
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 07 November 2022
Sec. Virus and Host
Volume 12 - 2022 | https://doi.org/10.3389/fcimb.2022.988694
This article is part of the Research Topic Pathogenesis, Vaccines, and Antivirals Against Respiratory Viruses View all 5 articles
 Hongru Li1,2†
Hongru Li1,2† Xiongpeng Zhu3†
Xiongpeng Zhu3† Rongguo Yu4†
Rongguo Yu4† Xin Qian5†
Xin Qian5† Yu Huang6†
Yu Huang6† Xiaoping Chen7†
Xiaoping Chen7† Haibin Lin8
Haibin Lin8 Huiming Zheng9
Huiming Zheng9 Yi Zhang10
Yi Zhang10 Jiarong Lin11
Jiarong Lin11 Yanqin Deng12
Yanqin Deng12 Wen Zhong1
Wen Zhong1 Yuejiao Ji7
Yuejiao Ji7 Qing Li1
Qing Li1 Jiabin Fang1
Jiabin Fang1 Xiaojie Yang1
Xiaojie Yang1 Rong Lin13
Rong Lin13 Fangsu Chen1
Fangsu Chen1 Zhijun Su13*
Zhijun Su13* Baosong Xie1*
Baosong Xie1* Hong Li2,14*
Hong Li2,14*Object: This study attempted to explore the effects of vaccination on disease severity and the factors for viral clearance and hospitalization in omicron-infected patients.
Methods: The clinical manifestations of 3,265 Omicron-infected patients (BA.2 lineage variant; the Omicron group) were compared with those of 226 Delta-infected patients (the Delta group). A Multi-class logistic regression model was employed to analyze the impacts of vaccination doses and intervals on disease severity; a logistic regression model to evaluate the risk factors for hospitalization; R 4.1.2 data analysis to investigate the factors for time for nucleic acid negativization (NAN).
Results: Compared with the Delta group, the Omicron group reported a fast transmission, mild symptoms, and lower severity incidence, and a significant inverse correlation of vaccination dose with clinical severity (OR: 0.803, 95%CI: 0.742-0.868, p<0.001). Of the 7 or 5 categories of vaccination status, the risk of severity significantly decreased only at ≥21 days after three doses (OR: 0.618, 95% CI: 0.475-0.803, p<0.001; OR: 0.627, 95% CI: 0.482-0.815, p<0.001, respectively). The Omicron group also reported underlying illness as an independent factor for hospitalization, sore throat as a protective factor, and much shorter time for NAN [15 (12,19) vs. 16 (12,22), p<0.05]. NAN was associated positively with age, female gender, fever, cough, and disease severity, but negatively with vaccination doses.
Conclusion: Booster vaccination should be advocated for COVID-19 pandemic-related control and prevention policies and adequate precautions should be taken for patients with underlying conditions.
The COVID-19 outbreak in early 2020 has brought about a global pandemic, with a toll of tens of thousands of deaths (6,294,252 in total as of May 30, 2022). Of the new variants of the novel coronavirus, the Omicron mutant was first identified in Botswana on November 9, 2021 and soon spread worldwide from South Africa. (Viana et al., 2022) It was imported to Hong Kong and Chinese mainland Tianjin in November 2021, and has spread to many regions. The BA.2 lineage variant has infected over 600,000 people in Shanghai (Zhang et al., 2022) and a total of 3,265 patients in Quanzhou.
Compared with the Delta variant, the Omicron variant features a higher transmissibility (R0 value: 9.5 vs. 3.4) (Liu and Rocklöv, 2022) and a shorter incubation period (3.6 d vs. 4.6 d) (Du et al., 2022). It is also characterized by more noticeable upper respiratory symptoms (strong nasal tropism) and lower incidence of pneumonia (less obvious lung tropism). (Hui et al., 2022) Due to the decreased virulence, the Omicron variant infection displays milder clinical manifestations, reduced hospitalization risk (about 50-70% of that of the Delta variant) (Mahase, 2021), and decreased mortality rate (about half of the Delta variant) (Sigal et al., 2022).
To date, vaccination provides effective protection (Andrews et al., 2022) against the Omicron variant and reduces hospitalization risk. However, mutations in S protein compromise the effectiveness of neutralizing antibodies and vaccination, leading to immune escape and breakthrough infections. (Cele et al., 2022; Planas et al., 2022) Given that booster vaccines can only reinforce the protection temporarily, (Andrews et al., 2022) the COVID-19 pandemic is far from over. A South African study has predicted 100 million infections on the 100th day of Omicron prevalence, 160,000 deaths and a case fatality rate of about 0.58%. (Sigal et al., 2022) WHO has recently announced that the Omicron strain has caused 830,000 excess deaths. (Wang et al., 2022) The outbreak of Hong Kong Omicron epidemic from January to March 2022 has claimed 9,115 deaths, with a mortality rate of 0.76%. (Smith et al., 2022) Therefore, the gravity of the Omicron variant should not be underestimated and it remains urgent to formulate effective epidemic prevention strategies.
Currently, available research has probed into the efficacy of different vaccines against Omicron (Andrews et al., 2022), the symptomatic differences between Omicron and Delta infection and the relationship between the vaccine doses and the hospitalization risk (Menni et al., 2022). However, little relevant literature is available to elucidate the effects of vaccination on the disease severity of the Omicron variant infection and the hazard factors for hospitalization and time for viral clearance. Therefore, the current study tackled this very issue by comparing two recent regional COVID-19 surges in China (one local Omicron and one local Delta outbreak), so as to provide evidence for drafting effective public health policies in coping with the pandemic.
The flow chart of the study design can be found in the supplementary data (Figure S1). All the SARS-CoV-2 cases during two recent regional outbreaks in Fujian, China (from 2021 to the present) were confirmed by fluorescence real-time PCR (RT-PCR). They were divided into the Omicron group: 3,265 Omicron BA.2-infected patients in Quanzhou City, Fujian, China between March 13 and May 6, 2022; and the Delta group: 226 Delta-infected patients (Delta B.1.167.2) in Putian City, Fujian, China from September 10 to October 20, 2021 (Li H. et al., 2022). The two locations were geographically adjacent to each other, with the Han ethnics accounting for over 99% of the total population. Different from previous other overseas studies (Herrmann et al., 2021) that only included symptomatic populations, this study enrolled all nucleic acid-positive cases in the regional outbreaks, including asymptomatic and mild patients. Informed consent was granted by all patients and the study protocol was approved by the Ethics Committee of the First Hospital of Quanzhou City (No. 202212) and of the Affiliated Hospital of Putian University (No. 202152).
Clinical and experimental data were collected from electronic medical records using standardized data collection forms. The clinical data of the patients included symptoms, signs, and treatment prognosis. The laboratory data included the cycle threshold (Ct) value of SARS-CoV-2 RT-PCR upon admission and serological results of whole blood cells, biochemical tests, blood coagulation function, C-reactive protein, and IL-6 within 24 hours after admission (Test kits and apparatus were described in Table S1, normal value of test results were shown in Table S2). The information of vaccination time and doses of the patients was obtained from Chinese Health Organization, most patients (99.47%) received inactivated vaccination (including Sinopharm and CoronaVac vaccine), and a few (0.46%) received Recombinant novel coronavirus protein vaccine (Zhifei Longcom) and 0.07% received mRNA vaccine (CanSino). Nasopharyngeal swabs were collected every day from the 7th day of the disease course until the nucleic acid negativization (NAN). The time for NAN was defined as the duration from the beginning of the patient’s disease course or the day of a positive SARS-CoV-2 nucleic acid swab test to the recovery of the patient when two consecutive negative nucleic acid swab tests were obtained at an interval of more than 24 hours. The NAN standard was defined as a Ct value of over 35 in the Omicron group [“Corona Virus Disease-19 Prevention and Control: Consensus Diagnosis and treatment of corona virus disease-19 (9th trial edition),” 2022] and of over 40 in the Delta group (Li H. et al., 2022).
As reported in the Delta variant (B.1.617.2) group (Li H. et al., 2022), the second-generation whole genome sequencing was performed by Fujian Provincial Center for Disease Control and Prevention (CDC) for the positive SARS-CoV-2 specimens by fluorescence real-time PCR. The epidemic strain was confirmed to be the Omicron variant (BA.2 lineage), with which all patients were infected. All of the sequences of virus were uploaded to Chinese CDC, who finalized the accessibility of the data.
The disease severity was classified into asymptomatic, mild (no pneumonia on chest X-ray), moderate (pneumonia by chest imaging), severe (SpO2<93%, supplemental oxygen needed) or critical (requiring RICU or mechanical ventilation) categories. In the Omicron group, the asymptomatic and mild patients were quarantined in the mobile cabin hospital (MCH) while patients with moderate or severer symptoms were hospitalized for treatment (as the latter group of patients were hospitalized and there were few severe/critical cases, patients with moderate symptoms were recruited for the analysis of hospitalization risks). All Delta patients were treated in hospitals. Most patients in the Omicron group received traditional Chinese medicine (TCM) treatment and were monitored for vital signs, body temperature and respiration. Oxygen inhalation treatment was prescribed for patients with SpO2 below 93%; Nematevir/Ritonavir antiviral treatment was administered to patients showing unstable disease progression within 3-5 days and no contraindications; specific immunotherapies, such as COVID-19 immunoglobulin, neutralizing antibody or convalescent plasma, were initiated for high risk patients with high viral load or a rapid disease progression in the early stage of the disease course; glucocorticoids were only used for people with specific needs and antibiotics were for patients complicated with bacterial infection [“Corona Virus Disease-19 Prevention and Control: Consensus Diagnosis and treatment of corona virus disease-19 (9th trial edition),” 2022]. The Delta group received the same treatment schemes, except for nematevir/ritonavir, which was not yet available then (Li H. et al., 2022).
For descriptive analysis, we presented data as mean (variance) or median [interquartile range (IQR)] for continuous parameters and as frequency (percentage) for categorical variables using IBM SPSS Stastistic25 data analysis software. Wilcoxon rank sum testing was employed for numerical variables and Fisher’s precision probability test was adopted for categorical variables, if a sample size was less than 5 in the cells of the contingency table of the corresponding indicators, and the Chi Square test was used for the rest. An ordered multivariate logistic regression model was used to analyze the effects of vaccine doses and intervals on disease severity of patients. A disordered multi-category logistic regression model to analyze the hospitalization risk factors. The factors affecting the time for NAN which illuminated the viral clearance was examined by the least-squares regression of the R 4.1.2 data analysis. A two-tailed P value of less than 0.05 was considered statistically significant.
As for the effect of vaccination on disease severity, since the principle of multivariate-ordered logistic regression is to categorize the multiple dependent variables and analyze them in turn by multiple binary logistic regression, the three categories of clinical classification, i.e., asymptomatic, mild, and moderate, were imported into two binary logistic regression equations, namely “asymptomatic vs. mild+moderate” and “asymptomatic + mild vs. moderate”. The two estimated values corresponded to the threshold and were the constant terms of the two equations, respectively, rather than a reflection of the situation of the three categories of clinical classification. Meanwhile, since the independent variable coefficients in both equations were the same, only one set of independent variable coefficients was presented in the result.
The demographic characteristics of the patients are shown in Table 1. The total number of infected persons in the Omicron group and the Delta group was 3,265 and 266, respectively. The median age of the Omicron-infected patients was higher than that of the Delta-infected patients [36 (25,48) vs. 32 (9,46)] (Li H. et al., 2022); the most common primary diseases in both groups were hypertension (5.61% vs. 2.65%) and diabetes (2.62% vs. 4.87%); 16 pregnant women were found in the Omicron group.
Due to the specific vaccination strategy and policies in China, in the Omicron group, children aged 0-2 years old were not vaccinated while patients under 12 years old were mostly vaccinated with two doses (53.09%) and those over 12 years old were mostly vaccinated with three doses (49.23%). In the Delta group, none of the children under 12 years old was vaccinated while most of the patients older than 12 years were vaccinated with two doses (88.59%) (Table 1).
Compared with the Delta group, the Omicron group reported fewer severe symptoms (p<0.05), including asymptomatic [61.13% (1996/3265) vs. 2.21% (5/226)], mild [31.82% (1039/3265) vs. 36.73% (83/226)], moderate [2.76% (90/3265) vs. 58.41% (132/226)], severe [0.06% (2/3265) vs. 1.77% (4/226)], and critical [0.03% (1/3265) vs. 0.88% (2/226)] categories, respectively, with the incidence of severe critical symptoms paralleling the increasing age (Table 2). Furthermore, the Omicron group showed significantly lower rates of pneumonia (2.85% vs. 59.73%, p<0.001) and respiratory failure (0.06% vs. 2.65%, p<0.001), and less ICU occupancy (0.09% vs. 2.21%, p<0.05) (Table 2) No deaths were reported in both groups.
The results showed that disease severity was markedly reduced by the increased vaccination doses (OR: 0.803; 95%CI: 0.742-0.868, p<0.01) (Table 3). The association between vaccination doses and intervals and disease severity was referred to the unvaccinated Omicron-infected individuals. The vaccination status was divided into seven categories (No vaccination, <21 days post dose one, ≥21 days post dose one, but no dose two, <21 days post dose two, ≥21 days post dose two, but no dose three, <21 days post dose three, ≥21 days post dose three) (Figure 1; Table S3) or five categories (No vaccination, <21 days post dose one, ≥21 days post dose one to <21 days post dose two, ≥21 days post dose two to <21 days post dose three, ≥21 days post dose three) (Figure 1; Table S4) (Paredes et al., 2022). The analyses showed that regardless of the 7 or 5 categories of vaccination status, the disease severity was significantly reduced only at ≥21 days after 3 doses (OR: 0.618; 95% CI: 0.475-0.803, p<0.001; OR: 0.627, 95% CI: 0.482-0.815, p<0.001 respectively), with no significant difference found for the other categories (Figure 1).
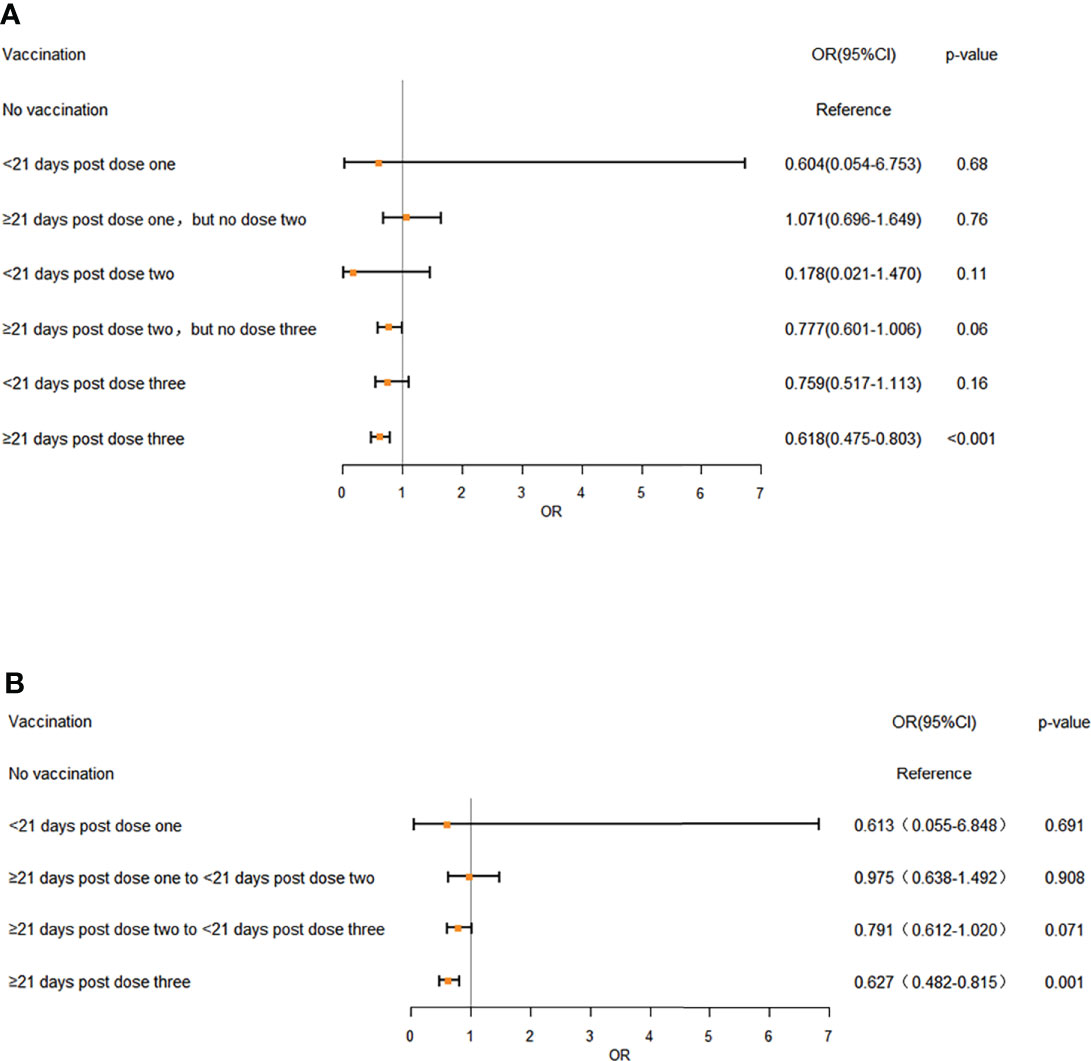
Figure 1 The effects of different vaccination doses and intervals on disease severity in Omicron infection (A) 7 different vaccination doses and intervals, (B) 5 different vaccination doses and intervals. OR: odds ratio. Error bars represent 95% CI. Unvaccinated individuals infected with Omicron served as the reference category. A multivariate ordered logistic regression model was used to analyze the effects of vaccination doses and intervals on disease severity of the patients. Doses of vaccines were treated as independent variable and three clinical symptom categories, “asymptomatic”, “mild” and “moderate”, were treated as dependent variables and designated to indicate the increasing disease severity, with 3 severe and critical patients excluded from analyses. The analyses showed that regardless of the 7 (A) or 5 categories (B) of vaccination status, the disease severity was significantly reduced only at ≥ 21 days after 3 doses (OR: 0.618; 95% CI: 0.475-0.803, p < 0.001; OR: 0.627, 95% CI: 0.482-0.815, p < 0.001 respectively), with no significant difference found for the other categories.
The results showed that the Omicron group reported a much shorter NAN time when compared with the Delta group [15 (12,19) vs. 16 (12,22), p<0.05] (Table 2). For the influencing factors, the NAN time of the Omicron group was negatively correlated with fever and disease severity, which was similar to that of the Delta group (Li H. et al., 2022) Moreover, the NAN time was negatively correlated with vaccination, but positively with female gender, age, cough, and underlying disease (Table 4), which was different from the Delta group (Li H. et al., 2022). In the Omicron group, the Ct value of nucleic acid on admission did not affect the time of NAN. The relationship between COVID-19 antibody and the time for NAN was not analyzed because the titer of COVID-19 antibody was not detected in the omicron surge.
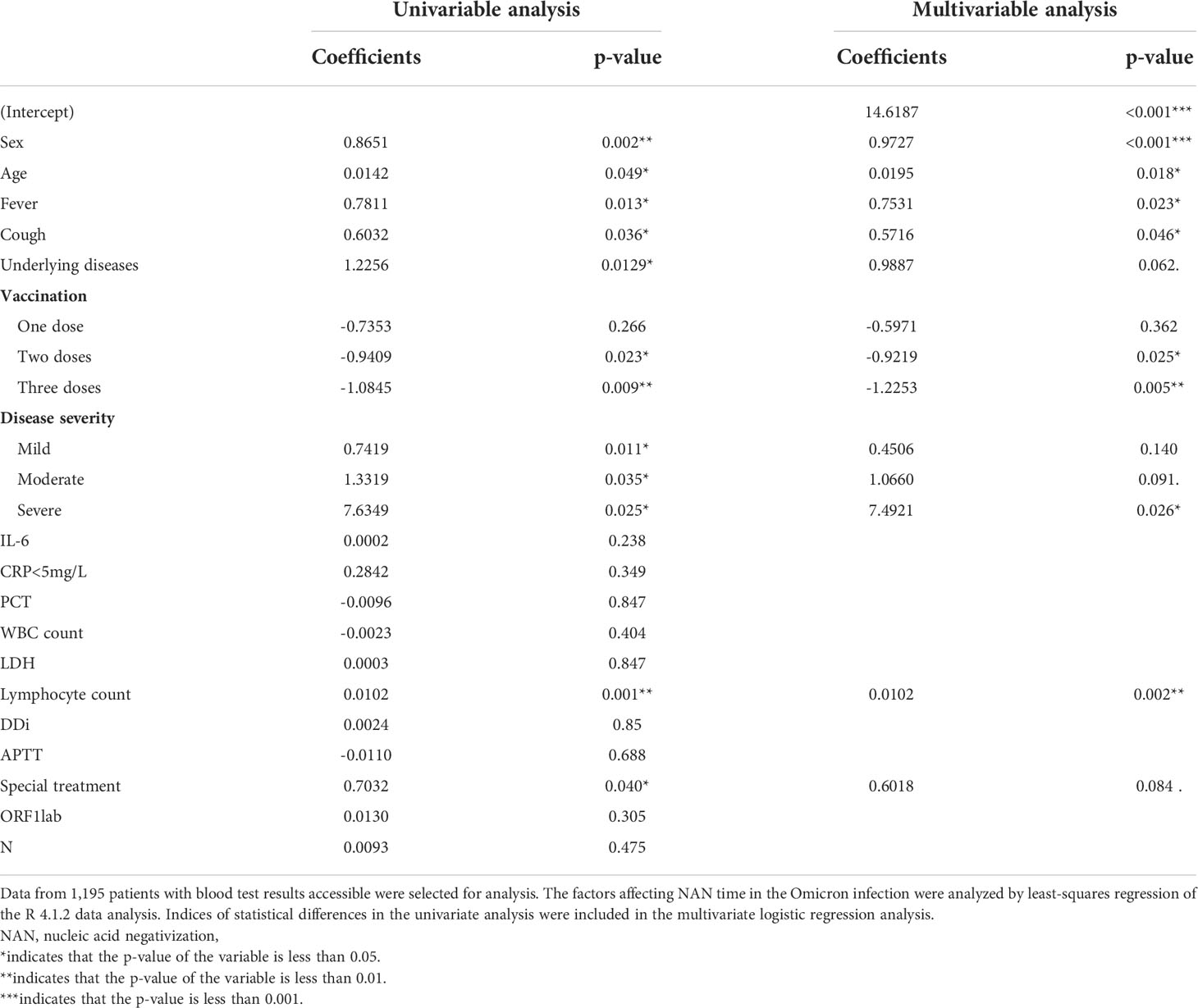
Table 4 Analysis of influencing factors for the time for nucleic acid negativization (NAN) in Omicron infection.
The results showed that the underlying disease was the most significant independent risk factor (OR: 1.299, 95% CI: 0.601-2.811, p<0.05) and sore throat was a protective factor (OR: 0.367, 95% CI: 0.16-0.84, p<0.05), with mild patients as the reference category (Table 5). The above results were significantly different from those of the Delta group (Table S5). In the latter group, an advanced age and cough were risk factors for the moderate patients while the two-dose vaccination acted as a protective factor.
Omicron variants have become the dominant VOC in the world, including multiple subfamilies known as BA.1, BA.2 and BA.3, BA.4, BA.5 lineage (Callaway, 2022; Viana et al., 2022). Compared with the Delta counterpart, the disease severity and mortality of the Omicron group decreased significantly. The data obtained in this study are consistent with the results of previous studies (Nyberg et al., 2022). The findings from the current study provide strong evidence for the timely adjustments to the pandemic control and prevention policies, especially for the developing countries that are challenged with a large population basis but inadequate healthcare resources.
Different countries have responded to the COVID-19 pandemic with vaccination, but it is still obscured whether vaccination is effective in reducing the transmission and severity of Omicron infection. Our study showed that despite the vaccination, many breakthrough infections still occurred, of which more than 45% of patients received a third booster vaccine and more than 86% of patients received two, which is consistent with previous studies (Cele et al., 2022; Planas et al., 2022). Of interest, the number of vaccinations, however, was significantly negatively correlated with the severity of Omicron infection, suggesting that vaccination can prevent the infection from exacerbation. Further analysis showed that compared with the two-dose regimen, which was found statistically ineffective in the current study, a booster vaccine fared much better and provided the strongest protection at ≥21 days. This finding is consistent with that of previous study (Andrews et al., 2022) reporting the best effect at 2-4 weeks after the booster vaccination. A potential explanation may lie in that the booster vaccine restores the Omicron-weakened neutralization of the antibodies (Andrews et al., 2022) and enhances the protection by activating the specific T cell immunity (Schultz et al., 2022). In addition, the vaccination-induced memory B cells also play an important role in fighting against the Omicron variant (Kotaki et al., 2022). Therefore, the vaccination with a booster vaccine is of great significance in the prevention of the Omicron epidemic. As the effectiveness of the booster shots markedly wanes after 9 weeks (Andrews et al., 2022), a fourth booster dose surfaces as a potential option in the response to the Omicron variant epidemic. Recent studies (Magen et al., 2022; Mallapaty, 2022) have produced inconsistent conclusions regarding the necessity of a fourth dose however. Meanwhile, the development of new vaccines (Qu et al., 2022) against specific Omicron variants is currently under way in some countries, which may offer an important means to deal with the Omicron outbreak, but their effectiveness awaits further verification.
In the current study, the hospitalization risks in the Omicron group were defined as the factors in patients of the moderate clinical category who needed to be hospitalized. We found that underlying diseases were an independent risk factor and that sore throat was a significant protective factor. Pharyngeal pain, one of the manifestations of the upper respiratory tract infection, serves as one of the significant clinical manifestations of Omicron infection, which differs from those of Delta infection (Li H. et al., 2022). The discrepancy may be related to the strong characteristic nasal tropism but less apparent lung tropism of the Omicron variant (Li H. et al., 2022). In the current study, we found that, different from the Delta group (Li H. et al., 2022), the underlying disease was an independent risk factor for hospitalization. The potential explanation may lie in the fact that despite the weakened virulence of the Omicron strain, the underlying diseases in patients can be aggravated by the Omicron infection, resulting in a higher hospitalization rate, severer or life-threatening illnesses (Zhang et al., 2022). Therefore, in preventing the Omicron epidemic, due attention should focus on the population with underlying diseases. Adequate measures, such as vaccination, priority healthcare, and early intervention for primary conditions, should be taken to minimize the hospitalization risk and spare the healthcare system from overwhelming demands.
In the current study, the overall time of NAN in the Omicron group was much shorter than that of the Delta group (Li H. et al., 2022). This difference may be attributed to the adoption of a booster vaccine in the Omicron group and the recent policy adjustments (“Corona Virus Disease-19 Prevention and Control: Consensus Diagnosis and treatment of corona virus disease-19(9th trial edition),” 2022), in which the Ct value of NAN standard was adjusted from greater than 40 to greater than 35, resulting in an early discharge, in that no infectious viral particles are detected in patients with a Ct value of 34 or over (La Scola et al., 2020) and the measures adopted in containing the local surges in China have demonstrated that the lowered NAN standard has not aggravated the epidemic and has shortened the quarantine time and eased the demands of medical resources.
Ever since the pandemic, vaccination has long been controversial in the elderly population, who are more prone to underlying conditions and thus resultant impaired immunity. This very group of people are particularly reluctant to accept vaccination due to concerns over the side effects. In the current study, further analyses found that the time for NAN was positively correlated with female gender, advanced age, and disease severity, but negatively with vaccination, in a dose-dependent manner. This finding is of particular significance for the elderly population, who may have great difficulties in viral clearance due to the underlying diseases and impaired immunity (Chen et al., 2021). They may benefit enormously from active vaccination (McMenamin et al., 2022), which can stimulate the production of neutralizing antibodies and promote viral clearance. Recent evidence demonstrates the safety and good tolerance of vaccines in the elderly with primary conditions (Li C. et al., 2022) and a necessity of vaccinating the elderly (Mwimanzi et al., 2022).
In addition, the overall NAN median time of 15 days suggests that it is necessary to quarantine the infected people for at least 15 days in order to minimize the possibility of transmission, which may be prolonged for elderly people with primary conditions. The policy of a five-day home quarantine plus a mask for additional five days, which was once implemented in the United States, has turned out quite inadequate and ineffective in inhibiting the viral transmission (Bouton et al., 2022).
Altogether, the findings from the current study evidence that vaccination reduces disease severity and accelerates viral clearance, which signifies an urgency of vaccinating the elderly population with underlying illnesses to lower the risks of hospitalization. The median time of NAN suggests that the infected people should be quarantined for at least 15 days to minimize the transmission of the virus.
Firstly, some statistical deviations are inevitable due to the limited patient population and the presence of objective confounding factors such as different vaccination doses and varied sample sizes between the Omicron and Delta groups. Secondly, for an imbalance and deviation may be present in analyzing the hospitalization risks of patients when the asymptomatic and mild patients in the Omicron group were randomly selected to compare with the patients of the moderate category. Thirdly, according to the vaccination policy in China, at the time of Delta surge, most of the patients aged ≥12y were vaccinated with two doses of vaccine and patients aged<12y were not eligible for vaccination. Therefore, the data available were insufficient for statistical analysis of the association between vaccination and severity. During the Omicron surge, sufficient data regarding different vaccination status were available for evaluating the potential association between vaccination and severity. Therefore, due to these objectivities, no comparison of the impact of vaccination on severity was conducted between Delta and Omicron groups. Fourthly, since the number of severely and critically ill patients in the two regional surges were too small (3 vs. 6 in Omicron and Delta groups, respectively) to be statistically analyzed, the effect of vaccination on disease severity only involved asymptomatic, mild, and moderate types. Finally, the COVID-19 antibody was not detected in the enrolled population of this study, so it is impossible to study the impacts of vaccination on the status of natural immunity and virus clearance.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Hospital of Quanzhou City (No. 202212) and of the Affiliated Hospital of Putian University (No. 202152). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
HL, BX, and XPC were responsible for funding acquisition. HRL, HL and ZS were responsible for design conception. XPC and YJ were responsible for formal analysis. JL, YD, WZ, QL, JF, XY, FC, RL performed data curation. XZ, YZ, ZS, HBL and YH were responsible for resources. JL and RY, XQ verified the underlying data. HRL, WZ, QL and JF prepared the original draft. All authors contributed to manuscript reviewing and editing. JL, XZ, YZ, ZS, and HBL had access to the raw data. All authors had access to all data in the study and the corresponding author had final responsibility for the decision to submit the paper for publication.
This work was supported by the following Fundings : Central Government Guiding Local Science and Technology Development of Fujian Province (2021L3018). Fujian Science and Technology Guidance Project(2021Y0100), The Major Health Research Project of Fujian Province(2021ZD01001),Natural Science Foundation of Fujian Province (2021J01658、2019J01178), We thank Professor Hongzhi Huang from Fujian Medical University for proofreading and polishing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2022.988694/full#supplementary-material
Andrews, N., Stowe, J., Kirsebom, F., Toffa, S., Rickeard, T., Gallagher, E., et al. (2022). Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl. J. Med. 386 (16), 1532–1546. doi: 10.1056/NEJMoa2119451
Bouton, T. C., Atarere, J., Turcinovic, J., Seitz, S., Sher-Jan, C., Gilbert, M., et al. (2022). Viral dynamics of omicron and delta SARS-CoV-2 variants with implications for timing of release from isolation: A longitudinal cohort study. medRxiv 2022, 2022.04.04.22273429. doi: 10.1101/2022.04.04.22273429
Callaway, E. (2022). Are COVID surges becoming more predictable? new omicron variants offer a hint. Nature 605 (7909), 204–206. doi: 10.1038/d41586-022-01240-x
Cele, S., Jackson, L., Khoury, D. S., Khan, K., Moyo-Gwete, T., Tegally, H., et al. (2022). Omicron extensively but incompletely escapes pfizer BNT162b2 neutralization. Nature 602 (7898), 654–656. doi: 10.1038/s41586-021-04387-1
Chen, Y., Klein, S. L., Garibaldi, B. T., Li, H., Wu, C., Osevala, N. M., et al. (2021). Aging in COVID-19: Vulnerability, immunity and intervention. Ageing Res. Rev. 65, 101205. doi: 10.1016/j.arr.2020.101205
Corona Virus Disease-19 Prevention and Control (2022). Consensus diagnosis and treatment of corona virus disease-19(9th trial edition). Chin. Med. 17 (04), 481–487. doi: 10.16505/j.2095-0136.2022.0023.
Du, Z., Liu, C., Wang, L., Bai, Y., Lau, E. H. Y., Wu, P., et al. (2022). Shorter serial intervals and incubation periods in SARS-CoV-2 variants than the SARS-CoV-2 ancestral strain. J. Travel Med 29 (6), taac052. doi: 10.1093/jtm/taac052
Herrmann, Y., Starck, T., Brindl, N., Kitchen, P. J., Rädeker, L., Sebastian, J., et al. (2021). Description and analysis of representative COVID-19 cases-a retrospective cohort study. PloS One 16 (7), e0255513. doi: 10.1371/journal.pone.0255513
Hui, K. P. Y., Ho, J. C. W., Cheung, M. C., Ng, K. C., Ching, R. H. H., Lai, K. L., et al. (2022). SARS-CoV-2 omicron variant replication in human bronchus and lung ex vivo. Nature 603 (7902), 715–720. doi: 10.1038/s41586-022-04479-6
Kotaki, R., Adachi, Y., Moriyama, S., Onodera, T., Fukushi, S., Nagakura, T., et al. (2022). SARS-CoV-2 omicron-neutralizing memory b cells are elicited by two doses of BNT162b2 mRNA vaccine. Sci. Immunol. 7 (70), eabn8590. doi: 10.1126/sciimmunol.abn8590
La Scola, B., Le Bideau, M., Andreani, J., Hoang, V. T., Grimaldier, C., Colson, P., et al. (2020). Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 39 (6), 1059–1061. doi: 10.1007/s10096-020-03913-9
Li, C., Li, A., Bi, H., Hu, J., Yang, F., Zhou, T., et al. (2022). Immunogenicity and safety of the CoronaVac inactivated SARS-CoV-2 vaccine in people with underlying medical conditions: a retrospective study. medRxiv 2022, 2004.2028.22274402. doi: 10.1101/2022.04.28.22274402
Li, H., Lin, H., Chen, X., Li, H., Li, H., Lin, S., et al. (2022). Unvaccinated children are an important link in the transmission of SARS-CoV-2 delta variant (B1.617.2): Comparative clinical evidence from a recent community surge. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.814782
Liu, Y., Rocklöv, J. (2022). The effective reproduction number for the omicron SARS-CoV-2 variant of concern is several times higher than delta. J. Travel Med 29 (3), taac037. doi: 10.1093/jtm/taac037
Magen, O., Waxman, J. G., Makov-Assif, M., Vered, R., Dicker, D., Hernán, M. A., et al. (2022). Fourth dose of BNT162b2 mRNA covid-19 vaccine in a nationwide setting. N Engl. J. Med. 386 (17), 1603–1614. doi: 10.1056/NEJMoa2201688
Mahase, E. (2021). Covid-19: Hospital admission 50-70% less likely with omicron than delta, but transmission a major concern. Bmj 375, n3151. doi: 10.1136/bmj.n3151
Mallapaty, S. (2022). Fourth dose of COVID vaccine offers only slight boost against omicron infection. Nature. doi: 10.1038/d41586-022-00486-9
McMenamin, M. E., Nealon, J., Lin, Y., Wong, J. Y., Cheung, J. K., Lau, E. H. Y., et al. (2022). Vaccine effectiveness of two and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong. medRxiv 2022, 2003.2022.22272769. doi: 10.1101/2022.03.22.22272769
Menni, C., Valdes, A. M., Polidori, L., Antonelli, M., Penamakuri, S., Nogal, A., et al. (2022). Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID study. Lancet 399 (10335), 1618–1624. doi: 10.1016/s0140-6736(22)00327-0
Mwimanzi, F., Lapointe, H. R., Cheung, P. K., Sang, Y., Yaseen, F., Umviligihozo, G., et al. (2022). Older adults mount less durable humoral responses to two doses of COVID-19 mRNA vaccine, but strong initial responses to a third dose. J. Infect. Dis 226 (6), 983–994. doi: 10.1093/infdis/jiac199
Nyberg, T., Ferguson, N. M., Nash, S. G., Webster, H. H., Flaxman, S., Andrews, N., et al. (2022). Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet 399 (10332), 1303–1312. doi: 10.1016/s0140-6736(22)00462-7
Paredes, M. I., Lunn, S. M., Famulare, M., Frisbie, L. A., Painter, I., Burstein, R., et al. (2022). Associations between SARS-CoV-2 variants and risk of COVID-19 hospitalization among confirmed cases in Washington state: A retrospective cohort study. Clin. Infect. Dis 75 (1), e536–e544. doi: 10.1093/cid/ciac279
Planas, D., Saunders, N., Maes, P., Guivel-Benhassine, F., Planchais, C., Buchrieser, J., et al. (2022). Considerable escape of SARS-CoV-2 omicron to antibody neutralization. Nature 602 (7898), 671–675. doi: 10.1038/s41586-021-04389-z
Qu, L., Yi, Z., Shen, Y., Lin, L., Chen, F., Xu, Y., et al. (2022). Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185 (10), 1728–1744.e16. doi: 10.1016/j.cell.2022.03.044
Schultz, B. M., Melo-González, F., Duarte, L. F., Gálvez, N. M., Pacheco, G. A., Soto, J. A., et al. (2022). A booster dose of an inactivated SARS-CoV-2 vaccine increases neutralizing antibodies and T cells that recognize delta and omicron variants of concern. medRxiv. doi: 10.1101/2021.11.16.21266350
Sigal, A., Milo, R., Jassat, W. (2022). Estimating disease severity of omicron and delta SARS-CoV-2 infections. Nat. Rev. Immunol. 22 (5), 267–269. doi: 10.1038/s41577-022-00720-5
Smith, D. J., Hakim, A. J., Leung, G. M., Xu, W., Schluter, W. W., Novak, R. T., et al. (2022). COVID-19 mortality and vaccine coverage - Hong Kong special administrative region, China, January 6, 2022-march 21, 2022. MMWR Morb Mortal Wkly Rep. 71 (15), 545–548. doi: 10.15585/mmwr.mm7115e1
Viana, R., Moyo, S., Amoako, D. G., Tegally, H., Scheepers, C., Althaus, C. L., et al. (2022). Rapid epidemic expansion of the SARS-CoV-2 omicron variant in southern Africa. Nature 603 (7902), 679–686. doi: 10.1038/s41586-022-04411-y
Wang, H., Paulson, K. R., Pease, S. A., Watson, S., Comfort, H., Zheng, P., et al(2022). Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality 2020-21. Lancet 399 (10334), 1513–1536. doi: 10.1016/s0140-6736(21)02796-3
Keywords: COVID-19, vaccination, hospitalization, viral clearance, public health policy
Citation: Li H, Zhu X, Yu R, Qian X, Huang Y, Chen X, Lin H, Zheng H, Zhang Y, Lin J, Deng Y, Zhong W, Ji Y, Li Q, Fang J, Yang X, Lin R, Chen F, Su Z, Xie B and Li H (2022) The effects of vaccination on the disease severity and factors for viral clearance and hospitalization in Omicron-infected patients: A retrospective observational cohort study from recent regional outbreaks in China. Front. Cell. Infect. Microbiol. 12:988694. doi: 10.3389/fcimb.2022.988694
Received: 07 July 2022; Accepted: 14 October 2022;
Published: 07 November 2022.
Edited by:
Leonardo Susta, University of Guelph, CanadaReviewed by:
Partha Chattopadhyay, Council of Scientific and Industrial Research (CSIR), IndiaCopyright © 2022 Li, Zhu, Yu, Qian, Huang, Chen, Lin, Zheng, Zhang, Lin, Deng, Zhong, Ji, Li, Fang, Yang, Lin, Chen, Su, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Li, bGVlaG9uZzk5QDEyNi5jb20=; Zhijun Su, c3UyMzY2QHNpbmEuY29t; Baosong Xie, eGJhb3NvbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.