and δ-9 desaturase, making them unable to synthesize lipids thus suggesting that they might need to inhabit lipid-rich skin or use exogenous lipids (Vijaya Chandra et al., 2021). Colonization of M. furfur on the surfaces of skin and catheter might increase the chance of invasive infections, and parenteral lipid emulsions could play an important role in their growth. Contamination during catheter placement, injection of contaminated lipids, hematogenous seeding of the catheter from a distant source, migration of M. furfur from the exit site on skin along the outer wall of the catheter and from the catheter hub through the lumen have been suggested as possible infection pathways (Marcon and Powell, 1987).
This study showed that invasive infections caused by M. furfur were special in that most of them occurred in prematurity, immunocompromised hosts, and patients requiring TPN via CVADs due to severe gastrointestinal disease and/or bowel resection. Given that these risk factors were largely unmodifiable, M. furfur invasive infections were likely to continue in the future. Studies have found that there were no differences in clinical features or laboratory markers between candida and Malassezia fungemia (Iatta et al., 2014). However, M. furfur fungemia appeared earlier and lasted longer than candidemia, which undoubtedly increased its mortality (Rhimi et al., 2020). In addition, infections caused by M. furfur seemed to be more complicated and more likely to lead to disseminated infections of the lungs, heart, kidneys, liver, spleen, and brain than M. pachyderm as another Malassezia causing fungemia (Huang et al., 2020). Hence, early diagnosis and timely and effective treatment were the key to reduce the mortality of invasive infections caused by M. furfur.
Isolation and accurate identification of M. furfur from the site of infection is currently the gold standard for the diagnosis of related infections. Unlike other studies, M. furfur grew on SDA plates in our case, which might differ in nutrient composition from plates from different manufacturers, or the small amount of lipids remaining in the specimen might have contributed to its growth. However, the dust-like colonies were easily overlooked, and its morphological characteristics were not typical due to poor growth. M. furfur did not grow well on SDA plate, but colony morphology could be clearly observed after cultured on SDA covered with sterile olive oil. Olive oil was composed of oleic acid and triglyceride esters of palmitic acid, which were good for promoting the growth of M. furfur (Devlin, 2006). It grew at temperatures between 25°C and 37°C, with optimal growth at 35°C-37°C (Dankner et al., 1987). If M. furfur infection was suspected, the clinician should alert the microbiology personnel at the time of culture submission. In our case, pink colonies of M. furfur were clearly visible on our modified chromogenic Candida medium (covered with sterile olive oil). MALDI-TOF MS was increasingly used and provided faster and more reliable identification at the Malassezia species level (Rhimi et al., 2021). In recent years, the development of molecular methods such as next-generation sequencing (NGS) had made it possible to identify yeasts directly from clinical specimens without the need to cultivate. Moreover, DNA sequencing and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) could differentiate the vast majority of Malassezia species (Didehdar et al., 2014; Lian et al., 2014).
Thus far, the antifungal susceptibility testing recommended by the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) was not suitable for Malassezia species due to their lipid-dependent nature, slow growth and a tendency to form cell clusters (Rhimi et al., 2021). A variety of alternative procedures for the broth micro-dilution have been proposed in recent years, such as modified LN broth FastFung broth and modified Dixon broth (Far et al., 2018; Abdillah et al., 2021). However, there is neither standard parameters including incubation time, culture media and inoculation amount established for the determination of MIC values of Malassezia spp. nor susceptibility/resistance breakpoints of antifungal drugs. Thereby, standard procedures for antimicrobial susceptibility profiles of Malassezia species needed to be further established.
Azoles, polyenes and echinocandins were the main antifungal agents used to manage various types of fungal infections. The first two antifungal drugs were frequently employed to treat Malassezia-related infections in humans and animals. Topical antifungal agents (mainly azoles) were adequate for the treatment of localized skin lesions, while systemic fluconazole (FLZ) or itraconazole (ITZ) for severe skin diseases. Intravenous AmB or its formulations was recommended for the treatment of systemic infections caused by M. furfur (Chen et al., 2020; Chen et al., 2021). The antifungal activity of l-AmB was higher than deoxycholate AmB, which might be contributed to the lipophilic nature of this yeast (Iatta et al., 2015). Usually, approximately 24 days of AmB treatment might be useful for a positive outcome of M. furfur fungemia (Rhimi et al., 2020). Furthermore, the observed in vitro susceptibility values suggested that Malassezia might be intrinsically resistant to echinocandins such as caspofungin and micafungin (Rhimi et al., 2021). If echinocandins were used to prevent fungal infection in hospitalized patients, they might be selective for Malassezia, potentially leading to Malassezia-related infections.
The formation of fungal biofilms not only played an important role in the pathogenesis, but also increased the resistance to immune defense and antifungal therapy (Le Mauff, 2020; Pathakumari et al., 2020). Successful eradication of biofilm-associated fungi was difficult, mainly because it required higher concentrations of antifungal agents than those needed to kill planktonic cells, which were generally toxic to the host. Removal or exchange of medical devices containing biofilms was the most important treatment for biofilm infections (Muakkassa and Ghannoum, 2016). In patients with catheter-related M. furfur infections, catheter removal, discontinuation of parenteral lipid nutrition and the institution of systemic antifungal treatment were recommended (Pedrosa et al., 2018).
All-cause mortality rate in this study was 27.9% (24/86). All deaths had CVADs, and 20 patients received TPN infusion. As risk factors for M. furfur infection, whether indwelling catheter and TPN infusion were associated with the mortality needed further investigation. Although 17 patients received the recommended AmB or l-AmB treatment, the cause of death was likely related to delayed diagnosis, delayed administration of medication, and failure to combine the removal of the indwelling catheter. However, this was a retrospective study, and the data about the time from hospitalization to diagnosis and the time from diagnosis to medication were missing, so the cause of death could not be further discussed, which was also the limitation of this study.
In summary, we provide a case report and literature review on invasive infections caused by M. furfur, demonstrating the epidemiology, risk factors, pathogenesis, diagnosis and treatment. M. furfur invasive infections generally present with nonspecific symptoms and occur in preterm infants or patients with immunocompromised or underlying gastrointestinal diseases. Indwelling catheter and TPN infusion are the main risk factors and involved in the pathogenesis of infection. Pathogenic diagnosis requires special attention to the lipophilic characteristics of M. furfur. Standard procedures for in vitro antimicrobial susceptibility testing of M. furfur needed to be further established. Currently, the most commonly used anti-M. furfur drugs in clinical practice are azoles and polyenes, especially AmB or l-AMB. Removal of the catheter and termination of TPN infusion are effective in the treatment of M. furfur invasive infections.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Jiangsu Province Hospital. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
XZ designed the study, collected the data, and wrote the manuscript. FJ and FN contributed to the fungal identification and manuscript writing. FJ, YX, YL contributed to extract data from literature and drawing of figures and tables in the manuscript. WX and YL contributed to manuscript revisions. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdillah, A., Packeu, A., Khelaifia, S., Ranque, S. (2021). Intra- and inter-laboratory comparison of mDixon and FastFung broths for Malassezia antifungal susceptibility testing. Mycoses 64 (7), 716–720. doi: 10.1111/myc.13270
Abdillah, A., Ranque, S. (2021). Chronic diseases associated with Malassezia yeast. J. Fungi (Basel) 7 (10), 855. doi: 10.3390/jof7100855
Alpert, G., Bell, L. M., Campos, J. M. (1987). Malassezia furfur fungemia in infancy. Clin. Pediatr. (Phila) 26 (10), 528–531. doi: 10.1177/000992288702601007
Arnow, P. M., Kushner, R. (1991). Malassezia furfur catheter infection cured with antibiotic lock therapy. Am. J. Med. 90 (1), 128–130. doi: 10.1016/0002-9343(91)90518-3
Baker, R. M., Stegink, R. J., Manaloor, J. J., Schmitt, B. H., Stevens, J. C., Christenson, J. C. (2016). Malassezia pneumonia: a rare complication of parenteral nutrition therapy. JPEN J. Parenter Enteral Nutr. 40 (8), 1194–1196. doi: 10.1177/0148607115595224
Barber, G. R., Brown, A. E., Kiehn, T. E., Edwards, F. F., Armstrong, D. (1993). Catheter-related Malassezia furfur fungemia in immunocompromised patients. Am. J. Med. 95 (4), 365–370. doi: 10.1016/0002-9343(93)90304-8
Bassetti, M., Azoulay, E., Kullberg, B. J., Ruhnke, M., Shoham, S., Vazquez, J., et al. (2021). EORTC/MSGERC definitions of invasive fungal diseases: summary of activities of the intensive care unit working group. Clin. Infect. Dis. 72 (Suppl 2), S121–S127. doi: 10.1093/cid/ciaa1751
Bhargava, P., Longhi, L. P. (2007). Images in clinical medicine. peripheral smear with Malassezia furfur. N Engl. J. Med. 356 (24), e25. doi: 10.1056/NEJMicm063672
Blaes, A. H., Cavert, W. P., Morrison, V. A. (2009). Malassezia: is it a pulmonary pathogen in the stem cell transplant population? Transpl. Infect. Dis. 11 (4), 313–317. doi: 10.1111/j.1399-3062.2009.00404.x
Boisvert, A. A., Cheng, M. P., Sheppard, D. C., Nguyen, D. (2016). Microbial biofilms in pulmonary and critical care diseases. Ann. Am. Thorac. Soc. 13 (9), 1615–1623. doi: 10.1513/AnnalsATS.201603-194FR
Borghi, E., Sciota, R., Biassoni, C., Cirasola, D., Cappelletti, L., Vizzini, L., et al. (2011). Cell surface hydrophobicity: a predictor of biofilm production in candida isolates? J. Med. Microbiol. 60 (Pt 5), 689–690. doi: 10.1099/jmm.0.026898-0
Brooks, R., Brown, L. (1987). Systemic infection with Malassezia furfur in an adult receiving long-term hyperalimentation therapy. J. Infect. Dis. 156 (2), 410–411. doi: 10.1093/infdis/156.2.410
Chen, I. T., Chen, C.-C., Huang, H.-C., Kuo, K.-C. (2020). Malassezia furfur emergence and candidemia trends in a neonatal intensive care unit during 10 years the experience of fluconazole prophylaxis in a single hospital. Adv. Neonatal Care 20 (1), E3–E8. doi: 10.1097/ANC.0000000000000640
Chen, S. C., Perfect, J., Colombo, A. L., Cornely, O. A., Groll, A. H., Seidel, D., et al. (2021). Global guideline for the diagnosis and management of rare yeast infections: an initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 21 (12), e375–e386. doi: 10.1016/s1473-3099(21)00203-6
Chu, C. M., Lai, R. W. (2002). Malassezia furfur fungaemia in a ventilator-dependent patient without known risk factors. Hong Kong Med. J. 8 (3), 212–214.
Dankner, W. M., Spector, S. A. (1985). Malasezzia furfur sepsis in neonates. J. Pediatr. 107 (4), 643–644. doi: 10.1016/s0022-3476(85)80044-5
Dankner, W. M., Spector, S. A., Fierer, J., Davis, C. E. (1987). Malassezia fungemia in neonates and adults: complication of hyperalimentation. Rev. Infect. Dis. 9 (4), 743–753. doi: 10.1093/clinids/9.4.743
Devlin, R. K. (2006). Invasive fungal infections caused by Candida and Malassezia species in the neonatal intensive care unit. Adv. Neonatal Care 6 (2), 68–77. doi: 10.1016/j.adnc.2006.01.005
Didehdar, M., Mehbod, A. S. A., Eslamirad, Z., Mosayebi, M., Hajihossein, R., Ghorbanzade, B., et al. (2014). Identification of Malassezia species isolated from patients with pityriasis versicolor using PCR-RFLP method in markazi province, central Iran. Iran J. Public Health 43 (5), 682–686.
Doerr, C. A., Demmler, G. J., Garcia-Prats, J. A., Brandt, M. L. (1994). Solitary pyogenic liver abscess in neonates: report of three cases and review of the literature. Pediatr. Infect. Dis. J. 13 (1), 64–69. doi: 10.1097/00006454-199401000-00014
Far, F. E., Al-Obaidi, M. M. J., Desa, M. N. M. (2018). Efficacy of modified leeming-notman media in a resazurin microtiter assay in the evaluation of in-vitro activity of fluconazole against Malassezia furfur ATCC 14521. J. Mycol. Med. 28 (3), 486–491. doi: 10.1016/j.mycmed.2018.04.007
Garcia, C. R., Johnston, B. L., Corvi, G., Walker, L. J., George, W. L. (1987). Intravenous catheter-associated Malassezia furfur fungemia. Am. J. Med. 83 (4), 790–792. doi: 10.1016/0002-9343(87)90917-x
Gidding, H., Hawes, L., Dwyer, B. (1989). The isolation of Malassezia furfur from an episode of peritonitis. Med. J. Aust. 151 (10), 603. doi: 10.5694/j.1326-5377.1989.tb101300.x
Glaser, C. A., Atwater, S. K. (1995). Febrile infant with a percutaneous vascular catheter. Pediatr. Infect. Dis. J. 14 (2), 163, 165–166.
Granok, A. B. (2020). Successful treatment of Malassezia furfur endocarditis. Open Forum Infect. Dis. 7 (8), ofaa029. doi: 10.1093/ofid/ofaa029
Hassall, E., Ulich, T., Ament, M. E. (1983). Pulmonary embolus and Malassezia pulmonary infection related to urokinase therapy. J. Pediatr. 102 (5), 722–725. doi: 10.1016/s0022-3476(83)80244-3
Huang, C. Y., Peng, C. C., Hsu, C. H., Chang, J. H., Chiu, N. C., Chi, H. (2020). Systemic infection caused by Malassezia pachydermatis in infants: case series and review of the literature. Pediatr. Infect. Dis. J. 39 (5), 444–448. doi: 10.1097/INF.0000000000002591
Iatta, R., Cafarchia, C., Cuna, T., Montagna, O., Laforgia, N., Gentile, O., et al. (2014). Bloodstream infections by Malassezia and Candida species in critical care patients. Med. Mycol. 52 (3), 264–269. doi: 10.1093/mmy/myt004
Iatta, R., Immediato, D., Montagna, M. T., Otranto, D., Cafarchia, C. (2015). In vitro activity of two amphotericin b formulations against Malassezia furfur strains recovered from patients with bloodstream infections. Med. Mycol. 53 (3), 269–274. doi: 10.1093/mmy/myu089
Inigo, M., Del Pozo, J. L. (2018). Fungal biofilms: from bench to bedside. Rev. Esp. Quimioter. 31 Suppl 1, 35–38.
Jenks, J. D., Cornely, O. A., Chen, S. C., Thompson, G. R., 3rd, Hoenigl, M. (2020). Breakthrough invasive fungal infections: who is at risk? Mycoses 63 (10), 1021–1032. doi: 10.1111/myc.13148
Johnson, A. S., Bailey, E., Wright, P. A., Solomon, L. (1996). Malassezia furfur: a possible cause of culture-negative CAPD peritonitis. Perit. Dial. Int. 16 (2), 187–188. doi: 10.1177/089686089601600222
Kessler, A. T., Kourtis, A. P., Simon, N. (2002). Peripheral thromboembolism associated with Malassezia furfur sepsis. Pediatr. Infect. Dis. J. 21 (4), 356–357. doi: 10.1097/00006454-200204000-00022
Kim, E. H., Cohen, R. S., Ramachandran, P., Glasscock, G. F. (1993). Adhesion of percutaneously inserted silastic central venous lines to the vein wall associated with Malassezia furfur infection. JPEN J. Parenter. Enteral. Nutr. 17 (5), 458–460. doi: 10.1177/0148607193017005458
Lagree, K., Mitchell, A. P. (2017). Fungal biofilms: inside out. Microbiol. Spectr. 5 (2), 10. doi: 10.1128/microbiolspec.FUNK-0024-2016
Le Mauff, F. (2020). Exopolysaccharides and biofilms. Curr. Top. Microbiol. Immunol. 425, 225–254. doi: 10.1007/82_2020_199
Lian, C.H., Shen, L.l., Gao, Q.Y., Jiang, M., Zhao, Z.J., Zhao, J.J. (2014). Identification of malassezia species in the facial lesions of Chinese seborrhoeic dermatitis patients based on DNA sequencing. Mycoses 57 (12), 759–764. doi: 10.1111/myc.12229
Limon, J. J., Tang, J., Li, D., Wolf, A. J., Michelsen, K. S., Funari, V., et al. (2019). Malassezia is associated with crohn's disease and exacerbates colitis in mouse models. Cell Host Microbe 25 (3), 377–388 e376. doi: 10.1016/j.chom.2019.01.007
Long, J. G., Keyserling, H. L. (1985). Catheter-related infection in infants due to an unusual lipophilic yeast–Malassezia furfur. Pediatrics 76 (6), 896–900. doi: 10.1542/peds.76.6.896
Marcon, M. J., Powell, D. A. (1987). Epidemiology, diagnosis, and management of Malassezia furfur systemic infection. Diagn. Microbiol. Infect. Dis. 7 (3), 161–175. doi: 10.1016/0732-8893(87)90001-0
Masure, O., Leostic, C., Abalain, M. L., Chastel, C., Yakoub-Agha, I., Berthou, C., et al. (1991). Malassezia furfur septicaemia in a child with leukaemia. J. Infect. 23 (3), 335–336. doi: 10.1016/0163-4453(91)93296-o
Miceli, M. H., Díaz, J. A., Lee, S. A. (2011). Emerging opportunistic yeast infections. Lancet Infect. Dis. 11 (2), 142–151. doi: 10.1016/s1473-3099(10)70218-8
Middleton, C., Lowenthal, R. M. (1987). Malassezia furfur fungemia as a treatable cause of obscure fever in a leukemia patient receiving parenteral nutrition. Aust. N. Z. J. Med. 17 (6), 603–604. doi: 10.1111/j.1445-5994.1987.tb01270.x
Muakkassa, F. K., Ghannoum, M. (2016). Updates on therapeutic strategies against Candida (and Aspergillus) biofilm related infections. Adv. Exp. Med. Biol. 931, 95–103. doi: 10.1007/5584_2016_11
Myers, J. W., Smith, R. J., Youngberg, G., Gutierrez, C., Berk, S. L. (1992). Fungemia due to Malassezia furfur in patients without the usual risk factors. Clin. Infect. Dis. 14 (2), 620–621. doi: 10.1093/clinids/14.2.620
Nguyen, S. T., Lund, C. H., Durand, D. J. (2001). Thrombolytic therapy for adhesion of percutaneous central venous catheters to vein intima associated with Malassezia furfur infection. J. Perinatol. 21 (5), 331–333. doi: 10.1038/sj.jp.7200517
Oliveri, S., Trovato, L., Betta, P., Romeo, M. G., Nicoletti, G. (2011). Malassezia furfur fungaemia in a neonatal patient detected by lysis-centrifugation blood culture method: first case reported in Italy. Mycoses 54 (5), e638–e640. doi: 10.1111/j.1439-0507.2010.01955.x
Pathakumari, B., Liang, G., Liu, W. (2020). Immune defence to invasive fungal infections: a comprehensive review. BioMed. Pharmacother. 130, 110550. doi: 10.1016/j.biopha.2020.110550
Pedrosa, A. F., Lisboa, C., Rodrigues, A. G. (2018). Malassezia infections with systemic involvement: figures and facts. J. Dermatol. 45 (11), 1278–1282. doi: 10.1111/1346-8138.14653
Petrokilidou, C., Pavlou, E., Gaitanis, G., Bassukas, I. D., Saridomichelakis, M. N., Velegraki, A., et al. (2019). The lipid profile of three Malassezia species assessed by raman spectroscopy and discriminant analysis. Mol. Cell Probes 46, 101416. doi: 10.1016/j.mcp.2019.06.006
Powell, D. A., Aungst, J., Snedden, S., Hansen, N., Brady, M. (1984). Broviac catheter-related Malassezia furfur sepsis in five infants receiving intravenous fat emulsions. J. Pediatr. 105 (6), 987–990. doi: 10.1016/s0022-3476(84)80096-7
Powell, D. A., Marcon, M. J. (1987). Failure to eradicate Malassezia furfur broviac catheter infection with antifungal therapy. Pediatr. Infect. Dis. J. 6 (6), 579–580. doi: 10.1097/00006454-198706000-00022
Puerta-Alcalde, P., Garcia-Vidal, C. (2021). Changing epidemiology of invasive fungal disease in allogeneic hematopoietic stem cell transplantation. J. Fungi (Basel) 7 (10), 848. doi: 10.3390/jof7100848
Redline, R. W., Dahms, B. B. (1981). Malassezia pulmonary vasculitis in an infant on long-term intralipid therapy. N. Engl. J. Med. 305 (23), 1395–1398. doi: 10.1056/nejm198112033052307
Redline, R. W., Redline, S. S., Boxerbaum, B., Dahms, B. B. (1985). Systemic Malassezia furfur infections in patients receiving intralipid therapy. Hum. Pathol. 16 (8), 815–822. doi: 10.1016/s0046-8177(85)80253-7
Rhimi, W., Theelen, B., Boekhout, T., Aneke, C. I., Otranto, D., Cafarchia, C. (2021). Conventional therapy and new antifungal drugs against Malassezia infections. Med. Mycol. 59 (3), 215–234. doi: 10.1093/mmy/myaa087
Rhimi, W., Theelen, B., Boekhout, T., Otranto, D., Cafarchia, C. (2020). Malassezia spp. yeasts of emerging concern in fungemia. Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00370
Rosales, C. M., Jackson, M. A., Zwick, D. (2004). Malassezia furfur meningitis associated with total parenteral nutrition subdural effusion. Pediatr. Dev. Pathol. 7 (1), 86–90. doi: 10.1007/s10024-003-4030-5
Schleman, K. A., Tullis, G., Blum, R. (2000). Intracardiac mass complicating Malassezia furfur fungemia. Chest 118 (6), 1828–1829. doi: 10.1378/chest.118.6.1828
Shek, Y. H., Tucker, M. C., Viciana, A. L., Manz, H. J., Connor, D. H. (1989). Malassezia furfur–disseminated infection in premature infants. Am. J. Clin. Pathol. 92 (5), 595–603. doi: 10.1093/ajcp/92.5.595
Surmont, I., Gavilanes, A., Vandepitte, J., Devlieger, H., Eggermont, E. (1989). Malassezia furfur fungaemia in infants receiving intravenous lipid emulsions. a rarity or just underestimated? Eur. J. Pediatr. 148 (5), 435–438. doi: 10.1007/bf00595906
Tetsuka, N., Muramatsu, H., Iguchi, M., Oka, K., Morioka, H., Takahashi, Y., et al. (2022). Difficulties in diagnosing Malassezia furfur bloodstream infection and possibility of spontaneous resolution in a patient undergoing chemotherapy for neuroblastoma: a case report. J. Infect. Chemother. 28 (7), 987–990. doi: 10.1016/j.jiac.2022.02.026
Theelen, B., Cafarchia, C., Gaitanis, G., Bassukas, I. D., Boekhout, T., Dawson, T. L., Jr. (2018). Malassezia ecology, pathophysiology, and treatment. Med. Mycol. 56, S10–S25. doi: 10.1093/mmy/myx134
Theelen, B., Christinaki, A. C., Dawson, T. L., Boekhout, T., Kouvelis, V. N. (2021). Comparative analysis of Malassezia furfur mitogenomes and the development of a mitochondria-based typing approach. FEMS Yeast Res. 21 (7), foab051. doi: 10.1093/femsyr/foab051
Vavala, E., Colone, M., Passariello, C., Celestino, I., Toccacieli, L., Stringaro, A., et al. (2013). Characterization of biofilms in drug-sensitive and drug-resistant strains of Candida albicans. J. Chemother. 25 (2), 87–95. doi: 10.1179/1973947812Y.0000000047
Vijaya Chandra, S. H., Srinivas, R., Dawson, T. L., Jr., Common, J. E. (2021). Cutaneous Malassezia: commensal, pathogen, or protector? Front. Cell. Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.614446
von Lilienfeld-Toal, M., Wagener, J., Einsele, H., Cornely, O. A., Kurzai, O. (2019). Invasive fungal infection. Dtsch. Arztebl. Int. 116 (16), 271–278. doi: 10.3238/arztebl.2019.0271
Weimer, K. E. D., Smith, P. B., Puia-Dumitrescu, M., Aleem, S. (2022). Invasive fungal infections in neonates: a review. Pediatr. Res. 91 (2), 404–412. doi: 10.1038/s41390-021-01842-7
Keywords: Malassezia furfur, invasive infection, fungemia, pulmonary infection, premature infant, indwelling catheter, amphotericin B
Citation: Zhang X, Jin F, Ni F, Xu Y, Lu Y and Xia W (2023) Clinical data analysis of 86 patients with invasive infections caused by Malassezia furfur from a tertiary medical center and 37 studies. Front. Cell. Infect. Microbiol. 13:1079535. doi: 10.3389/fcimb.2023.1079535
Received: 11 November 2022; Accepted: 12 June 2023;
Published: 29 June 2023.
Edited by:
Anna Katrina Walduck, Charles Sturt University, AustraliaReviewed by:
Peralam Yegneswaran Prakash, Manipal Academy of Higher Education, IndiaCopyright © 2023 Zhang, Jin, Ni, Xu, Lu and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanfei Lu, NTQ5NzkzNTQ2QHFxLmNvbQ==; Wenying Xia, eGlhd2VueWluZzIxMTA2ODkxQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.